CN103897221A - Chitosan/phosphorylated silicon dioxide particle hybrid film as well as preparation and application thereof - Google Patents
Chitosan/phosphorylated silicon dioxide particle hybrid film as well as preparation and application thereofDownload PDFInfo
- Publication number
- CN103897221A CN103897221ACN201410093433.6ACN201410093433ACN103897221ACN 103897221 ACN103897221 ACN 103897221ACN 201410093433 ACN201410093433 ACN 201410093433ACN 103897221 ACN103897221 ACN 103897221A
- Authority
- CN
- China
- Prior art keywords
- phosphorylated
- chitosan
- mesoporous hollow
- particles
- membrane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical classO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000titleclaimsabstractdescription77
- 239000002245particleSubstances0.000titleclaimsabstractdescription23
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription17
- 239000012528membraneSubstances0.000claimsabstractdescription81
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000claimsabstractdescription63
- 239000000377silicon dioxideSubstances0.000claimsabstractdescription35
- 229920001661ChitosanPolymers0.000claimsabstractdescription25
- 239000012798spherical particleSubstances0.000claimsabstractdescription25
- QAOWNCQODCNURD-UHFFFAOYSA-NSulfuric acidChemical compoundOS(O)(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-N0.000claimsabstractdescription18
- 238000005266castingMethods0.000claimsabstractdescription15
- 239000000446fuelSubstances0.000claimsabstractdescription14
- XHXFXVLFKHQFAL-UHFFFAOYSA-Nphosphoryl trichlorideChemical compoundClP(Cl)(Cl)=OXHXFXVLFKHQFAL-UHFFFAOYSA-N0.000claimsabstractdescription12
- 235000012239silicon dioxideNutrition0.000claimsabstractdescription10
- 238000000034methodMethods0.000claimsabstractdescription8
- 239000011521glassSubstances0.000claimsabstractdescription6
- 239000004593EpoxySubstances0.000claimsabstractdescription4
- 230000026731phosphorylationEffects0.000claimsabstractdescription4
- 238000006366phosphorylation reactionMethods0.000claimsabstractdescription4
- 239000006087Silane Coupling AgentSubstances0.000claimsabstractdescription3
- 239000000243solutionSubstances0.000claimsdescription35
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterChemical compoundOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsdescription23
- LZZYPRNAOMGNLH-UHFFFAOYSA-MCetrimonium bromideChemical compound[Br-].CCCCCCCCCCCCCCCC[N+](C)(C)CLZZYPRNAOMGNLH-UHFFFAOYSA-M0.000claimsdescription18
- 239000008367deionised waterSubstances0.000claimsdescription17
- 229910021641deionized waterInorganic materials0.000claimsdescription17
- QTBSBXVTEAMEQO-UHFFFAOYSA-NAcetic acidChemical compoundCC(O)=OQTBSBXVTEAMEQO-UHFFFAOYSA-N0.000claimsdescription15
- CDBYLPFSWZWCQE-UHFFFAOYSA-LSodium CarbonateChemical compound[Na+].[Na+].[O-]C([O-])=OCDBYLPFSWZWCQE-UHFFFAOYSA-L0.000claimsdescription12
- 238000005406washingMethods0.000claimsdescription12
- YXFVVABEGXRONW-UHFFFAOYSA-NTolueneChemical compoundCC1=CC=CC=C1YXFVVABEGXRONW-UHFFFAOYSA-N0.000claimsdescription9
- 239000007864aqueous solutionSubstances0.000claimsdescription9
- BOTDANWDWHJENH-UHFFFAOYSA-NTetraethyl orthosilicateChemical compoundCCO[Si](OCC)(OCC)OCCBOTDANWDWHJENH-UHFFFAOYSA-N0.000claimsdescription7
- 239000006185dispersionSubstances0.000claimsdescription7
- 229910000029sodium carbonateInorganic materials0.000claimsdescription6
- YDONNITUKPKTIG-UHFFFAOYSA-N[Nitrilotris(methylene)]trisphosphonic acidChemical compoundOP(O)(=O)CN(CP(O)(O)=O)CP(O)(O)=OYDONNITUKPKTIG-UHFFFAOYSA-N0.000claimsdescription5
- 238000010907mechanical stirringMethods0.000claimsdescription5
- 239000007788liquidSubstances0.000claimsdescription3
- 239000011159matrix materialSubstances0.000claimsdescription3
- BPSIOYPQMFLKFR-UHFFFAOYSA-Ntrimethoxy-[3-(oxiran-2-ylmethoxy)propyl]silaneChemical compoundCO[Si](OC)(OC)CCCOCC1CO1BPSIOYPQMFLKFR-UHFFFAOYSA-N0.000claimsdescription3
- 238000004132cross linkingMethods0.000claims1
- 238000001035dryingMethods0.000claims1
- 238000005530etchingMethods0.000claims1
- 239000008187granular materialSubstances0.000claims1
- 230000007062hydrolysisEffects0.000claims1
- 238000006460hydrolysis reactionMethods0.000claims1
- FUKUFMFMCZIRNT-UHFFFAOYSA-Nhydron;methanol;chlorideChemical compoundCl.OCFUKUFMFMCZIRNT-UHFFFAOYSA-N0.000claims1
- 239000011256inorganic fillerSubstances0.000claims1
- 229910003475inorganic fillerInorganic materials0.000claims1
- 238000006068polycondensation reactionMethods0.000claims1
- 239000000463materialSubstances0.000abstractdescription17
- 229910010272inorganic materialInorganic materials0.000abstractdescription9
- 239000011147inorganic materialSubstances0.000abstractdescription9
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical compoundOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000abstractdescription6
- 238000011049fillingMethods0.000abstractdescription5
- 229910000147aluminium phosphateInorganic materials0.000abstractdescription3
- 230000008569processEffects0.000abstractdescription2
- 229920002521macromoleculePolymers0.000abstract1
- 230000000865phosphorylative effectEffects0.000abstract1
- 230000002194synthesizing effectEffects0.000abstract1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description14
- 230000000052comparative effectEffects0.000description12
- 238000003756stirringMethods0.000description10
- 229910019142PO4Inorganic materials0.000description9
- 239000010452phosphateSubstances0.000description9
- 239000000047productSubstances0.000description9
- 229910004298SiO 2Inorganic materials0.000description7
- 230000035699permeabilityEffects0.000description7
- VEXZGXHMUGYJMC-UHFFFAOYSA-NHydrochloric acidChemical compoundClVEXZGXHMUGYJMC-UHFFFAOYSA-N0.000description6
- NBIIXXVUZAFLBC-UHFFFAOYSA-KphosphateChemical compound[O-]P([O-])([O-])=ONBIIXXVUZAFLBC-UHFFFAOYSA-K0.000description6
- 238000000445field-emission scanning electron microscopyMethods0.000description5
- 238000005119centrifugationMethods0.000description4
- 238000001000micrographMethods0.000description4
- 238000000926separation methodMethods0.000description4
- 239000012265solid productSubstances0.000description4
- VHUUQVKOLVNVRT-UHFFFAOYSA-NAmmonium hydroxideChemical compound[NH4+].[OH-]VHUUQVKOLVNVRT-UHFFFAOYSA-N0.000description3
- 235000011114ammonium hydroxideNutrition0.000description3
- 238000006243chemical reactionMethods0.000description3
- 238000000635electron micrographMethods0.000description3
- 230000007935neutral effectEffects0.000description3
- 238000002047photoemission electron microscopyMethods0.000description3
- 229920001483poly(ethyl methacrylate) polymerPolymers0.000description3
- QGZKDVFQNNGYKY-UHFFFAOYSA-NAmmoniaChemical compoundNQGZKDVFQNNGYKY-UHFFFAOYSA-N0.000description2
- BLRPTPMANUNPDV-UHFFFAOYSA-NSilaneChemical compound[SiH4]BLRPTPMANUNPDV-UHFFFAOYSA-N0.000description2
- 239000002253acidSubstances0.000description2
- 230000004888barrier functionEffects0.000description2
- 230000005540biological transmissionEffects0.000description2
- 125000003700epoxy groupChemical group0.000description2
- UQSQSQZYBQSBJZ-UHFFFAOYSA-Nfluorosulfonic acidChemical compoundOS(F)(=O)=OUQSQSQZYBQSBJZ-UHFFFAOYSA-N0.000description2
- 238000010438heat treatmentMethods0.000description2
- 238000002173high-resolution transmission electron microscopyMethods0.000description2
- 230000003100immobilizing effectEffects0.000description2
- 125000002467phosphate groupChemical group[H]OP(=O)(O[H])O[*]0.000description2
- 238000010992refluxMethods0.000description2
- 229910000077silaneInorganic materials0.000description2
- 239000000126substanceSubstances0.000description2
- BDHFUVZGWQCTTF-UHFFFAOYSA-MsulfonateChemical compound[O-]S(=O)=OBDHFUVZGWQCTTF-UHFFFAOYSA-M0.000description2
- NLXLAEXVIDQMFP-UHFFFAOYSA-NAmmonium chlorideSubstances[NH4+].[Cl-]NLXLAEXVIDQMFP-UHFFFAOYSA-N0.000description1
- 229920000557Nafion®Polymers0.000description1
- 229910021529ammoniaInorganic materials0.000description1
- 230000015572biosynthetic processEffects0.000description1
- 238000002485combustion reactionMethods0.000description1
- 239000004020conductorSubstances0.000description1
- 238000010276constructionMethods0.000description1
- 239000007822coupling agentSubstances0.000description1
- 230000007423decreaseEffects0.000description1
- 238000010586diagramMethods0.000description1
- 238000009792diffusion processMethods0.000description1
- 230000000694effectsEffects0.000description1
- 238000003487electrochemical reactionMethods0.000description1
- 238000005516engineering processMethods0.000description1
- 230000007613environmental effectEffects0.000description1
- IDGUHHHQCWSQLU-UHFFFAOYSA-Nethanol;hydrateChemical compoundO.CCOIDGUHHHQCWSQLU-UHFFFAOYSA-N0.000description1
- 239000010954inorganic particleSubstances0.000description1
- 230000009191jumpingEffects0.000description1
- 230000007246mechanismEffects0.000description1
- 239000000203mixtureSubstances0.000description1
- 229920005597polymer membranePolymers0.000description1
- 239000011148porous materialSubstances0.000description1
- 238000010248power generationMethods0.000description1
- 239000002243precursorSubstances0.000description1
- 239000002994raw materialSubstances0.000description1
- 229910052710siliconInorganic materials0.000description1
- 239000010703siliconSubstances0.000description1
- 239000007787solidSubstances0.000description1
- 230000008961swellingEffects0.000description1
- 238000003786synthesis reactionMethods0.000description1
- 230000007723transport mechanismEffects0.000description1
- 238000003868zero point energyMethods0.000description1
Images
Classifications
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Landscapes
- Conductive Materials (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及一种壳聚糖/磷酸化介孔中空二氧化硅亚微米球形颗粒杂化膜及其制备方法和应用,属于有机-无机杂化直接甲醇燃料电池用质子交换膜领域,尤其是涉及磷酸化介孔中空二氧化硅亚微米球形颗粒的制备方法和应用。 The invention relates to a chitosan/phosphorylated mesoporous hollow silicon dioxide submicron spherical particle hybrid membrane and a preparation method and application thereof, belonging to the field of proton exchange membranes for organic-inorganic hybrid direct methanol fuel cells, in particular to Preparation method and application of phosphorylated mesoporous hollow silica submicron spherical particles.
背景技术Background technique
燃料电池是一种新型化学能源,它是一种不经过燃烧,直接将燃料的化学能以电化学反应的方式转变为电能的装置,具有发电效率高、适用于多种燃料及环境友好等优点。其中,直接甲醇燃料电池(direct methanol fuel cell,DMFC)是以甲醇水溶液为燃料,除具有一般燃料电池的优点以外,还具有常温快速启动、能量密度高、燃料廉价丰富易储存、电池结构简单、成本低等突出优点,被誉为21世纪最有希望的新型便携式电源。质子交换膜(proton exchange membrane,PEM)是DMFC最关键部件之一,是提高燃料电池能量转化效率的关键组件。目前,商业化的PEM主要为全氟磺酸膜,如杜邦DuPont公司开发的Nafion®系列膜。但此类磺酸化膜用于DMFC时,在高温操作条件下由于膜中水分散失会导致性能衰退。此外,全氟磺酸膜价格昂贵(600-1200 $ m-2),甲醇渗透率较高,极大地限制了DMFC技术推广及其应用。因此,适用于高温低湿DMFC操作条件、质子传导率高、成本低的新型PEM的制备成为当前研究热点。Fuel cell is a new type of chemical energy. It is a device that directly converts the chemical energy of fuel into electrical energy in the form of electrochemical reaction without combustion. It has the advantages of high power generation efficiency, applicable to various fuels and environmental friendliness. . Among them, direct methanol fuel cell (DMFC) uses methanol aqueous solution as fuel. In addition to the advantages of general fuel cells, it also has the advantages of rapid start-up at room temperature, high energy density, cheap and abundant fuel and easy storage, and simple battery structure. Low cost and other outstanding advantages, known as the most promising new portable power supply in the 21st century. Proton exchange membrane (proton exchange membrane, PEM) is one of the most critical components of DMFC, and it is a key component to improve the energy conversion efficiency of fuel cells. At present, commercial PEMs are mainly perfluorosulfonic acid membranes, such as the Nafion® series membranes developed by DuPont. However, when this type of sulfonated membrane is used in DMFC, the performance will decline due to the loss of water in the membrane under high temperature operating conditions. In addition, the perfluorosulfonic acid membrane is expensive (600-1200 $ m-2 ), and the methanol permeability is high, which greatly limits the promotion and application of DMFC technology. Therefore, the preparation of new PEMs suitable for high-temperature and low-humidity DMFC operating conditions, high proton conductivity, and low cost has become a current research hotspot.
作为质子传递位点,磷酸根具有两性(质子受体、质子供体)、介电常数高的优点,可以形成较高的质子密度,这种作用在高温低湿条件下尤为显著。磷酸根的水结合能较高、平均零点能低于磺酸根,可以为膜材料提供较高的保水能力以及较低的质子跳跃能垒。另外磷酸根的热稳定性也优于磺酸根。正因为这些优势,磷酸根类质子交换膜逐渐成为研究热点。由于磷酸化高分子膜材料种类少、合成条件苛刻,一般在膜中引入磷酸根的途径是通过将膜基质浸泡于磷酸水溶液当中,但是这种操作往往导致磷酸在DMFC工作的水环境中流失。将磷酸根固定在无机材料上就成为一种切实可行的途径。填充具有吸湿性颗粒的有机-无机杂化膜可以将具有导质子能力的无机颗粒引入膜中,从而提高膜的质子传导率等性能。到目前为止,壳聚糖/磷酸化介孔中空二氧化硅杂化膜用于直接甲醇燃料电池的质子交换膜未见文献报道。As a proton transfer site, phosphate has the advantages of amphoteric (proton acceptor, proton donor) and high dielectric constant, which can form a higher proton density. This effect is especially significant under high temperature and low humidity conditions. Phosphate has higher water binding energy and lower average zero-point energy than sulfonate, which can provide membrane materials with higher water retention capacity and lower proton jumping energy barrier. In addition, the thermal stability of phosphate is better than that of sulfonate. Because of these advantages, phosphate-based proton exchange membranes have gradually become a research hotspot. Due to the few types of phosphorylated polymer membrane materials and harsh synthesis conditions, the general way to introduce phosphate into the membrane is to soak the membrane matrix in aqueous phosphoric acid solution, but this operation often leads to the loss of phosphoric acid in the water environment where DMFC works. Fixing phosphate groups on inorganic materials has become a practical way. The organic-inorganic hybrid membrane filled with hygroscopic particles can introduce proton-conducting inorganic particles into the membrane, thereby improving the proton conductivity and other properties of the membrane. So far, chitosan/phosphorylated mesoporous hollow silica hybrid membranes have not been reported as proton exchange membranes for direct methanol fuel cells.
发明内容Contents of the invention
本发明的目的在于提供一种有机-无机杂化直接甲醇燃料电池用质子交换膜,其通过如下方法制备:首先制备介孔中空二氧化硅亚微米球形颗粒(HMSS),然后通过环氧基硅烷偶联剂对其表面进行改性引入环氧基,再使用三氯氧磷进行磷酸化制备磷酸化介孔中空二氧化硅亚微米球形颗粒(P-HMSS),最后将其与壳聚糖(CS)通过流延法合成壳聚糖/磷酸化介孔中空二氧化硅亚微米球形颗粒(CS/P-HMSS)杂化膜。The object of the present invention is to provide an organic-inorganic hybrid direct methanol fuel cell proton exchange membrane, which is prepared by the following method: first prepare mesoporous hollow silica submicron spherical particles (HMSS), and then pass epoxy silane The surface of the coupling agent is modified to introduce epoxy groups, and then phosphorus oxychloride is used for phosphorylation to prepare phosphorylated mesoporous hollow silica submicron spherical particles (P-HMSS), and finally combined with chitosan ( CS) Chitosan/phosphorylated mesoporous hollow silica submicron spherical particles (CS/P-HMSS) hybrid membranes were synthesized by casting.
本发明提供的磷酸化介孔中空二氧化硅亚微米球形颗粒通过如下步骤制备。The phosphorylated mesoporous hollow silica submicron spherical particles provided by the present invention are prepared through the following steps.
1)二氧化硅亚微米球形颗粒内核的制备1) Preparation of the inner core of silica submicron spherical particles
使用硅烷前躯体(正硅酸乙酯,tetraethyl orthosilicate,TEOS)和氨水作为原料,采用Stöber法合成二氧化硅,所得产品为直径约130 nm单分散的二氧化硅球形颗粒,作为内核sSiO2。Using silane precursor (tetraethyl orthosilicate, TEOS) and ammonia as raw materials, the Stöber method was used to synthesize silica. The obtained product was monodispersed silica spherical particles with a diameter of about 130 nm, which were used as the core sSiO2 .
2) 介孔中空二氧化硅亚微米球形颗粒(HMSS)的制备2) Preparation of Mesoporous Hollow Silica Submicron Spherical Particles (HMSS)
首先,向sSiO2外层包覆以CTAB(十六烷基三甲基溴化铵,hexadecyl trimethyl ammonium bromide)为模板的介孔壳层:将sSiO2充分分散于去离子水中形成分散液,之后向此分散液中加入混合均匀的CTAB、去离子水、乙醇和氨水溶液,逐滴向溶液中加入TEOS,配置的溶液中sSiO2的质量/体积分数为0.124 wt./vol.%,CTAB的质量/体积分数为0.186 wt./vol.%, TEOS的体积分数为0.309 vol.%,保持剧烈搅拌2-12小时。离心洗涤得到固体产品,所得产品即外表包覆CTAB为模板介孔壳层的实心二氧化硅亚微米球形颗粒,记为CTAB@SiO2/sSiO2。First, coat the mesoporous shell with CTAB (hexadecyl trimethyl ammonium bromide) as template on the outer layer of sSiO2 : fully disperse sSiO2 in deionized water to form a dispersion liquid, and then Add uniformly mixed CTAB, deionized water, ethanol and ammonia solution to this dispersion, add TEOS dropwise to the solution, the mass/volume fraction ofsSiO in the configured solution is 0.124 wt./vol.%, CTAB The mass/volume fraction is 0.186 wt./vol.%, and the volume fraction of TEOS is 0.309 vol.%. Keep stirring vigorously for 2-12 hours. The solid product was obtained by centrifuging and washing, and the obtained product was solid silica submicron spherical particles coated with CTAB as a template mesoporous shell, which was recorded as CTAB@SiO2 /sSiO2 .
将CTAB@SiO2/sSiO2分散于过量的0.2 mol/L碳酸钠水溶液中,溶液保持于50oC剧烈搅拌6-38小时,用碳酸钠水溶液刻蚀颗粒内核。离心分离洗涤,所得产品即CTAB为模板介孔空心二氧化硅亚微米球形颗粒,记为CTAB@SiO2。Disperse CTAB@SiO2 /sSiO2 in an excess of 0.2 mol/L sodium carbonate aqueous solution, keep the solution at 50o C and stir vigorously for 6-38 hours, and etch the particle core with aqueous sodium carbonate solution. After centrifugal separation and washing, the obtained product, namely CTAB, is a template mesoporous hollow silica submicron spherical particle, which is recorded as CTAB@SiO2 .
最后,将CTAB@SiO2颗粒分散于过量甲醇盐酸溶液中,回流48小时去除颗粒的CTAB模板。离心分离洗涤,得到介孔中空二氧化硅亚微米球形颗粒HMSS。Finally, the CTAB@SiO2 particles were dispersed in excess methanolic hydrochloric acid solution and refluxed for 48 hours to remove the CTAB template of the particles. Centrifugal separation and washing to obtain mesoporous hollow silica submicron spherical particles HMSS.
3) 磷酸化介孔中空二氧化硅亚微米球形颗粒(P-HMSS)的制备:3)Preparation of Phosphorylated Mesoporous Hollow Silica Submicron Spherical Particles (P-HMSS):
将如上方法合成的HMSS颗粒分散于过量的无水甲苯中,加入等质量的环氧基硅烷偶联剂(3-缩水甘油基氧丙基三甲氧基硅烷,3-glycidyloxypropyltrimethoxysilane,GPTMS),回流反应24小时,对HMSS进行环氧基改性。离心洗涤,将所得环氧基改性颗粒分散于过量的三氯氧磷中,回流24小时对颗粒进行磷酸化。离心洗涤,得到磷酸化的介孔中空二氧化硅亚微米球形颗粒P-HMSS。Disperse the HMSS particles synthesized by the above method in excess anhydrous toluene, add an equal mass of epoxy silane coupling agent (3-glycidyloxypropyltrimethoxysilane, 3-glycidyloxypropyltrimethoxysilane, GPTMS), reflux reaction At 24 hours, the HMSS was epoxy-modified. Wash by centrifugation, disperse the obtained epoxy-modified particles in excess phosphorus oxychloride, and reflux for 24 hours to phosphorylate the particles. After centrifugation and washing, phosphorylated mesoporous hollow silicon dioxide submicron spherical particles P-HMSS are obtained.
本发明提供的壳聚糖/磷酸化介孔中空二氧化硅亚微米球形颗粒杂化膜(CS/P-HMSS)通过如下步骤制备。The chitosan/phosphorylated mesoporous hollow silica submicron spherical particle hybrid membrane (CS/P-HMSS) provided by the invention is prepared through the following steps.
将一定量的P-HMSS超声分散于含有氨基三亚甲基膦酸(aminotrimethylene phosphonic acid,ATMP,占铸膜液总质量分数为0.12 wt.%-0.44 wt.%)的去离子水中。向分散液中加入占铸膜液总质量分数为2 wt.%的醋酸,并加热至80oC。机械搅拌条件下向溶液中加入壳聚糖,其中壳聚糖占铸膜液总质量分数为2 wt.%,P-HMSS用量为壳聚糖的1.25 wt.%-10 wt.%。在80oC下剧烈搅拌溶解2小时得到铸膜液。将铸膜液脱泡后在玻璃板上流延,干燥,用硫酸水溶液交联,而后洗涤干燥得到杂化膜。所得膜的厚度在80 μm左右。A certain amount of P-HMSS was ultrasonically dispersed in deionized water containing aminotrimethylene phosphonic acid (ATMP, accounting for 0.12 wt.%-0.44 wt.% of the total mass fraction of the casting solution). Add 2 wt.% acetic acid to the dispersion liquid and heat to 80o C. Chitosan was added to the solution under mechanical stirring, where chitosan accounted for 2 wt.% of the total mass fraction of the casting solution, and the amount of P-HMSS was 1.25 wt.%-10 wt.% of chitosan. Dissolve under vigorous stirring at 80o C for 2 hours to obtain a casting solution. The casting solution was defoamed and cast on a glass plate, dried, cross-linked with sulfuric acid aqueous solution, washed and dried to obtain a hybrid membrane. The thickness of the obtained film is about 80 μm.
本发明的优点在于:通过在介孔中空二氧化硅亚微米球形颗粒表面引入环氧基的方法实现颗粒磷酸化,过程温和简单,克服了二氧化硅表面硅羟基惰性不易反应的缺点。将磷酸根成功固定于二氧化硅上、同时中空二氧化硅的内部空腔作为固定磷酸根的酸池,向膜内高效引入磷酸根作为质子传递基团。填充入CS膜之后,可以得到均匀的磷酸化有机-无机杂化膜。磷酸化的介孔中空二氧化硅亚微米球形颗粒作为质子导体,提高了杂化膜质子传导性能。本发明方法所制得的磷酸化杂化膜CS/P-HMSS,用于直接甲醇燃料电池时,在110oC、相对湿度100%的条件下,传导率最高达到0.0934 S cm-1,在浓度为2 M的甲醇水溶液中,室温25oC条件下,甲醇渗透率最低达到8.42×10-7 cm2 s-1。The invention has the advantages of: realizing particle phosphorylation by introducing epoxy groups on the surface of mesoporous hollow silicon dioxide submicron spherical particles, the process is mild and simple, and overcomes the disadvantage that silicon hydroxyl groups on the surface of silicon dioxide are inert and difficult to react. Phosphate radicals are successfully immobilized on the silica, and the inner cavity of the hollow silica serves as an acid pool for immobilizing phosphate radicals, and phosphate radicals are efficiently introduced into the membrane as proton transfer groups. After filling into the CS film, a uniform phosphorylated organic-inorganic hybrid film can be obtained. Phosphorylated mesoporous hollow silica submicron spherical particles act as proton conductors to improve the proton conduction performance of the hybrid membrane. When the phosphorylated hybrid membrane CS/P-HMSS prepared by the method of the present invention is used in a direct methanol fuel cell, under the conditions of 110o C and 100% relative humidity, the conductivity can reach up to 0.0934 S cm-1 . In 2 M methanol aqueous solution, at room temperature 25o C, the methanol permeability reaches the lowest of 8.42×10-7 cm2 s-1 .
附图说明Description of drawings
图1 实施例和对比例中膜于30oC、100% RH的质子传导率比较图。Figure 1 Comparison of the proton conductivity of the membranes in Examples and Comparative Examples at 30o C and 100% RH.
图2 实施例和对比例中膜于升温过程(100oC-110oC)、100% RH的质子传导率比较图。Figure 2 Comparison of the proton conductivity of the membranes in the examples and comparative examples during the heating process (100o C-110o C) and 100% RH.
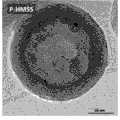
图3 实施例2中所制的磷酸化介孔中空二氧化硅亚微米球形颗粒的透射电镜图(HRTEM-JEM-2100F型HRTEM,JEOL公司)。Fig. 3 Transmission electron microscope image of phosphorylated mesoporous hollow silica submicron spherical particles prepared in Example 2 (HRTEM-JEM-2100F type HRTEM, JEOL Company).
图4 实施例1中所制的介孔中空二氧化硅亚微米球形颗粒的透射电镜图(HRTEM-JEM-2100F型HRTEM,JEOL公司)。Fig. 4 Transmission electron microscope image of mesoporous hollow silica submicron spherical particles prepared in Example 1 (HRTEM-JEM-2100F type HRTEM, JEOL Company).
图5 实施例3中所制的膜CS/P-HMSS-1.25的断面电镜图(Nanosem 430型FESEM,FEI公司)。Fig. 5 Cross-sectional electron microscope image of the film CS/P-HMSS-1.25 prepared in Example 3 (Nanosem 430 FESEM, FEI Company).
图6 实施例3中所制的膜CS/P-HMSS-7.5的断面电镜图(Nanosem 430型FESEM,FEI公司)。Fig. 6 Electron micrograph of the cross-section of the film CS/P-HMSS-7.5 prepared in Example 3 (Nanosem 430 FESEM, FEI Company).
图7 对比例1中所制的对比膜CS的断面电镜图(Nanosem 430型FESEM,FEI公司)。The cross-sectional electron micrograph of the comparative membrane CS prepared in Fig. 7 Comparative Example 1 (Nanosem430 FESEM, FEI Corporation).
图8 对比例2中所制的对比膜CS/HMSS-1.25的断面电镜图(Nanosem 430型FESEM,FEI公司)。Fig. 8 Electron microscope image of the cross-section of the comparative film CS/HMSS-1.25 prepared in Comparative Example 2 (Nanosem 430 FESEM, FEI Company).
图9 对比例2中所制的对比膜CS/HMSS-7.5的断面电镜图(Nanosem 430型FESEM,FEI公司)。Fig. 9 Cross-sectional electron micrograph of the comparative film CS/HMSS-7.5 prepared in Comparative Example 2 (Nanosem 430 FESEM, FEI Company).
具体实施方式Detailed ways
实施例1 HMSS的制备Example 1Preparation of HMSS
TEOS(9.5 mL)、氨水(7 mL)分散于乙醇-水混合液(乙醇200 mL,去离子水20 mL)中,室温下搅拌24小时。离心并用乙醇、水洗涤三次,室温真空干燥至恒重后得到sSiO2。TEOS (9.5 mL) and ammonia water (7 mL) were dispersed in ethanol-water mixture (ethanol 200 mL, deionized water 20 mL), and stirred at room temperature for 24 hours. Centrifuge, wash with ethanol and water three times, and vacuum dry at room temperature to constant weight to obtain sSiO2 .
将250 mg sSiO2充分分散于50 mL去离子水中,保持搅拌30分钟。之后将此分散液加入到混合均匀的CTAB(375 mg)、去离子水(75 mL)、乙醇(75 mL)、氨水(1.375 mL)溶液中,保持室温下剧烈搅拌30分钟。逐滴向溶液中加入TEOS(0.625 mL),室温保持搅拌6小时。离心分离得到固体产品,用去离子水洗涤三次之后,将产品于室温真空干燥至恒重,得到CTAB@SiO2/sSiO2。Thoroughly disperse 250 mg ofsSiO2 in 50 mL of deionized water and keep stirring for 30 min. Afterwards, this dispersion was added to a solution of CTAB (375 mg), deionized water (75 mL), ethanol (75 mL), and ammonia water (1.375 mL) that had been mixed well, and kept stirring vigorously at room temperature for 30 minutes. TEOS (0.625 mL) was added dropwise to the solution and kept stirring at room temperature for 6 hours. The solid product was obtained by centrifugal separation. After washing three times with deionized water, the product was vacuum-dried at room temperature to constant weight to obtain CTAB@SiO2 /sSiO2 .
将上述制得的CTAB@SiO2/sSiO2用5 mL去离子水分散,而后加入5 mL碳酸钠溶液,配置成碳酸钠占总体溶液浓度为0.2 mol/L的碳酸钠水溶液中,溶液保持于50oC剧烈搅拌19小时。离心分离得到固体产品,用去离子水洗涤三次之后,产品于室温真空干燥至恒重,所得产品为CTAB@SiO2。Disperse the CTAB@SiO2 /sSiO2 prepared above with 5 mL of deionized water, then add 5 mL of sodium carbonate solution, and configure it into an aqueous solution of sodium carbonate whose concentration of sodium carbonate in the total solution is 0.2 mol/L, and keep the solution in Stir vigorously at 50o C for 19 hours. The solid product was obtained by centrifugal separation. After washing three times with deionized water, the product was vacuum-dried at room temperature to constant weight, and the obtained product was CTAB@SiO2 .
最后,将1.2 g CTAB@SiO2颗粒分散于由9 mL 37.4%的浓盐酸溶解于160 mL无水甲醇中形成的甲醇盐酸溶液中,回流48小时。离心分离得到固体产品,用去离子水洗涤三次之后,产品于室温真空干燥至恒重,所得产品为HMSS。Finally, 1.2 g of CTAB@SiOparticles were dispersed in a methanolic hydrochloric acid solution formed by dissolving 9 mL of 37.4% concentrated hydrochloric acid in 160 mL of anhydrous methanol and refluxed for 48 h. The solid product was obtained by centrifugation. After washing three times with deionized water, the product was vacuum-dried at room temperature to constant weight, and the obtained product was HMSS.
实施例2 P-HMSS的制备Example 2Preparation of P-HMSS
制得的HMSS分散于无水甲苯(质量为HMSS的60倍)中,加入等质量的GPTMS,回流反应24小时。离心并用乙醇、水洗涤三次,产品于室温真空干燥至恒重,得到环氧基改性HMSS。将上述环氧基改性HMSS颗粒分散于过量的三氯氧磷中(三氯氧磷质量是颗粒质量的60倍),回流24小时。离心并用乙醇、水洗涤三次,干燥后得到磷酸化介孔中空二氧化硅亚微米球形颗粒P-HMSS。The prepared HMSS was dispersed in anhydrous toluene (60 times the mass of HMSS), and an equal mass of GPTMS was added, and the reaction was refluxed for 24 hours. After centrifugation and washing with ethanol and water three times, the product was vacuum-dried to constant weight at room temperature to obtain epoxy-modified HMSS. The above epoxy-modified HMSS particles were dispersed in excess phosphorus oxychloride (the mass of phosphorus oxychloride was 60 times the mass of the particles), and refluxed for 24 hours. Centrifuge, wash with ethanol and water three times, and dry to obtain phosphorylated mesoporous hollow silica submicron spherical particles P-HMSS.
实施例3 CS/P-HMSS杂化膜的制备Example 3Preparation of CS/P-HMSS hybrid membrane
称取一定量的如实施例2制备的P-HMSS颗粒超声分散于含有0.088 g的ATMP的20 mL去离子水中。向分散液中加入0.3810 mL醋酸,并加热至80oC。机械搅拌条件下向溶液中溶解0.4 g壳聚糖。在80oC下剧烈搅拌溶解2小时。脱泡后将铸膜液流延在玻璃板上,在25oC下干燥至恒重。随后在2 M的硫酸水溶液中交联24小时,用去离子水洗去残留的硫酸,直到洗涤液呈中性,最后在25oC下真空干燥膜至恒重。所制备膜的编号分别为CS/P-HMSS-1.25、CS/P-HMSS-2.5、CS/P-HMSS-5、CS/P-HMSS-7.5、CS/P-HMSS-10,其中无机材料P-HMSS用量分别为壳聚糖用量的1.25 wt.%、2.5 wt.%、5 wt.%、7.5 wt.%、10 wt.%。A certain amount of P-HMSS particles prepared as in Example 2 was weighed and ultrasonically dispersed in 20 mL of deionized water containing 0.088 g of ATMP. Add 0.3810 mL of acetic acid to the dispersion and heat to 80o C. Dissolve 0.4 g of chitosan into the solution under mechanical stirring. Dissolve with vigorous stirring at 80o C for 2 hours. After defoaming, the casting solution was cast on a glass plate and dried to constant weight at 25o C. Then cross-link in 2 M sulfuric acid aqueous solution for 24 hours, wash away the residual sulfuric acid with deionized water until the washing solution is neutral, and finally dry the membrane under vacuum at 25o C to constant weight. The numbers of the prepared membranes are CS/P-HMSS-1.25, CS/P-HMSS-2.5, CS/P-HMSS-5, CS/P-HMSS-7.5, CS/P-HMSS-10, in which the inorganic materials The amount of P-HMSS was 1.25 wt.%, 2.5 wt.%, 5 wt.%, 7.5 wt.%, 10 wt.% of the amount of chitosan, respectively.
对比例1 纯CS膜的制备Comparative Example 1 Preparation of Pure CS Membrane
将0.3810 mL醋酸溶解于20 mL去离子水中,并加热至80oC,机械搅拌条件下向溶液中溶解0.4 g壳聚糖。在80oC下剧烈搅拌溶解2小时。脱泡后将铸膜液流延在玻璃板上,在25oC下干燥至恒重。随后在2 M的硫酸水溶液中交联24小时,用去离子水洗去残留的硫酸,直到洗涤液呈中性,最后在25oC下真空干燥膜至恒重,所得CS膜中不含有无机材料。Dissolve 0.3810 mL of acetic acid in 20 mL of deionized water and heat to 80o C. Dissolve 0.4 g of chitosan into the solution under mechanical stirring. Dissolve with vigorous stirring at 80o C for 2 hours. After defoaming, the casting solution was cast on a glass plate and dried to constant weight at 25o C. Then, it was cross-linked in 2 M sulfuric acid aqueous solution for 24 hours, and the residual sulfuric acid was washed with deionized water until the washing solution was neutral, and finally the film was vacuum-dried at 25o C to constant weight, and the obtained CS film did not contain inorganic materials .
对比例2 CS/HMSS杂化膜的制备Comparative example 2 Preparation of CS/HMSS hybrid membrane
称取一定量如实施例1制备的HMSS颗粒超声分散于20 mL去离子水中。向分散液中加入0.3810 mL醋酸,并加热至80oC。机械搅拌条件下向溶液中溶解0.4 g壳聚糖。在80oC下剧烈搅拌溶解2小时。脱泡后将铸膜液流延在玻璃板上,在25oC下干燥至恒重。随后在2 M的硫酸水溶液中交联24小时,用去离子水洗去残留的硫酸,直到洗涤液呈中性,最后在25oC下真空干燥膜至恒重。所制备膜的编号分别为CS/HMSS-1.25、CS/HMSS-2.5、CS/HMSS-5、CS/HMSS-7.5、CS/HMSS-10,其中无机材料HMSS用量分别为壳聚糖用量的1.25 wt.%、2.5 wt.%、5 wt.%、7.5 wt.%、10 wt.%。Weigh a certain amount of HMSS particles prepared as in Example 1 and ultrasonically disperse them in 20 mL of deionized water. Add 0.3810 mL of acetic acid to the dispersion and heat to 80o C. Dissolve 0.4 g of chitosan into the solution under mechanical stirring. Dissolve with vigorous stirring at 80o C for 2 hours. After defoaming, the casting solution was cast on a glass plate and dried to constant weight at 25o C. Then cross-link in 2 M sulfuric acid aqueous solution for 24 hours, wash away the residual sulfuric acid with deionized water until the washing solution is neutral, and finally dry the membrane in vacuum at 25o C to constant weight. The numbers of the prepared membranes are CS/HMSS-1.25, CS/HMSS-2.5, CS/HMSS-5, CS/HMSS-7.5, CS/HMSS-10, and the amount of the inorganic material HMSS is 1.25% of that of chitosan. wt.%, 2.5 wt.%, 5 wt.%, 7.5 wt.%, 10 wt.%.
测试条件Test Conditions
实施例及对比例中制得的膜材料质子传导率测试条件为:控制膜材料的湿度为100% RH,由室温升温过程中使用电化学工作站(electrochemical workstation,PARSTAT 2273,Princeton)测试膜材料的水平向质子传导率。在测试过程中,温度升高到一定数值,即达到膜材料在100% RH下的最高耐受温度,超过此温度时,膜材料发生结构性的破坏,无法再测量出质子传导率,因此膜材料质子传导的结束温度即为100% RH下最高耐受温度,反映了测试条件下膜材料的水热稳定性。膜材料的甲醇渗透率测试条件为2 mol/L甲醇溶液中、室温条件下测试甲醇透过膜的扩散系数,即膜的甲醇渗透率。The membrane material proton conductivity test condition that makes in embodiment and comparative example is: the humidity of control membrane material is 100%RH, by using an electrochemical workstation (electrochemicalworkstation, PARSTAT 2273, Princeton) to test the horizontal proton conductivity of membrane materials. During the test, the temperature rises to a certain value, that is, the membrane material reaches 100%The maximum withstand temperature under RH. When the temperature exceeds this temperature, the membrane material will be structurally damaged, and the proton conductivity can no longer be measured. Therefore, the end temperature of the membrane material proton conduction is 100%.The maximum withstand temperature under RH reflects the hydrothermal stability of the membrane material under the test conditions. The methanol permeability test condition of the membrane material is 2In the mol/L methanol solution, the diffusion coefficient of methanol permeating the membrane is tested at room temperature, that is, the methanol permeability of the membrane.
实施例和对比例制备的各个膜材料中无机材料含量、最高耐受温度、质子传导率和甲醇渗透率见表1。Table 1 shows the content of inorganic materials, maximum temperature resistance, proton conductivity and methanol permeability in each membrane material prepared in Examples and Comparative Examples.
表1Table 1
a 无机材料占壳聚糖的质量分数aInorganic materials account for the mass fraction of chitosan
b 100% RH质子传导率测试条件下膜材料最高耐受温度b The maximum withstand temperature of the membrane material under the test condition of 100% RH proton conductivity
从图1中可以看出向膜内填充P-HMSS与HMSS颗粒,30oC条件下杂化膜质子传导率均得到提升,且CS/P-HMSS杂化膜质子传导率提升幅度最大。这是由于HMSS颗粒的填入向膜内引入更多保水位点,有利于通过运载机理传递质子;P-HMSS的填入进一步在孔壁引入质子传递位点磷酸根、并在中空酸池中固定质子传递位点磷酸根,降低了通过跳跃机理传递质子的能垒。30oC、100% RH条件下CS/P-HMSS杂化膜质子传导率提升幅度最大可达到98%。图2是100% RH升温测试条件下膜的质子传导率图,膜材料测试的结束温度反映了膜在100% RH下可耐受最高温度。100oC时,HMSS颗粒的填充基本维持了杂化膜质子传导率与CS纯膜相同,P-HMSS颗粒的引入提高了杂化膜CS/P-HMSS的质子传导率,高于纯膜。超过100oC,CS纯膜溶胀过度被破坏,在105oC时,CS/P-HMSS具有高于CS/HMSS的质子传导能力。超过105oC,CS/HMSS杂化膜同样溶胀过度被破坏,而CS/P-HMSS杂化膜仍然具有水热稳定性、和优异的质子传导能力。由此说明,CS纯膜在100% RH测试条件下可耐受最高温度为100oC,填入HMSS最高耐受温度可提升至105oC,P-HMSS的填充将可耐受温度进一步提高至110oC,这说明无机材料具有较强的稳定性结构,杂化膜的构建增强了膜材料的水热稳定性,提高了膜在测试条件下的适应性。It can be seen from Figure 1 that the proton conductivity of the hybrid membrane is improved when the membrane is filled with P-HMSS and HMSS particles at 30o C, and the proton conductivity of the CS/P-HMSS hybrid membrane is the largest. This is because the filling of HMSS particles introduces more water retention sites into the membrane, which is conducive to the transfer of protons through the transport mechanism; the filling of P-HMSS further introduces the proton transfer site phosphate in the pore wall, and in the hollow acid pool Immobilizing the phosphate group at the proton transfer site lowers the energy barrier for proton transfer via a hopping mechanism. Under the conditions of 30o C and 100% RH, the proton conductivity of CS/P-HMSS hybrid membrane can be increased by up to 98%. Figure 2 is a diagram of the proton conductivity of the membrane under 100% RH heating test conditions. The end temperature of the membrane material test reflects the maximum temperature that the membrane can withstand under 100% RH. At 100o C, the filling of HMSS particles basically maintained the same proton conductivity of the hybrid membrane as that of the pure CS membrane, and the introduction of P-HMSS particles improved the proton conductivity of the hybrid membrane CS/P-HMSS, which was higher than that of the pure membrane. Above 100o C, the CS pure membrane was destroyed due to excessive swelling, and at 105o C, CS/P-HMSS had a higher proton conductivity than CS/HMSS. Above 105o C, the CS/HMSS hybrid membrane also swells excessively and is destroyed, while the CS/P-HMSS hybrid membrane still has hydrothermal stability and excellent proton conductivity. This shows that the CS pure membrane can withstand a maximum temperature of 100o C under the test condition of 100% RH, and the maximum temperature can be increased to 105o C when filled with HMSS, and the temperature can be further increased when filled with P-HMSS to 110o C, which shows that the inorganic material has a strong stable structure, and the construction of the hybrid membrane enhances the hydrothermal stability of the membrane material and improves the adaptability of the membrane under the test conditions.
在测试条件下,CS/HMSS的甲醇渗透率低于CS纯膜,说明无机材料的引入起到了阻碍甲醇分子物质传递的作用,提高了膜材料的阻醇性能,尽管CS/P-HMSS的甲醇渗透率相比于CS纯膜略有增大,但基本维持在CS纯膜的水平,并且随着无机材料在膜中质量分数的增加,而呈现甲醇渗透得到抑制的趋势。综上所述,将P-HMSS引入CS膜基质后得到的CS/P-HMSS杂化膜具有增强的质子传导率,优异的水热稳定性,应用于DMFC中具有一定的优势,其最高质子传导率可达0.0934 S cm-1(CS/P-HMSS-7.5,110oC、100% RH),水热稳定性可达110oC,及较好的阻醇特性(8.42×10-7 cm2 s-1)。Under the test conditions, the methanol permeability of CS/HMSS is lower than that of CS pure membrane, indicating that the introduction of inorganic materials has played a role in hindering the transport of methanol molecules and improving the alcohol resistance of the membrane material, although the methanol of CS/P-HMSS Compared with the CS pure membrane, the permeability slightly increased, but basically maintained at the level of the CS pure membrane, and with the increase of the mass fraction of inorganic materials in the membrane, the methanol permeation tended to be inhibited. In summary, the CS/P-HMSS hybrid membrane obtained by introducing P-HMSS into the CS membrane matrix has enhanced proton conductivity and excellent hydrothermal stability, and has certain advantages when applied to DMFC. Conductivity up to 0.0934 S cm-1 (CS/P-HMSS-7.5, 110o C, 100% RH), hydrothermal stability up to 110o C, and good alcohol resistance properties (8.42×10-7 cm2 s-1 ).
Claims (4)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201410093433.6ACN103897221A (en) | 2014-03-13 | 2014-03-13 | Chitosan/phosphorylated silicon dioxide particle hybrid film as well as preparation and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201410093433.6ACN103897221A (en) | 2014-03-13 | 2014-03-13 | Chitosan/phosphorylated silicon dioxide particle hybrid film as well as preparation and application thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN103897221Atrue CN103897221A (en) | 2014-07-02 |
Family
ID=50988820
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201410093433.6APendingCN103897221A (en) | 2014-03-13 | 2014-03-13 | Chitosan/phosphorylated silicon dioxide particle hybrid film as well as preparation and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN103897221A (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107834089A (en)* | 2017-10-19 | 2018-03-23 | 武汉氢阳能源有限公司 | A kind of high temperature proton exchange film available for membrane cell and its preparation method and application |
| CN108155406A (en)* | 2017-12-26 | 2018-06-12 | 中国科学院青岛生物能源与过程研究所 | Temperature proton exchange film and preparation method thereof in a kind of phosphorosilicate glass doping chitosan |
| CN109569323A (en)* | 2018-11-27 | 2019-04-05 | 华南理工大学 | A kind of modified TiO of organic phosphoric acid2The preparation method of/PVC cross blend cation exchange membrane and the application in electrodialysis |
| CN109777037A (en)* | 2019-02-18 | 2019-05-21 | 山东星火科学技术研究院 | Preparation method of phosphorylated sulfonated polyether ether ketone and silica nanoparticle composite membrane |
| CN110197911A (en)* | 2018-02-27 | 2019-09-03 | 湖南省银峰新能源有限公司 | A kind of porous septum used for all-vanadium redox flow battery and its preparation method and application |
| CN114292689A (en)* | 2021-12-07 | 2022-04-08 | 广州崃克保新材料科技有限公司 | Release agent composition |
| CN114524978A (en)* | 2021-12-20 | 2022-05-24 | 华南理工大学 | Chitosan/silicon dioxide nano hybrid material and biomimetic mineralization preparation method and application thereof |
| CN114899463A (en)* | 2022-05-24 | 2022-08-12 | 湖北工程学院 | Organic-inorganic composite medium-temperature proton exchange membrane and preparation method thereof |
| CN115558169A (en)* | 2022-09-27 | 2023-01-03 | 南开大学 | Preparation method of bell-type zwitterionic microsphere chitosan hybrid membrane with high proton conductivity |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101624449A (en)* | 2009-07-23 | 2010-01-13 | 天津大学 | Organic phosphorylated titanium dioxide micrballon-filled chitosan hybrid membrane as well as preparation and application |
| US20110160319A1 (en)* | 2009-12-31 | 2011-06-30 | Industrial Technology Research Institute | Organic/inorganic hybrid composite proton exchange membrane |
| CN103521100A (en)* | 2013-10-29 | 2014-01-22 | 天津大学 | High-molecular-functional mesoporous silica hybrid membrane, preparation method and applications |
- 2014
- 2014-03-13CNCN201410093433.6Apatent/CN103897221A/enactivePending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101624449A (en)* | 2009-07-23 | 2010-01-13 | 天津大学 | Organic phosphorylated titanium dioxide micrballon-filled chitosan hybrid membrane as well as preparation and application |
| US20110160319A1 (en)* | 2009-12-31 | 2011-06-30 | Industrial Technology Research Institute | Organic/inorganic hybrid composite proton exchange membrane |
| CN103521100A (en)* | 2013-10-29 | 2014-01-22 | 天津大学 | High-molecular-functional mesoporous silica hybrid membrane, preparation method and applications |
Non-Patent Citations (3)
| Title |
|---|
| HONG WU等: ""Preparation and Properties of hybrid direct methanol fuel cell membranes by embedding organophosphorylated titania submicrospheres into a chitosan polymer matrix"", 《JOURNAL OF POWER SOURCES》* |
| JUNJIE YUAN等: ""Preparation and properties of Nafion/hollow silica spheres composite membranes"", 《JOURNAL OF MEMBRANE SCIENCE》* |
| XIAOLIANG FANG等: ""A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres"", 《NANOSCALE》* |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107834089B (en)* | 2017-10-19 | 2022-02-18 | 武汉氢阳能源有限公司 | High-temperature proton exchange membrane for membrane fuel cell and preparation method and application thereof |
| CN107834089A (en)* | 2017-10-19 | 2018-03-23 | 武汉氢阳能源有限公司 | A kind of high temperature proton exchange film available for membrane cell and its preparation method and application |
| CN108155406A (en)* | 2017-12-26 | 2018-06-12 | 中国科学院青岛生物能源与过程研究所 | Temperature proton exchange film and preparation method thereof in a kind of phosphorosilicate glass doping chitosan |
| CN108155406B (en)* | 2017-12-26 | 2021-04-06 | 中国科学院青岛生物能源与过程研究所 | Phosphosilicate glass doped chitosan intermediate-temperature proton exchange membrane and preparation method thereof |
| CN110197911A (en)* | 2018-02-27 | 2019-09-03 | 湖南省银峰新能源有限公司 | A kind of porous septum used for all-vanadium redox flow battery and its preparation method and application |
| CN110197911B (en)* | 2018-02-27 | 2021-03-26 | 湖南省银峰新能源有限公司 | Porous diaphragm for all-vanadium redox flow battery and preparation method and use thereof |
| CN109569323A (en)* | 2018-11-27 | 2019-04-05 | 华南理工大学 | A kind of modified TiO of organic phosphoric acid2The preparation method of/PVC cross blend cation exchange membrane and the application in electrodialysis |
| CN109569323B (en)* | 2018-11-27 | 2021-09-03 | 华南理工大学 | Organic phosphoric acid modified TiO2Preparation method of/PVC (polyvinyl chloride) blended cation exchange membrane and application of cation exchange membrane in electrodialysis |
| CN109777037A (en)* | 2019-02-18 | 2019-05-21 | 山东星火科学技术研究院 | Preparation method of phosphorylated sulfonated polyether ether ketone and silica nanoparticle composite membrane |
| CN114292689A (en)* | 2021-12-07 | 2022-04-08 | 广州崃克保新材料科技有限公司 | Release agent composition |
| CN114292689B (en)* | 2021-12-07 | 2023-03-31 | 广州崃克保新材料科技有限公司 | Release agent composition |
| CN114524978A (en)* | 2021-12-20 | 2022-05-24 | 华南理工大学 | Chitosan/silicon dioxide nano hybrid material and biomimetic mineralization preparation method and application thereof |
| CN114899463A (en)* | 2022-05-24 | 2022-08-12 | 湖北工程学院 | Organic-inorganic composite medium-temperature proton exchange membrane and preparation method thereof |
| CN114899463B (en)* | 2022-05-24 | 2024-04-26 | 湖北工程学院 | Organic-inorganic composite medium-temperature proton exchange membrane and preparation method thereof |
| CN115558169A (en)* | 2022-09-27 | 2023-01-03 | 南开大学 | Preparation method of bell-type zwitterionic microsphere chitosan hybrid membrane with high proton conductivity |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103897221A (en) | Chitosan/phosphorylated silicon dioxide particle hybrid film as well as preparation and application thereof | |
| Zakaria et al. | A review of progressive advanced polymer nanohybrid membrane in fuel cell application | |
| CN103897336A (en) | Sulfonated polyether-ether-ketone/phosphorylated mesoporous silica hybrid membrane preparation and application | |
| CN103570960B (en) | A kind of preparation method of the compound proton exchange membrane for high temperature resistant fuel cell | |
| CN101440167B (en) | Preparation method of SiO2/organic polymer composite proton exchange membrane | |
| CN103531831B (en) | Temperature proton exchange film material and preparation method thereof in a kind of soda acid type aminopolyphosphonic acid polysiloxanes | |
| Zhao et al. | Enhanced proton conductivity of hybrid membranes by incorporating phosphorylated hollow mesoporous silica submicrospheres | |
| CN103304979B (en) | Phosphorylation titanium dioxide hollow sphere-filled sulfonated polyether ether ketone film as well as preparation and application | |
| Seo et al. | Polybenzimidazole/inorganic composite membrane with advanced performance for high temperature polymer electrolyte membrane fuel cells | |
| CN101624449B (en) | Organic phosphorylated titanium dioxide micrballon-filled chitosan hybrid membrane as well as preparation and application | |
| WO2012045335A1 (en) | Proton exchange membrane | |
| CN105742677A (en) | Nafion/phosphorylation graphene oxide hybrid membrane and preparation and application thereof | |
| He et al. | Effect of clay modification on the structure and properties of sulfonated poly (ether ether ketone)/clay nanocomposites | |
| CN103408760B (en) | A kind of middle temperature proton exchange film material and preparation method thereof and the fuel cell using this material to prepare | |
| CN105390721B (en) | A kind of preparation method of boron phosphate enveloped carbon nanometer tube compound proton exchange membrane | |
| CN101510617B (en) | Method for preparing proton exchange film based on con-radiation technology | |
| CN103224639A (en) | Macromolecule-microcapsule composite membrane, and preparation method and application thereof | |
| Panawong et al. | Composite proton conducting membranes from crosslinked poly (vinyl alcohol)/chitosan and silica particles containing poly (2‐acrylamido‐2‐methyl‐1‐propansulfonic acid) | |
| CN111100328A (en) | Modified inorganic nano particle, polymer mixed slurry, composite membrane and preparation method | |
| CN103865231B (en) | Sulfonated polyether-ether-ketone-original position phosphorylation titania hybrid film and Synthesis and applications | |
| CN117728110B (en) | Preparation method of sodium ion battery diaphragm | |
| CN116178766B (en) | A kind of perfluorosulfonic acid nanocomposite film and preparation method thereof | |
| KR101209155B1 (en) | Organic-inorganic hybrid electrolyte membrane, method for fabricating the same and fuel cell | |
| CN108376791B (en) | A kind of organic-inorganic composite proton exchange membrane containing double proton conductor and preparation method thereof | |
| CN109585889A (en) | A kind of preparation method of ionic gel matrix proton exchange |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | Application publication date:20140702 |