CN103378355A - Alkali metal secondary battery as well as negative active substance, negative material and negative electrode thereof, and preparation method of negative active substance - Google Patents
Alkali metal secondary battery as well as negative active substance, negative material and negative electrode thereof, and preparation method of negative active substanceDownload PDFInfo
- Publication number
- CN103378355A CN103378355ACN2012101071363ACN201210107136ACN103378355ACN 103378355 ACN103378355 ACN 103378355ACN 2012101071363 ACN2012101071363 ACN 2012101071363ACN 201210107136 ACN201210107136 ACN 201210107136ACN 103378355 ACN103378355 ACN 103378355A
- Authority
- CN
- China
- Prior art keywords
- negative electrode
- active material
- electrode active
- negative
- alkali metal
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Battery Electrode And Active Subsutance (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及一种用于碱金属二次电池的钛酸盐的负极物质、负极材料、负极和负极物质的制备方法,以及采用该负极物质的碱金属二次电池。 The invention relates to a negative electrode material of titanate used in an alkali metal secondary battery, a negative electrode material, a negative electrode and a preparation method for the negative electrode material, and an alkali metal secondary battery using the negative electrode material. the
背景技术Background technique
碱金属二次电池主要包括锂离子二次电池和钠离子二次电池等。 Alkali metal secondary batteries mainly include lithium ion secondary batteries, sodium ion secondary batteries, and the like. the
自80年代末Sony公司制造出第一个锂离子电池以来,以其高能量密度,长循环寿命,对环境污染小等特点,在小型电子设备上已经得到了广泛的应用,并且近年来开始应用于电动车和大型储能设备。但是随着锂资源的日益消耗,人们开始将目光投向资源更为丰富的钠,近来对钠离子电池的研究越来越多。 Since Sony manufactured the first lithium-ion battery in the late 1980s, it has been widely used in small electronic devices due to its high energy density, long cycle life, and low environmental pollution, and has begun to be used in recent years. for electric vehicles and large energy storage devices. However, with the increasing consumption of lithium resources, people began to turn their attention to sodium, which is more abundant in resources, and more and more researches have been done on sodium-ion batteries recently. the
从目前的报道中,碱金属二次电池负极材料主要有碱金属、碳材料中的石墨碳、无定形和非多孔的碳黑、碳合金、以及金属氧化物。碱金属作为负极材料在充放电循环中均易产生金属枝晶而出现短路等安全问题。对于碳材料中的石墨作为负极与自身石墨化程度和自身性质有很大关系,无定形碳与比表面积程度非常大。碱金属合金作为负极体积膨胀比较大,循环稳定性不好,况且至今还没发现很好的合金循环性能和容量非常的好。金属氧化物作为碱金属二次电池负极材料的问题也很多,例如充放电极化大,循环不稳定,库仑效率低等。 From the current reports, the anode materials of alkali metal secondary batteries mainly include alkali metals, graphitic carbon in carbon materials, amorphous and non-porous carbon black, carbon alloys, and metal oxides. Alkali metals as anode materials are prone to produce metal dendrites during the charge-discharge cycle and cause safety problems such as short circuits. For graphite in carbon materials, as a negative electrode, it has a great relationship with its own graphitization degree and its own properties, and amorphous carbon has a very large specific surface area. Alkali metal alloys have relatively large volume expansion as the negative electrode, and the cycle stability is not good. Moreover, no good alloy has been found so far with very good cycle performance and capacity. There are also many problems with metal oxides as anode materials for alkali metal secondary batteries, such as large charge and discharge polarization, unstable cycle, and low Coulombic efficiency. the
现在主要实用化的负极材料锂离子电池主要集中在石墨材料,对于Li4Ti5O12则存在胀气等问题,而钠离子电池一直没有循环稳定,库仑效率高的负极材料。 At present, the main practical anode materials for lithium-ion batteries are mainly graphite materials. For Li4 Ti5 O12 , there are problems such as flatulence, while sodium-ion batteries have no negative electrode materials with stable cycle and high Coulombic efficiency.
因此,寻找一种容量高、库仑效率高、循环性能好、价格便宜的负极材 料,是碱金属二次电池在储能和实用化走向实用的关键。 Therefore, finding a negative electrode material with high capacity, high coulombic efficiency, good cycle performance and low price is the key to the practicality of alkali metal secondary batteries in energy storage and practical application. the
发明内容Contents of the invention
本发明的目的在于提供一种具有单相反应的碱金属二次电池负极活性物质及其制备方法,能够克服目前碱金属二次电池负极材料电位较低且易形成碱金属沉积的缺陷。 The object of the present invention is to provide a negative electrode active material of an alkali metal secondary battery with a single-phase reaction and a preparation method thereof, which can overcome the defects of low potential and easy formation of alkali metal deposition in the current negative electrode material of an alkali metal secondary battery. the
本发明的另一目的在于提供采用该负极物质的负极材料、负极和碱金属二次电池。Another object of the present invention is to provide a negative electrode material, a negative electrode and an alkali metal secondary battery using the negative electrode material.
本发明提供了一种碱金属二次电池负极活性物质,该负极活性物质的化学式为:[A(x-y)By][Bx/3Ti(1-x/3)]O2-δ,其中,A、B分别采用K、Na和Li其中的一种;0<x≤1,0≤y<x,0≤δ≤1。 The invention provides an alkali metal secondary battery negative electrode active material, the chemical formula of the negative electrode active material is: [A(xy) By ][Bx/3 Ti(1-x/3) ]O2-δ , Wherein, A and B respectively adopt one of K, Na and Li; 0<x≤1, 0≤y<x, 0≤δ≤1.
优选地,A为Na,B为Li;0.5≤x≤0.8,0≤y<x,0≤δ≤0.1。 Preferably, A is Na, B is Li; 0.5≤x≤0.8, 0≤y<x, 0≤δ≤0.1. the
本发明还提供了所述负极活性物质的制备方法,所述制备方法可以选自固相法和溶胶-凝胶法中的任一种。 The present invention also provides a preparation method of the negative electrode active material, and the preparation method may be selected from any one of solid-phase method and sol-gel method. the
所述溶胶-凝胶法包括如下步骤: Described sol-gel method comprises the steps:
1)按照负极活性物质的化学计量比称取适量碱金属的乙酸盐和钛酸四丁酯并分别溶于无水乙醇,在搅拌过程中将碱金属乙酸的无水乙醇溶液缓慢加入到钛酸四丁酯的无水乙醇溶液中,并加入柠檬酸,形成前驱体凝胶; 1) Weigh an appropriate amount of alkali metal acetate and tetrabutyl titanate according to the stoichiometric ratio of the negative electrode active material and dissolve them in absolute ethanol respectively, and slowly add the absolute ethanol solution of alkali metal acetic acid to the titanium In the dehydrated ethanol solution of acid tetrabutyl ester, and add citric acid, form precursor gel;
2)将所得前驱体凝胶置于坩埚中于250-500℃预处理两个小时,再在750-1000℃下处理8~20小时,研磨即得所述负极活性物质。 2) Pretreating the obtained precursor gel in a crucible at 250-500° C. for two hours, then treating at 750-1000° C. for 8-20 hours, and grinding to obtain the negative active material. the
所述固相法包括如下步骤: Described solid-phase method comprises the steps:
1)将碱金属的碳酸盐、钛的氧化物、按照负极活性物质的化学计量比混合,研磨均匀后得前驱体粉末; 1) Mix the alkali metal carbonate, titanium oxide, and the stoichiometric ratio of the negative electrode active material, and grind them evenly to obtain the precursor powder;
2)将所得前驱体粉末置于坩埚内于650~1000℃下处理8~25小时,研磨即得所述负极活性物质。 2) The obtained precursor powder is placed in a crucible, treated at 650-1000° C. for 8-25 hours, and ground to obtain the negative electrode active material. the
根据本发明的制备方法,其中,可以采用以下方法中的任一种对所述负极活性物质包覆碳层、金属层、氮化物层、氧化物层和高分子聚合物层的一 种或多种:(1)在所述前驱体粉末或凝胶中加入蔗糖、葡萄糖、有机聚合物、离子液体或金属盐,并在Ar或者N2惰性气体保护下加热处理;(2)向所述负极活性物质中加入蔗糖、葡萄糖、有机聚合物、离子液体或金属盐,并在Ar或者N2气体保护下加热处理;(3)采用热气相沉积法对所述前驱体或所述负极活性物质进行包覆。 According to the preparation method of the present invention, any one of the following methods can be used to coat the negative electrode active material with one or more of a carbon layer, a metal layer, a nitride layer, an oxide layer and a polymer layer Kinds: (1) adding sucrose, glucose, organic polymer, ionic liquid or metal salt to the precursor powder or gel, and heat treatment under the protection of Ar orN2 inert gas; (2) adding to the negative electrode Adding sucrose, glucose, organic polymer, ionic liquid or metal salt to the active material, and heat treatment under the protection of Ar orN2 gas; clad.
本发明提供了一种碱金属二次电池负极材料,所述负极材料可以包含导电添加剂和粘结剂,还可以包含本发明的负极活性物质或按照本发明的制备方法而制得的负极活性物质。 The invention provides a negative electrode material for an alkali metal secondary battery, the negative electrode material may contain a conductive additive and a binder, and may also contain the negative electrode active material of the present invention or the negative electrode active material prepared according to the preparation method of the present invention . the
本发明提供了一种碱金属二次电池负极,所述负极可以包含本发明的负极材料和集流体。 The present invention provides a negative electrode of an alkali metal secondary battery, and the negative electrode may include the negative electrode material and the current collector of the present invention. the
本发明提供了一种碱金属二次电池,所述碱金属二次电池可以包含正极和本发明的负极,以及置于所述正极和所述负极之间的电解液。 The present invention provides an alkali metal secondary battery, which may include a positive electrode, the negative electrode of the present invention, and an electrolytic solution placed between the positive electrode and the negative electrode. the
将所述负极活性物质用于制备碱金属二次电池负极材料及负极时,可采用现有锂离子电池或者钠离子电池的通用制作方法。即,将本发明的负极活性物质与作为导电添加剂的粉体(如碳黑、乙炔黑、石墨粉、碳纳米管、石墨稀等)研磨混合,所述导电添加剂占0~30wt%。然后与通用的粘结剂溶液(PVDF(聚偏二氟乙烯),Sodium alginate(海藻酸钠),CMC(羧甲基纤维素钠),SBR(丁苯橡胶)等),例如可以为PVDF(聚偏二氟乙烯)的NMP(N-甲基吡咯烷酮)溶液,混合成均匀浆料,涂覆于集流体上(如铜箔、钛箔、镍网、泡沫镍等)制备电极片,涂覆后所得薄膜的厚度可以为2~500μm。将所得电极片裁剪成适合形状,在基本上为真空的环境中100~150℃下烘干后备用。 When the negative electrode active material is used to prepare the negative electrode material and negative electrode of the alkali metal secondary battery, the general production method of the existing lithium ion battery or sodium ion battery can be adopted. That is, the negative electrode active material of the present invention is ground and mixed with powders as conductive additives (such as carbon black, acetylene black, graphite powder, carbon nanotubes, graphene, etc.), and the conductive additives account for 0-30 wt%. Then with a common binder solution (PVDF (polyvinylidene fluoride), Sodium alginate (sodium alginate), CMC (sodium carboxymethyl cellulose), SBR (styrene-butadiene rubber), etc.), for example, it can be PVDF ( NMP (N-methylpyrrolidone) solution of polyvinylidene fluoride), mixed into a uniform slurry, coated on the current collector (such as copper foil, titanium foil, nickel mesh, foamed nickel, etc.) to prepare electrode sheets, coated The thickness of the obtained thin film may be 2-500 μm. The obtained electrode sheet is cut into a suitable shape, dried at 100-150° C. in a substantially vacuum environment, and then used. the
所述碱金属二次电池中的改进之处在于使用本发明提供的负极活性物质,其它组成部分及制备方法为本领域技术人员所公知,此处不再赘述。所述碱金属二次电池可以是水系、非水或全固态的碱金属二次电池。 The improvement in the alkali metal secondary battery lies in the use of the negative electrode active material provided by the present invention, and other components and preparation methods are well known to those skilled in the art, and will not be repeated here. The alkali metal secondary battery may be an aqueous, non-aqueous or all-solid alkali metal secondary battery. the
本发明碱金属二次电池中的钠离子电池具有成本低、循环寿命长、能量密度高等特点,可广泛应用于太阳能、风力发电所需的大规模储能设备,以及智能电网调峰、分布电站、后备电源、通讯基站等领域,尤其适合作为大规模储能设备。 The sodium ion battery in the alkali metal secondary battery of the present invention has the characteristics of low cost, long cycle life, and high energy density, and can be widely used in large-scale energy storage equipment required for solar energy and wind power generation, as well as peak regulation and distributed power stations in smart grids , backup power supply, communication base station and other fields, especially suitable as large-scale energy storage equipment. the
本发明的碱金属二次电池的负极活性物质具有单相反应,在钠离子电池中电压范围0.5~1.2V之间,在锂离子电池中电压范围0.7-1.5V,可有效避免钠金属在负极上沉积的现象,还具有较高的容量(在C/10下首周放电容量大于120mAh/g)。 The negative electrode active material of the alkali metal secondary battery of the present invention has a single-phase reaction, and the voltage range is between 0.5-1.2V in the sodium-ion battery, and the voltage range is 0.7-1.5V in the lithium-ion battery, which can effectively avoid the sodium metal in the negative electrode. It also has a higher capacity (discharge capacity in the first week under C/10 is greater than 120mAh/g). the
附图说明Description of drawings
以下,结合附图来详细说明本发明的实施方案,其中: Below, describe embodiment of the present invention in detail in conjunction with accompanying drawing, wherein:
图1示出了本发明实施例1的负极活性物质的X射线衍射(XRD)图谱; Fig. 1 shows the X-ray diffraction (XRD) collection of illustrative plates of the negative electrode active material of the embodiment of the
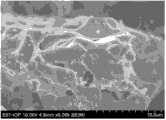
图2示出了本发明实施例1的负极活性物质的扫描电镜(SEM)图; Fig. 2 shows the scanning electron microscope (SEM) figure of the negative electrode active material of the embodiment of the
图3示出了本发明实施例1的钠离子电池的前5周充放电曲线; Fig. 3 shows the first 5 week charge and discharge curves of the sodium ion battery of the embodiment of the
图4示出了本发明实施例2的锂离子电池的前4周充放电曲线; Fig. 4 has shown the first 4 week charge-discharge curves of the lithium-ion battery of the embodiment of the present invention 2;
图5示出了本发明实施例3的未包覆碳和包覆碳钠离子电池的充放电曲线。 Fig. 5 shows the charge and discharge curves of the uncoated carbon and coated carbon sodium ion batteries in Example 3 of the present invention. the
图6示出了本发明实施例4的钠离子电池的首周充放电曲线; Fig. 6 shows the first cycle charge and discharge curve of the sodium ion battery of the embodiment of the present invention 4;
图7示出了本发明实施例5的锂离子电池的首周充放电曲线。 FIG. 7 shows the charge and discharge curves of the lithium ion battery of Example 5 of the present invention in the first cycle. the
具体实施方式Detailed ways
下面通过具体的实施例进一步说明本发明,但是,应当理解为,这些实施例仅仅是用于更详细具体地说明之用,而不应理解为用于以任何形式限制本发明。 The present invention will be further illustrated by specific examples below, but it should be understood that these examples are only used for more detailed description, and should not be construed as limiting the present invention in any form. the
本部分对本发明试验中所使用到的材料以及试验方法进行一般性的描述。虽然为实现本发明目的所使用的许多材料和操作方法是本领域公知的,但是本发明仍然在此作尽可能详细描述。本领域技术人员清楚,在上下文中,如果未特别说明,本发明所用材料和操作方法是本领域公知的。 This section provides a general description of the materials and test methods used in the tests of the present invention. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible. It will be apparent to those skilled in the art that, in the context and context, the materials used and methods of operation used in the present invention are known in the art unless otherwise indicated. the
实施例1 Example 1
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质Na0.66Li0.22Ti0.78O2,具体步骤为: 将纳米锐钛矿TiO2(颗粒粒径为50~100nm),Li2CO3(分析纯)与Na2CO3按化学计量比混合,在玛瑙研钵中混合研磨半小时,得到前驱体,对所得前驱体在20MPa压片,所得圆片直径约1.5cm,厚度0.4cm,将前驱体片转移到Al2O3坩埚内,在马弗炉中1000℃下处理20小时,所得白色粉末片经研磨后备用,即为本发明的负极活性物质Na0.66Li0.22Ti0.78O2,其XRD图谱及SEM图见图1和图2。由图1和图2可以看出,该负极活性物质是粒径为2~10μm的颗粒,且为Na0.66Li0.22Ti0.78O2纯相。 In this example, the negative electrode active material Na0.66 Li0.22 Ti0.78 O2 was prepared by the solid phase method, and the specific steps were as follows: Nano anatase TiO2 (particle size 50-100nm), Li2 CO3 (analytical pure) and Na2 CO3 was mixed according to the stoichiometric ratio, mixed and ground in an agate mortar for half an hour to obtain a precursor, and the obtained precursor was pressed at 20MPa. The obtained disc was about 1.5cm in diameter and 0.4cm in thickness. into an Al2 O3 crucible, and treated in a muffle furnace at 1000°C for 20 hours, and the resulting white powder sheet is ground and ready for use, which is the negative electrode active material Na0.66 Li0.22 Ti0.78 O2 of the present invention, and its XRD pattern and See Figure 1 and Figure 2 for SEM images. It can be seen from FIG. 1 and FIG. 2 that the negative electrode active material is a particle with a particle size of 2-10 μm, and is a pure phase of Na0.66 Li0.22 Ti0.78 O2 .
将上述负极活性物质制备成钠离子电池。具体步骤为:将制备好的负极活性物质Na0.66Li0.22Ti0.78O2粉末与乙炔黑、粘结剂PVDF按照60∶30∶10的重量比混合,加入适量的NMP溶液,在常温干燥的环境中研磨形成浆料,然后把浆料均匀涂覆于集流体铜箔或铝箔上,干燥后裁成8×8mm的极片,在真空条件下于100℃干燥10小时,随即转移入手套箱备用。模拟电池的装配在Ar气氛的手套箱内进行,以金属钠片作为对电极,1M的NaClO4/PC(丙烯碳酸酯)溶液作为电解液,装配成CR2032扣式电池。使用恒流充放电模式进行测试,放电截至电压为0V,充电截至电压为3.0V,所有测试均在C/10电流密度下进行。测试结果见图3,其中a1、a2、b1、b2分别为第一周充电曲线、第一周放电曲线、第五周充电曲线、第五周放电曲线。由图3看出,其首周放电容量可达290mAh/g,首周库仑效率约为62%,充、放电电位约为0.5~1.2V。 The above-mentioned negative electrode active material is prepared into a sodium ion battery. The specific steps are as follows: mix the prepared negative electrode active material Na0.66 Li0.22 Ti0.78 O2 powder with acetylene black and binder PVDF according to the weight ratio of 60:30:10, add an appropriate amount of NMP solution, and dry it at room temperature Grind to form a slurry, then evenly coat the slurry on the current collector copper foil or aluminum foil, cut it into 8×8mm pole pieces after drying, dry at 100°C for 10 hours under vacuum conditions, and then transfer it to the glove box for later use . The assembly of the simulated battery was carried out in a glove box with an Ar atmosphere, and a metal sodium sheet was used as a counter electrode, and 1M NaClO4 /PC (propylene carbonate) solution was used as an electrolyte, and a CR2032 button battery was assembled. The constant current charge and discharge mode is used for testing, the discharge cut-off voltage is 0V, and the charge cut-off voltage is 3.0V. All tests are carried out at C/10 current density. The test results are shown in Figure 3, where a1, a2, b1, and b2 are the charging curve of the first week, the discharging curve of the first week, the charging curve of the fifth week, and the discharging curve of the fifth week. It can be seen from Figure 3 that the discharge capacity in the first week can reach 290mAh/g, the coulombic efficiency in the first week is about 62%, and the charge and discharge potential is about 0.5-1.2V.
实施例2 Example 2
本实施例用于说明本发明的负极活性物质在锂离子电池中的应用。 This example is used to illustrate the application of the negative electrode active material of the present invention in lithium ion batteries. the
本实施例采用实施例1固相法制备负极活性物质Na0.66Li0.22Ti0.78O2。 In this example, the negative electrode active material Na0.66 Li0.22 Ti0.78 O2 was prepared by using the solid phase method in Example 1.
将上述负极活性物质制备成锂离子电池。具体步骤为:将制备好的负极活性物质Na0.66Li0.22Ti0.78O2粉末与乙炔黑、粘结剂PVDF按照60∶30∶10的重量比混合,加入适量的NMP溶液,在常温干燥的环境中研磨形成浆料,然后把浆料均匀涂覆于集流体铜箔上,干燥后裁成8×8mm的极片,在真空条件下于100℃干燥10小时,随即转移入手套箱备用。模拟电池的装配在Ar气氛的手套箱内进行,以金属锂片作为对电极,1M的LiBF6/PC溶液作为电解 液,装配成CR2032扣式电池。使用恒流充放电模式进行测试,放电截至电压为0V,充电截至电压为3.0V,所有测试均在C/10电流密度下进行。测试结果见图4,其中c1、c2、d1、d2分别为第一周充电曲线、第一周放电曲线、第四周充电曲线、第四周放电曲线。由图3看出,其首周放电容量可达440mAh/g,首周库仑效率约为75%,充、放电电位约为0.8~1.5V。 The above-mentioned negative electrode active material is prepared into a lithium ion battery. The specific steps are as follows: mix the prepared negative electrode active material Na0.66 Li0.22 Ti0.78 O2 powder with acetylene black and binder PVDF according to the weight ratio of 60:30:10, add an appropriate amount of NMP solution, and dry it at room temperature The slurry was formed by medium grinding, and then the slurry was evenly coated on the current collector copper foil, dried and cut into 8×8mm pole pieces, dried at 100°C for 10 hours under vacuum conditions, and then transferred to the glove box for later use. The assembly of the simulated battery was carried out in a glove box with an Ar atmosphere, and a metal lithium sheet was used as a counter electrode, and 1M LiBF6 /PC solution was used as an electrolyte, and a CR2032 button battery was assembled. The constant current charge and discharge mode is used for testing, the discharge cut-off voltage is 0V, and the charge cut-off voltage is 3.0V. All tests are carried out at C/10 current density. The test results are shown in Figure 4, where c1, c2, d1, and d2 are the charging curve of the first week, the discharging curve of the first week, the charging curve of the fourth week, and the discharging curve of the fourth week. It can be seen from Figure 3 that the discharge capacity in the first week can reach 440mAh/g, the coulombic efficiency in the first week is about 75%, and the charging and discharging potential is about 0.8-1.5V.
实施例3 Example 3
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用溶胶-凝胶法制备负极活性物质Na0.66Li0.22Ti0.78O2,并对其进行碳包覆处理。具体步骤为:将钛酸四丁酯(Ti(C4H9O)4)、乙酸锂(CH3COOLi)乙酸钠((CH3COONa)按照化学计量比称取适量,并分别溶于无水乙醇。在搅拌过程中将乙酸锂的无水乙醇溶液逐渐加入到碳酸四丁酯的无水乙醇溶液中,并加入适量柠檬酸以抑制水解,逐渐形成前驱体凝胶,将所得前驱体凝胶转移到Al2O3坩埚中于900℃下处理20小时,研磨后得到白色粉末备用。将该白色粉末与离子液体[EMIm][N(CN)2](1-ethyl-3-methy limidazoliumdicyanamide)混合均匀,并在Ar气氛中于600℃加热4h进行热解,冷却后即得到碳包覆的负极活性物质Na0.66Li0.22Ti0.78O2/C,其中氮掺杂碳层的厚度基本上为1~10nm。此处也可以使用其他可行方法将所述负极活性物质包覆碳层,或者包覆金属层、氮化物层、氧化物层和高分子聚合物层等。 In this example, a negative electrode active material Na0.66 Li0.22 Ti0.78 O2 was prepared by a sol-gel method, and carbon coating treatment was performed on it. The specific steps are: take an appropriate amount of tetrabutyl titanate (Ti(C4 H9 O)4 ), lithium acetate (CH3 COOLi) sodium acetate ((CH3 COONa) according to the stoichiometric ratio, and dissolve them in Water ethanol.In the process of stirring, the dehydrated ethanol solution of lithium acetate is gradually added to the dehydrated ethanol solution of tetrabutyl carbonate, and an appropriate amount of citric acid is added to inhibit hydrolysis, and the precursor gel is gradually formed, and the gained precursor is coagulated The glue was transferred to an Al2 O3 crucible and treated at 900°C for 20 hours. After grinding, a white powder was obtained for future use. The white powder was mixed with ionic liquid [EMIm][N(CN)2 ](1-ethyl-3-methyl limidazolium dicyanamide ) mixed evenly, and heated at 600°C for 4h in an Ar atmosphere for pyrolysis. After cooling, a carbon-coated negative electrode active material Na0.66 Li0.22 Ti0.78 O2 /C was obtained, wherein the thickness of the nitrogen-doped carbon layer was basically 1-10nm. Here, other feasible methods can also be used to coat the negative electrode active material with a carbon layer, or coat a metal layer, a nitride layer, an oxide layer, a polymer layer, and the like.
将上述碳包覆的负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1,对电池进行C/10放电,测试结果见图5,其中e1、e2分别为第一周充电曲线和第一周放电曲线,f1和f2为未包碳处理的。由图5可以看出,首周库仑效率由未包碳的68%,提高到了80%。 The above carbon-coated negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. The preparation process and test method are the same as in Example 1, and the battery is discharged at C/10. The test results are shown in Figure 5, where e1 and e2 are the charging curve and discharge curve of the first week, respectively, and f1 and f2 are carbon-free processed. It can be seen from Figure 5 that the Coulombic efficiency in the first week increased from 68% without carbon coating to 80%. the
实施例4 Example 4
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质Na0.51Li0.17Ti0.83O2,具体步骤为:将TiO2、Li2CO3和Na2CO3按照化学计量比混合,在玛瑙球磨罐中900转/分 钟干磨混合4小时,得到白色的前驱体粉末;将所得粉末在20MPa的压力下压片,将所得前驱体片转移到Al2O3坩埚内,在空气气氛下1000℃热处理15小时,所得片经研磨后得到粉末备用,即为本发明的负极活性物质Na0.51Li0.17Ti0.83O2。 In this example, the negative electrode active material Na0.51 Li0.17 Ti0.83 O2 was prepared by the solid phase method. The specific steps were as follows: mix TiO2 , Li2 CO3 and Na2 CO3 according to the stoichiometric ratio, and place them in an agate ball mill jar at 900 rpm /min dry milling and mixing for 4 hours to obtain a white precursor powder; the obtained powder was pressed under a pressure of 20MPa, and the obtained precursor sheet was transferred to anAl2O3 crucible, and heat-treated at1000 °C for 15 hours in an air atmosphere, The obtained flakes are ground and powdered for use, which is the negative electrode active material Na0.51 Li0.17 Ti0.83 O2 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.4V-2.5V,测试结果见图6。图6为第一周充放电曲线。由图6可以看出,其首周放电容量可达147mAh/g,首周库仑效率约为73.5%,循环五周后容量为108mAh/g。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and testing method are the same as in Example 1. The test voltage range is 0.4V-2.5V, and the test results are shown in Figure 6. Figure 6 is the charge and discharge curve for the first week. It can be seen from Figure 6 that the discharge capacity in the first week can reach 147mAh/g, the coulombic efficiency in the first week is about 73.5%, and the capacity after five cycles is 108mAh/g. the
实施例5 Example 5
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例4所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。电压测试范围为0.7-2.5V,测试结果见图7,其首周放电容量可达223mAh/g,首周库仑效率约为69%,循环五周后容量为153mAh/g。 In this example, the material synthesized in Example 4 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The voltage test range is 0.7-2.5V. The test results are shown in Figure 7. The discharge capacity in the first week can reach 223mAh/g, the coulombic efficiency in the first week is about 69%, and the capacity after five weeks of cycling is 153mAh/g. the
实施例6 Example 6
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质Na0.54Li0.18Ti0.82O2,具体步骤为:将纳米锐钛矿TiO2(颗粒粒径为50~100nm),Li2CO3(分析纯)与Na2CO3按化学计量比混合,在玛瑙研钵中混合研磨半小时,得到前驱体,将前驱体粉末转移到Al2O3坩埚内,在马弗炉中1000℃下处理20h,所得白色粉末片经研磨后备用,即为本发明的负极活性物质Na0.54Li0.18Ti0.82O2。 In this example, the solid-phase method is used to prepare the negative electrode active material Na0.54 Li0.18 Ti0.82 O2 , and the specific steps are as follows: nano anatase TiO2 (particle size is 50-100nm), Li2 CO3 (analytical pure) and Na2 CO3 was mixed according to the stoichiometric ratio, mixed and ground in an agate mortar for half an hour to obtain a precursor, the precursor powder was transferred to an Al2 O3 crucible, and treated in a muffle furnace at 1000°C for 20 hours, the obtained white The powder sheet is ground and ready for use, which is the negative electrode active material Na0.54 Li0.18 Ti0.82 O2 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.4V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test voltage range is 0.4V-2.5V, and the results are shown in Table 1 below. the
实施例7 Example 7
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例6所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试结果见表1。 In this example, the material synthesized in Example 6 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test results are shown in Table 1. the
实施例8 Example 8
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质Na0.6Li0.2Ti0.8O2,具体步骤为:将纳米锐钛矿TiO2(颗粒粒径为50~100nm),Li2CO3(分析纯)与Na2CO3按化学计量比混合,在玛瑙研钵中混合研磨半小时,得到前驱体,,将前驱体粉末转移到Al2O3坩埚内,在马弗炉中1000℃下处理20小时,所得白色粉末片经研磨后备用,即为本发明的负极活性物质Na0.6Li0.2Ti0.8O2。 In this example, the solid-phase method is used to prepare the negative electrode active material Na0.6 Li0.2 Ti0.8 O2 , and the specific steps are as follows: nano-anatase TiO2 (particle size is 50-100nm), Li2 CO3 (analytical pure) and Na2 CO3 was mixed according to the stoichiometric ratio, mixed and ground in an agate mortar for half an hour to obtain a precursor, and the precursor powder was transferred to an Al2 O3 crucible, and treated in a muffle furnace at 1000°C for 20 hours, The obtained white powder flakes are ground and ready for use, which is the negative electrode active material Na0.6 Li0.2 Ti0.8 O2 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.4V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test voltage range is 0.4V-2.5V, and the results are shown in Table 1 below. the
实施例9 Example 9
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例8所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试结果见表1。 In this example, the material synthesized in Example 8 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test results are shown in Table 1. the
实施例10 Example 10
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质Na0.75Li0.25Ti0.75O2,具体步骤为:将纳米锐钛矿TiO2(颗粒粒径为50~100nm),Li2CO3(分析纯)与Na2CO3按化学计量比混合,在玛瑙研钵中混合研磨半小时,得到前驱体,将前驱体粉末转移到Al2O3坩埚内,在马弗炉中1000℃下处理20h,所得白色粉末片 经研磨后备用,即为本发明的负极活性物质Na0.75Li0.25Ti0.75O2。 In this example, the solid-phase method is used to prepare the negative electrode active material Na0.75 Li0.25 Ti0.75 O2 , and the specific steps are as follows: nano anatase TiO2 (particle size is 50-100nm), Li2 CO3 (analytical pure) and Na2 CO3 was mixed according to the stoichiometric ratio, mixed and ground in an agate mortar for half an hour to obtain a precursor, the precursor powder was transferred to an Al2 O3 crucible, and treated in a muffle furnace at 1000°C for 20 hours, the obtained white The powder sheet is ground and ready for use, which is the negative electrode active material Na0.75 Li0.25 Ti0.75 O2 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.4V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test voltage range is 0.4V-2.5V, and the results are shown in Table 1 below. the
实施例11 Example 11
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例10所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例2。测试结果见表1。 In this example, the material synthesized in Example 10 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and testing method are the same as in Example 2. The test results are shown in Table 1. the
实施例12 Example 12
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用溶胶-凝胶法制备负极活性物质Na0.81Li0.27Ti0.73O2,并对其进行碳包覆处理。具体步骤为:将钛酸四丁酯(Ti(C4H9O)4)、乙酸锂(CH3COOLi)乙酸钠((CH3COONa)按照化学计量比称取适量,并分别溶于无水乙醇。在搅拌过程中将乙酸锂的无水乙醇溶液逐渐加入到碳酸四丁酯的无水乙醇溶液中,并加入适量柠檬酸以抑制水解,逐渐形成前驱体凝胶,将所得前驱体凝胶转移到Al2O3坩埚中于900℃下处理20小时,研磨后得到白色粉末备用。即为本发明的负极活性物质Na0.81Li0.27Ti0.73O2。 In this example, a negative electrode active material Na0.81 Li0.27 Ti0.73 O2 was prepared by a sol-gel method, and carbon coating treatment was performed on it. The specific steps are: take an appropriate amount of tetrabutyl titanate (Ti(C4 H9 O)4 ), lithium acetate (CH3 COOLi) sodium acetate ((CH3 COONa) according to the stoichiometric ratio, and dissolve them in Water ethanol.In the process of stirring, the dehydrated ethanol solution of lithium acetate is gradually added to the dehydrated ethanol solution of tetrabutyl carbonate, and an appropriate amount of citric acid is added to inhibit hydrolysis, and the precursor gel is gradually formed, and the gained precursor is coagulated The glue was transferred to an Al2 O3 crucible and treated at 900°C for 20 hours, and the white powder was obtained after grinding, which was the negative electrode active material Na0.81 Li0.27 Ti0.73 O2 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.5V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test voltage range is 0.5V-2.5V, and the results are shown in Table 1 below. the
实施例13 Example 13
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例12所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例2。测试结果见表1。 In this example, the material synthesized in Example 12 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and testing method are the same as in Example 2. The test results are shown in Table 1. the
实施例14 Example 14
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质[Na2/7Li1/7][Li1/7Ti2/7]O2具体步骤为:将纳米锐钛矿TiO2(颗粒粒径为50~100nm),Li2CO3(分析纯)与Na2CO3按化学计量比混合,在玛瑙研钵中混合研磨半小时,得到前驱体,,将前驱体粉末转移到Al2O3坩埚内,在马弗炉中1000℃下处理15h,所得白色粉末片经研磨后备用,即为本发明的负极活性物质[Na2/7Li1/7][Li1/7Ti2/7]O2。 In this example, the solid-phase method is used to prepare the negative electrode active material [Na2/7 Li1/7 ][Li1/7 Ti2/7 ]O2. The specific steps are as follows: nano anatase TiO2 (particle size of 50 ~100nm), Li2 CO3 (analytically pure) and Na2 CO3 were mixed according to the stoichiometric ratio, mixed and ground in an agate mortar for half an hour to obtain a precursor, and the precursor powder was transferred to an Al2 O3 crucible , treated in a muffle furnace at 1000°C for 15 hours, the resulting white powder flakes are ground and ready for use, which is the negative electrode active material of the present invention [Na2/7 Li1/7 ][Li1/7 Ti2/7 ]O2 .
将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例2。测试电压范围为0.7V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a lithium ion battery, and an electrochemical test was performed. Its preparation process and testing method are the same as in Example 2. The test voltage range is 0.7V-2.5V, and the results are shown in Table 1 below. the
实施例15 Example 15
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用固相法制备负极活性物质Na0.77Li0.31Ti0.73O1.97具体步骤为:将纳米锐钛矿TiO2(颗粒粒径为50~100nm),Li2CO3(分析纯)与Na2CO3按化学计量比混合,在玛瑙研钵中混合研磨半小时,得到前驱体,,将前驱体粉末转移到Al2O3坩埚内,在马弗炉中950℃下处理18小时,所得白色粉末片经研磨后备用,即为本发明的负极活性物质Na0.77Li0.31Ti0.73O1.97。 In this example, the solid-phase method is used to prepare the negative active material Na0.77 Li0.31 Ti0.73 O1.97 . The specific steps are as follows: nano anatase TiO2 (particle size is 50-100nm), Li2 CO3 (analytical pure) and Na2 CO3 was mixed according to the stoichiometric ratio, mixed and ground in an agate mortar for half an hour to obtain a precursor, and the precursor powder was transferred to an Al2 O3 crucible, and treated in a muffle furnace at 950°C for 18 hours to obtain The white powder flakes are ground and ready for use, which is the negative electrode active material Na0.77 Li0.31 Ti0.73 O1.97 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.3V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 1. The test voltage range is 0.3V-2.5V, and the results are shown in Table 1 below. the
实施例16 Example 16
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例13所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例2。测试结果见表1。 In this example, the material synthesized in Example 13 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and testing method are the same as in Example 2. The test results are shown in Table 1. the
实施例17 Example 17
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用溶胶-凝胶法制备负极活性物质Na0.69Li0.23Ti0.77O1.99。具体步骤为:将钛酸四丁酯(Ti(C4H9O)4)、乙酸锂(CH3COOLi)乙酸钠((CH3COONa)按照化学计量比称取适量,并分别溶于无水乙醇。在搅拌过程中将乙酸锂的无水乙醇溶液逐渐加入到碳酸四丁酯的无水乙醇溶液中,并加入适量柠檬酸以抑制水解,逐渐形成前驱体凝胶,将所得前驱体凝胶转移到Al2O3坩埚中于850℃下处理25小时,研磨后得到白色粉末备用。即为本发明的负极活性物质Na0.69Li0.23Ti0.77O1.99。 In this example, the negative electrode active material Na0.69 Li0.23 Ti0.77 O1.99 was prepared by a sol-gel method. The specific steps are: take an appropriate amount of tetrabutyl titanate (Ti(C4 H9 O)4 ), lithium acetate (CH3 COOLi) sodium acetate ((CH3 COONa) according to the stoichiometric ratio, and dissolve them in Water ethanol.In the process of stirring, the dehydrated ethanol solution of lithium acetate is gradually added to the dehydrated ethanol solution of tetrabutyl carbonate, and an appropriate amount of citric acid is added to inhibit hydrolysis, and the precursor gel is gradually formed, and the gained precursor is coagulated The glue was transferred to an Al2 O3 crucible and treated at 850°C for 25 hours, and the white powder was obtained after grinding, which was the negative electrode active material Na0.69 Li0.23 Ti0.77 O1.99 of the present invention.
将上述负极活性物质制备成钠离子电池,并进行电化学测试。其制备过程和测试方法同实施例1。测试电压范围为0.5V-2.5V,结果见下表1。 The above-mentioned negative electrode active material was prepared into a sodium ion battery, and an electrochemical test was performed. Its preparation process and testing method are the same as in Example 1. The test voltage range is 0.5V-2.5V, and the results are shown in Table 1 below. the
实施例18 Example 18
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例17所合成的物质装成锂离子电池,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其制备过程和测试方法同实施例2。测试结果见表1。 In this example, the material synthesized in Example 17 was used to assemble a lithium-ion battery, and the above-mentioned negative electrode active material was prepared into a lithium-ion battery, and an electrochemical test was performed. Its preparation process and test method are the same as in Example 2. The test results are shown in Table 1. the
实施例19 Example 19
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例17所合成的活性材料,利用原子层沉积(ALD)2nm的Al2O3,将上述负极活性物质制备成锂离子电池,并进行电化学测试。其测试方法同实施例2。测试结果见表1。 In this example, the active material synthesized in Example 17 was used to prepare the above-mentioned negative electrode active material into a lithium-ion battery by atomic layer deposition (ALD) of 2nm Al2 O3 , and an electrochemical test was performed. Its testing method is with embodiment 2. The test results are shown in Table 1.
实施例20 Example 20
本实施例用于说明本发明的负极活性物质的制备及其应用。 This example is used to illustrate the preparation and application of the negative electrode active material of the present invention. the
本实施例采用实施例17所合成的活性材料,直接将上述材料和PAM(聚 丙烯酰胺)按照6%∶94%混合,在Ar气得保护气氛中750度处理,得到氮掺杂碳的化合物,将所得负极活性物质制备成锂离子电池,并进行电化学测试。其测试方法同实施例2。测试结果见表1。 In this example, the active material synthesized in Example 17 is used, and the above material is directly mixed with PAM (polyacrylamide) at a rate of 6%:94%, and treated at 750 degrees in an Ar gas protective atmosphere to obtain a nitrogen-doped carbon compound. , preparing the obtained negative electrode active material into a lithium ion battery, and performing an electrochemical test. Its testing method is with embodiment 2. The test results are shown in Table 1. the
尽管本发明已进行了一定程度的描述,明显地,在不脱离本发明的精神和范围的条件下,可进行各个条件的适当变化。可以理解,本发明不限于所述实施方案,而归于权利要求的范围,其包括所述每个因素的等同替换。 While the invention has been described to a certain extent, it will be obvious that various changes may be made in various conditions without departing from the spirit and scope of the invention. It is to be understood that the invention is not limited to the described embodiments, but rather falls within the scope of the claims, which include equivalents to each of the elements described. the
Claims (7)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210107136.3ACN103378355B (en) | 2012-04-12 | 2012-04-12 | Alkali metal secondary battery and the preparation method of negative electrode active material, negative material, negative pole and negative electrode active material |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210107136.3ACN103378355B (en) | 2012-04-12 | 2012-04-12 | Alkali metal secondary battery and the preparation method of negative electrode active material, negative material, negative pole and negative electrode active material |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN103378355Atrue CN103378355A (en) | 2013-10-30 |
| CN103378355B CN103378355B (en) | 2016-03-23 |
Family
ID=49463159
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201210107136.3AActiveCN103378355B (en) | 2012-04-12 | 2012-04-12 | Alkali metal secondary battery and the preparation method of negative electrode active material, negative material, negative pole and negative electrode active material |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN103378355B (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105914354A (en)* | 2016-05-09 | 2016-08-31 | 北京工业大学 | Sodium-rich type titanium matrix layered solid solution electrode material for room-temperature sodium ion battery and preparation method |
| JP2016537294A (en)* | 2014-07-17 | 2016-12-01 | インスティチュート・オブ・フィジックス,ザ・チャイニーズ・アカデミー・オブ・サイエンシズ | Layered copper-containing oxide material, its preparation process and its use |
| CN106256033A (en)* | 2014-05-02 | 2016-12-21 | 3M创新有限公司 | Anode composition for sodium ion accumulator and preparation method thereof |
| EP3237334A4 (en)* | 2014-12-23 | 2017-11-01 | Sharp Kabushiki Kaisha | Layered oxide compositions |
| CN109244441A (en)* | 2018-08-29 | 2019-01-18 | 浙江大学 | Non-newtonian flow posture Na-K alloy electrode and its preparation method and application |
| CN112038581A (en)* | 2014-01-03 | 2020-12-04 | 溧阳天目先导电池材料科技有限公司 | Method for preliminary alkali metallization and application of method in battery material |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101071853A (en)* | 2007-06-01 | 2007-11-14 | 河南大学 | Nano lithium titanate for Negative electrode material of cell or electrochemical vessel, and its and titanium dioxide composite preparing method |
| EP2437342A2 (en)* | 2010-09-30 | 2012-04-04 | Sanyo Electric Co., Ltd. | Nonaqueous electrolyte secondary battery and method for manufacturing the same |
| CN102414882A (en)* | 2009-04-24 | 2012-04-11 | 锂电池科技有限公司 | Electrochemical cell with lithium titanate |
- 2012
- 2012-04-12CNCN201210107136.3Apatent/CN103378355B/enactiveActive
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101071853A (en)* | 2007-06-01 | 2007-11-14 | 河南大学 | Nano lithium titanate for Negative electrode material of cell or electrochemical vessel, and its and titanium dioxide composite preparing method |
| CN102414882A (en)* | 2009-04-24 | 2012-04-11 | 锂电池科技有限公司 | Electrochemical cell with lithium titanate |
| EP2437342A2 (en)* | 2010-09-30 | 2012-04-04 | Sanyo Electric Co., Ltd. | Nonaqueous electrolyte secondary battery and method for manufacturing the same |
Non-Patent Citations (1)
| Title |
|---|
| 尹盛玉: ""锂离子电池负极材料钛酸锂的合成及电化学性能研究"", 《中国博士学位论文全文数据库(工程科技I辑)》* |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112038581A (en)* | 2014-01-03 | 2020-12-04 | 溧阳天目先导电池材料科技有限公司 | Method for preliminary alkali metallization and application of method in battery material |
| CN106256033A (en)* | 2014-05-02 | 2016-12-21 | 3M创新有限公司 | Anode composition for sodium ion accumulator and preparation method thereof |
| JP2016537294A (en)* | 2014-07-17 | 2016-12-01 | インスティチュート・オブ・フィジックス,ザ・チャイニーズ・アカデミー・オブ・サイエンシズ | Layered copper-containing oxide material, its preparation process and its use |
| EP3237334A4 (en)* | 2014-12-23 | 2017-11-01 | Sharp Kabushiki Kaisha | Layered oxide compositions |
| CN105914354A (en)* | 2016-05-09 | 2016-08-31 | 北京工业大学 | Sodium-rich type titanium matrix layered solid solution electrode material for room-temperature sodium ion battery and preparation method |
| CN109244441A (en)* | 2018-08-29 | 2019-01-18 | 浙江大学 | Non-newtonian flow posture Na-K alloy electrode and its preparation method and application |
| CN109244441B (en)* | 2018-08-29 | 2020-06-05 | 浙江大学 | non-Newtonian fluid Na-K alloy electrode and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103378355B (en) | 2016-03-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103456936B (en) | Sodium ion secondary battery and the preparation method of layered titanate active substance, electrode material, both positive and negative polarity and active substance | |
| CN103579605B (en) | The preparation method of active material, both positive and negative polarity and the active material of sodium ion secondary battery and use thereof | |
| CN103066265B (en) | Sodium ion battery negative pole active substance and preparation method and application thereof | |
| CN108470903B (en) | Modification method of negative electrode material titanium dioxide of sodium ion battery | |
| CN104900862B (en) | The P2 phase layered electrode materials and preparation method of symmetrical sodium ion secondary battery | |
| CN105810914B (en) | A kind of sodium-ion battery sulfur doping porous carbon materials and preparation method thereof | |
| CN102738458B (en) | Surface modification method of lithium-rich cathode material | |
| CN102394305B (en) | Foamy copper oxide/copper lithium ion battery anode and preparation method thereof | |
| CN105870447B (en) | Sodium-ion battery N doping rutile TiO2The preparation method of/C negative materials | |
| CN102820458A (en) | Synthetic method for preparing nitrogen-carbon-containing coated lithium titanate composite material by introducing ionic liquid as carbon source | |
| CN106099113A (en) | A kind of nucleocapsid structure Si-C composite material and preparation method thereof | |
| CN108598405B (en) | Preparation method of three-dimensional graphene tin oxide carbon composite negative electrode material | |
| CN105883940B (en) | Preparation method of block NiS2 and application of block NiS2 to sodium-ion battery | |
| CN103219168A (en) | A kind of Li4Ti5O12/graphene composite electrode material and preparation method thereof | |
| CN101580273A (en) | High energy density spinel structural lithium titanate material and preparation method thereof | |
| CN103378355B (en) | Alkali metal secondary battery and the preparation method of negative electrode active material, negative material, negative pole and negative electrode active material | |
| CN104638261A (en) | A kind of high rate LiFePO4/C cathode material and its preparation method | |
| CN107240678A (en) | A kind of preparation method of lithium ion battery metal sulfide negative material | |
| CN114520323A (en) | Double-strategy modified layered oxide sodium ion battery positive electrode material and preparation method and application thereof | |
| WO2024145994A1 (en) | Method for preparing hard carbon negative electrode material from waste thermosetting plastic | |
| CN105226244A (en) | Three-dimensional porous silicon-nano silver composite material and preparation thereof and the application as lithium ion battery negative material | |
| CN103413918B (en) | A kind of synthetic method of anode material for lithium ion battery cobalt phosphate lithium | |
| CN101533912A (en) | Method for preparing lithium iron phosphate used as positive active material of lithium ion secondary battery | |
| CN113540428A (en) | 3DOM graphene carbon supported monodisperse NiO nanocrystalline material, preparation and application | |
| CN103996823B (en) | A kind of rapid microwave reaction method for preparing of power lithium-ion battery ternary polyanion phosphate/carbon positive electrode |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right | Effective date of registration:20180712 Address after:100094 4 floor 258, block D, 24 building, 68 Beiqing Road, Haidian District, Beijing. Patentee after:Beijing Zhong Ke sea sodium Technology Co., Ltd. Address before:100190 South Third Street, Zhongguancun, Haidian District, Haidian District, Beijing Patentee before:Research Institute of Physics, Chinese Academy of Sciences | |
| TR01 | Transfer of patent right |