CN114533660B - Periodontal local delivery preparation, preparation method and application thereof - Google Patents
Periodontal local delivery preparation, preparation method and application thereofDownload PDFInfo
- Publication number
- CN114533660B CN114533660BCN202210238196.2ACN202210238196ACN114533660BCN 114533660 BCN114533660 BCN 114533660BCN 202210238196 ACN202210238196 ACN 202210238196ACN 114533660 BCN114533660 BCN 114533660B
- Authority
- CN
- China
- Prior art keywords
- solution
- polymer
- periodontal
- polyvinyl alcohol
- preparation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription49
- 230000003239periodontal effectEffects0.000titleclaimsabstractdescription46
- NUJOXMJBOLGQSY-UHFFFAOYSA-Nmanganese dioxideChemical compoundO=[Mn]=ONUJOXMJBOLGQSY-UHFFFAOYSA-N0.000claimsabstractdescription94
- 239000004372Polyvinyl alcoholSubstances0.000claimsabstractdescription41
- 229920002451polyvinyl alcoholPolymers0.000claimsabstractdescription41
- 229920000642polymerPolymers0.000claimsabstractdescription39
- 239000002135nanosheetSubstances0.000claimsabstractdescription35
- WTDRDQBEARUVNC-UHFFFAOYSA-NL-DopaNatural productsOC(=O)C(N)CC1=CC=C(O)C(O)=C1WTDRDQBEARUVNC-UHFFFAOYSA-N0.000claimsabstractdescription34
- 201000001245periodontitisDiseases0.000claimsabstractdescription29
- WTDRDQBEARUVNC-LURJTMIESA-NL-DOPAChemical compoundOC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1WTDRDQBEARUVNC-LURJTMIESA-N0.000claimsabstractdescription26
- 238000006243chemical reactionMethods0.000claimsabstractdescription24
- 229910001437manganese ionInorganic materials0.000claimsabstractdescription12
- 229910052751metalInorganic materials0.000claimsabstractdescription10
- 239000002184metalSubstances0.000claimsabstractdescription10
- ISWSIDIOOBJBQZ-UHFFFAOYSA-Nphenol groupChemical groupC1(=CC=CC=C1)OISWSIDIOOBJBQZ-UHFFFAOYSA-N0.000claimsabstractdescription10
- 125000002887hydroxy groupChemical group[H]O*0.000claimsabstractdescription9
- 238000005886esterification reactionMethods0.000claimsabstractdescription4
- 239000000017hydrogelSubstances0.000claimsdescription84
- 239000000243solutionSubstances0.000claimsdescription67
- 238000000034methodMethods0.000claimsdescription28
- OAEZRJNGCVFHCM-UHFFFAOYSA-Ntetramethylazanium;pentahydrateChemical compoundO.O.O.O.O.C[N+](C)(C)COAEZRJNGCVFHCM-UHFFFAOYSA-N0.000claimsdescription22
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsdescription21
- MHAJPDPJQMAIIY-UHFFFAOYSA-NHydrogen peroxideChemical compoundOOMHAJPDPJQMAIIY-UHFFFAOYSA-N0.000claimsdescription19
- 150000002696manganeseChemical class0.000claimsdescription16
- 238000011282treatmentMethods0.000claimsdescription12
- 238000009472formulationMethods0.000claimsdescription11
- 239000000203mixtureSubstances0.000claimsdescription11
- 239000007864aqueous solutionSubstances0.000claimsdescription10
- 239000003054catalystSubstances0.000claimsdescription10
- 239000000499gelSubstances0.000claimsdescription9
- 238000003756stirringMethods0.000claimsdescription9
- 230000008569processEffects0.000claimsdescription8
- 230000035484reaction timeEffects0.000claimsdescription8
- 239000003814drugSubstances0.000claimsdescription7
- 238000001035dryingMethods0.000claimsdescription4
- 239000011261inert gasSubstances0.000claimsdescription4
- 239000007787solidSubstances0.000claimsdescription4
- 238000002156mixingMethods0.000claimsdescription3
- 230000000699topical effectEffects0.000claimsdescription3
- 238000005406washingMethods0.000claimsdescription3
- 238000004108freeze dryingMethods0.000claims1
- 238000004519manufacturing processMethods0.000claims1
- 239000002064nanoplateletSubstances0.000claims1
- 238000002390rotary evaporationMethods0.000claims1
- 239000013049sedimentSubstances0.000claims1
- 230000000694effectsEffects0.000abstractdescription14
- 229960004502levodopaDrugs0.000abstractdescription10
- MHUWZNTUIIFHAS-CLFAGFIQSA-Ndioleoyl phosphatidic acidChemical compoundCCCCCCCC\C=C/CCCCCCCC(=O)OCC(COP(O)(O)=O)OC(=O)CCCCCCC\C=C/CCCCCCCCMHUWZNTUIIFHAS-CLFAGFIQSA-N0.000abstractdescription8
- 241000237536Mytilus edulisSpecies0.000abstractdescription5
- 235000020638musselNutrition0.000abstractdescription5
- 241000894006BacteriaSpecies0.000abstractdescription4
- 239000002131composite materialSubstances0.000abstractdescription3
- 230000004044responseEffects0.000abstractdescription3
- 239000012567medical materialSubstances0.000abstractdescription2
- 239000003642reactive oxygen metaboliteSubstances0.000abstract3
- 239000011664nicotinic acidSubstances0.000abstract1
- 239000000463materialSubstances0.000description27
- 238000012360testing methodMethods0.000description21
- 230000015572biosynthetic processEffects0.000description15
- 230000000844anti-bacterial effectEffects0.000description14
- 230000008859changeEffects0.000description14
- 241001234580PalioSpecies0.000description12
- 210000004027cellAnatomy0.000description12
- 239000008367deionised waterSubstances0.000description12
- 229910021641deionized waterInorganic materials0.000description12
- 238000003786synthesis reactionMethods0.000description12
- 239000000047productSubstances0.000description11
- IAZDPXIOMUYVGZ-UHFFFAOYSA-NDimethylsulphoxideChemical compoundCS(C)=OIAZDPXIOMUYVGZ-UHFFFAOYSA-N0.000description10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description9
- 230000003078antioxidant effectEffects0.000description9
- HHEAADYXPMHMCT-UHFFFAOYSA-NdpphChemical compound[O-][N+](=O)C1=CC([N+](=O)[O-])=CC([N+]([O-])=O)=C1[N]N(C=1C=CC=CC=1)C1=CC=CC=C1HHEAADYXPMHMCT-UHFFFAOYSA-N0.000description9
- 238000010586diagramMethods0.000description8
- 239000002244precipitateSubstances0.000description8
- 239000011572manganeseSubstances0.000description7
- 230000003110anti-inflammatory effectEffects0.000description6
- 230000004043responsivenessEffects0.000description6
- 210000001519tissueAnatomy0.000description6
- 241000588724Escherichia coliSpecies0.000description5
- 229910021380Manganese ChlorideInorganic materials0.000description5
- GLFNIEUTAYBVOC-UHFFFAOYSA-LManganese chlorideChemical compoundCl[Mn]ClGLFNIEUTAYBVOC-UHFFFAOYSA-L0.000description5
- 241000700159RattusSpecies0.000description5
- 241000191967Staphylococcus aureusSpecies0.000description5
- 239000008280bloodSubstances0.000description5
- 210000004369bloodAnatomy0.000description5
- 239000013522chelantSubstances0.000description5
- 239000011565manganese chlorideSubstances0.000description5
- 235000002867manganese chlorideNutrition0.000description5
- JPXMTWWFLBLUCD-UHFFFAOYSA-Nnitro blue tetrazolium(2+)Chemical compoundCOC1=CC(C=2C=C(OC)C(=CC=2)[N+]=2N(N=C(N=2)C=2C=CC=CC=2)C=2C=CC(=CC=2)[N+]([O-])=O)=CC=C1[N+]1=NC(C=2C=CC=CC=2)=NN1C1=CC=C([N+]([O-])=O)C=C1JPXMTWWFLBLUCD-UHFFFAOYSA-N0.000description5
- 230000002000scavenging effectEffects0.000description5
- 210000000988bone and boneAnatomy0.000description4
- 238000004445quantitative analysisMethods0.000description4
- 239000000126substanceSubstances0.000description4
- WTJXVDPDEQKTCV-UHFFFAOYSA-N4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide;hydron;chlorideChemical compoundCl.C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2C1CC1C(N(C)C)C(=O)C(C(N)=O)=C(O)C1(O)C2=OWTJXVDPDEQKTCV-UHFFFAOYSA-N0.000description3
- 230000009471actionEffects0.000description3
- 238000005119centrifugationMethods0.000description3
- 230000000052comparative effectEffects0.000description3
- 239000002552dosage formSubstances0.000description3
- 150000002500ionsChemical class0.000description3
- 229960002421minocycline hydrochlorideDrugs0.000description3
- 239000002674ointmentSubstances0.000description3
- 230000003647oxidationEffects0.000description3
- 238000007254oxidation reactionMethods0.000description3
- 238000001878scanning electron micrographMethods0.000description3
- 230000001954sterilising effectEffects0.000description3
- 238000004659sterilization and disinfectionMethods0.000description3
- VIYKYVYAKVNDPS-HKGPVOKGSA-N(2s)-2-azanyl-3-[3,4-bis(oxidanyl)phenyl]propanoic acidChemical compoundOC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1.OC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1VIYKYVYAKVNDPS-HKGPVOKGSA-N0.000description2
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000description2
- OUUQCZGPVNCOIJ-UHFFFAOYSA-MSuperoxideChemical compound[O-][O]OUUQCZGPVNCOIJ-UHFFFAOYSA-M0.000description2
- 239000003242anti bacterial agentSubstances0.000description2
- 229940088710antibiotic agentDrugs0.000description2
- 239000003963antioxidant agentSubstances0.000description2
- 230000009286beneficial effectEffects0.000description2
- 230000003592biomimetic effectEffects0.000description2
- YCIMNLLNPGFGHC-UHFFFAOYSA-NcatecholChemical compoundOC1=CC=CC=C1OYCIMNLLNPGFGHC-UHFFFAOYSA-N0.000description2
- 230000004663cell proliferationEffects0.000description2
- 238000001514detection methodMethods0.000description2
- 201000010099diseaseDiseases0.000description2
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description2
- 229940079593drugDrugs0.000description2
- 230000002255enzymatic effectEffects0.000description2
- 239000007850fluorescent dyeSubstances0.000description2
- 238000011010flushing procedureMethods0.000description2
- AMWRITDGCCNYAT-UHFFFAOYSA-Lhydroxy(oxo)manganese;manganeseChemical compound[Mn].O[Mn]=O.O[Mn]=OAMWRITDGCCNYAT-UHFFFAOYSA-L0.000description2
- 230000006872improvementEffects0.000description2
- 238000001727in vivoMethods0.000description2
- 230000002757inflammatory effectEffects0.000description2
- 210000002540macrophageAnatomy0.000description2
- 230000014759maintenance of locationEffects0.000description2
- 238000013507mappingMethods0.000description2
- 239000011259mixed solutionSubstances0.000description2
- 230000004048modificationEffects0.000description2
- 238000012986modificationMethods0.000description2
- 210000000214mouthAnatomy0.000description2
- 230000001590oxidative effectEffects0.000description2
- 230000036542oxidative stressEffects0.000description2
- 102000004169proteins and genesHuman genes0.000description2
- 108090000623proteins and genesProteins0.000description2
- 239000002994raw materialSubstances0.000description2
- 210000003296salivaAnatomy0.000description2
- 230000028327secretionEffects0.000description2
- 238000001228spectrumMethods0.000description2
- 230000035882stressEffects0.000description2
- 230000002459sustained effectEffects0.000description2
- 230000009747swallowingEffects0.000description2
- 230000009885systemic effectEffects0.000description2
- 230000001225therapeutic effectEffects0.000description2
- 239000012049topical pharmaceutical compositionSubstances0.000description2
- UAIUNKRWKOVEES-UHFFFAOYSA-N3,3',5,5'-tetramethylbenzidineChemical compoundCC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1UAIUNKRWKOVEES-UHFFFAOYSA-N0.000description1
- -13,4-dihydroxyphenylalanine Amino acidChemical class0.000description1
- 208000024172Cardiovascular diseaseDiseases0.000description1
- 102400000888Cholecystokinin-8Human genes0.000description1
- 101800005151Cholecystokinin-8Proteins0.000description1
- 206010020772HypertensionDiseases0.000description1
- WAEMQWOKJMHJLA-UHFFFAOYSA-NManganese(2+)Chemical compound[Mn+2]WAEMQWOKJMHJLA-UHFFFAOYSA-N0.000description1
- 229910002651NO3Inorganic materials0.000description1
- NHNBFGGVMKEFGY-UHFFFAOYSA-NNitrateChemical compound[O-][N+]([O-])=ONHNBFGGVMKEFGY-UHFFFAOYSA-N0.000description1
- 241000605862Porphyromonas gingivalisSpecies0.000description1
- PLXBWHJQWKZRKG-UHFFFAOYSA-NResazurinChemical compoundC1=CC(=O)C=C2OC3=CC(O)=CC=C3[N+]([O-])=C21PLXBWHJQWKZRKG-UHFFFAOYSA-N0.000description1
- 208000008312Tooth LossDiseases0.000description1
- 230000003044adaptive effectEffects0.000description1
- 230000002411adverseEffects0.000description1
- 125000003277amino groupChemical group0.000description1
- 238000004458analytical methodMethods0.000description1
- 230000010072bone remodelingEffects0.000description1
- 230000007681cardiovascular toxicityEffects0.000description1
- CETPSERCERDGAM-UHFFFAOYSA-Nceric oxideChemical compoundO=[Ce]=OCETPSERCERDGAM-UHFFFAOYSA-N0.000description1
- 229910000422cerium(IV) oxideInorganic materials0.000description1
- 238000012512characterization methodMethods0.000description1
- 239000003153chemical reaction reagentSubstances0.000description1
- 208000037976chronic inflammationDiseases0.000description1
- 208000037893chronic inflammatory disorderDiseases0.000description1
- 208000001277chronic periodontitisDiseases0.000description1
- 238000003501co-cultureMethods0.000description1
- 230000001332colony forming effectEffects0.000description1
- 238000002591computed tomographyMethods0.000description1
- 238000010276constructionMethods0.000description1
- 238000004132cross linkingMethods0.000description1
- 231100000135cytotoxicityToxicity0.000description1
- 230000003013cytotoxicityEffects0.000description1
- 238000001804debridementMethods0.000description1
- 230000007547defectEffects0.000description1
- 210000003298dental enamelAnatomy0.000description1
- 206010012601diabetes mellitusDiseases0.000description1
- 238000001704evaporationMethods0.000description1
- 125000000524functional groupChemical group0.000description1
- 239000003292glueSubstances0.000description1
- 230000036541healthEffects0.000description1
- 210000002216heartAnatomy0.000description1
- 238000000338in vitroMethods0.000description1
- 238000010348incorporationMethods0.000description1
- 230000007794irritationEffects0.000description1
- 210000003734kidneyAnatomy0.000description1
- 210000004185liverAnatomy0.000description1
- 229910021645metal ionInorganic materials0.000description1
- 238000001000micrographMethods0.000description1
- 239000002105nanoparticleSubstances0.000description1
- 229930014626natural productNatural products0.000description1
- 229910052757nitrogenInorganic materials0.000description1
- 210000000056organAnatomy0.000description1
- 239000003960organic solventSubstances0.000description1
- 230000002028prematureEffects0.000description1
- 230000035755proliferationEffects0.000description1
- 230000001681protective effectEffects0.000description1
- 238000003908quality control methodMethods0.000description1
- 206010039073rheumatoid arthritisDiseases0.000description1
- 238000007493shaping processMethods0.000description1
- IZTQOLKUZKXIRV-YRVFCXMDSA-NsincalideChemical compoundC([C@@H](C(=O)N[C@@H](CCSC)C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](N)CC(O)=O)C1=CC=C(OS(O)(=O)=O)C=C1IZTQOLKUZKXIRV-YRVFCXMDSA-N0.000description1
- 239000002904solventSubstances0.000description1
- 238000010186stainingMethods0.000description1
- 238000010025steamingMethods0.000description1
- 230000002195synergetic effectEffects0.000description1
- 238000001308synthesis methodMethods0.000description1
- 238000010998test methodMethods0.000description1
- 231100000331toxicToxicity0.000description1
- 230000002588toxic effectEffects0.000description1
- 230000001988toxicityEffects0.000description1
- 231100000419toxicityToxicity0.000description1
- 238000012546transferMethods0.000description1
- 230000001052transient effectEffects0.000description1
- 238000011269treatment regimenMethods0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0063—Periodont
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
- A61K33/32—Manganese; Compounds thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/32—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. carbomers, poly(meth)acrylates, or polyvinyl pyrrolidone
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/52—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an inorganic compound, e.g. an inorganic ion that is complexed with the active ingredient
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/02—Stomatological preparations, e.g. drugs for caries, aphtae, periodontitis
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/02—Local antiseptics
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
- C08F8/30—Introducing nitrogen atoms or nitrogen-containing groups
- C08F8/32—Introducing nitrogen atoms or nitrogen-containing groups by reaction with amines
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Inorganic Chemistry (AREA)
- Oncology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Communicable Diseases (AREA)
- Nutrition Science (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Physiology (AREA)
- Polymers & Plastics (AREA)
- Medicinal Preparation (AREA)
- Cosmetics (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及医用材料技术领域,具体而言,涉及一种牙周局部递送制剂、其制备方法及应用。The invention relates to the technical field of medical materials, in particular to a periodontal local delivery preparation, its preparation method and application.
背景技术Background technique
牙周炎是一种由细菌引起的慢性炎症性疾病,是世界第六大流行性疾病,患病率高达50%。牙周炎不仅是成人失牙的主要原因,且还可能增加心血管疾病、糖尿病、类风湿性关节炎、高血压等许多严重系统性疾病的风险,严重威胁人类健康。然而,超过30%的重度慢性牙周炎患者对治疗反应不佳,甚至发展为难治性牙周炎,使本就复杂的牙周治疗变得更为棘手。Periodontitis, a chronic inflammatory disease caused by bacteria, is the sixth most prevalent disease in the world, with a prevalence rate as high as 50%. Periodontitis is not only the main cause of tooth loss in adults, but also may increase the risk of many serious systemic diseases such as cardiovascular disease, diabetes, rheumatoid arthritis, hypertension, etc., seriously threatening human health. However, more than 30% of patients with severe chronic periodontitis do not respond well to treatment, and even develop refractory periodontitis, which makes the already complicated periodontal treatment more difficult.
牙周炎目前的主要治疗方案为机械去除局部刺激或辅以抗生素。然而,机械清创后菌斑生物膜的快速重新定植仍会导致易感患者牙周炎的复发,而抗生素的大量使用则易引起多种全身不良反应。现有常见市售牙周炎局部治疗剂型盐酸米诺环素软膏虽然具备一定的抗菌和抗炎作用,但在口腔独特的环境下,唾液的持续分泌、冲洗和吞咽等活动极易导致局部递送的牙周治疗剂型被过早消耗,使炎症部位难以达到持续有效的治疗浓度。若使用浓度过高的剂型虽可达到一过性的治疗效果,但容易导致潜在的毒副作用。The current main treatment options for periodontitis are mechanical removal of local irritation or supplemented with antibiotics. However, the rapid recolonization of plaque biofilm after mechanical debridement still leads to recurrence of periodontitis in susceptible patients, and the heavy use of antibiotics is prone to cause multiple systemic adverse effects. Minocycline hydrochloride ointment is a common commercially available topical formulation of periodontitis Although it has certain antibacterial and anti-inflammatory effects, in the unique environment of the oral cavity, activities such as continuous secretion of saliva, flushing and swallowing can easily lead to premature consumption of locally delivered periodontal treatment dosage forms, making it difficult to achieve sustained effectiveness at the inflammatory site therapeutic concentration. If the dosage form with too high concentration can achieve a transient therapeutic effect, it will easily lead to potential toxic and side effects.
因此,亟需探寻更加新型有效的治疗策略,使局部递送的剂型能够长期稳固粘附于牙周组织,同时能够高效、持续地清除牙周组织产生的过量自由基并进行持续有效的杀菌,进而纠正牙周炎性微环境,改善牙周炎的结局。Therefore, it is urgent to explore more new and effective treatment strategies, so that the locally delivered dosage form can adhere to the periodontal tissue for a long time, and at the same time, it can efficiently and continuously remove the excess free radicals produced by the periodontal tissue and carry out continuous and effective sterilization. Correcting the periodontal inflammatory microenvironment improves periodontitis outcomes.
发明内容Contents of the invention
本发明的目的在于提供一种牙周局部递送制剂及其制备方法,旨在有效清除牙周炎导致的过量ROS,并进行持续有效的杀菌,改善牙周炎的进展,阻遏牙周炎导致的牙周支持组织丧失等问题。The purpose of the present invention is to provide a periodontal local delivery preparation and its preparation method, which aims to effectively remove the excessive ROS caused by periodontitis, and carry out continuous and effective sterilization, improve the progress of periodontitis, and prevent the Periodontal support tissue loss and other issues.
本发明的另一目的在于提供上述牙周局部递送制剂在制备用于治疗牙周炎的药剂中的应用。Another object of the present invention is to provide the application of the above-mentioned periodontal local delivery formulation in the preparation of a medicament for treating periodontitis.
本发明是这样实现的:The present invention is achieved like this:
第一方面,本发明提供一种牙周局部递送制剂的制备方法,包括:利用聚乙烯醇和3,4-二羟基苯丙氨酸进行酯化反应得到PD聚合物,再利用PD聚合物与二氧化锰纳米片反应,利用PD聚合物上的酚羟基螯合金属锰离子。In the first aspect, the present invention provides a method for preparing a periodontal local delivery preparation, comprising: using polyvinyl alcohol and 3,4-dihydroxyphenylalanine to carry out esterification reaction to obtain PD polymer, and then using PD polymer and dihydroxyphenylalanine The reaction of manganese oxide nanosheets utilizes the phenolic hydroxyl groups on the PD polymer to chelate metal manganese ions.
在可选的实施方式中,聚乙烯醇和3,4-二羟基苯丙氨酸的摩尔比为4-8:1;优选为5-7:1;In an optional embodiment, the molar ratio of polyvinyl alcohol and 3,4-dihydroxyphenylalanine is 4-8:1; preferably 5-7:1;
优选地,所述聚乙烯醇的分子量为85-124kDa。Preferably, the molecular weight of the polyvinyl alcohol is 85-124kDa.
在可选的实施方式中,PD聚合物的制备过程包括:将聚乙烯醇溶解后在催化剂存在的条件下与3,4-二羟基苯丙氨酸反应,控制反应温度为75-85℃,反应时间为12-15h;In an optional embodiment, the preparation process of the PD polymer includes: dissolving polyvinyl alcohol and reacting with 3,4-dihydroxyphenylalanine in the presence of a catalyst, controlling the reaction temperature to 75-85°C, The reaction time is 12-15h;
优选地,在反应过程中通入惰性气体。Preferably, an inert gas is introduced during the reaction.
在可选的实施方式中,催化剂为NaHSO4·H2O;In an alternative embodiment, the catalyst is NaHSO4 ·H2 O;
优选地,催化剂与聚乙烯醇的质量比为2-4:1。Preferably, the mass ratio of catalyst to polyvinyl alcohol is 2-4:1.
在可选的实施方式中,在聚乙烯醇和3,4-二羟基苯丙氨酸反应完成之后,将产品制备成凝胶状态;In an optional embodiment, after the reaction between polyvinyl alcohol and 3,4-dihydroxyphenylalanine is completed, the product is prepared into a gel state;
优选地,在聚乙烯醇和3,4-二羟基苯丙氨酸反应后得到的溶液进行透析2-4天,之后进行旋蒸、冻干以得到凝胶状态的产品。Preferably, the solution obtained after the reaction of polyvinyl alcohol and 3,4-dihydroxyphenylalanine is dialyzed for 2-4 days, and then rotary evaporated and freeze-dried to obtain a gel state product.
在可选的实施方式中,利用PD聚合物上的酚羟基螯合金属锰离子的过程包括:将PD聚合物的形成溶液与二氧化锰溶液反应,反应时间为0.5-1h,使二者充分交联形成PDMO水凝胶。In an optional embodiment, the process of using the phenolic hydroxyl group on the PD polymer to chelate metal manganese ions includes: reacting the formation solution of the PD polymer with the manganese dioxide solution, and the reaction time is 0.5-1h, so that the two are fully Cross-linking forms PDMO hydrogels.
在可选的实施方式中,控制PD聚合物与二氧化锰的质量比为100:2-6;In an optional embodiment, the mass ratio of controlling PD polymer to manganese dioxide is 100:2-6;
优选地,PD聚合物的形成溶液的浓度为150-250mg/mL。Preferably, the concentration of the forming solution of PD polymer is 150-250 mg/mL.
在可选的实施方式中,二氧化锰溶液的制备过程包括:将锰盐的水溶液与四甲基五水合铵溶液混合反应10-15h,反应完后进行离心,将得到的固态中间品进行洗涤、干燥后溶于水中,再进行离心以去除沉淀;其中,四甲基五水合铵溶液是由四甲基五水合铵溶于双氧水而得;In an optional embodiment, the preparation process of the manganese dioxide solution includes: mixing the aqueous solution of the manganese salt with the tetramethylammonium pentahydrate solution for 10-15h, centrifuging after the reaction, and washing the obtained solid intermediate , Dissolve in water after drying, and then centrifuge to remove the precipitate; wherein, the tetramethylammonium pentahydrate solution is obtained by dissolving tetramethylammonium pentahydrate in hydrogen peroxide;
优选地,将四甲基五水合铵溶液加入至锰盐的水溶液中,在1200-1500rpm的转速下搅拌0.5-3min后,继续以600-800rpm搅拌10-14h;Preferably, the tetramethylammonium pentahydrate solution is added to the aqueous solution of the manganese salt, stirred at a speed of 1200-1500rpm for 0.5-3min, and then stirred at 600-800rpm for 10-14h;
优选地,锰盐的水溶液的制备过程包括:将0.755-0.805g锰盐溶解于20-30mL水;更优选地,锰盐为MnCl2;Preferably, the preparation process of the aqueous solution of manganese salts comprises: dissolving 0.755-0.805 g of manganese salts in 20-30 mL of water; more preferably, the manganese salts are MnCl2 ;
优选地,四甲基五水合铵溶液的制备过程包括:将4.3-4.5g四甲基五水合铵溶解于40-50mL、质量分数为2-4%的双氧水中。Preferably, the preparation process of the tetramethylammonium pentahydrate solution includes: dissolving 4.3-4.5 g of tetramethylammonium pentahydrate in 40-50 mL of hydrogen peroxide with a mass fraction of 2-4%.
第二方面,本发明提供一种牙周局部递送制剂,通过前述实施方式中任一项的制备方法制备而得;In the second aspect, the present invention provides a periodontal local delivery preparation prepared by the preparation method in any one of the foregoing embodiments;
优选地,牙周局部递送制剂为水凝胶的形态。Preferably, the periodontal topical delivery formulation is in the form of a hydrogel.
第三方面,本发明提供前述实施方式中的牙周局部递送制剂在制备用于治疗牙周炎的药剂中的应用。In a third aspect, the present invention provides the application of the periodontal local delivery formulation in the foregoing embodiments in the preparation of a medicament for treating periodontitis.
本发明具有以下有益效果:通过以聚乙烯醇(PVA)为载体,在聚乙烯醇上接枝贻贝粘附蛋白3,4-二羟基苯丙氨酸(DOPA),并利用DOPA上的酚羟基螯合金属锰离子,利用DOPA优良的湿粘接性能与二氧化锰纳米片的拟酶活性,构建一种ROS集联响应性和NIR光热响应性的MnO2纳米片贻贝仿生复合材料作为牙周局部递送制剂,能够有效清除牙周炎导致的过量ROS,并进行持续有效的杀菌,改善牙周炎的进展,遏制牙周炎导致的牙周支持组织丧失的问题。The present invention has the following beneficial effects: by using polyvinyl alcohol (PVA) as a carrier, graft
附图说明Description of drawings
为了更清楚地说明本发明实施例的技术方案,下面将对实施例中所需要使用的附图作简单地介绍,应当理解,以下附图仅示出了本发明的某些实施例,因此不应被看作是对范围的限定,对于本领域普通技术人员来讲,在不付出创造性劳动的前提下,还可以根据这些附图获得其他相关的附图。In order to illustrate the technical solutions of the embodiments of the present invention more clearly, the accompanying drawings used in the embodiments will be briefly introduced below. It should be understood that the following drawings only show some embodiments of the present invention, and thus It should be regarded as a limitation on the scope, and those skilled in the art can also obtain other related drawings based on these drawings without creative work.
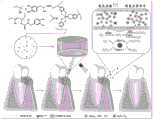
图1为PDMO水凝胶的构建及清除ROS治疗牙周炎的示意图;Figure 1 is a schematic diagram of the construction of PDMO hydrogel and the removal of ROS for the treatment of periodontitis;
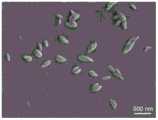
图2为MnO2纳米片的扫描电镜图;Fig. 2 is the scanning electron micrograph of MnONanosheet ;
图3为PD和PDMO水凝胶的扫描电镜图;Figure 3 is a scanning electron micrograph of PD and PDMO hydrogel;
图4为PDMO1水凝胶的Mapping图;Figure 4 is the Mapping diagram of PDMO1 hydrogel;
图5为PD、PDMO1、PDMO2和PDMO3的EDS能谱图和不同元素的重量百分比;Figure 5 is the EDS energy spectrum of PD, PDMO1, PDMO2 and PDMO3 and the weight percentage of different elements;
图6为PDMO水凝胶的XPS图谱;Fig. 6 is the XPS pattern of PDMO hydrogel;
图7为利用DPPH测试PDMO水凝胶清除ROS能力的结果图;Fig. 7 is the result figure that utilizes DPPH test PDMO hydrogel to scavenge ROS ability;
图8为利用TMB测试PDMO水凝胶清除羟基自由基能力的结果图;Figure 8 is a result diagram of testing the ability of PDMO hydrogel to scavenge hydroxyl radicals by using TMB;
图9为利用NBT测试PDMO水凝胶清除超氧阴离子能力的结果图;Fig. 9 is the result figure of using NBT to test the ability of PDMO hydrogel to scavenge superoxide anion;
图10为利用DCFH-DA检测100ng/mL LPS刺激巨噬细胞并加入不同组别水凝胶作用2h后的细胞荧光强度;Figure 10 is the fluorescence intensity of macrophages stimulated by 100ng/mL LPS detected by DCFH-DA and added to different groups of hydrogels for 2 hours;
图11为利用Ru(dpp)3Cl2检测100ng/mL LPS刺激巨噬细胞并加入不同组别水凝胶作用2h后的细胞荧光强度;Figure 11 is the fluorescence intensity of macrophages stimulated by 100ng/mL LPS detected by Ru(dpp)3 Cl2 and added to different groups of hydrogels for 2 hours;
图12为不同组别水凝胶在10min内热图变化;Figure 12 is the heat map change of different groups of hydrogels within 10 minutes;
图13为不同组别水凝胶在10min内温度变化图;Figure 13 is a diagram of the temperature change of different groups of hydrogels within 10 minutes;
图14为不同组别水凝胶在50分钟内的光热稳定性;Figure 14 shows the photothermal stability of different groups of hydrogels within 50 minutes;
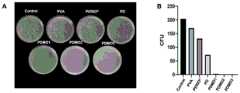
图15为SA与不同组别水凝胶共培养8h后的菌落图和CFU定量计数结果;Figure 15 is the colony diagram and CFU quantitative counting results after SA and different groups of hydrogels were co-cultured for 8 hours;
图16为EC与不同组别水凝胶共培养8h后的菌落图和CFU定量计数结果;Figure 16 is the colony diagram and CFU quantitative counting results after EC and different groups of hydrogels were co-cultured for 8 hours;
图17为不同组别水凝胶与P.g共培养24h后的阿尔玛蓝染色定量分析结果;Figure 17 is the quantitative analysis results of Almar blue staining after different groups of hydrogels were co-cultured with P.g for 24 hours;
图18为Raw264.7细胞与不同组别水凝胶共培养1天和3天后细胞增殖情况;Figure 18 shows the cell proliferation of Raw264.7 cells co-cultured with different groups of hydrogels for 1 day and 3 days;
图19为SD大鼠牙周炎模型建立过程的示意图;Figure 19 is a schematic diagram of the SD rat periodontitis model establishment process;
图20为将不同组别水凝胶应用于SD大鼠四周后的血常规结果;Figure 20 is the blood routine results after applying different groups of hydrogels to SD rats for four weeks;
图21为将不同组别水凝胶应用于SD大鼠四周后的血生化结果;Figure 21 is the blood biochemical results after applying different groups of hydrogels to SD rats for four weeks;
图22为将不同组别水凝胶用于治疗SD大鼠牙周炎后的牙槽骨CT三维重建结果;Figure 22 is the three-dimensional reconstruction results of alveolar bone CT after different groups of hydrogels are used to treat periodontitis in SD rats;
图23为PDMO水凝胶与PD-CeO2水凝胶分别与DPPH作用30min后的颜色变化和DPPH清除率对比结果。Figure 23 shows the comparison results of the color change and DPPH clearance rate of PDMO hydrogel and PD-CeO2 hydrogel respectively after they reacted with DPPH for 30 minutes.
具体实施方式Detailed ways
为使本发明实施例的目的、技术方案和优点更加清楚,下面将对本发明实施例中的技术方案进行清楚、完整地描述。实施例中未注明具体条件者,按照常规条件或制造商建议的条件进行。所用试剂或仪器未注明生产厂商者,均为可以通过市售购买获得的常规产品。In order to make the purpose, technical solutions and advantages of the embodiments of the present invention clearer, the technical solutions in the embodiments of the present invention will be clearly and completely described below. Those who do not indicate the specific conditions in the examples are carried out according to the conventional conditions or the conditions suggested by the manufacturer. The reagents or instruments used were not indicated by the manufacturer, and they were all conventional products that could be purchased from the market.
本发明实施例提供一种牙周局部递送制剂的制备方法,请参照图1,包括以下步骤:The embodiment of the present invention provides a preparation method for periodontal local delivery preparation, please refer to Figure 1, including the following steps:
S1、PD聚合物的合成S1. Synthesis of PD polymer
利用聚乙烯醇和3,4-二羟基苯丙氨酸进行酯化反应,将3,4-二羟基苯丙氨酸接枝在聚乙烯醇长链上得到PD聚合物。Polyvinyl alcohol and 3,4-dihydroxyphenylalanine are used for esterification reaction, and 3,4-dihydroxyphenylalanine is grafted on the long chain of polyvinyl alcohol to obtain PD polymer.
具体地,3,4-二羟基苯丙氨酸(DOPA)中丰富的还原官能基团(儿茶酚基和氨基)使其在清除ROS方面也表现出优异的性能,与金属离子发生螯合反应后还可表现出协同的抗氧化活性和光热响应性,且作为天然化合物,具备优异的生物安全性,在生物学领域拥有可观的应用前景。Specifically, the abundant reducing functional groups (catechol and amino groups) in 3,4-dihydroxyphenylalanine (DOPA) make it also exhibit excellent performance in scavenging ROS, chelating with metal ions After the reaction, it can also show synergistic antioxidant activity and photothermal responsiveness, and as a natural compound, it has excellent biological safety and has considerable application prospects in the field of biology.
在一些实施例中,聚乙烯醇和3,4-二羟基苯丙氨酸的摩尔比为4-8:1;优选为5-7:1,通过控制聚乙烯醇和3,4-二羟基苯丙氨酸的摩尔比,以进一步提升产品的持续抗菌能力,能够有效清除牙周炎导致的过量ROS。具体地,聚乙烯醇和3,4-二羟基苯丙氨酸的摩尔比可以为4:1、5:1、6:1、7:1、8:1等,也可以为以上相邻比例值之间的任意值。In some embodiments, the molar ratio of polyvinyl alcohol and 3,4-dihydroxyphenylalanine is 4-8:1; preferably 5-7:1, by controlling the ratio of polyvinyl alcohol and 3,4-dihydroxyphenylalanine Amino acid molar ratio to further enhance the product's continuous antibacterial ability, which can effectively remove excess ROS caused by periodontitis. Specifically, the molar ratio of polyvinyl alcohol and 3,4-dihydroxyphenylalanine can be 4:1, 5:1, 6:1, 7:1, 8:1, etc., or it can be the above adjacent ratio values any value in between.
在一些实施例中,聚乙烯醇的分子量为85-124kDa,聚乙烯醇的分子量控制在上述范围内为宜,分子量过大过小均不利于产品持续抗菌能力的提升。In some embodiments, the molecular weight of the polyvinyl alcohol is 85-124kDa, and it is advisable to control the molecular weight of the polyvinyl alcohol within the above-mentioned range. Too large or too small a molecular weight is not conducive to the improvement of the product's continuous antibacterial ability.
进一步地,PD聚合物的制备过程包括:将聚乙烯醇溶解后在催化剂存在的条件下与3,4-二羟基苯丙氨酸反应,控制反应温度为75-85℃,反应时间为12-15h。通过进一步控制反应温度和时间以使聚乙烯醇与3,4-二羟基苯丙氨酸充分反应,提升3,4-二羟基苯丙氨酸的接枝率。具体地,反应温度可以为75℃、76℃、77℃、78℃、79℃、80℃、81℃、82℃、83℃、84℃、85℃等,也可以为以上相邻温度值之间的任意值;反应时间可以为12h、13h、14h、15h等,也可以为以上相邻时间值之间的任意值。Further, the preparation process of the PD polymer includes: dissolving polyvinyl alcohol and reacting with 3,4-dihydroxyphenylalanine in the presence of a catalyst, controlling the reaction temperature to be 75-85°C, and the reaction time to be 12- 15h. By further controlling the reaction temperature and time to fully react polyvinyl alcohol with 3,4-dihydroxyphenylalanine, the grafting rate of 3,4-dihydroxyphenylalanine is improved. Specifically, the reaction temperature can be 75°C, 76°C, 77°C, 78°C, 79°C, 80°C, 81°C, 82°C, 83°C, 84°C, 85°C, etc., or it can be between the above adjacent temperature values Any value between; the reaction time can be 12h, 13h, 14h, 15h, etc., or any value between the above adjacent time values.
在一些实施例中,在反应过程中通入惰性气体,惰性气体可以为氮气等,可以在反应过程中起到保护作用,避免氧化。In some embodiments, an inert gas is introduced during the reaction, and the inert gas can be nitrogen, etc., which can play a protective role during the reaction and avoid oxidation.
具体地,用于溶解聚乙烯醇的溶剂可以为二甲基亚砜(DMSO),也可以为其他有机溶剂,在此不做限定。Specifically, the solvent used to dissolve the polyvinyl alcohol may be dimethyl sulfoxide (DMSO), or other organic solvents, which are not limited herein.
在一些实施例中,催化剂为NaHSO4·H2O;催化剂与聚乙烯醇的质量比为2-4:1。具体地,催化剂与聚乙烯醇的质量比可以为2:1、3:1、4:1等。In some embodiments, the catalyst is NaHSO4 ·H2 O; the mass ratio of catalyst to polyvinyl alcohol is 2-4:1. Specifically, the mass ratio of catalyst to polyvinyl alcohol may be 2:1, 3:1, 4:1, etc.
在一些实施例中,在聚乙烯醇和3,4-二羟基苯丙氨酸反应完成之后,将产品制备成凝胶状态,以去除未反应的原料,同时有利于制备凝胶状态的产品。In some embodiments, after the reaction between polyvinyl alcohol and 3,4-dihydroxyphenylalanine is completed, the product is prepared into a gel state, so as to remove unreacted raw materials and facilitate the preparation of a gel state product.
凝胶的制备工艺不做限定,可以采用现有技术中的常见方法。在一些实施例中,在聚乙烯醇和3,4-二羟基苯丙氨酸反应后得到的溶液进行透析2-4天,之后进行旋蒸、冻干以得到凝胶状态的产品。The preparation process of the gel is not limited, and common methods in the prior art can be used. In some embodiments, the solution obtained after the reaction between polyvinyl alcohol and 3,4-dihydroxyphenylalanine is dialyzed for 2-4 days, and then rotary evaporated and freeze-dried to obtain a gel state product.
S2、MnO2纳米片的合成S2, Synthesis ofMnO2 nanosheets
MnO2纳米片可以采用市购材料,也可以为采用本发明实施例中所提供的合成方法进行制备,使MnO2纳米片以溶液的形式存在为宜,有利于后续和酚羟基的反应。MnO2 nanosheets can be commercially available materials, or can be prepared by using the synthesis method provided in the examples of the present invention. It is advisable to make MnO2 nanosheets exist in the form of a solution, which is beneficial to the subsequent reaction with phenolic hydroxyl groups.
在一些实施例中,二氧化锰溶液的制备过程包括:将锰盐的水溶液与四甲基五水合铵溶液混合反应10-15h,反应完后进行离心,将得到的固态中间品进行洗涤、干燥后溶于水中,再进行离心以去除沉淀得到MnO2溶液;其中,四甲基五水合铵溶液是由四甲基五水合铵溶于双氧水而得。In some embodiments, the preparation process of the manganese dioxide solution includes: mixing and reacting the aqueous solution of the manganese salt and the tetramethylammonium pentahydrate solution for 10-15 hours, centrifuging after the reaction, washing and drying the obtained solid intermediate Dissolve in water, then centrifuge to remove precipitates to obtain MnO2 solution; wherein, tetramethyl ammonium pentahydrate solution is obtained by dissolving tetramethyl ammonium pentahydrate in hydrogen peroxide.
需要说明的是,利用四甲基五水合铵溶液和双氧水的双重氧化作用,将锰离子氧化为二氧化锰。It should be noted that manganese ions are oxidized to manganese dioxide by double oxidation of tetramethylammonium pentahydrate solution and hydrogen peroxide.
在一些实施例中,反应过程是将四甲基五水合铵溶液加入至锰盐的水溶液中,在1200-1500rpm的转速下搅拌0.5-3min后,继续以600-800rpm搅拌10-14h。In some embodiments, the reaction process is to add tetramethylammonium pentahydrate solution to the aqueous solution of manganese salt, stir at 1200-1500rpm for 0.5-3min, and then continue to stir at 600-800rpm for 10-14h.
在一些实施例中,锰盐的水溶液的制备过程包括:将0.755-0.805g锰盐溶解于20-30mL水;优选地,锰盐为MnCl2。在其他实施例中,锰盐也可以为硝酸盐。In some embodiments, the preparation process of the aqueous solution of the manganese salt includes: dissolving 0.755-0.805 g of the manganese salt in 20-30 mL of water; preferably, the manganese salt is MnCl2 . In other embodiments, the manganese salt may also be a nitrate.
在一些实施例中,四甲基五水合铵溶液的制备过程包括:将4.3-4.5g四甲基五水合铵溶解于40-50mL、质量分数为2-4%的双氧水中。In some embodiments, the preparation process of the tetramethylammonium pentahydrate solution includes: dissolving 4.3-4.5 g of tetramethylammonium pentahydrate in 40-50 mL of hydrogen peroxide with a mass fraction of 2-4%.
S3、PDMO水凝胶的合成S3, Synthesis of PDMO hydrogel
再利用PD聚合物与二氧化锰纳米片反应,利用PD聚合物上的酚羟基螯合金属锰离子,二氧化锰纳米片具有拟酶活性,赋予材料良好的ROS清除活性。Then, the PD polymer is used to react with manganese dioxide nanosheets, and the phenolic hydroxyl group on the PD polymer is used to chelate metal manganese ions. The manganese dioxide nanosheets have enzyme-mimicking activity, which endows the material with good ROS scavenging activity.
需要补充的是,二氧化锰纳米片具备良好的光热效应,但是二氧化锰纳米片分布不均匀,且难以在作用部位长期停留发挥作用,这大大限制了其应用。本申请中由于水凝胶可以保护其内封装的物质,延长封装物质保留时间并在作用部位持续释放。因此,将二氧化锰纳米片整合入适当的可注射水凝胶可实现更好的抗氧化活性和抗菌效果。What needs to be added is that manganese dioxide nanosheets have good photothermal effect, but the distribution of manganese dioxide nanosheets is uneven, and it is difficult to stay in the action site for a long time, which greatly limits its application. In this application, because the hydrogel can protect the substances encapsulated in it, the retention time of the encapsulated substances can be extended and the release can be sustained at the action site. Therefore, the incorporation of MnO2 nanosheets into appropriate injectable hydrogels can achieve better antioxidant activity and antibacterial effect.
在一些实施例中,利用PD聚合物上的酚羟基螯合金属锰离子的过程包括:将PD聚合物的形成溶液与二氧化锰溶液反应,反应时间为0.5-1h使PD聚合物与锰离子交联成胶。In some embodiments, the process of using the phenolic hydroxyl group on the PD polymer to chelate metal manganese ions includes: reacting the formation solution of the PD polymer with the manganese dioxide solution, and the reaction time is 0.5-1h to make the PD polymer and the manganese ion Cross-linked into gel.
在实际操作过程中,将冻干的凝胶状态的PD聚合物在去离子水中加热溶解,使PD聚合物的形成溶液的浓度为150-250mg/mL(如150mg/mL、160mg/mL、170mg/mL、180mg/mL、190mg/mL、200mg/mL、210mg/mL、220mg/mL、230mg/mL、240mg/mL、250mg/mL)的水溶液;控制PD聚合物与二氧化锰的质量比为100:2-6(如100:2、100:3、100:4、100:5、100:6),二氧化锰的质量控制需要测试MnO2溶液中二氧化锰的含量,进而计算所需MnO2溶液的体积。具体地,MnO2溶液中二氧化锰含量的测定是将MnO2溶液蒸干后进行称量。In the actual operation process, the PD polymer in the lyophilized gel state is heated and dissolved in deionized water, so that the concentration of the forming solution of the PD polymer is 150-250mg/mL (such as 150mg/mL, 160mg/mL, 170mg /mL, 180mg/mL, 190mg/mL, 200mg/mL, 210mg/mL, 220mg/mL, 230mg/mL, 240mg/mL, 250mg/mL) aqueous solution; control the mass ratio of PD polymer and manganese dioxide to be 100:2-6 (such as 100:2, 100:3, 100:4, 100:5, 100:6), the quality control of manganese dioxide needs to test the content of manganese dioxide inMnO2 solution, and then calculate the required Volume ofMnO2 solution. Specifically, the determination of the manganese dioxide content in theMnO2 solution is to weigh theMnO2 solution after evaporating it to dryness.
本发明实施例还提供一种牙周局部递送制剂,通过上述制备方法制备而得,其在PD聚合物上螯合有金属锰离子。The embodiment of the present invention also provides a periodontal local delivery preparation prepared by the above preparation method, which has metal manganese ions chelated on the PD polymer.
需要说明的是,本发明实施例中所提供的牙周局部递送制剂相比现有现有常见市售牙周炎局部治疗剂型,如盐酸米诺环素软膏本研究所构建的牙周局部递送制剂,在口腔独特的环境下,唾液的持续分泌、冲洗和吞咽等活动下仍能够长期牢固粘附于牙周组织,继而发挥抗ROS和抗炎、抗菌等多重作用,且生物安全性良好,临床应用前景非常好。It should be noted that the periodontal local delivery formulation provided in the embodiments of the present invention is compared with the existing common commercially available local treatment formulations for periodontitis, such as minocycline hydrochloride ointment The periodontal local delivery preparation constructed in this study can still firmly adhere to the periodontal tissue for a long time under the unique environment of the oral cavity, such as continuous secretion of saliva, flushing and swallowing, and then exert anti-ROS, anti-inflammatory, antibacterial, etc. It has multiple functions, good biological safety, and very good prospects for clinical application.
在一些实施例中,牙周局部递送制剂为水凝胶的形态,水凝胶可以保护其内封装的物质,延长封装物质保留时间并在作用部位持续释放。In some embodiments, the periodontal local delivery preparation is in the form of a hydrogel, which can protect the substance encapsulated in it, prolong the retention time of the encapsulated substance and release it continuously at the site of action.
本发明实施例还提供了上述牙周局部递送制剂在制备用于治疗牙周炎的药剂中的应用,可以复配其他原料形成药剂,在此不做限定。The embodiment of the present invention also provides the application of the above-mentioned periodontal local delivery preparation in the preparation of a medicament for treating periodontitis, which can be compounded with other raw materials to form a medicament, which is not limited here.
以下结合实施例对本发明的特征和性能作进一步的详细描述。The characteristics and performance of the present invention will be described in further detail below in conjunction with the examples.
实施例1Example 1
本实施例提供一种牙周局部递送制剂的制备方法,包括以下步骤:This embodiment provides a preparation method for periodontal local delivery preparation, comprising the following steps:
(1)PD聚合物的合成:将50mmol、分子量为85-124kDa的PVA在100℃条件下溶于110mL的DMSO溶液中,完全溶解后,加入12g的NaHSO4·H2O催化,待温度降至80℃时,加入3,4-二羟基苯丙氨酸(DOPA)8mmol,在N2的保护下反应13h,反应结束后,待温度降至室温后,在透析袋中透析3天,旋蒸仪去除多余水分后,冻干机中冻干3天。(1) Synthesis of PD polymer: Dissolve 50mmol of PVA with a molecular weight of 85-124kDa in 110mL of DMSO solution at 100°C. At 80°C, add 8 mmol of 3,4-dihydroxyphenylalanine (DOPA), and react for 13 hours under the protection of N2 . After removing excess water in a steamer, freeze-dry in a freeze dryer for 3 days.
(2)MnO2纳米片的合成:将0.755g MnCl2溶解于20mL去离子水中,将4.3g四甲基五水合铵(TMAOH)溶解于40mL 3%的H2O2,将TMAOH溶液加入MnCl2溶液中,在1300rpm的转速下快速搅拌1min后,继续以700rpm搅拌12h,离心后用乙醇和去离子水分别洗三次。将黑色沉淀物材料冻干,再将冻干物溶解在去离子水中,浓度为10mg/mL,超声10h,最后将溶液以10000g离心10min,去除沉淀,得到MnO2溶液,取出1mL的MnO2溶液充分干燥后进行称重定量,得到MnO2的浓度为16mg/ml。(2) Synthesis ofMnO2 nanosheets: Dissolve 0.755gMnCl2 in 20mL deionized water, dissolve 4.3g tetramethylammonium pentahydrate (TMAOH) in
(3)PDMO水凝胶的合成:将冻干的PD聚合物在去离子水中加热溶解,使其浓度为200mg/mL,在0.25mL的PD溶液中分别加入MnO2溶液,控制MnO2纳米片的加入量为1mg(PD聚合物与MnO2纳米片的质量比为100:2),将二者在常温下充分搅拌反应1h,得到PDMO水凝胶记为PDMO1。(3) Synthesis of PDMO hydrogel: heat and dissolve the lyophilized PD polymer in deionized water to make the
实施例2Example 2
与实施例1的区别仅在于:控制MnO2纳米片的加入量为2mg,PD聚合物与MnO2纳米片的质量比为100:4,得到的水凝胶记为PDMO2。The only difference from Example 1 is that the amount ofMnO2 nanosheets is controlled to be 2 mg, the mass ratio of PD polymer toMnO2 nanosheets is 100:4, and the obtained hydrogel is denoted as PDMO2.
实施例3Example 3
与实施例1的区别仅在于:控制MnO2纳米片的加入量为3mg,PD聚合物与MnO2纳米片的质量比为100:6,得到的水凝胶记为PDMO3。The only difference from Example 1 is that the amount ofMnO2 nanosheets is controlled to be 3 mg, the mass ratio of PD polymer toMnO2 nanosheets is 100:6, and the obtained hydrogel is denoted as PDMO3.
实施例4Example 4
本实施例提供一种牙周局部递送制剂的制备方法,包括以下步骤:This embodiment provides a preparation method for periodontal local delivery preparation, comprising the following steps:
(1)PD聚合物的合成:将45mmol、分子量为146-186kDa的PVA在100℃条件下溶于100mL的DMSO溶液中,完全溶解后,加入5g的NaHSO4·H2O催化,待温度降至75℃时,加入3,4-二羟基苯丙氨酸(DOPA)6mmol,在N2的保护下反应12h,反应结束后,待温度降至室温后,在透析袋中透析2天,旋蒸仪去除多余水分后,冻干机中冻干2天。(1) Synthesis of PD polymer: Dissolve 45mmol of PVA with a molecular weight of146-186kDa in 100mL of DMSO solution at 100°C . At 75°C, add 6 mmol of 3,4-dihydroxyphenylalanine (DOPA), and react for 12 hours under the protection of N2 . After steaming to remove excess water, freeze-dry in a freeze dryer for 2 days.
(2)MnO2纳米片的合成:将0.755g MnCl2溶解于20mL去离子水中,将4.3g四甲基五水合铵(TMAOH)溶解于50mL3%的H2O2,将TMAOH溶液加入MnCl2溶液中,在1200rpm的转速下快速搅拌1min后,继续以600rpm搅拌12h,离心后用乙醇和去离子水分别洗三次。将黑色沉淀物材料冻干,再将冻干物溶解在去离子水中,浓度为10mg/mL,超声10h,最后将溶液以8800g离心10min,去除沉淀,得到MnO2溶液。(2) Synthesis ofMnO2 nanosheets: Dissolve 0.755gMnCl2 in 20mL deionized water, dissolve4.3g tetramethylammonium pentahydrate (TMAOH) in 50mL3%H2O2 , add TMAOH solution toMnCl2 In the solution, after stirring rapidly at 1200 rpm for 1 min, continue stirring at 600 rpm for 12 h, wash with ethanol and deionized water three times after centrifugation. Freeze-dry the black precipitate material, then dissolve the freeze-dried product in deionized water at a concentration of 10mg/mL, sonicate for 10h, and finally centrifuge the solution at 8800g for 10min to remove the precipitate and obtain aMnO2 solution.
(3)PDMO水凝胶的合成:具体过程参照实施例1,仅改变反应时间为0.5h。(3) Synthesis of PDMO hydrogel: refer to Example 1 for the specific process, only changing the reaction time to 0.5h.
实施例5Example 5
本实施例提供一种牙周局部递送制剂的制备方法,包括以下步骤:This embodiment provides a preparation method for periodontal local delivery preparation, comprising the following steps:
(1)PD聚合物的合成:将55mmol、分子量为89-98kDa的PVA在100℃条件下溶于120mL的DMSO溶液中,完全溶解后,加入8g的NaHSO4·H2O催化,待温度降至85℃时,加入3,4-二羟基苯丙氨酸(DOPA)11mmol,在N2的保护下反应15h,反应结束后,待温度降至室温后,在透析袋中透析4天,旋蒸仪去除多余水分后,冻干机中冻干3天。(1) Synthesis of PD polymer: Dissolve 55mmol of PVA with a molecular weight of 89-98kDa in120mL of DMSO solution at 100°C. At 85°C, add 11 mmol of 3,4-dihydroxyphenylalanine (DOPA), and react for 15 hours under the protection of N2 . After removing excess water in a steamer, freeze-dry in a freeze dryer for 3 days.
(2)MnO2纳米片的合成:将0.805g MnCl2溶解于30mL去离子水中,将4.5g四甲基五水合铵(TMAOH)溶解于45mL3%的H2O2,将TMAOH溶液加入MnCl2溶液中,在1500rpm的转速下快速搅拌1min后,继续以800rpm搅拌12h,离心后用乙醇和去离子水分别洗三次。将黑色沉淀物材料冻干,再将冻干物溶解在去离子水中,浓度为10mg/mL,超声10h,最后将溶液以10000g离心10min,去除沉淀,得到MnO2溶液。(2) Synthesis ofMnO2 nanosheets: Dissolve 0.805gMnCl2 in 30mL deionized water, dissolve4.5g tetramethylammonium pentahydrate (TMAOH) in 45mL3%H2O2 , add TMAOH solution toMnCl2 In the solution, after stirring rapidly at 1500 rpm for 1 min, continue stirring at 800 rpm for 12 h, wash with ethanol and deionized water three times after centrifugation. Freeze-dry the black precipitate material, then dissolve the freeze-dried product in deionized water at a concentration of 10 mg/mL, sonicate for 10 h, and finally centrifuge the solution at 10,000 g for 10 min to remove the precipitate and obtain a MnO2 solution.
(3)PDMO水凝胶的合成:具体过程参照实施例1,仅改变反应时间为1.0h。(3) Synthesis of PDMO hydrogel: refer to Example 1 for the specific process, only changing the reaction time to 1.0 h.
对比例1Comparative example 1
与实施例1的区别仅在于:将MnO2纳米片替换为等量的CeO2纳米颗粒。The only difference from Example 1 is that theMnO2 nanosheets are replaced by the same amount ofCeO2 nanoparticles.
对比例2Comparative example 2
将不同配比的PDMO水凝胶与现有常见市售牙周炎局部治疗剂型盐酸米诺环素软膏进行对比,比较他们抗炎、抗菌、牙周治疗效果等的差异。Combine different ratios of PDMO hydrogel with minocycline hydrochloride ointment For comparison, compare the differences in their anti-inflammatory, antibacterial, and periodontal treatment effects.
试验例1Test example 1
对实施例中制备得到的PD和PDMO进行材料表征。Material characterization was performed on the PD and PDMO prepared in the examples.
(1)测试实施例中制备的MnO2纳米片的扫描电镜图,如图2所示,可以看出MnO2纳米片呈现均匀的纳米级片装结构,直径约为200-300nm。(1) The scanning electron microscope image of theMnO2 nanosheets prepared in the test example, as shown in FIG. 2, it can be seen that theMnO2 nanosheets present a uniform nanoscale sheet structure with a diameter of about 200-300nm.
(2)测试实施例1中制备得到的PD和PDMO1水凝胶的扫描电镜图,如图3所示。(2) Scanning electron micrographs of the PD and PDMO1 hydrogels prepared in Test Example 1, as shown in FIG. 3 .
从图3可以看出,PD和PDMO1水凝胶均为疏松的多孔结构,且可以看到MnO2纳米片均匀附着于PD上。It can be seen from Figure 3 that both PD and PDMO1 hydrogels are loose porous structures, andMnO2 nanosheets can be seen to be evenly attached to PD.
(3)测试实施例1中制备得到的PDMO1水凝胶的Mapping图,如图4所示。(3) The Mapping diagram of the PDMO1 hydrogel prepared in Test Example 1 is shown in FIG. 4 .
从图4可以看出,Mn元素均匀分散于PDMO水凝胶中,证明MnO2纳米片均匀分散于PDMO水凝胶。It can be seen from Figure 4 that the Mn element is uniformly dispersed in the PDMO hydrogel, which proves that theMnO2 nanosheets are uniformly dispersed in the PDMO hydrogel.
(4)测试实施例1中制备得到的PD和实施例1-3中制备的PDMO1、PDMO2和PDMO3水凝胶的能谱图以及不同元素所占的重量百分比,结果如图5所示。图5中,A表示PD的测试结果,B表示PDMO1水凝胶的测试结果,C表示PDMO2水凝胶的测试结果,D表示PDMO3水凝胶的测试结果。(4) Test the energy spectrum of the PD prepared in Example 1 and the PDMO1, PDMO2 and PDMO3 hydrogels prepared in Examples 1-3 and the weight percentages of different elements. The results are shown in Figure 5. In Fig. 5, A represents the test result of PD, B represents the test result of PDMO1 hydrogel, C represents the test result of PDMO2 hydrogel, and D represents the test result of PDMO3 hydrogel.
从图5可以看出,三种PDMO水凝胶均含有Mn元素,证明MnO2纳米片均存在于PDMO水凝胶中,且其含量逐渐增加。It can be seen from Figure 5 that all three PDMO hydrogels contain Mn element, proving thatMnO2 nanosheets all exist in PDMO hydrogels, and their content gradually increases.
(5)测试实施例1中制备得到的PDMO1水凝胶的XPS衍射图谱,结果如图6所示。(5) The XPS diffraction pattern of the PDMO1 hydrogel prepared in Example 1 was tested, and the results are shown in FIG. 6 .
从图6可以看出,MnO2纳米片中Mn离子表现为三价和四价共存,且其比例为Mn3+:Mn4+=84%:16%,以上结果证明Mn离子具备良好的电子转移能力,具备良好的抗氧化性能。It can be seen from Figure 6 that Mn ions in MnO2 nanosheets exhibit trivalent and tetravalent coexistence, and the ratio is Mn3+ : Mn4+ = 84%: 16%. The above results prove that Mn ions have good electronic Transfer ability, with good antioxidant properties.
试验例2Test example 2
测试实施例中制备得到材料的抗氧化性能。The oxidation resistance of the materials prepared in the examples was tested.
(1)用1,1-二苯基-2-三硝基苯肼(DPPH)、无水乙醇和稀释后的材料放入EP管中,观察颜色的变化,并与常用市售牙周制剂派力奥进行对比。结果如图7所示,图7中A表示加入不同材料后DPPH溶液颜色变化,B表示利用酶标仪分析加入不同材料后DPPH溶液颜色的OD值(****P<0.0001)。(1) Put 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH), absolute ethanol and diluted materials into EP tubes, observe the color change, and compare with common commercially available periodontal preparations Palio comparing. The results are shown in Figure 7. In Figure 7, A represents the color change of the DPPH solution after adding different materials, and B represents the OD value of the color of the DPPH solution analyzed by a microplate reader after adding different materials (****P<0.0001).
从图7中A可以看出,PD、PDMO组颜色发生了变化,PDMO组比PD组颜色变化更明显。图7中B为每个组的定量分析,证明PDMO水凝胶清除ROS的能力明显增加,且优于市售牙周制剂派力奥It can be seen from A in Figure 7 that the color of the PD and PDMO groups has changed, and the color change of the PDMO group is more obvious than that of the PD group. B in Figure 7 is the quantitative analysis of each group, which proves that the ability of PDMO hydrogel to scavenge ROS is significantly increased, and it is better than the commercially available periodontal preparation Palio
(2)利用3,3',5,5'-四甲基联苯胺(TMB)验证材料的清除羟基自由基(OH-)的能力:称取0.02g的TMB溶于10ml的无水乙醇,称取0.002g的FeSO4溶于10ml的去离子水中,将3%过氧化氢1Mm,材料稀释至20mg/ml,在EP管分别加入H2O2、FeSO4溶液和TMB,观察溶液颜色的变化,并用酶标仪获取OD值。结果如图8所示,图8中A表示加入不同材料后TMB溶液颜色变化,图8中B表示利用酶标仪分析加入不同材料后TMB溶液颜色的OD值。(2)
从图8中A可以看出,PDMO组比PD组颜色变化更明显,并且PDMO1、PDMO2、PDMO3的DPPH溶液颜色依次变浅。图8中B为每个组的定量分析,证明PDMO水凝胶清除OH-的能力较PD明显增加,且随加入MnO2量增加而增加,且均优于市售牙周制剂派力奥It can be seen from A in Figure 8 that the color change of the PDMO group is more obvious than that of the PD group, and the color of the DPPH solution of PDMO1, PDMO2, and PDMO3 becomes lighter in turn. B in Figure 8 is the quantitative analysis of each group, which proves that the ability of PDMO hydrogel to scavenge OH- is significantly higher than that of PD, and it increases with the increase of the amount of MnO2 added, and it is better than the commercially available periodontal preparation Palio
(3)采用硝基四氮唑蓝(NBT)验证材料的清除超氧阴离子(O2-)的能力,结果如图9所示。图9中A表示加入不同材料后NBT溶液颜色变化,B表示利用酶标仪分析加入不同材料后NBT溶液颜色的OD值(**P<0.01,****P<0.0001)。(3) Nitro blue tetrazolium (NBT) was used to verify the ability of the material to scavenge superoxide anion (O2− ), and the results are shown in FIG. 9 . In Figure 9, A shows the color change of NBT solution after adding different materials, and B shows the OD value of NBT solution color after adding different materials analyzed by microplate reader (**P<0.01, ****P<0.0001).
从图9可以看出,PD溶液具备一定的清除O2-的能力,加入MnO2后的PDMO水凝胶的O2-清除能力逐渐增强。It can be seen from Figure 9 that the PD solution has a certain ability to scavenge O2- , and the O2- scavenging ability of the PDMO hydrogel after adding MnO2 is gradually enhanced.
(4)为了验证材料对于细胞的抗氧化应激效果,分别将Raw264.7细胞以1×105铺板后,用LPS刺激细胞将材料共培养6h后,用DCFH-DA荧光探针观察ROS的清除效果,结果见图10。(4) In order to verify the anti-oxidative stress effect of the material on the cells, Raw264.7 cells were plated at 1×105 , and the cells were stimulated with LPS. After the materials were co-cultured for 6 hours, the ROS was observed with the DCFH-DA fluorescent probe. Clearing effect, the results are shown in Figure 10.
如图10所示,加入派力奥和PD水凝胶后可在一定程度上改善细胞的氧化状态,在加入PDMO水凝胶后可进一步改善细胞的氧化应激状态,证明PDMO水凝胶具备良好的抗氧化能力。As shown in Figure 10, add Palio After adding PDMO hydrogel, the oxidative state of cells can be improved to a certain extent, and the oxidative stress state of cells can be further improved after adding PDMO hydrogel, which proves that PDMO hydrogel has good antioxidant capacity.
(5)验证材料对于细胞的抗氧化应激效果:分别将细胞Raw264.7以1×105铺板后,用LPS刺激细胞将材料共培养后,进一步用Ru(dpp)3Cl2荧光探针观察ROS的清除效果,结果如图11所示。(5) To verify the anti-oxidative stress effect of the material on the cells: the cells Raw264.7 were plated at 1×105 respectively, and the cells were stimulated with LPS to co-culture the materials, and then Ru(dpp)3 Cl2 fluorescent probe was used to Observe the scavenging effect of ROS, the results are shown in Figure 11.
从图11可以看出,加入派力奥和PD水凝胶后可在一定程度上改善细胞的氧化状态,在加入PDMO水凝胶后可进一步改善细胞的氧化应激状态,进一步证明PDMO水凝胶具备良好的抗氧化能力。As can be seen from Figure 11, adding Palio After adding PDMO hydrogel, the oxidative state of cells can be improved to a certain extent, and the oxidative stress state of cells can be further improved after adding PDMO hydrogel, which further proves that PDMO hydrogel has good antioxidant capacity.
试验例3Test example 3
测试本发明实施例制备得到PDMO水凝胶的近红外光热响应性。The near-infrared photothermal responsiveness of the PDMO hydrogel prepared in the embodiment of the present invention was tested.
测试方法:采用近红外激发器(波长为808nm)进行检测,将材料放入24孔板中,分组后分别在近红外光下照射10min,在每一分钟用近红外相机拍照记录,图12为不同组别水凝胶在1.4W/cm2的808nm近红外光下照射10min内每分钟的热图变化。图13为对应的每个时间点温度变化的定量分析,图14为重复5个周期不同组别水凝胶的温度变化,以上结果表明PDMO水凝胶具备良好的近红外光热响应性能。Test method: Use a near-infrared exciter (wavelength of 808nm) for detection, put the materials into a 24-well plate, irradiate them under near-infrared light for 10 minutes after grouping, and take pictures and records with a near-infrared camera every minute, as shown in Figure 12 The heat map changes per minute of different groups of hydrogels irradiated with 1.4W/cm2 808nm near-infrared light for 10min. Figure 13 is the quantitative analysis of the temperature change at each corresponding time point, and Figure 14 is the temperature change of different groups of hydrogels repeated for 5 cycles. The above results show that the PDMO hydrogel has good near-infrared photothermal response performance.
试验例4Test example 4
测试本发明实施例制备得到PDMO水凝胶的抗菌作用。The antibacterial effect of the PDMO hydrogel prepared in the embodiment of the present invention was tested.
(1)将不同组别水凝胶分别与金黄色葡糖球菌(SA)和大肠杆菌(EC)共培养8小时后,然后将材料和细菌混合溶液进行铺板,观察菌落形成单位(CFU),结果如图15和图16所示。(1) After co-cultivating different groups of hydrogels with Staphylococcus aureus (SA) and Escherichia coli (EC) for 8 hours, the mixed solution of materials and bacteria was plated, and the colony forming units (CFU) were observed. The results are shown in Figure 15 and Figure 16.
从图15和图16可以看出,在三种组别的PDMO水凝胶的CFU明显少于control、PVA、PD和商用派力奥组,而PD组CFU少于control、PVA以及派力奥组。以上实验结果证明PD具备一定的抗菌能力,而PDMO水凝胶具备更加优异的抗菌性能。It can be seen from Figure 15 and Figure 16 that the CFU of the PDMO hydrogel in the three groups is significantly less than that of the control, PVA, PD and commercial Palio groups, while the CFU of the PD group is less than that of the control, PVA and Palio Group. The above experimental results prove that PD has a certain antibacterial ability, and PDMO hydrogel has more excellent antibacterial performance.
(2)将不同组别水凝胶和牙龈卟啉单胞菌(P.g)共培养24小时后,对材料和细菌混合溶液进行阿尔玛蓝检测,结果见图17。(2) After co-cultivating different groups of hydrogels and Porphyromonas gingivalis (P.g) for 24 hours, the mixed solution of materials and bacteria was tested for alamar blue, and the results are shown in Figure 17.
从图17可以看出,三组PDMO水凝胶和派力奥中P.g增殖数量明显少于control、PVA、PD组,也证明PDMO水凝胶具备优异的抗菌性能。It can be seen from Figure 17 that the number of P.g proliferation in the three groups of PDMO hydrogel and Palio was significantly less than that of the control, PVA, and PD groups, which also proves that PDMO hydrogel has excellent antibacterial properties.
试验例5Test example 5
测试本发明实施例制备得到PDMO水凝胶的体外生物相容性。The in vitro biocompatibility of the PDMO hydrogel prepared in the embodiment of the present invention was tested.
对不同组别水凝胶的生物安全性能进行了评估,将材料与Raw264.7细胞共培养1天和3天后利用CCK8观察细胞增殖情况,如图18所示,1天和3天的结果均显示无明显的细胞毒性,证明PDMO水凝胶具备良好的生物相容性。The biosafety performance of different groups of hydrogels was evaluated. The materials were co-cultured with Raw264.7 cells for 1 day and 3 days, and the cell proliferation was observed by CCK8. As shown in Figure 18, the results of 1 day and 3 days were all It shows no obvious cytotoxicity, which proves that PDMO hydrogel has good biocompatibility.
试验例6Test example 6
测试本发明实施例制备得到的PDMO水凝胶的体内生物安全性和牙周炎治疗效果。The in vivo biological safety and periodontitis treatment effect of the PDMO hydrogel prepared in the embodiment of the present invention were tested.
选择4-6周雄性SD大鼠,适应性喂养一周后,在左侧上颌第一磨牙用0.2mm的正畸丝围绕牙颈部一圈,并固定后,建立牙周炎模型,如图19所示。建模10天后,进行给药,每三天给药一次,给药后进行近红外照射。给药四周后进行取材,并且取每只鼠的血液进行血常规、血生化检测和分析,评价PDMO水凝胶的体内生物安全性。实验结果如图20、图21所示,可以看出,PDMO水凝胶无明显的血液毒性,对心脏、肝脏、肾脏等主要脏器均无明显毒性,证明PDMO水凝胶具备良好的生物相容性。Select 4-6 week old male SD rats, after one week of adaptive feeding, use 0.2mm orthodontic wire around the neck of the tooth on the left maxillary first molar, and fix it to establish a periodontitis model, as shown in Figure 19 shown. After 10 days of modeling, the drug was administered once every three days, and near-infrared irradiation was performed after the drug was administered. After four weeks of administration, the samples were taken, and the blood of each mouse was taken for blood routine and blood biochemical detection and analysis, so as to evaluate the in vivo biological safety of PDMO hydrogel. The experimental results are shown in Figure 20 and Figure 21. It can be seen that the PDMO hydrogel has no obvious blood toxicity, and has no obvious toxicity to the heart, liver, kidney and other major organs, which proves that the PDMO hydrogel has a good biological phase. Capacitance.
然后取材进行牙槽骨CT扫描,并进行三维重建,比较釉牙骨质界到牙槽嵴顶的距离,实验结果如图22所示,可以看出,PDMO2水凝胶具备最佳的牙槽骨重建能力。Then take the alveolar bone CT scan and perform three-dimensional reconstruction to compare the distance from the enamel cementum junction to the alveolar crest. The experimental results are shown in Figure 22. It can be seen that PDMO2 hydrogel has the best alveolar Bone remodeling capacity.
试验例7Test Example 7
测试实施例1和对比例1中制备得到水凝胶材料的抗氧化性能。利用DPPH、无水乙醇和稀释后的材料放入EP管中,观察颜色的变化。结果如图23所示,PDMO1水凝胶组颜色发生的变化比PD-CeO2组颜色变化更明显,且PDMO1水凝胶组的DPPH清除率较PD-CeO2组更高,证明PDMO水凝胶清除ROS的能力优于PD-CeO2。The antioxidant properties of the hydrogel materials prepared in Example 1 and Comparative Example 1 were tested. Put DPPH, absolute ethanol and diluted materials into EP tubes, and observe the color change. The results are shown in Figure 23. The color change of the PDMO1 hydrogel group is more obvious than that of the PD-CeO2 group, and the DPPH clearance rate of the PDMO1 hydrogel group is higher than that of the PD-CeO2 group, which proves that the PDMO hydrogel group The ability of glue to scavenge ROS is better than that of PD-CeO2 .
试验例8Test example 8
测试实施例1-3和市售牙周局部制剂派力奥的抗炎、抗菌、牙周炎治疗效果等进行对比。如图7,图8,图10,图11所示,3种PDMO水凝胶的抗氧化性能均优于派力奥。如图15,图16所示,3种PDMO水凝胶对金黄色葡糖球菌(SA)和大肠杆菌(EC)的抗菌能力均优于派力奥。如图22所示,PDMO2水凝胶较派力奥具备更佳的牙槽骨重建能力。以上结果证明,本发明所构建PDMO水凝胶较常见市售牙周制剂具备更佳的抗炎、抗菌、牙周炎治疗效果。Test Examples 1-3 and the commercially available periodontal topical formulation Palio The anti-inflammatory, antibacterial, and periodontitis treatment effects were compared. As shown in Figure 7, Figure 8, Figure 10, and Figure 11, the antioxidant properties of the three PDMO hydrogels are all better than those of Palio. As shown in Figure 15 and Figure 16, the antibacterial ability of the three PDMO hydrogels against Staphylococcus aureus (SA) and Escherichia coli (EC) is better than that of Palio. As shown in Figure 22, PDMO2 hydrogel has better alveolar bone reconstruction ability than Palio. The above results prove that the PDMO hydrogel constructed by the present invention has better anti-inflammatory, antibacterial and periodontitis treatment effects than common commercially available periodontal preparations.
综上所述,本发明提供一种牙周局部递送制剂、其制备方法及应用,通过以聚乙烯醇(PVA)为载体,在聚乙烯醇上接枝贻贝粘附蛋白3,4-二羟基苯丙氨酸(DOPA),并利用DOPA上的酚羟基螯合金属锰离子,利用DOPA优良的湿粘接性能与二氧化锰纳米片的拟酶活性,构建一种ROS集联响应性和NIR光热响应性的MnO2纳米片贻贝仿生复合材料作为牙周局部递送制剂,具有以下优点:In summary, the present invention provides a periodontal local delivery preparation, its preparation method and application, by using polyvinyl alcohol (PVA) as a carrier, grafting
(1)具有良好的可注射性和塑形能力,操作便捷,适用于不同形状的牙周缺损;(1) It has good injectability and shaping ability, convenient operation, and is suitable for periodontal defects of different shapes;
(2)具有良好的粘接性,可牢固粘附于牙周组织持续缓释Mn离子;(2) It has good adhesion and can firmly adhere to the periodontal tissue to continuously release Mn ions;
(3)具有高效的ROS清除活性和抗炎作用;(3) have efficient ROS scavenging activity and anti-inflammatory effect;
(4)具有良好的近红外光热响应性和抗菌效果;(4) It has good near-infrared photothermal responsiveness and antibacterial effect;
(5)具有良好的生物安全性;(5) have good biological safety;
(6)具有良好的牙周治疗效果和牙槽骨重建能力。(6) It has good periodontal treatment effect and alveolar bone reconstruction ability.
以上仅为本发明的优选实施例而已,并不用于限制本发明,对于本领域的技术人员来说,本发明可以有各种更改和变化。凡在本发明的精神和原则之内,所作的任何修改、等同替换、改进等,均应包含在本发明的保护范围之内。The above are only preferred embodiments of the present invention, and are not intended to limit the present invention. For those skilled in the art, the present invention may have various modifications and changes. Any modifications, equivalent replacements, improvements, etc. made within the spirit and principles of the present invention shall be included within the protection scope of the present invention.
Claims (21)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210238196.2ACN114533660B (en) | 2022-03-11 | 2022-03-11 | Periodontal local delivery preparation, preparation method and application thereof |
| PCT/CN2023/070598WO2023169073A1 (en) | 2022-03-11 | 2023-01-05 | Periodontal topical delivery preparation, and preparation method therefor and use thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210238196.2ACN114533660B (en) | 2022-03-11 | 2022-03-11 | Periodontal local delivery preparation, preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114533660A CN114533660A (en) | 2022-05-27 |
| CN114533660Btrue CN114533660B (en) | 2023-06-16 |
Family
ID=81664426
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210238196.2AActiveCN114533660B (en) | 2022-03-11 | 2022-03-11 | Periodontal local delivery preparation, preparation method and application thereof |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN114533660B (en) |
| WO (1) | WO2023169073A1 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114533660B (en)* | 2022-03-11 | 2023-06-16 | 重庆医科大学 | Periodontal local delivery preparation, preparation method and application thereof |
| WO2024108469A1 (en)* | 2022-11-24 | 2024-05-30 | 深圳先进技术研究院 | Shape memory material with chelating system and preparation method therefor |
| CN116327713B (en)* | 2023-03-31 | 2024-08-23 | 四川大学 | Functional particles with antibacterial and mineralization repairing functions as well as preparation method and application thereof |
| CN118717709B (en)* | 2024-06-05 | 2025-06-24 | 中国人民解放军空军军医大学 | Composite bionic nanomaterial with oral periodontitis targeting and inflammatory neutralization functions and preparation method and application thereof |
| CN118831065A (en)* | 2024-06-17 | 2024-10-25 | 中国人民解放军空军军医大学 | Preparation method and application of functional nano-enzyme with periodontitis antioxidation stress effect |
| CN118512333B (en)* | 2024-07-18 | 2024-12-10 | 中国人民解放军联勤保障部队第九二〇医院 | A mussel bionic monomer modified acid etching agent and its preparation method and application |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016202907A1 (en)* | 2015-06-16 | 2016-12-22 | Straumann Holding Ag | Two-component system |
| CN111265497A (en)* | 2020-03-26 | 2020-06-12 | 四川大学 | Oral drug-loaded adhesive film and preparation method thereof, oral drug-loaded adhesive film component and preparation method thereof |
| CN111671899A (en)* | 2020-06-16 | 2020-09-18 | 西北工业大学 | A kind of preparation method and antitumor application of manganese dioxide nanosheet hybrid hydrogel |
| CN112546288A (en)* | 2020-11-30 | 2021-03-26 | 西北工业大学 | Hydrogel dressing capable of dissolving according to needs and preparation method thereof |
| CN114099416A (en)* | 2021-10-28 | 2022-03-01 | 四川大学 | Multifunctional injectable hydrogel with microenvironment response and preparation method and application thereof |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101103423B1 (en)* | 2009-09-04 | 2012-01-06 | 아주대학교산학협력단 | Bio-injectable tissue adhesive hydrogels and their biomedical uses |
| KR101522462B1 (en)* | 2012-09-19 | 2015-05-21 | 아주대학교산학협력단 | Preparation Method of In situ forming Hydrogel Using Enzyme-Immobilized Supports and Biomedical Use Thereof |
| CN111150888B (en)* | 2018-11-07 | 2022-03-15 | 财团法人工业技术研究院 | Double-effect film and preparation method thereof |
| CN113049563B (en)* | 2021-03-25 | 2024-06-25 | 湖南师范大学 | Method for detecting formaldehyde in air and food based on manganese dioxide nano-sheet |
| CN114533660B (en)* | 2022-03-11 | 2023-06-16 | 重庆医科大学 | Periodontal local delivery preparation, preparation method and application thereof |
- 2022
- 2022-03-11CNCN202210238196.2Apatent/CN114533660B/enactiveActive
- 2023
- 2023-01-05WOPCT/CN2023/070598patent/WO2023169073A1/ennot_activeCeased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016202907A1 (en)* | 2015-06-16 | 2016-12-22 | Straumann Holding Ag | Two-component system |
| CN111265497A (en)* | 2020-03-26 | 2020-06-12 | 四川大学 | Oral drug-loaded adhesive film and preparation method thereof, oral drug-loaded adhesive film component and preparation method thereof |
| CN111671899A (en)* | 2020-06-16 | 2020-09-18 | 西北工业大学 | A kind of preparation method and antitumor application of manganese dioxide nanosheet hybrid hydrogel |
| CN112546288A (en)* | 2020-11-30 | 2021-03-26 | 西北工业大学 | Hydrogel dressing capable of dissolving according to needs and preparation method thereof |
| CN114099416A (en)* | 2021-10-28 | 2022-03-01 | 四川大学 | Multifunctional injectable hydrogel with microenvironment response and preparation method and application thereof |
Non-Patent Citations (3)
| Title |
|---|
| A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery;Shanshan Hu et al.;NATURE COMMUNICATIONS;第1-17页* |
| MoS2@PDA@Ag/PVA Hybrid Hydrogel with Excellent Light-Responsive Antibacterial Activity and Enhanced Mechanical Properties for Wound Dressing;Yan Pengfei et al.;MACROMOLECULAR MATERIALS AND ENGINEERING;第307卷(第2期);第1-13页* |
| 含儿茶酚基聚合物的制备及其性能研究;刘蓉瑾;中国优秀硕士学位论文全文数据库工程科技I辑(第2015年第12期期);第B014-225页* |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2023169073A1 (en) | 2023-09-14 |
| CN114533660A (en) | 2022-05-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN114533660B (en) | Periodontal local delivery preparation, preparation method and application thereof | |
| CN110354295B (en) | Photo-thermal conversion material and preparation method thereof | |
| Li et al. | A photothermal-response oxygen release platform based on a hydrogel for accelerating wound healing | |
| Yan et al. | pH Switchable Nanozyme Platform for Healing Skin Tumor Wound Infected with Drug‐Resistant Bacteria | |
| Wang et al. | Nanodot-doped peptide hydrogels for antibacterial phototherapy and wound healing | |
| CN111773429A (en) | Hydrogel dressing and preparation method thereof and multifunctional nanocomposite dressing and preparation method and application thereof | |
| CN111440349B (en) | Preparation method of sodium alginate antibacterial dressing | |
| CN111632040A (en) | A kind of manganese dioxide wrapped drug-loaded mesoporous titanium dioxide nanoparticles and preparation method and application thereof | |
| Liu et al. | Local photothermal/photodynamic synergistic antibacterial therapy based on two-dimensional BP@ CQDs triggered by single NIR light source | |
| Wu et al. | Enhanced diabetic foot ulcer treatment with a chitosan-based thermosensitive hydrogel loaded self-assembled multi-functional nanoparticles for antibacterial and angiogenic effects | |
| CN116650710A (en) | Mussel inspired multifunctional double-network crosslinked hydrogel wound dressing | |
| Weng et al. | Electrical and visible light dual-responsive ZnO nanocomposite with multiple wound healing capability | |
| Sun et al. | Engineering ag-decorated graphene oxide nano-photothermal platforms with enhanced antibacterial properties for promoting infectious wound healing | |
| CN115501122A (en) | Antibacterial mineralized hydrogel and precursor material, and preparation and application methods thereof | |
| CN104013960A (en) | Water-soluble complex for targeting photo-thermal treatment, and preparation method and application thereof | |
| CN115554270A (en) | Drug-loaded composite material with pH stimulation response and slow release functions and preparation method and application thereof | |
| CN118286487B (en) | Preparation method of multifunctional nanofiber membrane for promoting healing of complex infected wound | |
| CN119386256A (en) | A self-healing double-layer hydrogel for promoting diabetic wound healing and its preparation method and application | |
| Xiong et al. | The Three‐Pronged Strategy: A Bilayer Hydrogel Treats Diabetic Chronic Wound through Microalgae Oxygen Therapy, Ag─ FA NP Antibacterial, and Synergistic Scavenging of ROS | |
| CN114652679B (en) | Delivery system based on nano hydroxyapatite, preparation method, application, pharmaceutical composition, spray and hydrogel | |
| CN108752357B (en) | Preparation method and application of water-soluble phthalocyanine nanomaterial with high light-to-heat conversion efficiency | |
| HK40076893B (en) | Periodontal topical delivery formulation, preparation method and application thereof | |
| HK40076893A (en) | Periodontal topical delivery formulation, preparation method and application thereof | |
| CN116983424A (en) | Preparation method of targeting near-infrared carbon dot-based antibacterial composite material and application of material | |
| Tian et al. | Nanoparticle-mediated photothermal and photodynamic antibacterial therapy for the treatment of periodontitis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code | Ref country code:HK Ref legal event code:DE Ref document number:40076893 Country of ref document:HK | |
| GR01 | Patent grant | ||
| GR01 | Patent grant |