CN115645531A - A kind of magnetic graphene nanocapsule and its preparation method and application - Google Patents
A kind of magnetic graphene nanocapsule and its preparation method and applicationDownload PDFInfo
- Publication number
- CN115645531A CN115645531ACN202211286538.4ACN202211286538ACN115645531ACN 115645531 ACN115645531 ACN 115645531ACN 202211286538 ACN202211286538 ACN 202211286538ACN 115645531 ACN115645531 ACN 115645531A
- Authority
- CN
- China
- Prior art keywords
- magnetic
- nanocapsule
- magnetic graphene
- graphene
- preparation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Carbon And Carbon Compounds (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及纳米材料的生物医学运用技术领域,尤其涉及一种磁性石墨烯纳米囊及其制备方法与应用。The invention relates to the technical field of biomedical application of nanomaterials, in particular to a magnetic graphene nanocapsule and its preparation method and application.
背景技术Background technique
热疗作为一种肿瘤治疗手段,通过改变细胞环境温度,使温度范围维持在42℃到47℃之间,从而诱导癌细胞凋亡,已取得了良好的临床治疗效果,目前广泛应用于治疗乳腺癌、膀胱癌、头颈癌、黑色素瘤和直肠癌等。但胃部相较于其他器官,有其独特的生理特性:1、它深埋于人的腹腔,常规手段难以作用于深层组织;2、胃腔内含有丰富的消化酶和强腐蚀性胃酸,极大限制了常规产热试剂的应用。目前传统的热疗方法如灌注热水、射频、微波、高强度聚焦超声和光热转换等,都难以大规模地从内而外地对胃部进行热疗。磁热疗是一种创伤性极小的热疗手段,它没有穿透深度的限制,使其成为胃部热疗的一种有竞争力的方法。其发热原理是利用超顺磁性纳米颗粒在交变磁场中的弛豫现象,完成磁能向热能的转化。磁热疗在生物医学领域一般使用氧化铁纳米颗粒(化学式为Fe3O4),其用于胃部磁热疗的挑战是:1、磁热转换效率低,其比损耗功率(SLP)难以突破100W/g;2、现常用的氧化铁纳米颗粒在酸性条件下不稳定,从而无法达到指定的治疗温度并且可能产生潜在生物毒性。因此,开发能在胃内稳定提供高效热疗效果的磁热疗技术具有重要的科学意义与实际应用价值。As a tumor treatment method, hyperthermia can induce the apoptosis of cancer cells by changing the temperature of the cell environment to maintain the temperature range between 42°C and 47°C. It has achieved good clinical therapeutic effects and is currently widely used in the treatment of breast cancer. cancer, bladder cancer, head and neck cancer, melanoma, and rectal cancer. However, compared with other organs, the stomach has its unique physiological characteristics: 1. It is deeply buried in the human abdominal cavity, and it is difficult for conventional methods to act on deep tissues; 2. The stomach cavity is rich in digestive enzymes and strong corrosive gastric acid. This greatly limits the application of conventional thermogenic reagents. At present, traditional hyperthermia methods, such as perfusion of hot water, radio frequency, microwave, high-intensity focused ultrasound, and photothermal conversion, are difficult to perform hyperthermia on the stomach from the inside out on a large scale. Magnetic hyperthermia is a minimally invasive means of hyperthermia with no limitation of penetration depth, making it a competitive method of hyperthermia for the stomach. Its heating principle is to use the relaxation phenomenon of superparamagnetic nanoparticles in an alternating magnetic field to complete the conversion of magnetic energy into thermal energy. Magnetic hyperthermia generally uses iron oxide nanoparticles (chemical formula Fe3 O4 ) in the biomedical field. The challenges of its use in gastric magnetic hyperthermia are: 1. The magnetothermal conversion efficiency is low, and its specific power loss (SLP) is difficult Breakthrough 100W/g; 2. The commonly used iron oxide nanoparticles are unstable under acidic conditions, so they cannot reach the specified treatment temperature and may cause potential biological toxicity. Therefore, it is of great scientific significance and practical application value to develop a magnetic hyperthermia technology that can stably provide high-efficiency hyperthermia effects in the stomach.
发明内容Contents of the invention
有鉴于此,本发明提供了一种磁性石墨烯纳米囊及其制备方法与应用,以解决现有磁热疗所用氧化铁纳米颗粒存在的磁热转换效率低,同时在胃腔内不能稳定存在,无法达到指定的治疗温度并且可能产生潜在生物毒性的问题。In view of this, the present invention provides a magnetic graphene nanocapsule and its preparation method and application to solve the problem of low magnetic-to-thermal conversion efficiency of iron oxide nanoparticles used in existing magnetic hyperthermia, and at the same time cannot exist stably in the gastric cavity , can not reach the specified treatment temperature and may cause potential biotoxicity problems.
为了达到上述目的,本发明采用如下技术方案:In order to achieve the above object, the present invention adopts following technical scheme:
本发明提供了一种磁性石墨烯纳米囊的制备方法,包括如下步骤:The invention provides a method for preparing magnetic graphene nanocapsules, comprising the steps of:
(1)将磁性金属盐溶液和气相二氧化硅溶液混合后进行蒸发,之后顺次进行热处理和原位生长得到催化剂;(1) Evaporating the magnetic metal salt solution and the fumed silica solution after mixing, followed by heat treatment and in-situ growth to obtain the catalyst;
(2)将催化剂与氢氟酸溶液混合进行刻蚀,得到磁性石墨烯纳米囊。(2) The catalyst is mixed with a hydrofluoric acid solution for etching to obtain magnetic graphene nanocapsules.
作为优选,所述步骤(1)中,磁性金属盐为氯化铁、硝酸铁、氯化钴、硝酸钴、氯化镍或硝酸镍;磁性金属盐溶液的溶剂为甲醇;磁性金属盐和甲醇的摩尔体积比为0.0036mol:10~20mL。As preferably, in the step (1), the magnetic metal salt is ferric chloride, ferric nitrate, cobalt chloride, cobalt nitrate, nickel chloride or nickel nitrate; the solvent of the magnetic metal salt solution is methanol; the magnetic metal salt and methanol The molar volume ratio is 0.0036mol:10-20mL.
作为优选,所述步骤(1)中,气相二氧化硅的粒径为200~500nm;气相二氧化硅和甲醇超声混合,得到气相二氧化硅溶液;气相二氧化硅和甲醇的质量体积比为1~2g:200~500mL;超声混合的频率为40~50kHz,超声混合的时间为30~60min。As a preference, in the step (1), the particle diameter of fumed silica is 200-500nm; ultrasonically mixed fumed silica and methanol to obtain a fumed silica solution; the mass-volume ratio of fumed silica and methanol is 1~2g: 200~500mL; the frequency of ultrasonic mixing is 40~50kHz, and the time of ultrasonic mixing is 30~60min.
作为优选,所述磁性金属盐溶液和气相二氧化硅溶液中的甲醇的纯度独立的为≥99.8%。Preferably, the purity of methanol in the magnetic metal salt solution and the fumed silica solution is independently ≥99.8%.
作为优选,所述步骤(1)中,蒸发的温度为50~60℃,蒸发的转速为80~100r/min。Preferably, in the step (1), the evaporation temperature is 50-60° C., and the evaporation speed is 80-100 r/min.
作为优选,所述步骤(1)中,热处理在还原气体的条件下进行,还原气体的流量为50~100cfm;热处理的温度为810~900℃,热处理的时间为20~30min,升温至热处理温度的升温速率为10~20℃/min;原位生长在甲烷的气氛下进行,甲烷的通入流量为200~300cfm,原位生长的温度为810~900℃,原位生长的时间为5~10min。As preferably, in the step (1), the heat treatment is carried out under the condition of reducing gas, the flow rate of the reducing gas is 50-100cfm; the temperature of heat treatment is 810-900°C, the time of heat treatment is 20-30min, and the temperature is raised to the heat treatment temperature The heating rate is 10-20°C/min; the in-situ growth is carried out under the atmosphere of methane, the flow rate of methane is 200-300cfm, the temperature of in-situ growth is 810-900°C, and the time of in-situ growth is 5-5 10min.
作为优选,所述步骤(1)中,进行热处理前,对蒸发所得产物进行研磨。As a preference, in the step (1), before heat treatment, the evaporated product is ground.
作为优选,步骤(1)所述磁性金属盐和步骤(2)中所述氢氟酸溶液的摩尔体积比为0.0012mol:8~15mL;所述氢氟酸溶液为氢氟酸的水溶液,氢氟酸溶液的体积浓度为10~20%。As a preference, the molar volume ratio of the magnetic metal salt in step (1) to the hydrofluoric acid solution in step (2) is 0.0012mol: 8-15mL; the hydrofluoric acid solution is an aqueous solution of hydrofluoric acid, hydrogen The volume concentration of the hydrofluoric acid solution is 10-20%.
本发明还提供了所述磁性石墨烯纳米囊的制备方法制备得到的磁性石墨烯纳米囊。The present invention also provides the magnetic graphene nanocapsule prepared by the preparation method of the magnetic graphene nanocapsule.
本发明还提供了所述磁性石墨烯纳米囊在磁热疗中的应用。The invention also provides the application of the magnetic graphene nanocapsule in magnetic hyperthermia.
经由上述的技术方案可知,与现有技术相比,本发明有益效果如下:It can be seen through the above-mentioned technical solution that, compared with the prior art, the beneficial effects of the present invention are as follows:
(1)本发明通过化学气相沉积法原位生长内核为磁性金属,壳层为石墨烯的磁性石墨烯纳米囊,相较于传统磁性金属氧化物,产热能力和耐酸能力显著增强,能够在胃内提供稳定的热疗条件;(1) In the present invention, the magnetic graphene nanocapsules whose inner core is magnetic metal and the shell layer is graphene are grown in situ by chemical vapor deposition. Stable heat treatment conditions are provided in the stomach;
(2)本发明原料为金属氯化盐和硝酸盐等廉价易得的原材料,合成条件简单,适合大批量生产;(2) The raw materials of the present invention are cheap and easy-to-get raw materials such as metal chlorides and nitrates, and the synthesis conditions are simple and suitable for mass production;
(3)本发明所得磁性石墨烯纳米囊通过口服进入指定部位,这样的给药方式可以避免纳米颗粒进入血液后在肝脏富集会产生副作用等问题,并且使绝大部分磁热试剂富集在胃部,从而可以降低磁热试剂的使用剂量,规避纳米粒子进入人体循环后所产生的潜在毒性。(3) The magnetic graphene nanocapsules obtained by the present invention enter the designated site orally. Such an administration method can avoid problems such as side effects caused by the accumulation of nanoparticles in the liver after entering the blood, and make most of the magnetocaloric reagents enriched in the blood. Stomach, so that the dosage of magnetocaloric reagents can be reduced, and the potential toxicity of nanoparticles entering the human circulation can be avoided.
附图说明Description of drawings
为了更清楚地说明本发明实施例或现有技术中的技术方案,下面将对实施例或现有技术描述中所需要使用的附图作简单地介绍,显而易见地,下面描述中的附图仅仅是本发明的实施例,对于本领域普通技术人员来讲,在不付出创造性劳动的前提下,还可以根据提供的附图获得其他的附图。In order to more clearly illustrate the technical solutions in the embodiments of the present invention or the prior art, the following will briefly introduce the drawings that need to be used in the description of the embodiments or the prior art. Obviously, the accompanying drawings in the following description are only It is an embodiment of the present invention, and those skilled in the art can also obtain other drawings according to the provided drawings without creative work.
图1为本发明实施例1、3和5所得磁性石墨烯纳米囊的表面形貌的扫描投射电子显微镜图像,其中,a为实施例1所得Fe@G磁性石墨烯纳米囊,b为实施例3所得Co@G磁性石墨烯纳米囊,c为实施例5所得Ni@G磁性石墨烯纳米囊;Figure 1 is a scanning projection electron microscope image of the surface morphology of the magnetic graphene nanocapsules obtained in Examples 1, 3 and 5 of the present invention, wherein a is the Fe@G magnetic graphene nanocapsules obtained in Example 1, and b is the embodiment 3 obtained Co@G magnetic graphene nanocapsules, c is Ni@G magnetic graphene nanocapsules obtained in Example 5;
图2为本发明实施例1、3和5所得磁性石墨烯纳米囊的元素分布图像和能量色散图谱,其中,a为实施例1、3和5所得磁性石墨烯纳米囊的元素分布图像,b为实施例1、3和5所得磁性石墨烯纳米囊的能量色散图谱;Fig. 2 is the element distribution image and the energy dispersive spectrum of magnetic graphene nanocapsule obtained in embodiment 1, 3 and 5 of the present invention, wherein, a is the element distribution image of magnetic graphene nanocapsule obtained in embodiment 1, 3 and 5, b It is the energy dispersive spectrum of magnetic graphene nanocapsule of embodiment 1,3 and 5 gained;
图3为本发明实施例1、3和5所得磁性石墨烯纳米囊的磁滞回线测试曲线;Fig. 3 is the magnetic hysteresis loop test curve of the obtained magnetic graphene nanocapsule of the embodiment of the present invention 1, 3 and 5;
图4为本发明实施例1、3和5所得磁性石墨烯纳米囊在磁场强度为20kA/m、频率为340kHz的交变磁场下的产热速率图像和SLP值,其中,a对应实施例1所得磁性石墨烯纳米囊,b对应实施例3所得磁性石墨烯纳米囊,c对应实施例5所得磁性石墨烯纳米囊;Fig. 4 is the heat production rate image and the SLP value of the magnetic graphene nanocapsule obtained in Examples 1, 3 and 5 of the present invention under an alternating magnetic field with a magnetic field strength of 20kA/m and a frequency of 340kHz, wherein a corresponds to Example 1 Gained magnetic graphene nanocapsules, b corresponds to the magnetic graphene nanocapsules gained in Example 3, and c corresponds to the magnetic graphene nanocapsules gained in Example 5;
图5为本发明实施例1所得磁性石墨烯纳米囊灌胃前后小鼠胃部的MRI-T2加权成像图,其中,a为灌胃前小鼠胃部的MRI-T2加权成像图,b为灌胃后小鼠胃部的MRI-T2加权成像图;Fig. 5 is the MRI-T2 weighted imaging diagram of the mouse stomach before and after gavage of the magnetic graphene nanocapsule obtained in Example 1 of the present invention, wherein, a is the MRI-T2 weighted imaging diagram of the mouse stomach before gavage, b is the MRI-T2- weighted imaging image of the mouse stomach after gavage;
图6为小鼠胃部H&E染色切片图,其中,a为未经磁热疗的小鼠胃部H&E染色切片图,b为经实施例1所得磁性石墨烯纳米囊进行磁热疗后小鼠胃部H&E染色切片图;Figure 6 is a H&E stained slice of the stomach of a mouse, wherein, a is a H&E stained slice of the stomach of a mouse without magnetic hyperthermia, and b is a mouse after the magnetic graphene nanocapsule obtained in Example 1 is subjected to magnetic hyperthermia H&E stained section of stomach;
图7为四氧化三铁和本发明实施例1所得磁性石墨烯纳米囊在浓度为1M盐酸溶液中磁热升温情况对比图,其中,在图a、b和c中,右边为本发明实施例1所得磁性石墨烯纳米囊,左边为四氧化三铁;在图d中,左边为本发明实施例1所得磁性石墨烯纳米囊,右边为四氧化三铁;a为处于磁场10min后的热成像图,b为处于磁场20min后的热成像图,c为处于磁场30min后的热成像图,d为处于磁场30min后的明场图。Fig. 7 is a comparison diagram of ferroferric oxide and magnetic graphene nanocapsules obtained in Example 1 of the present invention in a concentration of 1M hydrochloric acid solution, where the magnetic-caloric temperature rise is compared, wherein, in Figures a, b and c, the right side is the embodiment of the present invention 1 The obtained magnetic graphene nanocapsule, the left side is ferric oxide; in Figure d, the left side is the magnetic graphene nanocapsule obtained in Example 1 of the present invention, and the right side is ferric oxide; a is the thermal imaging after being in the magnetic field for 10min In the picture, b is the thermal imaging image after being in the magnetic field for 20 minutes, c is the thermal imaging image after being in the magnetic field for 30 minutes, and d is the bright field image after being in the magnetic field for 30 minutes.
具体实施方式Detailed ways
本发明提供了一种磁性石墨烯纳米囊的制备方法,包括如下步骤:The invention provides a method for preparing magnetic graphene nanocapsules, comprising the steps of:
(1)将磁性金属盐溶液和气相二氧化硅溶液混合后进行蒸发,之后顺次进行热处理和原位生长得到催化剂;(1) Evaporating the magnetic metal salt solution and the fumed silica solution after mixing, followed by heat treatment and in-situ growth to obtain the catalyst;
(2)将催化剂与氢氟酸溶液混合进行刻蚀,得到磁性石墨烯纳米囊。(2) The catalyst is mixed with a hydrofluoric acid solution for etching to obtain magnetic graphene nanocapsules.
在本发明中,所述步骤(1)中,磁性金属盐优选为氯化铁、硝酸铁、氯化钴、硝酸钴、氯化镍或硝酸镍,进一步优选为硝酸铁、氯化钴或硝酸镍;磁性金属盐溶液的溶剂优选为甲醇;磁性金属盐和甲醇的摩尔体积比优选为0.0036mol:10~20mL,进一步优选为0.0036mol:12~15mL。In the present invention, in the step (1), the magnetic metal salt is preferably ferric chloride, ferric nitrate, cobalt chloride, cobalt nitrate, nickel chloride or nickel nitrate, more preferably ferric nitrate, cobalt chloride or nitric acid Nickel; the solvent of the magnetic metal salt solution is preferably methanol; the molar volume ratio of the magnetic metal salt to methanol is preferably 0.0036 mol: 10-20 mL, more preferably 0.0036 mol: 12-15 mL.
在本发明中,所述步骤(1)中,气相二氧化硅的粒径优选为200~500nm,进一步优选为250~400nm;气相二氧化硅和甲醇超声混合,得到气相二氧化硅溶液;气相二氧化硅和甲醇的质量体积比优选为1~2g:200~500mL,进一步优选为1.2~1.6g:300~400mL;超声混合的频率优选为40~50kHz,进一步优选为42~45kHz;超声混合的时间优选为30~60min,进一步优选为40~50min。In the present invention, in the step (1), the particle size of the fumed silica is preferably 200-500 nm, more preferably 250-400 nm; the fumed silica and methanol are ultrasonically mixed to obtain a fumed silica solution; the gas-phase The mass volume ratio of silicon dioxide and methanol is preferably 1-2g: 200-500mL, more preferably 1.2-1.6g: 300-400mL; the frequency of ultrasonic mixing is preferably 40-50kHz, more preferably 42-45kHz; ultrasonic mixing The time is preferably 30 to 60 minutes, more preferably 40 to 50 minutes.
在本发明中,所述磁性金属盐溶液和气相二氧化硅溶液中的甲醇的纯度独立的优选为≥99.8%,进一步优选为≥99.9%。In the present invention, the purity of methanol in the magnetic metal salt solution and the fumed silica solution is independently preferably ≥99.8%, more preferably ≥99.9%.
在本发明中,所述步骤(1)中,蒸发的温度优选为50~60℃,进一步优选为55~58℃;蒸发的转速优选为80~100r/min,进一步优选为85~95r/min;In the present invention, in the step (1), the evaporation temperature is preferably 50-60°C, more preferably 55-58°C; the evaporation speed is preferably 80-100r/min, more preferably 85-95r/min ;
所述蒸发在旋转蒸发仪上进行,蒸发过程中的加热方式为水浴加热。The evaporation is carried out on a rotary evaporator, and the heating mode in the evaporation process is water bath heating.
在本发明中,所述步骤(1)中,热处理在还原气体的条件下进行;还原气体优选为氢气;还原气体的流量优选为50~100cfm,进一步优选为60~90cfm;热处理的温度优选为810~900℃,进一步优选为820~880℃;热处理的时间优选为20~30min,进一步优选为25~28min;升温至热处理温度的升温速率优选为10~20℃/min,进一步优选为12~18℃/min;原位生长在甲烷的气氛下进行,甲烷的通入流量优选为200~300cfm,进一步优选为220~260cfm;原位生长的温度优选为810~900℃,进一步优选为850~880℃;原位生长的时间优选为5~10min,进一步优选为6~8min。In the present invention, in the step (1), the heat treatment is carried out under the condition of a reducing gas; the reducing gas is preferably hydrogen; the flow rate of the reducing gas is preferably 50 to 100 cfm, more preferably 60 to 90 cfm; the temperature of the heat treatment is preferably 810-900°C, more preferably 820-880°C; the heat treatment time is preferably 20-30min, more preferably 25-28min; the heating rate to the heat treatment temperature is preferably 10-20°C/min, more preferably 12-28min 18°C/min; the in-situ growth is carried out under the atmosphere of methane, the flow rate of methane is preferably 200-300cfm, more preferably 220-260cfm; the temperature of in-situ growth is preferably 810-900°C, more preferably 850- 880°C; the time for in-situ growth is preferably 5-10 minutes, more preferably 6-8 minutes.
在本发明中,所述步骤(1)中,进行热处理前,对蒸发所得产物进行研磨;研磨所得产物的粒径优选为10~100μm,进一步优选为20~70μm。In the present invention, in the step (1), before heat treatment, the evaporated product is ground; the particle size of the ground product is preferably 10-100 μm, more preferably 20-70 μm.
在本发明中,所述步骤(1)中,得到催化剂前,对原位生长所得产物进行冷却处理,冷却的速率优选为20~50℃/min,进一步优选为30~40℃/min。In the present invention, in the step (1), before obtaining the catalyst, the product grown in situ is cooled, and the cooling rate is preferably 20-50°C/min, more preferably 30-40°C/min.
在本发明中,步骤(1)所述磁性金属盐和步骤(2)中所述氢氟酸溶液的摩尔体积比优选为0.0012mol:8~15mL,进一步优选为0.0012mol:9~12mL;所述氢氟酸溶液为氢氟酸的水溶液,氢氟酸溶液的体积浓度优选为10~20%,进一步优选为11~15%。In the present invention, the molar volume ratio of the magnetic metal salt in step (1) to the hydrofluoric acid solution in step (2) is preferably 0.0012mol: 8-15mL, more preferably 0.0012mol: 9-12mL; The hydrofluoric acid solution is an aqueous solution of hydrofluoric acid, and the volume concentration of the hydrofluoric acid solution is preferably 10-20%, more preferably 11-15%.
在本发明中,所述步骤(2)中,刻蚀的时间优选为10~14h,进一步优选为11~13h。In the present invention, in the step (2), the etching time is preferably 10-14 hours, more preferably 11-13 hours.
在本发明中,所述步骤(2)中,氢氟酸溶液的作用为刻蚀气相二氧化硅。In the present invention, in the step (2), the function of the hydrofluoric acid solution is to etch fumed silicon dioxide.
在本发明中,所述步骤(2)中,刻蚀结束后,对刻蚀所得产物磁吸回收磁性粉体,将磁性粉体洗涤至中性,再经磁吸回收得到磁性石墨烯纳米囊。In the present invention, in the step (2), after the etching is completed, magnetically absorb the magnetic powder to the product obtained by etching, wash the magnetic powder to neutrality, and then recover the magnetic graphene nanocapsule through magnetic absorption. .
本发明通过化学气相沉积法实现磁性石墨纳米囊的制备,以磁性金属盐(铁、钴、镍的氯化盐或硝酸盐)为原料,在管式炉中利用氢气的还原性,形成磁性金属纳米粒子,磁性金属纳米粒子相较于磁性金属氧化物具有更高饱和磁化强度,而饱和磁化强度与SLP值成正比关系,所以选择金属纳米粒子比金属氧化物磁热转换效率更好。另外,本发明通过原位生长致密的石墨烯层,形成核壳结构,将磁性金属纳米颗粒与外界腐蚀性酸与酶隔绝开,从而达到保护的目的。The present invention realizes the preparation of magnetic graphite nanocapsules by chemical vapor deposition, using magnetic metal salts (iron, cobalt, nickel chloride or nitrate) as raw materials, and utilizing the reducibility of hydrogen in a tube furnace to form magnetic metal Nanoparticles, magnetic metal nanoparticles have higher saturation magnetization than magnetic metal oxides, and the saturation magnetization is proportional to the SLP value, so choosing metal nanoparticles has better magnetothermal conversion efficiency than metal oxides. In addition, the present invention grows a dense graphene layer in situ to form a core-shell structure, and isolates the magnetic metal nanoparticles from external corrosive acids and enzymes, thereby achieving the purpose of protection.
本发明还提供了所述磁性石墨烯纳米囊的制备方法制备得到的磁性石墨烯纳米囊。The present invention also provides the magnetic graphene nanocapsule prepared by the preparation method of the magnetic graphene nanocapsule.
本发明还提供了所述磁性石墨烯纳米囊在磁热疗中的应用。The invention also provides the application of the magnetic graphene nanocapsule in magnetic hyperthermia.
在本发明中,所述磁性石墨烯纳米囊在磁热疗中的应用包括如下步骤:In the present invention, the application of the magnetic graphene nanocapsule in magnetic hyperthermia comprises the following steps:
将磁性石墨烯纳米囊灌入胃囊中;将待治疗部分置于磁热线圈中,施加磁场。The magnetic graphene nanocapsules are poured into the gastric pouch; the part to be treated is placed in the magnetic heating coil and a magnetic field is applied.
在本发明中,所述磁性石墨烯纳米囊的灌入体积优选为胃囊容积的50~70%,进一步优选为55~66%;In the present invention, the filling volume of the magnetic graphene nanocapsule is preferably 50-70% of the gastric pouch volume, more preferably 55-66%;
所述磁性石墨烯纳米囊的灌入体积的确定是为了保证其足够充盈胃囊。The determination of the pouring volume of the magnetic graphene nanocapsule is to ensure that it is enough to fill the gastric sac.
在本发明中,所述磁场的频率优选为280~370kHz,进一步优选为300~350kHz;磁场的强度优选为1300~1700A/m,进一步优选为1400~1600A/m;磁场的强度和频率的乘积优选为≤4.85×108A/m,进一步优选为≤4.84×108A/m;In the present invention, the frequency of the magnetic field is preferably 280-370kHz, more preferably 300-350kHz; the intensity of the magnetic field is preferably 1300-1700A/m, more preferably 1400-1600A/m; the product of the intensity of the magnetic field and the frequency Preferably ≤4.85×108 A/m, more preferably ≤4.84×108 A/m;
所述磁场的强度和频率的乘积的确定可以避免产生涡流热,保证对生物组织的安全性。The determination of the product of the intensity and frequency of the magnetic field can avoid the generation of eddy current heat and ensure the safety of biological tissues.
在本发明中,所述施加磁场的时间优选为20~40min,进一步优选为25~35min。In the present invention, the time for applying the magnetic field is preferably 20-40 minutes, more preferably 25-35 minutes.
下面结合实施例对本发明提供的技术方案进行详细的说明,但是不能把它们理解为对本发明保护范围的限定。The technical solutions provided by the present invention will be described in detail below in conjunction with the examples, but they should not be interpreted as limiting the protection scope of the present invention.
实施例1Example 1
Fe@G磁性石墨烯纳米囊的制备:Preparation of Fe@G magnetic graphene nanocapsules:
将0.0036molFe(NO3)3·9H2O加入到10mL纯度为99.8%的甲醇中,溶解得硝酸铁的甲醇溶液。再取1g气相二氧化硅(粒径为250nm)加入到300mL纯度为99.8%的甲醇中,以45kHz的频率超声分散30min,使得溶液中无沉淀,得气相二氧化硅甲醇溶液;后将硝酸铁的甲醇溶液加入气相二氧化硅甲醇溶液中混合,随后在50℃下,以80r/min的转速在旋转蒸发仪上蒸干甲醇溶剂,得到粉末。将所得粉末研磨至粒径为10μm后放入管式炉中,在流量为100cfm的氢气保护下,设置升温速率为20℃/min,由常温升温至860℃,保持20min后,通入流量为300cfm的甲烷进行原位生长10min,原位生长的温度为860℃,随后以20℃/min的降温速率降温至室温,得到催化剂;将所得催化剂加入30mL体积浓度为20%氢氟酸的水溶液中刻蚀12h,反应完成后磁吸回收磁性粉体,用超纯水洗涤至中性,再经磁吸回收得到Fe@G磁性石墨烯纳米囊。Add 0.0036mol Fe(NO3 )3 ·9H2 O into 10 mL of methanol with a purity of 99.8%, and dissolve to obtain a methanol solution of ferric nitrate. Take 1g of fumed silica (250nm in particle size) and add it to 300mL of methanol with a purity of 99.8%, and disperse it ultrasonically at a frequency of 45kHz for 30min, so that there is no precipitation in the solution to obtain a methanol solution of fumed silica; The methanol solution was added into the fumed silica methanol solution and mixed, and then the methanol solvent was evaporated to dryness on a rotary evaporator at a speed of 80 r/min at 50° C. to obtain a powder. Grind the obtained powder to a particle size of 10 μm and put it into a tube furnace. Under the protection of hydrogen gas with a flow rate of 100 cfm, set the heating rate to 20°C/min, raise the temperature from normal temperature to 860°C, and keep it for 20 minutes. 300cfm of methane was used for in-situ growth for 10 minutes, the temperature of in-situ growth was 860°C, and then the temperature was lowered to room temperature at a cooling rate of 20°C/min to obtain a catalyst; the obtained catalyst was added to 30mL of an aqueous solution with a volume concentration of 20% hydrofluoric acid After etching for 12 hours, the magnetic powder was recovered by magnetic suction after the reaction was completed, washed with ultrapure water until neutral, and then recovered by magnetic suction to obtain Fe@G magnetic graphene nanocapsules.
本实施例所得Fe@G磁性石墨烯纳米囊的性能测试结果如下:The performance test results of Fe@G magnetic graphene nanocapsules obtained in this example are as follows:
对制备的Fe@G磁性石墨烯纳米囊进行扫描投射电镜的表征,结果如图1a所示。由图1a可知,本发明所得Fe@G磁性石墨烯纳米囊可以明显的金属晶格与外层的石墨烯层,说明所得Fe@G磁性石墨烯纳米囊为核壳结构;进行元素EDS分析,结果如图2所示。由图2可知,所得Fe@G磁性石墨烯纳米囊的组成主要为铁元素,纯度高,未产生其它杂质。The prepared Fe@G magnetic graphene nanocapsules were characterized by scanning transmission electron microscopy, and the results are shown in Figure 1a. It can be seen from Fig. 1a that the obtained Fe@G magnetic graphene nanocapsules of the present invention can have obvious metal lattice and outer graphene layer, indicating that the obtained Fe@G magnetic graphene nanocapsules have a core-shell structure; elemental EDS analysis is performed, The result is shown in Figure 2. It can be seen from Figure 2 that the composition of the obtained Fe@G magnetic graphene nanocapsules is mainly iron element, with high purity and no other impurities.
对制备的Fe@G磁性石墨烯纳米囊进行磁滞回线测试,结果如图3所示。由图3可知,本实施例所得Fe@G磁性石墨烯纳米囊的饱和磁化强度为104emu/g。Hysteresis loop tests were performed on the prepared Fe@G magnetic graphene nanocapsules, and the results are shown in Figure 3. It can be seen from Figure 3 that the saturation magnetization of Fe@G magnetic graphene nanocapsules obtained in this example is 104emu/g.
用水稀释制备得到的Fe@G磁性石墨烯纳米囊至浓度为5mg/mL,放置在交变磁场中(施加的磁场的强度为20kA/m、频率为340kHz),进行体外磁热升温测试,结果如图4所示。由图4可知,通过计算得到Fe@G磁性石墨烯纳米囊的SLP值为418.2W/g,说明所得Fe@G磁性石墨烯纳米囊的磁热性能优异。The prepared Fe@G magnetic graphene nanocapsules were diluted with water to a concentration of 5 mg/mL, placed in an alternating magnetic field (the strength of the applied magnetic field was 20kA/m, and the frequency was 340kHz), and the in vitro magnetocaloric heating test was carried out. As shown in Figure 4. It can be seen from Figure 4 that the calculated SLP value of Fe@G magnetic graphene nanocapsules is 418.2 W/g, indicating that the obtained Fe@G magnetic graphene nanocapsules have excellent magnetocaloric properties.
实施例2Example 2
本实施例与实施例1的区别为将Fe(NO3)3·9H2O替换为FeCl3·6H2O,其它同实施例1。The difference between this embodiment and embodiment 1 is that Fe(NO3 )3 ·9H2 O is replaced by FeCl3 ·6H2 O, and the others are the same as embodiment 1.
实施例3Example 3
Co@G磁性石墨烯纳米囊的制备:Preparation of Co@G magnetic graphene nanocapsules:
将0.0036molCo(NO3)2·6H2O加入到15mL纯度为99.9%的甲醇中,溶解得硝酸钴的甲醇溶液。再取1g气相二氧化硅(粒径为400nm)加入到350mL纯度为99.8%的甲醇中,以46kHz的频率超声分散40min,使得溶液中无沉淀,得气相二氧化硅甲醇溶液;后将硝酸钴的甲醇溶液加入气相二氧化硅甲醇溶液中混合,随后在55℃下,以90r/min的转速在旋转蒸发仪上蒸干甲醇溶剂,得到粉末;将所得粉末研磨至粒径为50μm后放入管式炉中,在流量为80cfm的氢气保护下,设置升温速率为19℃/min,由常温升温至850℃,保持25min后,通入流量为250cfm的甲烷进行原位生长15min,原位生长的温度为850℃,随后以35℃/min的降温速率降温至室温,得到催化剂;将所得催化剂加入32mL体积浓度为18%氢氟酸的水溶液中刻蚀12h,反应完成后磁吸回收磁性粉体,用超纯水洗涤至中性,再经磁吸回收得到Co@G磁性石墨烯纳米囊。0.0036mol Co(NO3 )2 ·6H2 O was added into 15 mL of methanol with a purity of 99.9%, and dissolved to obtain a methanol solution of cobalt nitrate. Then take 1g of fumed silica (400nm in particle size) and add it to 350mL of methanol with a purity of 99.8%, and disperse it ultrasonically for 40min at a frequency of 46kHz, so that there is no precipitation in the solution to obtain a methanol solution of fumed silica; Add the methanol solution of fumed silica to the methanol solution and mix, and then evaporate the methanol solvent to dryness on a rotary evaporator at a speed of 90r/min at 55°C to obtain a powder; grind the obtained powder to a particle size of 50μm and put it into In the tube furnace, under the protection of hydrogen with a flow rate of 80cfm, set the heating rate at 19°C/min, increase the temperature from room temperature to 850°C, and keep it for 25min. The temperature was 850°C, and then cooled to room temperature at a cooling rate of 35°C/min to obtain a catalyst; the obtained catalyst was added to 32 mL of an aqueous solution with a volume concentration of 18% hydrofluoric acid and etched for 12 hours, and the magnetic powder was recovered by magnetic absorption after the reaction was completed. body, washed with ultrapure water until neutral, and recovered by magnetic absorption to obtain Co@G magnetic graphene nanocapsules.
本实施例所得Co@G磁性石墨烯纳米囊的性能测试结果如下:The performance test results of Co@G magnetic graphene nanocapsules obtained in this example are as follows:
对制备的Co@G磁性石墨烯纳米囊进行扫描投射电镜的表征,结果如图1b所示。由图1b可知,本发明所得Co@G磁性石墨烯纳米囊可以明显的金属晶格与外层的石墨烯层,说明所得Co@G磁性石墨烯纳米囊为核壳结构;进行元素EDS分析,结果如图2所示。由图2可知,所得Co@G磁性石墨烯纳米囊的组成主要为钴元素,纯度高,未产生其它杂质。The prepared Co@G magnetic graphene nanocapsules were characterized by scanning transmission electron microscopy, and the results are shown in Figure 1b. It can be seen from Figure 1b that the Co@G magnetic graphene nanocapsules obtained in the present invention can have obvious metal lattice and outer graphene layer, indicating that the obtained Co@G magnetic graphene nanocapsules have a core-shell structure; elemental EDS analysis is performed, The result is shown in Figure 2. It can be seen from Figure 2 that the obtained Co@G magnetic graphene nanocapsules are mainly composed of cobalt element, with high purity and no other impurities.
对制备的Co@G磁性石墨烯纳米囊进行磁滞回线测试,结果如图3所示。由图3可知,本实施例所得Co@G磁性石墨烯纳米囊的饱和磁化强度为116emu/g。Hysteresis loop tests were performed on the prepared Co@G magnetic graphene nanocapsules, and the results are shown in Figure 3. It can be seen from Figure 3 that the saturation magnetization of Co@G magnetic graphene nanocapsules obtained in this example is 116 emu/g.
用水稀释制备得到的Co@G磁性石墨烯纳米囊至浓度为5mg/mL,放置在交变磁场中(施加的磁场的强度为20kA/m、频率为340kHz),进行体外磁热升温测试,结果如图4所示。由图4可知,通过计算得到Co@G磁性石墨烯纳米囊的SLP值为398.2W/g,说明所得Co@G磁性石墨烯纳米囊的磁热性能优异。The prepared Co@G magnetic graphene nanocapsules were diluted with water to a concentration of 5 mg/mL, placed in an alternating magnetic field (the strength of the applied magnetic field was 20kA/m, and the frequency was 340kHz), and the in vitro magnetocaloric heating test was performed. As shown in Figure 4. It can be seen from Figure 4 that the calculated SLP value of Co@G magnetic graphene nanocapsules is 398.2 W/g, indicating that the obtained Co@G magnetic graphene nanocapsules have excellent magnetocaloric properties.
实施例4Example 4
本实施例与实施例3的区别为将Co(NO3)2·6H2O替换为CoCl2·6H2O,其它同实施例3。The difference between this embodiment and Embodiment 3 is that Co(NO3 )2 ·6H2 O is replaced by CoCl2 ·6H2 O, and the others are the same as Embodiment 3.
实施例5Example 5
Ni@G磁性石墨烯纳米囊的制备:Preparation of Ni@G magnetic graphene nanocapsules:
将0.0036molNi(NO3)2·6H2O加入到13mL纯度为99.8%的甲醇中,溶解得硝酸镍的甲醇溶液。再取1g气相二氧化硅(粒径为500nm)加入到360mL纯度为99.9%的甲醇中,以43kHz的频率超声分散50min,使得溶液中无沉淀,得气相二氧化硅甲醇溶液;后将硝酸镍的甲醇溶液加入气相二氧化硅甲醇溶液中混合,随后在60℃下,以100r/min的转速在旋转蒸发仪上蒸干甲醇溶剂,得到粉末;将所得粉末研磨至粒径为80μm后放入管式炉中,在流量为90cfm的氢气保护下,设置升温速率为20℃/min,由常温升温至900℃,保持20min后,通入流量为300cfm的甲烷进行原位生长10min,原位生长的温度为900℃,随后以50℃/min的降温速率降温至室温,得到催化剂;将所得催化剂加入30mL体积浓度为15%氢氟酸的水溶液中刻蚀12h,反应完成后磁吸回收磁性粉体,用超纯水洗涤至中性,再经磁吸回收得到Ni@G磁性石墨烯纳米囊。Add 0.0036 mol of Ni(NO3 )2 ·6H2 O into 13 mL of methanol with a purity of 99.8%, and dissolve to obtain a methanol solution of nickel nitrate. Take 1g of fumed silica (500nm in particle size) and add it to 360mL of methanol with a purity of 99.9%, and ultrasonically disperse it at a frequency of 43kHz for 50min, so that there is no precipitation in the solution to obtain a methanol solution of fumed silica; Add the methanol solution of fumed silica to the methanol solution and mix, then evaporate the methanol solvent to dryness on a rotary evaporator at 60°C at a speed of 100r/min to obtain a powder; grind the obtained powder to a particle size of 80μm and put it into In the tube furnace, under the protection of hydrogen with a flow rate of 90cfm, set the heating rate at 20°C/min, raise the temperature from room temperature to 900°C, and keep it for 20min. The temperature is 900°C, and then cooled to room temperature at a cooling rate of 50°C/min to obtain a catalyst; the obtained catalyst is added to 30 mL of an aqueous solution with a volume concentration of 15% hydrofluoric acid for etching for 12 hours, and the magnetic powder is recovered by magnetic absorption after the reaction is completed. body, washed with ultrapure water until neutral, and then recovered by magnetic absorption to obtain Ni@G magnetic graphene nanocapsules.
本实施例所得Ni@G磁性石墨烯纳米囊的性能测试结果如下:The performance test results of Ni@G magnetic graphene nanocapsules obtained in this example are as follows:
对制备的Ni@G磁性石墨烯纳米囊进行扫描投射电镜的表征,结果如图1c所示。由图1c可知,本发明所得Ni@G磁性石墨烯纳米囊可以明显的金属晶格与外层的石墨烯层,说明所得Ni@G磁性石墨烯纳米囊为核壳结构;进行元素EDS分析,结果如图2所示。由图2可知,所得Ni@G磁性石墨烯纳米囊的组成主要为镍元素,纯度高,未产生其它杂质。The prepared Ni@G magnetic graphene nanocapsules were characterized by scanning transmission electron microscopy, and the results are shown in Figure 1c. It can be seen from Figure 1c that the Ni@G magnetic graphene nanocapsules obtained in the present invention can have obvious metal lattice and outer graphene layer, indicating that the obtained Ni@G magnetic graphene nanocapsules have a core-shell structure; elemental EDS analysis is performed, The result is shown in Figure 2. It can be seen from Figure 2 that the obtained Ni@G magnetic graphene nanocapsules are mainly composed of nickel element, with high purity and no other impurities.
对制备的Ni@G磁性石墨烯纳米囊进行磁滞回线测试,结果如图3所示。由图3可知,本实施例所得Ni@G磁性石墨烯纳米囊的饱和磁化强度为116emu/g。Hysteresis loop tests were performed on the prepared Ni@G magnetic graphene nanocapsules, and the results are shown in Figure 3. It can be seen from Figure 3 that the saturation magnetization of Ni@G magnetic graphene nanocapsules obtained in this example is 116 emu/g.
用水稀释制备得到的Ni@G磁性石墨烯纳米囊至浓度为5mg/mL,放置在交变磁场中(施加的磁场的强度为20kA/m、频率为340kHz),进行体外磁热升温测试,结果如图4所示。由图4可知,通过计算得到Ni@G磁性石墨烯纳米囊的SLP值为223.1W/g,说明所得Ni@G磁性石墨烯纳米囊的磁热性能优异。The prepared Ni@G magnetic graphene nanocapsules were diluted with water to a concentration of 5 mg/mL, placed in an alternating magnetic field (the strength of the applied magnetic field was 20kA/m, and the frequency was 340kHz), and an in vitro magnetocaloric heating test was performed. As shown in Figure 4. It can be seen from Figure 4 that the calculated SLP value of Ni@G magnetic graphene nanocapsules is 223.1 W/g, indicating that the obtained Ni@G magnetic graphene nanocapsules have excellent magnetocaloric properties.
实施例6Example 6
本实施例与实施例5的区别为将Ni(NO3)2·6H2O替换为NiCl2·6H2O,其它同实施例5。The difference between this embodiment and Embodiment 5 is that Ni(NO3 )2 ·6H2 O is replaced by NiCl2 ·6H2 O, and the others are the same as Embodiment 5.
对实施例1所得Fe@G磁性石墨烯纳米囊进行性能测试,具体如下:The performance test of Fe@G magnetic graphene nanocapsules obtained in Example 1 is as follows:
1、为验证磁性石墨烯纳米囊作为磁热试剂能通过口服的方式滞留在胃部,对实施例1所得Fe@G磁性石墨烯纳米囊通过小鼠灌胃实验进行验证。该验证经湖南大学动物伦理委员会批准。1. In order to verify that the magnetic graphene nanocapsules can be retained in the stomach by oral administration as a magnetocaloric reagent, the Fe@G magnetic graphene nanocapsules obtained in Example 1 were verified by gavage experiments in mice. This validation was approved by the Animal Ethics Committee of Hunan University.
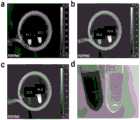
验证方法为:对BALB/C小鼠进行灌胃操作(Fe@G磁性石墨烯纳米囊的水溶液浓度为0.2mg/mL,灌胃体积为200uL/只),通过核磁共振成像表征Fe@G磁性石墨烯纳米囊的胃部滞留状态,结果如图5所示。The verification method is as follows: intragastric administration of BALB/C mice (the aqueous solution concentration of Fe@G magnetic graphene nanocapsules is 0.2mg/mL, and the intragastric volume is 200uL/mouse), and the magnetic resonance imaging of Fe@G is used to characterize the The gastric retention state of graphene nanocapsules, the results are shown in Figure 5.
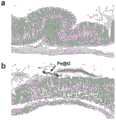
由图5可知,图5a为灌胃前小鼠成像图,红圈位置为胃部,此时胃部信号为亮信号。图5b为灌胃后20min小鼠成像图,红圈位置为胃部,此时胃部信号因为磁性石墨烯纳米囊的存在变成暗信号。表明本发明所得磁性石墨烯纳米囊可以在胃内长时间滞留。It can be seen from Fig. 5 that Fig. 5a is the imaging image of the mouse before gavage, the position of the red circle is the stomach, and the signal of the stomach is bright at this time. Figure 5b is the imaging image of the
2、为验证磁性石墨烯纳米囊在胃部热疗的能力,对实施例1所得Fe@G磁性石墨烯纳米囊通过小鼠胃部热疗实验进行验证。该实验经湖南大学动物伦理委员会批准。2. In order to verify the ability of the magnetic graphene nanocapsules in stomach hyperthermia, the Fe@G magnetic graphene nanocapsules obtained in Example 1 were verified by a mouse stomach hyperthermia experiment. This experiment was approved by the Animal Ethics Committee of Hunan University.
验证方法为:对BALB/C小鼠进行灌胃操作(Fe@G磁性石墨烯纳米囊的水溶液浓度为0.2mg/mL,灌胃体积为200uL/只),等Fe@G磁性石墨烯纳米囊滞留在小鼠胃部后,将小鼠放入交变磁场中(施加的磁场强度为20kA/m、频率为340kHz),进行体内磁热升温测试。加热时长为30min。小鼠牺牲后取出小鼠胃部,进行切片和H&E染色,结果如图6所示。The verification method is as follows: intragastric administration of BALB/C mice (the aqueous solution concentration of Fe@G magnetic graphene nanocapsules is 0.2mg/mL, and the intragastric volume is 200uL/mouse), and the Fe@G magnetic graphene nanocapsules After staying in the stomach of the mouse, the mouse was placed in an alternating magnetic field (the applied magnetic field strength was 20kA/m, and the frequency was 340kHz), and the in vivo magnetocaloric heating test was carried out. The heating time is 30min. After the mice were sacrificed, the stomachs of the mice were taken out, sectioned and stained with H&E. The results are shown in Figure 6.
由图6可知,图6a是正常小鼠胃切片图,粘膜层胃上皮结构完整,胃小凹结构清晰,上皮细胞排列紧密,固有层胃腺丰富,主细胞、壁细胞形态结构正常。图6b为经磁热疗30min后的切片图,粘膜层可见多处上皮细胞脱落,并混合Fe@G磁性石墨烯纳米囊(黑色箭头)。表明本发明所得磁性石墨烯纳米囊能在胃部进行磁热疗,为高性能胃部磁热疗试剂的研发奠定了基础。It can be seen from Figure 6 that Figure 6a is a normal mouse stomach slice. The gastric epithelium in the mucosa layer is intact, the structure of the gastric pit is clear, the epithelial cells are closely arranged, the lamina propria is rich in gastric glands, and the morphology and structure of the principal cells and parietal cells are normal. Figure 6b is a sliced image after 30 min of magnetic hyperthermia. Multiple epithelial cells can be seen in the mucosal layer shedding and mixed with Fe@G magnetic graphene nanocapsules (black arrows). It shows that the magnetic graphene nanocapsule obtained in the present invention can perform magnetic hyperthermia in the stomach, which lays the foundation for the research and development of high-performance gastric magnetothermotherapy reagents.
3、对实施例1所得Fe@G磁性石墨烯纳米囊与四氧化三铁的稳定性进行测试。3. The stability of the Fe@G magnetic graphene nanocapsules obtained in Example 1 and ferroferric oxide was tested.
测试方法:将Fe@G磁性石墨烯纳米囊和四氧化三铁分别置于20mL浓度为1M的盐酸溶液中,将二者放入交变磁场中(施加的磁场强度为20kA/m、频率为340kHz),进行磁热升温测试。所得结果如图7所示。Test method: Place Fe@G magnetic graphene nanocapsules and ferroferric oxide in 20mL hydrochloric acid solution with a concentration of 1M, respectively, and put them in an alternating magnetic field (applied magnetic field strength is 20kA/m, frequency is 340kHz), for the magnetocaloric temperature rise test. The results obtained are shown in Figure 7.
由图7可知,图7a显示处于磁场10min后,两支试管升到了相同温度。图7b显示处于磁场20min后,左侧装有四氧化三铁的试管温度有所下降,颗粒开始分解,右侧试管无影响。图7c显示处于磁场30min后,左侧装有四氧化三铁的试管温度大幅下降,颗粒分解,右侧试管无影响。图7d显示处于磁场30min后,四氧化三铁已经大部分分解,呈现绿色澄清溶液,而Fe@G磁性石墨烯纳米囊维持原样。表明本发明所得磁性石墨烯纳米囊在酸性条件下磁热升温比常用磁热试剂四氧化三铁更加稳定。It can be seen from Fig. 7, Fig. 7a shows that after being in the magnetic field for 10 minutes, the two test tubes rose to the same temperature. Figure 7b shows that after being in the magnetic field for 20 minutes, the temperature of the test tube with ferroferric oxide on the left dropped, and the particles began to decompose, while the test tube on the right had no effect. Figure 7c shows that after being in the magnetic field for 30 minutes, the temperature of the test tube with ferric oxide on the left dropped sharply, and the particles decomposed, while the test tube on the right had no effect. Figure 7d shows that after being exposed to the magnetic field for 30 min, the ferroferric oxide has been mostly decomposed, showing a green clear solution, while the Fe@G magnetic graphene nanocapsules remain intact. It shows that the magnetic graphene nanocapsule obtained in the present invention is more stable in magnetocaloric heating under acidic conditions than the commonly used magnetocaloric reagent ferric oxide.
以上所述仅是本发明的优选实施方式,应当指出,对于本技术领域的普通技术人员来说,在不脱离本发明原理的前提下,还可以做出若干改进和润饰,这些改进和润饰也应视为本发明的保护范围。The above is only a preferred embodiment of the present invention, and it should be pointed out that for those of ordinary skill in the art, some improvements and modifications can also be made without departing from the principles of the present invention. It should be regarded as the protection scope of the present invention.
Claims (10)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211286538.4ACN115645531A (en) | 2022-10-20 | 2022-10-20 | A kind of magnetic graphene nanocapsule and its preparation method and application |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211286538.4ACN115645531A (en) | 2022-10-20 | 2022-10-20 | A kind of magnetic graphene nanocapsule and its preparation method and application |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN115645531Atrue CN115645531A (en) | 2023-01-31 |
Family
ID=84989653
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211286538.4APendingCN115645531A (en) | 2022-10-20 | 2022-10-20 | A kind of magnetic graphene nanocapsule and its preparation method and application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115645531A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118976106A (en)* | 2024-07-31 | 2024-11-19 | 湖南大学 | An alginate-based thermogenetic CXCL12 releaser and its preparation method and application |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102500295A (en)* | 2011-10-26 | 2012-06-20 | 天津大学 | Preparation method of carbon-coated metallic nano-particles |
| CN103632793A (en)* | 2013-12-03 | 2014-03-12 | 南昌航空大学 | Preparation method for carbon-coated Ni-Zn-Fe magnetic nanometer material taking chitosan as carbon source |
| KR20140136638A (en)* | 2013-05-21 | 2014-12-01 | 연세대학교 산학협력단 | Nano-capsuled organic-inorganic complex both for diagnosing and treating disease and manufacturing method thereof |
| CN107537438A (en)* | 2017-08-23 | 2018-01-05 | 湖南大学 | Magnetic composite nano material of graphene parcel and its preparation method and application |
| CN113020590A (en)* | 2021-03-02 | 2021-06-25 | 湖南大学 | Graphene-coated cobalt-platinum composite nano material and preparation process and application thereof |
| CN114377690A (en)* | 2022-01-19 | 2022-04-22 | 中国科学技术大学 | PtFe-SiO2Nanocomposite material, preparation method and application thereof |
- 2022
- 2022-10-20CNCN202211286538.4Apatent/CN115645531A/enactivePending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102500295A (en)* | 2011-10-26 | 2012-06-20 | 天津大学 | Preparation method of carbon-coated metallic nano-particles |
| KR20140136638A (en)* | 2013-05-21 | 2014-12-01 | 연세대학교 산학협력단 | Nano-capsuled organic-inorganic complex both for diagnosing and treating disease and manufacturing method thereof |
| CN103632793A (en)* | 2013-12-03 | 2014-03-12 | 南昌航空大学 | Preparation method for carbon-coated Ni-Zn-Fe magnetic nanometer material taking chitosan as carbon source |
| CN107537438A (en)* | 2017-08-23 | 2018-01-05 | 湖南大学 | Magnetic composite nano material of graphene parcel and its preparation method and application |
| CN113020590A (en)* | 2021-03-02 | 2021-06-25 | 湖南大学 | Graphene-coated cobalt-platinum composite nano material and preparation process and application thereof |
| CN114377690A (en)* | 2022-01-19 | 2022-04-22 | 中国科学技术大学 | PtFe-SiO2Nanocomposite material, preparation method and application thereof |
Non-Patent Citations (3)
| Title |
|---|
| 宋志灵: "新型金属石墨纳米囊的合成及其生化传感性能研究", 《中国博士学位论文全文数据库》, no. 02, 15 February 2017 (2017-02-15), pages 014 - 267* |
| 张鲁凤: "新型石墨烯纳米囊材料的制备及其细菌诊疗研究", 《中国博士学位论文数据库 工程科技I辑》, no. 08, 15 August 2022 (2022-08-15), pages 014 - 42* |
| 朱兆田等: "多功能金属石墨纳米囊的 生物医学应用进展", 《高等学校化学学报》, vol. 42, no. 9, 9 September 2021 (2021-09-09), pages 2701 - 2716* |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118976106A (en)* | 2024-07-31 | 2024-11-19 | 湖南大学 | An alginate-based thermogenetic CXCL12 releaser and its preparation method and application |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102659191B (en) | Method for controlling morphology and performance of ferriferrous oxide | |
| CN101337695B (en) | Method for preparing nano ferric oxide microspheres with adjustable particle size by microwave | |
| CN101625919A (en) | Preparation method of meso-porous nanometer magnetic material in novel structure | |
| Tadic et al. | Synthesis of metastable hard-magnetic ε-Fe 2 O 3 nanoparticles from silica-coated akaganeite nanorods | |
| CN104072762B (en) | The preparation method of the magnetic carbon nano-tube that the poly-Dopamine HCL in a kind of surface is modified | |
| CN105355866B (en) | A kind of preparation method of cobaltosic oxide composite graphite alkene three-dimensional aeroge | |
| CN111072070B (en) | A kind of preparation method of high saturation magnetization superparamagnetic porous ferrite microspheres | |
| CN107601461A (en) | A kind of magnetic composite of Fe 3 O 4 coating carbon nanotube and preparation method thereof | |
| CN105832699B (en) | A kind of Fe3O4@SiO2The preparation method and application of yolk-eggshell structure hollow complex microsphere | |
| CN106474473A (en) | Preparation of a photothermal diagnostic agent based on gadolinium-modified Fe3O4@PDA nanomaterials | |
| CN115645531A (en) | A kind of magnetic graphene nanocapsule and its preparation method and application | |
| CN105741996B (en) | A kind of preparation method of the superparamagnetic nano particle based on low temperature plasma | |
| CN116038653B (en) | Magnetic micro-robot with microalgae as template and preparation method thereof | |
| CN100497186C (en) | Method for preparing Fe2O3 Nano particles clad by Fe2O3 | |
| CN107452457A (en) | A kind of magnetic nanoparticle, preparation method and applications | |
| CN112175618B (en) | Green simple regulation and control synthesis method of highly uniform gadolinium phosphate micro-nano luminescent material | |
| CN110436529A (en) | A kind of Fe can be used for magnetic thermotherapy3O4The preparation method of nano-bar material | |
| Aquino et al. | New synthesis route for high quality iron oxide-based nanorings: Structural and magnetothermal evaluations | |
| CN104001173A (en) | A water-soluble multifunctional CoFe2O4@MnFe2O4@polypyrrole satellite structure nanomaterial and its preparation method and application | |
| CN105540676B (en) | Magnetic ball chain and preparation method thereof | |
| CN103303981A (en) | Ferroferric oxide nanoparticle as well as preparation method and application thereof | |
| CN101748497B (en) | Preparation method of one-dimensional monodisperse superparamagnetic nanometer composite fiber material | |
| CN104689345B (en) | A kind of NaGdF4@CaF2The brilliant preparation method of core-shell nano | |
| CN105950148A (en) | Preparation method for preparing ferroferric oxide hollow ball-based fluorescent magnetic composite material | |
| CN117776276A (en) | Ferric oxide nano particle and application thereof in magnetic particle imaging |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |