CN113185557B - Iridium complex organic photovoltaic material and preparation method and application thereof - Google Patents
Iridium complex organic photovoltaic material and preparation method and application thereofDownload PDFInfo
- Publication number
- CN113185557B CN113185557BCN202110517256.XACN202110517256ACN113185557BCN 113185557 BCN113185557 BCN 113185557BCN 202110517256 ACN202110517256 ACN 202110517256ACN 113185557 BCN113185557 BCN 113185557B
- Authority
- CN
- China
- Prior art keywords
- organic photovoltaic
- preparation
- donor
- iridium complex
- acceptor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F15/00—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic Table
- C07F15/0006—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic Table compounds of the platinum group
- C07F15/0033—Iridium compounds
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K30/00—Organic devices sensitive to infrared radiation, light, electromagnetic radiation of shorter wavelength or corpuscular radiation
- H10K30/10—Organic devices sensitive to infrared radiation, light, electromagnetic radiation of shorter wavelength or corpuscular radiation comprising heterojunctions between organic semiconductors and inorganic semiconductors
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
- H10K85/342—Transition metal complexes, e.g. Ru(II)polypyridine complexes comprising iridium
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Electromagnetism (AREA)
- Photovoltaic Devices (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及有机光伏领域,具体涉及一类铱配合物有机光伏材料及其制备方法与应用。The invention relates to the field of organic photovoltaics, in particular to a class of iridium complex organic photovoltaic materials and a preparation method and application thereof.
背景技术Background technique
随着人们对传统化石能源的大量开采和使用,当今世界面临着严重的能源危机和环境污染问题,开发和利用新型的清洁可持续能源是解决这一问题的有效途径。在众多的清洁能源中,太阳能由于其取之不尽用之不竭的优点,作为一种源源不断的清洁可再生能源,备受大家关注,如何有效的将其进行利用也是目前众多领域的研究热点。其中,太阳能电池可以通过光生伏打效应有效地将光能转化为电能从而实现这种绿色能源的有效利用,这符合社会发展的需要,并且具有极大的市场应用前景和长远的意义。相比于传统的无机硅基太阳能电池,有机太阳能电池由于具有质量轻,材料来源广,成本低,结构易修饰且可柔性大面积制备等优点而受到学术界及工业界越来越多的关注。有机太阳能电池的工作原理主要由四个步骤组成,它们协同运作,缺一不可,具体为:1、光照条件下,活性层吸收光子并产生激子;2、产生的激子在给受体界面进行扩散;3、在驱动力的推动下,激子在给受体界面发生分离形成自由电荷;4、形成的电荷进行传输并被电极所收集形成光电流。With the massive exploitation and use of traditional fossil energy, the world today is facing serious energy crisis and environmental pollution problems. The development and utilization of new clean and sustainable energy is an effective way to solve this problem. Among the many clean energy sources, solar energy has attracted much attention due to its inexhaustible advantages as a steady stream of clean and renewable energy. hot spot. Among them, solar cells can effectively convert light energy into electrical energy through the photovoltaic effect to realize the effective utilization of this green energy, which meets the needs of social development and has great market application prospects and long-term significance. Compared with traditional inorganic silicon-based solar cells, organic solar cells have attracted more and more attention from academia and industry due to their advantages of light weight, wide material sources, low cost, easy structure modification and flexible large-area fabrication. . The working principle of organic solar cells is mainly composed of four steps. They work together and are indispensable. Specifically: 1. Under illumination conditions, the active layer absorbs photons and generates excitons; 2. The generated excitons are at the donor-acceptor interface. Diffusion; 3. Driven by the driving force, the excitons are separated at the interface of the acceptor to form free charges; 4. The formed charges are transported and collected by the electrode to form a photocurrent.
从材料角度来看,目前广泛应用的光伏材料大部分都是由稠环的梯形结构,非稠环的类梯形结构或以苯并二噻吩(BDT)为核心单元所构成,在材料合成中往往会面临一些繁琐的合成步骤,产率较低,提纯困难。而且,绝大多数都是基于单线态激子主导的纯有机体系,其相应的激子寿命短(10-100ps),进而导致激子扩散长度不足,仅有5-10nm,该激子扩散长度明显短于有效光吸收所需的范围,限制了给体/受体的域尺寸和活性层厚度,在一定层面上限制了器件性能的改善。因此在材料设计方面除了考虑吸光能力,还需考虑激子的寿命和扩散长度,从而实现有机太阳能电池光伏效率的提升。重金属铱配合物被广泛应用于磷光有机发光二极管(PHOLED)中,研究表明其可以利用重金属原子铱诱导强的自旋-轨道耦合(SOC),通过有效地隙间蹿越(ISC)将单线态激子转换成三线态激子。对有机太阳能电池而言,三线态激子比单线态激子具有更长的寿命(高2-3个数量级)和激子扩散长度(10-140nm),有望于提升激子的解离效率和抑制电荷的复合过程,并且铱配合物具有独特的八面体空间构型,将其作为光伏材料,在一定程度上可以优化共混膜形貌,调节分子堆积,促进电荷的产生和传输,最终在器件效率上实现突破。因此,开发具有新型分子结构的铱配合物并作为给体材料应用于有机太阳能电池中,探究其在有机光伏领域中的应用前景具有十分重要的意义。From the point of view of materials, most of the widely used photovoltaic materials are composed of fused-ring ladder structures, non-fused-ring ladder-like structures or benzodithiophene (BDT) as the core unit, which are often used in material synthesis. It will face some tedious synthesis steps, the yield is low, and the purification is difficult. Moreover, most of them are based on pure organic systems dominated by singlet excitons, and their corresponding exciton lifetimes are short (10-100ps), which in turn leads to insufficient exciton diffusion length, which is only 5-10nm. Significantly shorter than the range required for effective light absorption, limiting the domain size of the donor/acceptor and the thickness of the active layer, limiting the improvement of device performance to a certain level. Therefore, in addition to the light absorption ability, the exciton lifetime and diffusion length need to be considered in material design, so as to improve the photovoltaic efficiency of organic solar cells. Heavy metal iridium complexes are widely used in phosphorescent organic light-emitting diodes (PHOLEDs), and studies have shown that they can utilize heavy metal atoms iridium to induce strong spin-orbit coupling (SOC), which can effectively convert the singlet state by interstitial jumping (ISC). The excitons are converted into triplet excitons. For organic solar cells, triplet excitons have longer lifetimes (2-3 orders of magnitude higher) and exciton diffusion lengths (10-140 nm) than singlet excitons, which are expected to improve exciton dissociation efficiency and The recombination process of charge is suppressed, and the iridium complex has a unique octahedral spatial configuration. Using it as a photovoltaic material can optimize the morphology of the blend film to a certain extent, adjust the molecular packing, and promote the generation and transport of charges. Breakthroughs in device efficiency. Therefore, it is of great significance to develop iridium complexes with novel molecular structures and use them as donor materials in organic solar cells, and to explore their application prospects in the field of organic photovoltaics.
2012年,李健等人报道了一种铱配合物给体材料APIr(DOI:10.1021/ic3023453),以C60作为受体材料制备了一种双层异质结光伏器件,获得了2.8%的器件效率;2014年,甄宏宇等人通过改变主配体设计合成了两个具有红光发射的小分子铱配合物(DOI:10.1039/C4TA01526F),将其作为给体,富勒烯衍生物PC71BM为电子受体来制备光伏器件,分别获得了1.2%和2%的器件效率;2019年,申请人课题组设计合成了一种新型的具有八面体空间构型、以乙酰丙酮为辅助配体的异配位结构铱配合物TBzIr(DOI:10.1039/c9cc00173e),以富勒烯衍生物PC71BM为受体材料,PDINO为电子传输层制备光伏器件,其器件效率(PCE)最大可以达到3.81%,远远高于其基于纯有机配体TBz的器件效率(≈0%);同年,张凤玲课题组通过改变主配体的共轭长度报道了两个同配体型铱配合物Ir(Ftbpa)3和Ir(FOtbpa)3(DOI:10.1039/c9tc04914b)来研究其在有机光伏中的电压损失,将其作为给体,富勒烯PC71BM为受体制备了体异质结器件,分别获得了3.17%和3.56%的器件效率。In 2012, Li Jian et al. reported an iridium complex donor material APIr (DOI: 10.1021/ic3023453), and prepared a double-layer heterojunction photovoltaic device withC60 as the acceptor material, and obtained 2.8%. device efficiency; in 2014, Zhen Hongyu et al. synthesized two small-molecule iridium complexes (DOI: 10.1039/C4TA01526F) with red light emission by changing the main ligand design, using it as the donor, the fullerene derivative PC71 BM was used as an electron acceptor to prepare photovoltaic devices, and device efficiencies of 1.2% and 2% were obtained, respectively; in 2019, the applicant's research group designed and synthesized a new type of octahedral spatial configuration with acetylacetone as an auxiliary ligand. The heterocoordinate structure of iridium complex TBzIr (DOI: 10.1039/c9cc00173e), using fullerene derivative PC71 BM as acceptor material, PDINO as electron transport layer to prepare photovoltaic devices, the maximum device efficiency (PCE) can reach 3.81 %, which is much higher than its device efficiency (≈0%) based on pure organic ligand TBz; in the same year, Zhang Fengling’s group reported two isoligand-type iridium complexes Ir(Ftbpa) by changing the conjugation length of the main ligand.3 and Ir(FOtbpa)3 (DOI: 10.1039/c9tc04914b) to study its voltage loss in organic photovoltaics, using it as the donor and fullerene PC71 BM as the acceptor to prepare bulk heterojunction devices, respectively obtained device efficiencies of 3.17% and 3.56%.
然而目前报导的小分子铱配合物光伏材料大部分都是基于带有辅助配体的异配体型铱配合物,而具有更好空间立体结构的同配体型铱配合物鲜有报导且器件效率并不理想。因此为了更好调节光伏器件的光吸收能力、共混薄膜的形貌以及提供更长的激子寿命,设计合成具有更好立体空间构型的新型同配体铱配合物,以进一步研究分子结构对光伏性能的影响具有十分重要的意义。However, most of the reported small-molecule iridium complex photovoltaic materials are based on heteroligand iridium complexes with auxiliary ligands, while homoligand iridium complexes with better steric structure are rarely reported and the device efficiency is not high. not ideal. Therefore, in order to better adjust the light absorption capacity of photovoltaic devices, the morphology of the blended films and provide longer exciton lifetimes, new isoligand iridium complexes with better steric configuration were designed and synthesized to further study the molecular structure. The impact on photovoltaic performance is of great significance.
发明内容SUMMARY OF THE INVENTION
发明目的:本发明所要解决的技术问题是针对现有技术的不足,提供一类铱配合物有机光伏材料。Purpose of the invention: The technical problem to be solved by the present invention is to provide a class of iridium complex organic photovoltaic materials for the deficiencies of the prior art.
本发明还要解决的技术问题是提供上述铱配合物有机光伏材料的制备方法。The technical problem to be solved by the present invention is to provide a preparation method of the above-mentioned iridium complex organic photovoltaic material.
本发明进一步要解决的技术问题是提供上述铱配合物有机光伏材料的应用。The further technical problem to be solved by the present invention is to provide the application of the above-mentioned iridium complex organic photovoltaic material.
发明思路:本发明通过在主配体上引入重金属原子铱进行环金属化,设计合成了具有八面体空间结构的铱配合物,并改变主配体的共轭长度和烷基链来调节材料的光电性能,研究分子结构-性能之间的相互关系。Invention idea: The present invention designs and synthesizes iridium complexes with octahedral space structure by introducing heavy metal atom iridium on the main ligand for cyclometallation, and changes the conjugation length and alkyl chain of the main ligand to adjust the material properties. Optoelectronic properties, study the relationship between molecular structure and properties.
为了解决上述第一个技术问题,本发明公开了一类铱配合物有机光伏材料(一种具有空间立体结构的同配体型铱配合物小分子材料),其结构通式如式I所示:In order to solve the above-mentioned first technical problem, the present invention discloses a kind of iridium complex organic photovoltaic material (a kind of isoligand type iridium complex small molecule material with steric structure), and its general structural formula is as shown in formula I:
其中,R为噻吩联己基(式II1所示结构式)、噻吩联叔丁基(式II2所示结构式)和正己基(式II3所示结构式)中的任意一种;优选地,R为噻吩联己基(式II1所示结构式)或噻吩联叔丁基(式II2所示结构式);进一步优选地,R为噻吩联己基(式II1所示结构式);Wherein, R is any one of thiophene bihexyl (structural formula shown in formula II1), thiophene bi-tert-butyl (structural formula shown in formula II2) and n-hexyl (structural formula shown in formula II3); preferably, R is thiophene biphenyl Hexyl (structural formula shown in formula II1) or thiophenebi-tert-butyl (structural formula shown in formula II2); more preferably, R is thiophenebihexyl (structural formula shown in formula II1);
为了解决上述第二个技术问题,本发明公开了上述铱配合物有机光伏材料的制备方法,将Ir(BzBr)3、噻吩类化合物、催化剂和溶剂的混合溶液进行偶联反应。In order to solve the above second technical problem, the present invention discloses a preparation method of the above-mentioned iridium complex organic photovoltaic material, wherein a mixed solution of Ir(BzBr)3 , a thiophene compound, a catalyst and a solvent is subjected to a coupling reaction.
其中,当R为噻吩联叔丁基(式II2所示结构式)和正己基(式II3所示结构式),所述反应需要加入碱;当R为噻吩联己基(式II1所示结构式),所述反应不需要加入碱。Wherein, when R is thiophenebi-tert-butyl (structural formula shown in formula II2) and n-hexyl (structural formula shown in formula II3), the reaction needs to add a base; when R is thiophenebihexyl (structural formula shown in formula II1), so The above reaction does not require the addition of a base.
其中,所述噻吩类化合物为2-(5-己基噻吩-2-基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷、2-(5'-(叔丁基)-[2,2'-联噻吩]-5-基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷和三丁基(5'-己基-[2,2'-联噻吩]-5-基)锡烷中的任意一种或几种组合。Wherein, the thiophene compound is 2-(5-hexylthiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane, 2-(5' -(tert-butyl)-[2,2'-bithiophene]-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane and tributyl ( Any one or several combinations of 5'-hexyl-[2,2'-bithiophene]-5-yl)stannane.
其中,所述催化剂为四三苯基膦钯。Wherein, the catalyst is tetrakistriphenylphosphine palladium.
其中,所述溶剂为甲苯、乙醇、N,N-二甲基甲酰胺和水中的任意一种或几种组合;优选地,所述溶剂为甲苯、乙醇和水的混合溶剂或甲苯与N,N-二甲基甲酰胺的混合溶剂;进一步优选地,所述溶剂为体积比为3-7:1:1-3的甲苯、乙醇和水的混合溶剂或体积比为8-12:1的甲苯与N,N-二甲基甲酰胺的混合溶剂;更进一步优选地,所述溶剂为体积比为5:1:2的甲苯、乙醇和水的混合溶剂或体积比为10:1的甲苯与N,N-二甲基甲酰胺的混合溶剂。Wherein, the solvent is any one or several combinations of toluene, ethanol, N,N-dimethylformamide and water; preferably, the solvent is a mixed solvent of toluene, ethanol and water or toluene and N, A mixed solvent of N-dimethylformamide; further preferably, the solvent is a mixed solvent of toluene, ethanol and water with a volume ratio of 3-7:1:1-3 or a mixed solvent with a volume ratio of 8-12:1 A mixed solvent of toluene and N,N-dimethylformamide; further preferably, the solvent is a mixed solvent of toluene, ethanol and water with a volume ratio of 5:1:2 or toluene with a volume ratio of 10:1 Mixed solvent with N,N-dimethylformamide.
其中,所述Ir(BzBr)3和噻吩类化合物的质量比为1:2-6;优选地,所述Ir(BzBr)3和噻吩类化合物的质量比为1:4。Wherein, the mass ratio of the Ir(BzBr)3 and the thiophene compound is 1:2-6; preferably, the mass ratio of the Ir(BzBr)3 and the thiophene compound is 1:4.
其中,所述催化剂的用量为反应体系中固体反应物总质量的0.2%-2.3%;优选地,所述催化剂的用量为反应体系中固体反应物总质量的0.5%-2%;进一步优选地,所述催化剂的用量为反应体系中固体反应物总质量的0.5%或2%;其中,所述反应体系中固体反应物为Ir(BzBr)3、噻吩类化合物和碱(当体系不含有碱的情况下,不包括碱)。Wherein, the dosage of the catalyst is 0.2%-2.3% of the total mass of the solid reactants in the reaction system; preferably, the dosage of the catalyst is 0.5%-2% of the total mass of the solid reactants in the reaction system; further preferably , the dosage of the catalyst is 0.5% or 2% of the total mass of the solid reactants in the reaction system; wherein, the solid reactants in the reaction system are Ir(BzBr)3 , thiophene compounds and alkali (when the system does not contain alkali , excluding bases).
其中,所述Ir(BzBr)3和溶剂的质量体积比为10-15mg/mL。Wherein, the mass volume ratio of the Ir(BzBr)3 and the solvent is 10-15 mg/mL.
其中,所述碱包括但不限于碳酸钾。Wherein, the base includes but not limited to potassium carbonate.
其中,所述Ir(BzBr)3和碱的质量比为1:8-16;优选地,所述Ir(BzBr)3和碱的质量比为1:10-14;进一步优选地,所述Ir(BzBr)3和碱的质量比为1:12。Wherein, the mass ratio of the Ir(BzBr)3 and the base is 1:8-16; preferably, the mass ratio of the Ir(BzBr)3 and the base is 1:10-14; further preferably, the Ir The mass ratio of (BzBr)3 and base was 1:12.
其中,所述反应为在惰性氛围下反应;优选地,所述反应为在氮气氛围下反应。Wherein, the reaction is a reaction under an inert atmosphere; preferably, the reaction is a reaction under a nitrogen atmosphere.
其中,所述反应的温度为80-120℃;优选地,所述反应的温度为100℃。Wherein, the temperature of the reaction is 80-120°C; preferably, the temperature of the reaction is 100°C.
其中,所述反应的时间为2-34h;优选地,所述反应的时间为7-29h;进一步优选地,所述反应的时间为12-24h。Wherein, the reaction time is 2-34h; preferably, the reaction time is 7-29h; further preferably, the reaction time is 12-24h.
其中,所述反应结束后,将反应液冷却,萃取收集有机相,干燥,过滤,旋干,柱层析,即得。Wherein, after the reaction is completed, the reaction solution is cooled, the organic phase is extracted and collected, dried, filtered, spin-dried, and subjected to column chromatography to obtain the final product.
其中,所述冷却为冷却至室温。Wherein, the cooling is cooling to room temperature.
其中,所述萃取为二氯甲烷萃取。Wherein, the extraction is dichloromethane extraction.
其中,所述干燥为无水硫酸钠干燥。Wherein, the drying is anhydrous sodium sulfate drying.
其中,所述柱层析为采用二氯甲烷/石油醚(v/v)=1:2作为洗脱剂进行柱层析。Wherein, the column chromatography is performed by using dichloromethane/petroleum ether (v/v)=1:2 as an eluent for column chromatography.
为了解决上述第三个技术问题,本发明公开了上述铱配合物有机光伏材料在有机光伏器件(OPV)中的应用。In order to solve the above-mentioned third technical problem, the present invention discloses the application of the above-mentioned iridium complex organic photovoltaic material in an organic photovoltaic device (OPV).
其中,所述有机光伏器件包括但不限于有机太阳能电池光伏器件,器件结构为经典的体异质结(BHJ)正向器件。Wherein, the organic photovoltaic device includes, but is not limited to, an organic solar cell photovoltaic device, and the device structure is a classical bulk heterojunction (BHJ) forward device.
其中,所述有机光伏器件包括由电子给体材料(D)和电子受体材料(A)构成的给受体共混活性层(D:A)。Wherein, the organic photovoltaic device comprises a donor-acceptor blended active layer (D:A) composed of an electron-donor material (D) and an electron-acceptor material (A).
其中,所述有机光伏器件包括玻璃基板、附着在玻璃基板上的空穴传输层、附着在空穴传输层上的给受体共混活性层、附着在给受体共混活性层上的电子传输层、附着在电子传输层上的阴极层。Wherein, the organic photovoltaic device comprises a glass substrate, a hole transport layer attached to the glass substrate, a donor-acceptor blended active layer attached to the hole transport layer, and an electron donor attached to the acceptor blended active layer. Transport layer, cathode layer attached to the electron transport layer.
其中,所述玻璃基板包括但不限于氧化铟锡(ITO)玻璃基板。Wherein, the glass substrate includes but is not limited to indium tin oxide (ITO) glass substrate.
其中,所述空穴传输层为PEDOT:PSS;优选地,所述空穴传输层为30nm。Wherein, the hole transport layer is PEDOT:PSS; preferably, the hole transport layer is 30 nm.
其中,所述空穴传输层旋涂在玻璃基板上。Wherein, the hole transport layer is spin-coated on the glass substrate.
其中,所述给受体共混活性层中的电子给体材料为所述铱配合物有机光伏材料。Wherein, the electron donor material in the donor-acceptor blending active layer is the iridium complex organic photovoltaic material.
其中,所述给受体共混活性层中的电子受体材料包括但不限于富勒烯衍生物PC71BM,稠环芳香烃非富勒烯受体Y6;优选地,所述给受体共混活性层中的电子受体材料为稠环芳香烃非富勒烯受体Y6。Wherein, the electron acceptor material in the blending active layer of the acceptor includes but is not limited to fullerene derivative PC71 BM, fused-ring aromatic hydrocarbon non-fullerene acceptor Y6; preferably, the acceptor The electron acceptor material in the blended active layer is a fused-ring aromatic hydrocarbon non-fullerene acceptor Y6.
其中,所述给受体共混活性层中的电子给体材料和受体材料质量比为1:0.5-2.5;优选地,所述电子给体材料和受体材料质量比为1:1-2;进一步优选地,所述电子给体材料和受体材料质量比为1:1。Wherein, the mass ratio of the electron donor material to the acceptor material in the active layer of the donor-acceptor blend is 1:0.5-2.5; preferably, the mass ratio of the electron donor material to the acceptor material is 1:1- 2; Further preferably, the mass ratio of the electron donor material and the acceptor material is 1:1.
其中,所述给受体共混活性层旋涂在空穴传输层。Wherein, the donor-acceptor blending active layer is spin-coated on the hole transport layer.
其中,所述电子传输层为PDINO;优选地,所述电子传输层为10nm。Wherein, the electron transport layer is PDINO; preferably, the electron transport layer is 10 nm.
其中,所述电子传输层旋涂在给受体共混活性层上。Wherein, the electron transport layer is spin-coated on the donor-acceptor blending active layer.
其中,所述阴极层为Al。Wherein, the cathode layer is Al.
其中,所述阴极层通过真空蒸镀沉积在电子传输层上。Wherein, the cathode layer is deposited on the electron transport layer by vacuum evaporation.
优选地,所述有机光伏器件为玻璃基板/空穴传输层/给受体共混活性层/电子传输层/阴极;进一步优选地,所述有机光伏器件为玻璃基板(ITO)/空穴传输层(PEDOT:PSS)/活性层(D:A)/电子传输层(PDINO)/阴极(Al)。Preferably, the organic photovoltaic device is a glass substrate/hole transport layer/donor-acceptor blended active layer/electron transport layer/cathode; further preferably, the organic photovoltaic device is a glass substrate (ITO)/hole transport layer (PEDOT: PSS)/active layer (D:A)/electron transport layer (PDINO)/cathode (Al).
其中,当所述电子受体材料为富勒烯衍生物PC71BM,玻璃基板为ITO,空穴传输层为PEDOT:PSS,电子传输层为PDINO,阴极层为金属Al,器件结构为传统的正向本体异质结器件,即:玻璃基板(ITO)/空穴传输层(PEDOT:PSS)/活性层(D:PC71BM)/电子传输层(PDINO)/阴极(Al)。Wherein, when the electron acceptor material is fullerene derivative PC71 BM, the glass substrate is ITO, the hole transport layer is PEDOT:PSS, the electron transport layer is PDINO, the cathode layer is metal Al, and the device structure is traditional Forward bulk heterojunction device, ie: glass substrate (ITO)/hole transport layer (PEDOT: PSS)/active layer (D:PC71BM )/electron transport layer (PDINO)/cathode (Al).
其中,当所述电子受体材料为稠环芳香烃非富勒烯受体Y6,玻璃基板为ITO,空穴传输层为PDOT:PSS,电子传输层为PDINO,阴极层为金属Al,器件结构为传统的正向本体异质结器件,即:玻璃基板(ITO)/空穴传输层(PEDOT:PSS)/活性层(D:Y6)/电子传输层(PDINO)/阴极(Al)。Wherein, when the electron acceptor material is fused ring aromatic hydrocarbon non-fullerene acceptor Y6, the glass substrate is ITO, the hole transport layer is PDOT:PSS, the electron transport layer is PDINO, the cathode layer is metal Al, and the device structure It is a traditional forward bulk heterojunction device, namely: glass substrate (ITO)/hole transport layer (PEDOT: PSS)/active layer (D: Y6)/electron transport layer (PDINO)/cathode (Al).
有益效果:与现有技术相比,本发明具有如下优势Beneficial effects: Compared with the prior art, the present invention has the following advantages
(1)本发明提供一种新型有机光伏材料,将其作为给体材料应用于有机太阳能电池中,可获得相比于纯有机主配体为给体的更高器件效率。(1) The present invention provides a novel organic photovoltaic material, which can be used as a donor material in an organic solar cell to obtain a higher device efficiency than that of a pure organic main ligand as the donor.
(2)本发明通过引入重金属原子铱,将主配体进行环金属化得到一种具有八面体空间构型的铱配合物,探究该类铱配合物的分子结构与能量转换效率之间的结构-性质相互关系,通过设计这种八面体构型的铱配合物可有效提高基于配合物光伏材料的器件性能,为设计新型的有机光伏材料提供了一种有效策略,实现在有机光伏领域中的有效应用。(2) The present invention obtains an iridium complex with an octahedral spatial configuration by introducing a heavy metal atom iridium, cyclometallates the main ligand, and explores the structure between the molecular structure and energy conversion efficiency of this type of iridium complex -The interrelationship of properties, the device performance of complex-based photovoltaic materials can be effectively improved by designing iridium complexes in this octahedral configuration, which provides an effective strategy for designing new organic photovoltaic materials, and realizes the application in the field of organic photovoltaics. Effective application.
(3)本发明中涉及的分子化学结构简单,通过简单的化学反应即可制得,合成简便,反应条件温和,易于纯化。(3) The molecule involved in the present invention has a simple chemical structure, can be prepared by a simple chemical reaction, is easy to synthesize, has mild reaction conditions and is easy to purify.
(4)本发明将铱配合物有机光伏材料与富勒烯受体共混制备光伏器件,可实现短路电流密度,开路电压以及填充因子的全面提高,从而实现更高的器件效率(4) In the present invention, the photovoltaic device is prepared by blending the iridium complex organic photovoltaic material and the fullerene acceptor, which can realize the overall improvement of the short-circuit current density, open-circuit voltage and filling factor, thereby realizing higher device efficiency.
附图说明Description of drawings
下面结合附图和具体实施方式对本发明做更进一步的具体说明,本发明的上述和/或其他方面的优点将会变得更加清楚。The present invention will be further described in detail below with reference to the accompanying drawings and specific embodiments, and the advantages of the above-mentioned and/or other aspects of the present invention will become clearer.
图1为本发明中涉及的有机光伏器件的器件结构。FIG. 1 is the device structure of the organic photovoltaic device involved in the present invention.
图2为相应的铱配合物光伏给体材料在薄膜状态下的吸收光谱。Figure 2 is the absorption spectrum of the corresponding iridium complex photovoltaic donor material in the thin film state.
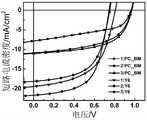
图3为以相应的铱配合物(Ir(BzT)3(化合物1),Ir(tTBz)3(化合物2),Ir(TBz)3(化合物3)为给体材料,PC71BM及Y6为受体材料的正向结构的有机光伏器件(器件1,2,3,4,5,6)的短路电流密度-电压(J-V)曲线。Figure 3 shows the corresponding iridium complexes (Ir(BzT)3 (Compound 1), Ir(tTBz)3 (Compound 2), Ir(TBz)3 (Compound 3) as donor materials, PC71 BM and Y6 as donor materials Short-circuit current density-voltage (JV) curves of organic photovoltaic devices (
具体实施方式Detailed ways
下述实施例中所述实验方法,如无特殊说明,均为常规方法;所述试剂和材料,如无特殊说明,均可从商业途径获得。The experimental methods described in the following examples are conventional methods unless otherwise specified; the reagents and materials can be obtained from commercial sources unless otherwise specified.
本发明中,所述Ir(BzBr)3和噻吩类化合物2-(5-己基噻吩-2-基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷、2-(5'-(叔丁基)-[2,2'-联噻吩]-5-基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷和三丁基(5'-己基-[2,2'-联噻吩]-5-基)锡烷均从苏州纳凯科技有限公司(Suna Tech Inc.)购买获得。In the present invention, the Ir(BzBr)3 and the thiophene compound 2-(5-hexylthiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborole Alkane, 2-(5'-(tert-butyl)-[2,2'-bithiophene]-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxa Borane and tributyl(5'-hexyl-[2,2'-bithiophene]-5-yl)stannane were purchased from Suna Tech Inc., Suzhou.
下述实施例中,所述固体反应物为Ir(BzBr)3、噻吩类化合物和碱(当体系不含有碱的情况下,不包括碱)。In the following examples, the solid reactants are Ir(BzBr)3 , a thiophene compound and a base (if the system does not contain a base, the base is not included).
实施例1:Example 1:
在本实例中,以苯并噻唑联双噻吩为主配体,末端侧链为正己基的铱配合物Ir(BzT)3(简称化合物1)的合成,其结构式为:In this example, the synthesis of the iridium complex Ir(BzT)3 (referred to as compound 1) with benzothiazole-linked dithiophene as the main ligand and the terminal side chain as n-hexyl group, its structural formula is:
将化合物(Ir(BzBr)3),2-(5-己基噻吩-2-基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷和碳酸钾以质量比为1:4:12加入到体积比为5:1:2的甲苯,乙醇和水的混合溶剂中,化合物(Ir(BzBr)3)的浓度为12.5mg/mL,在氮气氛围下,快速加入固体反应物总质量0.5%的四三苯基膦钯,在氮气氛围、100℃下反应24小时。反应结束冷却到室温用二氯甲烷萃取,收集有机相,无水硫酸钠干燥,过滤,旋干。采用二氯甲烷/石油醚(v/v)=1:2作为洗脱剂进行柱层分析,得到目标产物化合物(1)Ir(BzT)3。产率:60%。1H NMR(400MHz,CDCl3,δppm):7.69(d,3H),7.08(t,3H),6.97(d,3H),6.84(t,3H),6.59(t,6H),6.38(s,3H),2.70(t,6H),1.59(t,6H),1.23-1.32(m,18H),0.83(t,9H)。MALDI-TOF(m/z):M+calculated at 1339.19,found at 1339.234Compound (Ir(BzBr)3 ), 2-(5-hexylthiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane and potassium carbonate were The mass ratio of 1:4:12 was added to a mixed solvent of toluene, ethanol and water with a volume ratio of 5:1:2. The concentration of the compound (Ir(BzBr)3 ) was 12.5 mg/mL. Under a nitrogen atmosphere, Quickly add tetrakistriphenylphosphine palladium with a total mass of 0.5% of the solid reactant, and react under nitrogen atmosphere at 100° C. for 24 hours. After the reaction was completed, the mixture was cooled to room temperature and extracted with dichloromethane. The organic phase was collected, dried over anhydrous sodium sulfate, filtered and spin-dried. Using dichloromethane/petroleum ether (v/v)=1:2 as the eluent for column analysis, the target product compound (1) Ir(BzT)3 was obtained. Yield: 60%.1 H NMR (400 MHz, CDCl3 , δppm): 7.69(d,3H), 7.08(t,3H), 6.97(d,3H), 6.84(t,3H), 6.59(t,6H), 6.38(s , 3H), 2.70 (t, 6H), 1.59 (t, 6H), 1.23-1.32 (m, 18H), 0.83 (t, 9H). MALDI-TOF(m/z): M+ calculated at 1339.19, found at 1339.234
实施例2:Example 2:
在本实例中,以苯并噻唑联三噻吩为主配体,末端侧链为叔丁基的铱配合物Ir(tTBz)3(简称化合物2)的合成,其结构式为:In this example, the synthesis of the iridium complex Ir(tTBz)3 (referred to as compound 2) with benzothiazole-linked trithiophene as the main ligand and the terminal side chain of tert-butyl (referred to as compound 2) is as follows:
将化合物(Ir(BzBr)3),2-(5'-(叔丁基)-[2,2'-联噻吩]-5-基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷和碳酸钾以质量比1:4:12加入到体积比为5:1:2的甲苯,乙醇和水的混合溶剂中,化合物(Ir(BzBr)3)的浓度为12.5mg/mL,在氮气氛围下,快速加入固体反应物总质量0.5%的催化剂四三苯基膦钯,在氮气氛围、100℃下反应24小时。反应结束冷却到室温,用二氯甲烷萃取,收集有机相,无水硫酸钠干燥,过滤,旋干。采用二氯甲烷/石油醚(v/v)=1:1作为洗脱剂进行柱层分析,得到目标产物化合物(2)Ir(tTBz)3。产率:65%。1H NMR(400MHz,CDCl3,δppm):7.71(d,3H),7.11(t,3H),7.06(d,3H),6.92(t,6H),6.86(t,3H),6.67(d,3H),6.60(d,3H),6.44(s,3H),1.35(s,27H)。MALDI-TOF(m/z):M+calculated at1501.06,found at 1501.1Compound (Ir(BzBr)3 ), 2-(5'-(tert-butyl)-[2,2'-bithiophene]-5-yl)-4,4,5,5-tetramethyl-1 ,3,2-dioxaborane and potassium carbonate were added to a mixed solvent of toluene, ethanol and water with a volume ratio of 5:1:2 in a mass ratio of 1:4:12, the compound (Ir(BzBr)3 ) The concentration of the solution was 12.5 mg/mL. Under a nitrogen atmosphere, the catalyst tetrakistriphenylphosphine palladium with a total mass of 0.5% of the solid reactant was rapidly added, and the reaction was carried out at 100° C. for 24 hours in a nitrogen atmosphere. After the reaction was completed, it was cooled to room temperature, extracted with dichloromethane, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and spin-dried. Using dichloromethane/petroleum ether (v/v)=1:1 as the eluent for column analysis, the target product compound (2) Ir(tTBz)3 was obtained. Yield: 65%.1 H NMR (400 MHz, CDCl3 , δppm): 7.71(d,3H), 7.11(t,3H), 7.06(d,3H), 6.92(t,6H), 6.86(t,3H), 6.67(d , 3H), 6.60 (d, 3H), 6.44 (s, 3H), 1.35 (s, 27H). MALDI-TOF(m/z): M+ calculated at 1501.06, found at 1501.1
实施例3Example 3
在本实例中,以苯并噻唑联三噻吩为主配体,末端侧链为正己基的铱配合物Ir(TBz)3(简称化合物3)的合成,其结构式为:In this example, the synthesis of the iridium complex Ir(TBz)3 (referred to as compound 3) with benzothiazole-linked trithiophene as the main ligand and the terminal side chain as n-hexyl group, its structural formula is:
将化合物(Ir(BzBr)3),三丁基(5'-己基-[2,2'-联噻吩]-5-基)锡烷以质量比1:4加入到体积比为10:1的甲苯和N,N-二甲基甲酰胺(DMF)的混合溶剂中,化合物(Ir(BzBr)3)的浓度为12.5mg/mL,在氮气氛围下,快速加入固体反应物总质量2%的催化剂四三苯基膦钯,在氮气氛围、110℃下反应12小时。反应结束冷却到室温,用二氯甲烷萃取,收集有机相,无水硫酸钠干燥,过滤,旋干。采用二氯甲烷/石油醚(v/v)=1:1作为洗脱剂进行柱层分析,得到目标产物化合物(3)Ir(TBz)3。产率:70%。1H NMR(400MHz,CDCl3,δppm):7.71(d,3H),7.11(t,3H),7.06(d,3H),6.92(d,J=4Hz,6H),6.86(t,3H),6.60(d,6H),6.44(s,3H),2.72(t,6H),1.61(t,6H),1.23-1.32(m,18H),0.84(t,9H)。MALDI-TOF(m/z):M+calculated at1585.15,found at 1585.738The compound (Ir(BzBr)3 ), tributyl(5'-hexyl-[2,2'-bithiophene]-5-yl)stannane was added in a mass ratio of 1:4 to a volume ratio of 10:1. In a mixed solvent of toluene and N,N-dimethylformamide (DMF), the concentration of compound (Ir(BzBr)3 ) was 12.5 mg/mL, and under nitrogen atmosphere, 2% of the total mass of the solid reactant was rapidly added. The catalyst, tetrakistriphenylphosphine palladium, was reacted at 110° C. for 12 hours in a nitrogen atmosphere. After the reaction was completed, it was cooled to room temperature, extracted with dichloromethane, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and spin-dried. Using dichloromethane/petroleum ether (v/v)=1:1 as the eluent for column analysis, the target product compound (3) Ir(TBz)3 was obtained. Yield: 70%.1 H NMR (400 MHz, CDCl3 , δppm): 7.71 (d, 3H), 7.11 (t, 3H), 7.06 (d, 3H), 6.92 (d, J=4Hz, 6H), 6.86 (t, 3H) , 6.60(d, 6H), 6.44(s, 3H), 2.72(t, 6H), 1.61(t, 6H), 1.23-1.32(m, 18H), 0.84(t, 9H). MALDI-TOF(m/z): M+ calculated at 1585.15, found at 1585.738
实施例4Example 4
(1)有机太阳能电池光伏器件的制备,其具体结构如图1所示,为导电玻璃基板(ITO)/空穴传输层(PEDOT:PSS)/给受体共混活性层(D:A)/电子传输层(PDINO)/阴极(Al);其中,以实施例1-3所制备的小分子铱配合物(化合物1-3)作为给受体共混活性层中的电子给体材料。(1) Preparation of organic solar cell photovoltaic device, the specific structure of which is shown in Figure 1, which is a conductive glass substrate (ITO)/hole transport layer (PEDOT:PSS)/donor-acceptor blending active layer (D:A) /electron transport layer (PDINO)/cathode (Al); wherein, the small molecule iridium complex (compound 1-3) prepared in Example 1-3 was used as the electron donor material in the donor-acceptor blending active layer.
其中,所述有机太阳能电池光伏器件是通过目前该领域文献中已报导的方法进行制备,其具体制备方法为:首先对ITO玻璃基板进行清洗预处理,然后在真空条件下通过旋涂的方法依次制备空穴传输层(30nm),给受体共混活性层和电子传输层(10nm),最后通过真空蒸镀沉积得到100nm的Al作为阴极层。采用该方法制备本发明中涉及的有机光伏器件的具体器件结构如下所示:Wherein, the organic solar cell photovoltaic device is prepared by a method that has been reported in the literature in this field. The specific preparation method is as follows: first, the ITO glass substrate is cleaned and pretreated, and then the method of spin coating is carried out under vacuum conditions. A hole transport layer (30 nm) was prepared, the acceptor was blended with an active layer and an electron transport layer (10 nm), and finally 100 nm of Al was obtained by vacuum evaporation deposition as a cathode layer. The specific device structure of the organic photovoltaic device involved in the present invention prepared by this method is as follows:
器件1:Device 1:
ITO/PEDOT:PSS(30nm)/化合物1:PC71BM(给受体质量比=1:2)/PDINO(10nm)/Al器件2:ITO/PEDOT: PSS (30 nm)/Compound 1:PC71BM (mass ratio of donor to acceptor=1:2)/PDINO (10 nm)/Al Device 2:
ITO/PEDOT:PSS(30nm)/化合物2:PC71BM(给受体质量比=1:2)/PDINO(10nm)/Al器件3:ITO/PEDOT: PSS (30 nm)/Compound 2:PC71BM (mass ratio of donor to acceptor=1:2)/PDINO (10 nm)/Al Device 3:
ITO/PEDOT:PSS(30nm)/化合物3:PC71BM(给受体质量比=1:2)/PDINO(10nm)/Al器件4:ITO/PEDOT: PSS (30 nm)/Compound 3:PC71BM (mass ratio of donor to acceptor=1:2)/PDINO (10 nm)/Al Device 4:
ITO/PEDOT:PSS(30nm)/化合物1:Y6(给受体质量比=1:1)/PDINO(10nm)/AlITO/PEDOT:PSS(30nm)/Compound 1:Y6(mass ratio of donor to acceptor=1:1)/PDINO(10nm)/Al
器件5:Device 5:
ITO/PEDOT:PSS(30nm)/化合物2:Y6(给受体质量比=1:1)/PDINO(10nm)/AlITO/PEDOT: PSS (30 nm)/Compound 2: Y6 (mass ratio of donor to acceptor=1:1)/PDINO (10 nm)/Al
器件6:Device 6:
ITO/PEDOT:PSS(30nm)/化合物3:Y6(给受体质量比=1:1)/PDINO(10nm)/AlITO/PEDOT:PSS(30nm)/Compound 3:Y6(mass ratio of donor to acceptor=1:1)/PDINO(10nm)/Al
(2)相应材料的紫外-可见吸收光谱(2) UV-Vis absorption spectra of corresponding materials
以三氯甲烷为溶剂,将实施例1-3所制备的三种化合物配置浓度为10mg/mL的三种材料溶液。在室温空气条件下将相应溶液以2000rpm的转速旋涂至石英片上制备相应的薄膜样品。薄膜状态下的吸收光谱是通过紫外可见分光光度计UV-1780进行测得,所有的测试均在大气环境中进行完成,检测结果如图2所示。三种材料在薄膜状态下均表现出以250-650nm为范围的较宽的光吸收。Using chloroform as a solvent, the three compounds prepared in Examples 1-3 were prepared with three material solutions with a concentration of 10 mg/mL. The corresponding thin film samples were prepared by spin-coating the corresponding solutions on quartz wafers at 2000 rpm under room temperature air conditions. The absorption spectrum in the thin film state was measured by an ultraviolet-visible spectrophotometer UV-1780. All the tests were done in the atmospheric environment. The test results are shown in Figure 2. All three materials exhibited broad light absorption in the range of 250-650 nm in the thin film state.
(3)有机太阳能电池(器件1-6)的光伏性能(3) Photovoltaic performance of organic solar cells (devices 1-6)
有机太阳能电池器件的电流密度-电压(J-V)曲线的绘制:将带有450W氙灯的Oriel Sol3A级的太阳光模拟器(Enlitech SS-F5-3A)作为光源,然后通过NewportOriel91150V标准硅电池将光强校准到100mW cm-2,最后使用NIKON LV100ND测试系统对器件1-6进行检测测得,如图3所示。其中,从相应的J-V曲线中可以得到器件的开路电压(Voc)、短路电流密度(Jsc)、填充因子(FF)。进而,器件的能量转换效率等于开路电压与短路电流密度以及填充因子三者之间的乘积(PCE=Voc*Jsc*FF),平均能量转换效率(PCEave)为在同一条件下制备的10个以上光伏器件所获得的平均器件效率。具体的器件数据如表1所示:The plotting of the current density-voltage (JV) curve of the organic solar cell device: using an Oriel Sol3A-class solar simulator (Enlitech SS-F5-3A) with a 450W xenon lamp as the light source, and then passing the Newport Oriel91150V standard silicon cell to the light intensity Calibrate to 100mW cm-2 , and finally use the NIKON LV100ND test system to test and measure devices 1-6, as shown in Figure 3. Among them, the open circuit voltage (Voc ), the short-circuit current density (Jsc ), and the fill factor (FF) of the device can be obtained from the corresponding JV curves. Furthermore, the energy conversion efficiency of the device is equal to the product of the open-circuit voltage, the short-circuit current density and the fill factor (PCE=Voc *Jsc *FF), and the average energy conversion efficiency (PCEave ) is obtained under the same conditions Average device efficiency obtained with more than 10 photovoltaic devices. The specific device data is shown in Table 1:
表1Table 1
基于苯并噻唑联噻吩的均三配体型铱配合物作为有机光伏中有效的电子给体材料,其与富勒烯受体(PC71BM)或稠环小分子非富勒烯受体(Y6)均可获得较高的能量转换效率。同样以PC71BM作为受体,对比文献基于乙酰丙酮辅助配体的异配体型铱配合物TBzIr小分子二元器件最高效率仅3.81%,(DOI:10.1039/c9cc00173e),本发明的均三配体型铱配合物给体材料PCE显著提升至6.15%,提升幅度超过60%。尤其基于Y6受体获得了高达9.68%的光电转化效率,是目前基于小分子铱配合物光伏材料的最高光电转化效率。三个同配体型铱配合物的成功合成和应用,表明该类型材料可作为电子给体材料应用于有机光伏领域,这为今后开发新型有机光伏材料提供了新思路和有利指导。Benzothiazole-bithiophene-based mes-triligand iridium complexes as efficient electron-donor materials in organic photovoltaics, which interact with fullerene acceptors (PC71 BM) or fused-ring small-molecule non-fullerene acceptors (Y6 ) can achieve higher energy conversion efficiency. Also using PC71 BM as the receptor, the highest efficiency of the heteroligand iridium complex TBzIr small molecule binary component based on the acetylacetone auxiliary ligand in the comparative literature is only 3.81%, (DOI: 10.1039/c9cc00173e). The PCE of the bulk iridium complex donor material was significantly increased to 6.15%, an increase of more than 60%. Especially based on the Y6 acceptor, the photoelectric conversion efficiency as high as 9.68% is obtained, which is the highest photoelectric conversion efficiency of photovoltaic materials based on small molecule iridium complexes. The successful synthesis and application of three isoligand-type iridium complexes indicate that this type of materials can be used as electron donor materials in the field of organic photovoltaics, which provides new ideas and favorable guidance for the development of new organic photovoltaic materials in the future.
本发明通过引入重金属原子铱将主配体进行环金属化,然后改变主配体的共轭长度和烷基链,设计合成了三个新型的铱配合物给体材料。由于重金属铱原子的引入,诱导了强的自旋轨道耦合,通过有效的系间窜越产生了具有长寿命的三线态激子,有利于增加激子扩散长度。环金属化后,相应的铱配合物表现出一种八面体空间构型,有效抑制了分子聚集,优化了共混薄膜形貌,促进激子的解离和载流子传输,使得短路电流和填充因子得到全面提高,从而实现更好的光伏性能。证明了这种具有八面体空间立体构型的新型同配体铱配合物不仅可作为一种有意义的结构-性质相关的有效电子给体材料,也为以后设计新型的有机光伏材料提供了一种有效途径。The present invention designs and synthesizes three novel iridium complex donor materials by introducing heavy metal atom iridium to cyclometallate the main ligand, and then changing the conjugated length and alkyl chain of the main ligand. Due to the introduction of heavy metal iridium atoms, strong spin-orbit coupling is induced, and triplet excitons with long lifetimes are generated through efficient intersystem crossing, which is beneficial to increase the exciton diffusion length. After cyclometallation, the corresponding iridium complexes exhibit an octahedral spatial configuration, which effectively suppresses molecular aggregation, optimizes the morphology of the blended films, and promotes exciton dissociation and carrier transport, enabling short-circuit current and The fill factor is improved across the board, resulting in better photovoltaic performance. It is demonstrated that this novel isoligand iridium complex with an octahedral steric configuration can not only serve as a meaningful structure-property related efficient electron-donor material, but also provide a promising future for the design of novel organic photovoltaic materials. an effective way.
本发明提供了一类铱配合物有机光伏材料及其制备方法与应用的思路及方法,具体实现该技术方案的方法和途径很多,以上所述仅是本发明的优选实施方式,应当指出,对于本技术领域的普通技术人员来说,在不脱离本发明原理的前提下,还可以做出若干改进和润饰,这些改进和润饰也应视为本发明的保护范围。本实施例中未明确的各组成部分均可用现有技术加以实现。The present invention provides a class of iridium complex organic photovoltaic materials and ideas and methods for their preparation and application. There are many specific methods and approaches for realizing the technical solution. The above are only the preferred embodiments of the present invention. It should be pointed out that for For those of ordinary skill in the art, without departing from the principle of the present invention, several improvements and modifications can also be made, and these improvements and modifications should also be regarded as the protection scope of the present invention. All components not specified in this embodiment can be implemented by existing technologies.
Claims (10)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110517256.XACN113185557B (en) | 2021-05-12 | 2021-05-12 | Iridium complex organic photovoltaic material and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110517256.XACN113185557B (en) | 2021-05-12 | 2021-05-12 | Iridium complex organic photovoltaic material and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113185557A CN113185557A (en) | 2021-07-30 |
| CN113185557Btrue CN113185557B (en) | 2022-08-30 |
Family
ID=76981436
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110517256.XAActiveCN113185557B (en) | 2021-05-12 | 2021-05-12 | Iridium complex organic photovoltaic material and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113185557B (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2007004380A1 (en)* | 2005-07-01 | 2009-01-22 | コニカミノルタホールディングス株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| CN103497219A (en)* | 2013-10-12 | 2014-01-08 | 北京科技大学 | Red-light iridium complexes and application thereof in organic white or red electroluminescence device |
| CN110003234A (en)* | 2019-04-10 | 2019-07-12 | 常州大学 | One kind is based on the miscellaneous condensed ring D (A-Ar) of dithieno benzisoxa virtue2Type conjugated compound and its application |
| CN110372721A (en)* | 2019-08-23 | 2019-10-25 | 杭州师范大学 | It take 3,4- disulphanes base thiophene as the photovoltaic small molecule receptor and its preparation method and application of π bridge |
- 2021
- 2021-05-12CNCN202110517256.XApatent/CN113185557B/enactiveActive
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2007004380A1 (en)* | 2005-07-01 | 2009-01-22 | コニカミノルタホールディングス株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| CN103497219A (en)* | 2013-10-12 | 2014-01-08 | 北京科技大学 | Red-light iridium complexes and application thereof in organic white or red electroluminescence device |
| CN110003234A (en)* | 2019-04-10 | 2019-07-12 | 常州大学 | One kind is based on the miscellaneous condensed ring D (A-Ar) of dithieno benzisoxa virtue2Type conjugated compound and its application |
| CN110372721A (en)* | 2019-08-23 | 2019-10-25 | 杭州师范大学 | It take 3,4- disulphanes base thiophene as the photovoltaic small molecule receptor and its preparation method and application of π bridge |
Non-Patent Citations (1)
| Title |
|---|
| Cyclometalating organic ligand with Iridium center toward dramatically improved photovoltaic performance in organic solar cells;Qingjing Wu等,;《Chem. Commun.》;20190113;参见方案1* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113185557A (en) | 2021-07-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Galvis et al. | Challenges in the design and synthesis of self-assembling molecules as selective contacts in perovskite solar cells | |
| CN108912140B (en) | Asymmetric A-D-A type conjugated small molecule and intermediate and application thereof | |
| Zhou et al. | Recent progress in ternary organic solar cells based on solution-processed non-fullerene acceptors | |
| JP5425338B2 (en) | Copolymer containing anthracene and pearselenol, its production method and its application | |
| CN102574867B (en) | Heterocyclic quinone-type thiophene organic photoelectric material, its preparation method and application | |
| Gu et al. | Recent advances in small molecular design for high performance non-fullerene organic solar cells | |
| JP2015196661A (en) | Organic material and photoelectric conversion element | |
| CN102686592B (en) | Fluorene-containing porphyrin-anthracene copolymer, its preparation method and application | |
| CN115028647A (en) | A kind of fused-ring triazole bis-lactam-based non-fullerene acceptor material and preparation method and application thereof | |
| CN110156616B (en) | Synthesis method of doping-free hole transport material based on fluorene ethylene bridged aromatic ring nucleus and application of doping-free hole transport material in perovskite battery | |
| Eom et al. | n-Type core effect on perylene diimide based acceptors for panchromatic fullerene-free organic solar cells | |
| CN116375732B (en) | A non-fullerene acceptor material and its preparation method and application | |
| JP2011165963A (en) | Organic dye and organic thin-film solar cell | |
| CN117417287A (en) | Carbazole organic compounds and their preparation methods and applications | |
| CN103601757B (en) | Low bandgap ruthenium-containing complexes for solution-processed organic solar cells | |
| CN114133385B (en) | Hole transport material with carbazole as core and thiophene or phenoxazine as end group, and synthesis method and application thereof | |
| Liu et al. | Dopant-free hole-transporting materials based on a simple nonfused core with noncovalent conformational locking for efficient perovskite solar cells | |
| CN110600612A (en) | P-i-n type perovskite battery hole transport layer based on self-assembly engineering | |
| CN106892917B (en) | A kind of fluoro imide derivative and its application | |
| CN113185557B (en) | Iridium complex organic photovoltaic material and preparation method and application thereof | |
| CN107964021B (en) | Perylene bisimide photoelectric micromolecule material containing boron-nitrogen bonds as well as preparation method and application thereof | |
| Lyons et al. | Porphyrin–oligothiophene conjugates as additives for P3HT/PCBM solar cells | |
| CN113234095B (en) | Bitriazine group-containing compound and application thereof as three-dimensional electron acceptor material | |
| CN110028488A (en) | Using indeno [1,2-b] fluorenes as A-D-A type photovoltaic small molecule receptor of core and its preparation method and application | |
| CN114349771B (en) | Hexabenzocoronene-based non-fullerene acceptor material and preparation and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |