CN111234013A - Hepatitis B virus neutralizing antibody B430 and its application - Google Patents
Hepatitis B virus neutralizing antibody B430 and its applicationDownload PDFInfo
- Publication number
- CN111234013A CN111234013ACN201811442794.1ACN201811442794ACN111234013ACN 111234013 ACN111234013 ACN 111234013ACN 201811442794 ACN201811442794 ACN 201811442794ACN 111234013 ACN111234013 ACN 111234013A
- Authority
- CN
- China
- Prior art keywords
- sequence
- hepatitis
- amino acid
- virus
- antibody
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/082—Hepadnaviridae, e.g. hepatitis B virus
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Virology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Molecular Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- Communicable Diseases (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Gastroenterology & Hepatology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Oncology (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及一种乙肝病毒的中和抗体B430及其应用。The invention relates to a neutralizing antibody B430 of hepatitis B virus and its application.
背景技术Background technique
乙型肝炎病毒(Hepatitis B Virus,HBV)感染在全世界广泛存在,在中国尤其严重。乙肝病毒属于嗜肝病毒科的一员,其感染诱发肝炎,在中国大约有8000万-1亿人慢性感染乙肝病毒,全球约有2.4亿慢性乙肝患者。如果考虑已经恢复的急性乙肝病毒感染者,乙肝病毒感染者累计更多。有研究表明35-62%中国人感染过乙肝病毒,56-98%撒哈拉以南非洲人感染过乙肝病毒,全球约有20亿人感染过乙肝病毒,乙肝病毒感染是全球性的卫生问题。Hepatitis B virus (HBV) infection is widespread in the world, especially in China. Hepatitis B virus is a member of the family Hepadnaviridae, and its infection induces hepatitis. About 80 million to 100 million people in China are chronically infected with hepatitis B virus, and there are about 240 million chronic hepatitis B patients in the world. If the patients with acute hepatitis B virus infection who have recovered are considered, the cumulative number of patients with hepatitis B virus infection is higher. Studies have shown that 35-62% of Chinese people have been infected with hepatitis B virus, and 56-98% of sub-Saharan Africans have been infected with hepatitis B virus. About 2 billion people in the world have been infected with hepatitis B virus. HBV infection is a global health problem.
乙肝病毒在感染过程中诱发免疫炎症损伤患者肝脏。中国乙肝患者大部分为母婴传播或者小孩时期感染乙肝病毒,绝大部分为慢性乙肝感染,终生携带乙肝病毒。成年人感染乙肝病毒,大部分可依靠自身免疫系统清除乙肝病毒,为乙肝病毒的急性感染。乙肝病毒感染导致急性慢性肝炎、肝硬化乃至肝癌,其诱发肝炎机制主要是肝细胞内乙肝病毒诱发机体免疫系统的肝脏炎症,乙肝病毒的复制模板共价闭合环状DNA(cccDNA)作为病毒储存库稳定且随时补充,非常难以清除,乙肝病毒复制诱发持续不断和多次反复的免疫杀伤过程中造成肝脏炎症。Hepatitis B virus induces immune inflammation and damages the liver of patients during infection. Most of the hepatitis B patients in China are mother-to-child transmission or infected with hepatitis B virus during childhood, and most of them have chronic hepatitis B infection and carry the hepatitis B virus for life. Most adults infected with HBV can rely on their own immune system to clear HBV, which is an acute infection of HBV. Hepatitis B virus infection leads to acute chronic hepatitis, liver cirrhosis and even liver cancer. The mechanism of hepatitis B virus induction is mainly liver inflammation of the immune system induced by hepatitis B virus in hepatocytes. The replication template of hepatitis B virus is covalently closed circular DNA (cccDNA) as the virus reservoir. It is stable and replenished at any time, and it is very difficult to remove. Hepatitis B virus replication induces liver inflammation during the continuous and repeated immune killing process.
目前乙肝病毒疫苗预防效果很好,临床治疗上只能控制乙肝病毒复制,不能治愈乙肝病毒感染。乙肝疫苗控制了乙肝病毒在中国的广泛传播,对乙肝妈妈导致的母婴传播,临床上通过核苷药物如恩替卡韦、替诺福韦降低乙肝妈妈怀孕期间乙肝病毒复制水平,新生儿出生后注射乙肝免疫球蛋白(HBIG)和及时免疫乙肝疫苗的措施综合干预,非常有效的降低了乙肝病毒的母婴传播。虽然乙肝疫苗控制了乙肝病毒的传播,但是对已感染乙肝病毒的数量众多的患者,临床治疗手段如核苷药物和干扰素,治愈不了乙肝病毒感染。核苷药物能有效控制乙肝病毒的DNA复制,但不能彻底清除乙肝病毒,很难降低乙肝表面抗原,乙肝患者停药之后,乙肝病毒仍然会复发。干扰素治疗周期长,副作用大,只能在治疗期间对相当少的一部分乙肝患者有作用,治疗效果性价比很低,目前乙肝感染治疗基本以核苷药物为主。At present, hepatitis B virus vaccine has a good preventive effect, and clinical treatment can only control hepatitis B virus replication, but cannot cure hepatitis B virus infection. The hepatitis B vaccine has controlled the wide spread of hepatitis B virus in China, and the mother-to-child transmission caused by hepatitis B mothers. Clinically, nucleoside drugs such as entecavir and tenofovir are used to reduce the level of hepatitis B virus replication in hepatitis B mothers during pregnancy, and the newborns are injected with hepatitis B after birth. The comprehensive intervention of immune globulin (HBIG) and timely immunization of hepatitis B vaccine is very effective in reducing mother-to-child transmission of hepatitis B virus. Although the hepatitis B vaccine has controlled the spread of the hepatitis B virus, for a large number of patients who have been infected with the hepatitis B virus, clinical treatments such as nucleoside drugs and interferon cannot cure the hepatitis B virus infection. Nucleoside drugs can effectively control the DNA replication of hepatitis B virus, but they cannot completely eliminate hepatitis B virus, and it is difficult to reduce hepatitis B surface antigen. After hepatitis B patients stop taking the drug, hepatitis B virus will still recur. Interferon has a long treatment period and great side effects. It can only have an effect on a relatively small number of hepatitis B patients during the treatment period. The cost-effectiveness of the treatment effect is very low. At present, the treatment of hepatitis B infection is basically based on nucleoside drugs.
发明内容SUMMARY OF THE INVENTION
本发明的目的是提供一种乙肝病毒的中和抗体B430及其应用。The purpose of the present invention is to provide a kind of neutralizing antibody B430 of hepatitis B virus and its application.
本发明提供了一种IgG抗体,命名为单克隆抗体B430,由轻链和重链组成;所述重链中的重链可变区中的CDR1、CDR2和CDR3依次为序列表的序列1自N末端第45-52位氨基酸残基、第70-77位氨基酸残基、第116-132位氨基酸残基;所述轻链中的轻链可变区中的CDR1、CDR2和CDR3依次为序列表的序列3自N末端第43-48位氨基酸残基、第66-68位氨基酸残基、第105-113位氨基酸残基。The present invention provides an IgG antibody, named as monoclonal antibody B430, which is composed of a light chain and a heavy chain; CDR1, CDR2 and CDR3 in the variable region of the heavy chain in the heavy chain are sequence 1 in the sequence table. N-terminal amino acid residues 45-52, amino acid residues 70-77, and amino acid residues 116-132; CDR1, CDR2 and CDR3 in the light chain variable region in the light chain are in
所述重链可变区由序列表的序列1自N末端第20-143位氨基酸残基组成。The heavy chain variable region consists of amino acid residues 20-143 from the N-terminus of Sequence 1 of the Sequence Listing.
所述轻链可变区由序列表的序列3自N末端第17-123位氨基酸残基组成。The light chain variable region consists of amino acid residues 17-123 from the N-terminus of
所述重链为如下(a)或(b):(a)序列表的序列1自N末端第20-473位氨基酸残基组成的蛋白质;(b)序列表的序列1所示的蛋白质。The heavy chain is the following (a) or (b): (a) a protein consisting of amino acid residues 20-473 of the N-terminal in SEQ ID NO: 1 of the sequence listing; (b) a protein shown in SEQ ID NO: 1 of the sequence listing.
所述轻链为如下(c)或(d):(c)序列表的序列3自N末端第17-230位氨基酸残基组成的蛋白质;(d)序列表的序列3所示的蛋白质。The light chain is the following (c) or (d): (c) a protein consisting of amino acid residues 17-230 of the N-terminal in SEQ ID NO: 3 of the sequence listing; (d) a protein shown in SEQ ID NO: 3 of the sequence listing.
编码所述IgG抗体的基因也属于本发明的保护范围。The gene encoding the IgG antibody also belongs to the protection scope of the present invention.
编码所述重链的基因为如下(1)或(2)或(3):The gene encoding the heavy chain is as follows (1) or (2) or (3):
(1)序列表的序列2自5’末端第972-2333位核苷酸所示的DNA分子;(1)
(2)序列表的序列2自5’末端第915-2336位核苷酸所示的DNA分子;(2) the DNA molecule shown by the 915th-2336th nucleotide of the
(3)序列表的序列2所示的DNA分子。(3) The DNA molecule shown in SEQ ID NO: 2 of the Sequence Listing.
编码所述轻链的基因为如下(4)或(5)或(6):The gene encoding the light chain is as follows (4) or (5) or (6):
(4)序列表的序列4自5’末端第963-1604位核苷酸所示的DNA分子;(4) The DNA molecule represented by the 963-1604th nucleotide of the sequence 4 of the sequence listing from the 5' end;
(5)序列表的序列4自5’末端第915-1607位核苷酸所示的DNA分子;(5) the DNA molecule shown by the 915-1607th nucleotide of the sequence 4 of the sequence listing from the 5' end;
(6)序列表的序列4所示的DNA分子。(6) The DNA molecule shown in SEQ ID NO: 4 of the Sequence Listing.
本发明还保护以上任一所述IgG抗体在制备用于抑制乙肝病毒的药物中的应用。The present invention also protects the application of any of the IgG antibodies described above in the preparation of a medicine for inhibiting hepatitis B virus.
本发明还保护一种用于抑制乙肝病毒的药物,其活性成分为以上任一所述IgG抗体。The present invention also protects a medicine for inhibiting hepatitis B virus, the active ingredient of which is any one of the above-mentioned IgG antibodies.
本发明还保护以上任一所述IgG抗体在制备用于中和乙肝病毒的药物中的应用。The present invention also protects the application of any of the above-mentioned IgG antibodies in the preparation of medicines for neutralizing hepatitis B virus.
本发明还保护一种用于中和乙肝病毒的药物,其活性成分为以上任一所述IgG抗体。The present invention also protects a medicine for neutralizing hepatitis B virus, the active ingredient of which is any one of the above-mentioned IgG antibodies.
本发明还保护以上任一所述IgG抗体在制备用于预防和/或治疗乙型肝炎的药物中的应用。The present invention also protects the application of any of the above-mentioned IgG antibodies in the preparation of a medicament for preventing and/or treating hepatitis B.
本发明还保护一种用于预防和/或治疗乙型肝炎的药物,其活性成分为以上任一所述IgG抗体。The present invention also protects a medicament for preventing and/or treating hepatitis B, the active ingredient of which is any of the above-mentioned IgG antibodies.
单克隆抗体B430能够识别不同基因型乙肝病毒,保证了抗体中和的广谱性。Monoclonal antibody B430 can recognize different genotypes of hepatitis B virus, ensuring a broad spectrum of antibody neutralization.
所述乙肝病毒为各个基因型的乙肝病毒。例如,A基因型的乙肝病毒、B基因型的乙肝病毒、C基因型的乙肝病毒、D基因型的乙肝病毒。A基因型乙肝病毒即基因分型为A型的乙肝病毒。B基因型乙肝病毒即基因分型为B型的乙肝病毒。C基因型乙肝病毒即基因分型为C型的乙肝病毒。D基因型乙肝病毒即基因分型为D型的乙肝病毒。The hepatitis B virus is hepatitis B virus of each genotype. For example, hepatitis B virus of genotype A, hepatitis B virus of genotype B, hepatitis B virus of genotype C, and hepatitis B virus of genotype D. Genotype A hepatitis B virus is the hepatitis B virus genotyped to type A. HBV genotype B is the HBV genotype B. Genotype C hepatitis B virus is the hepatitis B virus genotyped as C type. Genotype D hepatitis B virus is the hepatitis B virus genotyped as D type.
相比较血清多抗,单克隆抗体在中和活性以及来源方便性上具有非常大的优势。本发明的发明人利用人源记忆性B细胞培养技术,从乙肝疫苗免疫者分离到单克隆抗体B430,可用于乙肝慢性感染患者的治疗和临床替代血清纯化多抗预防母婴传播。本发明对治疗和预防乙肝病毒感染具有重要的应用前景。Compared with serum polyclonal antibodies, monoclonal antibodies have great advantages in neutralizing activity and convenience of source. The inventor of the present invention uses the human-derived memory B cell culture technology to isolate the monoclonal antibody B430 from hepatitis B vaccine immunized patients, which can be used for the treatment of patients with chronic hepatitis B infection and the clinical replacement of serum purified polyclonal antibody to prevent mother-to-child transmission. The invention has important application prospect for treating and preventing hepatitis B virus infection.
附图说明Description of drawings
图1为实施例3的步骤一的结果。FIG. 1 is the result of step 1 of Example 3. FIG.
图2为实施例3的步骤二的结果。FIG. 2 is the result of
图3为实施例4的步骤一的结果。FIG. 3 is the result of step 1 of Example 4. FIG.
图4为实施例4的步骤二的结果。FIG. 4 is the result of
具体实施方式Detailed ways
以下的实施例便于更好地理解本发明,但并不限定本发明。下述实施例中的实验方法,如无特殊说明,均为常规方法。下述实施例中所用的试验材料,如无特殊说明,均为自常规生化试剂商店购买得到的。以下实施例中的定量试验,均设置三次重复实验,结果取平均值。如无特殊说明,实施例中的PBS缓冲液均为pH7.2、10mM的PBS缓冲液。PBST溶液:含0.5%(体积百分含量)Triton X-100的PBS缓冲液。乙肝免疫球蛋白溶液(HBIG溶液):北京天坛生物,浓度约为120mg/ml。如无特殊说明,细胞培养条件为37℃、5%CO2环境。JC126载体含HDV复制子,提及JC126载体的文献:李颖等,丁型肝炎病毒复制包装载体的构建.中国现代医学杂志,2017(11):第31-35页。A型乙肝包膜蛋白表达质粒即pHBV1.5,记载于如下文献:Bruss,V.and D.Ganem,The role of envelope proteins in hepatitis B virusassembly.Proc Natl Acad Sci U S A,1991.88(3):p.1059-63.。B型乙肝包膜蛋白表达质粒即pHBV1.18-B,记载于如下文献:Hu,W.,et al.,CpG oligodeoxynucleotide inhibitsHBV replication in a hydrodynamic injection murine model.Antivir Ther,2014.。C型乙肝包膜蛋白表达质粒即pHBV1.18-C。记载于如下文献:Wang,X.J.,et al.,A simpleand efficient strategy for the de novo construction of greater-than-genome-length hepatitis B virus replicons.J Virol Methods,2014.207:p.158-62.。D型乙肝包膜蛋白表达质粒即pCMV-HBV,记载于如下文献:Li,J.,et al.,Inhibition ofhepatitis B virus replication by MyD88involves accelerated degradation ofpregenomic RNA and nuclear retention of pre-S/S RNAs.J Virol,2010.84(13):p.6387-99.。HBs抗原:GENBANK ACCESSION NO:P30019.1,VRL 07-NOV-2018。HBV转基因小鼠(高复制HBV转基因小鼠):记载于如下文献:刘光泽等,高复制HBV转基因小鼠模型对抗乙型肝炎病毒药物的效应研究,中国病理生理杂志,2007,23(1):99–102。兔抗人丁型肝炎病毒δ抗原多克隆抗体(简称HDVδ抗体):北京泽溪源有限公司。单克隆抗体MERS-27及其制备方法见专利201310565893.X(公开号为CN 104628848 A,公告号为CN 104628848 B)。乙型肝炎病毒表面抗原诊断试剂盒(HBsAg,货号30811010101)和乙型肝炎病毒e抗原检测试剂盒(HBeAg,货号30811010103)均为上海科华产品。The following examples facilitate a better understanding of the present invention, but do not limit the present invention. The experimental methods in the following examples are conventional methods unless otherwise specified. The test materials used in the following examples were purchased from conventional biochemical reagent stores unless otherwise specified. The quantitative tests in the following examples are all set to repeat the experiments three times, and the results are averaged. Unless otherwise specified, the PBS buffers in the examples are all PBS buffers with pH 7.2 and 10 mM. PBST solution: PBS buffer containing 0.5% (volume percent) Triton X-100. Hepatitis B immunoglobulin solution (HBIG solution): Beijing Tiantan Biological, the concentration is about 120mg/ml. Unless otherwise specified, the cell culture conditions were 37°C, 5% CO2 environment. The JC126 vector contains the HDV replicon, and the literature that mentions the JC126 vector: Li Ying et al., Construction of hepatitis D virus replication packaging vector. Chinese Journal of Modern Medicine, 2017(11): pp. 31-35. Type A hepatitis B envelope protein expression plasmid, pHBV1.5, is described in the following literature: Bruss, V. and D. Ganem, The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA, 1991.88(3):p. 1059-63.. Type B hepatitis B envelope protein expression plasmid, pHBV1.18-B, is described in the following literature: Hu, W., et al., CpG oligodeoxynucleotide inhibits HBV replication in a hydrodynamic injection murine model. Antivir Ther, 2014. Type C hepatitis B envelope protein expression plasmid is pHBV1.18-C. Described in the following literature: Wang, XJ, et al., A simple and efficient strategy for the de novo construction of greater-than-genome-length hepatitis B virus replicaons. J Virol Methods, 2014.207:p.158-62. The expression plasmid for hepatitis B envelope protein, pCMV-HBV, is described in the following literature: Li, J., et al., Inhibition of hepatitis B virus replication by MyD88involves accelerated degradation of pregenomic RNA and nuclear retention of pre-S/S RNAs.J Virol, 2010.84(13):p.6387-99. HBs antigen: GENBANK ACCESSION NO: P30019.1, VRL 07-NOV-2018. HBV transgenic mice (high-replication HBV transgenic mice): described in the following literature: Liu Guangzhuang et al., Study on the effect of anti-HBV drugs in a high-replication HBV transgenic mouse model, Chinese Journal of Pathophysiology, 2007, 23(1) :99–102. Rabbit anti-human hepatitis D virus delta antigen polyclonal antibody (referred to as HDVdelta antibody): Beijing Zexiyuan Co., Ltd. The monoclonal antibody MERS-27 and its preparation method are shown in Patent 201310565893.X (publication number CN 104628848 A, publication number CN 104628848 B). Hepatitis B virus surface antigen diagnostic kit (HBsAg, product number 30811010101) and hepatitis B virus e antigen detection kit (HBeAg, product number 30811010103) are products of Shanghai Kehua.
实施例1、抗体的发现Example 1. Discovery of Antibodies
利用人源记忆性B细胞培养,从乙肝疫苗免疫者血液中寻找乙肝病毒的单克隆抗体,然后将获得的各个抗体进行效果比较。Human-derived memory B cell culture was used to find monoclonal antibodies against hepatitis B virus from the blood of hepatitis B vaccine immunized persons, and then the effects of the obtained antibodies were compared.
B细胞培养筛选克隆乙肝小膜蛋白特异性B细胞抗体基因的方法:从乙肝疫苗免疫的正常人的外周血分离出外周血淋巴细胞PBMC,流式细胞仪从PBMC分选出记忆性B细胞,将记忆性B细胞和支持细胞3T3-CD40L共培养10天,利用Elisa检测分泌的细胞上清中是否含有乙肝病毒小膜蛋白HBs抗体,抗体阳性孔裂解B细胞反转录cDNA,利用抗体可变区特异性引物克隆出阳性孔所有抗体可变区基因,轻重链配对筛选出一对乙肝小膜蛋白特异性抗体基因。The method for screening and cloning of hepatitis B small membrane protein-specific B cell antibody gene by B cell culture: The peripheral blood lymphocytes PBMC were isolated from the peripheral blood of normal people immunized with hepatitis B vaccine, and the memory B cells were sorted from the PBMC by flow cytometry. Memory B cells and Sertoli cells 3T3-CD40L were co-cultured for 10 days. Elisa was used to detect whether the secreted cell supernatant contained HBs antibody to hepatitis B virus small membrane protein. Antibody-positive wells were used to lyse B cells to reverse transcribe cDNA. Region-specific primers were used to clone all antibody variable region genes in positive wells, and a pair of hepatitis B small membrane protein-specific antibody genes were screened by light and heavy chain pairing.
发现一个效果良好的单克隆抗体(结合抗体),将该单克隆抗体命名为单克隆抗体B430,简称B430抗体。A monoclonal antibody (binding antibody) with good effect was found, and the monoclonal antibody was named as monoclonal antibody B430, abbreviated as B430 antibody.
单克隆抗体B430为IgG抗体,重链如序列表的序列1所示(第1-19位氨基酸残基组成信号肽,第20-143位氨基酸残基组成可变区,第144-473位氨基酸残基组成恒定区;CDR1、CDR2和CDR3依次为第45-52位、第70-77位、第116-132位),轻链如序列表的序列3所示(第1-16位氨基酸残基组成信号肽,第17-123位氨基酸残基组成可变区,第124-230位氨基酸残基组成恒定区;CDR1、CDR2和CDR3依次为第43-48位、第66-68位、第105-113位)。Monoclonal antibody B430 is an IgG antibody, and the heavy chain is shown in Sequence 1 of the sequence table (the 1-19th amino acid residues form the signal peptide, the 20th-143rd amino acid residues form the variable region, and the 144th-473rd amino acid residues form the variable region. Residues make up the constant region; CDR1, CDR2 and CDR3 are 45-52, 70-77, 116-132 in sequence), and the light chain is shown in
实施例2、单克隆抗体B430的制备Example 2. Preparation of monoclonal antibody B430
一、制备重组质粒1. Preparation of recombinant plasmids
将序列表的序列2所示的DNA分子插入pLB-simple Vector,得到重组质粒。该重组质粒已经测序验证。该质粒又命名为重链表达质粒。The DNA molecule shown in SEQ ID NO: 2 of the Sequence Listing was inserted into pLB-simple Vector to obtain a recombinant plasmid. The recombinant plasmid has been verified by sequencing. This plasmid was also named heavy chain expression plasmid.
序列表的序列2中,第1-666位核苷酸为CMV启动子,第915-971位核苷酸为信号肽编码区,第972-1343位核苷酸为重链可变区编码区,1344-2333位核苷酸为重链恒定区编码区,第2334-2336为终止密码子,第2395-2543位核苷酸为polyA终止子。序列表的序列2所示DNA分子表达序列表的序列1所示的重链。In
将序列表的序列4所示的DNA分子插入pLB-simple Vector,得到重组质粒。该重组质粒已经测序验证。该质粒又命名为轻链表达质粒。The DNA molecule shown in SEQ ID NO: 4 of the Sequence Listing was inserted into pLB-simple Vector to obtain a recombinant plasmid. The recombinant plasmid has been verified by sequencing. This plasmid was also named light chain expression plasmid.
序列表的序列4中,第1-666位核苷酸为CMV启动子,第915-962位核苷酸为信号肽编码区,第963-1283位核苷酸为轻链可变区编码区,1284-1604位核苷酸为轻链恒定区编码区,第1605-1607位核苷酸为终止密码子,第1614-1762位核苷酸为polyA终止子。序列表的序列4所示DNA分子表达序列表的序列3所示的轻链。In Sequence 4 of the sequence listing, the 1-666 nucleotides are the CMV promoter, the 915-962 nucleotides are the signal peptide coding region, and the 963-1283 nucleotides are the light chain variable region coding region , 1284-1604 nucleotides are the coding region of the light chain constant region, 1605-1607 nucleotides are stop codons, and 1614-1762 nucleotides are polyA terminators. The DNA molecule shown in Sequence 4 of the Sequence Listing expresses the light chain shown in
pLB-simple Vector为天根生物货号为VT206的pLB零背景快速连接试剂盒的组件,http://www.tiangen.com/?productShow/t1/6/id/308.html。pLB-simple Vector is a component of Tiangen Biotech's pLB zero background quick ligation kit, catalog number VT206, http://www.tiangen.com/? productShow/t1/6/id/308.html.
二、制备抗体2. Preparation of antibodies
1、采用无血清DMEM培养基培养293T细胞,借助PEI转染试剂共转染重链表达质粒和轻链表达质粒并培养8小时,然后更换培养基为含2%胎牛血清的DMEM培养基并培养72小时,然后4℃、4000rpm离心30min,收集上清液。1. Culture 293T cells in serum-free DMEM medium, co-transfect heavy chain expression plasmid and light chain expression plasmid with PEI transfection reagent and culture for 8 hours, then replace the medium with DMEM medium containing 2% fetal bovine serum and After culturing for 72 hours, centrifugation was performed at 4°C and 4000 rpm for 30 min, and the supernatant was collected.
2、取步骤1得到的上清液,使用Econo-Pac聚丙烯层析柱进行纯化。2. Take the supernatant obtained in step 1 and use Econo-Pac polypropylene chromatography column for purification.
聚丙烯层析柱(Biorad,货号7321010),1.5×12cm,柱床体积约20ml。Polypropylene chromatography column (Biorad, Cat. No. 7321010), 1.5×12 cm, with a bed volume of about 20 ml.
操作步骤:①将300-500mL上清液和约1ml protein A beads(Thermo,货号10006D)混合,4℃振荡培养10小时,然后将整个体系加入层析柱中;②用60ml结合缓冲液洗涤;③用30mL洗脱缓冲液洗脱,收集过柱后溶液。Operation steps: ①Mix 300-500mL supernatant with about 1ml protein A beads (Thermo, Cat. No. 10006D), incubate with shaking at 4°C for 10 hours, then add the whole system to the chromatography column; ②Wash with 60ml binding buffer; ③ Elute with 30 mL of elution buffer and collect the post-column solution.
结合缓冲液(pH8.0):每升含甘氨酸112.6g、氯化钠175.2g,余量为水。Binding buffer (pH 8.0): 112.6 g of glycine and 175.2 g of sodium chloride per liter, the balance being water.
洗脱缓冲液(pH3.0):每500ml含甘氨酸7.5g,余量为水。Elution buffer (pH 3.0): 7.5 g of glycine per 500 ml, and the balance is water.
3、取步骤2得到的过柱后溶液,用超滤浓缩管浓缩并将体系置换为PBS缓冲液,得到抗体浓度约为2mg/ml的抗体溶液,命名为B430抗体溶液。3. Take the post-column solution obtained in
实施例3、抗体与乙肝病毒的结合能力Example 3. Binding ability of antibody to hepatitis B virus
一、ELISA检测1. ELISA detection
1、取酶标板,加入包被原溶液以进行包被(50ng包被原/孔)。1. Take the ELISA plate and add the original coating solution for coating (50 ng original coating/well).
包被原为HBs抗原。用PBS缓冲液作为溶剂制备包被原溶液。The coating was originally HBs antigen. Coating stock solutions were prepared using PBS buffer as solvent.
2、完成步骤1后,取所述酶标板,每孔加入200μl含2%BSA的PBS缓冲液,37℃孵育2小时以进行封闭,然后吸弃上清,用PBST溶液洗两次。2. After completing step 1, take the ELISA plate, add 200 μl PBS buffer containing 2% BSA to each well, incubate at 37°C for 2 hours for blocking, then aspirate the supernatant and wash twice with PBST solution.
3、完成步骤2后,取所述酶标板,每孔加入200μl抗体稀释液,37℃孵育1小时,然后吸弃上清,用PBST溶液洗两次。抗体稀释液是用PBS缓冲液稀释实施例2制备的B430抗体溶液或者HBIG溶液或者MERS-27抗体溶液得到的。每种抗体稀释液设置5个复孔。3. After completing
4、完成步骤3后,取所述酶标板,加入抗人IgG-HRP(山羊抗人IgG-HRP,Promega,货号W4038),37℃孵育1小时,然后吸弃上清,用PBST溶液洗6次。4. After completing
5、完成步骤4后,取所述酶标板,加入显色液进行显色,适时终止,读取450nm吸光度值。5. After completing step 4, take the ELISA plate, add color developing solution to develop color, stop in time, and read the absorbance value at 450 nm.
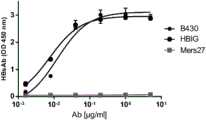
结果见图1。图1的横坐标显示的是抗体稀释液中B430抗体的浓度,HBIG浓度是B430抗体浓度的1000倍,MERS-27抗体浓度与B430抗体相同。例如,抗体稀释液中B430抗体的浓度为0.1μg/ml时,对应的HBIG浓度为0.1mg/ml,对应的MERS-27抗体浓度为0.1μg/ml。结果表明,B430抗体可以高效结合HBs抗原。The results are shown in Figure 1. The abscissa of Figure 1 shows the concentration of B430 antibody in the antibody diluent, the concentration of HBIG is 1000 times the concentration of B430 antibody, and the concentration of MERS-27 antibody is the same as that of B430 antibody. For example, when the concentration of B430 antibody in the antibody diluent is 0.1 μg/ml, the corresponding HBIG concentration is 0.1 mg/ml, and the corresponding MERS-27 antibody concentration is 0.1 μg/ml. The results showed that the B430 antibody could efficiently bind to the HBs antigen.
二、免疫荧光检测2. Immunofluorescence detection
供试质粒为A型乙肝包膜蛋白表达质粒或B型乙肝包膜蛋白表达质粒或C型乙肝包膜蛋白表达质粒或D型乙肝包膜蛋白表达质粒。The test plasmids are A-type hepatitis B envelope protein expression plasmid or B-type hepatitis B envelope protein expression plasmid or C-type hepatitis B envelope protein expression plasmid or D-type hepatitis B envelope protein expression plasmid.
供试抗体溶液为实施例2制备的B430抗体溶液或MERS-27抗体溶液。The test antibody solution was the B430 antibody solution or the MERS-27 antibody solution prepared in Example 2.
1、取6孔板,铺板前每孔先放入酒精消毒后的盖玻片,然后每孔加入约106个Huh7细胞,培养15小时。1. Take a 6-well plate, put alcohol-sterilized coverslips in each well before plating, then add about 106 Huh7 cells to each well, and culture for 15 hours.
2、完成步骤1后,取所述6孔板,每孔加入2μg供试质粒和2μg PEI转染试剂,培养72小时。2. After completing step 1, take the 6-well plate, add 2 μg of the test plasmid and 2 μg of PEI transfection reagent to each well, and culture for 72 hours.
3、完成步骤2后,取6孔板中的盖玻片,用PBS缓冲液浸洗3次,然后用4%多聚甲醛溶液固定15min,然后用PBS缓冲液浸洗3次,然后用PBST溶液室温穿透20min,然后用PBS缓冲液洗涤3次,然后滴加山羊血清并室温封闭30min,然后滴加一抗工作液(将供试抗体溶液稀释至1000倍体积,即为一抗工作液)并4℃孵育15小时,然后用PBST溶液洗涤3次,然后滴加荧光二抗工作液(荧光二抗为山羊抗人IgG-DyLight 594,货号CW0248,康为世纪)并37℃孵育1h,然后用PBST溶液浸洗3次,然后用DAPI复染核,然后用PBST溶液洗涤4次,然后用含抗荧光淬灭剂的封片液封片,在激光共聚焦显微镜下采集分析图像。3. After completing
结果见图2。结果表明,B430抗体可以结合A、B、C、D基因型的乙肝病毒表达的膜蛋白。The results are shown in Figure 2. The results showed that the B430 antibody could bind to the membrane proteins expressed by hepatitis B viruses of A, B, C, and D genotypes.
实施例4、抗体的中和活性Example 4. Neutralizing activity of antibodies
一、HBV感染及抗体中和试验1. HBV infection and antibody neutralization test
供试抗体溶液为实施例2制备的B430抗体溶液或MERS-27抗体溶液或HBIG溶液。The test antibody solution was the B430 antibody solution or the MERS-27 antibody solution or the HBIG solution prepared in Example 2.
1、培养HepAD38细胞,收集上清,向上清中加入PEG8000并使其在体系中的浓度为8%,然后离心沉淀病毒,用PMM培养基溶解,即为HBV病毒液。1. Cultivate HepAD38 cells, collect the supernatant, add PEG8000 to the supernatant to make the concentration in the system 8%, then centrifuge to precipitate the virus and dissolve it in PMM medium, which is the HBV virus liquid.
2、将HepG2-NTCP细胞接种到48孔板中,贴壁约5小时后,更换为PMM培养基继续培养16小时。2. Inoculate HepG2-NTCP cells into a 48-well plate, and after about 5 hours of adherence, change to PMM medium and continue to culture for 16 hours.
3、完成步骤2后,取所述48孔板,吸弃上清,加入步骤1制备的HBV病毒液(MOI=200)、供试抗体溶液和PEG8000(使PEG8000的终浓度为5%),培养16-20小时,然后吸弃上清,用PBS缓冲液洗细胞2次,然后用PMM培养基培养7-8天(两天换液一次)。3. After completing
3、完成步骤2后,吸取上清,采用乙型肝炎病毒e抗原检测试剂盒检测HBe含量,利用Graphpad拟合曲线比较抗体的中和活性。利用Prism 5软件计算抗体的IC50。3. After completing
每种供试抗体的每种浓度设置2个复孔。Two replicate wells were set up for each concentration of each test antibody.
结果见图3。图3的横坐标显示的是体系中B430抗体的浓度,HBIG浓度是B430抗体浓度的1000倍,MERS-27抗体浓度与B430抗体相同。例如,体系中B430抗体的浓度为0.1μg/ml时,对应的HBIG浓度为0.1mg/ml,对应的MERS-27抗体浓度为0.1μg/ml。结果表明,B430抗体可以高效中和HBV病毒。The results are shown in Figure 3. The abscissa of Figure 3 shows the concentration of B430 antibody in the system, the concentration of HBIG is 1000 times that of B430 antibody, and the concentration of MERS-27 antibody is the same as that of B430 antibody. For example, when the concentration of B430 antibody in the system is 0.1 μg/ml, the corresponding HBIG concentration is 0.1 mg/ml, and the corresponding MERS-27 antibody concentration is 0.1 μg/ml. The results showed that the B430 antibody could efficiently neutralize HBV virus.
B430抗体的IC50值为0.019μg/ml。The IC50 value of B430 antibody was 0.019 μg/ml.
HBIG的IC50值为15μg/ml。The IC50 value of HBIG was 15 μg/ml.
二、HDV感染及抗体中和试验2. HDV infection and antibody neutralization test
供试抗体溶液为实施例2制备的B430抗体溶液或MERS-27抗体溶液或HBIG溶液。设置用等体积PBS缓冲液代替供试抗体溶液的空白对照(NC)。The test antibody solution was the B430 antibody solution or the MERS-27 antibody solution or the HBIG solution prepared in Example 2. A blank control (NC) was set up with an equal volume of PBS buffer instead of the test antibody solution.
供试质粒为C型乙肝包膜蛋白表达质粒或D型乙肝包膜蛋白表达质粒。The tested plasmids are C-type hepatitis B envelope protein expression plasmid or D-type hepatitis B envelope protein expression plasmid.
1、Huh7细胞在10cm直径培养皿,培养至80%-90%汇合度。1. Huh7 cells were cultured to 80%-90% confluence in a 10cm diameter petri dish.
2、完成步骤1后,取所述培养皿,每个培养皿加入20μg供试质粒、10μg JC126载体和30μg PEI转染试剂,培养8天后收集上清液,加入PEG8000并使其在体系中的浓度为5%,然后离心沉淀病毒,用PMM培养基溶解,即为HDV病毒液。2. After completing step 1, take the petri dishes, add 20 μg test plasmid, 10 μg JC126 vector and 30 μg PEI transfection reagent to each petri dish, collect the supernatant after 8 days of culture, add PEG8000 and make it in the system. The concentration is 5%, and then the virus is precipitated by centrifugation and dissolved in PMM medium, which is the HDV virus liquid.
3、将HepG2-NTCP细胞接种到48孔板中,贴壁约5小时后,更换为PMM培养基继续培养16小时。3. The HepG2-NTCP cells were seeded into a 48-well plate, and after about 5 hours of adherence, the cells were replaced with PMM medium and continued to culture for 16 hours.
4、完成步骤3后,取所述48孔板,吸弃上清,加入步骤2制备的病毒液(MOI=500)、供试抗体溶液和PEG8000(体系中,PEG8000的终浓度为5%),培养16-20小时,然后吸弃上清,用PBS缓冲液洗细胞2次,然后用PMM培养基培养7天(两天换液一次)。4. After completing
体系中,B430抗体的浓度为0.5μg/ml。体系中,HBIG的浓度为0.5mg/ml,为单抗浓度1000倍。体系中,MERS-27抗体的浓度为0.5μg/ml。In the system, the concentration of B430 antibody was 0.5 μg/ml. In the system, the concentration of HBIG was 0.5 mg/ml, which was 1000 times the concentration of monoclonal antibody. In the system, the concentration of MERS-27 antibody was 0.5 μg/ml.
5、完成步骤4后,取所述48孔板,用PBS缓冲液浸洗3次,然后用4%多聚甲醛溶液固定15min,然后用PBS缓冲液浸洗3次,然后用PBST溶液室温穿透20min,然后用PBS缓冲液洗涤3次,然后滴加山羊血清并室温封闭30min,然后滴加一抗工作液(一抗为HDVδ抗体)并4℃孵育15小时,然后用PBST溶液洗涤3次,然后滴加荧光二抗工作液(二抗为驴抗兔AlexaFluor TM 594标记二抗,货号A-21207,美国Invitrogen公司)并37℃孵育1h,然后用PBST溶液浸洗3次,然后用DAPI复染核,然后用PBST溶液洗涤4次,然后用含抗荧光淬灭剂的封片液封片,在激光共聚焦显微镜下采集分析图像。5. After completing step 4, take the 48-well plate, wash it three times with PBS buffer, then fix it with 4% paraformaldehyde solution for 15 minutes, then wash it with PBS buffer three times, and then wash it with PBST solution at room temperature. Permeate for 20min, then wash 3 times with PBS buffer, then drop goat serum and block at room temperature for 30min, then dropwise add primary antibody working solution (primary antibody is HDVδ antibody) and incubate at 4°C for 15 hours, then wash 3 times with PBST solution , and then dropwise add fluorescent secondary antibody working solution (secondary antibody is donkey anti-rabbit AlexaFluor TM 594-labeled secondary antibody, Cat. No. A-21207, Invitrogen, USA) and incubate at 37°C for 1h, then wash with
结果见图4。与空白对照或者Mers27抗体比较,B430抗体能够有效阻断C型乙肝包膜质粒包装的HDV病毒和D型乙肝包膜质粒包装的HDV病毒的感染。The results are shown in Figure 4. Compared with the blank control or Mers27 antibody, B430 antibody can effectively block the infection of HDV virus packaged with C-type hepatitis B envelope plasmid and HDV virus packaged with D-type hepatitis B envelope plasmid.
SEQUENCE LISTINGSEQUENCE LISTING
<110> 清华大学<110> Tsinghua University
<120> 乙肝病毒的中和抗体B430及其应用<120> Hepatitis B virus neutralizing antibody B430 and its application
<130> CGGNQAYX186102<130> CGGNQAYX186102
<160> 4<160> 4
<170> PatentIn version 3.5<170> PatentIn version 3.5
<210> 1<210> 1
<211> 473<211> 473
<212> PRT<212> PRT
<213> Artificial sequence<213> Artificial sequence
<400> 1<400> 1
Met Gly Trp Ser Cys Ile Ile Leu Phe Leu Val Ala Thr Ala Thr GlyMet Gly Trp Ser Cys Ile Ile Leu Phe Leu Val Ala Thr Ala Thr Gly
1 5 10 151 5 10 15
Val His Ser Glu Val Gln Leu Val Glu Ser Gly Gly Gly Val Val ArgVal His Ser Glu Val Gln Leu Val Glu Ser Gly Gly Gly Val Val Arg
20 25 30 20 25 30
Pro Gly Gly Ser Val Arg Leu Ser Cys Thr Gly Ser Gly Phe Thr PhePro Gly Gly Ser Val Arg Leu Ser Cys Thr Gly Ser Gly Phe Thr Phe
35 40 45 35 40 45
Gly Asp Tyr Ala Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly LeuGly Asp Tyr Ala Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
50 55 60 50 55 60
Glu Trp Val Ser Phe Ile Ala Ser Asp Gly Ser Thr Thr Tyr Tyr AlaGlu Trp Val Ser Phe Ile Ala Ser Asp Gly Ser Thr Thr Tyr Tyr Ala
65 70 75 8065 70 75 80
Ser Ser Met Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Arg Lys LysSer Ser Met Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Arg Lys Lys
85 90 95 85 90 95
Ser Leu Tyr Leu Gln Met Thr Ser Leu Arg Pro Glu Asp Thr Ala LeuSer Leu Tyr Leu Gln Met Thr Ser Leu Arg Pro Glu Asp Thr Ala Leu
100 105 110 100 105 110
Tyr Tyr Cys Ala Lys Asp Met Gly Val Arg Asp Ser Tyr Tyr Gln TyrTyr Tyr Cys Ala Lys Asp Met Gly Val Arg Asp Ser Tyr Tyr Gln Tyr
115 120 125 115 120 125
Gly Met Asp Val Trp Gly Gln Gly Thr Ala Val Thr Val Ser Ser AlaGly Met Asp Val Trp Gly Gln Gly Thr Ala Val Thr Val Ser Ser Ala
130 135 140 130 135 140
Ser Thr Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys SerSer Thr Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser
145 150 155 160145 150 155 160
Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr PheThr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe
165 170 175 165 170 175
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser GlyPro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly
180 185 190 180 185 190
Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Ser LeuVal His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Ser Leu
195 200 205 195 200 205
Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln Thr TyrSer Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln Thr Tyr
210 215 220 210 215 220
Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys Val Asp Lys ArgIle Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys Val Asp Lys Arg
225 230 235 240225 230 235 240
Val Glu Pro Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys ProVal Glu Pro Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro
245 250 255 245 250 255
Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro LysAla Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys
260 265 270 260 265 270
Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys ValPro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val
275 280 285 275 280 285
Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp TyrVal Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr
290 295 300 290 295 300
Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu GluVal Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu Glu
305 310 315 320305 310 315 320
Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu HisGln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu His
325 330 335 325 330 335
Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn LysGln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys
340 345 350 340 345 350
Ala Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly GlnAla Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln
355 360 365 355 360 365
Pro Arg Glu Pro Gln Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu MetPro Arg Glu Pro Gln Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met
370 375 380 370 375 380
Thr Lys Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr ProThr Lys Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro
385 390 395 400385 390 395 400
Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn AsnSer Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn
405 410 415 405 410 415
Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe LeuTyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
420 425 430 420 425 430
Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn ValTyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn Val
435 440 445 435 440 445
Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr Thr GlnPhe Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr Thr Gln
450 455 460 450 455 460
Lys Ser Leu Ser Leu Ser Pro Gly LysLys Ser Leu Ser Leu Ser Pro Gly Lys
465 470465 470
<210> 2<210> 2
<211> 2543<211> 2543
<212> DNA<212> DNA
<213> Artificial sequence<213> Artificial sequence
<400> 2<400> 2
acattgatta ttgactagtt attaatagta atcaattacg gggtcattag ttcatagccc 60acattgatta ttgactagtt attaatagta atcaattacg gggtcattag ttcatagccc 60
atatatggag ttccgcgtta cataacttac ggtaaatggc ccgcctggct gaccgcccaa 120atatatggag ttccgcgtta cataacttac ggtaaatggc ccgcctggct gaccgcccaa 120
cgacccccgc ccattgacgt caataatgac gtatgttccc atagtaacgc caatagggac 180cgacccccgc ccattgacgt caataatgac gtatgttccc atagtaacgc caatagggac 180
tttccattga cgtcaatggg tggagtattt acggtaaact gcccacttgg cagtacatca 240tttccattga cgtcaatggg tggagtattt acggtaaact gcccacttgg cagtacatca 240
agtgtatcat atgccaagta cgccccctat tgacgtcaat gacggtaaat ggcccgcctg 300agtgtatcat atgccaagta cgccccctat tgacgtcaat gacggtaaat ggcccgcctg 300
gcattatgcc cagtacatga ccttatggga ctttcctact tggcagtaca tctacgtatt 360gcattatgcc cagtacatga ccttatggga ctttcctact tggcagtaca tctacgtatt 360
agtcatcgct attaccatgg tgatgcggtt ttggcagtac atcaatgggc gtggatagcg 420agtcatcgct attaccatgg tgatgcggtt ttggcagtac atcaatgggc gtggatagcg 420
gtttgactca cggggatttc caagtctcca ccccattgac gtcaatggga gtttgttttg 480gtttgactca cggggatttc caagtctcca ccccattgac gtcaatggga gtttgttttg 480
gcaccaaaat caacgggact ttccaaaatg tcgtaacaac tccgccccat tgacgcaaat 540gcaccaaaat caacgggact ttccaaaatg tcgtaacaac tccgccccat tgacgcaaat 540
gggcggtagg cgtgtacggt gggaggtcta tataagcaga gctcgtttag tgaaccgtca 600gggcggtagg cgtgtacggt gggaggtcta tataagcaga gctcgtttag tgaaccgtca 600
gatcgcctgg agacgccatc cacgctgttt tgacctccat agaagacacc gggaccgatc 660gatcgcctgg agacgccatc cacgctgttt tgacctccat agaagacacc gggaccgatc 660
cagcctccgc ggccgggaac ggtgcattgg aacgcggatt ccccgtgcca agagtgacgt 720cagcctccgc ggccgggaac ggtgcattgg aacgcggatt ccccgtgcca agagtgacgt 720
aagtaccgcc tatagagtct ataggcccac ccccttggct tcgttagaac gcggctacaa 780aagtaccgcc tatagagtct ataggcccac ccccttggct tcgttagaac gcggctacaa 780
ttaatacata accttatgta tcatacacat acgatttagg tgacactata gaataacatc 840ttaatacata accttatgta tcatacacat acgatttagg tgacactata gaataacatc 840
cactttgcct ttctctccac aggtgtccac tcccaggtcc aactgcacct cggttctatc 900cactttgcct ttctctccac aggtgtccac tcccaggtcc aactgcacct cggttctatc 900
gattgaattc caccatggga tggtcatgta tcatcctttt tctagtagca actgcaaccg 960gattgaattc caccatggga tggtcatgta tcatcctttt tctagtagca actgcaaccg 960
gtgtacattc tgaggtgcag ctggtggagt ctgggggagg cgtggtccgg ccgggggggt 1020gtgtacattc tgaggtgcag ctggtggagt ctgggggagg cgtggtccgg ccgggggggt 1020
cagtgagact ctcctgtaca ggctctggat tcacctttgg tgactatgcc atgagctggg 1080cagtgagact ctcctgtaca ggctctggat tcacctttgg tgactatgcc atgagctggg 1080
tccgtcaagc tccagggaag ggtctggagt gggtctcttt tattgcttcg gatggttcta 1140tccgtcaagc tccagggaag ggtctggagt gggtctcttt tattgcttcg gatggttcta 1140
cgacatacta tgcatcgtct atgaagggcc gattcaccat ctccagggac aacaggaaaa 1200cgacatacta tgcatcgtct atgaagggcc gattcaccat ctccagggac aacaggaaaa 1200
agtccctgta tcttcaaatg accagtctga gacctgagga caccgccctc tattactgtg 1260agtccctgta tcttcaaatg accagtctga gacctgagga caccgccctc tattactgtg 1260
caaaggacat gggcgtccgt gattcctact atcaatacgg aatggacgtc tggggccaag 1320caaaggacat gggcgtccgt gattcctact atcaatacgg aatggacgtc tggggccaag 1320
ggaccgcggt caccgtctcc tcagcgtcga ccaagggccc atcggtcttc cccctggcac 1380ggaccgcggt caccgtctcc tcagcgtcga ccaagggccc atcggtcttc cccctggcac 1380
cctcctccaa gagcacctct gggggcacag cggccctggg ctgcctggtc aaggactact 1440cctcctccaa gagcacctct gggggcacag cggccctggg ctgcctggtc aaggactact 1440
tccccgaacc tgtgacggtc tcgtggaact caggcgccct gaccagcggc gtgcacacct 1500tccccgaacc tgtgacggtc tcgtggaact caggcgccct gaccagcggc gtgcacacct 1500
tcccggctgt cctacagtcc tcaggactct actccctcag cagcgtggtg accgtgccct 1560tcccggctgt cctacagtcc tcaggactct actccctcag cagcgtggtg accgtgccct 1560
ccagcagctt gggcacccag acctacatct gcaacgtgaa tcacaagccc agcaacacca 1620ccagcagctt gggcacccag acctacatct gcaacgtgaa tcacaagccc agcaacacca 1620
aggtggacaa gagagttgag cccaaatctt gtgacaaaac tcacacatgc ccaccgtgcc 1680aggtggacaa gagagttgag cccaaatctt gtgacaaaac tcacacatgc ccaccgtgcc 1680
cagcacctga actcctgggg ggaccgtcag tcttcctctt ccccccaaaa cccaaggaca 1740cagcacctga actcctgggg ggaccgtcag tcttcctctt ccccccaaaa cccaaggaca 1740
ccctcatgat ctcccggacc cctgaggtca catgcgtggt ggtggacgtg agccacgaag 1800ccctcatgat ctcccggacc cctgaggtca catgcgtggt ggtggacgtg agccacgaag 1800
accctgaggt caagttcaac tggtacgtgg acggcgtgga ggtgcataat gccaagacaa 1860accctgaggt caagttcaac tggtacgtgg acggcgtgga ggtgcataat gccaagacaa 1860
agccgcggga ggagcagtac aacagcacgt accgtgtggt cagcgtcctc accgtcctgc 1920agccgcggga ggagcagtac aacagcacgt accgtgtggt cagcgtcctc accgtcctgc 1920
accaggactg gctgaatggc aaggagtaca agtgcaaggt ctccaacaaa gccctcccag 1980accaggactg gctgaatggc aaggagtaca agtgcaaggt ctccaacaaa gccctcccag 1980
cccccatcga gaaaaccatc tccaaagcca aagggcagcc ccgagaacca caggtgtaca 2040cccccatcga gaaaaccatc tccaaagcca aagggcagcc ccgagaacca caggtgtaca 2040
ccctgccccc atcccgggag gagatgacca agaaccaggt cagcctgacc tgcctggtca 2100ccctgccccc atcccgggag gagatgacca agaaccaggt cagcctgacc tgcctggtca 2100
aaggcttcta tcccagcgac atcgccgtgg agtgggagag caatgggcag ccggagaaca 2160aaggcttcta tcccagcgac atcgccgtgg agtgggagag caatgggcag ccggagaaca 2160
actacaagac cacgcctccc gtgctggact ccgacggctc cttcttcctc tatagcaagc 2220actacaagac cacgcctccc gtgctggact ccgacggctc cttcttcctc tatagcaagc 2220
tcaccgtgga caagagcagg tggcagcagg ggaacgtctt ctcatgctcc gtgatgcatg 2280tcaccgtgga caagagcagg tggcagcagg ggaacgtctt ctcatgctcc gtgatgcatg 2280
aggctctgca caaccactac acgcagaaga gcctctccct gtccccgggt aaatgagtgc 2340aggctctgca caaccactac acgcagaaga gcctctccct gtccccgggt aaatgagtgc 2340
gacggccggc aagcccccgc tccccgggct ctcgcggtcg tacgaggaaa gcttggccgc 2400gacggccggc aagcccccgc tccccgggct ctcgcggtcg tacgaggaaa gcttggccgc 2400
catggcccaa cttgtttatt gcagcttata atggttacaa ataaagcaat agcatcacaa 2460catggcccaa cttgtttatt gcagcttata atggttacaa ataaagcaat agcatcacaa 2460
atttcacaaa taaagcattt ttttcactgc attctagttg tggtttgtcc aaactcatca 2520atttcacaaa taaagcattt ttttcactgc attctagttg tggtttgtcc aaactcatca 2520
atgtatctta tcatgtctgg atc 2543atgtatctta tcatgtctgg atc 2543
<210> 3<210> 3
<211> 230<211> 230
<212> PRT<212> PRT
<213> Artificial sequence<213> Artificial sequence
<400> 3<400> 3
Met Gly Trp Ser Cys Ile Ile Leu Phe Leu Val Ala Thr Ala Thr GlyMet Gly Trp Ser Cys Ile Ile Leu Phe Leu Val Ala Thr Ala Thr Gly
1 5 10 151 5 10 15
Glu Ile Val Leu Thr Gln Ser Pro Val Thr Leu Ser Leu Ser Pro GlyGlu Ile Val Leu Thr Gln Ser Pro Val Thr Leu Ser Leu Ser Pro Gly
20 25 30 20 25 30
Asp Arg Ala Thr Leu Ser Cys Lys Ala Ser Gln Thr Val Ser Gly LeuAsp Arg Ala Thr Leu Ser Cys Lys Ala Ser Gln Thr Val Ser Gly Leu
35 40 45 35 40 45
Leu Ala Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Arg Leu Val IleLeu Ala Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Arg Leu Val Ile
50 55 60 50 55 60
Tyr Glu Ser Ser Lys Arg Ala Ser Gly Ile Pro Ala Arg Phe Ser GlyTyr Glu Ser Ser Lys Arg Ala Ser Gly Ile Pro Ala Arg Phe Ser Gly
65 70 75 8065 70 75 80
Gly Gly Ser Gly Thr Asp Tyr Thr Leu Thr Ile Ser Gly Leu Glu AlaGly Gly Ser Gly Thr Asp Tyr Thr Leu Thr Ile Ser Gly Leu Glu Ala
85 90 95 85 90 95
Glu Asp Phe Ala Val Tyr Tyr Cys His His Arg Ser Ile Trp Pro LeuGlu Asp Phe Ala Val Tyr Tyr Cys His His Arg Ser Ile Trp Pro Leu
100 105 110 100 105 110
Ser Phe Gly Gly Gly Thr Arg Val Asp Ile Lys Arg Thr Val Ala AlaSer Phe Gly Gly Gly Thr Arg Val Asp Ile Lys Arg Thr Val Ala Ala
115 120 125 115 120 125
Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Gln Leu Lys Ser GlyPro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Gln Leu Lys Ser Gly
130 135 140 130 135 140
Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu AlaThr Ala Ser Val Val Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu Ala
145 150 155 160145 150 155 160
Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln Ser Gly Asn Ser GlnLys Val Gln Trp Lys Val Asp Asn Ala Leu Gln Ser Gly Asn Ser Gln
165 170 175 165 170 175
Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser Thr Tyr Ser Leu SerGlu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser
180 185 190 180 185 190
Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys Val TyrSer Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys Val Tyr
195 200 205 195 200 205
Ala Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr Lys SerAla Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr Lys Ser
210 215 220 210 215 220
Phe Asn Arg Gly Glu CysPhe Asn Arg Gly Glu Cys
225 230225 230
<210> 4<210> 4
<211> 1762<211> 1762
<212> DNA<212> DNA
<213> Artificial sequence<213> Artificial sequence
<400> 4<400> 4
acattgatta ttgactagtt attaatagta atcaattacg gggtcattag ttcatagccc 60acattgatta ttgactagtt attaatagta atcaattacg gggtcattag ttcatagccc 60
atatatggag ttccgcgtta cataacttac ggtaaatggc ccgcctggct gaccgcccaa 120atatatggag ttccgcgtta cataacttac ggtaaatggc ccgcctggct gaccgcccaa 120
cgacccccgc ccattgacgt caataatgac gtatgttccc atagtaacgc caatagggac 180cgacccccgc ccattgacgt caataatgac gtatgttccc atagtaacgc caatagggac 180
tttccattga cgtcaatggg tggagtattt acggtaaact gcccacttgg cagtacatca 240tttccattga cgtcaatggg tggagtattt acggtaaact gcccacttgg cagtacatca 240
agtgtatcat atgccaagta cgccccctat tgacgtcaat gacggtaaat ggcccgcctg 300agtgtatcat atgccaagta cgccccctat tgacgtcaat gacggtaaat ggcccgcctg 300
gcattatgcc cagtacatga ccttatggga ctttcctact tggcagtaca tctacgtatt 360gcattatgcc cagtacatga ccttatggga ctttcctact tggcagtaca tctacgtatt 360
agtcatcgct attaccatgg tgatgcggtt ttggcagtac atcaatgggc gtggatagcg 420agtcatcgct attaccatgg tgatgcggtt ttggcagtac atcaatgggc gtggatagcg 420
gtttgactca cggggatttc caagtctcca ccccattgac gtcaatggga gtttgttttg 480gtttgactca cggggatttc caagtctcca ccccattgac gtcaatggga gtttgttttg 480
gcaccaaaat caacgggact ttccaaaatg tcgtaacaac tccgccccat tgacgcaaat 540gcaccaaaat caacgggact ttccaaaatg tcgtaacaac tccgccccat tgacgcaaat 540
gggcggtagg cgtgtacggt gggaggtcta tataagcaga gctcgtttag tgaaccgtca 600gggcggtagg cgtgtacggt gggaggtcta tataagcaga gctcgtttag tgaaccgtca 600
gatcgcctgg agacgccatc cacgctgttt tgacctccat agaagacacc gggaccgatc 660gatcgcctgg agacgccatc cacgctgttt tgacctccat agaagacacc gggaccgatc 660
cagcctccgc ggccgggaac ggtgcattgg aacgcggatt ccccgtgcca agagtgacgt 720cagcctccgc ggccgggaac ggtgcattgg aacgcggatt ccccgtgcca agagtgacgt 720
aagtaccgcc tatagagtct ataggcccac ccccttggct tcgttagaac gcggctacaa 780aagtaccgcc tatagagtct ataggcccac ccccttggct tcgttagaac gcggctacaa 780
ttaatacata accttatgta tcatacacat acgatttagg tgacactata gaataacatc 840ttaatacata accttatgta tcatacacat acgatttagg tgacactata gaataacatc 840
cactttgcct ttctctccac aggtgtccac tcccaggtcc aactgcacct cggttctatc 900cactttgcct ttctctccac aggtgtccac tcccaggtcc aactgcacct cggttctatc 900
gattgaattc caccatggga tggtcatgta tcatcctttt tctagtagca actgcaaccg 960gattgaattc caccatggga tggtcatgta tcatcctttt tctagtagca actgcaaccg 960
gtgaaattgt gctgacacag tctccagtca ccctgtcttt gtctccaggg gacagagcca 1020gtgaaattgt gctgacacag tctccagtca ccctgtcttt gtctccaggg gacagagcca 1020
ccctctcctg caaggccagc cagactgtta gcggcctctt agcctggtac caacaaaaac 1080ccctctcctg caaggccagc cagactgtta gcggcctctt agcctggtac caacaaaaac 1080
ctggccagag tccccggctc gtcatctatg aatcatccaa aagggcctct ggcatcccag 1140ctggccagag tccccggctc gtcatctatg aatcatccaa aagggcctct ggcatcccag 1140
ccaggttcag tggcggtggg tctgggacag actacaccct caccatcagc ggcctggagg 1200ccaggttcag tggcggtggg tctgggacag actacaccct caccatcagc ggcctggagg 1200
ctgaggattt tgcagtttat tactgtcacc accgtagcat ctggccgctc tctttcggcg 1260ctgaggattt tgcagtttat tactgtcacc accgtagcat ctggccgctc tctttcggcg 1260
gagggaccag ggtggacatc aaacgtacgg tggctgcacc atctgtcttc atcttcccgc 1320gagggaccag ggtggacatc aaacgtacgg tggctgcacc atctgtcttc atcttcccgc 1320
catctgatga gcagttgaaa tctggaactg cctctgttgt gtgcctgctg aataacttct 1380catctgatga gcagttgaaa tctggaactg cctctgttgt gtgcctgctg aataacttct 1380
atcccagaga ggccaaagta cagtggaagg tggataacgc cctccaatcg ggtaactccc 1440atcccagaga ggccaaagta cagtggaagg tggataacgc cctccaatcg ggtaactccc 1440
aggagagtgt cacagagcag gacagcaagg acagcaccta cagcctcagc agcaccctga 1500aggagagtgt cacagagcag gacagcaagg acagcaccta cagcctcagc agcaccctga 1500
cgctgagcaa agcagactac gagaaacaca aagtctacgc ctgcgaagtc acccatcagg 1560cgctgagcaa agcagactac gagaaacaca aagtctacgc ctgcgaagtc acccatcagg 1560
gcctgagctc gcccgtcaca aagagcttca acaggggaga gtgttagaag cttggccgcc 1620gcctgagctc gcccgtcaca aagagcttca acaggggaga gtgttagaag cttggccgcc 1620
atggcccaac ttgtttattg cagcttataa tggttacaaa taaagcaata gcatcacaaa 1680atggcccaac ttgtttattg cagcttataa tggttacaaa taaagcaata gcatcacaaa 1680
tttcacaaat aaagcatttt tttcactgca ttctagttgt ggtttgtcca aactcatcaa 1740tttcacaaat aaagcatttt tttcactgca ttctagttgt ggtttgtcca aactcatcaa 1740
tgtatcttat catgtctgga tc 1762tgtatcttat catgtctgga tc 1762
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811442794.1ACN111234013B (en) | 2018-11-29 | 2018-11-29 | Neutralizing antibody B430 of hepatitis B virus and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811442794.1ACN111234013B (en) | 2018-11-29 | 2018-11-29 | Neutralizing antibody B430 of hepatitis B virus and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN111234013Atrue CN111234013A (en) | 2020-06-05 |
| CN111234013B CN111234013B (en) | 2022-01-11 |
Family
ID=70868717
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201811442794.1AActiveCN111234013B (en) | 2018-11-29 | 2018-11-29 | Neutralizing antibody B430 of hepatitis B virus and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111234013B (en) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2815634A1 (en)* | 2000-10-20 | 2002-04-26 | Biomerieux Sa | New monoclonal antibody, useful for diagnosis and treatment of hepatitis B infection, is specific for highly conserved epitope |
| CN102757492A (en)* | 2011-04-26 | 2012-10-31 | 中国人民解放军第二军医大学 | Holistic hepatitis B surface protein monoclonal antibodies and application thereof to preparation of medicines for preventing HBV (hepatitis B virus) infection |
| CN104058996A (en)* | 2014-06-23 | 2014-09-24 | 清华大学 | Anti-hepatitis C virus compound, preparation method and application thereof |
| CN105001325A (en)* | 2015-07-31 | 2015-10-28 | 北京泰诺迪生物科技有限公司 | Total-humanized anti-hepatitis B virus neutralizing antibody and preparing method and application thereof |
| EP3070103A1 (en)* | 2015-03-19 | 2016-09-21 | Institut Hospitalier Universitaire De Strasbourg | Anti-Claudin 1 monoclonal antibodies for the prevention and treatment of hepatocellular carcinoma |
- 2018
- 2018-11-29CNCN201811442794.1Apatent/CN111234013B/enactiveActive
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2815634A1 (en)* | 2000-10-20 | 2002-04-26 | Biomerieux Sa | New monoclonal antibody, useful for diagnosis and treatment of hepatitis B infection, is specific for highly conserved epitope |
| CN102757492A (en)* | 2011-04-26 | 2012-10-31 | 中国人民解放军第二军医大学 | Holistic hepatitis B surface protein monoclonal antibodies and application thereof to preparation of medicines for preventing HBV (hepatitis B virus) infection |
| CN104058996A (en)* | 2014-06-23 | 2014-09-24 | 清华大学 | Anti-hepatitis C virus compound, preparation method and application thereof |
| EP3070103A1 (en)* | 2015-03-19 | 2016-09-21 | Institut Hospitalier Universitaire De Strasbourg | Anti-Claudin 1 monoclonal antibodies for the prevention and treatment of hepatocellular carcinoma |
| CN105001325A (en)* | 2015-07-31 | 2015-10-28 | 北京泰诺迪生物科技有限公司 | Total-humanized anti-hepatitis B virus neutralizing antibody and preparing method and application thereof |
Non-Patent Citations (2)
| Title |
|---|
| ALEXANDER WALZ等: "Vertical Transmission of Hepatitis B Virus (HBV) from Mothers Negative for HBV Surface Antigen and Positive for Antibody to HBV Core Antigen", 《THE JOURNAL OF INFECTIOUS DISEASES》* |
| 王金侠等: "乙型肝炎病毒e抗体在HBV感染中的作用", 《标记免疫分析与临床》* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111234013B (en) | 2022-01-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109666070B (en) | Monoclonal antibody MERS-4V2 and coding gene and application thereof | |
| AU2020340881B2 (en) | Anti-SARS-CoV-2-spike glycoprotein antibodies and antigen-binding fragments | |
| TWI891060B (en) | Nucleic acid molecule encoding an antibody specific for gastric inhibitory peptide receptor (gipr) and method for making the antibody | |
| CN110862454B (en) | anti-Claudin 18_2 antibody and application thereof | |
| KR102781241B1 (en) | Method of treating or ameliorating metabolic disorders using glp-1 receptor agonists conjugated to antagonists for gastric inhibitory peptide receptor(gipr) | |
| KR101567117B1 (en) | Interleukin-1 alpha antibodies and methods of use | |
| TW201738272A (en) | anti-PACAP antibody and use thereof | |
| US12331104B2 (en) | Hepatitis B antibodies | |
| CN110799537B (en) | Anti-PD-1 antibody and method for its preparation and use | |
| KR20170040249A (en) | Anti-cdh6 antibody drug conjugates | |
| KR20160021849A (en) | Lectin-like oxidized ldl receptor1 antibodies and methods of use | |
| CN113271974B (en) | Anti-HIV antibody 10-1074 variants | |
| RU2756101C2 (en) | Cys80-CONJUGATED IMMUNOGLOBULINS | |
| CN113493506A (en) | Novel coronavirus antibody and application thereof | |
| KR20230017815A (en) | Anti-SARS-COV-2 Spike Glycoprotein Antibodies and Antigen-Binding Fragments | |
| CN111234013B (en) | Neutralizing antibody B430 of hepatitis B virus and application thereof | |
| CN104610453A (en) | Anti-HER2 dual-targeting antibodies, and preparation method and application thereof | |
| CN113234150A (en) | Fully human novel crown IgG1 single-chain antibody and application thereof | |
| CN111499735B (en) | A bispecific antibody against HIV and its encoding gene and application | |
| AU2019279206A1 (en) | Anti-human TLR7 antibody | |
| KR20220149573A (en) | Anti-CD19 Antibodies and Methods of Use and Preparation thereof | |
| RU2834996C1 (en) | Anti-cd19 antibodies and methods for use and production thereof | |
| CN106832002A (en) | The fusion protein and its related application of a kind of targeting PD 1 | |
| EA044746B1 (en) | ANTIBODIES AND ANTIGENS-BINDING FRAGMENTS AGAINST SARS-CoV-2 SPIKE GLYCOPROOTEIN |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |