CN115808519A - Chemiluminescence kit for thyroxine detection and preparation method thereof - Google Patents
Chemiluminescence kit for thyroxine detection and preparation method thereofDownload PDFInfo
- Publication number
- CN115808519A CN115808519ACN202210828775.2ACN202210828775ACN115808519ACN 115808519 ACN115808519 ACN 115808519ACN 202210828775 ACN202210828775 ACN 202210828775ACN 115808519 ACN115808519 ACN 115808519A
- Authority
- CN
- China
- Prior art keywords
- compound
- solution
- thyroxine
- preparation
- detection according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及医用诊断试剂盒技术领域,具体是一种用于甲状腺素检测的化学发光试剂盒及其制备方法。The invention relates to the technical field of medical diagnostic kits, in particular to a chemiluminescent kit for detecting thyroxine and a preparation method thereof.
背景技术Background technique
血浆中的四碘甲状腺原氨酸(Tetraiodothyronine,T4)均来自甲状腺直接分泌,所以血清中T4浓度可代表甲状腺功能水平。血清中T4水平检测可直观评估出甲状腺分泌能力变化,是甲状腺相关疾病诊断监测的重要指标之一。Tetraiodothyronine (T4) in plasma is directly secreted from the thyroid gland, so the concentration of T4 in serum can represent the level of thyroid function. Serum T4 level detection can visually evaluate changes in thyroid secretion capacity, and is one of the important indicators for the diagnosis and monitoring of thyroid-related diseases.
目前临床上检测T4的常用方法有化学发光法、酶联免疫法、时间分辨荧光法等。其中化学发光免疫检测采用磁分离技术,其具有灵敏度高、精密度好、易于实现自动化操作等特点,近年来在临床上被大量推广使用。由于T4在储存期间,部分内环容易过早脱碘,从而转变成三碘甲状腺原氨酸(Triiodothyronine,T3),其转化的程度很难预测,其转化率易受各种环境条件影响,因而目前市场上此类临床诊断试剂盒普遍存在热稳定性不好、产品检测效价下降过快等质量问题,影响了产品的整体性能。At present, the commonly used methods for clinical detection of T4 include chemiluminescence, enzyme-linked immunoassay, and time-resolved fluorescence method. Among them, chemiluminescent immunoassay adopts magnetic separation technology, which has the characteristics of high sensitivity, good precision, and easy automatic operation. It has been widely promoted and used in clinical practice in recent years. During storage, part of the inner ring of T4 is prone to premature deiodination, thus converting into triiodothyronine (T3), the degree of conversion is difficult to predict, and the conversion rate is easily affected by various environmental conditions, so At present, such clinical diagnostic kits on the market generally have quality problems such as poor thermal stability and rapid decline in product testing titer, which affect the overall performance of the product.
因此,建立一种热稳定好的用于甲状腺素检测的化学发光试剂盒,对实现产业化生产、降低产品成本至关重要。Therefore, it is very important to establish a thermally stable chemiluminescent kit for thyroxine detection to realize industrial production and reduce product cost.
发明内容Contents of the invention
本发明提供了一种用于甲状腺素检测的化学发光试剂盒及其制备方法,其解决了此类临床诊断试剂盒热稳定性不好、产品检测效价下降过快等质量问题。The invention provides a chemiluminescent reagent kit for thyroxine detection and a preparation method thereof, which solves the quality problems of such clinical diagnostic reagent kits such as poor thermal stability and rapid decline in product detection titer.
本发明一种用于甲状腺激素检测的化学发光试剂盒,包括下述试剂:A kind of chemiluminescence reagent kit for thyroid hormone detection of the present invention comprises following reagents:
R1浓缩液:由甲状腺素T4抗体偶联制得;R1 concentrate: prepared by coupling thyroxine T4 antibody;
R2浓缩液:由甲状腺素T4化合物和碱性磷酸酶(ALP)标记纯化制得;R2 Concentrate: It is prepared by labeling and purifying thyroxine T4 compound and alkaline phosphatase (ALP);
试剂缓冲液:由4-羟乙基哌嗪乙磺酸 (HEPES)、氯化钠、蔗糖、BSA、甘氨酸、聚乙烯吡咯烷酮K30(PVP K30)、甘露醇、海藻糖、5-溴-5-硝基-1,3-二恶烷(5-Bromo-5-nitro-1,3-dioxane,BND或者BRONIDOX-L)、吐温-20(Tween-20)组成。Reagent buffer: composed of 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), sodium chloride, sucrose, BSA, glycine, polyvinylpyrrolidone K30 (PVP K30), mannitol, trehalose, 5-bromo-5- Nitro-1,3-dioxane (5-Bromo-5-nitro-1,3-dioxane, BND or BRONIDOX-L), Tween-20 (Tween-20).
进一步地,所述R1浓缩液T4抗体偶联时使用的偶联剂为N-羟基丁二酰亚胺(NHS)和1-乙基-(3-二甲基氨基丙基)碳酰二亚胺(EDC)。Further, the coupling agent used in the coupling of the T4 antibody in the R1 concentrate is N-hydroxysuccinimide (NHS) and 1-ethyl-(3-dimethylaminopropyl)carbodiimide Amine (EDC).
进一步地,所述R2浓缩液标记碱性磷酸酶(ALP)的T4化合物活化时,使用了双琥珀酰亚胺辛二酸酯(DSS)、二甲基亚砜(DMSO)和N-甲基吗啉。Further, when the T4 compound of the R2 concentrate labeled alkaline phosphatase (ALP) is activated, disuccinimide suberate (DSS), dimethyl sulfoxide (DMSO) and N-methyl Morpholine.
进一步地,所述T4化合物通过对酚羟基的烷基化成醚制备而成。Further, the T4 compound is prepared by alkylating phenolic hydroxyl groups into ethers.
本发明一种用于甲状腺素检测的化学发光试剂盒的制备方法,包括以下步骤:A kind of preparation method of the chemiluminescent reagent kit for the detection of thyroxine of the present invention comprises the following steps:
T4化合物的合成;Synthesis of T4 compound;
称量T4化合物和碳酸钾,并用N,N-二甲基甲酰胺溶液溶解;Weigh T4 compound and potassium carbonate, and dissolve with N,N-dimethylformamide solution;
在通惰性气体条件下,混合溶液中加入三聚氰胺并在一定温度下反应一段时间;Under the condition of passing an inert gas, add melamine to the mixed solution and react at a certain temperature for a period of time;
反应后,在溶液中加入去离子水,调节pH=6.0;After the reaction, add deionized water to the solution to adjust the pH=6.0;
然后,溶液用乙酸乙酯萃取并真空干燥,得到白色粉末,即为合成的T4化合物,4℃保存备用;Then, the solution was extracted with ethyl acetate and dried in vacuum to obtain a white powder, which was the synthesized T4 compound, which was stored at 4°C for future use;
R1浓缩液的制备:Preparation of R1 Concentrate:
利用磁性微球进行T4抗体偶联,用4-吗啉乙磺酸(MES)溶解的N-羟基丁二酰亚胺(NHS)、1-乙基-(3-二甲基氨基丙基)碳酰二亚胺(EDC)进行抗体偶联,终浓度为5.0mg/mL,于2~8℃冰箱保存备用;T4 antibody coupling using magnetic microspheres, N-hydroxysuccinimide (NHS), 1-ethyl-(3-dimethylaminopropyl) dissolved in 4-morpholineethanesulfonic acid (MES) Carbodiimide (EDC) was used for antibody coupling, the final concentration was 5.0mg/mL, and it was stored in a refrigerator at 2-8°C for later use;
R2浓缩液的制备:Preparation of R2 Concentrate:
称量一定量的T4化合物标记碱性磷酸酶,用双琥珀酰亚胺辛二酸酯,二甲基亚砜,N-甲基吗啉活化T4化合物,得到T4活化液待用;Weigh a certain amount of T4 compound labeled alkaline phosphatase, activate the T4 compound with disuccinimide suberate, dimethyl sulfoxide, and N-methylmorpholine, and obtain the T4 activation solution for use;
上紫外分光光度计测280nm下吸光度值,确定浓度;The upper ultraviolet spectrophotometer measures the absorbance value at 280nm to determine the concentration;
试剂缓冲液的配制:由HEPES、氯化钠、蔗糖、BSA、甘氨酸、PVP K30、甘露醇、海藻糖、BRONIDOX-L和吐温-20组成,该缓冲液的pH为7-8,优选为7.2-7.4。Preparation of reagent buffer: composed of HEPES, sodium chloride, sucrose, BSA, glycine, PVP K30, mannitol, trehalose, BRONIDOX-L and Tween-20, the pH of the buffer is 7-8, preferably 7.2-7.4.
进一步地,T4化合物的具体合成方法是:Further, the specific synthetic method of T4 compound is:
称量T4化合物770~777mg和碳酸钾276mg-280mg,并用N,N-二甲基甲酰胺溶液溶解;Weigh 770~777mg of T4 compound and 276mg-280mg of potassium carbonate, and dissolve it with N,N-dimethylformamide solution;
在通惰性气体条件下,混合溶液中加入三聚氰胺150~156mg并在70~80℃反应5~6h。Under the condition of passing an inert gas, add 150~156mg of melamine into the mixed solution and react at 70~80°C for 5~6h.
进一步地,R2浓缩液具体制备方法是:Further, the specific preparation method of R2 concentrate is:
称量5mg的T4化合物,用12mg的双琥珀酰亚胺辛二酸酯,200ul的二甲基亚砜,10uL的N-甲基吗啉活化T4化合物,混合均匀,37℃反应90min,反应完成之后再加入800 µL的二甲基亚砜,得到T4活化液待用;Weigh 5mg of T4 compound, activate T4 compound with 12mg of disuccinimide suberate, 200ul of dimethyl sulfoxide, and 10uL of N-methylmorpholine, mix well, react at 37°C for 90min, and the reaction is complete Then add 800 µL of dimethyl sulfoxide to obtain T4 activation solution for use;
取1mg 碱性磷酸酶溶解在浓度为0.1mol/L,pH=9.3的三羟甲基氨基甲烷的缓冲液中,加入200µL T4活化液,反应30min;Dissolve 1 mg of alkaline phosphatase in tris buffer solution with a concentration of 0.1mol/L and pH=9.3, add 200µL T4 activation solution, and react for 30 minutes;
反应完成之后,加入100µL,浓度为1mol/L的甘氨酸,反应15min;After the reaction is complete, add 100 µL of glycine with a concentration of 1 mol/L and react for 15 min;
上紫外分光光度计测280nm下吸光度值,确定浓度。Measure the absorbance value at 280nm with a UV spectrophotometer to determine the concentration.
进一步地,所述试剂缓冲液由HEPES为0.01M、氯化钠为0.15M、蔗糖为1M、BSA为0.5M、甘氨酸为0.05M、PVP K30为0.2M、甘露醇为0.5M、海藻糖为0.5M、BRONIDOX-L为0.01M、吐温-20为0.05%组成,该缓冲液pH=7.2-7.4。Further, the reagent buffer is 0.01M for HEPES, 0.15M for sodium chloride, 1M for sucrose, 0.5M for BSA, 0.05M for glycine, 0.2M for PVP K30, 0.5M for mannitol, and 0.5M for trehalose. 0.5M, BRONIDOX-L 0.01M, Tween-20 0.05%, the pH of the buffer solution is 7.2-7.4.
T4化合物、R2浓缩液和试剂缓冲液在实际制备时,可按制备方法中原料具体配比的倍数添加原料。During the actual preparation of T4 compound, R2 concentrated solution and reagent buffer solution, the raw materials can be added according to the multiple of the specific ratio of raw materials in the preparation method.
T4在自然状态下因为端基的酚羟基容易被氧化为醌而不稳定,导致邻位的碘容易脱落。有两种方式供选择。In the natural state, T4 is unstable because the phenolic hydroxyl group at the terminal group is easily oxidized to quinone, which leads to the easy loss of iodine at the adjacent position. There are two ways to choose from.
方法1:通过对酚羟基的改性可以有效的增加其稳定性。其中最常用的方式为酚羟基的甲酰化或者乙酰化变成活性酯。但是该方法下生成的活性酯本身在酸或碱性条件下也相对较不稳定,很容易水解脱除。Method 1: By modifying the phenolic hydroxyl group, its stability can be effectively increased. The most commonly used method is the formylation or acetylation of phenolic hydroxyl groups into active esters. However, the active ester itself generated under this method is relatively unstable under acid or alkaline conditions, and is easy to be removed by hydrolysis.
方法2:通过对酚羟基的烷基化成醚,可有效提高其稳定性,且生成的醚稳定性也很高,需要强酸或高温等较为严苛的条件才能脱出,自然状态下稳定性极高。Method 2: Alkylation of phenolic hydroxyl groups into ethers can effectively improve its stability, and the resulting ethers are also highly stable, requiring harsh conditions such as strong acid or high temperature to be released, and the stability is extremely high in the natural state .
本发明中,通过对酚羟基的烷基化成醚制备T4化合物,提高其热稳定性,因此选择方法2。In the present invention, the T4 compound is prepared by alkylating the phenolic hydroxyl group into an ether to improve its thermal stability, so method 2 is selected.
本发明在使用时,T4化合物直接标记ALP,和样本中的抗原竞争包被有磁性微球的特异性抗体,形成磁性微球-抗体-酶标抗原免疫复合物。经化学发光分析仪进行测量,通过标准曲线把光信号转换为T4的浓度。When the present invention is used, the T4 compound directly labels ALP, and competes with the antigen in the sample for the specific antibody coated with magnetic microspheres to form a magnetic microsphere-antibody-enzyme-labeled antigen immune complex. It is measured by a chemiluminescence analyzer, and the light signal is converted into the concentration of T4 through a standard curve.
本发明提供了一种用于甲状腺素检测的化学发光试剂盒及其制备方法,采用新的催化系统,缩短反应路径和生产周期,缩减了反应成本。通过对酚羟基烷基化成醚制备T4化合物,可有效提高其稳定性,且生成的醚稳定性也很高,需要强酸或高温等较为严苛的条件才能脱出,自然状态下稳定性极高。有利于此类临床诊断试剂的大范围推广。The invention provides a chemiluminescent reagent kit for detecting thyroxine and a preparation method thereof, which adopts a new catalytic system, shortens the reaction path and production cycle, and reduces the reaction cost. The preparation of T4 compound by alkylating phenolic hydroxyl groups into ethers can effectively improve its stability, and the resulting ethers are also highly stable, requiring harsh conditions such as strong acid or high temperature to be released, and the stability is extremely high in the natural state. It is beneficial to the large-scale popularization of such clinical diagnostic reagents.
附图说明Description of drawings
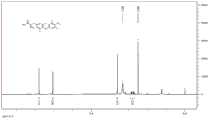
图1为实施例1合成的T4化合物的核磁共振图。Fig. 1 is the NMR figure of the T4 compound synthesized in Example 1.
图2为实施例5试剂盒稳定性测试阴性样本的发光值对照图。Fig. 2 is a control chart of the luminescence value of the negative sample in the stability test of the kit in Example 5.
图3为实施例5试剂盒稳定性测试阳性样本在10ng/mL 、50ng/mL 和150ng/mL的发光值对照图。Figure 3 is a control chart of the luminescence values of the positive samples in the stability test of the kit in Example 5 at 10 ng/mL, 50 ng/mL and 150 ng/mL.
具体实施方式Detailed ways
下面结合附图和实施例对本发明内容作进一步的说明,但不是对本发明的限定。The content of the present invention will be further described below in conjunction with the accompanying drawings and embodiments, but the present invention is not limited thereto.
实施例中的实验方法,如无特殊说明,均为常规方法;所用的实验材料,如无特殊说明,均来自常规生化试剂厂商购买得到的。The experimental methods in the examples, unless otherwise specified, are conventional methods; the experimental materials used, unless otherwise specified, were purchased from conventional biochemical reagent manufacturers.
实施例1:T4化合物的合成Embodiment 1: the synthesis of T4 compound
制备T4化合物最常用的制备方法为:酚羟基的甲酰化或者乙酰化变成活性酯。但是该方法下生成的活性酯本身在酸或碱性条件下也相对较不稳定,很容易水解脱除。本发明通过对酚羟基的烷基化成醚,可有效提高其稳定性,且生成的醚稳定性也很高,需要强酸或高温等较为严苛的条件才能脱出,自然状态下稳定性极高。The most commonly used preparation method for preparing T4 compound is: formylation or acetylation of phenolic hydroxyl group into active ester. However, the active ester itself generated under this method is relatively unstable under acid or alkaline conditions, and is easy to be removed by hydrolysis. The present invention can effectively improve its stability by alkylating phenolic hydroxyl groups into ethers, and the generated ethers are also highly stable, requiring harsh conditions such as strong acid or high temperature to be released, and have extremely high stability in a natural state.
本发明中,通过对酚羟基的烷基化成醚制备T4化合物,提高其热稳定性,本实施例具体制备步骤为:称量777mg的T4化合物和276mg碳酸钾,并用N,N-二甲基甲酰胺(DMF)溶液溶解;在通惰性气体条件下,混合溶液中加入156mg三聚氰胺(Melamine,Mel)并在80℃反应5h;随后,将溶液中加入200mL去离子水,并调节pH=6.0;然后,溶液用乙酸乙酯萃取并真空干燥,得到白色粉末,即为合成的T4化合物,保存4℃备用。In the present invention, the T4 compound is prepared by alkylating the phenolic hydroxyl group into an ether to improve its thermal stability. The specific preparation steps of this embodiment are: weigh 777 mg of the T4 compound and 276 mg of potassium carbonate, and use N,N-dimethyl Formamide (DMF) solution was dissolved; under the condition of inert gas, 156mg of melamine (Melamine, Mel) was added to the mixed solution and reacted at 80°C for 5h; then, 200mL of deionized water was added to the solution, and the pH was adjusted to 6.0; Then, the solution was extracted with ethyl acetate and dried under vacuum to obtain a white powder, which was the synthesized T4 compound, which was stored at 4°C for future use.
对合成的T4化合物进行核磁共振检测(NMR),如图1所示,结果表明合成的T4和天然的T4之间没有结构差异。Nuclear magnetic resonance detection (NMR) was performed on the synthesized T4 compound, as shown in Figure 1, the results showed that there was no structural difference between the synthesized T4 and natural T4.
实施例2:R1浓缩液的制备Embodiment 2: the preparation of R1 concentrate
利用磁性微球进行T4抗体偶联,用MES溶解的NHS、EDC进行抗体偶联,终浓度为5.0mg/mL,于2~8℃冰箱保存备用。Use magnetic microspheres for T4 antibody coupling, use MES-dissolved NHS and EDC for antibody coupling, the final concentration is 5.0mg/mL, and store in a refrigerator at 2-8°C for later use.
实施例3:R2浓缩液的制备Embodiment 3: the preparation of R2 concentrate
称量5mg的T4化合物,用12mg的双琥珀酰亚胺辛二酸酯,200ul的二甲基亚砜,10uL的N-甲基吗啉活化T4化合物,混合均匀,37℃反应90min,反应完成之后再加入800 µL的二甲基亚砜,得到T4活化液待用;Weigh 5mg of T4 compound, activate T4 compound with 12mg of disuccinimide suberate, 200ul of dimethyl sulfoxide, and 10uL of N-methylmorpholine, mix well, react at 37°C for 90min, and the reaction is complete Then add 800 µL of dimethyl sulfoxide to obtain T4 activation solution for use;
取1mg 碱性磷酸酶溶解在浓度为0.1mol/L,pH=9.3的三羟甲基氨基甲烷的缓冲液中,加入200µL T4活化液,反应30min;Dissolve 1 mg of alkaline phosphatase in tris buffer solution with a concentration of 0.1mol/L and pH=9.3, add 200µL T4 activation solution, and react for 30 minutes;
反应完成之后,加入100µL,浓度为1mol/L的甘氨酸,反应15min;After the reaction is complete, add 100 µL of glycine with a concentration of 1 mol/L and react for 15 min;
上紫外分光光度计测280nm下吸光度值(optical density,OD),确定浓度。The concentration was determined by measuring the absorbance value (optical density, OD) at 280 nm with a UV spectrophotometer.
实施例4:试剂缓冲液的配制Embodiment 4: the preparation of reagent buffer
R1浓缩液、R2浓缩液制备时所用到的试剂缓冲液由R1浓缩液、R2浓缩液制备时所用到的试剂缓冲液HEPES为0.01M、氯化钠为0.15M、蔗糖为1M、BSA为0.5M、甘氨酸为0.05M、PVP K30为0.2M、甘露醇为0.5M、海藻糖为0.5M、BRONIDOX-L为0.01M、Tween-20为0.05%组成,该缓冲液pH=7.4。The reagent buffer used in the preparation of R1 concentrate and R2 concentrate is 0.01M HEPES, 0.15M sodium chloride, 1M sucrose, and 0.5 BSA. M, glycine is 0.05M, PVP K30 is 0.2M, mannitol is 0.5M, trehalose is 0.5M, BRONIDOX-L is 0.01M, Tween-20 is 0.05%, and the pH of the buffer is 7.4.
实施例5:试剂盒稳定性测试Embodiment 5: kit stability test
将制备好的R1/R2浓缩液分别用缓冲液按1:50稀释,配制成工作液,进行37℃加速老化7天实验后,用化学发光免疫分析仪测试阴阳性质控品,选择3批不同批次的不含T4的人血清作为阴性样本,对测试结果的发光值进行对照,结果显示,本发明试剂盒免疫测定在阴性样品中表现出很好的稳定性,发光值仅降低了约3.7%,如图2所示,图中合成是指试剂盒中按照本发明方法合成的T4。天然就是血清中的T4。Dilute the prepared R1/R2 concentrates with buffer solution at a ratio of 1:50 to prepare a working solution. After the accelerated aging experiment at 37°C for 7 days, use a chemiluminescence immunoassay analyzer to test negative and positive quality control products, and select 3 batches of different Batches of human serum not containing T4 were used as negative samples, and the luminescence value of the test results was compared. The results showed that the immunoassay of the kit of the present invention showed good stability in negative samples, and the luminescence value was only reduced by about 3.7 %, as shown in Figure 2, the synthesis in the figure refers to the T4 synthesized in the kit according to the method of the present invention. Naturally, it is T4 in serum.
将中国国家阳性标准品(YJ-150551)稀释至10 ng/mL、50 ng/mL和150 ng/mL作为阳性样本,在阳性样品中,免疫测定仅在150ng/mL时显示出良好的稳定性,但本发明试剂盒的免疫测定在3个不同批次上均表现出优异的检测性能,发光值仅下降3.9%、2.9%和5.9%,如图3所示。The Chinese National Positive Standard (YJ-150551) was diluted to 10 ng/mL, 50 ng/mL and 150 ng/mL as positive samples, and in the positive samples, the immunoassay showed good stability only at 150 ng/mL , but the immunoassay of the kit of the present invention showed excellent detection performance on three different batches, and the luminescence value only decreased by 3.9%, 2.9% and 5.9%, as shown in Figure 3.
Claims (9)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210828775.2ACN115808519A (en) | 2022-07-15 | 2022-07-15 | Chemiluminescence kit for thyroxine detection and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210828775.2ACN115808519A (en) | 2022-07-15 | 2022-07-15 | Chemiluminescence kit for thyroxine detection and preparation method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN115808519Atrue CN115808519A (en) | 2023-03-17 |
Family
ID=85482331
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210828775.2APendingCN115808519A (en) | 2022-07-15 | 2022-07-15 | Chemiluminescence kit for thyroxine detection and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115808519A (en) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4467030A (en)* | 1978-06-12 | 1984-08-21 | Boehringer Mannheim Gmbh | Determination of the thyroxine-binding index in serum |
| KR20020065698A (en)* | 2001-02-07 | 2002-08-14 | 주식회사 바이오라인 | Detection kit of thyroid hormone using ria |
| CN103048476A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Thyroxine nanometer magnetic particle chemiluminiscence determinstion kit as well as preparation method and detection method thereof |
| CN103951704A (en)* | 2013-12-10 | 2014-07-30 | 云南民族大学 | A preparation method of chemiluminescent substance AMPPD for immunoassay |
| CN113214380A (en)* | 2021-05-06 | 2021-08-06 | 三诺生物传感股份有限公司 | Preparation method of alkaline phosphatase-labeled thyroxine |
- 2022
- 2022-07-15CNCN202210828775.2Apatent/CN115808519A/enactivePending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4467030A (en)* | 1978-06-12 | 1984-08-21 | Boehringer Mannheim Gmbh | Determination of the thyroxine-binding index in serum |
| KR20020065698A (en)* | 2001-02-07 | 2002-08-14 | 주식회사 바이오라인 | Detection kit of thyroid hormone using ria |
| CN103048476A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Thyroxine nanometer magnetic particle chemiluminiscence determinstion kit as well as preparation method and detection method thereof |
| CN103951704A (en)* | 2013-12-10 | 2014-07-30 | 云南民族大学 | A preparation method of chemiluminescent substance AMPPD for immunoassay |
| CN113214380A (en)* | 2021-05-06 | 2021-08-06 | 三诺生物传感股份有限公司 | Preparation method of alkaline phosphatase-labeled thyroxine |
Non-Patent Citations (2)
| Title |
|---|
| EVELYN SKOPEK 等: "Detection of autoantibodies against thyroid peroxidase in serum samples of hypothyroid dogs", AJVR, vol. 67, no. 5, 31 May 2006 (2006-05-31), pages 809 - 814* |
| 沈亚: "基于纳米复合材料的免疫传感检测机理研究", 中国优秀硕士学位论文全文数据库 医药卫生科技辑, no. 6, 15 June 2024 (2024-06-15), pages 059 - 39* |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100464189C (en) | Magnetic separation direct chemiluminescence reagent and test method using the same | |
| CN108484622B (en) | Synthesis of Multi-Signal Fluorescent Probes and Its Application in Simultaneous Differential Detection of Hcy, Cys and GSH | |
| CN103588872B (en) | A kind of vitamins D synthetic antigen, its preparation method and application | |
| CN101377500A (en) | Free prostate gland specificity antigen chemiluminescence immune analysis determination reagent kit and preparing method thereof | |
| CN101819206B (en) | AFP (Alpha-Fetoprotein) testing kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof | |
| CN105652018A (en) | C-peptide quantitative determination kit | |
| CN101377514A (en) | Insulin autoantibody chemiluminescence immune analysis determination reagent kit and preparing method thereof | |
| CN111175491A (en) | sBCMA magnetic particle chemiluminescence immunoassay kit and preparation method and application thereof | |
| WO2023015795A1 (en) | Ratiometric polysulfane fluorescent probe, and preparation method therefor and use thereof | |
| EP0656005B1 (en) | Biotinylated chemiluminescent label, conjugates, assays, assay kits | |
| CN101949945B (en) | Kit for detecting free thyroxin by using magnetic particles as solid-phase carriers and preparation method thereof | |
| Pratt et al. | Chemiluminescence-linked immunoassay | |
| CN110092771A (en) | A kind of fluorescence probe and preparation method thereof for human serum albumins detection, Fluorescence kit | |
| CN117105950A (en) | A fluorescent probe for myeloperoxidase detection and its preparation method and application | |
| CN110988333A (en) | Serum tissue metalloproteinase inhibitor-1 chemiluminescence immunoassay kit and preparation method thereof | |
| CN115808519A (en) | Chemiluminescence kit for thyroxine detection and preparation method thereof | |
| CN114778816A (en) | Acridinium ester labeling compound and preparation method thereof | |
| CN112305228B (en) | Myoglobin direct chemiluminescence detection kit, preparation method and application | |
| CN105785019B (en) | A kind of detection method for prostate specific antigen | |
| CN110618280A (en) | Thyrotropin determination kit and preparation method thereof | |
| CN112858678B (en) | Creatine kinase isoenzyme detection kit, preparation method and application | |
| CN101101294B (en) | Colour-developing agent preparation for enzyme-linked immunoassay in vitro diagnosis agent | |
| CN113376378A (en) | D-dimer detection kit, preparation method and application | |
| CN110398589B (en) | Kit for quantitatively detecting content of anti-mullerian hormone and detection method thereof for non-disease diagnosis purpose | |
| CN105842447B (en) | A kind of detection method for hepatitis B surface antigen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |