CN115513473A - A polyphenylene sulfide-based hollow fiber with controllable wall thickness and its preparation method and application - Google Patents
A polyphenylene sulfide-based hollow fiber with controllable wall thickness and its preparation method and applicationDownload PDFInfo
- Publication number
- CN115513473A CN115513473ACN202211204278.1ACN202211204278ACN115513473ACN 115513473 ACN115513473 ACN 115513473ACN 202211204278 ACN202211204278 ACN 202211204278ACN 115513473 ACN115513473 ACN 115513473A
- Authority
- CN
- China
- Prior art keywords
- acid
- hollow fiber
- pps
- fiber
- wall thickness
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/88—Processes of manufacture
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/8636—Inert electrodes with catalytic activity, e.g. for fuel cells with a gradient in another property than porosity
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/90—Selection of catalytic material
- H01M4/9008—Organic or organo-metallic compounds
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/90—Selection of catalytic material
- H01M4/9075—Catalytic material supported on carriers, e.g. powder carriers
- H01M4/9083—Catalytic material supported on carriers, e.g. powder carriers on carbon or graphite
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Catalysts (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及PPS中空纤维制备领域,具体是一种壁厚可控的聚苯硫醚基中空纤维及其制备方法和应用。The invention relates to the field of PPS hollow fiber preparation, in particular to a polyphenylene sulfide-based hollow fiber with controllable wall thickness, a preparation method and application thereof.
背景技术Background technique
由于化石燃料的过度使用导致了地球环境的恶化和能源枯竭问题,开发绿色可持续发展的新能源是21世纪人类面临的最大挑战之一。具有极高理论能量密度的金属空气电池引发研究人员的广泛关注,然而目前驱动金属空气电池阴极的催化剂主要是贵金属Pt和Ru,其储量低、成本高、稳定性差等缺点限制了金属空气电池的大规模使用。因此,开发高效、低成本的功能化碳基催化剂来替代贵金属催化剂是实现金属空气电池大规模使用的重点。Due to the deterioration of the earth's environment and energy depletion caused by the excessive use of fossil fuels, the development of green and sustainable new energy sources is one of the greatest challenges facing mankind in the 21st century. Metal-air batteries with extremely high theoretical energy density have attracted widespread attention of researchers. However, the catalysts currently driving the cathodes of metal-air batteries are mainly noble metals Pt and Ru. Their shortcomings such as low reserves, high cost, and poor stability limit the development of metal-air batteries. Used on a large scale. Therefore, the development of efficient and low-cost functionalized carbon-based catalysts to replace noble metal catalysts is the focus of realizing the large-scale use of metal-air batteries.
聚苯硫醚(PPS)是一种高含硫量的工程塑料材料,其理论含S量高达30%,因此将PPS材料热解后所形成的多孔碳用作金属空气电池的阴极的氧还原催化剂具有重大研究价值。然而,PPS材料在受热之后会发生融滴,严重影响了所制备碳材料孔道结构而无法起到优异的催化效果。申请号为201510631820.5的专利公开了一种具有自熄性和无融滴的聚苯硫醚纤维,这种新型PPS材料克服了受热融滴的缺点,可以在热解过程中保持形貌结构不变。基于此技术,文献《M.Wang,K.Su,M.Zhang,X.Du,Z.Li,Advanced TrifunctionalElectrocatalysis with Cu-,N-,S-Doped Defect-Rich Porous Carbon forRechargeable Zn-Air Batteries and Self-Driven Water Splitting,ACS SustainableChem.Eng.9(2021)13324-13336》利用PPS材料成功构筑了多孔的N、S共掺杂碳基氧还原催化剂,但是受限于PPS本身较大的微米级尺寸,所制备的催化剂比表面积较小,催化性能还有待进一步提升。Polyphenylene sulfide (PPS) is an engineering plastic material with a high sulfur content, and its theoretical S content is as high as 30%, so the porous carbon formed after pyrolysis of the PPS material is used as the oxygen reduction of the cathode of the metal-air battery Catalysts have great research value. However, the PPS material will melt and drip after being heated, which seriously affects the pore structure of the prepared carbon material and cannot achieve an excellent catalytic effect. The patent with the application number 201510631820.5 discloses a polyphenylene sulfide fiber with self-extinguishing properties and no melt drops. This new type of PPS material overcomes the shortcomings of heat melt drops and can keep the shape and structure unchanged during the pyrolysis process. . Based on this technology, the literature "M.Wang, K.Su, M.Zhang, X.Du, Z.Li, Advanced Trifunctional Electrocatalysis with Cu-,N-,S-Doped Defect-Rich Porous Carbon for Rechargeable Zn-Air Batteries and Self -Driven Water Splitting, ACS SustainableChem.Eng.9(2021) 13324-13336 "Using PPS material to successfully construct a porous N, S co-doped carbon-based oxygen reduction catalyst, but limited by the large micron size of PPS itself , the specific surface area of the prepared catalyst is small, and the catalytic performance needs to be further improved.
发明内容Contents of the invention
针对现有技术的不足,本发明拟解决的技术问题是,提供一种壁厚可控的聚苯硫醚基中空纤维及其制备方法和应用。Aiming at the deficiencies of the prior art, the technical problem to be solved by the present invention is to provide a polyphenylene sulfide-based hollow fiber with controllable wall thickness and its preparation method and application.
本发明解决所述方法技术问题的技术方案是,提供一种壁厚可控的聚苯硫醚基中空纤维的制备方法,其特征在于,该方法包括以下步骤:The technical solution of the present invention to solve the technical problem of the method is to provide a method for preparing a polyphenylene sulfide-based hollow fiber with controllable wall thickness, which is characterized in that the method includes the following steps:
步骤1、将去离子水、氧化剂与酸共混形成氧化处理溶液;
步骤2、将PPS纤维均匀分散在氧化处理溶液中,然后在20~100℃下氧化反应0.01~48h;取出后,冲洗至中性;干燥后,得到氧化程度可控的氧化PPS纤维;
步骤3、将步骤2得到的氧化PPS纤维放入氧化性酸溶液中进行反应,直至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维。
本发明解决所述中空纤维技术问题的技术方案是,提供一种所述方法制备得到的壁厚可控的聚苯硫醚基中空纤维。The technical solution of the present invention to solve the technical problem of the hollow fiber is to provide a polyphenylene sulfide-based hollow fiber with controllable wall thickness prepared by the method.
本发明解决所述应用技术问题的技术方案是,提供一种所述的壁厚可控的聚苯硫醚基中空纤维的应用,其特征在于,将所述PPS基中空纤维浸渍在金属盐溶液中来吸附金属离子,取出后蒸干水分;再置于密闭加热环境中在500℃~1200℃的氨气氛围中碳化0.5h~5h,得到金属、N、S共掺杂的中空纤维状碳基氧还原催化剂,用于锌-空气电池或氢燃料电池的氧还原催化剂。The technical solution of the present invention to solve the application technical problem is to provide an application of the polyphenylene sulfide-based hollow fiber with controllable wall thickness, which is characterized in that the PPS-based hollow fiber is immersed in a metal salt solution The metal ions are adsorbed in the medium, and the water is evaporated after taking it out; then placed in a closed heating environment and carbonized in an ammonia atmosphere at 500°C to 1200°C for 0.5h to 5h to obtain a hollow fiber-shaped carbon co-doped with metal, N, and S Oxygen reduction catalysts for zinc-air batteries or hydrogen fuel cells.
与现有技术相比,本发明的有益效果在于:Compared with prior art, the beneficial effect of the present invention is:
(1)本发明以PPS纤维为基体,通过纤维皮层可控氧化和芯层可控氧化溶出法相结合的工艺制备了一种壁厚可控的PPS基中空纤维。(1) The present invention uses PPS fibers as a matrix, and prepares a PPS-based hollow fiber with controllable wall thickness by combining the process of controlled oxidation of the fiber skin layer and controlled oxidation and dissolution of the core layer.
(2)本发明通过纤维皮层可控氧化法靶向调控PPS纤维的皮层性质,氧化进程是由外向内的,进而使纤维形成芯层仍为PPS成分而皮层为抗氧化性酸成分的皮芯结构复合纤维,同时,通过调控PPS纤维皮层的氧化处理深度,能够得到不同氧化深度的皮芯结构复合纤维,随后再使用芯层可控氧化溶出法去除纤维芯层的未氧化的PPS成分,从而形成中空结构,得到一系列不同壁厚的PPS基中空纤维。(2) The present invention regulates the properties of the cortex of the PPS fiber through the controllable oxidation method of the fiber cortex, and the oxidation process is from the outside to the inside, so that the core layer of the fiber is still composed of PPS and the cortex is an anti-oxidative acid component. At the same time, by adjusting the oxidation treatment depth of the PPS fiber skin layer, skin-core structure composite fibers with different oxidation depths can be obtained, and then the unoxidized PPS component of the fiber core layer is removed by the core layer controllable oxidation dissolution method, thereby A hollow structure is formed to obtain a series of PPS-based hollow fibers with different wall thicknesses.
(3)本发明所制备的中空纤维具有极强的耐酸性,可以在浓盐酸、浓硝酸甚至王水中长期稳定使用,是一种理想的过滤和分离材料,可以用于极端环境下的有机溶剂回收(强溶解性)和高浓污水处理(强腐蚀性)等。同时,其具有优异的耐高温稳定性,并且内部的孔腔结构大幅提高了与反应物的接触面积,未来可以广泛用于高温废气(含腐蚀性)处理以及细颗粒型工业危险废物高效分离、过滤及回收等领域。(3) The hollow fiber prepared by the present invention has extremely strong acid resistance, can be used stably for a long time in concentrated hydrochloric acid, concentrated nitric acid or even aqua regia, is an ideal filtration and separation material, and can be used for organic solvents in extreme environments Recovery (strong solubility) and high-concentration sewage treatment (strong corrosiveness), etc. At the same time, it has excellent high temperature stability, and the internal pore structure greatly increases the contact area with the reactants. In the future, it can be widely used in the treatment of high-temperature waste gas (including corrosive) and the efficient separation of fine-grained industrial hazardous waste. Filtration and recycling and other fields.
(4)本发明所制备的中空纤维作为前驱体所制备的碳材料,具有独特的中空结构,可以暴露出大量的活性位点,具有更高比表面积和孔隙率,在热解过程中将极大的提高气体与前驱体的接触面积。其可以作为催化剂用于能源存储和转换领域(例如:锂硫电池、金属空气电池和超级电容器)。特别是将其作为金属空气电池阴极的氧还原催化剂,表现出了极其优异的催化活性和稳定性,能够推动金属空气电池的商业化发展。(4) The hollow fiber prepared by the present invention is used as the carbon material prepared by the precursor, which has a unique hollow structure, can expose a large number of active sites, has a higher specific surface area and porosity, and will be extremely The contact area between the gas and the precursor is greatly improved. It can be used as a catalyst in the field of energy storage and conversion (eg: lithium-sulfur batteries, metal-air batteries, and supercapacitors). Especially when it is used as an oxygen reduction catalyst for the cathode of metal-air batteries, it shows extremely excellent catalytic activity and stability, which can promote the commercial development of metal-air batteries.
附图说明Description of drawings
图1为本发明实施例1-6中使用的PPS纤维的扫描电镜图;Fig. 1 is the scanning electron micrograph of the PPS fiber used in the embodiment of the present invention 1-6;
图2为本发明实施例1中制得的HOPPS-1的扫描电镜图;Fig. 2 is the scanning electron micrograph of the HOPPS-1 that makes in the embodiment of the
图3为本发明实施例1中的PPS、OPPS-1和HOPPS-1的傅里叶红外图谱;Fig. 3 is the Fourier transform infrared spectrum of PPS, OPPS-1 and HOPPS-1 in the
图4为本发明实施例1中制得的Ni@NS-HCF的扫描电镜图(a为600倍,b为5000倍);Fig. 4 is a scanning electron microscope image of Ni@NS-HCF prepared in Example 1 of the present invention (a is 600 times, b is 5000 times);
图5为本发明实施例1中制得的Ni@NS-HCF的截面的透射电镜图(a为3000倍,b为100000倍,c为1000000倍);5 is a transmission electron microscope image of the cross section of Ni@NS-HCF prepared in Example 1 of the present invention (a is 3000 times, b is 100000 times, and c is 1000000 times);
图6为本发明实施例1中制得的Ni@NS-HCF的XRD图谱;Fig. 6 is the XRD spectrum of Ni@NS-HCF prepared in Example 1 of the present invention;
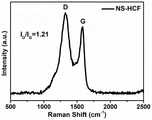
图7为本发明实施例1中制得的Ni@NS-HCF的Raman图谱;Figure 7 is the Raman spectrum of Ni@NS-HCF prepared in Example 1 of the present invention;
图8为本发明实施例1中制得的Ni@NS-HCF的XPS图谱;Figure 8 is the XPS spectrum of Ni@NS-HCF prepared in Example 1 of the present invention;
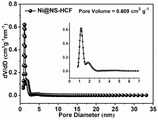
图9为本发明实施例1中制得的Ni@NS-HCF的氮气-吸脱附曲线图;Fig. 9 is a nitrogen-adsorption-desorption curve diagram of Ni@NS-HCF prepared in Example 1 of the present invention;
图10为本发明实施例1中制得的Ni@NS-HCF的孔径分布曲线图;Fig. 10 is a pore size distribution curve diagram of Ni@NS-HCF prepared in Example 1 of the present invention;
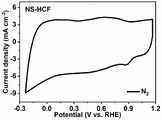
图11为本发明实施例1中制得的Ni@NS-HCF在O2饱和的0.1M的KOH中的循环伏安曲线图;11 is a cyclic voltammetry curve of Ni@NS-HCF prepared in Example 1 of the present invention in O2 saturated 0.1M KOH;
图12为本发明实施例1中制得的Ni@NS-HCF在O2饱和的0.1M的KOH中的线性扫描伏安曲线图;12 is a linear sweep voltammetry curve of Ni@NS-HCF prepared in Example 1 of the present invention in 0.1M KOH saturated with O2 ;
图13为本发明实施例1中制得的Ni@NS-HCF的I-t稳定性测试结果图;Figure 13 is a graph showing the I-t stability test results of Ni@NS-HCF prepared in Example 1 of the present invention;
图14为本发明对比例1中制得的NS-HCF的扫描电镜图(a为600倍,b为5000倍);Fig. 14 is the scanning electron microscope picture (a is 600 times, b is 5000 times) of the NS-HCF that makes in comparative example 1 of the present invention;
图15为本发明对比例1中制得的NS-HCF的XRD图谱;Figure 15 is the XRD spectrum of the NS-HCF prepared in Comparative Example 1 of the present invention;
图16为本发明对比例1中制得的NS-HCF的Raman图谱;Figure 16 is the Raman spectrum of the NS-HCF prepared in Comparative Example 1 of the present invention;
图17为本发明对比例1中制得的NS-HCF的XPS图谱;Figure 17 is the XPS spectrum of the NS-HCF prepared in Comparative Example 1 of the present invention;
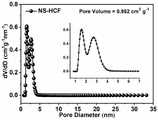
图18为本发明对比例1中制得的NS-HCF的氮气-吸脱附曲线图;Fig. 18 is the nitrogen-adsorption-desorption curve diagram of the NS-HCF prepared in comparative example 1 of the present invention;
图19为本发明对比例1中制得的NS-HCF的孔径分布曲线图;Fig. 19 is a pore size distribution curve diagram of NS-HCF prepared in Comparative Example 1 of the present invention;
图20为本发明对比例1中制得的NS-HCF在O2饱和的0.1M的KOH中的循环伏安曲线图;Fig. 20 is the cyclic voltammetry curve of NS-HCF prepared in Comparative Example 1 of the present invention inO2 saturated 0.1M KOH;
图21为本发明对比例1中制得的NS-HCF在O2饱和的0.1M的KOH中的线性扫描伏安曲线图;Fig. 21 is the linear sweep voltammetry curve graph of NS-HCF prepared in Comparative Example 1 of the present invention inO2 saturated 0.1M KOH;
图22为本发明对比例1中制得的NS-HCF的I-t稳定性测试结果图;Fig. 22 is the I-t stability test result figure of the NS-HCF that makes in comparative example 1 of the present invention;
图23为本发明实施例2中制得的HOPPS-2的扫描电镜图;Figure 23 is a scanning electron micrograph of HOPPS-2 prepared in Example 2 of the present invention;
图24为本发明实施例3中制得的HOPPS-3的扫描电镜图;Figure 24 is a scanning electron micrograph of HOPPS-3 prepared in Example 3 of the present invention;
图25为本发明实施例4中制得的HOPPS-4的扫描电镜图;Figure 25 is a scanning electron micrograph of HOPPS-4 prepared in Example 4 of the present invention;
图26为本发明实施例5中制得的HOPPS-5的扫描电镜图;Figure 26 is a scanning electron micrograph of HOPPS-5 prepared in Example 5 of the present invention;
图27为本发明实施例6中制得的HOPPS-6的扫描电镜图。Fig. 27 is a scanning electron micrograph of HOPPS-6 prepared in Example 6 of the present invention.
具体实施方式detailed description
下面给出本发明的具体实施例。具体实施例仅用于进一步详细说明本发明,不限制本申请权利要求的保护范围。Specific examples of the present invention are given below. The specific embodiments are only used to further describe the present invention in detail, and do not limit the protection scope of the claims of the present application.
本发明提供了一种壁厚可控的聚苯硫醚基中空纤维的制备方法(简称方法),其特征在于,该方法包括以下步骤:The invention provides a method for preparing a polyphenylene sulfide-based hollow fiber with controllable wall thickness (method for short), characterized in that the method comprises the following steps:
步骤1、将去离子水、氧化剂与酸共混形成氧化处理溶液;
优选地,步骤1中,氧化剂为过氧乙酸、双氧水、过甲酸、HClO、NaClO、过硫酸钠或过氧化苯甲酸(优选双氧水)。Preferably, in
优选地,步骤1中,酸为硫酸、盐酸、硝酸、磷酸、杂多酸、甲酸、乙酸、苯甲酸、苯磺酸、三氟乙酸、酸性分子筛、固体超强酸或醋酸(优选盐酸)。Preferably, in
优选地,步骤1中,氧化剂的质量分数为20~70wt%(优选30wt%),酸的质量分数为5~20wt%(优选10wt%)。Preferably, in
优选地,步骤1中,氧化处理溶液的pH控制在3~9,其中碱性环境优选pH=8,酸性环境优选pH=5。Preferably, in
步骤2、PPS纤维皮层的可控氧化:将PPS纤维均匀分散在氧化处理溶液中,然后在20~100℃(优选70℃)下氧化反应0.01~48h(优选1min);取出后,冲洗至中性;干燥后,得到氧化程度可控的氧化PPS纤维(简称氧化PPS纤维,命名为OPPS);
优选地,步骤2中,PPS纤维与氧化处理溶液的质量体积比为5~20g:100ml。Preferably, in
优选地,步骤2中,PPS纤维是PPS短纤维、PPS长纤维、熔喷PPS纤维、超细PPS纤维、PPS膈膜或PPS针刺毡(优选PPS短纤维)。Preferably, in
优选地,步骤2中,20~100℃为水浴加热、烘箱加热、真空加热、微波炉加热、油浴加热或酒精灯加热。干燥温度为0~200℃。Preferably, in
步骤3、氧化PPS纤维芯层的可控氧化溶出:将步骤2得到的氧化PPS纤维放入氧化性酸溶液中进行反应,直至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(简称PPS基中空纤维,命名为HOPPS)。
优选地,步骤3中,氧化性酸溶液为NMP、浓硝酸、浓硫酸或王水中的至少一种(优选浓硝酸、浓硫酸或王水,更优选浓硝酸)。Preferably, in
优选地,步骤3中,氧化性酸溶液的质量分数为40~80wt%(优选65wt%的浓硝酸)。Preferably, in
优选地,步骤3中,反应温度为20℃~100℃(优选70℃),反应时间为1~48h(优选24h)。Preferably, in
本发明同时提供了一种所述方法制备得到的壁厚可控的聚苯硫醚基中空纤维。The invention also provides a polyphenylene sulfide-based hollow fiber with controllable wall thickness prepared by the method.
本发明同时提供了一种所述壁厚可控的聚苯硫醚基中空纤维的应用,其特征在于,将所述PPS基中空纤维浸渍在金属盐溶液中1~4h来吸附金属离子,取出后蒸干水分;再置于密闭加热环境中在500℃~1200℃(优选800~1000℃,更优选950℃)的氨气氛围中碳化0.5h~5h(优选2.5h),得到金属、N、S共掺杂的中空纤维状碳基氧还原催化剂,用于锌-空气电池或氢燃料电池的氧还原催化剂(命名为Ni@NS-HCF)。The present invention also provides an application of the polyphenylene sulfide-based hollow fiber with controllable wall thickness, which is characterized in that the PPS-based hollow fiber is immersed in a metal salt solution for 1-4 hours to adsorb metal ions, and Afterwards, the water is evaporated to dryness; then placed in a closed heating environment and carbonized in an ammonia atmosphere at 500°C to 1200°C (preferably 800°C to 1000°C, more preferably 950°C) for 0.5h to 5h (preferably 2.5h) to obtain metal, N , S co-doped hollow fiber carbon-based oxygen reduction catalyst for zinc-air battery or hydrogen fuel cell oxygen reduction catalyst (named Ni@NS-HCF).
优选地,金属盐溶液为硝酸镍溶液、乙酸镍溶液、硝酸铁溶液、硫酸铁溶液、硫酸钴溶液或硫酸铜溶液(优选硝酸镍溶液)。Preferably, the metal salt solution is nickel nitrate solution, nickel acetate solution, iron nitrate solution, iron sulfate solution, cobalt sulfate solution or copper sulfate solution (preferably nickel nitrate solution).
优选地,蒸干溶液中的水分是放入20℃~200℃(优选120℃)的环境中蒸干。Preferably, the water in the evaporated solution is evaporated to dryness in an environment of 20° C. to 200° C. (preferably 120° C.).
实施例1Example 1
(1)将50ml去离子水、50ml双氧水与10ml盐酸共混形成氧化处理溶液;(1) 50ml deionized water, 50ml hydrogen peroxide and 10ml hydrochloric acid are blended to form an oxidation treatment solution;
(2)将5g的PPS短纤维分散在氧化处理溶液中,然后在70℃反应1min;然后取出纤维,用去离子水冲洗至中性,再在70℃干燥2h,得到氧化PPS纤维(命名为OPPS-1);(2) Disperse 5 g of PPS short fibers in the oxidation treatment solution, and then react at 70 ° C for 1 min; then take out the fibers, rinse them with deionized water until they are neutral, and then dry them at 70 ° C for 2 h to obtain oxidized PPS fibers (named as OPPS-1);
(3)将步骤2得到的OPPS-1放入浓硝酸溶液中,在70℃下反应24h至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(命名为HOPPS-1)。(3) Put the OPPS-1 obtained in
由图1可以看出,PPS纤维是一种实心纤维,表面光滑平整,直径约为15μm。It can be seen from Figure 1 that the PPS fiber is a solid fiber with a smooth surface and a diameter of about 15 μm.
由图2可以看出,实施例1成功制备了具有中空结构的纤维,该中空纤维表面粗糙,直径约为15μm,壁厚约1μm,其直径与PPS纤维相一致。这说明OPPS-1纤维芯层的PPS成分被浓硝酸所溶解;而经过氧化处理的皮层结构可以在浓硝酸中保持结构不变,具有极强的耐酸性。It can be seen from Figure 2 that Example 1 successfully prepared a fiber with a hollow structure. The hollow fiber has a rough surface, a diameter of about 15 μm, and a wall thickness of about 1 μm, which is consistent with the diameter of the PPS fiber. This shows that the PPS component of the OPPS-1 fiber core layer is dissolved by concentrated nitric acid; while the oxidized skin layer structure can remain unchanged in concentrated nitric acid, and has strong acid resistance.
表1为实施例1中PPS、OPPS-1和HOPPS-1纤维的元素分析结果。由表1可以看出,相对于PPS,经过氧化处理的OPPS-1中O元素的含量(16.66wt%)大幅提升,尤其是在溶解PPS芯层后得到的HOPPS-1中O元素的含量高达23.36wt%。同时,由图3可以看出,OPPS-1的皮层与HOPPS-1的表面成分高度一致,并且相对于PPS额外增加了S=O与O=S=O的含O官能团,对应了表1的元素分析结果。Table 1 shows the elemental analysis results of PPS, OPPS-1 and HOPPS-1 fibers in Example 1. It can be seen from Table 1 that compared with PPS, the content of O element (16.66wt%) in the oxidized OPPS-1 is greatly increased, especially in HOPPS-1 obtained after dissolving the PPS core layer. 23.36 wt%. At the same time, it can be seen from Figure 3 that the skin layer of OPPS-1 is highly consistent with the surface composition of HOPPS-1, and compared with PPS, additional O-containing functional groups of S=O and O=S=O are added, corresponding to those in Table 1. Elemental analysis results.
表1Table 1
应用:将步骤3得到的HOPPS-1浸渍在硝酸镍溶液中1h,取出后放入120℃烘箱中蒸干水分;再置于密闭加热环境(管式炉)中在氨气氛围下950℃碳化2.5h,得到Ni、N、S共掺杂的中空纤维状碳基氧还原催化剂(命名为Ni@NS-HCF)。Application: Immerse the HOPPS-1 obtained in
由图4可以看出,实施例1所制备的Ni@NS-HCF与HOPPS-1的形貌高度相似,没有明显变化。It can be seen from Fig. 4 that the morphology of Ni@NS-HCF prepared in Example 1 is highly similar to that of HOPPS-1 without significant change.
由图5可以看出,在Ni@NS-HCF中空纤维的内外表面以及碳基体中弥散分布了金属纳米粒子,在图5c的高倍透射电镜下观察到金属颗粒上存在晶面间距为0.20nm的晶格条纹,与Ni的(111)晶面相一致。同时,在图6的XRD图谱中进一步证实了Ni的存在,这些弥散分布Ni金属纳米粒子将作为高效活性中心来提高催化剂的催化活性。It can be seen from Figure 5 that metal nanoparticles are diffusely distributed on the inner and outer surfaces of the Ni@NS-HCF hollow fiber and in the carbon matrix. The lattice fringes are consistent with the (111) crystal plane of Ni. At the same time, the existence of Ni is further confirmed in the XRD pattern of Figure 6, and these dispersed Ni metal nanoparticles will serve as efficient active centers to improve the catalytic activity of the catalyst.
由图7可以看出,在1330cm-1和1580cm-1处显示了碳材料的典型D峰和G峰,其ID/IG值为1.12,证实了催化剂中存在大量的缺陷位点。It can be seen from Figure 7 that the typical D peak andG peak of carbon materials are displayed at 1330cm-1 and 1580cm-1 , and its ID/IG value is 1.12, which confirms that there are a large number of defect sites in the catalyst.
由图8可以看出,C、N、O、S及Ni元素的存在,证实了Ni、N、S元素成功掺杂到了碳基体中。It can be seen from Figure 8 that the presence of C, N, O, S and Ni elements proves that Ni, N, and S elements have been successfully doped into the carbon matrix.
由图9可以看出,经计算,Ni@NS-HCF比表面积高达1321.439m2·g-1。并且通过图10的孔径分布曲线可以得知,Ni@NS-HCF主要以微孔形式存在,其孔体积为0.609cm3·g-1。It can be seen from Fig. 9 that the calculated specific surface area of Ni@NS-HCF is as high as 1321.439m2 ·g-1 . And it can be seen from the pore size distribution curve in Figure 10 that Ni@NS-HCF mainly exists in the form of micropores, and its pore volume is 0.609cm3 ·g-1 .
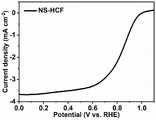
由图11可以看出,在0.9V处出现一个明显的还原峰,证实了催化剂的催化活性。由图12可以看出,Ni@NS-HCF可以在1600的转速下达到0.86V的半波电位和5.5mA·cm-2的极限电流密度。循环伏安曲线及线性扫描伏安曲线结果充分说明Ni@NS-HCF表现了优异的氧还原催化性能。It can be seen from Figure 11 that an obvious reduction peak appears at 0.9V, which confirms the catalytic activity of the catalyst. It can be seen from Figure 12 that Ni@NS-HCF can reach a half-wave potential of 0.86V and a limiting current density of 5.5mA·cm-2 at a rotational speed of 1600. The results of cyclic voltammetry curves and linear sweep voltammetry curves fully demonstrate that Ni@NS-HCF exhibits excellent catalytic performance for oxygen reduction.
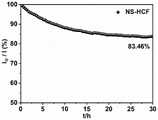
由图13可以看出,在经过30个小时的测试后,Ni@NS-HCF的电流密度仅仅衰减了15.28%,证明了其良好的稳定性。It can be seen from Figure 13 that after 30 hours of testing, the current density of Ni@NS-HCF only decayed by 15.28%, proving its good stability.
对比例1Comparative example 1
应用:将实施例1得到的HOPPS-1置于密闭加热环境(管式炉)中在氨气氛围下950℃碳化2.5h,得到N、S共掺杂的中空纤维状碳基氧还原催化剂(命名为NS-HCF)。Application: the HOPPS-1 obtained in Example 1 was placed in a closed heating environment (tube furnace) and carbonized at 950° C. for 2.5 h under an ammonia atmosphere to obtain a hollow fiber-shaped carbon-based oxygen reduction catalyst co-doped with N and S ( named NS-HCF).
由图14可以看出,NS-CHF与HOPPS-1是一致的中空纤维形貌,碳化并不对其结构产生影响。It can be seen from Figure 14 that NS-CHF and HOPPS-1 have the same hollow fiber morphology, and carbonization does not affect its structure.
由图15可以看出,在23°和44°附近显示出2个宽峰,对应了典型石墨碳的(002)和(100)晶面。It can be seen from Figure 15 that there are two broad peaks around 23° and 44°, corresponding to the (002) and (100) crystal planes of typical graphitic carbon.
由图16可以看出,其ID/IG值为1.21,说明了催化剂存在缺陷的特征。It can be seen from Fig. 16 that the ID/IG value is1.21 , indicating that the catalyst has defects.
由图17可以看出,催化剂中存在C、N、O和S元素,说明N和S元素成功引入到了碳基体中。It can be seen from Figure 17 that C, N, O and S elements exist in the catalyst, indicating that N and S elements have been successfully introduced into the carbon matrix.
由图18可以看出,经计算,NS-HCF比表面积高达1642.382m2·g-1。由图19可以看出,催化剂中同时存在微孔和介孔结构,其孔体积高达0.952cm3·g-1。对比例1的比表面积和孔体积均大于实施例1的Ni@NS-HCF。It can be seen from Fig. 18 that the calculated specific surface area of NS-HCF is as high as 1642.382m2 ·g-1 . It can be seen from Figure 19 that both micropores and mesoporous structures exist in the catalyst, and the pore volume is as high as 0.952 cm3 ·g-1 . Both the specific surface area and pore volume of Comparative Example 1 are larger than that of Ni@NS-HCF of Example 1.
由图20可以看出,在0.89V处观察到了一个微弱的还原峰,同时在图21的线性扫描伏安曲线中观察到0.82V的半波电位及3.6mA·cm-2的极限电流密度,这显示出NS-HCF的氧还原催化活性。此外,由图22证实了NS-HCF催化剂优异的稳定性。It can be seen from Figure 20 that a weak reduction peak was observed at 0.89V, and at the same time, a half-wave potential of 0.82V and a limiting current density of 3.6mA·cm-2 were observed in the linear sweep voltammetry curve of Figure 21, This shows the oxygen reduction catalytic activity of NS-HCF. In addition, the excellent stability of the NS-HCF catalyst was confirmed from FIG. 22 .
因此,与对比例1所制备的NS-HCF相比,实施例1所制备的Ni@NS-HCF在碱性电解液中表现出优异的氧还原催化性能,说明Ni的引入大幅提高了催化剂的氧还原性能。Therefore, compared with the NS-HCF prepared in Comparative Example 1, the Ni@NS-HCF prepared in Example 1 showed excellent oxygen reduction catalytic performance in alkaline electrolyte, indicating that the introduction of Ni greatly improved the catalytic performance of the catalyst. Oxygen reduction performance.
实施例2Example 2
(1)将50ml去离子水、50ml双氧水与10ml盐酸共混形成氧化处理溶液;(1) 50ml deionized water, 50ml hydrogen peroxide and 10ml hydrochloric acid are blended to form an oxidation treatment solution;
(2)将5g的PPS短纤维分散在氧化处理溶液中,然后在70℃反应5min;然后取出纤维,用去离子水冲洗至中性,再在70℃干燥2h,得到氧化PPS纤维(命名为OPPS-2);(2) Disperse 5 g of PPS short fibers in the oxidation treatment solution, and then react at 70 ° C for 5 min; then take out the fibers, rinse them with deionized water until neutral, and then dry them at 70 ° C for 2 h to obtain oxidized PPS fibers (named as OPPS-2);
(3)将步骤2得到的OPPS-2放入浓硝酸溶液中,在70℃下反应24h至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(命名为HOPPS-2)。(3) Put the OPPS-2 obtained in
由图23可以看出,实施例2同样成功制备了具有中空结构的HOPPS纤维,HOPPS-2的壁厚约为2μm,说明随着步骤2的氧化处理时间的延长,氧化处理深度逐渐增加,同时在溶解芯层PPS后得到中空纤维的壁厚也在逐渐增加。而该中空纤维外壁的直径保持不变,约为15μm,说明本发明仅仅是调控了PPS纤维的皮层结构,不影响纤维直径。It can be seen from Figure 23 that Example 2 also successfully prepared HOPPS fibers with a hollow structure, and the wall thickness of HOPPS-2 is about 2 μm, indicating that with the prolongation of the oxidation treatment time in
实施例3Example 3
(1)将50ml去离子水、50ml双氧水与10ml盐酸共混形成氧化处理溶液;(1) 50ml deionized water, 50ml hydrogen peroxide and 10ml hydrochloric acid are blended to form an oxidation treatment solution;
(2)将5g的PPS短纤维分散在氧化处理溶液中,然后在70℃反应20min;然后取出纤维,用去离子水冲洗至中性,再在70℃干燥2h,得到皮芯结构复合纤维(命名为OPPS-3);(2) Disperse 5 g of PPS short fibers in the oxidation treatment solution, and then react at 70 ° C for 20 min; then take out the fibers, rinse them with deionized water until neutral, and then dry them at 70 ° C for 2 h to obtain a skin-core structure composite fiber ( named OPPS-3);
(3)将步骤2得到的OPPS-3纤维放入浓硝酸溶液中,在70℃下反应24h至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(命名为HOPPS-3)。(3) Put the OPPS-3 fiber obtained in
由图24可以看出,实施例3所制备的HOPPS-3纤维的壁厚约为3.5μm,随着氧化处理时间的延长,氧化处理深度进一步增加,在溶解芯层PPS后得到中空纤维的壁厚也逐渐增加。It can be seen from Figure 24 that the wall thickness of the HOPPS-3 fiber prepared in Example 3 is about 3.5 μm, and as the oxidation treatment time prolongs, the oxidation treatment depth further increases, and the hollow fiber wall is obtained after dissolving the core PPS. Thickness also gradually increases.
实施例4Example 4
(1)将50ml去离子水、50ml双氧水与10ml盐酸共混形成氧化处理溶液;(1) 50ml deionized water, 50ml hydrogen peroxide and 10ml hydrochloric acid are blended to form an oxidation treatment solution;
(2)将5g的PPS短纤维分散在氧化处理溶液中,然后在70℃反应1h;然后取出纤维,用去离子水冲洗至中性,再在70℃干燥2h,得到皮芯结构复合纤维(命名为OPPS-4);(2) Disperse 5 g of PPS short fibers in the oxidation treatment solution, then react at 70 ° C for 1 h; then take out the fibers, rinse them with deionized water until neutral, and then dry them at 70 ° C for 2 h to obtain a skin-core structure composite fiber ( named OPPS-4);
(3)将步骤2得到的OPPS-4纤维放入浓硝酸溶液中,在70℃下反应24h至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(命名为HOPPS-4)。(3) Put the OPPS-4 fiber obtained in
由图25可以看出,实施例4所制备的HOPPS-4纤维的壁厚约为4.7μm,随着氧化处理时间的延长,氧化处理深度进一步增加,在溶解芯层PPS后得到中空纤维的壁厚也逐渐增加,纤维壁厚明显大于HOPPS-1、HOPPS-2和HOPPS-3。It can be seen from Figure 25 that the wall thickness of the HOPPS-4 fiber prepared in Example 4 is about 4.7 μm, and as the oxidation treatment time prolongs, the oxidation treatment depth further increases, and the hollow fiber wall is obtained after dissolving the core PPS. The thickness also increased gradually, and the fiber wall thickness was significantly greater than that of HOPPS-1, HOPPS-2 and HOPPS-3.
实施例5Example 5
(1)将50ml去离子水、50ml双氧水与10ml盐酸共混形成氧化处理溶液;(1) 50ml deionized water, 50ml hydrogen peroxide and 10ml hydrochloric acid are blended to form an oxidation treatment solution;
(2)将5g的PPS短纤维分散在氧化处理溶液中,然后在70℃反应4h;然后取出纤维,用去离子水冲洗至中性,再在70℃干燥2h,得到皮芯结构复合纤维(命名为OPPS-5);(2) Disperse 5 g of PPS short fibers in the oxidation treatment solution, and then react at 70 ° C for 4 h; then take out the fibers, rinse them with deionized water until neutral, and then dry them at 70 ° C for 2 h to obtain a skin-core composite fiber ( named OPPS-5);
(3)将步骤2得到的OPPS-5纤维放入浓硝酸溶液中,在70℃下反应24h至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(命名为HOPPS-5)。(3) Put the OPPS-5 fiber obtained in
由图26可以看出,实施例5所制备的HOPPS-5纤维的壁厚约为6μm,其壁厚明显大于HOPPS-1、HOPPS-2、HOPPS-3和HOPPS-4。It can be seen from Fig. 26 that the wall thickness of the HOPPS-5 fiber prepared in Example 5 is about 6 μm, which is obviously larger than that of HOPPS-1, HOPPS-2, HOPPS-3 and HOPPS-4.
实施例6Example 6
(1)将50ml去离子水、50ml双氧水与10ml盐酸共混形成氧化处理溶液;(1) 50ml deionized water, 50ml hydrogen peroxide and 10ml hydrochloric acid are blended to form an oxidation treatment solution;
(2)将5g的PPS短纤维分散在氧化处理溶液中,然后在70℃反应10h;然后取出纤维,用去离子水冲洗至中性,再在70℃干燥2h,得到皮芯结构复合纤维(命名为OPPS-6);(2) Disperse 5 g of PPS short fibers in the oxidation treatment solution, and then react at 70 ° C for 10 h; then take out the fibers, rinse them with deionized water until neutral, and then dry them at 70 ° C for 2 h to obtain a skin-core structure composite fiber ( named OPPS-6);
(3)将步骤2得到的OPPS-5纤维放入浓硝酸溶液中,在70℃下反应24h至不再产生棕红色气体并且在溶液中不再产生白色乳状物质;取出后,冲洗至中性;干燥后,得到壁厚可控的PPS基中空纤维(命名为HOPPS-6)。(3) Put the OPPS-5 fiber obtained in
由图27可以看出,实施例6所制备HOPPS-6纤维的壁厚约为7.2μm,纤维壁厚明显大于HOPPS-1、HOPPS-2、HOPPS-3、HOPPS-4和HOPPS-5。随着氧化处理时间的延长,氧化处理深度不断增加,而芯层的PPS比例越来越少,在溶解芯层PPS后得到中空纤维的直径只有一个很小的孔,孔直径约200nm。这说明随着步骤2的氧化处理时间的延长,PPS纤维由外向内逐渐被完全氧化。It can be seen from Figure 27 that the wall thickness of the HOPPS-6 fiber prepared in Example 6 is about 7.2 μm, which is significantly thicker than that of HOPPS-1, HOPPS-2, HOPPS-3, HOPPS-4 and HOPPS-5. With the prolongation of oxidation treatment time, the depth of oxidation treatment increases continuously, while the proportion of PPS in the core layer decreases. After dissolving the PPS in the core layer, the diameter of the hollow fiber obtained is only a small hole, and the diameter of the hole is about 200nm. This shows that with the prolongation of the oxidation treatment time in
本发明未述及之处适用于现有技术。What is not mentioned in the present invention is applicable to the prior art.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211204278.1ACN115513473B (en) | 2022-09-29 | 2022-09-29 | A polyphenylene sulfide-based hollow fiber with controllable wall thickness and its preparation method and application |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211204278.1ACN115513473B (en) | 2022-09-29 | 2022-09-29 | A polyphenylene sulfide-based hollow fiber with controllable wall thickness and its preparation method and application |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN115513473Atrue CN115513473A (en) | 2022-12-23 |
| CN115513473B CN115513473B (en) | 2024-04-26 |
Family
ID=84508677
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211204278.1AActiveCN115513473B (en) | 2022-09-29 | 2022-09-29 | A polyphenylene sulfide-based hollow fiber with controllable wall thickness and its preparation method and application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115513473B (en) |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5246647A (en)* | 1989-03-28 | 1993-09-21 | The Dow Chemical Company | Process of making microporous hollow fiber or film membrane of poly(phenylene sulfide) (PPS) |
| CN103572398A (en)* | 2012-07-24 | 2014-02-12 | 上海杜为化纤有限公司 | Preparation method of microporous hollow polyphenylene sulfide monofilament for filtration |
| CN108905655A (en)* | 2018-07-06 | 2018-11-30 | 天津工业大学 | A kind of preparation method of micropore polyphenylene sulfide hollow-fibre membrane |
| CN113058618A (en)* | 2021-03-25 | 2021-07-02 | 福州大学 | In situ supported sulfur-doped graphene denitration and sulfur-resistant catalyst polyphenylene sulfide composite material and preparation method thereof |
| KR20210113780A (en)* | 2020-03-09 | 2021-09-17 | 도레이첨단소재 주식회사 | Spinning solution for flexible PPS porous hollow fiber having hydrophilicity, flexible PPS porous hollow fiber membrane having hydrophilicity and Manufacturing method thereof |
| CN113481626A (en)* | 2021-07-14 | 2021-10-08 | 天津工业大学 | Preparation method of polyphenylene sulfide sulfone ketone fiber |
| CN113818246A (en)* | 2021-09-08 | 2021-12-21 | 安徽元琛环保科技股份有限公司 | Preparation method of antioxidant PPS fiber |
- 2022
- 2022-09-29CNCN202211204278.1Apatent/CN115513473B/enactiveActive
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5246647A (en)* | 1989-03-28 | 1993-09-21 | The Dow Chemical Company | Process of making microporous hollow fiber or film membrane of poly(phenylene sulfide) (PPS) |
| CN103572398A (en)* | 2012-07-24 | 2014-02-12 | 上海杜为化纤有限公司 | Preparation method of microporous hollow polyphenylene sulfide monofilament for filtration |
| CN108905655A (en)* | 2018-07-06 | 2018-11-30 | 天津工业大学 | A kind of preparation method of micropore polyphenylene sulfide hollow-fibre membrane |
| KR20210113780A (en)* | 2020-03-09 | 2021-09-17 | 도레이첨단소재 주식회사 | Spinning solution for flexible PPS porous hollow fiber having hydrophilicity, flexible PPS porous hollow fiber membrane having hydrophilicity and Manufacturing method thereof |
| CN113058618A (en)* | 2021-03-25 | 2021-07-02 | 福州大学 | In situ supported sulfur-doped graphene denitration and sulfur-resistant catalyst polyphenylene sulfide composite material and preparation method thereof |
| CN113481626A (en)* | 2021-07-14 | 2021-10-08 | 天津工业大学 | Preparation method of polyphenylene sulfide sulfone ketone fiber |
| CN113818246A (en)* | 2021-09-08 | 2021-12-21 | 安徽元琛环保科技股份有限公司 | Preparation method of antioxidant PPS fiber |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115513473B (en) | 2024-04-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Nie et al. | Key issues facing electrospun carbon nanofibers in energy applications: on-going approaches and challenges | |
| Wu et al. | Graphene-based hollow spheres as efficient electrocatalysts for oxygen reduction | |
| Zhang et al. | ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction | |
| JP5660917B2 (en) | Air electrode catalyst for fuel cell and production method thereof | |
| CN110540216A (en) | A kind of carbon-based Prussian blue analog composite material and its preparation method and application | |
| WO2009098812A1 (en) | Carbon catalyst, slurry containing the carbon catalyst, process for producing carbon catalyst, and fuel cell, storage device, and environmental catalyst each employing carbon catalyst | |
| CN111354952B (en) | A kind of graphite felt composite electrode and preparation method thereof | |
| CN114300702B (en) | Fuel cell gas diffusion layer structure containing cerium oxide modified carbon nanofiber and preparation method thereof | |
| CN112938930B (en) | A carbon aerogel derived from bacterial cellulose composite metal-organic framework material and its preparation method and application | |
| CN106784877B (en) | A kind of preparation method of microbial fuel cell cathode composite material and microbial fuel cell reactor | |
| Li et al. | Fluorine and phosphorus atoms cooperated on an N-doped 3D porous carbon network for enhanced ORR performance toward the zinc–air batteries | |
| CN103762091A (en) | Cellular porous manganese dioxide nanofiber preparing method and application of cellular porous manganese dioxide nanofiber in supercapacitor | |
| Li et al. | Recent advances and perspectives of practical modifications of vanadium redox flow battery electrodes | |
| Cheng et al. | High-graphitization, large-surface area, and porous carbon nanofiber: A superior bi-functional electrode for vanadium redox flow battery | |
| CN110648854A (en) | Boron-nitrogen co-doped carbon/manganese oxide composite nanosheet material, and preparation method and application thereof | |
| Schmidt et al. | Properties of mesoporous carbon modified carbon felt for anode of all-vanadium redox flow battery | |
| Wang et al. | Aqueous Zn-ion batteries using amorphous Zn-buserite with high activity and stability | |
| CN111252863A (en) | Mn-MOF (manganese-metal organic framework) derived carbon modified electrode for enhanced removal of organic pollutants and preparation method thereof | |
| CN110752378A (en) | Biomass-based active carbon-coated iron carbide three-dimensional porous microbial fuel cell anode material, anode and preparation method thereof | |
| CN111170307B (en) | Nanocarbon modified hollow activated carbon microtube and preparation method and application thereof | |
| CN111744527A (en) | A high-performance carbon-based electrocatalytic oxygen reduction material based on mesoporous silica molecular sieve and preparation method thereof | |
| CN104332637A (en) | Preparation method of catalyst of porous graphene loading precious metal nano particles | |
| Jing et al. | In situ pore-making and heteroatom doping of carbon nanofibers electrode for high performance vanadium redox flow battery | |
| CN112079352A (en) | Preparation method and application of biomass-based porous nitrogen-doped carbon material | |
| CN113871212A (en) | Manganese dioxide/carbon film composite material with core-shell structure and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |