CN114369623B - A kind of Synaptotagmin2-RNAi based on Cre-lox recombination system and its application - Google Patents
A kind of Synaptotagmin2-RNAi based on Cre-lox recombination system and its applicationDownload PDFInfo
- Publication number
- CN114369623B CN114369623BCN202210030878.4ACN202210030878ACN114369623BCN 114369623 BCN114369623 BCN 114369623BCN 202210030878 ACN202210030878 ACN 202210030878ACN 114369623 BCN114369623 BCN 114369623B
- Authority
- CN
- China
- Prior art keywords
- rnai
- cre
- expression
- aav2itr
- expression vector
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/30—Vector systems comprising sequences for excision in presence of a recombinase, e.g. loxP or FRT
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2830/00—Vector systems having a special element relevant for transcription
- C12N2830/34—Vector systems having a special element relevant for transcription being a transcription initiation element
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biophysics (AREA)
- Virology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明属于生物医学技术领域,尤其涉及一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用。The invention belongs to the technical field of biomedicine, and in particular relates to a Cre-lox recombination system-based Synaptotagmin2-RNAi and an application thereof.
背景技术Background technique
1.RNA干扰技术:1. RNA interference technology:
RNA干扰(RNA interference,RNAi)是指在进化过程中高度保守的、由双链RNA(double-stranded RNA,dsRNA)诱发的、和同源mRNA高效特异性降解的现象。由于使用RNAi技术可以特异性降低或关闭特定基因在细胞或有机体中的表达,所以该技术已被广泛用于探索基因功能和传染性疾病及恶性肿瘤的基因治疗领域。人们也基于这一现象研究了一种反向调控基因表达的遗传学机制。在动物中,RNAi可以通过U6启动表达shRNA来实现。目前的U6载体可以通过2种方式在动物细胞内表达:瞬时表达以及稳定表达。稳定表达主要是通过插入基因组中实现的。RNA interference (RNA interference, RNAi) refers to the highly conserved phenomenon in the evolution process, induced by double-stranded RNA (double-stranded RNA, dsRNA), and the efficient and specific degradation of homologous mRNA. Since the use of RNAi technology can specifically reduce or shut down the expression of specific genes in cells or organisms, this technology has been widely used in the field of gene therapy for exploring gene functions and infectious diseases and malignant tumors. Based on this phenomenon, people have also studied a genetic mechanism of negative regulation of gene expression. In animals, RNAi can be achieved by expressing shRNA from the U6 promoter. The current U6 vector can be expressed in animal cells in two ways: transient expression and stable expression. Stable expression is mainly achieved by insertion into the genome.
2.Cre-lox技术:2. Cre-lox technology:
Cre-lox技术是20世纪80年代从P1噬菌体中发现的一种位点特异性重组技术。该技术可通过Cre重组酶和lox位点的相互作用,实现目的片段的删除、翻转、插入和易位等。该技术不需要借助任何辅助因子,可作用于多种结构的DNA底物,如线形、环状甚至超螺旋DNA等,操作简单、快速、高效,因此被广泛应用于真核生物和原核生物的基因敲除、插入、翻转和易位等研究中。目前,Cre-lox系统应用最热门的领域是基因打靶,已被证明是哺乳动物细胞和小鼠遗传操作最有用的工具,该系统和基因打靶的结合提供了实现条件基因敲除或激活的手段。Cre-lox technology is a site-specific recombination technology discovered from P1 phage in the 1980s. This technology can realize the deletion, flipping, insertion and translocation of the target fragment through the interaction of Cre recombinase and lox site. This technology does not need any cofactors, and can act on DNA substrates with various structures, such as linear, circular or even supercoiled DNA. It is simple, fast and efficient, so it is widely used in eukaryotic and prokaryotic In the study of gene knockout, insertion, flipping and translocation. At present, the most popular field of application of the Cre-lox system is gene targeting, which has been proved to be the most useful tool for genetic manipulation of mammalian cells and mice. The combination of this system and gene targeting provides a means to achieve conditional gene knockout or activation .
3.病毒依赖的基因重组3. Virus-dependent gene recombination
由于转基因动物依赖的基因重组存在耗时长、成本高、区域或者组织特异性不高等不足。采用比较灵活的方式使用Cre-lox系统,例如通过病毒引入Cre或loxP元件,从而实现基因重组。借助病毒表达的cre-lox重组系统,可以特异性对某些细胞的标记和操控。当前运用神经示踪技术对大脑特定神经环路的结构和功能进行解析时,运用 Cre重组酶系统和 AAV血清型(比如 rAAV2/9、rAAV2/retro、rAAV2/1)结合,达到特异性对神经环路标记和功能研究的目的:病毒可以通过局部注射的方式保证区域特异性感染,再加上驱动 Cre基因的特异性启动子,能够实现更强的区域和细胞特异性的基因重组。但是对于如何时空特异性地干扰特定的神经环路的功能,需要涉及一种更优的时空特异调控相关重要基因的表达工具的开发。Due to the gene recombination that transgenic animals rely on, there are disadvantages such as time-consuming, high cost, and low regional or tissue specificity. Use the Cre-lox system in a more flexible way, such as introducing Cre or loxP elements through viruses to achieve gene recombination. With the help of the cre-lox recombinant system expressed by the virus, certain cells can be specifically marked and manipulated. When using neural tracing technology to analyze the structure and function of specific neural circuits in the brain, the Cre recombinase system is combined with AAV serotypes (such as rAAV2/9, rAAV2/retro, rAAV2/1) to achieve specific neural The purpose of loop labeling and functional research: The virus can ensure region-specific infection through local injection, coupled with the specific promoter driving the Cre gene, it can achieve stronger region and cell-specific gene recombination. However, how to specifically interfere with the function of specific neural circuits in time and space needs to involve the development of a better expression tool for time and space specific regulation of related important genes.
发明内容Contents of the invention
本发明实施例的目的在于提供一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用,旨在解决背景技术提出的问题。The purpose of the embodiments of the present invention is to provide a Cre-lox recombination system-based Synaptotagmin2-RNAi and its application, aiming to solve the problems raised by the background technology.
本发明实施例是这样实现的,一种基于Cre-lox重组系统的Synaptotagmin2-RNAi,包括一种Cre酶依赖的rAAV表达的RNAi表达载体,所述的Cre酶依赖的rAAV表达的RNAi表达载体包含顺次连接的:AAV2ITR,兴奋性神经元表达的启动子CaMKIIα,Cre酶依赖的RNAi表达盒,Syt2 RNAi发卡结构插入位点,WPRE,bGHpoly(A)signal和AAV2ITR。The embodiment of the present invention is achieved in this way, a Synaptotagmin2-RNAi based on the Cre-lox recombination system includes a Cre enzyme-dependent RNAi expression vector for rAAV expression, and the Cre enzyme-dependent rAAV expression RNAi expression vector contains Linked in sequence: AAV2ITR, excitatory neuron-expressed promoter CaMKIIα, Cre-dependent RNAi expression cassette, Syt2 RNAi hairpin insertion site, WPRE, bGHpoly(A) signal, and AAV2ITR.
进一步的技术方案,所使用的的Syt2 shRNA 的具体序列为:In a further technical solution, the specific sequence of the Syt2 shRNA used is:
5’-CCCTTTGACCCTCAGTGAT-3’。5'-CCCTTTGACCCTCAGTGAT-3'.
对照组Scramble shRNA的具体序列为:The specific sequence of the control group Scramble shRNA is:
5’-GGTTTATATCGCGGTTATT -3’。5'-GGTTTATATCGCGGTTATT-3'.
本发明实施例的另一目的在于,一种基于Cre-lox重组系统的Synaptotagmin2-RNAi的应用,将所述的Synaptotagmin2-RNAi应用于重组病毒pAAV2-CaMKIIα-DIO-(mCherry-bGH polyA-U6)-shRNA(Syt2/Scramble)-WPRE -hGH polyA的制备。Another object of the embodiments of the present invention is an application of Synaptotagmin2-RNAi based on the Cre-lox recombination system, and the Synaptotagmin2-RNAi is applied to the recombinant virus pAAV2-CaMKIIα-DIO-(mCherry-bGH polyA-U6) - Preparation of shRNA(Syt2/Scramble)-WPRE-hGH polyA.
进一步的技术方案,将所述的重组病毒pAAV2-CaMKIIα-DIO-(mCherry-bGHpolyA-U6)-shRNA(Syt2/Scramble)-WPRE-hGH polyA 注射到小鼠岛叶,并将辅助病毒AAV2/R-hSyn-cre-WPRE-hGH polyA注射到小鼠基底外侧杏仁核,待病毒表达四周后,观察腓总神经结扎模型小鼠的后足底机械痛阈值。In a further technical scheme, the recombinant virus pAAV2-CaMKIIα-DIO-(mCherry-bGHpolyA-U6)-shRNA(Syt2/Scramble)-WPRE-hGH polyA was injected into the mouse insula, and the helper virus AAV2/R -hSyn-cre-WPRE-hGH polyA was injected into the basolateral amygdala of mice, and after the virus was expressed for four weeks, the mechanical pain threshold of the rear foot of the common peroneal nerve ligation model mice was observed.
本发明实施例提供的一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用,通过腺相关病毒依赖的Cre-lox重组系统,对特定脑区Synaptotagmin-2 分子进行shRNA干扰,从而应用于镇痛治疗。本发明在生物信息学分析的基础上,发现小鼠痛模型中高表达的突触传递相关蛋白Synaptotagmin 2 (简称Syt2:是一种钙结合蛋白,有助于钙离子触发中枢和神经肌肉突触的快速神经递质释放,在神经系统的多个脑区都有表达),因此构建了shRNA干扰策略,可有效缓解痛模型动物的伤害性感受,本发明将为靶向镇痛治疗提供方向。A Cre-lox recombination system-based Synaptotagmin2-RNAi and its application provided in the embodiment of the present invention, through the adeno-associated virus-dependent Cre-lox recombination system, perform shRNA interference on the Synaptotagmin-2 molecule in a specific brain region, so as to be applied to the town pain treatment. On the basis of bioinformatics analysis, the present invention finds that the highly expressed synaptic transmission-related protein Synaptotagmin 2 (Syt2 for short) in the mouse pain model is a calcium-binding protein that helps calcium ions trigger the activation of the central and neuromuscular synapses. Rapid neurotransmitter release, expressed in multiple brain regions of the nervous system), so the shRNA interference strategy was constructed, which can effectively relieve the nociception of pain model animals, and the present invention will provide direction for targeted analgesic therapy.
附图说明Description of drawings
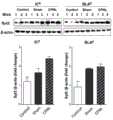
图1为本发明实施例提供的一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用中的痛模型小鼠IC和BLA脑区Syt2分子表达情况。Figure 1 shows the expression of Syt2 molecules in the IC and BLA brain regions of pain model mice in a Cre-lox recombination system-based Synaptotagmin2-RNAi and its application provided by the embodiment of the present invention.
图2为本发明实施例提供的一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用中的Syt2荧光素酶检测图。Fig. 2 is a detection diagram of Syt2 luciferase in a Cre-lox recombination system-based Synaptotagmin2-RNAi and its application provided by the embodiment of the present invention.
图3为本发明实施例提供的一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用中的病毒注射示意图。Fig. 3 is a schematic diagram of a Cre-lox recombination system-based Synaptotagmin2-RNAi and virus injection in its application provided by the embodiment of the present invention.
图4为本发明实施例提供的一种基于Cre-lox重组系统的Synaptotagmin2-RNAi及其应用中的行为学检测结果。Fig. 4 is a Cre-lox recombination system-based Synaptotagmin2-RNAi provided in the embodiment of the present invention and the behavioral detection results in its application.
具体实施方式Detailed ways
为了使本发明的目的、技术方案及优点更加清楚明白,以下结合附图及实施例,对本发明进行进一步详细说明。应当理解,此处所描述的具体实施例仅仅用以解释本发明,并不用于限定本发明。In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
以下结合具体实施例对本发明的具体实现进行详细描述。The specific implementation of the present invention will be described in detail below in conjunction with specific embodiments.
本发明一个实施例提供的一种基于Cre-lox重组系统的Synaptotagmin2-RNAi,包括一种Cre酶依赖的rAAV表达的RNAi表达载体,所述的Cre酶依赖的rAAV表达的RNAi表达载体包含顺次连接的:AAV2ITR,兴奋性神经元表达的启动子CaMKIIα,Cre酶依赖的RNAi表达盒,Syt2 RNAi发卡结构插入位点,WPRE,bGHpoly(A)signal和AAV2ITR。A Cre-lox recombinant system-based Synaptotagmin2-RNAi provided by an embodiment of the present invention includes a Cre enzyme-dependent RNAi expression vector for rAAV expression, and the Cre enzyme-dependent RNAi expression vector for rAAV expression contains sequential Linked: AAV2ITR, excitatory neuron-expressed promoter CaMKIIα, Cre-dependent RNAi expression cassette, Syt2 RNAi hairpin insertion site, WPRE, bGHpoly(A) signal, and AAV2ITR.
所使用的Syt2 shRNA 的具体序列为:5’-CCCTTTGACCCTCAGTGAT-3’,对照Scramble shRNA的具体序列为:5’-GGTTTATATCGCGGTTATT -3’。The specific sequence of the Syt2 shRNA used is: 5'-CCCTTTGACCCCAGTGAT-3', and the specific sequence of the control Scramble shRNA is: 5'-GGTTTATATCGCGGTTATT-3'.
小鼠痛觉模型实验的具体步骤包括:The specific steps of the mouse pain model experiment include:
1.建立小鼠腓总神经结扎(common peroneal nerve ligation,CPNL)模型:小鼠通过2%异氟醚麻醉后,在左腿做切口暴露腓总神经,无菌手术线结扎腓总神经后局部缝合消毒,待动物清醒后放回笼中饲养。假手术组仅暴露腓总神经,但不进行结扎。1. Establish the common peroneal nerve ligation (CPNL) model in mice: after mice were anesthetized with 2% isoflurane, an incision was made in the left leg to expose the common peroneal nerve, and the common peroneal nerve was ligated with sterile surgical thread locally The sutures were sterilized, and the animals were returned to their cages for feeding after they regained consciousness. In the sham operation group, only the common peroneal nerve was exposed without ligation.
2.机械痛检测:动物手术前使用von-frey丝(克数为0.008g,0.02g,0.04g,0.16g,0.4g,0.6g,1g,1.4g以及2g)检测左足机械痛阈值,记为第0天数据,手术后从第1天开始,每天固定时间检测左足机械痛阈值,并进行统计学分析。2. Mechanical pain detection: Use von-frey wire (0.008g, 0.02g, 0.04g, 0.16g, 0.4g, 0.6g, 1g, 1.4g and 2g) to detect the mechanical pain threshold of the left foot before the animal operation, record It is the data of the 0th day. From the 1st day after the operation, the mechanical pain threshold of the left foot is detected at a fixed time every day, and the statistical analysis is carried out.
3.蛋白印迹分析:动物术后第7天,麻醉分离大脑,对右侧IC和BLA脑区进行取材提蛋白,对Syt2等蛋白表达进行半定量分析(结果如图1所示)。3. Western blot analysis: On the 7th day after the operation, the animals were anesthetized to separate the brain, and the right IC and BLA brain regions were extracted to extract proteins, and the expression of Syt2 and other proteins was semi-quantitatively analyzed (results shown in Figure 1).
4.病毒构建与注射:委托武汉枢密公司构建重组腺相关病毒pAAV2-CaMKIIα-DIO-(mCherry-bGH-polyA-U6)-shRNA(Syt2/Scramble)-WPRE-hGH polyA和辅助病毒,在HEK293细胞对shRNA进行验证,结果如图2所示。随后将该病毒注射在小鼠右侧IC (注射量为200nl,病毒滴度>1012),将辅助病毒AAV2/R-hSyn-cre-WPRE-hGH polyA注射在小鼠右侧BLA(注射量为200nl,病毒滴度>1012),注射方法如图3所示。4. Virus construction and injection: entrust Wuhan Privy Company to construct recombinant adeno-associated virus pAAV2-CaMKIIα-DIO-(mCherry-bGH-polyA-U6)-shRNA(Syt2/Scramble)-WPRE-hGH polyA and helper virus in HEK293 cells The shRNA was verified, and the results are shown in Figure 2. Then the virus was injected IC on the right side of the mouse (the injection volume was 200nl, the virus titer>1012 ), and the helper virus AAV2/R-hSyn-cre-WPRE-hGH polyA was injected into the BLA on the right side of the mouse (injection volume 200nl, virus titer>1012 ), the injection method is shown in Figure 3.
5.动物行为学检测:病毒表达4周后,建立腓总神经结扎模型,检测小鼠左后足机械痛阈值,对各组动物痛阈进行统计学分析,如图4所示:注射shRNA(Syt2)的痛觉模型动物痛阈明显上升,而注射shRNA(Scramble)的痛觉模型动物痛阈相比于无病毒注射组没有明显改变,通过shRNA降低IC-BLA神经通路上的Syt2分子表达,可以有效镇痛。5. Animal behavior test: After 4 weeks of virus expression, the ligation model of the common peroneal nerve was established, the mechanical pain threshold of the left hind foot of the mouse was detected, and the pain threshold of animals in each group was statistically analyzed, as shown in Figure 4: injection of shRNA ( The pain threshold of the pain model animals of Syt2) was significantly increased, while the pain threshold of the pain model animals injected with shRNA (Scramble) was not significantly changed compared with the non-virus injection group. Reducing the expression of Syt2 molecules on the IC-BLA neural pathway by shRNA can be effective analgesia.
以上所述仅为本发明的较佳实施例而已,并不用以限制本发明,凡在本发明的精神和原则之内所作的任何修改、等同替换和改进等,均应包含在本发明的保护范围之内。The above descriptions are only preferred embodiments of the present invention, and are not intended to limit the present invention. Any modifications, equivalent replacements and improvements made within the spirit and principles of the present invention should be included in the protection of the present invention. within range.
Claims (2)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210030878.4ACN114369623B (en) | 2022-01-12 | 2022-01-12 | A kind of Synaptotagmin2-RNAi based on Cre-lox recombination system and its application |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210030878.4ACN114369623B (en) | 2022-01-12 | 2022-01-12 | A kind of Synaptotagmin2-RNAi based on Cre-lox recombination system and its application |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114369623A CN114369623A (en) | 2022-04-19 |
| CN114369623Btrue CN114369623B (en) | 2023-07-07 |
Family
ID=81144076
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210030878.4AExpired - Fee RelatedCN114369623B (en) | 2022-01-12 | 2022-01-12 | A kind of Synaptotagmin2-RNAi based on Cre-lox recombination system and its application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114369623B (en) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5919676A (en)* | 1993-06-24 | 1999-07-06 | Advec, Inc. | Adenoviral vector system comprising Cre-loxP recombination |
| CN101460634A (en)* | 2006-04-13 | 2009-06-17 | 康乃尔研究基金会有限公司 | Methods and compositions for targeting C-REL |
| CN104093830A (en)* | 2011-04-15 | 2014-10-08 | 吉恩勒克斯公司 | Cloned strains of attenuated vaccinia virus and methods of use thereof |
| CN104736166A (en)* | 2012-05-30 | 2015-06-24 | 哈佛大学校长及研究员协会 | Engineered Botulinum Neurotoxin |
| CN107630009A (en)* | 2016-07-19 | 2018-01-26 | 中国科学院武汉物理与数学研究所 | It is a kind of to be attenuated blast, replicate controllable HSV recombinant viruses and preparation method and application |
| CN109476713A (en)* | 2016-06-08 | 2019-03-15 | 儿童医学中心公司 | Engineered botulinum neurotoxin |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2560897A (en)* | 2017-03-23 | 2018-10-03 | Lotvall Jan | Tissue-derived extracellular vesicles and their use as diagnostics |
- 2022
- 2022-01-12CNCN202210030878.4Apatent/CN114369623B/ennot_activeExpired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5919676A (en)* | 1993-06-24 | 1999-07-06 | Advec, Inc. | Adenoviral vector system comprising Cre-loxP recombination |
| CN101460634A (en)* | 2006-04-13 | 2009-06-17 | 康乃尔研究基金会有限公司 | Methods and compositions for targeting C-REL |
| CN104093830A (en)* | 2011-04-15 | 2014-10-08 | 吉恩勒克斯公司 | Cloned strains of attenuated vaccinia virus and methods of use thereof |
| CN104736166A (en)* | 2012-05-30 | 2015-06-24 | 哈佛大学校长及研究员协会 | Engineered Botulinum Neurotoxin |
| CN109476713A (en)* | 2016-06-08 | 2019-03-15 | 儿童医学中心公司 | Engineered botulinum neurotoxin |
| CN107630009A (en)* | 2016-07-19 | 2018-01-26 | 中国科学院武汉物理与数学研究所 | It is a kind of to be attenuated blast, replicate controllable HSV recombinant viruses and preparation method and application |
Non-Patent Citations (1)
| Title |
|---|
| Glutamatergic synapses from the insular cortex to the basolateral amygdala encode observational pain;Ming-Ming Zhang et al;《Neuron》;第第110卷卷;第1-16页* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN114369623A (en) | 2022-04-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Ravindra Kumar et al. | Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types | |
| Griffin et al. | Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury | |
| El-Shamayleh et al. | Strategies for targeting primate neural circuits with viral vectors | |
| Lin et al. | Directed evolution of adeno-associated virus for efficient gene delivery to microglia | |
| Duan et al. | The deletion of mutant SOD1 via CRISPR/Cas9/sgRNA prolongs survival in an amyotrophic lateral sclerosis mouse model | |
| Saunders et al. | Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons | |
| US20190270980A1 (en) | Treating cancer | |
| JP2022530457A (en) | Genetically engineered AAV | |
| Himič et al. | Evaluating the potential of novel genetic approaches for the treatment of Duchenne muscular dystrophy | |
| Yu et al. | Intraganglionic AAV6 results in efficient and long-term gene transfer to peripheral sensory nervous system in adult rats | |
| Xu et al. | In vivo gene knockdown in rat dorsal root ganglia mediated by self-complementary adeno-associated virus serotype 5 following intrathecal delivery | |
| Qiu et al. | Lighting up neural circuits by viral tracing | |
| Klaw et al. | Intraspinal AAV injections immediately rostral to a thoracic spinal cord injury site efficiently transduces neurons in spinal cord and brain | |
| CN120569476A (en) | Methods and compositions for targeting trans-splicing | |
| Inquimbert et al. | Stereotaxic injection of a viral vector for conditional gene manipulation in the mouse spinal cord | |
| CN114369623B (en) | A kind of Synaptotagmin2-RNAi based on Cre-lox recombination system and its application | |
| Boehm et al. | Translational PET applications for brain circuit mapping with transgenic neuromodulation tools | |
| Pichler et al. | Generation of a highly inducible Gal4→ Fluc universal reporter mouse for in vivo bioluminescence imaging | |
| Ball et al. | Use of adeno-associated viruses for transgenic modulation of microglia structure and function: A review of technical considerations and challenges | |
| CN114410683B (en) | A kind of RIM3-RNAi based on Cre-lox recombination system and its application | |
| Grinevich et al. | Somatic transgenesis (viral vectors) | |
| Imai et al. | Pentatricopeptide repeat protein targeting CUG repeat RNA ameliorates RNA toxicity in a myotonic dystrophy type 1 mouse model | |
| Zelena et al. | Considerations for the use of virally delivered genetic tools for in-vivo circuit analysis and behavior in mutant mice: a practical guide to optogenetics | |
| Maturana | Engineered compact pan-neuronal promoter from Alphaherpesvirus LAP2 enhances target gene expression in the mouse brain and reduces tropism in the liver | |
| Ishibashi et al. | Gene targeting in adult organs using in vivo cleavable donor plasmids for CRISPR-Cas9 and CRISPR-Cas12a |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20230707 | |
| CF01 | Termination of patent right due to non-payment of annual fee |