CN112121828A - Preparation of hydrotalcite-based three-dimensional core-shell heterogeneous nano-array water oxidation electrocatalyst by electrodeposition method - Google Patents
Preparation of hydrotalcite-based three-dimensional core-shell heterogeneous nano-array water oxidation electrocatalyst by electrodeposition methodDownload PDFInfo
- Publication number
- CN112121828A CN112121828ACN202010648040.2ACN202010648040ACN112121828ACN 112121828 ACN112121828 ACN 112121828ACN 202010648040 ACN202010648040 ACN 202010648040ACN 112121828 ACN112121828 ACN 112121828A
- Authority
- CN
- China
- Prior art keywords
- cop
- nico
- ldh
- electrode
- precursor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000titleclaimsabstractdescription56
- 229910001868waterInorganic materials0.000titleclaimsabstractdescription36
- 238000007254oxidation reactionMethods0.000titleclaimsabstractdescription29
- 238000004070electrodepositionMethods0.000titleclaimsabstractdescription28
- 238000000034methodMethods0.000titleclaimsabstractdescription22
- 239000010411electrocatalystSubstances0.000titleclaimsabstractdescription21
- 239000011258core-shell materialSubstances0.000titleclaimsabstractdescription16
- 230000003647oxidationEffects0.000titleclaimsdescription20
- GDVKFRBCXAPAQJ-UHFFFAOYSA-Adialuminum;hexamagnesium;carbonate;hexadecahydroxideChemical compound[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Al+3].[Al+3].[O-]C([O-])=OGDVKFRBCXAPAQJ-UHFFFAOYSA-A0.000titleclaimsdescription5
- 229960001545hydrotalciteDrugs0.000titleclaimsdescription5
- 229910001701hydrotalciteInorganic materials0.000titleclaimsdescription5
- 238000002360preparation methodMethods0.000titleabstractdescription14
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000claimsabstractdescription28
- 239000002243precursorSubstances0.000claimsabstractdescription27
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000claimsabstractdescription14
- 229940075397calomelDrugs0.000claimsabstractdescription14
- 229910052799carbonInorganic materials0.000claimsabstractdescription14
- ZOMNIUBKTOKEHS-UHFFFAOYSA-Ldimercury dichlorideChemical compoundCl[Hg][Hg]ClZOMNIUBKTOKEHS-UHFFFAOYSA-L0.000claimsabstractdescription14
- 239000003792electrolyteSubstances0.000claimsabstractdescription14
- 239000004744fabricSubstances0.000claimsabstractdescription14
- 229910052697platinumInorganic materials0.000claimsabstractdescription14
- QGUAJWGNOXCYJF-UHFFFAOYSA-Ncobalt dinitrate hexahydrateChemical compoundO.O.O.O.O.O.[Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=OQGUAJWGNOXCYJF-UHFFFAOYSA-N0.000claimsabstractdescription8
- DDFHBQSCUXNBSA-UHFFFAOYSA-N5-(5-carboxythiophen-2-yl)thiophene-2-carboxylic acidChemical compoundS1C(C(=O)O)=CC=C1C1=CC=C(C(O)=O)S1DDFHBQSCUXNBSA-UHFFFAOYSA-N0.000claimsabstractdescription5
- XSQUKJJJFZCRTK-UHFFFAOYSA-NUreaChemical compoundNC(N)=OXSQUKJJJFZCRTK-UHFFFAOYSA-N0.000claimsabstractdescription5
- 239000004202carbamideSubstances0.000claimsabstractdescription5
- 239000002070nanowireSubstances0.000claimsabstractdescription5
- YCKRFDGAMUMZLT-UHFFFAOYSA-NFluorine atomChemical compound[F]YCKRFDGAMUMZLT-UHFFFAOYSA-N0.000claimsabstractdescription4
- OAICVXFJPJFONN-UHFFFAOYSA-NPhosphorusChemical compound[P]OAICVXFJPJFONN-UHFFFAOYSA-N0.000claimsabstractdescription4
- 229910017052cobaltInorganic materials0.000claimsabstractdescription4
- 239000010941cobaltSubstances0.000claimsabstractdescription4
- GUTLYIVDDKVIGB-UHFFFAOYSA-Ncobalt atomChemical compound[Co]GUTLYIVDDKVIGB-UHFFFAOYSA-N0.000claimsabstractdescription4
- 239000011737fluorineSubstances0.000claimsabstractdescription4
- 229910052731fluorineInorganic materials0.000claimsabstractdescription4
- AOPCKOPZYFFEDA-UHFFFAOYSA-Nnickel(2+);dinitrate;hexahydrateChemical compoundO.O.O.O.O.O.[Ni+2].[O-][N+]([O-])=O.[O-][N+]([O-])=OAOPCKOPZYFFEDA-UHFFFAOYSA-N0.000claimsabstractdescription4
- 229910052698phosphorusInorganic materials0.000claimsabstractdescription4
- 239000011574phosphorusSubstances0.000claimsabstractdescription4
- 239000000758substrateSubstances0.000claimsabstractdescription4
- 239000000463materialSubstances0.000claimsdescription22
- 238000006243chemical reactionMethods0.000claimsdescription20
- 239000008367deionised waterSubstances0.000claimsdescription20
- 229910021641deionized waterInorganic materials0.000claimsdescription20
- 238000005406washingMethods0.000claimsdescription16
- 238000001035dryingMethods0.000claimsdescription12
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000claimsdescription11
- -1polytetrafluoroethylenePolymers0.000claimsdescription11
- 229920001343polytetrafluoroethylenePolymers0.000claimsdescription11
- 239000004810polytetrafluoroethyleneSubstances0.000claimsdescription11
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000claimsdescription10
- 239000012300argon atmosphereSubstances0.000claimsdescription10
- 238000001291vacuum dryingMethods0.000claimsdescription10
- 238000001027hydrothermal synthesisMethods0.000claimsdescription9
- 239000012298atmosphereSubstances0.000claimsdescription6
- 229910052573porcelainInorganic materials0.000claimsdescription6
- 239000000126substanceSubstances0.000claimsdescription6
- GRYLNZFGIOXLOG-UHFFFAOYSA-NNitric acidChemical compoundO[N+]([O-])=OGRYLNZFGIOXLOG-UHFFFAOYSA-N0.000claimsdescription5
- 238000012512characterization methodMethods0.000claimsdescription5
- 229910017604nitric acidInorganic materials0.000claimsdescription5
- 238000011144upstream manufacturingMethods0.000claimsdescription5
- 230000001351cycling effectEffects0.000claimsdescription4
- 229910052759nickelInorganic materials0.000claimsdescription3
- 230000001105regulatory effectEffects0.000claimsdescription2
- 229910021205NaH2PO2Inorganic materials0.000claims1
- LDDQLRUQCUTJBB-UHFFFAOYSA-Nammonium fluorideChemical compound[NH4+].[F-]LDDQLRUQCUTJBB-UHFFFAOYSA-N0.000claims1
- 238000001816coolingMethods0.000claims1
- 238000010438heat treatmentMethods0.000claims1
- KBJMLQFLOWQJNF-UHFFFAOYSA-Nnickel(II) nitrateInorganic materials[Ni+2].[O-][N+]([O-])=O.[O-][N+]([O-])=OKBJMLQFLOWQJNF-UHFFFAOYSA-N0.000claims1
- 238000007789sealingMethods0.000claims1
- 238000009210therapy by ultrasoundMethods0.000claims1
- 230000003197catalytic effectEffects0.000abstractdescription4
- 238000011065in-situ storageMethods0.000abstractdescription4
- 239000002135nanosheetSubstances0.000abstractdescription2
- 229910017855NH 4 FInorganic materials0.000description4
- 239000000919ceramicSubstances0.000description4
- 230000000694effectsEffects0.000description4
- 238000000840electrochemical analysisMethods0.000description4
- UFHFLCQGNIYNRP-UHFFFAOYSA-NHydrogenChemical compound[H][H]UFHFLCQGNIYNRP-UHFFFAOYSA-N0.000description3
- 239000001257hydrogenSubstances0.000description3
- 229910052739hydrogenInorganic materials0.000description3
- 239000003054catalystSubstances0.000description2
- 238000009792diffusion processMethods0.000description2
- 238000005868electrolysis reactionMethods0.000description2
- 239000002803fossil fuelSubstances0.000description2
- 239000007789gasSubstances0.000description2
- 238000001308synthesis methodMethods0.000description2
- 238000002441X-ray diffractionMethods0.000description1
- 239000000654additiveSubstances0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 230000009286beneficial effectEffects0.000description1
- 239000011230binding agentSubstances0.000description1
- 230000015572biosynthetic processEffects0.000description1
- 238000006555catalytic reactionMethods0.000description1
- 239000006258conductive agentSubstances0.000description1
- 238000010276constructionMethods0.000description1
- 230000008878couplingEffects0.000description1
- 238000010168coupling processMethods0.000description1
- 238000005859coupling reactionMethods0.000description1
- 238000002484cyclic voltammetryMethods0.000description1
- 230000002542deteriorative effectEffects0.000description1
- 238000010586diagramMethods0.000description1
- HTXDPTMKBJXEOW-UHFFFAOYSA-NdioxoiridiumChemical compoundO=[Ir]=OHTXDPTMKBJXEOW-UHFFFAOYSA-N0.000description1
- 238000004146energy storageMethods0.000description1
- 230000007613environmental effectEffects0.000description1
- 238000011156evaluationMethods0.000description1
- 230000001747exhibiting effectEffects0.000description1
- 230000003993interactionEffects0.000description1
- 239000011229interlayerSubstances0.000description1
- 229910000457iridium oxideInorganic materials0.000description1
- 238000004519manufacturing processMethods0.000description1
- 229910000000metal hydroxideInorganic materials0.000description1
- 150000004692metal hydroxidesChemical class0.000description1
- 239000000203mixtureSubstances0.000description1
- 239000002114nanocompositeSubstances0.000description1
- 229910000510noble metalInorganic materials0.000description1
- 230000001590oxidative effectEffects0.000description1
- 239000001301oxygenSubstances0.000description1
- 229910052760oxygenInorganic materials0.000description1
- 239000002245particleSubstances0.000description1
- 239000000843powderSubstances0.000description1
- 239000010970precious metalSubstances0.000description1
- 239000002994raw materialSubstances0.000description1
- 230000027756respiratory electron transport chainEffects0.000description1
- 229910001925ruthenium oxideInorganic materials0.000description1
- WOCIAKWEIIZHES-UHFFFAOYSA-Nruthenium(iv) oxideChemical compoundO=[Ru]=OWOCIAKWEIIZHES-UHFFFAOYSA-N0.000description1
- 238000001878scanning electron micrographMethods0.000description1
- 230000026683transductionEffects0.000description1
- 238000010361transductionMethods0.000description1
- 238000004832voltammetryMethods0.000description1
Images
Classifications
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/14—Phosphorus; Compounds thereof
- B01J27/185—Phosphorus; Compounds thereof with iron group metals or platinum group metals
- B01J27/1853—Phosphorus; Compounds thereof with iron group metals or platinum group metals with iron, cobalt or nickel
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J21/00—Catalysts comprising the elements, oxides, or hydroxides of magnesium, boron, aluminium, carbon, silicon, titanium, zirconium, or hafnium
- B01J21/18—Carbon
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/70—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper
- B01J23/74—Iron group metals
- B01J23/755—Nickel
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/20—Catalysts, in general, characterised by their form or physical properties characterised by their non-solid state
- B01J35/23—Catalysts, in general, characterised by their form or physical properties characterised by their non-solid state in a colloidal state
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/33—Electric or magnetic properties
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/396—Distribution of the active metal ingredient
- B01J35/398—Egg yolk like
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/08—Heat treatment
- B01J37/10—Heat treatment in the presence of water, e.g. steam
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/28—Phosphorising
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/34—Irradiation by, or application of, electric, magnetic or wave energy, e.g. ultrasonic waves ; Ionic sputtering; Flame or plasma spraying; Particle radiation
- B01J37/348—Electrochemical processes, e.g. electrochemical deposition or anodisation
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/02—Hydrogen or oxygen
- C25B1/04—Hydrogen or oxygen by electrolysis of water
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Inorganic Chemistry (AREA)
- Metallurgy (AREA)
- Health & Medical Sciences (AREA)
- Plasma & Fusion (AREA)
- Toxicology (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
- Inert Electrodes (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及纳米阵列核壳结构CoP@NiCo-LDH纳米复合物的制备和应用于电催化碱性水氧化反应的方法,特别是涉及先在碳布(CC)上原位生长Co(OH)F前驱,再通过低温保形磷化法处理Co(OH)F/CC以制备CoP/CC,再在CoP/CC上电沉积NiCo-LDH以制备CoP@NiCo-LDH/CC,以及该材料在电催化换能领域中的应用。仅采用简单可控、环保经济的合成方法便可制备高性能水氧化电催化剂。The invention relates to a method for preparing a nano-array core-shell structure CoP@NiCo-LDH nanocomposite and applying it to an electrocatalytic alkaline water oxidation reaction, in particular to in-situ growth of Co(OH)F on carbon cloth (CC) Precursor, then treated Co(OH)F/CC by low temperature conformal phosphating method to prepare CoP/CC, and then electrodeposited NiCo-LDH on CoP/CC to prepare CoP@NiCo-LDH/CC, and the material was electro-deposited. Applications in the field of catalytic conversion. High-performance electrocatalysts for water oxidation can be prepared using only a simple, controllable, environmentally friendly and economical synthesis method.
背景技术Background technique
全球环境的日渐恶化及传统化石燃料的不断消耗迫使人们不断寻求清洁可持续的能源,氢能因其高能量密度及环境友好性等优点被认为是化石燃料的理想替代品(Nat.Chem., 2009, 1, 112-117,Science, 2004, 305, 972-974)。电解水是一种简单的制氢方法,包括析氧反应(OER)和析氢反应(HER)两个半反应,其中析氧反应涉及四电子传递过程,是一个动力学缓慢的过程,被认为是电解水的瓶颈。因此,急需高效水氧化电催化剂以实现较快的动力学过程及减小较大的过电位(Science, 2011, 334, 1383-1385,EnergyEnviron. Sci., 2013, 6, 2921-2924)。目前,氧化钌和氧化铱等贵金属基电催化剂被认为是最好的水氧化电催化剂,但其高成本及低储量限制了其大规模的应用(J. Phys.Chem. Lett., 2012, 3, 339–404)。因此,开发非贵金属基的水氧化电催化剂实现高效稳定的水氧化电催化反应尤其重要和紧迫。The deteriorating global environment and the continuous consumption of traditional fossil fuels force people to continuously seek clean and sustainable energy. Hydrogen energy is considered to be an ideal substitute for fossil fuels due to its high energy density and environmental friendliness (Nat.Chem ., 2009, 1, 112-117,Science , 2004, 305, 972-974). Water electrolysis is a simple method for hydrogen production, including two half-reactions, oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). Bottleneck of electrolyzed water. Therefore, high-efficiency electrocatalysts for water oxidation are urgently needed to achieve faster kinetic processes and reduce larger overpotentials (Science , 2011, 334, 1383-1385,EnergyEnviron. Sci. , 2013, 6, 2921-2924). Currently, noble metal-based electrocatalysts such as ruthenium oxide and iridium oxide are considered to be the best electrocatalysts for water oxidation, but their high cost and low reserves limit their large-scale applications (J. Phys.Chem. Lett. , 2012, 3 , 339–404). Therefore, it is particularly important and urgent to develop non-precious metal-based electrocatalysts for water oxidation to achieve efficient and stable electrocatalytic reactions for water oxidation.
层状双金属氢氧化物(LDHs)由于其低成本,高活性且易于扩展的层间结构等优点,在催化和储能方面都具有很大的应用前景(Energy Environ. Sci., 2017, 10, 1820–1827)。特别是镍基LDHs,因其易于调整的化学组分,高活性等展现出优异的水氧化电催化活性(Chem. Rev., 2016, 116, 14120–14136,Sci. Rep., 2016, 6, 18737–18746)。但是,大多数镍基LDHs都是团聚颗粒,具有有限的比表面积和较差的稳定性(Electrochim.Acta, 2014, 135, 513–518)。为克服这些缺点,已采用了各种策略,其中界面工程被认为是最重要和最有效的方法之一(Adv. Funct. Mater., 2013, 23, 3513–3518)。特别是核-壳异质结构,由于其结构相互作用,电子耦合,较大的表面积以及与电解质的紧密接触,表现出比其单相结构更强的电催化活性(ACS Appl. Energy Mater., 2018, 1, 3929−3936)。此外,在碳布上生长的纳米阵列结构由于其有效的电子转移,高电导率及大表面积显示出优异的电解水性能(Nanoscale, 2017, 9, 16632–16637,Langmuir, 2015, 31,5220–5227)。因此,在碳布上构建水滑石基核-壳纳米阵列材料有望获得很好的电化学性能,这鲜有报道。Layered double metal hydroxides (LDHs) have great application prospects in both catalysis and energy storage due to their low cost, high activity, and easily extended interlayer structure (Energy Environ. Sci. , 2017, 10 , 1820–1827). In particular, nickel-based LDHs exhibit excellent electrocatalytic activity for water oxidation due to their easily tunable chemical composition, high activity, etc. (Chem. Rev. , 2016, 116, 14120–14136,Sci. Rep. , 2016, 6, 18737–18746). However, most Ni-based LDHs are agglomerated particles with limited specific surface area and poor stability (Electrochim.Acta , 2014, 135, 513–518). To overcome these shortcomings, various strategies have been adopted, among which interface engineering is considered to be one of the most important and effective methods (Adv. Funct. Mater. , 2013, 23, 3513–3518). In particular, core-shell heterostructures exhibit stronger electrocatalytic activity than their single-phase structures due to their structural interactions, electronic coupling, larger surface area, and close contact with the electrolyte (ACS Appl. Energy Mater. , 2018, 1, 3929−3936). In addition, nanoarray structures grown on carbon cloth exhibit excellent water electrolysis performance due to their efficient electron transfer, high electrical conductivity and large surface area (Nanoscale , 2017, 9, 16632–16637,Langmuir , 2015, 31, 5220– 5227). Therefore, the construction of hydrotalcite-based core-shell nanoarray materials on carbon cloth is expected to achieve good electrochemical performance, which is rarely reported.
本发明的目的是提供一种水滑石基三维核壳异质纳米阵列的简单可控,环保经济的合成方法,并将其用作高活性的水氧化电催化剂。The purpose of the present invention is to provide a simple, controllable, environment-friendly and economical synthesis method of hydrotalcite-based three-dimensional core-shell heterogeneous nanoarrays, and to use it as a highly active electrocatalyst for water oxidation.
本发明的基本构思是:以六水合硝酸钴为钴源,采用氟化铵为氟源并和尿素共同调控前驱体溶液的pH值,碳布为导电基底,采用水热法先制备Co(OH)F/CC前驱,再以NaH2PO2·H2O为磷源,采用低温保形磷化法以Co(OH)F/CC为前驱以合成CoP/CC,再以六水合硝酸镍和六水合硝酸钴为电解液,甘汞电极为参比电极,CoP/CC为工作电极,铂片为对电极,采用电沉积法以合成CoP@NiCo-LDH/CC,并将其用于水氧化反应电催化剂。The basic concept of the present invention is as follows: using cobalt nitrate hexahydrate as the cobalt source, using ammonium fluoride as the fluorine source and regulating the pH value of the precursor solution together with urea, carbon cloth as the conductive substrate, and using the hydrothermal method to first prepare Co(OH )F/CC precursor, and then using NaH2 PO2 ·H2 O as the phosphorus source, the low temperature conformal phosphating method was used to synthesize CoP/CC with Co(OH)F/CC as the precursor, and then nickel nitrate hexahydrate and Cobalt nitrate hexahydrate was used as electrolyte, calomel electrode as reference electrode, CoP/CC as working electrode, and platinum sheet as counter electrode. Electrodeposition method was used to synthesize CoP@NiCo-LDH/CC and use it for water oxidation Reacting Electrocatalysts.
发明内容SUMMARY OF THE INVENTION
本发明提出一种简单可控,环保经济的水热法,低温保形磷化法及电沉积法以原位制备三维核壳异质结构CoP@NiCo-LDH纳米阵列,并将其作为高活性的水氧化电催化剂。The present invention proposes a simple, controllable, environmentally friendly and economical hydrothermal method, low-temperature conformal phosphating method and electrodeposition method to in situ prepare a three-dimensional core-shell heterostructure CoP@NiCo-LDH nanoarray, and use it as a highly active water oxidation electrocatalyst.
本发明主要解决的技术问题是克服一般LDHs基水氧化电催化剂由于稳定性和导电性较差而导致的活性位点利用率不高和催化活性降低的缺点,避免了常规粉末电极制备过程中由于导电剂和粘结剂的引入而导致活性位点被覆盖和接触电阻增大,制备了原位生长于碳布上具有纳米线阵列核壳结构在CoP纳米线上电沉积NiCo-LDH纳米片(CoP@NiCo-LDH/CC)水氧化电催化剂,利用三维核壳异质纳米阵列的形成降低系列电阻,暴露更多活性位点和促进电解质和析出气体的扩散,作为水氧化电催化剂,展示出极高的电化学换能性质。具体来讲,本发明是以六水合硝酸钴为钴源,采用氟化铵为氟源并和尿素共同调控前驱体溶液的pH值,碳布为导电基底,采用水热法先制备Co(OH)F/CC前驱,再以NaH2PO2·H2O为磷源,采用低温保形磷化法以Co(OH)F/CC为前驱以合成CoP/CC,再以六水合硝酸镍和六水合硝酸钴为电解液,甘汞电极为参比电极,CoP/CC为工作电极,铂片为对电极,采用电沉积法以合成CoP@NiCo-LDH/CC三维核壳异质纳米阵列水氧化电催化剂,展现出低的水氧化反应过电位和塔菲尔斜率,以及优异的循环稳定性能。The main technical problem solved by the present invention is to overcome the shortcomings of low active site utilization and reduced catalytic activity of general LDHs-based water oxidation electrocatalysts due to poor stability and conductivity, and to avoid the problems in the preparation process of conventional powder electrodes. The introduction of conductive agent and binder leads to the coverage of active sites and the increase of contact resistance. NiCo-LDH nanosheets with nanowire array core-shell structure grown on carbon cloth in situ were prepared by electrodeposition on CoP nanowires ( CoP@NiCo-LDH/CC) water oxidation electrocatalyst, utilizing the formation of 3D core-shell heteronanoarrays to reduce series resistance, expose more active sites and promote diffusion of electrolyte and evolved gas, as water oxidation electrocatalyst, exhibiting Extremely high electrochemical transduction properties. Specifically, the present invention uses cobalt nitrate hexahydrate as the cobalt source, ammonium fluoride as the fluorine source and controls the pH value of the precursor solution together with urea, carbon cloth as the conductive substrate, and firstly prepares Co(OH) by hydrothermal method. )F/CC precursor, and then using NaH2 PO2 ·H2 O as the phosphorus source, the low temperature conformal phosphating method was used to synthesize CoP/CC with Co(OH)F/CC as the precursor, and then nickel nitrate hexahydrate and Using cobalt nitrate hexahydrate as electrolyte, calomel electrode as reference electrode, CoP/CC as working electrode, and platinum sheet as counter electrode, electrodeposition method was used to synthesize CoP@NiCo-LDH/CC three-dimensional core-shell heterogeneous nanoarray water. Oxidative electrocatalysts exhibit low overpotentials and Tafel slopes for water oxidation reactions, as well as excellent cycling stability.
本发明具体工序步骤如下:The concrete process steps of the present invention are as follows:
(1). 备料:Co (NO3)2·6H2O(0.582 g),CO(NH2)2(0.61 g)和NH4F(0.186 g),溶解于40mL去离子水中,搅拌均匀,一片尺寸为2×4 cm2的碳布(CC),对其先使用69 %浓硝酸在120℃下处理2 h,后分别使用去离子水和无水乙醇超声10 min进行预处理;(1). Preparation: Co(NO3 )2 ·6H2 O (0.582 g), CO(NH2 )2 (0.61 g) and NH4 F (0.186 g), dissolved in 40 mL of deionized water, stirred well, A piece of carbon cloth (CC) with a size of 2 × 4 cm2 was first treated with 69 % concentrated nitric acid at 120 °C for 2 h, and then pretreated with deionized water and absolute ethanol for 10 min by ultrasonic wave respectively;
(2). 水热反应:将步骤(1)中的溶液和处理好的CC移至50 mL聚四氟乙烯高压釜中,并密封高压釜,将其放置在真空干燥箱中,于120 ℃下反应6 h;(2). Hydrothermal reaction: transfer the solution in step (1) and the treated CC to a 50 mL polytetrafluoroethylene autoclave, seal the autoclave, and place it in a vacuum drying oven at 120 °C The next reaction is 6 h;
(3). 洗涤干燥:待步骤(2)的反应完成后,置聚四氟乙烯高压釜于空气中冷却至室温,取出被红色物质均匀覆盖的CC,用去离子水和无水乙醇先后超声洗涤3-5次后于60 ℃下真空干燥2 h,制得Co(OH)F/CC前驱;(3). Washing and drying: After the reaction in step (2) is completed, set the polytetrafluoroethylene autoclave to cool to room temperature in the air, take out the CC evenly covered by the red substance, and use deionized water and anhydrous ethanol to sonicate successively After washing 3-5 times, vacuum drying at 60 °C for 2 h to obtain Co(OH)F/CC precursor;
(4). 备料:一片步骤(3)所获得的Co(OH)F/CC前驱,NaH2PO2·H2O(摩尔比Co:P =1:3);(4). Material preparation: a piece of Co(OH)F/CC precursor obtained in step (3), NaH2 PO2 ·H2 O (molar ratio Co:P = 1:3);
(5). 低温保形磷化:将步骤(4)中的NaH2PO2·H2O和步骤(3)所制备的Co(OH)F/CC前驱分别放在两个瓷舟里置于管式气氛炉中,装有NaH2PO2·H2O的瓷舟放在管的上游,在氩气气氛下升温至300 ℃并保温2 h,然后在氩气气氛下冷却至室温,制得黑色的CoP/CC;(5). Low-temperature conformal phosphating: place the NaH2 PO2 ·H2 O in step (4) and the Co(OH)F/CC precursor prepared in step (3) in two ceramic boats respectively. In a tubular atmosphere furnace, a porcelain boat filled with NaH2 PO2 ·H2 O was placed upstream of the tube, heated to 300 °C under an argon atmosphere and kept for 2 h, and then cooled to room temperature under an argon atmosphere. Obtained black CoP/CC;
(6). 备料:Co (NO3)2·6H2O(0.15 M),Ni(NO3)2·6H2O(0.15 M),甘汞电极,铂片,一片步骤(5)所获得的CoP/CC;(6). Materials: Co(NO3 )2 ·6H2 O (0.15 M), Ni(NO3 )2 ·6H2 O (0.15 M), calomel electrode, platinum sheet, one piece obtained in step (5) CoP/CC;
(7). 电沉积:将步骤(6)的溶液作为电解液,甘汞电极为参比电极,铂片为对电极,步骤(5)制得的CoP/CC为工作电极,采用三电极系统在CoP/CC上电沉积NiCo-LDH,电沉积电位为 -1.0 V vs SCE,时间为180-220 s;(7). Electrodeposition: the solution in step (6) is used as the electrolyte, the calomel electrode is used as the reference electrode, the platinum sheet is used as the counter electrode, the CoP/CC obtained in step (5) is used as the working electrode, and a three-electrode system is used. Electrodeposition of NiCo-LDH on CoP/CC with electrodeposition potential of -1.0 V vs SCE for 180-220 s;
(8). 洗涤干燥:待步骤(7)电沉积结束后,取出被产物均匀覆盖的CC,用去离子水洗涤3-5次后于40 ℃下真空干燥24 h,制得CoP@NiCo-LDH/CC;(8). Washing and drying: After the electrodeposition in step (7), take out the CC evenly covered by the product, wash it with deionized water for 3-5 times, and then vacuum dry it at 40 °C for 24 h to obtain CoP@NiCo- LDH/CC;
(9). 表征及电化学测试:采用XRD,SEM,EDX,TEM和XPS表征CoP@NiCo-LDH/CC材料的结构和微观形貌,并使用DH7000电化学工作站,评价CoP@NiCo-LDH/CC的水氧化反应过电位,塔菲尔斜率和循环稳定性能,评价结果见表一。(9). Characterization and electrochemical tests: XRD, SEM, EDX, TEM and XPS were used to characterize the structure and microscopic morphology of CoP@NiCo-LDH/CC materials, and DH7000 electrochemical workstation was used to evaluate CoP@NiCo-LDH/CC materials. The water oxidation reaction overpotential, Tafel slope and cycle stability performance of CC are shown in Table 1.
本发明所需的反应装置简单,仅需聚四氟乙烯高压反应釜,真空干燥箱及管式气氛炉;所涉及的原料来源广泛,价格低廉;操作步骤简单,制备周期短,直接通过水热反应,低温保形磷化及电沉积即可获得所需的催化剂材料,如此设计的CoP@NiCo-LDH/CC具有三维核壳异质纳米阵列结构,将其用作水氧化电催化剂,在1 M KOH电解质中表现出优异的催化活性(η20 mA cm–2 = 259 mV,塔菲尔斜率 = 98 mV dec–1)和突出的循环稳定性能。The reaction device required by the invention is simple, and only needs a polytetrafluoroethylene high-pressure reaction kettle, a vacuum drying box and a tubular atmosphere furnace; the raw materials involved are widely sourced, and the price is low; the operation steps are simple, the preparation period is short, and the hydrothermal The desired catalyst materials can be obtained by reaction, low-temperature conformal phosphating and electrodeposition. The thus designed CoP@NiCo-LDH/CC has a three-dimensional core-shell heterogeneous nanoarray structure, which is used as an electrocatalyst for water oxidation. It exhibits excellent catalytic activity (η20 mA cm–2 = 259 mV, Tafel slope = 98 mV dec-1 ) and outstanding cycling stability in M KOH electrolyte.
本发明与现有技术及合成路线相比,具有如下的优点和有益效果:Compared with the prior art and the synthetic route, the present invention has the following advantages and beneficial effects:
1.CoP@NiCo-LDH/CC制备过程简单可控,环保经济,且反应条件温和,反应周期短;1. The preparation process of CoP@NiCo-LDH/CC is simple and controllable, environmentally friendly and economical, and the reaction conditions are mild and the reaction cycle is short;
2.CoP@NiCo-LDH/CC表现为三维核壳异质纳米阵列,可避免引入其他添加剂,有效减小系列电阻,简化催化剂电极的制备过程,并且能提供更多暴露的活性位点,促进电解质和析出气体的扩散;2. CoP@NiCo-LDH/CC behaves as a three-dimensional core-shell heterogeneous nanoarray, which can avoid the introduction of other additives, effectively reduce the series resistance, simplify the preparation process of catalyst electrodes, and can provide more exposed active sites, promote electrolyte and Diffusion of evolved gases;
3.CoP@NiCo-LDH/CC水氧化电催化剂展示出优异的电化学换能性质,拥有低的水氧化反应过电位(η20 mA cm–2 = 259 mV)和突出的循环稳定性能。3. The CoP@NiCo-LDH/CC water oxidation electrocatalyst exhibits excellent electrochemical energy conversion properties with low water oxidation reaction overpotential (η20 mA cm–2 = 259 mV) and outstanding cycling stability.
附图说明Description of drawings
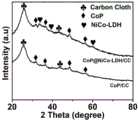
1. 图1是CoP/CC和CoP@NiCo-LDH/CC的XRD图;1. Figure 1 is the XRD pattern of CoP/CC and CoP@NiCo-LDH/CC;
2. 图2是 CoP@NiCo-LDH/CC的EDX图;2. Figure 2 is the EDX diagram of CoP@NiCo-LDH/CC;
3. 图3是CoP@NiCo-LDH/CC的SEM图;3. Figure 3 is the SEM image of CoP@NiCo-LDH/CC;
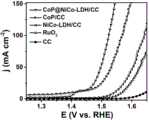
4. 图4是CoP/CC,CoP@NiCo-LDH/CC,NiCo-LDH/CC,CC及Ru2O/CC扫描伏安(LSV)曲线;4. Figure 4 is the scanning voltammetry (LSV) curves of CoP/CC, CoP@NiCo-LDH/CC, NiCo-LDH/CC, CC and Ru2 O/CC;
5. 图5是CoP/CC,CoP@NiCo-LDH/CC,NiCo-LDH/CC及Ru2O/CC的塔菲尔(Tafel)曲线;5. Figure 5 is the Tafel curve of CoP/CC, CoP@NiCo-LDH/CC, NiCo-LDH/CC and Ru2 O/CC;
6. 图6是CoP@NiCo-LDH/CC经1000次循环伏安测试前后的LSV曲线。6. Figure 6 shows the LSV curves of CoP@NiCo-LDH/CC before and after 1000 cyclic voltammetry tests.
具体实施方式Detailed ways
实例一Example 1
(1). 备料:Co (NO3)2·6H2O(0.582 g),CO(NH2)2(0.61 g)和NH4F(0.186 g),溶解于40mL去离子水中,搅拌均匀,一片尺寸为2×4 cm2的碳布(CC),对其先使用69 %浓硝酸在120℃下处理2 h,后分别使用去离子水和无水乙醇超声10 min进行预处理;(1). Preparation: Co(NO3 )2 ·6H2 O (0.582 g), CO(NH2 )2 (0.61 g) and NH4 F (0.186 g), dissolved in 40 mL of deionized water, stirred well, A piece of carbon cloth (CC) with a size of 2 × 4 cm2 was first treated with 69 % concentrated nitric acid at 120 °C for 2 h, and then pretreated with deionized water and absolute ethanol for 10 min by ultrasonic wave respectively;
(2). 水热反应:将步骤(1)中的溶液和处理好的CC移至50 mL聚四氟乙烯高压釜中,并密封高压釜,将其放置在真空干燥箱中,于120 ℃下反应6 h;(2). Hydrothermal reaction: transfer the solution in step (1) and the treated CC to a 50 mL polytetrafluoroethylene autoclave, seal the autoclave, and place it in a vacuum drying oven at 120 °C The next reaction is 6 h;
(3). 洗涤干燥:待步骤(2)的反应完成后,置聚四氟乙烯高压釜于空气中冷却至室温,取出被红色物质均匀覆盖的CC,用去离子水和无水乙醇先后超声洗涤3-5次后于60 ℃下真空干燥2 h,制得Co(OH)F/CC前驱;(3). Washing and drying: After the reaction in step (2) is completed, set the polytetrafluoroethylene autoclave to cool to room temperature in the air, take out the CC evenly covered by the red substance, and use deionized water and anhydrous ethanol to sonicate successively After washing 3-5 times, vacuum drying at 60 °C for 2 h to obtain Co(OH)F/CC precursor;
(4). 备料:一片步骤(3)所获得的Co(OH)F/CC前驱,NaH2PO2·H2O(摩尔比Co:P = 1:3);(4). Material preparation: a piece of Co(OH)F/CC precursor obtained in step (3), NaH2 PO2 ·H2 O (molar ratio Co:P = 1:3);
(5). 低温保形磷化:将步骤(4)中的NaH2PO2·H2O和步骤(3)所制备的Co(OH)F/CC前驱分别放在两个瓷舟里置于管式气氛炉中,装有NaH2PO2·H2O的瓷舟放在管的上游,在氩气气氛下升温至300 ℃并保温2 h,然后在氩气气氛下冷却至室温,制得黑色的CoP/CC;(5). Low-temperature conformal phosphating: place the NaH2 PO2 ·H2 O in step (4) and the Co(OH)F/CC precursor prepared in step (3) in two ceramic boats respectively. In a tubular atmosphere furnace, a porcelain boat filled with NaH2 PO2 ·H2 O was placed upstream of the tube, heated to 300 °C under an argon atmosphere and kept for 2 h, and then cooled to room temperature under an argon atmosphere. Obtained black CoP/CC;
(6). 备料:Co (NO3)2·6H2O(0.15 M),Ni(NO3)2·6H2O(0.15 M),甘汞电极,铂片,一片步骤(5)所获得的CoP/CC;(6). Materials: Co(NO3 )2 ·6H2 O (0.15 M), Ni(NO3 )2 ·6H2 O (0.15 M), calomel electrode, platinum sheet, one piece obtained in step (5) CoP/CC;
(7). 电沉积:将步骤(6)的溶液作为电解液,甘汞电极为参比电极,铂片为对电极,步骤(5)制得的CoP/CC为工作电极,采用三电极系统在CoP/CC上电沉积NiCo-LDH,电沉积电位为-1.0 V vs SCE,时间为180 s;(7). Electrodeposition: the solution in step (6) is used as the electrolyte, the calomel electrode is used as the reference electrode, the platinum sheet is used as the counter electrode, the CoP/CC obtained in step (5) is used as the working electrode, and a three-electrode system is used. Electrodeposition of NiCo-LDH on CoP/CC with electrodeposition potential of -1.0 V vs SCE for 180 s;
(8). 洗涤干燥:待步骤(7)电沉积结束后,取出被产物均匀覆盖的CC,用去离子水洗涤3-5次后于40 ℃下真空干燥24 h,制得CoP@NiCo-LDH/CC;(8). Washing and drying: After the electrodeposition in step (7), take out the CC evenly covered by the product, wash it with deionized water for 3-5 times, and then vacuum dry it at 40 °C for 24 h to obtain CoP@NiCo- LDH/CC;
(9). 表征及电化学测试:采用XRD,SEM,EDX,TEM和XPS表征CoP@NiCo-LDH/CC材料的结构和微观形貌,并使用DH7000电化学工作站,评价CoP@NiCo-LDH/CC的水氧化反应过电位,塔菲尔斜率和循环稳定性能,评价结果见表一。(9). Characterization and electrochemical tests: XRD, SEM, EDX, TEM and XPS were used to characterize the structure and microscopic morphology of CoP@NiCo-LDH/CC materials, and DH7000 electrochemical workstation was used to evaluate CoP@NiCo-LDH/CC materials. The water oxidation reaction overpotential, Tafel slope and cycle stability performance of CC are shown in Table 1.
实例二Example 2
(1). 备料:Co (NO3)2·6H2O(0.582 g),CO(NH2)2(0.61 g)和NH4F(0.186 g),溶解于40mL去离子水中,搅拌均匀,一片尺寸为2×4 cm2的碳布(CC),对其先使用69 %浓硝酸在120℃下处理2 h,后分别使用去离子水和无水乙醇超声10 min进行预处理;(1). Preparation: Co(NO3 )2 ·6H2 O (0.582 g), CO(NH2 )2 (0.61 g) and NH4 F (0.186 g), dissolved in 40 mL of deionized water, stirred well, A piece of carbon cloth (CC) with a size of 2 × 4 cm2 was first treated with 69 % concentrated nitric acid at 120 °C for 2 h, and then pretreated with deionized water and absolute ethanol for 10 min by ultrasonic wave respectively;
(2). 水热反应:将步骤(1)中的溶液和处理好的CC移至50 mL聚四氟乙烯高压釜中,并密封高压釜,将其放置在真空干燥箱中,于120 ℃下反应6 h;(2). Hydrothermal reaction: transfer the solution in step (1) and the treated CC to a 50 mL polytetrafluoroethylene autoclave, seal the autoclave, and place it in a vacuum drying oven at 120 °C The next reaction is 6 h;
(3). 洗涤干燥:待步骤(2)的反应完成后,置聚四氟乙烯高压釜于空气中冷却至室温,取出被红色物质均匀覆盖的CC,用去离子水和无水乙醇先后超声洗涤3-5次后于60 ℃下真空干燥2 h,制得Co(OH)F/CC前驱;(3). Washing and drying: After the reaction in step (2) is completed, set the polytetrafluoroethylene autoclave to cool to room temperature in the air, take out the CC evenly covered by the red substance, and use deionized water and anhydrous ethanol to sonicate successively After washing 3-5 times, vacuum drying at 60 °C for 2 h to obtain Co(OH)F/CC precursor;
(4). 备料:一片步骤(3)所获得的Co(OH)F/CC前驱,NaH2PO2·H2O(摩尔比Co:P = 1:3);(4). Material preparation: a piece of Co(OH)F/CC precursor obtained in step (3), NaH2 PO2 ·H2 O (molar ratio Co:P = 1:3);
(5). 低温保形磷化:将步骤(4)中的NaH2PO2·H2O和步骤(3)所制备的Co(OH)F/CC前驱分别放在两个瓷舟里置于管式气氛炉中,装有NaH2PO2·H2O的瓷舟放在管的上游,在氩气气氛下升温至300 ℃并保温2 h,然后在氩气气氛下冷却至室温,制得黑色的CoP/CC;(5). Low-temperature conformal phosphating: place the NaH2 PO2 ·H2 O in step (4) and the Co(OH)F/CC precursor prepared in step (3) in two ceramic boats respectively. In a tubular atmosphere furnace, a porcelain boat filled with NaH2 PO2 ·H2 O was placed upstream of the tube, heated to 300 °C under an argon atmosphere and kept for 2 h, and then cooled to room temperature under an argon atmosphere. Obtained black CoP/CC;
(6). 备料:Co (NO3)2·6H2O(0.15 M),Ni(NO3)2·6H2O(0.15 M),甘汞电极,铂片,一片步骤(5)所获得的CoP/CC;(6). Materials: Co(NO3 )2 ·6H2 O (0.15 M), Ni(NO3 )2 ·6H2 O (0.15 M), calomel electrode, platinum sheet, one piece obtained in step (5) CoP/CC;
(7). 电沉积:将步骤(6)的溶液作为电解液,甘汞电极为参比电极,铂片为对电极,步骤(5)制得的CoP/CC为工作电极,采用三电极系统在CoP/CC上电沉积NiCo-LDH,电沉积电位为-1.0 V vs SCE,时间为200 s;(7). Electrodeposition: the solution in step (6) is used as the electrolyte, the calomel electrode is used as the reference electrode, the platinum sheet is used as the counter electrode, the CoP/CC obtained in step (5) is used as the working electrode, and a three-electrode system is used. Electrodeposition of NiCo-LDH on CoP/CC with electrodeposition potential of -1.0 V vs SCE for 200 s;
(8). 洗涤干燥:待步骤(7)电沉积结束后,取出被产物均匀覆盖的CC,用去离子水洗涤3-5次后于40 ℃下真空干燥24 h,制得CoP@NiCo-LDH/CC;(8). Washing and drying: After the electrodeposition in step (7), take out the CC evenly covered by the product, wash it with deionized water for 3-5 times, and then vacuum dry it at 40 °C for 24 h to obtain CoP@NiCo- LDH/CC;
(9).表征及电化学测试:采用XRD,SEM,EDX,TEM和XPS表征CoP@NiCo-LDH/CC材料的结构和微观形貌,并使用DH7000电化学工作站,评价CoP@NiCo-LDH/CC的水氧化反应过电位,塔菲尔斜率和循环稳定性能,评价结果见表一。(9). Characterization and electrochemical test: The structure and microstructure of CoP@NiCo-LDH/CC were characterized by XRD, SEM, EDX, TEM and XPS, and the CoP@NiCo-LDH/CC was evaluated by DH7000 electrochemical workstation. The water oxidation reaction overpotential, Tafel slope and cycle stability performance of CC are shown in Table 1.
实例三Example three
(1). 备料:Co (NO3)2·6H2O(0.582 g),CO(NH2)2(0.61 g)和NH4F(0.186 g),溶解于40mL去离子水中,搅拌均匀,一片尺寸为2×4 cm2的碳布(CC),对其先使用69 %浓硝酸在120℃下处理2 h,后分别使用去离子水和无水乙醇超声10 min进行预处理;(1). Preparation: Co(NO3 )2 ·6H2 O (0.582 g), CO(NH2 )2 (0.61 g) and NH4 F (0.186 g), dissolved in 40 mL of deionized water, stirred well, A piece of carbon cloth (CC) with a size of 2 × 4 cm2 was first treated with 69 % concentrated nitric acid at 120 °C for 2 h, and then pretreated with deionized water and absolute ethanol for 10 min by ultrasonic wave respectively;
(2). 水热反应:将步骤(1)中的溶液和处理好的CC移至50 mL聚四氟乙烯高压釜中,并密封高压釜,将其放置在真空干燥箱中,于120 ℃下反应6 h;(2). Hydrothermal reaction: transfer the solution in step (1) and the treated CC to a 50 mL polytetrafluoroethylene autoclave, seal the autoclave, and place it in a vacuum drying oven at 120 °C The next reaction is 6 h;
(3). 洗涤干燥:待步骤(2)的反应完成后,置聚四氟乙烯高压釜于空气中冷却至室温,取出被红色物质均匀覆盖的CC,用去离子水和无水乙醇先后超声洗涤3-5次后于60 ℃下真空干燥2 h,制得Co(OH)F/CC前驱;(3). Washing and drying: After the reaction in step (2) is completed, set the polytetrafluoroethylene autoclave to cool to room temperature in the air, take out the CC evenly covered by the red substance, and use deionized water and anhydrous ethanol to sonicate successively After washing 3-5 times, vacuum drying at 60 °C for 2 h to obtain Co(OH)F/CC precursor;
(4). 备料:一片步骤(3)所获得的Co(OH)F/CC前驱,NaH2PO2·H2O(摩尔比Co:P = 1:3);(4). Material preparation: a piece of Co(OH)F/CC precursor obtained in step (3), NaH2 PO2 ·H2 O (molar ratio Co:P = 1:3);
(5). 低温保形磷化:将步骤(4)中的NaH2PO2·H2O和步骤(3)所制备的Co(OH)F/CC前驱分别放在两个瓷舟里置于管式气氛炉中,装有NaH2PO2·H2O的瓷舟放在管的上游,在氩气气氛下升温至300 ℃并保温2 h,然后在氩气气氛下冷却至室温,制得黑色的CoP/CC;(5). Low-temperature conformal phosphating: place the NaH2 PO2 ·H2 O in step (4) and the Co(OH)F/CC precursor prepared in step (3) in two ceramic boats respectively. In a tubular atmosphere furnace, a porcelain boat filled with NaH2 PO2 ·H2 O was placed upstream of the tube, heated to 300 °C under an argon atmosphere and kept for 2 h, and then cooled to room temperature under an argon atmosphere. Obtained black CoP/CC;
(6). 备料:Co (NO3)2·6H2O(0.15 M),Ni(NO3)2·6H2O(0.15 M),甘汞电极,铂片,一片步骤(5)所获得的CoP/CC;(6). Materials: Co(NO3 )2 ·6H2 O (0.15 M), Ni(NO3 )2 ·6H2 O (0.15 M), calomel electrode, platinum sheet, one piece obtained in step (5) CoP/CC;
(7). 电沉积:将步骤(6)的溶液作为电解液,甘汞电极为参比电极,铂片为对电极,步骤(5)制得的CoP/CC为工作电极,采用三电极系统在CoP/CC上电沉积NiCo-LDH,电沉积电位为-1.0 V vs SCE,时间为220 s;(7). Electrodeposition: the solution in step (6) is used as the electrolyte, the calomel electrode is used as the reference electrode, the platinum sheet is used as the counter electrode, the CoP/CC obtained in step (5) is used as the working electrode, and a three-electrode system is used. Electrodeposition of NiCo-LDH on CoP/CC with electrodeposition potential of -1.0 V vs SCE for 220 s;
(8). 洗涤干燥:待步骤(7)电沉积结束后,取出被产物均匀覆盖的CC,用去离子水洗涤3-5次后于40 ℃下真空干燥24 h,制得CoP@NiCo-LDH/CC;(8). Washing and drying: After the electrodeposition in step (7), take out the CC evenly covered by the product, wash it with deionized water for 3-5 times, and then vacuum dry it at 40 °C for 24 h to obtain CoP@NiCo- LDH/CC;
(9). 表征及电化学测试:采用XRD,SEM,EDX,TEM和XPS表征CoP@NiCo-LDH/CC材料的结构和微观形貌,并使用DH7000电化学工作站,评价CoP@NiCo-LDH/CC的水氧化反应过电位,塔菲尔斜率和循环稳定性能,评价结果见表一。(9). Characterization and electrochemical tests: XRD, SEM, EDX, TEM and XPS were used to characterize the structure and microscopic morphology of CoP@NiCo-LDH/CC materials, and DH7000 electrochemical workstation was used to evaluate CoP@NiCo-LDH/CC materials. The water oxidation reaction overpotential, Tafel slope and cycle stability performance of CC are shown in Table 1.
表一 各实例CoP@NiCo-LDH/CC的水氧化电催化性能评价Table 1 Evaluation of electrocatalytic performance for water oxidation of CoP@NiCo-LDH/CC for each example
Claims (3)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010648040.2ACN112121828A (en) | 2020-07-07 | 2020-07-07 | Preparation of hydrotalcite-based three-dimensional core-shell heterogeneous nano-array water oxidation electrocatalyst by electrodeposition method |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010648040.2ACN112121828A (en) | 2020-07-07 | 2020-07-07 | Preparation of hydrotalcite-based three-dimensional core-shell heterogeneous nano-array water oxidation electrocatalyst by electrodeposition method |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN112121828Atrue CN112121828A (en) | 2020-12-25 |
Family
ID=73851804
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010648040.2APendingCN112121828A (en) | 2020-07-07 | 2020-07-07 | Preparation of hydrotalcite-based three-dimensional core-shell heterogeneous nano-array water oxidation electrocatalyst by electrodeposition method |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112121828A (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113026031A (en)* | 2021-02-25 | 2021-06-25 | 澳门大学 | Electrode material, preparation method and application thereof, and assembled water electrolysis device |
| CN113201752A (en)* | 2021-03-30 | 2021-08-03 | 北京化工大学 | Preparation method and application of CoNiP-P nano catalyst with rich heterojunction |
| CN113421781A (en)* | 2021-06-25 | 2021-09-21 | 上海理工大学 | Preparation method of nickel-cobalt oxide @ nickel-cobalt hydroxide core-shell structure electrode material |
| CN114122416A (en)* | 2021-11-29 | 2022-03-01 | 东莞理工学院 | A three-dimensional porous cobalt nitride-poly(3,4-ethylenedioxythiophene) flexible composite electrode and preparation method thereof |
| CN114438544A (en)* | 2021-12-22 | 2022-05-06 | 山东师范大学 | Preparation method of nickel-cobalt alloy @ nickel-cobalt oxide solid solution core-shell structure dual-functional electrocatalyst |
| CN114477170A (en)* | 2022-01-12 | 2022-05-13 | 大连海事大学 | A method for improving intrinsic properties and recycling of biomass-derived carbon materials |
| CN116005128A (en)* | 2021-10-18 | 2023-04-25 | 天津理工大学 | Construction method of vertical two-dimensional heterojunction |
| CN117070990A (en)* | 2023-08-15 | 2023-11-17 | 陕西科技大学 | Ru/CoP/CC composite nanowire array electrocatalyst and preparation method and application thereof |
| CN118756211A (en)* | 2024-06-18 | 2024-10-11 | 三峡大学 | Preparation method of platinum quantum dots loaded on cobalt phosphide nanowire composite electrode and its application in hydrogen evolution reaction at all pH values |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104941674A (en)* | 2015-06-18 | 2015-09-30 | 西南大学 | Catalyst for loading cobalt phosphide on activated carbon as well as preparation and application of catalyst |
- 2020
- 2020-07-07CNCN202010648040.2Apatent/CN112121828A/enactivePending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104941674A (en)* | 2015-06-18 | 2015-09-30 | 西南大学 | Catalyst for loading cobalt phosphide on activated carbon as well as preparation and application of catalyst |
Non-Patent Citations (2)
| Title |
|---|
| GUANGZHI ZHANG等: ""Cobalt phosphide nanowire arrays grown on carbon cloth as novel electrode material for supercapacitors"", 《MATERIALS SCIENCE IN SEMICONDUCTOR PROCESSING》* |
| KAI HE等: ""Utilizing the Space-charge Region of FeNi-LDH/CoP p-n Junction to Promote the Performance in Oxygen Evolution Electrocatalysis"", 《ANGEW. CHEM. INT. ED.》* |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113026031A (en)* | 2021-02-25 | 2021-06-25 | 澳门大学 | Electrode material, preparation method and application thereof, and assembled water electrolysis device |
| CN113201752A (en)* | 2021-03-30 | 2021-08-03 | 北京化工大学 | Preparation method and application of CoNiP-P nano catalyst with rich heterojunction |
| CN113421781A (en)* | 2021-06-25 | 2021-09-21 | 上海理工大学 | Preparation method of nickel-cobalt oxide @ nickel-cobalt hydroxide core-shell structure electrode material |
| CN116005128A (en)* | 2021-10-18 | 2023-04-25 | 天津理工大学 | Construction method of vertical two-dimensional heterojunction |
| CN116005128B (en)* | 2021-10-18 | 2024-03-26 | 天津理工大学 | Construction method of vertical two-dimensional heterostructure |
| CN114122416A (en)* | 2021-11-29 | 2022-03-01 | 东莞理工学院 | A three-dimensional porous cobalt nitride-poly(3,4-ethylenedioxythiophene) flexible composite electrode and preparation method thereof |
| WO2023092630A1 (en)* | 2021-11-29 | 2023-06-01 | 东莞理工学院 | Three-dimensional porous cobalt nitride-poly(3,4-ethylenedioxythiophene) flexible composite electrode and manufacturing method therefor |
| CN114438544A (en)* | 2021-12-22 | 2022-05-06 | 山东师范大学 | Preparation method of nickel-cobalt alloy @ nickel-cobalt oxide solid solution core-shell structure dual-functional electrocatalyst |
| CN114477170A (en)* | 2022-01-12 | 2022-05-13 | 大连海事大学 | A method for improving intrinsic properties and recycling of biomass-derived carbon materials |
| CN117070990A (en)* | 2023-08-15 | 2023-11-17 | 陕西科技大学 | Ru/CoP/CC composite nanowire array electrocatalyst and preparation method and application thereof |
| CN118756211A (en)* | 2024-06-18 | 2024-10-11 | 三峡大学 | Preparation method of platinum quantum dots loaded on cobalt phosphide nanowire composite electrode and its application in hydrogen evolution reaction at all pH values |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN112121828A (en) | Preparation of hydrotalcite-based three-dimensional core-shell heterogeneous nano-array water oxidation electrocatalyst by electrodeposition method | |
| CN109652822B (en) | Preparation of Layered Metal-Organic Framework Material Nanoarray Electrocatalysts for Water Oxidation Using LDH as a Template | |
| CN112108163A (en) | Preparation of CoFe-LDH nanosheet coated CoP nanowire core-shell nano array water oxidation electrocatalyst | |
| CN110055557B (en) | A three-dimensional nickel-doped iron-based oxygen evolution catalyst and its preparation method and application | |
| CN108325539B (en) | A kind of synthetic method of rod-shaped self-assembled into curd-shaped vanadium-modified Ni3S2 electrocatalyst | |
| CN108118362A (en) | A kind of molybdenum disulfide electro-catalysis production hydrogen electrode and its preparation method and application | |
| CN108654658B (en) | High-efficiency water decomposition dual-function electrocatalyst NiCoP and preparation method thereof | |
| CN108796535A (en) | One kind having three metallic coppers-cobalt-molybdenum/nickel foam porous electrode material and the preparation method and application thereof | |
| CN111636074B (en) | Preparation and application of a copper electrode for electrochemical reduction of carbon dioxide | |
| CN113955728B (en) | Preparation of cobalt phosphide/cobalt manganese phosphide with hollow grade structure and application of electrolytic water | |
| CN105688958A (en) | Polyhedron cobalt phosphide/graphite carbon hybrid material and preparing method and application thereof | |
| CN110270353A (en) | The preparation and application of load transitions bimetallic chalcogen compound nano material in situ | |
| CN112647092B (en) | A supported nickel-based composite hydrogen evolution catalyst and its preparation method and application | |
| CN110820006B (en) | A MoS2 nanoribbon-embedded VS2 microflora self-supporting electrode and its preparation method and application | |
| CN113789543B (en) | A three-dimensional layered nano-array structure copper-based material and its preparation method and application | |
| Yan et al. | Interfacial electronic regulation on NiCo2O4 nanowires@ NiFe-LDH nanosheets heterostructure for efficient oxygen evolution | |
| Li et al. | A novel 3D CoNiCu-LDH@ CuO micro-flowers on copper foam as efficient electrocatalyst for overall water splitting | |
| CN111155146B (en) | Preparation method of vanadium-doped nickel phosphide composite nitrogen-sulfur double-doped reduced graphene oxide electrocatalytic material | |
| CN111974415A (en) | Copper sulfide/brass mesh electrode material with nanosheet array structure and preparation method and application thereof | |
| Xiao et al. | Co-Mn-S nanosheets decorated with CeO2: a highly active electrocatalyst toward oxygen evolution reaction | |
| CN115094475B (en) | Electrode material with high-performance oxygen evolution catalytic activity and preparation method thereof | |
| CN108855139B (en) | Titanium sheet with surface-modified sulfur-doped titanium dioxide nanosheet, preparation method and application thereof | |
| CN110773202A (en) | Preparation method of yolk-shell structured nickel-molybdenum bimetallic sulfide applied to water cracking | |
| CN114990619B (en) | An amorphous NiOOH/Ni3S2 heterostructure nickel-based composite catalyst and its preparation method and application | |
| CN114457369B (en) | A kind of preparation method and application of CP@MoS2-PtNi catalyst |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| WD01 | Invention patent application deemed withdrawn after publication | Application publication date:20201225 | |
| WD01 | Invention patent application deemed withdrawn after publication |