CN111320228A - Self-cleaning carbon nitride gel and preparation method thereof - Google Patents
Self-cleaning carbon nitride gel and preparation method thereofDownload PDFInfo
- Publication number
- CN111320228A CN111320228ACN202010171276.1ACN202010171276ACN111320228ACN 111320228 ACN111320228 ACN 111320228ACN 202010171276 ACN202010171276 ACN 202010171276ACN 111320228 ACN111320228 ACN 111320228A
- Authority
- CN
- China
- Prior art keywords
- carbon nitride
- hydrogel
- self
- aqueous solution
- aerogel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- JMANVNJQNLATNU-UHFFFAOYSA-NoxalonitrileChemical compoundN#CC#NJMANVNJQNLATNU-UHFFFAOYSA-N0.000titleclaimsabstractdescription82
- 238000004140cleaningMethods0.000titleclaimsabstractdescription8
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription7
- 238000001879gelationMethods0.000title1
- 239000000178monomerSubstances0.000claimsabstractdescription14
- 229920002643polyglutamic acidPolymers0.000claimsabstractdescription13
- 229920000642polymerPolymers0.000claimsabstractdescription12
- 239000003431cross linking reagentSubstances0.000claimsabstractdescription9
- 239000000243solutionSubstances0.000claimsdescription24
- 239000007864aqueous solutionSubstances0.000claimsdescription21
- 238000000034methodMethods0.000claimsdescription16
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000claimsdescription8
- 239000011259mixed solutionSubstances0.000claimsdescription7
- 238000001035dryingMethods0.000claimsdescription6
- 239000000843powderSubstances0.000claimsdescription6
- 238000004108freeze dryingMethods0.000claimsdescription4
- 229910052757nitrogenInorganic materials0.000claimsdescription4
- XSQUKJJJFZCRTK-UHFFFAOYSA-NUreaChemical compoundNC(N)=OXSQUKJJJFZCRTK-UHFFFAOYSA-N0.000claimsdescription3
- 238000001354calcinationMethods0.000claimsdescription3
- 239000004202carbamideSubstances0.000claimsdescription3
- 239000002243precursorSubstances0.000claimsdescription3
- 239000007787solidSubstances0.000claimsdescription2
- 238000003756stirringMethods0.000claimsdescription2
- PQDJYEQOELDLCP-UHFFFAOYSA-NtrimethylsilaneChemical groupC[SiH](C)CPQDJYEQOELDLCP-UHFFFAOYSA-N0.000claimsdescription2
- 239000004964aerogelSubstances0.000abstractdescription29
- 239000000017hydrogelSubstances0.000abstractdescription19
- 239000000975dyeSubstances0.000abstractdescription17
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000abstractdescription12
- 239000000499gelSubstances0.000abstractdescription8
- JOPOVCBBYLSVDA-UHFFFAOYSA-Nchromium(6+)Chemical compound[Cr+6]JOPOVCBBYLSVDA-UHFFFAOYSA-N0.000abstractdescription7
- 239000000463materialSubstances0.000abstractdescription6
- YUYCVXFAYWRXLS-UHFFFAOYSA-NtrimethoxysilaneChemical compoundCO[SiH](OC)OCYUYCVXFAYWRXLS-UHFFFAOYSA-N0.000abstractdescription6
- 229910001385heavy metalInorganic materials0.000abstractdescription5
- 239000004065semiconductorSubstances0.000abstractdescription4
- 238000010521absorption reactionMethods0.000abstractdescription3
- 230000005284excitationEffects0.000abstract2
- 230000003020moisturizing effectEffects0.000abstract1
- 238000006116polymerization reactionMethods0.000abstract1
- 238000011084recoveryMethods0.000abstract1
- PYWVYCXTNDRMGF-UHFFFAOYSA-Nrhodamine BChemical compound[Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=OPYWVYCXTNDRMGF-UHFFFAOYSA-N0.000description12
- 229940043267rhodamine bDrugs0.000description12
- 230000000694effectsEffects0.000description8
- 230000015556catabolic processEffects0.000description5
- 238000006731degradation reactionMethods0.000description5
- 238000013033photocatalytic degradation reactionMethods0.000description5
- 230000000593degrading effectEffects0.000description4
- 238000010586diagramMethods0.000description4
- 238000001000micrographMethods0.000description4
- 230000001699photocatalysisEffects0.000description4
- 229910052724xenonInorganic materials0.000description4
- FHNFHKCVQCLJFQ-UHFFFAOYSA-Nxenon atomChemical compound[Xe]FHNFHKCVQCLJFQ-UHFFFAOYSA-N0.000description4
- 238000005259measurementMethods0.000description3
- 238000001179sorption measurementMethods0.000description3
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description2
- 238000005033Fourier transform infrared spectroscopyMethods0.000description2
- 238000002441X-ray diffractionMethods0.000description2
- 229910001430chromium ionInorganic materials0.000description2
- 230000018044dehydrationEffects0.000description2
- 238000006297dehydration reactionMethods0.000description2
- 238000002474experimental methodMethods0.000description2
- 229910002804graphiteInorganic materials0.000description2
- 239000010439graphiteSubstances0.000description2
- LELOWRISYMNNSU-UHFFFAOYSA-Nhydrogen cyanideChemical compoundN#CLELOWRISYMNNSU-UHFFFAOYSA-N0.000description2
- -1Cr(VI) ionsChemical class0.000description1
- 238000001157Fourier transform infrared spectrumMethods0.000description1
- 238000010170biological methodMethods0.000description1
- 239000002977biomimetic materialSubstances0.000description1
- 230000003197catalytic effectEffects0.000description1
- 238000005345coagulationMethods0.000description1
- 230000015271coagulationEffects0.000description1
- 238000011161developmentMethods0.000description1
- 238000002848electrochemical methodMethods0.000description1
- 238000005516engineering processMethods0.000description1
- 230000007613environmental effectEffects0.000description1
- 239000003344environmental pollutantSubstances0.000description1
- 238000003912environmental pollutionMethods0.000description1
- 238000000605extractionMethods0.000description1
- 238000001125extrusionMethods0.000description1
- 150000002500ionsChemical class0.000description1
- 239000012528membraneSubstances0.000description1
- 239000000203mixtureSubstances0.000description1
- 239000002086nanomaterialSubstances0.000description1
- 230000003647oxidationEffects0.000description1
- 238000007254oxidation reactionMethods0.000description1
- 231100000719pollutantToxicity0.000description1
- 238000012805post-processingMethods0.000description1
- 238000004321preservationMethods0.000description1
- 239000002994raw materialSubstances0.000description1
- 238000004064recyclingMethods0.000description1
- 238000011160researchMethods0.000description1
- 238000004062sedimentationMethods0.000description1
- 238000000926separation methodMethods0.000description1
- 238000002798spectrophotometry methodMethods0.000description1
- 238000012360testing methodMethods0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/30—Treatment of water, waste water, or sewage by irradiation
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J13/00—Colloid chemistry, e.g. the production of colloidal materials or their solutions, not otherwise provided for; Making microcapsules or microballoons
- B01J13/0052—Preparation of gels
- B01J13/0056—Preparation of gels containing inorganic material and water
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J13/00—Colloid chemistry, e.g. the production of colloidal materials or their solutions, not otherwise provided for; Making microcapsules or microballoons
- B01J13/0091—Preparation of aerogels, e.g. xerogels
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/24—Nitrogen compounds
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/20—Catalysts, in general, characterised by their form or physical properties characterised by their non-solid state
- B01J35/23—Catalysts, in general, characterised by their form or physical properties characterised by their non-solid state in a colloidal state
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/39—Photocatalytic properties
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/70—Treatment of water, waste water, or sewage by reduction
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/10—Inorganic compounds
- C02F2101/20—Heavy metals or heavy metal compounds
- C02F2101/22—Chromium or chromium compounds, e.g. chromates
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/30—Organic compounds
- C02F2101/308—Dyes; Colorants; Fluorescent agents
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2305/00—Use of specific compounds during water treatment
- C02F2305/10—Photocatalysts
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Hydrology & Water Resources (AREA)
- Life Sciences & Earth Sciences (AREA)
- Dispersion Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- Water Supply & Treatment (AREA)
- Inorganic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Toxicology (AREA)
- Catalysts (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及一种氮化碳水凝胶和氮化碳气凝胶材料及其制备方法和应用,属于纳米材料应用领域。The invention relates to a carbon nitride hydrogel and a carbon nitride aerogel material, a preparation method and application thereof, and belongs to the application field of nanomaterials.
背景技术Background technique
环境污染一直是影响人类生存环境的主要因素,其中,染料污染、重金属污染的处理方式主要有吸附法、萃取法、膜分离法、混凝沉降法、催化氧化法、电化学法、生物法。然而这些方法多数会引起二次污染,所以采取有良好的生物相容性的处理方法是亟需解决的问题。近年来兴起的光催化技术在环境治理中有很大的潜能。石墨相氮化碳(g-C3N4)作为一种新型聚合物半导体材料,可以吸收可见光,有较好的稳定性,它被公认为是一种具有发展潜力的环境友好的新型光催化半导体材料。现有的技术中利用g-C3N4粉末直接进行染料降解存在一定的效果,但是对g-C3N4后处理以及循环利用没有很好地解决方案。Environmental pollution has always been the main factor affecting human living environment. Among them, dye pollution and heavy metal pollution are mainly treated by adsorption method, extraction method, membrane separation method, coagulation sedimentation method, catalytic oxidation method, electrochemical method and biological method. However, most of these methods will cause secondary pollution, so it is an urgent problem to adopt a treatment method with good biocompatibility. The photocatalytic technology emerging in recent years has great potential in environmental governance. Graphitic carbon nitride (gC3 N4 ), as a new type of polymer semiconductor material, can absorb visible light and has good stability. It is recognized as an environmentally friendly new photocatalytic semiconductor material with development potential. . In the prior art, the use of gC3 N4 powder to directly degrade dyes has certain effects, but there is no good solution for the post-treatment and recycling of gC3 N4 .
发明内容SUMMARY OF THE INVENTION
本发明的目的在于提供一种氮化碳凝胶材料及其制备方法。The purpose of the present invention is to provide a carbon nitride gel material and a preparation method thereof.
实现本发明目的的技术解决方案为:一种可自清洁氮化碳凝胶,采用以下步骤制备:The technical solution for realizing the object of the present invention is: a self-cleaning carbon nitride gel, prepared by adopting the following steps:
第一步,将氮化碳均匀地分散在水溶液中;In the first step, the carbon nitride is uniformly dispersed in the aqueous solution;
第二步,将聚合物单体均匀地分散在水溶液中;In the second step, the polymer monomers are uniformly dispersed in the aqueous solution;
第三步,将第一步所得氮化碳水溶液滴加到第二步所得聚合物单体水溶液中;In the third step, the carbon nitride aqueous solution obtained in the first step is added dropwise to the polymer monomer aqueous solution obtained in the second step;
第四步,向第三步所得混合溶液中滴加交联剂,并搅拌分散均匀;In the fourth step, the cross-linking agent is added dropwise to the mixed solution obtained in the third step, and the mixture is stirred and dispersed evenly;
第五步,将分散均匀的溶液移入模具,干燥。The fifth step is to move the evenly dispersed solution into the mold and dry it.
较佳的,第一步中,氮化碳通过煅烧富氮前驱体所得,其具体制备步骤是:将尿素装入带盖的坩埚中,将其置于管式炉中程序升温至500-550℃并保温2-4h,所得固体粉末即为氮化碳。Preferably, in the first step, carbon nitride is obtained by calcining a nitrogen-rich precursor, and the specific preparation steps are: put urea into a crucible with a lid, and place it in a tube furnace to program the temperature to 500-550 ℃. ℃ and heat preservation for 2-4h, the obtained solid powder is carbon nitride.
较佳的,第二步中,聚合物单体为γ-聚谷氨酸。Preferably, in the second step, the polymer monomer is γ-polyglutamic acid.
较佳的,第三步中,氮化碳和聚合物单体的质量比为1:10-1:40。Preferably, in the third step, the mass ratio of carbon nitride and polymer monomer is 1:10-1:40.
较佳的,第四步中,交联剂为三甲基硅烷,聚合物单体和交联剂的质量比为1:1-4:1。Preferably, in the fourth step, the cross-linking agent is trimethylsilane, and the mass ratio of the polymer monomer and the cross-linking agent is 1:1-4:1.
较佳的,第五步中,所述的干燥是指置于35-40℃下烘干,或冷冻干燥。Preferably, in the fifth step, the drying refers to drying at 35-40° C., or freeze-drying.
本发明与现有技术相比,其优点在于:(1)以尿素为前驱体煅烧所得的氮化碳质地较轻,带隙能合适展现出更好的光催化性能,并且该方法制得的氮化碳易于分散在水溶液中;(2)制备氮化碳凝胶所用的原料y-聚谷氨酸、氮化碳都有很好的生物相容性,对环境,人体都无害,有希望应用在仿生材料的研究。(3)制备的氮化碳气凝胶密度小,质地轻,有很好的吸水性和自清洁能力。(4)利用氮化碳凝胶进行染料降解和重金属还原存在后处理方便的优势。Compared with the prior art, the present invention has the following advantages: (1) the carbon nitride obtained by calcining urea as a precursor is lighter in texture, and has a suitable band gap to exhibit better photocatalytic performance, and the carbon nitride obtained by the method Carbon nitride is easy to disperse in aqueous solution; (2) The raw materials y-polyglutamic acid and carbon nitride used in the preparation of carbon nitride gel have good biocompatibility, and are harmless to the environment and human body. Hope to apply it in the research of biomimetic materials. (3) The prepared carbon nitride aerogel has low density, light texture, good water absorption and self-cleaning ability. (4) The use of carbon nitride gel for dye degradation and heavy metal reduction has the advantage of convenient post-processing.
附图说明Description of drawings
图1为实施例1所制备的氮化碳水凝胶的数码照片。Figure 1 is a digital photograph of the carbon nitride hydrogel prepared in Example 1.
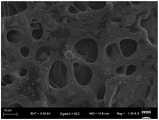
图2为实施例1所制备的氮化碳水凝胶的扫描电镜图。2 is a scanning electron microscope image of the carbon nitride hydrogel prepared in Example 1.
图3为实施例2所制备的氮化碳气凝胶的扫描电镜图。3 is a scanning electron microscope image of the carbon nitride aerogel prepared in Example 2.
图4为石墨相氮化碳粉末和实施例2所制备的氮化碳气凝胶的X射线衍射图谱。4 is the X-ray diffraction pattern of the graphite phase carbon nitride powder and the carbon nitride aerogel prepared in Example 2.
图5为石墨相氮化碳粉末、实施例1所制备的氮化碳水凝胶、实施例2所制备的氮化碳气凝胶的傅里叶红外光谱图。FIG. 5 is the Fourier transform infrared spectrogram of graphitic carbon nitride powder, carbon nitride hydrogel prepared in Example 1, and carbon nitride aerogel prepared in Example 2.
图6为实施例3所制备的氮化碳水凝胶还原六价铬离子的效果图。6 is a graph showing the effect of reducing hexavalent chromium ions by carbon nitride hydrogel prepared in Example 3.
图7为实施例4所制备的氮化碳气凝胶降解罗丹明B染料溶液的效果图。7 is an effect diagram of the carbon nitride aerogel prepared in Example 4 for degrading the rhodamine B dye solution.
图8为实施例4所制备的氮化碳气凝胶降解罗丹明B染料溶液的过程图。8 is a process diagram of the carbon nitride aerogel prepared in Example 4 for degrading the rhodamine B dye solution.
具体实施方式Detailed ways
本发明制备的氮化碳水凝胶和氮化碳气凝胶,都对染料污染物有突出的降解作用,对重金属Cr(Ⅵ)有明显的还原作用,并且氮化碳凝胶有自清洁功能。The carbon nitride hydrogel and carbon nitride aerogel prepared by the invention have outstanding degradation effect on dye pollutants, obvious reduction effect on heavy metal Cr(VI), and carbon nitride gel has self-cleaning function .
本发明的氮化碳水凝胶、氮化碳气凝胶采用以下步骤制备而得:The carbon nitride hydrogel and carbon nitride aerogel of the present invention are prepared by the following steps:
第一步,将单体(γ-聚谷氨酸,0.5-2g)均匀地分散在水溶液中;In the first step, the monomer (γ-polyglutamic acid, 0.5-2g) is uniformly dispersed in the aqueous solution;
第二步,将石墨相氮化碳粉末(0.05-0.1g)均匀地分散在水溶液中;In the second step, the graphite phase carbon nitride powder (0.05-0.1g) is uniformly dispersed in the aqueous solution;
第三步,将分散均匀的氮化碳水溶液滴加到分散均匀的单体溶液中;The third step is to drop the uniformly dispersed carbon nitride aqueous solution into the uniformly dispersed monomer solution;
第四步,向第三步的混合溶液中滴加交联剂(三甲氧基硅烷,0.5-1g),并搅拌分散均匀;In the fourth step, dropwise add the crosslinking agent (trimethoxysilane, 0.5-1 g) to the mixed solution in the third step, and stir and disperse evenly;
第五步,将分散均匀的溶液移入模具,烘干。In the fifth step, the uniformly dispersed solution is moved into the mold and dried.
实施例1:Example 1:
第一步,将0.5g γ-聚谷氨酸均匀地分散在10mL水中,搅拌1h;In the first step, 0.5 g of γ-polyglutamic acid was evenly dispersed in 10 mL of water, and stirred for 1 h;
第二步,将0.05g氮化碳均匀地分散在10mL水中,搅拌2h;In the second step, 0.05g of carbon nitride was evenly dispersed in 10mL of water, and stirred for 2h;
第三步,将分散均匀的氮化碳水溶液滴加到分散均匀的γ-聚谷氨酸水溶液中;The third step is to drop the uniformly dispersed carbon nitride aqueous solution into the uniformly dispersed γ-polyglutamic acid aqueous solution;
第四步,向第三步的混合溶液中滴加1g三甲氧基硅烷,并搅拌3h;In the fourth step, 1 g of trimethoxysilane was added dropwise to the mixed solution in the third step, and stirred for 3h;
第五步,将分散均匀的溶液移入模具,真空烘箱38℃烘干12h得氮化碳水凝胶。In the fifth step, the uniformly dispersed solution was moved into the mold, and dried in a vacuum oven at 38° C. for 12 hours to obtain carbon nitride hydrogel.
第六步,对所制备的氮化碳水凝胶进行表征,图1为氮化碳水凝胶的数码照片,图2为氮化碳水凝胶的扫描电镜图,图5为氮化碳水凝胶的傅里叶红外光谱图。The sixth step is to characterize the prepared carbon nitride hydrogel. Figure 1 is a digital photo of the carbon nitride hydrogel, Figure 2 is a scanning electron microscope image of the carbon nitride hydrogel, and Figure 5 is the carbon nitride hydrogel. Fourier transform infrared spectrogram.
实施例2:Example 2:
第一步,将0.5g γ-聚谷氨酸均匀地分散在10mL水中,搅拌1h;In the first step, 0.5 g of γ-polyglutamic acid was evenly dispersed in 10 mL of water, and stirred for 1 h;
第二步,将0.05g氮化碳均匀地分散在10mL水中,搅拌2h;In the second step, 0.05g of carbon nitride was evenly dispersed in 10mL of water, and stirred for 2h;
第三步,将分散均匀的氮化碳水溶液滴加到分散均匀的γ-聚谷氨酸水溶液中;The third step is to drop the uniformly dispersed carbon nitride aqueous solution into the uniformly dispersed γ-polyglutamic acid aqueous solution;
第四步,向第三步的混合溶液中滴加1g三甲氧基硅烷,并搅拌3h;In the fourth step, 1 g of trimethoxysilane was added dropwise to the mixed solution in the third step, and stirred for 3h;
第五步,将分散均匀的溶液移入模具,冷冻干燥脱水得氮化碳气凝胶。In the fifth step, the uniformly dispersed solution is moved into the mold, and the carbon nitride aerogel is obtained by freeze-drying and dehydration.
第六步,对所制备的氮化碳气凝胶进行表征,图3为氮化碳气凝胶的扫描电镜图,图4为氮化碳气凝胶的X射线衍射图谱,图5为氮化碳气凝胶的傅里叶红外光谱图。The sixth step is to characterize the prepared carbon nitride aerogel. Figure 3 is the scanning electron microscope image of the carbon nitride aerogel, Figure 4 is the X-ray diffraction pattern of the carbon nitride aerogel, and Figure 5 is the nitrogen Fourier transform infrared spectra of carbonized aerogels.
实施例3:Example 3:
第一步,将0.5g γ-聚谷氨酸均匀地分散在10mL水中,搅拌1h;In the first step, 0.5 g of γ-polyglutamic acid was evenly dispersed in 10 mL of water, and stirred for 1 h;
第二步,将0.05g氮化碳均匀地分散在10mL水中,搅拌2h;In the second step, 0.05g of carbon nitride was evenly dispersed in 10mL of water, and stirred for 2h;
第三步,将分散均匀的氮化碳水溶液滴加到分散均匀的γ-聚谷氨酸水溶液中;The third step is to drop the uniformly dispersed carbon nitride aqueous solution into the uniformly dispersed γ-polyglutamic acid aqueous solution;
第四步,向第三步的混合溶液中滴加1g三甲氧基硅烷,并搅拌3h;In the fourth step, 1 g of trimethoxysilane was added dropwise to the mixed solution in the third step, and stirred for 3h;
第五步,将分散均匀的溶液移入模具,真空烘箱38℃烘干12h得氮化碳水凝胶。;In the fifth step, the uniformly dispersed solution was moved into the mold, and dried in a vacuum oven at 38° C. for 12 hours to obtain carbon nitride hydrogel. ;
第六步,将制得的氮化碳水凝胶置于装有50mL50ppmCr(Ⅵ)溶液的烧杯中,将氙灯光源置于烧杯上方25cm处进行光催化还原重金属离子实验。每隔20min取1mL溶液用于紫外分光光度计的测量,所测得的结果即为溶液中Cr(Ⅵ)的浓度。In the sixth step, the prepared carbon nitride hydrogel was placed in a beaker containing 50 mL of 50 ppm Cr(VI) solution, and the xenon lamp light source was placed 25 cm above the beaker to carry out the photocatalytic reduction experiment of heavy metal ions. Take 1mL of solution every 20min for measurement by UV spectrophotometer, and the measured result is the concentration of Cr(VI) in the solution.
实施例3所制备的氮化碳水凝胶还原六价铬离子的效果如图6所示。紫外分光光度计测量的结果表明,在80min内,0.5g氮化碳水凝胶可将50mL 50ppm的Cr(Ⅵ)溶液中的Cr(Ⅵ)离子完全还原。The effect of reducing hexavalent chromium ions by the carbon nitride hydrogel prepared in Example 3 is shown in FIG. 6 . The results of UV spectrophotometer measurements showed that within 80 min, 0.5 g of carbon nitride hydrogel could completely reduce Cr(VI) ions in 50 mL of 50 ppm Cr(VI) solution.
实施例4:Example 4:
第一步,将0.5g γ-聚谷氨酸均匀地分散在10mL水中,搅拌1h;In the first step, 0.5 g of γ-polyglutamic acid was evenly dispersed in 10 mL of water, and stirred for 1 h;
第二步,将0.05g氮化碳均匀地分散在10mL水中,搅拌2h;In the second step, 0.05g of carbon nitride was evenly dispersed in 10mL of water, and stirred for 2h;
第三步,将分散均匀的氮化碳水溶液滴加到分散均匀的y-聚谷氨酸水溶液中;In the third step, the uniformly dispersed carbon nitride aqueous solution is added dropwise to the uniformly dispersed y-polyglutamic acid aqueous solution;
第四步,向第三步的混合溶液中滴加1g三甲氧基硅烷,并搅拌3h;In the fourth step, 1 g of trimethoxysilane was added dropwise to the mixed solution in the third step, and stirred for 3h;
第五步,将分散均匀的溶液移入模具,冷冻干燥脱水得氮化碳气凝胶;The fifth step, the uniformly dispersed solution is moved into the mold, freeze-drying and dehydration to obtain carbon nitride aerogel;
第六步,将制得的氮化碳气凝胶置于装有50mL50ppm罗丹明B溶液的烧杯中,将氙灯光源置于烧杯上方25cm处进行光催化降解染料实验;In the sixth step, the prepared carbon nitride aerogel was placed in a beaker containing 50 mL of 50 ppm Rhodamine B solution, and the xenon lamp light source was placed 25 cm above the beaker to perform a photocatalytic degradation dye experiment;
第七步,静置半小时后,氮化碳气凝胶将50mL50ppm的罗丹明B溶液全部吸附,并在外光上表现出罗丹明B染料的颜色,表明只是单纯地吸附了染料溶液;The seventh step, after standing for half an hour, the carbon nitride aerogel adsorbed all the 50mL 50ppm Rhodamine B solution, and showed the color of Rhodamine B dye on the external light, indicating that the dye solution was simply adsorbed;
第八步,打开氙灯光源后,进行20min的光催化降解后,氮化碳气凝胶的颜色完全褪去,表明成功地降解了罗丹明B染料。In the eighth step, after turning on the xenon lamp light source and carrying out photocatalytic degradation for 20 minutes, the color of the carbon nitride aerogel completely faded, indicating that the rhodamine B dye was successfully degraded.
第九步,通过物理挤压的方式将降解后气凝胶中的水溶液进行紫外分光光度法检测,对比降解前后的测试结果成功验证了氮化碳气凝胶对罗丹明B染料的降解。In the ninth step, the aqueous solution in the degraded aerogel is detected by ultraviolet spectrophotometry by means of physical extrusion, and the degradation of rhodamine B dye by carbon nitride aerogel is successfully verified by comparing the test results before and after the degradation.
实施例4所制备的氮化碳气凝胶降解罗丹明B染料溶液的效果如图7所示。紫外分光光度计测量的结果表明,在经过20min的光催化降解后,氮化碳气凝胶对50mL50ppm的罗丹明B溶液降解效果显著,从而说明了所制备的氮化碳气凝胶有很好的自清洁能力。The effect of the carbon nitride aerogel prepared in Example 4 for degrading the rhodamine B dye solution is shown in FIG. 7 . The results of UV spectrophotometer measurement showed that after 20min of photocatalytic degradation, the carbon nitride aerogel had a significant degradation effect on 50mL50ppm Rhodamine B solution, which indicated that the prepared carbon nitride aerogel had good performance. self-cleaning ability.
图8为实施例4所制备的氮化碳气凝胶降解罗丹明B染料溶液的过程图。a是将氮化碳气凝胶置于罗丹明B染料溶液进行吸附;b表明经过30min的吸附,50mL的罗丹明B染料溶液完全被氮化碳气凝胶吸附,这也表明了制备的氮化碳气凝胶有很好的吸水性;c是通过氙灯进行光催化降解的装置示意图;d是经过光催化降解后的凝胶的数码照片。8 is a process diagram of the carbon nitride aerogel prepared in Example 4 for degrading the rhodamine B dye solution. a is carbon nitride aerogel placed in rhodamine B dye solution for adsorption; b shows that after 30min of adsorption, 50 mL of rhodamine B dye solution is completely adsorbed by carbon nitride aerogel, which also shows that the prepared nitrogen Carbonized aerogel has good water absorption; c is the schematic diagram of the device for photocatalytic degradation by xenon lamp; d is the digital photo of the gel after photocatalytic degradation.
Claims (8)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010171276.1ACN111320228A (en) | 2020-03-12 | 2020-03-12 | Self-cleaning carbon nitride gel and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010171276.1ACN111320228A (en) | 2020-03-12 | 2020-03-12 | Self-cleaning carbon nitride gel and preparation method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111320228Atrue CN111320228A (en) | 2020-06-23 |
Family
ID=71165656
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010171276.1APendingCN111320228A (en) | 2020-03-12 | 2020-03-12 | Self-cleaning carbon nitride gel and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111320228A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114570424A (en)* | 2022-03-30 | 2022-06-03 | 东北石油大学 | Double-modified carbon nitride and preparation method and application thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008120596A1 (en)* | 2007-04-03 | 2008-10-09 | Konica Minolta Opto, Inc. | Cellulose ester optical film, polarizing plate and liquid crystal display using the cellulose ester optical film, method for producing cellulose ester optical film, and copolymer |
| US20100103352A1 (en)* | 2007-04-10 | 2010-04-29 | Takayuki Suzuki | Optical film, polarizing plate, liquid crystal display and ultraviolet absorbing polymer |
| CN103347904A (en)* | 2011-02-08 | 2013-10-09 | 葛非亚公司 | Carbocatalysts for polymerization |
| CN106582461A (en)* | 2016-12-14 | 2017-04-26 | 南京理工大学 | Carbon nitride hydrogel and preparation method thereof |
| CN110538578A (en)* | 2019-09-06 | 2019-12-06 | 苏州清溪环保科技有限公司 | Sewage treatment membrane with high decontamination efficiency and preparation method thereof |
- 2020
- 2020-03-12CNCN202010171276.1Apatent/CN111320228A/enactivePending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008120596A1 (en)* | 2007-04-03 | 2008-10-09 | Konica Minolta Opto, Inc. | Cellulose ester optical film, polarizing plate and liquid crystal display using the cellulose ester optical film, method for producing cellulose ester optical film, and copolymer |
| US20100103352A1 (en)* | 2007-04-10 | 2010-04-29 | Takayuki Suzuki | Optical film, polarizing plate, liquid crystal display and ultraviolet absorbing polymer |
| CN103347904A (en)* | 2011-02-08 | 2013-10-09 | 葛非亚公司 | Carbocatalysts for polymerization |
| CN106582461A (en)* | 2016-12-14 | 2017-04-26 | 南京理工大学 | Carbon nitride hydrogel and preparation method thereof |
| CN110538578A (en)* | 2019-09-06 | 2019-12-06 | 苏州清溪环保科技有限公司 | Sewage treatment membrane with high decontamination efficiency and preparation method thereof |
Non-Patent Citations (2)
| Title |
|---|
| 刘宗梅等: "不同前驱体制备g-C_3N_4光催化性能及稳定性", 《应用化工》* |
| 高彦祥: "《食品添加剂》", 31 July 2019, 北京:中国轻工业出版社* |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114570424A (en)* | 2022-03-30 | 2022-06-03 | 东北石油大学 | Double-modified carbon nitride and preparation method and application thereof |
| CN114570424B (en)* | 2022-03-30 | 2024-02-09 | 东北石油大学 | Double-modified carbon nitride and preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN108816292B (en) | Preparation method and application of polydopamine/nano molybdenum disulfide photocatalyst | |
| CN110479350A (en) | A kind of preparation method of thin layer nitride porous carbon photochemical catalyst | |
| CN113600164B (en) | Preparation method and application of a kind of iron-doped carbon quantum dot/graphite phase carbon nitride composite photocatalyst | |
| CN107715855A (en) | A kind of porous carbon materials for being used for organic dyestuff and fluoride wastewater treatment and preparation method thereof | |
| CN108499602B (en) | A kind of preparation method of conjugated polyvinyl alcohol modified nano molybdenum disulfide | |
| CN112973795A (en) | Modified sodium alginate-based photocatalytic reducing agent, preparation method and application thereof | |
| CN108097313A (en) | A kind of carbonitride/chitosan aeroge composite photo-catalyst and its preparation method and application | |
| CN113181949A (en) | Cobalt-iron alloy/nitrogen-sulfur co-doped carbon nano composite material and preparation method and application thereof | |
| CN110624534A (en) | A kind of biorelic WO3 photocatalyst and its preparation method and application | |
| CN111320228A (en) | Self-cleaning carbon nitride gel and preparation method thereof | |
| CN106044842A (en) | Preparation method and application of sector hydroxyl zinc fluoride | |
| CN115414958A (en) | A kind of melamine-doped biochar material and its application | |
| CN115445646A (en) | Carbon nitride composite photocatalyst and its preparation method and application | |
| CN116459860A (en) | Preparation method and application of a cyano-functionalized porous carbon nitride photocatalyst | |
| CN110201718B (en) | Preparation and application of red phosphorus/iron-based metal organic framework composite material | |
| CN111192703B (en) | A method for treating radioactive wastewater by using KGM-rGO sponge | |
| CN116408011A (en) | MXene@PDA and oxidized sodium alginate modified collagen composite aerogel as well as preparation method and application thereof | |
| CN109092245A (en) | A kind of tripolite loading carbon nanotube adsorption agent and preparation method thereof | |
| CN103433078B (en) | A kind of conductive polymer/BiPO4 composite photocatalyst and preparation method thereof | |
| CN113680366B (en) | A graphite-phase carbon nitride-based composite photocatalyst and its preparation method and application | |
| CN111097472B (en) | Mesoporous ultrathin g-C 3 N 4 Photocatalytic nanosheet and preparation method thereof | |
| CN105056998B (en) | A kind of zinc oxide nano-particle is uniformly distributed in the preparation method of nano zine oxide/cyclization polyacrylonitrile composite microsphere material in polymer | |
| CN117205956B (en) | Simple preparation method and application of modified carbon nitride | |
| CN117531497B (en) | Preparation method of efficient composite carbon source and application of efficient composite carbon source in sewage treatment | |
| CN117482996A (en) | Preparation method of efficient and stable self-floating core-shell gel catalyst |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication | Application publication date:20200623 |