CN114177346B - Hemostatic composition and hemostatic patch and application thereof - Google Patents
Hemostatic composition and hemostatic patch and application thereofDownload PDFInfo
- Publication number
- CN114177346B CN114177346BCN202111603820.6ACN202111603820ACN114177346BCN 114177346 BCN114177346 BCN 114177346BCN 202111603820 ACN202111603820 ACN 202111603820ACN 114177346 BCN114177346 BCN 114177346B
- Authority
- CN
- China
- Prior art keywords
- hemostatic
- collagen
- fibrinogen
- thrombin
- parts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/04—Surgical adhesives or cements; Adhesives for colostomy devices containing macromolecular materials
- A61L24/10—Polypeptides; Proteins
- A61L24/106—Fibrin; Fibrinogen
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/18—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing inorganic materials
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/32—Proteins, polypeptides; Degradation products or derivatives thereof, e.g. albumin, collagen, fibrin, gelatin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/44—Medicaments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/64—Use of materials characterised by their function or physical properties specially adapted to be resorbable inside the body
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0015—Medicaments; Biocides
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0042—Materials resorbable by the body
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/02—Surgical adhesives or cements; Adhesives for colostomy devices containing inorganic materials
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/04—Surgical adhesives or cements; Adhesives for colostomy devices containing macromolecular materials
- A61L24/10—Polypeptides; Proteins
- A61L24/108—Specific proteins or polypeptides not covered by groups A61L24/102 - A61L24/106
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/10—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing inorganic materials
- A61L2300/102—Metals or metal compounds, e.g. salts such as bicarbonates, carbonates, oxides, zeolites, silicates
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/20—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials
- A61L2300/252—Polypeptides, proteins, e.g. glycoproteins, lipoproteins, cytokines
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/20—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials
- A61L2300/252—Polypeptides, proteins, e.g. glycoproteins, lipoproteins, cytokines
- A61L2300/254—Enzymes, proenzymes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/04—Materials for stopping bleeding
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Engineering & Computer Science (AREA)
- Surgery (AREA)
- Hematology (AREA)
- Inorganic Chemistry (AREA)
- Materials For Medical Uses (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及医用止血材料技术领域,特别是涉及一种止血组合物,由该止血组合物制得的止血贴,及该止血组合物和止血贴的应用。The invention relates to the technical field of medical hemostatic materials, in particular to a hemostatic composition, a hemostatic patch made from the hemostatic composition, and applications of the hemostatic composition and the hemostatic patch.
背景技术Background technique
出血是外科手术和外伤中最常见、最难控制的问题之一,特别是重要脏器的出血,如肝脏、脾脏、肾脏破裂出血常常直接威胁生命。传统的止血方式(如结扎、压迫止血法)仅适用于大血管出血的止血,对弥漫性出血和内脏出血的止血效果有限。创伤出血也是一大致死因素,伤后快速有效止血是挽救伤病员生命的最重要手段。Bleeding is one of the most common and difficult to control problems in surgery and trauma, especially the bleeding of important organs, such as liver, spleen, and kidney rupture and bleeding are often life-threatening. Traditional hemostasis methods (such as ligation and compression hemostasis) are only suitable for hemostasis of large blood vessel bleeding, and have limited hemostatic effect on diffuse bleeding and visceral bleeding. Trauma bleeding is also a major cause of death, and rapid and effective hemostasis after injury is the most important means to save the lives of the wounded and sick.
然而当机体遭受重创时,自体凝血不足以迅速凝血,因此需要在创伤处外加止血材料控制出血。理想的止血材料在性能上有以下要求:(1)止血迅速(即凝固时间短);(2)具有良好的生物相容性,无短期或长期的不良影响;(3)无需二次清创,不会对伤员造成二次伤害;(4)应用方便,即使非专业人员也易于使用;(5)在各种环境条件下稳定,保质期长。However, when the body is severely injured, autologous blood coagulation is not enough to quickly coagulate blood, so it is necessary to add hemostatic materials to the wound to control bleeding. An ideal hemostatic material has the following performance requirements: (1) rapid hemostasis (ie short coagulation time); (2) good biocompatibility without short-term or long-term adverse effects; (3) no need for secondary debridement , will not cause secondary injury to the wounded; (4) easy to apply, even non-professionals are easy to use; (5) stable under various environmental conditions, and has a long shelf life.
目前,可用于外科手术和创伤救治的止血材料种类繁多,如纤维蛋白类、壳聚糖类天然生物止血材料,沸石、高岭土和蒙脱土类等矿物质止血材料,明胶-间苯二酚-甲醛类或氰化丙烯类等人工合成止血材料,其止血机制各不相同。这些止血材料存在着各自的缺点,如常用的壳聚糖类止血材料在创伤处的粘附性差,容易被血液冲走,大大降低了其止血效果;矿物质类止血材料不可吸收、需要二次清创,用于内脏出血的止血会对伤员造成二次伤害,而且安全性差;人工合成止血材料的生物相容性和降解性都较差,安全隐患多。At present, there are many kinds of hemostatic materials that can be used in surgical operations and trauma treatment, such as natural biological hemostatic materials such as fibrin and chitosan, mineral hemostatic materials such as zeolite, kaolin and montmorillonite, gelatin-resorcinol- Synthetic hemostatic materials such as formaldehyde or cyanide have different hemostatic mechanisms. These hemostatic materials have their own shortcomings. For example, the commonly used chitosan hemostatic materials have poor adhesion at the wound and are easily washed away by blood, greatly reducing their hemostatic effect; mineral hemostatic materials are not absorbable and require secondary For debridement, hemostasis for visceral bleeding will cause secondary damage to the wounded, and the safety is poor; the biocompatibility and degradability of synthetic hemostatic materials are poor, and there are many safety hazards.
综上,纤维蛋白类止血材料制成的止血贴产品与以上这些材料相比,具有粘附性强、不需二次清创、不会造成二次伤害、可降解、低免疫原性和使用方便等优点,可用于轻中度和无固定出血点的出血,特别适用于内脏出血的止血。In summary, compared with the above materials, hemostatic patch products made of fibrin hemostatic materials have strong adhesion, no need for secondary debridement, no secondary damage, degradable, low immunogenicity and ease of use. Convenient and other advantages, it can be used for mild to moderate bleeding and no fixed bleeding point, especially suitable for hemostasis of visceral bleeding.
公开号为CN1507358A的中国专利申请中公开了一种具有固体纤维蛋白原和固体凝血酶的载体,载体上均匀分散并固定有固体纤维蛋白原和固体凝血酶。该产品中发挥止血作用的仅有纤维蛋白原和凝血酶两种成分,凝固时间过长,止血效果不理想。而且该产品的药液层(药液层为含有固体纤维蛋白原和固体凝血酶的层)在使用过程中易脱落(见图3),无法保证其止血效果。The Chinese patent application with publication number CN1507358A discloses a carrier with solid fibrinogen and solid thrombin, on which solid fibrinogen and solid thrombin are evenly dispersed and immobilized. In this product, only two components, fibrinogen and thrombin, play a hemostatic effect. The coagulation time is too long, and the hemostatic effect is not ideal. And the liquid medicine layer of this product (the liquid medicine layer is the layer that contains solid fibrinogen and solid thrombin) is easy to come off in use (see Fig. 3), can't guarantee its hemostatic effect.
发明内容Contents of the invention
本发明的目的是针对现有技术中存在的技术缺陷,第一方面,提供一种凝固时间短的止血组合物,包括16-46份(优选16-20份)纤维蛋白原、38-42份凝血酶和100-121份氯化钙,其中,纤维蛋白原以mg计为一份,凝血酶以IU计为一份,氯化钙以μg计为一份。The purpose of the present invention is to address the technical defects in the prior art. In the first aspect, a hemostatic composition with a short coagulation time is provided, comprising 16-46 parts (preferably 16-20 parts) of fibrinogen, 38-42 parts Thrombin and 100-121 parts of calcium chloride, wherein one part of fibrinogen is calculated in mg, one part of thrombin is calculated in IU, and one part of calcium chloride is calculated in μg.
包括16-19份纤维蛋白原、39-41份凝血酶和103-118份氯化钙;优选的,包括17份纤维蛋白原、40份凝血酶、115份氯化钙(CaCl2)。It includes 16-19 parts of fibrinogen, 39-41 parts of thrombin and 103-118 parts of calcium chloride; preferably, it includes 17 parts of fibrinogen, 40 parts of thrombin and 115 parts of calcium chloride (CaCl2 ).
所述止血组合物遇水后形成的纤维蛋白多聚体的黏弹特性值tanδ<1,优选为0.13-0.31。The viscoelastic characteristic value of the fibrin polymer formed by the hemostatic composition when it meets water is tanδ<1, preferably 0.13-0.31.
第二方面,本发明提供一种止血贴,包括由胶原蛋白制备得到的胶原载体和均匀分散并固定在胶原载体上的药液层,所述药液层由上述止血组合物制备而成;胶原载体为片状。In a second aspect, the present invention provides a hemostatic patch, comprising a collagen carrier prepared from collagen and a drug solution layer uniformly dispersed and fixed on the collagen carrier, the drug solution layer being prepared from the above-mentioned hemostatic composition; collagen The carrier is in the form of a sheet.
每平方厘米止血贴中含有纤维蛋白原16-46mg,凝血酶38-42IU,氯化钙100-121μg,胶原蛋白>0.5mg。Each hemostatic patch contains 16-46 mg of fibrinogen, 38-42 IU of thrombin, 100-121 μg of calcium chloride, and >0.5 mg of collagen.
所述药液层是将所述止血组合物分散于乙醇中制成混悬液,然后喷涂在胶原载体上形成的;可选的,将所述止血组合物分散于乙醇中后,置于分散机中进行均质搅拌,搅拌速度为(10-20)×103rpm,搅拌次数5-10次,每次搅拌2min,搅拌时间共计10-20min。The liquid medicine layer is formed by dispersing the hemostatic composition in ethanol to form a suspension, and then spraying it on the collagen carrier; optionally, after dispersing the hemostatic composition in ethanol, place the suspension Carry out homogeneous stirring in the machine, the stirring speed is (10-20)×103 rpm, the stirring frequency is 5-10 times, each stirring is 2 minutes, and the total stirring time is 10-20 minutes.
喷涂时,每平方厘米胶原载体上喷涂的药液量为0.049-0.563ml,形成的药液层的厚度为0.049-0.247mm。When spraying, the amount of liquid medicine sprayed on the collagen carrier per square centimeter is 0.049-0.563ml, and the thickness of the formed liquid medicine layer is 0.049-0.247mm.
所述混悬液中止血组合物颗粒的平均直径为33-50μm(优选37-42μm),和/或所述混悬液的5min沉淀体积占比均值>90%(优选≥95%)。The average diameter of the hemostatic composition particles in the suspension is 33-50 μm (preferably 37-42 μm), and/or the 5-minute sedimentation volume ratio of the suspension is >90% (preferably ≥95%) on average.
喷涂前所述胶原载体是在20℃降至-50℃的过程中将胶原蛋白冷冻19-23h得到的表面光滑、内部结构致密的玻璃体;可选的,其厚度为0.3-0.5cm。The collagen carrier before spraying is a vitreous body with a smooth surface and a dense inner structure obtained by freezing the collagen for 19-23 hours during the process of cooling from 20°C to -50°C; optionally, its thickness is 0.3-0.5cm.
第三方面,本发明提供上述止血组合物和止血贴在制备止血产品中的应用,优选的,所述止血产品为内脏出血止血产品。In a third aspect, the present invention provides the application of the above-mentioned hemostatic composition and hemostatic patch in the preparation of a hemostatic product, preferably, the hemostatic product is a hemostatic product for visceral bleeding.
本发明提供的止血组合物包括纤维蛋白原、凝血酶和氯化钙,并通过大量的实验研究发现纤维蛋白原、凝血酶和氯化钙在特定的配比下可协同发挥止血功效以降低血液的凝固时间,该止血组合物中纤维蛋白原、凝血酶和氯化钙的特定配比分别为16-46份、38-42份和100-121份(纤维蛋白原以mg计为一份,凝血酶以IU计为一份,氯化钙以μg计为一份),实验证实在该特定配比下的止血组合物能够达到良好的止血效果。The hemostatic composition provided by the present invention includes fibrinogen, thrombin and calcium chloride, and a large number of experimental studies have found that fibrinogen, thrombin and calcium chloride can synergistically exert a hemostatic effect in a specific ratio to reduce blood pressure. The specific ratio of fibrinogen, thrombin and calcium chloride in the hemostatic composition is 16-46 parts, 38-42 parts and 100-121 parts respectively (fibrinogen is calculated as one part in mg, Thrombin is counted as one part in IU, and calcium chloride is counted as one part in μg), and experiments have confirmed that the hemostatic composition with this specific ratio can achieve a good hemostatic effect.
本发明还提供了一种止血贴,包括胶原载体和上述止血组合物形成的药液层,药液层由含有上述止血组合物的混悬液均匀分散并固定在胶原载体上形成。该止血贴可用于内脏出血时的止血,使用时直接将其贴在内脏上的无固定出血点即可,操作简单、粘附性强、止血效果好、具有良好的生物相容性,在体内能完全降解,无需二次清创,避免对伤员造成二次伤害。该止血贴性能稳定、药液层不易脱落,可应用于各种突发事件、自然灾害(如强烈地震)等情况的伤员止血(特别是内脏出血的止血),具有良好的商用价值和广阔的应用前景。The present invention also provides a hemostatic patch, which includes a collagen carrier and a drug solution layer formed by the above-mentioned hemostatic composition, and the drug solution layer is formed by uniformly dispersing and fixing the suspension containing the above-mentioned hemostatic composition on the collagen carrier. The hemostatic patch can be used for hemostasis when visceral bleeding occurs. When in use, it can be directly pasted on the internal organs without fixed bleeding points. It is easy to operate, strong in adhesion, good in hemostatic effect, and has good biocompatibility. It can be completely degraded without secondary debridement, avoiding secondary damage to the wounded. The hemostatic patch has stable performance and the liquid layer is not easy to fall off, and can be applied to hemostasis (especially hemostasis of visceral bleeding) of wounded in various emergencies, natural disasters (such as strong earthquakes), etc., and has good commercial value and broad application Application prospect.
附图说明Description of drawings
图1所示为实施例1的止血组合物制备得到的止血贴的侧视图;Fig. 1 shows the side view of the hemostatic patch prepared by the hemostatic composition of Example 1;
图2所示为图1止血贴的俯视图;Figure 2 is a top view of the hemostatic patch in Figure 1;
图3所示为现有技术中载体的俯视图;Figure 3 shows a top view of the carrier in the prior art;
图4是自动喷涂装置的立体结构示意图;Fig. 4 is the three-dimensional structure schematic diagram of automatic spraying device;
图5是喷液机构5的结构示意图。FIG. 5 is a schematic structural view of the
具体实施方式Detailed ways
本发明提供了一种纤维蛋白类的止血组合物,包括16-46份纤维蛋白原(来自人、哺乳动物或重组纤维蛋白原)、38-42份凝血酶(来自人、哺乳动物或重组凝血酶)和100-121份氯化钙,其中,纤维蛋白原以mg计为一份,凝血酶以IU计为一份,氯化钙以μg计为一份;优选的,包括17份纤维蛋白原、40份凝血酶、115份氯化钙(CaCl2)。其中,The invention provides a hemostatic composition of fibrin, comprising 16-46 parts of fibrinogen (from human, mammal or recombinant fibrinogen), 38-42 parts of thrombin (from human, mammal or recombinant coagulation enzyme) and 100-121 parts of calcium chloride, wherein, fibrinogen is one part in mg, thrombin is one part in IU, and calcium chloride is one part in μg; preferably, it includes 17 parts of fibrin Original, 40 parts of thrombin, 115 parts of calcium chloride (CaCl2 ). in,
纤维蛋白原(Fibrinogen),即凝血因子Ⅰ,是一种含2964个氨基酸的大分子糖蛋白,相对分子质量为340kDa,由两个亚基(纤维蛋白肽A和B)组成,呈两侧对称性排列,每个亚基含有α、β、γ三条肽链,中间由29个二硫键连接构成(AαBβγ)2。纤维蛋白原是纤维蛋白的前体,足够的纤维蛋白原水平是形成有效凝血的基本要求。本发明的止血组合物含有足量的纤维蛋白原,能向失血处补充额外的纤维蛋白原,纤维蛋白原经凝血酶酶解成纤维蛋白,纤维蛋白在凝血酶、血液中的凝血因子XIII和XIIIa的共同作用下聚合形成呈坚实血凝块状的纤维蛋白多聚体,堵塞出血血管或创伤表面,阻止出血血管或创伤继续出血,达到止血的目的。本发明可使用人纤维蛋白原,也可使用哺乳动物纤维蛋白原和重组的纤维蛋白原,可购自华兰生物工程股份有限公司、上海源叶科技有限公司、南京赛泓瑞生物科技有限公司;人纤维蛋白原为白色、灰白色或淡黄色疏松体,可粉碎成粉末状。Fibrinogen, namely blood coagulation factor Ⅰ, is a macromolecular glycoprotein containing 2964 amino acids, with a relative molecular mass of 340kDa, composed of two subunits (fibrinopeptide A and B), which are bilaterally symmetrical Each subunit contains three peptide chains α, β, and γ, and the middle is composed of 29 disulfide bonds (AαBβγ)2. Fibrinogen is the precursor of fibrin, and adequate levels of fibrinogen are an essential requirement for efficient coagulation. The hemostatic composition of the present invention contains a sufficient amount of fibrinogen, which can supplement additional fibrinogen to the blood loss site. Under the combined action of XIIIa, it polymerizes to form a solid blood clot-like fibrin polymer, which blocks the surface of bleeding vessels or wounds, prevents bleeding vessels or wounds from continuing to bleed, and achieves the purpose of hemostasis. The present invention can use human fibrinogen, and can also use mammalian fibrinogen and recombinant fibrinogen, which can be purchased from Hualan Bioengineering Co., Ltd., Shanghai Yuanye Technology Co., Ltd., Nanjing Saihongrui Biotechnology Co., Ltd. ; Human fibrinogen is white, off-white or light yellow loose body, which can be crushed into powder.
凝血酶(Thrombin)是一种丝氨酸蛋白酶,主要功能在于将可溶于水的纤维蛋白原水解成不溶于水的纤维蛋白,因此在止血和凝血、组织修复和创伤愈合等方面有重要作用。凝血酶是由凝血酶原(凝血因子Ⅱ)激活产生,主要转换成具有活化形式的α-凝血酶(在凝血酶中含量>97%,余量是少量的β-和γ-凝血酶)。本发明可使用人凝血酶,也可使用哺乳动物凝血酶和重组的凝血酶可购自华兰生物工程股份有限公司、上海源叶科技有限公司、南京赛泓瑞生物科技有限公司,为白色、灰白色或淡黄色疏松体,可粉碎成粉末状。Thrombin is a serine protease whose main function is to hydrolyze water-soluble fibrinogen into water-insoluble fibrin, so it plays an important role in hemostasis and coagulation, tissue repair and wound healing. Thrombin is activated by prothrombin (coagulation factor II), and is mainly converted into α-thrombin in an activated form (>97% in thrombin, with a small amount of β- and γ-thrombin in the balance). The present invention can use human thrombin, and can also use mammalian thrombin and recombinant thrombin, which can be purchased from Hualan Bioengineering Co., Ltd., Shanghai Yuanye Technology Co., Ltd., Nanjing Saihongrui Biotechnology Co., Ltd., white, Off-white or light yellow loose body, which can be crushed into powder.
钙离子(Ca2+),即凝血因子Ⅳ。一般用氯化钙提供Ca2+,Ca2+在各个凝血途径中均不可或缺。如在内源性凝血途径中,Ca2+可以协助激活凝血因子Ⅺ(存在于血液中),凝血因子Ⅺ会参与其他凝血途径进而发挥凝血作用;而且在Ca2+和血小板磷脂存在的条件下,凝血因子Ⅹ(存在于血液中)也会被激活,进而在共同凝血途径中发挥作用。在外源性凝血途径中,Ca2+、凝血因子Ⅲ(存在于血液中)和凝血因子Ⅶ(存在于血液中)可一同激活凝血因子Ⅹ,进而在共同凝血途径中发挥作用。在共同凝血途径中,Ca2+结合凝血酶、凝血因子Ⅴ(存在于血液中)和激活的凝血因子Ⅹ(内、外源性凝血途径中激活的)能将纤维蛋白原转变为纤维蛋白单体,发挥止血和凝血作用。另外,Ca2+还可以协助激活凝血因子XIII(存在于血液中),进而激活凝血因子XIIIa(存在于血液中),凝血因子XIIIa再作用于纤维蛋白单体,将其变成稳定的纤维蛋白多聚体血凝块。本发明使用的氯化钙购自北京红星化工厂,呈白色颗粒状。Calcium ion (Ca2+ ), that is, coagulation factor IV. Calcium chloride is generally used to provide Ca2+ , and Ca2+ is indispensable in every blood coagulation pathway. For example, in the intrinsic blood coagulation pathway, Ca2+ can assist in the activation of coagulation factor Ⅺ (present in the blood), and coagulation factor Ⅺ will participate in other coagulation pathways to play a coagulation role; and in the presence of Ca2+ and platelet phospholipids , coagulation factor X (present in the blood) is also activated, which in turn plays a role in the common coagulation pathway. In the extrinsic coagulation pathway, Ca2+ , coagulation factor III (existing in blood) and coagulation factor VII (existing in blood) can activate coagulation factor X together, and then play a role in the common coagulation pathway. In the common coagulation pathway, Ca2+ binds thrombin, coagulation factor V (present in the blood) and activated coagulation factor X (activated in the intrinsic and extrinsic coagulation pathway) to convert fibrinogen into fibrin mono Body, play a role in hemostasis and blood coagulation. In addition, Ca2+ can also assist in the activation of coagulation factor XIII (present in blood), which in turn activates coagulation factor XIIIa (present in blood), and coagulation factor XIIIa acts on fibrin monomers to turn them into stable fibrin Polymeric blood clot. The calcium chloride used in the present invention is purchased from Beijing Hongxing Chemical Factory, and is in the form of white granules.
本发明提供的止血组合物以纤维蛋白原、凝血酶和钙离子为主要止血成分,止血原理是模拟生物凝血级联反应的最后阶段,即:止血组合物与出血部位接触时,纤维蛋白原在凝血酶及Ca2+的作用下,形成纤维蛋白单体;然后在凝血因子XIIIa的作用下纤维蛋白单体进一步聚合形成纤维蛋白多聚体,多聚体以血凝块形式存在,作为障碍物堵塞住出血血管或创伤表面,可有效地阻止出血血管或创伤表面继续出血。同时,凝血酶能促进血小板的粘附、活化和聚集,聚集活化的血小板进一步释放凝血活性物质,如二磷酸腺苷(ADP)、三磷酸腺苷(ATP)、血栓素A2、五羟色胺等;同时Ca2+能激活某些凝血因子,这些凝血活性物质和凝血因子可以增加凝血酶的生成,进而增加且加快纤维蛋白及多聚体的形成,如此良性循环,正向地加速凝血和止血。The hemostatic composition provided by the present invention uses fibrinogen, thrombin and calcium ions as the main hemostatic components. Under the action of thrombin and Ca2+ , fibrin monomers are formed; then under the action of coagulation factor XIIIa, the fibrin monomers are further polymerized to form fibrin polymers, which exist in the form of blood clots as obstacles Blocking the bleeding blood vessel or wound surface can effectively prevent the bleeding blood vessel or wound surface from continuing to bleed. At the same time, thrombin can promote the adhesion, activation and aggregation of platelets, and the activated platelets can further release coagulation active substances, such as adenosine diphosphate (ADP), adenosine triphosphate (ATP), thromboxane A2, serotonin, etc.; at the same time, Ca2+ Can activate certain coagulation factors, these coagulation active substances and coagulation factors can increase the generation of thrombin, and then increase and accelerate the formation of fibrin and polymers, such a virtuous cycle, positively accelerate coagulation and hemostasis.
在此基础上,本发明还提供了一种止血贴,包括上述止血组合物和固体载体。固体载体需要具有可降解性,常用的可降解固体载体材料有氧化再生纤维素、聚乳酸蛋白和胶原蛋白等。本发明使用胶原蛋白作为止血贴的固体载体,这是因为胶原蛋白(Collagen)具有发达的四级结构,能够形成支架状结构,可用作载体。此外,胶原蛋白还可通过促进血小板聚集发挥止血作用,能够促进血管的形成和生长,对局部组织具有修复功能。常用的胶原蛋白有牛胶原蛋白、猪胶原蛋白、马胶原蛋白等,这些胶原蛋白均来源于哺乳动物,免疫原性低。本发明的具体实施例中使用牛胶原蛋白作为止血贴的固体载体,因此也称该固体载体为胶原载体。牛胶原蛋白液购自天津世纪康泰生物医学工程有限公司,呈透明凝胶状。该止血贴的原料包括:16-46mg/cm2的纤维蛋白原,38-42IU/cm2的凝血酶,100-121μg/cm2的氯化钙,≥0.5mg/cm2的胶原蛋白。On this basis, the present invention also provides a hemostatic patch, comprising the above hemostatic composition and a solid carrier. The solid carrier needs to be degradable. Commonly used degradable solid carrier materials include oxidized regenerated cellulose, polylactic acid protein, and collagen. The present invention uses collagen as the solid carrier of the hemostatic patch, because the collagen (Collagen) has a well-developed quaternary structure, can form a scaffold-like structure, and can be used as a carrier. In addition, collagen can also play a hemostatic effect by promoting platelet aggregation, promote the formation and growth of blood vessels, and have a repair function for local tissues. Commonly used collagens include bovine collagen, porcine collagen, horse collagen, etc. These collagens are all derived from mammals and have low immunogenicity. In the specific embodiment of the present invention, bovine collagen is used as the solid carrier of the hemostatic patch, so the solid carrier is also called collagen carrier. Bovine collagen solution was purchased from Tianjin Century Kangtai Biomedical Engineering Co., Ltd. and was in the form of a transparent gel. The raw materials of the hemostatic patch include: 16-46 mg/cm2 of fibrinogen, 38-42 IU/cm2 of thrombin, 100-121 μg/cm2 of calcium chloride, and ≥0.5 mg/cm2 of collagen.
制备该止血贴的过程,包括以下步骤:The process of preparing the hemostatic patch comprises the following steps:
(1)、将方形模具(医用级PVC制成)放进316不锈钢药液盘中,取少量牛胶原蛋白(透明凝胶状,购买后不作处理,直接使用)于模具中,用胶原刮板(316不锈钢制成)刮平,使胶原蛋白在模具上形成厚度为0.3-0.5cm的胶原蛋白层;除去多余的牛胶原蛋白后,再将压膜片(医用级PVC制成)贴在刮平的胶原蛋白层表面,贴的过程中要避免压膜片与胶原蛋白层之间出现大的气泡,最后在20~-50℃下梯度(即:温度从20℃梯度降至-50℃)冷冻19-23h,揭去压膜片,冷冻的胶原蛋白层作为止血贴的固体载体,也叫胶原载体。(1) Put the square mold (made of medical grade PVC) into the 316 stainless steel liquid medicine tray, take a small amount of bovine collagen (transparent gel, not processed after purchase, use directly) in the mold, and use a collagen scraper (made of 316 stainless steel) scrape flat, so that the collagen forms a collagen layer with a thickness of 0.3-0.5cm on the mold; after removing excess bovine collagen, stick the pressure film (made of medical grade PVC) on the scraper For a flat collagen layer surface, avoid large air bubbles between the pressure film and the collagen layer during the pasting process, and finally apply a gradient at 20 to -50°C (ie: temperature gradient from 20°C to -50°C) Freeze for 19-23 hours, remove the pressure film, and the frozen collagen layer is used as the solid carrier of the hemostatic patch, also called collagen carrier.
梯度冷冻具体可以为:1h内温度从20℃降至0℃,再在18-22h内降温至-50℃,并维持该温度2h。Gradient freezing can specifically be as follows: the temperature drops from 20°C to 0°C within 1 hour, then drops to -50°C within 18-22 hours, and maintains the temperature for 2 hours.
(2)、将纤维蛋白原、凝血酶、氯化钙和低温无水乙醇(-20℃预冷)混合在一起形成药液,置于分散机(IKA,T 25easy clean)中进行均质搅拌,均质过程中的搅拌速度为(10-20)×103rpm,搅拌药液的次数为5-10次,搅拌时间共计10-20min。均质后,即得到含止血组合物的混悬液,置于低温环境(-50℃~-20℃)中备用。(2) Mix fibrinogen, thrombin, calcium chloride and low-temperature absolute ethanol (pre-cooled at -20°C) to form a medicinal solution, and place it in a disperser (IKA, T 25easy clean) for homogeneous stirring , the stirring speed during the homogenization process is (10-20)×103 rpm, the frequency of stirring the liquid medicine is 5-10 times, and the total stirring time is 10-20 minutes. After homogenization, a suspension containing the hemostatic composition is obtained, which is placed in a low-temperature environment (-50° C. to -20° C.) for use.
(3)、设置自动喷涂装置(自动喷涂装置的结构记载于授权公告号为CN214021579U的专利中)的参数:蠕动泵流速为50-80ml/min,喷液管摆动1-6次,喷涂的药液量为0.049-0.563ml/cm2,Y轴单次移动距离3000-4500cmm(1cmm(忽米)=1×10-5m);X轴总移动距离3000-4500cmm。(3), setting the parameters of the automatic spraying device (the structure of the automatic spraying device is recorded in the patent of CN214021579U with the authorized announcement number): the flow rate of the peristaltic pump is 50-80ml/min, the liquid spray pipe swings 1-6 times, and the sprayed medicine The liquid volume is 0.049-0.563ml/cm2 , the single movement distance of the Y axis is 3000-4500cmm (1cmm (meter)=1×10-5 m); the total movement distance of the X axis is 3000-4500cmm.
(4)、步骤(3)的参数设置完成后,将泵管(19#,硅胶制,内径2.4mm,外径5.6mm,购自保定兰格恒流泵有限公司)进口放入步骤(2)得到的混悬液中,利用自动喷涂装置将混悬液均匀地喷涂在冷冻后的胶原载体表面,混悬液在胶原载体表面形成一层厚度为1-5mm的药液层。(4), after the parameter setting of step (3) is completed, put the inlet of the pump tube (19#, made of silica gel, with an inner diameter of 2.4 mm and an outer diameter of 5.6 mm, purchased from Baoding Lange Constant Flow Pump Co., Ltd.) into step (2) ) in the suspension obtained by using an automatic spraying device to evenly spray the suspension on the surface of the frozen collagen carrier, and the suspension forms a liquid medicine layer with a thickness of 1-5mm on the surface of the collagen carrier.
(5)、喷涂完成后将不锈钢药液盘放进提前预冷至-50℃的冻干机(CHRIST,Epsilon 2-4 LSC plus)中,冷冻干燥49h40min-63h(冻干时间过短,胶原载体和药液层内的水分含量过高,无法形成片状的贴剂产品;冻干时间过长,胶原载体和药液层内的水分含量过低,药液层会因过于干燥开裂,不仅影响产品外观,还会影响止血效果),药液层在冷冻干燥的过程中会附在胶原载体表面,并与胶原载体形成一体的止血贴,至止血贴的含水率不高于10%,停止冻干,取出、包装并灭菌,得到止血贴产品。(5) After the spraying is completed, put the stainless steel chemical solution tray into a freeze dryer (CHRIST, Epsilon 2-4 LSC plus) pre-cooled to -50°C in advance, and freeze-dry for 49h40min-63h (the freeze-drying time is too short, the collagen The moisture content in the carrier and the liquid medicine layer is too high to form a flaky patch product; if the freeze-drying time is too long, the moisture content in the collagen carrier and the liquid medicine layer is too low, and the liquid medicine layer will crack due to being too dry, not only affect the appearance of the product, and also affect the hemostatic effect), the liquid medicine layer will be attached to the surface of the collagen carrier during the freeze-drying process, and form an integrated hemostatic patch with the collagen carrier, until the moisture content of the hemostatic patch is not higher than 10%, stop Freeze-dried, taken out, packaged and sterilized to obtain a hemostatic patch product.
该方法中,与现有技术不同的是,步骤(1)胶原蛋白经冷冻(而不是冻干)形成胶原载体,这使得形成的胶原载体中没有海绵状结构,而是表面光滑、内部致密的玻璃状固体。再进行步骤(4)的混悬液喷涂时,混悬液不会渗入胶原载体内部,而是位于胶原载体光滑的表面上。最后进行步骤(5)的冻干时,胶原载体内部的水分和药液层中的溶剂共同挥发,在此过程中胶原载体和药液层中留下的物质相互渗透,使得两者紧密连接在一起,使得药液层不易从胶原载体上脱落下来。In this method, different from the prior art, in step (1), the collagen is frozen (instead of freeze-dried) to form a collagen carrier, which makes the formed collagen carrier not have a spongy structure, but a smooth surface and a dense interior. Glassy solid. When spraying the suspension in step (4), the suspension will not penetrate into the inside of the collagen carrier, but be located on the smooth surface of the collagen carrier. When finally carrying out the freeze-drying of step (5), the moisture in the collagen carrier and the solvent in the liquid medicine layer volatilize together, and the substances left in the collagen carrier and the liquid medicine layer penetrate each other during this process, so that the two are closely connected Together, the liquid medicine layer is not easy to fall off from the collagen carrier.
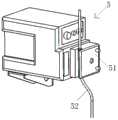
该方法中使用的自动喷涂装置用于向316不锈钢药液盘中的方形模具的每一区域喷涂止血组合物以制成止血贴。图4为该自动喷涂装置的结构示例。该自动喷涂装置包括试剂料盘平移机构3、喷液机构5和喷嘴横移机构6,试剂料盘平移机构3设有用于驱动设置在试剂料盘平移机构3上的试剂料盘平移往复运动(即左右水平移动,X轴)的平移驱动电机,喷嘴横移机构6设有用于驱动设置在喷嘴横移机构6的喷嘴65横移往复移动(即前后水平移动,Y轴)的横移驱动电机,喷嘴位于试剂料盘4的上方,喷液机构5设有用于将药液通过一喷管52推进至喷嘴65中的蠕动泵51(参见图5),使药液定量喷涂在布设有底板的试剂料盘的每一区域。其中,平移驱动电机能够正反转动,使安装在试剂料盘平移机构3上的试剂料盘4平移往复运动,横移驱动电机能够正反转动,使安装在喷嘴横移机构6上的喷嘴65沿与试剂料盘4平移方向相垂直的方向作线性往复运动;喷液机构5的蠕动泵针对试剂料盘4的每一区域抽取预定量的药液,从而实现向试剂料盘4的每一区域内按预定路线快速、均匀的喷涂药液,保持了药物活性,减少了药液损失,保证喷涂的均匀性。The automatic spraying device used in this method is used to spray the hemostatic composition to each area of the square mold in the 316 stainless steel liquid medicine pan to make a hemostatic patch. Figure 4 is a structural example of the automatic spraying device. The automatic spraying device includes a reagent
以下结合具体实施例,更具体地说明本发明的内容,并对本发明作进一步阐述,但这些实施例绝非对本发明进行限制。The content of the present invention will be described in more detail below in conjunction with specific examples, and the present invention will be further elaborated, but these examples are by no means limiting the present invention.
实施例1Example 1
本实施例的止血组合物由17mg纤维蛋白原、40IU凝血酶、115μg氯化钙(CaCl2)组成。The hemostatic composition of this example consists of 17 mg of fibrinogen, 40 IU of thrombin, and 115 μg of calcium chloride (CaCl2 ).
实施例2Example 2
本实施例的止血组合物由16mg纤维蛋白原、38IU凝血酶、100μg氯化钙(CaCl2)组成。The hemostatic composition of this example consists of 16 mg of fibrinogen, 38 IU of thrombin, and 100 μg of calcium chloride (CaCl2 ).
实施例3Example 3
本实施例的止血组合物由20mg纤维蛋白原、42IU凝血酶、121μg氯化钙(CaCl2)组成。The hemostatic composition of this example consists of 20 mg of fibrinogen, 42 IU of thrombin, and 121 μg of calcium chloride (CaCl2 ).
实施例4Example 4
本实施例的止血组合物由18mg纤维蛋白原、39IU凝血酶、110μg氯化钙(CaCl2)组成。The hemostatic composition of this example consists of 18 mg of fibrinogen, 39 IU of thrombin, and 110 μg of calcium chloride (CaCl2 ).
实施例5Example 5
本实施例的止血组合物由17mg纤维蛋白原、40IU凝血酶、103μg氯化钙(CaCl2)组成。The hemostatic composition of this example consists of 17 mg of fibrinogen, 40 IU of thrombin, and 103 μg of calcium chloride (CaCl2 ).
实施例6Example 6
本实施例的止血组合物由46mg纤维蛋白原、40IU凝血酶、115μg氯化钙(CaCl2)组成。The hemostatic composition of this example consists of 46 mg of fibrinogen, 40 IU of thrombin, and 115 μg of calcium chloride (CaCl2 ).
比较例1Comparative example 1
本比较例的止血组合物由10.3mg纤维蛋白原、28.6IU凝血酶、2500μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 10.3 mg of fibrinogen, 28.6 IU of thrombin, and 2500 μg of calcium chloride (CaCl2 ).
比较例2Comparative example 2
本比较例的止血组合物由15mg纤维蛋白原、37.5IU凝血酶、117μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 15 mg of fibrinogen, 37.5 IU of thrombin, and 117 μg of calcium chloride (CaCl2 ).
比较例3Comparative example 3
本比较例的止血组合物由13mg纤维蛋白原、40IU凝血酶、115μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 13 mg of fibrinogen, 40 IU of thrombin, and 115 μg of calcium chloride (CaCl2 ).
比较例4Comparative example 4
本比较例的止血组合物由47mg纤维蛋白原、40IU凝血酶、115μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 47 mg of fibrinogen, 40 IU of thrombin, and 115 μg of calcium chloride (CaCl2 ).
比较例5Comparative Example 5
本比较例的止血组合物由17mg纤维蛋白原、45IU凝血酶、115μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 17 mg of fibrinogen, 45 IU of thrombin, and 115 μg of calcium chloride (CaCl2 ).
比较例6Comparative Example 6
本比较例的止血组合物由17mg纤维蛋白原、30IU凝血酶、115μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 17 mg of fibrinogen, 30 IU of thrombin, and 115 μg of calcium chloride (CaCl2 ).
比较例7Comparative Example 7
本比较例的止血组合物由17mg纤维蛋白原、40IU凝血酶、80μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 17 mg of fibrinogen, 40 IU of thrombin, and 80 μg of calcium chloride (CaCl2 ).
比较例8Comparative Example 8
本比较例的止血组合物由17mg纤维蛋白原、40IU凝血酶、130μg氯化钙(CaCl2)组成。The hemostatic composition of this comparative example consists of 17 mg of fibrinogen, 40 IU of thrombin, and 130 μg of calcium chloride (CaCl2 ).
实验一、力学性能的检测
本实验旨在模拟止血组合物与血液接触后形成纤维蛋白多聚体血凝块,再检测其力学性能。以力学性能指标:储能模量(G')、损耗模量(G")和黏弹特性值tanδ(G"/G')为检测指标。This experiment aims to simulate the formation of fibrin polymer blood clot after the hemostatic composition contacts with blood, and then detect its mechanical properties. The mechanical performance indicators: storage modulus (G'), loss modulus (G") and viscoelastic property value tanδ (G"/G') are used as detection indicators.
1.样品的制备1. Sample Preparation
取实施例1-6止血组合物中的纤维蛋白原溶于1ml水中制成水溶液A,再取实施例1-6止血组合物中的凝血酶和氯化钙溶于1ml水中制成水溶液B,最后将水溶液A和水溶液B混合,形成凝块样品,检测其力学性能指标。该过程中加入的水用来模拟血液,形成的凝块用来模拟形成的纤维蛋白多聚体血凝块。The fibrinogen in the hemostatic composition of Example 1-6 was dissolved in 1 ml of water to make aqueous solution A, and then the thrombin and calcium chloride in the hemostatic composition of Example 1-6 were dissolved in 1 ml of water to make aqueous solution B, Finally, the aqueous solution A and the aqueous solution B are mixed to form a clot sample, and its mechanical property index is tested. The water added during this process is used to simulate blood and the clot formed is used to simulate the formation of a fibrin polymer blood clot.
2.力学性能指标的检测2. Detection of mechanical performance indicators
在流变仪(安东帕,MCR302)的测试板上放置凝块样品,加热到37℃,测试转子的直径为12mm,测试转子与测试板之间的距离为1mm。采用小振幅(1%)的动态方法,即:在频率为0.1~10Hz的小振幅振荡下剪切凝块样品,然后用频率扫描测量样品的储能模量(G')和损耗模量(G"),并计算得出黏弹特性值tanδ(G"/G');tanδ<1,说明样品趋向于凝胶体,不易发生形变,即:止血组合物在使用时不易被血液冲破,可维持其原本的形态;tanδ>1,说明样品趋向于流体,容易发生形变,即:止血组合物在使用时容易被血液冲破,无法维持其原本的形态。以实施例1和实施例5为例,结果见表1。The clot sample was placed on a test plate of a rheometer (Anton Paar, MCR302), heated to 37° C., the diameter of the test rotor was 12 mm, and the distance between the test rotor and the test plate was 1 mm. The dynamic method with small amplitude (1%) is adopted, that is, the clot sample is sheared under small amplitude oscillation with a frequency of 0.1-10 Hz, and then the storage modulus (G') and loss modulus (G') of the sample are measured by frequency sweeping ( G"), and calculate the viscoelastic property value tanδ(G"/G'); tanδ<1, indicating that the sample tends to be a gel and is not easily deformed, that is, the hemostatic composition is not easily broken by blood during use, It can maintain its original form; tanδ>1 indicates that the sample tends to be fluid and easily deformed, that is, the hemostatic composition is easily broken by blood during use and cannot maintain its original form. Taking Example 1 and Example 5 as examples, the results are shown in Table 1.
表1 实施例1和5止血组合物的力学性能测试结果Table 1 The mechanical property test results of the hemostatic compositions of Examples 1 and 5
表1的结果表明,实施例1和实施例5止血组合物形成的凝块样品的tanδ<1,说明形成的凝块样品趋向于凝胶体,不易发生形变,即:本发明的止血组合物在使用时不易被血液冲破,可维持其原本的形态。The results in Table 1 show that the tanδ of the clot samples formed by the hemostatic compositions of Example 1 and Example 5 <1, indicating that the formed clot samples tend to be gels and are not easily deformed, that is: the hemostatic composition of the present invention It is not easy to be broken by blood when used, and can maintain its original shape.
实验二、平均粒径和沉淀体积占比的测定Experiment 2. Determination of Average Particle Size and Precipitation Volume Proportion
本发明发现制备止血贴方法的步骤(2)中,得到的混悬液中颗粒的平均直径和5min沉淀体积占比(混悬液制备完成后静置5min,测量静置前混悬液中颗粒的整体初始高度(记为H0)和静置后混悬液中颗粒的高度(记为H),5min沉淀体积占比=H/H0)会影响后续喷涂的均匀效果:颗粒直径大时,混悬液中颗粒的沉降速度快,容易沉积堵住泵管,导致混悬液无法喷出或者喷涂不均匀。因此本实验通过调整制备方法中步骤(2)的参数以得到粒径和5min沉淀体积占比不同的含止血组合物的混悬液。步骤(2)的药液用分散机均质后,温度会发生变化,温度过高会对纤维蛋白原和凝血酶的蛋白活性产生影响,因此本实验还检测了均质前后混悬液的温度。The present invention finds that in the step (2) of the method for preparing the hemostatic patch, the average diameter of the particles in the obtained suspension and the 5min sedimentation volume ratio (the suspension is left to stand for 5 minutes after the suspension is prepared, and the particles in the suspension before the measurement is measured) The overall initial height (denoted as H0 ) and the height of the particles in the suspension after standing (denoted as H), 5min sedimentation volume ratio=H/H0 ) will affect the uniform effect of subsequent spraying: when the particle diameter is large , The sedimentation speed of the particles in the suspension is fast, and it is easy to deposit and block the pump tube, resulting in the suspension being unable to be sprayed or sprayed unevenly. Therefore, in this experiment, the parameters of step (2) in the preparation method were adjusted to obtain suspensions containing hemostatic compositions with different particle sizes and 5-min sedimentation volume ratios. After the medicinal solution in step (2) is homogenized with a disperser, the temperature will change. If the temperature is too high, it will affect the protein activity of fibrinogen and thrombin. Therefore, this experiment also detected the temperature of the suspension before and after homogenization. .
本实验的具体过程为:将实施例1-6的止血组合物分别加入预先冷却至-20℃的无水乙醇,搅拌制成药液,药液中各物质的终浓度为纤维蛋白原140.5mg/ml、凝血酶330.6IU/ml、氯化钙950.5μg/ml。然后用分散机(IKA,T 25easy clean)对药液进行均质搅拌处理,搅拌速度为15×103rpm,搅拌5-10次,单次搅拌时间为2min(搅拌时间为30s时,后续喷涂会堵塞泵管),均质过程的时间共计10-20min。每一次均质后取三个样品,并记录样品均质前和均质停止时的温度,以及5min沉淀体积占比。以实施例1的止血组合物为例,进行了两组实验,两组实验(实验A和实验B)的不同之处,仅在于搅拌次数不同。实验A中搅拌次数为5次,结果见表2;实验B中搅拌次数为10次,结果见表3。The specific process of this experiment is: add the hemostatic compositions of Examples 1-6 to absolute ethanol pre-cooled to -20°C, and stir to make a medicinal solution. The final concentration of each substance in the medicinal solution is 140.5 mg of fibrinogen /ml, thrombin 330.6IU/ml, calcium chloride 950.5μg/ml. Then use a disperser (IKA, T 25easy clean) to homogeneously stir the liquid medicine at a stirring speed of 15×103 rpm for 5-10 times, with a single stirring time of 2 minutes (when the stirring time is 30s, subsequent spraying will block the pump tube), the homogenization process takes a total of 10-20min. Take three samples after each homogenization, and record the temperature of the samples before homogenization and when the homogenization stops, as well as the 5-min precipitation volume ratio. Taking the hemostatic composition of Example 1 as an example, two sets of experiments were carried out. The difference between the two sets of experiments (Experiment A and Experiment B) lies in the number of times of stirring. In experiment A, the stirring frequency was 5 times, and the results were shown in Table 2; in experiment B, the stirring frequency was 10 times, and the results were shown in Table 3.
表2 实验A均质前后的温度、平均直径和5min沉淀体积占比Table 2 Temperature, average diameter and 5min sedimentation volume ratio before and after homogenization in experiment A
由表2的结果显示,混悬液在步骤(2)的均质前后温度差不超过1.5℃,不会影响蛋白的活性;均质后混悬液中止血组合物颗粒的平均直径为37.78μm,每次均质后的5min沉淀体积占比均高于90%,均质结束后得到的混悬液的5min沉淀体积占比能达到95%,表明该均质过程得到的混悬液可达到更高的5min沉淀体积占比。The results in Table 2 show that the temperature difference before and after the homogenization of the suspension in step (2) does not exceed 1.5°C, which will not affect the activity of the protein; the average diameter of the hemostatic composition particles in the suspension after homogenization is 37.78 μm , the 5min sedimentation volume ratio after each homogenization is higher than 90%, and the 5min sedimentation volume ratio of the suspension obtained after homogenization can reach 95%, indicating that the suspension obtained in the homogenization process can reach Higher 5min sedimentation volume ratio.
表3 实验B均质前后的温度、平均直径和凝固时间Table 3 Temperature, average diameter and solidification time before and after homogenization in experiment B
表3的结果显示,均质后混悬液中止血组合物颗粒的平均直径为41.63μm,每次均质后的5min沉淀体积占比平均值高于90%(表中未示出),表明该均质过程得到的混悬液可达到更高的5min沉淀体积占比。混悬液在步骤(2)的均质前后温度差不超过2.5℃,对蛋白活性的影响较低。The results in Table 3 show that the average diameter of the hemostatic composition particles in the suspension after homogenization is 41.63 μm, and the average value of the 5min sedimentation volume after each homogenization is higher than 90% (not shown in the table), indicating that The suspension obtained in this homogenization process can achieve a higher 5-min sedimentation volume ratio. The temperature difference of the suspension before and after homogenization in step (2) does not exceed 2.5° C., which has a low impact on protein activity.
此外,本实验还用凝固时间表征均质过程对蛋白活性的影响,以纤维蛋白原的凝固时间为例,结果见表3。表3的结果显示,每次均质后纤维蛋白原的凝固时间均不超过22s,符合《中国药典》(2020版)对人纤维蛋白原活性标准中凝固时间﹤60s的规定。In addition, this experiment also used the coagulation time to characterize the effect of the homogenization process on the protein activity. Taking the coagulation time of fibrinogen as an example, the results are shown in Table 3. The results in Table 3 show that the clotting time of fibrinogen after each homogenization does not exceed 22s, which is in line with the regulation of the clotting time of human fibrinogen activity in the "Chinese Pharmacopoeia" (2020 edition) < 60s.
表2和表3的结果显示,制备止血贴方法的步骤(2)得到的混悬液中止血组合物颗粒的平均直径为37.78-41.63μm,5min沉淀体积占比均高于90%,表明得到的混悬液颗粒粒径较集中,跨度小,颗粒的沉降速度慢,不容易沉积堵住泵管,在后续的喷涂中能达到喷涂均匀的效果。混悬液均质处理前后的温度差<10℃,均质后纤维蛋白原凝固时间<60s,符合《中国药典》(2020版)的要求,均表明均质过程不会对纤维蛋白原和凝血酶的蛋白活性产生影响。The results in Table 2 and Table 3 show that the average diameter of the hemostatic composition particles in the suspension obtained in step (2) of the method for preparing the hemostatic patch is 37.78-41.63 μm, and the proportion of the sedimentation volume in 5 minutes is higher than 90%, indicating that the obtained The particle size of the suspension is relatively concentrated, the span is small, the sedimentation speed of the particles is slow, it is not easy to deposit and block the pump tube, and the effect of uniform spraying can be achieved in the subsequent spraying. The temperature difference before and after homogenization of the suspension is less than 10°C, and the coagulation time of fibrinogen after homogenization is less than 60 s, which meets the requirements of the "Chinese Pharmacopoeia" (2020 edition), indicating that the homogenization process will not affect fibrinogen and blood coagulation. Enzyme protein activity is affected.
由于原料中各物质的含量与均质的效果无关,因此本实验中用实施例1的结果就可表明其他实施例具有与实施例1相同的结果,在此不一一赘述。Because the content of each substance in the raw material has nothing to do with the effect of homogeneity, the results of Example 1 can be used to show that other examples have the same results as Example 1 in this experiment, and details will not be repeated here.
实验三、凝固时间的测定
样品:sample:
对照样品1:按照公开号为CN1507358A的中国专利申请中公开的内容制备得到,其止血成分由纤维蛋白原5.5mg/cm2和凝血酶2.0IU/cm2组成。Control sample 1: Prepared according to the content disclosed in the Chinese patent application publication number CN1507358A, its hemostatic component consists of 5.5 mg/cm2 of fibrinogen and 2.0 IU/cm2 of thrombin.
对照样品2:止血成分由纤维蛋白原15mg/cm2,凝血酶37.5IU/cm2和氯化钙117μg/cm2组成。Control sample 2: the hemostatic component consisted of fibrinogen 15 mg/cm2 , thrombin 37.5 IU/cm2 and calcium chloride 117 μg/cm2 .
实验样品:实施例1-6的止血组合物。Experimental sample: the hemostatic composition of Examples 1-6.
比较样品:比较例1、3-8的止血组合物。Comparative samples: the hemostatic compositions of Comparative Examples 1, 3-8.
本实验将单位平方厘米中含有的止血组合物/止血成分加入水中,用来模拟在血液中的凝固情况。根据文献报道,单位面积对照样品1和2的吸血量不超过4ml。因此本实验利用磁珠法,将单位平方厘米含有的止血组合物/止血成分中的纤维蛋白原加入5ml水中形成溶液A,再把剩余止血成分(凝血酶或凝血酶和氯化钙)加入5ml水中形成溶液B,取100μl溶液A和100μl溶液B等体积加样于血凝仪的反应杯中,血凝仪自动检测出溶液A和溶液B反应生成纤维蛋白多聚体凝块的时间,记作凝固时间。以实施例1、2、5和6为例,结果见表4。In this experiment, the hemostatic composition/hemostatic component contained in a unit square centimeter is added to water to simulate coagulation in blood. According to literature reports, the blood absorption volume per unit area of
表4 样品的凝固时间Table 4 The solidification time of samples
表5的结果显示,同等稀释比例下,实施例1、2、5和6的止血组合物在凝固时间上均优于对照样品1和2,且差异显著;表明本发明的止血组合物在止血效果上显著优异于对照样品1和2。另外,实施例1、2、5和6的止血组合物在凝固时间上也均优于比较例1-8,且差异显著;表明本发明的止血组合物在止血效果上显著优异于比较例1-8;特别是比较例4,仅是纤维蛋白原的含量略高于实施例6,在凝固时间上有显著提高,表明本发明提供的止血组合物中通过调整纤维蛋白原、凝血酶和氯化钙的含量,可使三者协同发挥更优异的止血效果。The results in Table 5 show that under the same dilution ratio, the hemostatic compositions of Examples 1, 2, 5 and 6 are better than
以上所述仅是本发明的优选实施方式,应当指出的是,对于本技术领域的普通技术人员来说,在不脱离本发明原理的前提下,还可以做出若干改进和润饰,这些改进和润饰也应视为本发明的内容。The above is only a preferred embodiment of the present invention, it should be pointed out that, for those of ordinary skill in the art, without departing from the principle of the present invention, some improvements and modifications can also be made, these improvements and Retouching should also be considered as part of the invention.
Claims (12)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111603820.6ACN114177346B (en) | 2021-12-24 | 2021-12-24 | Hemostatic composition and hemostatic patch and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111603820.6ACN114177346B (en) | 2021-12-24 | 2021-12-24 | Hemostatic composition and hemostatic patch and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114177346A CN114177346A (en) | 2022-03-15 |
| CN114177346Btrue CN114177346B (en) | 2023-05-26 |
Family
ID=80544950
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202111603820.6AActiveCN114177346B (en) | 2021-12-24 | 2021-12-24 | Hemostatic composition and hemostatic patch and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114177346B (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002102276A2 (en)* | 2001-06-14 | 2002-12-27 | Providence Health System-Oregon | Wound dressing and method for controlling severe, life-threatening bleeding |
| CN106110367A (en)* | 2016-07-29 | 2016-11-16 | 北京化工大学常州先进材料研究院 | Based on natural polymer MULTILAYER COMPOSITE medical dressing and preparation method thereof |
| CN107405427A (en)* | 2016-02-15 | 2017-11-28 | 现代牧场股份有限公司 | Biology manufacture composite |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| UA73028C2 (en)* | 2001-01-25 | 2005-05-16 | Нікомед Фарма Ас | A method of preparing a collagen sponge (variants), a device for extracting a part of a collagen foam and an elongated collagen sponge |
| ATE333907T1 (en)* | 2001-05-09 | 2006-08-15 | Baxter Int | FIBRIN MATERIAL AND METHOD FOR THE PRODUCTION AND USE THEREOF |
| CN1314446C (en)* | 2002-06-24 | 2007-05-09 | 张清 | High-solidifiability fibrinogen gelatin |
| CN1552465A (en)* | 2003-06-05 | 2004-12-08 | 胡庆柳 | Fiber protein stopping bleeding paste |
| CN1234425C (en)* | 2003-06-05 | 2006-01-04 | 胡庆柳 | Method for preparing absorbent fibrin hemostatic plaster |
| CN102250253A (en)* | 2010-05-17 | 2011-11-23 | 中国人民解放军军事医学科学院军事兽医研究所 | Fusogenic peptide containing thrombin fragment |
| CN102178975B (en)* | 2011-04-25 | 2013-10-23 | 福建南生科技有限公司 | Fibrous protein hemostatic patch and making method thereof |
| CN106432810B (en)* | 2016-09-09 | 2019-11-15 | 湖北科技学院 | A high surface tension hydrogel vitreous substitute and its radiation preparation method |
| JOP20190200A1 (en)* | 2017-02-28 | 2019-08-27 | Univ Pennsylvania | Compositions useful in treatment of spinal muscular atrophy |
| CN112972755B (en)* | 2021-03-25 | 2022-07-05 | 哈尔滨瀚邦医疗科技有限公司 | A kind of preparation method of biological hemostatic material based on pig-derived fibrinogen and thrombin |
- 2021
- 2021-12-24CNCN202111603820.6Apatent/CN114177346B/enactiveActive
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002102276A2 (en)* | 2001-06-14 | 2002-12-27 | Providence Health System-Oregon | Wound dressing and method for controlling severe, life-threatening bleeding |
| CN107405427A (en)* | 2016-02-15 | 2017-11-28 | 现代牧场股份有限公司 | Biology manufacture composite |
| CN106110367A (en)* | 2016-07-29 | 2016-11-16 | 北京化工大学常州先进材料研究院 | Based on natural polymer MULTILAYER COMPOSITE medical dressing and preparation method thereof |
Non-Patent Citations (1)
| Title |
|---|
| 组织工程支架材料的研究现状;汪海滨,罗盛康;中国临床康复(20);161-163* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN114177346A (en) | 2022-03-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230381288A1 (en) | Hemostatic compositions | |
| Li et al. | Preparation and the hemostatic property study of porous gelatin microspheres both in vitro and in vivo | |
| SK10362003A3 (en) | A method of preparing a collagen sponge, a device for extracting a part of a collagen foam, and en elongated collagen sponge | |
| CN106913900B (en) | Silk fibroin hemostatic material and preparation method thereof | |
| US20130096082A1 (en) | Hemostatic compositions | |
| KR20180091035A (en) | Thrombin microcapsule | |
| Mu et al. | Thrombin immobilized polydopamine–diatom biosilica for effective hemorrhage control | |
| CN113289050A (en) | Hemostatic sponge and preparation method thereof | |
| CN112300418B (en) | Adhesive high-efficiency hemostatic microsphere and preparation method thereof | |
| CN111729126B (en) | Hemostatic material based on combination of chitosan and tissue factor and preparation method thereof | |
| US20160121017A1 (en) | SINGLE SOLUTION of Gel-LIKE FIBRIN HEMOSTAT | |
| Hu et al. | Sodium alginate/carboxycellulose/polydopamine composite microspheres for rapid hemostasis of deep irregular wounds | |
| US11903961B2 (en) | Hemostatic agent and method of production thereof | |
| Şeker et al. | Current trends in the design and fabrication of PRP-based scaffolds for tissue engineering and regenerative medicine | |
| EP3074055B1 (en) | Dry pad comprising thrombin and pectin | |
| CN114177346B (en) | Hemostatic composition and hemostatic patch and application thereof | |
| CN114246974B (en) | Preparation method of hemostatic patch | |
| US11311643B2 (en) | Fibrin and/or dialdehyde starch hydrolysate materials, and preparation and use thereof | |
| Xu et al. | Preparation and application of collagen-based hemostatic materials: a review | |
| Hou et al. | A facile way to fabricate a thrombin immobilized composite sponge with dual hemostatic effects for acute hemorrhage control | |
| US20250256005A1 (en) | Flowable paste with ellagic acid-nickel complex and uses thereof for activating coagulation | |
| Sun et al. | Sodium alginate hydrogel platelet rich plasma exosome loaded for synergistic hemostasis | |
| CN115300664A (en) | Spray hemostatic film based on chitosan and sodium polyphosphate | |
| HK40011498A (en) | Hemostatic compositions and methods of making thereof | |
| Team et al. | ZEOLITE-LOADED ALGINATE-CHITOSAN HYDROGEL BEADS AS A TOPICAL HEMOSTAT |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |