CN111215074B - NiFeS water oxidation electrocatalyst supported by a nickel carrier and preparation method thereof - Google Patents
NiFeS water oxidation electrocatalyst supported by a nickel carrier and preparation method thereofDownload PDFInfo
- Publication number
- CN111215074B CN111215074BCN201811411703.8ACN201811411703ACN111215074BCN 111215074 BCN111215074 BCN 111215074BCN 201811411703 ACN201811411703 ACN 201811411703ACN 111215074 BCN111215074 BCN 111215074B
- Authority
- CN
- China
- Prior art keywords
- nickel
- water oxidation
- reaction
- carrier
- nifes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000titleclaimsabstractdescription210
- 229910052759nickelInorganic materials0.000titleclaimsabstractdescription97
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000titleclaimsabstractdescription93
- 238000007254oxidation reactionMethods0.000titleclaimsabstractdescription65
- 230000003647oxidationEffects0.000titleclaimsabstractdescription63
- 239000010411electrocatalystSubstances0.000titleclaimsabstractdescription47
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription33
- 239000003054catalystSubstances0.000claimsabstractdescription61
- 238000000034methodMethods0.000claimsabstractdescription21
- 238000011065in-situ storageMethods0.000claimsabstractdescription20
- 229910052742ironInorganic materials0.000claimsabstractdescription18
- 229910052717sulfurInorganic materials0.000claimsabstractdescription18
- 238000006243chemical reactionMethods0.000claimsdescription85
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000claimsdescription36
- 239000002243precursorSubstances0.000claimsdescription35
- UMGDCJDMYOKAJW-UHFFFAOYSA-NthioureaChemical compoundNC(N)=SUMGDCJDMYOKAJW-UHFFFAOYSA-N0.000claimsdescription22
- 239000003153chemical reaction reagentSubstances0.000claimsdescription17
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical compoundOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000claimsdescription16
- KWGKDLIKAYFUFQ-UHFFFAOYSA-Mlithium chlorideChemical compound[Li+].[Cl-]KWGKDLIKAYFUFQ-UHFFFAOYSA-M0.000claimsdescription16
- NINIDFKCEFEMDL-UHFFFAOYSA-NSulfurChemical compound[S]NINIDFKCEFEMDL-UHFFFAOYSA-N0.000claimsdescription11
- XSQUKJJJFZCRTK-UHFFFAOYSA-NUreaNatural productsNC(N)=OXSQUKJJJFZCRTK-UHFFFAOYSA-N0.000claimsdescription11
- 239000001509sodium citrateSubstances0.000claimsdescription11
- 239000011593sulfurSubstances0.000claimsdescription11
- 239000012018catalyst precursorSubstances0.000claimsdescription10
- 229960002089ferrous chlorideDrugs0.000claimsdescription10
- NMCUIPGRVMDVDB-UHFFFAOYSA-Liron dichlorideChemical compoundCl[Fe]ClNMCUIPGRVMDVDB-UHFFFAOYSA-L0.000claimsdescription10
- 239000000463materialSubstances0.000claimsdescription10
- 239000000376reactantSubstances0.000claimsdescription10
- 239000007868Raney catalystSubstances0.000claimsdescription9
- 229910000564Raney nickelInorganic materials0.000claimsdescription9
- 230000035484reaction timeEffects0.000claimsdescription9
- 229910000147aluminium phosphateInorganic materials0.000claimsdescription8
- CWYNVVGOOAEACU-UHFFFAOYSA-NFe2+Chemical compound[Fe+2]CWYNVVGOOAEACU-UHFFFAOYSA-N0.000claimsdescription4
- 229910002651NO3Inorganic materials0.000claimsdescription4
- NHNBFGGVMKEFGY-UHFFFAOYSA-NNitrateChemical compound[O-][N+]([O-])=ONHNBFGGVMKEFGY-UHFFFAOYSA-N0.000claimsdescription4
- BAUYGSIQEAFULO-UHFFFAOYSA-Liron(2+) sulfate (anhydrous)Chemical compound[Fe+2].[O-]S([O-])(=O)=OBAUYGSIQEAFULO-UHFFFAOYSA-L0.000claimsdescription4
- 239000006260foamSubstances0.000claimsdescription3
- QTBSBXVTEAMEQO-UHFFFAOYSA-MAcetateChemical compoundCC([O-])=OQTBSBXVTEAMEQO-UHFFFAOYSA-M0.000claimsdescription2
- VEXZGXHMUGYJMC-UHFFFAOYSA-MChloride anionChemical compound[Cl-]VEXZGXHMUGYJMC-UHFFFAOYSA-M0.000claimsdescription2
- KRKNYBCHXYNGOX-UHFFFAOYSA-KCitrateChemical compound[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OKRKNYBCHXYNGOX-UHFFFAOYSA-K0.000claimsdescription2
- DGAQECJNVWCQMB-PUAWFVPOSA-MIlexoside XXIXChemical compoundC[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+]DGAQECJNVWCQMB-PUAWFVPOSA-M0.000claimsdescription2
- 229910019142PO4Inorganic materials0.000claimsdescription2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-NPotassiumChemical compound[K]ZLMJMSJWJFRBEC-UHFFFAOYSA-N0.000claimsdescription2
- QAOWNCQODCNURD-UHFFFAOYSA-LSulfateChemical compound[O-]S([O-])(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-L0.000claimsdescription2
- MCDLETWIOVSGJT-UHFFFAOYSA-Nacetic acid;ironChemical compound[Fe].CC(O)=O.CC(O)=OMCDLETWIOVSGJT-UHFFFAOYSA-N0.000claimsdescription2
- FRHBOQMZUOWXQL-UHFFFAOYSA-Lammonium ferric citrateChemical compound[NH4+].[Fe+3].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OFRHBOQMZUOWXQL-UHFFFAOYSA-L0.000claimsdescription2
- 229960004642ferric ammonium citrateDrugs0.000claimsdescription2
- 229940116007ferrous phosphateDrugs0.000claimsdescription2
- 239000011790ferrous sulphateSubstances0.000claimsdescription2
- 235000003891ferrous sulphateNutrition0.000claimsdescription2
- 239000004313iron ammonium citrateSubstances0.000claimsdescription2
- 235000000011iron ammonium citrateNutrition0.000claimsdescription2
- 229910000155iron(II) phosphateInorganic materials0.000claimsdescription2
- 229910000359iron(II) sulfateInorganic materials0.000claimsdescription2
- SDEKDNPYZOERBP-UHFFFAOYSA-Hiron(ii) phosphateChemical compound[Fe+2].[Fe+2].[Fe+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OSDEKDNPYZOERBP-UHFFFAOYSA-H0.000claimsdescription2
- NBIIXXVUZAFLBC-UHFFFAOYSA-KphosphateChemical compound[O-]P([O-])([O-])=ONBIIXXVUZAFLBC-UHFFFAOYSA-K0.000claimsdescription2
- 239000010452phosphateSubstances0.000claimsdescription2
- IEQIEDJGQAUEQZ-UHFFFAOYSA-NphthalocyanineChemical classN1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1IEQIEDJGQAUEQZ-UHFFFAOYSA-N0.000claimsdescription2
- 229910052700potassiumInorganic materials0.000claimsdescription2
- 229960003975potassiumDrugs0.000claimsdescription2
- 239000011591potassiumSubstances0.000claimsdescription2
- 239000001508potassium citrateSubstances0.000claimsdescription2
- 229960002635potassium citrateDrugs0.000claimsdescription2
- QEEAPRPFLLJWCF-UHFFFAOYSA-Kpotassium citrate (anhydrous)Chemical compound[K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OQEEAPRPFLLJWCF-UHFFFAOYSA-K0.000claimsdescription2
- 235000011082potassium citratesNutrition0.000claimsdescription2
- 229910052708sodiumInorganic materials0.000claimsdescription2
- 239000011734sodiumSubstances0.000claimsdescription2
- NLJMYIDDQXHKNR-UHFFFAOYSA-Ksodium citrateChemical compoundO.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=ONLJMYIDDQXHKNR-UHFFFAOYSA-K0.000claimsdescription2
- DHCDFWKWKRSZHF-UHFFFAOYSA-Nsulfurothioic S-acidChemical compoundOS(O)(=O)=SDHCDFWKWKRSZHF-UHFFFAOYSA-N0.000claimsdescription2
- BYGYBSHPLSVNGL-UHFFFAOYSA-Ktrisodium trithiocyanateChemical compound[Na+].[Na+].[Na+].[S-]C#N.[S-]C#N.[S-]C#NBYGYBSHPLSVNGL-UHFFFAOYSA-K0.000claimsdescription2
- QGZKDVFQNNGYKY-UHFFFAOYSA-OAmmoniumChemical compound[NH4+]QGZKDVFQNNGYKY-UHFFFAOYSA-O0.000claims1
- ZGSDJMADBJCNPN-UHFFFAOYSA-N[S-][NH3+]Chemical class[S-][NH3+]ZGSDJMADBJCNPN-UHFFFAOYSA-N0.000claims1
- YRKCREAYFQTBPV-UHFFFAOYSA-NacetylacetoneChemical classCC(=O)CC(C)=OYRKCREAYFQTBPV-UHFFFAOYSA-N0.000claims1
- UETZVSHORCDDTH-UHFFFAOYSA-Niron(2+);hexacyanideChemical compound[Fe+2].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-]UETZVSHORCDDTH-UHFFFAOYSA-N0.000claims1
- ZFSLODLOARCGLH-UHFFFAOYSA-Nisocyanuric acidChemical compoundOC1=NC(O)=NC(O)=N1ZFSLODLOARCGLH-UHFFFAOYSA-N0.000claims1
- 230000003197catalytic effectEffects0.000abstractdescription13
- 239000000243solutionSubstances0.000description46
- 230000000694effectsEffects0.000description11
- HRXKRNGNAMMEHJ-UHFFFAOYSA-Ktrisodium citrateChemical compound[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OHRXKRNGNAMMEHJ-UHFFFAOYSA-K0.000description9
- 229940038773trisodium citrateDrugs0.000description9
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description8
- 229910052760oxygenInorganic materials0.000description8
- 239000001301oxygenSubstances0.000description8
- 238000001035dryingMethods0.000description7
- 238000002525ultrasonicationMethods0.000description7
- 238000005406washingMethods0.000description7
- 238000004519manufacturing processMethods0.000description6
- 229910052751metalInorganic materials0.000description6
- 239000002184metalSubstances0.000description6
- 229910001092metal group alloyInorganic materials0.000description6
- 238000012360testing methodMethods0.000description6
- UFHFLCQGNIYNRP-UHFFFAOYSA-NHydrogenChemical compound[H][H]UFHFLCQGNIYNRP-UHFFFAOYSA-N0.000description5
- 150000001875compoundsChemical class0.000description5
- 229910052739hydrogenInorganic materials0.000description5
- 239000001257hydrogenSubstances0.000description5
- 238000005868electrolysis reactionMethods0.000description4
- 238000004502linear sweep voltammetryMethods0.000description4
- 230000009286beneficial effectEffects0.000description3
- 239000000203mixtureSubstances0.000description3
- YGHCWPXPAHSSNA-UHFFFAOYSA-Nnickel subsulfideChemical compound[Ni].[Ni]=S.[Ni]=SYGHCWPXPAHSSNA-UHFFFAOYSA-N0.000description3
- 229910002555FeNiInorganic materials0.000description2
- 238000002441X-ray diffractionMethods0.000description2
- 230000000875corresponding effectEffects0.000description2
- 238000011161developmentMethods0.000description2
- 238000010586diagramMethods0.000description2
- 239000006185dispersionSubstances0.000description2
- 229910000510noble metalInorganic materials0.000description2
- 238000013112stability testMethods0.000description2
- 239000000126substanceSubstances0.000description2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-Nthiocyanic acidChemical compoundSC#NZMZDMBWJUHKJPS-UHFFFAOYSA-N0.000description2
- POILWHVDKZOXJZ-ARJAWSKDSA-M(z)-4-oxopent-2-en-2-olateChemical compoundC\C([O-])=C\C(C)=OPOILWHVDKZOXJZ-ARJAWSKDSA-M0.000description1
- ZOKXTWBITQBERF-UHFFFAOYSA-NMolybdenumChemical compound[Mo]ZOKXTWBITQBERF-UHFFFAOYSA-N0.000description1
- 229910000990Ni alloyInorganic materials0.000description1
- 239000011149active materialSubstances0.000description1
- UYJXRRSPUVSSMN-UHFFFAOYSA-Pammonium sulfideChemical compound[NH4+].[NH4+].[S-2]UYJXRRSPUVSSMN-UHFFFAOYSA-P0.000description1
- 238000004458analytical methodMethods0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 239000000969carrierSubstances0.000description1
- 238000006555catalytic reactionMethods0.000description1
- 238000012512characterization methodMethods0.000description1
- 229910017052cobaltInorganic materials0.000description1
- 239000010941cobaltSubstances0.000description1
- GUTLYIVDDKVIGB-UHFFFAOYSA-Ncobalt atomChemical compound[Co]GUTLYIVDDKVIGB-UHFFFAOYSA-N0.000description1
- 230000008878couplingEffects0.000description1
- 238000010168coupling processMethods0.000description1
- 238000005859coupling reactionMethods0.000description1
- GDVKFRBCXAPAQJ-UHFFFAOYSA-Adialuminum;hexamagnesium;carbonate;hexadecahydroxideChemical group[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Al+3].[Al+3].[O-]C([O-])=OGDVKFRBCXAPAQJ-UHFFFAOYSA-A0.000description1
- ZOMNIUBKTOKEHS-UHFFFAOYSA-Ldimercury dichlorideChemical classCl[Hg][Hg]ClZOMNIUBKTOKEHS-UHFFFAOYSA-L0.000description1
- 238000009510drug designMethods0.000description1
- 238000002149energy-dispersive X-ray emission spectroscopyMethods0.000description1
- 238000005516engineering processMethods0.000description1
- 230000002708enhancing effectEffects0.000description1
- 238000003912environmental pollutionMethods0.000description1
- 230000007774longtermEffects0.000description1
- WPBNNNQJVZRUHP-UHFFFAOYSA-Lmanganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioateChemical compound[Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OCWPBNNNQJVZRUHP-UHFFFAOYSA-L0.000description1
- 229910044991metal oxideInorganic materials0.000description1
- 150000004706metal oxidesChemical class0.000description1
- 229910052750molybdenumInorganic materials0.000description1
- 239000011733molybdenumSubstances0.000description1
- 239000002994raw materialSubstances0.000description1
- 238000011160researchMethods0.000description1
- 238000013341scale-upMethods0.000description1
- 238000001878scanning electron micrographMethods0.000description1
- 229960001790sodium citrateDrugs0.000description1
- ZXQVPEBHZMCRMC-UHFFFAOYSA-Rtetraazanium;iron(2+);hexacyanideChemical compound[NH4+].[NH4+].[NH4+].[NH4+].[Fe+2].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-]ZXQVPEBHZMCRMC-UHFFFAOYSA-R0.000description1
- WFKWXMTUELFFGS-UHFFFAOYSA-NtungstenChemical compound[W]WFKWXMTUELFFGS-UHFFFAOYSA-N0.000description1
- 229910052721tungstenInorganic materials0.000description1
- 239000010937tungstenSubstances0.000description1
Images
Classifications
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/02—Sulfur, selenium or tellurium; Compounds thereof
- B01J27/04—Sulfides
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/70—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper
- B01J23/74—Iron group metals
- B01J23/755—Nickel
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/02—Sulfur, selenium or tellurium; Compounds thereof
- B01J27/04—Sulfides
- B01J27/047—Sulfides with chromium, molybdenum, tungsten or polonium
- B01J27/049—Sulfides with chromium, molybdenum, tungsten or polonium with iron group metals or platinum group metals
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- C01B3/04—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by decomposition of inorganic compounds, e.g. ammonia
- C01B3/042—Decomposition of water
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Combustion & Propulsion (AREA)
- Inorganic Chemistry (AREA)
- Catalysts (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及一种催化剂及其制备方法,具体为一种用于电催化分解水的水氧化镍基自支撑电催化剂及其制备方法。The invention relates to a catalyst and a preparation method thereof, in particular to a nickel water oxide-based self-supporting electrocatalyst for electrocatalytic water splitting and a preparation method thereof.
背景技术Background technique
利用太阳能分解水制氢是将太阳能转化储存为化学能的重要方式之一,也是解决能源危机和环境污染的理想途径之一。其中太阳能光伏发电-电解水耦合分解水制氢因其发展较成熟、太阳能转化效率高、易规模化等优势受到广泛关注和研究并取得了一定的进展。但是目前商业电解水中所广泛使用的电解体系仍是较早发展的镍网(阳极)-雷尼镍(阴极)体系,为了进一步提高太阳能分解水制氢效率,降低能耗,发展廉价、高效、稳定的新一代电解水催化剂和电解水设备是至关重要的。电化学水氧化反应是电催化分解水中非常重要和挑战的反应,为降低制氢成本,许多研究者致力于发展铁、钴、镍、锰、钼、钨等非贵金属基的高性能产氧电催化剂。如公开号为CN 104415758 A,CN 104607191 A,及106861699A等专利中所述的单元或者双元金属氧化物、水滑石结构的产氧电催化剂均被报道为优秀的产氧电催化剂。然而,为实现更高效的水分解反应,非贵金属产氧电催化剂的活性以及稳定性仍需进一步提升,此外,已有的技术中制备得到的催化剂组成固定,为了满足大面积、大规模、不同需求的商业应用,催化剂的制备方法仍需进一步改善和简化。The use of solar energy to split water to produce hydrogen is one of the important ways to convert solar energy into chemical energy, and it is also one of the ideal ways to solve the energy crisis and environmental pollution. Among them, solar photovoltaic power generation-electrolyzed water coupling splitting water to produce hydrogen has received extensive attention and research due to its relatively mature development, high solar conversion efficiency, and easy scale-up, and some progress has been made. However, the electrolysis system widely used in commercial water electrolysis is still an earlier developed nickel mesh (anode)-Raney nickel (cathode) system. Stable next-generation electrolyzed water catalysts and electrolyzed devices are crucial. Electrochemical water oxidation is a very important and challenging reaction for electrocatalytic water splitting. In order to reduce the cost of hydrogen production, many researchers have devoted themselves to the development of high-performance oxygen-generating electrocatalysts based on non-noble metals such as iron, cobalt, nickel, manganese, molybdenum, and tungsten. catalyst. For example, the oxygen-generating electrocatalysts with unit or binary metal oxides and hydrotalcite structures described in patents such as CN 104415758 A, CN 104607191 A, and 106861699A have been reported as excellent oxygen-generating electrocatalysts. However, in order to achieve a more efficient water splitting reaction, the activity and stability of non-noble metal electrocatalysts for oxygen generation still need to be further improved. In addition, the catalysts prepared in the existing technologies have a fixed composition. The commercial application of the demand, the preparation method of the catalyst still needs to be further improved and simplified.
基于此,本发明以一种简单易行的低温溶液法制备得到了含有Ni,S,Fe的镍基自支撑产氧电催化剂。本发明所述的制备方法可以通过调变反应溶液的组分,浓度,反应温度,反应时间等参数来获得高活性高载量的电极体系,从而满足对于催化性能的不同需求。同时这种原位的制备方法有利于催化剂和载体之间的紧密结合,从而提高其电荷传输特性和机械稳定性,有一定的工业应用前景。Based on this, the present invention prepares a nickel-based self-supporting oxygen-generating electrocatalyst containing Ni, S, and Fe by a simple and easy low-temperature solution method. The preparation method of the present invention can obtain a highly active and high-capacity electrode system by adjusting the components, concentration, reaction temperature, reaction time and other parameters of the reaction solution, thereby meeting different requirements for catalytic performance. At the same time, this in-situ preparation method is conducive to the close combination between the catalyst and the carrier, thereby improving its charge transport characteristics and mechanical stability, and has certain industrial application prospects.
发明内容Contents of the invention
本发明的目的在于为水分解制氢过程中非常重要和挑战的产氧反应提供一种镍基自支撑的产氧电催化剂体系及其制备方法。The purpose of the present invention is to provide a nickel-based self-supporting oxygen-generating electrocatalyst system and a preparation method thereof for the very important and challenging oxygen-generating reaction in the process of water splitting and hydrogen production.
为实现上述目的,本发明采用了原位生长的方式在低温水溶液中将Fe,S,Ni组装在镍基材料上,得到了具有优异活性和稳定性的产氧电催化剂。其中:In order to achieve the above purpose, the present invention adopts the method of in-situ growth to assemble Fe, S, and Ni on nickel-based materials in a low-temperature aqueous solution, and obtains an oxygen-producing electrocatalyst with excellent activity and stability. in:
所制备得到的催化剂体系为镍基自支撑的层状多孔结构,催化剂活性物质与载体之间结合紧密,机械稳定性高。催化剂中Fe占比为5-36%,Ni占比5-18%,S占比0.2-40%。The prepared catalyst system is a nickel-based self-supporting layered porous structure, the catalyst active material is closely combined with the carrier, and the mechanical stability is high. In the catalyst, Fe accounts for 5-36%, Ni accounts for 5-18%, and S accounts for 0.2-40%.
为得到上述的催化剂体系,本发明通过以下具体的技术方案来实现:In order to obtain the above-mentioned catalyst system, the present invention is realized through the following specific technical solutions:
步骤1,采用低温溶液反应的方法,以镍基材料为载体和反应物,在硫源以及辅助试剂组成的前驱反应溶液中反应,原位得到镍基自支撑水氧化催化剂的前驱体;Step 1, using a low-temperature solution reaction method, using nickel-based materials as carriers and reactants, reacting in a precursor reaction solution composed of a sulfur source and auxiliary reagents, and obtaining a precursor of a nickel-based self-supporting water oxidation catalyst in situ;
步骤2,将得到的镍基自支撑水氧化催化剂前驱体放入含有铁源和辅助缓冲试剂的反应溶液中反应,原位得到镍基自支撑水氧化电催化剂。In step 2, the obtained nickel-based self-supporting water oxidation catalyst precursor is put into a reaction solution containing an iron source and an auxiliary buffer reagent for reaction, and a nickel-based self-supporting water oxidation electrocatalyst is obtained in situ.
优选的,所述的镍基材料采用镍板、镍片、镍网、泡沫镍、雷尼镍的任意一种或二种以上,优选镍网。Preferably, the nickel-based material is any one or more of nickel plate, nickel sheet, nickel mesh, nickel foam, and Raney nickel, preferably nickel mesh.
优选的,所述的硫源采用硫脲、钠、钾、铵的硫化物、硫代硫酸盐、三聚硫氰酸、三聚硫氰酸三钠中的任意一种或二种以上,优选硫脲;所述的辅助试剂采用磷酸、氯化锂中的任意一种或二种。Preferably, the sulfur source is any one or two or more of thiourea, sodium, potassium, ammonium sulfide, thiosulfate, thiocyanic acid, and trisodium thiocyanate, preferably Thiourea; the auxiliary reagent adopts any one or two of phosphoric acid and lithium chloride.
优选的,所述的铁源采用柠檬酸铁铵、六氰合铁(II)酸亚铁铵、氯化亚铁、硝酸亚铁、硫酸亚铁、磷酸亚铁、醋酸亚铁、以及铁(III)的酞菁类化合物、乙酰丙酮盐、柠檬酸盐、乙酸盐、氯化盐、硝酸盐、硫酸盐、磷酸盐中的任意一种或二种以上,优选亚铁盐。所述的辅助缓冲试剂采用柠檬酸钾,柠檬酸钠,柠檬酸三钠中的任意一种或二种以上。Preferably, the iron source adopts ferric ammonium citrate, ferrous ammonium hexacyanoferrate (II), ferrous chloride, ferrous nitrate, ferrous sulfate, ferrous phosphate, ferrous acetate, and iron ( III) Any one or two or more of phthalocyanine compounds, acetylacetonate, citrate, acetate, chloride, nitrate, sulfate, and phosphate, preferably ferrous salt. The auxiliary buffer reagent adopts any one or two or more of potassium citrate, sodium citrate and trisodium citrate.
优选的,所述的制备镍基自支撑水氧化催化剂的前驱体的反应溶液中硫源和辅助试剂的浓度范围在0.1-5mol/L(优选1mol/L),反应温度为70-150℃,反应时间为0.5-5h。Preferably, the concentration range of the sulfur source and the auxiliary reagent in the reaction solution for preparing the precursor of the nickel-based self-supporting water oxidation catalyst is 0.1-5mol/L (preferably 1mol/L), and the reaction temperature is 70-150°C, The reaction time is 0.5-5h.
优选的,所述的铁源的浓度范围在0.001-2mol/L(优选0.1mol/L),辅助缓冲试剂的浓度范围在0.0001-1mol/L(优选0.00625mol/L),反应温度为30-120℃,反应时间为0.2-20h。Preferably, the concentration range of the iron source is 0.001-2mol/L (preferably 0.1mol/L), the concentration range of the auxiliary buffer reagent is 0.0001-1mol/L (preferably 0.00625mol/L), and the reaction temperature is 30- 120°C, the reaction time is 0.2-20h.
一种镍载体支撑的NiFeS水氧化电催化剂,由本发明所述的任意一种制备方法制得。该水氧化电催化剂可用于电解水制氢的反应中。根据本发明的具体实施方案(实施例1-7),辅助试剂以及硫的加入能够显著提升电催化剂的催化性能,此外,制备条件的变化也会对电催化剂的产氧性能产生明显影响。A NiFeS water oxidation electrocatalyst supported by a nickel carrier is prepared by any one of the preparation methods described in the present invention. The water oxidation electrocatalyst can be used in the reaction of electrolyzing water to produce hydrogen. According to specific embodiments of the present invention (Example 1-7), the addition of auxiliary reagents and sulfur can significantly improve the catalytic performance of the electrocatalyst, and in addition, changes in the preparation conditions will also have a significant impact on the oxygen production performance of the electrocatalyst.
与现有技术相比,本发明具有如下有益的技术效果:Compared with the prior art, the present invention has the following beneficial technical effects:
本发明所述的水氧化电催化剂为自支撑的片层状多孔结构,分散度高,比表面积大,导电性好,具有优异的电荷传输特性和稳定性。在催化电化学水氧化时,显示出了优异的活性和稳定性。其中活性最好的催化剂在1mol/L的KOH溶液和室温条件下,电流密度为100mA/cm2,500mA/cm2和1000mA/cm2时仅需过电位207,285和324mV,而且在反应140小时之后,体系仍保持稳定。在同样的条件下,以商用雷尼镍为阴极,制备得到的催化剂为阳极组装得到的两电极体系在电流密度为100mA/cm2和500mA/cm2时需加槽压1.68和1.86V。The water oxidation electrocatalyst of the invention is a self-supporting lamellar porous structure with high dispersion, large specific surface area, good electrical conductivity, and excellent charge transport characteristics and stability. It shows excellent activity and stability in catalyzing electrochemical water oxidation. Among them, the catalyst with the best activity only needs overpotentials of 207, 285 and 324mV when the current density is 100mA/cm2 , 500mA/cm2 and 1000mA/cm2 under the condition of 1mol/L KOH solution and room temperature, and in the reaction of 140 After hours, the system remained stable. Under the same conditions, the commercial Raney nickel is used as the cathode, and the prepared catalyst is used as the anode to assemble the two-electrode system. When the current density is 100mA/cm2 and 500mA/cm2 , the cell pressure needs to be 1.68 and 1.86V.
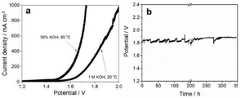
本发明所述的镍基自支撑水氧化电催化剂也可适用于高温,强碱性环境,在模拟商业电解槽的工况运行条件即30%的KOH溶液和80℃的条件下,在1.56和1.68V的槽压下便能得到100和500mA/cm2的电流密度,有工业应用基础。The nickel-based self-supporting water oxidation electrocatalyst of the present invention is also suitable for high temperature and strong alkaline environment. Under the conditions of 30% KOH solution and 80 °C under the conditions of simulating the operating conditions of commercial electrolyzers, the electrocatalyst can be used at 1.56 and 80 °C. The current density of 100 and 500mA/cm2 can be obtained under the cell voltage of 1.68V, which has a basis for industrial application.
本发明所述的制备方法操作简单,所需的原料均廉价易得,具有快速大量制备的优势,工业适应性较好。The preparation method of the invention is simple to operate, and the required raw materials are all cheap and easy to obtain, and has the advantages of fast mass production and good industrial adaptability.
本发明所述的制备方法通过调变反应溶液的组分,浓度,反应温度,反应时间等参数来获得高活性高稳定性的电极体系,从而满足对于催化性能的不同需求。同时原位制备方法有利于催化剂和导电载体之间的紧密结合,从而提高其电荷传输特性和机械稳定性,这在工业应用中是至关重要的。The preparation method of the present invention obtains an electrode system with high activity and high stability by adjusting the components, concentration, reaction temperature, reaction time and other parameters of the reaction solution, thereby meeting different requirements for catalytic performance. Meanwhile, the in situ preparation method is beneficial for the tight combination between the catalyst and the conductive support, thereby enhancing its charge transport properties and mechanical stability, which are crucial for industrial applications.
附图说明Description of drawings
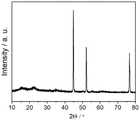
图1为实施例5中制得样品的XRD图,表明金属相Ni,金属合金相FeNi3和化合物Ni3S2相在制得的催化剂中共存。Figure 1 is the XRD pattern of the sample prepared in Example 5, which shows that the metal phase Ni, the metal alloy phase FeNi3 and the compound Ni3 S2 phase coexist in the prepared catalyst.
图2(a)、2(b)为实施例5中制得样品的SEM图。2(a), 2(b) are SEM images of the samples prepared in Example 5.
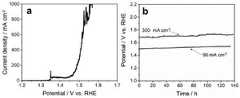
图3(a)为实施例5中制得的镍基自支撑水氧化电催化剂在1mol/L KOH溶液中的线性扫描伏安(LSV)曲线。测试采用三电极体系:制备得到的镍基电极为工作电极;Pt片为对电极;饱和甘汞电极为参比电极。测试采用的扫速为5mV/s;图3(b)为实施例9中样品的稳定性测试图。Figure 3(a) is the linear sweep voltammetry (LSV) curve of the nickel-based self-supporting water oxidation electrocatalyst prepared in Example 5 in 1 mol/L KOH solution. A three-electrode system was used in the test: the prepared nickel-based electrode was used as the working electrode; the Pt sheet was used as the counter electrode; and the saturated calomel electrode was used as the reference electrode. The scan rate used in the test is 5mV/s; FIG. 3(b) is the stability test diagram of the sample in Example 9.
图4(a)为实施例10中镍基自支撑水氧化电催化剂和商业雷尼镍构建的两电极体系在实验室条件(1mol/L KOH,室温)以及工况条件(30%KOH,80℃)下的线性扫描伏安(LSV)曲线。测试采用两电极体系:制备得到的镍基电极作为阳极,为工作电极;商业雷尼镍作为阴极,为对电极。测试采用的扫速为5mV/s;图4(b)为实施例10中的两电极体系在工况条件下的稳定性测试图。Figure 4(a) shows the two-electrode system constructed by nickel-based self-supporting water oxidation electrocatalyst and commercial Raney nickel in Example 10 under laboratory conditions (1mol/L KOH, room temperature) and working conditions (30% KOH, 80 ℃) linear sweep voltammetry (LSV) curve. The test uses a two-electrode system: the prepared nickel-based electrode is used as the anode, which is the working electrode; commercial Raney nickel is used as the cathode, which is the counter electrode. The scan rate used in the test was 5mV/s; FIG. 4(b) is a stability test diagram of the two-electrode system in Example 10 under working conditions.
具体实施方式Detailed ways
为了进一步说明本发明,列举以下实施实例,但并不因此而限制本发明。In order to further illustrate the present invention, the following implementation examples are listed, but the present invention is not limited thereby.
实施例1Example 1
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L磷酸,1mol/L硫脲)以120度的反应温度反应2h,原位得到镍基自支撑水氧化催化剂的前驱体;Adopt the preparation method of low-temperature solution reaction, use nickel mesh as carrier and reactant, in the newly prepared reaction precursor solution (the components of the reaction precursor solution are 1mol/L phosphoric acid, 1mol/L thiourea) with a reaction temperature of 120 degrees After reacting for 2 hours, the precursor of nickel-based self-supporting water oxidation catalyst was obtained in situ;
将得到的镍基自支撑水氧化催化剂前驱体放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂。Put the obtained nickel-based self-supporting water oxidation catalyst precursor into a reaction solution containing 0.1 mol/L ferrous chloride and 0.00625 mol/L trisodium citrate, react at a reaction temperature of 90 degrees for 6 hours, and the reaction ends After ultrasonication, water washing and drying, a nickel-based self-supporting electrocatalyst for water oxidation was obtained.
制得的催化剂由金属相Ni,金属合金相FeNi3和化合物Ni3S2组成。The prepared catalyst consists of metal phase Ni, metal alloy phaseFeNi3 andcompoundNi3S2 .
实施例2Example 2
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L氯化锂,1mol/L硫脲)以120度的反应温度反应2h,原位得到镍基自支撑水氧化催化剂的前驱体;Adopt the preparation method of low-temperature solution reaction, take nickel net as carrier and reactant, in the reaction precursor solution (the component of reaction precursor solution is 1mol/L lithium chloride, 1mol/L thiourea) of newly prepared reaction, with 120 degree React at the reaction temperature for 2 hours to obtain the precursor of the nickel-based self-supporting water oxidation catalyst in situ;
将得到的镍基自支撑水氧化催化剂前驱体放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂。Put the obtained nickel-based self-supporting water oxidation catalyst precursor into a reaction solution containing 0.1 mol/L ferrous chloride and 0.00625 mol/L trisodium citrate, react at a reaction temperature of 90 degrees for 6 hours, and the reaction ends After ultrasonication, water washing and drying, a nickel-based self-supporting electrocatalyst for water oxidation was obtained.
制得的催化剂由金属相Ni,金属合金相FeNi3和化合物Ni3S2组成。The prepared catalyst consists of metal phase Ni, metal alloy phaseFeNi3 andcompoundNi3S2 .
实施例3Example 3
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L硫脲)以120度的反应温度反应2h,原位得到镍基自支撑水氧化催化剂的前驱体;The preparation method of low-temperature solution reaction is adopted, with nickel mesh as the carrier and reactant, in the newly prepared reaction precursor solution (the composition of the reaction precursor solution is 1mol/L thiourea) for 2 hours at a reaction temperature of 120 degrees, in situ Obtain the precursor of nickel-based self-supporting water oxidation catalyst;
将得到的镍基自支撑水氧化催化剂前驱体放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂。Put the obtained nickel-based self-supporting water oxidation catalyst precursor into a reaction solution containing 0.1 mol/L ferrous chloride and 0.00625 mol/L trisodium citrate, react at a reaction temperature of 90 degrees for 6 hours, and the reaction ends After ultrasonication, water washing and drying, a nickel-based self-supporting electrocatalyst for water oxidation was obtained.
制得的催化剂由金属相Ni,金属合金相FeNi3和化合物Ni3S2组成。The prepared catalyst consists of metal phase Ni, metal alloy phaseFeNi3 andcompoundNi3S2 .
实施例4Example 4
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L氯化锂,1mol/L磷酸)以120度的反应温度反应2h,原位得到镍基自支撑水氧化催化剂的前驱体;The preparation method of low-temperature solution reaction is adopted, with nickel mesh as the carrier and reactant, in the newly prepared reaction precursor solution (the components of the reaction precursor solution are 1mol/L lithium chloride, 1mol/L phosphoric acid) at 120 degrees. Temperature reaction for 2 hours, the precursor of nickel-based self-supporting water oxidation catalyst was obtained in situ;
将得到的镍基自支撑水氧化催化剂前驱体放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂。Put the obtained nickel-based self-supporting water oxidation catalyst precursor into a reaction solution containing 0.1 mol/L ferrous chloride and 0.00625 mol/L trisodium citrate, react at a reaction temperature of 90 degrees for 6 hours, and the reaction ends After ultrasonication, water washing and drying, a nickel-based self-supporting electrocatalyst for water oxidation was obtained.
制得的催化剂由金属相Ni和金属合金相FeNi3组成。The as-prepared catalyst consists of metal phase Ni and metal alloy phaseFeNi3 .
实施例5Example 5
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L氯化锂,1mol/L磷酸,1mol/L硫脲)以120度的反应温度反应2h,原位得到镍基自支撑水氧化催化剂的前驱体,记为Ni-S;Adopt the preparation mode of low-temperature solution reaction, take nickel net as carrier and reactant, in the reaction precursor solution newly prepared (the component of reaction precursor solution is 1mol/L lithium chloride, 1mol/L phosphoric acid, 1mol/L thiourea ) react at a reaction temperature of 120 degrees for 2 hours, and obtain the precursor of a nickel-based self-supporting water oxidation catalyst in situ, denoted as Ni-S;
将得到的镍基自支撑水氧化催化剂前驱体Ni-S放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥,原位得到镍基自支撑水氧化电催化剂,记作Ni-S-Fe。该催化剂表现出了非常优异的电催化水氧化催化性能,如图3a中的线性扫描伏安(LSV)曲线所示,在100mA/cm2时具体的过电位数据如表1所示。Put the obtained nickel-based self-supporting water oxidation catalyst precursor Ni-S into the reaction solution containing 0.1mol/L ferrous chloride and 0.00625mol/L trisodium citrate, and react at a reaction temperature of 90 degrees for 6h , after the reaction was finished by ultrasonication, washing with water, and drying, a nickel-based self-supporting electrocatalyst for water oxidation was obtained in situ, denoted as Ni-S-Fe. The catalyst exhibited excellent electrocatalytic water oxidation catalytic performance, as shown in the linear sweep voltammetry (LSV) curve in Figure 3a, and the specific overpotential data at 100mA/cm2 are shown in Table 1.
结合XRD和EDX等分析,证明制得的催化剂由金属相Ni,金属合金相FeNi3和化合物Ni3S2组成。SEM表征表明得到的催化剂具有片层状多孔结构的形貌特征。其中实施例1-5中所述催化剂的过电位如表1所示,表中数据表明了辅助试剂以及硫的加入能够显著提升催化剂的催化性能。Combined with XRD and EDX analysis, it is proved that the prepared catalyst is composed of metal phase Ni, metal alloy phase FeNi3 and compound Ni3 S2 . SEM characterization showed that the obtained catalyst had the morphology characteristics of lamellar porous structure. The overpotentials of the catalysts described in Examples 1-5 are shown in Table 1, and the data in the table show that the addition of auxiliary reagents and sulfur can significantly improve the catalytic performance of the catalyst.
表1:实施例1-5所述催化剂体系的产氧过电位表Table 1: Oxygen production overpotential table of the catalyst system described in Examples 1-5
由上表可以看出我们发展的Ni-S-Fe催化剂作为电解水产氧催化剂表现出了非常优异的性能,这表明加入合适的辅助试剂,合理的设计和组装不同的元素能够显著的提升催化剂的催化性能,有利于获得高性能的优异水氧化催化剂。It can be seen from the above table that the Ni-S-Fe catalyst we developed has shown excellent performance as an oxygen-generating catalyst for electrolysis of water, which shows that adding suitable auxiliary reagents, rational design and assembly of different elements can significantly improve the performance of the catalyst. Catalytic properties are beneficial to obtain high-performance excellent water oxidation catalysts.
实施例6Example 6
本实施例说明催化剂制备过程中前驱体制备温度(T)的控制实例:This embodiment illustrates the control example of the precursor preparation temperature (T) in the catalyst preparation process:
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L氯化锂,1mol/L磷酸,1mol/L硫脲)分别在80,90,100,110,120,130度的反应温度反应2h,原位得到镍基自支撑水氧化催化剂的前驱体,记为Ni-S-T;Adopt the preparation mode of low-temperature solution reaction, take nickel net as carrier and reactant, in the reaction precursor solution newly prepared (the component of reaction precursor solution is 1mol/L lithium chloride, 1mol/L phosphoric acid, 1mol/L thiourea ) were reacted at reaction temperatures of 80, 90, 100, 110, 120, and 130 degrees for 2 hours to obtain a precursor of a nickel-based self-supporting water oxidation catalyst in situ, denoted as Ni-S-T;
将得到的镍基自支撑水氧化催化剂前驱体Ni-S-T放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂,记作Ni-S-Fe-T。不同制备温度得到的产氧电催化剂相应的活性数据见表2。Put the obtained nickel-based self-supporting water oxidation catalyst precursor Ni-S-T into the reaction solution containing 0.1mol/L ferrous chloride and 0.00625mol/L trisodium citrate, and react at a reaction temperature of 90 degrees for 6h , after the reaction, ultrasonication, water washing, and drying were performed to obtain a nickel-based self-supporting electrocatalyst for water oxidation, denoted as Ni-S-Fe-T. The corresponding activity data of oxygen-producing electrocatalysts obtained at different preparation temperatures are shown in Table 2.
表2不同制备温度得到的催化剂的催化活性表The catalytic activity table of the catalyst that table 2 different preparation temperature obtains
由上表可以看出,制备前驱体的反应温度对得到的催化剂的产氧性能有一定的影响,当温度低于100度时,随着反应温度的升高,催化剂的催化性能依次增加,而当温度高于100度以后,再升高温度对催化剂的催化性能影响不大。It can be seen from the above table that the reaction temperature for preparing the precursor has a certain influence on the oxygen production performance of the obtained catalyst. When the temperature is lower than 100 degrees, the catalytic performance of the catalyst increases sequentially with the increase of the reaction temperature, while When the temperature is higher than 100 degrees, increasing the temperature has little effect on the catalytic performance of the catalyst.
实施例7Example 7
本实施例说明催化剂制备过程中前驱体制备时间(t)的控制实例:This embodiment illustrates the control example of the precursor preparation time (t) in the catalyst preparation process:
采用低温溶液反应的制备方式,以镍网为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L氯化锂,1mol/L磷酸,1mol/L硫脲)在120度的反应温度下分别反应0.5,1,1.5,2,2.5h,原位得到镍基自支撑水氧化催化剂的前驱体,记为Ni-S-120-t;Adopt the preparation mode of low-temperature solution reaction, take nickel net as carrier and reactant, in the reaction precursor solution newly prepared (the component of reaction precursor solution is 1mol/L lithium chloride, 1mol/L phosphoric acid, 1mol/L thiourea ) were reacted at a reaction temperature of 120 degrees for 0.5, 1, 1.5, 2, and 2.5 hours respectively, and the precursor of the nickel-based self-supporting water oxidation catalyst was obtained in situ, denoted as Ni-S-120-t;
将得到的镍基自支撑水氧化催化剂前驱体Ni-S-120-t放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂,记作Ni-S-Fe-120-t。不同制备温度得到的产氧电催化剂相应的活性数据见表3。Put the obtained nickel-based self-supporting water oxidation catalyst precursor Ni-S-120-t into the reaction solution containing 0.1mol/L ferrous chloride and 0.00625mol/L trisodium citrate, and react at 90 degrees React at high temperature for 6 hours. After the reaction, ultrasonic, water wash and dry to obtain a nickel-based self-supporting electrocatalyst for water oxidation, denoted as Ni-S-Fe-120-t. The corresponding activity data of oxygen-producing electrocatalysts obtained at different preparation temperatures are shown in Table 3.
表3不同制备时间得到的催化剂的催化活性表The catalytic activity table of the catalyst that table 3 different preparation time obtains
由上表可以看出,当制备前驱体的反应时间低于2h时随着制备时间的增加,最终得到的催化剂的催化性能有所提升,而当反应时间超过2h后再增加反应时间则会使最终得到的催化剂的催化活性降低。It can be seen from the above table that when the reaction time for preparing the precursor is less than 2 hours, the catalytic performance of the final catalyst is improved with the increase of the preparation time, and when the reaction time exceeds 2 hours, increasing the reaction time will make the The catalytic activity of the resulting catalyst is reduced.
实施例8Example 8
本实施例说明镍基自支撑水氧化电催化剂制备的镍基材料的控制实例:This embodiment illustrates the control example of the nickel-based material prepared by the nickel-based self-supporting water oxidation electrocatalyst:
采用低温溶液反应的制备方式,分别以镍板、镍片、镍网、泡沫镍、雷尼镍为载体和反应物,在新配的反应前驱溶液中(反应前驱溶液的组分为1mol/L氯化锂,1mol/L磷酸,1mol/L硫脲)在120度的反应温度下反应2h,原位得到镍基自支撑水氧化催化剂的前驱体;Adopt the preparation mode of low-temperature solution reaction, take nickel plate, nickel sheet, nickel net, foam nickel, Raney nickel as carrier and reactant respectively, in the newly prepared reaction precursor solution (the component of reaction precursor solution is 1mol/L Lithium chloride, 1mol/L phosphoric acid, 1mol/L thiourea) were reacted for 2h at a reaction temperature of 120 degrees, and the precursor of the nickel-based self-supporting water oxidation catalyst was obtained in situ;
将得到的镍基自支撑水氧化催化剂前驱体放入含有0.1mol/L氯化亚铁和0.00625mol/L的柠檬酸三钠的反应溶液中,在90度的反应温度下反应6h,反应结束后超声、水洗、干燥后得到镍基自支撑水氧化电催化剂。Put the obtained nickel-based self-supporting water oxidation catalyst precursor into a reaction solution containing 0.1 mol/L ferrous chloride and 0.00625 mol/L trisodium citrate, react at a reaction temperature of 90 degrees for 6 hours, and the reaction ends After ultrasonication, water washing and drying, a nickel-based self-supporting electrocatalyst for water oxidation was obtained.
分析表明催化剂的组成和物化性质基本不受镍基材料形式的影响,但催化剂的活性和稳定性则与镍基材料本身的比表面积,孔隙率以及机械强度有关。The analysis shows that the composition and physical and chemical properties of the catalyst are basically not affected by the form of the nickel-based material, but the activity and stability of the catalyst are related to the specific surface area, porosity and mechanical strength of the nickel-based material itself.
实施例9Example 9
本实施例说明镍基自支撑水氧化电催化剂Ni-S-Fe的稳定性。This example illustrates the stability of nickel-based self-supporting water oxidation electrocatalyst Ni-S-Fe.
按照实施例5的方法制备得到Ni-S-Fe催化剂体系,通过恒电流密度法测试其在电流密度为50和300mA/cm2时的长时间稳定性,如图3b所示,其在催化反应140h后仍然没有衰减,表明我们制得的镍基自支撑水氧化电催化剂能够长时间稳定的催化产氧反应的进行。Prepare Ni-S-Fe catalyst system according to the method for embodiment5 , test its long-term stability when current density is 50 and 300mA/cm by constant current density method, as shown in Figure 3b, it is in catalytic reaction There is still no decay after 140h, indicating that the nickel-based self-supporting water oxidation electrocatalyst we prepared can stably catalyze the oxygen evolution reaction for a long time.
实施例10Example 10
本实施例说明镍基自支撑水氧化电催化剂Ni-S-Fe和雷尼镍组成的两电极体系在实验室条件和工况条件下的电解水性能。This example illustrates the water electrolysis performance of a two-electrode system composed of nickel-based self-supporting water oxidation electrocatalyst Ni-S-Fe and Raney nickel under laboratory conditions and working conditions.
按照实施例5的方法制备得到Ni-S-Fe催化剂体系,将其作为电解水阳极,以商用雷尼镍作为电解水阴极,构筑得到两电极水分解体系,分别在实验室条件(1mol/L KOH,室温)和工况条件(30%KOH,80℃)下测试其电解水性能。该两电极体系表现出了非常优异的水分解性能,如图4a中的线性扫描伏安(LSV)曲线所示,在实验室条件下电流密度为100mA/cm2和500mA/cm2时需加槽压1.68和1.86V,工况条件下则在1.56和1.68V的槽压下便能得到100和500mA/cm2的电流密度。同时该两电极体系在500mA/cm2的电流密度下电解时间长达350h以上仍能保持其水分解性能,如图4b所示。The Ni-S-Fe catalyst system was prepared according to the method of Example 5, and it was used as the electrolyzed water anode, and the commercial Raney nickel was used as the electrolyzed water cathode, and the two-electrode water splitting system was constructed, respectively under laboratory conditions (1mol/L KOH, room temperature) and operating conditions (30% KOH, 80°C) to test its electrolytic water performance. Thetwo- electrode system exhibits excellent water splitting performance, as shown by the linear sweep voltammetry (LSV) curve in Figure 4a. The cell pressure is 1.68 and 1.86V, and the current density of 100 and 500mA/cm2 can be obtained under the cell pressure of 1.56 and 1.68V under working conditions. At the same time, the two-electrode system can still maintain its water splitting performance at a current density of 500mA/cm2 for a period of more than 350h, as shown in Figure 4b.
本发明提供一种镍载体支撑的NiFeS水氧化电催化剂及其制备方法。所述的水氧化电催化剂是通过两步低温溶液的方法将S和Fe原位组装至Ni基导电载体上制备而成。原位制备得到的水氧化电极由Fe,Ni和S元素组成。电极表面Fe占比为5-36%,Ni占比5-18%,S占比0.2-40%。所制备得到的催化剂体系为自支撑的片层状多孔结构,分散度高,比表面积大,物理稳定性和结构稳定性好。在催化电化学水氧化反应时,显示出了优异的活性和稳定性。其中活性最好的催化剂在1mol/L的KOH溶液和室温条件下,电流密度为100mA/cm2,500mA/cm2和1000mA/cm2时仅需过电位207,285和324mV,而且在反应140小时之后,活性仍保持。在同样的条件下,以商用雷尼镍为阴极,制备得到的催化剂为阳极组装得到的两电极体系在电流密度为100mA/cm2和500mA/cm2时需加槽压1.68和1.86V。本发明所述的镍基自支撑水氧化电催化剂也可适用于高温,强碱性环境,在模拟商业电解槽的工况运行条件即30%的KOH溶液和80℃的条件下,在1.56和1.68V的槽压下便能得到100和500mA/cm2的电流密度,同时该两电极体系在500mA/cm2的电流密度下电解时间长达350h以上仍能保持其水分解性能,有工业应用基础。The invention provides a NiFeS water oxidation electrocatalyst supported by a nickel carrier and a preparation method thereof. The water oxidation electrocatalyst is prepared by in-situ assembly of S and Fe onto a Ni-based conductive carrier through a two-step low-temperature solution method. The in situ prepared water oxidation electrode is composed of Fe, Ni and S elements. The proportion of Fe on the electrode surface is 5-36%, that of Ni is 5-18%, and that of S is 0.2-40%. The prepared catalyst system is a self-supporting lamellar porous structure with high dispersion, large specific surface area, good physical stability and structural stability. It shows excellent activity and stability in catalyzing electrochemical water oxidation reaction. Among them, the catalyst with the best activity only needs overpotentials of 207, 285 and 324mV when the current density is 100mA/cm2 , 500mA/cm2 and 1000mA/cm2 under the condition of 1mol/L KOH solution and room temperature, and in the reaction of 140 After hours, the activity is still maintained. Under the same conditions, the commercial Raney nickel is used as the cathode, and the prepared catalyst is used as the anode to assemble the two-electrode system. When the current density is 100mA/cm2 and 500mA/cm2 , the cell pressure needs to be 1.68 and 1.86V. The nickel-based self-supporting water oxidation electrocatalyst of the present invention is also suitable for high temperature and strong alkaline environment. Under the conditions of 30% KOH solution and 80 °C under the conditions of simulating the operating conditions of commercial electrolyzers, the electrocatalyst can be used at 1.56 and 80 °C. The current density of 100 and 500mA/cm2 can be obtained under the cell voltage of 1.68V. At the same time, the two-electrode system can maintain its water splitting performance for more than 350h at a current density of 500mA/cm2 . It has industrial applications Base.
本领域技术人员容易理解在不脱离上述说明书中公开的材料和方法的思想的条件下可对本发明进行组合或改变,认为这种改变包括在本发明的范围内。因此,在上文具体描述的特别实施方案仅是说明性的,而不限制本发明的范围,由附加权利要求和其任何及全部等同方式给出本发明的完全范围。Those skilled in the art can easily understand that the present invention can be combined or changed without departing from the concept of the materials and methods disclosed in the above specification, and such changes are considered to be included in the scope of the present invention. Accordingly, the particular embodiments described above in detail are illustrative only and not limiting as to the scope of the invention which is to be given the full scope of the appended claims and any and all equivalents thereof.
Claims (8)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811411703.8ACN111215074B (en) | 2018-11-25 | 2018-11-25 | NiFeS water oxidation electrocatalyst supported by a nickel carrier and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811411703.8ACN111215074B (en) | 2018-11-25 | 2018-11-25 | NiFeS water oxidation electrocatalyst supported by a nickel carrier and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN111215074A CN111215074A (en) | 2020-06-02 |
| CN111215074Btrue CN111215074B (en) | 2023-01-24 |
Family
ID=70808746
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201811411703.8AActiveCN111215074B (en) | 2018-11-25 | 2018-11-25 | NiFeS water oxidation electrocatalyst supported by a nickel carrier and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111215074B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115825179A (en)* | 2022-12-05 | 2023-03-21 | 嘉庚创新实验室 | Method for evaluating activity of catalyst for water electrolysis and electrolytic cell |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105013512A (en)* | 2015-06-08 | 2015-11-04 | 中国科学院长春应用化学研究所 | Self-supporting transitional metal sulfide catalyst and preparation methods and applications thereof |
| CN107904614A (en)* | 2017-10-17 | 2018-04-13 | 华南理工大学 | A kind of Ni3S2@Ni Fe LDH analysis oxygen electro catalytic electrodes and preparation method and application |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180023199A1 (en)* | 2016-07-19 | 2018-01-25 | Utah State University | Electrocatalytic hydrogen evolution and biomass upgrading |
- 2018
- 2018-11-25CNCN201811411703.8Apatent/CN111215074B/enactiveActive
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105013512A (en)* | 2015-06-08 | 2015-11-04 | 中国科学院长春应用化学研究所 | Self-supporting transitional metal sulfide catalyst and preparation methods and applications thereof |
| CN107904614A (en)* | 2017-10-17 | 2018-04-13 | 华南理工大学 | A kind of Ni3S2@Ni Fe LDH analysis oxygen electro catalytic electrodes and preparation method and application |
Non-Patent Citations (2)
| Title |
|---|
| Enhanced Catalysis of Electrochemical Overall Water Splitting in Alkaline Media by Fe Doping in Ni3S2 Nanosheet Arrays;Geng Zhang et al.;《ACS Catal.》;20180503;第8卷;5431-5441* |
| Ni3S2 nanowires grown on nickel foam as an efficient bifunctional electrocatalyst for water splitting with greatly practical prospects;Dawei Zhang et al.;《Nanotechnology》;20180416;第29卷(第24期);245402:1-10* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111215074A (en) | 2020-06-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN113445072B (en) | Foamed nickel composite electrode and preparation method and application thereof | |
| CN103715436B (en) | Carbon dioxide electrochemical reduction catalyst as well as preparation method and application thereof | |
| CN113437314B (en) | Nitrogen-doped carbon-supported low-content ruthenium and Co 2 Three-function electrocatalyst of P nano particle and preparation method and application thereof | |
| CN111001428B (en) | A kind of metal-free carbon-based electrocatalyst and preparation method and application | |
| CN111167480B (en) | Novel oxygen evolution electrocatalyst and preparation method and application thereof | |
| CN113981487B (en) | High-entropy carbonate electrocatalyst and preparation method thereof | |
| CN110838588A (en) | Rechargeable zinc-air battery bifunctional catalyst and preparation method and application thereof | |
| CN110197909A (en) | Ferronickel catalysis material, preparation method and the application in water electrolysis hydrogen production gas, preparation liquid sun fuel | |
| CN109954503A (en) | A kind of nickel selenide and ternary nickel-iron selenide composite electrocatalyst and preparation method and application | |
| CN112951623B (en) | Copper-cobalt-zinc composite self-supporting nano array electrode material and preparation method and application thereof | |
| CN112080759A (en) | Preparation method of bismuth-doped bimetallic sulfide electrode for electrocatalytic oxidation of urea | |
| Wu et al. | CoWO4/CoP2 nanoflakes grown on carbon nanotube film as an efficient electrocatalyst for water splitting in alkaline media | |
| CN110711597B (en) | A kind of Co-Mo-P-O electrocatalyst and its preparation method and application | |
| CN115505961A (en) | A low-cost catalytic electrode for rapid full-electrolysis hydrogen production from seawater, its preparation and application | |
| CN113104862A (en) | Method for rapidly preparing Prussian blue or analogues thereof in batches and application of method | |
| CN115770621A (en) | Preparation method and application of bimetallic MOF (metal organic framework) anchored Pt nanocluster catalyst | |
| CN115852429A (en) | Ni3Fe-LDH@NiCoP/NF heterojunction high-efficiency total water splitting electrocatalyst and its preparation method and application | |
| CN114892203A (en) | A kind of method of electrochemical catalytic conversion of carbon dioxide to synthesize carbon monoxide | |
| CN115821319B (en) | Octahedral Cu2O/CuO heterojunction catalyst and preparation method and application thereof | |
| CN117568862A (en) | A kind of electrolysis water hydrogen production catalyst and its preparation method and application | |
| CN110075925B (en) | Preparation method of oxygen evolution catalyst based on metal organic framework material | |
| CN114921803A (en) | Preparation method and application of transition metal sulfide composite hydroxide electrode | |
| CN110508292A (en) | Preparation method of metal-doped rhenium disulfide nanosheet arrays for electrocatalytic total water splitting | |
| CN111215074B (en) | NiFeS water oxidation electrocatalyst supported by a nickel carrier and preparation method thereof | |
| CN113774428A (en) | A kind of preparation method of high-efficiency cobalt rhodium hydroxide nanoparticle/carbon cloth electrode, product and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |