CN111617267A - A nano-scale ultrasound contrast agent encapsulated with perfluorocarbon - Google Patents
A nano-scale ultrasound contrast agent encapsulated with perfluorocarbonDownload PDFInfo
- Publication number
- CN111617267A CN111617267ACN202010649043.8ACN202010649043ACN111617267ACN 111617267 ACN111617267 ACN 111617267ACN 202010649043 ACN202010649043 ACN 202010649043ACN 111617267 ACN111617267 ACN 111617267A
- Authority
- CN
- China
- Prior art keywords
- contrast agent
- ultrasonic
- ultrasound contrast
- perfluorocarbon
- nano
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/22—Echographic preparations; Ultrasound imaging preparations ; Optoacoustic imaging preparations
- A61K49/221—Echographic preparations; Ultrasound imaging preparations ; Optoacoustic imaging preparations characterised by the targeting agent or modifying agent linked to the acoustically-active agent
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/24—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing atoms other than carbon, hydrogen, oxygen, halogen, nitrogen or sulfur, e.g. cyclomethicone or phospholipids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/28—Steroids, e.g. cholesterol, bile acids or glycyrrhetinic acid
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/22—Echographic preparations; Ultrasound imaging preparations ; Optoacoustic imaging preparations
- A61K49/222—Echographic preparations; Ultrasound imaging preparations ; Optoacoustic imaging preparations characterised by a special physical form, e.g. emulsions, liposomes
- A61K49/227—Liposomes, lipoprotein vesicles, e.g. LDL or HDL lipoproteins, micelles, e.g. phospholipidic or polymeric
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Physics & Mathematics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Acoustics & Sound (AREA)
- Radiology & Medical Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Medicinal Preparation (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及一种用于超声成像诊断的超声造影剂及其制备方法,属于生物医用材料领域。The invention relates to an ultrasonic contrast agent for ultrasonic imaging diagnosis and a preparation method thereof, belonging to the field of biomedical materials.
背景技术Background technique
超声成像是一种利用超声的物理特性和人体器官组织声学性质上的差异来进行疾病诊断的影像技术,由于其非侵袭性、花费低、操作简单及广泛的诊断应用而受到特别的关注。超声造影剂常被应用于超声诊断,有效改善超声图像的对比质量,明显提高超声波对于病变区形态和类型的分辨能力,增强超声诊断的敏感性和特异性。Ultrasound imaging is an imaging technology that utilizes the physical properties of ultrasound and the differences in the acoustic properties of human organs and tissues for disease diagnosis. It has received special attention due to its non-invasiveness, low cost, simple operation, and wide range of diagnostic applications. Ultrasound contrast agents are often used in ultrasound diagnosis, which can effectively improve the contrast quality of ultrasound images, significantly improve the ability of ultrasound to distinguish the shape and type of lesions, and enhance the sensitivity and specificity of ultrasound diagnosis.
随着超声成像技术与生物纳米技术的迅猛发展,纳米级超声造影剂发展迅速。与传统微米级超声造影剂相比,纳米级超声造影剂粒径小,组织穿透能力更强,更容易穿透血管到达组织病灶部位。此外,纳米级粒子表面积大,吸附能力强,可以聚集显像,在靶区可显著增强信号,降低背景噪声。With the rapid development of ultrasound imaging technology and bio-nanotechnology, nano-scale ultrasound contrast agents have developed rapidly. Compared with traditional micron-sized ultrasound contrast agents, nano-sized ultrasound contrast agents have smaller particle size and stronger tissue penetration ability, making it easier to penetrate blood vessels to reach tissue lesions. In addition, the nano-sized particles have large surface area and strong adsorption capacity, which can be aggregated and imaged, which can significantly enhance the signal in the target area and reduce the background noise.
超声造影剂的壳膜材料主要包括脂质、高分子聚合物、表面活性剂、无机非金属等。其中高分子聚合物制备的壳层较硬,弹性差,在超声爆破或自然溶蚀下易形成碎片,产生非治疗性损伤;表面活性剂制备的造影剂稳定性较差,不易被修饰;无机纳米颗粒材料刚性强,需要较高的超声输出。而脂质材料有较高的韧性,壳膜在水介质中可形成液晶,稳定性好,显像效果佳,是一种较为理想的超声造影剂壳膜材料。采用混合脂质材料制备的双分子层壳膜,生物相容性更好,稳定性更佳,包载能力更强,可以很好的包载成像剂并进行体内输送,具有很高的研究价值。The shell membrane materials of ultrasound contrast agents mainly include lipids, high molecular polymers, surfactants, inorganic non-metals, etc. Among them, the shell layer prepared by high molecular polymer is relatively hard and has poor elasticity, and it is easy to form fragments under ultrasonic blasting or natural erosion, resulting in non-therapeutic damage; the contrast agent prepared by surfactant has poor stability and is not easy to be modified; Granular materials are rigid and require high ultrasound output. The lipid material has high toughness, the shell membrane can form liquid crystal in aqueous medium, has good stability and good imaging effect, and is an ideal ultrasound contrast agent shell membrane material. The bilayer shell membrane prepared with mixed lipid materials has better biocompatibility, better stability, and stronger entrapment capacity. It can well encapsulate imaging agents and transport them in vivo, and has high research value. .
全氟化碳是一种氟化的脂肪族类复合物,其沸点低,进入体内后在体温及超声效应的作用下进行液-气相转变,形成微泡,增强显像效果。与直接包载气态氟碳化合物相比,包载全氟化碳的造影剂具有更好的储存稳定性、更长的组织内循环时间、更高的生物安全性、更持久地抵抗外界压力及机械应力变化的能力,能够实现体内长效超声成像效果,更有利于检测观察病灶部位。Perfluorocarbon is a fluorinated aliphatic compound with a low boiling point. After entering the body, it undergoes a liquid-gas phase transformation under the action of body temperature and ultrasonic effects to form microbubbles and enhance the imaging effect. Compared with the direct encapsulation of gaseous fluorocarbons, perfluorocarbon-encapsulated contrast agents have better storage stability, longer intra-tissue circulation time, higher biosafety, longer resistance to external pressure and The ability to change mechanical stress can achieve long-term ultrasound imaging effects in vivo, which is more conducive to the detection and observation of lesions.
采用纳米级脂质体包载全氟化碳,可利用纳米粒子的强穿透力穿过血管内皮细胞,使之到达血管外病变组织器官,经过超声作用后全氟化碳进行相转变形成微泡,产生较强的超声显像效果;另外,形成的多层磷脂稳定层可以有效地提高超声造影剂的储存稳定性,但不影响体内的超声响应性,方便临床使用。Nano-scale liposomes are used to encapsulate perfluorocarbon, which can use the strong penetrating force of nanoparticles to penetrate vascular endothelial cells and make them reach extravascular diseased tissues and organs. After ultrasonication, perfluorocarbon undergoes phase transformation to form microscopic In addition, the formed multi-layer phospholipid stable layer can effectively improve the storage stability of the ultrasonic contrast agent, but does not affect the ultrasonic responsiveness in vivo, which is convenient for clinical use.
发明内容SUMMARY OF THE INVENTION
本发明针对现有技术的不足,目的在于提供一种包载全氟化碳的纳米级超声造影剂。该纳米级超声造影剂由脂质包膜包裹全氟化碳及其它有效成分组成。制备得到的纳米级超声造影剂生物相容性更好、穿透能力更强,体内循环时间更长。包载的全氟化碳在体温与超声效应的作用下通过液-气相转变产生微泡,可以达到增强的超声显影的效果。Aiming at the deficiencies of the prior art, the present invention aims to provide a nano-scale ultrasonic contrast agent loaded with perfluorocarbon. The nano-scale ultrasonic contrast agent is composed of perfluorocarbon and other effective components wrapped in lipid envelope. The prepared nano-scale ultrasound contrast agent has better biocompatibility, stronger penetrating ability and longer circulation time in vivo. The encapsulated perfluorocarbon generates microbubbles through liquid-gas phase transition under the action of body temperature and ultrasonic effect, which can achieve the effect of enhanced ultrasonic imaging.
所述脂质包膜由一种或多种脂质材料组成,例如氢化大豆磷脂,PEG化磷脂及胆固醇等,但不限于举例范围。The lipid envelope is composed of one or more lipid materials, such as hydrogenated soybean phospholipids, PEGylated phospholipids and cholesterol, etc., but not limited to the scope of examples.
所述全氟化碳为全氟戊烷、全氟己烷中任意一种,但不限于举例范围。The perfluorocarbon is any one of perfluoropentane and perfluorohexane, but is not limited to the scope of examples.
本发明采用如下技术方案:The present invention adopts following technical scheme:
一种包载全氟化碳的纳米级超声造影剂,所述纳米级超声造影剂以氢化大豆磷脂,PEG化磷脂及胆固醇为脂质材料,制备壳膜,内部包有全氟化碳。制得的脂质体粒径分布范围为80~400nm,透射电镜可观察到明显磷脂层,表面荷负电,电位绝对值约为30mV。A perfluorocarbon-encapsulated nano-scale ultrasonic contrast agent, wherein the nano-scale ultrasonic contrast agent uses hydrogenated soybean phospholipid, PEGylated phospholipid and cholesterol as lipid materials to prepare a shell membrane, and the inside is encapsulated with perfluorocarbon. The particle size distribution range of the prepared liposomes is 80-400 nm, and an obvious phospholipid layer can be observed by transmission electron microscope. The surface is negatively charged, and the absolute value of the potential is about 30 mV.
本发明优选的内部包载的全氟化碳为全氟戊烷、全氟己烷中任意一种。The preferred perfluorocarbon contained in the present invention is any one of perfluoropentane and perfluorohexane.
本发明优选的形成壳膜的脂质材料为氢化大豆磷脂、PEG化磷脂与胆固醇的混合物,氢化大豆磷脂、PEG化磷脂与胆固醇三者的质量比为10∶3∶1。The preferred lipid material for forming the shell membrane in the present invention is a mixture of hydrogenated soybean phospholipid, PEGylated phospholipid and cholesterol, and the mass ratio of hydrogenated soybean phospholipid, PEGylated phospholipid and cholesterol is 10:3:1.
本发明优选的全氟化碳加入量在1%-10%(v∶v)。The preferred amount of perfluorocarbon added in the present invention is 1%-10% (v:v).
本发明制得的脂质体,其内部包载的全氟化碳在体温与超声效应的作用下气化产生微泡,接收超声能量后发生共振并散射超声信号,具有增强显像的能力。In the liposome prepared by the invention, the perfluorocarbon contained in the liposome is vaporized under the action of body temperature and ultrasonic effect to generate microbubbles, and after receiving ultrasonic energy, it resonates and scatters ultrasonic signals, and has the ability to enhance imaging.
上述载全氟化碳的纳米级超声造影剂的制备方法,包括以下步骤:The preparation method of the above-mentioned perfluorocarbon-loaded nano-scale ultrasonic contrast agent comprises the following steps:
(1)将脂质材料氢化大豆磷脂、PEG化磷脂及胆固醇在有机溶剂中进行分散,旋蒸除去有机溶剂后得薄膜,37℃下进行薄膜生理盐水水化,30min后得脂质囊泡;(1) Disperse the lipid material hydrogenated soybean phospholipid, PEGylated phospholipid and cholesterol in an organic solvent, remove the organic solvent by rotary evaporation to obtain a film, hydrate the film with physiological saline at 37°C, and obtain lipid vesicles after 30 min;
(2)取步骤(1)制得的脂质囊泡,加入全氟化碳,采用探头超声进行粉碎,得粗混悬液;(2) taking the lipid vesicles obtained in step (1), adding perfluorocarbon, and pulverizing by ultrasonic probe to obtain a coarse suspension;
(3)将步骤(2)制得的粗混悬液与步骤(1)制得的脂质囊泡混合后,按顺序挤出通过400nm,200nm,100nm的纤维素膜,每层挤出次数不少于13次,取续滤液,稀释后即得所述超声造影剂。(3) After mixing the crude suspension prepared in step (2) with the lipid vesicles prepared in step (1), extrude through cellulose membranes of 400 nm, 200 nm, and 100 nm in sequence, and extrude each layer for the number of times No less than 13 times, the continuous filtrate is taken and diluted to obtain the ultrasonic contrast agent.
本发明优选的步骤(1)中所述有机溶剂为甲醇,乙醇,丙酮、二氯甲烷、三氯甲烷中的一种或多种,但不限于举例范围。The organic solvent described in the preferred step (1) of the present invention is one or more of methanol, ethanol, acetone, dichloromethane and chloroform, but is not limited to the scope of examples.
本发明优选的步骤(2)中所述探头超声强度为50%,超声时间为10min,但不限于举例范围。本发明优选的步骤(3)中所述稀释倍数为3~6倍。In the preferred step (2) of the present invention, the ultrasonic intensity of the probe is 50%, and the ultrasonic time is 10 minutes, but it is not limited to the scope of examples. In the preferred step (3) of the present invention, the dilution ratio is 3 to 6 times.
与现有技术相比,本发明具有如下优点:Compared with the prior art, the present invention has the following advantages:
(1)采用氢化大豆磷脂、PEG化磷脂及胆固醇的混合物制备壳膜,制备方法成熟,工艺简单,具有体内生物相容性好,体外稳定性佳的特点,有利于该制剂的临床应用。(1) Using the mixture of hydrogenated soybean phospholipid, PEGylated phospholipid and cholesterol to prepare shell membrane, the preparation method is mature, the process is simple, and it has the characteristics of good in vivo biocompatibility and good in vitro stability, which is beneficial to the clinical application of the preparation.
(2)纳米级超声造影剂通过静脉注射后,具有强穿透能力,可穿过血管内皮细胞到达血管外病变组织器官。(2) Nano-scale ultrasound contrast agent has strong penetrating ability after intravenous injection, and can pass through vascular endothelial cells to reach extravascular diseased tissues and organs.
(3)包载全氟化碳化合物,相较于气态氟碳具有更高的安全性,更长的组织内循环时间、更持久地抵抗外界压力及机械应力变化的能力。在体内进行相转变后形成微泡,增强纳米氟碳造影剂的显像效果,气态氟碳化合物最终可通过呼吸快速排出体外,使干扰信号达到最小。(3) Encapsulating perfluorocarbon compounds, compared with gaseous fluorocarbons, has higher safety, longer circulation time in tissues, and more durable resistance to changes in external pressure and mechanical stress. After the phase transition in the body, microbubbles are formed to enhance the imaging effect of nano-fluorocarbon contrast agents. The gaseous fluorocarbons can finally be quickly excreted through breathing, so as to minimize the interference signal.
(4)可以形成多层磷脂层包覆的纳米制剂,提高了储存稳定性和超声响应稳定性。(4) Nano-formulations coated with multi-layer phospholipid layers can be formed, and the storage stability and ultrasonic response stability are improved.
附图说明Description of drawings
图1为包载全氟化碳的纳米级超声造影剂的透射电镜图。FIG. 1 is a transmission electron microscope image of a nano-scale ultrasound contrast agent encapsulated with perfluorocarbon.
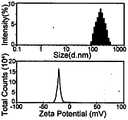
图2为包载全氟化碳的纳米级超声造影剂的粒径电位分布图。FIG. 2 is a particle size potential distribution diagram of a nano-scale ultrasound contrast agent encapsulated with perfluorocarbon.
图3为包载全氟化碳的纳米级超声造影剂的储存稳定性。Figure 3 shows the storage stability of perfluorocarbon-encapsulated nanoscale ultrasound contrast agents.
图4为超声仪器进行无造影剂的肝门静脉造影成像图。Fig. 4 is an imaging diagram of hepatic portal vein angiography without contrast agent performed by an ultrasound instrument.
图5为静脉注射包载全氟化碳的纳米级超声造影剂的肝门静脉造影成像图。FIG. 5 is an angiographic image of the hepatic portal vein by intravenous injection of perfluorocarbon-loaded nano-sized ultrasound contrast agent.
具体实施方式Detailed ways
本发明具体实施方式列举的实施例子仅用于对本发明进行说明,并不对本发明内容进行限制。The examples listed in the specific embodiments of the present invention are only used to illustrate the present invention, and do not limit the content of the present invention.
实施例1Example 1
一种包载全氟化碳的纳米级超声造影剂制备方式包括以下步骤:A preparation method of a perfluorocarbon-loaded nano-scale ultrasonic contrast agent comprises the following steps:
(1)将氢化大豆磷脂、PEG化磷脂及胆固醇按摩尔比溶于三氯甲烷,超声使完全溶解,40℃减压蒸发除去有机溶剂得脂质薄膜。加入适量生理盐水作为水合介质,37℃水合30min得脂质囊泡。(1) Dissolving hydrogenated soybean phospholipid, PEGylated phospholipid and cholesterol in chloroform in molar ratio, ultrasonically dissolving completely, and evaporating under reduced pressure at 40° C. to remove the organic solvent to obtain a lipid film. An appropriate amount of normal saline was added as a hydration medium, and lipid vesicles were obtained by hydration at 37°C for 30 min.
(2)取步骤(1)制得的脂质体,加入全氟化碳,以50%强度进行探头超声粉碎,超声10min后即得粗混悬液。(2) Take the liposomes prepared in step (1), add perfluorocarbon, carry out ultrasonic pulverization with probes at 50% strength, and obtain a coarse suspension after ultrasonication for 10 minutes.
(3)将步骤(2)制得的粗混悬液与步骤(1)制得的脂质囊泡混合后,按顺序挤出通过400nm,200nm,100nm的纤维素膜,每层挤出次数不少于13次,取续滤液,稀释后即得所述超声造影剂。(3) After mixing the crude suspension prepared in step (2) with the lipid vesicles prepared in step (1), extrude through cellulose membranes of 400 nm, 200 nm, and 100 nm in sequence, and extrude each layer for the number of times No less than 13 times, the continuous filtrate is taken and diluted to obtain the ultrasonic contrast agent.
对上述制得的制剂进行透射电镜扫描,其外观呈现大小均一的球形,可见明显壳核结构,磷脂壳有明显多层结构,见附图1。进行粒径测定和Zeta电位评价,该制剂粒径均值为200nm,粒径分布范围为80~400nm,表面荷负电,电位绝对值约为25mV,粒径电位分布见附图2。The above-prepared preparation was scanned by transmission electron microscope, and its appearance showed a spherical shape of uniform size, obvious shell-core structure was visible, and the phospholipid shell had obvious multi-layer structure, as shown in FIG. 1 . The particle size measurement and Zeta potential evaluation were carried out. The average particle size of the preparation was 200 nm, the particle size distribution range was 80-400 nm, the surface was negatively charged, and the absolute value of the potential was about 25 mV. The particle size potential distribution is shown in Figure 2.
实施例2Example 2
实施例1制备的超声造影剂的储存稳定性评价主要包括如下步骤:The storage stability evaluation of the ultrasound contrast agent prepared in Example 1 mainly includes the following steps:
将制得的造影剂置于4℃条件下,于第1,2,3,4,5,6,7天取样,对样品进行粒径测定及Zeta电位评价。The prepared contrast agent was placed at 4°C, and samples were taken on the 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th days, and the samples were subjected to particle size determination and Zeta potential evaluation.
评价结果见图3The evaluation results are shown in Figure 3
实施例3Example 3
实施例1制备的超声造影剂活体成像效果评价主要包括如下步骤:The evaluation of the in vivo imaging effect of the ultrasound contrast agent prepared in Example 1 mainly includes the following steps:
(1)选取雄性SD大鼠,将腹部毛发剔除,腹腔注射巴比妥类药物用于麻醉。在腹部涂上超声耦合剂,用超声仪器进行无造影剂的肝门静脉造影,储存图像见附图4。(1) Male SD rats were selected, the abdominal hair was removed, and barbiturates were injected intraperitoneally for anesthesia. Apply ultrasound coupling agent on the abdomen, and use an ultrasound instrument to perform contrast agent-free hepatic portal vein angiography. The stored images are shown in Figure 4.
(2)将实施例1所述造影剂用生理盐水稀释5-10倍,经尾静脉注射造影剂,对其进行肝门静脉造影,储存图像见附图5。(2) The contrast agent described in Example 1 was diluted 5-10 times with normal saline, and the contrast agent was injected through the tail vein to perform hepatic portal venography. The stored images are shown in FIG. 5 .
Claims (7)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010649043.8ACN111617267A (en) | 2020-07-07 | 2020-07-07 | A nano-scale ultrasound contrast agent encapsulated with perfluorocarbon |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010649043.8ACN111617267A (en) | 2020-07-07 | 2020-07-07 | A nano-scale ultrasound contrast agent encapsulated with perfluorocarbon |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111617267Atrue CN111617267A (en) | 2020-09-04 |
Family
ID=72255799
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010649043.8APendingCN111617267A (en) | 2020-07-07 | 2020-07-07 | A nano-scale ultrasound contrast agent encapsulated with perfluorocarbon |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111617267A (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114588125A (en)* | 2022-02-25 | 2022-06-07 | 北京科技大学 | Targeted drug-loaded thrombolytic microvesicles and preparation method thereof |
| CN115944752A (en)* | 2022-12-27 | 2023-04-11 | 南京邮电大学 | Engineered fused membrane bubble, preparation method and application thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1125389A (en)* | 1993-06-11 | 1996-06-26 | ImaRx药物公司 | Methods of preparing gas and gaseous precursor-filled microspheres |
| CN101732343A (en)* | 2009-12-24 | 2010-06-16 | 上海纳米技术及应用国家工程研究中心有限公司 | Nano perfluocarbon liposome particles and preparation method thereof |
| CN110237276A (en)* | 2019-07-10 | 2019-09-17 | 香港大学深圳医院 | A kind of nanoparticle and its preparation method and application |
| CN111330025A (en)* | 2020-03-03 | 2020-06-26 | 中山大学附属第三医院 | Bionic microbubble ultrasound contrast agent and preparation method thereof |
- 2020
- 2020-07-07CNCN202010649043.8Apatent/CN111617267A/enactivePending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1125389A (en)* | 1993-06-11 | 1996-06-26 | ImaRx药物公司 | Methods of preparing gas and gaseous precursor-filled microspheres |
| CN101732343A (en)* | 2009-12-24 | 2010-06-16 | 上海纳米技术及应用国家工程研究中心有限公司 | Nano perfluocarbon liposome particles and preparation method thereof |
| CN110237276A (en)* | 2019-07-10 | 2019-09-17 | 香港大学深圳医院 | A kind of nanoparticle and its preparation method and application |
| CN111330025A (en)* | 2020-03-03 | 2020-06-26 | 中山大学附属第三医院 | Bionic microbubble ultrasound contrast agent and preparation method thereof |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114588125A (en)* | 2022-02-25 | 2022-06-07 | 北京科技大学 | Targeted drug-loaded thrombolytic microvesicles and preparation method thereof |
| CN115944752A (en)* | 2022-12-27 | 2023-04-11 | 南京邮电大学 | Engineered fused membrane bubble, preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Zhang et al. | Bioinspired multifunctional melanin-based nanoliposome for photoacoustic/magnetic resonance imaging-guided efficient photothermal ablation of cancer | |
| Dai et al. | Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging-guided photothermal tumor ablation | |
| Li et al. | Imaging guided photothermal therapy using iron oxide loaded poly (lactic acid) microcapsules coated with graphene oxide | |
| Liu et al. | Low-intensity focused ultrasound (LIFU)-activated nanodroplets as a theranostic agent for noninvasive cancer molecular imaging and drug delivery | |
| Zha et al. | Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging | |
| Wang et al. | A theranostic nanoplatform: magneto-gold@ fluorescence polymer nanoparticles for tumor targeting T 1 & T 2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy | |
| Fan et al. | Experimental investigation of the penetration of ultrasound nanobubbles in a gastric cancer xenograft | |
| Xiong et al. | Zwitterionic modification of nanomaterials for improved diagnosis of cancer cells | |
| CN109453398B (en) | A kind of mesoporous polydopamine-encapsulated liquid fluorocarbon ultrasound contrast agent and preparation method thereof | |
| JP2001527547A (en) | Microparticles useful as ultrasound contrast agents and for drug delivery to the bloodstream | |
| CN1303273A (en) | Use of particulate contrast agents in diagnostic imaging for studying physiological parameters | |
| Wu et al. | pH-sensitive black phosphorous–incorporated hydrogel as novel implant for cancer treatment | |
| CN108653754B (en) | A hyaluronic acid-targeted polydopamine-coated phase-change liquid fluorocarbon nano-ultrasound contrast agent | |
| Liu et al. | PEGylated hybrid ytterbia nanoparticles as high-performance diagnostic probes for in vivo magnetic resonance and X-ray computed tomography imaging with low systemic toxicity | |
| JP2009508924A (en) | Contrast agent containing silicon | |
| CN103908682A (en) | Application of poly-dopamine nano-particles | |
| Cheng et al. | Ultrasound-triggered phase transition sensitive magnetic fluorescent nanodroplets as a multimodal imaging contrast agent in rat and mouse model | |
| CN108379600A (en) | A kind of multi-functional contrast agent of oxygen carrier liquid fluorocarbon and preparation method thereof | |
| CN103495185B (en) | Preparation method of functionalized polyethyleneimine-modified multi-wall carbon nano-tube magnetic resonance imaging contrast agent | |
| CN111617267A (en) | A nano-scale ultrasound contrast agent encapsulated with perfluorocarbon | |
| CN111632154A (en) | A kind of phase transition nanobubble, its preparation method and use | |
| Luo et al. | Novel DiR and SPIO nanoparticles embedded PEG-PLGA nanobubbles as a multimodalimaging contrast agent | |
| Ma et al. | Three-dimensional angiography fused with CT/MRI for multimodal imaging of nanoparticles based on Ba 4 Yb 3 F 17: Lu 3+, Gd 3+ | |
| CN110604824B (en) | Ultrasonic imaging nanocapsule and preparation method thereof | |
| CN103638534B (en) | A kind of nano-lipid acoustic contrast agent and preparation method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | Application publication date:20200904 | |
| RJ01 | Rejection of invention patent application after publication |