CN108192095B - Preparation method of polymer with amido bond in main chain - Google Patents
Preparation method of polymer with amido bond in main chainDownload PDFInfo
- Publication number
- CN108192095B CN108192095BCN201810100749.1ACN201810100749ACN108192095BCN 108192095 BCN108192095 BCN 108192095BCN 201810100749 ACN201810100749 ACN 201810100749ACN 108192095 BCN108192095 BCN 108192095B
- Authority
- CN
- China
- Prior art keywords
- reaction
- polymer
- main chain
- monomer
- trimethylphosphine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 229920000642polymerPolymers0.000titleclaimsabstractdescription65
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription16
- 238000006243chemical reactionMethods0.000claimsabstractdescription82
- YWWDBCBWQNCYNR-UHFFFAOYSA-NtrimethylphosphineChemical compoundCP(C)CYWWDBCBWQNCYNR-UHFFFAOYSA-N0.000claimsabstractdescription60
- 239000000178monomerSubstances0.000claimsabstractdescription52
- IVRMZWNICZWHMI-UHFFFAOYSA-Nazide groupChemical group[N-]=[N+]=[N-]IVRMZWNICZWHMI-UHFFFAOYSA-N0.000claimsabstractdescription33
- 125000003178carboxy groupChemical group[H]OC(*)=O0.000claimsabstractdescription24
- 239000003054catalystSubstances0.000claimsabstractdescription11
- OFOBLEOULBTSOW-UHFFFAOYSA-NMalonic acidChemical compoundOC(=O)CC(O)=OOFOBLEOULBTSOW-UHFFFAOYSA-N0.000claimsabstractdescription9
- YXFVVABEGXRONW-UHFFFAOYSA-NTolueneNatural productsCC1=CC=CC=C1YXFVVABEGXRONW-UHFFFAOYSA-N0.000claimsdescription18
- -1dipyridine diselenideChemical compound0.000claimsdescription17
- 239000003960organic solventSubstances0.000claimsdescription5
- 125000003944tolyl groupChemical group0.000claimsdescription3
- 150000001735carboxylic acidsChemical class0.000claims1
- 150000001875compoundsChemical class0.000claims1
- CBQLVACGEMGVMI-UHFFFAOYSA-N2-(pyridin-2-yldiselanyl)pyridineChemical compoundC=1C=CC=NC=1[Se][Se]C1=CC=CC=N1CBQLVACGEMGVMI-UHFFFAOYSA-N0.000abstractdescription8
- 238000000655nuclear magnetic resonance spectrumMethods0.000description19
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000description18
- 239000000243solutionSubstances0.000description17
- ZMXDDKWLCZADIW-UHFFFAOYSA-NN,N-DimethylformamideChemical compoundCN(C)C=OZMXDDKWLCZADIW-UHFFFAOYSA-N0.000description15
- UFHFLCQGNIYNRP-UHFFFAOYSA-NHydrogenChemical compound[H][H]UFHFLCQGNIYNRP-UHFFFAOYSA-N0.000description14
- PXIPVTKHYLBLMZ-UHFFFAOYSA-NSodium azideChemical compound[Na+].[N-]=[N+]=[N-]PXIPVTKHYLBLMZ-UHFFFAOYSA-N0.000description14
- 239000001257hydrogenSubstances0.000description14
- 229910052739hydrogenInorganic materials0.000description14
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description13
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000description12
- 125000000217alkyl groupChemical group0.000description12
- 238000000034methodMethods0.000description10
- 238000006116polymerization reactionMethods0.000description10
- 239000004952PolyamideSubstances0.000description9
- HEMHJVSKTPXQMS-UHFFFAOYSA-MSodium hydroxideChemical compound[OH-].[Na+]HEMHJVSKTPXQMS-UHFFFAOYSA-M0.000description9
- 239000008367deionised waterSubstances0.000description9
- 229910021641deionized waterInorganic materials0.000description9
- 229910052757nitrogenInorganic materials0.000description9
- 229920002647polyamidePolymers0.000description9
- 239000002904solventSubstances0.000description9
- 238000003756stirringMethods0.000description9
- 239000000047productSubstances0.000description8
- LXAVFOAOGZWQKT-UHFFFAOYSA-N11-azidoundecanoic acidChemical compoundOC(=O)CCCCCCCCCCN=[N+]=[N-]LXAVFOAOGZWQKT-UHFFFAOYSA-N0.000description7
- 125000001931aliphatic groupChemical group0.000description7
- 230000015572biosynthetic processEffects0.000description7
- 230000010354integrationEffects0.000description7
- 239000007787solidSubstances0.000description7
- 238000003786synthesis reactionMethods0.000description7
- YMWUJEATGCHHMB-UHFFFAOYSA-NDichloromethaneChemical compoundClCClYMWUJEATGCHHMB-UHFFFAOYSA-N0.000description6
- XEKOWRVHYACXOJ-UHFFFAOYSA-NEthyl acetateChemical compoundCCOC(C)=OXEKOWRVHYACXOJ-UHFFFAOYSA-N0.000description6
- BUGBHKTXTAQXES-UHFFFAOYSA-NSeleniumChemical compound[Se]BUGBHKTXTAQXES-UHFFFAOYSA-N0.000description6
- 125000002915carbonyl groupChemical group[*:2]C([*:1])=O0.000description6
- 238000005227gel permeation chromatographyMethods0.000description6
- 239000011261inert gasSubstances0.000description6
- 239000012074organic phaseSubstances0.000description6
- 238000005481NMR spectroscopyMethods0.000description5
- 125000003118aryl groupChemical group0.000description5
- 150000001540azidesChemical class0.000description5
- 238000000921elemental analysisMethods0.000description5
- NWZSZGALRFJKBT-KNIFDHDWSA-N(2s)-2,6-diaminohexanoic acid;(2s)-2-hydroxybutanedioic acidChemical compoundOC(=O)[C@@H](O)CC(O)=O.NCCCC[C@H](N)C(O)=ONWZSZGALRFJKBT-KNIFDHDWSA-N0.000description4
- BYEAHWXPCBROCE-UHFFFAOYSA-N1,1,1,3,3,3-hexafluoropropan-2-olChemical compoundFC(F)(F)C(O)C(F)(F)FBYEAHWXPCBROCE-UHFFFAOYSA-N0.000description4
- ZJJATABWMGVVRZ-UHFFFAOYSA-N1,12-dibromododecaneChemical compoundBrCCCCCCCCCCCCBrZJJATABWMGVVRZ-UHFFFAOYSA-N0.000description4
- CSCPPACGZOOCGX-UHFFFAOYSA-NAcetoneChemical compoundCC(C)=OCSCPPACGZOOCGX-UHFFFAOYSA-N0.000description4
- WKBOTKDWSSQWDR-UHFFFAOYSA-NBromine atomChemical compound[Br]WKBOTKDWSSQWDR-UHFFFAOYSA-N0.000description4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-NDimethylsulphoxideChemical compoundCS(C)=OIAZDPXIOMUYVGZ-UHFFFAOYSA-N0.000description4
- GDTBXPJZTBHREO-UHFFFAOYSA-NbromineSubstancesBrBrGDTBXPJZTBHREO-UHFFFAOYSA-N0.000description4
- 229910052794bromiumInorganic materials0.000description4
- IKDUDTNKRLTJSI-UHFFFAOYSA-Nhydrazine monohydrateSubstancesO.NNIKDUDTNKRLTJSI-UHFFFAOYSA-N0.000description4
- JJOJFIHJIRWASH-UHFFFAOYSA-Nicosanedioic acidChemical compoundOC(=O)CCCCCCCCCCCCCCCCCCC(O)=OJJOJFIHJIRWASH-UHFFFAOYSA-N0.000description4
- 238000002329infrared spectrumMethods0.000description4
- GKTNLYAAZKKMTQ-UHFFFAOYSA-Nn-[bis(dimethylamino)phosphinimyl]-n-methylmethanamineChemical compoundCN(C)P(=N)(N(C)C)N(C)CGKTNLYAAZKKMTQ-UHFFFAOYSA-N0.000description4
- 238000007151ring opening polymerisation reactionMethods0.000description4
- LPXPTNMVRIOKMN-UHFFFAOYSA-Msodium nitriteChemical compound[Na+].[O-]N=OLPXPTNMVRIOKMN-UHFFFAOYSA-M0.000description4
- HQHCYKULIHKCEB-UHFFFAOYSA-Ntetradecanedioic acidChemical compoundOC(=O)CCCCCCCCCCCCC(O)=OHQHCYKULIHKCEB-UHFFFAOYSA-N0.000description4
- IMRWILPUOVGIMU-UHFFFAOYSA-N2-bromopyridineChemical compoundBrC1=CC=CC=N1IMRWILPUOVGIMU-UHFFFAOYSA-N0.000description3
- NLXLAEXVIDQMFP-UHFFFAOYSA-NAmmonia chlorideChemical class[NH4+].[Cl-]NLXLAEXVIDQMFP-UHFFFAOYSA-N0.000description3
- JUJWROOIHBZHMG-UHFFFAOYSA-NPyridineChemical groupC1=CC=NC=C1JUJWROOIHBZHMG-UHFFFAOYSA-N0.000description3
- PMZURENOXWZQFD-UHFFFAOYSA-LSodium SulfateChemical compound[Na+].[Na+].[O-]S([O-])(=O)=OPMZURENOXWZQFD-UHFFFAOYSA-L0.000description3
- 238000010521absorption reactionMethods0.000description3
- 238000005452bendingMethods0.000description3
- 230000003197catalytic effectEffects0.000description3
- 239000000706filtrateSubstances0.000description3
- 239000007789gasSubstances0.000description3
- 125000004435hydrogen atomChemical group[H]*0.000description3
- 125000000325methylidene groupChemical group[H]C([H])=*0.000description3
- 239000011669seleniumSubstances0.000description3
- 229910052711seleniumInorganic materials0.000description3
- HPALAKNZSZLMCH-UHFFFAOYSA-Msodium;chloride;hydrateChemical classO.[Na+].[Cl-]HPALAKNZSZLMCH-UHFFFAOYSA-M0.000description3
- 238000001228spectrumMethods0.000description3
- 238000000967suction filtrationMethods0.000description3
- CZDYPVPMEAXLPK-UHFFFAOYSA-NtetramethylsilaneChemical compoundC[Si](C)(C)CCZDYPVPMEAXLPK-UHFFFAOYSA-N0.000description3
- GWRANYLMPAJNOJ-UHFFFAOYSA-N1,12-diazidododecaneChemical group[N-]=[N+]=NCCCCCCCCCCCCN=[N+]=[N-]GWRANYLMPAJNOJ-UHFFFAOYSA-N0.000description2
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description2
- RTZKZFJDLAIYFH-UHFFFAOYSA-NDiethyl etherChemical compoundCCOCCRTZKZFJDLAIYFH-UHFFFAOYSA-N0.000description2
- VEXZGXHMUGYJMC-UHFFFAOYSA-NHydrochloric acidChemical compoundClVEXZGXHMUGYJMC-UHFFFAOYSA-N0.000description2
- 238000012695Interfacial polymerizationMethods0.000description2
- XYFCBTPGUUZFHI-UHFFFAOYSA-NPhosphineChemical compoundPXYFCBTPGUUZFHI-UHFFFAOYSA-N0.000description2
- HEDRZPFGACZZDS-MICDWDOJSA-NTrichloro(2H)methaneChemical compound[2H]C(Cl)(Cl)ClHEDRZPFGACZZDS-MICDWDOJSA-N0.000description2
- 239000002253acidSubstances0.000description2
- 238000005273aerationMethods0.000description2
- PNEYBMLMFCGWSK-UHFFFAOYSA-Naluminium oxideInorganic materials[O-2].[O-2].[O-2].[Al+3].[Al+3]PNEYBMLMFCGWSK-UHFFFAOYSA-N0.000description2
- 125000003277amino groupChemical group0.000description2
- 239000006227byproductSubstances0.000description2
- 229910052799carbonInorganic materials0.000description2
- 238000004440column chromatographyMethods0.000description2
- 239000012043crude productSubstances0.000description2
- 238000010528free radical solution polymerization reactionMethods0.000description2
- 229910052734heliumInorganic materials0.000description2
- 239000001307heliumSubstances0.000description2
- SWQJXJOGLNCZEY-UHFFFAOYSA-Nhelium atomChemical compound[He]SWQJXJOGLNCZEY-UHFFFAOYSA-N0.000description2
- 239000007788liquidSubstances0.000description2
- 239000005267main chain polymerSubstances0.000description2
- 239000011259mixed solutionSubstances0.000description2
- 239000000203mixtureSubstances0.000description2
- 230000004048modificationEffects0.000description2
- 238000012986modificationMethods0.000description2
- 229910052754neonInorganic materials0.000description2
- GKAOGPIIYCISHV-UHFFFAOYSA-Nneon atomChemical compound[Ne]GKAOGPIIYCISHV-UHFFFAOYSA-N0.000description2
- 230000007935neutral effectEffects0.000description2
- 238000006053organic reactionMethods0.000description2
- 229920003229poly(methyl methacrylate)Polymers0.000description2
- 238000006068polycondensation reactionMethods0.000description2
- 239000004926polymethyl methacrylateSubstances0.000description2
- 238000012805post-processingMethods0.000description2
- 239000002994raw materialSubstances0.000description2
- 230000035484reaction timeEffects0.000description2
- 235000010288sodium nitriteNutrition0.000description2
- 159000000000sodium saltsChemical class0.000description2
- 230000002194synthesizing effectEffects0.000description2
- 2380000051601H NMR spectroscopyMethods0.000description1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N2-[2,4-di(pentan-2-yl)phenoxy]acetyl chlorideChemical compoundCCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1NGNBDVOYPDDBFK-UHFFFAOYSA-N0.000description1
- 101710141544Allatotropin-related peptideProteins0.000description1
- 239000004677NylonSubstances0.000description1
- 150000007513acidsChemical class0.000description1
- 125000003368amide groupChemical group0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 238000010560atom transfer radical polymerization reactionMethods0.000description1
- 150000001732carboxylic acid derivativesChemical class0.000description1
- 239000003795chemical substances by applicationSubstances0.000description1
- 125000004122cyclic groupChemical group0.000description1
- 150000004985diaminesChemical class0.000description1
- XIMIGUBYDJDCKI-UHFFFAOYSA-NdiseleniumChemical compound[Se]=[Se]XIMIGUBYDJDCKI-UHFFFAOYSA-N0.000description1
- 150000002148estersChemical class0.000description1
- 239000000835fiberSubstances0.000description1
- 239000012467final productSubstances0.000description1
- 239000003365glass fiberSubstances0.000description1
- 239000003999initiatorSubstances0.000description1
- 150000003951lactamsChemical class0.000description1
- 239000000463materialSubstances0.000description1
- 229910052751metalInorganic materials0.000description1
- 239000002184metalSubstances0.000description1
- 150000002825nitrilesChemical class0.000description1
- 229920001778nylonPolymers0.000description1
- 239000003208petroleumSubstances0.000description1
- 150000003003phosphinesChemical class0.000description1
- 229910000073phosphorus hydrideInorganic materials0.000description1
- 239000002244precipitateSubstances0.000description1
- 102000004196processed proteins & peptidesHuman genes0.000description1
- 108090000765processed proteins & peptidesProteins0.000description1
- 238000011084recoveryMethods0.000description1
- 230000002787reinforcementEffects0.000description1
- 239000013557residual solventSubstances0.000description1
- 239000011347resinSubstances0.000description1
- 229920005989resinPolymers0.000description1
- 238000012712reversible addition−fragmentation chain-transfer polymerizationMethods0.000description1
- 238000002390rotary evaporationMethods0.000description1
- 238000007086side reactionMethods0.000description1
- 150000003384small moleculesChemical class0.000description1
- 239000000126substanceSubstances0.000description1
- 239000000758substrateSubstances0.000description1
- 238000001308synthesis methodMethods0.000description1
- 239000004753textileSubstances0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G69/00—Macromolecular compounds obtained by reactions forming a carboxylic amide link in the main chain of the macromolecule
- C08G69/42—Polyamides containing atoms other than carbon, hydrogen, oxygen, and nitrogen
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Polyamides (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及高分子聚合领域,尤其涉及一种主链含酰胺键的聚合物的制备方法。The invention relates to the field of polymer polymerization, in particular to a method for preparing a polymer with an amide bond in the main chain.
背景技术Background technique
聚酰胺俗称尼龙,它是大分子主链重复单元中含有酰胺基团的高聚物的总称。最初是由美国DuPont公司开发用于纤维的树脂,并最早于1939年实现工业化。聚酰胺具有良好的机械性能、耐热、耐磨损、耐化学性、阻燃性和自润滑性,而且易加工、特别适于玻璃纤维及其他材料增强改性等。因此,聚酰胺产品广泛应用于汽车、仪表、机械、纺织、军工、体育休闲,日用消费品等众多领域。Polyamide, commonly known as nylon, is a general term for polymers containing amide groups in the repeating units of the macromolecular main chain. Resin for fiber was originally developed by DuPont Company of the United States, and was industrialized as early as 1939. Polyamide has good mechanical properties, heat resistance, wear resistance, chemical resistance, flame retardancy and self-lubricating properties, and is easy to process, especially suitable for the reinforcement and modification of glass fiber and other materials. Therefore, polyamide products are widely used in many fields such as automobiles, instruments, machinery, textiles, military industry, sports and leisure, and daily consumer goods.
目前,聚酰胺主要二元胺与二元酸缩聚或者由内酰胺开环聚合制得。聚合方法主要有界面聚合、溶液聚合、熔融聚合和开环聚合。界面聚合优点是缩聚温度较低,不必严格等基团比,分子量较高,但是原料要用到酰氯较贵,并且溶剂回收成本高。而溶液聚合虽然所需要的聚合温度较低,副反应也较少,但是需要回收溶剂,聚合物中残留溶剂的脱出比较困难。熔融聚合具有产率高,产物纯净,应用范围广的优点,但是聚合温度高,对基团比要求严格,单体纯度要高。开环聚合用水作引发剂,可以制备大型机械部件,但是要在高温下进行连续聚合,并且产物中含有环状单体。基于聚酰胺的广泛用途以及目前合成方法的缺点,有必要发展一种合成聚酰胺的新方法。而目前把高效的小分子有机反应应用到聚合物的合成中是一个非常普遍的思路。其中最为典型的是点击化学反应。由于其具有原料易得,反应操作简单,反应产率高,副产物少,后处理过程简单等优点。科学家们很快将这个高效的有机反应应用到聚合物的合成中,将这些反应与ATRP,RAFT,开环聚合相结合,可以构建不同的拓扑结构。At present, polyamides are mainly obtained by polycondensation of diamines and dibasic acids or by ring-opening polymerization of lactams. The main polymerization methods are interfacial polymerization, solution polymerization, melt polymerization and ring-opening polymerization. The advantages of interfacial polymerization are that the polycondensation temperature is lower, the group ratio does not need to be strictly equal, and the molecular weight is higher, but the acid chloride used as the raw material is more expensive, and the solvent recovery cost is high. While solution polymerization requires lower polymerization temperature and fewer side reactions, it needs to recover the solvent, and it is difficult to remove the residual solvent in the polymer. Melt polymerization has the advantages of high yield, pure product, and wide application range, but high polymerization temperature, strict requirements on group ratio, and high monomer purity. The ring-opening polymerization uses water as an initiator to prepare large mechanical parts, but the continuous polymerization is carried out at high temperature, and the product contains cyclic monomers. Based on the wide use of polyamides and the shortcomings of current synthesis methods, it is necessary to develop a new method for synthesizing polyamides. At present, it is a very common idea to apply efficient small-molecule organic reactions to the synthesis of polymers. One of the most typical is the click chemistry reaction. Because it has the advantages of easy availability of raw materials, simple reaction operation, high reaction yield, few by-products, and simple post-processing process. Scientists soon applied this highly efficient organic reaction to the synthesis of polymers, combining these reactions with ATRP, RAFT, and ring-opening polymerization to construct different topologies.
羧酸化合物与有机叠氮化物的反应被称为Staudinger-Vilarrasa反应,简称S-V反应。而目前催化的S-V反应主要被用到合成多肽以及含有酰胺键的天然大分子反应中。The reaction of a carboxylic acid compound with an organic azide is called the Staudinger-Vilarrasa reaction, or S-V reaction for short. The currently catalyzed S-V reactions are mainly used in the synthesis of peptides and natural macromolecular reactions containing amide bonds.
发明内容SUMMARY OF THE INVENTION
为解决上述技术问题,本发明的目的是提供一种主链含酰胺键的聚合物的制备方法,本发明利用二吡啶二硒醚催化的Staudinger-Vilarrasa反应使各种结构的二元羧酸与两端为叠氮基团的单体发生聚合反应,或者使两端分别为叠氮基团和羧基的分子发生自聚合,从而制备出一系列主链含酰胺键聚合物。In order to solve the above-mentioned technical problems, the object of the present invention is to provide a kind of preparation method of the polymer of the main chain containing amide bond, the present invention utilizes the Staudinger-Vilarrasa reaction catalyzed by dipyridyl diselenide to make the dibasic carboxylic acid of various structures and Polymerization of monomers with azide groups at both ends, or self-polymerization of molecules with azide groups and carboxyl groups at both ends, to prepare a series of polymers containing amide bonds in the main chain.
在一方面,本发明提供了一种主链含酰胺键的聚合物的制备方法,包括以下步骤:On the one hand, the present invention provides a kind of preparation method of the main chain containing amide bond polymer, comprises the following steps:
将两端为叠氮基团的单体、二元羧酸以及催化剂二吡啶二硒醚混匀,在0-5℃下向其中加入三甲基膦的有机溶液进行反应,当反应无气泡产生时,然后在25-40℃下反应2-24h,得到主链含酰胺键的聚合物,其中,二元羧酸、二吡啶二硒醚、两端为叠氮基团的单体和三甲基膦之间的摩尔比为1:0.4-2:1:4.8。Mix the monomer with azide groups at both ends, the dicarboxylic acid and the catalyst dipyridine diselenide evenly, and add the organic solution of trimethylphosphine to it at 0-5°C for the reaction, when no bubbles are generated in the reaction , and then react at 25-40°C for 2-24h to obtain a polymer containing amide bonds in the main chain, wherein dicarboxylic acid, dipyridine diselenide, monomers with azide groups at both ends, and trimethyl The molar ratio between the base phosphines is 1:0.4-2:1:4.8.
优选地,二元羧酸、二吡啶二硒醚、两端为叠氮基团的单体和三甲基膦之间的摩尔比为1:0.4:1:4.8。Preferably, the molar ratio between the dicarboxylic acid, dipyridine diselenide, the monomer having azide groups at both ends and trimethylphosphine is 1:0.4:1:4.8.
进一步地,两端为叠氮基团的单体的分子式为N3-R1-N3,其中R1表示脂肪族烷基或其衍生物,或者芳香族烷基或其衍生物。Further, the molecular formula of the monomer having azide groups at both ends is N3 -R1 -N3 , wherein R1 represents an aliphatic alkyl group or a derivative thereof, or an aromatic alkyl group or a derivative thereof.
进一步地,两端为叠氮基团的单体选自以下分子式中的任意一种:Further, the monomer whose two ends are azide groups is selected from any one of the following molecular formulas:
其中,n=6-12; Among them, n=6-12;
进一步地,二元羧酸的分子式为HCOO-R2-COOH,其中R2表示脂肪族烷基或其衍生物,或者芳香族烷基或其衍生物。Further, the molecular formula of the dicarboxylic acid is HCOO-R2 -COOH, wherein R2 represents an aliphatic alkyl group or a derivative thereof, or an aromatic alkyl group or a derivative thereof.
进一步地,两端为羧基基团的单体选自以下分子式中的任意一种:Further, the monomer that both ends are carboxyl groups is selected from any one of the following molecular formulas:
其中n=2-20; where n=2-20;
进一步地,三甲基膦的有机溶液中的有机溶剂为甲苯。Further, the organic solvent in the organic solution of trimethylphosphine is toluene.
进一步地,三甲基膦的有机溶液的浓度为1mol/L。Further, the concentration of the organic solution of trimethylphosphine was 1 mol/L.
进一步地,当反应无气泡时,在25-40℃下反应,优选的反应温度是40℃。Further, when the reaction is free of bubbles, the reaction is carried out at 25-40°C, and the preferred reaction temperature is 40°C.
进一步地,反应均在惰性气体保护下进行。具体是将惰性气体通入反应溶液中。Further, the reactions were carried out under the protection of inert gas. Specifically, an inert gas is passed into the reaction solution.
进一步地,惰性气体为氮气、氦气或氖气中的任意一种,优选氮气。Further, the inert gas is any one of nitrogen, helium or neon, preferably nitrogen.
进一步地,两端为叠氮基团的单体的制备方法包括以下步骤:Further, the preparation method of the monomer whose two ends are azide groups comprises the following steps:
将两端为溴的单体、叠氮化钠和溶剂加入到反应容器中,在25-60℃下反应24h,得到两端为叠氮基团的单体。其中两端为溴的单体和叠氮化钠的摩尔比为1:2-4,优选地,摩尔比为1:2.4。溶剂为丙酮和水的混合溶液、N,N’-二甲基甲酰胺或二甲亚砜中的一种,优选N,N’-二甲基甲酰胺。反应温度为25-60℃,优选60℃。A monomer with bromine at both ends, sodium azide and a solvent are added to a reaction vessel, and the reaction is carried out at 25-60° C. for 24 hours to obtain a monomer with azide groups at both ends. The molar ratio of the monomer having bromine at both ends and sodium azide is 1:2-4, preferably, the molar ratio is 1:2.4. The solvent is one of a mixed solution of acetone and water, N,N'-dimethylformamide or dimethyl sulfoxide, preferably N,N'-dimethylformamide. The reaction temperature is 25-60°C, preferably 60°C.
或or
将两端为氨基的单体、浓盐酸和水加入到反应容器中,在0-5℃下加入亚硝酸钠的水溶液,搅拌反应1-2h,再向其中加入叠氮化钠进行反应,得到两端为叠氮基团的单体。其中两端为氨基的单体、亚硝酸钠和叠氮化钠的摩尔比为1:2.1-2.5:2.3-2.5,优选地,摩尔比为1:2.1:2.3。优选地,反应温度为0℃。The monomer with amino groups at both ends, concentrated hydrochloric acid and water are added into the reaction vessel, and the aqueous solution of sodium nitrite is added at 0-5°C, and the reaction is stirred for 1-2 hours, and then sodium azide is added to the reaction vessel to obtain A monomer with azide groups at both ends. The molar ratio of the monomer having amino groups at both ends, sodium nitrite and sodium azide is 1:2.1-2.5:2.3-2.5, preferably, the molar ratio is 1:2.1:2.3. Preferably, the reaction temperature is 0°C.
以上主链含酰胺键聚合物的制备方法的反应路线如下:The reaction scheme of the preparation method of the above main chain containing amide bond polymer is as follows:
其中,R1表示脂肪族烷基或其衍生物,R2表示脂肪族烷基或其衍生物,Me3P表示三甲基膦,PySeSePy表示二吡啶二硒醚。Wherein, R1 represents an aliphatic alkyl group or a derivative thereof, R2 represents an aliphatic alkyl group or a derivative thereof, Me3 P represents trimethylphosphine, and PySeSePy represents dipyridine diselenide.
在以上反应中,加入三甲基膦的有机溶液后,两端为叠氮基团的单体与三甲基膦反应生成膦腈,同时两端为羧基的单体与催化剂反应生成活化的单体,膦腈与活化的单体反应生成中间体,剩余的二元羧酸与中间体反应生成目标产物主链含酰胺键的聚合物以及活性酯,再以此循环下去,直到底物消耗完全。反应原理如下:In the above reaction, after adding the organic solution of trimethylphosphine, the monomer with azide groups at both ends reacts with trimethylphosphine to generate phosphazene, and the monomer with carboxyl groups at both ends reacts with the catalyst to generate activated mono- The phosphazene reacts with the activated monomer to form an intermediate, and the remaining dicarboxylic acid reacts with the intermediate to generate the target product main chain containing amide bond polymer and active ester, and then repeat this cycle until the substrate is completely consumed . The reaction principle is as follows:
在另一方面,本发明还提供了另外一种主链含酰胺键的聚合物的制备方法,包括以下步骤:On the other hand, the present invention also provides a preparation method of another main chain containing amide bond polymer, comprising the following steps:
将一端为叠氮基团一端为羧基的单体以及催化剂二吡啶二硒醚混匀,在0℃下向其中加入三甲基膦的有机溶液进行反应,当反应无气泡产生时,然后在40℃下反应2-24h,得到主链含酰胺键的聚合物,其中,一端为叠氮基团一端为羧基的单体、二吡啶二硒醚和三甲基膦之间的摩尔比为1:0.2-1:2.4。Mix the monomer with azide group at one end and carboxyl group at the other end and catalyst dipyridine diselenide, and add the organic solution of trimethylphosphine to it for reaction at 0 °C. When no bubbles are generated in the reaction, then at 40 The reaction is carried out at ℃ for 2-24h to obtain a polymer containing an amide bond in the main chain, wherein the molar ratio between the monomer having an azide group at one end and a carboxyl group at the other end, dipyridine diselenide and trimethylphosphine is 1: 0.2-1:2.4.
优选地,一端为叠氮基团一端为羧基的单体、二吡啶二硒醚和三甲基膦之间的摩尔比为1:0.2:2.4。Preferably, the molar ratio between the monomer having an azide group at one end and a carboxyl group at the other end, dipyridine diselenide and trimethylphosphine is 1:0.2:2.4.
进一步地,一端为叠氮基团一端为羧基的单体分子式为N3-R3-COOH,其中R3表示脂肪族烷基或其衍生物,或者芳香族烷基或其衍生物。Further, the molecular formula of the monomer having an azide group at one end and a carboxyl group at the other end is N3 -R3 -COOH, wherein R3 represents an aliphatic alkyl group or a derivative thereof, or an aromatic alkyl group or a derivative thereof.
进一步地,一端为叠氮基团一端为羧基的单体分子式如下:Further, the molecular formula of the monomer whose one end is an azide group and one end is a carboxyl group is as follows:
其中n=5-10。 where n=5-10.
进一步地,三甲基膦的有机溶液中的有机溶剂为甲苯。Further, the organic solvent in the organic solution of trimethylphosphine is toluene.
进一步地,三甲基膦的有机溶液的浓度为1mol/L。Further, the concentration of the organic solution of trimethylphosphine was 1 mol/L.
进一步地,反应均在惰性气体保护下进行。具体是将惰性气体通入反应溶液中。Further, the reactions were carried out under the protection of inert gas. Specifically, an inert gas is passed into the reaction solution.
进一步地,惰性气体为氮气、氦气或氖气中的任意一种,优选氮气。Further, the inert gas is any one of nitrogen, helium or neon, preferably nitrogen.
进一步地,一端叠氮基团一端为羧基的单体的制备方法包括以下步骤:Further, the preparation method of the monomer whose one end azide group is one end carboxyl group comprises the following steps:
将一端为溴一端为羧基的单体、叠氮化钠和溶剂加入到反应容器中,在25-65℃下反应24h,得到一端叠氮基团一端为羧基的单体。其中一端为溴一端为羧基的单体和叠氮化钠的摩尔比为1:1.2-2,优选地,摩尔比为1:1.2。溶剂为丙酮和水的混合溶液、N,N’-二甲基甲酰胺或二甲亚砜中的一种,优选N,N’-二甲基甲酰胺。反应温度为25-65℃,优选65℃。A monomer whose one end is bromine and one end is a carboxyl group, sodium azide and a solvent are added to the reaction vessel, and the reaction is carried out at 25-65° C. for 24 hours to obtain a monomer whose one end is an azide group and one end is a carboxyl group. The molar ratio of the monomer whose one end is bromine and the other end is a carboxyl group and sodium azide is 1:1.2-2, preferably, the molar ratio is 1:1.2. The solvent is one of a mixed solution of acetone and water, N,N'-dimethylformamide or dimethyl sulfoxide, preferably N,N'-dimethylformamide. The reaction temperature is 25-65°C, preferably 65°C.
以上单体的制备方法的反应路线如下:The reaction scheme of the preparation method of the above monomers is as follows:
其中,R3表示肪族烷基或其衍生物,或者芳香族烷基或其衍生物,DMF表示N,N’-二甲基甲酰胺,NaN3表示叠氮化钠。Wherein, R3 represents an aliphatic alkyl group or a derivative thereof, or an aromatic alkyl group or a derivative thereof, DMF represents N,N'-dimethylformamide, and NaN3 represents sodium azide.
在50mL的反应管中,加1mmol的一端为叠氮一端为羧基的单体和0.2mmol的PySeSePy,搅拌并通入氮气,再将2.4mL温度为0℃的三甲基膦的甲苯溶液缓慢注入到体系中,当体系中无气体产生时,将体系温度升到40℃,通气状态下继续反应24h。反应停止后加入适量的去离子水搅拌,再离心,收集固体放入真空箱中干燥,再用六氟异丙醇溶解,沉淀在过量的甲醇中,抽滤,真空干燥,最终得到主链含酰胺键的聚合物。In a 50 mL reaction tube, add 1 mmol of a monomer whose one end is azide and one end is a carboxyl group and 0.2 mmol of PySeSePy, stir and pass nitrogen, and then slowly inject 2.4 mL of a toluene solution of trimethylphosphine at a temperature of 0 °C. Into the system, when no gas is produced in the system, the temperature of the system is raised to 40°C, and the reaction is continued for 24 hours in a ventilated state. After the reaction is stopped, add an appropriate amount of deionized water to stir, and then centrifuge, collect the solid and put it into a vacuum box to dry, then dissolve it in hexafluoroisopropanol, precipitate in excess methanol, suction filtration, and vacuum dry to finally obtain the main chain containing polymers of amide bonds.
以上制备方法的反应路线如下:The reaction scheme of the above preparation method is as follows:
其中,R3表示肪族烷基或其衍生物,或者芳香族烷基或其衍生物,Me3P表示三甲基膦,PySeSePy表示二吡啶二硒醚。Wherein, R3 represents an aliphatic alkyl group or a derivative thereof, or an aromatic alkyl group or a derivative thereof, Me3 P represents trimethylphosphine, and PySeSePy represents dipyridine diselenide.
三甲基膦加入到体系中后,单体与催化剂、三甲基膦反应生成活化的单体,随后活化的单体之间反应生成中间体,由于三甲基膦是过量的,并且生成膦腈的反应可以在几分钟内完成,所以能快速地把全部的叠氮基团转化为膦腈,一端为膦腈一端为羧基的部分活化的单体(由于催化剂PySeSePy与单体的摩尔比为0.2-1:1,只能活化部分的单体)在与中间体反应生成目标产物主链含酰胺键的聚合物以及活化的单体,再循环下去直到单体反应完全。以上反应原理如下:After trimethyl phosphine is added to the system, the monomer reacts with the catalyst and trimethyl phosphine to form an activated monomer, and then the activated monomer reacts to form an intermediate. Since trimethyl phosphine is in excess, and phosphine is generated The reaction of the nitrile can be completed in a few minutes, so the entire azide group can be quickly converted into a phosphazene, a partially activated monomer with a phosphazene at one end and a carboxyl group at the other end (due to the molar ratio of catalyst PySeSePy to monomer is 0.2-1:1, which can only activate part of the monomer) reacts with the intermediate to generate a polymer containing an amide bond in the main chain of the target product and an activated monomer, which is recycled until the monomer reaction is complete. The above reaction principle is as follows:
以上两种制备方法中,所使用的催化剂二吡啶二硒醚的制备方法如下:In the above two preparation methods, the preparation method of the used catalyst dipyridyl diselenide is as follows:
将氢氧化钠、硒粉、水合肼溶于有机溶剂,在25℃下反应2h,向其中加入2-溴吡啶,然后在120℃下反应24h,得到催化剂二吡啶二硒醚。Dissolve sodium hydroxide, selenium powder and hydrazine hydrate in an organic solvent, react at 25° C. for 2 hours, add 2-bromopyridine, and react at 120° C. for 24 hours to obtain the catalyst dipyridyl diselenide.
进一步地,氢氧化钠、硒粉、水合肼和2-溴吡啶之间的摩尔比为1.5:1:1:1。Further, the molar ratio between sodium hydroxide, selenium powder, hydrazine hydrate and 2-bromopyridine is 1.5:1:1:1.
进一步地,有机溶剂为N,N’-二甲基甲酰胺。Further, the organic solvent is N,N'-dimethylformamide.
进一步地,水合肼的浓度为85%。Further, the concentration of hydrazine hydrate was 85%.
借由上述方案,本发明至少具有以下优点:By means of the above scheme, the present invention has at least the following advantages:
(1)本发明首次将二吡啶二硒醚催化的Staudinger-Vilarrasa反应用于主链含酰胺键聚合物的合成,通过各种二元羧酸与两端为叠氮基团的单体缩聚生成聚合物,或通过两端分别为叠氮基团和羧基的分子的自聚合,为主链含酰胺键的聚合物的合成提供了新方法。(1) For the first time in the present invention, the Staudinger-Vilarrasa reaction catalyzed by dipyridine diselenide is used for the synthesis of polymers containing amide bonds in the main chain. Polymers, or through the self-polymerization of molecules with azide groups and carboxyl groups at both ends, provide a new method for the synthesis of polymers containing amide bonds in the main chain.
(2)本发明在制备聚合物时采用了二吡啶二硒醚催化的Staudinger-Vilarrasa反应,具有适用范围广、不需要高温、反应条件温和、反应速率快、副产物少、后处理简单、无需金属催化剂等优点,提供了一种高效的合成主链含酰胺键聚合物的方法。(2) The present invention adopts the Staudinger-Vilarrasa reaction catalyzed by dipyridine diselenide when preparing the polymer, which has the advantages of wide application range, no need for high temperature, mild reaction conditions, fast reaction rate, few by-products, simple post-processing, no need for The advantages of metal catalysts and the like provide an efficient method for synthesizing polymers containing amide bonds in the main chain.
上述说明仅是本发明技术方案的概述,为了能够更清楚了解本发明的技术手段,并可依照说明书的内容予以实施,以下以本发明的较佳实施例并配合附图详细说明如后。The above description is only an overview of the technical solution of the present invention. In order to understand the technical means of the present invention more clearly, and implement it according to the content of the description, the preferred embodiments of the present invention are described in detail below with the accompanying drawings.
附图说明Description of drawings
图1为实施例1制备的二吡啶二硒醚的核磁共振氢谱;Fig. 1 is the hydrogen nuclear magnetic resonance spectrum of the dipyridyl diselenide prepared in Example 1;
图2为实施例1制备的二吡啶二硒醚的核磁共振硒谱;Fig. 2 is the nuclear magnetic resonance selenium spectrum of the dipyridine diselenide prepared in Example 1;
图3为实施例1制备的1,12-二叠氮十二烷的核磁共振氢谱;Fig. 3 is the hydrogen nuclear magnetic resonance spectrum of 1,12-diazidedodecane prepared in Example 1;
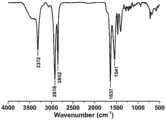
图4为实施例1中1,12-二溴十二烷以及1,12-二叠氮十二烷的红外光谱图;Fig. 4 is the infrared spectrogram of 1,12-dibromododecane and 1,12-diazidedodecane in Example 1;
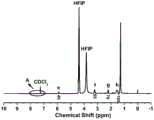
图5是实施例1制备的主链含酰胺键的聚合物PA1214的局部放大的核磁共振氢谱;Fig. 5 is the partially amplified hydrogen nuclear magnetic resonance spectrum of the main chain amide bond-containing polymer PA1214 prepared in Example 1;
图6是实施例1制备的主链含酰胺键的聚合物PA1214的核磁共振氢谱;Fig. 6 is the hydrogen nuclear magnetic resonance spectrum of the main chain containing amide bond polymer PA1214 prepared in Example 1;
图7是实施例1制备的主链含酰胺键的聚合物PA1214的红外光谱图;Fig. 7 is the infrared spectrogram of the polymer PA1214 of the main chain containing amide bond prepared in Example 1;
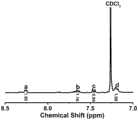
图8是实施例2制备的主链含酰胺键的聚合物PA1220的局部放大的核磁共振氢谱;Fig. 8 is the partially amplified hydrogen nuclear magnetic resonance spectrum of the main chain amide bond-containing polymer PA1220 prepared in Example 2;
图9是实施例2制备的主链含酰胺键的聚合物PA1220的核磁共振氢谱;Fig. 9 is the hydrogen nuclear magnetic resonance spectrum of the main chain containing amide bond polymer PA1220 prepared in Example 2;
图10是实施例2制备的主链含酰胺键的聚合物PA1220的红外光谱图;Fig. 10 is the infrared spectrogram of the polymer PA1220 of the main chain containing amide bond prepared in Example 2;
图11为实施例3制备的11-叠氮十一烷酸的核磁共振氢谱;Figure 11 is the hydrogen nuclear magnetic resonance spectrum of 11-azidoundecanoic acid prepared in Example 3;
图12为实施例3制备的11-叠氮十一烷酸的核磁共振碳谱;Figure 12 is the carbon nuclear magnetic resonance spectrum of 11-azidoundecanoic acid prepared in Example 3;
图13是实施例3制备的主链含酰胺键的聚合物PA11的局部放大的核磁共振氢谱;Fig. 13 is the partially amplified hydrogen nuclear magnetic resonance spectrum of the main chain amide bond-containing polymer PA11 prepared in Example 3;
图14是实施例3制备的主链含酰胺键的聚合物PA11的核磁共振氢谱;Figure 14 is the hydrogen nuclear magnetic resonance spectrum of the main chain containing amide bond polymer PA11 prepared in Example 3;
图15是实施例3制备的主链含酰胺键的聚合物PA11的红外光谱图;Fig. 15 is the infrared spectrogram of the main chain containing amide bond polymer PA11 prepared in Example 3;
图16是实施例3制备的主链含酰胺键的聚合物PA11的GPC流出曲线。FIG. 16 is the GPC efflux curve of the polymer PA11 containing amide bonds in the main chain prepared in Example 3. FIG.
具体实施方式Detailed ways
下面结合附图和实施例,对本发明的具体实施方式作进一步详细描述。以下实施例用于说明本发明,但不用来限制本发明的范围。The specific embodiments of the present invention will be described in further detail below with reference to the accompanying drawings and embodiments. The following examples are intended to illustrate the present invention, but not to limit the scope of the present invention.
本发明以下实施例中,所使用的测试仪器及测试条件如下:In the following examples of the present invention, the used test instrument and test conditions are as follows:
核磁共振氢谱(1H-NMR):使用Bruker 300MHz核磁仪,以CDCl3(氘代氯仿)为溶剂,TMS(四甲基硅烷)为内标,室温下测定;Hydrogen nuclear magnetic resonance spectrum (1 H-NMR): use a Bruker 300MHz nuclear magnetometer, with CDCl3 (deuterated chloroform) as solvent, TMS (tetramethylsilane) as internal standard, measured at room temperature;
核磁共振硒谱(77Se-NMR):使用Bruker 600MHz核磁仪,以CDCl3为溶剂,TMS为内标,室温下测定;Nuclear magnetic resonance selenium spectrum (77 Se-NMR): use Bruker 600MHz nuclear magnetic instrument, with CDCl3 as solvent, TMS as internal standard, measured at room temperature;
元素分析(EA):使用EA1110-CHNO-S微量分析仪测定;Elemental analysis (EA): determined using EA1110-CHNO-S microanalyzer;
凝胶渗透色谱(GPC):使用英国Malvern生产的Viscotek TDA305max多检测器凝胶渗透色谱测定,色谱柱为HFIPGuard+1x HFIP6000M,以六氟异丙醇(HFIP)为流动相,流速为0.7mL/min,在40℃下进行测试,用日本Shodex生产的聚甲基丙烯酸甲酯(PMMA)对聚合物分子量进行标定。Gel permeation chromatography (GPC): determined by Viscotek TDA305max multi-detector gel permeation chromatography produced by Malvern, UK. min, the test was carried out at 40°C, and the molecular weight of the polymer was calibrated with polymethyl methacrylate (PMMA) produced by Shodex in Japan.
实施例1Example 1
(1)将6.1g(152mmol)的氢氧化钠、7.9g(100mmol)的硒粉和250mL的N,N’-二甲基甲酰胺溶剂加入到500mL的三颈烧瓶中,在通气状态下搅拌,再将加入4.9g的水合肼慢慢加入到体系中,然后在室温下反应2h。随后再将2-溴吡啶15.8g(100mmol)缓慢滴加到反应瓶中,然后升温到120℃下反应24h,停止反应后恢复到室温。抽滤,滤液用大量乙酸乙酯进行萃取。有机相再用分别用饱和氯化铵溶液、去离子水饱和食盐水各洗一次,再用无水硫酸钠搅拌干燥2h,浓缩得粗产品,再用柱层析进行提纯。最终得到9.7g最终产物PySeSePy,产率为62%。以上反应的路线如下:(1) Add 6.1 g (152 mmol) of sodium hydroxide, 7.9 g (100 mmol) of selenium powder and 250 mL of N,N'-dimethylformamide solvent to a 500 mL three-necked flask, and stir under aeration , and then add 4.9 g of hydrazine hydrate slowly into the system, and then react at room temperature for 2 h. Subsequently, 15.8 g (100 mmol) of 2-bromopyridine was slowly added dropwise to the reaction flask, and then the temperature was raised to 120° C. to react for 24 h, and the reaction was stopped and returned to room temperature. Suction filtration, and the filtrate was extracted with a large amount of ethyl acetate. The organic phase was washed with saturated ammonium chloride solution and saturated brine with deionized water, respectively, and then stirred and dried with anhydrous sodium sulfate for 2 h, and concentrated to obtain a crude product, which was purified by column chromatography. Finally, 9.7 g of the final product PySeSePy was obtained with a yield of 62%. The route of the above reaction is as follows:
对产物分别进行核磁共振氢谱(图1)、核磁共振硒谱(图2)和元素分析测试(表1),图1中各峰都能找到归属,并且积分正确,图2为单峰,表明合成的为单硒醚或二硒醚,再结合表1中元素分析的结果,表明成功合成了二吡啶二硒醚PySeSePy。The product was subjected to H NMR spectrum (Fig. 1), NMR selenium spectrum (Fig. 2) and elemental analysis test (Table 1). All peaks in Fig. 1 can be assigned and the integration is correct. Fig. 2 is a single peak, It is shown that the synthesis is monoselenide or diselenide. Combined with the results of elemental analysis in Table 1, it is shown that the dipyridine diselenide PySeSePy has been successfully synthesized.
表1 PySeSePy的元素分析测试结果Table 1 Elemental analysis test results of PySeSePy
(2)将3.2813g(10mmol)的1,12-二溴十二烷、1.5602g(24mmol)NaN3和30mL的N,N-二甲基甲酰胺加入到50mL的圆底烧瓶中,在60℃的油浴锅中搅拌反应24h。停止反应恢复到室温后,再用中性氧化铝柱除去各种钠盐,在滤液中加入200mL的去离子水,用二氯甲烷进行萃取,收集有机相,然后分别用饱和氯化铵溶液、去离子水和饱和食盐水洗有机相,再用无水硫酸钠干燥有机相,抽滤,旋蒸,真空干燥。最后得到2.4480g的淡黄色液体,即为两端为叠氮基团的单体,其收率为97.0%。其反应路线如下:(2) 3.2813g (10mmol) of 1,12-dibromododecane, 1.5602g (24mmol)of NaN and 30mL of N,N-dimethylformamide were added to a 50mL round-bottomed flask at 60 The reaction was stirred in an oil bath at ℃ for 24h. After stopping the reaction and returning to room temperature, use a neutral alumina column to remove various sodium salts, add 200 mL of deionized water to the filtrate, extract with dichloromethane, collect the organic phase, and then use saturated ammonium chloride solution, The organic phase was washed with deionized water and saturated brine, then dried over anhydrous sodium sulfate, suction filtered, rotary evaporated, and dried in vacuo. Finally, 2.4480 g of light yellow liquid was obtained, which was a monomer with azide groups at both ends, and the yield was 97.0%. The reaction route is as follows:
以上产物1,12-二叠氮十二烷的核磁共振氢谱如图3所示,图中各峰都能找到对应的归属,并且积分正确。图4为1,12-二溴十二烷叠氮化前后的红外光谱图,图中a曲线表示1,12-二溴十二烷,b曲线表示1,12-二叠氮十二烷,反应之后在2100cm-1处出现叠氮基团的特征峰。由图3和图4可知,以上方法成功的合成了两端叠氮化的单体1,12-二叠氮十二烷。The H NMR spectrum of the
(3)在50mL的反应管中,加0.0997g(0.3920mmol)的十四烷二酸,0.0989g(0.3920mmol)的1,12-二叠氮十二烷和0.0493g(0.1568mmol)PySeSePy,搅拌并通入氮气,再将2mL温度为0℃的三甲基膦的甲苯溶液缓慢注入到反应管中,当反应管中无气泡产生时,将体系温度升到40℃,通氮气状态下继续反应2-24h,每隔几小时取样,测其核磁共振氢谱。反应停止后加入适量的去离子水搅拌,再离心,收集固体放入真空箱中干燥,再用六氟异丙醇溶解,沉淀在过量的甲醇中,抽滤,真空干燥,其中反应24h后,最终得到0.1507g的黄色固体,即为主链含酰胺键的聚合物,将其命名为PA1214,其收率为92%。以上反应的路线如下:(3) In a 50mL reaction tube, add 0.0997g (0.3920mmol) of tetradecanedioic acid, 0.0989g (0.3920mmol) of 1,12-diazidedodecane and 0.0493g (0.1568mmol) of PySeSePy, Stir and pass nitrogen, and then slowly inject 2 mL of toluene solution of trimethylphosphine with a temperature of 0 °C into the reaction tube. When no bubbles are generated in the reaction tube, raise the temperature of the system to 40 °C, and continue under nitrogen. The reaction was carried out for 2-24 h, and samples were taken every few hours to measure the H NMR spectrum. After the reaction was stopped, an appropriate amount of deionized water was added to stir, and then centrifuged. The collected solid was put into a vacuum box to dry, dissolved in hexafluoroisopropanol, precipitated in excess methanol, filtered with suction, and dried in vacuum. After 24 hours of reaction, the Finally, 0.1507 g of yellow solid was obtained, which was a polymer containing amide bonds in the main chain, which was named PA1214, and the yield was 92%. The route of the above reaction is as follows:
图5和图6为PA1214的核磁共振氢谱,其中图5为图6中的圈A处的局部的放大图。在图5中可以找到聚合物PA1214分子链末端基吡啶环的特征峰,各峰都能找到归属且积分正确。图6中5.90ppm处为酰胺键的特征峰,表明含酰胺键的聚合物的成功合成,聚合物主链上其余质子氢都能找到对应归属,并且积分正确,由图5和图6可以计算出聚合物PA1214的分子量,如表2所示。图7为聚合物PA1214的红外谱图,在3313和1546cm-1处的特征峰分别为酰胺键中N-H键的伸缩和弯曲振动峰,在2923和2852cm-1的特征峰是主链上亚甲基(CH2)的吸收峰,而羰基(C=O)的特征峰则在1642cm-1处,聚酰胺所有的特征峰都能找到,证明成功合成了主链含酰胺键聚合物PA1214。在本实施例中,通过催化的Staudinger-Vilarrasa反应十四烷二酸和1,12-二叠氮十二烷发生了聚合反应,成功的合成了主链含酰胺键的聚合物PA1214。表2为通过核磁氢谱计算的不同反应时间下PA1214的分子量和收率。FIG. 5 and FIG. 6 are the hydrogen nuclear magnetic resonance spectra of PA1214, wherein FIG. 5 is an enlarged view of the part at the circle A in FIG. 6 . In Figure 5, the characteristic peaks of the pyridine ring at the end group of the polymer PA1214 molecular chain can be found, and each peak can be assigned and integrated correctly. The characteristic peak of amide bond at 5.90ppm in Figure 6 indicates that the polymer containing amide bond was successfully synthesized. The rest of the proton hydrogens on the main chain of the polymer can be assigned correspondingly, and the integration is correct. It can be calculated from Figure 5 and Figure 6 The molecular weight of the polymer PA1214 was obtained, as shown in Table 2. Figure 7 is the infrared spectrum of polymer PA1214, the characteristic peaks at 3313 and 1546 cm-1 are the stretching and bending vibration peaks of the NH bond in the amide bond, respectively, and the characteristic peaks at 2923 and 2852 cm-1 are methylene on the main chain. The absorption peak of carbonyl group (CH2 ) and the characteristic peak of carbonyl group (C═O) are at 1642cm-1 . All the characteristic peaks of polyamide can be found, which proves that the main chain polymer PA1214 containing amide bond has been successfully synthesized. In this example, tetradecanedioic acid and 1,12-diazidedodecane were polymerized through the catalytic Staudinger-Vilarrasa reaction, and the polymer PA1214 with an amide bond in the main chain was successfully synthesized. Table 2 shows the molecular weight and yield of PA1214 at different reaction times calculated by hydrogen NMR.
表2不同反应时间下PA1412的分子量和收率Table 2 Molecular weight and yield of PA1412 under different reaction times
实施例2Example 2
在50mL的反应管中,加0.1350g(0.3941mmol)的二十烷二酸,0.0995g(0.3941mmol)的1,12-二叠氮十二烷和0.0495g(0.1577mmol)PySeSePy,搅拌并通入氮气,再将2mL温度为0℃的三甲基膦的甲苯溶液缓慢注入到体系中,当体系中无气体产生时,将体系温度升到40℃,通气状态下继续反应24h。反应停止后加入适量的去离子水搅拌,再离心,收集固体放入真空箱中干燥,再用六氟异丙醇溶解,沉淀在过量的甲醇中,抽滤,真空干燥,最终得到0.1358g的黄色固体,即为主链含酰胺键的聚合物,将其命名为PA1220,其收率为68%。以上反应的路线如下:In a 50 mL reaction tube, add 0.1350 g (0.3941 mmol) of eicosanedioic acid, 0.0995 g (0.3941 mmol) of 1,12-diazidedodecane and 0.0495 g (0.1577 mmol) of PySeSePy, stir and pass through Nitrogen was introduced, and then 2 mL of toluene solution of trimethylphosphine at 0°C was slowly injected into the system. When no gas was produced in the system, the temperature of the system was raised to 40°C, and the reaction was continued for 24h under aeration. After the reaction was stopped, an appropriate amount of deionized water was added to stir, and then centrifuged, and the solid was collected and put into a vacuum box to dry, then dissolved in hexafluoroisopropanol, precipitated in excess methanol, suction filtered, and vacuum-dried to finally obtain 0.1358g of The yellow solid, namely the polymer with amide bond in the main chain, was named PA1220, and its yield was 68%. The route of the above reaction is as follows:
图8和图9为PA1220的核磁共振氢谱,其中图8为图9中的圈A处的局部的放大图。在图8中可以找到聚合物PA1220分子链末端基吡啶环的出峰,各峰都能找到归属积分正确。图9中5.89ppm为酰胺键的特征峰,表明含酰胺键的聚合物的成功合成,聚合物主链上其余质子氢都能找到对应归属,并且积分正确,由图9中聚合物的末端基和图9可以计算出聚合物PA1220的分子量为Mn=10800Da。图10为聚合物PA1220的红外光谱图,在3424和1546cm-1处的特征峰分别为酰胺键中N-H键的伸缩和弯曲振动峰,在2923和2847cm-1的特征峰是主链上亚甲基(CH2)的吸收峰,而羰基(C=O)的特征峰则在1642cm-1处,聚酰胺所有的特征峰都能找到,证明成功合成了主链含酰胺键聚合物PA1220。在本实施例中,通过催化的Staudinger-Vilarrasa反应二十烷二酸和1,12-二叠氮十二烷发生了聚合反应,成功的合成了主链含酰胺键的聚合物PA2012。FIG. 8 and FIG. 9 are the hydrogen nuclear magnetic resonance spectra of PA1220, wherein FIG. 8 is an enlarged view of the part at the circle A in FIG. 9 . In Figure 8, the peaks of the pyridine ring at the end group of the polymer PA1220 molecular chain can be found, and each peak can be found and integrated correctly. In Figure 9, 5.89ppm is the characteristic peak of amide bond, indicating that the polymer containing amide bond was successfully synthesized. The rest of the proton hydrogens on the main chain of the polymer can be assigned to the corresponding attribution, and the integration is correct. From the end group of the polymer in Figure 9 And Figure 9 can calculate the molecular weight of the polymer PA1220 isMn = 10800Da. Figure 10 is the infrared spectrum of the polymer PA1220. The characteristic peaks at 3424 and 1546 cm-1 are the stretching and bending vibration peaks of the NH bond in the amide bond, respectively, and the characteristic peaks at 2923 and 2847 cm-1 are methylene on the main chain. The absorption peak of carbonyl group (CH2 ) and the characteristic peak of carbonyl group (C═O) are at 1642cm-1 , all the characteristic peaks of polyamide can be found, which proves that the main chain polymer PA1220 containing amide bond has been successfully synthesized. In this example, eicosanedioic acid and 1,12-diazidedodecane were polymerized through the catalytic Staudinger-Vilarrasa reaction, and the polymer PA2012 with an amide bond in the main chain was successfully synthesized.
实施例3Example 3
(1)将3.9779g(15mmol)的11-溴十一烷酸、1.1702g(18mmol)NaN3和20mL的N,N-二甲基甲酰胺加入到50mL的圆底烧瓶中,在65℃的油浴锅中搅拌反应24h。停止反应恢复到室温后,再用中性氧化铝柱除去各种钠盐,在滤液中加入100mL的去离子水,用二氯甲烷进行萃取,收集有机相,然后分别用饱和氯化铵溶液、去离子水和饱和食盐水洗涤有机相,再用无水硫酸钠干燥有机相,抽滤,旋蒸,用柱层析法提纯粗产物,选用的展开剂为石油醚和乙酸乙酯(V/V=4/1),浓缩,真空干燥。最后得到2.800g的淡黄色液体,即一端为叠氮基团另一端为羧基的单体,其收率为82.4%。其反应路线如下:(1) 3.9779g (15mmol) of 11-bromonodecanoic acid, 1.1702g (18mmol)of NaN and 20mL of N,N-dimethylformamide were added to a 50mL round-bottomed flask, and the flask was heated at 65°C. The reaction was stirred in an oil bath for 24h. After stopping the reaction and returning to room temperature, use a neutral alumina column to remove various sodium salts, add 100 mL of deionized water to the filtrate, extract with dichloromethane, collect the organic phase, and then use saturated ammonium chloride solution, Deionized water and saturated brine wash the organic phase, then dry the organic phase with anhydrous sodium sulfate, suction filtration, rotary evaporation, purify the crude product by column chromatography, and the selected developing agent is petroleum ether and ethyl acetate (V/ V=4/1), concentrated and dried in vacuo. Finally, 2.800 g of light yellow liquid was obtained, that is, a monomer with an azide group at one end and a carboxyl group at the other end, and the yield was 82.4%. The reaction route is as follows:
以上产物11-叠氮十一烷酸的核磁共振氢谱如图11所示,图中各峰都能找到对应的归属,并且积分正确。图12为11-叠氮十一烷酸的核磁共振碳谱,各峰都能找到对应的归属。由图11和图12可知,以上方法成功的合成了一端为叠氮一端为羧基的单体11-叠氮十一烷酸。The H NMR spectrum of the above product 11-azidoundecanoic acid is shown in Figure 11. Each peak in the figure can be found to have a corresponding attribution, and the integration is correct. Figure 12 is the carbon nuclear magnetic resonance spectrum of 11-azidoundecanoic acid, and each peak can be assigned a corresponding assignment. It can be seen from Fig. 11 and Fig. 12 that the above method successfully synthesized the monomer 11-azidoundecanoic acid whose one end is azide and the other end is carboxyl group.
(2)在50mL的反应管中,加0.2580g(1.1350mmol)的11-叠氮十一烷酸和0.0713g(0.2270mmol)PySeSePy,搅拌并通入氮气,再将2.7mL温度为0℃的三甲基膦的甲苯溶液缓慢注入到体系中,当体系中无气体产生时,将体系温度升到40℃,通气状态下继续反应24h。反应停止后加入适量的去离子水搅拌,再离心,收集固体放入真空箱中干燥,再用六氟异丙醇溶解,沉淀在过量的甲醇中,抽滤,真空干燥,最终得到0.1700g的黄色固体,即为主链含酰胺键的聚合物,将其命名为PA11,其收率为82.0%。以上反应的路线如下:(2) In a 50 mL reaction tube, add 0.2580 g (1.1350 mmol) of 11-azidoundecanoic acid and 0.0713 g (0.2270 mmol) of PySeSePy, stir and pass nitrogen, and then add 2.7 mL of 0. The toluene solution of trimethylphosphine was slowly injected into the system, and when no gas was produced in the system, the temperature of the system was raised to 40°C, and the reaction was continued for 24 hours in a ventilated state. After the reaction was stopped, an appropriate amount of deionized water was added to stir, then centrifuged, and the solid was collected and put into a vacuum box to dry, then dissolved in hexafluoroisopropanol, precipitated in excess methanol, suction filtered, and vacuum-dried to finally obtain 0.1700g of The yellow solid, namely the polymer with amide bond in the main chain, was named PA11, and its yield was 82.0%. The route of the above reaction is as follows:
图13和图14为PA11的核磁共振氢谱,其中图13为图14中的圈A处的局部的放大图。在图13中可以找到聚合物PA11分子链末端基吡啶环的特征峰,各峰都能找到归属,并且积分正确。图14中6.04ppm处为酰胺键的特征峰,表明含酰胺键的聚合物的成功合成,聚合物主链上其余质子氢都能找到对应归属,并且积分正确,由图13中末端基和图14可以计算出聚合物PA11的分子量为Mn=4900Da。图15为聚合物PA11的红外光谱图,在3312和1541cm-1处的特征峰分别为酰胺键中N-H键的伸缩和弯曲振动峰,在2918和2852cm-1的特征峰是主链上亚甲基(CH2)的吸收峰,而羰基(C=O)的特征峰则在1637cm-1处,聚酰胺所有的特征峰都能找到,证明成功合成了主链含酰胺键聚合物PA11。图16为聚合物PA11的GPC流出曲线,由GPC流出曲线计算出聚合物的数均分子量为Mn=3100Da,分子量分布PDI=1.13。在本实施例中,通过催化的Staudinger-Vilarrasa反应11-叠氮十一烷酸发生了聚合反应,成功的合成了主链含酰胺键的聚合物PA11。13 and 14 are hydrogen NMR spectra of PA11, wherein FIG. 13 is an enlarged view of a part at the circle A in FIG. 14 . In Figure 13, the characteristic peaks of the pyridine ring of the terminal group of the polymer PA11 molecular chain can be found, and each peak can be assigned and the integration is correct. The characteristic peak of amide bond at 6.04ppm in Figure 14 indicates that the polymer containing amide bond was successfully synthesized. The rest of the proton hydrogens on the main chain of the polymer can be assigned corresponding assignments, and the integration is correct. 14 The molecular weight of polymer PA11 can be calculated to beMn = 4900 Da. Figure 15 is the infrared spectrum of polymer PA11, the characteristic peaks at 3312 and 1541 cm-1 are the stretching and bending vibration peaks of the NH bond in the amide bond, respectively, and the characteristic peaks at 2918 and 2852 cm-1 are methylene on the main chain. The absorption peak of the carbonyl group (CH2 ) and the characteristic peak of the carbonyl group (C=O) are at 1637 cm-1 . All the characteristic peaks of the polyamide can be found, which proves the successful synthesis of the main chain amide bond-containing polymer PA11. Figure 16 is the GPC efflux curve of the polymer PA11. The number average molecular weight of the polymer calculated from the GPC efflux curve isMn = 3100 Da, and the molecular weight distribution PDI = 1.13. In this example, 11-azidoundecanoic acid was polymerized through the catalytic Staudinger-Vilarrasa reaction, and the polymer PA11 with an amide bond in the main chain was successfully synthesized.
以上所述仅是本发明的优选实施方式,并不用于限制本发明,应当指出,对于本技术领域的普通技术人员来说,在不脱离本发明技术原理的前提下,还可以做出若干改进和变型,这些改进和变型也应视为本发明的保护范围。The above are only the preferred embodiments of the present invention and are not intended to limit the present invention. It should be pointed out that for those skilled in the art, some improvements can be made without departing from the technical principles of the present invention. These improvements and modifications should also be regarded as the protection scope of the present invention.
Claims (4)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810100749.1ACN108192095B (en) | 2018-02-01 | 2018-02-01 | Preparation method of polymer with amido bond in main chain |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810100749.1ACN108192095B (en) | 2018-02-01 | 2018-02-01 | Preparation method of polymer with amido bond in main chain |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN108192095A CN108192095A (en) | 2018-06-22 |
| CN108192095Btrue CN108192095B (en) | 2020-07-31 |
Family
ID=62592326
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201810100749.1AActiveCN108192095B (en) | 2018-02-01 | 2018-02-01 | Preparation method of polymer with amido bond in main chain |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN108192095B (en) |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7812051B2 (en)* | 2004-08-11 | 2010-10-12 | Arqule, Inc. | Pharmaceutical compositions of β-lapachone and β-lapachone analogs with improved tumor targeting potential |
| US8173765B2 (en)* | 2007-07-30 | 2012-05-08 | Valorisation-Recherche, Limited Partnership | Polymers, uses and methods of manufacture thereof |
| KR20130072513A (en)* | 2011-12-22 | 2013-07-02 | 제일모직주식회사 | Modified polyamide resin with excellent heat resistance, moldability and whiteness and method for preparing the same using polyester resin |
| CN103242495B (en)* | 2012-12-27 | 2015-07-01 | 苏州大学 | Preparation method of diblock copolymer containing polyamide chain segment |
| EP4001342B1 (en)* | 2013-09-05 | 2024-08-21 | The Penn State Research Foundation | Bioelastomers and applications thereof |
| CN103467746B (en)* | 2013-09-27 | 2015-12-09 | 中国科学院长春应用化学研究所 | A kind of amphipathic nature polyalcohol and preparation method thereof |
| CN104530417B (en)* | 2014-10-01 | 2017-09-08 | 厦门赛诺邦格生物科技股份有限公司 | A kind of multiple functionalized H types polyethyleneglycol derivative and preparation method thereof |
- 2018
- 2018-02-01CNCN201810100749.1Apatent/CN108192095B/enactiveActive
Non-Patent Citations (1)
| Title |
|---|
| Catalytic Staudingers Vilarrasa Reaction for the Direct Ligation of Carboxylic Acids and Azides;Jordi Bure s et al.;《Journal of Organic Chemistry》;20090209(第74期);第2203-2206页* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN108192095A (en) | 2018-06-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN108976402B (en) | Polyester polymer and method for preparing polyester polymer by using binary catalytic system | |
| CN106366309B (en) | A kind of polyamide-based compound and preparation method thereof | |
| CN101323662B (en) | Biodegradable hyperbranched aliphatic polyamide and preparation method thereof | |
| Gao et al. | Synthesis and characterization of poly (hexamethylene terephthalate/hexamethylene oxamide) alternating copolyamide (alt‐PA6T/62) | |
| Ali et al. | pH and reduction sensitive bio-based polyamides derived from renewable dicarboxylic acid monomers and cystine amino acid | |
| CN108192095B (en) | Preparation method of polymer with amido bond in main chain | |
| CN113105631A (en) | Sulfonamide polymer and preparation method thereof | |
| JPH0693100A (en) | Production of azo group-containing polysiloxane amide | |
| Ghaemy et al. | Fluorene‐ring‐containing diamine and resultant soluble thermally stable polyamides | |
| CN105348542A (en) | Synthesis method of aromatic hyperbranched polyamidoamine compound | |
| Lee et al. | Rearrangement of the main chain of the organocobalt polymers: 2. Synthesis of novel poly (pyridine-diyl-alt-biphenyl-4, 4′-diyl) by the reaction with nitriles (1) | |
| Namgoong et al. | Micro-chemical structure of polyaniline synthesized by self-stabilized dispersion polymerization | |
| CN103467353B (en) | Containing the bismaleimides and preparation method thereof of fluorenyl and aryl oxide bond structure | |
| JP4873570B2 (en) | POLY [2] CATENAN COMPOUND AND MONOMER THEREOF AND METHOD FOR PRODUCING THEM | |
| CN116496480B (en) | A polyarylate containing polyphenylene ether structure and preparation method thereof | |
| Bheda et al. | Main chain polyamide rotaxanes from aliphatic crown ethers | |
| JP3258116B2 (en) | Polyamide polymer and method for producing the same | |
| JPH0313252B2 (en) | ||
| JP4936580B2 (en) | Method for producing azo group-containing polyamide | |
| JP3858458B2 (en) | Azo group-containing polyamide and process for producing the same | |
| US5576417A (en) | Method for synthesizing aromatic polymers | |
| Toktonov et al. | Synthesis of polyamidines based on aromatic bis (imidoyl) chlorides in solution | |
| EP3896112A1 (en) | Polyalkyleneimine-modified polyamide 4 | |
| Wang et al. | Functionalized Polyphosphoester via Living Ring-opening Polymerization and Photochemical Thiol-ene Click Reaction | |
| Popa et al. | Some polyamides, polyesters, and polyurethanes containing azo groups |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |