CN111763955A - Superaerophobic platinum hydrogen evolution electrode, its preparation method and hydrogen preparation method - Google Patents
Superaerophobic platinum hydrogen evolution electrode, its preparation method and hydrogen preparation methodDownload PDFInfo
- Publication number
- CN111763955A CN111763955ACN202010662834.4ACN202010662834ACN111763955ACN 111763955 ACN111763955 ACN 111763955ACN 202010662834 ACN202010662834 ACN 202010662834ACN 111763955 ACN111763955 ACN 111763955A
- Authority
- CN
- China
- Prior art keywords
- platinum
- electrode
- hydrogen
- superaerophobic
- hydrogen evolution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/02—Hydrogen or oxygen
- C25B1/04—Hydrogen or oxygen by electrolysis of water
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D3/00—Electroplating: Baths therefor
- C25D3/02—Electroplating: Baths therefor from solutions
- C25D3/50—Electroplating: Baths therefor from solutions of platinum group metals
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明通常涉及无机催化材料制备技术领域。The present invention generally relates to the technical field of preparation of inorganic catalytic materials.
背景技术Background technique
氢气作为一种可再生的清洁燃料,其能量密度高、产能效率好,是能量储存的理想载体,可以解决可持续性,环境排放和能源安全问题,因其在未来可能取代化石燃料而备受世界各国的关注。As a renewable and clean fuel with high energy density and high production efficiency, hydrogen is an ideal carrier for energy storage and can address sustainability, environmental emissions and energy security issues, and has received much attention due to its potential to replace fossil fuels in the future. the world's attention.
电催化水分解是制取氢气的可持续途径之一。氢还原反应包括质子在电极表面的还原以及产物氢气在电极表面的脱附。传统的薄片铂析氢电极只有表面才能与电解液接触,电极的有效反应面积小。而且,由于氢气在电极表面的粘附力大,氢气气泡粘附在电极表面,阻碍了电解液溶液与催化剂表面的接触,降低了催化活性,不能满足高效氢还原反应制氢气的需求。Electrocatalytic water splitting is one of the sustainable ways to produce hydrogen. The hydrogen reduction reaction includes the reduction of protons on the electrode surface and the desorption of the product hydrogen on the electrode surface. Only the surface of the traditional thin platinum hydrogen evolution electrode can be in contact with the electrolyte, and the effective reaction area of the electrode is small. Moreover, due to the strong adhesion of hydrogen on the electrode surface, hydrogen bubbles adhere to the electrode surface, which hinders the contact between the electrolyte solution and the catalyst surface, reduces the catalytic activity, and cannot meet the needs of high-efficiency hydrogen reduction reaction for hydrogen production.
现有技术中有利用循环伏安沉积法在钛片表面制备出了铂纳米针析氢电极的报道。另外,也有通过在TiO2纳米管上光催化沉积铂颗粒制得析氢电极的报道。但是这些方法制备出来的析氢电极是在薄片载体上形成铂纳米针或者纳米颗粒,导致析氢反应时的有效反应面积小。In the prior art, there are reports that platinum nanoneedle hydrogen evolution electrodes are prepared on the surface of titanium sheets by cyclic voltammetry deposition. In addition, there have also been reports on the preparation of hydrogen-evolution electrodes by photocatalytic deposition of platinum particles onTiO2 nanotubes. However, the hydrogen evolution electrodes prepared by these methods form platinum nanoneedles or nanoparticles on the sheet carrier, resulting in a small effective reaction area during the hydrogen evolution reaction.
因此,如何提高析氢电极的有效反应面积小、降低电极表面的粘附力是铂析氢电极这个领域亟待解决的技术问题。Therefore, how to improve the small effective reaction area of the hydrogen evolution electrode and reduce the adhesion of the electrode surface are the technical problems to be solved urgently in the field of platinum hydrogen evolution electrode.
发明内容SUMMARY OF THE INVENTION
为了解决上述问题,本发明人等经过深入研究后发现,通过使用网状的导电载体,利用电沉积技术在这样的呈网状的导电载体表面生长出来的铂呈现为尖锐的纳米片状,这样的纳米片状结构的铂电极作为析氢电极使用时,电极表面与电解液的接触面积大,从而大大提高了析氢反应时氢还原反应的催化活性。另外,铂呈尖锐的纳米片状结构,使得电极表面的气泡粘附力进一步降低,从而有利于析氢反应产生的氢气从电极表面脱附。这两个作用协同发挥效果,从而大大增强了电极的催化活性。从而完成了本发明。In order to solve the above-mentioned problems, the inventors have found after intensive research that by using a mesh-shaped conductive carrier, the platinum grown on the surface of such a mesh-shaped conductive carrier by electrodeposition technology presents a sharp nano-sheet shape. When the platinum electrode with the nano-sheet structure is used as a hydrogen evolution electrode, the contact area between the electrode surface and the electrolyte is large, thereby greatly improving the catalytic activity of the hydrogen reduction reaction during the hydrogen evolution reaction. In addition, platinum has a sharp nano-sheet structure, which further reduces the adhesion of bubbles on the electrode surface, which is conducive to the desorption of hydrogen produced by the hydrogen evolution reaction from the electrode surface. These two effects work synergistically, thereby greatly enhancing the catalytic activity of the electrode. Thus, the present invention has been completed.
本发明提供以下的技术方案。The present invention provides the following technical solutions.
本发明一方面,提供一种超疏气铂析氢电极,其具有网状导电载体以及沉积在所述网状导电载体上的铂纳米片,所述铂纳米片分布在所述导电载体的两面。In one aspect of the present invention, a superaerophobic platinum hydrogen evolution electrode is provided, which has a reticulated conductive carrier and platinum nanosheets deposited on the reticulated conductive carrier, and the platinum nanosheets are distributed on both sides of the conductive carrier.
优选地,前述导电载体的材质为金、银、镍、钛或者碳。Preferably, the material of the aforementioned conductive carrier is gold, silver, nickel, titanium or carbon.
优选地,前述网状导电载体为100~1500目。Preferably, the aforementioned mesh conductive carrier is 100-1500 mesh.
优选地,前述铂纳米片的尺寸为10~400纳米,厚度为1~30nm。更优选铂纳米片的尺寸为30~300纳米,厚度为5~20nm。需要说明的是,铂纳米片的尺寸是指,其横向的尺寸。例如,铂纳米片的尺寸为10nm是指,其为10nm见方的片状的结构。Preferably, the size of the aforementioned platinum nanosheets is 10-400 nm, and the thickness is 1-30 nm. More preferably, the size of the platinum nanosheets is 30-300 nm, and the thickness is 5-20 nm. It should be noted that the size of the platinum nanosheet refers to the size in the lateral direction. For example, that the size of the platinum nanosheet is 10 nm means that it has a 10 nm square sheet-like structure.
本发明的另一方面,提供本发明的超疏气铂析氢电极的制备方法,其包括:Another aspect of the present invention provides the preparation method of the superaerophobic platinum hydrogen evolution electrode of the present invention, which comprises:
在作为电解液的含有H2PtCl6和KCl的水溶液中,利用电沉积法在网状导电载体上形成所述铂纳米片,所述水溶液中,H2PtCl6的浓度为1×10-3mol/L~50×10-3mol/L,KCl的浓度为10×10-3mol/L~500×10-3mol/L。In an aqueous solution containing H2 PtCl6 and KCl as an electrolyte, the platinum nanosheets were formed on a mesh-like conductive carrier by an electrodeposition method. In the aqueous solution, the concentration of H2 PtCl6 was 1×10-3 mol/L~50×10-3 mol/L, and the concentration of KCl is 10×10-3 mol/L~500×10-3 mol/L.
优选地,前述电沉积的电压为-0.2~-0.4V,沉积时间为10~50min。Preferably, the voltage of the aforementioned electrodeposition is -0.2~-0.4V, and the deposition time is 10~50min.
本发明的又一方面,提供一种氢气的制备方法,其使用了本发明的超疏气铂析氢电极。In yet another aspect of the present invention, a method for preparing hydrogen gas is provided, which uses the superaerophobic platinum hydrogen evolution electrode of the present invention.
本发明的超疏气铂析氢电极使用网状导电载体,其表面形成有呈尖锐纳米片状的铂催化剂。导电载体的网格结构有利于电解质在电极表面的良好浸润,铂催化剂的尖锐纳米片状结构也增加了电极与电解液的接触表面积,从而大大提高了析氢反应时氢还原反应的催化活性。另外,铂催化剂的尖锐纳米片状结构使得电极表面的气泡粘附力进一步降低,从而有利于析氢反应产生的氢气从电极表面脱附。气体快速从电极表面离开又使催化剂与电解质的接触更充分,增加了电极的有效活性面积。这两个作用协同发挥效果,从而大大增强了电极的催化活性。The superaerophobic platinum hydrogen evolution electrode of the present invention uses a mesh-shaped conductive carrier, and a platinum catalyst in the shape of sharp nano-sheets is formed on the surface thereof. The grid structure of the conductive carrier is conducive to the good infiltration of the electrolyte on the electrode surface, and the sharp nanosheet structure of the platinum catalyst also increases the contact surface area between the electrode and the electrolyte, thereby greatly improving the catalytic activity of the hydrogen reduction reaction during the hydrogen evolution reaction. In addition, the sharp nanosheet-like structure of the platinum catalyst further reduces the adhesion of bubbles on the electrode surface, which is beneficial to the desorption of hydrogen produced by the hydrogen evolution reaction from the electrode surface. The rapid departure of the gas from the electrode surface makes the contact between the catalyst and the electrolyte more sufficient, and increases the effective active area of the electrode. These two effects work synergistically, thereby greatly enhancing the catalytic activity of the electrode.
本发明的超疏气铂析氢电极的制备方法中,通过使用网状导电载体作为载体,由于载体中带有大量的网状的孔,通过电沉积生成的铂沿着网格的骨架生长。与使用不带网的导电载体进行电沉积来获得的电极相比,含铂前驱体的电解质更容易浸润网格的骨架,生长的铂催化剂为垂直于载体骨架表面的呈尖锐的纳米片状结构,使得其与电解液的接触表面积大,从而大大提高了析氢反应时氢还原反应的催化活性。另外,呈尖锐的纳米片状结构的铂催化剂使得电极表面的气泡粘附力进一步降低,从而有利于析氢反应产生的氢气从电极表面脱附。这两个作用协同发挥效果,从而大大增强了电极的催化活性。In the preparation method of the superaerophobic platinum hydrogen evolution electrode of the present invention, by using a mesh conductive carrier as the carrier, since the carrier has a large number of mesh holes, the platinum generated by electrodeposition grows along the skeleton of the mesh. Compared with electrodes obtained by electrodeposition using conductive supports without meshes, electrolytes containing platinum precursors are more likely to infiltrate the mesh framework, and the grown platinum catalysts are sharp nanosheet-like structures perpendicular to the surface of the support framework. , so that its contact surface area with the electrolyte is large, thereby greatly improving the catalytic activity of the hydrogen reduction reaction during the hydrogen evolution reaction. In addition, the platinum catalyst with a sharp nanosheet structure further reduces the adhesion of bubbles on the electrode surface, which is conducive to the desorption of hydrogen produced by the hydrogen evolution reaction from the electrode surface. These two effects work synergistically, thereby greatly enhancing the catalytic activity of the electrode.
附图说明Description of drawings
图1是本发明的实施例中制备的超疏气铂析氢电极的环境扫描电镜图。1 is an environmental scanning electron microscope image of the superaerophobic platinum hydrogen evolution electrode prepared in the embodiment of the present invention.
图2是图1所使用的超疏气铂析氢电极的高倍放大的环境扫描电镜图。FIG. 2 is a high-magnification environmental scanning electron microscope image of the superaerophobic platinum hydrogen evolution electrode used in FIG. 1 .
图3是图1所使用的超疏气铂析氢电极的进一步高倍放大的环境扫描电镜图。FIG. 3 is a further high-magnification environmental scanning electron microscope image of the superaerophobic platinum hydrogen evolution electrode used in FIG. 1 .
图4是本发明的实施例(实施例2和5)中制备的超疏气铂析氢电极与空白金网在0.5M硫酸中的线性扫描伏安图。4 is a linear sweep voltammogram of the superaerophobic platinum hydrogen evolution electrode and blank gold mesh prepared in the examples of the present invention (Examples 2 and 5) in 0.5M sulfuric acid.
图5是本发明的实施例2与比较例1中制备的超疏气铂析氢电极的线性扫描伏安图。5 is a linear scan voltammogram of the superaerophobic platinum hydrogen evolution electrodes prepared in Example 2 and Comparative Example 1 of the present invention.
图6是本发明的实施例2中制备的超疏气铂析氢电极的水下气泡接触角照片。6 is a photo of the underwater bubble contact angle of the superaerophobic platinum hydrogen evolution electrode prepared in Example 2 of the present invention.
图7是使用本发明的实施例2中制备的超疏气铂析氢电极进行电化学析氢时的电极表面的照片。7 is a photograph of the electrode surface during electrochemical hydrogen evolution using the superaerophobic platinum hydrogen evolution electrode prepared in Example 2 of the present invention.
具体实施方式Detailed ways
[超疏气铂析氢电极][Superaerophobic platinum hydrogen evolution electrode]
本发明的超疏气铂析氢电极具有网状导电载体以及沉积在前述导电载体上的铂纳米片,所述铂纳米片分布在所述导电载体的两面。The superaerophobic platinum hydrogen evolution electrode of the present invention has a mesh conductive carrier and platinum nanosheets deposited on the aforementioned conductive carrier, and the platinum nanosheets are distributed on both sides of the conductive carrier.
前述的网状导电载体的尺寸没有特别的限定,可以为例如100~1500目,优选为200~1000目,更优选为400~600目。The size of the aforementioned mesh-like conductive carrier is not particularly limited, and may be, for example, 100 to 1500 mesh, preferably 200 to 1000 mesh, and more preferably 400 to 600 mesh.
前述的铂纳米片是指为纳米尺寸的片状,前述铂纳米片的尺寸是指横向尺寸,为10~400纳米见方的片状,更优选为30~300见方的片状,铂纳米片厚度优选为1~30nm。The aforementioned platinum nanosheet refers to a nano-sized sheet, and the aforementioned size of the platinum nanosheet refers to the lateral dimension, which is a sheet of 10 to 400 nanometers, more preferably a sheet of 30 to 300 square, and the thickness of the platinum nanosheets. It is preferably 1 to 30 nm.
前述超疏气是电极表面在水下对气体的浸润性,通常通过测量电极在水下对气泡的接触角来进行表征。“超疏气”指的是电极在水下对气泡的接触角为140度以上,优选为150度以上。The aforementioned superaerophobicity is the wettability of the electrode surface to gas under water, which is usually characterized by measuring the contact angle of the electrode to bubbles under water. "Superaerophobic" means that the contact angle of the electrode to air bubbles under water is 140 degrees or more, preferably 150 degrees or more.
作为网状导电载体的材质,可以列举出例如金、银、镍、钛或者碳。Examples of the material of the mesh-like conductive carrier include gold, silver, nickel, titanium, or carbon.
[超疏气铂析氢电极的制备方法][Preparation method of superaerophobic platinum hydrogen evolution electrode]
本发明的超疏气铂析氢电极的制备方法包括,在作为电解液的含有H2PtCl6和KCl的水溶液中,利用电沉积法在网状导电载体上形成所述铂纳米片,所述水溶液中,H2PtCl6的浓度为1×10-3mol/L~50×10-3mol/L,KCl的浓度为10×10-3mol/L~500×10-3mol/L。The preparation method of the superaerophobic platinum hydrogen evolution electrode of the present invention comprises: in an aqueous solution containing H2 PtCl6 and KCl as an electrolyte, the platinum nanosheets are formed on a mesh conductive carrier by an electrodeposition method, and the aqueous solution is Among them, the concentration of H2 PtCl6 is 1×10-3 mol/L~50×10-3 mol/L, and the concentration of KCl is 10×10-3 mol/L~500×10-3 mol/L.
前述的H2PtCl6的浓度更优选为1×10-3mol/L~20×10-3mol/L,进一步优选为1×10-3mol/L~10×10-3mol/L。The concentration of the aforementioned H2 PtCl6 is more preferably 1×10-3 mol/L to 20×10-3 mol/L, and still more preferably 1×10-3 mol/L to 10×10-3 mol/L.
前述的KCl的浓度更优选为10×10-3mol/L~300×10-3mol/L,进一步优选为10×10-3mol/L~200×10-3mol/L。The concentration of the aforementioned KCl is more preferably 10×10-3 mol/L to 300×10-3 mol/L, and still more preferably 10×10-3 mol/L to 200×10-3 mol/L.
前述电沉积法中所使用的电压范围为-0.2~-0.4V。例如可以为-0.2V、-0.3V、或者-0.4V。前述电沉积法中的沉积时间为5~80min,更优选为10~50min,进一步优选为20min~40min。The voltage range used in the aforementioned electrodeposition method is -0.2 to -0.4V. For example, it may be -0.2V, -0.3V, or -0.4V. The deposition time in the aforementioned electrodeposition method is 5 to 80 minutes, more preferably 10 to 50 minutes, and further preferably 20 to 40 minutes.
本发明的超疏气铂析氢电极的制备方法中,还可以包括下述步骤:在电沉积之后,利用去离子水冲洗沉积在电极表面的铂纳米片,以除去电极表面残留的KCl等无机盐。In the preparation method of the superaerophobic platinum hydrogen evolution electrode of the present invention, the following steps may also be included: after the electrodeposition, the platinum nanosheets deposited on the electrode surface are rinsed with deionized water to remove the remaining inorganic salts such as KCl on the electrode surface. .
[氢气的制备方法][Preparation method of hydrogen]
本发明的氢气的制备方法中,使用前述的本发明的超疏气铂析氢电极。除此之外,本发明的氢气的制备方法中的其他条件可以参照本领域通常使用的条件,本领域技术人员能够根据需要适宜的选择。例如,可以采用下述的制备方法。In the method for preparing hydrogen of the present invention, the aforementioned superaerophobic platinum hydrogen evolution electrode of the present invention is used. In addition, other conditions in the hydrogen preparation method of the present invention can refer to the conditions commonly used in the art, and those skilled in the art can appropriately select according to needs. For example, the following production methods can be employed.
将本发明的超疏气铂析氢电极作为工作电极,碳棒作为对电极,参比电极为Ag/AgCl电极,采用1M硫酸作为电解质。The superaerophobic platinum hydrogen evolution electrode of the present invention is used as the working electrode, the carbon rod is used as the counter electrode, the reference electrode is an Ag/AgCl electrode, and 1M sulfuric acid is used as the electrolyte.
本发明的氢气的制备方法中,通过使用本发明的超疏气铂析氢电极,与电解液的接触表面积大,大大提高了析氢反应时氢还原反应的催化活性。另外,电极表面的气泡粘附力低,从而有利于析氢反应产生的氢气从电极表面脱附。这两个作用协同发挥效果,从而大大增强了电极的催化活性。In the hydrogen preparation method of the present invention, by using the superaerophobic platinum hydrogen evolution electrode of the present invention, the contact surface area with the electrolyte is large, and the catalytic activity of the hydrogen reduction reaction during the hydrogen evolution reaction is greatly improved. In addition, the adhesion of bubbles on the electrode surface is low, which facilitates the desorption of hydrogen gas generated by the hydrogen evolution reaction from the electrode surface. These two effects work synergistically, thereby greatly enhancing the catalytic activity of the electrode.
实施例Example
超疏气析氢电极的表征和性能测试有:The characterization and performance tests of the super gas-repellent hydrogen evolution electrode are as follows:
(1)形貌表征:采用美国FEI公司Quanta FEG 250环境扫描电子显微镜观察样品的微观形貌。具体地,利用环境扫描电子显微镜对实施例制备的具有超疏气性质的析氢电极进行形貌表征。(1) Morphology characterization: The microscopic morphologies of the samples were observed using a Quanta FEG 250 environmental scanning electron microscope from FEI, USA. Specifically, the morphology of the hydrogen evolution electrode with super gas-repellent properties prepared in the example was characterized by environmental scanning electron microscope.
(2)氢还原测试:采用上海辰华电器有限公司CHI 660D电化学工作站三电极体系来测试制备的超疏气铂析氢电极在0.5M硫酸中的电催化活性。(2) Hydrogen reduction test: The three-electrode system of CHI 660D electrochemical workstation of Shanghai Chenhua Electric Co., Ltd. was used to test the electrocatalytic activity of the prepared superaerophobic platinum hydrogen evolution electrode in 0.5M sulfuric acid.
(3)气泡接触角测试:采用上海中晨JC2000D1接触角系统来测试制备的超疏气铂析氢电极的浸润性。(3) Bubble contact angle test: Shanghai Zhongchen JC2000D1 contact angle system was used to test the wettability of the prepared superaerophobic platinum hydrogen evolution electrode.
(4)气泡脱附测:采用摄像机对氢气泡在制备的超疏气铂析氢电极表面的的演化过程进行了现场成像。(4) Bubble desorption measurement: The evolution process of hydrogen bubbles on the surface of the prepared superaerophobic platinum hydrogen evolution electrode was imaged on-site by a camera.
实施例1Example 1
利用三电极系统,使用直径约为3mm、目数为600目的金网作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.3V,电沉积10min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的超疏气铂析氢电极1。Using a three-electrode system, a gold mesh with a diameter of about3 mm and a mesh number of600 was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. , 100×10-3 mol/L KCl mixed solution, set the electrodeposition voltage to -0.3V, electrodeposit for 10min, after the reaction is completed, rinse the surface with deionized water and dry it to obtain the gold mesh with both positive and negative sides. Superaerophobic platinum
实施例2Example 2
利用三电极系统,使用直径约为3mm、目数为600目的金网作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.3V,电沉积30min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的超疏气铂析氢电极2。Using a three-electrode system, a gold mesh with a diameter of about3 mm and a mesh number of600 was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. , 100×10-3 mol/L KCl mixed solution, set the electrodeposition voltage to -0.3V, and electrodeposit for 30min. After the reaction is completed, rinse the surface with deionized water and dry it to obtain the gold mesh with both positive and negative sides. Superaerophobic platinum hydrogen evolution electrode 2 deposited with metallic platinum.
实施例3Example 3
利用三电极系统,使用直径约为3mm、目数为600目的金网作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.3V,电沉积50min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的超疏气铂析氢电极3。Using a three-electrode system, a gold mesh with a diameter of about3 mm and a mesh number of600 was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. , 100 × 10-3 mol/L KCl mixed solution, set the electrodeposition voltage to -0.3V, and electrodeposit for 50min. After the reaction is completed, rinse the surface with deionized water and dry it to obtain the gold mesh. Superaerophobic platinum hydrogen evolution electrode 3 deposited with metallic platinum.
实施例4Example 4
利用三电极系统,使用直径约为3mm、目数为600目的金网作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.2V,电沉积30min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的超疏气铂析氢电极4。Using a three-electrode system, a gold mesh with a diameter of about3 mm and a mesh number of600 was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. , 100×10-3 mol/L KCl mixed solution, set the electrodeposition voltage to -0.2V, and electrodeposit for 30min. After the reaction is completed, rinse the surface with deionized water and then dry it to get the gold mesh. Superaerophobic platinum hydrogen evolution electrode 4 deposited with metallic platinum.
实施例5Example 5
利用三电极系统,使用直径约为3mm、目数为600目的金网作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.4V,电沉积30min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的超疏气铂析氢电极5。Using a three-electrode system, a gold mesh with a diameter of about3 mm and a mesh number of600 was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. , 100×10-3 mol/L KCl mixed solution, set the electrodeposition voltage to -0.4V, and electrodeposit for 30min. After the reaction is completed, rinse the surface with deionized water and dry it to obtain the gold mesh. Superaerophobic platinum hydrogen evolution electrode 5 deposited with metallic platinum.
实施例6Example 6
利用三电极系统,使用直径约为3mm、目数为1500目的金网作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.3V,电沉积30min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的超疏气铂析氢电极6。Using a three-electrode system, a gold mesh with a diameter of about3 mm and a mesh number of 1500 was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgClelectrode was used as the reference electrode. , 100×10-3 mol/L KCl mixed solution, set the electrodeposition voltage to -0.3V, and electrodeposit for 30min. After the reaction is completed, rinse the surface with deionized water and dry it to obtain the gold mesh with both positive and negative sides. Superaerophobic platinum hydrogen evolution electrode 6 deposited with metallic platinum.
比较例1Comparative Example 1
金箔表面沉积铂纳米片制备铂析氢电极:Preparation of platinum hydrogen evolution electrode by depositing platinum nanosheets on gold foil:
利用三电极系统,使用直径约为3mm的金箔作为工作电极,铂片作为辅助电极,Ag/AgCl电极作为参比电极,置于3×10-3mol/L H2PtCl6、100×10-3mol/L KCl的混合溶液中,设置电沉积电压为-0.3V,电沉积50min,反应完成后,用去离子水冲洗其表面后晾干便得到金网的正反面均沉积上金属铂的铂析氢电极7。Using athree- electrode system, a gold foil with a diameter of about3 mm was used as the working electrode, the platinum sheet was used as the auxiliary electrode, and the Ag/AgCl electrode was used as the reference electrode. In the mixed solution of mol/L KCl, the electrodeposition voltage was set to -0.3V, and the electrodeposition was carried out for 50min. After the reaction was completed, the surface was rinsed with deionized water and dried to obtain platinum with platinum metal deposited on both sides of the gold mesh. Hydrogen evolution electrode 7.
对上述实施例以及比较例中得到的超疏气铂析氢电极进行形貌表征、气泡接触角测试以及氢还原测试。通过形貌表征可以看出,实施例1~6中制备得到的超疏气铂析氢电极具有宏观的多孔结构,在电极表面有致密均匀分布的尖锐的铂纳米片。泡接触角测试结果表明实施例1~6得到的超疏气铂析氢电极在水下表现出超疏气性,有利于产物气体的脱附;氢还原活性测试结果表明,超疏气铂析氢电极在较低的过电位下,表现出较大的电流密度。The superaerophobic platinum hydrogen evolution electrodes obtained in the above examples and comparative examples were subjected to morphology characterization, bubble contact angle test and hydrogen reduction test. It can be seen from the morphological characterization that the superaerophobic platinum hydrogen evolution electrodes prepared in Examples 1 to 6 have a macroscopic porous structure, and there are sharp platinum nanosheets densely and uniformly distributed on the electrode surface. The bubble contact angle test results show that the superaerophobic platinum hydrogen evolution electrodes obtained in Examples 1 to 6 exhibit superaerophobicity under water, which is conducive to the desorption of product gases; the hydrogen reduction activity test results show that the superaerophobic platinum hydrogen evolution electrodes At lower overpotentials, larger current densities are exhibited.
(1)形貌表征(1) Morphological characterization
利用环境扫描电镜对实施例2制备的超疏气铂析氢电极2进行形貌观察。如图1~3所示,可明显观察到超疏气铂析氢电极2的表面沉积有尖锐的铂纳米片,且致密均匀。The morphology of the superaerophobic platinum hydrogen evolution electrode 2 prepared in Example 2 was observed by environmental scanning electron microscope. As shown in FIGS. 1 to 3 , it can be clearly observed that sharp platinum nanosheets are deposited on the surface of the superaerophobic platinum hydrogen evolution electrode 2 , which are dense and uniform.
(2)氢还原活性测试(2) Hydrogen reduction activity test
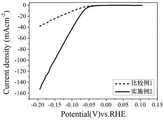
室温下,在0.5mol/L的H2SO4电解液中,利用CHI660E电化学工作站对实施例2制备的超疏气析氢铂电极2、实施例5制备的超疏气析氢铂电极5进行氢还原活性测试,并用空白金网作为对照,采用三电极工作系统,将饱和Ag/AgCl电极为参比电极,碳棒作为辅助电极,所制得的超疏气铂析氢电极2或5作为工作电极,进行线性扫描伏安测试。根据方程,将可逆氢电极(RHE)与标准电极相对应,方程为:电位(vs.Ag/AgCl)量程从-0.4V到-0.1V,扫描速率设置为5mV/s。由图4可知,本发明实施例2制备的超疏气析氢铂电极2、实施例5制备的超疏气析氢铂电极5表现出极好的氢还原催化活性。进一步,对比较例1中得到的铂析氢电极7也进行上述氢还原活性测试,得到的结果与实施例2一并示于图5中。由图5可知,本发明实施例2制备的超疏气析氢铂电极1与比较例1相比也表现出极好的氢还原催化活性。At room temperature, in 0.5mol/L H2 SO4 electrolyte, using CHI660E electrochemical workstation, the super gas-repellent hydrogen-evolution platinum electrode 2 prepared in Example 2 and the super-aerophobic hydrogen-evolving platinum electrode 5 prepared in Example 5 were subjected to hydrogenation. The reduction activity was tested, and a blank gold mesh was used as a control. A three-electrode working system was used. The saturated Ag/AgCl electrode was used as the reference electrode, the carbon rod was used as the auxiliary electrode, and the prepared superaerophobic platinum hydrogen evolution electrode 2 or 5 was used as the working electrode. , perform a linear sweep voltammetry test. Corresponding the reversible hydrogen electrode (RHE) to the standard electrode according to the equation, the equation is: The potential (vs. Ag/AgCl) range was from -0.4V to -0.1V, and the scan rate was set to 5mV/s. It can be seen from FIG. 4 that the super gas-repellent hydrogen-evolution platinum electrode 2 prepared in Example 2 of the present invention and the super-gas-repellent hydrogen-evolution platinum electrode 5 prepared in Example 5 show excellent hydrogen reduction catalytic activity. Further, the above-mentioned hydrogen reduction activity test was also performed on the platinum hydrogen evolution electrode 7 obtained in Comparative Example 1, and the obtained results are shown in FIG. 5 together with Example 2. It can be seen from FIG. 5 that the super gas-repellent hydrogen-evolving
(3)气泡接触角的测试(3) Test of bubble contact angle
采用(德国Dataphysics)0CA20接触角系统对实施例2制得的超疏气析氢铂电极2在水下气泡接触角(CAs)进行了测试。将超疏气析氢铂电极2悬挂固定好放入一定大小的石英水槽中,调整浸入的位置,直到在屏幕上清晰的看到超疏气析氢铂电极2为一条直线,调节焦距,使成像清晰,然后轻轻地在水下打出一个气泡,使气泡完全附着在金网表面,最后测试气泡接触角。如图6所示,实施例2制备出的超疏气析氢铂电极2,在水下具有较大的气泡接触角,为148°,表现出超疏气性,气泡很容易从电极表面脱附出来,从而极大的提高了电催化活性。The contact angle (CAs) of the super gas-repellent hydrogen-evolving platinum electrode 2 prepared in Example 2 was tested under water by using the OCA20 contact angle system (Dataphysics, Germany). Hang the super-gas-phobic hydrogen-evolution platinum electrode 2 into a quartz water tank of a certain size, and adjust the immersion position until the super-gas-phobic hydrogen-evolution platinum electrode 2 is clearly seen on the screen as a straight line, and adjust the focal length to make the image clear. , and then gently blow a bubble under water to make the bubble completely adhere to the surface of the gold mesh, and finally test the contact angle of the bubble. As shown in Figure 6, the super-gas-repellent hydrogen-evolving platinum electrode 2 prepared in Example 2 has a large bubble contact angle under water, which is 148°, showing super-gas-repellent properties, and the bubbles are easily desorbed from the electrode surface out, which greatly improves the electrocatalytic activity.
(4)气泡脱附测试(4) Bubble Desorption Test
采用摄像机对氢气泡在其释放过程中的演化过程进行了现场成像。将实施例2中制备的超疏气析氢铂电极2作为工作电极,几何面积为0.071cm-2,碳棒为对电极,3.5M Ag/AgCl电极为参比电极,置于0.5MH2SO4电解液中,如图7所示,超疏气析氢铂电极2在氢还原过程中,在水下具有较大的气泡接触角,即水下超疏气。氢气气泡的尺寸很小,能及时离开电极表面,而没有粘附在电极表面,保证了电解质溶液与催化剂的充分接触,从而极大的提高了电催化活性。The evolution of the hydrogen bubble during its release was imaged in situ using a camera. The super-gas-repellent hydrogen-evolving platinum electrode 2 prepared in Example 2 was used as the working electrode, the geometric area was 0.071cm-2 , the carbon rod was the counter electrode, and the 3.5M Ag/AgCl electrode was the reference electrode, placed in 0.5MH2SO4In the electrolyte , as shown in Figure 7, the super-gas-repellent hydrogen-evolving platinum electrode 2 has a large bubble contact angle underwater during the hydrogen reduction process, that is, underwater super-gas-repellent. The size of the hydrogen gas bubbles is small and can leave the electrode surface in time without adhering to the electrode surface, which ensures the full contact between the electrolyte solution and the catalyst, thereby greatly improving the electrocatalytic activity.
在不背离本发明的范围或精神的情况下,可对本发明说明书的具体实施方式做多种改进和变化,这对本领域技术人员而言是显而易见的。由本发明的说明书得到的其他实施方式对技术人员而言是显而易见得的。本申请说明书和实施例仅是示例性的。It will be apparent to those skilled in the art that various modifications and variations can be made in the specific embodiments of the present invention without departing from the scope or spirit of the invention. Other embodiments will be apparent to those skilled in the art from the description of the present invention. The description and examples of the present application are only exemplary.
Claims (6)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010662834.4ACN111763955A (en) | 2020-07-10 | 2020-07-10 | Superaerophobic platinum hydrogen evolution electrode, its preparation method and hydrogen preparation method |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010662834.4ACN111763955A (en) | 2020-07-10 | 2020-07-10 | Superaerophobic platinum hydrogen evolution electrode, its preparation method and hydrogen preparation method |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111763955Atrue CN111763955A (en) | 2020-10-13 |
Family
ID=72726634
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010662834.4APendingCN111763955A (en) | 2020-07-10 | 2020-07-10 | Superaerophobic platinum hydrogen evolution electrode, its preparation method and hydrogen preparation method |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111763955A (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113061922A (en)* | 2021-03-15 | 2021-07-02 | 北京航空航天大学 | Super-hydrophobic manganese oxide oxygen evolution electrode, preparation method thereof and electrochemical preparation method of oxygen |
| CN113355689A (en)* | 2021-05-07 | 2021-09-07 | 北京仿生界面科学未来技术研究院 | Qinqi-dispelling and qi-dispelling cooperative confinement electrode and preparation method thereof |
| CN115125572A (en)* | 2022-06-22 | 2022-09-30 | 北京航空航天大学 | Preparation method and application of mushroom-shaped array electrode |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2307708C1 (en)* | 2006-01-31 | 2007-10-10 | Федеральное государственное унитарное предприятие "Производственное объединение "Маяк" | Method of preparation of the platinum hydrophobic catalyst of the isotopic exchange of hydrogen with water |
| CN102703953A (en)* | 2012-06-07 | 2012-10-03 | 北京工业大学 | Method for preparing nanometer platinum/titanium dioxide nanotube electrode through cyclic voltammetry electrodeposition |
| CN104617307A (en)* | 2015-01-29 | 2015-05-13 | 北京化工大学 | Electrode material with surface nanometer-micrometer structure, preparation method thereof and hydrazine hydrate fuel battery containing electrode material |
| CN109289835A (en)* | 2018-10-15 | 2019-02-01 | 天津工业大学 | A kind of Pt catalyst synthesis method of hydrogen evolution reaction |
| CN109382118A (en)* | 2018-11-21 | 2019-02-26 | 黑龙江省科学院技术物理研究所 | A kind of network structure Pt-Ni alloy nano-material and preparation method thereof |
| CN110252348A (en)* | 2019-07-01 | 2019-09-20 | 青岛科技大学 | Preparation method of a superhydrophilic and superaerophobic transition metal molybdenum-sulfur gel electrocatalyst |
| CN110724966A (en)* | 2019-10-23 | 2020-01-24 | 北京化工大学 | Directional gas transport electrode, preparation method and use thereof, and electrolytic cell comprising the same |
| CN110965076A (en)* | 2019-12-06 | 2020-04-07 | 吉林大学 | A kind of preparation method of bifunctional three-dimensional layered core-shell structure water electrolysis electrode |
| CN111188056A (en)* | 2020-02-24 | 2020-05-22 | 北京化工大学 | Porous array electrode with secondary structure and preparation method and application thereof |
| US20200325586A1 (en)* | 2019-04-15 | 2020-10-15 | Unist (Ulsan National Institute Of Science And Technology) | Electrode for Gas Generation, Method of Preparing the Electrode and Device Including the Electrode for Gas Generation |
- 2020
- 2020-07-10CNCN202010662834.4Apatent/CN111763955A/enactivePending
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2307708C1 (en)* | 2006-01-31 | 2007-10-10 | Федеральное государственное унитарное предприятие "Производственное объединение "Маяк" | Method of preparation of the platinum hydrophobic catalyst of the isotopic exchange of hydrogen with water |
| CN102703953A (en)* | 2012-06-07 | 2012-10-03 | 北京工业大学 | Method for preparing nanometer platinum/titanium dioxide nanotube electrode through cyclic voltammetry electrodeposition |
| CN104617307A (en)* | 2015-01-29 | 2015-05-13 | 北京化工大学 | Electrode material with surface nanometer-micrometer structure, preparation method thereof and hydrazine hydrate fuel battery containing electrode material |
| CN109289835A (en)* | 2018-10-15 | 2019-02-01 | 天津工业大学 | A kind of Pt catalyst synthesis method of hydrogen evolution reaction |
| CN109382118A (en)* | 2018-11-21 | 2019-02-26 | 黑龙江省科学院技术物理研究所 | A kind of network structure Pt-Ni alloy nano-material and preparation method thereof |
| US20200325586A1 (en)* | 2019-04-15 | 2020-10-15 | Unist (Ulsan National Institute Of Science And Technology) | Electrode for Gas Generation, Method of Preparing the Electrode and Device Including the Electrode for Gas Generation |
| CN110252348A (en)* | 2019-07-01 | 2019-09-20 | 青岛科技大学 | Preparation method of a superhydrophilic and superaerophobic transition metal molybdenum-sulfur gel electrocatalyst |
| CN110724966A (en)* | 2019-10-23 | 2020-01-24 | 北京化工大学 | Directional gas transport electrode, preparation method and use thereof, and electrolytic cell comprising the same |
| CN110965076A (en)* | 2019-12-06 | 2020-04-07 | 吉林大学 | A kind of preparation method of bifunctional three-dimensional layered core-shell structure water electrolysis electrode |
| CN111188056A (en)* | 2020-02-24 | 2020-05-22 | 北京化工大学 | Porous array electrode with secondary structure and preparation method and application thereof |
Non-Patent Citations (6)
| Title |
|---|
| JIAO JIN等: ""Superaerophobic Platinum Nanosheets Arrays on Conductive Microgrids: A Highly Efficient Electrocatalytic Electrode for Hydrogen Evolution Reaction"", 《CHEMCATCHEM》* |
| LI, LIN等: ""Underwater superoleophobic porous membrane based on hierarchical TiO2 nanotubes: multifunctional integration of oil-water separation, flow-through photocatalysis and self-cleaning"", 《JOURNAL OF MATERIALS CHEMISTRY A》* |
| LI, YINGJIE等: ""Under-Water Superaerophobic Pine-Shaped Pt Nanoarray Electrode for Ultrahigh-Performance Hydrogen Evolution"", 《ADVANCED FUNCTIONAL MATERIALS》* |
| TIAN, YUMENG等: ""3D porous Ni-Co-P nanosheets on carbon fiber cloth for efficient hydrogen evolution reaction"", 《ELECTROCHIMICA ACTA》* |
| 姜茗: ""纳米阵列超疏气电极在析气反应中的应用"", 《中国优秀硕士学位论文全文数据库 工程科技I辑》* |
| 李英杰: ""新型纳米阵列电极的构建及其在气体参与的电催化反应中的应用"", 《中国博士学位论文全文数据库 工程科技I辑》* |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113061922A (en)* | 2021-03-15 | 2021-07-02 | 北京航空航天大学 | Super-hydrophobic manganese oxide oxygen evolution electrode, preparation method thereof and electrochemical preparation method of oxygen |
| CN113355689A (en)* | 2021-05-07 | 2021-09-07 | 北京仿生界面科学未来技术研究院 | Qinqi-dispelling and qi-dispelling cooperative confinement electrode and preparation method thereof |
| CN113355689B (en)* | 2021-05-07 | 2023-03-31 | 北京蕴超仿生智能科技发展有限公司 | Qinqi-dispelling and qi-dispelling cooperative confinement electrode and preparation method thereof |
| CN115125572A (en)* | 2022-06-22 | 2022-09-30 | 北京航空航天大学 | Preparation method and application of mushroom-shaped array electrode |
| CN115125572B (en)* | 2022-06-22 | 2023-12-19 | 北京航空航天大学 | Preparation method and application of mushroom-shaped array electrode |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110205636B (en) | Preparation method of self-supporting three-dimensional porous structure bifunctional catalytic electrode | |
| CN104846397B (en) | One kind being used for electrochemical reduction CO2The electrode and its preparation method and application of formic acid processed | |
| CN111575729A (en) | Nickel phosphide compound with multi-level hole structure and preparation method and application thereof | |
| CN107858701B (en) | A kind of titanium-based hydrogen-precipitating electrode and preparation method thereof for solid polymer water electrolyzer | |
| CN105297107B (en) | A kind of method of cyclic voltammetric electrodeposited nanocrystalline platinum nickel/titanium dioxide nanotube electrode | |
| CN103165908B (en) | A kind of preparation method of ordering electrode | |
| CN102806093B (en) | Preparation method of high-efficiency low-platinum catalyst for direct methanol fuel cell | |
| CN106669739A (en) | Transition metal sulfide/carbon nanotube composite material as well as preparation method and application thereof | |
| CN111763955A (en) | Superaerophobic platinum hydrogen evolution electrode, its preparation method and hydrogen preparation method | |
| CN108950593A (en) | For electrochemical reduction CO2Copper nano-wire tin supported catalysis electrode and method | |
| CN102925923A (en) | Preparation method of nano-palladium or palladium-nickel alloy catalyst having three-dimensional porous structure | |
| CN113136597B (en) | Copper-tin composite material and preparation method and application thereof | |
| CN110711597B (en) | A kind of Co-Mo-P-O electrocatalyst and its preparation method and application | |
| CN113174600A (en) | Porous nickel screen electrolytic water catalytic material and preparation method thereof | |
| CN102703942A (en) | Method for preparing nano-platinum/palladium titanium dioxide nanotube composite electrode by pulse electrodeposition | |
| CN111647909A (en) | Dendritic copper electrode with hydrophobic surface and preparation method and application thereof | |
| CN106191945A (en) | A kind of pulse electrodeposition prepares the method for titania nanotube immobilized platinum nickel bimetal combination electrode | |
| CN221895142U (en) | Hydrogen production electrolytic tank and water electrolysis device | |
| CN110724966A (en) | Directional gas transport electrode, preparation method and use thereof, and electrolytic cell comprising the same | |
| CN102534742A (en) | Titanium dioxide nano thin film composite material and constant-current preparation method thereof | |
| Cheng et al. | Dynamic hydrogen bubble template electrodeposited Bi on graphite felt and the effect of its post-processing in vanadium redox flow batteries | |
| CN114807967B (en) | A kind of preparation method of Ir modified Ni/NiO porous nanorod array total water splitting catalyst | |
| Jahani et al. | Modified carbon cloth with cobalt oxide and palladium for efficient electrochemical hydrogen production | |
| CN107490652B (en) | A single-orientation iridium oxide nano-array preparation and membrane electrode construction method | |
| CN108878912A (en) | A kind of network-like TiOx nano wire material and preparation method and application of the hydrogenation of original position carbon coating |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | Application publication date:20201013 | |
| RJ01 | Rejection of invention patent application after publication |