CN115584536A - A kind of ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction and preparation method thereof - Google Patents
A kind of ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction and preparation method thereofDownload PDFInfo
- Publication number
- CN115584536A CN115584536ACN202211297373.0ACN202211297373ACN115584536ACN 115584536 ACN115584536 ACN 115584536ACN 202211297373 ACN202211297373 ACN 202211297373ACN 115584536 ACN115584536 ACN 115584536A
- Authority
- CN
- China
- Prior art keywords
- ruthenium
- catalyst
- preparation
- active agent
- carbon carrier
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/073—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material
- C25B11/091—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds
- C25B11/093—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds at least one noble metal or noble metal oxide and at least one non-noble metal oxide
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/02—Hydrogen or oxygen
- C25B1/04—Hydrogen or oxygen by electrolysis of water
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B9/00—Cells or assemblies of cells; Constructional parts of cells; Assemblies of constructional parts, e.g. electrode-diaphragm assemblies; Process-related cell features
- C25B9/17—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof
- C25B9/19—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms
- C25B9/23—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms comprising ion-exchange membranes in or on which electrode material is embedded
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Catalysts (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明属于电析氢催化剂技术领域,更具体地,涉及一种用于碱性电析氢反应的纳米团簇催化剂及其制备方法。The invention belongs to the technical field of electrolytic hydrogen evolution catalysts, and more specifically relates to a nano-cluster catalyst for alkaline electrolytic hydrogen evolution reaction and a preparation method thereof.
背景技术Background technique
目前,全球主流的能源依然是以化石能源为代表的一次能源。化石能源的使用带来了一系列复杂问题。第一,作为不可再生能源,化石能源的储量有限,无法满足人类社会的高质量可持续发展;第二,化石能源的大量使用造成了诸多环境问题,对全球生态系统构成了严重威胁。在此背景下,世界各国都积极努力的开发能够替代化石能源的新能源。在诸多新能源中,氢能由于其特殊的优势受到了广泛关注。首先,氢元素是宇宙中储量最丰富的元素,地球海水中的氢元素就能完全满足人类的使用;其次,氢能是零排放的环境友好型能源,氢能在使用的过程中仅生产无毒无害的水而不产生其它任何废弃物;最后,氢能是可再生的能源,氢能在使用过程中转化成水,而水可以通过太阳能等其它可再生能源产生的电能电解再生成氢气,如此构建成氢能的循环利用体系。但要构建氢能为核心的能源体系,首先要解决的是规模化制氢的难题。目前,主流的制氢方式为化石能源制氢法和电解水制氢法。前者技术成熟但消耗大量化石能源,而电解水制氢的设备简单,制得的氢气纯度高,可以满足燃料电池等能源器件的使用要求。相比于酸性条件电解水制氢,碱性条件下的电解水制氢工艺更成熟、成本更低。但碱性电解水依然面临阴极析氢催化剂活性低的问题。目前,铂基材料是活性最优的电析氢催化剂,但是铂的高成本限制了其规模化应用。At present, the world's mainstream energy is still the primary energy represented by fossil energy. The use of fossil energy brings a series of complex problems. First, as non-renewable energy, fossil energy reserves are limited and cannot meet the high-quality and sustainable development of human society; second, the extensive use of fossil energy has caused many environmental problems and poses a serious threat to the global ecosystem. In this context, countries all over the world are making active efforts to develop new energy sources that can replace fossil energy sources. Among many new energy sources, hydrogen energy has received widespread attention due to its special advantages. First of all, hydrogen is the most abundant element in the universe, and the hydrogen in the seawater of the earth can fully meet the needs of human beings; second, hydrogen energy is an environmentally friendly energy source with zero emissions. Toxic and harmless water does not produce any other waste; finally, hydrogen energy is a renewable energy source, hydrogen energy is converted into water during use, and water can be regenerated into hydrogen by electrolysis of electricity generated by solar energy and other renewable energy sources , thus constructing a recycling system for hydrogen energy. But to build an energy system with hydrogen energy as the core, the first thing to solve is the problem of large-scale hydrogen production. At present, the mainstream hydrogen production methods are fossil energy hydrogen production method and electrolysis water hydrogen production method. The former technology is mature but consumes a lot of fossil energy, while the equipment for hydrogen production by electrolysis of water is simple, and the hydrogen produced has high purity, which can meet the requirements of energy devices such as fuel cells. Compared with hydrogen production by electrolysis of water under acidic conditions, the process of hydrogen production by electrolysis of water under alkaline conditions is more mature and lower in cost. However, alkaline electrolyzed water still faces the problem of low activity of cathodic hydrogen evolution catalyst. At present, platinum-based materials are the most active electrohydrogen evolution catalysts, but the high cost of platinum limits its large-scale application.
钌作为铂的同族元素,由于对H*的吸附能力与铂接近,但价格远低于铂而受到了广泛关注。现有技术中通过将钌金属颗粒负载在多孔碳中获得的复合材料的电析氢活性接近Pt/C。相比于纳米颗粒催化剂,团簇的分散性更好,设计团簇催化剂可以大幅度提高金属位点的利用率,降低钌的用量。另外,碱性电析氢的第一步是水分子的吸附和解离,而氧化物对水分子具有较低的吸附能和解离能。因此,合理的设想是设计钌氧化物纳米团簇复合材料可以获得高电析氢活性。但目前,如何制备低钌载量、高活性的氧化钌纳米团簇催化剂依然是一个难题。As a congener element of platinum, ruthenium has attracted extensive attention because its adsorption capacity for H* is close to that of platinum, but its price is much lower than that of platinum. In the prior art, the electrohydrogen evolution activity of the composite material obtained by loading ruthenium metal particles in porous carbon is close to that of Pt/C. Compared with nanoparticle catalysts, the dispersion of clusters is better, and the design of cluster catalysts can greatly improve the utilization of metal sites and reduce the amount of ruthenium. In addition, the first step of alkaline electrolysis of hydrogen is the adsorption and dissociation of water molecules, while oxides have lower adsorption energy and dissociation energy for water molecules. Therefore, it is reasonable to assume that designing ruthenium oxide nanocluster composites can obtain high electrohydrogen evolution activity. However, how to prepare low ruthenium loading and high activity ruthenium oxide nanocluster catalysts is still a difficult problem.
发明内容Contents of the invention
本发明的目的在于克服现有技术中的不足,提供一种用于催化碱性条件下电化学析氢反应的钌纳米团簇催化剂,所述催化剂由于纳米团簇结构可以充分暴露金属活性位点,同时钌和氧的配位形式提高了对水分子的亲和性和结合能力,促使其具有优异的碱性电析氢活性。The purpose of the present invention is to overcome the deficiencies in the prior art, to provide a ruthenium nanocluster catalyst for electrochemical hydrogen evolution reaction under catalyzed alkaline conditions, the catalyst can fully expose metal active sites due to the nanocluster structure, At the same time, the coordination form of ruthenium and oxygen improves the affinity and binding ability to water molecules, which makes it have excellent alkaline electrolytic hydrogen evolution activity.
本发明的又一目的在于提供所述催化剂的制备方法。Another object of the present invention is to provide a preparation method of the catalyst.
为实现上述目的,本发明采用了如下技术方案:To achieve the above object, the present invention adopts the following technical solutions:
一种用于碱性电析氢反应的钌纳米团簇催化剂,所述催化剂是钌氧化物以纳米团簇形式负载在碳载体上的复合材料。A ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction, the catalyst is a composite material in which ruthenium oxide is loaded on a carbon carrier in the form of nano-clusters.
本发明中,所述催化剂为钌氧化物以纳米团簇形式负载在碳载体上,纳米团簇的结构使金属活性位点充分暴露,同时钌氧化物的形式提高了对水分子的亲和性和解离能力,有助于提高其催化活性。In the present invention, the catalyst is ruthenium oxide loaded on the carbon support in the form of nano-clusters, the structure of the nano-clusters fully exposes the metal active sites, while the form of ruthenium oxide improves the affinity for water molecules And dissociation ability, help to improve its catalytic activity.
进一步地,所述纳米团簇是由3~30个金属原子构成的金属核,金属核与氧配位,其中金属原子为钌原子,所述钌原子的直径为0.268nm。Further, the nano-cluster is a metal core composed of 3 to 30 metal atoms, and the metal core is coordinated with oxygen, wherein the metal atom is a ruthenium atom, and the diameter of the ruthenium atom is 0.268nm.
所述催化剂的制备方法,包括如下步骤:The preparation method of described catalyst comprises the steps:
S1.将干燥的农业废弃物粉碎后与活性剂混合煅烧得到生物炭;S1. Grinding the dry agricultural waste and mixing it with an active agent to obtain biochar;
S2.将S1.得到的生物炭在盐酸水溶液中搅拌、过滤、洗净得碳载体;S2. Stirring, filtering, and washing the biochar obtained in S1. in an aqueous hydrochloric acid solution to obtain a carbon carrier;
S3.将钌盐分散在溶剂中,再加入S2.得到的碳载体混合分散均匀后旋蒸干燥得前驱体材料;S3. Disperse the ruthenium salt in the solvent, and then add the carbon carrier obtained in S2. Mix and disperse evenly, then spin evaporate and dry to obtain the precursor material;
S4.将S3.得到的前驱体材料进行热处理得到钌纳米团簇催化剂。S4. Heat-treating the precursor material obtained in S3. to obtain a ruthenium nano-cluster catalyst.
农业废弃物一般是指粮食作物收获后废弃的枝叶秸秆。本发明中,选用农业废弃物作为碳载体的前驱体,制得的碳载体含有较多的含氧官能团,利用其氧元素富电子性对钌离子吸附作用,结合热处理工艺获得低钌载量的钌纳米团簇催化剂,同时钌和氧的配位形式提高了催化剂对水分子的亲和性和解离能力。另外,这种制备过程简单易控。Agricultural waste generally refers to the discarded branches, leaves and stalks of food crops after harvesting. In the present invention, agricultural waste is selected as the precursor of the carbon carrier, and the prepared carbon carrier contains more oxygen-containing functional groups, and the electron-rich nature of oxygen element is used to adsorb ruthenium ions, and the carbon carrier with low ruthenium loading is obtained in combination with the heat treatment process. Ruthenium nanocluster catalyst, meanwhile, the coordination form of ruthenium and oxygen improves the affinity and dissociation ability of the catalyst to water molecules. In addition, the preparation process is simple and easy to control.
进一步地,步骤S1.中,所述农业废弃物为水稻秸秆、玉米秸秆、小麦秸秆、油菜秸秆、高粱秸秆中的一种或几种。Further, in step S1., the agricultural waste is one or more of rice straw, corn straw, wheat straw, rape straw, and sorghum straw.
具体地,步骤S1.中,将干燥的农业废弃物粉碎后先与第一活性剂混合,再与第二活性剂混合,然后煅烧得到生物炭。Specifically, in step S1., the dried agricultural waste is pulverized, mixed with the first active agent, and then mixed with the second active agent, and then calcined to obtain biochar.
更进一步地,所述第一活性剂为碱式碳酸镁、氢氧化钾或氢氧化钠中的一种。Furthermore, the first active agent is one of basic magnesium carbonate, potassium hydroxide or sodium hydroxide.
更进一步地,所述农业废弃物与第一活性剂的质量比为1:0.5~5。Furthermore, the mass ratio of the agricultural waste to the first active agent is 1:0.5-5.
更进一步地,所述第二活性剂为二水合氯化锌和/或六水合硝酸锌。Furthermore, the second active agent is zinc chloride dihydrate and/or zinc nitrate hexahydrate.
更进一步地,所述农业废弃物与第二活性剂的质量比为1:1~12。Furthermore, the mass ratio of the agricultural waste to the second active agent is 1:1-12.
进一步地,步骤S1.中,所述煅烧的温度为700~1200℃。Further, in step S1., the calcination temperature is 700-1200°C.
进一步地,步骤S1.中,所述煅烧的气氛为氮气、氩气或氢气中的一种或几种。Further, in step S1., the calcination atmosphere is one or more of nitrogen, argon or hydrogen.
进一步地,步骤S1.中,所述煅烧的时间为0.5~10h。Further, in step S1., the calcination time is 0.5-10 hours.
进一步地,步骤S2.中,所述盐酸水溶液的浓度为0.5~8mol/L。Further, in step S2., the concentration of the hydrochloric acid aqueous solution is 0.5-8 mol/L.
进一步地,步骤S2.中,所述搅拌时间为24h。Further, in step S2., the stirring time is 24h.
进一步地,步骤S3.中,所述溶剂为水和/或醇。Further, in step S3., the solvent is water and/or alcohol.
具体的,所述醇为乙醇、异丙醇、甲醇或正丙醇中的一种或几种。Specifically, the alcohol is one or more of ethanol, isopropanol, methanol or n-propanol.
进一步地,步骤S3.中,所述钌盐为三水合氯化钌和/或硝酸钌。Further, in step S3., the ruthenium salt is ruthenium chloride trihydrate and/or ruthenium nitrate.
进一步地,步骤S3.中,所述碳载体和钌盐的质量比为10~100:1。Further, in step S3., the mass ratio of the carbon support to the ruthenium salt is 10-100:1.
进一步地,步骤S4.中,所述热处理的温度为200~900℃。Further, in step S4., the temperature of the heat treatment is 200-900°C.
进一步地,步骤S4.中,所述热处理的时间为0.5~12h。Further, in step S4., the time for the heat treatment is 0.5-12 hours.
进一步地,步骤S4.中,所述热处理的气氛为氢气、氮气、氩气或一氧化碳中的一种或几种。Further, in step S4., the heat treatment atmosphere is one or more of hydrogen, nitrogen, argon or carbon monoxide.
与现有技术相比,本发明具有如下有益效果:Compared with the prior art, the present invention has the following beneficial effects:
本发明提供了一种用于碱性电析氢反应的钌纳米团簇催化剂,所述催化剂中的钌以氧化物纳米团簇的形式存在,纳米团簇的高分散性提高了钌原子利用率,钌氧化物形式提高了催化剂吸附水分子和解离水分子的能力,加快了Volmer过程,提高了电催化析氢活性。所述催化剂的制备过程中钌盐用量较低,且涉及的材料处理过程简单,可行性高,规模化应用前景较好。The invention provides a ruthenium nanocluster catalyst for alkaline electrolysis hydrogen reaction, the ruthenium in the catalyst exists in the form of oxide nanoclusters, and the high dispersion of the nanoclusters improves the utilization rate of ruthenium atoms, The ruthenium oxide form improves the ability of the catalyst to absorb and dissociate water molecules, accelerate the Volmer process, and improve the electrocatalytic hydrogen evolution activity. The amount of ruthenium salt used in the preparation process of the catalyst is relatively low, and the material processing process involved is simple, the feasibility is high, and the prospect of large-scale application is good.
附图说明Description of drawings
图1a为实施例2制得的钌纳米团簇催化剂的TEM图;Fig. 1 a is the TEM figure of the ruthenium nanocluster catalyst that
图1b为实施例2制得的钌纳米团簇催化剂的HAADF-STEM图;Fig. 1 b is the HAADF-STEM figure of the ruthenium nanocluster catalyst that
图1c为实施例2制得的钌纳米团簇催化剂的N2吹扫的1mol/L KOH中的CV曲线图;Fig. 1c is the N of the ruthenium nanocluster catalyst that embodiment2 makes The CV curve figure of purging in the 1mol/L KOH;
图1d为实施例2制得的钌纳米团簇催化剂的Ru K-edge XANES谱图;Fig. 1 d is the Ru K-edge XANES spectrogram of the ruthenium nanocluster catalyst that
图1e为实施例2制得的钌纳米团簇催化剂的FT-EXAFS谱图;Fig. 1 e is the FT-EXAFS spectrogram of the ruthenium nanocluster catalyst that
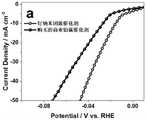
图2a为实施例7制得的钌纳米团簇催化剂在1mol/L的KOH水溶液中的电析氢活性图;Fig. 2 a is the electrolytic hydrogen evolution activity figure of the ruthenium nanocluster catalyst that embodiment 7 makes in the KOH aqueous solution of 1mol/L;
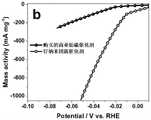
图2b为实施例7制得的钌纳米团簇催化剂在1mol/L的KOH水溶液中的金属质量活性图;Fig. 2b is the metal mass activity figure of the ruthenium nanocluster catalyst that embodiment 7 makes in the KOH aqueous solution of 1mol/L;
图2c为实施例7制得的钌纳米团簇催化剂在1mol/L的KOH水溶液中线性循环10000圈前后的极化曲线图;Fig. 2c is the polarization curve before and after the ruthenium nanocluster catalyst that embodiment 7 makes in the KOH aqueous solution of 1mol/L linear cycle 10000 circles;
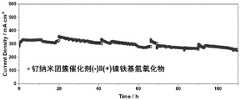
图3为实施例8制得的钌纳米团簇催化剂为阴极匹配镍铁氢氧化物为阳极组装的基于阴离子交换膜的碱性电解水电解槽20℃下恒电位2V得到的i-t曲线图。Fig. 3 is the i-t graph obtained at 20° C. of an alkaline electrolyzed water electrolyzer based on an anion exchange membrane with the ruthenium nanocluster catalyst prepared in Example 8 as the cathode and the nickel-iron hydroxide as the anode assembly.
具体实施方式detailed description
下面结合具体实施例对本发明做出进一步地详细阐述,所述实施例只用于解释本发明,并非用于限定本发明的范围。除非特别说明,本发明采用的试剂、方法和设备为本技术领域常规试剂、方法和设备。The present invention will be further described in detail below in conjunction with specific embodiments, which are only used to explain the present invention, and are not intended to limit the scope of the present invention. Unless otherwise specified, the reagents, methods and equipment used in the present invention are conventional reagents, methods and equipment in the technical field.
实施例1Example 1
S1.将干燥至恒重并粉碎后的4g水稻秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至900℃并保持2h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 4 g of rice straw dried to constant weight and pulverized to obtain a light yellow powder A; then grind the light yellow powder A with 24 g of zinc chloride dihydrate to obtain a light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 2h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在3mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 3mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将20mg三水合氯化钌溶于100mL正丙醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 20 mg of ruthenium chloride trihydrate in 100 mL of n-propanol to obtain liquid C, then add 1000 mg of the carbon carrier obtained in S2, and disperse evenly by ultrasonication for 20 minutes, then dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持氮气气氛,以5℃/min速率升温至300℃并保持2h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a nitrogen atmosphere, raise the temperature to 300° C. at a rate of 5° C./min and keep it for 2 hours to obtain a ruthenium nanocluster catalyst.
实施例2Example 2
S1.将干燥至恒重并粉碎后的4g油菜秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至900℃并保持4h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 4 g of rape stalks dried to constant weight and pulverized, and grind evenly to obtain light yellow powder A; then grind light yellow powder A and 24 g of zinc chloride dihydrate evenly to obtain light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 4h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在3mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 3mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将30mg三水合氯化钌溶于100mL正丙醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 30 mg of ruthenium chloride trihydrate in 100 mL of n-propanol to obtain liquid C, then add 1000 mg of the carbon carrier obtained in S2, and disperse evenly by ultrasonication for 20 minutes, then dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持5%(5%VH2/95%VAr)氢气氩气混合气氛气氛,以5℃/min速率升温至400℃并保持2h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a 5% (5% VH2 /95% VAr ) mixed atmosphere of hydrogen and argon, and raise the temperature to 5°C/min. Keep at 400°C for 2h to obtain ruthenium nanocluster catalyst.
实施例3Example 3
S1.将干燥至恒重并粉碎后的4g小麦秸秆,加入4g氢氧化钾,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与48g六水合硝酸锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持5%(5%VH2/95%VAr)氢气氩气混合气氛,以10℃/min速率升温至700℃并保持9h,材料由淡黄色变为黑色,得到生物炭;S1. Add 4 g of potassium hydroxide to the 4 g of wheat straw dried to constant weight and pulverized, and grind to obtain a light yellow powder A; then grind the light yellow powder A with 48 g of zinc nitrate hexahydrate to obtain a light yellow powder B; The powder B was transferred to the quartz furnace tube of the tube furnace, maintained a mixed atmosphere of 5% (5% VH2 /95% VAr ) hydrogen and argon, and raised the temperature to 700 ° C at a rate of 10 ° C / min and kept it for 9 hours. The material changed from light yellow to Turn black to get biochar;
S2.将S1.得到的生物炭在1mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 1mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将50mg硝酸钌溶于100mL水中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 50 mg of ruthenium nitrate in 100 mL of water to obtain liquid C, and then add 1000 mg of the carbon carrier obtained in S2. After ultrasonication for 20 minutes to disperse evenly, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持氮气气氛,以5℃/min速率升温至200℃并保持10h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a nitrogen atmosphere, raise the temperature to 200° C. at a rate of 5° C./min and keep it for 10 hours to obtain a ruthenium nanocluster catalyst.
实施例4Example 4
S1.将干燥至恒重并粉碎后的4g油菜秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至900℃并保持2h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 4 g of rape stalks dried to constant weight and pulverized, and grind evenly to obtain light yellow powder A; then grind light yellow powder A and 24 g of zinc chloride dihydrate evenly to obtain light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 2h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在3mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 3mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将40mg三水合氯化钌溶于100mL乙醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 40 mg of ruthenium chloride trihydrate in 100 mL of ethanol to obtain liquid C, and then add 1000 mg of the carbon carrier obtained in S2. After ultrasonication for 20 minutes to disperse evenly, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持5%(5%VH2/95%VAr)氢气氩气混合气氛,以5℃/min速率升温至300℃并保持2h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a 5% (5% VH2 /95% VAr ) hydrogen and argon mixed atmosphere, and raise the temperature to 300 at a rate of 5°C/min. ℃ and kept for 2h to obtain ruthenium nano-cluster catalyst.
实施例5Example 5
S1.将干燥至恒重并粉碎后的4g玉米秸秆,加入18g氢氧化钠,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g六水合硝酸锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至1200℃并保持1h,材料由淡黄色变为黑色,得到生物炭;S1. Add 18g of sodium hydroxide to the 4g of corn stalks dried to constant weight and pulverized, and grind uniformly to obtain a light yellow powder A; then grind the light yellow powder A and 24g of zinc nitrate hexahydrate to obtain a light yellow powder B; Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 1200°C at a rate of 10°C/min and keep it for 1h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在2mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 2mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将100mg硝酸钌溶于100mL异丙醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 100mg of ruthenium nitrate in 100mL of isopropanol to obtain liquid C, then add 1000mg of the carbon carrier obtained in S2. After ultrasonication for 20 minutes to disperse evenly, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持5%(5%VH2/95%VAr)氢气氩气混合气氛,以5℃/min速率升温至800℃并保持5h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a 5% (5% VH2 /95% VAr ) hydrogen and argon mixed atmosphere, and raise the temperature to 800 at a rate of 5°C/min. ℃ and kept for 5h to obtain ruthenium nano-cluster catalyst.
实施例6Example 6
S1.将干燥至恒重并粉碎后的8g油菜秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氩气气氛,以10℃/min速率升温至900℃并保持2h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 8 g of rape stalks dried to constant weight and pulverized, and grind evenly to obtain light yellow powder A; then grind light yellow powder A and 24 g of zinc chloride dihydrate evenly to obtain light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the argon atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 2h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在3mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 3mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将100mg硝酸钌溶于100mL甲醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 100mg of ruthenium nitrate in 100mL of methanol to obtain liquid C, then add 1000mg of the carbon carrier obtained in S2. After ultrasonication for 20 minutes to disperse evenly, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持氮气气氛,以5℃/min速率升温至500℃并保持5h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a nitrogen atmosphere, raise the temperature to 500° C. at a rate of 5° C./min and keep it for 5 hours to obtain a ruthenium nanocluster catalyst.
实施例7Example 7
S1.将干燥至恒重并粉碎后的4g油菜秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至900℃并保持4h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 4 g of rape stalks dried to constant weight and pulverized, and grind evenly to obtain light yellow powder A; then grind light yellow powder A and 24 g of zinc chloride dihydrate evenly to obtain light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 4h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在3mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 3mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将40mg三水合氯化钌溶于100mL乙醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 40 mg of ruthenium chloride trihydrate in 100 mL of ethanol to obtain liquid C, and then add 1000 mg of the carbon carrier obtained in S2. After ultrasonication for 20 minutes to disperse evenly, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持氮气气氛,以5℃/min速率升温至400℃并保持4h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a nitrogen atmosphere, raise the temperature to 400° C. at a rate of 5° C./min and keep it for 4 hours to obtain a ruthenium nanocluster catalyst.
实施例8Example 8
S1.将干燥至恒重并粉碎后的4g玉米秸秆,加入18g氢氧化钠,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g六水合硝酸锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至1200℃并保持1h,材料由淡黄色变为黑色,得到生物炭;S1. Add 18g of sodium hydroxide to the 4g of corn stalks dried to constant weight and pulverized, and grind uniformly to obtain a light yellow powder A; then grind the light yellow powder A and 24g of zinc nitrate hexahydrate to obtain a light yellow powder B; Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 1200°C at a rate of 10°C/min and keep it for 1h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在2mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 2mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将80mg三水合氯化钌溶于100mL异丙醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 80 mg of ruthenium chloride trihydrate in 100 mL of isopropanol to obtain liquid C, and then add 1000 mg of the carbon carrier obtained in S2. After dispersing evenly by ultrasonication for 20 min, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持5%(5%VCO/95%VAr)一氧化碳氩气混合气氛,以5℃/min速率升温至600℃并保持3h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a 5% (5% VCO /95% VAr ) carbon monoxide-argon mixed atmosphere, and raise the temperature to 600 at a rate of 5 °C/min. ℃ and kept for 3h to obtain ruthenium nanocluster catalyst.
实施例9Example 9
S1.将干燥至恒重并粉碎后的4g水稻秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至900℃并保持2h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 4 g of rice straw dried to constant weight and pulverized to obtain a light yellow powder A; then grind the light yellow powder A with 24 g of zinc chloride dihydrate to obtain a light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 2h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在3mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 3mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered and washed with deionized water after the end, and then dried to obtain a black powder carbon carrier;
S3.将40mg硝酸钌溶于100mL甲醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 40mg of ruthenium nitrate in 100mL of methanol to obtain liquid C, then add 1000mg of the carbon carrier obtained in S2. After ultrasonication for 20 minutes to disperse evenly, dry on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持氮气气氛,以5℃/min速率升温至500℃并保持1h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a tube furnace quartz furnace tube, maintain a nitrogen atmosphere, raise the temperature to 500° C. at a rate of 5° C./min and keep it for 1 hour to obtain a ruthenium nanocluster catalyst.
实施例10Example 10
S1.将干燥至恒重并粉碎后的4g高粱秸秆,加入8g碱式碳酸镁,研磨均匀得到淡黄色粉末A;再将淡黄色粉末A与24g二水合氯化锌研磨均匀得到淡黄色粉末B;将粉末B转移至管式炉石英炉管中,维持氮气气氛,以10℃/min速率升温至900℃并保持5h,材料由淡黄色变为黑色,得到生物炭;S1. Add 8 g of basic magnesium carbonate to 4 g of sorghum stalks dried to constant weight and pulverized, and grind evenly to obtain a light yellow powder A; then grind the light yellow powder A and 24 g of zinc chloride dihydrate evenly to obtain a light yellow powder B ;Transfer the powder B to the quartz furnace tube of the tube furnace, maintain the nitrogen atmosphere, raise the temperature to 900°C at a rate of 10°C/min and keep it for 5h, the material changes from light yellow to black, and biochar is obtained;
S2.将S1.得到的生物炭在7mol/L的盐酸溶液中,磁力搅拌24h,结束后过滤用去离子水洗净再烘干得到黑色粉末碳载体;S2. The biochar obtained in S1. was placed in a 7mol/L hydrochloric acid solution, magnetically stirred for 24 hours, filtered, washed with deionized water, and then dried to obtain a black powder carbon carrier;
S3.将40mg三水合氯化钌溶于100mL甲醇中得C液,再加入1000mg S2.得到的碳载体,超声20min分散均匀后,在旋转蒸发仪上干燥得到前驱体材料;S3. Dissolve 40 mg of ruthenium chloride trihydrate in 100 mL of methanol to obtain liquid C, and then add 1000 mg of the carbon carrier obtained in S2. After dispersing evenly by ultrasonication for 20 min, dry it on a rotary evaporator to obtain a precursor material;
S4.将步骤S3.得到的前驱体材料转移至管式炉石英炉管中,维持氩气气氛,以5℃/min速率升温至300℃并保持8h,得到钌纳米团簇催化剂。S4. Transfer the precursor material obtained in step S3. to a quartz furnace tube of a tube furnace, maintain an argon atmosphere, raise the temperature to 300° C. at a rate of 5° C./min and keep it for 8 hours to obtain a ruthenium nanocluster catalyst.
性能测试:Performance Testing:
将上述实施例2制得的催化剂进行测试,具体测试项目和测试条件如下:The catalyst that above-mentioned
(1)TEM、HAADF-STEM图:通过美国FEI公司的Titan Cubed Themis G2 300型球差电子显微镜对材料进行透射电子显微镜(TEM)和高角环形暗场扫描透射电子显微镜(HAADF-STEM)表征;(1) TEM and HAADF-STEM images: the materials were characterized by transmission electron microscopy (TEM) and high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) through Titan
(2)CV曲线图:取2mg催化剂、400μL无水乙醇和10μL Nafion溶液混合后超声震荡30min得到催化剂浆料。将4μL催化剂浆料滴涂在直径为5mm的玻碳电极上,自然干燥后作为工作电极。以可逆氢电极(RHE)为参比电极,石墨棒为对电极,1mol/L KOH为电解液。在CHI-760E电化学工作站(上海辰华仪器有限公司)上进行测试。将工作电极放入测试液中,使用高纯N2通气30min去除测试液中的其它气体,继续通入10%(10%VCO/90%VAr)的气体使CO饱和,继续通入高纯N2通气30min去除测试液中的CO,以1mV/s的扫速在0.1-1V电压区间内做线性循环伏安测试;(2) CV curve diagram: 2 mg of catalyst, 400 μL of absolute ethanol and 10 μL of Nafion solution were mixed and ultrasonically oscillated for 30 minutes to obtain a catalyst slurry. 4 μL of the catalyst slurry was drop-coated on a glassy carbon electrode with a diameter of 5 mm, and it was used as a working electrode after natural drying. A reversible hydrogen electrode (RHE) was used as the reference electrode, a graphite rod was used as the counter electrode, and 1mol/L KOH was used as the electrolyte. Tests were performed on a CHI-760E electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd.). Put the working electrode into the test solution, use high-purityN2 gas for 30 minutes to remove other gases in the test solution, continue to flow 10% (10% VCO /90% VAr ) gas to saturate CO, and continue to flow high Pure N2 was ventilated for 30 minutes to remove CO in the test solution, and a linear cyclic voltammetry test was performed in the voltage range of 0.1-1V at a scan rate of 1mV/s;
(3)Ru K-edge XANES和FT-EXAFS谱图:通过日本同步辐射光源SPring-8的高能线站对材料进行X射线吸收近边结构(XANES)和X射线吸收精细结构(EXAFS)吸收谱进行表征,进一步拟合分析得到FT-EXAFS。(3) Ru K-edge XANES and FT-EXAFS spectra: The X-ray absorption near-edge structure (XANES) and X-ray absorption fine structure (EXAFS) absorption spectra of materials are carried out by the high-energy line station of the Japanese synchrotron radiation source SPring-8 Characterization was carried out, and FT-EXAFS was obtained by further fitting analysis.
测试结果如图1a~1e所示。从图1a中可以看出制得的催化剂呈现片状结构,具有较大的比表面积,有利于暴露金属催化活性位点,且TEM图中未发现金属纳米颗粒;图1b可以发现钌以纳米团簇的形式均匀分散在碳载体上,团簇平均的粒径约为1nm,约3.73个钌原子直径大小,属于纳米团簇范围;图1c的CV曲线中,在0.52V处并未出现金属钌的特征剥离峰,说明钌在该催化剂中不以金属的形式存在;从图1d中可以看出该催化剂中的钌更接近二氧化钌的价态,从图1e中可以看出该催化剂具有明显Ru-O配位结构,单不存在Ru-Ru配位结构。以上结果充分说明该催化剂是碳载纳米团簇催化剂,钌以氧化物纳米团簇形式存在。The test results are shown in Figures 1a-1e. It can be seen from Figure 1a that the prepared catalyst presents a sheet-like structure with a large specific surface area, which is conducive to exposing the metal catalytic active sites, and no metal nanoparticles are found in the TEM image; The form of clusters is evenly dispersed on the carbon support. The average particle size of the clusters is about 1nm, about 3.73 ruthenium atoms in diameter, which belongs to the range of nano-clusters; in the CV curve of Figure 1c, metal ruthenium does not appear at 0.52V The characteristic stripping peak of , shows that ruthenium does not exist in the form of metal in this catalyst; As can be seen from Figure 1d, the ruthenium in this catalyst is closer to the valence state of ruthenium dioxide, and from Figure 1e, it can be seen that this catalyst has obvious Ru-O coordination structure, single Ru-Ru coordination structure does not exist. The above results fully demonstrate that the catalyst is a carbon-supported nanocluster catalyst, and ruthenium exists in the form of oxide nanoclusters.
将上述实施例7制得的催化剂进行测试,具体测试项目和测试条件如下:The catalyst that above-mentioned embodiment 7 is made is tested, and concrete test item and test condition are as follows:
采用三电极体系对材料的电析氢活性和稳定性性能进行评价,获得电析氢活性后进一步通过金属含量将其转化为金属质量活性。具体为取2mg催化剂、400μL无水乙醇和10μL Nafion溶液混合后超声震荡30min得到催化剂浆料。将40μL催化剂浆料滴涂在直径为5mm的玻碳电极上,自然干燥后作为工作电极。以RHE为参比电极,石墨棒为对电极,1mol/L KOH为电解液。在CHI-760E电化学工作站上进行测试,LSV极化曲线测试前先用CV模式反复扫描至曲线稳定后再进行测试,扫速为5mV/s,电极电位均为IR校正后的电位(|Etrue|):|Etrue|=|Emeasure|–|Imeasure|×R,其中Imeasure和Emeasure是测试得到的电流和电位,R是EIS测试得到的欧姆阻抗。A three-electrode system was used to evaluate the electrohydrogen evolution activity and stability performance of the material. After obtaining the electrohydrogen evolution activity, it was further converted into a metal mass activity through the metal content. Specifically, 2 mg of catalyst, 400 μL of absolute ethanol and 10 μL of Nafion solution were mixed and ultrasonically oscillated for 30 minutes to obtain a catalyst slurry. 40 μL of the catalyst slurry was drop-coated on a glassy carbon electrode with a diameter of 5 mm and dried naturally as a working electrode. RHE is used as the reference electrode, graphite rod is used as the counter electrode, and 1mol/L KOH is used as the electrolyte. The test was carried out on the CHI-760E electrochemical workstation. Before the LSV polarization curve test, the CV mode was used to scan repeatedly until the curve was stable, and then the test was performed. The scan speed was 5mV/s, and the electrode potentials were all potentials after IR correction (|Etrue |): |Etrue |=|Emeasure |–|Imeasure |×R, where Imeasure and Emeasure are the current and potential obtained from the test, and R is the ohmic impedance obtained from the EIS test.
测试结果如图2a~2c所示。从图2a可以看出,在相同的1mg/cm-2的负载量下,1mol/LKOH的电解液中,本发明制得的催化剂的电析氢催化活性明显优于购买的商业铂碳催化剂,且随着电流密度的增大,本发明的催化剂的优势愈发明显;从图2b可以看出,本发明制得的催化剂的金属质量活性远远高于购买的商业铂碳催化剂;从图2c中可以看出本发明制得的催化剂1mol/L KOH的电解液中动电位循环10000圈后极化曲线与初始状态基本相同。这些结果表明,本发明制得的催化剂比商业的铂碳材料表现出更优异的表观活性和金属质量活性,更具有成本优势,同时也具有良好的电化学稳定性,具有规模化应用为碱性电解水阴极的潜力。The test results are shown in Figures 2a-2c. As can be seen from Figure 2a, under the same loading of 1mg/cm-2 , in the electrolyte of 1mol/LKOH, the catalytic activity of the catalyst prepared by the present invention is significantly better than that of the purchased commercial platinum-carbon catalyst, and Along with the increase of electric current density, the advantage of catalyst of the present invention is more and more obvious; As can be seen from Fig. 2 b, the metal quality activity of the catalyst that the present invention makes is far higher than the commercial platinum carbon catalyst of purchase; From Fig. 2 c It can be seen that the polarization curve after 10,000 cycles of potentiodynamic cycles in the electrolytic solution of 1mol/L KOH of the catalyst prepared by the present invention is basically the same as the initial state. These results show that the catalyst prepared by the present invention exhibits more excellent apparent activity and metal quality activity than commercial platinum carbon materials, and has more cost advantages, and also has good electrochemical stability, and has large-scale application as alkali Potential for electrolytic water cathodes.
将实施例8制得的催化剂进行i-t曲线图稳定性测试,测试具体条件为:将镍铁基氢氧化物和洗净的泡沫镍裁成2cm×2cm大小后在0.15Mpa下压30s备用。取10mg的催化剂,分别加入异丙醇和阴离子交换树脂,超声均匀后将其涂在泡沫镍上烘干备用。将德国Fumasep公司的FAA-3-50阴离子交换膜裁成3cm×3cm大小,置于1mol/L NaOH水溶液中常温浸泡24h实现离子交换,取出用纯水洗净后保存在纯水中备用。以镍铁基氢氧化物为阳极,该催化剂分别为阴极,FAA-3-50阴离子交换膜为隔膜组装基于阴离子交换树脂的电解水电解槽,其中端板为具有蛇形流场的镀金钢板,电解液为1mol/LKOH,蠕动泵输入电解液,电解液用水浴锅控温。组装完成后,由ITCEH公司的IT-6832A型直流稳压电源进行相关测试并记录数据。采用恒电压的方式评价电解池的稳定性,控温20℃,施加电压为2V,每24h替换新的电解液,记录电流随时间的变化。测试结果如图3所示。The catalyst prepared in Example 8 was subjected to the i-t curve stability test, and the specific conditions of the test were: the nickel-iron-based hydroxide and the cleaned foamed nickel were cut into 2cm×2cm in size and pressed at 0.15Mpa for 30s for later use. Take 10mg of the catalyst, add isopropanol and anion exchange resin respectively, apply it on the nickel foam after ultrasonic evenly, and dry it for later use. Cut the FAA-3-50 anion exchange membrane from Fumasep, Germany into a size of 3cm×3cm, soak it in 1mol/L NaOH aqueous solution at room temperature for 24h to realize ion exchange, take it out and wash it with pure water, and store it in pure water for later use. The nickel-iron-based hydroxide is used as the anode, the catalyst is used as the cathode, and the FAA-3-50 anion-exchange membrane is used as the diaphragm to assemble the electrolyzed water electrolyzer based on anion-exchange resin. The end plate is a gold-plated steel plate with a serpentine flow field. The electrolyte is 1mol/LKOH, the peristaltic pump inputs the electrolyte, and the temperature of the electrolyte is controlled by a water bath. After the assembly is completed, IT-6832A DC regulated power supply of ITCEH Company conducts relevant tests and records the data. The stability of the electrolytic cell was evaluated by means of constant voltage, the temperature was controlled at 20°C, the applied voltage was 2V, the electrolyte was replaced every 24 hours, and the change of current with time was recorded. The test results are shown in Figure 3.
从图3中可以看出,采用本发明制得催化剂为阴极,镍铁氢氧化物为阳极,1mol/LKOH为电解液的构建的基于阴离子交换膜的电解水电解池系统中,2V下可达到0.3A/cm-2的电流密度,且能够稳定运行超110h,表现出良好的实用化前景。As can be seen from Fig. 3, the catalyst prepared by the present invention is used as the negative electrode, the nickel-iron hydroxide is the anode, and 1mol/LKOH is the electrolyzed water electrolysis cell system based on the anion exchange membrane of the construction of the electrolyte, which can be reached under 2V. 0.3A/cm-2 current density, and can run stably for more than 110h, showing a good practical prospect.
本发明的上述实施例仅仅是为清楚地说明本发明所作的举例,而并非是对本发明的实施方式的限定。对于所属领域的普通技术人员来说,在上述说明的基础上还可以做出其它不同形式的变化或变动。这里无需也无法对所有的实施方式予以穷举。凡在本发明的精神和原则之内所作的任何修改、等同替换和改进等,均应包含在本发明权利要求的保护范围之内。The above-mentioned embodiments of the present invention are only examples for clearly illustrating the present invention, rather than limiting the implementation of the present invention. For those of ordinary skill in the art, other changes or changes in different forms can be made on the basis of the above description. It is not necessary and impossible to exhaustively list all the implementation manners here. All modifications, equivalent replacements and improvements made within the spirit and principles of the present invention shall be included within the protection scope of the claims of the present invention.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211297373.0ACN115584536A (en) | 2022-10-21 | 2022-10-21 | A kind of ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211297373.0ACN115584536A (en) | 2022-10-21 | 2022-10-21 | A kind of ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction and preparation method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN115584536Atrue CN115584536A (en) | 2023-01-10 |
Family
ID=84780978
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211297373.0APendingCN115584536A (en) | 2022-10-21 | 2022-10-21 | A kind of ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115584536A (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116505007A (en)* | 2023-06-08 | 2023-07-28 | 浙江大学 | Preparation and application of single-atom/nanocluster composite anode catalyst for hydrogen fuel cell |
| CN116786114A (en)* | 2023-06-16 | 2023-09-22 | 北京同威新材技术有限公司 | A RuOx/C nanocomposite material and its preparation method and application |
| CN119900051A (en)* | 2025-02-25 | 2025-04-29 | 江西师范大学 | Cobalt-based nanosheet-anchored noble metal oxide cluster catalyst and preparation method and application thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113235115A (en)* | 2021-04-20 | 2021-08-10 | 深圳大学 | High-stability metal nanocluster catalyst and preparation method and application thereof |
| CN113667995A (en)* | 2021-08-24 | 2021-11-19 | 西北工业大学深圳研究院 | Two-dimensional flaky dopamine pyrolytic carbon-coated ruthenium nanocluster catalyst and preparation and use method thereof |
| CN114318362A (en)* | 2021-12-24 | 2022-04-12 | 复旦大学 | A kind of ruthenium nanocluster hydrogen evolution electrocatalyst and superassembly method thereof |
- 2022
- 2022-10-21CNCN202211297373.0Apatent/CN115584536A/enactivePending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113235115A (en)* | 2021-04-20 | 2021-08-10 | 深圳大学 | High-stability metal nanocluster catalyst and preparation method and application thereof |
| CN113667995A (en)* | 2021-08-24 | 2021-11-19 | 西北工业大学深圳研究院 | Two-dimensional flaky dopamine pyrolytic carbon-coated ruthenium nanocluster catalyst and preparation and use method thereof |
| CN114318362A (en)* | 2021-12-24 | 2022-04-12 | 复旦大学 | A kind of ruthenium nanocluster hydrogen evolution electrocatalyst and superassembly method thereof |
Non-Patent Citations (3)
| Title |
|---|
| HAN-SAEM PARK ET AL.: "Bifunctional hydrous RuO2 nanocluster electrocatalyst embedded in carbon matrix for efficient and durable operation of rechargeable zinc–air batteries", SCIENTIFIC REPORTS, 27 June 2017 (2017-06-27), pages 1 - 9* |
| QI HU ET AL.: "General Synthesis of Ultrathin Metal Borate Nanomeshes Enabled by 3D Bark-Like N-Doped Carbon for Electrocatalysis", ADV. ENERGY MATER., vol. 9, 6 July 2019 (2019-07-06), pages 1901130* |
| QI HU ET AL.: "Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction", NATURE COMMUNICATIONS, vol. 13, 8 July 2022 (2022-07-08), pages 3958* |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116505007A (en)* | 2023-06-08 | 2023-07-28 | 浙江大学 | Preparation and application of single-atom/nanocluster composite anode catalyst for hydrogen fuel cell |
| CN116505007B (en)* | 2023-06-08 | 2024-08-09 | 浙江大学 | Preparation and application of single-atom/nanocluster composite anode catalyst for hydrogen fuel cell |
| CN116786114A (en)* | 2023-06-16 | 2023-09-22 | 北京同威新材技术有限公司 | A RuOx/C nanocomposite material and its preparation method and application |
| CN119900051A (en)* | 2025-02-25 | 2025-04-29 | 江西师范大学 | Cobalt-based nanosheet-anchored noble metal oxide cluster catalyst and preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103638925B (en) | A kind of fuel cell catalyst with core-casing structure and pulse electrodeposition preparation method thereof | |
| CN110252335B (en) | Carbon-coated nickel-ruthenium nano material and preparation method and application thereof | |
| CN113437314B (en) | Nitrogen-doped carbon-supported low-content ruthenium and Co 2 Three-function electrocatalyst of P nano particle and preparation method and application thereof | |
| CN104923204B (en) | A kind of preparation method and applications of graphene coated catalyst with metal nanoparticles | |
| CN110838588B (en) | A kind of rechargeable zinc-air battery bifunctional catalyst and its preparation method and application | |
| CN115584536A (en) | A kind of ruthenium nano-cluster catalyst for alkaline electrolysis hydrogen reaction and preparation method thereof | |
| CN106328960A (en) | ZIF-67 template method for preparing cobalt-platinum core-shell particle/porous carbon composite material and catalytic application of composite material in cathode of fuel cell | |
| CN111001428B (en) | A kind of metal-free carbon-based electrocatalyst and preparation method and application | |
| CN112899723B (en) | Metal organic framework derived iron-nickel metal sulfide catalyst, preparation and application thereof | |
| CN111545250A (en) | A ruthenium catalyst with efficient electrocatalytic total water splitting performance and its application | |
| CN114875442A (en) | Ruthenium-modified molybdenum-nickel nanorod composite catalyst and preparation method and application thereof | |
| CN111841598B (en) | S-doped Co @ NC composite material with high oxygen evolution catalytic activity and preparation method thereof | |
| Liu et al. | Valence regulation of Ru/Mo2C heterojunction for efficient acidic overall water splitting | |
| CN111346642A (en) | High-dispersion metal nanoparticle/biomass carbon composite electrode material and preparation method and application thereof | |
| Li et al. | Preparation of carbon coated hyperdispersed Ru nanoparticles supported on TiO 2 HER electrocatalysts by dye-sensitization | |
| Ren et al. | Zeolitic-imidazolate-framework-derived Fe-NC catalysts towards efficient oxygen reduction reaction | |
| Chen et al. | Hollow CoVOx/Ag nanoprism with tailored electronic structure for high efficiency oxygen evolution reaction | |
| Du et al. | Sulfur-deficient CoNi2S4 nanoparticles-anchored porous carbon nanofibers as bifunctional electrocatalyst for overall water splitting | |
| Lv et al. | N/C doped nano-size IrO2 catalyst of high activity and stability in proton exchange membrane water electrolysis | |
| Dai et al. | Facile fabrication of self-supporting porous CuMoO 4@ Co 3 O 4 nanosheets as a bifunctional electrocatalyst for efficient overall water splitting | |
| CN108585044A (en) | The simple preparation and electro-catalysis application of a kind of Co-MoO2 nanospheres with mylikes structures | |
| CN114808026A (en) | A two-dimensional metal-organic framework nanosheet-supported noble metal single-atom catalyst and its preparation method and application | |
| CN119465239A (en) | A supported iridium oxide water electrolysis catalyst and its preparation method and application | |
| CN110354870B (en) | Preparation method and application of high-performance silver-doped cobalt sulfide oxygen evolution catalyst | |
| CN112779550B (en) | A three-dimensional micro-tubular hydrogen evolution reaction electrocatalyst and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |