CN108148808B - Induction medium for the induction of neural precursor cells - Google Patents
Induction medium for the induction of neural precursor cellsDownload PDFInfo
- Publication number
- CN108148808B CN108148808BCN201611101061.2ACN201611101061ACN108148808BCN 108148808 BCN108148808 BCN 108148808BCN 201611101061 ACN201611101061 ACN 201611101061ACN 108148808 BCN108148808 BCN 108148808B
- Authority
- CN
- China
- Prior art keywords
- cells
- medium
- neural precursor
- gibco
- induction
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000001537neural effectEffects0.000titleclaimsabstractdescription83
- 239000002243precursorSubstances0.000titleclaimsabstractdescription82
- 230000006698inductionEffects0.000titleclaimsabstractdescription78
- 210000004027cellAnatomy0.000claimsabstractdescription166
- 239000002609mediumSubstances0.000claimsabstractdescription91
- 108090000379Fibroblast growth factor 2Proteins0.000claimsabstractdescription30
- 102000009024Epidermal Growth FactorHuman genes0.000claimsabstractdescription29
- 239000006144Dulbecco’s modified Eagle's mediumSubstances0.000claimsabstractdescription19
- HJCMDXDYPOUFDY-WHFBIAKZSA-NAla-GlnChemical compoundC[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=OHJCMDXDYPOUFDY-WHFBIAKZSA-N0.000claimsabstractdescription15
- 102000003974Fibroblast growth factor 2Human genes0.000claimsabstractdescription15
- 102100024785Fibroblast growth factor 2Human genes0.000claimsabstractdescription15
- 101800003838Epidermal growth factorProteins0.000claimsabstractdescription14
- 238000005516engineering processMethods0.000claimsabstractdescription14
- 229940116977epidermal growth factorDrugs0.000claimsabstractdescription14
- 230000001939inductive effectEffects0.000claimsabstractdescription14
- VBEQCZHXXJYVRD-GACYYNSASA-NuroantheloneChemical compoundC([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1VBEQCZHXXJYVRD-GACYYNSASA-N0.000claimsabstractdescription14
- 239000000203mixtureSubstances0.000claimsabstractdescription12
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N13-cis retinolNatural productsOCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)CFPIPGXGPPPQFEQ-UHFFFAOYSA-N0.000claimsabstractdescription10
- HTTJABKRGRZYRN-UHFFFAOYSA-NHeparinChemical compoundOC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1HTTJABKRGRZYRN-UHFFFAOYSA-N0.000claimsabstractdescription10
- FPIPGXGPPPQFEQ-BOOMUCAASA-NVitamin ANatural productsOC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)CFPIPGXGPPPQFEQ-BOOMUCAASA-N0.000claimsabstractdescription10
- FPIPGXGPPPQFEQ-OVSJKPMPSA-Nall-trans-retinolChemical compoundOC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)CFPIPGXGPPPQFEQ-OVSJKPMPSA-N0.000claimsabstractdescription10
- 229960002897heparinDrugs0.000claimsabstractdescription10
- 229920000669heparinPolymers0.000claimsabstractdescription10
- 235000019155vitamin ANutrition0.000claimsabstractdescription10
- 239000011719vitamin ASubstances0.000claimsabstractdescription10
- 229940045997vitamin aDrugs0.000claimsabstractdescription10
- 210000000130stem cellAnatomy0.000claimsabstractdescription9
- 210000001082somatic cellAnatomy0.000abstractdescription14
- 102000004127CytokinesHuman genes0.000abstractdescription9
- 108090000695CytokinesProteins0.000abstractdescription9
- 238000000338in vitroMethods0.000abstractdescription5
- 210000002950fibroblastAnatomy0.000description68
- 238000000034methodMethods0.000description27
- 230000014509gene expressionEffects0.000description18
- 108090000623proteins and genesProteins0.000description18
- 230000009466transformationEffects0.000description17
- 230000008569processEffects0.000description15
- 108010043121Green Fluorescent ProteinsProteins0.000description12
- 239000005090green fluorescent proteinSubstances0.000description11
- 239000012091fetal bovine serumSubstances0.000description10
- 239000003102growth factorSubstances0.000description10
- 239000003550markerSubstances0.000description7
- 108010088225NestinProteins0.000description6
- 238000011529RT qPCRMethods0.000description6
- 230000001464adherent effectEffects0.000description6
- 238000004113cell cultureMethods0.000description6
- 239000003814drugSubstances0.000description6
- 210000005155neural progenitor cellAnatomy0.000description6
- 108091003079Bovine Serum AlbuminProteins0.000description5
- 101100247004Rattus norvegicus Qsox1 geneProteins0.000description5
- 239000007640basal mediumSubstances0.000description5
- 238000002659cell therapyMethods0.000description5
- 210000001178neural stem cellAnatomy0.000description5
- 230000001737promoting effectEffects0.000description5
- KCXVZYZYPLLWCC-UHFFFAOYSA-NEDTAChemical compoundOC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=OKCXVZYZYPLLWCC-UHFFFAOYSA-N0.000description4
- ZDXPYRJPNDTMRX-VKHMYHEASA-NL-glutamineChemical compoundOC(=O)[C@@H](N)CCC(N)=OZDXPYRJPNDTMRX-VKHMYHEASA-N0.000description4
- 101100310657Mus musculus Sox1 geneProteins0.000description4
- 238000003559RNA-seq methodMethods0.000description4
- 102000004142TrypsinHuman genes0.000description4
- 108090000631TrypsinProteins0.000description4
- 201000010099diseaseDiseases0.000description4
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description4
- 230000000977initiatory effectEffects0.000description4
- 238000007481next generation sequencingMethods0.000description4
- 230000001172regenerating effectEffects0.000description4
- 230000008672reprogrammingEffects0.000description4
- 239000012588trypsinSubstances0.000description4
- 102000004144Green Fluorescent ProteinsHuman genes0.000description3
- 102000008730NestinHuman genes0.000description3
- 230000004069differentiationEffects0.000description3
- 239000001963growth mediumSubstances0.000description3
- 239000003112inhibitorSubstances0.000description3
- 210000004962mammalian cellAnatomy0.000description3
- 238000011160researchMethods0.000description3
- 239000012679serum free mediumSubstances0.000description3
- 210000001519tissueAnatomy0.000description3
- 230000007704transitionEffects0.000description3
- AQGNHMOJWBZFQQ-UHFFFAOYSA-NCT 99021Chemical compoundCC1=CNC(C=2C(=NC(NCCNC=3N=CC(=CC=3)C#N)=NC=2)C=2C(=CC(Cl)=CC=2)Cl)=N1AQGNHMOJWBZFQQ-UHFFFAOYSA-N0.000description2
- 229930182816L-glutamineNatural products0.000description2
- 101150052863THY1 geneProteins0.000description2
- 102000040945Transcription factorHuman genes0.000description2
- 108091023040Transcription factorProteins0.000description2
- 238000004458analytical methodMethods0.000description2
- 230000009286beneficial effectEffects0.000description2
- 150000001875compoundsChemical class0.000description2
- 238000012258culturingMethods0.000description2
- 230000003247decreasing effectEffects0.000description2
- 238000001514detection methodMethods0.000description2
- 230000000694effectsEffects0.000description2
- 238000000684flow cytometryMethods0.000description2
- 238000002073fluorescence micrographMethods0.000description2
- 238000010166immunofluorescenceMethods0.000description2
- 238000001727in vivoMethods0.000description2
- 238000003780insertionMethods0.000description2
- 230000037431insertionEffects0.000description2
- 230000010354integrationEffects0.000description2
- 230000001404mediated effectEffects0.000description2
- 210000000944nerve tissueAnatomy0.000description2
- 210000000653nervous systemAnatomy0.000description2
- 230000004770neurodegenerationEffects0.000description2
- 208000015122neurodegenerative diseaseDiseases0.000description2
- 230000037361pathwayEffects0.000description2
- 230000035755proliferationEffects0.000description2
- 239000012474protein markerSubstances0.000description2
- 102000004169proteins and genesHuman genes0.000description2
- 230000001105regulatory effectEffects0.000description2
- 150000003384small moleculesChemical class0.000description2
- 230000006641stabilisationEffects0.000description2
- 238000011105stabilizationMethods0.000description2
- 238000010186stainingMethods0.000description2
- 239000000126substanceSubstances0.000description2
- 230000001225therapeutic effectEffects0.000description2
- 230000026683transductionEffects0.000description2
- 238000010361transductionMethods0.000description2
- 230000003612virological effectEffects0.000description2
- HPTXLHAHLXOAKV-INIZCTEOSA-N(2S)-2-(1,3-dioxo-2-isoindolyl)-3-(1H-indol-3-yl)propanoic acidChemical compoundO=C1C2=CC=CC=C2C(=O)N1[C@H](C(=O)O)CC1=CNC2=CC=CC=C12HPTXLHAHLXOAKV-INIZCTEOSA-N0.000description1
- HIJMSZGHKQPPJS-UHFFFAOYSA-N3-(6-methylpyridin-2-yl)-n-phenyl-4-quinolin-4-ylpyrazole-1-carbothioamideChemical compoundCC1=CC=CC(C=2C(=CN(N=2)C(=S)NC=2C=CC=CC=2)C=2C3=CC=CC=C3N=CC=2)=N1HIJMSZGHKQPPJS-UHFFFAOYSA-N0.000description1
- RXZDWPYJFCAZCW-UHFFFAOYSA-N3-chloro-4,7-difluoro-n-[4-(methylamino)cyclohexyl]-n-[(3-pyridin-4-ylphenyl)methyl]-1-benzothiophene-2-carboxamideChemical compoundC1CC(NC)CCC1N(C(=O)C1=C(C2=C(F)C=CC(F)=C2S1)Cl)CC1=CC=CC(C=2C=CN=CC=2)=C1RXZDWPYJFCAZCW-UHFFFAOYSA-N0.000description1
- CDOVNWNANFFLFJ-UHFFFAOYSA-N4-[6-[4-(1-piperazinyl)phenyl]-3-pyrazolo[1,5-a]pyrimidinyl]quinolineChemical compoundC1CNCCN1C1=CC=C(C2=CN3N=CC(=C3N=C2)C=2C3=CC=CC=C3N=CC=2)C=C1CDOVNWNANFFLFJ-UHFFFAOYSA-N0.000description1
- 208000024827Alzheimer diseaseDiseases0.000description1
- 101150008656COL1A1 geneProteins0.000description1
- 241000196324EmbryophytaSpecies0.000description1
- 102000001267GSK3Human genes0.000description1
- 108060006662GSK3Proteins0.000description1
- 102000003964Histone deacetylaseHuman genes0.000description1
- 108090000353Histone deacetylaseProteins0.000description1
- 206010021143HypoxiaDiseases0.000description1
- 241000713666LentivirusSpecies0.000description1
- 102000007354PAX6 Transcription FactorHuman genes0.000description1
- 101150081664PAX6 geneProteins0.000description1
- 208000018737Parkinson diseaseDiseases0.000description1
- BCPOLXUSCUFDGE-UHFFFAOYSA-NSMER 28Chemical compoundN1=CN=C(NCC=C)C2=CC(Br)=CC=C21BCPOLXUSCUFDGE-UHFFFAOYSA-N0.000description1
- 102000004887Transforming Growth Factor betaHuman genes0.000description1
- 108090001012Transforming Growth Factor betaProteins0.000description1
- 241000700605VirusesSpecies0.000description1
- BKPRVQDIOGQWTG-ICOOEGOYSA-N[(1s,2r)-2-phenylcyclopropyl]azanium;[(1r,2s)-2-phenylcyclopropyl]azanium;sulfateChemical compound[O-]S([O-])(=O)=O.[NH3+][C@H]1C[C@@H]1C1=CC=CC=C1.[NH3+][C@@H]1C[C@H]1C1=CC=CC=C1BKPRVQDIOGQWTG-ICOOEGOYSA-N0.000description1
- 230000009471actionEffects0.000description1
- 230000032683agingEffects0.000description1
- SHGAZHPCJJPHSC-YCNIQYBTSA-Nall-trans-retinoic acidChemical compoundOC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)CSHGAZHPCJJPHSC-YCNIQYBTSA-N0.000description1
- 206010002026amyotrophic lateral sclerosisDiseases0.000description1
- 238000013459approachMethods0.000description1
- 230000008901benefitEffects0.000description1
- 210000004556brainAnatomy0.000description1
- 239000006143cell culture mediumSubstances0.000description1
- 239000002771cell markerSubstances0.000description1
- 230000010307cell transformationEffects0.000description1
- 230000008859changeEffects0.000description1
- 230000000973chemotherapeutic effectEffects0.000description1
- 238000011281clinical therapyMethods0.000description1
- 230000029087digestionEffects0.000description1
- 230000007783downstream signalingEffects0.000description1
- 238000005206flow analysisMethods0.000description1
- 238000009472formulationMethods0.000description1
- 239000012737fresh mediumSubstances0.000description1
- 230000036541healthEffects0.000description1
- XPXMKIXDFWLRAA-UHFFFAOYSA-NhydrazinideChemical compound[NH-]NXPXMKIXDFWLRAA-UHFFFAOYSA-N0.000description1
- 230000001146hypoxic effectEffects0.000description1
- 238000011065in-situ storageMethods0.000description1
- 210000004263induced pluripotent stem cellAnatomy0.000description1
- 210000005171mammalian brainAnatomy0.000description1
- 230000007246mechanismEffects0.000description1
- 230000000877morphologic effectEffects0.000description1
- 230000035772mutationEffects0.000description1
- NFVJNJQRWPQVOA-UHFFFAOYSA-Nn-[2-chloro-5-(trifluoromethyl)phenyl]-2-[3-(4-ethyl-5-ethylsulfanyl-1,2,4-triazol-3-yl)piperidin-1-yl]acetamideChemical compoundCCN1C(SCC)=NN=C1C1CN(CC(=O)NC=2C(=CC=C(C=2)C(F)(F)F)Cl)CCC1NFVJNJQRWPQVOA-UHFFFAOYSA-N0.000description1
- 210000005055nestinAnatomy0.000description1
- 210000003061neural cellAnatomy0.000description1
- 210000003757neuroblastAnatomy0.000description1
- 210000002569neuronAnatomy0.000description1
- 210000000056organAnatomy0.000description1
- 229940087824parnateDrugs0.000description1
- 210000001778pluripotent stem cellAnatomy0.000description1
- 229930002330retinoic acidNatural products0.000description1
- -1small molecule compoundsChemical class0.000description1
- 230000000392somatic effectEffects0.000description1
- 210000000278spinal cordAnatomy0.000description1
- 230000001131transforming effectEffects0.000description1
- 238000011830transgenic mouse modelMethods0.000description1
- 229960001727tretinoinDrugs0.000description1
- 241001430294unidentified retrovirusSpecies0.000description1
- 238000011144upstream manufacturingMethods0.000description1
- 230000009750upstream signalingEffects0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0618—Cells of the nervous system
- C12N5/0623—Stem cells
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/38—Vitamins
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/11—Epidermal growth factor [EGF]
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/115—Basic fibroblast growth factor (bFGF, FGF-2)
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/998—Proteins not provided for elsewhere
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/02—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from embryonic cells
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/13—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from connective tissue cells, from mesenchymal cells
- C12N2506/1307—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from connective tissue cells, from mesenchymal cells from adult fibroblasts
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Neurology (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Neurosurgery (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Developmental Biology & Embryology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及再生医学领域,特别是一种有助于诱导生成神经前体细胞的诱导培养基。The present invention relates to the field of regenerative medicine, in particular to an induction medium for inducing the generation of neural precursor cells.
背景技术Background technique
神经退行性疾病是大脑或脊髓的神经细胞的结构和功能丧失导致的神经系统疾病。此类疾病,包括病程较长(20年或更长)的如阿兹海默症、帕金森症及少数病程进展较快(2-3年)的肌萎缩性脊髓侧索硬化症等,对人类特别是老年人的健康构成了巨大的威胁。现有的化学药物治疗手段虽然也可以缓解这些病症,但都没有取得十分明显的根治效果。因此,通过再生医学的手段重建病变的神经组织和器官的治疗策略显得尤为重要。Neurodegenerative diseases are diseases of the nervous system caused by the loss of structure and function of nerve cells in the brain or spinal cord. Such diseases, including those with a longer course (20 years or more) such as Alzheimer's disease, Parkinson's disease, and a few amyotrophic lateral sclerosis with a rapid course (2-3 years), etc. Human health, especially the elderly, poses a huge threat. Although the existing chemotherapeutic methods can also alleviate these diseases, they have not achieved a very obvious radical effect. Therefore, the therapeutic strategy of reconstructing diseased nerve tissues and organs by means of regenerative medicine is particularly important.
再生医学的最终目标为替换老化、病变或损伤的组织,目前可通过两大途径来实现,即细胞重编程技术和细胞转分化技术。细胞重编程技术的革新为细胞治疗带来了希望,现有研究表明已建立的多种疾病特异的多能性细胞系,可以在体外分化为多种类型的体细胞,为细胞治疗提供足够的种子来源。与此同时,细胞转分化技术的出现也引起了人们的极大关注。通过细胞转分化,可将病人的成纤维细胞直接转变成其他类型的体细胞,无需通过多能干细胞的阶段。因此,该方法更为直接和高效,这一技术同样为细胞治疗点燃了新的希望。目前,通过细胞重编程和细胞转分化这两种技术均可获得神经退行性疾病病人病变或损伤神经组织的细胞类型,为修复受损的组织功能提供了强有力的手段和全新的治疗策略,在细胞治疗方面具有潜在的应用价值。在未来的应用中,甚至可能通过原位改变附近细胞的基因表达或微环境来补充受损组织的细胞类型。The ultimate goal of regenerative medicine is to replace aging, diseased or damaged tissues, which can be achieved through two approaches, namely, cell reprogramming technology and cell transdifferentiation technology. The innovation of cell reprogramming technology has brought hope for cell therapy. Existing studies have shown that a variety of disease-specific pluripotent cell lines have been established that can differentiate into various types of somatic cells in vitro, providing sufficient cell therapy. seed source. At the same time, the emergence of cell transdifferentiation technology has also attracted great attention. Through cell transdifferentiation, a patient's fibroblasts can be directly transformed into other types of somatic cells without going through the pluripotent stem cell stage. Therefore, the method is more direct and efficient, and this technique also sparks new hope for cell therapy. At present, the cell types of diseased or damaged nerve tissue in patients with neurodegenerative diseases can be obtained through the two technologies of cell reprogramming and cell transdifferentiation, which provide a powerful means and a new therapeutic strategy for repairing damaged tissue function. It has potential application value in cell therapy. In future applications, it might even be possible to replenish damaged tissue cell types by altering the gene expression or microenvironment of nearby cells in situ.
尽管传统的转录因子介导的重编程和转分化技术为细胞治疗带来了前所未有的曙光,但是从新类型细胞的获得到临床治疗的应用还有诸多问题亟待解决。由于转录因子介导的诱导过程中常借助于逆转录病毒、慢病毒等方式将外源基因导入细胞,虽然这类方式具有较高的基因转导效率,但是由于病毒序列整合到细胞的基因组中,会导致基因插入突变发生,甚至具有致瘤性,所以这种具有潜在危险性的基因导入方法显然不利于其在再生医学领域的应用,因而不同的研究小组将如何采用非整合型诱导方法实现转分化作为研究热点。Although traditional transcription factor-mediated reprogramming and transdifferentiation technologies have brought unprecedented light to cell therapy, there are still many problems to be solved from the acquisition of new types of cells to the application of clinical therapy. In the process of transcription factor-mediated induction, exogenous genes are often introduced into cells by means of retroviruses, lentiviruses, etc. Although such methods have high gene transduction efficiency, due to the integration of viral sequences into the genome of cells, It can lead to gene insertion mutations and even tumorigenicity, so this potentially dangerous gene introduction method is obviously not conducive to its application in the field of regenerative medicine, so how different research groups will use non-integrative induction methods to achieve transduction? differentiation as a research hotspot.
不同于诱导多能干细胞的致瘤性和传统转分化的外源基因插入,化合物诱导生成神经前体细胞成功规避了传统方法的诸多应用限制,更适合直接用于临床治疗。2014年,同济大学裴钢教授组的研究人员发现,通过纯粹的化学小分子诱导的方法,可以将体细胞转分化成神经前体细胞。他们通过特定化学组合物的组合,即VCR(VPA,HDAC的抑制剂;CHIR99021,GSK3的抑制剂;Repsox,TGFβ通路的抑制剂),再加上低氧环境,可以将鼠的胚胎成纤维细胞诱导成神经前体细胞。近期,华人科学家丁胜教授及其同事发现并从机制上鉴定了多个全新的小分子化合物,将他们组合成一种名为M9的培养基,包括CHIR99021,A83-01,RG108,Parnate,SMER28,Hh-Ag1.5,retinoic acid,LDN193189和bFGF,可以促进哺乳动物成纤维细胞向神经前体细胞的诱导分化。这类化合物方法诱导的神经前体细胞和哺乳动物脑来源的神经前体细胞在很多方面相似,例如增值和自我更新能力,基因表达谱以及体内外的分化潜能等。Different from the tumorigenicity of induced pluripotent stem cells and the traditional transdifferentiation of exogenous gene insertion, the compound-induced neural progenitor cells successfully circumvent many application limitations of traditional methods and are more suitable for direct clinical treatment. In 2014, researchers from Professor Pei Gang's group at Tongji University discovered that somatic cells can be transdifferentiated into neural precursor cells by a purely chemical small molecule induction method. Through a combination of specific chemical compositions, namely VCR (VPA, an inhibitor of HDAC; CHIR99021, an inhibitor of GSK3; Repsox, an inhibitor of the TGFβ pathway), coupled with a hypoxic environment, mouse embryonic fibroblasts can be transformed into Induce neuroblasts. Recently, Chinese scientist Prof. Sheng Ding and his colleagues discovered and identified a number of new small molecule compounds from the mechanism, and combined them into a medium called M9, including CHIR99021, A83-01, RG108, Parnate, SMER28, Hh-Ag1.5, retinoic acid, LDN193189 and bFGF can promote the differentiation of mammalian fibroblasts into neural precursor cells. The neural progenitor cells induced by this compound method are similar to mammalian brain-derived neural progenitor cells in many aspects, such as proliferation and self-renewal capacity, gene expression profile, and differentiation potential in vitro and in vivo.
上述研究结果都证实了无需通过外源病毒的导入,就可以通过一定方式将哺乳动物的体细胞诱导转变为神经前体细胞,而小分子的主要作用方式,是激活细胞内源神经前体基因的表达。因此,我们提出通过特定细胞因子的组合,来激活分化细胞内神经前体标志基因的再表达,也可以实现将哺乳动物成纤维细胞向神经前体细胞的诱导转化。此外,由于细胞因子价格相对低廉,而且操作简便,便于在细胞培养基中进行添加。因此,这种添加特定细胞因子后,获得的有助于诱导生成神经前体细胞的培养基,将是一种安全、高效、又操作简便的培养系统,不仅能够促使分化的成体细胞转变为神经前体细胞,更适合应用于临床转化医学的应用。The above research results have confirmed that mammalian somatic cells can be induced into neural precursor cells in a certain way without the introduction of exogenous viruses, and the main mode of action of small molecules is to activate endogenous neural precursor genes in cells. expression. Therefore, we propose that the re-expression of neural precursor marker genes in differentiated cells can be activated by the combination of specific cytokines, and the inducible transformation of mammalian fibroblasts into neural precursor cells can also be achieved. In addition, because cytokines are relatively inexpensive and easy to handle, they are easy to add to cell culture media. Therefore, after adding specific cytokines, the medium that is helpful for inducing the generation of neural precursor cells will be a safe, efficient and easy-to-operate culture system, which can not only promote the transformation of differentiated adult cells into neural cells Precursor cells are more suitable for clinical translational medicine applications.
发明内容SUMMARY OF THE INVENTION
本发明的目的在于提供一种有助于诱导生成神经前体细胞的诱导培养基,主要解决上述现有技术所存在的问题,该培养基中包含谱系特异性细胞因子,以一定浓度进行组合。通过该诱导培养基,可以在体外环境下,将哺乳动物体细胞诱导形成有功能的神经前体细胞。The purpose of the present invention is to provide an induction medium that is helpful for inducing the generation of neural precursor cells, and mainly solves the problems existing in the prior art. The medium contains lineage-specific cytokines, which are combined in a certain concentration. With this induction medium, mammalian somatic cells can be induced to form functional neural precursor cells in an in vitro environment.
为实现上述目的,本发明的技术方案是:For achieving the above object, technical scheme of the present invention is:
一种有助于诱导生成神经前体细胞的诱导培养基,其特征在于:其成份和含量是:An induction medium for inducing the generation of neural precursor cells is characterized in that: its composition and content are:
87%DMEM/F12培养基(Gibco),87% DMEM/F12 medium (Gibco),
1%GlutaMAX(Gibco),1% GlutaMAX (Gibco),
2%B27minus vitamin A(Gibco),2% B27minus vitamin A (Gibco),
1~10μg/mL heparin(Stem Cell Technologies),1~10μg/mL heparin (Stem Cell Technologies),
1000units/mL LIF(mLIF或hLIF)(Merck Millipore),1000units/mL LIF (mLIF or hLIF) (Merck Millipore),
10~20ng/mL basic fibroblast growth factor(bFGF;R&D Systems),10~20ng/mL basic fibroblast growth factor (bFGF; R&D Systems),
10~40ng/mL epidermal growth factor(EGF;Peprotech)。10~40ng/mL epidermal growth factor (EGF; Peprotech).
此配方中,以上生长因子的特定组合,可以起到使体细胞转变为神经前体细胞状态的作用。In this formulation, a specific combination of the above growth factors can act to transform the somatic cells into a state of neural precursor cells.
为实现利用上述诱导培养基进行哺乳动物体细胞转分化为神经前体细胞的效果,具体操作步骤如下:In order to realize the effect of using the above-mentioned induction medium to transdifferentiate mammalian somatic cells into neural precursor cells, the specific operation steps are as follows:
1.利于哺乳动物成纤维细胞培养系统培养小鼠胚胎或成体成纤维细胞,或人成纤维细胞。1. It is beneficial to the mammalian fibroblast culture system to cultivate mouse embryonic or adult fibroblasts, or human fibroblasts.
2.以一定比例将成纤维细胞进行接种,并通过添加了特殊细胞因子的上述诱导培养基进行培养,完成将哺乳动物体细胞转分化为神经前体细胞状态的过程,该过程需要约2-4周的时间。2. Inoculate fibroblasts in a certain proportion, and culture them in the above-mentioned induction medium supplemented with special cytokines to complete the process of transdifferentiation of mammalian somatic cells into neural precursor cells. This process requires about 2-4 week time.
3.此后采用神经前体细胞稳定培养系统,包括N2、B27(含维他命A)、FGF2、EGF等将这种转分化获得的神经前体细胞,稳定在功能性的神经前体细胞状态。该过程需要约1-2周的时间。3. Afterwards, a neural precursor cell stable culture system, including N2, B27 (containing vitamin A), FGF2, EGF, etc., is used to stabilize the neural precursor cells obtained by transdifferentiation in a functional neural precursor cell state. The process takes about 1-2 weeks.
4.最终可将这类神经前体细胞进行扩增或者保存。4. Finally, such neural precursor cells can be expanded or preserved.
通过诱导培养基获得的神经前体细胞,具有与传统体内获得的神经前体细胞非常相似的分子特征,表现为具有一定的自我更新能力,和特异性的转录组以分化为神经系统细胞的潜能。Neural precursor cells obtained by induction medium have molecular characteristics very similar to those obtained by traditional in vivo neural precursor cells, showing a certain self-renewal ability, and a specific transcriptome to differentiate into nervous system cells. .
本发明的诱导培养基适合的细胞优选的是哺乳动物细胞,更优选小鼠胚胎成纤维细胞和小鼠成体成纤维细胞。The cells suitable for the induction medium of the present invention are preferably mammalian cells, more preferably mouse embryonic fibroblasts and mouse adult fibroblasts.
本发明的有益效果是通过调整培养基中的生长因子来调控上下游的信号通路,从而实现非整合型的体外诱导生成神经前体细胞。这种通过培养基实现细胞转分化的方式,不仅操作方面,更加安全和高效,而且稳定度高,适合哺乳动物细胞在转化医学方面的应用,能够促使分化的成体细胞转变为神经前体细胞。The beneficial effect of the present invention is that the upstream and downstream signaling pathways are regulated by adjusting the growth factors in the culture medium, so as to realize the non-integrated in vitro induction of neural precursor cells. This method of transdifferentiation of cells through the medium is not only safer and more efficient in operation, but also has high stability, which is suitable for the application of mammalian cells in translational medicine, and can promote the transformation of differentiated adult cells into neural precursor cells.
附图说明Description of drawings
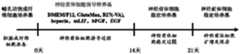
图1显示了通过添加特定生长因子的诱导培养基,进行小鼠胚胎成纤维细胞转变为神经前体细胞的流程,包括培养基的选择及时间点。Figure 1 shows the process of converting mouse embryonic fibroblasts into neural precursor cells by adding specific growth factor induction medium, including medium selection and time points.
图2显示了添加特定生长因子的诱导培养基,诱导小鼠胚胎成纤维细胞生成神经前体细胞各时期的形态照片。Figure 2 shows the morphological photos of each stage of inducing the generation of neural precursor cells from mouse embryonic fibroblasts by adding specific growth factors to the induction medium.
图3显示了添加特定生长因子的诱导培养基,诱导Nestin-GFP小鼠胚胎成纤维细胞生成神经前体细胞若干时间点的荧光显微照片。Figure 3 shows fluorescence micrographs at several time points of the induction of Nestin-GFP mouse embryonic fibroblasts to generate neural precursor cells in induction medium supplemented with specific growth factors.
图4显示了添加特定生长因子的诱导培养基,诱导Nestin-GFP小鼠胚胎成纤维细胞生成神经前体细胞的流式分析效率统计图。Figure 4 shows the statistics of flow analysis efficiency of inducing the generation of neural precursor cells from Nestin-GFP mouse embryonic fibroblasts by adding specific growth factors to the induction medium.
图5显示了RNA-Seq二代测序结果,表明该诱导培养基可以促使诱导中的成纤维细胞表达神经前体细胞特异性基因,促进其向神经前体细胞转变。Figure 5 shows the results of RNA-Seq next-generation sequencing, indicating that the induction medium can promote the expression of neural precursor cell-specific genes in induced fibroblasts and promote their transformation into neural precursor cells.
图6显示了RNA-Seq二代测序结果,表明该诱导培养基可以抑制诱导中的成纤维细胞本有的成纤维特异性基因,促使其打破固有体细胞基因表达谱,从而向神经前体细胞转变。Figure 6 shows the results of RNA-Seq next-generation sequencing, indicating that the induction medium can inhibit the inherent fibroblast-specific genes of the inducing fibroblasts, prompting them to break the gene expression profile of the intrinsic somatic cells, thereby promoting neural precursor cells. change.
图7显示了实时定量PCR结果,表明该诱导培养基生成的神经前体细胞表达神经干细胞特异性基因。Figure 7 shows real-time quantitative PCR results showing that neural precursor cells generated from the induction medium express neural stem cell-specific genes.
图8显示了免疫荧光的结果,表明该诱导培养基诱导生成的神经前体细胞表达神经干细胞特异性基因Nestin,Sox1和Sox2。Figure 8 shows the results of immunofluorescence, indicating that the neural precursor cells induced by the induction medium express neural stem cell-specific genes Nestin, Sox1 and Sox2.
图9显示了通过添加特定生长因子的诱导培养基,进行小鼠成体成纤维细胞转变为神经前体细胞的流程。Figure 9 shows the process of converting mouse adult fibroblasts to neural precursor cells by adding specific growth factor induction medium.
图10显示了添加特定生长因子的诱导培养基,诱导Nestin-GFP小鼠成体成纤维细胞生成神经前体细胞若干时间点的荧光显微照片。Figure 10 shows fluorescence micrographs at several time points of the induction of Nestin-GFP mouse adult fibroblasts to generate neural precursors in induction medium supplemented with specific growth factors.
图11显示了实时定量PCR结果,表明该诱导培养基可以促使诱导中的成体成纤维细胞表达神经前体细胞特异性基因,促进其向神经前体细胞转变。Figure 11 shows the results of real-time quantitative PCR, indicating that the induction medium can induce the induced adult fibroblasts to express neural progenitor cell-specific genes and promote their transformation into neural progenitor cells.
图12显示了实时定量PCR结果,表明该诱导培养基可以抑制诱导中的成纤维细胞本有的成纤维特异性基因,促使其打破固有成体细胞基因表达谱,从而向神经前体细胞转变。Figure 12 shows the results of real-time quantitative PCR, indicating that the induction medium can inhibit the inherent fibroblast-specific genes of the fibroblasts in the induction, and cause them to break the gene expression profile of the resident adult cells, thereby transforming into neural precursor cells.
图13显示了免疫荧光的结果,表明该诱导培养基诱导生成的神经前体细胞表达神经干细胞特异性基因Nestin,Sox1和Sox2。Figure 13 shows the results of immunofluorescence, indicating that the neural precursor cells induced by the induction medium express neural stem cell-specific genes Nestin, Sox1 and Sox2.
图14显示了通过添加特定生长因子的诱导培养基,进行人成纤维细胞转变为神经前体细胞的流程。Figure 14 shows the process of converting human fibroblasts into neural progenitor cells by adding specific growth factors to the induction medium.
图15显示了添加特定生长因子的诱导培养基,诱导人成纤维细胞转变为神经前体细胞的形态图。Figure 15 shows the morphology of human fibroblasts converted into neural precursor cells induced by the addition of specific growth factors to induction medium.
具体实施方式Detailed ways
有关本发明的详细说明和技术内容,配合图式说明如下,然而所附图式仅提供参考与说明用,非用以限制本发明。The detailed description and technical content of the present invention are described below in conjunction with the drawings. However, the accompanying drawings are only for reference and description, and are not intended to limit the present invention.
1、培养基1. Culture medium
(1)成纤维细胞的培养基组成为:(1) The culture medium of fibroblasts is composed of:
89%基础培养基DMEM(Gibco),89% basal medium DMEM (Gibco),
10%胎牛血清(FBS;Gibco),10% fetal bovine serum (FBS; Gibco),
1%L-谷氨酰胺(Glutamax;Gibco)。1% L-Glutamine (Glutamax; Gibco).
(2)发明所述有助于诱导生成神经前体细胞的诱导培养基,适用于小鼠胚胎成纤维细胞向神经前体细胞转变,其培养基成份及含量:(2) The induction medium that is helpful for inducing the generation of neural precursor cells according to the invention is suitable for the transformation of mouse embryonic fibroblasts to neural precursor cells. The composition and content of the medium are:
87%DMEM/F12培养基(Gibco),87% DMEM/F12 medium (Gibco),
1%GlutaMAX(Gibco),1% GlutaMAX (Gibco),
2%B27minus vitamin A(Gibco),2% B27minus vitamin A (Gibco),
1-2.5μg/mL heparin(Stem Cell Technologies),1-2.5 μg/mL heparin (Stem Cell Technologies),
1000units/mL mLIF(Merck Millipore),1000units/mL mLIF (Merck Millipore),
10-15ng/mL basic fibroblast growth factor(bFGF;R&D Systems),10-15ng/mL basic fibroblast growth factor (bFGF; R&D Systems),
10-15ng/mL epidermal growth factor(EGF;Peprotech)。10-15ng/mL epidermal growth factor (EGF; Peprotech).
(3)发明所述有助于诱导生成神经前体细胞的诱导培养基,适用于小鼠成体成纤维细胞向神经前体细胞转变,其培养基成份及含量:(3) The induction medium that is helpful for inducing the generation of neural precursor cells according to the invention is suitable for the transformation of mouse adult fibroblasts to neural precursor cells. The composition and content of the medium are:
87%DMEM/F12培养基(Gibco),87% DMEM/F12 medium (Gibco),
1%GlutaMAX(Gibco),1% GlutaMAX (Gibco),
2%B27minus vitamin A(Gibco),2% B27minus vitamin A (Gibco),
2.5-5μg/mL heparin(Stem Cell Technologies),2.5-5 μg/mL heparin (Stem Cell Technologies),
1000uni ts/mL mLIF(Merck Millipore),1000uni ts/mL mLIF (Merck Millipore),
15-20ng/mL basic fibroblast growth factor(bFGF;R&D Systems),15-20ng/mL basic fibroblast growth factor (bFGF; R&D Systems),
15-20ng/mL epidermal growth factor(EGF;Peprotech)。15-20ng/mL epidermal growth factor (EGF; Peprotech).
(4)发明所述有助于诱导生成神经前体细胞的诱导培养基,适用于人成纤维细胞向神经前体细胞转变,其培养基成份及含量:(4) The induction medium that is helpful for inducing the generation of neural precursor cells according to the invention is suitable for the transformation of human fibroblasts to neural precursor cells. The composition and content of the medium are:
87%DMEM/F12培养基(Gibco),87% DMEM/F12 medium (Gibco),
1%GlutaMAX(Gibco),1% GlutaMAX (Gibco),
2%B27minus vitamin A(Gibco),2% B27minus vitamin A (Gibco),
10μg/mL heparin(Stem Cell Technologies),10 μg/mL heparin (Stem Cell Technologies),
1000units/mL hLIF(Merck Millipore),1000units/mL hLIF (Merck Millipore),
20ng/mL basic fibroblast growth factor(bFGF;R&D Systems),20ng/mL basic fibroblast growth factor (bFGF; R&D Systems),
40ng/mL epidermal growth factor(EGF;Peprotech)。40ng/mL epidermal growth factor (EGF; Peprotech).
(5)小鼠神经前体细胞稳定培养基组成为:DMEM/F12培养基(Gibco),外加1%N2(Invitrogen),2%B27(Invitrogen),20ng/mL basic fibroblast growth factor(bFGF;R&D Systems)和10ng/mL epidermal growth factor(EGF;Peprotech)。(5) The stable medium of mouse neural precursor cells is composed of: DMEM/F12 medium (Gibco), plus 1% N2 (Invitrogen), 2% B27 (Invitrogen), 20ng/mL basic fibroblast growth factor (bFGF; R&D Systems) and 10 ng/mL epidermal growth factor (EGF; Peprotech).
(6)人源神经干细胞无血清培养基:采用Angecon公司的AngCell人源神经干细胞无血清培养基。(6) Serum-free medium for human neural stem cells: AngCell human-derived neural stem cell serum-free medium from Angecon Company was used.
2、用于转分化诱导的细胞2. Cells used for transdifferentiation induction
转分化诱导采用的体细胞类型分别为小鼠胚胎成纤维细胞,小鼠成体成纤维细胞,以及人成纤维细胞,这三类细胞均为发明人自行制备。此外,发明人还优选出一种Nestin-GFP转基因小鼠的成纤维细胞,该细胞在转变为神经前体细胞时,可表达绿色荧光蛋白(GFP),在荧光显微镜下呈现绿色,而在成纤维细胞中该绿色荧光蛋白不表达。通过流式细胞仪分析绿色荧光细胞的比例,可以证实该诱导培养基的诱导效率。The somatic cell types used for transdifferentiation induction are mouse embryonic fibroblasts, mouse adult fibroblasts, and human fibroblasts, and these three types of cells are prepared by the inventors themselves. In addition, the inventors also preferred a Nestin-GFP transgenic mouse fibroblasts, which can express green fluorescent protein (GFP) when transformed into neural precursor cells, which appear green under a fluorescence microscope, while in the This green fluorescent protein is not expressed in fibroblasts. The induction efficiency of the induction medium can be confirmed by analyzing the ratio of green fluorescent cells by flow cytometry.
实施例1Example 1
准备普通小鼠胚胎成纤维细胞,采用成纤维细胞培养基进行培养,该培养基组成为:89%基础培养基DMEM(Gibco),10%胎牛血清(FBS;Gibco)和1%L-谷氨酰胺(Glutamax;Gibco)。Ordinary mouse embryonic fibroblasts were prepared and cultured in fibroblast medium consisting of 89% basal medium DMEM (Gibco), 10% fetal bovine serum (FBS; Gibco) and 1% L-Valley Aminoamide (Glutamax; Gibco).
(1)以150000个细胞/孔的密度种植细胞于24孔细胞培养板内,采用发明所述适用于小鼠胚胎成纤维细胞向神经前体细胞转变的诱导培养基:87%DMEM/F12培养基(Gibco),1%GlutaMAX(Gibco),2%B27minus vitamin A(Gibco),1μg/mL heparin(Stem CellTechnologies),1000units/mL mLIF(Merck Millipore),10ng/mL basic fibroblastgrowth factor(bFGF;R&D Systems),10ng/mL epidermal growth factor(EGF;Peprotech)。诱导起始的7天内,每天一次轻轻吹打细胞,目的是使成团块聚集的细胞悬浮起来,形成较好的细胞球形,每两天更换新鲜诱导培养基一次。7天后,不再吹打细胞,细胞开始贴壁,继续使用小鼠胚胎成纤维细胞诱导培养基,每两天更换新鲜小鼠胚胎成纤维细胞诱导培养基一次。此时,细胞从贴壁的团块中迅速扩增起来,可观察到细胞呈区域性增殖。(1) Plant cells at a density of 150,000 cells/well in a 24-well cell culture plate, and use the induction medium described in the invention for the transformation of mouse embryonic fibroblasts into neural precursor cells: 87% DMEM/F12 culture base (Gibco), 1% GlutaMAX (Gibco), 2% B27minus vitamin A (Gibco), 1 μg/mL heparin (Stem CellTechnologies), 1000 units/mL mLIF (Merck Millipore), 10 ng/mL basic fibroblastgrowth factor (bFGF; R&D Systems) ), 10ng/mL epidermal growth factor (EGF; Peprotech). During the first 7 days of induction, the cells were gently pipetted once a day in order to suspend the aggregated cells and form better cell spheres, and fresh induction medium was replaced every two days. After 7 days, the cells were no longer pipetted, and the cells began to adhere. The mouse embryonic fibroblast induction medium was continued to be used, and the fresh mouse embryonic fibroblast induction medium was replaced every two days. At this point, cells rapidly expanded from adherent clumps, and regional proliferation of cells was observed.
(2)诱导起始14天后,用trypsin/EDTA(Invitrogen)将24孔培养板中每一个孔的细胞分别消化下来,铺到对应的一个35mm细胞培养皿中,此时更换为小鼠神经前体细胞稳定培养基:DMEM/F12培养基(Gibco),外加1%N2(Invitrogen),2%B27(Invitrogen),20ng/mL basic fibroblast growth factor(bFGF;R&D Systems)和10ng/mL epidermal growthfactor(EGF;Peprotech)。继续培养7天后,可观察到贴壁细胞的边缘爬出很多类似神经上皮样细胞,形成神经网络状结构,这些细胞经消化后进行扩增,最终可形成成熟的神经前体细胞,具有典型神经前体细胞形态。(2) 14 days after the initiation of induction, the cells in each well of the 24-well culture plate were digested with trypsin/EDTA (Invitrogen) and plated into a corresponding 35mm cell culture dish. Somatic cell stabilization medium: DMEM/F12 medium (Gibco), plus 1% N2 (Invitrogen), 2% B27 (Invitrogen), 20ng/mL basic fibroblast growth factor (bFGF; R&D Systems) and 10ng/mL epidermal growthfactor ( EGF; Peprotech). After culturing for 7 days, it can be observed that many neuroepithelial-like cells crawled out from the edge of the adherent cells to form a neural network-like structure. These cells were digested and expanded, and finally formed mature neural precursor cells with typical neural Precursor cell morphology.
(3)培养基成份及使用时间如图1所示。细胞变化形态如图2所示。(3) The composition of the medium and the use time are shown in Figure 1. The cell morphology is shown in Figure 2.
实施例2Example 2
准备Nestin-GFP小鼠胚胎成纤维细胞,采用成纤维细胞培养基进行培养,该培养基组成为:89%基础培养基DMEM(Gibco),10%胎牛血清(FBS;Gibco)和1%L-谷氨酰胺(Glutamax;Gibco)。Nestin-GFP mouse embryonic fibroblasts were prepared and cultured in fibroblast medium consisting of 89% basal medium DMEM (Gibco), 10% fetal bovine serum (FBS; Gibco) and 1% L - Glutamine (Glutamax; Gibco).
(1)以150000个细胞/孔的密度,将Nestin-GFP小鼠胚胎成纤维细胞接种于24孔细胞培养板内,采用发明所述适用于小鼠胚胎成纤维细胞向神经前体细胞转变的诱导培养基:87%DMEM/F12培养基(Gibco),1%GlutaMAX(Gibco),2%B27minus vitamin A(Gibco),2.5μg/mL heparin(Stem Cell Technologies),1000units/mL mLIF(Merck Millipore),15ng/mL basic fibroblast growth factor(bFGF;R&D Systems),15ng/mL epidermalgrowth factor(EGF;Peprotech)。诱导起始的7天内,每天一次轻轻吹打细胞,目的是使成团块聚集的细胞悬浮起来,形成较好的细胞球形,每两天更换该新鲜诱导培养基一次,此时可见细胞逐渐出现绿色荧光。7天后,不再吹打细胞,细胞开始贴壁,继续使用该诱导培养基,每两天更换新鲜培养基一次。此时,细胞从贴壁的团块中迅速扩增起来,可观察到细胞呈区域性增殖,并且绿色荧光细胞变多,绿色荧光信号变强。(1) Inoculate Nestin-GFP mouse embryonic fibroblasts in a 24-well cell culture plate at a density of 150,000 cells/well, using the method described in the invention that is suitable for the transformation of mouse embryonic fibroblasts to neural precursor cells Induction medium: 87% DMEM/F12 medium (Gibco), 1% GlutaMAX (Gibco), 2% B27minus vitamin A (Gibco), 2.5 μg/mL heparin (Stem Cell Technologies), 1000 units/mL mLIF (Merck Millipore) , 15ng/mL basic fibroblast growth factor (bFGF; R&D Systems), 15ng/mL epidermal growth factor (EGF; Peprotech). During the first 7 days of induction, the cells were gently pipetted once a day to suspend the aggregated cells and form a better cell sphere. The fresh induction medium was replaced every two days. At this time, cells gradually appeared. Green fluorescence. After 7 days, the cells were no longer pipetted, and the cells began to adhere. Continue to use the induction medium and replace with fresh medium every two days. At this time, the cells rapidly expanded from the adherent clumps, and it was observed that the cells proliferated regionally, and the green fluorescent cells increased, and the green fluorescent signal became stronger.
(2)诱导起始14天后,用trypsin/EDTA(Invitrogen)将24孔培养板中每一个孔的细胞分别消化下来,铺到对应的一个35mm细胞培养皿中,此时更换为小鼠神经前体细胞稳定培养基:DMEM/F12培养基(Gibco),外加1%N2(Invitrogen),2%B27(Invitrogen),20ng/mL basic fibroblast growth factor(bFGF;R&D Systems)和10ng/mL epidermal growthfactor(EGF;Peprotech)。继续培养7天后,可观察到贴壁细胞的边缘爬出很多类似神经上皮样细胞,形成神经网络状结构,呈现强绿色荧光信号。这些细胞经消化后进行扩增,最终可形成成熟的神经前体细胞,具有典型神经前体细胞形态。(2) 14 days after the initiation of induction, the cells in each well of the 24-well culture plate were digested with trypsin/EDTA (Invitrogen) and plated into a corresponding 35mm cell culture dish. Somatic cell stabilization medium: DMEM/F12 medium (Gibco), plus 1% N2 (Invitrogen), 2% B27 (Invitrogen), 20ng/mL basic fibroblast growth factor (bFGF; R&D Systems) and 10ng/mL epidermal growthfactor ( EGF; Peprotech). After culturing for 7 days, it was observed that many neuroepithelial-like cells crawled out from the edge of the adherent cells, forming a neural network-like structure, showing a strong green fluorescent signal. These cells are digested and expanded to eventually form mature neural precursor cells with typical neural precursor cell morphology.
(3)培养基成份、使用时间及操作流程如图1所示。细胞变化形态如图3所示。(3) The composition of the medium, the use time and the operation process are shown in Figure 1. The cell morphology is shown in Figure 3.
(4)由于选用Nestin-GFP小鼠胚胎成纤维细胞作为转分化诱导的起始细胞,在荧光显微镜下,可见成功实现转分化的细胞形成神经球样的形态,以及呈现绿色荧光的表达,如图3所示。在整个操作过程中的不同时间点,采用流式细胞分析技术对表达绿色荧光细胞的比例进行分析,可见在操作14天时,绿色荧光细胞比例超过80%,而此时采用的是发明所述的诱导培养基,高度证明该培养基可以促进成纤维细胞向神经前体细胞的转变。如图4所示。(4) Since Nestin-GFP mouse embryonic fibroblasts were selected as the starting cells for transdifferentiation induction, under the fluorescence microscope, the successfully transdifferentiated cells can be seen to form neurosphere-like morphology, and the expression of green fluorescence, such as shown in Figure 3. At different time points in the whole operation process, the proportion of green fluorescent cells was analyzed by flow cytometry. It can be seen that at the 14th day of operation, the proportion of green fluorescent cells exceeded 80%. Induction medium, which is highly demonstrated to promote the transition of fibroblasts to neural precursor cells. As shown in Figure 4.
(5)在细胞进行转变的过程中,提取细胞的RNA,通过二代测序技术--RNA-Seq对转变过程中的细胞进行基因表达谱分析,证明这些使用了含发明所述的特定细胞因子的诱导培养基进行培养的细胞,逐渐开始表达神经前体细胞的标志基因,并且表达量可维持在较高水平,证明该诱导培养基可以促进成纤维细胞向神经前体细胞的转变,激活内源的神经前体细胞的基因调控通路。如图5所示。(5) In the process of cell transformation, extract the RNA of the cells, and analyze the gene expression profile of the cells in the transformation process by the next-generation sequencing technology-RNA-Seq, which proves that these cells contain the specific cytokines described in the invention. The cells cultured in the induction medium gradually begin to express the marker genes of neural precursor cells, and the expression level can be maintained at a high level, which proves that the induction medium can promote the transformation of fibroblasts to neural precursor cells, activate the internal Gene regulatory pathways in derived neural precursor cells. As shown in Figure 5.
(6)此外,进一步通过二代测序技术--RNA-Seq对转变过程中的细胞进行基因表达谱分析,发现这些使用了含发明所述的特定细胞因子的诱导培养基进行培养的细胞,其原本的成纤维细胞特有的表达谱被逐渐抹除,进一步证明了该诱导培养基可以抑制成纤维细胞本有的体细胞表达谱,并激活神经前体细胞的表达谱,从而促进成纤维细胞向神经前体细胞的转变。如图6所示。(6) In addition, the gene expression profile of the cells in the transition process was further analyzed by the next-generation sequencing technology-RNA-Seq, and it was found that these cells were cultured using the induction medium containing the specific cytokines described in the invention, which The original fibroblast-specific expression profile was gradually erased, which further proved that the induction medium could inhibit the original somatic expression profile of fibroblasts and activate the expression profile of neural precursor cells, thereby promoting fibroblast growth. Transformation of neural precursor cells. As shown in Figure 6.
(7)通过实时定量PCR检测,证实采用了诱导培养基后,成纤维细胞特异性的标志基因Thy1的表达量逐渐降低,而神经前体细胞的标志基因Nestin的表达量逐渐升高,证明了该发明的诱导培养基具有促进成纤维细胞向神经前体细胞转变的功能。如图7所示。(7) Through real-time quantitative PCR detection, it was confirmed that the expression of fibroblast-specific marker gene Thy1 gradually decreased after the induction medium was used, while the expression of neural precursor cell marker gene Nestin gradually increased, proving that The induction medium of the invention has the function of promoting the transformation of fibroblasts into neural precursor cells. As shown in Figure 7.
(8)对该诱导培养基获得的神经前体细胞进行蛋白标志染色分析,证实其从蛋白水平,已高表达神经前体标志蛋白Nestin、Sox1和Sox2。如图8所示。(8) The neural precursor cells obtained from the induction medium were subjected to protein marker staining analysis, and it was confirmed that the neural precursor marker proteins Nestin, Sox1 and Sox2 were highly expressed at the protein level. As shown in Figure 8.
实施例3Example 3
准备Nestin-GFP小鼠成体成纤维细胞,采用成纤维细胞培养基进行培养,该培养基组成为:89%基础培养基DMEM(Gibco),10%胎牛血清(FBS;Gibco)和1%L-谷氨酰胺(Glutamax;Gibco)。Nestin-GFP mouse adult fibroblasts were prepared and cultured in fibroblast medium consisting of 89% basal medium DMEM (Gibco), 10% fetal bovine serum (FBS; Gibco) and 1% L - Glutamine (Glutamax; Gibco).
(1)以200000个细胞/孔的密度种植细胞于24孔培养板中,将培养体系更换为适用于小鼠成体成纤维细胞向神经前体细胞转变的诱导培养基:87%DMEM/F12培养基(Gibco),1%GlutaMAX(Gibco),2%B27minus vitamin A(Gibco),5μg/mL heparin(Stem CellTechnologies),1000units/mL mLIF(Merck Millipore),20ng/mL basic fibroblastgrowth factor(bFGF;R&D Systems),20ng/mL epidermal growth factor(EGF;Peprotech)。诱导起始的7天内,每天一次轻轻吹打细胞,目的是使成团块聚集的细胞悬浮起来,形成较好的细胞球形,每两天更换新鲜诱导培养基一次,此时可见细胞逐渐出现绿色荧光。此后,不再吹打细胞,细胞开始贴壁,继续使用诱导培养基,每两天更换该诱导培养基一次。此时,细胞从贴壁的团块中迅速扩增起来,可观察到细胞呈区域性增殖,并且绿色荧光细胞变多,绿色荧光信号变强。诱导起始21天后,用trypsin/EDTA(Invitrogen)将24孔培养板中每一个孔的细胞分别消化下来,铺到对应的一个35mm细胞培养皿中,此时更换为小鼠神经前体细胞稳定培养基:DMEM/F12培养基(Gibco),外加1%N2(Invitrogen),2%B27(Invitrogen),20ng/mL basic fibroblast growth factor(bFGF;R&D Systems)和10ng/mL epidermal growth factor(EGF;Peprotech)。继续培养7天后,细胞呈现强绿色荧光信号。这些细胞经消化后进行扩增,最终可形成成熟的神经前体细胞,具有典型神经前体细胞形态。(1) The cells were seeded in a 24-well culture plate at a density of 200,000 cells/well, and the culture system was replaced with an induction medium suitable for the transformation of mouse adult fibroblasts to neural precursor cells: 87% DMEM/F12 culture base (Gibco), 1% GlutaMAX (Gibco), 2% B27minus vitamin A (Gibco), 5 μg/mL heparin (Stem CellTechnologies), 1000 units/mL mLIF (Merck Millipore), 20 ng/mL basic fibroblastgrowth factor (bFGF; R&D Systems) ), 20ng/mL epidermal growth factor (EGF; Peprotech). During the first 7 days of induction, the cells were gently pipetted once a day to suspend the aggregated cells and form a better cell sphere. The fresh induction medium was replaced every two days. At this time, the cells gradually appeared green. Fluorescence. Thereafter, the cells were no longer pipetted, and the cells began to adhere, and induction medium was continued, which was changed every two days. At this time, the cells rapidly expanded from the adherent clumps, and it was observed that the cells proliferated regionally, and the green fluorescent cells increased, and the green fluorescent signal became stronger. 21 days after the initiation of induction, the cells in each well of the 24-well culture plate were digested with trypsin/EDTA (Invitrogen) and plated into a corresponding 35mm cell culture dish. At this time, it was replaced with stable mouse neural precursor cells. Medium: DMEM/F12 medium (Gibco), plus 1% N2 (Invitrogen), 2% B27 (Invitrogen), 20ng/mL basic fibroblast growth factor (bFGF; R&D Systems) and 10ng/mL epidermal growth factor (EGF; Peprotech). After continuing to culture for 7 days, the cells showed strong green fluorescent signal. These cells are digested and expanded to eventually form mature neural precursor cells with typical neural precursor cell morphology.
(2)培养基成份、使用时间及操作流程如图9所示。细胞变化形态如图10所示。(2) The composition of the medium, the use time and the operation process are shown in Figure 9. The cell morphology is shown in Figure 10.
(3)在成体成纤维细胞向神经前体细胞进行转变的过程中,提取细胞的RNA,通过实时定量PCR检测,证实采用了诱导培养基后,成体成纤维细胞逐渐高水平表达神经前体细胞的标志基因如Pax6、Sox2和Nestin,证明了该发明的诱导培养基具有促进成纤维细胞向神经前体细胞转变的功能。如图11所示。(3) During the process of the transformation of adult fibroblasts into neural precursor cells, the RNA of the cells was extracted and detected by real-time quantitative PCR. It was confirmed that after the induction medium was used, the adult fibroblasts gradually expressed high levels of neural precursor cells. The marker genes of , such as Pax6, Sox2 and Nestin, proved that the induction medium of the invention has the function of promoting the transformation of fibroblasts to neural precursor cells. As shown in Figure 11.
(4)通过实时定量PCR检测,证实采用了诱导培养基后,成体成纤维细胞特异性的标志基因Thy1和Col1a1的表达量逐渐降低,进一步证明了该诱导培养基可以抑制成纤维细胞本有的体细胞表达谱,并激活神经前体细胞的表达谱,从而促进成纤维细胞向神经前体细胞的转变。如图12所示。(4) Through real-time quantitative PCR detection, it was confirmed that the expression of adult fibroblast-specific marker genes Thy1 and Col1a1 gradually decreased after the induction medium was used, which further proved that the induction medium could inhibit the original fibroblasts. Somatic cell expression profile, and activate the expression profile of neural precursor cells, thereby promoting the transition of fibroblasts to neural precursor cells. As shown in Figure 12.
(5)对该诱导培养基获得的神经前体细胞进行蛋白标志染色分析,证实其从蛋白水平,已高水平表达神经前体标志蛋白Nestin、Sox1和Sox2。如图13所示。(5) The neural precursor cells obtained from the induction medium were subjected to protein marker staining analysis to confirm that the neural precursor marker proteins Nestin, Sox1 and Sox2 were expressed at a high level from the protein level. As shown in Figure 13.
实施例4Example 4
准备人成纤维细胞,采用成纤维细胞培养基进行培养,该培养基组成为:89%基础培养基DMEM(Gibco),10%胎牛血清(FBS;Gibco)和1%L-谷氨酰胺(Glutamax;Gibco)。Human fibroblasts were prepared and cultured in fibroblast medium consisting of 89% basal medium DMEM (Gibco), 10% fetal bovine serum (FBS; Gibco) and 1% L-glutamine ( Glutamax; Gibco).
(1)以350000个细胞/孔的密度种植细胞于24孔培养板中,将培养体系更换为人成纤维细胞诱导培养基:87%DMEM/F12培养基(Gibco),1%GlutaMAX(Gibco),2%B27minusvitamin A(Gibco),10μg/mL heparin(Stem Cell Technologies),1000units/mL hLIF(Merck Millipore),20ng/mL basic fibroblast growth factor(bFGF;R&D Systems),40ng/mL epidermal growth factor(EGF;Peprotech)。诱导起始的14天内,每天一次轻轻吹打细胞,目的是使成团块聚集的细胞悬浮起来,形成较好的细胞球形,每两天更换新鲜人成纤维细胞诱导培养基一次。此后,不再吹打细胞,细胞开始贴壁,继续使用人成纤维细胞诱导培养基,每两天更换该诱导培养基一次。此时,细胞从贴壁的团块中慢慢扩增起来,可观察到部分细胞呈区域性增殖。诱导起始28天后,用trypsin/EDTA(Invitrogen)将24孔培养板中每一个孔的细胞分别消化下来,铺到对应的一个35mm细胞培养皿中,此时更换为Angecon公司的AngCell人源神经干细胞无血清培养基。继续培养14天后,最终可形成成熟的神经前体细胞。这些细胞经消化后进行扩增,具有典型神经前体细胞形态。(1) Cells were seeded in a 24-well culture plate at a density of 350,000 cells/well, and the culture system was replaced with human fibroblast induction medium: 87% DMEM/F12 medium (Gibco), 1% GlutaMAX (Gibco), 2% B27minusvitamin A (Gibco), 10μg/mL heparin (Stem Cell Technologies), 1000units/mL hLIF (Merck Millipore), 20ng/mL basic fibroblast growth factor (bFGF; R&D Systems), 40ng/mL epidermal growth factor (EGF; Peprotech). During the first 14 days of induction, the cells were gently pipetted once a day to suspend the aggregated cells and form a better cell sphere. Fresh human fibroblast induction medium was replaced every two days. After that, the cells were no longer pipetted and the cells started to adhere, and the human fibroblast induction medium was continued, which was changed every two days. At this time, the cells slowly expanded from the adherent clumps, and some cells were observed to proliferate regionally. 28 days after the initiation of induction, the cells in each well of the 24-well culture plate were digested with trypsin/EDTA (Invitrogen) and plated into a corresponding 35mm cell culture dish. Stem cell serum-free medium. After a further 14 days of culture, mature neural precursor cells can eventually be formed. These cells were expanded after digestion and had typical neural precursor cell morphology.
(2)培养基成份、使用时间及操作流程如图14所示。诱导获得的人神经前体细胞形态如图15所示。(2) The composition of the medium, the use time and the operation process are shown in Figure 14. The morphology of the induced human neural precursor cells is shown in Figure 15.
因此,本发明得出,运用本发明所述的含特定细胞因子的诱导培养基,可以将包括小鼠胚胎成纤维细胞、小鼠成体成纤维细胞和人成纤维细胞在内的哺乳哺乳动物成纤维细胞,转变为神经前体细胞状态,并通过对应的神经前体培养系统稳定地维持在这一状态。本发明所述的有助于诱导生成神经前体细胞的培养基进行细胞转分化的优势在于,其没有病毒外源基因的整合,使用更加安全,同时操作方便,效率高很大程度上提高了临床应用的潜能。Therefore, the present invention draws that, using the induction medium containing specific cytokines described in the present invention, mammalian mammalian cells including mouse embryonic fibroblasts, mouse adult fibroblasts and human fibroblasts can be Fibroblasts are transformed into a state of neural precursor cells, and are stably maintained in this state by the corresponding neural precursor culture system. The advantage of the medium for inducing the generation of neural precursor cells for cell transdifferentiation is that it does not have the integration of viral exogenous genes, so it is safer to use, and at the same time, it is easy to operate and has high efficiency, which greatly improves the potential for clinical application.
以上所述者,仅为本发明的较佳可行实施例而已,非因此即局限本发明的专利范围,举凡运用本发明说明书及图式内容所为的等效结构变化,均理同包含于本发明的权利范围内,合予陈明。The above descriptions are only the preferred feasible embodiments of the present invention, which do not limit the scope of the present invention. Any equivalent structural changes made by using the contents of the description and drawings of the present invention are all deemed to be included in the present invention. Within the scope of the rights of the invention, it is hereby stated.
Claims (1)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201611101061.2ACN108148808B (en) | 2016-12-05 | 2016-12-05 | Induction medium for the induction of neural precursor cells |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201611101061.2ACN108148808B (en) | 2016-12-05 | 2016-12-05 | Induction medium for the induction of neural precursor cells |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN108148808A CN108148808A (en) | 2018-06-12 |
| CN108148808Btrue CN108148808B (en) | 2020-12-11 |
Family
ID=62469900
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201611101061.2AActiveCN108148808B (en) | 2016-12-05 | 2016-12-05 | Induction medium for the induction of neural precursor cells |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN108148808B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114574441A (en)* | 2022-03-16 | 2022-06-03 | 中山大学·深圳 | Novel biological application of black phosphorus nanosheet |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010057039A2 (en)* | 2008-11-14 | 2010-05-20 | Cythera, Inc. | Encapsulation of pancreatic cells derived from human pluripotent stem cells |
| WO2012018933A2 (en)* | 2010-08-04 | 2012-02-09 | Cellular Dynamics International, Inc. | Reprogramming immortalized b cells |
| CN103740757A (en)* | 2014-01-21 | 2014-04-23 | 中国科学院生物物理研究所 | Method for preparing pig neural stem cells by reprogramming |
| CN104894060A (en)* | 2014-03-03 | 2015-09-09 | 中国科学院上海生命科学研究院 | Method for inducing transdifferentiation of somatic cells into neural stem cells and application thereof |
- 2016
- 2016-12-05CNCN201611101061.2Apatent/CN108148808B/enactiveActive
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010057039A2 (en)* | 2008-11-14 | 2010-05-20 | Cythera, Inc. | Encapsulation of pancreatic cells derived from human pluripotent stem cells |
| WO2012018933A2 (en)* | 2010-08-04 | 2012-02-09 | Cellular Dynamics International, Inc. | Reprogramming immortalized b cells |
| CN103740757A (en)* | 2014-01-21 | 2014-04-23 | 中国科学院生物物理研究所 | Method for preparing pig neural stem cells by reprogramming |
| CN104894060A (en)* | 2014-03-03 | 2015-09-09 | 中国科学院上海生命科学研究院 | Method for inducing transdifferentiation of somatic cells into neural stem cells and application thereof |
Non-Patent Citations (1)
| Title |
|---|
| Engineering cell fate: Spotlight on cell-activation and signaling-directed lineage conversion;Behnam Ebrahimi;《Tissue and Cell》;20160726(第48期);第478-481页表1* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN108148808A (en) | 2018-06-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Conti et al. | Neural stem cell systems: physiological players or in vitro entities? | |
| Otsu et al. | Differentiation of induced pluripotent stem cells into dental mesenchymal cells | |
| CN110396499B (en) | A method for inducing neural stem cells and its application | |
| CN104419661B (en) | The preparation method of NSC | |
| CN103088065B (en) | Method capable of forming hematopoietic stem cells by quickly inducing reversal decision of mesenchymal stem cells in large scale with high purity | |
| Dai et al. | Non-genetic direct reprogramming and biomimetic platforms in a preliminary study for adipose-derived stem cells into corneal endothelia-like cells | |
| CN105219729B (en) | Method for inducing neural stem cells by using non-integrative plasmid vector and application thereof | |
| Dai et al. | The Human Skin‐Derived Precursors for Regenerative Medicine: Current State, Challenges, and Perspectives | |
| Sauerzweig et al. | A population of serumdeprivation-induced bone marrow stem cells (SD-BMSC) expresses marker typical for embryonic and neural stem cells | |
| Vasyliev et al. | Comparative analysis of biological properties of large‐scale expanded adult neural crest‐derived stem cells isolated from human hair follicle and skin dermis | |
| CN116694567A (en) | Culture solution and method for indirectly inducing human induced pluripotent stem cells into mesenchymal stem cells | |
| Cui et al. | Biological Characterization and Pluripotent Identification of Sheep Dermis‐Derived Mesenchymal Stem/Progenitor Cells | |
| Yamasaki et al. | Long-term serial cultivation of mouse induced pluripotent stem cells in serum-free and feeder-free defined medium | |
| CN108148808B (en) | Induction medium for the induction of neural precursor cells | |
| CN106754657A (en) | A serum-free medium for monkey embryonic stem cells | |
| CN119391631A (en) | Umbilical cord mesenchymal stem cell adipogenic differentiation medium and culture method | |
| CN105087475B (en) | A kind of method that cell culture fluid and its application and induction dental pulp stem cell break up to neural-like cells | |
| CN101935635A (en) | Method for in vitro expansion of human bone marrow pluripotent stem cells and directed differentiation to dopaminergic neurons | |
| JP2011211956A (en) | Undifferentiation-maintaining agent for stem cell and growth-promoting agent | |
| CN116970557A (en) | Culture solution and method for directly inducing human induced pluripotent stem cells into mesenchymal stem cells | |
| Keshteli et al. | Study of the differentiation of rat omentum stem cells to nerve cells using brain tissue extract of Wistar rats | |
| CN110628712B (en) | Preparation methods and applications of therapeutic grade mesenchymal stem cells based on induced pluripotent stem cells | |
| CN108148807B (en) | A method of growth factor-induced generation of neural precursor cells | |
| WO2021227573A1 (en) | Xeno-free culture medium and method for expansion of mesenchymal stem cells by means of using same | |
| Ernst et al. | An improved, standardised protocol for the isolation, enrichment and targeted neural differentiation of Nestin+ progenitors from adult human dermis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |