CN113599537B - A kind of nano-aggregate and its preparation method and application - Google Patents
A kind of nano-aggregate and its preparation method and applicationDownload PDFInfo
- Publication number
- CN113599537B CN113599537BCN202110911464.8ACN202110911464ACN113599537BCN 113599537 BCN113599537 BCN 113599537BCN 202110911464 ACN202110911464 ACN 202110911464ACN 113599537 BCN113599537 BCN 113599537B
- Authority
- CN
- China
- Prior art keywords
- nano
- cpul1
- solution
- aggregates
- aggregate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription14
- HTMNMHIFUPOACL-UHFFFAOYSA-N3,5-diamino-1-(3,4-dichlorophenyl)-1H-pyrano[3,2-a]phenazine-2-carbonitrileChemical compoundNC1=C(C#N)C(c2ccc(Cl)c(Cl)c2)c3c(O1)c(N)cc4nc5ccccc5nc34HTMNMHIFUPOACL-UHFFFAOYSA-N0.000claimsabstractdescription75
- 239000000243solutionSubstances0.000claimsabstractdescription60
- 239000002245particleSubstances0.000claimsabstractdescription35
- 239000003814drugSubstances0.000claimsabstractdescription28
- RIOQSEWOXXDEQQ-UHFFFAOYSA-NtriphenylphosphineChemical classC1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1RIOQSEWOXXDEQQ-UHFFFAOYSA-N0.000claimsabstractdescription22
- 206010028980NeoplasmDiseases0.000claimsabstractdescription20
- 238000003756stirringMethods0.000claimsabstractdescription20
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterChemical compoundOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsabstractdescription12
- 239000008367deionised waterSubstances0.000claimsabstractdescription9
- 229910021641deionized waterInorganic materials0.000claimsabstractdescription9
- 239000011259mixed solutionSubstances0.000claimsabstractdescription6
- 239000003960organic solventSubstances0.000claimsabstractdescription5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-NDimethylsulphoxideChemical compoundCS(C)=OIAZDPXIOMUYVGZ-UHFFFAOYSA-N0.000claimsdescription26
- 238000000034methodMethods0.000claimsdescription19
- 238000003760magnetic stirringMethods0.000claimsdescription12
- CSCPPACGZOOCGX-UHFFFAOYSA-NAcetoneChemical compoundCC(C)=OCSCPPACGZOOCGX-UHFFFAOYSA-N0.000claimsdescription6
- YMWUJEATGCHHMB-UHFFFAOYSA-NDichloromethaneChemical compoundClCClYMWUJEATGCHHMB-UHFFFAOYSA-N0.000claimsdescription6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000claimsdescription6
- XEKOWRVHYACXOJ-UHFFFAOYSA-NEthyl acetateChemical compoundCCOC(C)=OXEKOWRVHYACXOJ-UHFFFAOYSA-N0.000claimsdescription6
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000claimsdescription6
- HEDRZPFGACZZDS-UHFFFAOYSA-NChloroformChemical compoundClC(Cl)ClHEDRZPFGACZZDS-UHFFFAOYSA-N0.000claimsdescription4
- FKRCODPIKNYEAC-UHFFFAOYSA-Nethyl propionateChemical compoundCCOC(=O)CCFKRCODPIKNYEAC-UHFFFAOYSA-N0.000claimsdescription4
- YKYONYBAUNKHLG-UHFFFAOYSA-Nn-Propyl acetateNatural productsCCCOC(C)=OYKYONYBAUNKHLG-UHFFFAOYSA-N0.000claimsdescription2
- 229940090181propyl acetateDrugs0.000claimsdescription2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-Nvaleric acidChemical compoundCCCCC(O)=ONQPDZGIKBAWPEJ-UHFFFAOYSA-N0.000claimsdescription2
- 238000004519manufacturing processMethods0.000claims1
- 229940079593drugDrugs0.000abstractdescription26
- 230000000694effectsEffects0.000abstractdescription16
- 239000002105nanoparticleSubstances0.000abstractdescription12
- 230000002209hydrophobic effectEffects0.000abstractdescription6
- 230000000259anti-tumor effectEffects0.000abstractdescription4
- 210000004027cellAnatomy0.000description57
- 101000600434Homo sapiens Putative uncharacterized protein encoded by MIR7-3HGProteins0.000description22
- 102100037401Putative uncharacterized protein encoded by MIR7-3HGHuman genes0.000description22
- CURLTUGMZLYLDI-UHFFFAOYSA-NCarbon dioxideChemical compoundO=C=OCURLTUGMZLYLDI-UHFFFAOYSA-N0.000description16
- 238000002296dynamic light scatteringMethods0.000description9
- 108091003079Bovine Serum AlbuminProteins0.000description8
- 239000006144Dulbecco’s modified Eagle's mediumSubstances0.000description8
- 230000006907apoptotic processEffects0.000description8
- 229910002092carbon dioxideInorganic materials0.000description8
- 239000001569carbon dioxideSubstances0.000description8
- 239000012091fetal bovine serumSubstances0.000description8
- 230000008685targetingEffects0.000description8
- 238000010586diagramMethods0.000description7
- 239000002609mediumSubstances0.000description7
- 210000003470mitochondriaAnatomy0.000description7
- 201000011510cancerDiseases0.000description6
- 239000002246antineoplastic agentSubstances0.000description5
- 210000000170cell membraneAnatomy0.000description5
- 230000002438mitochondrial effectEffects0.000description5
- 239000000203mixtureSubstances0.000description5
- 210000001519tissueAnatomy0.000description5
- TZCPCKNHXULUIY-RGULYWFUSA-N1,2-distearoyl-sn-glycero-3-phosphoserineChemical compoundCCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCCTZCPCKNHXULUIY-RGULYWFUSA-N0.000description4
- 1087000128137-aminoactinomycin DProteins0.000description4
- YXHLJMWYDTXDHS-IRFLANFNSA-N7-aminoactinomycin DChemical compoundC[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=C(N)C=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21YXHLJMWYDTXDHS-IRFLANFNSA-N0.000description4
- ZWZWYGMENQVNFU-UHFFFAOYSA-NGlycerophosphorylserinNatural productsOC(=O)C(N)COP(O)(=O)OCC(O)COZWZWYGMENQVNFU-UHFFFAOYSA-N0.000description4
- 230000001640apoptogenic effectEffects0.000description4
- 229940044683chemotherapy drugDrugs0.000description4
- 238000012377drug deliveryMethods0.000description4
- 239000000975dyeSubstances0.000description4
- 238000000684flow cytometryMethods0.000description4
- 238000011534incubationMethods0.000description4
- 230000005764inhibitory processEffects0.000description4
- 230000002147killing effectEffects0.000description4
- 230000008569processEffects0.000description4
- 102000004121Annexin A5Human genes0.000description3
- 108090000672Annexin A5Proteins0.000description3
- 230000005540biological transmissionEffects0.000description3
- 230000015556catabolic processEffects0.000description3
- 238000004113cell cultureMethods0.000description3
- 238000006731degradation reactionMethods0.000description3
- 239000007788liquidSubstances0.000description3
- 239000002539nanocarrierSubstances0.000description3
- 125000001791phenazinyl groupChemical classC1(=CC=CC2=NC3=CC=CC=C3N=C12)*0.000description3
- 238000012360testing methodMethods0.000description3
- 238000004448titrationMethods0.000description3
- BDXJANJAHYKTMI-UHFFFAOYSA-N2,3,4,5-tetramethyl-1h-pyrroleChemical classCC=1NC(C)=C(C)C=1CBDXJANJAHYKTMI-UHFFFAOYSA-N0.000description2
- 102000004190EnzymesHuman genes0.000description2
- 108090000790EnzymesProteins0.000description2
- 102000001639Thioredoxin Reductase 1Human genes0.000description2
- 108010093836Thioredoxin Reductase 1Proteins0.000description2
- 230000035508accumulationEffects0.000description2
- 238000009825accumulationMethods0.000description2
- 239000007864aqueous solutionSubstances0.000description2
- 239000012876carrier materialSubstances0.000description2
- 239000003795chemical substances by applicationSubstances0.000description2
- 230000006870functionEffects0.000description2
- 239000003112inhibitorSubstances0.000description2
- 238000002347injectionMethods0.000description2
- 239000007924injectionSubstances0.000description2
- 230000003993interactionEffects0.000description2
- 239000012528membraneSubstances0.000description2
- 230000025608mitochondrion localizationEffects0.000description2
- 238000001338self-assemblyMethods0.000description2
- 230000001225therapeutic effectEffects0.000description2
- 231100000331toxicToxicity0.000description2
- 230000002588toxic effectEffects0.000description2
- 231100000419toxicityToxicity0.000description2
- 230000001988toxicityEffects0.000description2
- 238000004627transmission electron microscopyMethods0.000description2
- 210000004881tumor cellAnatomy0.000description2
- FWBHETKCLVMNFS-UHFFFAOYSA-N4',6-Diamino-2-phenylindolChemical compoundC1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1FWBHETKCLVMNFS-UHFFFAOYSA-N0.000description1
- 102000000412AnnexinHuman genes0.000description1
- 108050008874AnnexinProteins0.000description1
- 208000024172Cardiovascular diseaseDiseases0.000description1
- 230000004543DNA replicationEffects0.000description1
- 206010059866Drug resistanceDiseases0.000description1
- 206010061218InflammationDiseases0.000description1
- 239000000232Lipid BilayerSubstances0.000description1
- 208000012902Nervous system diseaseDiseases0.000description1
- 208000025966Neurological diseaseDiseases0.000description1
- 102000004316OxidoreductasesHuman genes0.000description1
- 108090000854OxidoreductasesProteins0.000description1
- 229930040373ParaformaldehydeNatural products0.000description1
- BUGBHKTXTAQXES-UHFFFAOYSA-NSeleniumChemical compound[Se]BUGBHKTXTAQXES-UHFFFAOYSA-N0.000description1
- 102000004142TrypsinHuman genes0.000description1
- 108090000631TrypsinProteins0.000description1
- 238000002835absorbanceMethods0.000description1
- 239000000980acid dyeSubstances0.000description1
- 230000001093anti-cancerEffects0.000description1
- 229940124599anti-inflammatory drugDrugs0.000description1
- 229940041181antineoplastic drugDrugs0.000description1
- 238000013459approachMethods0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 230000009286beneficial effectEffects0.000description1
- 230000008901benefitEffects0.000description1
- 230000000975bioactive effectEffects0.000description1
- 239000000969carrierSubstances0.000description1
- 150000001768cationsChemical class0.000description1
- 230000030833cell deathEffects0.000description1
- 230000010261cell growthEffects0.000description1
- 230000003833cell viabilityEffects0.000description1
- 238000003570cell viability assayMethods0.000description1
- 230000001413cellular effectEffects0.000description1
- 230000004700cellular uptakeEffects0.000description1
- 238000006243chemical reactionMethods0.000description1
- 238000002512chemotherapyMethods0.000description1
- 230000008045co-localizationEffects0.000description1
- 238000004737colorimetric analysisMethods0.000description1
- 238000007398colorimetric assayMethods0.000description1
- 239000013078crystalSubstances0.000description1
- 210000004748cultured cellAnatomy0.000description1
- 238000012258culturingMethods0.000description1
- 231100000433cytotoxicToxicity0.000description1
- 230000001472cytotoxic effectEffects0.000description1
- 230000001419dependent effectEffects0.000description1
- 238000011161developmentMethods0.000description1
- 201000010099diseaseDiseases0.000description1
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description1
- 238000009510drug designMethods0.000description1
- 230000002526effect on cardiovascular systemEffects0.000description1
- 230000012202endocytosisEffects0.000description1
- 238000002474experimental methodMethods0.000description1
- 238000002073fluorescence micrographMethods0.000description1
- 230000036541healthEffects0.000description1
- 238000010438heat treatmentMethods0.000description1
- 238000003384imaging methodMethods0.000description1
- 238000000338in vitroMethods0.000description1
- 230000004054inflammatory processEffects0.000description1
- 230000003902lesionEffects0.000description1
- 150000002632lipidsChemical class0.000description1
- 201000007270liver cancerDiseases0.000description1
- 208000014018liver neoplasmDiseases0.000description1
- 230000007246mechanismEffects0.000description1
- 230000004060metabolic processEffects0.000description1
- 238000000386microscopyMethods0.000description1
- IKEOZQLIVHGQLJ-UHFFFAOYSA-MmitoTracker RedChemical compound[Cl-].C1=CC(CCl)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2IKEOZQLIVHGQLJ-UHFFFAOYSA-M0.000description1
- 210000001700mitochondrial membraneAnatomy0.000description1
- 239000002086nanomaterialSubstances0.000description1
- 230000017074necrotic cell deathEffects0.000description1
- 230000001338necrotic effectEffects0.000description1
- 239000002547new drugSubstances0.000description1
- 102000039446nucleic acidsHuman genes0.000description1
- 108020004707nucleic acidsProteins0.000description1
- 150000007523nucleic acidsChemical class0.000description1
- 230000036542oxidative stressEffects0.000description1
- 229910052760oxygenInorganic materials0.000description1
- 239000001301oxygenSubstances0.000description1
- 229920002866paraformaldehydePolymers0.000description1
- 230000035699permeabilityEffects0.000description1
- 125000001997phenyl groupChemical group[H]C1=C([H])C([H])=C(*)C([H])=C1[H]0.000description1
- 150000003904phospholipidsChemical class0.000description1
- 238000010837poor prognosisMethods0.000description1
- 239000000843powderSubstances0.000description1
- 238000012545processingMethods0.000description1
- 230000035755proliferationEffects0.000description1
- 102000004169proteins and genesHuman genes0.000description1
- 108090000623proteins and genesProteins0.000description1
- 229910052711seleniumInorganic materials0.000description1
- 239000011669seleniumSubstances0.000description1
- 239000002904solventSubstances0.000description1
- 238000000638solvent extractionMethods0.000description1
- 230000002269spontaneous effectEffects0.000description1
- 238000013112stability testMethods0.000description1
- 229940126585therapeutic drugDrugs0.000description1
- 230000036962time dependentEffects0.000description1
- 238000000954titration curveMethods0.000description1
- 239000012588trypsinSubstances0.000description1
- 230000005740tumor formationEffects0.000description1
- 230000035899viabilityEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6927—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores
- A61K47/6929—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4985—Pyrazines or piperazines ortho- or peri-condensed with heterocyclic ring systems
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0013—Luminescence
- A61K49/0017—Fluorescence in vivo
- A61K49/0019—Fluorescence in vivo characterised by the fluorescent group, e.g. oligomeric, polymeric or dendritic molecules
- A61K49/0021—Fluorescence in vivo characterised by the fluorescent group, e.g. oligomeric, polymeric or dendritic molecules the fluorescent group being a small organic molecule
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0013—Luminescence
- A61K49/0017—Fluorescence in vivo
- A61K49/005—Fluorescence in vivo characterised by the carrier molecule carrying the fluorescent agent
- A61K49/0052—Small organic molecules
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0063—Preparation for luminescence or biological staining characterised by a special physical or galenical form, e.g. emulsions, microspheres
- A61K49/0069—Preparation for luminescence or biological staining characterised by a special physical or galenical form, e.g. emulsions, microspheres the agent being in a particular physical galenical form
- A61K49/0089—Particulate, powder, adsorbate, bead, sphere
- A61K49/0091—Microparticle, microcapsule, microbubble, microsphere, microbead, i.e. having a size or diameter higher or equal to 1 micrometer
- A61K49/0093—Nanoparticle, nanocapsule, nanobubble, nanosphere, nanobead, i.e. having a size or diameter smaller than 1 micrometer, e.g. polymeric nanoparticle
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Nanotechnology (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Medical Informatics (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Immunology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及药物领域,尤其涉及一种纳米聚集体及其制备方法和应用。The invention relates to the field of medicine, in particular to a nano-aggregate and a preparation method and application thereof.
背景技术Background technique
近年来恶性肿瘤的发病率逐年攀升,已经成为威胁人类生命健康的严重疾病。目前,有很多恶性肿瘤仍然没有很好的药物进行针对性的治疗,化疗是目前临床上最广泛使用的癌症治疗方式之一。然而,大多数化疗药物通常遇到水溶性差和缺乏选择性或特异性的不足,这可能导致不令人满意的治疗结果和严重的副作用,例如在肿瘤组织中的低积累、生物利用度差、严重的耐药性、对健康组织的副作用等。In recent years, the incidence of malignant tumor has been increasing year by year, and it has become a serious disease that threatens human life and health. At present, many malignant tumors still do not have good drugs for targeted treatment. Chemotherapy is one of the most widely used cancer treatment methods in clinical practice. However, most chemotherapeutic drugs usually suffer from poor water solubility and lack of selectivity or specificity, which can lead to unsatisfactory treatment outcomes and serious side effects, such as low accumulation in tumor tissues, poor bioavailability, Severe drug resistance, side effects on healthy tissue, etc.
TrxR1(thioredoxin reductase 1,硫氧还蛋白还原酶1),是一种可用于支持细胞生长,并保护它们免受导致氧化应激的氧自由基伤害的含硒酶。TrxR1在多种原发性肿瘤(如肝癌)细胞内高度表达,且与多种肿瘤的预后不良相关联;对其抑制能阻止肿瘤形成,抑制肿瘤增殖和DNA复制,对肿瘤发生与发展具有重要的生理意义。CPUL1的结构记载在专利CN201510894070.0中,专利中的吡喃并[3,2-α]吩嗪衍生物即为CPUL1。在Liao J,Wang L,Wu Z,et al.Identification of phenazine analogue as a novel scaffold forthioredoxin reductase I inhibitors against Hep G2 cancer cell lines[J].Journal of Enzyme Inhibition&Medicinal Chemistry,2019,34(1):1158.中研究表明,吩嗪衍生物CPUL1是一种TrxR1抑制剂。TrxR1 (
线粒体被认为是癌症、心血管和神经系统疾病新药设计的最重要靶标之一。体外线粒体膜电位180~200mV时,内侧带负电;在活细胞和机体内略低,为130~150mV。利用亲脂阳离子进行药物递送,其中研究最为成功的是三苯基膦衍生物(TPP)。TPP所含三个苯环可增大分子表面积,并形成离域正电荷,可穿过线粒体双层疏水膜。许多生物活性分子经TPP修饰后,显示出线粒体靶向性。Mitochondria are considered to be one of the most important targets for new drug design in cancer, cardiovascular and neurological diseases. When the mitochondrial membrane potential in vitro is 180-200mV, the inner side is negatively charged; in living cells and organisms, it is slightly lower, at 130-150mV. Using lipophilic cations for drug delivery, triphenylphosphine derivatives (TPP) have been the most successfully studied. The three benzene rings contained in TPP can increase the molecular surface area and form a delocalized positive charge, which can pass through the mitochondrial bilayer hydrophobic membrane. Many bioactive molecules have been modified with TPP to show mitochondrial targeting.
为了保留化疗药物的高效抗癌活性,同时减少其对机体其他部位的毒副作用,通常将化疗药物与纳米载药递送系统联合使用。这些纳米载体辅助药物递送系统建立了一种新型的癌症治疗方法,并在近年来取得了巨大的成就。然而,纳米载体辅助药物递送系统仍然有其不可避免的缺点:例如,化疗药物的载药率并不理想,普遍低于10%(w/w),导致为了达到药效,需要注射大量的纳米载体,使病人的顺应性降低,同时大多数载体不但没有直接的治疗效果,还会导致在人体内的降解和新陈代谢过程中引起毒性和炎症;此外,不同形貌的纳米载体的药物装载能力以及降解速率是不确定的,因此,整体治疗效果是不可知的。In order to retain the highly effective anticancer activity of chemotherapeutic drugs while reducing their toxic and side effects on other parts of the body, chemotherapeutic drugs are usually used in combination with nano-drug delivery systems. These nanocarrier-assisted drug delivery systems have established a novel approach to cancer treatment and have achieved great success in recent years. However, nanocarrier-assisted drug delivery systems still have their unavoidable shortcomings: for example, the drug loading rate of chemotherapeutic drugs is not ideal, generally lower than 10% (w/w), resulting in the need to inject a large amount of nanomaterials in order to achieve drug efficacy. The carrier reduces the patient's compliance. At the same time, most carriers not only have no direct therapeutic effect, but also cause toxicity and inflammation in the process of degradation and metabolism in the human body; in addition, the drug loading capacity of nanocarriers with different morphologies and The rate of degradation is uncertain and, therefore, the overall therapeutic effect is unknown.
发明内容SUMMARY OF THE INVENTION
发明目的:本发明的目的是提供一种载药率高、粒径均一的纳米聚集体及其制备方法和应用;本发明的另一目的是提供一种纳米聚集体的制备方法;本发明的另一目的是提供一种纳米聚集体在抗肿瘤药物中的应用。Purpose of the invention: The purpose of the present invention is to provide a nano-aggregate with high drug loading rate and uniform particle size and its preparation method and application; another purpose of the present invention is to provide a preparation method of the nano-aggregate; Another object is to provide the application of a nano-aggregate in anti-tumor drugs.
技术方案:本发明的纳米聚集体,所述纳米聚集体包括CPUL1和三苯基膦衍生物,CPUL1和TPP相互作用形成稳定的纳米聚集体,兼具自组装、线粒体靶向和细胞成像功能,纳米聚集体能够使难溶性活性分子CPUL1很好地分散在水溶液中,TPP使纳米聚集体能够更快、更多地聚集在肿瘤细胞线粒体中,在病变部位能够更快更多的释放出有效的药物分子,实现靶向性。Technical solution: the nano-aggregates of the present invention, the nano-aggregates include CPUL1 and triphenylphosphine derivatives, CPUL1 and TPP interact to form stable nano-aggregates, which have the functions of self-assembly, mitochondrial targeting and cell imaging, Nano-aggregates can make the poorly soluble active molecule CPUL1 well dispersed in aqueous solution, and TPP enables nano-aggregates to accumulate faster and more in tumor cell mitochondria, and release more effective molecules in the lesion site faster. Drug molecules to achieve targeting.
进一步地,所述纳米聚集体为球形形貌,所述纳米聚集体的粒径在纳米级别,粒径大小为5-500nm,优选90-250nm。Further, the nano-aggregates are spherical in shape, and the particle sizes of the nano-aggregates are in the nanometer level, and the particle size is 5-500 nm, preferably 90-250 nm.
上述的纳米聚集体的制备方法,包括以下步骤:The preparation method of above-mentioned nano-aggregates, comprises the following steps:
(1)CPUL1和三苯基膦衍生物分别溶于有机溶剂中,得到CPUL1溶液和三苯基膦衍生物溶液;(1) CPUL1 and triphenylphosphine derivative are respectively dissolved in organic solvent to obtain CPUL1 solution and triphenylphosphine derivative solution;
(2)三苯基膦衍生物溶液中加入去离子水,初次搅拌得到混合溶液,在混合溶液中加入步骤(1)中的CPUL1溶液,再次搅拌均匀后得到纳米聚集体。(2) adding deionized water to the triphenylphosphine derivative solution, stirring for the first time to obtain a mixed solution, adding the CPUL1 solution in step (1) to the mixed solution, and stirring again to obtain nano-aggregates.
进一步地,步骤(1)中,所述CPUL1溶液和三苯基膦衍生物溶液的摩尔浓度比为1-100:1-100。Further, in step (1), the molar concentration ratio of the CPUL1 solution and the triphenylphosphine derivative solution is 1-100:1-100.
进一步地,步骤(1)中,所述有机溶剂为乙酸乙酯、丙酮、甲醇、二氯甲烷、三氯甲烷、丙酸乙酯、乙酸丙酯、二甲基亚砜或乙醇中任意一种。Further, in step (1), the organic solvent is any one of ethyl acetate, acetone, methanol, dichloromethane, chloroform, ethyl propionate, propyl acetate, dimethyl sulfoxide or ethanol .
进一步地,步骤(2)中,所述初次搅拌和/或再次搅拌为磁力搅拌。Further, in step (2), the initial stirring and/or the re-stirring is magnetic stirring.
更进一步地,步骤(2)中,初次搅拌温度为20-80℃,搅拌速度为50-2000rpm。Further, in step (2), the initial stirring temperature is 20-80° C., and the stirring speed is 50-2000 rpm.
更进一步地,步骤(2)中,再次搅拌温度为25-60℃,搅拌速度为100-2500rpm,搅拌时间为0.5-24h。Further, in step (2), the stirring temperature is 25-60° C. again, the stirring speed is 100-2500 rpm, and the stirring time is 0.5-24 h.
进一步地,步骤(2)中,纳米聚集体中CPUL1和/或TPP的摩尔浓度为0.16-16mM/L。Further, in step (2), the molar concentration of CPUL1 and/or TPP in the nano-aggregates is 0.16-16 mM/L.
上述纳米聚集体在肿瘤治疗药物中的应用。本发明提供的纳米聚集体的粒径在纳米级别,可以延长体内循环周期。纳米粒粒径较小,易通过肿瘤部位的EPR效应,在肿瘤部位积聚,降低对正常组织的暴露,更好地发挥抗肿瘤效果。Application of the above-mentioned nano-aggregates in tumor therapeutic drugs. The particle size of the nano-aggregates provided by the present invention is at the nanometer level, which can prolong the circulation period in the body. Nanoparticles have a small particle size, which is easy to pass through the EPR effect of the tumor site, accumulate in the tumor site, reduce the exposure to normal tissues, and better exert the anti-tumor effect.
有益效果:与现有技术相比,本发明具有如下显著优点:(1)载药率达100%,在纳米聚集体中,不含有其他载体材料,避免了载药率低而需要大量注射的问题和载体材料可能带来的额外毒性;Beneficial effects: Compared with the prior art, the present invention has the following significant advantages: (1) The drug loading rate reaches 100%, and the nano-aggregates do not contain other carrier materials, which avoids the need for a large amount of injection due to low drug loading rate. problems and possible additional toxicity of the carrier material;
(2)粒径均一的纳米粒子,可以延长体内循环周期、提高疏水药物的生物利用度,且易通过肿瘤部位的EPR效应,在肿瘤部位积聚,降低对正常组织的暴露,更好地发挥抗肿瘤效果;(2) Nanoparticles with uniform particle size can prolong the circulation period in the body, improve the bioavailability of hydrophobic drugs, and easily accumulate in the tumor site through the EPR effect of the tumor site, reduce the exposure to normal tissues, and play a better role in anti-inflammatory drugs. tumor effect;
(3)具有靶向性,相对比单独用药而言,纳米聚集体提高了活性分子的抗肿瘤药效,且在细胞内发出显著荧光,较大程度地降低药物对正常组织的毒副作用;(3) It is targeted. Compared with the single drug, the nano-aggregate improves the anti-tumor efficacy of the active molecule, and emits significant fluorescence in the cell, which greatly reduces the toxic and side effects of the drug on normal tissues;
(4)纳米药物的制备方法简单易行,可以节省成本,安全无污染。(4) The preparation method of the nanomedicine is simple and feasible, can save cost, is safe and pollution-free.
附图说明Description of drawings
图1是本发明实例1的纳米聚集体的透射电镜的图;Fig. 1 is the figure of the transmission electron microscope of the nano-aggregate of Example 1 of the present invention;
图2是本发明实例1的CPUL1的透射电镜的图;Fig. 2 is the figure of the transmission electron microscope of CPUL1 of example 1 of the present invention;
图3是本发明实例1的纳米聚集体的动态光散射测试图;Fig. 3 is the dynamic light scattering test chart of the nano-aggregate of Example 1 of the present invention;
图4是本发明实例1的CPUL1的动态光散射测试图;Fig. 4 is the dynamic light scattering test chart of CPUL1 of Example 1 of the present invention;
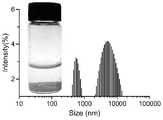
图5是本发明实例2的纳米聚集体的水合粒径分布图;Fig. 5 is the hydrated particle size distribution diagram of the nano-aggregate of the present invention example 2;
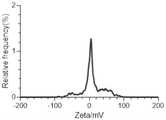
图6是本发明实例2的纳米聚集体的Zeta电位图;Fig. 6 is the Zeta potential figure of the nano-aggregate of Example 2 of the present invention;
图7是本发明实例3的纳米聚集体的水合粒径分布图;Fig. 7 is the hydrated particle size distribution diagram of the nano-aggregate of Example 3 of the present invention;
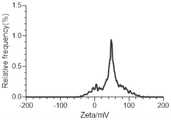
图8是本发明实例3的纳米聚集体的Zeta电位图;Fig. 8 is the Zeta potential diagram of the nano-aggregate of Example 3 of the present invention;
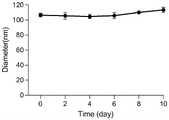
图9是本发明实例3的纳米聚集体的水合粒径稳定性的图;9 is a graph of the hydrated particle size stability of the nano-aggregates of Example 3 of the present invention;
图10是本发明实例4的纳米聚集体的水合粒径分布图;Fig. 10 is the hydrated particle size distribution diagram of the nano-aggregate of the present invention example 4;
图11是本发明实例4的纳米聚集体的Zeta电位图;Figure 11 is the Zeta potential diagram of the nano-aggregate of Example 4 of the present invention;
图12是本发明实例5的纳米聚集体的水合粒径分布图;Figure 12 is the hydrated particle size distribution diagram of the nano-aggregates of Example 5 of the present invention;
图13是本发明实例5的纳米聚集体的Zeta电位图;Figure 13 is the Zeta potential diagram of the nano-aggregates of Example 5 of the present invention;
图14是本发明实例6的纳米聚集体溶液的等温微量热滴定法测定的TPP溶液滴定CPUL1溶液的加热曲线;Figure 14 is the heating curve of the TPP solution titration CPUL1 solution measured by the isothermal microcalorimetry method of the nano-aggregate solution of Example 6 of the present invention;
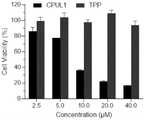
图15A是实例7的CPUL1和TPP对HUH7细胞的杀伤作用结果图;Figure 15A is a graph showing the killing effect of CPUL1 and TPP of Example 7 on HUH7 cells;
图15B是本发明提供的不同配比的纳米聚集体对HUH7细胞的杀伤作用结果图;Figure 15B is a graph showing the killing effect of different ratios of nano-aggregates provided by the present invention on HUH7 cells;
图16A是实例8提供的游离的CPUL1和CPUL1-TPP纳米聚集体在HUH7细胞荧光强度随时间变化的曲线;Figure 16A is a curve of the fluorescence intensity of free CPUL1 and CPUL1-TPP nanoaggregates in HUH7 cells provided by Example 8 as a function of time;
图16B是实例8用流式细胞仪比较同一时间点的游离CPUL1和CPUL1-TPP纳米聚集体在HUH7细胞内的荧光强度;Figure 16B shows the fluorescence intensity of free CPUL1 and CPUL1-TPP nanoaggregates in HUH7 cells at the same time point using flow cytometry in Example 8;
图17是实例9用流式细胞仪检测不同浓度(Q1,死亡细胞;Q2,晚期凋亡或坏死细胞;Q3,早期凋亡细胞;Q4,活细胞)CPUL1、CPUL1-TPP纳米聚集体诱导的HUH7细胞凋亡比率;Figure 17 is a flow cytometer to detect different concentrations (Q1, dead cells; Q2, late apoptotic or necrotic cells; Q3, early apoptotic cells; Q4, live cells) induced by CPUL1, CPUL1-TPP nano-aggregates HUH7 cell apoptosis ratio;
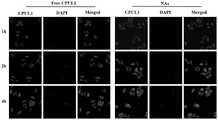
图18是实例10采用激光共聚焦显微镜检测CPUL1、CPUL1-TPP纳米聚集体在HUH7细胞不同时间点的线粒体定位图片。Figure 18 is a picture of mitochondrial localization of CPUL1 and CPUL1-TPP nanoaggregates at different time points in HUH7 cells detected by laser confocal microscope in Example 10.
具体实施方式Detailed ways
下面结合附图对本发明的技术方案作进一步说明。The technical solutions of the present invention will be further described below with reference to the accompanying drawings.
实施例1Example 1
本实施例提供的纳米聚集体包括CPUL1和TPP,纳米聚集体的水合粒径为104nm。制备方法如下:The nano-aggregates provided in this example include CPUL1 and TPP, and the hydrated particle size of the nano-aggregates is 104 nm. The preparation method is as follows:
(1)将4.34mg的CPUL1溶于100μL二甲基亚砜中,得到CPUL1摩尔浓度为100mM/L的溶液A;将4.43mg的TPP溶于100μL二甲基亚砜中,得到TPP摩尔浓度为100mM/L的溶液B;(1) Dissolve 4.34 mg of CPUL1 in 100 μL of dimethyl sulfoxide to obtain solution A with a molar concentration of CPUL1 of 100 mM/L; dissolve 4.43 mg of TPP in 100 μL of dimethyl sulfoxide to obtain a TPP molar concentration of 100mM/L solution B;
(2)取100μL溶液B注入到4.14mL去离子水中,在20℃下以50rpm的转速进行磁力搅拌,得到溶液C;取100μL溶液A注入到溶液C中混合,得到溶液D,在20℃下以50rpm的转速进行磁力搅拌0.5h,得到纳米聚集体。(2) Take 100 μL of solution B and inject it into 4.14 mL of deionized water, and perform magnetic stirring at 50 rpm at 20°C to obtain solution C; take 100 μL of solution A and inject it into solution C and mix to obtain solution D, at 20°C Magnetic stirring was performed at 50 rpm for 0.5 h to obtain nano-aggregates.
对本实施例提供的纳米聚集体进行透射电子显微镜观察,纳米聚集体的粒径指的是纳米药物干燥之后呈粉末状下的药物粒径,一般在透射电子显微镜下测得的粒子粒径为纳米药物的粒径;而纳米药物的水合粒径指的是纳米药物分散在水中形成的水合物的粒径,一般用动态光散射测得的粒径为水合粒径。如图1-4所示,纳米聚集体和游离CPUL1的动态光散射(DLS)测试图与透射电镜结果一致,本实施例提供的纳米聚集体粒径小,在纳米级别且粒径均匀。The nano-aggregates provided in this example are observed by transmission electron microscopy. The particle size of the nano-aggregates refers to the particle size of the drug in powder form after the nano-drug is dried. Generally, the particle size measured under a transmission electron microscope is nanometers. The particle size of the drug; the hydrated particle size of the nano-drug refers to the particle size of the hydrate formed by the nano-drug dispersed in water. Generally, the particle size measured by dynamic light scattering is the hydrated particle size. As shown in Figures 1-4, the dynamic light scattering (DLS) test charts of the nano-aggregates and free CPUL1 are consistent with the results of transmission electron microscopy. The nano-aggregates provided in this example have small particle sizes, and are at the nanoscale and uniform in particle size.
实施例2Example 2
本实施例提供的纳米聚集体包括CPUL1和TPP,纳米聚集体的水合粒径为120nm。制备方法如下:The nano-aggregates provided in this example include CPUL1 and TPP, and the hydrated particle size of the nano-aggregates is 120 nm. The preparation method is as follows:
(1)将4.34mg的CPUL1溶于100μL二甲基亚砜中,得到CPUL1摩尔浓度为100mM/L的溶液A;将2.22mg的TPP溶于100μL二甲基亚砜中,得到TPP摩尔浓度为50mM/L的溶液B;(1) Dissolve 4.34 mg of CPUL1 in 100 μL of dimethyl sulfoxide to obtain solution A with a molar concentration of CPUL1 of 100 mM/L; dissolve 2.22 mg of TPP in 100 μL of dimethyl sulfoxide to obtain a TPP molar concentration of 50mM/L solution B;
(2)取100μL溶液B注入到4.14mL去离子水中,在20℃下以150rpm的转速进行磁力搅拌,得到溶液C;取100μL溶液A注入到步骤(3)得到的溶液C中混合,得到溶液D,在25℃下以150rpm的转速进行磁力搅拌1h,得到纳米聚集体;(2) inject 100 μL of solution B into 4.14 mL of deionized water, and perform magnetic stirring at 150 rpm at 20° C. to obtain solution C; inject 100 μL of solution A into solution C obtained in step (3) and mix to obtain a solution D, magnetic stirring at 150 rpm for 1 h at 25 °C to obtain nano-aggregates;
通过DLS检测合成的纳米聚集体,如图5所示,CPUL1和TPP形成了粒径均匀的纳米颗粒。如图6所示,纳米聚集体的Zeta电位为4.7mV。The synthesized nanoaggregates were detected by DLS, and as shown in Figure 5, CPUL1 and TPP formed nanoparticles with uniform particle size. As shown in Figure 6, the Zeta potential of the nanoaggregates was 4.7 mV.
实施例3Example 3
本实施例提供的纳米聚集体包括CPUL1和TPP,纳米聚集体的水合粒径为136nm。制备方法如下:The nano-aggregates provided in this example include CPUL1 and TPP, and the hydrated particle size of the nano-aggregates is 136 nm. The preparation method is as follows:
(1)将4.34mg的CPUL1溶于100μL二甲基亚砜中,得到CPUL1摩尔浓度为100mM/L的溶液A;将3.55mg的TPP溶于100μL二甲基亚砜中,得到TPP摩尔浓度为80mM/L的溶液B;(1) Dissolve 4.34 mg of CPUL1 in 100 μL of dimethyl sulfoxide to obtain solution A with a molar concentration of CPUL1 of 100 mM/L; dissolve 3.55 mg of TPP in 100 μL of dimethyl sulfoxide to obtain a TPP molar concentration of 80mM/L solution B;
(2)取100μL的溶液B注入到4.14mL去离子水中,在30℃下以400rpm的转速进行磁力搅拌,得到溶液C;取100μL溶液A注入到溶液C中混合,得到溶液D,在30℃下以400rpm的转速进行磁力搅拌2h,得到纳米聚集体;(2) Take 100 μL of solution B and inject it into 4.14 mL of deionized water, and perform magnetic stirring at 400 rpm at 30°C to obtain solution C; take 100 μL of solution A and inject it into solution C and mix to obtain solution D. Magnetic stirring was carried out at a rotating speed of 400 rpm for 2 h to obtain nano-aggregates;
通过DLS检测合成的纳米聚集体,如图7所示,CPUL1和TPP形成了粒径均匀的纳米颗粒,图8是纳米聚集体的Zeta电位图。接着对本实施例提供的纳米药物进行稳定性测试,测试在放置过程中本实施例提供的纳米聚集体的水合粒径。如图9所示,经过10天放置的纳米聚集体依然保持稳定的水合粒径。The synthesized nano-aggregates were detected by DLS, as shown in Figure 7, CPUL1 and TPP formed nanoparticles with uniform particle size, and Figure 8 is the Zeta potential map of the nano-aggregates. Next, a stability test is performed on the nanomedicine provided in this example, and the hydrated particle size of the nanoaggregate provided in this example is tested during the placing process. As shown in Figure 9, the nano-aggregates kept stable hydrated particle size after 10 days.
实施例4Example 4
本实施例提供的纳米聚集体包括CPUL1和TPP,纳米聚集体的水合粒径为208nm。制备方法如下:The nano-aggregates provided in this example include CPUL1 and TPP, and the hydrated particle size of the nano-aggregates is 208 nm. The preparation method is as follows:
(1)将4.34mg的CPUL1溶于100μL二甲基亚砜中,得到CPUL1摩尔浓度为100mM/L的溶液A;将6.65mg的TPP溶于100μL二甲基亚砜中,得到TPP摩尔浓度为150mM/L的溶液B;(1) Dissolve 4.34 mg of CPUL1 in 100 μL of dimethyl sulfoxide to obtain solution A with a molar concentration of CPUL1 of 100 mM/L; dissolve 6.65 mg of TPP in 100 μL of dimethyl sulfoxide to obtain a TPP molar concentration of 150mM/L solution B;
(2)取100μL的溶液B注入到4.14mL去离子水中,在35℃下以500rpm的转速进行磁力搅拌,得到溶液C;取100μL的溶液A注入到溶液C中混合,得到溶液D,在35℃下以500rpm的转速进行磁力搅拌3h,得到纳米聚集体;(2) Take 100 μL of solution B and inject it into 4.14 mL of deionized water, and perform magnetic stirring at 500 rpm at 35°C to obtain solution C; take 100 μL of solution A and inject it into solution C and mix to obtain solution D. Magnetic stirring was performed at 500 rpm for 3 h at ℃ to obtain nano-aggregates;
通过DLS检测合成的纳米聚集体,如图10所示,CPUL1和TPP形成了粒径均匀的纳米颗粒,图11是纳米聚集体的Zeta电位图,结果显示Zeta电位为41.35mV。The synthesized nano-aggregates were detected by DLS. As shown in Figure 10, CPUL1 and TPP formed nanoparticles with uniform particle size. Figure 11 is the Zeta potential map of the nano-aggregates, and the results showed that the Zeta potential was 41.35mV.
实施例5Example 5
本实施例提供的纳米聚集体包括CPUL1和TPP,纳米聚集体的水合粒径为208nm。制备方法如下:The nano-aggregates provided in this example include CPUL1 and TPP, and the hydrated particle size of the nano-aggregates is 208 nm. The preparation method is as follows:
(1)将4.34mg的CPUL1溶于100μL二甲基亚砜中,得到CPUL1摩尔浓度为100mM/L的溶液A;将8.87mg的TPP溶于100μL二甲基亚砜中,得到TPP摩尔浓度为200mM/L的溶液B;(1) Dissolve 4.34 mg of CPUL1 in 100 μL of dimethyl sulfoxide to obtain solution A with a molar concentration of CPUL1 of 100 mM/L; dissolve 8.87 mg of TPP in 100 μL of dimethyl sulfoxide to obtain a TPP molar concentration of 200mM/L solution B;
(2)取100μL溶液B注入到4.14mL去离子水中,在40℃下以700rpm的转速进行磁力搅拌,得到溶液C;取100μL溶液A注入到步骤(3)得到的溶液C中混合,得到溶液D,在40℃下以700rpm的转速进行磁力搅拌3h,得到纳米聚集体;(2) Take 100 μL of solution B and inject it into 4.14 mL of deionized water, perform magnetic stirring at 700 rpm at 40° C. to obtain solution C; take 100 μL of solution A and inject it into solution C obtained in step (3) and mix to obtain a solution D, magnetic stirring at 700 rpm for 3 h at 40 °C to obtain nano-aggregates;
通过DLS检测合成的纳米聚集体,如图12所示,CPUL1和TPP形成了粒径均匀的纳米颗粒,如图13所示,纳米聚集体的Zeta电位为46.31mV。The synthesized nano-aggregates were detected by DLS, as shown in Figure 12, CPUL1 and TPP formed nanoparticles with uniform particle size, as shown in Figure 13, the Zeta potential of the nano-aggregates was 46.31 mV.
实施例6Example 6
用MicroCal ITC200(Malven)对其热力学机理进行了研究。在样品池中加入0.4mM的CPUL1水溶液。将2mM TPP水溶液装入注射器中。参比池含有去离子水。为避免滴定过程中产生气泡,所有样品均彻底脱气30分钟。所有实验均在25℃下进行。TPP水溶液注入样品池20次,每次2.5μL,每次注射在等温线上产生一个峰。第一个数据点由于不准确而被忽略。注射器的转速为250rpm。获得原始数据以生成能量曲线。确保CPUL1和TPP体系中的溶液完全一致,以消除溶剂的影响。The thermodynamic mechanism was investigated with MicroCal ITC200 (Malven). A 0.4 mM aqueous solution of CPUL1 was added to the sample cell. A 2 mM aqueous TPP solution was loaded into a syringe. The reference cell contains deionized water. To avoid air bubbles during titration, all samples were thoroughly degassed for 30 minutes. All experiments were performed at 25°C. The aqueous TPP solution was injected into the
如图14所示,TPP滴定到CPUL1中放出大量热量。通过对滴定曲线的拟合,得到了TPP和CPUL1的结合热力学参数(ΔS、ΔH、ΔG、n、Ka和Kd)。Ka=1.7×103,ΔG=-36.5KJ,表明TPP与CPUL1的反应是自发的,可解释为相反带电分子之间的相互作用。负的ΔS表明体系能量的均匀性降低,这可能归因于疏水相互作用驱动的有序自组装。拟合的化学计量比(n)为1.092,表明TPP与CPUL1的结合比为1:1,这是CPUL1和TPP衍生物可以在水中自组装形成纳米粒子的直接证据。As shown in Figure 14, titration of TPP into CPUL1 released a large amount of heat. By fitting the titration curves, the binding thermodynamic parameters (ΔS, ΔH, ΔG, n, Ka and Kd) of TPP and CPUL1 were obtained. Ka=1.7×103 , ΔG=-36.5KJ, indicating that the reaction between TPP and CPUL1 is spontaneous, which can be explained by the interaction between oppositely charged molecules. A negative ΔS indicates a reduced energy homogeneity of the system, which may be attributed to ordered self-assembly driven by hydrophobic interactions. The fitted stoichiometric ratio (n) was 1.092, indicating a 1:1 binding ratio of TPP to CPUL1, which is direct evidence that CPUL1 and TPP derivatives can self-assemble to form nanoparticles in water.
实施例7Example 7
在本实施例中,通过对HUH7细胞进行MTT比色法实验来考察实施例1提供的纳米药物对细胞活力的影响,其方法如下:In this example, the effect of the nanomedicine provided in Example 1 on cell viability was investigated by carrying out the MTT colorimetric assay on HUH7 cells, and the method was as follows:
(1)细胞培养:将HUH7细胞培养于含有10%胎牛血清的DMEM中,置于37℃、5%二氧化碳的培养箱中培养。(1) Cell culture: HUH7 cells were cultured in DMEM containing 10% fetal bovine serum, and cultured in an incubator at 37°C and 5% carbon dioxide.
(2)细胞活力测定:用四甲基偶氮唑盐(MTT)比色法检测CPUL1和CPUL1-TPP纳米聚集体对HUH7细胞的细胞毒作用。以6000个细胞/孔的接种于96孔板中,孵育12h后,加入10μL不同浓度(2.5、5、10、20、40μM)的CPUL1-TPP纳米聚集体样品。培养48h后加入10μL的MTT(5.0mg/mL)。孵育4h后,取出培养基,加入150μL二甲基亚砜溶解紫色晶体。用多功能酶标仪测定溶液在490nm处的吸光度。以未经任何处理培养的细胞作为对照。每组设置3个平行复孔。(2) Cell viability assay: The cytotoxic effects of CPUL1 and CPUL1-TPP nanoaggregates on HUH7 cells were detected by tetramethylazolium salt (MTT) colorimetric method. 6000 cells/well were seeded in a 96-well plate, and after 12 hours of incubation, 10 μL of different concentrations (2.5, 5, 10, 20, 40 μM) of CPUL1-TPP nanoaggregate samples were added. After 48 h of culture, 10 μL of MTT (5.0 mg/mL) was added. After 4 h of incubation, the medium was removed, and 150 μL of dimethyl sulfoxide was added to dissolve the purple crystals. The absorbance of the solution at 490 nm was measured with a multi-function microplate reader. Cells cultured without any treatment were used as controls.
如图15A和15B所示,与对照组相比,实施例1制备的纳米聚集体对HUH7细胞的活力有显著性影响,且与单独CPUL1处理和单独TPP处理相比,本发明提供的CPUL1-TPP纳米聚集体对HUH7细胞的抑制率最高,说明通过该方法制备的自组装纳米聚集体能够以更低的药物浓度达到更好的肿瘤抑制效果。As shown in Figures 15A and 15B, compared with the control group, the nano-aggregates prepared in Example 1 had a significant effect on the viability of HUH7 cells, and compared with the CPUL1 treatment alone and the TPP treatment alone, the CPUL1- The inhibition rate of TPP nano-aggregates on HUH7 cells was the highest, indicating that the self-assembled nano-aggregates prepared by this method could achieve better tumor inhibition effect with lower drug concentration.
实施例8Example 8
在本实施例中,通过流式细胞仪观察HUH7细胞对实施例1提供的CPUL1-TPP纳米聚集体的内吞作用进行观察,其方法如下:In this example, the endocytosis of CPUL1-TPP nano-aggregates provided in Example 1 by HUH7 cells was observed by flow cytometry, and the method was as follows:
将培养的细胞接种于12孔板,每孔10万细胞,加入1mL DMEM液体培养基(含10%胎牛血清),置于37℃、5%二氧化碳培养箱中培养24h。HUH7细胞分别用CPUL1和提供的纳米聚集体处理1h、2h和4h,其中CPUL1的药物浓度分别为2.5μM、5μM和10μM。4h后,用PBS缓冲液洗三次,用4%多聚甲醛固定,再用PBS洗涤细胞3次,然后用DAPI染细胞核。荧光显微镜下获取细胞荧光图像。流式细胞仪进一步测定细胞内游离CPUL1的积累量。在其他条件相同的情况下,还研究了CPUL1-TPP纳米聚集体对细胞的摄取,分析细胞荧光强度。The cultured cells were seeded in a 12-well plate, 100,000 cells per well, 1 mL of DMEM liquid medium (containing 10% fetal bovine serum) was added, and cultured in a 37° C., 5% carbon dioxide incubator for 24 hours. HUH7 cells were treated with CPUL1 and the provided nanoaggregates for 1 h, 2 h and 4 h, respectively, where the drug concentration of CPUL1 was 2.5 μM, 5 μM and 10 μM, respectively. After 4 h, the cells were washed three times with PBS buffer, fixed with 4% paraformaldehyde, washed three times with PBS, and then stained with DAPI. Fluorescence images of cells were acquired under a fluorescence microscope. The accumulation of free CPUL1 in cells was further determined by flow cytometry. Under other conditions being the same, the uptake of CPUL1-TPP nanoaggregates into cells was also investigated, and the cellular fluorescence intensity was analyzed.
如图16所示,用提供的纳米聚集体处理的HUH7细胞的荧光强度会比只用CPUL1处理的细胞荧光强度高。说明与同样浓度的CPUL1相比,本发明提供的纳米聚集体能被HUH7细胞更多摄入。摄入的药量越多,起药效的药物越多,更利于杀死肿瘤细胞达到抗肿瘤的目的。As shown in Figure 16, the fluorescence intensity of HUH7 cells treated with the provided nanoaggregates will be higher than that of cells treated with CPUL1 alone. It shows that compared with the same concentration of CPUL1, the nano-aggregates provided by the present invention can be more taken up by HUH7 cells. The more the amount of drugs ingested, the more effective drugs will be, which is more conducive to killing tumor cells to achieve the purpose of anti-tumor.
实施例9Example 9
在本实施例中,通过对CPUL1和实施例1提供的CPUL1-TPP纳米聚集体组对HUH7细胞的杀伤作用来测试本发明提供的纳米聚集体,其方法如下:In the present embodiment, the nano-aggregates provided by the present invention are tested by the killing effect of CPUL1 and the CPUL1-TPP nano-aggregate group provided in Example 1 on HUH7 cells, and the method is as follows:
(1)细胞培养:将HUH7细胞培养于含有10%胎牛血清的DMEM中,置于37℃、5%二氧化碳的培养箱中培养。(1) Cell culture: HUH7 cells were cultured in DMEM containing 10% fetal bovine serum, and cultured in an incubator at 37°C and 5% carbon dioxide.
(2)细胞活性观察:将步骤(1)中培养的细胞接种于12孔板,10万细胞每孔,加入1mL DMEM液体培养基(含10%胎牛血清),置于37℃、5%二氧化碳培养箱中培养12h,分为3组,分别为空白对照组、CPUL1组、提供的CPUL1-TPP纳米聚集体组。然后在新配置的含有10%胎牛血清的DMEM培养基中分别加入浓度为2.5、5、10μM的CPUL1和CPUL1-TPP纳米聚集体,置于37℃、5%二氧化碳培养箱中培养12h,空白对照组不做任何处理。弃去培养基,用不含EDTA的胰蛋白酶消化细胞,2000rpm离心5min,用PBS洗涤细胞两次,用Annexin V-APC/7-AAD细胞凋亡试剂盒进行染色;在正常细胞中,磷脂酰丝氨酸只分布在细胞膜脂质双层的内侧,而在细胞凋亡早期,细胞膜的磷脂酰丝氨酸由脂膜内侧翻向外侧。Annexin V是一种依赖于磷脂结合的蛋白,与磷脂酰丝氨酸有高度的亲和力,故可以通过细胞外侧暴露的磷脂酰丝氨酸与凋亡早期细胞的细胞膜结合。因此Annexin V被作为检测细胞早期凋亡的灵敏指标之一。7-AAD是一种核酸染料,它无法通过正常的质膜,随着细胞凋亡、细胞死亡过程,质膜对7-AAD的通透性增加,结合细胞凋亡过程中DNA的有控降解,在合适波长激发下可发出明亮的红光。因此将Annexin V与7-AAD联合使用,就可以将处于不同凋亡时期的细胞区分开来)。然后用流式细胞仪检测。采集一万个细胞进行分区,统计每一个区域细胞百分比。分析细胞死亡的方式。(2) Observation of cell activity: inoculate the cells cultured in step (1) into a 12-well plate, 100,000 cells per well, add 1 mL of DMEM liquid medium (containing 10% fetal bovine serum), and place at 37°C, 5% After culturing in a carbon dioxide incubator for 12 h, they were divided into 3 groups, namely the blank control group, the CPUL1 group, and the provided CPUL1-TPP nanoaggregate group. Then, CPUL1 and CPUL1-TPP nanoaggregates at concentrations of 2.5, 5, and 10 μM were added to the newly prepared DMEM medium containing 10% fetal bovine serum, respectively, and placed in a 37 °C, 5% carbon dioxide incubator for 12 h, blank The control group did not receive any treatment. The medium was discarded, cells were digested with EDTA-free trypsin, centrifuged at 2000 rpm for 5 min, cells were washed twice with PBS, and stained with Annexin V-APC/7-AAD apoptosis kit; in normal cells, phosphatidyl Serine is only distributed in the inner side of the lipid bilayer of the cell membrane, and in the early stage of apoptosis, the phosphatidylserine of the cell membrane is turned from the inside to the outside of the lipid membrane. Annexin V is a protein dependent on phospholipid binding and has a high affinity for phosphatidylserine, so it can bind to the cell membrane of early apoptotic cells through phosphatidylserine exposed on the outside of the cell. Therefore, Annexin V is used as one of the sensitive indicators to detect early apoptosis of cells. 7-AAD is a nucleic acid dye that cannot pass through the normal plasma membrane. With the process of apoptosis and cell death, the permeability of the plasma membrane to 7-AAD increases, combined with the controlled degradation of DNA in the process of apoptosis. , which can emit bright red light when excited at a suitable wavelength. Therefore, the combination of Annexin V and 7-AAD can distinguish cells in different apoptotic stages). and then detected by flow cytometry. Collect 10,000 cells for partitioning, and count the percentage of cells in each area. Analyze the way cells die.
如图17所示,本发明所述的纳米聚集体可以造成细胞凋亡和坏死,且与单独CPUL1组相比,本发明提供的纳米对HUH7细胞的造成的凋亡率最高,说明通过本发明提供的方法合成的纳米聚集体能够以更低的药物浓度达到更好的肿瘤抑制效果。As shown in Figure 17, the nano-aggregates of the present invention can cause apoptosis and necrosis, and compared with the CPUL1 group alone, the nano-aggregates provided by the present invention have the highest apoptosis rate on HUH7 cells, indicating that the present invention The nano-aggregates synthesized by the provided method can achieve better tumor suppressing effect with lower drug concentration.
实施例10Example 10
在本实施例中,通过对CPUL1和实施例1提供的CPUL1-TPP纳米聚集体组对HUH7细胞线粒体的靶向作用来测试本发明提供的纳米聚集体,其方法如下:In the present embodiment, the nano-aggregates provided by the present invention are tested by the targeting effect of CPUL1 and the CPUL1-TPP nano-aggregate group provided in Example 1 on the mitochondria of HUH7 cells, and the method is as follows:
(1)细胞培养:将HUH7细胞培养于含有10%胎牛血清的DMEM中,置于37℃、5%二氧化碳的培养箱中培养。(1) Cell culture: HUH7 cells were cultured in DMEM containing 10% fetal bovine serum, and cultured in an incubator at 37°C and 5% carbon dioxide.
(2)纳米聚集体靶向线粒体观察:将步骤(1)中培养的细胞接种于12孔板,10万细胞每孔,加入1mL含10%胎牛血清的DMEM液体培养基,置于37℃、5%二氧化碳培养箱中培养12h,分为3组,分别为空白对照组,CPUL1组、提供的CPUL1-TPP纳米聚集体组。然后在新配置的含有10%胎牛血清的DMEM培养基中分别加入2.5μM CPUL1和CPUL1-TPP纳米聚集体,置于37℃、5%二氧化碳培养箱中培养2h和4h,空白对照组不做任何处理。采用商品染料MitoTracker Red对HUH7细胞的线粒体进行染色检测。在纳米聚集体孵育2h或4h和染料处理30min后,用激光共聚焦显微镜(CLSM)检测线粒体在细胞内的定位。CPUL1自身的绿色荧光与染料的红色荧光重叠产生黄色荧光。线粒体共定位程度用Pearson相关系数表示。(2) Observation of nano-aggregates targeting mitochondria: inoculate the cells cultured in step (1) in a 12-well plate, 100,000 cells per well, add 1 mL of DMEM liquid medium containing 10% fetal bovine serum, and place at 37°C , 5% carbon dioxide incubator for 12h, divided into 3 groups, respectively blank control group, CPUL1 group, provided CPUL1-TPP nano-aggregate group. Then, 2.5μM CPUL1 and CPUL1-TPP nano-aggregates were added to the newly prepared DMEM medium containing 10% fetal bovine serum, respectively, and incubated in a 37°C, 5% carbon dioxide incubator for 2h and 4h. any processing. Mitochondria of HUH7 cells were stained with MitoTracker Red, a commercial dye. After 2h or 4h of nanoaggregate incubation and dye treatment for 30min, mitochondrial localization in cells was detected by confocal laser microscopy (CLSM). The green fluorescence of CPUL1 itself overlaps with the red fluorescence of the dye to produce yellow fluorescence. The degree of mitochondrial colocalization was expressed by the Pearson correlation coefficient.
如图18所示,本发明所述的CPUL1-TPP纳米聚集体和游离CPUL1可在2h或4h与染料共定位于HUH7细胞的线粒体中,沿白线随机绘制的荧光强度定量剖面进一步证实CPUL1-TPP纳米聚集体比游离CPUL1对线粒体的靶向性更好。孵育2h的纳米聚集体的Pearson相关系数为0.76,高于孵育2h的游离CPUL1的0.65,与孵育4h的结果一致,在同一时间点,纳米聚集体的共定位荧光强度明显高于游离CPUL1。这些结果表明CPUL1-TPP纳米聚集体具有良好的线粒体靶向能力和快速的时间依赖性细胞摄取特性。As shown in Figure 18, the CPUL1-TPP nanoaggregates and free CPUL1 of the present invention could co-localize with the dye in the mitochondria of HUH7 cells at 2h or 4h, and the fluorescence intensity quantitative profiles randomly drawn along the white line further confirmed that CPUL1- TPP nanoaggregates target mitochondria better than free CPUL1. The Pearson correlation coefficient of nanoaggregates incubated for 2h was 0.76, which was higher than that of free CPUL1 incubated for 2h, 0.65, which was consistent with the results of incubation for 4h. At the same time point, the co-localized fluorescence intensity of nanoaggregates was significantly higher than that of free CPUL1. These results suggest that CPUL1-TPP nanoaggregates have good mitochondrial targeting ability and rapid time-dependent cellular uptake properties.
本发明的纳米聚集体,所述纳米聚集体是由疏水性药物和靶向剂共组装而得的纳米粒子。在共组装纳米药物中,疏水性药物和靶向剂的比例可调。这样,可通过调整药物比例实现更好的协同作用,提高药物利用率。纳米粒子可为球形形貌,纳米粒子的粒径可调,为5-500nm,且纳米粒子可以高度分散。In the nano-aggregate of the present invention, the nano-aggregate is a nano-particle co-assembled by a hydrophobic drug and a targeting agent. In co-assembled nanodrugs, the ratio of hydrophobic drug and targeting agent can be tunable. In this way, better synergy can be achieved by adjusting the ratio of drugs, and the utilization rate of drugs can be improved. The nanoparticles can be spherical in shape, the particle size of the nanoparticles can be adjusted from 5 to 500 nm, and the nanoparticles can be highly dispersed.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110911464.8ACN113599537B (en) | 2021-08-10 | 2021-08-10 | A kind of nano-aggregate and its preparation method and application |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110911464.8ACN113599537B (en) | 2021-08-10 | 2021-08-10 | A kind of nano-aggregate and its preparation method and application |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113599537A CN113599537A (en) | 2021-11-05 |
| CN113599537Btrue CN113599537B (en) | 2022-07-12 |
Family
ID=78307830
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110911464.8AActiveCN113599537B (en) | 2021-08-10 | 2021-08-10 | A kind of nano-aggregate and its preparation method and application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113599537B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115844892B (en)* | 2022-09-21 | 2024-04-19 | 中国药科大学 | Disulfide bond-modified nanoaggregates and preparation method and application thereof |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105481869B (en)* | 2015-12-04 | 2018-04-27 | 中国药科大学 | A kind of pyrans simultaneously [3,2- α] phenazene derivative and its preparation method and application |
| CN105664176B (en)* | 2016-03-24 | 2018-08-24 | 浙江大学 | A kind of Mitochondrially targeted polysaccharide nanometer formulation and preparation method thereof |
| CN110156703B (en)* | 2019-05-21 | 2022-08-05 | 中国药科大学 | Phenazine derivatives as TrxR1 inhibitors, intermediates, and methods of making and using the same |
- 2021
- 2021-08-10CNCN202110911464.8Apatent/CN113599537B/enactiveActive
Also Published As
| Publication number | Publication date |
|---|---|
| CN113599537A (en) | 2021-11-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Yang et al. | Light-activatable dual-source ROS-responsive prodrug nanoplatform for synergistic chemo-photodynamic therapy | |
| Zhang et al. | Nanogels as novel nanocarrier systems for efficient delivery of CNS therapeutics | |
| Fu et al. | Co-delivery of anticancer drugs and cell penetrating peptides for improved cancer therapy | |
| Cao et al. | Polymeric prodrugs conjugated with reduction-sensitive dextran–camptothecin and pH-responsive dextran–doxorubicin: an effective combinatorial drug delivery platform for cancer therapy | |
| Zhou et al. | Oxygenated theranostic nanoplatforms with intracellular agglomeration behavior for improving the treatment efficacy of hypoxic tumors | |
| Wang et al. | Smart multifunctional core–shell nanospheres with drug and gene co-loaded for enhancing the therapeutic effect in a rat intracranial tumor model | |
| CN108295046A (en) | The preparation method and albumin nanoparticle obtained of a kind of albumin nanoparticle and application | |
| Lee et al. | Folic acid-modified bovine serum albumin nanoparticles with doxorubicin and chlorin e6 for effective combinational chemo-photodynamic therapy | |
| CN113004536A (en) | A metal-amino acid/peptide coordination polymer and its application | |
| CN105534896A (en) | Polypeptide and chemotherapy drug combined drug-loaded micelle and preparation method and application thereof | |
| CN110403916A (en) | A kind of nano therapeutic agent and its preparation method and application | |
| Zhang et al. | Heat-induced manganese-doped magnetic nanocarriers combined with Yap-siRNA for MRI/NIR-guided mild photothermal and gene therapy of hepatocellular carcinoma | |
| You et al. | Co-delivery of cisplatin and CJM-126 via photothermal conversion nanoparticles for enhanced synergistic antitumor efficacy | |
| Das et al. | Preparation of a size selective nanocomposite through temperature assisted co-assembly of gelatin and pluronic F127 for passive targeting of doxorubicin | |
| KR102525384B1 (en) | Manufacturing method for extracellular vesicles containing dual drugs | |
| CN113599537B (en) | A kind of nano-aggregate and its preparation method and application | |
| CN114224823B (en) | A glioma drug delivery system integrating chemotherapy/photodynamic therapy/chemodynamic therapy and its preparation method | |
| Shi et al. | Amphiphilic copolymer and TPGS mixed magnetic hybrid micelles for stepwise targeted co-delivery of DOX/TPP–DOX and image-guided chemotherapy with enhanced antitumor activity in liver cancer | |
| CN118806724A (en) | Drug delivery carrier for inhibiting glycogen release and treating large liposarcoma and preparation method thereof | |
| Yan et al. | Hollow chitosan–silica nanospheres for doxorubicin delivery to cancer cells with enhanced antitumor effect in vivo | |
| CN116370654B (en) | A dual-targeted nano-drug delivery system for combined anti-glutamine metabolism and sensitized photodynamic therapy, and its preparation and application | |
| CN107007550A (en) | A kind of amphipathic copolymer of redox response and its preparation method and application | |
| CN111450265A (en) | A kind of targeted pH-sensitive polymer vesicles loaded with gold-drug complexes and preparation method thereof | |
| CN114409729B (en) | A rapeseed peptide and its application in the preparation of drug nanocarriers | |
| Liao et al. | Preparation of galactosyl nanoparticles and their targeting efficiency to hepatocellular carcinoma |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |