CN115483375A - Method for applying silicon-carbon composite material to lithium ion battery cathode material - Google Patents
Method for applying silicon-carbon composite material to lithium ion battery cathode materialDownload PDFInfo
- Publication number
- CN115483375A CN115483375ACN202211078114.9ACN202211078114ACN115483375ACN 115483375 ACN115483375 ACN 115483375ACN 202211078114 ACN202211078114 ACN 202211078114ACN 115483375 ACN115483375 ACN 115483375A
- Authority
- CN
- China
- Prior art keywords
- silicon
- negative electrode
- carbon composite
- composite material
- lithium
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
- H01M4/1395—Processes of manufacture of electrodes based on metals, Si or alloys
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/386—Silicon or alloys based on silicon
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/624—Electric conductive fillers
- H01M4/625—Carbon or graphite
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Composite Materials (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Silicon Compounds (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明属于复合材料技术领域,涉及复合电极材料,尤其涉及一种硅碳复合材料的制备方法及应用于储锂应用。The invention belongs to the technical field of composite materials, and relates to a composite electrode material, in particular to a method for preparing a silicon-carbon composite material and its application to lithium storage.
背景技术Background technique
能源危机是二十一世纪最紧迫的问题之一,面对能源枯竭和环境污染带来的压力,对能源的迫切需求随经济的迅速发展下不断增加,人类开始将目光放到新能源的开发与利用上,这其中就包括太阳能产业的快速发展,但是随之而来产生大量的太阳能晶硅切割留下的废料硅造成大量的资源浪费的一些问题,现存的针对废料硅处理的手段操作都比较复杂,而且利用率低,因此如何有效地回收并利用好废料硅达到资源利用最大化有着十分重要的意义。The energy crisis is one of the most urgent problems in the 21st century. Facing the pressure brought by energy depletion and environmental pollution, the urgent demand for energy continues to increase with the rapid development of the economy. Human beings have begun to focus on the development of new energy. In terms of utilization, this includes the rapid development of the solar energy industry, but there will be a large amount of waste silicon left by the cutting of solar crystal silicon, resulting in a large amount of waste of resources. The existing methods for the treatment of waste silicon are all It is relatively complicated and has a low utilization rate, so how to effectively recycle and utilize waste silicon to maximize resource utilization is of great significance.
硅元素从1970年就开始被应用到离子电池的负极,硅的理论比容量甚至高达4200mA h g-1,是碳基材料的10倍以上,基于硅材料的高比容量,它被认为是下一代最有竞争力的负极材料。然而硅基作为负极材料也并非十全十美,它在充放电过程中,体积膨胀可达300%,导致硅颗粒的粉碎和SEI膜的破裂,大大影响了电池的循环性能,从而影响电池的使用性能。现在有一种办法可以通过碳包覆不但可以减缓硅体积膨胀产生的应力,还可以增加硅负极材料的导电性。此外,商业化的第一步就是要降低成本,从而实现商业化应用生产。因此,寻求简单价廉、无污染的硅原料和开发简单有效的制备工艺就显得十分重要。Silicon has been applied to the negative electrode of ion batteries since 1970. The theoretical specific capacity of silicon is even as high as 4200mA hg-1 , which is more than 10 times that of carbon-based materials. Based on the high specific capacity of silicon materials, it is considered to be the next generation The most competitive anode material. However, the silicon-based negative electrode material is not perfect. During the charging and discharging process, its volume expansion can reach 300%, resulting in the crushing of silicon particles and the rupture of the SEI film, which greatly affects the cycle performance of the battery, thereby affecting the performance of the battery. Now there is a way to not only slow down the stress caused by the volume expansion of silicon through carbon coating, but also increase the conductivity of silicon anode materials. In addition, the first step in commercialization is to reduce costs so as to achieve commercial application production. Therefore, it is very important to seek simple, cheap and pollution-free silicon raw materials and to develop simple and effective preparation processes.
发明内容Contents of the invention
本发明解决的技术问题是:提出一种废料硅的资源利用以及作为电极材料解决锂离子电池体积膨胀导致的循环稳定性差的问题。本发明采用的太阳能晶硅切割废料与PY碳源复合,不仅制备过程简单,操作方便,价格低廉并且环保,有效实现太阳能晶硅废料再利用的问题,实现晶硅废料再利用同时,其在第一圈放电比容量可达2400mAh g-1,其在0.1、0.25、0.5、1A g-1电流密度下的初始可逆容量分别高达1431、941、747、590mAh g-1,经过100个循环后,材料的可逆容量仍可以达到451mAh g-1,具有出色的倍率和循环性能。The technical problem solved by the invention is to propose a resource utilization of waste silicon and as an electrode material to solve the problem of poor cycle stability caused by volume expansion of lithium-ion batteries. The solar crystalline silicon cutting waste used in the present invention is compounded with PY carbon source, not only the preparation process is simple, the operation is convenient, the price is low, and it is environmentally friendly, and the problem of reusing solar crystalline silicon waste is effectively realized. One cycle discharge specific capacity can reach 2400mAh g-1 , and its initial reversible capacity at current density of 0.1, 0.25, 0.5, 1A g-1 is as high as 1431, 941, 747, 590mAh g -1, after 100 cycles, The reversible capacity of the material can still reach 451mAh g-1 , with excellent rate and cycle performance.
为了解决上述技术问题,本发明提出的技术方案是:一种硅碳复合材料用于锂离子电池负极材料的方法,包括如下步骤:In order to solve the above-mentioned technical problems, the technical solution proposed by the present invention is: a method for using a silicon-carbon composite material as an anode material for a lithium-ion battery, comprising the steps of:
A.硅源前驱体晶硅切割废料硅进行球磨,球/固质量比为10~20:1,球磨后的废料硅进行酸洗,浸出温度15~25℃,浸出时间3~12h,浸出液体积与切割废料质量比为20~200mL:1g,搅拌速度为100~500r/min;将酸洗水洗后的废料硅干燥研磨,得到的粉末在惰性气氛中600~900℃煅烧1~5h,得预处理后的硅粉;A. The silicon source precursor crystal silicon cutting waste silicon is ball milled, the ball/solid mass ratio is 10-20:1, the waste silicon after ball milling is pickled, the leaching temperature is 15-25 ℃, the leaching time is 3-12h, the volume of the leaching solution The mass ratio to the cutting waste is 20-200mL:1g, and the stirring speed is 100-500r/min; the waste silicon after pickling and water washing is dried and ground, and the obtained powder is calcined at 600-900°C for 1-5h in an inert atmosphere to obtain the pre- Treated silicon powder;
B.将酸和双氧水按照体积比1~3:1混合,冷却至室温后,在油浴锅中加热到80℃,将步骤A中得到的硅粉加入上述溶液中并搅拌3~6h后得到悬浮液;将悬浮液水洗、离心后,烘干,得到的表面附有羟基硅产物;B. Mix the acid and hydrogen peroxide in a volume ratio of 1 to 3:1, cool to room temperature, heat to 80°C in an oil bath, add the silicon powder obtained in step A to the above solution and stir for 3 to 6 hours to obtain Suspension; after washing the suspension with water, centrifuging, and drying, the obtained surface is attached with hydroxyl silicon products;
C.步骤B中得到的表面附有羟基硅产物、吡咯PY、去离子水分别按照100mg:400ul:50ml,在烧杯中加入冰块搅拌均匀,再将过硫酸铵和PY按照1:2的量加入其中,继续冰浴搅拌12h,等完成后温度恢复至室温后水洗、离心、烘干后管式炉在惰性气氛下从室温升温至400℃保温1h,即得硅碳复合材料;C. The silicon product with hydroxyl on the surface obtained in step B, pyrrole PY, and deionized water are respectively 100mg: 400ul: 50ml, add ice cubes to the beaker and stir well, and then mix ammonium persulfate and PY according to the amount of 1:2 Add it, continue to stir in an ice bath for 12 hours, after the temperature returns to room temperature after completion, wash with water, centrifuge, and dry, then heat the tube furnace from room temperature to 400°C for 1 hour under an inert atmosphere to obtain a silicon-carbon composite material;
D、将步骤C中制得的硅碳复合材料与导电剂和粘结剂按8:1:1混合研磨,加入适量分散剂进行溶解研磨,待研磨至无颗粒之后,涂覆在铜箔上,烘干8~12h,裁剪1*1cm2尺寸大小作为负极片,通过在手套箱中和正极片、负极片、隔膜、垫片、垫圈以及电解液进行组装,即得锂离子半电池。D. Mix and grind the silicon-carbon composite material prepared in step C with the conductive agent and the binder at a ratio of 8:1:1, add an appropriate amount of dispersant for dissolving and grinding, and coat it on the copper foil after grinding until there are no particles , dry for 8-12 hours, cut the size of 1*1cm2 as the negative electrode sheet, and assemble it with the positive electrode sheet, negative electrode sheet, separator, gasket, gasket and electrolyte in the glove box to obtain the lithium-ion half-cell.
优选的,步骤A中硅源前驱体为晶硅切割废料硅。Preferably, the silicon source precursor in step A is crystalline silicon cutting waste silicon.
优选的,步骤C中吡咯为碳源前驱体。Preferably, in step C, pyrrole is a carbon source precursor.
优选的,步骤B中所述的酸质量分数5~15%的硝酸或硫酸。Preferably, the acid mass fraction in step B is 5-15% nitric acid or sulfuric acid.
优选的,步骤C中所述惰性气体为氩气或氮气。Preferably, the inert gas in step C is argon or nitrogen.
优选的,步骤A和C中的碳化,所用设备为管式炉或箱式炉,升温速率为3~15℃/min-1。Preferably, for the carbonization in steps A and C, the equipment used is a tube furnace or a box furnace, and the heating rate is 3-15°C/min-1 .
优选的,所述负极片是在铜箔表面涂覆上由负极活性材料、导电剂、分散剂以及粘结剂,其中,所述负极活性材料为硅碳复合材料;所述导电剂为科琴导电炭黑KetjenblackEC-600JD;所述分散剂为氮甲基吡咯烷酮(NMP);所述粘结剂为油性粘结剂聚偏氟乙烯(PVDF);所述电解液为1M六氟磷酸锂(LiPF6)。Preferably, the negative electrode sheet is coated with a negative electrode active material, a conductive agent, a dispersant and a binding agent on the surface of the copper foil, wherein the negative electrode active material is a silicon-carbon composite material; the conductive agent is Ketjen Conductive carbon black Ketjenblack EC-600JD; the dispersant is nitrogen methylpyrrolidone (NMP); the binder is oily binder polyvinylidene fluoride (PVDF); the electrolyte is 1M lithium hexafluorophosphate (LiPF6 ).
优选的,将上述涂覆完的负极材料在手套箱中以锂片为对电极,Celgerd2400为隔膜,组装成2032纽扣电池,在0.01~1.5V电势窗口下,以不同的扫描速率测得循环伏安曲线,在不同的电流密度进行倍率性能以及长循环性能测试。Preferably, the above-mentioned coated negative electrode material is assembled into a 2032 button battery in a glove box with a lithium sheet as a counter electrode and Celgerd2400 as a diaphragm, and the cyclic volts are measured at different scan rates under a potential window of 0.01 to 1.5V. Ampere curves, rate performance and long cycle performance tests at different current densities.
有益效果Beneficial effect
本发明采用的太阳能晶硅切割废料与PY碳源复合,不仅制备过程简单,操作方便,价格低廉并且环保,有效实现太阳能晶硅废料再利用的问题,并且碳包裹后的硅基负极材料具有良好的导电性,实现晶硅废料再利用同时,电极材料也表现出良好的倍率性能长循环稳定性。The solar crystalline silicon cutting waste used in the present invention is compounded with PY carbon source, not only the preparation process is simple, the operation is convenient, the price is low, and it is environmentally friendly, and the problem of reusing solar crystalline silicon waste can be effectively realized, and the silicon-based negative electrode material after carbon wrapping has good Excellent electrical conductivity, realizing the reuse of crystalline silicon waste, at the same time, the electrode material also shows good rate performance and long cycle stability.
本发明以采用简单的在废料硅表面包覆上一层致密的PPy碳材料,反应操作简单,前驱体成本低,同时可以实现资源循环利用,复合绿色环保的理念,有着很大的应用潜能。合成的复合材料可以为锂离子提供更多的反应位点,并且具备较大的比表面积还可以帮助电极材料与电解质提供更多的接触面积,实现提高储存锂离子的能力,并与锂片组装成半电池,进行性能测试。The present invention simply coats the surface of waste silicon with a layer of dense PPy carbon material, the reaction operation is simple, the cost of the precursor is low, and at the same time, resource recycling can be realized, and the concept of compounding green and environmental protection has great application potential. The synthesized composite material can provide more reaction sites for lithium ions, and has a larger specific surface area, which can also help electrode materials and electrolytes provide more contact area, improve the ability to store lithium ions, and assemble with lithium sheets into a half battery for performance testing.
实施例1中将PY和过硫酸铵分别按照400μL和200mg进行反应,得到的负极材料组装成的半电池在进行充放电测试时,在第一圈放电比容量可达2400mAh g-1,同时具有最佳的倍率性能,即当电流密度在0.1A g-1、0.25Ag-1、0.5A g-1、1A g-1时初始可逆容量可以分别达到1431、941、747、590mAh g-1,经过100个循环后,材料的可逆容量仍可以稳定在451mAhg-1。In Example 1, PY and ammonium persulfate were reacted at 400 μL and 200 mg respectively, and the half-cell assembled with the obtained negative electrode material had a discharge specific capacity of 2400 mAh g-1 in the first lap during the charge and discharge test, and had The best rate performance, that is, the initial reversible capacity can reach 1431, 941, 747, 590mAh g-1 when the current density is 0.1A g-1 , 0.25Ag-1 , 0.5A g-1 , 1A g-1 , respectively, After 100 cycles, the reversible capacity of the material is still stable at 451mAhg-1 .
实施例2中将PY和过硫酸铵分别按照200μL和100mg进行反应,得到的负极材料组装成的半电池在进行充放电测试时,在第一圈放电比容量可达2083mAh g-1,同时具有最佳的倍率性能,即当电流密度在0.1、0.25、0.5、1Ag-1时初始可逆容量可以分别达到1259、832、641、504mAh g-1,其经过100个循环后,材料的可逆容量稳定在406mAh g-1。In Example 2, PY and ammonium persulfate were reacted at 200 μL and 100 mg, respectively, and the half-cell assembled with the obtained negative electrode material had a specific capacity of 2083 mAh g-1 in the first cycle when the charge and discharge test was performed. The best rate performance, that is, when the current density is 0.1, 0.25, 0.5, 1Ag-1 , the initial reversible capacity can reach 1259, 832, 641, 504mAh g-1 respectively, after 100 cycles, the reversible capacity of the material is stable At 406mAh g-1 .
实施例3中将PY和过硫酸铵分别按照800μL和400mg进行反应,得到的负极材料组装成的半电池在进行充放电测试时,在第一圈放电比容量可达1950mAh g-1,同时具有最佳的倍率性能,即当电流密度在0.1、0.25、0.5、1A g-1时初始可逆容量可以分别达到1083、695、556、450mAh g-1,其经过100个循环后,材料的可逆容量稳定在396mAh g-1。In Example 3, 800 μL and 400 mg of PY and ammonium persulfate were reacted respectively, and the half-cell assembled with the obtained negative electrode material had a discharge specific capacity of 1950 mAh g-1 in the first lap during the charge and discharge test, and had The best rate performance, that is, when the current density is 0.1, 0.25, 0.5, 1A g-1 , the initial reversible capacity can reach 1083, 695, 556, 450mAh g-1 respectively, after 100 cycles, the reversible capacity of the material stabilized at 396mAh g-1 .
三个硅碳复合材料组装成都的半电池进行测试之后显示出的倍率性能、较高的可逆比容量和长循环寿命均比原始硅基负极材料更加优异,从而有利于满足实际需求。实施例1中的倍率性能长循环稳定性最佳。The rate performance, high reversible specific capacity and long cycle life of three silicon-carbon composite materials assembled into half cells in Chengdu are all better than the original silicon-based anode materials, which is conducive to meeting actual needs. The rate performance in Example 1 has the best long-term cycle stability.
附图说明Description of drawings
图1.实施例1制备的硅碳复合负极材料的扫描电镜图片(SEM);The scanning electron microscope picture (SEM) of the silicon-carbon composite negative electrode material that Fig. 1. embodiment 1 prepares;
图2.实施例2制备的硅碳复合负极材料的扫描电镜图片(SEM);Fig. 2. the scanning electron microscope picture (SEM) of the silicon-carbon composite negative electrode material that embodiment 2 prepares;
图3.实施例1制备的硅碳复合负极材料的X射线粉末衍射图(XRD);The X-ray powder diffraction pattern (XRD) of the silicon-carbon composite negative electrode material that Fig. 3. embodiment 1 prepares;
图4.实施例1制备的硅碳复合负极材料的拉曼光谱图(Raman);The Raman spectrogram (Raman) of the silicon-carbon composite negative electrode material that Fig. 4. embodiment 1 prepares;
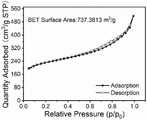
图5.实施例1制备的硅碳复合负极材料的N2吸脱附曲线;Fig. 5. the N of the silicon-carbon composite negative electrode material that embodiment1 prepares Adsorption-desorption curve;
图6.实施例1制备的硅碳复合负极材料用于锂离子电池后的倍率和循环性能曲线;Figure 6. The rate and cycle performance curves of the silicon-carbon composite negative electrode material prepared in Example 1 for lithium-ion batteries;
图7.实施例2制备的硅碳复合负极材料用于锂离子电池后的倍率和循环性能曲线;Figure 7. The rate and cycle performance curves of the silicon-carbon composite negative electrode material prepared in Example 2 for lithium-ion batteries;
图8.实施例3制备的硅碳复合负极材料用于锂离子电池后的倍率和循环性能曲线;Figure 8. The rate and cycle performance curves of the silicon-carbon composite negative electrode material prepared in Example 3 for lithium-ion batteries;
图9.实施例4制备的硅碳复合负极材料用于锂离子电池后的倍率和循环性能曲线;Figure 9. The silicon-carbon composite negative electrode material prepared in Example 4 is used for the rate and cycle performance curves of lithium-ion batteries;
具体实施方式detailed description
下面结合实施例对本发明进行详细说明,以使本领域技术人员更好地理解本发明,但本发明并不局限于以下实施例。The present invention will be described in detail below in conjunction with the examples, so that those skilled in the art can better understand the present invention, but the present invention is not limited to the following examples.
实施例1Example 1
一种硅碳复合材料的制备方法,包括:太阳能晶硅废料进行球磨,球/料质量比为20:1,球磨后的废料硅在HCL和HNO3体积比为2:1的条件下进行酸洗,酸洗条件浸出温度为室温,浸出时间12h,切割废料与浸出液体积按照1g:4ml的固液比进行混合,搅拌速度为300r/min,搅拌时间为30h;将酸洗水洗后的废料硅干燥研磨,并在N2氛围下700℃煅烧1h,得预处理后的硅粉;将硫酸和双氧水按照体积比3:1混合,冷却至室温后,在油浴锅中加热80℃,将预处理后硅粉加入上述溶液中并搅拌6h后得到悬浮液;水洗、离心后、烘干,得到的表面附有羟基硅产物,实现预处理;A method for preparing a silicon-carbon composite material, comprising: ball milling solar crystalline silicon waste with a ball/material mass ratio of 20:1, and acidifying the waste silicon after ball milling under the condition that the volume ratio of HCL andHNO3 is 2:1. Washing, pickling conditions The leaching temperature is room temperature, the leaching time is 12h, the cutting waste and the leachate volume are mixed according to the solid-to-liquid ratio of 1g: 4ml, the stirring speed is 300r/min, and the stirring time is 30h; the waste silicon after pickling and water washing Dry and grind, and calcinate at 700°C for 1 hour under N2 atmosphere to obtain pretreated silicon powder; mix sulfuric acid and hydrogen peroxide at a volume ratio of 3:1, cool to room temperature, heat in an oil bath at 80°C, and pretreat After treatment, silicon powder is added to the above solution and stirred for 6 hours to obtain a suspension; after washing, centrifuging, and drying, the obtained surface is attached with hydroxyl silicon products to realize pretreatment;
分别取100mg上述预处理后的表面附有羟基硅产物和400ul的PY至于50ml的去离子水中,置于冰箱中冷冻1h,冰浴搅拌均匀后,再加入200mg的过硫酸铵,在烧杯中加入冰块进行冰浴,并搅拌12h,溶液恢复至室温时,水洗离心后,烘干收集固体、研磨后,N2气氛下,在管式炉煅烧样品,从室温开始升温至400℃,保温1h,得到PPY碳源/废料硅复合材料。Take 100mg of the above-mentioned pretreated surface-attached hydroxyl silicon product and 400ul of PY into 50ml of deionized water, freeze in the refrigerator for 1 hour, stir in the ice bath, then add 200mg of ammonium persulfate, and add to the beaker Ice cubes were placed in an ice bath and stirred for 12 hours. When the solution returned to room temperature, it was washed with water and centrifuged, dried to collect the solids, and ground. Then, the sample was calcined in a tube furnace underN2 atmosphere, and the temperature was raised from room temperature to 400°C, and kept for 1 hour. , to obtain PPY carbon source/scrap silicon composites.
将制得的硅碳复合材料与导电剂和粘结剂按8:1:1混合研磨,加入适量分散剂进行溶解研磨,待研磨至无颗粒之后,涂覆在铜箔上,烘干8~12h,裁剪1*1cm2尺寸大小作为负极片,通过在手套箱中将正极片、负极片、隔膜、垫片、垫圈以及电解液进行组装,即得锂离子半电池。Mix and grind the prepared silicon-carbon composite material with a conductive agent and a binder at a ratio of 8:1:1, add an appropriate amount of dispersant to dissolve and grind, and after grinding to no particles, coat it on a copper foil and dry it for 8 to 10 minutes. 12h, cut the size of 1*1cm2 as the negative electrode sheet, and assemble the positive electrode sheet, negative electrode sheet, separator, spacer, gasket and electrolyte in the glove box to obtain the lithium-ion half-battery.
所述负极片是在铜箔表面涂覆上由负极活性材料、导电剂、分散剂以及粘结剂,其中,所述负极活性材料为硅碳复合材料;所述导电剂为科琴导电炭黑Ketjenblack EC-600JD;所述分散剂为氮甲基吡咯烷酮(NMP);所述粘结剂为油性粘结剂聚偏氟乙烯(PVDF)。The negative electrode sheet is coated with a negative electrode active material, a conductive agent, a dispersant and a binding agent on the surface of the copper foil, wherein the negative electrode active material is a silicon-carbon composite material; the conductive agent is Ketjen conductive carbon black Ketjenblack EC-600JD; the dispersant is nitrogen methylpyrrolidone (NMP); the binder is oily binder polyvinylidene fluoride (PVDF).
商业锂片为对电极,电解液为LiPF6电解液,Celgard 2500为隔膜,制备的硅碳复合负极材料为工作电极,一起组装成纽扣电池,对其进行电化学性能测试。The commercial lithium sheet is used as the counter electrode, the electrolyte is LiPF6 electrolyte,
图1可以看出来制备的硅碳复合电极材料的颗粒尺寸是微米级别。It can be seen from Figure 1 that the particle size of the prepared silicon-carbon composite electrode material is in the order of microns.
图3可以看出制备的硅碳复合电极材料各衍射峰位置和相对强度均与JPCDS卡片(#27-1402)相吻合,表明产物为硅碳复合材料。It can be seen from Figure 3 that the positions and relative intensities of the diffraction peaks of the prepared silicon-carbon composite electrode material are consistent with those of the JPCDS card (#27-1402), indicating that the product is a silicon-carbon composite material.
图4中拉曼光谱图中可以观察到硅和碳的峰,也代表了碳的无序化程度,表明产物为硅碳复合材料。The peaks of silicon and carbon can be observed in the Raman spectrum in Figure 4, which also represents the degree of disordering of carbon, indicating that the product is a silicon-carbon composite material.
图5中N2吸脱附曲线显示该复合材料的比表面达到了737.3813m2/g,主要以微孔和介孔的形式存在。The N2 adsorption-desorption curve in Figure 5 shows that the specific surface of the composite material reaches 737.3813m2 /g, mainly in the form of micropores and mesopores.
图6中的电化学测试结果表明,将本实施例制得的硅碳复合材料与导电剂和粘结剂按8:1:1制作成电极,并与锂片组装成半电池,进行性能测试,其在第一圈放电比容量可达2400mAh g-1,其在0.1、0.25、0.5、1A g-1电流密度下的初始可逆容量分别高达1431、941、747、590mAh g-1,经过100个循环后,材料的可逆容量仍可以达到451mAh g-1,具有出色的倍率和循环性能。The electrochemical test results in Figure 6 show that the silicon-carbon composite material prepared in this example, the conductive agent and the binder are made into electrodes at a ratio of 8:1:1, and assembled into a half-cell with a lithium sheet for performance testing. , its specific discharge capacity in the first cycle can reach 2400mAh g-1 , and its initial reversible capacity at current densities of 0.1, 0.25, 0.5, 1A g-1 is as high as 1431, 941, 747, 590mAh g -1respectively , after 100 After three cycles, the reversible capacity of the material can still reach 451mAh g-1 , with excellent rate and cycle performance.
实施例2Example 2
一种硅碳复合材料的制备方法,包括:分别取100mg预处理后的硅产物和200ul的PY至于50ml的去离子水中,至于冰箱中冷冻1h,冰浴搅拌均匀后,再加入100mg的过硫酸铵,冰浴搅拌12h,溶液恢复至室温时,水洗离心后,烘干收集固体、研磨后,N2气氛下,在管式炉煅烧样品,从室温开始升温至400℃,保温1h,得到PPY碳源/废料硅复合材料。A method for preparing a silicon-carbon composite material, comprising: respectively taking 100 mg of pretreated silicon product and 200 ul of PY in 50 ml of deionized water, freezing in a refrigerator for 1 hour, stirring evenly in an ice bath, and then adding 100 mg of persulfuric acid Ammonium, stirred in an ice bath for 12 hours, when the solution returned to room temperature, washed with water and centrifuged, dried to collect the solid, and ground, then calcined the sample in a tube furnace underN2 atmosphere, raised the temperature from room temperature to 400°C, and kept it for 1 hour to obtain PPY Carbon source/scrap silicon composites.
将实例制得的硅碳复合材料与导电剂和粘结剂按8:1:1混合研磨,加入适量分散剂进行溶解研磨,待研磨至无颗粒之后,涂覆在铜箔上,烘干8~12h,通过在手套箱中和正极片、负极片、隔膜、垫片、垫圈以及电解液进行组装,即得锂离子半电池。Mix and grind the silicon-carbon composite material prepared in the example with a conductive agent and a binder at a ratio of 8:1:1, add an appropriate amount of dispersant to dissolve and grind, and after grinding to no particles, coat it on a copper foil and dry it for 8 ~12h, by assembling with the positive electrode sheet, negative electrode sheet, separator, spacer, gasket and electrolyte in a glove box, a lithium-ion half-battery is obtained.
所述负极片是在铜箔表面涂覆上由负极活性材料、导电剂、分散剂以及粘结剂,其中,所述负极活性材料为硅碳复合材料;所述导电剂为科琴导电炭黑Ketjenblack EC-600JD;所述分散剂为氮甲基吡咯烷酮(NMP);所述粘结剂为油性粘结剂聚偏氟乙烯(PVDF)。The negative electrode sheet is coated with a negative electrode active material, a conductive agent, a dispersant and a binding agent on the surface of the copper foil, wherein the negative electrode active material is a silicon-carbon composite material; the conductive agent is Ketjen conductive carbon black Ketjenblack EC-600JD; the dispersant is nitrogen methylpyrrolidone (NMP); the binder is oily binder polyvinylidene fluoride (PVDF).
商业锂片为对电极,电解液为LiPF6电解液,Celgard 2500为隔膜,制备的硅碳复合负极材料为工作电极,一起组装成纽扣电池,对其进行电化学性能测试。The commercial lithium sheet is used as the counter electrode, the electrolyte is LiPF6 electrolyte,
图2可以看出来制备的硅碳复合电极材料的颗粒尺寸是微米级别。It can be seen from Figure 2 that the particle size of the prepared silicon-carbon composite electrode material is in the order of microns.
图3可以看出制备的硅碳复合电极材料各衍射峰位置和相对强度均与JPCDS卡片(#27-1402)相吻合,表明产物为硅碳复合材料。It can be seen from Figure 3 that the positions and relative intensities of the diffraction peaks of the prepared silicon-carbon composite electrode material are consistent with those of the JPCDS card (#27-1402), indicating that the product is a silicon-carbon composite material.
图7中的测试结果表明,将本实施例制得的硅碳复合材料与导电剂和粘结剂按8:1:1制作成电极,并与锂片组装成半电池,进行性能测试,初始放电比容量可达到2083mAhg-1,其在0.1、0.25、0.5、1A g-1电流密度下的初始可逆容量分别高达1259、832、641、504mAhg-1,其经过100个循环后,材料的可逆容量仍可以达到406mAh g-1。The test results in Fig. 7 show that the silicon-carbon composite material prepared in this embodiment, the conductive agent and the binder are made into electrodes according to 8:1:1, and assembled into a half-cell with a lithium sheet, and the performance test is performed. The discharge specific capacity can reach 2083mAhg-1 , and its initial reversible capacity at current densities of 0.1, 0.25, 0.5, and 1A g-1 is as high as 1259, 832, 641, and 504mAhg-1 , respectively. After 100 cycles, the reversible capacity of the material The capacity can still reach 406mAh g-1 .
实施例3Example 3
一种硅碳复合材料的制备方法,包括:分别取100mg预处理后的硅产物和800ul的PY至于50ml的去离子水中,至于冰箱中冷冻1h,冰浴搅拌均匀后,再加入400mg的过硫酸铵,冰浴搅拌12h,溶液恢复至室温时,水洗离心后,烘干收集固体、研磨后,N2气氛下,在管式炉煅烧样品,从室温开始升温至400℃,保温1h,得到PPY碳源/废料硅复合材料。A method for preparing a silicon-carbon composite material, comprising: respectively taking 100 mg of pretreated silicon product and 800 ul of PY in 50 ml of deionized water, freezing in a refrigerator for 1 hour, stirring in an ice bath, and then adding 400 mg of persulfuric acid Ammonium, stirred in an ice bath for 12 hours, when the solution returned to room temperature, washed with water and centrifuged, dried to collect the solid, and ground, then calcined the sample in a tube furnace underN2 atmosphere, raised the temperature from room temperature to 400°C, and kept it for 1 hour to obtain PPY Carbon source/scrap silicon composites.
将实例制得的硅碳复合材料与导电剂和粘结剂按8:1:1混合研磨,加入适量分散剂进行溶解研磨,待研磨至无颗粒之后,涂覆在铜箔上,烘干8~12h,通过在手套箱中和正极片、负极片、隔膜、垫片、垫圈以及电解液进行组装,即得锂离子半电池。Mix and grind the silicon-carbon composite material prepared in the example with a conductive agent and a binder at a ratio of 8:1:1, add an appropriate amount of dispersant for dissolving and grinding, and after grinding until there are no particles, coat it on a copper foil and dry it for 8 ~12h, by assembling with the positive electrode sheet, negative electrode sheet, separator, spacer, gasket and electrolyte in a glove box, a lithium-ion half-battery is obtained.
所述负极片是在铜箔表面涂覆上由负极活性材料、导电剂、分散剂以及粘结剂,其中,所述负极活性材料为硅碳复合材料;所述导电剂为科琴导电炭黑Ketjenblack EC-600JD;所述分散剂为氮甲基吡咯烷酮(NMP);所述粘结剂为油性粘结剂聚偏氟乙烯(PVDF)。The negative electrode sheet is coated with a negative electrode active material, a conductive agent, a dispersant and a binding agent on the surface of the copper foil, wherein the negative electrode active material is a silicon-carbon composite material; the conductive agent is Ketjen conductive carbon black Ketjenblack EC-600JD; the dispersant is nitrogen methylpyrrolidone (NMP); the binder is oily binder polyvinylidene fluoride (PVDF).
商业锂片为对电极,电解液为LiPF6电解液,Celgard 2500为隔膜,制备的硅碳复合负极材料为工作电极,一起组装成纽扣电池,对其进行电化学性能测试。The commercial lithium sheet is used as the counter electrode, the electrolyte is LiPF6 electrolyte,
图3可以看出制备的硅碳复合电极材料各衍射峰位置和相对强度均与JPCDS卡片(#27-1402)相吻合,表明产物为硅碳复合材料。It can be seen from Figure 3 that the positions and relative intensities of the diffraction peaks of the prepared silicon-carbon composite electrode material are consistent with those of the JPCDS card (#27-1402), indicating that the product is a silicon-carbon composite material.
图8中的测试结果表明,将本实施例制得的硅碳复合材料与导电剂和粘结剂按8:1:1制作成电极,并与锂片组装成半电池,进行性能测试,其初始放电比容量为1950mAh g-1,在0.1、0.25、0.5、1A g-1电流密度下的初始可逆容量分别高达1083、695、556、450mAh g-1,经过100个循环后,材料的可逆容量仍可以达到396mAh g-1。The test results in Fig. 8 show that the silicon-carbon composite material prepared in this embodiment and the conductive agent and the binding agent are made into electrodes according to 8:1:1, and assembled into a half-cell with a lithium sheet, and the performance test is performed. The initial discharge specific capacity is 1950mAh g-1 , and the initial reversible capacity is as high as 1083, 695, 556, and 450mAh g-1 at current densities of 0.1, 0.25, 0.5, and 1A g-1 respectively. After 100 cycles, the reversible The capacity can still reach 396mAh g-1 .
实施例4Example 4
取来太阳能晶硅切割废料的硅材料(粒度范围在1-100μm),将乙醇作为分散剂和溶剂用来溶解样品残留的有机物,并采用球磨机将废料硅团聚物研磨成粉末状,球磨的转速设定为300r/min将其球磨30h;球磨后的废料硅进行酸洗水洗,通过酸洗去除废料硅中由于切割过程中残留的金属元素以及一些杂质,酸洗条件为HCl(体积分数为36.0%-38.0%)和HNO3(体积分数为65.0%-68.0%),体积比为2:1,清洗后的废料硅再经过离心干燥并进行研磨,得到的粉末状样品在N2气氛下进行热处理,温度升到700℃,保温1h,升温速率为10℃/min。经过管式炉煅烧样品,得到预处理硅粉。Take the silicon material (particle size range of 1-100μm) from solar crystal silicon cutting waste, use ethanol as a dispersant and solvent to dissolve the residual organic matter in the sample, and use a ball mill to grind the waste silicon agglomerate into powder. Set to 300r/min with its ball milling for 30h; the waste silicon after the ball milling is carried out pickling and water washing, and the metal elements and some impurities remaining in the cutting process in the waste silicon are removed by pickling, and the pickling condition is HCl (volume fraction is 36.0 %-38.0%) and HNO3 (volume fraction is 65.0%-68.0%), the volume ratio is 2:1, the waste silicon after cleaning is centrifuged and dried and ground, and the obtained powder sample is carried out under N2 atmosphere For heat treatment, the temperature was raised to 700°C, kept for 1 hour, and the heating rate was 10°C/min. The sample is calcined in a tube furnace to obtain pretreated silicon powder.
将实例制得的硅碳复合材料与导电剂和粘结剂按8:1:1混合研磨,加入适量分散剂进行溶解研磨,待研磨至无颗粒之后,涂覆在铜箔上,烘干8~12h,通过在手套箱中和正极片、负极片、隔膜、垫片、垫圈以及电解液进行组装,即得锂离子半电池。Mix and grind the silicon-carbon composite material prepared in the example with a conductive agent and a binder at a ratio of 8:1:1, add an appropriate amount of dispersant for dissolving and grinding, and after grinding until there are no particles, coat it on a copper foil and dry it for 8 ~12h, by assembling with the positive electrode sheet, negative electrode sheet, separator, spacer, gasket and electrolyte in a glove box, a lithium-ion half-battery is obtained.
所述负极片是在铜箔表面涂覆上由负极活性材料、导电剂、分散剂以及粘结剂,其中,所述负极活性材料为硅碳复合材料;所述导电剂为科琴导电炭黑Ketjenblack EC-600JD;所述分散剂为氮甲基吡咯烷酮(NMP);所述粘结剂为油性粘结剂聚偏氟乙烯(PVDF)。The negative electrode sheet is coated on the surface of copper foil by negative active material, conductive agent, dispersant and binding agent, wherein the negative active material is a silicon-carbon composite material; the conductive agent is Ketjen conductive carbon black Ketjenblack EC-600JD; the dispersant is nitrogen methylpyrrolidone (NMP); the binder is oily binder polyvinylidene fluoride (PVDF).
以商业锂片为对电极,电解液为LiPF6电解液,Celgard 2500为隔膜,以预处理的硅粉作为工作电极,一起组装成纽扣电池,对其进行电化学性能测试,进行对比。The commercial lithium sheet was used as the counter electrode, the electrolyte was LiPF6 electrolyte,
图9中的测试结果表明,将本实施例处理的硅粉与导电剂和粘结剂按8:1:1制作成电极,并与锂片组装成半电池,进行性能测试,其在0.1、0.25、0.5、1Ag-1电流密度下进行充放电,初始放电比容量可达1454、19、7、4mAh g-1,经过100个循环后,材料的可逆容量只能维持在19mAh g-1,表明未经处理的硅粉容量衰减较快,循环稳定性比较差。The test results in Fig. 9 show that the silicon powder processed in this embodiment and the conductive agent and the binding agent are made into electrodes according to 8:1:1, and assembled into a half-cell with a lithium sheet, and the performance test is carried out, and it is in the range of 0.1, Charge and discharge at current densities of 0.25, 0.5, 1Ag-1 , the initial discharge specific capacity can reach 1454, 19, 7, 4mAh g-1 , after 100 cycles, the reversible capacity of the material can only be maintained at 19mAh g-1 , It shows that the capacity of untreated silicon powder decays faster and the cycle stability is relatively poor.
以上所述仅为本发明的实施例,并非因此限制本发明的专利范围,凡是利用本发明说明书所作的等效结构或等效流程变换,或直接或间接运用在其他相关的技术领域,均同理包括在本发明的专利保护范围内。The above is only an embodiment of the present invention, and does not limit the patent scope of the present invention. Any equivalent structure or equivalent process transformation made by the description of the present invention, or directly or indirectly used in other related technical fields, shall be the same as The theory is included in the patent protection scope of the present invention.
Claims (8)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211078114.9ACN115483375B (en) | 2022-09-05 | 2022-09-05 | Method for applying silicon-carbon composite material to negative electrode material of lithium ion battery |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211078114.9ACN115483375B (en) | 2022-09-05 | 2022-09-05 | Method for applying silicon-carbon composite material to negative electrode material of lithium ion battery |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN115483375Atrue CN115483375A (en) | 2022-12-16 |
| CN115483375B CN115483375B (en) | 2024-01-30 |
Family
ID=84392555
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211078114.9AActiveCN115483375B (en) | 2022-09-05 | 2022-09-05 | Method for applying silicon-carbon composite material to negative electrode material of lithium ion battery |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115483375B (en) |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104868107A (en)* | 2015-03-11 | 2015-08-26 | 中国科学院化学研究所 | Spherical silicon/carbon composite material for lithium ion battery as well as preparation method and application thereof |
| CN107732200A (en)* | 2017-10-12 | 2018-02-23 | 西安交通大学 | A kind of method that lithium ion battery negative material is prepared using photovoltaic industry waste material |
| CN110474032A (en)* | 2019-08-21 | 2019-11-19 | 郑州中科新兴产业技术研究院 | It is a kind of to be given up the silicon-carbon cathode material and preparation method thereof of silicon based on photovoltaic |
| CN112110448A (en)* | 2020-09-21 | 2020-12-22 | 中山大学 | A kind of nitrogen-doped carbon and nano-silicon composite negative electrode material and preparation method thereof |
| CN113611858A (en)* | 2021-06-24 | 2021-11-05 | 中南大学 | Battery negative electrode active material and preparation method thereof |
| CN114180548A (en)* | 2021-11-12 | 2022-03-15 | 江苏大学 | Preparation method of silicon-carbon composite negative electrode material and lithium storage application |
| CN114975962A (en)* | 2022-06-24 | 2022-08-30 | 内蒙古瑞盛天然石墨应用技术研究院 | Method for preparing silicon carbon anode material using photovoltaic waste silicon powder and graphene oxide |
- 2022
- 2022-09-05CNCN202211078114.9Apatent/CN115483375B/enactiveActive
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104868107A (en)* | 2015-03-11 | 2015-08-26 | 中国科学院化学研究所 | Spherical silicon/carbon composite material for lithium ion battery as well as preparation method and application thereof |
| CN107732200A (en)* | 2017-10-12 | 2018-02-23 | 西安交通大学 | A kind of method that lithium ion battery negative material is prepared using photovoltaic industry waste material |
| CN110474032A (en)* | 2019-08-21 | 2019-11-19 | 郑州中科新兴产业技术研究院 | It is a kind of to be given up the silicon-carbon cathode material and preparation method thereof of silicon based on photovoltaic |
| CN112110448A (en)* | 2020-09-21 | 2020-12-22 | 中山大学 | A kind of nitrogen-doped carbon and nano-silicon composite negative electrode material and preparation method thereof |
| CN113611858A (en)* | 2021-06-24 | 2021-11-05 | 中南大学 | Battery negative electrode active material and preparation method thereof |
| CN114180548A (en)* | 2021-11-12 | 2022-03-15 | 江苏大学 | Preparation method of silicon-carbon composite negative electrode material and lithium storage application |
| CN114975962A (en)* | 2022-06-24 | 2022-08-30 | 内蒙古瑞盛天然石墨应用技术研究院 | Method for preparing silicon carbon anode material using photovoltaic waste silicon powder and graphene oxide |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115483375B (en) | 2024-01-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Yao et al. | 5, 7, 12, 14-Pentacenetetrone as a high-capacity organic positive-electrode material for use in rechargeable lithium batteries | |
| CN111146427A (en) | A method for preparing hollow core-shell nano-silicon carbon composite material using polyaniline as carbon source and secondary battery using the material | |
| CN101924211A (en) | A kind of graphene/silicon lithium ion battery negative electrode material and preparation method | |
| CN112652757B (en) | Modified silicon-carbon negative electrode material and preparation method and application thereof | |
| Gan et al. | Polymeric carbon encapsulated Si nanoparticles from waste Si as a battery anode with enhanced electrochemical properties | |
| CN110676447B (en) | A high-voltage workable composite positive electrode and preparation method thereof | |
| CN105742695A (en) | Lithium-ion battery and preparation method thereof | |
| CN109037606A (en) | Carbon-coated porous silicon-iron alloy composite negative electrode material and preparation and application thereof | |
| CN117476913A (en) | Fluorosilicate coated silicon-based anode material and preparation method and application thereof | |
| CN107293715A (en) | A kind of lithium-sulphur cell positive electrode S/CNT CeO2The preparation method of composite | |
| CN113299894A (en) | MnF2@ NC lithium ion battery cathode material and preparation method and application thereof | |
| CN115986069A (en) | A kind of positive electrode pre-lithiation material for lithium ion battery and preparation method thereof | |
| CN105742619B (en) | A kind of unformed Mn oxide cladding ferriferous oxide lithium/anode material of lithium-ion battery and preparation method thereof | |
| CN117175016B (en) | Negative-electrode-free sodium ion secondary battery, electrolyte and application thereof | |
| CN111825080A (en) | Three-dimensional porous graphene and application thereof in lithium ion battery | |
| CN109360947B (en) | Preparation method of porous carbon cathode material of quasi-solid-state lithium-sulfur battery | |
| CN115939359B (en) | Silicon-based negative electrode material, preparation method thereof and lithium ion secondary battery | |
| CN111293297A (en) | Carbon-coated MoSe2Black phosphorus composite material and preparation method thereof | |
| CN117393754A (en) | Sodium-supplementing and binding additive for positive electrode material of sodium-ion battery and application method of additive | |
| CN115483375B (en) | Method for applying silicon-carbon composite material to negative electrode material of lithium ion battery | |
| CN115995535A (en) | MoSe 2 MXene composite material, preparation method and application thereof | |
| CN115084468A (en) | An intercalated-transformed composite magnesium storage cathode active material and its preparation method and application | |
| CN115966656A (en) | Electrode preparation method based on lithium-rich manganese-based positive electrode material | |
| CN108682798B (en) | Preparation method of cubic carbon-coated vanadium-based positive electrode material | |
| CN115000385B (en) | Negative electrode material, preparation method thereof, negative electrode sheet and secondary battery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |