CN109803938B - Manufacturing method of chemically strengthened glass - Google Patents
Manufacturing method of chemically strengthened glassDownload PDFInfo
- Publication number
- CN109803938B CN109803938BCN201780060568.4ACN201780060568ACN109803938BCN 109803938 BCN109803938 BCN 109803938BCN 201780060568 ACN201780060568 ACN 201780060568ACN 109803938 BCN109803938 BCN 109803938B
- Authority
- CN
- China
- Prior art keywords
- glass
- chemically strengthened
- chemical strengthening
- strength
- acid treatment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C21/00—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface
- C03C21/001—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface in liquid phase, e.g. molten salts, solutions
- C03C21/002—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface in liquid phase, e.g. molten salts, solutions to perform ion-exchange between alkali ions
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C15/00—Surface treatment of glass, not in the form of fibres or filaments, by etching
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C21/00—Treatment of glass, not in the form of fibres or filaments, by diffusing ions or metals in the surface
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Surface Treatment Of Glass (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及一种化学强化玻璃的制造方法。The present invention relates to a manufacturing method of chemically strengthened glass.
背景技术Background technique
在数码相机、手机、便携信息终端PDA(Personal Digital Assistants)等平板显示器装置中,为了保护显示器以及提高美观,实施了以成为大于图像显示部分的区域的方式将薄板状的罩玻璃配置于显示器的前表面。玻璃虽然理论强度高,但由于损伤会导致强度大幅降低,因此,对于要求强度的罩玻璃,使用通过离子交换等在玻璃表面形成压缩应力层的化学强化玻璃。In flat panel display devices such as digital cameras, cell phones, and PDAs (Personal Digital Assistants), in order to protect the display and improve the appearance, a thin plate-shaped cover glass is placed on the display so as to have an area larger than the image display portion. front surface. Although glass has high theoretical strength, the strength is greatly reduced due to damage. Therefore, for cover glass requiring strength, chemically strengthened glass in which a compressive stress layer is formed on the glass surface by ion exchange or the like is used.
伴随着对平板显示器装置的轻量化和薄型化的要求,需要罩玻璃自身也变薄。因此,对于罩玻璃,为了满足该目的而对表面要求进一步的强度。With the demand for weight reduction and thinning of flat panel display devices, the cover glass itself needs to be thinned. Therefore, for cover glass, further strength is required for the surface in order to meet this purpose.
作为提高玻璃强度的方法之一,在专利文献1中公开了利用含有特定盐的无机盐进行化学强化后进行酸处理和碱处理的方法。As one of the methods for improving glass strength,
现有技术文献prior art literature
专利文献Patent Literature
专利文献1:国际公开第2015/008763号Patent Document 1: International Publication No. 2015/008763
发明内容SUMMARY OF THE INVENTION
然而,对于专利文献1中记载的方法,如果出于提高压缩应力层的深度(定义为压缩应力值成为零的深度,以下,也简称为DOC(Depth of Compression))以得到高强度的目的而在高温下进行长时间化学强化,则存在如下问题:作为副作用玻璃强度降低,并且化学强化的温度条件、时间受限制。However, in the method described in
另外,以往,通过在化学强化处理后进行研磨处理来谋求面强度的提高,但有可能由于研磨而导致玻璃表面损伤,面强度反而下降。进而,有可能由于研磨使玻璃的翘曲增大。Moreover, conventionally, the improvement of surface strength was aimed at by performing grinding|polishing process after a chemical strengthening process, but there exists a possibility that a glass surface may be damaged by grinding|polishing, and the surface strength may fall on the contrary. Furthermore, there exists a possibility that the curvature of glass may increase by grinding|polishing.
因此,本发明提供一种化学强化的温度条件、时间不受限制,即使在高温下进行长时间的化学强化处理也不会减弱玻璃的强度,显示较深的DOC并且面强度高的化学强化玻璃的制造方法。Therefore, the present invention provides a chemically strengthened glass that is not limited in terms of temperature and time for chemical strengthening, and exhibits a deep DOC and a high surface strength without weakening the strength of the glass even if the chemical strengthening treatment is performed at a high temperature for a long time. manufacturing method.
本发明人等反复进行了深入研究,结果发现通过实施使化学强化所使用的盐的pH为规定范围的化学强化工序以及对所述化学强化工序后的玻璃进行酸处理的酸处理工序,可得到化学强化的温度条件、时间不受限制,即使在高温下进行长时间的化学强化处理也显示较深的DOC并且面强度高的化学强化玻璃,从而完成了本发明。The inventors of the present invention have repeatedly conducted intensive studies, and as a result, have found that by implementing a chemical strengthening process in which the pH of the salt used for chemical strengthening is within a predetermined range, and an acid treatment process in which the glass after the chemical strengthening process is acid-treated, it is possible to obtain The temperature conditions and time for chemical strengthening are not limited, and the present invention has been completed as a chemically strengthened glass that exhibits deep DOC and high surface strength even if chemical strengthening treatment is performed at a high temperature for a long time.
即,本发明如下。That is, the present invention is as follows.
1.一种化学强化玻璃的制造方法,包括如下工序:1. A manufacturing method of chemically strengthened glass, comprising the following steps:
化学强化工序,使玻璃与制成10质量%水溶液时的氢离子指数(pH)为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐接触而进行离子交换;和a chemical strengthening step of contacting the glass with an inorganic salt containing at least one of sodium nitrate and potassium nitrate having a hydrogen ion index (pH) of 7.5 to 10.5 in a 10 mass % aqueous solution to perform ion exchange; and
酸处理工序,使所述化学强化工序后的玻璃与氢离子指数(pH)小于7.0的酸性溶液接触而进行酸处理。In the acid treatment step, acid treatment is performed by contacting the glass after the chemical strengthening step with an acidic solution having a hydrogen ion index (pH) of less than 7.0.
2.根据上述1所述的化学强化玻璃的制造方法,其中,进一步包括如下工序:碱处理工序,使所述酸处理工序后的玻璃与氢离子指数(pH)超过7.0的碱性溶液接触而进行碱处理。2. The method for producing a chemically strengthened glass according to the above 1, further comprising a step of: an alkali treatment step of contacting the glass after the acid treatment step with an alkaline solution having a hydrogen ion index (pH) exceeding 7.0 to obtain a Alkaline treatment.
3.根据上述1或2所述的化学强化玻璃的制造方法,其中,所述化学强化工序是使所述玻璃与400℃以上的所述无机盐接触2小时以上而进行离子交换的工序。3. The manufacturing method of the chemically strengthened glass according to the above 1 or 2, wherein the chemical strengthening step is a step of contacting the glass with the inorganic salt of 400° C. or higher for 2 hours or more to perform ion exchange.
4.根据上述1~3中任一项所述的化学强化玻璃的制造方法,其中,所述化学强化工序后的玻璃具有深度35μm以上的压缩应力层。4. The manufacturing method of chemically strengthened glass in any one of said 1-3 whose glass after the said chemical strengthening process has a compressive stress layer with a depth of 35 micrometers or more.
5.根据上述1~4中任一项所述的化学强化玻璃的制造方法,其中,所述化学强化工序后的玻璃通过球环试验(ボールオンリング試験)在下述条件下测得的面强度F(N)相对于玻璃板的板厚t(mm)为F≥1000×t2。5. The method for producing a chemically strengthened glass according to any one of 1 to 4 above, wherein the glass after the chemical strengthening step has a surface strength measured by a ball-and-ring test under the following conditions F(N) is F≧1000×t2 with respect to the plate thickness t (mm) of the glass plate.
球环试验条件:Ball ring test conditions:
将板厚t(mm)的玻璃板配置在直径30mm、接触部具有曲率半径2.5mm的圆角的由不锈钢构成的环上,在使直径10mm的由钢构成的球体与该玻璃板接触的状态下,使该球体以下降速度1mm/min下降而在该环的中心施加负载,将玻璃板被破坏时的破坏负载(单位N)设为BOR强度,将该BOR强度的20次的测定平均值设为面强度F(N)。其中,从用于计算平均值的数据中排除在玻璃板的破坏起点离开该球体的负载点2mm以上时所得的数据。A glass plate of plate thickness t (mm) is placed on a ring made of stainless steel with a diameter of 30 mm and a contact portion having a fillet with a radius of curvature of 2.5 mm, and a sphere made of steel with a diameter of 10 mm is brought into contact with the glass plate Then, the sphere was lowered at a lowering speed of 1 mm/min, a load was applied to the center of the ring, the breaking load (unit N) when the glass plate was broken was defined as the BOR strength, and the average value of the 20 measurements of the BOR strength was Let it be the surface strength F(N). However, data obtained when the starting point of failure of the glass plate is 2 mm or more away from the load point of the sphere are excluded from the data for calculating the average value.
本发明的化学强化玻璃的制造方法中,通过使用pH为规定范围的无机盐对玻璃进行化学强化,利用无机盐中的OH-将玻璃的Si-O-Si键适度地切断,在玻璃表面形成压缩应力层的表层被改性的低密度层。然后,通过进行酸处理,能够将该低密度层均匀地除去,即使不进行研磨处理,也能够有效地显著提高玻璃的面强度。In the manufacturing method of the chemically strengthened glass of the present invention, the glass is chemically strengthened using an inorganic salt having a pH in a predetermined range, and the Si—O—Si bond of the glass is appropriately cut by OH− in the inorganic salt, and the glass is formed on the surface of the glass. A low-density layer in which the surface layer of the compressive stress layer is modified. Then, by performing the acid treatment, the low-density layer can be uniformly removed, and the surface strength of the glass can be effectively and remarkably improved even without the polishing treatment.
因此,根据本发明的化学强化玻璃的制造方法,能够简单地得到化学强化的温度条件、时间不受限制,即使在高温下进行长时间的化学强化处理也显示较深的DOC并且面强度高的化学强化玻璃。Therefore, according to the manufacturing method of the chemically strengthened glass of the present invention, the temperature conditions and time for chemical strengthening can be easily obtained, and even if the chemical strengthening treatment is performed at a high temperature for a long time, a deep DOC and a high surface strength are exhibited. Chemically strengthened glass.
附图说明Description of drawings
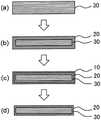
图1(a)~(d)是表示本发明的化学强化玻璃的制造工序的示意图。Fig.1 (a) - (d) are schematic diagrams which show the manufacturing process of the chemically strengthened glass of this invention.
图2是用于说明球环试验的方法的简图。FIG. 2 is a schematic diagram for explaining the method of the ball-and-ring test.
图3A是具有表面研磨伤的玻璃表面的AFM图像,图3B是不具有表面研磨伤的玻璃表面的AFM图像。FIG. 3A is an AFM image of a glass surface with surface abrasion damage, and FIG. 3B is an AFM image of a glass surface without surface abrasion damage.
图4A是表示在玻璃面内未产生白雾的状态的图,图4B是表示在玻璃面内产生有白雾的状态的图。FIG. 4A is a diagram showing a state in which white fog is not generated in the glass surface, and FIG. 4B is a diagram showing a state in which white fog is generated in the glass surface.
图5A表示实施例1和3以及比较例1中得到的化学强化玻璃的应力分布,图5B表示实施例7和8以及比较例6中得到的化学强化玻璃的应力分布,图5C表示实施例10和11以及比较例11中得到的化学强化玻璃的应力分布。5A shows the stress distribution of the chemically strengthened glasses obtained in Examples 1 and 3 and Comparative Example 1, FIG. 5B shows the stress distribution of the chemically strengthened glasses obtained in Examples 7 and 8 and Comparative Example 6, and FIG. 5C shows Example 10 and 11 and the stress distribution of the chemically strengthened glass obtained in Comparative Example 11.
图6A和图6B表示对实施例1和5以及比较例1、4和5中得到的化学强化玻璃的面强度进行评价而得的结果。6A and 6B show the results obtained by evaluating the surface strength of the chemically strengthened glasses obtained in Examples 1 and 5 and Comparative Examples 1, 4 and 5.
图7A和图7B表示对实施例7和8以及比较例6中得到的化学强化玻璃的面强度进行评价而得的结果。7A and 7B show the results obtained by evaluating the surface strength of the chemically strengthened glasses obtained in Examples 7 and 8 and Comparative Example 6. FIG.
图8A和图8B表示对实施例10和11以及比较例11中得到的化学强化玻璃的面强度进行评价而得的结果。8A and 8B show the results obtained by evaluating the surface strength of the chemically strengthened glasses obtained in Examples 10 and 11 and Comparative Example 11.
具体实施方式Detailed ways
以下,对本发明进行详细说明,但本发明并不限定于以下的实施方式,可以在不脱离本发明的主旨的范围内任意地变形而实施。Hereinafter, although this invention is demonstrated in detail, this invention is not limited to the following embodiment, It can deform|transform arbitrarily in the range which does not deviate from the summary of this invention, and can implement.
在此,本说明书中,“质量%”与“重量%”、“质量ppm”与“重量ppm”分别为相同含义。另外,在仅记载为“ppm”的情况下,表示“重量ppm”。Here, in this specification, "mass %" and "weight %", "mass ppm" and "weight ppm" have the same meaning, respectively. In addition, when describing only as "ppm", it shows "weight ppm".
另外,本说明书中,表示数值范围的“~”以包含其前后所记载的数值作为下限值和上限值的含义使用,只要没有特别说明,则以下在本说明书中“~”以同样的含义使用。In addition, in the present specification, "to" indicating a numerical range is used to include the numerical values before and after it as the lower limit value and the upper limit value. Unless otherwise specified, "to" will be used in the same description hereinafter in this specification. Meaning use.
<化学强化玻璃的制造方法><Manufacturing method of chemically strengthened glass>
以下,对制造本发明的化学强化玻璃的方法(以下,也称为本发明的方法)的一个方案进行说明,但本发明并不限定于此。应予说明,只要没有特别说明,则玻璃的组成以氧化物基准的摩尔百分率计。Hereinafter, one aspect of the method for producing the chemically strengthened glass of the present invention (hereinafter, also referred to as the method of the present invention) will be described, but the present invention is not limited to this. In addition, unless otherwise specified, the composition of glass is a molar percentage based on an oxide.
(化学强化工序)(chemical strengthening process)
本发明的方法中的化学强化工序是如下工序:使玻璃与制成10质量%水溶液时的氢离子指数(pH)为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐接触,将上述玻璃中的Na与上述无机盐中的K进行离子交换而在玻璃表面形成压缩应力层,并进一步形成该压缩应力层的表层发生改性而低密度化的低密度层。The chemical strengthening step in the method of the present invention is a step of bringing glass into contact with an inorganic salt containing at least one of sodium nitrate and potassium nitrate, which has a hydrogen ion index (pH) of 7.5 to 10.5 in a 10 mass % aqueous solution , Na in the glass is ion-exchanged with K in the inorganic salt to form a compressive stress layer on the glass surface, and further a low-density layer in which the surface layer of the compressive stress layer is modified and reduced in density is formed.
无机盐在制成10质量%水溶液时的氢离子指数(pH)为7.5以上,优选为8.0以上,更优选为8.5以上。另外,制成10质量%水溶液时的氢离子指数(pH)为10.5以下,优选为10.0以下,更优选为9.5以下。The hydrogen ion index (pH) of the inorganic salt in a 10 mass % aqueous solution is 7.5 or more, preferably 8.0 or more, and more preferably 8.5 or more. Moreover, the hydrogen ion index (pH) at the time of making into a 10 mass % aqueous solution is 10.5 or less, Preferably it is 10.0 or less, More preferably, it is 9.5 or less.
通过使无机盐的pH为上述范围,能够利用无机盐中的OH-将玻璃的Si-O-Si键适度地切断,在玻璃表面形成压缩应力层的表层发生改性的低密度层。无机盐的pH可在25℃下使用堀场制作所制造的便携式pH计D-71S等pH计进行测定。By setting the pH of the inorganic salt to be in the above range, the Si—O—Si bond of the glass is appropriately cut by OH− in the inorganic salt, and a low-density layer in which the surface layer of the compressive stress layer is modified can be formed on the glass surface. The pH of the inorganic salt can be measured at 25°C using a pH meter such as a portable pH meter D-71S manufactured by Horiba Corporation.
无机盐优选含有选自KNO2、NaNO2、K2CO3、Na2CO3、KHCO3、NaHCO3、KOH或NaOH中的至少一种盐,可通过上述盐的含量而适当调整无机盐的pH。The inorganic salt preferably contains at least one salt selected from KNO2 , NaNO2 , K2 CO3 , Na2 CO3 , KHCO3 , NaHCO3 , KOH and NaOH, and the content of the inorganic salt can be appropriately adjusted by the content of the above salt. pH.
无机盐含有硝酸钠和硝酸钾中的至少一者。通过使无机盐含有硝酸钠和硝酸钾中的至少一者,在玻璃的应变点以下成为熔融状态,且在实施化学强化处理时的一般的温度区域容易操作。通过使无机盐含有硝酸钠,可得到CTlimit值以下且DOC大的化学强化玻璃。应予说明,已知CTlimit值在经验上为-38.7×ln(t)+48.2[MPa]。在此,t表示玻璃的板厚,单位为mm。The inorganic salt contains at least one of sodium nitrate and potassium nitrate. By making the inorganic salt contain at least one of sodium nitrate and potassium nitrate, it becomes a molten state below the strain point of glass, and it becomes easy to handle in a general temperature range when chemical strengthening treatment is performed. By making the inorganic salt contain sodium nitrate, a chemically strengthened glass with a CT limit value or less and a large DOC can be obtained. In addition, it is known that the CT limit value is -38.7×ln(t)+48.2 [MPa] empirically. Here, t represents the thickness of the glass, and the unit is mm.
无机盐中的硝酸钠的含量优选为1质量%以上,更优选为5质量%以上。在此,无机盐中的硝酸钠的含量是指无机盐为液体状态的液相盐的钠浓度。应予说明,作为无机盐中的硝酸钠的含量的上限,没有特别限制。The content of sodium nitrate in the inorganic salt is preferably 1% by mass or more, and more preferably 5% by mass or more. Here, the content of sodium nitrate in the inorganic salt refers to the sodium concentration of the liquid-phase salt in which the inorganic salt is in a liquid state. In addition, there is no restriction|limiting in particular as an upper limit of content of sodium nitrate in an inorganic salt.
通过无机盐中的硝酸钠的含量为1质量%以上,在玻璃的应变点以下成为熔融状态,且在实施化学强化处理时的一般的温度区域容易操作。无机盐中的硝酸钠的含量是以可得到期望的表面压缩应力值(CS,单位为MPa)的方式适当调整而决定的。When the content of sodium nitrate in the inorganic salt is 1 mass % or more, it becomes a molten state below the strain point of glass, and it is easy to handle in a general temperature range when chemical strengthening treatment is performed. The content of sodium nitrate in the inorganic salt is appropriately adjusted so that a desired surface compressive stress value (CS, in MPa) can be obtained.
无机盐除硝酸钠或硝酸钾以外,还可以在不会损害本发明的效果的范围内含有其它化学种,例如可举出氯化钠、氯化钾、硼酸钠和硼酸钾等碱金属氯化盐以及碱金属硼酸盐等。它们可以单独添加,也可以组合添加多种。Inorganic salts other than sodium nitrate or potassium nitrate may contain other chemical species within the range that does not impair the effect of the present invention, for example, alkali metal chlorides such as sodium chloride, potassium chloride, sodium borate, and potassium borate can be mentioned. salts and alkali metal borates. They can be added individually or in combination.
在上述无机盐中含有KNO2的情况下,无机盐中的KNO2的含量优选为0.2质量%以上,更优选为0.4质量%以上,进一步优选为0.6质量%以上。另外,优选为10.0质量%以下,更优选为8.0质量%以下,进一步优选为6.0质量%以下。通过使KNO2的含量为上述范围,能够使制成10质量%水溶液时的无机盐的pH为7.5~10.5。WhenKNO2 is contained in the said inorganic salt, content ofKNO2 in an inorganic salt becomes like this. Preferably it is 0.2 mass % or more, More preferably, it is 0.4 mass % or more, More preferably, it is 0.6 mass % or more. Moreover, 10.0 mass % or less is preferable, 8.0 mass % or less is more preferable, and 6.0 mass % or less is still more preferable. By making content ofKNO2 into the said range, the pH of the inorganic salt at the time of making into a 10 mass % aqueous solution can be 7.5-10.5.
作为使玻璃与无机盐接触的方法,可以为涂布糊状的无机盐的方法、对玻璃喷射无机盐的水溶液的方法、将玻璃浸渍于加热至熔点以上的熔融盐的盐浴中的方法等,它们之中,优选在熔融盐中浸渍的方法。The method of bringing the glass into contact with the inorganic salt includes a method of applying a paste-like inorganic salt, a method of spraying an aqueous solution of the inorganic salt on the glass, a method of immersing the glass in a salt bath of a molten salt heated to a melting point or higher, and the like. , among them, the method of dipping in molten salt is preferable.
本发明的方法中使用的玻璃只要含有钠即可,只要具有能够进行成型、利用化学强化处理进行强化的组成就可以采用各种玻璃。具体而言,例如可举出铝硅酸盐玻璃、钠钙玻璃、硼硅酸盐玻璃、铅玻璃、碱钡玻璃和铝硼硅酸盐玻璃等。The glass used in the method of the present invention only needs to contain sodium, and any glass can be used as long as it has a composition capable of being molded and strengthened by chemical strengthening treatment. Specifically, aluminosilicate glass, soda lime glass, borosilicate glass, lead glass, alkali barium glass, aluminoborosilicate glass, etc. are mentioned, for example.
玻璃的制造方法没有特别限定,可以通过如下操作制造:向连续熔融炉投入期望的玻璃原料,将玻璃原料优选在1500~1600℃进行加热熔融,澄清后,供给到成型装置,然后将熔融玻璃成型为板状并缓慢冷却。The production method of glass is not particularly limited, and it can be produced by charging a desired glass raw material into a continuous melting furnace, heating and melting the glass raw material preferably at 1500 to 1600° C., and clarifying it, supplying it to a molding apparatus, and then molding the molten glass. Plate and cool slowly.
应予说明,玻璃的成型可采用各种方法。例如可以采用下拉法(例如,溢流下拉法、流孔下引法和再曳引法等)、浮法、辊压法和压制法等各种成型方法。In addition, various methods can be employ|adopted for shaping|molding of glass. For example, various molding methods such as a down-draw method (for example, an overflow down-draw method, an orifice down-draw method, a redraw method, etc.), a float method, a rolling method, and a pressing method can be employed.
玻璃的厚度没有特别限制,为了有效地进行化学强化处理,优选为3mm以下,更优选为2mm以下,进一步优选为1mm以下。The thickness of the glass is not particularly limited, but in order to efficiently perform the chemical strengthening treatment, it is preferably 3 mm or less, more preferably 2 mm or less, and even more preferably 1 mm or less.
另外,本发明的方法中使用的玻璃的形状没有特别限定。例如可以采用具有均匀板厚的平板形状、在表面和背面中的至少一面具有曲面的形状和具有弯曲部等的立体形状等各种形状的玻璃。In addition, the shape of the glass used in the method of this invention is not specifically limited. For example, glass of various shapes such as a flat plate shape having a uniform plate thickness, a shape having a curved surface on at least one of the front and rear surfaces, and a three-dimensional shape having a curved portion or the like can be used.
作为本发明的方法中使用的玻璃的组成的具体例,例如可举出以下的玻璃的组成。As a specific example of the composition of the glass used by the method of this invention, the following glass composition is mentioned, for example.
(i)以氧化物基准的摩尔百分率计,含有56~72%的SiO2、5~18%的Al2O3、0~15%的B2O3和0.1~10%的P2O5,并且Na2O与K2O的合计含量为3~30%的玻璃。(i) 56-72% SiO2 , 5-18% Al2 O3 , 0-15% B2 O3 , and 0.1-10% P2 O5 in molar percentages based on oxides , and the total content of Na2 O and K2 O is 3 to 30% glass.
(ii)以氧化物基准的摩尔百分率计,含有55.5~80%的SiO2、12~20%的Al2O3、8~25%的Na2O、2.5%以上的P2O5和1%以上的碱土金属RO(RO为MgO+CaO+SrO+BaO)的玻璃。(ii) 55.5 to 80% of SiO2 , 12 to 20% of Al2 O3 , 8 to 25% of Na2 O , 2.5% or more of P2 O5 and 1 in molar percentages based on oxides % or more of alkaline earth metal RO (RO is MgO+CaO+SrO+BaO) glass.
(iii)以氧化物基准的摩尔百分率计,含有57~76.5%的SiO2、12~18%的Al2O3、8~25%的Na2O、2.5~10%的P2O5和1%以上的碱土金属RO的玻璃。(iii) 57-76.5% of SiO2 , 12-18% of Al2 O3 , 8-25% of Na2 O , 2.5-10% of P2 O5 and Glass with more than 1% alkaline earth metal RO.
(iv)以氧化物基准的摩尔百分率计,含有56~72%的SiO2、8~20%的Al2O3、3~20%的B2O3、8~25%的Na2O、0~5%的K2O、0~15%的MgO、0~15%的CaO、0~15%的SrO2、0~15%的BaO和0~8%的ZrO2的玻璃。(iv) 56-72% of SiO2 , 8-20% of Al2 O3 , 3-20% of B2 O3 , 8-25% of Na2 O , Glass of 0-5% K2 O, 0-15% MgO, 0-15% CaO, 0-15% SrO2 , 0-15% BaO and 0-8% ZrO2 .
(v)以氧化物基准的摩尔百分率计,含有50~80%的SiO2、2~25%的Al2O3、0~10%的Li2O、0~18%的Na2O、0~10%的K2O、0~15%的MgO、0~5%的CaO和0~5%的ZrO2的玻璃。(v) 50-80% SiO2 , 2-25% Al2 O3 , 0-10% Li2 O, 0-18% Na2 O, 0 Glass of ~10 % K2O, 0-15% MgO, 0-5% CaO, and 0-5% ZrO2.
(vi)以氧化物基准的摩尔百分率计,含有50~74%的SiO2、1~10%的Al2O3、6~14%的Na2O、3~11%的K2O、2~15%的MgO、0~6%的CaO和0~5%的ZrO2,并且SiO2与Al2O3的合计含量为75%以下,Na2O与K2O的合计含量为12~25%,MgO与CaO的合计含量为7~15%的玻璃。(vi) 50-74% of SiO2 , 1-10% of Al2 O3 , 6-14% of Na2 O, 3-11% of K2 O, 2 ~15% of MgO, 0 to 6% of CaO, and 0 to 5% of ZrO2 , and the total content of SiO2 and Al2 O3 is 75% or less, and the total content of Na2 O and K2 O is 12- 25%, and the total content of MgO and CaO is 7 to 15% of the glass.
(vii)以氧化物基准的摩尔百分率计,含有68~80%的SiO2、4~10%的Al2O3、5~15%的Na2O、0~1%的K2O、4~15%的MgO和0~1%的ZrO2的玻璃。(vii) 68-80% of SiO2 , 4-10% of Al2 O3 , 5-15% of Na2 O, 0-1% of K2 O, 4 Glass of ~15% MgO and 0~1 % ZrO2.
(viii)以氧化物基准的摩尔百分率计,含有67~75%的SiO2、0~4%的Al2O3、7~15%的Na2O、1~9%的K2O、6~14%的MgO和0~1.5%的ZrO2,并且SiO2与Al2O3的合计含量为71~75%,Na2O与K2O的合计含量为12~20%,在含有CaO的情况下其含量小于1%的玻璃。(viii) 67-75% of SiO2 , 0-4% of Al2 O3 , 7-15% of Na2 O, 1-9% of K2 O, 6 ~14% of MgO and 0 to 1.5% of ZrO2 , and the total content of SiO2 and Al2 O3 is 71 to 75%, and the total content of Na2 O and K2 O is 12 to 20%. In the case of glass whose content is less than 1%.
(ix)以氧化物基准的质量%计,含有65~75%的SiO2、0.1~5%的Al2O3、1~6%的MgO和1~15%的CaO,并且Na2O+K2O为10~18%的玻璃。(ix) 65-75% of SiO2 , 0.1-5% of Al2 O3 , 1-6% of MgO and 1-15% of CaO in mass % based on oxide, and Na2 O+ K2 O is 10-18% glass.
(x)以氧化物基准的质量%计,含有60~72%的SiO2、1~10%的Al2O3、5~12%的MgO、0.1~5%的CaO、13~19%的Na2O和0~5%的K2O,并且RO/(RO+R2O)为0.20~0.42(式中,RO表示碱土金属氧化物,R2O表示碱金属氧化物)的玻璃。(x) 60-72% of SiO2 , 1-10% of Al2 O3 , 5-12% of MgO, 0.1-5% of CaO, 13-19% of Glass with Na2 O and 0 to 5% of K2 O, and RO/(RO+R2 O) of 0.20 to 0.42 (in the formula, RO represents an alkaline earth metal oxide, and R2 O represents an alkali metal oxide).
化学强化处理通过在熔融盐浴内将玻璃浸渍于无机盐的熔融盐中,将玻璃中的金属离子(Na离子)置换为熔融盐中的离子半径大的金属离子(K离子)而进行。通过该离子交换,能够改变玻璃表面的组成,形成玻璃表面高密度化的压缩应力层20[图1(a)~(b)]。通过该玻璃表面的高密度化而产生压缩应力,因此,能够将玻璃强化。The chemical strengthening treatment is performed by immersing glass in a molten salt of inorganic salt in a molten salt bath, and substituting metal ions (Na ions) in the glass with metal ions (K ions) having a large ionic radius in the molten salt. By this ion exchange, the composition of the glass surface can be changed, and the
本发明的方法中的化学强化工序中,在进行化学强化时,通过使用制成10质量%水溶液时的氢离子指数(pH)为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐进行化学强化处理,从而利用无机盐中的OH-将玻璃的Si-O-Si键适度地切断,形成压缩应力层的表层改性而低密度化的低密度层10[图1(b)~(c)]。In the chemical strengthening step in the method of the present invention, when chemical strengthening is performed, a hydrogen ion index (pH) when a 10 mass % aqueous solution is used is 7.5 to 10.5 and contains at least one of sodium nitrate and potassium nitrate. The inorganic salt is chemically strengthened, so that the Si-O-Si bond of the glass is appropriately cut by theOH- in the inorganic salt, and the surface layer of the compressive stress layer is modified and the low-
应予说明,实际上,化学强化玻璃的密度从存在于玻璃中心的中间层30(本体)的外缘向压缩应力层表面缓慢地高密度化,因此,在中间层30与压缩应力层20之间没有密度急剧变化的明确的边界。在此,中间层是指存在于玻璃中心部且被压缩应力层夹持的层。该中间层与压缩应力层不同,是不进行离子交换的层。In fact, the density of the chemically strengthened glass gradually increases from the outer edge of the intermediate layer 30 (main body) existing in the center of the glass toward the surface of the compressive stress layer. Therefore, between the
化学强化工序具体而言可如下进行。在化学强化工序中,将玻璃预热,将熔融盐调整到化学强化的处理温度。接着,将已预热的玻璃在熔融盐中浸渍规定时间后,从熔融盐中提起玻璃,放冷。应予说明,优选在化学强化处理之前根据用途对玻璃进行形状加工,例如切断、端面加工和开孔加工等机械加工。Specifically, the chemical strengthening step can be performed as follows. In a chemical strengthening process, glass is preheated, and molten salt is adjusted to the processing temperature of chemical strengthening. Next, after immersing the preheated glass in molten salt for a predetermined time, the glass is lifted from the molten salt and left to cool. In addition, it is preferable to subject glass to shape processing, for example, mechanical processing, such as cutting, end surface processing, and drilling processing, according to the application before the chemical strengthening process.
玻璃的预热温度依赖于在熔融盐中浸渍的温度,一般优选为100℃以上。The preheating temperature of the glass depends on the temperature at which it is immersed in molten salt, but is generally preferably 100°C or higher.
从得到具备较深的DOC的化学强化玻璃的观点考虑,进行化学强化的温度优选为400℃以上,更优选为450℃以上,进一步优选为470℃以上。进行化学强化的温度的上限没有特别限制,典型而言,优选为被强化玻璃的应变点(通常500~600℃)以下。From the viewpoint of obtaining a chemically strengthened glass having a deep DOC, the temperature at which chemical strengthening is performed is preferably 400°C or higher, more preferably 450°C or higher, and further preferably 470°C or higher. The upper limit of the temperature at which chemical strengthening is performed is not particularly limited, but typically, it is preferably not more than the strain point (usually 500 to 600° C.) of the tempered glass.
玻璃在熔融盐中的浸渍时间取决于化学强化温度,但从得到具备较深的DOC的化学强化玻璃的观点考虑,优选为2小时以上,更优选为4小时以上,进一步优选为8小时以上。上限没有特别限制,通常为48小时以下,如果为24小时以下,则从生产率的观点考虑更优选。The immersion time of the glass in the molten salt depends on the chemical strengthening temperature, but from the viewpoint of obtaining a chemically strengthened glass having a deep DOC, it is preferably 2 hours or more, more preferably 4 hours or more, and even more preferably 8 hours or more. The upper limit is not particularly limited, but it is usually 48 hours or less, and it is more preferable from the viewpoint of productivity if it is 24 hours or less.
从对玻璃赋予充分的强度的观点考虑,化学强化工序后的形成于玻璃的表层的压缩应力层的深度(DOC)优选为35μm以上,更优选为45μm以上,进一步优选为55μm以上。From the viewpoint of imparting sufficient strength to the glass, the depth (DOC) of the compressive stress layer formed on the surface layer of the glass after the chemical strengthening step is preferably 35 μm or more, more preferably 45 μm or more, and still more preferably 55 μm or more.
通过本发明的方法制造的化学强化玻璃的压缩应力值优选为100MPa以上,更优选为200MPa以上,进一步优选为300MPa以上。另外,上限没有特别限制,典型而言为1200MPa以下。The compressive stress value of the chemically strengthened glass produced by the method of the present invention is preferably 100 MPa or more, more preferably 200 MPa or more, and further preferably 300 MPa or more. In addition, the upper limit is not particularly limited, but is typically 1200 MPa or less.
压缩应力层的深度可使用EPMA(electron probe micro analyzer)或表面应力计(例如,折原制作所制造的FSM-6000)等进行测定。The depth of the compressive stress layer can be measured using an EPMA (electron probe micro analyzer) or a surface stress meter (for example, FSM-6000 manufactured by Orihara Corporation).
低密度层通过后述的酸处理工序而除去,因此,低密度层越厚越容易除去玻璃表面。因此,从玻璃表面除去量的观点考虑,低密度层的厚度优选为10nm以上,更优选为20nm以上。低密度层的厚度可通过化学强化工序的熔融盐中的钠浓度、温度或时间等进行控制。The low-density layer is removed by the acid treatment step described later, and therefore, the thicker the low-density layer, the easier it is to remove the glass surface. Therefore, the thickness of the low-density layer is preferably 10 nm or more, and more preferably 20 nm or more, from the viewpoint of the amount of removal from the glass surface. The thickness of the low-density layer can be controlled by the sodium concentration, temperature, time, etc. in the molten salt of the chemical strengthening process.
通过酸处理工序除去低密度层后,进行碱处理,由此,能够进一步除去低密度层。After removing the low-density layer by the acid treatment step, the low-density layer can be further removed by performing an alkali treatment.
从玻璃表面除去性的观点考虑,低密度层的密度优选低于比经离子交换的压缩应力层深的区域(本体)的密度。From the viewpoint of glass surface removability, the density of the low-density layer is preferably lower than the density of a region (bulk) deeper than the ion-exchanged compressive stress layer.
低密度层的厚度由通过X射线反射率法(X-ray-Reflectometry,XRR)测得的周期(Δθ)求出。低密度层的密度由通过XRR测得的临界角(θc)求出。应予说明,简单地通过利用扫描式电子显微镜(SEM)观察玻璃的截面,也能够确认低密度层的形成和层的厚度。The thickness of the low-density layer is determined from the period (Δθ) measured by X-ray-Reflectometry (XRR). The density of the low-density layer was determined from the critical angle (θc) measured by XRR. In addition, the formation of a low density layer and the thickness of a layer can also be confirmed simply by observing the cross section of glass with a scanning electron microscope (SEM).
在化学强化工序中,也可与利用制成10质量%水溶液时的氢离子指数(pH)为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐进行的上述化学强化处理组合,将上述的化学强化处理和变更了无机盐的组成、氢离子指数、化学强化的温度和化学强化的时间的条件中的至少一个条件的化学强化处理工序在上述的化学强化处理工序的前后进行多次。In the chemical strengthening step, the chemical strengthening treatment may be combined with the above-mentioned chemical strengthening treatment using an inorganic salt having a hydrogen ion index (pH) of 7.5 to 10.5 and containing at least one of sodium nitrate and potassium nitrate when prepared as a 10 mass % aqueous solution. The chemical strengthening treatment process described above and the chemical strengthening treatment process in which at least one of the conditions of the composition of the inorganic salt, the hydrogen ion index, the temperature for chemical strengthening, and the time for chemical strengthening is changed are performed before and after the chemical strengthening treatment process. repeatedly.
在化学强化工序后,使用工业用水、离子交换水等进行玻璃的清洗。其中,优选离子交换水。清洗的条件也根据使用的清洗液而不同,在使用离子交换水的情况下,如果在0~100℃进行清洗,则从完全地除去附着的盐的方面考虑优选。After the chemical strengthening process, the glass is washed using industrial water, ion-exchanged water, or the like. Among them, ion-exchanged water is preferable. The cleaning conditions also vary depending on the cleaning liquid to be used. In the case of using ion-exchanged water, cleaning at 0 to 100° C. is preferable in terms of completely removing the adhering salt.
(酸处理工序)(acid treatment process)
在酸处理工序中,对在化学强化工序后进行了清洗的玻璃进一步进行酸处理。玻璃的酸处理通过使玻璃与氢离子指数(pH)小于7.0的酸性溶液中接触而进行。In the acid treatment step, the glass washed after the chemical strengthening step is further subjected to acid treatment. The acid treatment of the glass is carried out by contacting the glass with an acidic solution having a hydrogen ion index (pH) of less than 7.0.
酸处理中使用的溶液只要为酸性就没有特别限制,只要pH小于7.0即可,所使用的酸可以为弱酸,也可以为强酸。具体而言,优选盐酸、硝酸、硫酸、磷酸、乙酸、草酸、碳酸或柠檬酸等酸。这些酸可以单独使用,也可以组合使用多种。The solution used for the acid treatment is not particularly limited as long as it is acidic, and the acid to be used may be a weak acid or a strong acid as long as the pH is less than 7.0. Specifically, acids such as hydrochloric acid, nitric acid, sulfuric acid, phosphoric acid, acetic acid, oxalic acid, carbonic acid, or citric acid are preferable. These acids may be used alone or in combination of two or more.
进行酸处理的温度也根据使用的酸的种类、浓度、时间而不同,优选100℃以下。另外,从容易除去低密度层的观点考虑,优选20℃以上。进行酸处理的时间虽然也根据使用的酸的种类、浓度、温度而不同,但从生产率的方面考虑,优选10秒~5小时,更优选1分钟~2小时。The temperature at which the acid treatment is performed also varies depending on the type, concentration, and time of the acid used, but is preferably 100° C. or lower. In addition, from the viewpoint of easy removal of the low density layer, it is preferably 20°C or higher. The time for performing the acid treatment varies depending on the type, concentration, and temperature of the acid to be used, but from the viewpoint of productivity, it is preferably 10 seconds to 5 hours, and more preferably 1 minute to 2 hours.
进行酸处理的溶液的浓度虽然根据使用的酸的种类、时间、温度而不同,但优选容器腐蚀的顾虑小的浓度,具体而言,优选0.1质量%~20质量%。The concentration of the solution for acid treatment varies depending on the type of acid used, time, and temperature, but is preferably a concentration with little concern for corrosion of the container, and specifically, is preferably 0.1% by mass to 20% by mass.
作为酸处理的条件,具体而言,例如可举出使化学强化工序后的玻璃与优选35~75℃的0.1质量%~10质量%硝酸水溶液接触1~15分钟的条件。As a condition of an acid treatment, for example, the conditions which contact the glass after a chemical strengthening process with the 0.1 mass % - 10 mass % nitric acid aqueous solution preferably at 35-75 degreeC are mentioned for 1 to 15 minutes.
通过上述酸处理,使玻璃表面的低密度化加速,露出低密度层的一部分或全部被除去的表层[图1(c)和(d)]。由此,可得到面强度显著提高的化学强化玻璃。进而,通过除去低密度层,存在于玻璃表面的损伤也同时被除去,认为在该方面也有助于提高强度。By the above-mentioned acid treatment, the dedensification of the glass surface is accelerated, and the surface layer from which a part or all of the low density layer has been removed is exposed [ FIGS. 1( c ) and ( d )]. Thereby, the chemically strengthened glass whose surface strength is remarkably improved can be obtained. Furthermore, by removing the low density layer, the damage existing on the glass surface is also removed at the same time, and it is considered that this also contributes to the improvement of the strength.
(碱处理工序)(alkali treatment step)
在本发明的方法中,可以接着酸处理后进行碱处理。通过进行碱处理,与仅酸处理的情况相比,能够增加低密度层的除去量而进一步提高面强度。In the method of the present invention, alkali treatment may be performed following acid treatment. By performing the alkali treatment, the removal amount of the low-density layer can be increased, and the surface strength can be further improved as compared with the case of the acid treatment alone.
碱处理中使用的溶液只要为碱性就没有特别限制,只要pH超过7.0即可,可以使用弱碱,也可以使用强碱。具体而言,优选氢氧化钠、氢氧化钾、碳酸钾或碳酸钠等碱。这些碱可以单独使用,也可以组合使用多种。The solution used for the alkali treatment is not particularly limited as long as the pH exceeds 7.0, and a weak base or a strong base may be used. Specifically, alkalis, such as sodium hydroxide, potassium hydroxide, potassium carbonate, or sodium carbonate, are preferable. These bases may be used alone or in combination of two or more.
进行碱处理的温度也根据使用的碱的种类、浓度、时间而不同,但优选为0~100℃,更优选为10~80℃,特别优选为20~60℃。如果为该温度范围,则没有玻璃腐蚀的顾虑,因此优选。The temperature at which the alkali treatment is performed also varies depending on the type, concentration, and time of the base used, but is preferably 0 to 100°C, more preferably 10 to 80°C, and particularly preferably 20 to 60°C. If it is this temperature range, since there is no concern of glass corrosion, it is preferable.
进行碱处理的时间虽然也根据使用的碱的种类、浓度、温度而不同,但从生产率的方面考虑,优选为10秒~5小时,更优选为1分钟~2小时。进行碱处理的溶液的浓度虽然根据使用的碱的种类、时间、温度而不同,但从玻璃表面除去性的观点考虑,优选为0.1质量%~20质量%。The time for performing the alkali treatment also varies depending on the type, concentration, and temperature of the alkali to be used, but from the viewpoint of productivity, it is preferably 10 seconds to 5 hours, and more preferably 1 minute to 2 hours. Although the density|concentration of the solution which performs an alkali treatment changes with the kind of alkali used, time, and temperature, it is preferable that it is 0.1 mass % - 20 mass % from the viewpoint of glass surface removability.
作为碱处理的条件,具体而言,例如可举出使酸处理工序后的玻璃与优选35~75℃的0.1质量%~10%质量%氢氧化钠水溶液接触1~15分钟的条件。Specific examples of the conditions for the alkali treatment include conditions in which the glass after the acid treatment step is brought into contact with a 0.1 to 10% by mass sodium hydroxide aqueous solution preferably at 35 to 75°C for 1 to 15 minutes.
通过上述碱处理,与酸处理工序后的玻璃相比,露出低密度层被进一步除去的表层。由此,可得到面强度进一步提高的化学强化玻璃。另外,存在于玻璃表面的损伤也被进一步除去,认为在该方面也有助于进一步提高面强度。By the above-mentioned alkali treatment, the surface layer from which the low-density layer was further removed is exposed compared to the glass after the acid treatment step. Thereby, the chemically strengthened glass whose surface strength is further improved can be obtained. In addition, the damage existing on the glass surface is also removed, and it is considered that it contributes to the further improvement of the surface strength also in this respect.
应予说明,优选在上述酸处理工序与碱处理工序之间或者碱处理工序结束后具有与化学强化工序后的清洗工序同样的清洗工序。In addition, it is preferable to have the same washing|cleaning process as the washing|cleaning process after a chemical strengthening process between the said acid-treatment process and an alkali-processing process or after completion|finish of an alkali-processing process.
应予说明,被除去的低密度层的量取决于酸处理工序、以及酸处理工序和碱处理工序中的至少一者的条件。图1(d)示出低密度层10被全部除去的样态,但也可以将低密度层10的一部分除去而残留一部分。从提高强度的观点考虑,即使低密度层未被全部去除也可得到效果。It should be noted that the amount of the removed low-density layer depends on the acid treatment step and the conditions of at least one of the acid treatment step and the alkali treatment step. FIG. 1( d ) shows a state in which the low-
<化学强化玻璃><Chemical Tempered Glass>
通过本发明的方法制造的化学强化玻璃的面强度可通过下述所示的球环试验进行评价。The surface strength of the chemically strengthened glass produced by the method of the present invention can be evaluated by the ball-and-ring test shown below.
(球环试验)(Ball Ring Test)
由通过球环[Ball on Ring(BOR)]试验测得的BOR强度F(N)进行评价,该球环试验是将玻璃板配置在直径30mm、接触部具有曲率半径2.5mm的圆角的由不锈钢构成的环上,在使直径10mm的由钢构成的球体与该玻璃板接触的状态下,将该球体在静负载条件下负载于该环的中心。The evaluation was performed by the BOR strength F(N) measured by the Ball on Ring (BOR) test in which a glass plate was arranged on a 30 mm diameter and a contact portion with a fillet having a 2.5 mm radius of curvature. On a ring made of stainless steel, a sphere made of steel having a diameter of 10 mm was placed in contact with the glass plate, and the sphere was loaded at the center of the ring under static load conditions.
由本发明制造的化学强化玻璃优选满足F≥1000×t2,更优选F≥1200×t2[式中,F为通过球环试验测得的BOR强度(N),t为玻璃板的板厚(mm)]。通过BOR强度F(N)为该范围,在进行薄板化时也显示优异的强度。The chemically strengthened glass produced by the present invention preferably satisfies F≥1000×t2 , more preferably F≥1200×t2 [wherein, F is the BOR strength (N) measured by the ball ring test, and t is the plate thickness of the glass plate (mm)]. When the BOR strength F(N) is in this range, it exhibits excellent strength even when thinning is performed.
图2表示用于说明球环试验的简图。球环[Ball on Ring(BOR)]试验中,在水平载置玻璃板1的状态下,使用SUS304制的加压夹具2(淬火钢,直径10mm,镜面抛光)对玻璃板1进行加压,测定玻璃板1的强度。FIG. 2 shows a schematic diagram for explaining the ball-and-ring test. In the Ball on Ring (BOR) test, in the state where the
图2中,在SUS304制的承受夹具3(直径30mm,接触部的曲率R为2.5mm,接触部为淬火钢,镜面抛光)上水平设置作为样品的玻璃板1。在玻璃板1的上方设置用于对玻璃板1进行加压的加压夹具2。在本实施的形态中,从玻璃板1的上方对玻璃板1的中央区域进行加压。In FIG. 2 , a
应予说明,试验条件如下。In addition, the test conditions are as follows.
加压夹具2的下降速度:1.0(mm/min)The descending speed of the pressing jig 2: 1.0 (mm/min)
此时,将玻璃板被破坏时的破坏负载(单位N)设为BOR强度,将该BOR强度的20次测定的平均值设为面强度F(N)。其中,在玻璃板的破坏起点离开该球体的负载点2mm以上的情况下,从用于计算平均值的数据中排除。At this time, the breaking load (unit N) at the time of breaking the glass plate was taken as the BOR strength, and the average value of 20 measurements of the BOR strength was taken as the surface strength F(N). However, when the starting point of failure of the glass plate was 2 mm or more away from the load point of the sphere, it was excluded from the data for calculating the average value.
由本发明的方法制造的化学强化玻璃的压缩应力层的深度(DOC)优选为35μm以上,更优选为45μm以上,进一步优选为55μm以上。The depth (DOC) of the compressive stress layer of the chemically strengthened glass produced by the method of the present invention is preferably 35 μm or more, more preferably 45 μm or more, and further preferably 55 μm or more.
通过酸处理工序、碱处理工序而被除去的低密度层的厚度如上所述,即使大于10nm左右,也如实施例那样为1000nm左右,因此,对于压缩应力层的深度(DOC)而言,化学强化工序中形成的深度(DOC)与酸处理工序、碱处理工序后的深度(DOC)大致相同。The thickness of the low-density layer removed by the acid treatment step and the alkali treatment step is, as described above, about 1000 nm, even if it is larger than about 10 nm, as in the example. Therefore, the depth of the compressive stress layer (DOC) is chemically The depth (DOC) formed in the strengthening step is substantially the same as the depth (DOC) after the acid treatment step and the alkali treatment step.
通过本发明的方法制造的化学强化玻璃的表面压缩应力值(CS)优选为100MPa以上,更优选为200MPa以上,进一步优选为300MPa以上。另外,上限没有特别限制,典型而言为1200MPa以下。The surface compressive stress value (CS) of the chemically strengthened glass produced by the method of the present invention is preferably 100 MPa or more, more preferably 200 MPa or more, and further preferably 300 MPa or more. In addition, the upper limit is not particularly limited, but is typically 1200 MPa or less.
压缩应力值可使用EPMA(Electron Probe Micro Analyzer)或表面应力计(例如,折原制作所制造的FSM-6000)等进行测定。压缩应力值可使用日本特开2016-142600号公报中公开的应力分布计算方法而计算。The compressive stress value can be measured using EPMA (Electron Probe Micro Analyzer) or a surface stress meter (for example, FSM-6000 manufactured by Orihara Corporation). The compressive stress value can be calculated using the stress distribution calculation method disclosed in Japanese Patent Laid-Open No. 2016-142600.
通过本发明的方法制造的化学强化玻璃的内部拉伸应力(CT)优选为72MPa以下,更优选为62MPa以下,进一步优选为52MPa以下。另外,下限没有特别限制,典型而言为20MPa以上。测定应力分布,将该应力分布用厚度进行积分,求出CT值。The internal tensile stress (CT) of the chemically strengthened glass produced by the method of the present invention is preferably 72 MPa or less, more preferably 62 MPa or less, and further preferably 52 MPa or less. In addition, the lower limit is not particularly limited, but is typically 20 MPa or more. The stress distribution was measured, and the stress distribution was integrated with the thickness to obtain the CT value.
另外,已知CTlimit值在经验上为-38.7×ln(t)+48.2[MPa]。在此,t表示玻璃的板厚,单位为mm。In addition, it is known that the CT limit value is empirically -38.7×ln(t)+48.2 [MPa]. Here, t represents the thickness of the glass, and the unit is mm.
通过本发明的方法制造的化学强化玻璃也可以在化学强化工序前进行对玻璃表面进行研磨的研磨工序而制造。在此,本发明中的研磨是指通过使用研磨粒切削玻璃表面而进行平滑化。The chemically strengthened glass manufactured by the method of this invention can also be manufactured by performing the grinding|polishing process of grinding|polishing the glass surface before a chemical strengthening process. Here, the grinding|polishing in this invention means smoothing by cutting the glass surface using abrasive grains.
另外,会因研磨工序而产生的研磨伤的有无可通过利用AFM(Atomic ForceMicroscope,原子力显微镜)的表面观察来进行辨别,在10μm×5μm区域内不存在2根以上的长度5μm以上、宽度0.1μm以上的划痕的情况下,可以说是在表面没有研磨伤的状态。图3A中示出具有表面研磨伤的状态,图3B中示出不具有表面研磨伤的状态。In addition, the presence or absence of polishing flaws that may be generated in the polishing process was identified by surface observation with AFM (Atomic Force Microscope), and there were no two or more 5 μm in length and 0.1 in width in a 10 μm×5 μm region. In the case of scratches of μm or more, it can be said that there is no polishing scratch on the surface. FIG. 3A shows a state with surface grinding flaws, and FIG. 3B shows a state without surface grinding flaws.
通过本发明的制造方法制造的化学强化玻璃,通过AFM表面观察测定的测定范围10μm×5μm的表面粗糙度Ra优选为0.2nm以上,更优选为0.25nm以上。另外,优选为1.5nm以下,更优选为1.2nm以下。应予说明,以往的未研磨的化学强化玻璃板的表面粗糙度通常为0.15nm以上且小于0.2nm。The chemically strengthened glass produced by the production method of the present invention preferably has a surface roughness Ra in a measurement range of 10 μm×5 μm measured by AFM surface observation of 0.2 nm or more, and more preferably 0.25 nm or more. In addition, it is preferably 1.5 nm or less, and more preferably 1.2 nm or less. In addition, the surface roughness of the conventional unpolished chemically strengthened glass plate is 0.15 nm or more and less than 0.2 nm normally.
实施例Example
以下,举出实施例和比较例对本发明进行具体说明,但本发明并不限定于这些实施例和比较例。Hereinafter, the present invention will be specifically described with reference to Examples and Comparative Examples, but the present invention is not limited to these Examples and Comparative Examples.
[化学强化玻璃的制作][Production of chemically strengthened glass]
根据下述所示的条件进行化学强化工序后,依次进行酸处理工序、碱处理工序和研磨工序,制作化学强化玻璃。应予说明,关于各实施例和比较例,将各工序的有无示于表1和2。After the chemical strengthening process was performed under the conditions shown below, the acid treatment process, the alkali treatment process, and the grinding process were sequentially performed to produce chemically strengthened glass. In addition, about each Example and a comparative example, the presence or absence of each process is shown in Tables 1 and 2.
(化学强化工序)(chemical strengthening process)
以成为表1和2所示的组成和pH的方式在SUS制的杯子中加入无机盐的材料,利用覆套式电阻加热器进行加热直至成为表1和2所示的温度,制备熔融盐。准备俯视时为50mm×50mm且表1和2所示的板厚的铝硅酸盐玻璃A~C,预热至200~400℃后,在表1和2所示的条件下进行离子交换处理后,冷却至室温附近,由此进行化学强化工序。得到的化学强化玻璃进行水洗,供至下一工序。应予说明,关于无机盐的组成,除表1和2所示的组成以外,KNO3合计为100质量%。另外,无机盐的pH是在25℃下利用堀场制作所制造的便携式pH计D-71S测定制成10质量%水溶液时的pH而得的值。An inorganic salt material was added to a cup made of SUS so that the composition and pH shown in Tables 1 and 2 were obtained, and the molten salt was prepared by heating with a jacketed resistance heater to the temperature shown in Tables 1 and 2. Aluminosilicate glasses A to C having a plan view of 50 mm × 50 mm and the plate thicknesses shown in Tables 1 and 2 were prepared, preheated to 200 to 400°C, and then subjected to ion exchange treatment under the conditions shown in Tables 1 and 2. Then, the chemical strengthening process is performed by cooling to room temperature vicinity. The obtained chemically strengthened glass is washed with water and used for the next step. In addition, regarding the composition of an inorganic salt, except for the composition shown in Tables 1 and 2,KNO3 is 100 mass % in total. In addition, the pH of an inorganic salt is the value obtained by measuring the pH at the time of making into a 10 mass % aqueous solution with the portable pH meter D-71S by Horiba Corporation Ltd. at 25 degreeC.
(酸处理工序)(acid treatment process)
在烧杯中准备6质量%的硝酸水溶液,使用水浴进行温度调整至40℃。将上述化学强化工序中得到的玻璃在已调整的硝酸水溶液中浸渍120秒钟而进行酸处理,然后利用纯水清洗多次后,通过鼓风进行干燥。将如此得到的玻璃供于下一工序。A 6 mass % nitric acid aqueous solution was prepared in a beaker, and the temperature was adjusted to 40 degreeC using a water bath. The glass obtained in the said chemical strengthening process was immersed in the adjusted nitric acid aqueous solution for 120 seconds, acid-treated, and after washing|cleaning several times with pure water, it was dried by a blower. The glass thus obtained is used for the next step.
(碱处理工序)(alkali treatment step)
在烧杯中准备4.0重量%的氢氧化钠水溶液,使用水浴进行温度调整至40℃。将酸处理工序中得到的玻璃在已调整的氢氧化钠水溶液中浸渍120秒钟而进行碱处理,然后利用纯水清洗多次后,通过鼓风进行干燥。A 4.0% by weight aqueous sodium hydroxide solution was prepared in a beaker, and the temperature was adjusted to 40°C using a water bath. The glass obtained in the acid treatment step was immersed in the adjusted sodium hydroxide aqueous solution for 120 seconds to perform an alkali treatment, then washed with pure water several times, and then dried by air blowing.
(研磨工序)(grinding process)
作为研磨浆料,使平均粒子直径(d50)为1μm的氧化铈分散于水中而制作浆料,使用得到的浆料,利用硬度(肖氏A硬度)为74的无纺布研磨垫,在压力0.1kPa的条件下,将平板玻璃的两面合计研磨约6μm。As a polishing slurry, cerium oxide having an average particle diameter (d50) of 1 μm was dispersed in water to prepare a slurry, and the obtained slurry was used with a non-woven polishing pad having a hardness (Shore A hardness) of 74 under pressure. Under the condition of 0.1 kPa, both sides of the plate glass were ground to about 6 μm in total.
<评价方法><Evaluation method>
本实施例中的各种评价通过以下所示的分析方法进行。Various evaluations in this Example were performed by the analysis method shown below.
(表面除去量)(Surface removal amount)
玻璃的除去量的厚度通过利用分析用电子天平(HR-202i,AND制造)测定试剂处理(酸处理和碱处理)前后的重量,使用下式进行厚度换算而求出。The thickness of the removed amount of glass was obtained by measuring the weight before and after the reagent treatment (acid treatment and alkali treatment) with an electronic balance for analysis (HR-202i, manufactured by AND), and calculating the thickness using the following formula.
(每个单面的除去量的厚度)=[(处理前重量)﹣(处理后重量)]/(玻璃比重)/处理面积/2(Thickness of removal amount per single side)=[(weight before treatment)-(weight after treatment)]/(specific gravity of glass)/treatment area/2
此时,玻璃材料(玻璃A、玻璃B和玻璃C)的玻璃比重如下,使用这些值进行计算。At this time, the glass specific gravity of the glass materials (glass A, glass B, and glass C) is as follows, and calculation is performed using these values.
玻璃A:2.42(g/cm3)Glass A: 2.42 (g/cm3 )
玻璃B:2.48(g/cm3)Glass B: 2.48 (g/cm3 )
玻璃C:2.39(g/cm3)Glass C: 2.39 (g/cm3 )
(面强度)(surface strength)
玻璃面强度通过球环试验而测得。图2示出用于说明本发明中使用的球环试验的简图。在将玻璃板1(在以下的实施例中为铝硅酸盐玻璃A)水平载置的状态下,使用SUS304制的加压夹具2(淬火钢,直径10mm,镜面抛光)对玻璃板1进行加压,测定玻璃板1的强度。The glass face strength is measured by the ball and ring test. FIG. 2 shows a schematic diagram for explaining the ball-and-ring test used in the present invention. In a state where the glass plate 1 (aluminosilicate glass A in the following examples) was placed horizontally, the
图2中,在SUS304制的承受夹具3(直径30mm,接触部的曲率R为2.5mm,接触部为淬火钢,镜面抛光)上水平设置作为样品的玻璃板1。在玻璃板1的上方设置用于对玻璃板1进行加压的加压夹具2。In FIG. 2 , a
从由实施例和比较例得到的玻璃板1的上方对玻璃板1的中央区域进行加压。应予说明,试验条件如下。The center area|region of the
加压夹具2的下降速度:1.0(mm/min)The descending speed of the pressing jig 2: 1.0 (mm/min)
此时,将玻璃被破坏时的破坏负载(单位N)设为BOR强度,将该BOR强度的20次测定的平均值设为面强度F(N)。其中,在玻璃板的破坏起点离开该球体(加压夹具)的负载点2mm以上的情况下,从用于计算平均值的数据中排除。At this time, the breaking load (unit N) when the glass is broken is defined as the BOR strength, and the average value of 20 measurements of the BOR strength is defined as the surface strength F(N). However, when the starting point of failure of the glass plate was 2 mm or more away from the load point of the sphere (pressing jig), it was excluded from the data for calculating the average value.
面强度F(N)依赖于玻璃板的板厚t(mm),因此,在此,通过利用玻璃板的板厚t(mm)进行标准化(基准化)而进行比较。将利用玻璃板的板厚t(mm)进行标准化(基准化)而得的值设为a(单位N/mm2)。a值由式:a=F/t2计算。Since the surface strength F(N) depends on the plate thickness t (mm) of the glass plate, here, the comparison is performed by normalizing (standardizing) using the plate thickness t (mm) of the glass plate. The value normalized (standardized) by the plate thickness t (mm) of a glass plate is made into a (unit N/mm2 ). The value of a is calculated by the formula: a=F/t2 .
(表面压缩应力、压缩应力层的深度)(surface compressive stress, depth of compressive stress layer)
表面压缩应力值(CS)和压缩应力层的深度(DOC,单位为μm)使用折原制作所公司制造的表面应力计(FSM-6000)而测定。压缩应力值(CS)和压缩应力层的深度(DOC)使用日本特开2016-142600号公报中公开的应力分布计算方法而计算。The surface compressive stress value (CS) and the depth (DOC, in μm) of the compressive stress layer were measured using a surface stress meter (FSM-6000) manufactured by Orihara, Ltd. The compressive stress value (CS) and the depth of the compressive stress layer (DOC) were calculated using the stress distribution calculation method disclosed in Japanese Patent Laid-Open No. 2016-142600.
(拉伸应力)(tensile stress)
拉伸应力值(CT,单位MPa)使用日本特开2016-142600号公报中公开的应力分布计算方法测定应力分布,将该应力分布用厚度进行积分而计算。The tensile stress value (CT, unit MPa) is calculated by measuring the stress distribution using the stress distribution calculation method disclosed in JP 2016-142600 A, and integrating the stress distribution with the thickness.
(研磨伤)(grinding wound)
研磨伤的有无通过利用AFM的表面观察进行辨别。在10μm×5μm区域内不存在2根以上的长度5μm以上、宽度0.1μm以上的划痕的情况下,作为在表面没有研磨伤的状态。The presence or absence of polishing scratches was discriminated by surface observation by AFM. When there are no two or more scratches with a length of 5 μm or more and a width of 0.1 μm or more in a 10 μm×5 μm area, it is assumed that there is no polishing scratch on the surface.
(外观品质)(Appearance quality)
在高亮度光源下且在成为照度100000Lux的条件下观察外观,通过下述评价基准对外观品质进行评价。图4A是表示在玻璃面内未产生白雾的状态的图,图4B是表示在玻璃面内产生白雾的状态的图。The appearance was observed under the conditions of a high-intensity light source and an illuminance of 100,000 Lux, and the appearance quality was evaluated by the following evaluation criteria. FIG. 4A is a diagram showing a state in which white fog is not generated in the glass surface, and FIG. 4B is a diagram showing a state in which white fog is generated in the glass surface.
○:在玻璃面内未产生白雾。○: No white fog was generated in the glass surface.
×:在玻璃面内产生白雾。×: White fog is generated in the glass surface.
将得到的结果示于表1和2以及图5~8。The obtained results are shown in Tables 1 and 2 and FIGS. 5 to 8 .
[表1][Table 1]
[表2][Table 2]
如表1所示,得到了通过本发明的制造方法得到的实施例1~12的化学强化玻璃,所述本发明的制造方法包括如下工序:使玻璃与pH为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐接触而进行离子交换的化学强化工序;以及使上述化学强化工序后的玻璃与pH小于7的酸性溶液接触而进行酸处理的酸处理工序。与比较例1~11中得到的化学强化玻璃相比,实施例1~12的化学强化玻璃即使在高温下进行长时间的化学强化处理,面强度也高,压缩应力层的深度(DOC)也深以及显示较高的表面压缩应力值(CS),并且在玻璃面内未产生白雾,在外观品质方面也优异。As shown in Table 1, the chemically strengthened glasses of Examples 1 to 12 obtained by the production method of the present invention including the steps of making glass with pH 7.5 to 10.5 and containing sodium nitrate and A chemical strengthening process of performing ion exchange by contacting at least one inorganic salt of potassium nitrate; and an acid treatment process of performing acid treatment by contacting the glass after the chemical strengthening process with an acidic solution having a pH of less than 7. Compared with the chemically strengthened glasses obtained in Comparative Examples 1 to 11, the chemically strengthened glasses of Examples 1 to 12 had higher surface strength and lower depth of compressive stress layer (DOC) even if they were chemically strengthened for a long time at high temperature. It is deep and shows a high surface compressive stress value (CS), and does not generate white fog in the glass surface, and is also excellent in appearance quality.
与实施例中得到的化学强化玻璃相比,在使玻璃与pH为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐接触而进行离子交换的化学强化工序后未进行酸处理的比较例1、2、4、6、7、10和11的化学强化玻璃的面强度低。另外,比较例1、2、4、8和9的化学强化玻璃在玻璃面内产生白雾。Compared with the chemically strengthened glass obtained in the Examples, the acid treatment was not performed after the chemical strengthening process in which the glass was brought into contact with an inorganic salt containing at least one of sodium nitrate and potassium nitrate at pH 7.5 to 10.5 to perform ion exchange. The chemically strengthened glasses of Comparative Examples 1, 2, 4, 6, 7, 10 and 11 had low areal strengths. In addition, in the chemically strengthened glasses of Comparative Examples 1, 2, 4, 8 and 9, white fog was generated in the glass surface.
另外,与其它比较例相比,在使玻璃与pH为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐接触而进行离子交换的化学强化工序后未进行酸处理而进行研磨处理的比较例5的化学强化玻璃的面强度略高。但是,在玻璃表面观察到研磨伤,与实施例中得到的化学强化玻璃相比,面强度低。In addition, compared with other comparative examples, the glass was ground without acid treatment after the chemical strengthening step of contacting the glass with an inorganic salt containing at least one of sodium nitrate and potassium nitrate to perform ion exchange with a pH of 7.5 to 10.5. The surface strength of the chemically strengthened glass of the treated comparative example 5 was slightly higher. However, polishing scratches were observed on the glass surface, and the surface strength was lower than that of the chemically strengthened glass obtained in the examples.
另外,与实施例中得到的化学强化玻璃相比,在使用pH小于7.5的无机盐进行化学强化工序后进行酸处理和碱处理的比较例3的化学强化玻璃、在使用pH超过10.5的无机盐进行化学强化工序后进行酸处理的比较例8以及在使用pH超过10.5的无机盐进行化学强化工序后进行酸处理和碱处理的比较例9的化学强化玻璃的面强度低,在玻璃面内产生有白雾。Moreover, compared with the chemically strengthened glass obtained in the Example, the chemically strengthened glass of Comparative Example 3 in which the acid treatment and the alkali treatment were performed after the chemical strengthening process using the inorganic salt with a pH of less than 7.5, the inorganic salt with a pH exceeding 10.5 was used in the chemically strengthened glass of the comparative example 3. The chemically strengthened glass of Comparative Example 8 in which the acid treatment was performed after the chemical strengthening process and the chemically strengthened glass in Comparative Example 9 in which the acid treatment and alkali treatment were performed after the chemical strengthening process using an inorganic salt with a pH exceeding 10.5 had a low surface strength and occurred in the glass surface. There is white fog.
图5A表示实施例1和3以及比较例1中得到的化学强化玻璃的应力分布,图5B表示实施例7和8以及比较例6中得到的化学强化玻璃的应力分布,图5C表示实施例10和11以及比较例11中得到的化学强化玻璃的应力分布。5A shows the stress distribution of the chemically strengthened glasses obtained in Examples 1 and 3 and Comparative Example 1, FIG. 5B shows the stress distribution of the chemically strengthened glasses obtained in Examples 7 and 8 and Comparative Example 6, and FIG. 5C shows Example 10 and 11 and the stress distribution of the chemically strengthened glass obtained in Comparative Example 11.
如图5A所示,实施例1和比较例1中得到的化学强化玻璃的应力分布大体一致。另外,如图5B所示,实施例7和8以及比较例6中得到的化学强化玻璃的应力分布大体一致。进而,如图5C所示,实施例10和11以及比较例11中得到的化学强化玻璃的应力分布大体一致。As shown in FIG. 5A , the stress distributions of the chemically strengthened glasses obtained in Example 1 and Comparative Example 1 were substantially the same. In addition, as shown in FIG. 5B , the stress distributions of the chemically strengthened glasses obtained in Examples 7 and 8 and Comparative Example 6 were substantially the same. Furthermore, as shown in FIG. 5C , the stress distributions of the chemically strengthened glasses obtained in Examples 10 and 11 and Comparative Example 11 were substantially the same.
图6A和图6B表示对实施例1和5以及比较例1、4和5中得到的化学强化玻璃的面强度进行评价而得的结果。如图6A和图6B所示,与在化学强化工序后未进行酸处理的比较例1和4以及在化学强化工序后未进行酸处理而进行研磨处理的比较例5相比,实施例1和5中得到的化学强化玻璃的面强度显著提高。6A and 6B show the results obtained by evaluating the surface strength of the chemically strengthened glasses obtained in Examples 1 and 5 and Comparative Examples 1, 4 and 5. As shown in FIGS. 6A and 6B , compared with Comparative Examples 1 and 4 in which no acid treatment was performed after the chemical strengthening process and Comparative Example 5 in which grinding treatment was performed without acid treatment after the chemical strengthening process, Examples 1 and 4 The surface strength of the chemically strengthened glass obtained in 5 was remarkably improved.
图7A和图7B表示对实施例7和8以及比较例6中得到的化学强化玻璃的面强度进行评价而得的结果。如图7A和图7B所示,与在化学强化工序后未进行酸处理的比较例6相比,实施例7和8中得到的化学强化玻璃的面强度显著提高。7A and 7B show the results obtained by evaluating the surface strength of the chemically strengthened glasses obtained in Examples 7 and 8 and Comparative Example 6. FIG. As shown in FIGS. 7A and 7B , the surface strength of the chemically strengthened glasses obtained in Examples 7 and 8 was significantly improved as compared with Comparative Example 6 in which the acid treatment was not performed after the chemical strengthening process.
图8A和图8B表示对实施例10和11以及比较例11中得到的化学强化玻璃的面强度进行评价而得的结果。如图8A和图8B所示,与在化学强化工序后未进行酸处理的比较例11相比,实施例10和11中得到的化学强化玻璃的面强度显著提高。8A and 8B show the results obtained by evaluating the surface strength of the chemically strengthened glasses obtained in Examples 10 and 11 and Comparative Example 11. As shown in FIGS. 8A and 8B , the surface strength of the chemically strengthened glasses obtained in Examples 10 and 11 was remarkably improved compared to Comparative Example 11 in which the acid treatment was not performed after the chemical strengthening process.
根据这些结果,通过在使用pH为7.5~10.5且含有硝酸钠和硝酸钾中的至少一者的无机盐的化学强化工序后进行酸处理,可得到即使在高温下进行长时间的化学强化处理也不会减弱玻璃的强度,显示较深的DOC并且面强度高的化学强化玻璃。Based on these results, by performing acid treatment after the chemical strengthening process using an inorganic salt containing at least one of sodium nitrate and potassium nitrate at pH 7.5 to 10.5, it is possible to obtain a chemical strengthening process that is stable even if the chemical strengthening treatment is performed at a high temperature for a long time. A chemically strengthened glass that exhibits deep DOC and high surface strength without weakening the glass.
参照特定的方案对本发明进行了详细说明,但在不脱离本发明的精神和范围的情况下进行各种变更和修正对本领域技术人员而言是清楚的。应予说明,本申请基于2016年9月30日提出申请的日本专利申请(日本特愿2016-193972号),通过引用而援引其整体。另外,引用于此的全部参照作为整体并入本说明书中。Although this invention was demonstrated in detail with reference to the specific aspect, it is clear for those skilled in the art to make various changes and correction without deviating from the mind and range of this invention. In addition, this application is based on the Japanese Patent Application (Japanese Patent Application No. 2016-193972) for which it applied on September 30, 2016, The whole is used by reference. In addition, all the references cited herein are incorporated into this specification as a whole.
符号说明Symbol Description
10 低密度层10 Low Density Layers
20 压缩应力层20 Compressive stress layer
30 中间层30 middle layer
1 玻璃板1 glass plate
2 加压夹具2 Pressing fixtures
3 承受夹具3 Bearing fixture
Claims (5)
Translated fromChineseApplications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-193972 | 2016-09-30 | ||
| JP2016193972 | 2016-09-30 | ||
| PCT/JP2017/034671WO2018062141A1 (en) | 2016-09-30 | 2017-09-26 | Method for producing chemically toughened glass |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109803938A CN109803938A (en) | 2019-05-24 |
| CN109803938Btrue CN109803938B (en) | 2022-07-12 |
Family
ID=61760563
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780060568.4AActiveCN109803938B (en) | 2016-09-30 | 2017-09-26 | Manufacturing method of chemically strengthened glass |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP6919658B2 (en) |
| KR (1) | KR102436191B1 (en) |
| CN (1) | CN109803938B (en) |
| TW (1) | TWI728189B (en) |
| WO (1) | WO2018062141A1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7395939B2 (en)* | 2019-10-09 | 2023-12-12 | Agc株式会社 | Manufacturing method of chemically strengthened glass |
| CN111122376A (en)* | 2020-01-06 | 2020-05-08 | 江苏奥天光学有限公司 | Tempering effect verification method of tempering mold |
| CN111807718A (en)* | 2020-07-24 | 2020-10-23 | 江苏铁锚玻璃股份有限公司 | Preparation method of high-light-transmission and high-strength antibacterial glass |
| CN112062480A (en)* | 2020-09-11 | 2020-12-11 | 河南卓金光电科技股份有限公司 | Surface strengthening treatment method for ultrathin large-plate-surface glass |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH04139034A (en)* | 1990-09-29 | 1992-05-13 | Nippon Sheet Glass Co Ltd | Formation of unevenness on surface of glass |
| CN1656037A (en)* | 2002-06-13 | 2005-08-17 | 国际商业机器公司 | pH adjustment of melts for microetching glass substrates |
| CN104736495A (en)* | 2013-07-19 | 2015-06-24 | 旭硝子株式会社 | Method for producing chemically strengthened glass |
| WO2015179345A1 (en)* | 2014-05-20 | 2015-11-26 | Corning Incorporated | Scratch resistant glass and method of making |
| CN105555731A (en)* | 2014-01-16 | 2016-05-04 | 旭硝子株式会社 | Chemically strengthened glass, and method for producing same |
| WO2016117478A1 (en)* | 2015-01-20 | 2016-07-28 | 旭硝子株式会社 | Float glass |
| WO2016117479A1 (en)* | 2015-01-20 | 2016-07-28 | 旭硝子株式会社 | Glass substrate production method |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5913455B2 (en)* | 1978-03-08 | 1984-03-29 | 石塚硝子株式会社 | How to manufacture frosted tempered glass products |
| US4687581A (en)* | 1984-01-30 | 1987-08-18 | Pedro B. Macedo | Method of separating and purifying cations by ion exchange with regenerable porous glass |
| US5232481A (en)* | 1991-12-26 | 1993-08-03 | Corning Incorporated | Glass dimensional control using ion exchange |
| US6638623B2 (en)* | 2001-12-18 | 2003-10-28 | International Business Machines Corporation | pH adjustment of a strengthening melt for use in strengthening glass substrates |
| WO2015027007A2 (en)* | 2013-08-23 | 2015-02-26 | Corning Incorporated | Strengthened glass articles, edge-strengthened laminated glass articles, and methods for making the same |
| TWI503184B (en)* | 2013-09-04 | 2015-10-11 | All Ring Tech Co Ltd | Dispenser and its material inspection of dust removal method |
| KR102302163B1 (en)* | 2013-10-14 | 2021-09-15 | 코닝 인코포레이티드 | Ion Exchange Processes and Chemically Strengthened Glass Substrates Resulting Therefrom |
| WO2017179360A1 (en)* | 2016-04-12 | 2017-10-19 | 日本電気硝子株式会社 | Method for manufacturing tempered glass and device for manufacturing tempered glass |
- 2017
- 2017-09-26WOPCT/JP2017/034671patent/WO2018062141A1/ennot_activeCeased
- 2017-09-26KRKR1020197008010Apatent/KR102436191B1/enactiveActive
- 2017-09-26CNCN201780060568.4Apatent/CN109803938B/enactiveActive
- 2017-09-26JPJP2018542579Apatent/JP6919658B2/enactiveActive
- 2017-09-28TWTW106133281Apatent/TWI728189B/enactive
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH04139034A (en)* | 1990-09-29 | 1992-05-13 | Nippon Sheet Glass Co Ltd | Formation of unevenness on surface of glass |
| CN1656037A (en)* | 2002-06-13 | 2005-08-17 | 国际商业机器公司 | pH adjustment of melts for microetching glass substrates |
| CN104736495A (en)* | 2013-07-19 | 2015-06-24 | 旭硝子株式会社 | Method for producing chemically strengthened glass |
| CN104812718A (en)* | 2013-07-19 | 2015-07-29 | 旭硝子株式会社 | Chemically strengthened glass |
| CN105669050A (en)* | 2013-07-19 | 2016-06-15 | 旭硝子株式会社 | Chemically strengthened glass |
| CN105555731A (en)* | 2014-01-16 | 2016-05-04 | 旭硝子株式会社 | Chemically strengthened glass, and method for producing same |
| WO2015179345A1 (en)* | 2014-05-20 | 2015-11-26 | Corning Incorporated | Scratch resistant glass and method of making |
| WO2016117478A1 (en)* | 2015-01-20 | 2016-07-28 | 旭硝子株式会社 | Float glass |
| WO2016117479A1 (en)* | 2015-01-20 | 2016-07-28 | 旭硝子株式会社 | Glass substrate production method |
Also Published As
| Publication number | Publication date |
|---|---|
| CN109803938A (en) | 2019-05-24 |
| KR20190065255A (en) | 2019-06-11 |
| TWI728189B (en) | 2021-05-21 |
| KR102436191B1 (en) | 2022-08-26 |
| JP6919658B2 (en) | 2021-08-18 |
| WO2018062141A1 (en) | 2018-04-05 |
| TW201817689A (en) | 2018-05-16 |
| JPWO2018062141A1 (en) | 2019-07-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN106277840B (en) | The manufacturing method of chemically reinforced glass | |
| JP6702470B2 (en) | Chemically tempered glass and method for manufacturing the same | |
| JP7056652B2 (en) | Chemically tempered glass | |
| CN109803938B (en) | Manufacturing method of chemically strengthened glass | |
| CN107207333B (en) | Chemically strengthened glass and method for producing the same | |
| CN107207335B (en) | Method for producing glass substrate | |
| CN106167357B (en) | Manufacturing method of chemically strengthened glass | |
| CN109095789A (en) | The manufacturing method of chemically reinforced glass | |
| CN107207311B (en) | Float glass | |
| CN107188398A (en) | The manufacture method of chemically reinforced glass | |
| CN109970361A (en) | Manufacturing method of chemically strengthened glass and chemically strengthened glass |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |