CN111969187A - Preparation method, material and application of high-performance CoSe/C-NS composite material - Google Patents
Preparation method, material and application of high-performance CoSe/C-NS composite materialDownload PDFInfo
- Publication number
- CN111969187A CN111969187ACN202010753861.2ACN202010753861ACN111969187ACN 111969187 ACN111969187 ACN 111969187ACN 202010753861 ACN202010753861 ACN 202010753861ACN 111969187 ACN111969187 ACN 111969187A
- Authority
- CN
- China
- Prior art keywords
- cose
- composite material
- zif
- precursor
- performance
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000002131composite materialSubstances0.000titleclaimsabstractdescription59
- 239000000463materialSubstances0.000titleclaimsabstractdescription28
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription22
- 239000002243precursorSubstances0.000claimsabstractdescription43
- UMGDCJDMYOKAJW-UHFFFAOYSA-NthioureaChemical compoundNC(N)=SUMGDCJDMYOKAJW-UHFFFAOYSA-N0.000claimsabstractdescription38
- 238000000137annealingMethods0.000claimsabstractdescription24
- BUGBHKTXTAQXES-UHFFFAOYSA-NSeleniumChemical compound[Se]BUGBHKTXTAQXES-UHFFFAOYSA-N0.000claimsabstractdescription21
- XSQUKJJJFZCRTK-UHFFFAOYSA-NUreaNatural productsNC(N)=OXSQUKJJJFZCRTK-UHFFFAOYSA-N0.000claimsabstractdescription19
- 238000000034methodMethods0.000claimsabstractdescription19
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000claimsabstractdescription18
- 238000002156mixingMethods0.000claimsabstractdescription15
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical group[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000claimsabstractdescription11
- HBBGRARXTFLTSG-UHFFFAOYSA-NLithium ionChemical compound[Li+]HBBGRARXTFLTSG-UHFFFAOYSA-N0.000claimsabstractdescription10
- 229910001416lithium ionInorganic materials0.000claimsabstractdescription10
- 229910052757nitrogenInorganic materials0.000claimsabstractdescription10
- 230000008569processEffects0.000claimsabstractdescription10
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000claimsdescription48
- 238000001035dryingMethods0.000claimsdescription16
- LXBGSDVWAMZHDD-UHFFFAOYSA-N2-methyl-1h-imidazoleChemical compoundCC1=NC=CN1LXBGSDVWAMZHDD-UHFFFAOYSA-N0.000claimsdescription9
- SECXISVLQFMRJM-UHFFFAOYSA-NN-MethylpyrrolidoneChemical compoundCN1CCCC1=OSECXISVLQFMRJM-UHFFFAOYSA-N0.000claimsdescription6
- 238000003756stirringMethods0.000claimsdescription4
- RYGMFSIKBFXOCR-UHFFFAOYSA-NCopperChemical compound[Cu]RYGMFSIKBFXOCR-UHFFFAOYSA-N0.000claimsdescription3
- 239000002033PVDF binderSubstances0.000claimsdescription3
- 239000006230acetylene blackSubstances0.000claimsdescription3
- 230000009471actionEffects0.000claimsdescription3
- 238000005119centrifugationMethods0.000claimsdescription3
- 239000011889copper foilSubstances0.000claimsdescription3
- 239000004570mortar (masonry)Substances0.000claimsdescription3
- 229920002981polyvinylidene fluoridePolymers0.000claimsdescription3
- 239000011248coating agentSubstances0.000claimsdescription2
- 238000000576coating methodMethods0.000claimsdescription2
- 238000000227grindingMethods0.000claimsdescription2
- 239000002904solventSubstances0.000claimsdescription2
- QGUAJWGNOXCYJF-UHFFFAOYSA-Ncobalt dinitrate hexahydrateChemical compoundO.O.O.O.O.O.[Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=OQGUAJWGNOXCYJF-UHFFFAOYSA-N0.000claims2
- 230000007547defectEffects0.000abstractdescription3
- 238000001027hydrothermal synthesisMethods0.000abstractdescription3
- 239000013078crystalSubstances0.000abstractdescription2
- 230000001351cycling effectEffects0.000abstractdescription2
- 239000000243solutionSubstances0.000description12
- UFMZWBIQTDUYBN-UHFFFAOYSA-Ncobalt dinitrateChemical compound[Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=OUFMZWBIQTDUYBN-UHFFFAOYSA-N0.000description6
- 238000010438heat treatmentMethods0.000description6
- 239000010410layerSubstances0.000description6
- 238000012360testing methodMethods0.000description5
- 230000015572biosynthetic processEffects0.000description4
- 238000003786synthesis reactionMethods0.000description4
- WHXSMMKQMYFTQS-UHFFFAOYSA-NLithiumChemical compound[Li]WHXSMMKQMYFTQS-UHFFFAOYSA-N0.000description3
- 238000002441X-ray diffractionMethods0.000description3
- 229910052744lithiumInorganic materials0.000description3
- 238000001291vacuum dryingMethods0.000description3
- XKRFYHLGVUSROY-UHFFFAOYSA-NArgonChemical group[Ar]XKRFYHLGVUSROY-UHFFFAOYSA-N0.000description2
- 239000010405anode materialSubstances0.000description2
- 229910052799carbonInorganic materials0.000description2
- 231100000357carcinogenToxicity0.000description2
- 239000003183carcinogenic agentSubstances0.000description2
- 239000003792electrolyteSubstances0.000description2
- 230000002708enhancing effectEffects0.000description2
- 239000007770graphite materialSubstances0.000description2
- 239000011259mixed solutionSubstances0.000description2
- 239000000203mixtureSubstances0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- 239000007773negative electrode materialSubstances0.000description2
- 239000000843powderSubstances0.000description2
- 239000002244precipitateSubstances0.000description2
- 239000011241protective layerSubstances0.000description2
- 238000001878scanning electron micrographMethods0.000description2
- 239000000126substanceSubstances0.000description2
- 238000005406washingMethods0.000description2
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description2
- 206010028980NeoplasmDiseases0.000description1
- 238000003917TEM imageMethods0.000description1
- 238000004833X-ray photoelectron spectroscopyMethods0.000description1
- 239000011149active materialSubstances0.000description1
- 150000001408amidesChemical class0.000description1
- 238000004458analytical methodMethods0.000description1
- 229910052786argonInorganic materials0.000description1
- 229910021383artificial graphiteInorganic materials0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 230000009286beneficial effectEffects0.000description1
- 201000011510cancerDiseases0.000description1
- 230000000711cancerogenic effectEffects0.000description1
- 238000012512characterization methodMethods0.000description1
- 238000006243chemical reactionMethods0.000description1
- 238000000975co-precipitationMethods0.000description1
- 238000010277constant-current chargingMethods0.000description1
- 239000010949copperSubstances0.000description1
- 238000002484cyclic voltammetryMethods0.000description1
- 230000007812deficiencyEffects0.000description1
- 238000007599dischargingMethods0.000description1
- 238000000445field-emission scanning electron microscopyMethods0.000description1
- 239000012467final productSubstances0.000description1
- 239000007789gasSubstances0.000description1
- 230000036541healthEffects0.000description1
- 230000006872improvementEffects0.000description1
- 230000001788irregularEffects0.000description1
- 150000002696manganeseChemical class0.000description1
- 238000013507mappingMethods0.000description1
- 230000000877morphologic effectEffects0.000description1
- 229910021382natural graphiteInorganic materials0.000description1
- 231100001081no carcinogenicityToxicity0.000description1
- 230000008520organizationEffects0.000description1
- 229910052760oxygenInorganic materials0.000description1
- 239000001301oxygenSubstances0.000description1
- 230000001681protective effectEffects0.000description1
- 230000005855radiationEffects0.000description1
- 239000002994raw materialSubstances0.000description1
- 238000011160researchMethods0.000description1
- 238000004626scanning electron microscopyMethods0.000description1
- 230000000087stabilizing effectEffects0.000description1
- 229910052717sulfurInorganic materials0.000description1
- 238000012546transferMethods0.000description1
- 238000004627transmission electron microscopyMethods0.000description1
Images
Classifications
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/581—Chalcogenides or intercalation compounds thereof
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/026—Electrodes composed of, or comprising, active material characterised by the polarity
- H01M2004/027—Negative electrodes
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Composite Materials (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及锂离子电池技术领域,特别涉及一种高性能CoSe/C-NS复合材料的制备方法及材料、应用。The invention relates to the technical field of lithium ion batteries, in particular to a preparation method, material and application of a high-performance CoSe/C-NS composite material.
背景技术Background technique
目前商用的锂离子电池负极材料主要是人造石墨和天然石墨材料,由于石墨材料的理论比容量仅为372mAh·g-1,因此无法满足日益上升的锂离子电池能量密度需求。经查询中国专利申请号为201911053422.4、名称为一种CO-Mn-S复合材料及其制备方法及应用的专利公开了通过制备ZIF-67后经过与硫脲和可溶锰盐进行水热反应得到了CO-Mn-S复合材料,其中硫脲用量约为ZIF-67用量的3-50倍,原料用量大,经济性较差,需要寻求用量更少更经济高效的方法,采用了硫代乙酰胺而这种物质在2017年10月27日世界卫生组织国际癌症研究机构公布的致癌物清单中,被归类为2B类致癌物,需要寻求致癌性更小或无致癌性的物质,应用于超级电容器中。现有的锂电池负极材料的结构稳定性不够强,在大电流密度下容量衰减过快;材料形貌结构不规则,会影响作为负极时的振实密度,需要不断改进寻找性能更高的替代材料。The current commercial lithium-ion battery anode materials are mainly artificial graphite and natural graphite materials. Since the theoretical specific capacity of graphite materials is only 372mAh·g-1, it cannot meet the increasing energy density requirements of lithium-ion batteries. After inquiring, the Chinese patent application number is 201911053422.4, and the patent named as a CO-Mn-S composite material and its preparation method and application discloses that it is obtained by preparing ZIF-67 and conducting hydrothermal reaction with thiourea and soluble manganese salt. The CO-Mn-S composite material, in which the amount of thiourea is about 3-50 times the amount of ZIF-67, the amount of raw materials is large, and the economy is poor. Amide and this substance is classified as a class 2B carcinogen in the list of carcinogens published by the World Health Organization International Agency for Research on Cancer on October 27, 2017. It is necessary to seek substances with less or no carcinogenicity. in supercapacitors. The structural stability of the existing negative electrode materials for lithium batteries is not strong enough, and the capacity decays too fast at high current density; the material morphology and structure are irregular, which will affect the tap density when used as a negative electrode, and continuous improvement is required to find alternatives with higher performance. Material.
有鉴于此,需要开发一种具有高能量密度、长循环寿命、高结构稳定性等优点的高性能锂离子电池负极材料。In view of this, it is necessary to develop a high-performance lithium-ion battery anode material with the advantages of high energy density, long cycle life, and high structural stability.
发明内容SUMMARY OF THE INVENTION
本发明的目的在于,针对现有技术的上述不足,提供一种高性能CoSe/C-NS复合材料的制备方法及材料、应用,是一种基于CoSe基外层具有碳骨架保护层的纳米多面体状的复合材料,是一步合成S掺杂的S-ZIF-67前驱体,无需水热反应,节能,省时,经过硒化退火处理后S元素均匀的分布在复合材料外层的表面,通过S引入的更多缺陷从而加宽了材料外层碳骨架的晶面间距,使得复合材料的整体结构更加稳固,增强了材料的导电性,从而使得CoSe/C-NS复合材料作为负极时拥有了更高的能量密度、更好的倍率性能、更强的循环稳定性。The object of the present invention is to provide a preparation method, material and application of a high-performance CoSe/C-NS composite material in view of the above-mentioned deficiencies of the prior art, which is a nano-polyhedron with a carbon skeleton protective layer based on a CoSe-based outer layer. The composite material is a one-step synthesis of S-doped S-ZIF-67 precursor, which does not require hydrothermal reaction, saves energy, and saves time. After selenization annealing, the S element is evenly distributed on the surface of the outer layer of the composite material. More defects introduced by S widen the interplanar spacing of the outer carbon skeleton of the material, making the overall structure of the composite more stable and enhancing the electrical conductivity of the material, thus making the CoSe/C-NS composite as a negative electrode. Higher energy density, better rate capability, and stronger cycle stability.
本发明为达到上述目的所采用的技术方案是:The technical scheme that the present invention adopts to achieve the above purpose is:
一种高性能CoSe/C-NS复合材料的制备方法,其包括以下步骤:A preparation method of high-performance CoSe/C-NS composite material, which comprises the following steps:
S1:在制备ZIF-67的过程中添加硫脲作为S源,制成有S掺杂的S-ZIF-67前驱体;S1: In the process of preparing ZIF-67, thiourea was added as the S source to make the S-ZIF-67 precursor with S doping;
S2:将S-ZIF-67前驱体与硒粉进行充分混合之后,在氮气保护下退火处理,得到CoSe/C-NS复合材料。S2: After fully mixing the S-ZIF-67 precursor and the selenium powder, annealing is performed under nitrogen protection to obtain a CoSe/C-NS composite material.
优选地,所述步骤S2中,S-ZIF-67前驱体与硒粉的质量比1:(0.8-1.2)。4.优选地,所述步骤S2中,S-ZIF-67前驱体与硒粉充分混合时间为10分钟以上,退火处理条件为:在温度550-650℃下退火处理4-5小时,升温速率设定为5℃/min。Preferably, in the step S2, the mass ratio of the S-ZIF-67 precursor to the selenium powder is 1:(0.8-1.2). 4. Preferably, in the step S2, the S-ZIF-67 precursor and the selenium powder are fully mixed for more than 10 minutes, and the annealing treatment conditions are: annealing treatment at a temperature of 550-650 ° C for 4-5 hours, the heating rate is Set to 5°C/min.
优选地,所述步骤S1中S-ZIF-67前驱体的制备过程包括:将Co(NO3)2·6H2O溶于甲醇中形成A溶液,将2-甲基咪唑和硫脲溶于甲醇中形成B溶液,然后将A溶液缓慢滴加到B溶液中混合形成C溶液,之后静置、离心、干燥后得到S-ZIF-67前驱体。Preferably, the preparation process of the S-ZIF-67 precursor in the step S1 includes: dissolving Co(NO3)2·6H2O in methanol to form A solution, dissolving 2-methylimidazole and thiourea in methanol to form B solution, then slowly drop A solution into B solution and mix to form C solution, then stand, centrifuge and dry to obtain S-ZIF-67 precursor.
优选地,所述的Co(NO3)2·6H2O、2-甲基咪唑、硫脲、甲醇的用量之比为5mmol:20mmol:15mmol:50-200ml。Preferably, the dosage ratio of the Co(NO3)2·6H2O, 2-methylimidazole, thiourea and methanol is 5mmol:20mmol:15mmol:50-200ml.
优选地,所述的静置时间为24小时以上;所述的离心采用甲醇为溶剂,离心洗涤三次以上;所述的干燥为在70℃下干燥24-48小时。Preferably, the standing time is more than 24 hours; the centrifugation adopts methanol as a solvent, and the centrifugal washing is performed more than three times; and the drying is drying at 70° C. for 24-48 hours.
一种上述的高性能CoSe/C-NS复合材料的制备方法得到CoSe/C-NS复合材料。A preparation method of the above-mentioned high-performance CoSe/C-NS composite material obtains the CoSe/C-NS composite material.
一种上述的CoSe/C-NS复合材料的应用,将所述的复合材料制备成负极极片,应用于锂离子电池中。优选地,所述的负极极片的制备过程包括:将CoSe/C-NS复合材料与乙炔黑、PVDF按照重量比为8:1:1在研钵中研磨10分钟以上充分混合之后,滴加适量的N-甲基吡络烷酮(NMP)并于室温下在磁力搅拌器的作用下搅拌10个小时得到浆糊状材料,将糊状材料均匀的倾倒到集流体铜箔上经涂布涂覆得到负极极片,经干燥后成为锂离子电池工作电极。An application of the above-mentioned CoSe/C-NS composite material, the composite material is prepared into a negative pole piece, which is used in a lithium ion battery. Preferably, the preparation process of the negative pole piece includes: after fully mixing the CoSe/C-NS composite material, acetylene black and PVDF in a mortar for more than 10 minutes in a weight ratio of 8:1:1, adding dropwise A proper amount of N-methylpyrrolidone (NMP) was stirred at room temperature under the action of a magnetic stirrer for 10 hours to obtain a paste-like material, which was uniformly poured onto the current collector copper foil and coated The negative electrode sheet is obtained by coating, and becomes the working electrode of the lithium ion battery after drying.
与现有技术相比,本发明具有如下有益效果:Compared with the prior art, the present invention has the following beneficial effects:
1.本发明的CoSe/C-NS复合材料是一种基于CoSe基外层具有碳骨架保护层的纳米多面体状的复合材料,是一步合成S掺杂的S-ZIF-67前驱体,无需水热反应,节能,省时,可以增加材料产率,具有更好的经济性,因S-ZIF-67是一步合成的,通过共沉淀合成,S-ZIF-67中的S均匀的分布在ZIF-67的外部碳层上的,保持了ZIF-67特有的形貌特点的同时,S元素的分布也更加均匀;而且,经过硒化退火处理后的CoSe/C-NS复合材料具有完美的中空结构,可以看到S元素均匀的分布在复合材料外层的表面,起到稳定材料结构的作用。1. The CoSe/C-NS composite material of the present invention is a nano-polyhedral-shaped composite material based on a CoSe-based outer layer with a carbon skeleton protective layer, which is a one-step synthesis of S-doped S-ZIF-67 precursor without water. Thermal reaction, energy saving, time saving, can increase material yield, and have better economy, because S-ZIF-67 is synthesized in one step, through co-precipitation synthesis, S in S-ZIF-67 is evenly distributed in ZIF On the outer carbon layer of -67, while maintaining the unique morphological characteristics of ZIF-67, the distribution of S element is also more uniform; moreover, the CoSe/C-NS composite after selenization annealing has a perfect hollow It can be seen that the S element is evenly distributed on the surface of the outer layer of the composite material, which plays a role in stabilizing the material structure.
2.本发明基于ZIF-67前驱体,经过硒化退火处理后的N掺杂的碳纳米多面体应用于锂离子电池负极材料,展现出优良的性能,通过使用S掺杂的ZIF-67作为前驱体,引入S元素掺杂复合材料外层的碳骨架,在之前已有的N掺杂CoSe复合材料的基础上研发了N、S共掺杂的CoSe/C复合材料,通过S引入的更多缺陷从而加宽了材料外层碳骨架的晶面间距,使得复合材料的整体结构更加稳固,增强了材料的导电性,从而使得CoSe/C-NS复合材料作为负极时拥有了更高的能量密度、更好的倍率性能、更强的循环稳定性。在200mA·g-1的电流密度下,300个循环过后依旧可以保持1494mAh·g-1的比容量;以及在高电流密度下(2A·g-1)也展现出了很好的循环性能(在500次充放电循环后依然保持513mAh·g-1以上的比容量);在100mA·g-1的电流密度下,首次充放电时库伦效率约为50.99%,190次循环过后剩余容量为1244mAh·g-1;期间最大容量出现在第157次循环,容量达到了1325mA·g-1。2. The present invention is based on ZIF-67 precursor, and the N-doped carbon nanopolyhedron after selenization annealing treatment is applied to the negative electrode material of lithium ion battery, showing excellent performance, by using S-doped ZIF-67 as a precursor The carbon skeleton of the outer layer of the S element-doped composite material was introduced, and the N and S co-doped CoSe/C composite material was developed on the basis of the previous N-doped CoSe composite material. More The defect thus widens the interplanar spacing of the outer carbon skeleton of the material, making the overall structure of the composite more stable and enhancing the electrical conductivity of the material, so that the CoSe/C-NS composite has a higher energy density when used as a negative electrode , better rate performance, stronger cycle stability. At a current density of 200mA·g-1, the specific capacity of 1494mAh·g-1 can still be maintained after 300 cycles; and at a high current density (2A·g-1), it also exhibits good cycling performance ( After 500 charge-discharge cycles, it still maintains a specific capacity of more than 513mAh·g-1); at a current density of 100mA·g-1, the Coulombic efficiency during the first charge and discharge is about 50.99%, and the remaining capacity after 190 cycles is 1244mAh. ·g-1; the maximum capacity during the period appeared in the 157th cycle, and the capacity reached 1325mA·g-1.
3.本发明的CoSe/C-NS复合材料获取纳米层面上更为规则的材料形貌,增强CoSe/C材料的结构稳定性;对CoSe/C材料进行进一步的改性以获得更好的能量密度;使CoSe/C复合材料的结构更加稳定以获得更好的倍率性能;改善CoSe/C复合材料作为负极时在大电流密度下的电化学性能。3. The CoSe/C-NS composite material of the present invention obtains a more regular material morphology at the nanometer level, and enhances the structural stability of the CoSe/C material; the CoSe/C material is further modified to obtain better energy Density; make the structure of CoSe/C composite more stable for better rate performance; improve the electrochemical performance of CoSe/C composite at high current density when it is used as anode.
上述是发明技术方案的概述,以下结合附图和具体实施方式,对本发明做进一步说明。The above is an overview of the technical solutions of the invention. The invention will be further described below with reference to the accompanying drawings and specific embodiments.
附图说明:Description of drawings:
图1为ZIF-67和S-ZIF-67的SEM图像(a、b)以及CoSe/C-NS和CoSe/C-N的SEM图像(c、d);Figure 1 shows the SEM images of ZIF-67 and S-ZIF-67 (a, b) and the SEM images of CoSe/C-NS and CoSe/C-N (c, d);
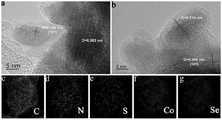
图2为CoSe/C-NS和CoSe/C-N的TEM图像(a、b)以及CoSe/C-NS的TEM Mapping图像(c-g);Figure 2 shows the TEM images of CoSe/C-NS and CoSe/C-N (a, b) and the TEM Mapping images of CoSe/C-NS (c-g);
图3为S-ZIF-67和ZIF-67的XRD图像(a)以及CoSe/C-NS和CoSe/C-N的XRD图像(b)。Figure 3 shows the XRD patterns (a) of S-ZIF-67 and ZIF-67 and the XRD patterns (b) of CoSe/C-NS and CoSe/C-N.
具体实施方式:Detailed ways:
为了使本发明的目的和技术方案及优点更加清楚明白,以下结合实施例作详细说明。应当理解,此处所描述的具体实施例仅仅用以解释本发明,并不用于限定本发明。In order to make the objectives, technical solutions and advantages of the present invention more clear, detailed descriptions are given below in conjunction with the embodiments. It should be understood that the specific embodiments described herein are only used to explain the present invention, but not to limit the present invention.
实施例1:本实施例提供的一种高性能CoSe/C-NS复合材料的制备方法及材料、应用,结合附图1-3,包括(1)S掺杂的ZIF-67前驱体(S-ZIF-67)的制备过程:将5mmol的Co(NO3)2·6H2O充分溶解于50ml甲醇中形成A溶液(洋红色);再将20mmol的2-甲基咪唑和15mmol的硫脲充分共溶于50ml甲醇中形成溶液B(无色透明);在磁力搅拌器的强力搅拌下,将A溶液使用胶头滴管非常缓慢的滴加到B溶液当中形成混合溶液C(深紫色);将混合溶液C同样在磁力搅拌器的强力搅拌下于室温下进行静置24个小时,之后将混合溶液C中的沉淀物使用甲醇进行反复的离心洗涤,这一过程通常需要持续3次以上,再将离心洗涤后的紫色沉淀物在70℃下置于真空干燥箱中干燥24个小时,最终收集到物质深紫色粉末S-ZIF-67前驱体。Embodiment 1: The preparation method, material and application of a high-performance CoSe/C-NS composite material provided in this embodiment, with reference to Figures 1-3, including (1) S-doped ZIF-67 precursor (S -ZIF-67) preparation process: fully dissolve 5mmol of Co(NO3)2·6H2O in 50ml of methanol to form A solution (magenta); then fully co-dissolve 20mmol of 2-methylimidazole and 15mmol of thiourea Solution B (colorless and transparent) was formed in 50ml methanol; under the strong stirring of a magnetic stirrer, A solution was very slowly added dropwise to solution B using a plastic tip dropper to form mixed solution C (dark purple); Solution C was also left to stand at room temperature for 24 hours under the strong stirring of a magnetic stirrer, and then the precipitate in mixed solution C was repeatedly centrifuged and washed with methanol. This process usually required more than 3 times. After centrifugation and washing, the purple precipitate was dried in a vacuum drying oven at 70 °C for 24 hours, and finally a dark purple powder S-ZIF-67 precursor was collected.
(2)将S-ZIF-67前驱体与硒粉按设定质量比充分混合之后,在氮气保护下退火处理,得到CoSe/C-NS复合材料,在合成ZIF-67(多孔晶体材料)的过程中添加硫脲作为S源,合成有S掺杂的ZIF-67(S-ZIF-67)前驱体(深紫色)。再将S-ZIF-67与硒粉进行充分混合10分钟以上,之后将混合充分的物质(紫黑色)移至管式炉中在氮气的保护下以600℃退火4小时,升温速率设定为5℃/min,收集最终产物黑色粉末CoSe/C-NS复合材料。(2) After fully mixing S-ZIF-67 precursor and selenium powder according to the set mass ratio, annealing treatment under nitrogen protection to obtain CoSe/C-NS composite material, in the synthesis of ZIF-67 (porous crystal material) In the process, thiourea was added as S source, and S-doped ZIF-67 (S-ZIF-67) precursor (dark purple) was synthesized. Then S-ZIF-67 and selenium powder were thoroughly mixed for more than 10 minutes, and then the fully mixed material (purple black) was moved to a tube furnace and annealed at 600 °C for 4 hours under the protection of nitrogen, and the heating rate was set as 5 °C/min, collect the final product black powder CoSe/C-NS composite.
(3)应用:负极极片的制备过程包括将活性材料(CoSe/C-NS)、乙炔黑、PVDF按照重量比为8:1:1在研钵中研磨10分钟以上,使三者充分混合,滴加适量的N-甲基吡络烷酮(NMP)并于室温下在磁力搅拌器的作用下搅拌10个小时得到浆糊状材料,将糊状材料均匀的倾倒到集流体(铜箔)上,使用用手工涂布器涂敷为厚度约为150μm的极片。使用鼓风干燥箱设定温度为80℃对极片进行干燥12个小时,最后转移至电热真空干燥箱中以120℃干燥12个小时;通过切片机切成直径约为1.2cm的圆形极片,继续以120℃在真空干燥箱中进行干燥,留待组装扣式电池。(3) Application: The preparation process of the negative pole piece includes grinding the active material (CoSe/C-NS), acetylene black, and PVDF in a mortar at a weight ratio of 8:1:1 for more than 10 minutes to fully mix the three , drop an appropriate amount of N-methylpyrrolidone (NMP) and stir at room temperature for 10 hours under the action of a magnetic stirrer to obtain a paste-like material, pour the paste-like material evenly into the current collector (copper foil) ), a pole piece with a thickness of about 150 μm was applied using a hand coater. Use a blast drying oven to set the temperature at 80 °C to dry the pole pieces for 12 hours, and finally transfer them to an electric vacuum drying oven for drying at 120 °C for 12 hours; cut them into circular poles with a diameter of about 1.2 cm by a microtome. Then, continue to dry at 120°C in a vacuum drying oven until the coin cell battery is assembled.
(4)模拟扣式电池的组装:扣式电池的规格为CR2016型,在手套箱中进行组装,手套箱内保护气为氩气,水氧分压均低于1ppm,按照顺序将CR2016配套的正极壳、垫片、锂片、隔膜、负极极片、垫片依次组装,并滴加适量电解液至锂片、隔膜、负极片之间使得电解液充分浸润隔膜和负极片;最后将组装好的模拟扣式电池在4Mpa左右的压强下进行封口压实;将组装好的电池于室温下静置8-12小时候进行测试。(4) Assembly of the simulated button battery: The specification of the button battery is CR2016, and it is assembled in a glove box. The protective gas in the glove box is argon, and the partial pressure of water and oxygen is lower than 1ppm. The positive electrode shell, gasket, lithium sheet, separator, negative electrode sheet, and gasket are assembled in sequence, and an appropriate amount of electrolyte is dripped between the lithium sheet, the separator, and the negative electrode sheet to make the electrolyte fully infiltrate the separator and the negative electrode sheet; finally, the assembled The simulated button battery is sealed and compacted under the pressure of about 4Mpa; the assembled battery is left for 8-12 hours at room temperature for testing.
(5)表征、测试:分别对于CoSe/C-NS复合材料采用如下方法进行表征,扫描电子显微镜表征(设备型号:FESEM,Hitachi S-4800),透射电子显微镜表征(设备型号:TEM,JEOL,JEM-2100F),X射线衍射分析(测试信息:采用阳极靶材Cu靶Kα辐射源,λ=0.15418nm,扫描角度为5-80°,扫描速率5°·min-1,工作电压40kV,工作电流40mA),X射线光电子能谱分析(设备型号:Thermo Fisher K-Alpha spectrometer)。采用BTS-5V/5mA-164型恒流充放电设备电压范围0.01V-3V并分别以50mA·g-1,100mA·g-1,200mA·g-1,500mA·g-1,1A·g-1,2A·g-1的电流密度得到充放电曲线,循环性能曲线和倍率性能曲线,并计算其库伦效率。采用CHI660C型电化学工作站进行复合材料的循环伏安测试(0.1mV·s-1,0.01-3V)和交流阻抗测试(0.01Hz-100kHz,5mV)。(5) Characterization and testing: CoSe/C-NS composites were characterized by the following methods, scanning electron microscopy (equipment model: FESEM, Hitachi S-4800), transmission electron microscopy (equipment model: TEM, JEOL, JEM-2100F), X-ray diffraction analysis (test information: using anode target Cu target Kα radiation source, λ=0.15418nm, scanning angle 5-80°,
实施例2:本实施例提供的一种高性能CoSe/C-NS复合材料的制备方法及材料、应用,其与实施例1基本相同,不同之处在于:Embodiment 2: The preparation method, material and application of a high-performance CoSe/C-NS composite material provided in this embodiment are basically the same as those in Embodiment 1, except that:
S1:在制备ZIF-67的过程中添加硫脲作为S源,制成有S掺杂的S-ZIF-67前驱体;S-ZIF-67前驱体的制备过程中,所述的Co(NO3)2·6H2O、2-甲基咪唑、硫脲、甲醇的用量之比为5mmol:20mmol:15mmol:50ml;所述的静置时间为24小时以上;所述的干燥为在70℃下干燥24小时。S1: In the process of preparing ZIF-67, thiourea was added as an S source to make S-ZIF-67 precursor; during the preparation of S-ZIF-67 precursor, the Co(NO3 ) The ratio of the consumption of 2.6H2O, 2-methylimidazole, thiourea, and methanol is 5mmol: 20mmol: 15mmol: 50ml; the standing time is more than 24 hours; the drying is drying at 70 ° C for 24 hours. Hour.
S2:将S-ZIF-67前驱体与硒粉进行充分混合之后,在氮气保护下退火处理,得到CoSe/C-NS复合材料。S-ZIF-67前驱体与硒粉的质量比为1:0.8;S-ZIF-67前驱体与硒粉充分混合时间为10分钟以上,退火处理条件为:在温度550℃下退火处理4小时,升温速率设定为5℃/min。S2: After fully mixing the S-ZIF-67 precursor and the selenium powder, annealing is performed under nitrogen protection to obtain a CoSe/C-NS composite material. The mass ratio of S-ZIF-67 precursor and selenium powder is 1:0.8; the time for fully mixing S-ZIF-67 precursor and selenium powder is more than 10 minutes, and the annealing treatment conditions are: annealing treatment at a temperature of 550 ° C for 4 hours , and the heating rate was set to 5 °C/min.
实施例3:本实施例提供的一种高性能CoSe/C-NS复合材料的制备方法及材料、应用,其与实施例1基本相同,不同之处在于:Embodiment 3: The preparation method, material and application of a high-performance CoSe/C-NS composite material provided in this embodiment are basically the same as those in Embodiment 1, except that:
S1:在制备ZIF-67的过程中添加硫脲作为S源,制成有S掺杂的S-ZIF-67前驱体;S-ZIF-67前驱体的制备过程中,所述的Co(NO3)2·6H2O、2-甲基咪唑、硫脲、甲醇的用量之比为5mmol:20mmol:15mmol:150ml;所述的静置时间为24小时以上;所述的干燥为在70℃下干燥24小时。S1: In the process of preparing ZIF-67, thiourea was added as an S source to make S-ZIF-67 precursor; during the preparation of S-ZIF-67 precursor, the Co(NO3 ) The ratio of the consumption of 2.6H2O, 2-methylimidazole, thiourea, and methanol is 5mmol: 20mmol: 15mmol: 150ml; the standing time is more than 24 hours; the drying is drying at 70 ° C for 24 hours. Hour.
S2:将S-ZIF-67前驱体与硒粉进行充分混合之后,在氮气保护下退火处理,得到CoSe/C-NS复合材料。S-ZIF-67前驱体与硒粉的质量比为1:0.8;S-ZIF-67前驱体与硒粉充分混合时间为10分钟以上,退火处理条件为:在温度650℃下退火处理5小时,升温速率设定为5℃/min。S2: After fully mixing the S-ZIF-67 precursor and the selenium powder, annealing is performed under nitrogen protection to obtain a CoSe/C-NS composite material. The mass ratio of S-ZIF-67 precursor and selenium powder is 1:0.8; the time for full mixing of S-ZIF-67 precursor and selenium powder is more than 10 minutes, and the annealing treatment conditions are: annealing treatment at a temperature of 650 ° C for 5 hours , and the heating rate was set to 5 °C/min.
实施例4:本实施例提供的一种高性能CoSe/C-NS复合材料的制备方法及材料、应用,其与实施例1基本相同,不同之处在于:Embodiment 4: The preparation method, material, and application of a high-performance CoSe/C-NS composite material provided in this embodiment are basically the same as those in Embodiment 1, except that:
S1:在制备ZIF-67的过程中添加硫脲作为S源,制成有S掺杂的S-ZIF-67前驱体;S-ZIF-67前驱体的制备过程中,所述的Co(NO3)2·6H2O、2-甲基咪唑、硫脲、甲醇的用量之比为5mmol:20mmol:15mmol:200ml;所述的静置时间为24小时以上;所述的干燥为在70℃下干燥48小时。S1: In the process of preparing ZIF-67, thiourea was added as an S source to make S-ZIF-67 precursor; during the preparation of S-ZIF-67 precursor, the Co(NO3 ) The ratio of the consumption of 2.6H2O, 2-methylimidazole, thiourea, and methanol is 5mmol: 20mmol: 15mmol: 200ml; the standing time is more than 24 hours; the drying is drying at 70 ° C for 48 Hour.
S2:将S-ZIF-67前驱体与硒粉进行充分混合之后,在氮气保护下退火处理,得到CoSe/C-NS复合材料。S-ZIF-67前驱体与硒粉的质量比为1:1;S-ZIF-67前驱体与硒粉充分混合时间为10分钟以上,退火处理条件为:在温度600℃下退火处理5小时,升温速率设定为5℃/min。S2: After fully mixing the S-ZIF-67 precursor and the selenium powder, annealing is performed under nitrogen protection to obtain a CoSe/C-NS composite material. The mass ratio of S-ZIF-67 precursor and selenium powder is 1:1; the time for full mixing of S-ZIF-67 precursor and selenium powder is more than 10 minutes, and the annealing treatment conditions are: annealing treatment at a temperature of 600 ℃ for 5 hours , and the heating rate was set to 5 °C/min.
实施例5:本实施例提供的一种高性能CoSe/C-NS复合材料的制备方法及材料、应用,其与实施例1基本相同,不同之处在于:Embodiment 5: The preparation method, material, and application of a high-performance CoSe/C-NS composite material provided in this embodiment are basically the same as those in Embodiment 1, except that:
S1:在制备ZIF-67的过程中添加硫脲作为S源,制成有S掺杂的S-ZIF-67前驱体;S-ZIF-67前驱体的制备过程中,所述的Co(NO3)2·6H2O、2-甲基咪唑、硫脲、甲醇的用量之比为5mmol:20mmol:15mmol:200ml;所述的静置时间为24小时以上;所述的干燥为在70℃下干燥48小时。S1: In the process of preparing ZIF-67, thiourea was added as an S source to make S-ZIF-67 precursor; during the preparation of S-ZIF-67 precursor, the Co(NO3 ) The ratio of the consumption of 2.6H2O, 2-methylimidazole, thiourea, and methanol is 5mmol: 20mmol: 15mmol: 200ml; the standing time is more than 24 hours; the drying is drying at 70 ° C for 48 Hour.
S2:将S-ZIF-67前驱体与硒粉进行充分混合之后,在氮气保护下退火处理,得到CoSe/C-NS复合材料。S-ZIF-67前驱体与硒粉的质量比为1:1.1;S-ZIF-67前驱体与硒粉充分混合时间为10分钟以上,退火处理条件为:在温度650℃下退火处理4小时,升温速率设定为5℃/min。S2: After fully mixing the S-ZIF-67 precursor and the selenium powder, annealing is performed under nitrogen protection to obtain a CoSe/C-NS composite material. The mass ratio of S-ZIF-67 precursor and selenium powder is 1:1.1; the time for full mixing of S-ZIF-67 precursor and selenium powder is more than 10 minutes, and the annealing treatment conditions are: annealing treatment at a temperature of 650 ° C for 4 hours , and the heating rate was set to 5 °C/min.
根据上述说明书的揭示和教导,本发明所属领域的技术人员还可以对上述实施方式进行变更和修改。因此,本发明并不局限于上面揭示和描述的具体实施方式,对发明的一些修改和变更也应当落入本发明的权利要求的保护范围内。Based on the disclosure and teaching of the above specification, those skilled in the art to which the present invention pertains can also make changes and modifications to the above embodiments. Therefore, the present invention is not limited to the specific embodiments disclosed and described above, and some modifications and changes to the invention should also fall within the protection scope of the claims of the present invention.
Claims (9)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010753861.2ACN111969187A (en) | 2020-07-30 | 2020-07-30 | Preparation method, material and application of high-performance CoSe/C-NS composite material |
| CN202110717068.1ACN113346065A (en) | 2020-07-30 | 2021-06-26 | Preparation method, material and application of high-performance CoSe/C-NS composite material |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010753861.2ACN111969187A (en) | 2020-07-30 | 2020-07-30 | Preparation method, material and application of high-performance CoSe/C-NS composite material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111969187Atrue CN111969187A (en) | 2020-11-20 |
Family
ID=73363340
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010753861.2AWithdrawnCN111969187A (en) | 2020-07-30 | 2020-07-30 | Preparation method, material and application of high-performance CoSe/C-NS composite material |
| CN202110717068.1APendingCN113346065A (en) | 2020-07-30 | 2021-06-26 | Preparation method, material and application of high-performance CoSe/C-NS composite material |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110717068.1APendingCN113346065A (en) | 2020-07-30 | 2021-06-26 | Preparation method, material and application of high-performance CoSe/C-NS composite material |
Country Status (1)
| Country | Link |
|---|---|
| CN (2) | CN111969187A (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116613318B (en)* | 2023-06-09 | 2024-02-20 | 广东格林赛福能源科技有限公司 | CoSe/Te composite material, preparation method and application |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107681142A (en)* | 2017-09-29 | 2018-02-09 | 合肥工业大学 | A kind of molybdenum disulfide cladding carbon nano-fiber as lithium ion battery negative material and preparation method thereof |
| CN110783549A (en)* | 2019-11-07 | 2020-02-11 | 吉林大学 | Polypyrrole-coated sulfur-doped cobalt-based carbon nanocage material, and preparation method and application thereof |
| CN110890534A (en)* | 2019-11-29 | 2020-03-17 | 中国石油大学(华东) | Cobalt selenide @ carbon composite material for high-performance potassium ion battery cathode, preparation method of cobalt selenide @ carbon composite material and matched electrolyte |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108183224B (en)* | 2017-12-30 | 2021-03-23 | 武汉理工大学 | In-situ nitrogen-doped porous core-shell carbon/selenium composite material and its preparation method and application |
| CN108383098B (en)* | 2018-01-12 | 2022-01-07 | 上海大学 | Hollow porous carbon material co-doped with various heteroatoms, and preparation method and application thereof |
| CN108686693A (en)* | 2018-04-19 | 2018-10-23 | 重庆大学 | A kind of preparation method of monatomic cobalt-based nitrogen sulphur codope carbon material catalyst |
| CN108649198B (en)* | 2018-05-08 | 2021-02-26 | 南开大学 | A kind of synthetic method of cobalt-embedded nitrogen and sulfur co-doped carbon nanomaterials |
| CN109037617B (en)* | 2018-07-10 | 2020-09-04 | 厦门理工学院 | Cobalt selenide/nitrogen-doped carbon composite material and preparation method and application thereof |
| CN109192949A (en)* | 2018-08-31 | 2019-01-11 | 扬州大学 | Suede shell hollow polyhedral Co is obtained by ZIF-67 multi-panel derivatization9S8@MoS2Method |
| CN109585823A (en)* | 2018-11-23 | 2019-04-05 | 重庆文理学院 | A kind of preparation method of cobaltous selenide/graphite carbon composite |
| CN109768237B (en)* | 2018-12-24 | 2020-11-27 | 肇庆市华师大光电产业研究院 | Lithium-sulfur battery positive electrode material, preparation method and application |
| CN109873134A (en)* | 2019-01-17 | 2019-06-11 | 华南师范大学 | In-situ carbon-encapsulated iron-based chalcogenide, electrode material, sodium-ion battery and preparation method thereof |
| CN110534738B (en)* | 2019-08-19 | 2021-02-09 | 中南大学 | A kind of dianion cobalt-based selenide sulfide and preparation method thereof |
- 2020
- 2020-07-30CNCN202010753861.2Apatent/CN111969187A/ennot_activeWithdrawn
- 2021
- 2021-06-26CNCN202110717068.1Apatent/CN113346065A/enactivePending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107681142A (en)* | 2017-09-29 | 2018-02-09 | 合肥工业大学 | A kind of molybdenum disulfide cladding carbon nano-fiber as lithium ion battery negative material and preparation method thereof |
| CN110783549A (en)* | 2019-11-07 | 2020-02-11 | 吉林大学 | Polypyrrole-coated sulfur-doped cobalt-based carbon nanocage material, and preparation method and application thereof |
| CN110890534A (en)* | 2019-11-29 | 2020-03-17 | 中国石油大学(华东) | Cobalt selenide @ carbon composite material for high-performance potassium ion battery cathode, preparation method of cobalt selenide @ carbon composite material and matched electrolyte |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113346065A (en) | 2021-09-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109004199B (en) | Preparation method of biomass hard carbon material for negative electrode of sodium-ion battery | |
| CN106099062B (en) | Silicon based composite material Si@C@TiO are covered in double-contracting2And preparation method thereof | |
| CN106450102B (en) | Graphite modified separator for lithium-sulfur battery, preparation method and lithium-sulfur battery | |
| CN107275587B (en) | A kind of lithium ion silicon-carbon composite cathode material and preparation method thereof | |
| CN110212162B (en) | A kind of flexible gel sulfur cathode for lithium-sulfur battery and preparation method thereof | |
| CN114583137B (en) | Method for modifying carbon surface by sulfur doped phosphorus and application thereof | |
| CN106876673B (en) | One-step preparation method of titanium dioxide and graphene double-layer co-coated core-shell structure lithium-sulfur battery cathode material | |
| CN107342421A (en) | A kind of high content pyridine N doping porous carbon negative material, preparation method and applications | |
| CN110176597A (en) | A kind of preparation and application of biomass carbon/sulphur composite material | |
| CN106374101A (en) | Preparation method and application of a Co3O4@Co@carbon nanocage | |
| CN109354015A (en) | A kind of activated carbon, electrode and test method for making lithium ion negative electrode with sunflower plate | |
| CN114751393A (en) | Nitrogen-sulfur co-doped porous carbon/sulfur composite material and preparation method and application thereof | |
| CN106654182B (en) | Manganese dioxide sulphur carbon anode and preparation method | |
| CN106848196A (en) | A kind of lithium-sulfur cell negative plate | |
| CN105742619B (en) | A kind of unformed Mn oxide cladding ferriferous oxide lithium/anode material of lithium-ion battery and preparation method thereof | |
| CN104241651A (en) | Method for preparing sulphur-supported porous carbon lithium battery electrode material by using waste polyurethane plastic | |
| CN111969187A (en) | Preparation method, material and application of high-performance CoSe/C-NS composite material | |
| CN112117502A (en) | Aqueous ion battery and application thereof | |
| CN110635093A (en) | A lithium-sulfur battery cathode and separator integrated structure and preparation method thereof | |
| CN106910883B (en) | Preparation method of lithium-sulfur battery | |
| CN112086629B (en) | Si @ C/ZnNb2O6Preparation method and application of negative electrode composite material | |
| CN114477305A (en) | Preparation method and application of ferrous disulfide positive electrode material of magnesium-lithium double-ion battery | |
| CN114447335A (en) | Silicon-tin-oxygen-carbon composite electrode material and preparation method and application thereof | |
| CN114804072A (en) | Preparation method of ZnO/CuO heterojunction-doped ultrathin carbon sheet lithium-sulfur battery anode material | |
| CN109860527B (en) | Carbon-based composite material for preparing lithium battery cathode and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| WW01 | Invention patent application withdrawn after publication | ||
| WW01 | Invention patent application withdrawn after publication | Application publication date:20201120 |