CN110257065B - A kind of red phosphor with waterproof performance and preparation method thereof - Google Patents
A kind of red phosphor with waterproof performance and preparation method thereofDownload PDFInfo
- Publication number
- CN110257065B CN110257065BCN201910571374.1ACN201910571374ACN110257065BCN 110257065 BCN110257065 BCN 110257065BCN 201910571374 ACN201910571374 ACN 201910571374ACN 110257065 BCN110257065 BCN 110257065B
- Authority
- CN
- China
- Prior art keywords
- solution
- fluorescent powder
- kmno
- mass percentage
- percentage concentration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- OAICVXFJPJFONN-UHFFFAOYSA-NPhosphorusChemical compound[P]OAICVXFJPJFONN-UHFFFAOYSA-N0.000titledescription49
- 238000002360preparation methodMethods0.000titledescription10
- 239000000843powderSubstances0.000claimsabstractdescription36
- 239000011159matrix materialSubstances0.000claimsabstractdescription22
- 238000000034methodMethods0.000claimsabstractdescription13
- 150000002500ionsChemical class0.000claimsabstractdescription3
- 229910016285MxNyInorganic materials0.000claimsabstract18
- 239000012286potassium permanganateSubstances0.000claimsabstract6
- 239000000758substrateSubstances0.000claimsabstract2
- 239000000243solutionSubstances0.000claimsdescription36
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsdescription19
- 239000000047productSubstances0.000claimsdescription16
- 238000003756stirringMethods0.000claimsdescription13
- 238000001291vacuum dryingMethods0.000claimsdescription13
- 238000000967suction filtrationMethods0.000claimsdescription12
- 239000012153distilled waterSubstances0.000claimsdescription10
- XAEFZNCEHLXOMS-UHFFFAOYSA-Mpotassium benzoateChemical compound[K+].[O-]C(=O)C1=CC=CC=C1XAEFZNCEHLXOMS-UHFFFAOYSA-M0.000claimsdescription10
- 238000006243chemical reactionMethods0.000claimsdescription9
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000claimsdescription8
- 239000011259mixed solutionSubstances0.000claimsdescription7
- 230000007935neutral effectEffects0.000claimsdescription6
- 238000002156mixingMethods0.000claimsdescription5
- 238000005406washingMethods0.000claimsdescription5
- 239000007864aqueous solutionSubstances0.000claimsdescription4
- 239000002244precipitateSubstances0.000claimsdescription3
- 230000035484reaction timeEffects0.000claimsdescription3
- 229910052700potassiumInorganic materials0.000claimsdescription2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-MPotassium chlorideChemical compound[Cl-].[K+]WCUXLLCKKVVCTQ-UHFFFAOYSA-M0.000claims6
- 239000001103potassium chlorideSubstances0.000claims6
- 235000011164potassium chlorideNutrition0.000claims6
- 238000001035dryingMethods0.000claims2
- 239000000203mixtureSubstances0.000claims2
- BWHMMNNQKKPAPP-UHFFFAOYSA-Lpotassium carbonateChemical compound[K+].[K+].[O-]C([O-])=OBWHMMNNQKKPAPP-UHFFFAOYSA-L0.000claims2
- 229910003638H2SiF6Inorganic materials0.000claims1
- 229910003708H2TiF6Inorganic materials0.000claims1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-NPotassiumChemical compound[K]ZLMJMSJWJFRBEC-UHFFFAOYSA-N0.000claims1
- 239000003054catalystSubstances0.000claims1
- 239000011591potassiumSubstances0.000claims1
- 229910000027potassium carbonateInorganic materials0.000claims1
- FGIUAXJPYTZDNR-UHFFFAOYSA-Npotassium nitrateInorganic materials[K+].[O-][N+]([O-])=OFGIUAXJPYTZDNR-UHFFFAOYSA-N0.000claims1
- ZEFWRWWINDLIIV-UHFFFAOYSA-Ntetrafluorosilane;dihydrofluorideChemical compoundF.F.F[Si](F)(F)FZEFWRWWINDLIIV-UHFFFAOYSA-N0.000claims1
- 238000004078waterproofingMethods0.000claims1
- 230000000694effectsEffects0.000abstractdescription10
- 239000011248coating agentSubstances0.000abstractdescription6
- 238000000576coating methodMethods0.000abstractdescription6
- 239000002245particleSubstances0.000abstractdescription3
- 238000004519manufacturing processMethods0.000abstractdescription2
- 238000000295emission spectrumMethods0.000description6
- 239000002253acidSubstances0.000description5
- 230000005284excitationEffects0.000description5
- 230000007423decreaseEffects0.000description4
- 239000005871repellentSubstances0.000description4
- 230000015572biosynthetic processEffects0.000description3
- 238000007654immersionMethods0.000description3
- 238000004020luminiscence typeMethods0.000description3
- 239000000376reactantSubstances0.000description3
- 238000009877renderingMethods0.000description3
- 239000012266salt solutionSubstances0.000description3
- 239000000725suspensionSubstances0.000description3
- 238000003786synthesis reactionMethods0.000description3
- 230000002238attenuated effectEffects0.000description2
- 230000003247decreasing effectEffects0.000description2
- 238000000695excitation spectrumMethods0.000description2
- 229910052761rare earth metalInorganic materials0.000description2
- 150000002910rare earth metalsChemical class0.000description2
- 229910019655synthetic inorganic crystalline materialInorganic materials0.000description2
- 238000003828vacuum filtrationMethods0.000description2
- 230000009286beneficial effectEffects0.000description1
- 230000015556catabolic processEffects0.000description1
- 229910019990cerium-doped yttrium aluminum garnetInorganic materials0.000description1
- 230000009849deactivationEffects0.000description1
- 238000006731degradation reactionMethods0.000description1
- 150000002222fluorine compoundsChemical class0.000description1
- 150000004820halidesChemical class0.000description1
- 238000003837high-temperature calcinationMethods0.000description1
- 150000004767nitridesChemical class0.000description1
- 238000002161passivationMethods0.000description1
- 230000000171quenching effectEffects0.000description1
- 230000009103reabsorptionEffects0.000description1
- 238000001228spectrumMethods0.000description1
- 230000000007visual effectEffects0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/66—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing germanium, tin or lead
- C09K11/664—Halogenides
- C09K11/665—Halogenides with alkali or alkaline earth metals
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/67—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing refractory metals
- C09K11/674—Halogenides
- C09K11/675—Halogenides with alkali or alkaline earth metals
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/67—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing refractory metals
- C09K11/676—Aluminates; Silicates
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/67—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing refractory metals
- C09K11/677—Germanates
Landscapes
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Luminescent Compositions (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及发光技术领域,具体涉及一种自带防水性能的红色荧光粉及其制备方法。The invention relates to the technical field of light-emitting, in particular to a red fluorescent powder with self-contained waterproof performance and a preparation method thereof.
背景技术Background technique
白光二极管(LED)的发展对节约全球能源具有重要的意义,因为其具有寿命长、能量转化效率高、稳定性良好、体积小、重量轻、结构紧凑等优点而被广泛应用于显示和照明领域。目前,最多商用WLED是通过将InGaN蓝色芯片和 YAG:Ce3+黄色荧光粉结合制成,GaN发出的蓝色光激发了YAG而得到黄色光,未被吸收的蓝光和黄光复合得到白光,因此在蓝光或紫光激发下发黄光的YAG 荧光粉是目前使用量最大的一类荧光粉,但由于YAG:Ce3+发射光谱中红色成分不足,导致WLED显色指数较低,相关色温较高,不能满足一般要求照明。实现高性能的具有高显色性和可调色温的大功率WLED,还需要红色荧光粉来补偿红色。现在,红色的荧光粉主要是稀土掺杂氮化物荧光粉,如Ca(Sr、 Ba)2Si5N8:Eu2+、CaAlSiN3:Eu2+等,但它们的合成条件苛刻会导致高成本,发生重吸收会导致发光效率低。此外,某些荧光粉明显超出了人眼的敏感范围,使 WLED的发光效率难以提高。因此,具有优良的性能的新型窄型红色荧光粉在简单或温和的合成条件下,发光性能被不断追求。The development of white light diodes (LEDs) is of great significance for saving global energy, and they are widely used in display and lighting fields because of their long life, high energy conversion efficiency, good stability, small size, light weight, and compact structure. . At present, most commercial WLEDs are made by combining InGaN blue chips and YAG:Ce3+ yellow phosphors. The blue light emitted by GaN excites YAG to obtain yellow light, and the unabsorbed blue light and yellow light are combined to obtain white light. Therefore, YAG phosphors thatemit yellow light under the excitation of blue light or violet light are the most widely used phosphors at present. High, can not meet the general requirements of lighting. Achieving high-performance high-power WLEDs with high color rendering and tunable color temperature also requires red phosphors to compensate for red. Now, red phosphors are mainly rare earth doped nitride phosphors, such as Ca(Sr, Ba)2 Si5 N8 :Eu2+ , CaAlSiN3 :Eu2+ , etc., but their harsh synthesis conditions will lead to high cost, the occurrence of reabsorption can lead to low luminous efficiency. In addition, some phosphors are obviously beyond the sensitive range of the human eye, making it difficult to improve the luminous efficiency of WLEDs. Therefore, new narrow-type red phosphors with excellent properties have been continuously pursued for luminescent properties under simple or mild synthesis conditions.
近年来,Mn4+掺杂氟化合物,如A2XF6:Mn4+(A=NH4,K,Na,Rb,Cs;X=Si, Ge,Sn,Ti,Zr)作为新型非稀土红色荧光粉出现,与氮化红色荧光粉相比,系列A2XF6:Mn4+红色荧光粉具有以下优点:首先,它们表现出高效的窄带红发射,大部分红发射小于650nm,这保证了更好的颜色纯度和视觉比色参数;其次,它们表现出很高的量子效率;第三,它们有非常好的热淬火性能,热稳定性高;最后是合成条件相对简单,这使得其成本低廉。但是荧光粉包括卤化物和碱土元素(如Cl-、F-、Na+、K+等)在内均容易受潮,使得它们的发光性能降低,虽有研究者提出对荧光粉表面进行涂层保护或者使用表面包膜方案,避免荧光粉直接与水接触,从而提高荧光粉抗水性能,但是这种方法使得荧光粉耐候性差,在高温或紫外线环境下性能衰减很快。也有研究表明通过H2O2或者H2C2O4等进行钝化处理构建失活层作为隔离膜,这始终不能解决根本性的问题,在一些潮湿的环境中照明还是需要荧光粉自身具有防水性的功能。In recent years, Mn4+ doped fluorine compounds, such as A2 XF6 : Mn4+ (A=NH4, K, Na, Rb, Cs; X=Si, Ge, Sn, Ti, Zr), have emerged as new types of non-rare earth reds The emergence of phosphors, compared with nitrided red phosphors, series A2 XF6 :Mn4+ red phosphors have the following advantages: First, they exhibit efficient narrow-band red emission, most of which is less than 650 nm, which guarantees better color purity and visual colorimetric parameters; secondly, they exhibit high quantum efficiency; thirdly, they have very good thermal quenching properties and high thermal stability; finally, the synthesis conditions are relatively simple, which makes their cost low. However, phosphors, including halides and alkaline earth elements (such as Cl- , F- , Na+ , K+ , etc.), are susceptible to moisture, which reduces their luminescent properties. Although some researchers propose to protect the surface of phosphors by coating Or use a surface coating solution to avoid direct contact of the phosphor with water, thereby improving the water resistance of the phosphor, but this method makes the phosphor poor weather resistance, and its performance decays rapidly in high temperature or ultraviolet environments. Some studies have also shown that passivation of H2 O2 or H2 C2 O4 is used to build a deactivation layer as a separator, which can never solve the fundamental problem. In some humid environments, the phosphor itself still needs to have Waterproof function.
发明内容SUMMARY OF THE INVENTION
本发明目的在于提供一种自带防水性能的红色荧光粉。The purpose of the present invention is to provide a red phosphor with self-contained waterproof performance.
本发明还有另一目的在于提供上述一种自带防水性能的红色荧光粉的制备方法。Another object of the present invention is to provide the above-mentioned preparation method of the red phosphor with self-contained waterproof performance.
本发明通过如下技术方案实现:The present invention is achieved through the following technical solutions:
一种自带防水性能的红色荧光粉,其特征在于:所述自带防水性能的红色荧光粉的通式为:K2MxNyF6:zMn4+,其中K2MxNyF6作为其基质,所述基质中MN 为Si、Ge、Ti离子中任意两种组合,其中x、y、z分别为M、N和Mn4+的摩尔分数,0<x<1、0<y<1,且x+y=1,0<z≦0.2。A red phosphor with self-contained waterproof performance, characterized in that: the general formula of the red phosphor with self-contained waterproof performance is: K2 Mx Ny F6 : zMn4+ , wherein K2 Mx Ny F6 as its matrix, MN in the matrix is any combination of Si, Ge, Ti ions, wherein x, y, z are the mole fractions of M, N and Mn4+ , 0<x<1, 0 <y<1, and x+y=1, 0<z≦0.2.
更优选地,上述自带防水性能的红色荧光粉通式为K2M0.3N0.7F6:0.1Mn4+。More preferably, the general formula of the above-mentioned red phosphor with waterproof performance is K2 M0.3 N0.7 F6 : 0.1Mn4+ .
上述K2MxNyF6作为基质在现有技术文献中没有报道过。发明人在研究中开发出了上述基质,并且掺杂Mn4+后得到的红色荧光粉K2MxNyF6:zMn4+具有发光效率高,显色效果好等特点,而且其本身自带非常好的防水效果,且防水稳定性高,可长期使用在潮湿环境中。The aforementioned K2 Mx Ny F6 as a matrix has not been reported in the prior art literature. The inventor has developed the above-mentioned matrix in the research, and the red phosphor K2 Mx Ny F6 : zMn4+ obtained after doping with Mn4+ has the characteristics of high luminous efficiency and good color rendering effect, and its own It has a very good waterproof effect and high waterproof stability, which can be used in a humid environment for a long time.
进一步,一种自带防水性能的红色荧光粉,其特征在于:所述荧光粉以 K2MxNyF6为基质,和KMnO4混合后与HF溶液反应制得;所述K2MxNyF6基质是由可溶性钾盐与H2AF6按照摩尔比为1:2~15配比反应制得,其中H2AF6溶液为H2SiF6、H2GeF6、H2TiF6溶液中两种混合的溶液,可溶性钾盐可以为KCl、 K2CO3、KNO3或者KOH其中之一。Further, a red phosphor with self-contained waterproof performance is characterized in that: the phosphor takes K2 Mx Ny F6 as a matrix, mixed with KMnO4 and then reacted with HF solution to prepare; the K2 Mx Ny F6 matrix is prepared by reacting soluble potassium salt and H2 AF6 according to the molar ratio of 1:2~15, wherein the H2 AF6 solution is H2 SiF6 , H2 GeF6 , H2 For the two mixed solutions in the TiF6 solution, the soluble potassium salt can be one of KCl, K2 CO3 , KNO3 or KOH.
更进一步,上述荧光粉是在常温常压下,以K2MxNyF6为基质,与KMnO4混合后与HF溶液按照K2MxNyF6:KMnO4:HF=1:0.1~0.5:1~300的摩尔比进行反应10~20h后,真空抽滤,无水乙醇清洗,再用80℃的真空干燥箱真空干燥8~ 10h,得到K2MxNyF6:zMn4+荧光粉。Further, the above phosphor powder is at normal temperature and pressure, with K2 Mx Ny F6 as the matrix, mixed with KMnO4 and then mixed with HF solution according to K2 Mx Ny F6 : KMnO4 : HF=1: After reacting with a molar ratio of 0.1-0.5:1-300 for 10-20 hours, vacuum filtration, washing with absolute ethanol, and then vacuum drying in a vacuum drying oven at 80°C for 8-10 hours to obtain K2 Mx Ny F6 : zMn4+ phosphor.
一种自带防水性能的红色荧光粉的制备方法,其特征在于:所述荧光粉是在常温常压下,由可溶性钾盐与H2AF6按照摩尔比为1:2~15配比反应制得 K2MxNyF6,其中H2AF6为H2SiF6、H2GeF6、H2TiF6溶液中任意两种混合的溶液;常温常压下,再以K2MxNyF6为基质,与KMnO4混合后与HF溶液按照K2MxNyF6: KMnO4:HF=1:0.1~0.5:1~300的摩尔比进行反应10~20h后,真空抽滤,无水乙醇清洗1~3次,再用80℃的真空干燥箱真空干燥8~10h,得到K2MxNyF6:zMn4+荧光粉。A method for preparing red fluorescent powder with self-contained waterproof performance, characterized in that: the fluorescent powder is reacted by soluble potassium salt and H2 AF6 in a molar ratio of 1:2 to 15 under normal temperature and pressure. K2 Mx Ny F6 is obtained, wherein H2 AF6 is a mixed solution of any two of H2 SiF6 , H2 GeF6 and H2 TiF6 solutions;x Ny F6 is used as the matrix. After mixing with KMnO4 and HF solution according to the molar ratio of K2 Mx Ny F6 : KMnO4 : HF=1:0.1~0.5:1~300, the reaction is carried out for 10~20h, Vacuum filtration, wash with absolute ethanol for 1 to 3 times, and then vacuum dry in a vacuum drying oven at 80° C. for 8 to 10 hours to obtain K2 Mx Ny F6 : zMn4+ phosphor.
更优选地说,上述可溶性钾盐与H2AF6的摩尔比为1:2~10;上述K2MxNyF6、 KMnO4和HF的摩尔比为K2MxNyF6:KMnO4:HF=1:0.1~0.2:50~150。More preferably, the molar ratio of the above soluble potassium salt to H2 AF6 is 1:2-10; the molar ratio of the above K2 Mx Ny F6 , KMnO4 and HF is K2 Mx Ny F6 : KMnO4 : HF=1:0.1 to 0.2:50 to 150.
进一步优先地,上述H2AF6溶液的质量百分浓度为30~50%,上述可溶性钾盐溶液的质量百分浓度为10~40%;上述HF的水溶液质量百分浓度为30~40%。Further preferably, the mass percentage concentration of the above-mentioned H2 AF6 solution is 30-50%, the mass percentage concentration of the above-mentioned soluble potassium salt solution is 10-40%; the mass percentage concentration of the above-mentioned HF aqueous solution is 30-40% .
若在防水性荧光粉制备过程中控制不好,不能得到本发明红色荧光粉,其制得的红色荧光粉浸在蒸馏水中,先接触水的表面那层荧光粉已经完全不发光了,虽还有一部分中间粉在发光,但是发光效果骤降,直至荧光粉全部变黑。采用上述制备方法成功地制得了本发明自带显著防水性以及稳定性好的红色荧光粉。If the control is not good in the preparation process of the waterproof phosphor, the red phosphor of the present invention cannot be obtained, and the prepared red phosphor is immersed in distilled water. A part of the intermediate powder is emitting light, but the luminous effect drops sharply until all the phosphor powders turn black. By adopting the above preparation method, the red phosphor of the present invention with excellent water resistance and good stability is successfully prepared.
同时,在研究中发现,在制备过程中控制不好,会导致制得的荧光粉粉末颗粒均匀性变差,对荧光粉在水中的发光效果有一定的影响,按照本发明方法制得的荧光粉很好的避免了该技术问题。At the same time, it is found in the research that poor control in the preparation process will lead to poor uniformity of the prepared phosphor powder particles, which will have a certain impact on the luminous effect of the phosphor powder in water. Powder avoids this technical problem very well.
最具体地说,一种自带防水性能的红色荧光粉的制备方法,它是包括以下步骤制得:Most specifically, a method for preparing a red phosphor with waterproof performance, which comprises the following steps:
(1)常温常压下将质量百分浓度为30~50%的H2AF6溶液缓慢滴入质量百分浓度为10~40%的可溶性钾盐溶液中,边滴加边进行同一方向规律搅拌,搅拌转速在60~80转/min,制得K2MxNyF6的基质溶液,其中可溶性钾盐与H2AF6的摩尔比为1:2~15;(1) Under normal temperature and pressure, slowly drop the H2 AF6 solution with a mass percentage concentration of 30-50% into a soluble potassium salt solution with a mass percentage concentration of 10-40%, and carry out the same direction rule while dropping. Stir, and the stirring speed is at 60~80 rev/min to prepare the matrix solution of K2 Mx Ny F6 , wherein the molar ratio of soluble potassium salt to H2 AF6 is 1:2~15;
(2)用真空泵抽滤机抽滤,蒸馏水清洗至中性,用烘箱在90℃下干燥8~ 10h,制得K2MxNyF6基质粉末;(2) Suction filtration with a vacuum pump suction filter, rinsed with distilled water until neutral, and dried in an oven at 90° C. for 8-10 hours to obtain K2 Mx Ny F6 matrix powder;
(3)将步骤(2)中制得的K2MxNyF6粉末和KMnO4混合均匀后,加入质量百分浓度为40%的HF水溶液中进行反应,其中K2MxNyF6、KMnO4和HF的摩尔比为1:0.1~0.5:1~300;常温常压下反应时间为10~20h,生成目标产物 K2MxNyF6:zMn4+沉淀物;(3) After mixing the K2 Mx Ny F6 powder prepared in step (2) and KMnO4 uniformly, add it to the HF aqueous solution with a mass percentage concentration of 40% to carry out the reaction, wherein K2 Mx Ny The molar ratio of F6 , KMnO4 and HF is 1:0.1-0.5:1-300; the reaction time under normal temperature and pressure is 10-20h, and the target product K2 Mx Ny F6 : zMn4+ precipitate is generated;
(4)使用真空泵抽滤机抽滤,无水乙醇清洗1~3次,再用80℃的真空干燥箱真空干燥8~10h,得到目标产物K2MxNyF6:zMn4+荧光粉。(4) Use a vacuum pump suction filter for suction filtration, wash with absolute ethanol for 1 to 3 times, and then use a vacuum drying oven at 80 ° C to vacuum dry for 8 to 10 hours to obtain the target product K2 Mx Ny F6 : zMn4+ fluorescence pink.
进一步,上述步骤(1)中H2AF6溶液滴入可溶性钾盐溶液完成后,还需继续搅拌30~40min。Further, after the H2 AF6 solution is dropped into the soluble potassium salt solution in the above step (1), it is necessary to continue stirring for 30-40 min.
本发明具有以下有益效果:The present invention has the following beneficial effects:
本发明提供了一种自带防水性能的红色荧光粉及其制备方法,使用本发明方法制备荧光粉简单,高效,不需要高温煅烧等步骤,常温常压条件下就可以制备;本发明红色荧光粉为窄带发射,颗粒均匀,其具有发光效率高,显色效果好,有很好防水效果等突出优点,很好的解决了传统红色荧光粉容易受潮水解的难题,而且荧光粉自身具有稳定的防水性,其在潮湿环境中保持长时间稳定不衰减,且最终发光强度可保持在原来的80%以上,不仅解决了传统涂层保护的防水手段中荧光粉性能衰减较快的问题,而且无需使用防水涂层,节约了生产成本,且广泛适用于潮湿环境中。The invention provides a red fluorescent powder with self-contained waterproof performance and a preparation method thereof. Using the method of the invention to prepare the fluorescent powder is simple and efficient, does not require high temperature calcination and other steps, and can be prepared under normal temperature and normal pressure conditions; the red fluorescent powder of the present invention is prepared. The powder has narrow-band emission and uniform particles. It has outstanding advantages such as high luminous efficiency, good color rendering effect, and good waterproof effect. Waterproof, it can remain stable for a long time without decay in a humid environment, and the final luminous intensity can be maintained at more than 80% of the original, which not only solves the problem of rapid degradation of phosphor performance in the waterproof method of traditional coating protection, but also does not require The use of a water-repellent coating saves production costs and is widely applicable in wet environments.
附图说明Description of drawings
图1:本发明实例1中所述红色荧光粉的XRD谱图;Fig. 1: XRD spectrum of the red phosphor powder described in Example 1 of the present invention;
图2:本发明实例1中所述红色荧光粉的激发光谱;Figure 2: The excitation spectrum of the red phosphor described in Example 1 of the present invention;
图3:本发明实例1中所述红色荧光粉的发射光谱;Figure 3: The emission spectrum of the red phosphor described in Example 1 of the present invention;
图4:本发明实例3中所述红色荧光粉的激发光谱;Figure 4: Excitation spectrum of the red phosphor described in Example 3 of the present invention;
图5:本发明实例3中所述红色荧光粉的发射光谱;Figure 5: The emission spectrum of the red phosphor powder described in Example 3 of the present invention;
图6:本发明实例1、2和3中所述红色荧光粉的浸水后发光强度对比图。FIG. 6 is a graph comparing the luminous intensity of the red phosphors described in Examples 1, 2 and 3 of the present invention after being immersed in water.
具体实施方式Detailed ways
下面通过实施例对本发明进行具体的描述,有必要在此指出的是以下实施例只用于对本发明进行进一步说明,不能理解为对本发明保护范围的限制,该领域技术人员可以根据本发明内容对本发明做出一些非本质的改进和调整。The present invention will be specifically described by the following examples. It is necessary to point out that the following examples are only used to further illustrate the present invention, and should not be construed as limiting the protection scope of the present invention. The invention makes some non-essential improvements and adjustments.
实施例1Example 1
一种自带防水性能的红色荧光粉的制备方法,按如下步骤:A preparation method of a red phosphor with self-contained waterproof performance, according to the following steps:
(1)常温常压下取质量百分浓度为50%的H2TiF6和H2GeF6组成的混合酸溶液,其中H2TiF6:H2GeF6的摩尔比为1:9,将混合溶液缓慢滴入质量百分浓度为 40%的KNO3溶液中,KNO3与混合酸溶液的施用量摩尔比1:2,边滴加边进行同一方向圆周规律搅拌,滴加完成后继续搅拌30min,搅拌转速为80转/min,制得含有K2Ge0.9Ti0.1F6的基质溶液;(1) Take a mixed acid solution composed of H2 TiF6 and H2 GeF6 with a mass percentage concentration of 50% at normal temperature and pressure, wherein the molar ratio of H2 TiF6 : H2 GeF6 is 1:9, and the The mixed solution was slowly dropped into the KNO3 solution with a mass percentage concentration of 40%, the molar ratio of KNO3 and the mixed acid solution was 1:2, and the circular stirring was carried out in the same direction while dropping, and the stirring was continued after the dropping was completed. 30min, the stirring speed is 80 rev/min, and the matrix solution containing K2 Ge0.9 Ti0.1 F6 is obtained;
(2)再经循环水式多用真空泵抽滤机抽滤,用蒸馏水清洗至中性,再用烘箱在90℃下干燥10h,制得K2Ge0.9Ti0.1F6基质;(2) Suction filtration through a circulating water-type multi-purpose vacuum pump suction filter, washed with distilled water until neutral, and then dried in an oven at 90° C. for 10 hours to obtain K2 Ge0.9 Ti0.1 F6 matrix;
(3)取步骤(2)制得的K2Ge0.9Ti0.1F6粉末和KMnO4混合均匀后,加入质量百分浓度为40%的HF溶液;常温常压下反应20h,生成目标产物K2Ge0.9Ti0.1F6: 0.15Mn4+,其中各反应物的摩尔比为K2Ge0.9Ti0.1F6:KMnO4:HF=1:0.15:150;(3) After mixing the K2 Ge0.9 Ti0.1 F6 powder obtained in step (2) and KMnO4 uniformly, add a HF solution with a mass percentage concentration of 40%; react under normal temperature and pressure for 20 hours to generate the target product K2 Ge0.9 Ti0.1 F6 : 0.15Mn4+ , wherein the molar ratio of each reactant is K2 Ge0.9 Ti0.1 F6 : KMnO4 : HF=1:0.15:150;
(4)去掉上层悬液,使用经循环水式多用真空泵抽滤机抽滤,无水乙醇清洗3次,用真空干燥箱在80℃下干燥10h,得到目标产物K2Ge0.9Ti0.1F6:0.15Mn4+荧光粉。(4) Remove the upper layer suspension, use a circulating water-type multi-purpose vacuum pump suction filter for suction filtration, wash with absolute ethanol for 3 times, and use a vacuum drying oven to dry at 80 ° C for 10 hours to obtain the target product K2 Ge0.9 Ti0.1 F6 : 0.15Mn4+ phosphor.
将实施例1中制得的K2Ge0.9Ti0.1F6:0.15Mn4+防水性红色荧光粉浸在蒸馏水中,静置一段时间,观察并检测该红色荧光粉在水中的发光情况如图6。The K2 Ge0.9 Ti0.1 F6 : 0.15Mn4+ water-repellent red phosphor obtained in Example 1 was immersed in distilled water, and allowed to stand for a period of time to observe and detect the luminescence of the red phosphor in water as shown in the figure 6.
根据图1、图2、图3和图6所示可知:实施例1中采用本发明方法制得的K2Ge0.9Ti0.1F6:0.15Mn4+红色荧光粉,其激发峰位于362.8nm和462.8nm,发射光谱是在580~680nm之间的窄带发射,有590.4nm、603.2nm、616.0nm、634.8nm 和650.6nm 5个交叉发射峰,其中634.8nm为最强峰,最强峰的半峰宽为10nm。荧光粉随着浸水时间增长,短时间内发光强度有略微降低,降低到一定程度后趋于稳定,仍然具有不错的发光效果,发光性能不再衰减。According to Fig. 1, Fig. 2, Fig. 3 and Fig. 6, it can be known that the K2 Ge0.9 Ti0.1 F6 : 0.15Mn4+ red phosphor obtained by the method of the present invention in Example 1 has an excitation peak at 362.8 nm and 462.8nm, the emission spectrum is a narrow-band emission between 580 and 680nm, with 5 cross-emission peaks at 590.4nm, 603.2nm, 616.0nm, 634.8nm and 650.6nm, of which 634.8nm is the strongest peak. The width at half maximum is 10 nm. As the immersion time increases, the luminous intensity of the phosphor decreases slightly in a short period of time, and then tends to be stable after decreasing to a certain extent, and still has a good luminous effect, and the luminous performance is no longer attenuated.
实施例2Example 2
一种自带防水性能的红色荧光粉的制备方法,按如下步骤:A preparation method of a red phosphor with self-contained waterproof performance, according to the following steps:
(1)常温常压下取质量百分浓度为30%的H2TiF6和H2SiF6组成的混合酸溶液,其摩尔比为H2TiF6:H2SiF6=1:1,缓慢滴入质量百分浓度为10%的KCl溶液中,KCl 与混合酸溶液的施用量摩尔比为1:10,边滴加边进行同一方向圆周规律搅拌,滴加完成后继续搅拌40min搅拌转速在60转/min,制得含有K2Ti0.5Si0.5F6的基质溶液;(1) Take a mixed acid solution composed of H2 TiF6 and H2 SiF6 with a mass percentage concentration of 30% under normal temperature and pressure, and the molar ratio is H2 TiF6 : H2 SiF6 =1:1, slowly Drop into the KCl solution with a mass percentage concentration of 10%, the molar ratio of KCl and the mixed acid solution is 1:10, while adding dropwise, carry out circular stirring in the same direction, and continue to stir for 40 minutes after the dropping is completed. 60 revolutions/min, to obtain the matrix solution containing K2 Ti0.5 Si0.5 F6 ;
(2)循环水式多用真空泵抽滤机抽滤,经蒸馏水清洗至中性,再使用90℃真空干燥箱干燥8h,制得基质K2Ti0.5Si0.5F6;(2) Suction filtration with a circulating water-type multi-purpose vacuum pump suction filter, washed with distilled water until neutral, and then dried in a 90°C vacuum drying oven for 8 hours to obtain a matrix K2 Ti0.5 Si0.5 F6 ;
(3)取步骤(2)制得的K2Ti0.5Si0.5F6粉末与KMnO4混合均匀后,加入质量百分浓度为30%的HF混合溶液中进行反应,各反应物摩尔比为K2Ti0.5Si0.5F6: KMnO4:HF=1:0.2:80,在常温常压下反应20h后,生成目标产物 K2Ti0.5Si0.5F6:0.2Mn4+;(3) After the K2 Ti0.5 Si0.5 F6 powder obtained in step (2) is mixed with KMnO4 uniformly, it is added to the HF mixed solution with a mass percentage concentration of 30% to carry out the reaction, and the molar ratio of each reactant is K2 Ti0.5 Si0.5 F6 : KMnO4 : HF=1:0.2:80, after 20 hours of reaction at normal temperature and pressure, the target product K2 Ti0.5 Si0.5 F6 : 0.2Mn4+ is generated;
(4)去掉上层悬液,用循环水式多用真空泵抽滤机抽滤,之后用无水乙醇清洗1次,在80℃的真空干燥箱中干燥8h,得到目标产物K2Ti0.5Si0.5F6:0.2Mn4+荧光粉。(4) Remove the upper layer suspension, use a circulating water-type multi-purpose vacuum pump suction filter for suction filtration, then wash with absolute ethanol once, and dry in a vacuum drying box at 80 ° C for 8 hours to obtain the target product K2 Ti0.5 Si0.5 F6 :0.2Mn4+ phosphor.
将实施例中制得的K2Ti0.5Si0.5F6:0.2Mn4+防水性红色荧光粉浸在蒸馏水中,静置一段时间,观察并检测该红色荧光粉在水中的发光情况如图6。The K2 Ti0.5 Si0.5 F6 : 0.2Mn4+ water-repellent red phosphor obtained in the example was immersed in distilled water, and allowed to stand for a period of time to observe and detect the luminescence of the red phosphor in water as shown in Figure 6 .
实施例2中采用本发明方法制得的K2Ti0.5Si0.5F6:0.2Mn4+红色荧光粉,其激发峰位于336.4.0nm和469.2nm,发射光谱在580~680nm之间窄带发射,有590.2nm、 605.6nm、617.2nm、636.2nm和651.4nm、5个交叉发射峰,其中636.2nm为最强峰,最强峰的半峰宽为10nm。如图6所示,荧光粉随着浸水时间增长,短时间内发光强度有略微降低,降低到一定程度后趋于稳定,仍然具有不错的发光效果,且发光性能不再衰减。In Example 2, the K2 Ti0.5 Si0.5 F6 :0.2Mn4+ red phosphor prepared by the method of the present invention has an excitation peak at 336.4.0 nm and 469.2 nm, and an emission spectrum in a narrow band between 580 and 680 nm. There are 5 cross emission peaks at 590.2 nm, 605.6 nm, 617.2 nm, 636.2 nm and 651.4 nm, of which 636.2 nm is the strongest peak, and the half-width of the strongest peak is 10 nm. As shown in Figure 6, as the immersion time increases, the luminous intensity of the phosphor decreases slightly in a short period of time, and then tends to be stable after decreasing to a certain extent, still has a good luminous effect, and the luminous performance no longer decays.
实施例3Example 3
一种自带防水性能的红色荧光粉的制备方法,按如下步骤:A preparation method of a red phosphor with self-contained waterproof performance, according to the following steps:
(1)常温常压下取质量百分浓度为40%的H2SiF6和H2GeF6组成的混合溶液,其摩尔比为H2GeF6:H2SiF6=3:7,缓慢滴入质量百分浓度为26%的KOH溶液, KOH与混合酸溶液的使用量摩尔比为1:6,边滴加边进行同一方向圆周规律搅拌,滴加完成后继续搅拌35min,搅拌搅拌转速为70转/min,制得含有K2Si0.7Ge0.3F6的基质溶液;(1) Under normal temperature and pressure, take a mixed solution of H2 SiF6 and H2 GeF6 with a mass percentage concentration of 40%, the molar ratio of which is H2 GeF6 : H2 SiF6 =3:7, slowly drip Add the KOH solution with a mass percentage concentration of 26%, the molar ratio of KOH and the mixed acid solution is 1:6, and stir regularly in the same direction while adding dropwise. 70 rev/min to prepare a matrix solution containing K2 Si0.7 Ge0.3 F6 ;
(2)采用循环水式多用真空泵抽滤机抽滤,用蒸馏水清洗至中性,再用90℃真空干燥箱干燥9h,制得基质K2Si0.7Ge0.3F6;(2) Suction filtration with a circulating water-type multi-purpose vacuum pump suction filter, washed with distilled water until neutral, and then dried in a vacuum drying oven at 90° C. for 9 hours to obtain the matrix K2 Si0.7 Ge0.3 F6 ;
(3)取步骤(2)中制得的K2Si0.7Ge0.3F6粉末与KMnO4混合均匀后,加入质量百分浓度为36%的HF混合溶液中进行反应,其中各反应物的摩尔比为 K2Si0.7Ge0.3F6:KMnO4:HF=1:0.1:50,常温常压下反应12h后,生成目标产物 K2Si0.7Ge0.3F6:0.1Mn4+;(3) After mixing the K2 Si0.7 Ge0.3 F6 powder obtained in step (2) with KMnO4 uniformly, add it into the HF mixed solution with a mass percentage concentration of 36% to carry out the reaction, wherein the moles of the reactants are The ratio is K2 Si0.7 Ge0.3 F6 : KMnO4 : HF=1:0.1:50, and the target product K2 Si0.7 Ge0.3 F6 : 0.1Mn4+ is generated after 12 hours of reaction under normal temperature and pressure;
(4)去掉上层悬液,用循环水式多用真空泵抽滤机抽滤,再用无水乙醇清洗3次,在80℃的真空干燥箱中干燥9h,得到目标产物K2Si0.7Ge0.3F6:0.1Mn4+荧光粉。(4) Remove the upper layer suspension, use a circulating water-type multi-purpose vacuum pump suction filter for suction filtration, wash with absolute ethanol for 3 times, and dry in a vacuum drying box at 80 ° C for 9 hours to obtain the target product K2 Si0.7 Ge0.3 F6 : 0.1Mn4+ phosphor.
将实施例3中制得的K2Si0.7Ge0.3F6:0.1Mn4+防水性红色荧光粉浸在蒸馏水中,静置一段时间,观察并检测该红色荧光粉在水中的发光情况如图6所示。The K2 Si0.7 Ge0.3 F6 : 0.1Mn4+ water-repellent red phosphor obtained in Example 3 was immersed in distilled water, and allowed to stand for a period of time to observe and detect the luminescence of the red phosphor in water as shown in the figure 6 shown.
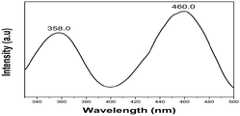
根据图4、图5和图6所示可知:实施例3中采用本发明方法制得的 K2Si0.7Ge0.3F6:0.1Mn4+红色荧光粉,其基质为K2Si0.7Ge0.3F6,其激发峰位于358.0nm和460.0nm,发射光谱在580~680nm之间窄带发射,有590.4nm、600.8nm、 615.2nm、623.8nm、633.6nm和649.8nm 6个交叉发射峰,其中633.6nm为最强峰,最强峰的半峰宽为10nm。荧光粉随着浸水时间增长,算时间内发光强度有略微降低,降低到一定程度后趋于稳定,仍然具有很好的发光效果,且发光性能不再衰减。According to Fig. 4, Fig. 5 and Fig. 6, it can be seen that the K2 Si0.7 Ge0.3 F6 : 0.1Mn4+ red phosphor prepared by the method of the present invention in Example 3 has a matrix of K2 Si0.7 Ge0.3 F6 , its excitation peaks are located at 358.0 nm and 460.0 nm, the emission spectrum is narrow-band emission between 580 and 680 nm, and there are 6 cross-emission peaks at 590.4 nm, 600.8 nm, 615.2 nm, 623.8 nm, 633.6 nm and 649.8 nm, of which 633.6 nm is the strongest peak, and the half-width of the strongest peak is 10 nm. As the immersion time increases, the luminous intensity of the phosphor decreases slightly during the calculation time, and becomes stable after it decreases to a certain extent, and still has a good luminous effect, and the luminous performance is no longer attenuated.
经多次试验检测,上述防水性红色荧光粉的激发峰为360nm±10nm和 460nm±10nm,其发射光谱在580~680nm之间窄带发射:有5~6个交叉的发射峰;After many tests, the excitation peaks of the above-mentioned waterproof red phosphor are 360nm±10nm and 460nm±10nm, and its emission spectrum is narrow-band emission between 580-680nm: there are 5-6 crossed emission peaks;
当0<z≦0.1时,会出现6个发射波峰,峰位分别为590nm±2nm、602nm± 2nm、616nm±2nm、625nm±2nm、635nm±2nm和650nm±2nm,其中最强峰为635nm±2nm,且最强峰的半峰宽为10nm±2nm。When 0<z≦0.1, there will be 6 emission peaks, the peak positions are 590nm±2nm, 602nm±2nm, 616nm±2nm, 625nm±2nm, 635nm±2nm and 650nm±2nm, of which the strongest peak is 635nm±
当0.1<z≦0.2时,会出现5个发射波峰,峰位分别为590nm±2nm、603nm ±3nm、616nm±2nm、635nm±2nm和650nm±2nm,其中最强峰为635nm±2nm,且最强峰的半峰宽为10nm±2nm。When 0.1<z≦0.2, there will be 5 emission peaks, the peak positions are 590nm±2nm, 603nm±3nm, 616nm±2nm, 635nm±2nm and 650nm±2nm, among which the strongest peak is 635nm±2nm, and the most The half-width of the strong peak was 10 nm ± 2 nm.
Claims (3)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910571374.1ACN110257065B (en) | 2019-06-28 | 2019-06-28 | A kind of red phosphor with waterproof performance and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910571374.1ACN110257065B (en) | 2019-06-28 | 2019-06-28 | A kind of red phosphor with waterproof performance and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN110257065A CN110257065A (en) | 2019-09-20 |
| CN110257065Btrue CN110257065B (en) | 2022-05-17 |
Family
ID=67922614
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201910571374.1AActiveCN110257065B (en) | 2019-06-28 | 2019-06-28 | A kind of red phosphor with waterproof performance and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN110257065B (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112410030B (en)* | 2020-12-03 | 2022-09-13 | 重庆文理学院 | A kind of preparation method of heterogeneous composite molybdate oxyfluoride nano fluorescent material |
| CN113097368A (en)* | 2021-03-26 | 2021-07-09 | 湖北三峡夷丰光电科技有限公司 | Production method of red fluorescent film |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013014715A (en)* | 2011-07-06 | 2013-01-24 | Nichia Corp | Fluoride phosphor and light-emitting device obtained using the fluoride phosphor |
| CN103003388A (en)* | 2010-07-27 | 2013-03-27 | 通用电气公司 | Moisture resistant phosphors and related methods |
| CN103980896A (en)* | 2014-04-29 | 2014-08-13 | 中国科学院福建物质结构研究所 | Preparation method of fluoride fluorescent powder material |
| CN105733575A (en)* | 2015-12-28 | 2016-07-06 | 温州大学 | Tetravalent manganese ion doped ammonium salt red light material and preparation method thereof |
| CN105980523A (en)* | 2014-01-30 | 2016-09-28 | 信越化学工业株式会社 | Method for producing and method for processing complex fluoride phosphor |
| CN106085428A (en)* | 2015-03-10 | 2016-11-09 | 重庆文理学院 | A kind of fluorescent material being applicable to LED illumination |
| JP2016210950A (en)* | 2015-04-28 | 2016-12-15 | 株式会社ネモト・ルミマテリアル | Fluoride phosphor and production method thereof, and semiconductor light-emitting device |
| CN106433626A (en)* | 2016-09-22 | 2017-02-22 | 陕西师范大学 | Method for preparing Mn(IV)-activated fluoride red fluorescent powder |
| CN106929015A (en)* | 2015-12-29 | 2017-07-07 | 有研稀土新材料股份有限公司 | Red fluorescence powder, its preparation method and the luminescent device comprising the red fluorescence powder |
| CN107236543A (en)* | 2017-06-15 | 2017-10-10 | 华南理工大学 | One kind improves Mn4+The method of doped fluoride red fluorescence powder material moisture resistance properties |
| WO2018215204A1 (en)* | 2017-05-23 | 2018-11-29 | Osram Opto Semiconductors Gmbh | Wavelength conversion element, light emitting device and method for producing a wavelength conversion element |
| CN109135739A (en)* | 2017-06-16 | 2019-01-04 | 隆达电子股份有限公司 | Manganese-doped red fluoride phosphor, light-emitting device and backlight module |
- 2019
- 2019-06-28CNCN201910571374.1Apatent/CN110257065B/enactiveActive
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103003388A (en)* | 2010-07-27 | 2013-03-27 | 通用电气公司 | Moisture resistant phosphors and related methods |
| JP2013014715A (en)* | 2011-07-06 | 2013-01-24 | Nichia Corp | Fluoride phosphor and light-emitting device obtained using the fluoride phosphor |
| CN105980523A (en)* | 2014-01-30 | 2016-09-28 | 信越化学工业株式会社 | Method for producing and method for processing complex fluoride phosphor |
| CN103980896A (en)* | 2014-04-29 | 2014-08-13 | 中国科学院福建物质结构研究所 | Preparation method of fluoride fluorescent powder material |
| CN106085428A (en)* | 2015-03-10 | 2016-11-09 | 重庆文理学院 | A kind of fluorescent material being applicable to LED illumination |
| JP2016210950A (en)* | 2015-04-28 | 2016-12-15 | 株式会社ネモト・ルミマテリアル | Fluoride phosphor and production method thereof, and semiconductor light-emitting device |
| CN105733575A (en)* | 2015-12-28 | 2016-07-06 | 温州大学 | Tetravalent manganese ion doped ammonium salt red light material and preparation method thereof |
| CN106929015A (en)* | 2015-12-29 | 2017-07-07 | 有研稀土新材料股份有限公司 | Red fluorescence powder, its preparation method and the luminescent device comprising the red fluorescence powder |
| CN106433626A (en)* | 2016-09-22 | 2017-02-22 | 陕西师范大学 | Method for preparing Mn(IV)-activated fluoride red fluorescent powder |
| WO2018215204A1 (en)* | 2017-05-23 | 2018-11-29 | Osram Opto Semiconductors Gmbh | Wavelength conversion element, light emitting device and method for producing a wavelength conversion element |
| CN107236543A (en)* | 2017-06-15 | 2017-10-10 | 华南理工大学 | One kind improves Mn4+The method of doped fluoride red fluorescence powder material moisture resistance properties |
| CN109135739A (en)* | 2017-06-16 | 2019-01-04 | 隆达电子股份有限公司 | Manganese-doped red fluoride phosphor, light-emitting device and backlight module |
Non-Patent Citations (3)
| Title |
|---|
| 20170621;陈文威;《厦门大学硕士学位论文》;20170621;第1-69页* |
| Synthesis and photoluminescence properties of octahedral;JIN Yuming et al.,;《JOURNAL OF RARE EARTHS》;20161230;第34卷(第12期);第1173-1178页* |
| 陈文威.20170621.《厦门大学硕士学位论文》.2017,第1-69页.* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN110257065A (en) | 2019-09-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102120931B (en) | Red fluorophor and preparation method thereof | |
| JP5005759B2 (en) | Fluorescent powder, method for producing the same, and light-emitting device using the same | |
| CN112251219B (en) | Moisture-resistant fluoride red fluorescent powder and preparation method thereof | |
| CN105733572A (en) | Red fluoride fluorescent powder as well as preparation method and application thereof | |
| CN108003872B (en) | Fluoride red phosphor for white light LED excited by blue light and method for its preparation and modification | |
| CN110257065B (en) | A kind of red phosphor with waterproof performance and preparation method thereof | |
| CN108276999A (en) | A kind of preparation method for mixing europium lanthanum molybdate red fluorescence powder | |
| WO2016065725A1 (en) | Fluorescent material and manufacturing method thereof and composition containing the same | |
| CN110157414B (en) | Red fluoromanganate fluorescent material and preparation method thereof | |
| CN102559173B (en) | Core-surface layer gradient nitrogen oxide fluorescent powder, manufacturing method thereof and light-emitting device adopting fluorescent powder | |
| CN109423281A (en) | Fluoride phosphor and light-emitting device | |
| CN109423280A (en) | Fluoride phosphor and light-emitting device | |
| JP4433793B2 (en) | Phosphor and light emitting device using the same | |
| CN108641715B (en) | A kind of barium sodium fluorogallate red light material for white light LED and preparation method thereof | |
| CN110157425A (en) | A kind of manganese ion activated oxyfluoride phosphor and preparation method thereof | |
| CN110184057A (en) | A kind of oxygen fluoride red fluorescence powder and preparation method thereof | |
| CN106318381B (en) | A kind of Mn4+Sodium bifluoride red light material of doping and preparation method thereof | |
| CN111978955B (en) | A kind of red phosphor and its preparation method and application | |
| CN114874774A (en) | Manganese ion activated tantalum-based oxyfluoride red luminescent material and preparation method thereof | |
| CN114989824A (en) | Short life spheroidal Mn 4+ Fluoride-doped red fluorescent powder, structure, preparation method and light-emitting device | |
| CN110172346A (en) | A kind of red fluorescence powder and preparation method thereof | |
| CN108130073A (en) | A kind of tetravalence manganese ion doping indium potassium sodium red fluorescence powder and preparation method | |
| CN115491200B (en) | A blue light-excited red phosphor and its preparation and white light LED device | |
| CN116333735B (en) | Tetravalent manganese doped fluoride red fluorescent material with homogeneous core-shell structure and preparation method thereof | |
| CN113214825B (en) | Multi-fluoride red-light material for solid-state lighting LED and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |