CN110672750A - Method for determining urushiol in rhus chinensis fruit oil by using UPLC-MS (ultra performance liquid chromatography-mass spectrometry) - Google Patents
Method for determining urushiol in rhus chinensis fruit oil by using UPLC-MS (ultra performance liquid chromatography-mass spectrometry)Download PDFInfo
- Publication number
- CN110672750A CN110672750ACN201911023896.4ACN201911023896ACN110672750ACN 110672750 ACN110672750 ACN 110672750ACN 201911023896 ACN201911023896 ACN 201911023896ACN 110672750 ACN110672750 ACN 110672750A
- Authority
- CN
- China
- Prior art keywords
- urushiol
- oil
- uplc
- saturated
- measure
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- QARRXYBJLBIVAK-UEMSJJPVSA-N3-[(8e,11e)-pentadeca-8,11-dienyl]benzene-1,2-diol;3-[(8e,11e)-pentadeca-8,11,14-trienyl]benzene-1,2-diol;3-[(8e,11e,13e)-pentadeca-8,11,13-trienyl]benzene-1,2-diol;3-[(e)-pentadec-8-enyl]benzene-1,2-diol;3-pentadecylbenzene-1,2-diolChemical compoundCCCCCCCCCCCCCCCC1=CC=CC(O)=C1O.CCCCCC\C=C\CCCCCCCC1=CC=CC(O)=C1O.CCC\C=C\C\C=C\CCCCCCCC1=CC=CC(O)=C1O.C\C=C\C=C\C\C=C\CCCCCCCC1=CC=CC(O)=C1O.OC1=CC=CC(CCCCCCC\C=C\C\C=C\CC=C)=C1OQARRXYBJLBIVAK-UEMSJJPVSA-N0.000titleclaimsabstractdescription167
- RMTXUPIIESNLPW-UHFFFAOYSA-N1,2-dihydroxy-3-(pentadeca-8,11-dienyl)benzeneNatural productsCCCC=CCC=CCCCCCCCC1=CC=CC(O)=C1ORMTXUPIIESNLPW-UHFFFAOYSA-N0.000titleclaimsabstractdescription61
- IYROWZYPEIMDDN-UHFFFAOYSA-N3-n-pentadec-8,11,13-trienyl catecholNatural productsCC=CC=CCC=CCCCCCCCC1=CC=CC(O)=C1OIYROWZYPEIMDDN-UHFFFAOYSA-N0.000titleclaimsabstractdescription61
- DQTMTQZSOJMZSF-UHFFFAOYSA-NurushiolNatural productsCCCCCCCCCCCCCCCC1=CC=CC(O)=C1ODQTMTQZSOJMZSF-UHFFFAOYSA-N0.000titleclaimsabstractdescription61
- 238000000034methodMethods0.000titleclaimsabstractdescription34
- 238000001946ultra-performance liquid chromatography-mass spectrometryMethods0.000titleclaimsabstractdescription24
- 235000013399edible fruitsNutrition0.000titleclaimsabstractdescription23
- 240000003152Rhus chinensisSpecies0.000titledescription5
- 235000014220Rhus chinensisNutrition0.000titledescription5
- 238000001514detection methodMethods0.000claimsabstractdescription14
- 241000007008Stachys grandidentataSpecies0.000claimsabstract5
- 244000153234Hibiscus abelmoschusSpecies0.000claimsabstract4
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000claimsdescription48
- 150000002500ionsChemical class0.000claimsdescription37
- 238000004949mass spectrometryMethods0.000claimsdescription26
- RTZKZFJDLAIYFH-UHFFFAOYSA-NDiethyl etherChemical compoundCCOCCRTZKZFJDLAIYFH-UHFFFAOYSA-N0.000claimsdescription24
- 238000001819mass spectrumMethods0.000claimsdescription10
- 238000001195ultra high performance liquid chromatographyMethods0.000claimsdescription10
- 238000000605extractionMethods0.000claimsdescription8
- 238000002360preparation methodMethods0.000claimsdescription8
- 238000005259measurementMethods0.000claimsdescription7
- 238000001035dryingMethods0.000claimsdescription6
- 238000010828elutionMethods0.000claimsdescription6
- 239000012086standard solutionSubstances0.000claimsdescription6
- 244000294611Punica granatumSpecies0.000claimsdescription4
- 235000014360Punica granatumNutrition0.000claimsdescription4
- 239000006228supernatantSubstances0.000claimsdescription4
- 239000012528membraneSubstances0.000claimsdescription3
- 239000007921spraySubstances0.000claimsdescription3
- 239000000706filtrateSubstances0.000claimsdescription2
- HQVFCQRVQFYGRJ-UHFFFAOYSA-Nformic acid;hydrateChemical compoundO.OC=OHQVFCQRVQFYGRJ-UHFFFAOYSA-N0.000claimsdescription2
- 238000003825pressingMethods0.000claimsdescription2
- 241000208422RhododendronSpecies0.000claims2
- 230000009286beneficial effectEffects0.000abstractdescription4
- 241001142677Artemisia serrataSpecies0.000abstractdescription2
- 239000003921oilSubstances0.000description47
- 235000019198oilsNutrition0.000description47
- 239000000523sampleSubstances0.000description19
- 230000000052comparative effectEffects0.000description11
- 239000004922lacquerSubstances0.000description11
- 239000000243solutionSubstances0.000description11
- 150000008442polyphenolic compoundsChemical class0.000description10
- 235000013824polyphenolsNutrition0.000description10
- 238000001228spectrumMethods0.000description10
- WEVYAHXRMPXWCK-UHFFFAOYSA-NAcetonitrileChemical compoundCC#NWEVYAHXRMPXWCK-UHFFFAOYSA-N0.000description9
- 240000003492Neolamarckia cadambaSpecies0.000description9
- 238000004458analytical methodMethods0.000description9
- 239000000047productSubstances0.000description9
- 230000014759maintenance of locationEffects0.000description7
- 238000004128high performance liquid chromatographyMethods0.000description6
- 150000002430hydrocarbonsChemical class0.000description6
- 229920006395saturated elastomerPolymers0.000description6
- -1diene unsaturated urushiolChemical class0.000description5
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical compoundOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000description4
- 241000049484Strobilanthes serrataSpecies0.000description4
- 150000001993dienesChemical class0.000description4
- 239000012535impuritySubstances0.000description4
- 238000002347injectionMethods0.000description4
- 239000007924injectionSubstances0.000description4
- 238000002386leachingMethods0.000description4
- BDAGIHXWWSANSR-UHFFFAOYSA-Nmethanoic acidNatural productsOC=OBDAGIHXWWSANSR-UHFFFAOYSA-N0.000description4
- 239000012488sample solutionSubstances0.000description4
- 230000035945sensitivityEffects0.000description4
- 239000000126substanceSubstances0.000description4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description3
- ISWSIDIOOBJBQZ-UHFFFAOYSA-NPhenolChemical compoundOC1=CC=CC=C1ISWSIDIOOBJBQZ-UHFFFAOYSA-N0.000description3
- 241000589516PseudomonasSpecies0.000description3
- HEMHJVSKTPXQMS-UHFFFAOYSA-MSodium hydroxideChemical compound[OH-].[Na+]HEMHJVSKTPXQMS-UHFFFAOYSA-M0.000description3
- 240000002383Vandopsis giganteaSpecies0.000description3
- 238000010521absorption reactionMethods0.000description3
- 239000003925fatSubstances0.000description3
- 239000012634fragmentSubstances0.000description3
- 238000004811liquid chromatographyMethods0.000description3
- 239000012982microporous membraneSubstances0.000description3
- OSWFIVFLDKOXQC-UHFFFAOYSA-N4-(3-methoxyphenyl)anilineChemical compoundCOC1=CC=CC(C=2C=CC(N)=CC=2)=C1OSWFIVFLDKOXQC-UHFFFAOYSA-N0.000description2
- 244000296825Amygdalus nanaSpecies0.000description2
- 235000003840Amygdalus nanaNutrition0.000description2
- 235000011432PrunusNutrition0.000description2
- 241000304195Salvia miltiorrhizaSpecies0.000description2
- 235000011135Salvia miltiorrhizaNutrition0.000description2
- GSEJCLTVZPLZKY-UHFFFAOYSA-NTriethanolamineChemical compoundOCCN(CCO)CCOGSEJCLTVZPLZKY-UHFFFAOYSA-N0.000description2
- 230000021736acetylationEffects0.000description2
- 238000006640acetylation reactionMethods0.000description2
- 229910000147aluminium phosphateInorganic materials0.000description2
- YCIMNLLNPGFGHC-UHFFFAOYSA-NcatecholChemical compoundOC1=CC=CC=C1OYCIMNLLNPGFGHC-UHFFFAOYSA-N0.000description2
- 239000003153chemical reaction reagentSubstances0.000description2
- 239000000284extractSubstances0.000description2
- 235000019197fatsNutrition0.000description2
- 235000019253formic acidNutrition0.000description2
- LNTHITQWFMADLM-UHFFFAOYSA-Ngallic acidChemical compoundOC(=O)C1=CC(O)=C(O)C(O)=C1LNTHITQWFMADLM-UHFFFAOYSA-N0.000description2
- 238000010438heat treatmentMethods0.000description2
- 239000007788liquidSubstances0.000description2
- 239000000203mixtureSubstances0.000description2
- 239000003973paintSubstances0.000description2
- 235000014774prunusNutrition0.000description2
- 238000004445quantitative analysisMethods0.000description2
- 238000011160researchMethods0.000description2
- 230000004044responseEffects0.000description2
- 229930195734saturated hydrocarbonNatural products0.000description2
- 241000894007speciesSpecies0.000description2
- QAIPRVGONGVQAS-DUXPYHPUSA-Ntrans-caffeic acidChemical compoundOC(=O)\C=C\C1=CC=C(O)C(O)=C1QAIPRVGONGVQAS-DUXPYHPUSA-N0.000description2
- 229930195735unsaturated hydrocarbonNatural products0.000description2
- 239000003643water by typeSubstances0.000description2
- PFTAWBLQPZVEMU-DZGCQCFKSA-N(+)-catechinChemical compoundC1([C@H]2OC3=CC(O)=CC(O)=C3C[C@@H]2O)=CC=C(O)C(O)=C1PFTAWBLQPZVEMU-DZGCQCFKSA-N0.000description1
- ACEAELOMUCBPJP-UHFFFAOYSA-N(E)-3,4,5-trihydroxycinnamic acidNatural productsOC(=O)C=CC1=CC(O)=C(O)C(O)=C1ACEAELOMUCBPJP-UHFFFAOYSA-N0.000description1
- CWVRJTMFETXNAD-FWCWNIRPSA-N3-O-Caffeoylquinic acidNatural productsO[C@H]1[C@@H](O)C[C@@](O)(C(O)=O)C[C@H]1OC(=O)\C=C\C1=CC=C(O)C(O)=C1CWVRJTMFETXNAD-FWCWNIRPSA-N0.000description1
- PZIRUHCJZBGLDY-UHFFFAOYSA-NCaffeoylquinic acidNatural productsCC(CCC(=O)C(C)C1C(=O)CC2C3CC(O)C4CC(O)CCC4(C)C3CCC12C)C(=O)OPZIRUHCJZBGLDY-UHFFFAOYSA-N0.000description1
- 239000004215Carbon black (E152)Substances0.000description1
- 241000209441CeratophyllumSpecies0.000description1
- 206010020751HypersensitivityDiseases0.000description1
- 229910021578Iron(III) chlorideInorganic materials0.000description1
- 241001465754MetazoaSpecies0.000description1
- CWVRJTMFETXNAD-KLZCAUPSSA-NNeochlorogenin-saeureNatural productsO[C@H]1C[C@@](O)(C[C@@H](OC(=O)C=Cc2ccc(O)c(O)c2)[C@@H]1O)C(=O)OCWVRJTMFETXNAD-KLZCAUPSSA-N0.000description1
- 241000233614PhytophthoraSpecies0.000description1
- 240000008334Pisonia albaSpecies0.000description1
- 240000000432Pistacia chinensisSpecies0.000description1
- 239000007868Raney catalystSubstances0.000description1
- NPXOKRUENSOPAO-UHFFFAOYSA-NRaney nickelChemical compound[Al].[Ni]NPXOKRUENSOPAO-UHFFFAOYSA-N0.000description1
- 229910000564Raney nickelInorganic materials0.000description1
- 235000017276SalviaNutrition0.000description1
- 240000007164Salvia officinalisSpecies0.000description1
- 238000000944Soxhlet extractionMethods0.000description1
- 241001420289Strobilanthes japonicaSpecies0.000description1
- 241001104043SyringaSpecies0.000description1
- 235000005811Viola aduncaNutrition0.000description1
- 240000009038Viola odorataSpecies0.000description1
- 235000013487Viola odorataNutrition0.000description1
- 235000002254Viola papilionaceaNutrition0.000description1
- BLAKAEFIFWAFGH-UHFFFAOYSA-Nacetyl acetate;pyridineChemical compoundC1=CC=NC=C1.CC(=O)OC(C)=OBLAKAEFIFWAFGH-UHFFFAOYSA-N0.000description1
- 239000002253acidSubstances0.000description1
- 150000001336alkenesChemical group0.000description1
- 208000026935allergic diseaseDiseases0.000description1
- 230000007815allergyEffects0.000description1
- 239000010775animal oilSubstances0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 238000003556assayMethods0.000description1
- 235000004883caffeic acidNutrition0.000description1
- 229940074360caffeic acidDrugs0.000description1
- 230000015556catabolic processEffects0.000description1
- ADRVNXBAWSRFAJ-UHFFFAOYSA-NcatechinNatural productsOC1Cc2cc(O)cc(O)c2OC1c3ccc(O)c(O)c3ADRVNXBAWSRFAJ-UHFFFAOYSA-N0.000description1
- 235000005487catechinNutrition0.000description1
- CWVRJTMFETXNAD-JUHZACGLSA-Nchlorogenic acidChemical compoundO[C@@H]1[C@H](O)C[C@@](O)(C(O)=O)C[C@H]1OC(=O)\C=C\C1=CC=C(O)C(O)=C1CWVRJTMFETXNAD-JUHZACGLSA-N0.000description1
- 235000001368chlorogenic acidNutrition0.000description1
- 229940074393chlorogenic acidDrugs0.000description1
- FFQSDFBBSXGVKF-KHSQJDLVSA-Nchlorogenic acidNatural productsO[C@@H]1C[C@](O)(C[C@@H](CC(=O)C=Cc2ccc(O)c(O)c2)[C@@H]1O)C(=O)OFFQSDFBBSXGVKF-KHSQJDLVSA-N0.000description1
- 229950001002cianidanolDrugs0.000description1
- BMRSEYFENKXDIS-KLZCAUPSSA-Ncis-3-O-p-coumaroylquinic acidNatural productsO[C@H]1C[C@@](O)(C[C@@H](OC(=O)C=Cc2ccc(O)cc2)[C@@H]1O)C(=O)OBMRSEYFENKXDIS-KLZCAUPSSA-N0.000description1
- QAIPRVGONGVQAS-UHFFFAOYSA-Ncis-caffeic acidNatural productsOC(=O)C=CC1=CC=C(O)C(O)=C1QAIPRVGONGVQAS-UHFFFAOYSA-N0.000description1
- 239000013068control sampleSubstances0.000description1
- 239000010779crude oilSubstances0.000description1
- 238000006731degradation reactionMethods0.000description1
- 238000011161developmentMethods0.000description1
- 238000010586diagramMethods0.000description1
- 239000008157edible vegetable oilSubstances0.000description1
- 238000001914filtrationMethods0.000description1
- 229940074391gallic acidDrugs0.000description1
- 235000004515gallic acidNutrition0.000description1
- 229930195733hydrocarbonNatural products0.000description1
- 239000004615ingredientSubstances0.000description1
- RBTARNINKXHZNM-UHFFFAOYSA-Kiron trichlorideChemical compoundCl[Fe](Cl)ClRBTARNINKXHZNM-UHFFFAOYSA-K0.000description1
- 238000004895liquid chromatography mass spectrometryMethods0.000description1
- 239000000463materialSubstances0.000description1
- 238000002844meltingMethods0.000description1
- 230000008018meltingEffects0.000description1
- 239000000401methanolic extractSubstances0.000description1
- 230000004048modificationEffects0.000description1
- 238000012986modificationMethods0.000description1
- 150000005673monoalkenesChemical group0.000description1
- VYQNWZOUAUKGHI-UHFFFAOYSA-NmonobenzoneChemical compoundC1=CC(O)=CC=C1OCC1=CC=CC=C1VYQNWZOUAUKGHI-UHFFFAOYSA-N0.000description1
- JRZJOMJEPLMPRA-UHFFFAOYSA-NolefinNatural productsCCCCCCCC=CJRZJOMJEPLMPRA-UHFFFAOYSA-N0.000description1
- 238000005457optimizationMethods0.000description1
- 150000002894organic compoundsChemical class0.000description1
- 150000002989phenolsChemical class0.000description1
- 239000002244precipitateSubstances0.000description1
- 238000000926separation methodMethods0.000description1
- 238000002798spectrophotometry methodMethods0.000description1
- 238000010561standard procedureMethods0.000description1
- 238000012360testing methodMethods0.000description1
- 238000004809thin layer chromatographyMethods0.000description1
- 229910021642ultra pure waterInorganic materials0.000description1
- 239000012498ultrapure waterSubstances0.000description1
- 238000000825ultraviolet detectionMethods0.000description1
- 238000000870ultraviolet spectroscopyMethods0.000description1
- 235000021122unsaturated fatty acidsNutrition0.000description1
- 150000004670unsaturated fatty acidsChemical class0.000description1
- 235000015112vegetable and seed oilNutrition0.000description1
- 235000019871vegetable fatNutrition0.000description1
- 239000008158vegetable oilSubstances0.000description1
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description1
Images
Classifications
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/72—Mass spectrometers
- G01N30/7233—Mass spectrometers interfaced to liquid or supercritical fluid chromatograph
- G01N30/724—Nebulising, aerosol formation or ionisation
- G01N30/7266—Nebulising, aerosol formation or ionisation by electric field, e.g. electrospray
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Dispersion Chemistry (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及漆酚测定技术领域,具体涉及一种采用UPLC-MS测定盐肤木果油中漆酚的方法。The invention relates to the technical field of urushiol determination, in particular to a method for using UPLC-MS to determine urushiol in S. chinensis oil.
背景技术Background technique
盐肤木果实及其种子均含丰富的油脂,初步研究表明,野生盐肤木果实中的油脂平均含量高达18%左右,其中以不饱和脂肪酸为主,含量可达 72%,极具开发应用价值。The fruit and its seeds are rich in oil. Preliminary research shows that the average content of oil in the wild fruit is as high as 18%, of which unsaturated fatty acids are the main ones, and the content can reach 72%, which is very suitable for development and application. value.
然而,盐肤木(Rhus chinensis Mill)为漆树科盐肤木属,与漆属的漆树同属于漆树族近缘物种,因此,盐肤木果实及果油中,是否含有漆树的生漆中可致敏性成分——漆酚,是盐肤木油脂作为食用油脂的主要隐患之一。However, Rhus chinensis Mill belongs to the genus Rhus chinensis, which belongs to the genus Rhus chinensis, which belongs to the same species as the Rhus chinensis family. The allergy ingredient, urushiol, is one of the main hidden dangers of the oil of salvia as edible oil.
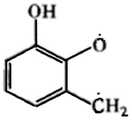
漆酚(Urushiol),在生漆中占其干重的50%~75%,是一类以邻苯二酚为主结构的烃基酚类化合物,其结构简图如图1所示,其侧链R为不同饱和度的直链C15~C17的烃基,是几个密切相关的有机化合物的混合物,由于其侧链R中包括饱和、单烯、双烯和三烯烃基的结构相近,极性差异小漆酚的测定方法包括可见分光光度计法、紫外光谱法、乙酰化测定法、薄层色谱法和高效液相色谱法。Urushiol, which accounts for 50% to 75% of its dry weight in raw lacquer, is a class of hydrocarbon-based phenolic compounds with catechol as the main structure. Its structural diagram is shown in Figure 1. Its side chain R is a straight-chain C15 -C17 hydrocarbon group with different degrees of saturation, which is a mixture of several closely related organic compounds. Because of the similar structure of the saturated, mono-, diene and tri-olefin groups in the side chain R, it is extremely Determination methods of urushiol with different properties include visible spectrophotometry, ultraviolet spectroscopy, acetylation assay, thin layer chromatography and high performance liquid chromatography.
国标《生漆》(GB4354-84)中确定,生漆中漆酚用可见分光光度计法:漆酚与三氯化铁和三乙醇胺(TEA)反应,生成TEA-Fe3+-漆酚蓝紫色络合物,在625nm波长处有最大吸收;该方法测定的灵敏度较低,由于生漆中漆酚含量高(可达生漆干重的50%左右),对于测定生漆中漆酚含量,方法适用,而对盐肤木油脂中极微量的含量,该国标方法的灵敏度不够。It is determined in the national standard "raw lacquer" (GB4354-84) that urushiol in raw lacquer is determined by visible spectrophotometer method: urushiol reacts with ferric chloride and triethanolamine (TEA) to generate TEA-Fe3+ -urushiol blue-violet complex. It has a maximum absorption at the wavelength of 625nm; the sensitivity of this method is low, due to the high content of urushiol in raw lacquer (up to about 50% of the dry weight of raw lacquer), the method is suitable for determining the content of urushiol in raw lacquer, while The sensitivity of the national standard method is not enough for the very small content of the oil of serrata.
漆酚的紫外光谱测定法:由于漆酚的乙醇溶液在紫外区206nm处有最大吸收峰;由于206nm波长太短,太接近紫外波长的下限,能量过高,干扰过大,尤其不宜用来分析盐肤木油脂中微量漆酚的测定。Ultraviolet spectroscopic determination of urushiol: because the ethanol solution of urushiol has a maximum absorption peak at 206nm in the ultraviolet region; because the wavelength of 206nm is too short, too close to the lower limit of the ultraviolet wavelength, the energy is too high, and the interference is too large, especially not suitable for analysis Determination of trace amounts of urushiol in the oil of A. serrata.
漆酚的乙酰化测定法:漆酚与乙酸酐-吡啶在无水介质中作用,使漆酚酯化并析出酸,用标准NaOH溶液滴定。该方法测定最适宜测定的漆酚含量100mg/mL以上浓度的样液(浓度过低,显色太浅,看不清),其灵敏度过低,不宜用于分析盐肤木油脂中微量漆酚。Acetylation determination method of urushiol: urushiol reacts with acetic anhydride-pyridine in anhydrous medium to esterify urushiol and precipitate acid, and titrate with standard NaOH solution. This method is the most suitable for the determination of the urushiol content of 100mg/mL or more in the sample solution (the concentration is too low, the color is too light, and it is not clear), its sensitivity is too low, and it is not suitable for the analysis of trace urushiol in the oil .
漆酚的高效液相色谱法;亦可在225nm虽不是漆酚的最灵敏线,但仍然有较强的吸收,常被用来做HPLC分离紫外检测器检测,但由于目前唯一可获得的漆酚标准品国标《生漆》(GB4354-84)附录中饱和漆酚的制备方法制备的饱和漆酚,即漆酚结构中R支链中的1~3个双键被加H饱和而成饱和漆酚,但盐肤木果油中漆酚多以不饱和漆酚(R支链中含1~3个双键),饱和漆酚含量较少,而HPLC紫外检测器检测对盐肤木果油中极微量的饱和漆酚含量的测定,灵敏度不够高。High performance liquid chromatography of urushiol; also at 225nm, although it is not the most sensitive line of urushiol, it still has strong absorption, and is often used for HPLC separation UV detector detection, but because the only available paint Saturated urushiol prepared by the preparation method of saturated urushiol in the appendix of the national standard "raw lacquer" (GB4354-84), that is, 1 to 3 double bonds in the R branch in the urushiol structure are saturated with H to form saturated lacquer However, most of the urushiol in A. chinensis oil is unsaturated urushiol (the R branch contains 1-3 double bonds), and the content of saturated urushiol is less. Determination of very small amounts of saturated urushiol content, the sensitivity is not high enough.
发明内容SUMMARY OF THE INVENTION
本发明所要解决的技术问题在于现有技术中没有对盐肤木果实中漆酚进行测定的方法,提供一种采用UPLC-MS测定盐肤木果油中漆酚的方法。The technical problem to be solved by the present invention is that there is no method for measuring urushiol in the fruit of A. chinensis in the prior art, and a method for measuring urushiol in the fruit of A. chinensis by UPLC-MS is provided.
本发明通过以下技术手段实现解决上述技术问题的:The present invention realizes and solves the above-mentioned technical problems through the following technical means:
一种采用UPLC-MS测定盐肤木果油中漆酚的方法,包括以下步骤:A kind of method that adopts UPLC-MS to measure urushiol in Fructus chinensis oil, comprising the following steps:
(1)盐肤木果油样品预处理;(1) sample pretreatment of pomegranate oil sample;
(2)采用UPLC-MS对步骤(1)中预处理后的样品进行测定:(2) UPLC-MS is used to measure the pretreated sample in step (1):
超高效液相色谱(UPLC)洗脱条件为:采用C18超高效液相色谱柱 (1.8μm,2.1×50mm)、流动相A为0.1%甲酸水溶液,流动相B为甲醇,洗脱梯度设置为:0~10min,A:B=90%:10%、10~15min,A:B=80%:20%、 15~25min,A:B=20%:80%、25~30min,A:B=10%:90%、30~35min,A:B=0%:100%,流速为0.25mL/min,柱温为30℃,检测波长为225nm;The ultra-high performance liquid chromatography (UPLC) elution conditions were as follows: a C18 ultra-high performance liquid chromatography column (1.8 μm, 2.1 × 50 mm) was used, the mobile phase A was 0.1% formic acid aqueous solution, the mobile phase B was methanol, and the elution gradient was set to : 0~10min, A:B=90%:10%, 10~15min, A:B=80%:20%, 15~25min, A:B=20%:80%, 25~30min, A:B =10%:90%, 30~35min, A:B=0%:100%, flow rate is 0.25mL/min, column temperature is 30℃, detection wavelength is 225nm;
质谱(MS)条件为:电子电离(EI),负离子模式,一级质谱电子电离毛细管出口电压为175V,二级质谱碰撞能为40eV。Mass spectrometry (MS) conditions were: electron ionization (EI), negative ion mode, 175V electron ionization capillary outlet voltage for primary mass spectrometry, and 40 eV collision energy for secondary mass spectrometry.
有益效果:本发明的检测方法可以检测出不同盐肤木样品中的饱和漆酚,检测盐肤木果油中微量的饱和漆酚,其可检测含量在ug/L级。Beneficial effects: the detection method of the present invention can detect the saturated urushiol in different samples of S. serrata, and detect the trace amount of saturated urushiol in S. serrata fruit oil, and its detectable content is at the ug/L level.
本发明的检测方法也可以检测不同盐肤木样品中的不饱和漆酚,尤其在无不饱和漆酚对照的情况下(其他方法均无法定量分析),依然能检测出微量不饱和漆酚含量,其可检测含量在ug/L级。The detection method of the present invention can also detect the unsaturated urushiol in the different samples of urushiol, especially in the case of no unsaturated urushiol control (all other methods cannot be quantitatively analyzed), still can detect the content of trace unsaturated urushiol, Its detectable content is at ug/L level.
本发明对超高效液相色谱、质谱条件进行优化,能够将盐肤木果中的微量漆酚进行分离,减少杂质信号。The invention optimizes the conditions of ultra-high performance liquid chromatography and mass spectrometry, and can separate the trace amount of urushiol in S.
优选的,所述步骤(2)中的进样量为2μL。Preferably, the injection volume in the step (2) is 2 μL.
优选的,所述步骤(2)质谱测定条件中分子分离器的雾化器喷雾压力为35PSI,干燥气流速10L/min,干燥气温度320℃,鞘气温度280℃,鞘气流速11L/min。Preferably, in the mass spectrometry conditions of the step (2), the atomizer spray pressure of the molecular separator is 35PSI, the drying gas flow rate is 10L/min, the drying gas temperature is 320°C, the sheath gas temperature is 280°C, and the sheath gas flow rate is 11L/min .
优选的,配制梯度浓度的饱和漆酚标准溶液,以质荷比为122.03的离子质谱峰测定离子流强度,以饱和漆酚标准溶液浓度为横坐标,以离子流强度为纵坐标,绘制标准曲线,将盐肤木果油样品在不同质荷比条件下的测定结果带入标准曲线,获得盐肤木果油中的饱和漆酚含量或不饱和漆酚含量。Preferably, a saturated urushiol standard solution with gradient concentration is prepared, and the ion current intensity is measured with the ion mass spectrum peak with a mass-to-charge ratio of 122.03, the concentration of the saturated urushiol standard solution is taken as the abscissa, and the ion current intensity is taken as the ordinate, and a standard curve is drawn , and the determination results of the A. chinensis oil samples under the conditions of different mass-to-charge ratios are brought into the standard curve to obtain the saturated urushiol content or the unsaturated urushiol content in the A. chinensis oil.
优选的,选择质荷比为122.03离子进行检测,测定盐肤木果油中的饱和漆酚含量。Preferably, ions with a mass-to-charge ratio of 122.03 are selected for detection, and the content of saturated urushiol in the oil is determined.
优选的,选择一级质谱中质荷比为319.2的二级质谱质荷比为122.03 的离子质谱峰测定离子流强度作为测定饱和漆酚的含量,用其一级质谱中质荷比为317.2、315.2、313.2、331.2、329.2、327.2和325.2各自的二级质谱质中荷比为122.03的离子质谱峰测定离子流强度作为测定不饱和漆酚的含量。Preferably, the mass-to-charge ratio of the mass-to-charge ratio in the primary mass spectrometer is 319.2, and the ion mass spectrum peak with a mass-to-charge ratio of 122.03 is selected to measure the ion current intensity as the content of the saturated urushiol, and the mass-to-charge ratio of the primary mass spectrometer is 317.2, 315.2, 313.2, 331.2, 329.2, 327.2 and 325.2 were measured by the ion current intensity of the ion mass spectrum peak of each secondary mass spectrum with a mass-to-charge ratio of 122.03 as the determination of the content of unsaturated urushiol.
有益效果:饱和漆酚标准品中的漆酚为分子式C21H36O2,其分子中R 为C15的饱和烃链,再以一级质谱中质荷比为319.2离子流中二级质谱的质荷比为122.037的分子碎片的离子流强度作为定量分析指标,以标准曲线法对样品中饱和漆酚进行定量测定,通过一级质谱对7中不同结构的不饱和漆酚在盐肤木果油中的含量进行不饱和漆酚含量的测定。Beneficial effects: the urushiol in the saturated urushiol standard product is of the molecular formula C21 H36 O2 , and R in the molecule is a saturated hydrocarbon chain of C15 . The ion current intensity of molecular fragments with a mass-to-charge ratio of 122.037 was used as a quantitative analysis index, and the saturated urushiol in the sample was quantitatively determined by the standard curve method. The content of the fruit oil was determined for the unsaturated urushiol content.
优选的,所述盐肤木果油样品预处理包括以下步骤:Preferably, the pretreatment of the oil sample of P. alba comprises the following steps:
(1)提取盐肤木果油;(1) Extraction of pomegranate oil;
(2)往步骤(1)提取的果油中加入甲醇萃取,2-6℃静置过夜后,取上清液,采用滤膜过滤,制得的滤液即为预处理后的样品。(2) Methanol is added to the fruit oil extracted in step (1) for extraction, and after standing at 2-6° C. overnight, the supernatant is taken and filtered through a membrane, and the obtained filtrate is the pretreated sample.
有益效果:盐肤木油脂粘稠度高,成分复杂,如果直接进样,一是油脂的成分出柱较慢,易残留在色谱柱中而洗脱困难;二是油脂成分在这里是杂质信号——噪音,多酚类成分信号相对较弱,很难达到分析所需的信噪比(S/N)≧3的要求,因此,对盐肤木油脂样品进行预处理时,取一定体积的油脂,加入甲醇(质谱纯)萃取其多酚类物质,进而测定甲醇萃取液中多酚。Beneficial effects: The oil and fat of S. chinensis have high viscosity and complex components. If the sample is injected directly, first, the oil components are slow to exit the column, and are easy to remain in the chromatographic column and elution is difficult; the second is that the oil components are impurity signals here. ——Noise, the signal of polyphenols is relatively weak, and it is difficult to meet the requirement of signal-to-noise ratio (S/N)≧3 required for analysis. Add methanol (mass spectrometry pure) to extract the polyphenols, and then determine the polyphenols in the methanol extract.
优选的,所述盐肤木果油的提取方法包括以下步骤:将盐肤木果油焙炒后,压榨制得果油。Preferably, the method for extracting the Fructus chinensis oil comprises the following steps: after roasting the Fructus chinensis oil, pressing to obtain the fruit oil.
优选的,所述盐肤木果油的提取方法包括以下步骤:采用无水乙醚提取盐肤木果油。Preferably, the method for extracting the Fructus alba oil comprises the following steps: using anhydrous diethyl ether to extract the Fructus Alba fruit oil.
优选的,所述果油与甲醇的体积比为1:1。Preferably, the volume ratio of the fruit oil to methanol is 1:1.
优选的,采用0.22μm的微孔滤膜过滤。Preferably, a 0.22 μm microporous membrane is used for filtration.
本发明的优点在于:The advantages of the present invention are:
(1)本发明的检测方法可以检测出不同盐肤木样品中的饱和漆酚,检测盐肤木果油中微量的饱和漆酚含量,能够测定含量在ug/L的极低含量;(1) the detection method of the present invention can detect the saturated urushiol in the different samples of S. serrata, detect the saturated urushiol content of a trace in S. serrata fruit oil, and can measure the very low content of ug/L;
(2)本发明的检测方法也可以检测不同盐肤木样品中的不饱和漆酚,尤其在无不饱和漆酚对照的情况下(其他方法均无法定量分析),依然能检测出微量不饱和漆酚含量,其可检测含量在ug/L级。(2) the detection method of the present invention can also detect the unsaturated urushiol in the different urushiol samples, especially in the case of no unsaturated urushiol control (all other methods can not be quantitatively analyzed), still can detect the trace amount of unsaturated lacquer Phenol content, its detectable content is at ug/L level.
(3)本发明对超高效液相色谱、质谱条件进行优化,能够将盐肤木果中的微量漆酚进行分离、分析,减少杂质信号的干扰。(3) The present invention optimizes the conditions of ultra-high performance liquid chromatography and mass spectrometry, and can separate and analyze the trace amount of urushiol in the fruit of A. chinensis, thereby reducing the interference of impurity signals.
附图说明Description of drawings
图1为本发明背景技术中漆酚的结构简图;Fig. 1 is the structural sketch of urushiol in the background technology of the present invention;
图2为本发明实施例5中五种多酚类标准品混标的总离子峰图谱;Fig. 2 is the total ion peak spectrum of the mixed standard of five kinds of polyphenols in the embodiment of the
图3为本发明实施例5中五种多酚类标准品混标的二级质谱图;Fig. 3 is the secondary mass spectrogram of the mixed standard of five kinds of polyphenols in the embodiment of the
图4为本发明实施例6中压榨盐肤木果油的部分离子峰和二级质谱图谱;Fig. 4 is the partial ion peak and the secondary mass spectrum of the pressed Syringa oleifera oil in the embodiment of the
图5为本发明实施例6中乙醚浸提盐肤木果油的部分离子峰和二级质谱图谱;Fig. 5 is the partial ion peak and secondary mass spectrum of the diethyl ether leaching of Prunus chinensis oil in the embodiment of the
图6为本发明实施例7中饱和漆酚的标准曲线;Fig. 6 is the standard curve of saturated urushiol in the embodiment of the
图7为本发明对比例1中0.1mg/mL的饱和漆酚标准品谱图;Fig. 7 is the saturated urushiol standard product spectrum of 0.1mg/mL in comparative example 1 of the present invention;
图8为本发明对比例1中0.2mg/mL的饱和漆酚标准品谱图;Fig. 8 is the 0.2mg/mL saturated urushiol standard product spectrum in Comparative Example 1 of the present invention;
图9为本发明对比例1中0.3mg/mL的饱和漆酚标准品谱图;Fig. 9 is the saturated urushiol standard product spectrogram of 0.3mg/mL in comparative example 1 of the present invention;
图10为本发明对比例1中压榨盐肤木果油的色谱图;Fig. 10 is the chromatogram of squeezing Fructus chinensis oil in Comparative Example 1 of the present invention;
图11为本发明对比例1中压榨盐肤木果油+饱和漆酚标准品的色谱图;Fig. 11 is the chromatogram of squeezing Fructus chinensis oil+saturated urushiol standard substance in Comparative Example 1 of the present invention;
图12为本发明对比例1中乙醚浸提盐肤木果油的色谱图;Fig. 12 is the chromatogram of the diethyl ether leaching of Prunus chinensis oil in Comparative Example 1 of the present invention;
图13为本发明对比例1中乙醚浸提盐肤木果油+饱和漆酚标准品的色谱图;Fig. 13 is the chromatogram of diethyl ether leaching of Fructus chinensis oil+saturated urushiol standard substance in Comparative Example 1 of the present invention;
图14为本发明对比例2中不同电子电离电压下的谱图;Figure 14 is the spectrogram under different electron ionization voltages in Comparative Example 2 of the present invention;
图15为本发明对比例2中不同碰撞能下的二级质谱图。FIG. 15 is the second mass spectrum under different collision energies in Comparative Example 2 of the present invention.
具体实施方式Detailed ways
为使本发明实施例的目的、技术方案和优点更加清楚,下面将结合本发明实施例,对本发明实施例中的技术方案进行清楚、完整地描述,显然,所描述的实施例是本发明一部分实施例,而不是全部的实施例。基于本发明中的实施例,本领域普通技术人员在没有作出创造性劳动前提下所获得的所有其他实施例,都属于本发明保护的范围。In order to make the purposes, technical solutions and advantages of the embodiments of the present invention clearer, the technical solutions in the embodiments of the present invention will be clearly and completely described below in conjunction with the embodiments of the present invention. Obviously, the described embodiments are part of the present invention. examples, but not all examples. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art without creative efforts shall fall within the protection scope of the present invention.
下述实施例中所用的试验材料和试剂等,如无特殊说明,均可从商业途径获得。The test materials and reagents used in the following examples can be obtained from commercial sources unless otherwise specified.
实施例中未注明具体技术或条件者,均可以按照本领域内的文献所描述的技术或条件或者按照产品说明书进行。If the specific technology or condition is not indicated in the embodiment, it can be carried out according to the technology or condition described in the literature in this field or according to the product specification.
饱和漆酚标准品:西安生漆研究所按照饱和漆酚的制备方法(GB/T 14703-2008)制备的饱和漆酚标准品;Saturated urushiol standard product: Saturated urushiol standard product prepared by Xi'an Raw Lacquer Research Institute according to the preparation method of saturated urushiol (GB/T 14703-2008);
甲醇(质谱纯)、甲酸(质谱纯)、超纯水、无水乙醇(分析纯);Methanol (mass spectrometry pure), formic acid (mass spectrometry pure), ultrapure water, absolute ethanol (analytical purity);
液-质联用仪(Agilent 1290II/6545Q-TOF)。Liquid-mass spectrometer (Agilent 1290II/6545Q-TOF).
实施例1Example 1
饱和漆酚标准溶液的配制Preparation of saturated urushiol standard solution
取2mL离心管,用甲醇清洗三次,晾干备用,精确称取饱和漆酚1.0mg,加入1mL甲醇,摇匀,用0.22μm的微孔滤膜过滤,得1mg/mL饱和漆酚标准溶液,再用甲醇稀释成所需浓度,供液相色谱-质谱分析用。Take a 2 mL centrifuge tube, wash it three times with methanol, and dry it for later use. Accurately weigh 1.0 mg of saturated urushiol, add 1 mL of methanol, shake well, and filter with a 0.22 μm microporous membrane to obtain a 1 mg/mL saturated urushiol standard solution. Diluted with methanol to the desired concentration for liquid chromatography-mass spectrometry analysis.
实施例2Example 2
盐肤木样液的制备Preparation of salvia miltiorrhiza solution
压榨盐肤木果油:盐肤木果实经焙炒(200℃,10-15min)后,小型榨油机压榨出的毛油,桶装冰柜内保存,样品制备前回温(加热升温热熔),吸取该油脂1.0mL,于5mL离心管中,加甲醇1.0mL,充分摇匀,萃取其中多酚类物质,4℃静置过夜,取上清液(甲醇层),用0.22μm的微孔滤膜过滤,得1#样液。Squeezing of S. chinensis fruit oil: After roasting (200℃, 10-15min) of S. japonicus fruit, the crude oil squeezed by a small oil press is stored in a barrel freezer, and the temperature is returned before sample preparation (heating, heating and melting) , suck 1.0 mL of the oil, add 1.0 mL of methanol to a 5 mL centrifuge tube, shake well, extract the polyphenols, let stand at 4 °C overnight, take the supernatant (methanol layer), use a 0.22 μm micropore Filter through membrane to get 1# sample solution.
实施例3Example 3
盐肤木样液的制备Preparation of salvia miltiorrhiza solution
乙醚萃取盐肤木果油:取用无水乙醚索氏抽提法(方法参照GB/T 5535.1-2008动植物油脂不皂化物测定第1部分:乙醚提取快速法)提取的盐肤木果油1.0mL,于5mL离心管中,加甲醇1.0mL,充分摇匀,萃取其中多酚类物质,4℃静置过夜,取上清液(甲醇层),用0.22μm的微孔滤膜过滤,得2#样液。Diethyl ether extraction of Ceratophyllum pratense oil: take the anhydrous diethyl ether Soxhlet extraction method (method refer to GB/T 5535.1-2008 Determination of Unsaponifiable Matter of Animal and Vegetable Oils and Fats Part 1: Diethyl ether extraction rapid method). 1.0mL, in a 5mL centrifuge tube, add 1.0mL methanol, shake well, extract the polyphenols, let stand at 4°C overnight, take the supernatant (methanol layer), filter with a 0.22μm microporous membrane, 2# sample solution was obtained.
实施例4Example 4
超高效液相色谱-质谱(UPLC-MS)联用分析的条件Conditions for Analysis by Ultra Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS)
(1)超高效液相色谱(UPLC)洗脱条件:Agilent Eclipse Plus C18超高效液相色谱柱(1.8μm,2.1×50mm)、流动相(A为0.1%甲酸水,B为甲醇),洗脱梯度设置为:0~10min,A:B=90%:10%、10~15min, A:B=80%:20%、15~25min,A:B=20%:80%、25~30min,A:B=10%:90%、 30~35min,A:B=0%:100%,流速0.25mL/min,柱温30℃,检测波长225nm,进样量2μL。(1) UPLC elution conditions: Agilent Eclipse Plus C18 UPLC column (1.8 μm, 2.1×50 mm), mobile phase (A is 0.1% formic acid water, B is methanol), wash Degradation settings are: 0~10min, A:B=90%:10%, 10~15min, A:B=80%:20%, 15~25min, A:B=20%:80%, 25~30min , A:B=10%:90%, 30~35min, A:B=0%:100%, flow rate 0.25mL/min,
(2)质谱(MS)条件分别是:电子电离(EI),负离子模式,电子电离电压175V(一级质谱),碰撞能40(二级质谱),分子分离器的雾化器喷雾压力为35PSI,干燥气流速10L/min,干燥气温度320℃,鞘气温度280℃,鞘气流速11L/min。(2) Mass spectrometry (MS) conditions are: electron ionization (EI), negative ion mode, electron ionization voltage 175V (first-order mass spectrometry), collision energy 40 (second-order mass spectrometry), and the atomizer spray pressure of the molecular separator is 35PSI , the drying gas flow rate is 10L/min, the drying gas temperature is 320°C, the sheath gas temperature is 280°C, and the sheath gas flow rate is 11L/min.
实施例5Example 5
对多酚类物质:没食子酸、儿茶素、绿原酸、咖啡酸及饱和漆酚标准品的混合样采用实施例5中的测定条件进行定性测定The polyphenols: gallic acid, catechin, chlorogenic acid, caffeic acid and the mixed sample of saturated urushiol standard substance were qualitatively determined using the measurement conditions in Example 5
测定结果:The measurement results:
如图2和图3所示,图2为五种多酚类标准品混标的总离子峰图谱,可以看出,其中最右侧一个峰,即保留时间为26.365min的离子峰即是饱和漆酚。As shown in Figure 2 and Figure 3, Figure 2 is the total ion peak spectrum of the mixed standard of five polyphenols standards. It can be seen that the rightmost peak, that is, the ion peak with a retention time of 26.365min, is saturated paint phenol.
图3为五种多酚类标准品混标二级质谱图,在漆酚标准品的制备过程中,侧链R中的不饱和键均在兰尼镍催化下加H成饱和键,故各种原侧链中含双键的一烯漆酚、二烯漆酚等不饱和漆酚,均成为饱和漆酚,从图2 和图3可知,饱和漆酚标准品经UPLC-MS,负离子模式下(即[M-H]-) 准分子离子的质荷比为319.2,即R为C15的饱和漆酚(总分子量320),其二级质谱峰质荷比为122.0377的碎片,其结构应为:Figure 3 is the MS spectrum of the mixed standard of five polyphenols. During the preparation of the urushiol standard, the unsaturated bonds in the side chain R were all catalyzed by Raney nickel to add H into saturated bonds, so each Unsaturated urushiols such as mono-urushiol and diene-urushiol containing double bonds in the side chain of the species are all saturated urushiols. It can be seen from Figure 2 and Figure 3 that the saturated urushiol standard product was tested by UPLC-MS in negative ion mode. The mass-to-charge ratio of the lower (ie [MH]- ) quasi-molecular ion is 319.2, that is, R is the saturated urushiol of C15 (total molecular weight 320), and its mass-to-charge ratio of the second mass spectrum peak is 122.0377 fragments, its structure should be :
实施例6Example 6
对实施例2-实施例4中盐肤木样液中饱和漆酚进行定性测定Qualitative determination is carried out to saturated urushiol in the sample solution of S.
测定结果:从图4、图5可以看出,盐肤木四种样品中,均有m/z为 122.03的分子碎片的离子峰,因此,可以确定盐肤木果油3种样品中均有饱和漆酚成分,只是盐肤木果油的提取方法不同,其中的漆酚含量不同。Measurement result: As can be seen from Figure 4 and Figure 5, in the four kinds of samples of Pseudomonas serrata, there are ion peaks of molecular fragments with m/z of 122.03. Therefore, it can be determined that there are 3 kinds of samples of Pseudomonas serrata oil. The content of saturated urushiol is different, but the extraction method of the oil is different, and the content of urushiol is different.
实施例7Example 7
对实施例2-实施例4中盐肤木样液中饱和漆酚进行定量测定Quantitative determination of saturated urushiol in Example 2-Example 4
将配制好的梯度浓度的漆酚标准品,用m/z为122.03的漆酚碎片峰面积(响应值)进行定量,通过饱和漆酚标准品的梯度浓度与峰面积(响应值)之间的关系,以浓度(ng/mL)为横坐标,以离子流强度(信号值)为纵坐标,绘制标准曲线,进而依其回归方程计算出1#-3#样品中饱和漆酚的含量。The prepared urushiol standard with gradient concentration was quantified with the peak area (response value) of urushiol fragments with m/z of 122.03, through the difference between the gradient concentration of saturated urushiol standard and the peak area (response value). Taking the concentration (ng/mL) as the abscissa and the ion current intensity (signal value) as the ordinate, draw a standard curve, and then calculate the content of saturated urushiol in the 1# -3# sample according to its regression equation.
测定结果:表1为饱和漆酚标准品浓度及峰面积,根据表1结果绘制的标准曲线如图6所示,其回归方程为:Y=5029.8X-44961;R2=0.9963。Measurement results: Table 1 shows the concentration and peak area of the saturated urushiol standard. The standard curve drawn according to the results in Table 1 is shown in Figure 6, and its regression equation is: Y=5029.8X-44961; R2 =0.9963.
将盐肤木果油等四种样品进行质谱分析,其中含饱和漆酚的同种离子的离子流强度(信号值),代入标准曲线的回归方程,可分别计算出样品中饱和漆酚的浓度,实施例2-实施例4样品中饱和漆酚含量测定结果如表2 所示。Mass spectrometry analysis was carried out on four kinds of samples, such as A. chinensis oil, and the ion current intensity (signal value) of the same ion containing saturated urushiol was substituted into the regression equation of the standard curve, and the concentration of saturated urushiol in the samples could be calculated respectively. , the measurement results of the saturated urushiol content in the samples of Example 2-Example 4 are shown in Table 2.
表1为饱和漆酚标准品浓度及峰面积Table 1 is the concentration and peak area of saturated urushiol standard
表2为3个样品中饱和漆酚含量测定结果Table 2 is the determination result of saturated urushiol content in 3 samples
从表2可以看出,用质谱分析法定量检测受试的盐肤木果油等样品中饱和漆酚的含量在27.5-42.6ng/mL,即27.5-42.6μg/L。As can be seen from Table 2, the content of saturated urushiol in the tested samples such as Pseudomonas serrata oil was quantitatively detected by mass spectrometry, and the content was 27.5-42.6 ng/mL, that is, 27.5-42.6 μg/L.
实验中对饱和漆酚标准品的UPLC-MS的一级质谱分析,该饱和漆酚在负离子模式下其离子流的质荷比为319.2,说明该饱和漆酚的分子式为 C21H36O2,式量为320,即其结构中R侧链应该为-C15H31的饱和烃链,因此,上述结果中测得的各样品中的漆酚即是该种饱和漆酚(C21H36O2)成分的含量。The mass-to-charge ratio of the ion current of the saturated urushiol in the negative ion mode is 319.2, indicating that the molecular formula of the saturated urushiol is C21 H36 O2 , the formula weight is 320, that is, the R side chain in its structure should be a saturated hydrocarbon chain of -C15 H31. Therefore, the urushiol in each sample measured in the above results is this saturated urushiol (C21 H36 O2 ) component content.
实施例8Example 8
对实施例2-实施例4中盐肤木样液中不饱和漆酚的测定Determination of Unsaturated Urushiol in the Example 2-Example 4
漆酚常见的除饱和漆酚外,尚有R侧链为C15的含一至三个双键的不饱和烃链,即-C15H29的一烯烃链、-C15H27的二烯烃链以及-C15H25的三烯烃链等,按照结构式的分子量计算,这三类不饱和漆酚的质荷比分别是:317.2、 315.2和313.2(均为负离子模式下,即-H模式下);以此类推,R侧链为 C16的含一至四个双键的不饱和烃链的不饱和漆酚的质荷比分别是:331.2、 329.2、327.2和325.2。根据这些推断的结构的质荷比,在3中样品的 UPLC-MS图谱的一级质谱的数据中,分别以各质荷比数值进行搜索,分析结果如表3和表4所示。In addition to saturated urushiol, urushiol also has unsaturated hydrocarbon chains with one to three double bonds whose R side chain is C15 , that is, a mono-olefin chain of -C15 H29 , a diolefin of -C15 H27 chain and the triolefin chain of -C15 H25 , etc., calculated according to the molecular weight of the structural formula, the mass-to-charge ratios of these three types of unsaturated urushiol are respectively: 317.2, 315.2 and 313.2 (all under the negative ion mode, namely under the -H mode) ); and so on, the mass-to-charge ratios of unsaturated urushiol with an unsaturated hydrocarbon chain containing one to four double bonds whose R side chain is C16 are: 331.2, 329.2, 327.2 and 325.2, respectively. According to the mass-to-charge ratios of these inferred structures, the data of the primary mass spectrometry of the UPLC-MS spectra of the samples in 3 were searched with each mass-to-charge ratio value, and the analysis results are shown in Table 3 and Table 4.
表3为2个样品中中不饱和漆酚的测定结果1Table 3 is the determination result of unsaturated urushiol in 2
表4为2个样品中中不饱和漆酚的测定结果2Table 4 is the
注:表中X表示离子流强度(信号值);tR表示离子流位置(即出峰时间/min)。Note: X in the table represents the ion current intensity (signal value); tR represents the ion current position (ie peak time/min).
从表3和表4可以看出,,与饱和漆酚的离子流强度(表中M/z=319.2 的一列)进行对比,M/z=317.2,(即含R=C15H29的一烯烃链)的不饱和漆酚的含量与饱和漆酚含量相近,甚至更高一些;M/z=315.2(即含R=C15H27的二烯烃链)的不饱和漆酚在2个样品中都有,只是其的含量只有饱和漆酚的1/10左右;M/z=313.2(即含R=C15H25的三烯烃链)的不饱和漆酚在3 个样品中都未检出。It can be seen from Table 3 and Table 4 that, compared with the ionic current intensity of saturated urushiol (a column of M/z=319.2 in the table), M/z=317.2, (that is, a column containing R=C15 H29 ) The content of unsaturated urushiol in the olefin chain) is similar to that of saturated urushiol, or even higher; the unsaturated urushiol with M/z=315.2 (that is, the diene chain containing R=C15 H27 ) is in 2 samples The content of urushiol is only about 1/10 of that of saturated urushiol; the unsaturated urushiol with M/z=313.2 (that is, the triolefin chain containing R=C15 H25 ) was not detected in all 3 samples out.
2个样品中一烯不饱和漆酚含量较高,二烯不饱和漆酚也都有,但含量稍低,三烯不饱和漆酚未检出。In the two samples, the content of monoethylenically unsaturated urushiol was higher, and the diene unsaturated urushiol was also present, but the content was slightly lower, and the triene unsaturated urushiol was not detected.
再比较侧链R为C16的各种不饱和漆酚,与R为C15的饱和漆酚含量对比,R中含一至四个双键的不饱和漆酚也都有,而且二烯和三烯不饱和漆酚含量较高,与饱和漆酚含量较近或更高;一稀和四烯不饱和漆酚的含量也与饱和漆酚含量相近或略低一些。Then compare the various unsaturated urushiols whose side chain R is C16. Compared with the content of saturated urushiol whose side chain R is C15 , there are also unsaturated urushiols containing one to four double bonds in R, and diene and three The content of ethylenically unsaturated urushiol is higher, which is close to or higher than that of saturated urushiol; the content of dilute and tetraene unsaturated urushiol is also similar to or slightly lower than that of saturated urushiol.
两者相较,不饱和漆酚种类较多,总含量也较饱和漆酚高,尤其是侧链R为C16的各种不饱和漆酚和侧链R为C15的一烯不饱和漆酚含量较高。Compared with the two, there are many kinds of unsaturated urushiol, and the total content is higher than that of saturated urushiol, especially the various unsaturated urushiols whose side chain R is C16 and the monoethylenic unsaturated lacquer whose side chain R is C15 . Phenol content is high.
因此,盐肤木果油及其油脂中漆酚成分是饱和漆酚和不饱和漆酚的混合物,且不饱和漆酚的种类和含量都较饱和漆酚多。所测的3个样品中总漆酚含量在165-345μg/L。Therefore, the urushiol component in the oil and its oil is a mixture of saturated urushiol and unsaturated urushiol, and the type and content of unsaturated urushiol are more than those of saturated urushiol. The total urushiol content in the three samples tested was 165-345 μg/L.
实施例9Example 9
精密度Precision
UPLC-MS检测的饱和漆酚适宜浓度为x ng/mL,即xμg/L(每升油脂中含量在10-9g)的痕量差异,即该方法的精密度可达10-9。The suitable concentration of saturated urushiol detected by UPLC-MS is x ng/mL, that is, the trace difference of xμg/L (10-9 g per liter of oil and fat), that is, the precision of this method can reach 10-9 .
对比例1Comparative Example 1
采用高效液相色谱对饱和漆酚标准品、实施例2-实施例4中盐肤木样液中饱和漆酚的测定Determination of saturated urushiol in saturated urushiol standard substance and in Example 2-Example 4 by high performance liquid chromatography
一、仪器、试剂:1. Instruments and reagents:
高效液相色谱仪(waters 1525,waters 2478紫外检测器)、超声清洗器、电子天平,饱和漆酚标准品、色谱纯乙腈、磷酸High performance liquid chromatograph (waters 1525, waters 2478 UV detector), ultrasonic cleaner, electronic balance, saturated urushiol standard, chromatographically pure acetonitrile, phosphoric acid
样品同UPLC-MS的样品处理方法。The samples were processed in the same way as UPLC-MS samples.
二、液相色谱条件:2. Liquid chromatography conditions:
ODS C18柱(5μm,4.6mm×150mm)、流动相(乙腈:0.1%磷酸水=90: 10)、流速为1mL/min、检测波长为225nm、进样量为10μL。ODS C18 column (5 μm, 4.6 mm×150 mm), mobile phase (acetonitrile: 0.1% phosphoric acid water=90:10), flow rate of 1 mL/min, detection wavelength of 225 nm, and injection volume of 10 μL.
三、液相色谱定量分析3. Quantitative Analysis by Liquid Chromatography
取0.1mg/mL、0.2mg/mL、0.3mg/mL的饱和漆酚标准品乙腈溶液,按照液相色谱分析条件进行设置和进样。Take 0.1mg/mL, 0.2mg/mL, 0.3mg/mL saturated urushiol standard acetonitrile solution, set and inject according to liquid chromatography analysis conditions.
图7-图9为0.1mg/mL、0.2mg/mL、0.3mg/mL的饱和漆酚标准品谱图,表5为漆酚的色谱图数据,可知饱和漆酚的出峰时间在36.9min左右。Figures 7-9 are the chromatograms of saturated urushiol standard products of 0.1mg/mL, 0.2mg/mL and 0.3mg/mL, and Table 5 is the chromatogram data of urushiol, it can be seen that the peak time of saturated urushiol is 36.9min about.
表5为漆酚的色谱图数据Table 5 is the chromatogram data of urushiol
同等HPLC条件下,测定2种盐肤木果油样品及各样品中分别添加漆酚标准品后的对照样品进行的进样分析,结果如图10-图15所示,由图10 可以看出,1#压榨盐肤木果油样品在饱和漆酚保留时间处没有出现色谱峰,向样品中加入饱和漆酚标准品后,在饱和漆酚保留时间处出现了色谱峰如图11。Under the same HPLC conditions, the sample injection analysis of the two samples of A. chinensis oil and the control sample after adding the urushiol standard in each sample was measured. The results are shown in Figure 10-Figure 15, and it can be seen from Figure 10 , No chromatographic peak appeared at the saturated urushiol retention time for the 1# pressed urushiol oil sample. After adding the saturated urushiol standard to the sample, a chromatographic peak appeared at the saturated urushiol retention time as shown in Figure 11.
由图12知,2#乙醚浸提盐肤木果油样品在饱和漆酚保留时间处没有出现色谱峰,当样品中加入饱和漆酚标准品后,在饱和漆酚保留时间处出现了色谱峰如图13。It is known from Figure 12 that the 2# ether leaching sample of P. chinensis oil does not have a chromatographic peak at the saturated urushiol retention time. When the saturated urushiol standard is added to the sample, a chromatographic peak appears at the saturated urushiol retention time. Figure 13.
由盐肤木2个样品的色谱图可知,2个样品在漆酚标准品的保留时间处均未出现色谱峰,在2个样品中分别加入高浓度的漆酚标准品后,再一次用液相色谱检测时,在饱和漆酚的保留时间内,均出现了一个色谱峰,检测到了饱和漆酚的存在。因此,初步判定可能是盐肤木样品中饱和漆酚的含量低,在紫外检测器225nm波长下,达不到紫外检测限,或者样品中杂质含量太高,杂质信号远大于微量饱和漆酚的信号,因此说明:HPLC紫外检测器检测的方法不适用于测定盐肤木果油中的漆酚。It can be seen from the chromatograms of the two samples of Phytophthora chinensis that no chromatographic peaks appear in the two samples at the retention time of the urushiol standard. After adding the high-concentration urushiol standard to the two samples, the liquid During phase chromatographic detection, a chromatographic peak appeared within the retention time of saturated urushiol, and the presence of saturated urushiol was detected. Therefore, the preliminary judgment may be that the content of saturated urushiol in the sample is low, and the UV detection limit cannot be reached at the wavelength of 225 nm of the UV detector, or the impurity content in the sample is too high, and the impurity signal is much larger than that of trace saturated urushiol. Therefore, it is indicated that the method of HPLC UV detector detection is not suitable for the determination of urushiol in A. chinensis oil.
对比例2Comparative Example 2
对一级质谱的电离电压高低和二级质谱的碰撞能的大小进行优化Optimize the ionization voltage of the primary mass spectrometer and the collision energy of the secondary mass spectrometer
电子电离电压的确定:在145V-175V之间取等梯度的四个值进行分析,结果如图14所示,从上至下依次为175V、165V、155V、145V四个电压的谱图,其中电压为175V对应的谱图漆酚离子流最大、出峰最好,最后选定175V。Determination of electron ionization voltage: take four values of isogradient between 145V-175V for analysis, the results are shown in Figure 14, from top to bottom are the four voltage spectra of 175V, 165V, 155V, 145V, among which The urushiol ion current of the spectrum corresponding to the voltage of 175V is the largest and the peak is the best. Finally, 175V is selected.
二级质谱碰撞能优化:175V电离漆酚后二级质谱的碰撞能优化,设置选择区间为10-40,进行二级质谱分析观察漆酚碎片离子流强度,结果如图15,由图15可知从上至下依次为10eV、20eV、30eV、40eV碰撞能谱图,其中碰撞能为40对应的谱图漆酚碎片离子流最大、出峰最好,最后选定40eV。Optimization of the collision energy of the secondary mass spectrometry: The collision energy of the secondary mass spectrometer after ionizing urushiol at 175V was optimized, and the selection interval was set to 10-40. The secondary mass spectrometry analysis was performed to observe the ion current intensity of the urushiol fragments. The results are shown in Figure 15. Figure 15 shows that From top to bottom are the 10eV, 20eV, 30eV, 40eV collision energy spectra, of which the collision energy of 40 corresponds to the spectrum of urushiol fragment ions with the largest ion flow and the best peak output, and 40eV is finally selected.
以上实施例仅用以说明本发明的技术方案,而非对其限制;尽管参照前述实施例对本发明进行了详细的说明,本领域的普通技术人员应当理解:其依然可以对前述各实施例所记载的技术方案进行修改,或者对其中部分技术特征进行等同替换;而这些修改或者替换,并不使相应技术方案的本质脱离本发明各实施例技术方案的精神和范围。The above embodiments are only used to illustrate the technical solutions of the present invention, but not to limit them; although the present invention has been described in detail with reference to the foregoing embodiments, those of ordinary skill in the art should understand that: The recorded technical solutions are modified, or some technical features thereof are equivalently replaced; and these modifications or replacements do not make the essence of the corresponding technical solutions deviate from the spirit and scope of the technical solutions of the embodiments of the present invention.
Claims (10)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911023896.4ACN110672750A (en) | 2019-10-25 | 2019-10-25 | Method for determining urushiol in rhus chinensis fruit oil by using UPLC-MS (ultra performance liquid chromatography-mass spectrometry) |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911023896.4ACN110672750A (en) | 2019-10-25 | 2019-10-25 | Method for determining urushiol in rhus chinensis fruit oil by using UPLC-MS (ultra performance liquid chromatography-mass spectrometry) |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN110672750Atrue CN110672750A (en) | 2020-01-10 |
Family
ID=69084313
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201911023896.4APendingCN110672750A (en) | 2019-10-25 | 2019-10-25 | Method for determining urushiol in rhus chinensis fruit oil by using UPLC-MS (ultra performance liquid chromatography-mass spectrometry) |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN110672750A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116271953A (en)* | 2023-03-07 | 2023-06-23 | 中国林业科学研究院林产化学工业研究所 | Green microextraction method of urushiol compound in lacquer tree |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040110847A1 (en)* | 2002-06-11 | 2004-06-10 | Pei-Lin Wu | Compounds extracted from Sap of Rhus succedanea |
| CN101297665A (en)* | 2007-04-30 | 2008-11-05 | 陈乃富 | Use of Rhus chinensis lipids as food extracted from Rhus chinensis fruit |
| CN101378765A (en)* | 2005-11-22 | 2009-03-04 | Rmi聚合体公司 | Hydrophobic elastomeric polymer chemistry device for inhibiting the growth of onychomycosis and urushiol-induced allergic contact dermatitis |
| CN102850189A (en)* | 2012-08-29 | 2013-01-02 | 中国林业科学研究院林产化学工业研究所 | Preparation method of high-purity saturated urushiol and its formal derivative |
| CN104407082A (en)* | 2014-11-27 | 2015-03-11 | 中国科学院上海药物研究所 | Method for detecting alkylphenol compounds in ginkgo leaf raw material and preparation |
| US20150284308A1 (en)* | 2012-11-21 | 2015-10-08 | Catherine F. YANG | Extraction and purification of urushiol from botanical sources |
| CN107653062A (en)* | 2017-09-01 | 2018-02-02 | 湖南中医药大学 | A kind of method of subcritical abstraction Rhus fruit grease |
- 2019
- 2019-10-25CNCN201911023896.4Apatent/CN110672750A/enactivePending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040110847A1 (en)* | 2002-06-11 | 2004-06-10 | Pei-Lin Wu | Compounds extracted from Sap of Rhus succedanea |
| CN101378765A (en)* | 2005-11-22 | 2009-03-04 | Rmi聚合体公司 | Hydrophobic elastomeric polymer chemistry device for inhibiting the growth of onychomycosis and urushiol-induced allergic contact dermatitis |
| CN101297665A (en)* | 2007-04-30 | 2008-11-05 | 陈乃富 | Use of Rhus chinensis lipids as food extracted from Rhus chinensis fruit |
| CN102850189A (en)* | 2012-08-29 | 2013-01-02 | 中国林业科学研究院林产化学工业研究所 | Preparation method of high-purity saturated urushiol and its formal derivative |
| US20150284308A1 (en)* | 2012-11-21 | 2015-10-08 | Catherine F. YANG | Extraction and purification of urushiol from botanical sources |
| CN104407082A (en)* | 2014-11-27 | 2015-03-11 | 中国科学院上海药物研究所 | Method for detecting alkylphenol compounds in ginkgo leaf raw material and preparation |
| CN107653062A (en)* | 2017-09-01 | 2018-02-02 | 湖南中医药大学 | A kind of method of subcritical abstraction Rhus fruit grease |
Non-Patent Citations (2)
| Title |
|---|
| 何源峰 等: "HPLC-MS法表征坝漆酚类化合物的结构", 《林产化学与工业》* |
| 李林 等: "生漆漆酚类化合物的HPLC-ESI-MSn 分析", 《中草药》* |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116271953A (en)* | 2023-03-07 | 2023-06-23 | 中国林业科学研究院林产化学工业研究所 | Green microextraction method of urushiol compound in lacquer tree |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Sun et al. | Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC–ELSD | |
| CN106950298B (en) | Method for simultaneously detecting mycotoxin and pesticide residue in Xinhui dried orange peel | |
| Giménez et al. | Pentacyclic triterpene in Olea europaea L: A simultaneous determination by high-performance liquid chromatography coupled to mass spectrometry | |
| CN110412183B (en) | Rose fragrance component analysis method by needle capture-gas chromatography-mass spectrometry | |
| CN106053675B (en) | A kind of analysis method of twin columns liquid chromatography tandem mass spectrometry to nitrosamine burst size in cigarette smoke | |
| Wang et al. | Simultaneous Determination of 15 Phenolic Constituents of Chinese Black Rice Wine by HPLC‐MS/MS with SPE | |
| Carretero et al. | A simplified method for HPLC‐MS analysis of sterols in vegetable oil | |
| CN106124604B (en) | A method for mass spectrometry analysis of free fatty acids in edible oils based on derivatization technology | |
| CN103983725A (en) | Quick measurement method for coumarin and safrole in essence and flavor | |
| Xu et al. | Characterization and determination of isomers in plants using trace matrix solid phase dispersion via ultrahigh performance liquid chromatography coupled with an ultraviolet detector and quadrupole time-of-flight tandem mass spectrometry | |
| Rigano et al. | Combining linear retention index and electron ionization mass spectrometry for a reliable identification in nano liquid chromatography | |
| CN105241965A (en) | Method of on-line quickly detecting total anti-oxidizing property of sample | |
| CN113267588B (en) | Method for simultaneously detecting 7 acrylic acid and acrylic ester monomers in plastic product | |
| CN110672750A (en) | Method for determining urushiol in rhus chinensis fruit oil by using UPLC-MS (ultra performance liquid chromatography-mass spectrometry) | |
| CN110632220B (en) | Method for analyzing sucrose ester in tobacco by multi-dimensional liquid chromatography-mass spectrometry | |
| Smith et al. | High-pressure liquid chromatography of cannabis: Quantitative analysis of acidic and neutral cannabinoids | |
| CN109444281B (en) | Method for detecting antioxidant components in cyclocarya paliurus leaves | |
| Duan et al. | Analysis of phenolic acids and their antioxidant activity by capillary electrophoresis-mass spectrometry with field-amplified sample injection | |
| Tong et al. | Liquid chromatographic-mass spectrometric method for detection of estrogen in commercial oils and in fruit seed oils | |
| Matos Cordeiro Borges et al. | Molecularly imprinted solid‐phase extraction coupled with LC–APCI–MS–MS for the selective determination of serum cholesterol | |
| CN104090061B (en) | The detection method of zeaxanthin list cis, two cis-isomer | |
| Niu et al. | Simultaneous analysis of eight phenolic compounds in Phyllanthus simplex Retz by HPLC-DAD-ESI/MS | |
| CN105467053B (en) | Method for rapidly detecting artificial musk residue of daily chemical products | |
| CN106290627A (en) | A kind of analysis method of nitrosamine burst size in cigarette smoke | |
| CN107228915A (en) | A kind of method for efficiently separating purification glycosyl phosphatidylinositol monoethanolamine |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication | Application publication date:20200110 |