CN113398325B - Fibrous membrane for enhancing screw stability and inducing bone regeneration and preparation method thereof - Google Patents
Fibrous membrane for enhancing screw stability and inducing bone regeneration and preparation method thereofDownload PDFInfo
- Publication number
- CN113398325B CN113398325BCN202110654923.9ACN202110654923ACN113398325BCN 113398325 BCN113398325 BCN 113398325BCN 202110654923 ACN202110654923 ACN 202110654923ACN 113398325 BCN113398325 BCN 113398325B
- Authority
- CN
- China
- Prior art keywords
- solution
- peptide
- screw
- nano
- preparation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/02—Inorganic materials
- A61L27/08—Carbon ; Graphite
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/18—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/56—Porous materials, e.g. foams or sponges
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01D—MECHANICAL METHODS OR APPARATUS IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS
- D01D5/00—Formation of filaments, threads, or the like
- D01D5/0007—Electro-spinning
- D01D5/0015—Electro-spinning characterised by the initial state of the material
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01D—MECHANICAL METHODS OR APPARATUS IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS
- D01D5/00—Formation of filaments, threads, or the like
- D01D5/0007—Electro-spinning
- D01D5/0061—Electro-spinning characterised by the electro-spinning apparatus
- D01D5/0076—Electro-spinning characterised by the electro-spinning apparatus characterised by the collecting device, e.g. drum, wheel, endless belt, plate or grid
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/70—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres characterised by the method of forming fleeces or layers, e.g. reorientation of fibres
- D04H1/72—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres characterised by the method of forming fleeces or layers, e.g. reorientation of fibres the fibres being randomly arranged
- D04H1/728—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres characterised by the method of forming fleeces or layers, e.g. reorientation of fibres the fibres being randomly arranged by electro-spinning
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/70—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres characterised by the method of forming fleeces or layers, e.g. reorientation of fibres
- D04H1/74—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres characterised by the method of forming fleeces or layers, e.g. reorientation of fibres the fibres being orientated, e.g. in parallel (anisotropic fleeces)
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/602—Type of release, e.g. controlled, sustained, slow
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/12—Nanosized materials, e.g. nanofibres, nanoparticles, nanowires, nanotubes; Nanostructured surfaces
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/02—Materials or treatment for tissue regeneration for reconstruction of bones; weight-bearing implants
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Dermatology (AREA)
- General Health & Medical Sciences (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Epidemiology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Textile Engineering (AREA)
- Mechanical Engineering (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Dispersion Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials For Medical Uses (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于生物材料技术领域,具体涉及一种用于增强螺钉稳定性和诱导骨再生的纤维膜及其制备方法。The invention belongs to the technical field of biomaterials, and in particular relates to a fibrous membrane for enhancing screw stability and inducing bone regeneration and a preparation method thereof.
背景技术Background technique
骨折时人类常见的损伤,是指由于外伤或病理等原因致使骨质部分或完全断裂的一种疾病。在目前骨折内固定治疗中,对于骨折的患者,在行手术切开复位和钢板螺钉内固定时,目前临床上使用的螺钉有时不能满足固定所有骨折块的要求,骨折内固定手术过程中可因螺钉多次进出、更换螺钉或骨质疏松导致螺钉部分松动或完全松动,进而引起内固定失效而引发一系列并发症,影响内固定的强度和效果,可能会造成内固定手术的失败,目前尚无良好解决办法。受安全套启发,如果在螺钉上也戴上“安全套”再拧入松动的孔道,也许会即刻增加螺钉固定的稳定性。Fracture is a common injury in humans, which refers to a disease in which the bone is partially or completely broken due to trauma or pathology. In the current internal fixation treatment of fractures, for patients with fractures, when performing open reduction and internal fixation with plates and screws, the screws currently used in clinical practice sometimes cannot meet the requirements for fixing all fracture fragments. Screws coming in and out multiple times, replacing screws or osteoporosis lead to partial or complete loosening of the screws, which in turn causes internal fixation failure and causes a series of complications, affecting the strength and effect of internal fixation, which may lead to the failure of internal fixation surgery. There is no good solution. Inspired by the condom, if you wear a "condom" on the screw and then screw it into the loose hole, it may immediately increase the stability of the screw fixation.
静电纺织技术一种高效低耗的纳米纤维制备技术,其原理是在装有聚合物溶液的喷头和接收装置之间施加高压静电,使溶液在高压电场作用下产生与表面张力相反的电场力,驱使溶液在喷头末端拉伸成一个泰勒锥,当电场力足够大时泰勒锥处液滴可克服表面张力而产生喷射流,在高速震荡中喷射流被迅速拉细,溶剂也快速挥发,最终形成纳微米直径的超细纤维,理论上任何可溶解或熔融的材料均可进行电纺加工。电纺纤维直径通常介于数十纳米至数微米之间,在微观结构上具有交错的网格结构,具有可控的力学性能和高摩擦性,同时高孔隙率和孔径的纤维排列可模拟细胞外基质的结构,进而促进细胞粘附、生长和分化。Electrospinning technology is a high-efficiency and low-consumption nanofiber preparation technology. Its principle is to apply high-voltage static electricity between the nozzle with polymer solution and the receiving device, so that the solution generates an electric field force opposite to the surface tension under the action of a high-voltage electric field. Drive the solution to stretch into a Taylor cone at the end of the nozzle. When the electric field force is large enough, the droplets at the Taylor cone can overcome the surface tension and generate a jet stream. The jet stream is rapidly thinned during high-speed oscillation, and the solvent is also quickly volatilized, eventually forming Ultrafine fibers with nanometer diameters, theoretically any soluble or meltable materials can be electrospun. The diameter of electrospun fibers usually ranges from tens of nanometers to several microns, and has a staggered grid structure on the microstructure, with controllable mechanical properties and high friction, while the fiber arrangement of high porosity and pore size can simulate cells The structure of the extracellular matrix, which in turn promotes cell adhesion, growth and differentiation.
聚3-羟基丁酸酯/4-羟基丁酸酯共聚物(poly(3-hydroxybutyrate-co-4-hydroxybutyrate), P34HB),除了具有良好的生物相容性、生物降解性和可纺性,还具有良好的力学性能,P34HB 电纺纤维材料可部分修复在SD大鼠颅骨缺损,但其亲水性较差,限制了其在生物医学方面的应用。氧化石墨烯(Graphene Oxide,GO)由于其特殊的sp2键合和六角形碳结构而具有较佳的机械强度和亲水性,能够有效调控细胞行为,为细胞提供定位点和诱导骨髓间充质干细胞成骨分化,并可作为涂层材料改善钛合金内固定与骨组织之间的相容性,另外,石墨烯基的材料具有良好抗菌性能,能使内固定植入物感染风险进一步降低,但GO本身不具备可纺性,需要和其它材料复合才能静电纺丝成为纳微米纤维而发挥作用。聚乙二醇(Polyethylene glycol,PEG)是亲水性生物材料,具备良好的生物相容性和可纺性而在生物制药领域广泛应用,与P34HB的复合纤维材料已证明了可促进骨再生,并且聚乙二醇作为粘结剂使氧化铝粉末形成固体,表明具有良好金属粘附性能。Poly 3-hydroxybutyrate/4-hydroxybutyrate copolymer (poly(3-hydroxybutyrate-co-4-hydroxybutyrate), P34HB), in addition to good biocompatibility, biodegradability and spinnability, Also has good mechanical properties, P34HB electrospun fiber material can partially repair the calvarial defect in SD rats, but its poor hydrophilicity limits its application in biomedicine. Graphene oxide (Graphene Oxide, GO) has better mechanical strength and hydrophilicity due to its special sp2 bonding and hexagonal carbon structure, which can effectively regulate cell behavior, provide cells with positioning points and induce bone marrow mesenchymal Osteogenic differentiation of stem cells can be used as a coating material to improve the compatibility between titanium alloy internal fixation and bone tissue. In addition, graphene-based materials have good antibacterial properties, which can further reduce the risk of infection of internal fixation implants. However, GO itself does not have spinnability, and it needs to be combined with other materials to be electrospun into nano-micron fibers to play a role. Polyethylene glycol (PEG) is a hydrophilic biomaterial with good biocompatibility and spinnability and is widely used in the field of biopharmaceuticals. The composite fiber material with P34HB has been proven to promote bone regeneration. And polyethylene glycol is used as a binder to make the alumina powder form a solid, indicating that it has good metal adhesion properties.
发明内容Contents of the invention
为克服以上技术问题,本发明提供了一种用于增强螺钉稳定性和诱导骨再生的纤维膜及其制备方法。该方法制备的纤维可成膜状、圆柱体状、以及螺钉套状,以解决现有骨科手术技术中螺钉固定不牢固和骨折愈合延迟或不愈合的技术问题。该纤维膜可以按需要实现可控的体内降解,不必进行二次手术取出。To overcome the above technical problems, the present invention provides a fibrous membrane for enhancing screw stability and inducing bone regeneration and a preparation method thereof. The fiber prepared by the method can be formed into a film shape, a cylinder shape, and a screw sleeve shape, so as to solve the technical problems of weak screw fixation and fracture healing delay or nonunion in the existing orthopedic surgery technology. The fibrous membrane can be degraded in vivo in a controlled manner as required, and no secondary surgery is required to remove it.
为实现以上目的,本发明提供的技术方案如下:To achieve the above object, the technical solution provided by the invention is as follows:
一种纳/微米纤维膜的制备方法,包括以下步骤:A preparation method of nano/micro fiber membrane, comprising the following steps:
S1:将氧化石墨烯分散于二氯甲烷或六福异丙醇中,制备成溶液,再把在聚羟基脂肪酸酯和聚乙二醇溶于溶液中得到溶液A;S1: Disperse graphene oxide in dichloromethane or hexafluoroisopropanol to prepare a solution, and then dissolve polyhydroxyalkanoate and polyethylene glycol in the solution to obtain solution A;
S2:取注射器、注射针头,使用锡箔纸滚轴为接收器,设置正电压,负电压,温度,推注速度,接收距离,制得薄膜,干燥、除菌即可。S2: Take the syringe and injection needle, use the tinfoil roller as the receiver, set the positive voltage, negative voltage, temperature, injection speed, receiving distance, make a film, dry and sterilize.
优选地,步骤S1中,所述聚羟基脂肪酸酯为第一代-聚羟基丁酸酯(PHB),第二代-羟基丁酸/戊酸共聚酯(PHBV),第三代-羟基丁酸已酸共聚酯(PHBHHx)或第四代-聚3-羟基丁酸酯/4-羟基丁酸酯共聚物(P34HB)中的任一种。Preferably, in step S1, the polyhydroxyalkanoate is the first generation-polyhydroxybutyrate (PHB), the second generation-hydroxybutyric acid/valeric acid copolyester (PHBV), the third generation-hydroxybutyrate Either of butyric caproic acid copolyester (PHBHHx) or fourth generation - poly 3-hydroxybutyrate/4-hydroxybutyrate copolymer (P34HB).
优选地,步骤S1中,所述溶液A,每10毫升二氯甲烷或六福异丙醇中,含有7%的在聚羟基脂肪酸酯,5%的聚乙二醇,以及1mg/mL的氧化石墨烯。Preferably, in step S1, the solution A contains 7% of polyhydroxyalkanoate, 5% of polyethylene glycol, and 1 mg/mL of oxidized Graphene.
优选地,步骤S2中,所述注射器的规格为10mL;所述注射针头为23G注射针头。Preferably, in step S2, the specification of the syringe is 10 mL; the injection needle is a 23G injection needle.
优选地,步骤S2中,所述正电压为10kV,负电压为10kV,所述温度为37℃,所述推注速度0.3mm/min,所述接收距离15-20cm。Preferably, in step S2, the positive voltage is 10 kV, the negative voltage is 10 kV, the temperature is 37° C., the injection speed is 0.3 mm/min, and the receiving distance is 15-20 cm.
优选地,步骤S2中,所述干燥为后放置于真空干燥机中干燥48小时,使残余有机溶剂蒸发。Preferably, in step S2, the drying is followed by drying in a vacuum dryer for 48 hours to evaporate the residual organic solvent.
本发明的目的还在于提供一种中空多孔纳/微米纤维膜的制备方法,包括以下步骤:The object of the present invention is also to provide a kind of preparation method of hollow porous nano/micro fiber membrane, comprises the following steps:
(1)将氧化石墨烯分散于二氯甲烷或六福异丙醇中,制备成溶液,再把聚羟基脂肪酸酯和聚乙二醇溶于溶液中得到溶液A;(1) Graphene oxide is dispersed in methylene chloride or hexafluoroisopropanol to prepare a solution, and polyhydroxyalkanoate and polyethylene glycol are dissolved in the solution to obtain solution A;
(2)将聚乙二醇与成骨活性肽或天然小分子化合物溶于水中,制得溶液B;(2) Dissolving polyethylene glycol and osteogenic active peptide or natural small molecular compound in water to prepare solution B;
(3)取注射器分别装上溶液A和B溶液,利用同轴喷头,将溶液A作为壳层,溶液B 作为芯层,使用锡箔纸滚轴为接收器,设置正电压,负电压,温度,推注速度分别,接收距离,制得纤维薄膜,放置于水溶液,超声震荡,得到具有中空多孔结构的微米纤维膜,真空干燥、除菌即可。(3) Take the syringe and install solution A and solution B respectively, use the coaxial nozzle, use solution A as the shell layer, solution B as the core layer, use tinfoil paper roller as the receiver, set positive voltage, negative voltage, temperature, Injection speed and receiving distance are respectively used to prepare a fiber film, placed in an aqueous solution, and ultrasonically oscillated to obtain a micron fiber film with a hollow porous structure, which can be vacuum-dried and sterilized.
本发明的另一目的在于提供一种有序-无序中空多孔的纳/微米纤维膜的制备方法,其特征在于,包括以下步骤:Another object of the present invention is to provide a method for preparing an ordered-disordered hollow porous nano/micro fiber membrane, characterized in that it comprises the following steps:
(1)将氧化石墨烯分散于二氯甲烷或六福异丙醇中,制备成溶液,再把聚羟基脂肪酸酯和聚乙二醇溶于溶液中得到溶液A;(1) Graphene oxide is dispersed in methylene chloride or hexafluoroisopropanol to prepare a solution, and polyhydroxyalkanoate and polyethylene glycol are dissolved in the solution to obtain solution A;
(2)将聚乙二醇溶于水中,制得溶液B;(2) Polyethylene glycol is dissolved in water to prepare solution B;
(3)取注射器分别装上溶液A和B溶液,利用同轴喷头,将溶液A作为壳层,溶液B 作为芯层,使用高速取向装置作为接收器,设置正电压,负电压,温度,推注速度分别,接收距离,制得纤维薄膜,放置于水溶液,超声震荡,得到具有有序-无序中空多孔结构的纳/ 微米纤维膜,再将纤维膜反复浸泡在具有成骨活性肽或天然小分子化合物的溶液中,真空干燥、除菌即可。(3) Take the syringe and install solution A and solution B respectively, use the coaxial nozzle, use solution A as the shell layer, solution B as the core layer, use the high-speed orientation device as the receiver, set the positive voltage, negative voltage, temperature, push The injection speed and the receiving distance are respectively used to prepare a fiber film, placed in an aqueous solution, and ultrasonically oscillated to obtain a nano/micron fiber membrane with an ordered-disordered hollow porous structure, and then repeatedly soak the fiber membrane in an osteogenic active peptide or natural In the solution of small molecule compounds, vacuum drying and sterilization are sufficient.
优选地,步骤(3)中,所述成骨活性肽或天然小分子化合物包括:Arg-Gly-Asp(RGD) 肽、胶原模拟肽P-15、肝素结合肽、骨形态发生蛋白-7衍生肽、骨形态发生蛋白-2衍生肽、胰高血糖素样肽-1(glucagon-like peptide-1,GLP-1)、成骨生长肽(osteogenicgrowth peptide, OGP)、甲状旁腺激素相关肽(PTHrP-1)、QK肽、Prominin-1衍生肽(prominin-1derived peptide,PR1P);槲皮素、葛根素、白藜芦醇、姜黄素、表没食子儿茶素没食子酸酯(EGCG)、和小檗碱中的任一种或多种。Preferably, in step (3), the osteogenic active peptide or natural small molecular compound includes: Arg-Gly-Asp (RGD) peptide, collagen mimic peptide P-15, heparin-binding peptide, bone morphogenetic protein-7 derivative peptide, bone morphogenetic protein-2 derived peptide, glucagon-like peptide-1 (GLP-1), osteogenic growth peptide (OGP), parathyroid hormone-related peptide ( PTHrP-1), QK peptide, Prominin-1 derived peptide (prominin-1derived peptide, PR1P); quercetin, puerarin, resveratrol, curcumin, epigallocatechin gallate (EGCG), and Any one or more of berberine.
优选地,步骤(3)中,所述注射器的规格为5mL。Preferably, in step (3), the specification of the syringe is 5mL.
优选地,步骤(3)中,所述正电压为15kV,负电压为5kV,所述温度为37℃,所述推注速度为0.5/0.1mm/min,所述接收距离15-20cm;接收装置转速为2000r/min。Preferably, in step (3), the positive voltage is 15kV, the negative voltage is 5kV, the temperature is 37°C, the injection speed is 0.5/0.1mm/min, and the receiving distance is 15-20cm; The rotating speed of the device is 2000r/min.
优选地,步骤(3)中,所述干燥后放置于真空干燥机中干燥48小时,使残余有机溶剂蒸发。Preferably, in step (3), after the drying, place it in a vacuum drier for 48 hours to evaporate the residual organic solvent.
本发明的另一目的在于提供一种所述的制备方法制备的纳/微米纤维膜。Another object of the present invention is to provide a nano/micro fiber membrane prepared by the preparation method.
优选地,所述的纳/微米纤维成膜状或成空心或实心的圆柱体状或成螺钉套状。Preferably, the nano/micro fibers are in the shape of a film or a hollow or solid cylinder or a screw sleeve.
优选地,所述纳/微米纤维具有中空多孔的微观结构。Preferably, the nano/micro fibers have a hollow porous microstructure.
本发明的另一目的在于提供一种功能型可吸收的骨科螺钉套,所述骨科螺钉套由所述纤维素膜或纳/微米纤维制备而成。Another object of the present invention is to provide a functional absorbable orthopedic screw sleeve, which is prepared from the cellulose film or nano/micron fibers.
本发明的另一目的在于所述功能型可吸收的骨科螺钉套的制备方法,包括以下步骤:Another object of the present invention is the preparation method of the functional absorbable orthopedic screw cover, comprising the following steps:
根据临床骨科螺钉直径、长度需要,利用智能滚轴将同一厚度的纤维膜做成不同直径、不同长度的套状,与螺钉本体形成一体。According to the diameter and length of clinical orthopedic screws, the fibrous membrane of the same thickness is made into sleeves of different diameters and lengths by using intelligent rollers, and is integrated with the screw body.
螺钉套与临床上骨科螺钉直径、长度相匹配,紧密结合于螺钉体表面,可有效增加术中螺钉的初试稳定性和抗拔出力。The screw sleeve matches the clinical orthopedic screw diameter and length, and is tightly combined with the surface of the screw body, which can effectively increase the initial stability and pull-out resistance of the screw during operation.
优选地,所述功能型可吸收的骨科螺钉套的制备还可以将骨科金属螺钉与电纺接收器相连,一次成型获得螺钉与螺钉套的一体结构。Preferably, the preparation of the functional absorbable orthopedic screw sleeve can also connect the orthopedic metal screw with the electrospun receiver, and obtain an integrated structure of the screw and the screw sleeve through one-time molding.
与现有技术比,本发明的技术优势在于:Compared with prior art, the technical advantage of the present invention is:
(1)本发明提供的纤维膜以具有生物相容性的可降解聚羟基脂肪酸酯及氧化石墨烯为主要材料,通过静电纺丝技术制备。其纤维具有中空多孔或取向结构,该方法制备的纤维膜具有良好的力学性能,与骨科螺钉结合后,可有效增强螺钉的初始稳定性和轴向抗拔出力,解决了术中螺钉松动的临床问题。(1) The fiber membrane provided by the present invention uses biocompatible degradable polyhydroxyalkanoate and graphene oxide as main materials, and is prepared by electrospinning technology. The fiber has a hollow porous or oriented structure. The fiber membrane prepared by this method has good mechanical properties. After being combined with orthopedic screws, it can effectively enhance the initial stability and axial pull-out resistance of the screw, and solve the problem of screw loosening during surgery. clinical question.
(2)本发明的通过同轴静电纺丝技术,结合氧化石墨烯、聚羟基脂肪酸酯和聚乙二醇三种材料的优点,构建具有控制缓释成分的中空多孔纤维膜,增加螺钉术中即刻和维持术后螺钉稳定性和轴向抗拔出力,并且促进骨折愈合,为临床螺钉松动提供有效的解决方案。(2) The coaxial electrospinning technology of the present invention combines the advantages of graphene oxide, polyhydroxyalkanoate and polyethylene glycol to construct a hollow porous fiber membrane with controlled release components, increasing the screw technology Immediately and maintain postoperative screw stability and axial pull-out resistance, and promote fracture healing, providing an effective solution for clinical screw loosening.
(3)同时在具有中空多孔微观结构的纤维中添加成骨诱导成分,在增加生物稳定性的同时诱导新生骨的生成,避免目前临床上螺钉固定不牢固、骨折愈合延迟,畸形愈合或骨折不愈合的技术问题;其在骨修复过程中缓慢释放,促进骨折愈合,在生物医药尤其骨科领域有着广泛的应用前景。(3) At the same time, osteogenic inductive components are added to the fibers with hollow porous microstructure, which can induce new bone formation while increasing biological stability, and avoid the current clinical screw fixation, delayed fracture healing, malunion or insufficient fracture. The technical problem of healing; it is slowly released in the process of bone repair to promote fracture healing, and has broad application prospects in the field of biomedicine, especially orthopedics.
(4)本发明中所述螺钉套,是将生物可吸收材料聚羟基脂肪酸酯、聚乙二醇、和氧化石墨烯配置成溶液,利用静电纺织技术,在高压电场下,将材料纺织为纳米级的膜装、三维套状、以及与螺钉一体。所采用的聚羟基脂肪酸酯、聚乙二醇、和氧化石墨烯材料,具有可完全降解的同时,无酸性代谢产物的产生,有效避免目前临床上内固定材料所产生的无菌性炎症反应并发症的发生,同时氧化石墨烯具有良好的生物活性和生物力学性能。使螺钉具有更好的早期生物稳定性,防止术后常见的螺钉松动问题。(4) The screw cover described in the present invention is that the bioabsorbable material polyhydroxyalkanoate, polyethylene glycol, and graphene oxide are configured into a solution, and the material is spun by electrostatic spinning technology under a high-voltage electric field. Nano-scale film packaging, three-dimensional sleeve shape, and integration with screws. The polyhydroxyalkanoate, polyethylene glycol, and graphene oxide materials used are completely degradable and have no acidic metabolites, effectively avoiding the aseptic inflammatory response produced by current clinical internal fixation materials complications, while graphene oxide has good bioactivity and biomechanical properties. Make the screw have better early biological stability and prevent common screw loosening problems after surgery.
进一步,所述的功能型螺钉套,是在聚羟基脂肪酸酯、聚乙二醇、和氧化石墨烯的溶液中在添加成骨活性肽或天然小分子化合物,利用静电纺织技术,在高压电场下,将材料纺织为纳微米级的纤维膜状、空心或实心的圆柱体状、和螺钉套状。在材料吸收过程中,成骨活性肽或天然小分子化合物具有成骨诱导作用,促进骨折愈合和骨再生。Further, the functional screw sleeve is added to a solution of polyhydroxyalkanoate, polyethylene glycol, and graphene oxide by adding bone-forming active peptide or natural small molecule compound, using electrospinning technology, in a high-voltage electric field Next, the material is woven into nano-micron-scale fiber membranes, hollow or solid cylinders, and screw sleeves. In the process of material absorption, osteogenic active peptides or natural small molecular compounds have osteogenic inductive effects, promoting fracture healing and bone regeneration.
(5)本发明制备的中空多孔纤维,可以调控纤维丝上孔的大小,以控制成骨成分释放的速度。(5) The hollow porous fiber prepared by the present invention can regulate the size of the hole on the fiber filament to control the release speed of the osteogenic component.
附图说明Description of drawings
图1:实施例1制成的纤维膜;Fig. 1: the fibrous film that
图2:实施例1纤维膜的电镜图;Fig. 2: the electron micrograph of
图3:实施例2制成的纤维膜;Fig. 3: the fibrous film that
图4:实施例2纤维膜的微观孔径结构图;其中,(4-1)为纤维中空结构图;(4-2)为纤维表面多孔结构图。Figure 4: Microscopic pore structure diagram of the fiber membrane of Example 2; wherein, (4-1) is a hollow fiber structure diagram; (4-2) is a fiber surface porous structure diagram.
图5:实施例3制成的纤维膜;Fig. 5: the fibrous film that
图6:实施例3纤维膜的纤维排列微观结构图;其中,(6-1)为纤维取向排列图;(6-2) 为纤维无序-有序排列图。Fig. 6: the fiber arrangement microstructure diagram of
图7:实施例4的制备示意图;其中,1为溶液A,2为溶液B,3为纤维膜,4为螺钉。Figure 7: Schematic diagram of the preparation of Example 4; wherein, 1 is solution A, 2 is solution B, 3 is fiber membrane, and 4 is screw.
图8:实施例4的螺钉套形状;Fig. 8: the screw cover shape of
图9:实施例5中纤维膜的力学性能图;其中,a为应力-位移图;b为弹性模量图;c为伸长率图;d为最大抗拉伸强度图;Fig. 9: The mechanical performance figure of fiber membrane in embodiment 5; Wherein, a is stress-displacement figure; B is elastic modulus figure; C is elongation rate figure; D is maximum tensile strength figure;
图10:实施例5的拔出曲线与拔出力对比图;其中,a为螺钉在体外标准试件中初次轴向拔出力、第二次轴向拔出力、完全松动情况下的拔出力曲线;b为部分松动情况下,将各组安全套螺钉结合后拧入原钉道进行拔出力测试,所得到的拔出力曲线;c为完全松动情况下,将各组安全套与螺钉结合后拧入钉道,进行拔出力测试,所得到的拔出力曲线;d为标准试件中初次轴向拔出力、第二次轴向拔出力、完全松动情况下的拔出力大小对比;e为部分松动情况下,各组安全套与螺钉结合后的轴向拔出力大小对比;f为完全松动情况下,各组安全套与螺钉结合后的轴向拔出力大小对比;Figure 10: Comparison diagram of the pull-out curve and pull-out force of Example 5; wherein, a is the initial axial pull-out force, the second axial pull-out force, and the pull-out force of the screw in the standard in vitro test piece under the condition of complete loosening Output force curve; b is the pull-out force curve obtained by combining the screws of each group of condoms and screwing them into the original nail channel under the condition of partial looseness; After being combined, screw it into the nail channel, and carry out the pull-out force test, and the obtained pull-out force curve; d is the initial axial pull-out force, the second axial pull-out force, and the pull-out force in the case of complete looseness in the standard test piece Force comparison; e is the comparison of the axial pull-out force of each group of condoms and screws in the case of partial loosening; f is the comparison of the axial pull-out force of each group of condoms and screws in the case of complete loosening;
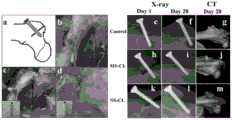
图11:体外抗拔出试验结果,其中,a为手术示意图;b为箭头钉道和骨折线位置示意图;c为常规组螺钉使用示意图;d为实验组螺钉使用示意图;e为无松动组骨折示意图;f为无松动组愈合后示意图;g为无松动组CBCT检查结果示意图;h为安全套组骨折示意图; i为安全套组愈合后示意图;j为安全套组CBCT检查结果示意图;k为完全松动组骨折示意图;l为完全松动组愈合后示意图;m为完全松动组CBCT检查结果示意图。Figure 11: The results of the in vitro pull-out resistance test, where a is a schematic diagram of the operation; b is a schematic diagram of the position of the arrowhead and the fracture line; c is a schematic diagram of the use of screws in the conventional group; d is a schematic diagram of the use of screws in the experimental group; e is a schematic diagram of the fracture in the non-loosening group Schematic diagram; f is the schematic diagram of the non-loosening group after healing; g is the schematic diagram of the CBCT examination results of the non-loosening group; h is the schematic diagram of the fracture of the condom group; i is the schematic diagram of the healing of the condom group; j is the schematic diagram of the CBCT examination results of the condom group; k is the complete loosening group Schematic diagram of the fracture; l is the schematic diagram of the completely loosened group after healing; m is the schematic diagram of the CBCT examination results of the completely loosened group.
现结合附图和实施例对本发明作进一步说明:Now in conjunction with accompanying drawing and embodiment the present invention will be further described:
具体实施方式Detailed ways
下面通过具体实施例对本发明进行说明,以使本发明技术方案更易于理解、掌握,但本发明并不局限于此。下述实施例中所述实验方法,如无特殊说明,均为常规方法;所述试剂和材料,如无特殊说明,均可从商业途径获得。The present invention will be described below through specific examples to make the technical solution of the present invention easier to understand and grasp, but the present invention is not limited thereto. The experimental methods described in the following examples, unless otherwise specified, are conventional methods; the reagents and materials, unless otherwise specified, can be obtained from commercial sources.
实施例1Example 1
一种纤维膜的制备方法,包括以下步骤:A method for preparing a fiber membrane, comprising the steps of:
(1)配制溶液:将氧化石墨烯在10mL二氯甲烷中避光超声分散3小时,制备w/v梯度为1mg/mL的溶液,再把0.7g P34HB和0.5g聚乙二醇4000溶于在上述溶液,增加六福异丙醇至10mL得到溶液A(溶液A表示10毫升六福异丙醇中,含有7%的P34HB,5%的聚乙二醇4000,以及1mg/mL的氧化石墨烯);(1) Preparation of solution: disperse graphene oxide in 10mL of dichloromethane by ultrasonic for 3 hours in the dark, prepare a solution with a w/v gradient of 1mg/mL, then dissolve 0.7g of P34HB and 0.5g of polyethylene glycol 4000 in In the above solution, add hexafluoro isopropanol to 10mL to obtain solution A (solution A means 10 ml of hexafelic isopropanol, containing 7% of P34HB, 5% of
(2)电纺:取10ml注射器、23G注射针头,使用锡箔纸滚轴为接收器,设置正电压约10kV,负电压10kV,温度37℃,推注速度0.3mm/min,接收距离15-20cm,获得薄膜后放置于真空干燥机中干燥48小时,使残余有机溶剂蒸发;双面紫外照射4h除菌,即得到纤维膜(直观图如图1),使用扫描电镜观察其纤维结构如图2。(2) Electrospinning: Take a 10ml syringe and a 23G injection needle, use a tinfoil roller as the receiver, set the positive voltage to about 10kV, the negative voltage to 10kV, the temperature at 37°C, the injection speed at 0.3mm/min, and the receiving distance at 15-20cm After obtaining the film, place it in a vacuum dryer to dry for 48 hours to evaporate the residual organic solvent; irradiate double-sided ultraviolet light for 4 hours to sterilize, and then obtain the fiber film (visual diagram as shown in Figure 1), and use a scanning electron microscope to observe its fiber structure as shown in Figure 2 .
实施例2Example 2
一种中空多孔纤维膜的制备方法,包括以下步骤:A method for preparing a hollow porous fiber membrane, comprising the following steps:
(1)配制溶液:将氧化石墨烯在10ml六福异丙醇中避光超声分散3小时,制备w/v梯度为1mg/mL的溶液,再把0.7g P34HB和0.5g聚乙二醇4000溶于在上述溶液,增加六福异丙醇至10mL得到溶液A,将0.5g聚乙二醇4000与成骨活性肽溶于10mL水中得到溶液 B;(1) Preparation of solution: disperse graphene oxide in 10ml of hexafluoroisopropanol by ultrasonic for 3 hours in the dark, prepare a solution with a w/v gradient of 1mg/mL, then dissolve 0.7g of P34HB and 0.5g of polyethylene glycol 4000 In the above solution, increase hexafluoroisopropanol to 10mL to obtain solution A, dissolve 0.5g polyethylene glycol 4000 and osteogenic active peptide in 10mL water to obtain solution B;
(2)电纺:取2个5ml注射器分别装上A和B溶液,利用同轴喷头,将溶液A作为壳层,溶液B作为芯层,使用锡箔纸滚轴为接收器,设置正电压约15kV,负电压5kV,温度 37℃,推注速度分别0.5/0.1mm/min,接收距离15-20cm,获得纤维薄膜后,放置于水溶液中,利用超声震荡仪充分震荡,得到具有中空多孔结构的微米纤维膜,真空干燥机中干燥48 小时,使残余有机溶剂蒸发;双面紫外照射4h除菌,即得到纤维膜(直观图如图3),使用扫描电镜观察其纤维结构如图4,其中,(4-1)为纤维中空结构图;(4-2)为纤维表面多孔结构图。(2) Electrospinning: Take two 5ml syringes and fill them with solutions A and B respectively. Using a coaxial nozzle, use solution A as the shell layer and solution B as the core layer. Use tinfoil rollers as the receiver, and set a positive voltage of about 15kV, negative voltage 5kV, temperature 37°C, injection speed 0.5/0.1mm/min, receiving distance 15-20cm, after obtaining the fiber film, place it in an aqueous solution, and use an ultrasonic oscillator to fully oscillate to obtain a hollow porous structure. The micron fiber membrane was dried in a vacuum dryer for 48 hours to evaporate the residual organic solvent; the double-sided ultraviolet radiation was sterilized for 4 hours to obtain the fiber membrane (visual diagram as shown in Figure 3), and the fiber structure was observed with a scanning electron microscope as shown in Figure 4, wherein , (4-1) is the fiber hollow structure diagram; (4-2) is the fiber surface porous structure diagram.
实施例3Example 3
一种有序-无序中空多孔纤维膜的制备方法,包括以下步骤:A method for preparing an ordered-disordered hollow porous fiber membrane, comprising the following steps:
(1)配制溶液:将氧化石墨烯在10ml六福异丙醇中避光超声分散3小时,制备w/v梯度为1mg/mL的溶液,再把0.7g P34HB和0.5g聚乙二醇4000溶于在上述溶液,增加六福异丙醇至10mL得到溶液A(溶液A表示10毫升六福异丙醇中,含有7%的P34HB,5%的聚乙二醇4000,以及1mg/mL的氧化石墨烯);(1) Preparation of solution: disperse graphene oxide in 10ml of hexafluoroisopropanol by ultrasonic for 3 hours in the dark, prepare a solution with a w/v gradient of 1mg/mL, then dissolve 0.7g of P34HB and 0.5g of polyethylene glycol 4000 In the above solution, increase hexafluoro isopropanol to 10mL to obtain solution A (solution A means 10 ml of hexafluoro isopropanol, containing 7% of P34HB, 5% of
(2)电纺:取注射器分别装上溶液A和B溶液,利用同轴喷头,将溶液A作为壳层,溶液B作为芯层,使用高速取向装置作为接收器,设置正电压约15kV,负电压5kV,温度 37℃,推注速度分别0.5/0.1mm/min,接收距离15-20cm,制得纤维薄膜,放置于水溶液,超声震荡,得到具有有序-无序中空多孔结构的纳/微米纤维膜,再将纤维膜反复浸泡在具有天然小分子化合物的溶液中,真空干燥机中干燥48小时,双面紫外照射4h除菌,即得到纤维膜(直观图如图5),使用扫描电镜观察其有序-无序排列的纤维结构,如图6,其中,(6-1) 为纤维取向排列图;(6-2)为纤维无序-有序排列图。(2) Electrospinning: take the syringe and install solution A and solution B respectively, use the coaxial nozzle, use solution A as the shell layer, solution B as the core layer, use a high-speed orientation device as the receiver, set the positive voltage to about 15kV, and the negative The voltage is 5kV, the temperature is 37°C, the injection speed is 0.5/0.1mm/min, and the receiving distance is 15-20cm. The fiber film is prepared, placed in an aqueous solution, and ultrasonically oscillated to obtain nano/micron with ordered-disordered hollow porous structure. Fiber membrane, then repeatedly soak the fiber membrane in the solution with natural small molecular compounds, dry it in a vacuum dryer for 48 hours, and sterilize it with ultraviolet radiation on both sides for 4 hours to obtain the fiber membrane (visual diagram as shown in Figure 5), use scanning electron microscope Observe its ordered-disordered fiber structure, as shown in Figure 6, where (6-1) is the fiber orientation arrangement diagram; (6-2) is the fiber disorder-ordered arrangement diagram.
实施例4Example 4
(1)配制溶液:将氧化石墨烯在10ml二氯甲烷中避光超声分散3小时,制备w/v梯度为1mg/mL的溶液,再把0.7g P34HB和0.5g聚乙二醇4000溶于在上述溶液,增加二氯甲烷至10mL得到溶液A,将0.5g聚乙二醇4000与成骨活性肽溶于10mL水中得到溶液B;(1) Preparation of solution: disperse graphene oxide in 10ml of dichloromethane by ultrasonic for 3 hours in the dark, prepare a solution with a w/v gradient of 1mg/mL, then dissolve 0.7g of P34HB and 0.5g of polyethylene glycol 4000 in In the above solution, increase dichloromethane to 10mL to obtain solution A, dissolve 0.5g polyethylene glycol 4000 and osteogenic active peptide in 10mL water to obtain solution B;
(2)螺钉套制备:将骨科金属螺钉与接收器相连,取2个5mL注射器分别装上A和B溶液,利用同轴喷头,将溶液A作为壳层,溶液B作为芯层,设置正电压约15kV,负电压5kV,温度37℃,推注速度分别0.5/0.1mm/min,接收距离15-20cm,获得螺钉-螺钉套一体后,真空干燥机中干燥48小时,使残余有机溶剂蒸发;双面紫外照射4h除菌。工艺图见图 7。(2) Screw sleeve preparation: Connect the orthopedic metal screw to the receiver, take two 5mL syringes and fill them with solutions A and B respectively, use a coaxial nozzle, use solution A as the shell layer, and solution B as the core layer, and set a positive voltage About 15kV, negative voltage 5kV, temperature 37°C, injection speed 0.5/0.1mm/min, receiving distance 15-20cm, after obtaining the screw-screw sleeve, dry it in a vacuum dryer for 48 hours to evaporate the residual organic solvent; Double-sided ultraviolet irradiation for 4 hours to sterilize. The process diagram is shown in Figure 7.
制成的螺钉套直观图如图8,该螺钉套为功能型可吸收骨科螺钉套,包括螺钉本身和由生物可吸收材料制成的螺钉套。所述的螺钉套,类似安全套一样套在螺钉钉体部位。螺钉套是将聚羟基脂肪酸酯、聚乙二醇、氧化石墨烯配置成溶液,同时溶液中添加成骨活性肽,利用静电纺织技术,在高压电场下,使用同轴喷头,将材料纺织为纳米级的三维套状。在材料吸收过程中,成骨活性肽的释放具有成骨诱导作用,可在增加生物稳定性的同时诱导新生骨的生成,避免目前临床上螺钉固定不牢固、骨折愈合延迟,畸形愈合或骨折不愈合的技术问题。The visual diagram of the fabricated screw sleeve is shown in Figure 8. The screw sleeve is a functional absorbable orthopedic screw sleeve, including the screw itself and the screw sleeve made of bioabsorbable material. The screw cover is set on the screw body like a condom. The screw sleeve is prepared by preparing polyhydroxyalkanoate, polyethylene glycol, and graphene oxide into a solution. At the same time, bone-forming active peptide is added to the solution. Using electrospinning technology, under a high-voltage electric field, using a coaxial nozzle, the material is spun into a Three-dimensional nesting at the nanoscale. In the process of material absorption, the release of osteogenic active peptide has an osteogenic induction effect, which can induce the formation of new bone while increasing biological stability, and avoid the current clinical screw fixation, delayed fracture healing, malunion or fracture failure. Technical issues of healing.
实施例5Example 5
参照实施例1的方法,在实验步骤不变的前提下,分别使用原料:P34HB和PEG并加入不同比例(0.5-2.5)氧化石墨烯,制成10mm×15mm×0.14mm大小、有效拉伸长度10mm的薄膜(n=5for each group);其中,单纯P34HB为(P),PEG加入后为(P-P),加入氧化石墨烯后为(P-P-G);制得产品:P、P-P、P-P-G0.5、P-P-G1、P-P-G1.5、P-P-G2、P-P-G2.5。Referring to the method of Example 1, under the premise that the experimental steps remain unchanged, use raw materials: P34HB and PEG respectively and add different proportions (0.5-2.5) of graphene oxide to make a size of 10mm×15mm×0.14mm and an effective stretching length 10mm film (n=5for each group); wherein, pure P34HB is (P), after PEG is added, it is (P-P), and after adding graphene oxide, it is (P-P-G); obtained products: P, P-P, P-P-G0. 5. P-P-G1, P-P-G1.5, P-P-G2, P-P-G2.5.
效果评价:Evaluation:
1、力学性能测试1. Mechanical performance test
实验方法:测试实施例5中产品的力学性能,包括应力-位移关系、弹性模量、伸长率、最大抗拉伸强度。Experimental method: test the mechanical properties of the product in Example 5, including stress-displacement relationship, elastic modulus, elongation, and maximum tensile strength.
经万能力学试验机(倾技科技有限公司,中国)进行拉伸测试(100N传感器,拉伸速度 1mm/min);得到拉伸强度、杨氏模量。Tensile test (100N sensor, tensile speed 1mm/min) was carried out by universal mechanical testing machine (Qingji Technology Co., Ltd., China); the tensile strength and Young's modulus were obtained.
结果如图9,经力学测试仪测得各组薄膜材料应力-位移关系,取各自平均水平曲线表示各组应力-位移(a),由力学测试仪得出各组弹性模量(b),伸长率(c),最大抗拉伸强度(d)(n=5for each group)*P<0.05(one-way ANOVA)(图中“*”表示P<0.05)。The results are shown in Figure 9. The stress-displacement relationship of each group of film materials is measured by the mechanical tester, and the respective average level curves are taken to represent the stress-displacement (a) of each group, and the elastic modulus (b) of each group is obtained by the mechanical tester. Elongation (c), maximum tensile strength (d) (n=5 for each group)*P<0.05 (one-way ANOVA) ("*" in the figure means P<0.05).
2、松动模型测试2. Loose model test
方法:硬质聚氨酯泡沫材料在材料性能、均匀性和可用性等方面差异小,因此被用作骨性材料的替代品。根据美国测试材料协会(ASTM:F1839-08)规范选择第20级(0.32g/cm3)标准硬质聚氨酯泡沫作为骨模型替代的标准试件。METHODS: Rigid polyurethane foams showed little variation in material properties, homogeneity, and availability, and were therefore used as a substitute for bony materials. According to the American Society for Testing Materials (ASTM: F1839-08) standard rigid polyurethane foam of grade 20 (0.32g/cm3 ) was selected as the standard specimen for bone model replacement.
样品:实施例5中产品。Sample: product in embodiment 5.
部分松动模型测试:直径1.0mm的钻头在试件(上海超群橡塑有限公司,中国)上钻孔,拧入直径1.5mm的自攻螺钉(广州华创医疗,中国),经力学测试仪轴向拔出测得空白组螺钉轴向拔出力;拧入螺钉后拧出,再次沿原钉道拧入,测出第二次轴向抗拔出力;同时将薄膜包覆螺钉再拧入,检测轴向抗拔出力。Partially loose model test: Drill with a diameter of 1.0mm to drill holes on the test piece (Shanghai Chaoqun Rubber and Plastic Co., Ltd., China), screw in a self-tapping screw with a diameter of 1.5mm (Guangzhou Huachuang Medical, China), and test the shaft of the mechanical tester. Measure the axial pull-out force of the blank group screw in the direction of pulling out; screw in the screw and then screw it out, screw it in again along the original screw path, and measure the second axial pull-out force; at the same time, screw the film-coated screw in again , to detect the axial pull-out resistance.
完全松动模型测试:直径1.5mm钻头钻孔,造成完全松动的钉道,拧入1.5mm的自攻螺钉,薄膜包覆螺钉再拧入,检测轴向拔出力;所有测试重复5次。Complete loosening model test: Drill with a 1.5mm diameter drill to create a completely loose nail track, screw in a 1.5mm self-tapping screw, and then screw in a film-coated screw to detect the axial pull-out force; all tests are repeated 5 times.
结果如图10,a为螺钉在体外标准试件中初次轴向拔出力、第二次轴向拔出力、完全松动情况下的拔出力曲线。b为部分松动情况下,将各组安全套螺钉结合后拧入原钉道进行拔出力测试,所得到的拔出力曲线。c为完全松动情况下,将各组安全套与螺钉结合后拧入钉道,进行拔出力测试,所得到的拔出力曲线。d为标准试件中初次轴向拔出力、第二次轴向拔出力、完全松动情况下的拔出力大小对比。e为部分松动情况下,各组安全套与螺钉结合后的轴向拔出力大小对比。f为完全松动情况下,各组安全套与螺钉结合后的轴向拔出力大小对比。(n=5for each group),*P<0.05,(one-way ANOVA)。The results are shown in Figure 10, a is the pull-out force curve of the screw in the standard test piece in vitro, the first axial pull-out force, the second axial pull-out force, and the complete loosening. b is the pull-out force curve obtained by combining the screws of each group of condoms and screwing them into the original screw channel for the pull-out force test in the case of partial looseness. c is the pull-out force curve obtained by combining each set of condoms with screws and then screwing them into the nail channel for a pull-out force test under the condition of complete looseness. d is the comparison of the initial axial pull-out force, the second axial pull-out force, and the pull-out force in the case of complete looseness in the standard test piece. e is the comparison of the axial pull-out force of each group of condoms and screws in the case of partial loosening. f is the comparison of the axial pull-out force of each group of condoms and screws in the case of complete loosening. (n=5 for each group), *P<0.05, (one-way ANOVA).
3、体内完全松动模型试验3. In vivo complete loosening model test
根据膜力学测试、细胞相容性测试和体外抗拔出试验的结果,选取实施例2的制成的膜进行体内完全松动模型试验。According to the results of the membrane mechanics test, the cytocompatibility test and the in vitro pull-out resistance test, the membrane prepared in Example 2 was selected for in vivo complete loosening model test.
新西兰白兔9只(雄性,平均2-2.5kg)。10%的水合氯醛,按3.5mL/kg腹腔注射麻醉后,双侧髋关节备皮,消毒铺敷,于股骨大转子处切开皮肤约3cm,逐层进入显露骨组织,电锯制备转子间不全性骨折,分为三组:1.5mm直径的电钻垂直于骨折线钻孔,分别用直径1.5mm 自攻螺钉拧入(完全松动组),包覆材料后行螺钉拧入(安全套组);无松动组以1.0mm直径钻头钻孔后直径1.5mm的自攻金属螺钉拧入。关闭切口后双下肢肌肉注射青霉素8万单位,持续3日,预防感染。结果见图11,其中,a为手术示意图;b为箭头钉道和骨折线位置示意图;c为常规组螺钉使用示意图;d为实验组螺钉使用示意图;9 New Zealand white rabbits (male, average 2-2.5kg). After anesthetized by intraperitoneal injection of 10% chloral hydrate at 3.5 mL/kg, the skin of the bilateral hip joints was prepared, disinfected and paved, and the skin was cut about 3 cm at the greater trochanter of the femur, and the bone tissue was exposed layer by layer, and the trochanter was prepared with an electric saw Divided into three groups for incomplete fractures: electric drill with a diameter of 1.5 mm is drilled perpendicular to the fracture line, screwed in with self-tapping screws with a diameter of 1.5 mm (completely loose group), screwed in after the coating material (safety set group) ; No loosening group is drilled with a 1.0mm diameter drill bit and then screwed in with a self-tapping metal screw with a diameter of 1.5mm. After the incision was closed, 80,000 units of penicillin were injected intramuscularly into both lower limbs for 3 days to prevent infection. The results are shown in Figure 11, in which, a is a schematic diagram of the operation; b is a schematic diagram of the position of the arrow nail track and the fracture line; c is a schematic diagram of the use of screws in the conventional group; d is a schematic diagram of the use of screws in the experimental group;
影像学检查:术后第一天(Day 1)及第四周(Day 28),并于术后4周CBCT检查,结果见图11,其中,e为无松动组(control)骨折示意图;f为无松动组愈合后示意图;g为无松动组CBCT检查结果示意图;h为安全套组(MS-CL)骨折示意图;i为安全套组愈合后示意图;j为安全套组CBCT检查结果示意图;k为完全松动组(NS-CL)骨折示意图;l为完全松动组愈合后示意图;m为完全松动组CBCT检查结果示意图。Imaging examination: the first day (Day 1) and the fourth week (Day 28) after the operation, and the CBCT examination at the 4th week after the operation, the results are shown in Figure 11, where e is the schematic diagram of the fracture in the non-loosening group (control); f g is the schematic diagram of the CBCT examination results of the non-loosening group; h is the schematic diagram of the fracture of the safety condom group (MS-CL); i is the schematic diagram of the healing of the condom group; j is the schematic diagram of the CBCT examination results of the condom group; Schematic diagram of the fracture in the loosening group (NS-CL); l is a schematic diagram of the healing of the complete loosening group; m is a schematic diagram of the CBCT examination results in the complete loosening group.
上述详细说明是针对本发明其中之一可行实施例的具体说明,该实施例并非用以限制本发明的专利范围,凡未脱离本发明所为的等效实施或变更,均应包含于本发明技术方案的范围内。The above detailed description is a specific description of one of the feasible embodiments of the present invention. This embodiment is not intended to limit the patent scope of the present invention. All equivalent implementations or changes that do not deviate from the present invention shall be included in the present invention. within the scope of the technical program.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110654923.9ACN113398325B (en) | 2021-06-11 | 2021-06-11 | Fibrous membrane for enhancing screw stability and inducing bone regeneration and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110654923.9ACN113398325B (en) | 2021-06-11 | 2021-06-11 | Fibrous membrane for enhancing screw stability and inducing bone regeneration and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113398325A CN113398325A (en) | 2021-09-17 |
| CN113398325Btrue CN113398325B (en) | 2023-04-21 |
Family
ID=77683618
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110654923.9AExpired - Fee RelatedCN113398325B (en) | 2021-06-11 | 2021-06-11 | Fibrous membrane for enhancing screw stability and inducing bone regeneration and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113398325B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114848887B (en)* | 2022-05-20 | 2023-08-25 | 诺一迈尔(苏州)生命科技有限公司 | A kind of nanofiber dressing and preparation method thereof |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102120871A (en)* | 2011-03-22 | 2011-07-13 | 暨南大学 | Preparation method of chitosan fiber reinforced polylactic acid composite material |
| CN104334095A (en)* | 2012-03-28 | 2015-02-04 | 伊西康内外科公司 | Tissue thickness compensator comprising fibers to produce a resilient load |
| CN104434291A (en)* | 2014-12-24 | 2015-03-25 | 叶川 | Functional anti-looseness absorbable screw used in orthopaedics department |
| CN105268033A (en)* | 2015-11-06 | 2016-01-27 | 杭州锐健马斯汀医疗器材有限公司 | Absorbable reticular reinforced interface screw and preparation method thereof |
| CN107106210A (en)* | 2014-12-26 | 2017-08-29 | 奥西奥有限公司 | Continuous Fiber Reinforced Biocomposite Medical Implants |
| CN110064076A (en)* | 2012-01-16 | 2019-07-30 | 麦瑞通医疗设备有限公司 | The medical instrument and manufacturing method covered by rotary spinning material |
| CN111050677A (en)* | 2017-09-07 | 2020-04-21 | 奥西西奥有限公司 | Fiber-reinforced biocomposite threaded implants |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8980300B2 (en)* | 2004-08-05 | 2015-03-17 | Advanced Cardiovascular Systems, Inc. | Plasticizers for coating compositions |
| CN102671244B (en)* | 2012-06-04 | 2015-01-21 | 广州迈普再生医学科技有限公司 | Micro/nano-fiber bone repairing scaffold and production method thereof |
| CN102961977B (en)* | 2012-12-17 | 2014-11-26 | 中国科学院宁波材料技术与工程研究所 | Preparation method of polylactic-acid hollow fiber dialysis membrane |
| TW201500066A (en)* | 2013-06-27 | 2015-01-01 | Far Eastern Memorial Hospital | Bone filling and bone repair composite material |
| WO2015048224A1 (en)* | 2013-09-25 | 2015-04-02 | Johnson Jed K | Fiber scaffolds for use creating implantable structures |
| CN105536051B (en)* | 2015-12-24 | 2018-08-24 | 杭州市第三人民医院 | A kind of core-shell type nano fibrous framework and its method with melanocyte structure tissue engineering material |
| CN106362206B (en)* | 2016-10-31 | 2019-08-13 | 四川大学 | A kind of high intensity high-hydrophilic graphene oxide-P34HB nano fiber scaffold and its preparation method and application |
| US20200345895A1 (en)* | 2017-12-20 | 2020-11-05 | Ossio Ltd | Fiber bundle reinforced biocomposite medical implants |
| CN108690199B (en)* | 2018-05-24 | 2020-09-04 | 暨南大学 | Segmented copolymer nano composite antibacterial material and preparation method and application thereof |
| CN108619580A (en)* | 2018-06-19 | 2018-10-09 | 佛山皖阳生物科技有限公司 | A kind of preparation method of hydrophilic coating coronary artery bracket material |
| ES2812048B2 (en)* | 2020-10-02 | 2022-05-05 | Univ Valencia Politecnica | BIODEGRADABLE BIOMATERIAL |
- 2021
- 2021-06-11CNCN202110654923.9Apatent/CN113398325B/ennot_activeExpired - Fee Related
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102120871A (en)* | 2011-03-22 | 2011-07-13 | 暨南大学 | Preparation method of chitosan fiber reinforced polylactic acid composite material |
| CN110064076A (en)* | 2012-01-16 | 2019-07-30 | 麦瑞通医疗设备有限公司 | The medical instrument and manufacturing method covered by rotary spinning material |
| CN104334095A (en)* | 2012-03-28 | 2015-02-04 | 伊西康内外科公司 | Tissue thickness compensator comprising fibers to produce a resilient load |
| CN104434291A (en)* | 2014-12-24 | 2015-03-25 | 叶川 | Functional anti-looseness absorbable screw used in orthopaedics department |
| CN107106210A (en)* | 2014-12-26 | 2017-08-29 | 奥西奥有限公司 | Continuous Fiber Reinforced Biocomposite Medical Implants |
| CN105268033A (en)* | 2015-11-06 | 2016-01-27 | 杭州锐健马斯汀医疗器材有限公司 | Absorbable reticular reinforced interface screw and preparation method thereof |
| CN111050677A (en)* | 2017-09-07 | 2020-04-21 | 奥西西奥有限公司 | Fiber-reinforced biocomposite threaded implants |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113398325A (en) | 2021-09-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Ranganathan et al. | Chitosan and gelatin-based electrospun fibers for bone tissue engineering | |
| Okamoto et al. | Synthetic biopolymer nanocomposites for tissue engineering scaffolds | |
| Subramanian et al. | Fabrication, characterization and in vitro evaluation of aligned PLGA–PCL nanofibers for neural regeneration | |
| Bottino et al. | A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration | |
| Badrossamay et al. | Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning | |
| Jose et al. | Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering | |
| Han et al. | Hydroxyapatite-doped polycaprolactone nanofiber membrane improves tendon–bone interface healing for anterior cruciate ligament reconstruction | |
| CN103394131B (en) | Novel double-layered composite transmitting tissue regeneration membrane and preparation method thereof | |
| CN105705172B (en) | Hydrophilic electrostatic spinning biological composite scaffold material for tissue regeneration and preparation method and application thereof | |
| Owida et al. | Recent applications of electrospun nanofibrous scaffold in tissue engineering | |
| JP4481994B2 (en) | Bioabsorbable porous material | |
| Li et al. | Bone substitute biomedical material of multi-(amino acid) copolymer: in vitro degradation and biocompatibility | |
| Wu et al. | Fabrication of Biologically Inspired Electrospun Collagen/Silk fibroin/bioactive glass composited nanofibrous scaffold to accelerate the treatment efficiency of bone repair | |
| WO2021077042A1 (en) | Fiber-based scaffolds for tendon cell migration and regeneration | |
| Karbasi et al. | Effect of multi-wall carbon nanotubes (MWNTs) on structural and mechanical properties of poly (3-hydroxybutirate) electrospun scaffolds for tissue engineering applications | |
| Laijun et al. | An enhanced periosteum structure/function dual mimicking membrane for in-situ restorations of periosteum and bone | |
| CN113398325B (en) | Fibrous membrane for enhancing screw stability and inducing bone regeneration and preparation method thereof | |
| CN114504680B (en) | Bone-ligament-bone integrated bracket and preparation method thereof | |
| Yin et al. | Preparation and characteristics of electrospinning PTH‐Fc/PLCL/SF membranes for bioengineering applications | |
| Liu et al. | Biomimetic fabrication of new bioceramics-introduced fibrous scaffolds: From physicochemical characteristics to in vitro biological properties | |
| Wu et al. | A novel 3D printed type II silk fibroin/polycaprolactone mesh for the treatment of pelvic organ prolapse | |
| CN114870070A (en) | An organic/inorganic composite three-dimensional porous nanofiber tissue engineering scaffold and its preparation method and application | |
| CN109663142A (en) | A kind of degradable operation sewing thread of load medicine and preparation method thereof | |
| Amnieh et al. | Evaluation of the effects of decellularized Wharton jelly nanoparticles on polyhydroxy butyrate-chitosan electrospun scaffolds for cartilage tissue engineering applications | |
| CN104434291A (en) | Functional anti-looseness absorbable screw used in orthopaedics department |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20230421 |