CN112322607B - Fusion type nitrile hydratase and application thereof - Google Patents
Fusion type nitrile hydratase and application thereofDownload PDFInfo
- Publication number
- CN112322607B CN112322607BCN202011307749.2ACN202011307749ACN112322607BCN 112322607 BCN112322607 BCN 112322607BCN 202011307749 ACN202011307749 ACN 202011307749ACN 112322607 BCN112322607 BCN 112322607B
- Authority
- CN
- China
- Prior art keywords
- subunit
- nitrile hydratase
- fusion
- pet24a
- nhase
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/88—Lyases (4.)
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/70—Vectors or expression systems specially adapted for E. coli
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P13/00—Preparation of nitrogen-containing organic compounds
- C12P13/02—Amides, e.g. chloramphenicol or polyamides; Imides or polyimides; Urethanes, i.e. compounds comprising N-C=O structural element or polyurethanes
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y402/00—Carbon-oxygen lyases (4.2)
- C12Y402/01—Hydro-lyases (4.2.1)
- C12Y402/01084—Nitrile hydratase (4.2.1.84)
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Medicinal Chemistry (AREA)
- Enzymes And Modification Thereof (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域Technical Field
本发明涉及一种融合型腈水合酶及其应用,属于基因工程领域和酶工程领域。The invention relates to a fusion type nitrile hydratase and application thereof, and belongs to the fields of genetic engineering and enzyme engineering.
背景技术Background Art
腈水合酶(Nitrle hydratase,NHase)能催化腈类化合物合成相应的酰胺类产物,在丙烯酰胺和烟酰胺的工业生产上已经被广泛应用,在工业生产中,NHase主要应用于高纯度烟酰胺和丙烯酰胺的生产,丙烯酰胺的酶法生产是绿色生物催化法代替化学合成法的典型案例,具有诸多优势如:工艺简单、反应条件温和、能耗低、三废少等,因此,Nhase在工业生产中是比较重要的酶。Nitrile hydratase (NHase) can catalyze the synthesis of corresponding amide products from nitrile compounds and has been widely used in the industrial production of acrylamide and nicotinamide. In industrial production, NHase is mainly used in the production of high-purity nicotinamide and acrylamide. The enzymatic production of acrylamide is a typical case of green biocatalysis replacing chemical synthesis. It has many advantages such as simple process, mild reaction conditions, low energy consumption, and less three wastes. Therefore, Nhase is a relatively important enzyme in industrial production.
但是,目前NHase在工业应用过程中普遍存在热稳定性差的问题,例如,来源于Pseudomonas chlororaphils B23、Rhodococcus rhodochrousJ1、Rhodococcus sp.N-774的NHase仅在20℃以下稳定,而腈类的水合过程属于放热反应,所以在工业催化过程中,需要用冷凝水来降低反应温度从而造成能源浪费;此外,腈水合酶的不稳定性还体现在对底物(腈类有机物)和产物(酰胺类有机物)的耐受性差,容易使腈水合酶失活;例如在丙烯酰胺的酶法制备过程中,由于腈水合酶对底物和产物的耐受性不高,导致终产物丙烯酰胺只能维持较低的水平,给后续的丙烯酰胺浓缩工艺带来影响。随着市场的不断拓展,酰胺类化合物的需求也日益剧增,因此如何提高腈水合酶的热稳定性及产物耐受性已成为研究重点。However, NHase generally has poor thermal stability in industrial applications. For example, NHase from Pseudomonas chlororaphils B23, Rhodococcus rhodochrous J1, and Rhodococcus sp. N-774 is only stable below 20°C, and the hydration process of nitriles is an exothermic reaction, so in the industrial catalytic process, condensed water is needed to reduce the reaction temperature, resulting in energy waste; in addition, the instability of nitrile hydratase is also reflected in its poor tolerance to substrates (nitrile organic matter) and products (amide organic matter), which easily inactivates nitrile hydratase; for example, in the enzymatic preparation of acrylamide, due to the low tolerance of nitrile hydratase to substrates and products, the final product acrylamide can only maintain a low level, which affects the subsequent acrylamide concentration process. With the continuous expansion of the market, the demand for amide compounds is also increasing rapidly, so how to improve the thermal stability and product tolerance of nitrile hydratase has become a research focus.
为了解决NHase热稳定性差的问题,许多课题组采取了各种各样的策略,包括催化工艺的优化、野生菌株的筛选和驯化,以及NHase的分子改造等等;发明人课题组在前期研究过程中,通过分子手段在基因水平上以共价键的方式融合两个亚基,消除了亚基解聚的可能性,虽然使得融合型腈水合酶的稳定性提高,但是融合型腈水合酶在50℃半衰期最高仅仅是26min,仍旧不能满足工业需求。In order to solve the problem of poor thermal stability of NHase, many research groups have adopted various strategies, including optimization of catalytic processes, screening and domestication of wild strains, and molecular modification of NHase. In the early research process, the inventor's research group used molecular means to covalently fuse two subunits at the genetic level, eliminating the possibility of subunit depolymerization. Although the stability of the fusion nitrile hydratase was improved, the half-life of the fusion nitrile hydratase at 50°C was only 26 minutes at most, which still could not meet industrial needs.
发明内容Summary of the invention
针对腈水合酶工业应用中对底物和产物的耐受性差、热稳定差的问题,本发明采用基因合策略,将Pseudomonas putida NRRL 1886来源的NHase的β和α两个亚基通过螺旋式,或柔性,或刚性的连接肽融合到一起,得到一种稳定性有很大提高的亚基融合型NHase,有望应用于工业生产。In view of the problems of poor tolerance to substrates and products and poor thermal stability of nitrile hydratase in industrial applications, the present invention adopts a gene synthesis strategy to fuse the β and α subunits of NHase from Pseudomonas putida NRRL 1886 through a spiral, flexible or rigid connecting peptide to obtain a subunit fusion NHase with greatly improved stability, which is expected to be applied to industrial production.
本发明首先提供了一种融合型腈水合酶,所述融合型腈水合酶同时表达了α亚基、β亚基及调控亚基,所述融合型腈水合酶的β亚基和α亚基之间通过Linker融合;所述Linker的通式为:A(EAAAK)nA,或(GGSG)n,或(PA)n,其中,n是至少为1的整数。The present invention first provides a fusion type nitrile hydratase, wherein the fusion type nitrile hydratase simultaneously expresses an α subunit, a β subunit and a regulatory subunit, and the β subunit and the α subunit of the fusion type nitrile hydratase are fused via a Linker; the general formula of the Linker is: A(EAAAK)n A, or (GGSG)n , or (PA)n , wherein n is an integer of at least 1.
在本发明的一种实施方式中,所述融合型腈水合酶的β亚基和α亚基之间通过Linker融合,所述融合是将β亚基的C端和α亚基的N端连接起来。In one embodiment of the present invention, the β subunit and the α subunit of the fusion-type nitrile hydratase are fused via a Linker, and the fusion is to connect the C-terminus of the β subunit and the N-terminus of the α subunit.
在本发明的一种实施方式中,所述Linker的通式中的n值为:1≤n≤15。In one embodiment of the present invention, the value of n in the general formula of the Linker is: 1≤n≤15.
在本发明的一种实施方式中,所述Linker的核苷酸序列为选自SEQ ID NO.5-22所示的序列。In one embodiment of the present invention, the nucleotide sequence of the Linker is selected from the sequences shown in SEQ ID NO.5-22.
在本发明的一种实施方式中,所述β亚基的氨基酸序列如SEQ ID NO.2所示,α亚基的氨基酸序列如SEQ ID NO.3所示,调控亚基的氨基酸序列如SEQ ID NO.4所示。In one embodiment of the present invention, the amino acid sequence of the β subunit is shown as SEQ ID NO.2, the amino acid sequence of the α subunit is shown as SEQ ID NO.3, and the amino acid sequence of the regulatory subunit is shown as SEQ ID NO.4.
在本发明的一种实施方式中,所述β亚基的核苷酸序列如SEQ ID NO.23所示,α亚基的核苷酸序列如SEQ ID NO.24所示,调控亚基的核苷酸序列如SEQ ID NO.25所示。In one embodiment of the present invention, the nucleotide sequence of the β subunit is shown as SEQ ID NO.23, the nucleotide sequence of the α subunit is shown as SEQ ID NO.24, and the nucleotide sequence of the regulatory subunit is shown as SEQ ID NO.25.
本发明还提供了编码上述融合型腈水合酶的基因。The present invention also provides a gene encoding the fusion type nitrile hydratase.
本发明还提供了携带上述融合型腈水合酶基因的重组载体。The present invention also provides a recombinant vector carrying the fusion type nitrile hydratase gene.
在本发明的一种实施方式中,所述重组载体以pET-24a质粒作为表达载体。In one embodiment of the present invention, the recombinant vector uses pET-24a plasmid as an expression vector.
本发明还提供了表达上述融合型腈水合酶的重组细胞。The present invention also provides a recombinant cell expressing the fusion nitrile hydratase.
在本发明的一种实施方式中,所述重组细胞以大肠杆菌为宿主细胞。In one embodiment of the present invention, the recombinant cell uses Escherichia coli as a host cell.
本发明还提供了上述融合型腈水合酶的制备方法,所述方法为:将上述重组细胞接种至LB培养基中,35~37℃培养至OD600为0.6-0.8时,加入诱导剂IPTG于25℃诱导12-18h,表达融合型腈水合酶。The present invention also provides a method for preparing the fusion type nitrile hydratase, which comprises: inoculating the recombinant cells into LB medium, culturing at 35-37°C untilOD600 is 0.6-0.8, adding inducer IPTG and inducing at 25°C for 12-18h to express the fusion type nitrile hydratase.
在本发明的一种实施方式中,所述方法是将所述重组细胞接种于含卡那霉素的LB表达培养基中,37℃、200r/min振荡培养至OD600为0.6-0.8时,加入诱导剂IPTG至0.1mM,Co2+至0.1mg/L,25℃诱导12-18h,表达融合型腈水合酶。In one embodiment of the present invention, the method is to inoculate the recombinant cells into an LB expression medium containing kanamycin, culture at 37°C and 200r/min with shaking untilOD600 is 0.6-0.8, add the inducer IPTG to 0.1mM, Co2+ to 0.1mg/L, induce at 25°C for 12-18h, and express the fusion nitrile hydratase.
在本发明的一种实施方式中,所述方法还包括收集上述重组细胞的菌体,将菌体破碎后收集上清,将上清膜过滤后,用Strep Trap HP柱分离得到融合型腈水合酶。In one embodiment of the present invention, the method further comprises collecting the bacterial cells of the recombinant cells, crushing the bacterial cells and collecting the supernatant, filtering the supernatant with a membrane, and separating the fusion nitrile hydratase using a Strep Trap HP column.
本发明还提供了一种提高腈水合酶热稳定性和/或耐受性的方法,所述方法为:融合了腈水合酶的β亚基和α亚基;所述融合型腈水合酶的β亚基和α亚基之间通过Linker连接;所述Linker的通式为:A(EAAAK)nA,或(GGSG)n,或(PA)n,其中,n是至少为1的整数。The present invention also provides a method for improving the thermal stability and/or tolerance of nitrile hydratase, the method comprising: fusing the β subunit and α subunit of nitrile hydratase; the β subunit and α subunit of the fusion nitrile hydratase are connected by a Linker; the general formula of the Linker is: A(EAAAK)n A, or (GGSG)n , or (PA)n , wherein n is an integer of at least 1.
在本发明的一种实施方式中,所述Linker的通式中的n值为:1≤n≤15。In one embodiment of the present invention, the value of n in the general formula of the Linker is: 1≤n≤15.
在本发明的一种实施方式中,所述Linker的核苷酸序列如SEQ ID NO.5-22所示。In one embodiment of the present invention, the nucleotide sequence of the Linker is shown as SEQ ID NO.5-22.
在本发明的一种实施方式中,所述β亚基的氨基酸序列如SEQ ID NO.2所示,α亚基的氨基酸序列如SEQ ID NO.3所示,调控亚基的氨基酸序列如SEQ ID NO.4所示。In one embodiment of the present invention, the amino acid sequence of the β subunit is shown as SEQ ID NO.2, the amino acid sequence of the α subunit is shown as SEQ ID NO.3, and the amino acid sequence of the regulatory subunit is shown as SEQ ID NO.4.
本发明还提供了一种生产酰胺类物质的方法,将所述的融合型腈水合酶添加至含有腈类有机物的反应体系中进行反应。The present invention also provides a method for producing amide substances, wherein the fusion nitrile hydratase is added into a reaction system containing nitrile organic matter to carry out reaction.
在本发明的一种实施方式中,向终浓度为200-400mmol/L的烟腈溶液中添加至少0.4mg/mL的融合型腈水合酶,得到反应体系,将反应体系置于20-28℃反应8-15min。In one embodiment of the present invention, at least 0.4 mg/mL of fusion nitrile hydratase is added to a nicotinonitrile solution with a final concentration of 200-400 mmol/L to obtain a reaction system, and the reaction system is placed at 20-28° C. for 8-15 min.
本发明还提供了上述融合型腈水合酶,或上述基因,或上述重组载体,或上述重组细胞在催化腈类化合物水合成相应的酰胺类产物中的应用。The present invention also provides the use of the fusion nitrile hydratase, or the gene, or the recombinant vector, or the recombinant cell in catalyzing the hydration of nitrile compounds into corresponding amide products.
有益效果Beneficial Effects
(1)采用本发明的融合型腈水合酶,相对于野生酶,其热稳定性得到了显著的提高,其中,融合型腈水合酶A8的半衰期高达139min,是野生酶的7.7倍;融合型腈水合酶B8的半衰期高达94min,是野生酶的5.2倍;融合型腈水合酶C8的半衰期高达53min,是野生酶的近3倍。本发明提供的热稳定性提高的融合型腈水合酶,可以克服工业生产中腈水合酶热稳定性低的缺陷,提高产率,节约成本。(1) The fusion nitrile hydratase of the present invention has a significantly improved thermal stability relative to the wild enzyme, wherein the half-life of the fusion nitrile hydratase A8 is as high as 139 min, which is 7.7 times that of the wild enzyme; the half-life of the fusion nitrile hydratase B8 is as high as 94 min, which is 5.2 times that of the wild enzyme; the half-life of the fusion nitrile hydratase C8 is as high as 53 min, which is nearly 3 times that of the wild enzyme. The fusion nitrile hydratase with improved thermal stability provided by the present invention can overcome the defect of low thermal stability of nitrile hydratase in industrial production, improve yield, and save costs.
(2)采用本发明提供的技术方案,底物耐受性及产物耐受性均得到了显著的提高,其中,采用底物3-氰基吡啶进行反应时,融合型腈水合酶A8、B8、C8的相对酶活由采用野生酶进行反应时的较低的相对酶活(30%)提高到84%、89%、86%;采用产物尼克酰胺下处理30min后,融合型腈水合酶A8、B8、C8的相对酶活由采用野生酶进行反应时的较低的相对酶活(77%)提高到80%、94%、86%。(2) By adopting the technical scheme provided by the present invention, the substrate tolerance and product tolerance are significantly improved. Among them, when the substrate 3-cyanopyridine is used for reaction, the relative enzyme activity of the fusion type nitrile hydratase A8, B8, and C8 is increased from the lower relative enzyme activity (30%) when the wild enzyme is used for reaction to 84%, 89%, and 86%; after being treated with the product nicotinamide for 30 minutes, the relative enzyme activity of the fusion type nitrile hydratase A8, B8, and C8 is increased from the lower relative enzyme activity (77%) when the wild enzyme is used for reaction to 80%, 94%, and 86%.
附图说明BRIEF DESCRIPTION OF THE DRAWINGS
图1:融合型腈水合酶野生酶及A1、A2、A3、A4、A6、A8的SDS-PAGE图。Figure 1: SDS-PAGE of wild-type fusion nitrile hydratase and A1, A2, A3, A4, A6, and A8.
图2:融合型腈水合酶野生酶及B1、B2、B3、B4、B6、B8的SDS-PAGE图。Figure 2: SDS-PAGE of wild-type fusion nitrile hydratase and B1, B2, B3, B4, B6, and B8.
图3:融合型腈水合酶野生酶及C1、C2、C3、C4、C6、C8的SDS-PAGE图。Figure 3: SDS-PAGE of wild-type fusion nitrile hydratase and C1, C2, C3, C4, C6, and C8.
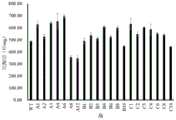
图4:野生型腈水合酶和融合型腈水合酶的比酶活。Figure 4: Specific enzyme activities of wild-type nitrile hydratase and fusion nitrile hydratase.
图5:野生型腈水合酶和融合型腈水合酶的底物耐受性。FIG. 5 : Substrate tolerance of wild-type nitrile hydratase and fusion nitrile hydratase.
图6:野生型腈水合酶和融合型腈水合酶的产物耐受性。Figure 6: Product tolerance of wild-type nitrile hydratase and fusion nitrile hydratase.
具体实施方式DETAILED DESCRIPTION
下述实施例中的酶A1-酶A16分别定义为:通过螺旋式Linker连接β亚基和α亚基的融合型腈水合酶,Linker的通式为A(EAAAK)nA;其中,A为丙氨酸,E为谷氨酸,K为赖氨酸;n=1-12,且n为整数。Enzymes A1-A16 in the following examples are respectively defined as: fusion-type nitrile hydratase in which β subunit and α subunit are connected by a spiral linker, and the general formula of the linker is A(EAAAK)n A; wherein A is alanine, E is glutamic acid, and K is lysine; n=1-12, and n is an integer.
下述实施例中的酶B1-酶B16分别定义为:通过柔性Linker连接β亚基和α亚基的融合型腈水合酶,Linker的通式为(GGSG)n;其中,G为甘氨酸,S为丝氨酸;n=1-16,且n为整数。Enzymes B1 to B16 in the following examples are respectively defined as: fusion-type nitrile hydratase in which β subunit and α subunit are connected by a flexible linker, and the general formula of the linker is (GGSG)n ; wherein G is glycine, S is serine, and n=1-16, and n is an integer.
下述实施例中的酶C1-酶C16分别定义为:通过刚性Linker连接β亚基和α亚基的融合型腈水合酶,Linker的通式为(PA)n;其中,P为脯氨酸,A为丙氨酸;n=1-16,且n为整数。Enzymes C1 to C16 in the following examples are respectively defined as: fusion-type nitrile hydratase in which β subunit and α subunit are connected by a rigid linker, and the general formula of the linker is (PA)n ; wherein P is proline, A is alanine, and n=1-16, and n is an integer.
下述实施例中所涉及的Linker汇总如表1所示。The linkers involved in the following embodiments are summarized in Table 1.
表1:融合型腈水合酶β亚基和α亚基的Linker汇总Table 1: Summary of linkers for fusion nitrile hydratase β and α subunits
下述实施例中所涉及的培养基如下:The culture medium involved in the following examples is as follows:
LB液体培养基:蛋白胨10g/L,酵母浸膏5g/L,NaCl 10g/L。LB liquid medium: peptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L.
2YT培养基:蛋白胨16g/L,酵母浸膏10g/L,NaCl 5g/L。2YT medium: peptone 16 g/L, yeast extract 10 g/L, NaCl 5 g/L.
下述实施例中涉及的检测方法如下:The detection methods involved in the following embodiments are as follows:
腈水合酶酶活检测:Nitrile hydratase activity assay:
在1.5mL的EP管中进行反应。取10μL浓度调到0.5mg/mL的腈水合酶,加入到490μL含有200mM烟腈及10mM磷酸缓冲溶液的混合液中,得到反应体系;将反应体系放置在25℃反应10min,加入500μL的乙腈终止反应。将反应产物过0.22μm的膜后,C18柱经HPLC测其酶活,流动相是为乙腈和水(水:乙腈=2:1)的混合液,检测波长为220nm,检测温度为40℃,流动相流速为0.6mL/min,进样量10μL,检测时间10min,每组实验数据至少重复3次。The reaction was carried out in a 1.5mL EP tube. 10μL of nitrile hydratase adjusted to a concentration of 0.5mg/mL was taken and added to a mixture of 490μL containing 200mM nicotinonitrile and 10mM phosphate buffer solution to obtain a reaction system; the reaction system was placed at 25℃ for 10min, and 500μL of acetonitrile was added to terminate the reaction. After the reaction product was passed through a 0.22μm membrane, the enzyme activity was measured by HPLC on a C18 column. The mobile phase was a mixture of acetonitrile and water (water: acetonitrile = 2:1), the detection wavelength was 220nm, the detection temperature was 40℃, the mobile phase flow rate was 0.6mL/min, the injection volume was 10μL, the detection time was 10min, and each set of experimental data was repeated at least 3 times.
酶活的定义(U):每分钟转化3-氰基吡啶(烟腈)生成1μmol/L尼克酰胺所需的酶量定义为1U。Definition of enzyme activity (U): The amount of enzyme required to convert 3-cyanopyridine (nicotinonitrile) to 1 μmol/L nicotinamide per minute is defined as 1 U.
比酶活(U/mg):每毫克NHase的酶活。Specific enzyme activity (U/mg): enzyme activity per mg of NHase.
相对酶活的定义:突变酶在pH=7.4,温度为30℃反应10分钟测得的酶活定义为100%。Definition of relative enzyme activity: The enzyme activity of the mutant enzyme measured at pH = 7.4 and temperature 30°C for 10 minutes is defined as 100%.
腈水合酶反应体系:向490μL 200mM的底物3-氰基吡啶溶液中,加入浓度为0.5mg/mL的纯酶溶液10μL,在25℃的温度下反应10min后,用500μL乙腈终止反应,过0.22μm的膜后作为液相测定的样品。Nitrile hydratase reaction system: add 10 μL of pure enzyme solution with a concentration of 0.5 mg/mL to 490 μL of 200 mM substrate 3-cyanopyridine solution, react at 25°C for 10 min, terminate the reaction with 500 μL of acetonitrile, and pass through a 0.22 μm membrane as a sample for liquid phase determination.
腈水合酶含量的检测:Detection of nitrile hydratase content:
用HPLC检测,流动相为水:乙腈=1:2;检测波长210nm,流速为0.6ml/min;色谱柱为C18柱。HPLC was used for detection, with the mobile phase being water:acetonitrile=1:2; the detection wavelength being 210 nm, the flow rate being 0.6 ml/min; and the chromatographic column being a C18 column.
烟腈消耗量的检测:Detection of nicotine nitrile consumption:
向含有250μL浓度为500mmol/L的烟酰胺溶液中添加10μL终浓度为0.5mg/mL纯酶溶液,得到反应体系;将反应体系置于25℃下孵育30min后,向反应体系中添加250μL浓度为40mM的烟腈溶液,在25℃条件下反应10min后,加入500μL的乙腈终止反应,得到反应产物;将反应产物过0.22μm的膜后,作为液相测定的样品测定烟腈剩余量;其中,烟腈的初始浓度为20mM,烟腈的初始浓度减去烟腈剩余量即为烟腈消耗量。To 250 μL of a 500 mmol/L nicotinamide solution, 10 μL of a pure enzyme solution with a final concentration of 0.5 mg/mL was added to obtain a reaction system; after incubating the reaction system at 25°C for 30 min, 250 μL of a 40 mM nicotinamide solution was added to the reaction system, and after reacting at 25°C for 10 min, 500 μL of acetonitrile was added to terminate the reaction to obtain a reaction product; after the reaction product was passed through a 0.22 μm membrane, the remaining amount of nicotinamide was determined as a sample for liquid phase determination; wherein, the initial concentration of nicotinamide was 20 mM, and the initial concentration of nicotinamide minus the remaining amount of nicotinamide was the nicotinamide consumption.
实施例1:融合型腈水合酶的制备Example 1: Preparation of fusion nitrile hydratase
(1)融合型腈水合酶的构建:(1) Construction of fusion nitrile hydratase:
化学合成核苷酸序列如SEQ ID NO.1所示的野生酶NHase基因WT,并将该基因克隆于pET24a质粒的NdeⅠ和EcoRⅠ酶切位点处,由苏州金唯智完成,获得pET24a-Nhase-WT重组质粒。以pET24a-Nhase-WT为模版,用表2所示引物,在表3所示条件下进行PCR反应,得到PCR产物,将PCR产物转化E.coli JM109感受态细胞后苏州金唯智测序,测序结果正确的质粒即为携带编码融合型腈水合酶基因的重组质粒:pET24a-NHase-A1、pET24a-NHase-A2、pET24a-NHase-A3、pET24a-NHase-A4、pET24a-NHase-A6、pET24a-NHase-A8、pET24a-NHase-A12、pET24a-NHase-B1、pET24a-NHase-B2、pET24a-NHase-B3、pET24a-NHase-B4、pET24a-NHase-B6、pET24a-NHase-B8、pET24a-NHase-B16、pET24a-NHase-C1、pET24a-NHase-C2、pET24a-NHase-C3、pET24a-NHase-C4、pET24a-NHase-C6、pET24a-NHase-C8、pET24a-NHase-C16;将上述携带编码融合型腈水合酶基因的重组质粒和上述携带野生酶的重组质粒pET24a-Nhase-WT转化E.coli BL21菌株进行表达,分别获得重组菌株BL21/pET24a-Nhase-WT、BL21/pET24a-NHase-A1、BL21/pET24a-pET24a-NHase-A2、BL21/pET24a-pET24a-NHase-A3、BL21/pET24a-pET24a-NHase-A4 、 BL21/pET24a-pET24a-NHase-A6 、BL21/pET24a-pET24a-NHase-A8 、 BL21/pET24a-pET24a-NHase-A12 、BL21/pET24a-pET24a-NHase-B1 、BL21/pET24a-pET24a-NHase-B2 、BL21/pET24a-pET24a-NHase-B3 、 BL21/pET24a-pET24a-NHase-B4 、BL21/pET24a-pET24a-NHase-B6 、 BL21/pET24a-pET24a-NHase-B8 、BL21/pET24a-pET24a-NHase-B16 、 BL21/pET24a-pET24a-NHase-C1 、BL21/pET24a-pET24a-NHase-C2 、 BL21/pET24a-pET24a-NHase-C3 、BL21/pET24a-pET24a-NHase-C4 、BL21/pET24a-pET24a-NHase-C6 、BL21/pET24a-pET24a-NHase-C8、BL21/pET24a-pET24a-NHase-C16。The wild enzyme NHase gene WT with a nucleotide sequence as shown in SEQ ID NO.1 was chemically synthesized and cloned into the NdeⅠ and EcoRⅠ restriction sites of the pET24a plasmid by Suzhou Jinweizhi to obtain the pET24a-Nhase-WT recombinant plasmid. Using pET24a-Nhase-WT as a template, the primers shown in Table 2 were used to perform PCR reaction under the conditions shown in Table 3 to obtain the PCR product, which was then transformed into E. coli JM109 competent cells were then sequenced by Suzhou Jinweizhi. The plasmids with correct sequencing results were the recombinant plasmids carrying the gene encoding the fusion nitrile hydratase: pET24a-NHase-A1, pET24a-NHase-A2, pET24a-NHase-A3, pET24a-NHase-A4, pET24a-NHase-A6, pET24a-NHase-A8, pET24a-NHase-A12, pET24a-NHase-B1, pET24a-NHase-B2, pET24a-NHase-B3, pET24a-NHase -B4, pET24a-NHase-B6, pET24a-NHase-B8, pET24a-NHase-B16, pET24a-NHase-C1, pET24a-NHase-C2, pET24a-NHase-C3, pET24a-NHase-C4, pET24a-NHase-C6, pET24a-NHase-C8, pET24a-NHase-C16; the above recombinant plasmids carrying the gene encoding the fusion nitrile hydratase and the above recombinant plasmid pET24a-NHase-WT carrying the wild enzyme were transformed into E. coli The BL21 strain was used for expression to obtain the recombinant strains BL21/pET24a-Nhase-WT, BL21/pET24a-NHase-A1, BL21/pET24a-pET24a-NHase-A2, BL21/pET24a-pET24a-NHase-A3, BL21/pET24a-pET24a-NHase-A4, BL21/pET24a-pET24a-NHase-A6, BL21/pET24a-pET24a-NHase-A8, BL21/pET24a-pET24a-NHase-A12, BL21/pET24a-pET24a-NHase-B1, BL21/pET24a-pET24a-NHase-B2. , BL21/pET24a-pET24a-NHase-B3, BL21/pET24a-pET24a-NHase-B4, BL21/pET24a-pET24a-NHase-B6, BL21/pET24a-pET24a-NHase-B8, BL21/pET24a-pET24a-NHase-B16, 1/pET24a-pET24a-NHase-C1, BL21/pET24a-pET24a-NHase-C2, BL21/pET24a-pET24a-NHase-C3, BL21/pET24a-pET24a-NHase-C4, BL21/pET24a-pET24a-NHase-C6 , BL21/pET24a-pET24a-NHase-C8, BL21/pET24a-pET24a-NHase-C16.
表2引物Table 2 Primers
表3全质粒PCR扩增反应体系Table 3 Whole plasmid PCR amplification reaction system
PCR扩增反应条件为:95℃预变性5min;95℃ 变性 1min,58℃退火30s,72 ℃ 延伸2min;(30个循环);72 ℃ 延伸10min。PCR产物用琼脂糖凝胶电泳方法鉴定。然后将PCR产物纯化、消化后转入大肠杆菌BL21感受态细胞。The PCR amplification reaction conditions were: 95℃ pre-denaturation for 5min; 95℃ denaturation for 1min, 58℃ annealing for 30s, 72℃ extension for 2min; (30 cycles); 72℃ extension for 10min. The PCR product was identified by agarose gel electrophoresis. The PCR product was then purified, digested, and transferred into E. coli BL21 competent cells.
(2)将步骤(1)获得的重组大肠杆菌分别接种于含卡那霉素浓度为50μg/mL的5mLLB液体培养基中,在37℃、200r/min条件下振荡过夜培养,得到各种子液。(2) The recombinant E. coli obtained in step (1) was inoculated into 5 mL of LB liquid culture medium containing kanamycin at a concentration of 50 μg/mL, and cultured overnight at 37° C. and 200 rpm to obtain various sub-liquids.
分别将上述种子液按1%(v/v)的接种量接种于含卡那霉素浓度为50μg/mL的100mL LB液体培养基中,在37℃、200r/min条件下振荡培养至OD600至0.6-0.8时,添加终浓度为0.1mM的诱导剂IPTG及终浓度为0.1mg/L的Co2+溶液,在25℃条件下诱导12-18h,得到发酵液,将得到的发酵液在12000rpm的转速下离心,弃上清分别得到重组大肠杆菌菌体。The above seed solution was inoculated at an inoculum rate of 1% (v/v) into 100 mL LB liquid culture medium containing kanamycin at a concentration of 50 μg/mL, and cultured at 37°C and 200 r/min with shaking until OD600 reached 0.6-0.8, and then an inducer IPTG with a final concentration of 0.1 mM and a Co2+ solution with a final concentration of 0.1 mg/L were added, and induced at 25°C for 12-18 h to obtain a fermentation broth, which was centrifuged at a speed of 12000 rpm, and the supernatant was discarded to obtain recombinant Escherichia coli cells.
(3)分别将步骤(2)得到重组大肠杆菌菌体用结合缓冲溶液(20mmol/L Na2HPO4、280mmol/L NaCl、6mmol/L KCl)浓缩5倍,超声破碎,得到细胞破碎液,将细胞破碎液在12000rpm条件下离心40min,将得到的上清液用0.22μm滤膜过滤,分别得到粗酶液。(3) The recombinant E. coli cells obtained in step (2) were concentrated 5 times with a binding buffer solution (20 mmol/L Na2 HPO4 , 280 mmol/L NaCl, 6 mmol/L KCl), and ultrasonically disrupted to obtain a cell disruption solution. The cell disruption solution was centrifuged at 12000 rpm for 40 min, and the obtained supernatant was filtered with a 0.22 μm filter membrane to obtain a crude enzyme solution.
(4)分别将步骤(3)得到的粗酶液纯化,步骤如下:用10倍柱体积的结合缓冲溶液平衡1mL的strep Trap HP柱,用15倍柱体积的结合缓冲溶液洗去非特异性吸附的蛋白,用8倍柱体积的20mM Na2HPO4,280mM NaCl,6mM KCl,2.5mM脱硫生物素缓冲液洗脱蛋白,分别收集样品,即为纯化后的野生型的腈水合酶WT、融合型腈水合酶A1、A2、A3、A4、A6、A8、A12、B1、B2、B3、B4、B6、B8、B16、C1、C2、C3、C4、C6、C8、C16,并用SDS-PAGE分析鉴定(其中,纯化后的野生型的腈水合酶WT、融合型腈水合酶A1、A2、A3、A4、A6、A8、B1、B2、B3、B4、B6、B8、C1、C2、C3、C4、C6、C8的SDS-PAGE如图1-图3所示),结果为:在49.2KDa处均有蛋白条带,证明腈水合酶蛋白得到表达。(4) Purify the crude enzyme solution obtained in step (3) by equilibrating 1 mL of strep Trap HP column with 10 column volumes of binding buffer solution, washing away nonspecifically adsorbed proteins with 15 column volumes of binding buffer solution, and washing with 8 column volumes of 20 mM Na2 HPO4 , 280 mM NaCl, 6 mM KCl, 2.5mM desulfurization biotin buffer was used to elute the protein, and samples were collected respectively, namely the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A1, A2, A3, A4, A6, A8, A12, B1, B2, B3, B4, B6, B8, B16, C1, C2, C3, C4, C6, C8, C16, and analyzed and identified by SDS-PAGE (wherein, the SDS-PAGE of the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A1, A2, A3, A4, A6, A8, B1, B2, B3, B4, B6, B8, C1, C2, C3, C4, C6, C8 is shown in Figures 1-3), and the results are: there are protein bands at 49.2KDa, indicating that the nitrile hydratase protein is expressed.
实施例2:融合型腈水合酶酶活的检测Example 2: Detection of fusion nitrile hydratase activity
分别测定实施例1得到的纯化后的野生型的腈水合酶WT、融合型腈水合酶A1、A2、A3、A4、A6、A8、A12、B1、B2、B3、B4、B6、B8、B16、C1、C2、C3、C4、C6、C8、C16的酶活,结果如表4所示;The enzyme activities of the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A1, A2, A3, A4, A6, A8, A12, B1, B2, B3, B4, B6, B8, B16, C1, C2, C3, C4, C6, C8, and C16 obtained in Example 1 were measured respectively, and the results are shown in Table 4;
表4:不同腈水合酶的酶活Table 4: Enzyme activities of different nitrile hydratases
如表4和图4所示,融合型腈水合酶A6的酶活为691.98,与野生酶相比,酶活提高了42%,融合型腈水合酶B4的酶活为607.42,与野生酶相比,酶活提高了24%,融合型腈水合酶C1的酶活为630.57,与野生酶相比,酶活提高了29%。As shown in Table 4 and Figure 4, the enzyme activity of fusion nitrile hydratase A6 was 691.98, which was 42% higher than that of wild enzyme; the enzyme activity of fusion nitrile hydratase B4 was 607.42, which was 24% higher than that of wild enzyme; and the enzyme activity of fusion nitrile hydratase C1 was 630.57, which was 29% higher than that of wild enzyme.
此外,由表4中可知,融合型腈水合酶A8和A12的酶活要低于野生酶,融合型腈水合酶B16及C16均低于野生酶;可见,Linker长度在一定范围内不同程度的提高腈水合酶酶活性,但是Linker过长则会引起酶活下降。In addition, it can be seen from Table 4 that the enzymatic activities of fusion nitrile hydratases A8 and A12 are lower than those of wild enzymes, and the enzymatic activities of fusion nitrile hydratases B16 and C16 are lower than those of wild enzymes. It can be seen that the Linker length improves the enzymatic activity of nitrile hydratase to varying degrees within a certain range, but excessive Linker length will cause a decrease in enzyme activity.
实施例3:融合型腈水合酶热稳定性的检测Example 3: Detection of thermal stability of fusion nitrile hydratase
本发明的融合型的腈水合酶的热稳定性均得到了提高,以融合型腈水合酶A1、A4、A8、B1、B4、B8、C1、C4、C8为例,测定其热稳定性,步骤如下:The thermal stability of the fusion nitrile hydratase of the present invention is improved. Taking the fusion nitrile hydratase A1, A4, A8, B1, B4, B8, C1, C4, and C8 as examples, the thermal stability is determined as follows:
取10μL终浓度为0.5mg/mL的实施例1纯化后的野生型的腈水合酶WT、融合型腈水合酶A1、A4、A8、B1、B4、B8、C1、C4、C8,于50℃金属浴中分别处理15、30、45、60、75、90min,后测定各腈水合酶的相对酶活,定义各酶在不做金属浴处理时的酶活为100%,计算出半衰期t1/2(min),结果如表5所示,分析其热稳定性情况。10 μL of the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A1, A4, A8, B1, B4, B8, C1, C4, C8 in Example 1 with a final concentration of 0.5 mg/mL were treated in a 50°C metal bath for 15, 30, 45, 60, 75, 90 min, respectively, and then the relative enzyme activity of each nitrile hydratase was determined, and the enzyme activity of each enzyme without metal bath treatment was defined as 100%, and the half-life t1/2 (min) was calculated. The results are shown in Table 5, and the thermal stability thereof was analyzed.
表5不同腈水合酶的半衰期Table 5 Half-life of different nitrile hydratases
由表5可知,酶A8的半衰期为139min,是野生酶WT的半衰期的7.7倍,酶B8的半衰期为94min,是野生酶WT的半衰期的7.7倍,酶C8的半衰期为53min,是野生酶WT的半衰期的2.9倍。可见不同种类Linker都是Linker越长腈水合酶热稳定性越好,螺旋式Linker提高稳定性效果更优。As shown in Table 5, the half-life of enzyme A8 is 139 min, which is 7.7 times that of wild enzyme WT, the half-life of enzyme B8 is 94 min, which is 7.7 times that of wild enzyme WT, and the half-life of enzyme C8 is 53 min, which is 2.9 times that of wild enzyme WT. It can be seen that the longer the linker is, the better the thermal stability of nitrile hydratase is, and the spiral linker has a better effect in improving stability.
实施例4:融合型腈水合酶底物耐受性的检测Example 4: Detection of substrate tolerance of fusion nitrile hydratase
本发明提供的融合型的腈水合酶的底物耐受性得到了提高,以融合型腈水合酶A8、B8、C8为例进行说明,具体步骤如下:The substrate tolerance of the fusion nitrile hydratase provided by the present invention is improved, and the fusion nitrile hydratase A8, B8, and C8 are used as examples for illustration, and the specific steps are as follows:
(1)向490μL含有200mmol/L烟腈溶液中分别添加10μL终浓度为0.5mg/mL的实施例1得到的纯化后的野生型的腈水合酶WT、融合型腈水合酶A8、B8、C8得到I型发应体系;(1) adding 10 μL of the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A8, B8, and C8 obtained in Example 1 at a final concentration of 0.5 mg/mL to 490 μL of a solution containing 200 mmol/L nicotinonitrile to obtain a type I reaction system;
(2)向490μL含有500mmol/L烟腈溶液中分别添加10μL终浓度为0.5mg/mL的实施例1得到的纯化后的野生型的腈水合酶WT、融合型腈水合酶A8、B8、C8得到II型发应体系;(2) adding 10 μL of the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A8, B8, and C8 obtained in Example 1 at a final concentration of 0.5 mg/mL to 490 μL of a 500 mmol/L nicotinonitrile solution to obtain a type II reaction system;
(3)将步骤(1)和(2)得到的各个反应体系分别置于25℃反应10min后,分别加入500μL的乙腈终止反应,通过测其酶活,反应其底物耐受性,将反应体系1中的200mM的底物反应时的酶活定义为100%,测定反应体系2中的500mM的底物反应时的酶活,计算其相对酶活,结果如表6和图5所示。(3) After the reaction systems obtained in steps (1) and (2) were placed at 25°C for 10 min, 500 μL of acetonitrile was added to terminate the reaction. The enzyme activity was measured to reflect the substrate tolerance. The enzyme activity of reaction system 1 at 200 mM substrate was defined as 100%. The enzyme activity of reaction system 2 at 500 mM substrate was measured and the relative enzyme activity was calculated. The results are shown in Table 6 and Figure 5.
表6:不同腈水合酶的相对酶活Table 6: Relative enzyme activities of different nitrile hydratases
由表6及图5可知,发现融合型腈水合酶用500mM的底物3-氰基吡啶(烟腈)反应,融合型腈水合酶A8、B8、C8的相对酶活由野生酶的30%提高到89%、90%、86%,可见,融合型腈水合酶的底物烟腈的耐受性有明显的提高。As can be seen from Table 6 and Figure 5, when the fusion nitrile hydratase reacted with 500mM substrate 3-cyanopyridine (nicotinonitrile), the relative enzyme activity of the fusion nitrile hydratase A8, B8, and C8 increased from 30% of the wild enzyme to 89%, 90%, and 86%, respectively. It can be seen that the tolerance of the fusion nitrile hydratase substrate nicotinonitrile was significantly improved.
实施例5:融合型腈水合酶产物耐受性的检测Example 5: Detection of tolerance of fusion nitrile hydratase products
本发明提供的融合型的腈水合酶的底物耐受性得到了提高,以融合型腈水合酶A8、B8、C8为例进行说明,具体步骤如下:The substrate tolerance of the fusion nitrile hydratase provided by the present invention is improved, and the fusion nitrile hydratase A8, B8, and C8 are used as examples for illustration, and the specific steps are as follows:
向含有250μL浓度为500mmol/L的烟酰胺溶液中,分别添加10μL终浓度为0.5mg/mL的实施例1得到的纯化后的野生型的腈水合酶WT、融合型腈水合酶融合型腈水合酶A8、B8、C8得到发应体系;将各个反应体系置于25℃下孵育30min后,向反应体系中添加250μL 40mM烟腈溶液,25℃反应10min,加入500μL的乙腈终止反应,通过测定烟腈消耗量反应其催化能力,得到产物耐受性结果;将0M处理的烟腈消耗量定义为100%,计算500mmol/L的烟酰胺溶液处理后的烟腈的相对消耗量,即相对酶活,结果如表7和图6所示。To 250 μL of a 500 mmol/L nicotinamide solution, 10 μL of the purified wild-type nitrile hydratase WT, fusion nitrile hydratase A8, B8, and C8 obtained in Example 1 were added at a final concentration of 0.5 mg/mL to obtain a reaction system; each reaction system was incubated at 25° C. for 30 min, and then 250 μL of a 40 mM nicotinamide solution was added to the reaction system, and the reaction was carried out at 25° C. for 10 min. 500 μL of acetonitrile was added to terminate the reaction, and the catalytic ability was reflected by measuring the consumption of nicotinamide to obtain the product tolerance result; the consumption of nicotinamide treated with 0 M was defined as 100%, and the relative consumption of nicotinamide after treatment with 500 mmol/L nicotinamide solution, i.e., the relative enzyme activity, was calculated, and the results are shown in Table 7 and FIG. 6 .
表7:不同腈水合酶对烟腈的相对消耗量Table 7: Relative consumption of nicotinonitrile by different nitrile hydratases
由表7及图6可知,在500mM的产物尼克酰胺下处理30min后,融合型腈水合酶A8、B8、C8的相对酶活由野生酶的77%提高到80%、94%、86%,融合型腈水合酶的产物耐受性有明显的提高。As shown in Table 7 and Figure 6, after being treated with 500 mM product nicotinamide for 30 min, the relative enzyme activities of the fusion nitrile hydratases A8, B8, and C8 increased from 77% of the wild enzyme to 80%, 94%, and 86%, respectively, and the product tolerance of the fusion nitrile hydratase was significantly improved.
虽然本发明已以较佳实施例公开如上,但其并非用以限定本发明,任何熟悉此技术的人,在不脱离本发明的精神和范围内,都可做各种的改动与修饰,因此本发明的保护范围应该以权利要求书所界定的为准。Although the present invention has been disclosed as above in the preferred embodiment, it is not intended to limit the present invention. Anyone familiar with this technology can make various changes and modifications without departing from the spirit and scope of the present invention. Therefore, the scope of protection of the present invention should be based on the definition of the claims.
SEQUENCE LISTINGSEQUENCE LISTING
<110> 江南大学<110> Jiangnan University
<120> 一种融合型腈水合酶及其应用<120> A fusion type nitrile hydratase and its application
<130> BAA201071A<130> BAA201071A
<160> 25<160> 25
<170> PatentIn version 3.3<170> PatentIn version 3.3
<210> 1<210> 1
<211> 1801<211> 1801
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 1<400> 1
atggcaagct ggagccaccc gcagttcgaa aagggtgcac atatgaatgg cattcacgat 60atggcaagct ggagccaccc gcagttcgaa aagggtgcac atatgaatgg cattcacgat 60
actggcggag cacatggtta tgggccggtt tacagagaac cgaacgaacc cgtctttcgc 120actggcggag cacatggtta tgggccggtt tacagagaac cgaacgaacc cgtctttcgc 120
tacgactggg aaaaaacggt catgtccctg ctcccggcgc tgctcgccaa cggcaacttc 180tacgactggg aaaaaacggt catgtccctg ctcccggcgc tgctcgccaa cggcaacttc 180
aacctcgatg aatttcggca ttcgatcgag cgaatgggcc cggcccacta tctggaggga 240aacctcgatg aatttcggca ttcgatcgag cgaatgggcc cggcccacta tctggaggga 240
acctactacg aacactggct tcatgtcttt gagaacctgc tggtcgagaa gggtgtgctc 300acctactacg aacactggct tcatgtcttt gagaacctgc tggtcgagaa gggtgtgctc 300
acggccacgg aagtcgcgac cggcaaggct gcgtctggca agacggcgac gccggtgctg 360acggccacgg aagtcgcgac cggcaaggct gcgtctggca agacggcgac gccggtgctg 360
acgccggcca tcgtggacgg actgctcagc accggggctt ctgccgcccg ggaggagggt 420acgccggcca tcgtggacgg actgctcagc accggggctt ctgccgcccg ggaggagggt 420
gcgcgggcgc ggttcgctgt gggggacaag gttcgcgtcc tcaacaagaa cccggtgggc 480gcgcgggcgc ggttcgctgt gggggacaag gttcgcgtcc tcaacaagaa cccggtgggc 480
catacccgca tgccgcgcta cacgcggggc aaagtgggga cagtggtcat cgaccatggt 540catacccgca tgccgcgcta cacgcggggc aaagtgggga cagtggtcat cgaccatggt 540
gtgttcgtga cgccggacac cgcggcacac ggaaagggcg agcaccccca gcacgtttac 600gtgttcgtga cgccggacac cgcggcacac ggaaagggcg agcaccccca gcacgtttac 600
accgtgagtt tcacgtcggt cgaactgtgg gggcaagacg cttcctcgcc gaaggacacg 660accgtgagtt tcacgtcggt cgaactgtgg gggcaagacg cttcctcgcc gaaggacacg 660
attcgcgtcg acttgtggga tgactacctg gagccagcgt gaaaggagac cgcaccatgg 720attcgcgtcg acttgtggga tgactacctg gagccagcgt gaaaggagac cgcaccatgg 720
ggcaatcaca cacgcatgac caccatcacg acgggtacca ggcaccgccc gaagacatcg 780ggcaatcaca cacgcatgac caccatcacg acgggtacca ggcaccgccc gaagacatcg 780
cgctgcgggt caaggccttg gagtctctgc tgatcgagaa aggtcttgtc gacccagcgg 840cgctgcgggt caaggccttg gagtctctgc tgatcgagaa aggtcttgtc gacccagcgg 840
ccatggactt ggtcgtccaa acgtatgaac acaaggtagg cccccgaaac ggcgccaaag 900ccatggactt ggtcgtccaa acgtatgaac acaaggtagg cccccgaaac ggcgccaaag 900
tcgtggccaa ggcctgggtg gaccctgcct acaaggcccg tctgctggca gacggcactg 960tcgtggccaa ggcctgggtg gaccctgcct acaaggcccg tctgctggca gacggcactg 960
ccggcattgc cgagctgggc ttctccgggg tacagggcga ggacatggtc attctggaaa 1020ccggcattgc cgagctgggc ttctccgggg tacagggcga ggacatggtc attctggaaa 1020
acacccccgc cgtccacaac gtcttcgttt gcaccttgtg ctcttgctac ccatggccga 1080acaccccccgc cgtccacaac gtcttcgttt gcaccttgtg ctcttgctac ccatggccga 1080
cgctgggctt gccccctgcc tggtacaagg ccgcgcccta ccggtcccgc atggtgagcg 1140cgctgggctt gccccctgcc tggtacaagg ccgcgcccta ccggtcccgc atggtgagcg 1140
acccgcgtgg ggttctcgcg gagttcggcc tggtgatccc cgccaacaag gaaatccgcg 1200acccgcgtgg ggttctcgcg gagttcggcc tggtgatccc cgccaacaag gaaatccgcg 1200
tctgggacac cacggccgaa ttgcgctaca tggtgctgcc ggaacggccc gcgggaactg 1260tctgggacac cacggccgaa ttgcgctaca tggtgctgcc ggaacggccc gcgggaactg 1260
aagcctacag cgaagaacaa ctggccgaac tcgttacccg cgattcgatg atcggcaccg 1320aagcctacag cgaagaacaa ctggccgaac tcgttacccg cgattcgatg atcggcaccg 1320
gcctgcccac ccaacccacc ccatctcatt aaaaggagat atagatatga aagacgaacg 1380gcctgcccac ccaacccacc ccatctcatt aaaaggagat atagatatga aagacgaacg 1380
gtttccattg ccagagggtt cgctgaagga cctcgatggc cctgtgtttg acgagccttg 1440gtttccattg ccagagggtt cgctgaagga cctcgatggc cctgtgtttg acgagccttg 1440
gcagtcccag gcgtttgcct tggtggtcag catgcacaag gccggtctct ttcagtggaa 1500gcagtcccag gcgtttgcct tggtggtcag catgcacaag gccggtctct ttcagtggaa 1500
agactgggcc gagaccttca ccgccgaaat cgacgcttcc ccggctctgc ccggcgaaag 1560agactgggcc gagaccttca ccgccgaaat cgacgcttcc ccggctctgc ccggcgaaag 1560
cgtcaacgac acctactacc ggcaatgggt gtcggcgctg gaaaagttgg tggcgtcgct 1620cgtcaacgac acctactacc ggcaatgggt gtcggcgctg gaaaagttgg tggcgtcgct 1620
ggggcttgtg acgggtggag acgtcaactc gcgcgcacag gagtggaaac aggcccacct 1680ggggcttgtg acgggtggag acgtcaactc gcgcgcacag gagtggaaac aggcccacct 1680
caacacccca catgggcacc cgatcctgct ggcccatgcg ctttgcccgc cagcgatcga 1740caacacccca catgggcacc cgatcctgct ggcccatgcg ctttgcccgc cagcgatcga 1740
ccccaagcac aagcacgagc cacaacgctc accgatcaag gtcgttgccg caatggcttg 1800ccccaagcac aagcacgagc cacaacgctc accgatcaag gtcgttgccg caatggcttg 1800
a 1801a 1801
<210> 2<210> 2
<211> 233<211> 233
<212> PRT<212> PRT
<213> 人工序列<213> Artificial sequence
<400> 2<400> 2
Met Ala Ser Trp Ser His Pro Gln Phe Glu Lys Gly Ala His Met AsnMet Ala Ser Trp Ser His Pro Gln Phe Glu Lys Gly Ala His Met Asn
1 5 10 151 5 10 15
Gly Ile His Asp Thr Gly Gly Ala His Gly Tyr Gly Pro Val Tyr ArgGly Ile His Asp Thr Gly Gly Ala His Gly Tyr Gly Pro Val Tyr Arg
20 25 3020 25 30
Glu Pro Asn Glu Pro Val Phe Arg Tyr Asp Trp Glu Lys Thr Val MetGlu Pro Asn Glu Pro Val Phe Arg Tyr Asp Trp Glu Lys Thr Val Met
35 40 4535 40 45
Ser Leu Leu Pro Ala Leu Leu Ala Asn Gly Asn Phe Asn Leu Asp GluSer Leu Leu Pro Ala Leu Leu Ala Asn Gly Asn Phe Asn Leu Asp Glu
50 55 6050 55 60
Phe Arg His Ser Ile Glu Arg Met Gly Pro Ala His Tyr Leu Glu GlyPhe Arg His Ser Ile Glu Arg Met Gly Pro Ala His Tyr Leu Glu Gly
65 70 75 8065 70 75 80
Thr Tyr Tyr Glu His Trp Leu His Val Phe Glu Asn Leu Leu Val GluThr Tyr Tyr Glu His Trp Leu His Val Phe Glu Asn Leu Leu Val Glu
85 90 9585 90 95
Lys Gly Val Leu Thr Ala Thr Glu Val Ala Thr Gly Lys Ala Ala SerLys Gly Val Leu Thr Ala Thr Glu Val Ala Thr Gly Lys Ala Ala Ser
100 105 110100 105 110
Gly Lys Thr Ala Thr Pro Val Leu Thr Pro Ala Ile Val Asp Gly LeuGly Lys Thr Ala Thr Pro Val Leu Thr Pro Ala Ile Val Asp Gly Leu
115 120 125115 120 125
Leu Ser Thr Gly Ala Ser Ala Ala Arg Glu Glu Gly Ala Arg Ala ArgLeu Ser Thr Gly Ala Ser Ala Ala Arg Glu Glu Gly Ala Arg Ala Arg
130 135 140130 135 140
Phe Ala Val Gly Asp Lys Val Arg Val Leu Asn Lys Asn Pro Val GlyPhe Ala Val Gly Asp Lys Val Arg Val Leu Asn Lys Asn Pro Val Gly
145 150 155 160145 150 155 160
His Thr Arg Met Pro Arg Tyr Thr Arg Gly Lys Val Gly Thr Val ValHis Thr Arg Met Pro Arg Tyr Thr Arg Gly Lys Val Gly Thr Val Val
165 170 175165 170 175
Ile Asp His Gly Val Phe Val Thr Pro Asp Thr Ala Ala His Gly LysIle Asp His Gly Val Phe Val Thr Pro Asp Thr Ala Ala His Gly Lys
180 185 190180 185 190
Gly Glu His Pro Gln His Val Tyr Thr Val Ser Phe Thr Ser Val GluGly Glu His Pro Gln His Val Tyr Thr Val Ser Phe Thr Ser Val Glu
195 200 205195 200 205
Leu Trp Gly Gln Asp Ala Ser Ser Pro Lys Asp Thr Ile Arg Val AspLeu Trp Gly Gln Asp Ala Ser Ser Pro Lys Asp Thr Ile Arg Val Asp
210 215 220210 215 220
Leu Trp Asp Asp Tyr Leu Glu Pro AlaLeu Trp Asp Asp Tyr Leu Glu Pro Ala
225 230225 230
<210> 3<210> 3
<211> 211<211> 211
<212> PRT<212> PRT
<213> 人工序列<213> Artificial sequence
<400> 3<400> 3
Met Gly Gln Ser His Thr His Asp His His His Asp Gly Tyr Gln AlaMet Gly Gln Ser His Thr His Asp His His His Asp Gly Tyr Gln Ala
1 5 10 151 5 10 15
Pro Pro Glu Asp Ile Ala Leu Arg Val Lys Ala Leu Glu Ser Leu LeuPro Pro Glu Asp Ile Ala Leu Arg Val Lys Ala Leu Glu Ser Leu Leu
20 25 3020 25 30
Ile Glu Lys Gly Leu Val Asp Pro Ala Ala Met Asp Leu Val Val GlnIle Glu Lys Gly Leu Val Asp Pro Ala Ala Met Asp Leu Val Val Gln
35 40 4535 40 45
Thr Tyr Glu His Lys Val Gly Pro Arg Asn Gly Ala Lys Val Val AlaThr Tyr Glu His Lys Val Gly Pro Arg Asn Gly Ala Lys Val Val Ala
50 55 6050 55 60
Lys Ala Trp Val Asp Pro Ala Tyr Lys Ala Arg Leu Leu Ala Asp GlyLys Ala Trp Val Asp Pro Ala Tyr Lys Ala Arg Leu Leu Ala Asp Gly
65 70 75 8065 70 75 80
Thr Ala Gly Ile Ala Glu Leu Gly Phe Ser Gly Val Gln Gly Glu AspThr Ala Gly Ile Ala Glu Leu Gly Phe Ser Gly Val Gln Gly Glu Asp
85 90 9585 90 95
Met Val Ile Leu Glu Asn Thr Pro Ala Val His Asn Val Phe Val CysMet Val Ile Leu Glu Asn Thr Pro Ala Val His Asn Val Phe Val Cys
100 105 110100 105 110
Thr Leu Cys Ser Cys Tyr Pro Trp Pro Thr Leu Gly Leu Pro Pro AlaThr Leu Cys Ser Cys Tyr Pro Trp Pro Thr Leu Gly Leu Pro Pro Ala
115 120 125115 120 125
Trp Tyr Lys Ala Ala Pro Tyr Arg Ser Arg Met Val Ser Asp Pro ArgTrp Tyr Lys Ala Ala Pro Tyr Arg Ser Arg Met Val Ser Asp Pro Arg
130 135 140130 135 140
Gly Val Leu Ala Glu Phe Gly Leu Val Ile Pro Ala Asn Lys Glu IleGly Val Leu Ala Glu Phe Gly Leu Val Ile Pro Ala Asn Lys Glu Ile
145 150 155 160145 150 155 160
Arg Val Trp Asp Thr Thr Ala Glu Leu Arg Tyr Met Val Leu Pro GluArg Val Trp Asp Thr Thr Ala Glu Leu Arg Tyr Met Val Leu Pro Glu
165 170 175165 170 175
Arg Pro Ala Gly Thr Glu Ala Tyr Ser Glu Glu Gln Leu Ala Glu LeuArg Pro Ala Gly Thr Glu Ala Tyr Ser Glu Glu Gln Leu Ala Glu Leu
180 185 190180 185 190
Val Thr Arg Asp Ser Met Ile Gly Thr Gly Leu Pro Thr Gln Pro ThrVal Thr Arg Asp Ser Met Ile Gly Thr Gly Leu Pro Thr Gln Pro Thr
195 200 205195 200 205
Pro Ser HisPro Ser His
210210
<210> 4<210> 4
<211> 144<211> 144
<212> PRT<212> PRT
<213> 人工序列<213> Artificial sequence
<400> 4<400> 4
Met Lys Asp Glu Arg Phe Pro Leu Pro Glu Gly Ser Leu Lys Asp LeuMet Lys Asp Glu Arg Phe Pro Leu Pro Glu Gly Ser Leu Lys Asp Leu
1 5 10 151 5 10 15
Asp Gly Pro Val Phe Asp Glu Pro Trp Gln Ser Gln Ala Phe Ala LeuAsp Gly Pro Val Phe Asp Glu Pro Trp Gln Ser Gln Ala Phe Ala Leu
20 25 3020 25 30
Val Val Ser Met His Lys Ala Gly Leu Phe Gln Trp Lys Asp Trp AlaVal Val Ser Met His Lys Ala Gly Leu Phe Gln Trp Lys Asp Trp Ala
35 40 4535 40 45
Glu Thr Phe Thr Ala Glu Ile Asp Ala Ser Pro Ala Leu Pro Gly GluGlu Thr Phe Thr Ala Glu Ile Asp Ala Ser Pro Ala Leu Pro Gly Glu
50 55 6050 55 60
Ser Val Asn Asp Thr Tyr Tyr Arg Gln Trp Val Ser Ala Leu Glu LysSer Val Asn Asp Thr Tyr Tyr Arg Gln Trp Val Ser Ala Leu Glu Lys
65 70 75 8065 70 75 80
Leu Val Ala Ser Leu Gly Leu Val Thr Gly Gly Asp Val Asn Ser ArgLeu Val Ala Ser Leu Gly Leu Val Thr Gly Gly Asp Val Asn Ser Arg
85 90 9585 90 95
Ala Gln Glu Trp Lys Gln Ala His Leu Asn Thr Pro His Gly His ProAla Gln Glu Trp Lys Gln Ala His Leu Asn Thr Pro His Gly His Pro
100 105 110100 105 110
Ile Leu Leu Ala His Ala Leu Cys Pro Pro Ala Ile Asp Pro Lys HisIle Leu Leu Ala His Ala Leu Cys Pro Pro Ala Ile Asp Pro Lys His
115 120 125115 120 125
Lys His Glu Pro Gln Arg Ser Pro Ile Lys Val Val Ala Ala Met AlaLys His Glu Pro Gln Arg Ser Pro Ile Lys Val Val Ala Ala Met Ala
130 135 140130 135 140
<210> 5<210> 5
<211> 21<211> 21
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 5<400> 5
tgcttctgct gctgcttttg c 21tgcttctgct gctgcttttg c 21
<210> 6<210> 6
<211> 36<211> 36
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 6<400> 6
tgcttctgct gctgcttttt ctgctgctgc ttttgc 36tgcttctgct gctgcttttt ctgctgctgc ttttgc 36
<210> 7<210> 7
<211> 51<211> 51
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 7<400> 7
tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttg c 51tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttg c 51
<210> 8<210> 8
<211> 66<211> 66
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 8<400> 8
tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 60tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 60
ttttgc 66ttttgc 66
<210> 9<210> 9
<211> 96<211> 96
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 9<400> 9
tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 60tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 60
tttttctgct gctgcttttt ctgctgctgc ttttgc 96tttttctgct gctgcttttt ctgctgctgc ttttgc 96
<210> 10<210> 10
<211> 126<211> 126
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 10<400> 10
tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 60tgcttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 60
tttttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 120tttttctgct gctgcttttt ctgctgctgc tttttctgct gctgcttttt ctgctgctgc 120
ttttgc 126ttttgc 126
<210> 11<210> 11
<211> 12<211> 12
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 11<400> 11
accgctacca cc 12accgctacca cc 12
<210> 12<210> 12
<211> 24<211> 24
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 12<400> 12
accgctacca ccaccgctac cacc 24accgctacca ccaccgctac cacc 24
<210> 13<210> 13
<211> 36<211> 36
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 13<400> 13
accgctacca ccaccgctac caccaccgct accacc 36accgctacca ccaccgctac caccaccgct accacc 36
<210> 14<210> 14
<211> 48<211> 48
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 14<400> 14
accgctacca ccaccgctac caccaccgct accaccaccg ctaccacc 48accgctacca ccaccgctac caccaccgct accaccaccg ctaccacc 48
<210> 15<210> 15
<211> 72<211> 72
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 15<400> 15
accgctacca ccaccgctac caccaccgct accaccaccg ctaccaccac cgctaccacc 60accgctacca ccaccgctac caccaccgct accaccaccg ctaccaccac cgctaccacc 60
accgctacca cc 72accgctacca cc 72
<210> 16<210> 16
<211> 96<211> 96
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 16<400> 16
accgctacca ccaccgctac caccaccgct accaccaccg ctaccaccac cgctaccacc 60accgctacca ccaccgctac caccaccgct accaccaccg ctaccaccac cgctaccacc 60
accgctacca ccaccgctac caccaccgct accacc 96accgctacca ccaccgctac caccaccgct accacc 96
<210> 17<210> 17
<211> 6<211> 6
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 17<400> 17
tgccgg 6tgccgg 6
<210> 18<210> 18
<211> 12<211> 12
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 18<400> 18
tgccggtgcc gg 12tgccggtgcc gg 12
<210> 19<210> 19
<211> 18<211> 18
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 19<400> 19
tgccggtgcc ggtgccgg 18
<210> 20<210> 20
<211> 24<211> 24
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 20<400> 20
tgccggtgcc ggtgccggtg ccgg 24tgccggtgcc ggtgccggtg ccgg 24
<210> 21<210> 21
<211> 36<211> 36
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 21<400> 21
tgccggtgcc ggtgccggtg ccggtgccgg tgccgg 36tgccggtgcc ggtgccggtg ccggtgccgg tgccgg 36
<210> 22<210> 22
<211> 48<211> 48
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 22<400> 22
tgccggtgcc ggtgccggtg ccggtgccgg tgccggtgcc ggtgccgg 48tgccggtgcc ggtgccggtg ccggtgccgg tgccggtgcc ggtgccgg 48
<210> 23<210> 23
<211> 702<211> 702
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 23<400> 23
atggcaagct ggagccaccc gcagttcgaa aagggtgcac atatgaatgg cattcacgat 60atggcaagct ggagccaccc gcagttcgaa aagggtgcac atatgaatgg cattcacgat 60
actggcggag cacatggtta tgggccggtt tacagagaac cgaacgaacc cgtctttcgc 120actggcggag cacatggtta tgggccggtt tacagagaac cgaacgaacc cgtctttcgc 120
tacgactggg aaaaaacggt catgtccctg ctcccggcgc tgctcgccaa cggcaacttc 180tacgactggg aaaaaacggt catgtccctg ctcccggcgc tgctcgccaa cggcaacttc 180
aacctcgatg aatttcggca ttcgatcgag cgaatgggcc cggcccacta tctggaggga 240aacctcgatg aatttcggca ttcgatcgag cgaatgggcc cggcccacta tctggaggga 240
acctactacg aacactggct tcatgtcttt gagaacctgc tggtcgagaa gggtgtgctc 300acctactacg aacactggct tcatgtcttt gagaacctgc tggtcgagaa gggtgtgctc 300
acggccacgg aagtcgcgac cggcaaggct gcgtctggca agacggcgac gccggtgctg 360acggccacgg aagtcgcgac cggcaaggct gcgtctggca agacggcgac gccggtgctg 360
acgccggcca tcgtggacgg actgctcagc accggggctt ctgccgcccg ggaggagggt 420acgccggcca tcgtggacgg actgctcagc accggggctt ctgccgcccg ggaggagggt 420
gcgcgggcgc ggttcgctgt gggggacaag gttcgcgtcc tcaacaagaa cccggtgggc 480gcgcgggcgc ggttcgctgt gggggacaag gttcgcgtcc tcaacaagaa cccggtgggc 480
catacccgca tgccgcgcta cacgcggggc aaagtgggga cagtggtcat cgaccatggt 540catacccgca tgccgcgcta cacgcggggc aaagtgggga cagtggtcat cgaccatggt 540
gtgttcgtga cgccggacac cgcggcacac ggaaagggcg agcaccccca gcacgtttac 600gtgttcgtga cgccggacac cgcggcacac ggaaagggcg agcaccccca gcacgtttac 600
accgtgagtt tcacgtcggt cgaactgtgg gggcaagacg cttcctcgcc gaaggacacg 660accgtgagtt tcacgtcggt cgaactgtgg gggcaagacg cttcctcgcc gaaggacacg 660
attcgcgtcg acttgtggga tgactacctg gagccagcgt ga 702attcgcgtcg acttgtggga tgactacctg gagccagcgt ga 702
<210> 24<210> 24
<211> 636<211> 636
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 24<400> 24
atggggcaat cacacacgca tgaccaccat cacgacgggt accaggcacc gcccgaagac 60atggggcaat cacacacgca tgaccaccat cacgacgggt accaggcacc gcccgaagac 60
atcgcgctgc gggtcaaggc cttggagtct ctgctgatcg agaaaggtct tgtcgaccca 120atcgcgctgc gggtcaaggc cttggagtct ctgctgatcg agaaaggtct tgtcgaccca 120
gcggccatgg acttggtcgt ccaaacgtat gaacacaagg taggcccccg aaacggcgcc 180gcggccatgg acttggtcgt ccaaacgtat gaacacaagg taggcccccg aaacggcgcc 180
aaagtcgtgg ccaaggcctg ggtggaccct gcctacaagg cccgtctgct ggcagacggc 240aaagtcgtgg ccaaggcctg ggtggaccct gcctacaagg cccgtctgct ggcagacggc 240
actgccggca ttgccgagct gggcttctcc ggggtacagg gcgaggacat ggtcattctg 300actgccggca ttgccgagct gggcttctcc ggggtacagg gcgaggacat ggtcattctg 300
gaaaacaccc ccgccgtcca caacgtcttc gtttgcacct tgtgctcttg ctacccatgg 360gaaaacaccc ccgccgtcca caacgtcttc gtttgcacct tgtgctcttg ctacccatgg 360
ccgacgctgg gcttgccccc tgcctggtac aaggccgcgc cctaccggtc ccgcatggtg 420ccgacgctgg gcttgccccc tgcctggtac aaggccgcgc cctaccggtc ccgcatggtg 420
agcgacccgc gtggggttct cgcggagttc ggcctggtga tccccgccaa caaggaaatc 480agcgacccgc gtggggttct cgcggagttc ggcctggtga tccccgccaa caaggaaatc 480
cgcgtctggg acaccacggc cgaattgcgc tacatggtgc tgccggaacg gcccgcggga 540cgcgtctggg acaccacggc cgaattgcgc tacatggtgc tgccggaacg gcccgcggga 540
actgaagcct acagcgaaga acaactggcc gaactcgtta cccgcgattc gatgatcggc 600actgaagcct acagcgaaga acaactggcc gaactcgtta cccgcgattc gatgatcggc 600
accggcctgc ccacccaacc caccccatct cattaa 636accggcctgc ccacccaacc cacccccatct cattaa 636
<210> 25<210> 25
<211> 435<211> 435
<212> DNA<212> DNA
<213> 人工序列<213> Artificial sequence
<400> 25<400> 25
atgaaagacg aacggtttcc attgccagag ggttcgctga aggacctcga tggccctgtg 60atgaaagacg aacggtttcc attgccagag ggttcgctga aggacctcga tggccctgtg 60
tttgacgagc cttggcagtc ccaggcgttt gccttggtgg tcagcatgca caaggccggt 120tttgacgagc cttggcagtc ccaggcgttt gccttggtgg tcagcatgca caaggccggt 120
ctctttcagt ggaaagactg ggccgagacc ttcaccgccg aaatcgacgc ttccccggct 180ctctttcagt ggaaagactg ggccgagacc ttcaccgccg aaatcgacgc ttccccggct 180
ctgcccggcg aaagcgtcaa cgacacctac taccggcaat gggtgtcggc gctggaaaag 240ctgcccggcg aaagcgtcaa cgacacctac taccggcaat gggtgtcggc gctggaaaag 240
ttggtggcgt cgctggggct tgtgacgggt ggagacgtca actcgcgcgc acaggagtgg 300ttggtggcgt cgctggggct tgtgacgggt ggagacgtca actcgcgcgc acaggagtgg 300
aaacaggccc acctcaacac cccacatggg cacccgatcc tgctggccca tgcgctttgc 360aaacaggccc acctcaacac cccacatggg cacccgatcc tgctggccca tgcgctttgc 360
ccgccagcga tcgaccccaa gcacaagcac gagccacaac gctcaccgat caaggtcgtt 420ccgccagcga tcgaccccaa gcacaagcac gagccacaac gctcaccgat caaggtcgtt 420
gccgcaatgg cttga 435gccgcaatgg cttga 435
Claims (8)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011307749.2ACN112322607B (en) | 2020-11-20 | 2020-11-20 | Fusion type nitrile hydratase and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011307749.2ACN112322607B (en) | 2020-11-20 | 2020-11-20 | Fusion type nitrile hydratase and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN112322607A CN112322607A (en) | 2021-02-05 |
| CN112322607Btrue CN112322607B (en) | 2023-03-28 |
Family
ID=74321363
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202011307749.2AActiveCN112322607B (en) | 2020-11-20 | 2020-11-20 | Fusion type nitrile hydratase and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112322607B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN119307478B (en)* | 2024-12-16 | 2025-04-15 | 华南理工大学 | A nitrile hydratase with high activity and thermal stability and its application |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102517271A (en)* | 2011-12-13 | 2012-06-27 | 清华大学 | Mutant nitrile hydratase |
| CN104450657A (en)* | 2014-11-06 | 2015-03-25 | 浙江大学 | Nitrile hydratase as well as encoding gene and application thereof |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116536293A (en)* | 2014-06-06 | 2023-08-04 | 三菱化学株式会社 | Improved nitrile hydratase |
| CN104862296B (en)* | 2015-04-22 | 2017-12-12 | 江南大学 | A kind of specific enzyme activity and stability-enhanced pattern of fusion nitrile hydratase |
| CN119120423A (en)* | 2016-07-01 | 2024-12-13 | 分解治疗有限责任公司 | Optimized dinuclease fusions and methods |
| CN106986922B (en)* | 2017-04-14 | 2020-03-06 | 江南大学 | A kind of self-assembled amphiphilic short peptide and its application |
- 2020
- 2020-11-20CNCN202011307749.2Apatent/CN112322607B/enactiveActive
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102517271A (en)* | 2011-12-13 | 2012-06-27 | 清华大学 | Mutant nitrile hydratase |
| CN104450657A (en)* | 2014-11-06 | 2015-03-25 | 浙江大学 | Nitrile hydratase as well as encoding gene and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112322607A (en) | 2021-02-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11525131B2 (en) | Recombinant vector constructed from an encoding gene of a nitrilase mutant, a recombinant genetic engineered strain and application thereof | |
| CN110791494B (en) | Aspartase mutant, recombinant expression vector comprising aspartase mutant, recombinant bacteria and application | |
| CN115322981B (en) | Nitrile hydratase mutant and application thereof in preparation of amide compounds | |
| CN108103120B (en) | A method for the synthesis of L-aspartic acid from maleic acid by double-enzyme-coupled whole-cell catalysis | |
| CN111748548A (en) | A kind of arginine decarboxylase mutant and its application in the production of agmatine | |
| CN113754726B (en) | A recombinant enzyme containing a polypeptide tag and its application in the synthesis of pharmaceutical chemicals | |
| CN112322607B (en) | Fusion type nitrile hydratase and application thereof | |
| CN110423787B (en) | Preparation method of uniform brown algae trisaccharide | |
| CN107937377A (en) | A kind of D N carbamyl hydrolysis enzymes and application | |
| CN110592045B (en) | A kind of recombinant esterase, gene, engineering bacteria and application of splitting (R,S)-indoline-2-ethyl carboxylate | |
| CN113373128B (en) | Epoxide hydrolase mutant with improved catalytic efficiency and preparation method thereof | |
| CN111269901A (en) | Epoxide hydrolase mutant and application thereof | |
| CN110699396A (en) | A kind of method for preparing D-aromatic amino acid by cascade reaction | |
| CN116949024A (en) | Phenylalanine ammonia lyase mutant and application thereof in synthesis of (S) -2-chloro-phenylalanine | |
| CN114164198B (en) | A (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid synthetic protein and its mutant and application | |
| CN104630194A (en) | (+)-gamma-lactamase from microbacterium as well as coding gene and application of (+)-gamma-lactamase | |
| CN114836401A (en) | Recombinant ssDNA (single-stranded deoxyribonucleic acid) nucleic acid cyclase, preparation method and application | |
| CN112481320A (en) | Method for preparing (-) gamma-lactam with high catalytic efficiency | |
| CN114990097B (en) | L-aspartic acid-α-decarboxylase mutant and its application | |
| CN105602922A (en) | Pantoea amidase, gene, vector, engineering bacterium and application thereof | |
| CN119391658B (en) | Method for preparing polypeptide ligase mutant and polypeptide | |
| CN113980948B (en) | High-activity tyrosine phenol lyase mutant | |
| CN111909913B (en) | Lipase mutant and application thereof | |
| CN111117979B (en) | Transaminase mutant, enzyme preparation, recombinant vector, recombinant cell and preparation method and application thereof | |
| CN112442474A (en) | Preparation method of (-) gamma-lactam |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |