CN114437128A - Choline phosphate modified paclitaxel medicine and preparation method and application thereof - Google Patents
Choline phosphate modified paclitaxel medicine and preparation method and application thereofDownload PDFInfo
- Publication number
- CN114437128A CN114437128ACN202210107371.4ACN202210107371ACN114437128ACN 114437128 ACN114437128 ACN 114437128ACN 202210107371 ACN202210107371 ACN 202210107371ACN 114437128 ACN114437128 ACN 114437128A
- Authority
- CN
- China
- Prior art keywords
- paclitaxel
- choline
- choline phosphate
- drug
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/547—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom
- C07F9/655—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having oxygen atoms, with or without sulfur, selenium, or tellurium atoms, as the only ring hetero atoms
- C07F9/6551—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having oxygen atoms, with or without sulfur, selenium, or tellurium atoms, as the only ring hetero atoms the oxygen atom being part of a four-membered ring
- C07F9/65512—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having oxygen atoms, with or without sulfur, selenium, or tellurium atoms, as the only ring hetero atoms the oxygen atom being part of a four-membered ring condensed with carbocyclic rings or carbocyclic ring systems
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/337—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having four-membered rings, e.g. taxol
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/683—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols
- A61K31/685—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols one of the hydroxy compounds having nitrogen atoms, e.g. phosphatidylserine, lecithin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/543—Lipids, e.g. triglycerides; Polyamines, e.g. spermine or spermidine
- A61K47/544—Phospholipids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及药物制剂技术领域,尤其涉及一种胆碱磷酸修饰的紫杉醇药物及其制备方法和应用。The invention relates to the technical field of pharmaceutical preparations, in particular to a choline phosphate-modified paclitaxel medicine and a preparation method and application thereof.
背景技术Background technique
紫杉醇(PTX)是从短叶红豆杉树皮中提取的天然产物,也可经半合成的方法获得。紫杉醇与细胞的微管蛋白结合,促进微管蛋白聚集并抑制其解离,细胞不能正常分裂,引起细胞周期阻滞和凋亡。紫杉醇是治疗乳腺癌、卵巢癌、非小细胞肺癌等的广谱抗肿瘤药物,疗效显著。然而紫杉醇几乎不溶于水,作为临床常用制剂的紫杉醇注射液是以聚氧乙烯蓖麻油和无水乙醇混合液作为溶剂的,而这种非水溶媒的使用会导致严重的过敏性反应;为减少过敏反应的产生,患者注射紫杉醇之前需给予地塞米松和苯海拉明等药物进行脱敏预处理,增加了患者和医护人员的负担;另外,紫杉醇缺乏靶向性,极易引起嗜中性白血球减少症、神经性疾病等全身性不良反应。因而,从紫杉醇应用于临床开始,对紫杉醇的结构修饰、改造以及剂型的改造在国内外一直受到重视。Paclitaxel (PTX) is a natural product extracted from the bark of Taxus breve and can also be obtained by semi-synthetic methods. Paclitaxel binds to the tubulin of cells, promotes tubulin aggregation and inhibits its dissociation, and cells cannot divide normally, causing cell cycle arrest and apoptosis. Paclitaxel is a broad-spectrum antitumor drug for the treatment of breast cancer, ovarian cancer, and non-small cell lung cancer, with significant curative effect. However, paclitaxel is almost insoluble in water. Paclitaxel injection, a commonly used clinical preparation, uses a mixture of polyoxyethylene castor oil and anhydrous ethanol as a solvent, and the use of this non-aqueous vehicle can lead to severe allergic reactions; in order to reduce For allergic reactions, patients need to be given dexamethasone and diphenhydramine and other drugs for desensitization pretreatment before injection of paclitaxel, which increases the burden on patients and medical staff; in addition, paclitaxel lacks targeting, which can easily cause neutropenia Systemic adverse reactions such as leukopenia and neurological diseases. Therefore, since the clinical application of paclitaxel, the structural modification, modification and dosage form modification of paclitaxel have been paid attention at home and abroad.
紫杉醇的结构改造主要是使用不同的化学基团,合成具有不同取代基的紫杉醇结构类似物;剂型的改造主要是指使用两性高分子聚合物对紫杉醇进行物理包覆,形成纳米胶束、纳米胶囊或纳米颗粒。紫杉醇的结构修饰主要是指在紫杉醇的基团上通过化学键连接小分子或者大分子化合物生成偶联物,偶联物在体内水解重新释放出紫杉醇,发挥紫杉醇原型药的抗肿瘤作用。目前,仍然有众多的紫杉醇结构修饰物处于临床前或者临床试验中。其中修饰基团的功能、连接片段的结构组成会影响紫杉醇的释放和肿瘤细胞的摄取,因此肿瘤临床治疗中亟需研发一种高效低毒且具有靶向作用的新型紫杉醇药物。The structural modification of paclitaxel mainly uses different chemical groups to synthesize taxol structural analogs with different substituents; the modification of dosage form mainly refers to the physical coating of paclitaxel with amphoteric polymers to form nanomicelles and nanocapsules. or nanoparticles. The structural modification of paclitaxel mainly refers to the formation of conjugates by linking small molecules or macromolecules on the groups of paclitaxel through chemical bonds, and the conjugates are hydrolyzed in vivo to re-release paclitaxel, and play the anti-tumor effect of the prototype drug of paclitaxel. At present, there are still many paclitaxel structural modifiers in preclinical or clinical trials. Among them, the function of the modified group and the structural composition of the connecting fragment will affect the release of paclitaxel and the uptake of tumor cells. Therefore, it is urgent to develop a new type of paclitaxel drug with high efficiency, low toxicity and targeting effect in the clinical treatment of tumors.
发明内容SUMMARY OF THE INVENTION
有鉴于此,本发明要解决的技术问题在于提供一种胆碱磷酸修饰的紫杉醇药物及其制备方法和应用,制备的胆碱磷酸修饰的紫杉醇药物具有较高的水溶性、体内循环时间以及肿瘤靶向能力。In view of this, the technical problem to be solved by the present invention is to provide a choline phosphate-modified paclitaxel drug and a preparation method and application thereof. The prepared choline phosphate-modified paclitaxel drug has higher water solubility, in vivo circulation time and tumor targeting ability.
本发明提供了一种胆碱磷酸修饰的紫杉醇药物,具有式Ⅰ所示结构:The invention provides a choline phosphate-modified paclitaxel medicine, which has the structure shown in formula I:
其中,R选自取代或非取代的C1-C8的烷基、C3-C8的烯基、C3-C8的炔基、C3-C8的环氧基、C2-C8的叠氮基、C1-C8的氨基、多元醇类物质中除去任一羟基后剩余的残基或选择保护基团;Wherein, R is selected from substituted or unsubstituted C1-C8 alkyl, C3-C8 alkenyl, C3-C8 alkynyl, C3-C8 epoxy, C2-C8 azido, C1-C8 The residues left after removing any hydroxyl group from the amino, polyols or select protective groups;
M为或O;M is or O;
当M为时,L选自氨基酸残基;When M is , L is selected from amino acid residues;
当M为O时,L选自羧酸及其衍生物的残基。When M is O, L is selected from the residues of carboxylic acids and derivatives thereof.
本发明中,上述羧酸及其衍生物至少具有两个羧基。In this invention, the said carboxylic acid and its derivative(s) have at least two carboxyl groups.
本发明中,所述R是胆碱磷酸末端可进一步修饰的基团,优选为取代或非取代的C1-C8的烷基、C3-C8的烯基、C3-C8的炔基、C3-C8的环氧基、C2-C8的叠氮基、C1-C8的氨基、多元醇类物质中除去任一羟基后剩余的残基或选择保护基团。In the present invention, the R is a group that can be further modified at the end of choline phosphate, preferably a substituted or unsubstituted C1-C8 alkyl group, C3-C8 alkenyl group, C3-C8 alkynyl group, C3-C8 group The epoxy group, the C2-C8 azide group, the C1-C8 amino group, the residual residue after removing any hydroxyl group from the polyols, or the selected protective group.
上述取代基团优选为氟取代的C1-C8的烷基、C3-C8的烯基、C3-C8的炔基、C3-C8的环氧基、C3-C8的叠氮基、C3-C8的氨基或氟取代的多元醇类。The above-mentioned substituent groups are preferably fluorine-substituted C1-C8 alkyl groups, C3-C8 alkenyl groups, C3-C8 alkynyl groups, C3-C8 epoxy groups, C3-C8 azido groups, C3-C8 Amino or fluorine substituted polyols.
更优选的,所述R选自C1-C5的烷基、C3-C5的烯基、C3-C5的炔基、C3-C5的环氧基、C2-C5的叠氮基、C1-C5的氨基、C3-C5的醇除去任一羟基后剩余的残基。More preferably, the R is selected from C1-C5 alkyl, C3-C5 alkenyl, C3-C5 alkynyl, C3-C5 epoxy, C2-C5 azido, C1-C5 Amino, C3-C5 alcohol residues remaining after any hydroxyl group is removed.
进一步优选的,所述R选自甲基、乙基、正丙基、异丙基、正丁基、异丁基、叔丁基、烯丙基、烯丁基、炔丙基、炔丁基、N3-(CH2)2-、N3-(CH2)3-、N3-(CH2)4-、NH2CH2-、NH2(CH2)2-、NH2(CH2)3-、NH2(CH2)4-、BOC-NH(CH2)2-、BOC-NH(CH2)3-、BOC-NH(CH2)4、HO-(CH2)2-、HO-(CH2)3-、HO-(CH2)4-、HO-(CH2)5-、HO-(CH2)2-O-(CH2)2-。Further preferably, the R is selected from methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, allyl, allyl, propargyl, propargyl , N3 -(CH2 )2 -, N3 -(CH2 )3 -, N3 -(CH2 )4 -, NH2 CH2 -, NH2 (CH2 )2 -, NH2 (CH 2 )2 )3 -, NH2 (CH2 )4 -, BOC-NH(CH2 )2 -, BOC-NH(CH2 )3 -, BOC-NH(CH2 )4 , HO-(CH2 )2 -, HO-(CH2 )3 -, HO-(CH2 )4 -, HO-(CH2 )5 -, HO-(CH2 )2 -O-(CH2 )2 -.
本发明中,所述L是将胆碱磷酸与紫杉醇相连的基团,L的一端与紫杉醇的羟基反应,另一端和胆碱磷酸基团反应。所述M是胆碱磷酸的修饰基团,能够和氨基或羧基反应,作为反应基团使胆碱磷酸与L反应,进而与紫杉醇相连。In the present invention, the L is a group connecting choline phosphate and paclitaxel, one end of L reacts with the hydroxyl group of paclitaxel, and the other end reacts with the choline phosphate group. The M is a modified group of choline phosphate, which can react with an amino group or a carboxyl group, and as a reactive group, the choline phosphate reacts with L, and then connects with paclitaxel.
当M为时,L优选为甘氨酸、丙氨酸、丝氨酸、苏氨酸、天冬氨酸、谷氨酸、赖氨酸或精氨酸的残基;上述残基指氨基酸的氨基失去一个氢原子,羧基失去一个OH后剩余的基团。上述M中的酯基端和胆碱磷酸端连接,乙基端和氨基酸残基的氨基连接,氨基酸残基的羰基端和紫杉醇的氧原子连接。When M is When L is preferably a residue of glycine, alanine, serine, threonine, aspartic acid, glutamic acid, lysine or arginine; the above-mentioned residues refer to the amino acid loss of a hydrogen atom, the carboxyl group The remaining group after the loss of one OH. In the above M, the ester group end is connected to the choline phosphate end, the ethyl end is connected to the amino group of the amino acid residue, and the carbonyl end of the amino acid residue is connected to the oxygen atom of paclitaxel.
当M为O时,L优选为丁二酸、戊二酸、己二酸、3,3’-二硫代二乙酸、3,3’-二硫代二丙酸、3,3’-二硫代二丁酸、二甘醇酸、三甘醇酸或四甘醇酸的残基;上述残基指羧酸及其衍生物的两个羧基分别失去OH后剩余的基团。When M is O, L is preferably succinic acid, glutaric acid, adipic acid, 3,3'-dithiodiacetic acid, 3,3'-dithiodipropionic acid, 3,3'-dithiodipropionic acid Residues of thiodibutyric acid, diglycolic acid, triglycolic acid or tetraglycolic acid; the above residues refer to the groups remaining after the two carboxyl groups of carboxylic acid and its derivatives respectively lose OH.
具体的,当M为时,L选自以下任一结构:Specifically, when M is , L is selected from any of the following structures:
当M为O时,L选自以下任一结构:When M is O, L is selected from any of the following structures:
在本发明的一些具体实施例中,所述胆碱磷酸修饰的紫杉醇药物具有以下任一结构:In some specific embodiments of the present invention, the choline phosphate-modified paclitaxel drug has any of the following structures:
本发明提供了一种胆碱磷酸修饰的紫杉醇药物的制备方法,包括以下步骤:The invention provides a preparation method of a choline phosphate-modified paclitaxel medicine, comprising the following steps:
将丙烯酰氧乙基胆碱磷酸乙酯、紫杉醇和氨基酸进行反应,得到胆碱磷酸修饰的紫杉醇药物。Acryloyloxyethyl choline ethyl phosphate, paclitaxel and amino acid are reacted to obtain a choline phosphate modified paclitaxel drug.
上述丙烯酰氧乙基胆碱磷酸乙酯的结构式如下:The structural formula of above-mentioned acryloyloxyethylcholine ethyl phosphate is as follows:
本发明提供了一种胆碱磷酸修饰的紫杉醇药物的制备方法,包括以下步骤:The invention provides a preparation method of a choline phosphate-modified paclitaxel medicine, comprising the following steps:
将羟乙基胆碱磷酸乙酯、紫杉醇和羧酸或其衍生物进行反应,得到胆碱磷酸修饰的紫杉醇药物。The choline phosphate modified paclitaxel drug is obtained by reacting ethyl hydroxyethylcholine phosphate, paclitaxel and carboxylic acid or its derivatives.
上述羟乙基胆碱磷酸乙酯的结构式如下:The structural formula of above-mentioned ethyl hydroxyethylcholine phosphate is as follows:
上述制备过程中,紫杉醇与连接基团L及丙烯酰氧乙基胆碱磷酸乙酯的反应可用一步法制得。紫杉醇C-2’位点上的羟基与连接基团L上的羧基进行酯化反应,与此同时,连接基团L上的氨基与胆碱磷酸上的丙烯酸酯双键进行迈克尔加成反应。本发明优选的,用DMAP、DIC作催化剂,在氮气保护下室温反应48小时,反应结束后通过柱层析即可得到终产物。类似的,紫杉醇与连接基团L及羟乙基胆碱磷酸乙酯的反应也可用一步法制得,优选的,用DMAP、DIC作为催化剂,优选的,所述反应的温度为室温,优选的,所述反应的时间为24小时,优选的,反应结束后在四氢呋喃中将产物沉析并干燥即可。In the above preparation process, the reaction of paclitaxel with the linking group L and acryloyloxyethylcholine ethyl phosphate can be prepared by one-step method. The hydroxyl group on the C-2' site of paclitaxel undergoes an esterification reaction with the carboxyl group on the linking group L, and at the same time, the amino group on the linking group L undergoes a Michael addition reaction with the acrylate double bond on the choline phosphate. Preferably, in the present invention, DMAP and DIC are used as catalysts, and the reaction is carried out at room temperature for 48 hours under nitrogen protection. After the reaction is completed, the final product can be obtained by column chromatography. Similarly, the reaction of paclitaxel with the linking group L and ethyl hydroxyethylcholine phosphate can also be prepared by one-step method, preferably, DMAP and DIC are used as catalysts, preferably, the temperature of the reaction is room temperature, preferably, The reaction time is 24 hours. Preferably, after the reaction is completed, the product is precipitated and dried in tetrahydrofuran.
实验结果表明,胆碱磷酸修饰后的紫杉醇显示出良好的水溶性及生物相容性,并且L基团可在肿瘤区域微酸性条件及酶环境下断裂,从而恢复紫杉醇的细胞毒性,实现对肿瘤细胞的靶向杀伤。The experimental results show that the paclitaxel modified by choline phosphate shows good water solubility and biocompatibility, and the L group can be cleaved under the slightly acidic conditions and enzyme environment in the tumor area, thereby restoring the cytotoxicity of paclitaxel and achieving anti-tumor effects. Targeted killing of cells.
本发明提供了上述胆碱磷酸修饰的紫杉醇药物或上述制备方法制备的胆碱磷酸修饰的紫杉醇药物在制备抗癌药物中的应用。更优选在制备靶向抗癌药物中的应用。The present invention provides the application of the choline phosphate-modified paclitaxel drug or the choline phosphate-modified paclitaxel drug prepared by the above preparation method in the preparation of an anticancer drug. More preferred is the application in the preparation of targeted anticancer drugs.
本发明优选的,所述抗癌药物为抗乳腺癌、卵巢癌的药物。Preferably, in the present invention, the anticancer drug is a drug against breast cancer and ovarian cancer.
与现有技术相比,本发明提供了一种胆碱磷酸修饰的紫杉醇药物,具有式Ⅰ所示结构。本发明所述的胆碱磷酸紫杉醇,其胆碱磷酸基团具有极强的亲水性,可以显著提升紫杉醇药物的水溶性,实现紫杉醇药物的注射给药;同时,胆碱磷酸基团具有较强的抗蛋白吸附性,可增强紫杉醇在体内的循环时间;连接基团,如甘氨酸,其与紫杉醇形成的酯键可以在肿瘤区域环境下被酯酶裂解,从而恢复紫杉醇的药性,实现靶向消灭癌细胞的目的。Compared with the prior art, the present invention provides a choline phosphate-modified paclitaxel drug, which has the structure shown in formula I. The choline phosphate paclitaxel of the present invention has extremely strong hydrophilicity in the choline phosphate group, which can significantly improve the water solubility of the paclitaxel drug and realize the injection and administration of the paclitaxel drug; meanwhile, the choline phosphate group has a relatively strong hydrophilicity. Strong anti-protein adsorption can enhance the circulation time of paclitaxel in the body; linking groups, such as glycine, the ester bond formed with paclitaxel can be cleaved by esterase in the tumor area environment, thereby restoring the pharmacological properties of paclitaxel and achieving targeting The purpose of destroying cancer cells.
附图说明Description of drawings
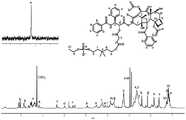
图1甘氨酸作为连接基团的胆碱磷酸紫杉醇的核磁共振氢谱和磷谱;Fig. 1 H NMR spectrum and phosphorus spectrum of choline phosphopaclitaxel with glycine as a linking group;
图2甘氨酸作为连接基团的胆碱磷酸紫杉醇的质谱谱图;Figure 2 is a mass spectrogram of choline phosphopaclitaxel with glycine as a linking group;
图3赖氨酸作为连接基团的胆碱磷酸紫杉醇的核磁共振氢谱和磷谱;Fig. 3 H NMR spectrum and phosphorus spectrum of choline phosphopaclitaxel with lysine as a linking group;
图4赖氨酸作为连接基团的胆碱磷酸紫杉醇的质谱谱图;Figure 4 is a mass spectrogram of choline phosphopaclitaxel with lysine as a linking group;
图5丁二酸作为连接基团的胆碱磷酸紫杉醇的核磁共振氢谱和磷谱;Fig. 5 H NMR spectrum and phosphorus spectrum of choline phosphopaclitaxel with succinic acid as a linking group;
图6丁二酸作为连接基团的胆碱磷酸紫杉醇的质谱图;Figure 6 is a mass spectrum of choline phosphopaclitaxel with succinic acid as a linking group;
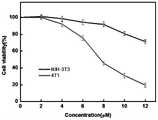
图7胆碱磷酸紫杉醇对小鼠成纤维细胞(NIH-3T3)和小鼠乳腺癌细胞(4T1)的细胞毒性测试结果;Fig. 7 Cytotoxicity test results of choline phosphate paclitaxel on mouse fibroblasts (NIH-3T3) and mouse breast cancer cells (4T1);
图8胆碱磷酸紫杉醇的溶血测试结果;Fig. 8 Hemolysis test results of choline phosphate paclitaxel;
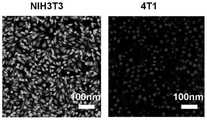
图9胆碱磷酸紫杉醇对小鼠成纤维细胞(NIH-3T3)和小鼠乳腺癌细胞(4T1)的活死染测试结果。Fig. 9 The results of live and dead staining test of choline phospho-paclitaxel on mouse fibroblasts (NIH-3T3) and mouse breast cancer cells (4T1).
具体实施方式Detailed ways
为了进一步说明本发明,下面结合实施例对本发明提供的胆碱磷酸修饰的紫杉醇药物及其制备方法和应用进行详细描述。In order to further illustrate the present invention, the choline phosphate-modified paclitaxel medicine provided by the present invention and its preparation method and application are described in detail below with reference to the examples.
实施例1以氨基酸作为连接基团的胆碱磷酸紫杉醇药物的制备Example 1 Preparation of choline phosphopaclitaxel drug with amino acid as linking group
将三乙胺(51g,0.51mol)、无水乙醇(22g,0.5mol)加入到装有300mL无水四氢呋喃的支口烧瓶中,氮气保护下磁力搅拌,在冰水浴中冷却30分钟后,从恒压滴液漏斗中滴加2-氯-2-氧-二氧磷杂环戊烷(71g,0.5mol),滴加过程中反应体系产生大量白色沉淀,1小时内滴加完毕,保持冰水浴反应2小时后缓慢升至室温,继续反应2小时,过滤,无水四氢呋喃洗涤滤饼两次,滤液合并收集,减压除去溶剂后得到粗品,粗品经短颈蒸馏,得纯品2-氧乙基-2-氧-二氧磷杂环戊烷(COP)49.4g,产率65%。产物结构如下:Triethylamine (51 g, 0.51 mol) and absolute ethanol (22 g, 0.5 mol) were added to a branch-mouthed flask containing 300 mL of anhydrous tetrahydrofuran, magnetically stirred under nitrogen protection, and cooled in an ice-water bath for 30 minutes. 2-Chloro-2-oxo-dioxaphosphalane (71g, 0.5mol) was added dropwise to the constant pressure dropping funnel. During the dropwise addition, the reaction system produced a large amount of white precipitates. The dropwise addition was completed within 1 hour, keeping the ice After 2 hours of water bath reaction, the reaction was slowly raised to room temperature, the reaction was continued for 2 hours, filtered, the filter cake was washed twice with anhydrous tetrahydrofuran, the filtrates were combined and collected, and the solvent was removed under reduced pressure to obtain the crude product, which was subjected to short-neck distillation to obtain pure 2-oxygen Ethyl-2-oxo-dioxaphosphalane (COP) 49.4 g, yield 65%. The product structure is as follows:
1H-NMR(500MHz,CDCl3):4.44-4.35(m,-OCH2CH2O-),4.15(m,-OCH2CH3),1.41(t,-OCH2CH3);31P-NMR(500MHz,CDCl3):δ(ppm)17.60(s).1 H-NMR (500 MHz, CDCl3 ): 4.44-4.35 (m, -OCH2 CH2 O-), 4.15 (m, -OCH2 CH3 ), 1.41 (t, -OCH2 CH3 );31 P -NMR (500MHz, CDCl3 ): δ(ppm) 17.60(s).
将COP(5.41g,30mmol),2-(二甲氨基)甲基丙烯酸乙酯(5.2g,33mmol)和100mg阻聚剂BHT加入到装有50mL无水乙腈的100毫升烧瓶中,加热到70℃反应56小时,反应结束后将溶液在500mL的四氢呋喃溶液中沉淀三次,最后除去四氢呋喃得到产物丙烯酰氧乙基胆碱磷酸乙酯5.6g,产率63%,产物结构如下:COP (5.41 g, 30 mmol), 2-(dimethylamino)ethyl methacrylate (5.2 g, 33 mmol) and 100 mg of inhibitor BHT were added to a 100-mL flask containing 50 mL of anhydrous acetonitrile and heated to 70 The reaction was carried out for 56 hours. After the reaction, the solution was precipitated three times in 500 mL of tetrahydrofuran solution. Finally, the tetrahydrofuran was removed to obtain 5.6 g of ethyl acryloyloxyethylcholine phosphate, with a yield of 63%. The product structure was as follows:
1H-NMR(500MHz,D2O):6.43 and 6.21(d and m,-OCCH=CH2),6.03(d,-OCCH=CH2),4.64(t,-CH2O-CO-),4.29(t,-CH2OP),3.86(m,-CH2N(CH3)2-CH2-),3.74(d,P-OCH2-CH2-),3.25(s,-N-(CH3)2),1.25(t,P-OCH2-CH3);31P-NMR(500MHz,D2O):δ(ppm)0.06(s).1 H-NMR (500 MHz, D2 O): 6.43 and 6.21 (d and m, -OCCH=CH2 ), 6.03 (d, -OCCH=CH2 ), 4.64 (t, -CH2 O-CO-) , 4.29(t, -CH2 OP), 3.86(m, -CH2 N(CH3 )2 -CH2 -), 3.74(d, P-OCH2 -CH2 -), 3.25(s, -N -(CH3 )2 ), 1.25 (t, P-OCH2 -CH3 );31 P-NMR (500 MHz, D2 O): δ (ppm) 0.06 (s).
将紫杉醇(2.0g,2.34mmol),丙烯酰氧乙基胆碱磷酸乙酯(0.7g,2.34mmol)和甘氨酸(0.18g,2.34mmol)加入到装有30mL无水乙腈的100毫升烧瓶中,室温反应48小时,反应结束后将溶液在500mL的四氢呋喃溶液中沉淀三次,最后除去四氢呋喃得到甘氨酸作为连接基团的胆碱磷酸紫杉醇1.73g,产率60%,产物结构如下:Paclitaxel (2.0 g, 2.34 mmol), ethyl acryloyloxyethylcholine phosphate (0.7 g, 2.34 mmol) and glycine (0.18 g, 2.34 mmol) were added to a 100 mL flask containing 30 mL of anhydrous acetonitrile, The reaction was carried out at room temperature for 48 hours. After the reaction, the solution was precipitated three times in 500 mL of tetrahydrofuran solution. Finally, the tetrahydrofuran was removed to obtain 1.73 g of choline phosphopaclitaxel with glycine as the linking group. The yield was 60%. The product structure was as follows:
1H-NMR(500MHz,CDCl3):7.3-8.2(d,m,-C6H5-),6.25(d,-NH-CH-CH-),5.8(d,-NH-CH-CH-),5.7(d,-C=C-CH-O-),5.0(s,-C-CH2-O-),4.64(t,-CH2O-CO-),4.29(t,-CH2OP),4.4(t,-O-CH-C-),4.0(d,C-CH-O-),3.86(m,-CH2N(CH3)2-CH2-),3.8(-C-CH-OH-),3.74(d,P-OCH2-CH2-),3.25(s,-N-(CH3)2),1.25(t,P-OCH2-CH3),2.4(s,-C-CH3),2.3(t,-CH-CH2-C-OH),2.2(s,-OOC-CH3),1.75(s,-OOCCH3),1.7(s,-C=C-CH3),1.6(m,-CH-CH2-CH-),1.25(s,-CO-C-CH3),1.24(s,-C=C-C-CH3);31P-NMR(500MHz,CDCl3):δ(ppm)-1.9(s).1 H-NMR (500 MHz, CDCl3 ): 7.3-8.2 (d, m, -C6 H5 -), 6.25 (d, -NH-CH-CH-), 5.8 (d, -NH-CH-CH -), 5.7(d, -C=C-CH-O-), 5.0(s, -C-CH2 -O-), 4.64(t, -CH2 O-CO-), 4.29(t, - CH2 OP), 4.4(t, -O-CH-C-), 4.0(d, C-CH-O-), 3.86(m, -CH2 N(CH3 )2 -CH2 -), 3.8 (-C-CH-OH-), 3.74(d, P-OCH2 -CH2 -), 3.25(s, -N-(CH3 )2 ), 1.25(t, P-OCH2 -CH3 ) , 2.4(s, -C-CH3 ), 2.3(t, -CH-CH2 -C-OH), 2.2(s, -OOC-CH3 ), 1.75(s, -OOCCH3 ), 1.7(s , -C=C-CH3 ), 1.6 (m, -CH-CH2 -CH-), 1.25 (s, -CO-C-CH3 ), 1.24 (s, -C=CC-CH3 );31 P-NMR (500MHz, CDCl3 ): δ(ppm)-1.9(s).
上述甘氨酸作为连接基团的胆碱磷酸紫杉醇的核磁共振氢谱和磷谱谱图如图1所示,质谱谱图如图2所示。The hydrogen nuclear magnetic resonance spectrum and phosphorus spectrum of the choline phosphopaclitaxel with glycine as the linking group are shown in FIG. 1 , and the mass spectrum is shown in FIG. 2 .
将紫杉醇(2.0g,2.34mmol),丙烯酰氧乙基胆碱磷酸乙酯(0.7g,2.34mmol)和赖氨酸(0.34g,2.34mmol)加入到装有30mL无水乙腈的100毫升烧瓶中,室温反应48小时,反应结束后将溶液在500mL的四氢呋喃溶液中沉淀三次,最后除去四氢呋喃得到赖氨酸作为连接基团的胆碱磷酸紫杉醇1.73g,产率60%,产物结构如下:Paclitaxel (2.0 g, 2.34 mmol), ethyl acryloyloxyethylcholine phosphate (0.7 g, 2.34 mmol) and lysine (0.34 g, 2.34 mmol) were added to a 100 mL flask containing 30 mL of anhydrous acetonitrile The reaction was carried out at room temperature for 48 hours. After the reaction, the solution was precipitated three times in 500 mL of tetrahydrofuran solution. Finally, the tetrahydrofuran was removed to obtain 1.73 g of choline phospho-paclitaxel with lysine as the linking group, and the yield was 60%. The product structure is as follows:
1H-NMR(500MHz,CDCl3):7.3-8.2(d,m,-C6H5-),6.25(d,-NH-CH-CH-),5.8(d,-NH-CH-CH-),5.7(d,-C=C-CH-O-),5.0(s,-C-CH2-O-),4.64(t,-CH2O-CO-),4.29(t,-CH2OP),4.4(t,-O-CH-C-),4.0(d,C-CH-O-),3.86(m,-CH2N(CH3)2-CH2-),3.8(-C-CH-OH-),3.74(d,P-OCH2-CH2-),3.25(s,-N-(CH3)2),1.25(t,P-OCH2-CH3),2.4(s,-C-CH3),2.3(t,-CH-CH2-C-OH),2.2(s,-OOC-CH3),1.75(s,-OOCCH3),1.7(s,-C=C-CH3),1.6(m,-CH-CH2-CH-),1.25(s,-CO-C-CH3),1.24(t,-NH-(CH2)4-CH-);31P-NMR(500MHz,CDCl3):δ(ppm)-1.9(s).1 H-NMR (500 MHz, CDCl3 ): 7.3-8.2 (d, m, -C6 H5 -), 6.25 (d, -NH-CH-CH-), 5.8 (d, -NH-CH-CH -), 5.7(d, -C=C-CH-O-), 5.0(s, -C-CH2 -O-), 4.64(t, -CH2 O-CO-), 4.29(t, - CH2 OP), 4.4(t, -O-CH-C-), 4.0(d, C-CH-O-), 3.86(m, -CH2 N(CH3 )2 -CH2 -), 3.8 (-C-CH-OH-), 3.74(d, P-OCH2 -CH2 -), 3.25(s, -N-(CH3 )2 ), 1.25(t, P-OCH2 -CH3 ) , 2.4(s, -C-CH3 ), 2.3(t, -CH-CH2 -C-OH), 2.2(s, -OOC-CH3 ), 1.75(s, -OOCCH3 ), 1.7(s , -C=C-CH3 ), 1.6(m, -CH-CH2 -CH-), 1.25(s, -CO-C-CH3 ), 1.24(t, -NH-(CH2 )4 - CH-);31 P-NMR (500MHz, CDCl3 ): δ(ppm)-1.9(s).
上述赖氨酸作为连接基团的胆碱磷酸紫杉醇的核磁共振氢谱和磷谱谱图如图3所示,质谱谱图如图4所示。The hydrogen nuclear magnetic resonance spectrum and phosphorus spectrum of the choline phosphopaclitaxel with the above-mentioned lysine as a linking group are shown in FIG. 3 , and the mass spectrum is shown in FIG. 4 .
实施例2以丁二酸作为连接基团的胆碱磷酸紫杉醇药物的制备Example 2 Preparation of choline phosphopaclitaxel medicine with succinic acid as linking group
2-氧乙基-2-氧-二氧磷杂环戊烷(COP)制备过程同实施例1。The preparation process of 2-oxoethyl-2-oxo-dioxaphosphalane (COP) is the same as that of Example 1.
将COP(5.41g,30mmol),二甲基氨基乙醇(2.94g,33mmol)加入到装有50mL无水乙腈的100毫升烧瓶中,加热到70℃反应56小时,反应结束后将溶液在500mL的四氢呋喃溶液中沉淀三次,最后除去四氢呋喃得到产物羟乙基胆碱磷酸乙酯5.6g,产率63%,产物结构如下:COP (5.41g, 30mmol) and dimethylaminoethanol (2.94g, 33mmol) were added to a 100-mL flask containing 50mL of anhydrous acetonitrile, heated to 70°C and reacted for 56 hours. After the reaction, the solution was placed in 500mL of Precipitate three times in the tetrahydrofuran solution, and finally remove the tetrahydrofuran to obtain the product 5.6g of ethyl hydroxyethylcholine phosphate, the yield is 63%, and the product structure is as follows:
1H-NMR(500MHz,D2O):4.64(t,-CH2O-CO-),4.29(t,-CH2OP),3.86(m,-CH2N(CH3)2-CH2-),3.74(d,P-OCH2-CH2-),3.25(s,-N-(CH3)2),1.25(t,P-OCH2-CH3);31P-NMR(500MHz,D2O):δ(ppm)0.06(s).1 H-NMR (500 MHz, D2 O): 4.64 (t, -CH2 O-CO-), 4.29 (t, -CH2 OP), 3.86 (m, -CH2 N(CH3 )2 -CH2- ), 3.74 (d, P-OCH2 -CH2 -), 3.25 (s, -N-(CH3 )2 ), 1.25 (t, P-OCH2 -CH3 );31 P-NMR ( 500MHz, D2 O): δ(ppm)0.06(s).
将紫杉醇(2.0g,2.34mmol),羟乙基胆碱磷酸乙酯(0.62g,2.34mmol)和丁二酸(0.28g,2.34mmol)加入到装有30mL无水乙腈的100毫升烧瓶中,室温反应48小时,反应结束后将溶液在500mL的四氢呋喃溶液中沉淀三次,最后除去四氢呋喃得到丁二酸作为连接基团的胆碱磷酸紫杉醇1.73g,产率60%,产物结构如下:Paclitaxel (2.0 g, 2.34 mmol), ethyl hydroxyethylcholine phosphate (0.62 g, 2.34 mmol) and succinic acid (0.28 g, 2.34 mmol) were added to a 100 mL flask containing 30 mL of anhydrous acetonitrile, The reaction was carried out at room temperature for 48 hours. After the reaction, the solution was precipitated three times in 500 mL of tetrahydrofuran solution. Finally, the tetrahydrofuran was removed to obtain 1.73 g of choline phosphate paclitaxel with succinic acid as the linking group. The yield was 60%. The product structure was as follows:
1H-NMR(500MHz,CDCl3):7.3-8.2(d,m,-C6H5-),6.25(d,-NH-CH-CH-),5.8(d,-NH-CH-CH-),5.7(d,-C=C-CH-O-),5.0(s,-C-CH2-O-),4.64(t,-CH2O-CO-),4.29(t,-CH2OP),4.4(t,-O-CH-C-),4.0(d,C-CH-O-),3.86(m,-CH2N(CH3)2-CH2-),3.8(-C-CH-OH-),3.74(d,P-OCH2-CH2-),3.25(s,-N-(CH3)2),2.8(t,-OOC-(CH2)2-COO-),2.4(s,-C-CH3),2.3(t,-CH-CH2-C-OH),2.2(s,-OOC-CH3),1.75(s,-OOCCH3),1.7(s,-C=C-CH3),1.6(m,-CH-CH2-CH-),1.25(s,-CO-C-CH3),1.25(t,P-OCH2-CH3),1.24(s,-C=C-C-CH3);31P-NMR(500MHz,CDCl3):δ(ppm)-1.9(s).1 H-NMR (500 MHz, CDCl3 ): 7.3-8.2 (d, m, -C6 H5 -), 6.25 (d, -NH-CH-CH-), 5.8 (d, -NH-CH-CH -), 5.7(d, -C=C-CH-O-), 5.0(s, -C-CH2 -O-), 4.64(t, -CH2 O-CO-), 4.29(t, - CH2 OP), 4.4(t, -O-CH-C-), 4.0(d, C-CH-O-), 3.86(m, -CH2 N(CH3 )2 -CH2 -), 3.8 (-C-CH-OH-), 3.74(d, P-OCH2 -CH2 -), 3.25(s, -N-(CH3 )2 ), 2.8(t, -OOC-(CH2 )2 -COO-), 2.4(s, -C-CH3 ), 2.3(t, -CH-CH2 -C-OH), 2.2(s, -OOC-CH3 ), 1.75(s, -OOCCH3 ) , 1.7(s, -C=C-CH3 ), 1.6(m, -CH-CH2 -CH-), 1.25(s, -CO-C-CH3 ), 1.25(t, P-OCH2 - CH3 ), 1.24 (s, -C=CC-CH3 );31 P-NMR (500 MHz, CDCl3 ): δ (ppm)-1.9 (s).
上述丁二酸作为连接基团的胆碱磷酸紫杉醇的核磁共振氢谱和磷谱谱图如图5所示,质谱谱图如图6所示。The hydrogen nuclear magnetic resonance spectrum and phosphorus spectrum of the choline phosphopaclitaxel with succinic acid as the linking group are shown in FIG. 5 , and the mass spectrum is shown in FIG. 6 .
实施例3胆碱磷酸紫杉醇作为抗癌药物的应用Example 3 Application of choline phosphate paclitaxel as anticancer drug
测试胆碱磷酸紫杉醇的水溶性:取一定量的实施例1、2制备的胆碱磷酸紫杉醇药物溶解于定量的蒸馏水中并连续搅拌至澄清,继续加入药物直至不再溶解为止,此时药物的加入量与水的用量的比值,即为胆碱磷酸紫杉醇在水中的最大溶解度。该溶解度是指导给药量的重要指标之一。Test the water solubility of choline phosphate paclitaxel: take a certain amount of the choline phosphate paclitaxel drug prepared in Examples 1 and 2 and dissolve it in quantitative distilled water and stir continuously until it becomes clear, and continue to add the drug until it is no longer dissolved. The ratio of the added amount to the amount of water is the maximum solubility of choline phosphate paclitaxel in water. The solubility is one of the important indicators to guide the dosage.
结果表明,以甘氨酸作为连接基团的胆碱磷酸紫杉醇药物在水中的最大溶解度为10mg/mL;以赖氨酸作为连接基团的胆碱磷酸紫杉醇药物在水中的最大溶解度为10.5mg/mL;以丁二酸作为连接基团的胆碱磷酸紫杉醇药物在水中的最大溶解度为8.5mg/mL。The results showed that the maximum solubility of the choline phospho-paclitaxel drug with glycine as the linking group in water was 10 mg/mL; the maximum solubility of the choline phospho-paclitaxel drug with lysine as the linking group in water was 10.5 mg/mL; The maximum solubility of the choline phosphopaclitaxel drug with succinic acid as the linking group in water is 8.5 mg/mL.
测试胆碱磷酸紫杉醇药物的细胞毒性:将小鼠成纤维细胞(NIH-3T3)和小鼠乳腺癌细胞(4T1)在96孔板中以1×104的密度孵育。孵育12小时后,分别用含2-12μM浓度的实施例1、2制备的胆碱磷酸紫杉醇药物的培养基将细胞孵育24小时,然后向每个孔中加入10μLCelltiter-Blue试剂。再温育4小时后,通过酶标仪(λex=560nm,λem=590nm)检测细胞存活率。通过以下公式计算细胞存活率:To test the cytotoxicity of the choline phospho-paclitaxel drug: Mouse fibroblasts (NIH-3T3) and mouse breast cancer cells (4T1) were incubated in 96-well plates at a density of1 x 104. After 12 hours of incubation, the cells were incubated for 24 hours with media containing the choline phospho-paclitaxel drug prepared in Examples 1 and 2 at a concentration of 2-12 μM, respectively, and then 10 μL of Celltiter-Blue reagent was added to each well. After an additional 4 hours of incubation, cell viability was detected by a microplate reader (λex=560 nm, λem=590 nm). Cell viability was calculated by the following formula:
细胞存活率(%)=(样品荧光强度/对照荧光强度)×100%Cell viability (%) = (sample fluorescence intensity/control fluorescence intensity) × 100%
胆碱磷酸紫杉醇对小鼠成纤维细胞(NIH-3T3)和小鼠乳腺癌细胞(4T1)的细胞毒性测试结果如图7所示。The cytotoxicity test results of choline phospho-paclitaxel on mouse fibroblasts (NIH-3T3) and mouse breast cancer cells (4T1) are shown in FIG. 7 .
测试胆碱磷酸紫杉醇药物的溶血率:从心脏收集新鲜的小鼠血液,并用PBS洗涤红细胞(RBC)3次。之后,将RBC稀释并用10mL PBS悬浮。首先,将0.3mL RBCs悬浮液与1.2mLPBS混合作为阴性对照组,并与1.2mL水混合作为阳性对照组。将溶解于1.2mL PBS中的不同浓度的药物添加到RBCs悬浮液(0.3mL)中,然后在37℃温育2小时。最后,将样品以12000r/min离心10分钟,收集上清液,并用微孔板分光光度计测量541nm处的吸光度。根据以下等式计算溶血百分比:To test the rate of hemolysis of the choline phospho-paclitaxel drug: Fresh mouse blood was collected from the heart and red blood cells (RBC) were washed 3 times with PBS. Afterwards, the RBCs were diluted and suspended with 10 mL of PBS. First, 0.3 mL of RBCs suspension was mixed with 1.2 mL of PBS as a negative control, and with 1.2 mL of water as a positive control. Various concentrations of drugs dissolved in 1.2 mL of PBS were added to the RBCs suspension (0.3 mL), followed by incubation at 37°C for 2 hours. Finally, the samples were centrifuged at 12,000 r/min for 10 minutes, the supernatant was collected, and the absorbance at 541 nm was measured with a microplate spectrophotometer. Calculate the percent hemolysis according to the following equation:
溶血百分比(%)=(样品的吸光度)/(阳性对照的吸光度)×100%Hemolysis percentage (%)=(absorbance of sample)/(absorbance of positive control)×100%
溶血测试结果如图8所示。The results of the hemolysis test are shown in Figure 8.
对小鼠成纤维细胞(NIH-3T3)和小鼠乳腺癌细胞(4T1)的活死染测试:NIH-3T3细胞和4T1细胞在24孔板中培养,密度为1×105个细胞。孵育24小时后,将培养基更换为含有10μg/mL胆碱磷酸紫杉醇药物的新鲜培养基。细胞继续培养24小时。然后,去除旧培养基,用PBS、0.25mL钙黄绿素乙酰氧基甲酯(Calcein AM,Ex/Em=494/517nm)和碘化丙啶(PI,Ex/Em=535/617nm)混合液洗涤细胞两次加入每孔中,避光培养40分钟,最后将制备好的样品在激光扫描共聚焦显微镜下成像,结果如图9所示。Live-dead staining assay on mouse fibroblasts (NIH-3T3) and mouse breast cancer cells (4T1): NIH-3T3 cells and 4T1 cells were cultured in 24-well plates at a density of 1×105 cells. After 24 hours of incubation, the medium was replaced with fresh medium containing 10 μg/mL of the drug choline phospho-paclitaxel. Cells were continued to culture for 24 hours. Then, the old medium was removed and washed with a mixture of PBS, 0.25 mL calcein acetoxymethyl ester (Calcein AM, Ex/Em=494/517 nm) and propidium iodide (PI, Ex/Em=535/617 nm) The cells were added to each well twice, and incubated in the dark for 40 minutes. Finally, the prepared samples were imaged under a laser scanning confocal microscope. The results are shown in Figure 9.
以上结果表明胆碱磷酸紫杉醇药物具有优异的水溶性,可靶向消灭癌细胞,且不会对正常细胞造成损伤,具有作为靶向抗癌药物的潜质。The above results show that the choline phospho-paclitaxel drug has excellent water solubility, can target and destroy cancer cells without causing damage to normal cells, and has the potential as a targeted anticancer drug.
以上实施例的说明只是用于帮助理解本发明的方法及其核心思想。应当指出,对于本技术领域的普通技术人员来说,在不脱离本发明原理的前提下,还可以对本发明进行若干改进和修饰,这些改进和修饰也落入本发明权利要求的保护范围内。The descriptions of the above embodiments are only used to help understand the method and the core idea of the present invention. It should be pointed out that for those skilled in the art, without departing from the principle of the present invention, several improvements and modifications can also be made to the present invention, and these improvements and modifications also fall within the protection scope of the claims of the present invention.
Claims (9)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210107371.4ACN114437128B (en) | 2022-01-28 | 2022-01-28 | Choline phosphate modified taxol medicine and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210107371.4ACN114437128B (en) | 2022-01-28 | 2022-01-28 | Choline phosphate modified taxol medicine and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114437128Atrue CN114437128A (en) | 2022-05-06 |
| CN114437128B CN114437128B (en) | 2023-12-19 |
Family
ID=81372207
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210107371.4AActiveCN114437128B (en) | 2022-01-28 | 2022-01-28 | Choline phosphate modified taxol medicine and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114437128B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111372587A (en)* | 2017-09-08 | 2020-07-03 | 里兰斯坦福初级大学理事会 | ENPP1 inhibitors and their use for the treatment of cancer |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5534499A (en)* | 1994-05-19 | 1996-07-09 | The University Of British Columbia | Lipophilic drug derivatives for use in liposomes |
| CN103096874A (en)* | 2010-04-15 | 2013-05-08 | 华盛顿大学 | Prodrug compositions, prodrug nanoparticles, and methods of use thereof |
| CN104225615A (en)* | 2014-09-24 | 2014-12-24 | 东南大学 | Taxol phospholipids compound, medicine composition and application thereof |
| CN105233298A (en)* | 2015-09-18 | 2016-01-13 | 东南大学 | Paclitaxel phospholipid compound and drug combination and application thereof |
| CN105457038A (en)* | 2015-11-09 | 2016-04-06 | 东南大学 | Quick release type medicine phosphatide compound and medicine composition thereof |
| CN107708702A (en)* | 2014-11-17 | 2018-02-16 | 塞勒克塔生物科学有限公司 | Phospholipid ether analogues as drug carriers targeting cancer |
| CN111662250A (en)* | 2019-03-05 | 2020-09-15 | 中国医学科学院药物研究所 | Quaternized modified taxane derivative, pharmaceutical composition, synthetic route and application thereof |
- 2022
- 2022-01-28CNCN202210107371.4Apatent/CN114437128B/enactiveActive
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5534499A (en)* | 1994-05-19 | 1996-07-09 | The University Of British Columbia | Lipophilic drug derivatives for use in liposomes |
| CN103096874A (en)* | 2010-04-15 | 2013-05-08 | 华盛顿大学 | Prodrug compositions, prodrug nanoparticles, and methods of use thereof |
| CN104225615A (en)* | 2014-09-24 | 2014-12-24 | 东南大学 | Taxol phospholipids compound, medicine composition and application thereof |
| CN107708702A (en)* | 2014-11-17 | 2018-02-16 | 塞勒克塔生物科学有限公司 | Phospholipid ether analogues as drug carriers targeting cancer |
| CN105233298A (en)* | 2015-09-18 | 2016-01-13 | 东南大学 | Paclitaxel phospholipid compound and drug combination and application thereof |
| CN105457038A (en)* | 2015-11-09 | 2016-04-06 | 东南大学 | Quick release type medicine phosphatide compound and medicine composition thereof |
| CN111662250A (en)* | 2019-03-05 | 2020-09-15 | 中国医学科学院药物研究所 | Quaternized modified taxane derivative, pharmaceutical composition, synthetic route and application thereof |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111372587A (en)* | 2017-09-08 | 2020-07-03 | 里兰斯坦福初级大学理事会 | ENPP1 inhibitors and their use for the treatment of cancer |
| CN111372587B (en)* | 2017-09-08 | 2024-01-09 | 里兰斯坦福初级大学理事会 | ENPP1 inhibitors and their use for the treatment of cancer |
Also Published As
| Publication number | Publication date |
|---|---|
| CN114437128B (en) | 2023-12-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3231043B2 (en) | Pharmacologically active nitrate and its preparation | |
| US5880131A (en) | High molecular weight polymer-based prodrugs | |
| RU2421443C2 (en) | Substituted beta-phenyl-alpha-hydroxyl propanoic acid, synthesis method and use | |
| US6277841B1 (en) | Quinoline ligands and metal complexes for diagnosis and therapy | |
| AU2010205816B2 (en) | Novel eicosanoid derivatives | |
| JP4819810B2 (en) | TRPV1 agonists, formulations containing them and uses thereof | |
| JP2002543171A (en) | Novel quinones as disease treatments | |
| CN106866572B (en) | Nitric oxide donator type β elemene derivatives and its production and use | |
| CN101100416B (en) | Small molecule inhibitor for preventing Alzheimer's disease Abeta polypeptide from fiberizing and its preparation method, pharmaceutical composition and application | |
| JP2023543069A (en) | Crystal of pyridinylphenyl compound and method for producing the same | |
| CN114437128B (en) | Choline phosphate modified taxol medicine and preparation method and application thereof | |
| CN108947949B (en) | Anxiolytic deuterated compounds and medical application thereof | |
| CN105641711B (en) | Using organic amine-modified vitamin C as the dual Brain targeting prodrug of carrier | |
| CN111362837A (en) | NQO1 activated Combretastatin A4 prodrug and synthesis method and application thereof | |
| JP2021512138A (en) | 2- (Α-Hydroxypentyl) benzoic acid organic amine ester derivative drug | |
| JPWO2008093655A1 (en) | Polyalcohol compounds and medicines | |
| CN114656453B (en) | Heptamethine indocyanine-TEMPO chemical even chain small molecule, preparation method and application thereof in preparation of radiation protection preparation | |
| CN112175014B (en) | Nitric oxide donor type tetravalent platinum derivative, preparation method and medical use thereof | |
| CN115137727A (en) | GSH/H 2 O 2 Application of double-response zwitter ion rhodamine-camptothecin nano prodrug in cancer chemotherapy | |
| CN114426538A (en) | Berberine canagliflozin derivative and preparation method and application thereof | |
| CN119874643B (en) | Paclitaxel prodrug, and preparation method and application thereof | |
| WO2011147254A1 (en) | Phenylbutyryl curcumin derivatives and uses for preparing anti-tumor drugs thereof | |
| CN115197113B (en) | Combretastatin A-4 derivative containing thiourea structure, its preparation method and use | |
| CN108440512A (en) | A kind of antitumoral compounds and preparation method thereof | |
| JP2700031B2 (en) | 0-phosphonocholine ester derivative |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |