CN103251983A - Method for preparing spliced artificial bone-filled sustained-release material with treatment effect - Google Patents
Method for preparing spliced artificial bone-filled sustained-release material with treatment effectDownload PDFInfo
- Publication number
- CN103251983A CN103251983ACN2013101702242ACN201310170224ACN103251983ACN 103251983 ACN103251983 ACN 103251983ACN 2013101702242 ACN2013101702242 ACN 2013101702242ACN 201310170224 ACN201310170224 ACN 201310170224ACN 103251983 ACN103251983 ACN 103251983A
- Authority
- CN
- China
- Prior art keywords
- artificial bone
- bone
- spliced
- therapeutic effect
- composite material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 210000000988bone and boneAnatomy0.000titleclaimsabstractdescription109
- 238000000034methodMethods0.000titleclaimsabstractdescription13
- 239000000463materialSubstances0.000titleclaimsabstractdescription11
- 238000013268sustained releaseMethods0.000titleclaimsabstractdescription7
- 239000012730sustained-release formSubstances0.000titleclaimsabstractdescription7
- 230000000694effectsEffects0.000titleclaimsdescription9
- 239000003814drugSubstances0.000claimsabstractdescription31
- 229940079593drugDrugs0.000claimsabstractdescription30
- 239000000843powderSubstances0.000claimsabstractdescription30
- 230000001225therapeutic effectEffects0.000claimsabstractdescription23
- 238000002360preparation methodMethods0.000claimsabstractdescription10
- 238000001523electrospinningMethods0.000claimsabstractdescription7
- 238000005516engineering processMethods0.000claimsabstractdescription7
- 230000007774longtermEffects0.000claimsabstractdescription6
- 239000002086nanomaterialSubstances0.000claimsabstractdescription6
- 239000010408filmSubstances0.000claimsdescription25
- 229920001610polycaprolactonePolymers0.000claimsdescription21
- 239000004632polycaprolactoneSubstances0.000claimsdescription21
- 239000002131composite materialSubstances0.000claimsdescription15
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000claimsdescription14
- 229910052588hydroxylapatiteInorganic materials0.000claimsdescription13
- XYJRXVWERLGGKC-UHFFFAOYSA-Dpentacalcium;hydroxide;triphosphateChemical compound[OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OXYJRXVWERLGGKC-UHFFFAOYSA-D0.000claimsdescription13
- 229960003350isoniazidDrugs0.000claimsdescription10
- QRXWMOHMRWLFEY-UHFFFAOYSA-NisoniazideChemical compoundNNC(=O)C1=CC=NC=C1QRXWMOHMRWLFEY-UHFFFAOYSA-N0.000claimsdescription10
- QORWJWZARLRLPR-UHFFFAOYSA-Htricalcium bis(phosphate)Chemical group[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OQORWJWZARLRLPR-UHFFFAOYSA-H0.000claimsdescription10
- JQXXHWHPUNPDRT-WLSIYKJHSA-NrifampicinChemical compoundO([C@](C1=O)(C)O/C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C=C(C)/C(=O)NC=2C(O)=C3C([O-])=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N1CC[NH+](C)CC1JQXXHWHPUNPDRT-WLSIYKJHSA-N0.000claimsdescription9
- 229960001225rifampicinDrugs0.000claimsdescription9
- 239000011780sodium chlorideSubstances0.000claimsdescription7
- 229930182555PenicillinNatural products0.000claimsdescription6
- JGSARLDLIJGVTE-MBNYWOFBSA-NPenicillin GChemical compoundN([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1JGSARLDLIJGVTE-MBNYWOFBSA-N0.000claimsdescription6
- 229940049954penicillinDrugs0.000claimsdescription6
- 238000003825pressingMethods0.000claimsdescription6
- 229920001661ChitosanPolymers0.000claimsdescription5
- 239000010409thin filmSubstances0.000claimsdescription4
- WEEMDRWIKYCTQM-UHFFFAOYSA-N2,6-dimethoxybenzenecarbothioamideChemical groupCOC1=CC=CC(OC)=C1C(N)=SWEEMDRWIKYCTQM-UHFFFAOYSA-N0.000claimsdescription3
- 229920002101ChitinPolymers0.000claimsdescription3
- 229920002307DextranPolymers0.000claimsdescription3
- 238000010030laminatingMethods0.000claimsdescription3
- 239000002121nanofiberSubstances0.000claimsdescription3
- 239000004626polylactic acidSubstances0.000claimsdescription3
- 229960002385streptomycin sulfateDrugs0.000claimsdescription3
- 230000008468bone growthEffects0.000claimsdescription2
- 229920001577copolymerPolymers0.000claimsdescription2
- 239000003102growth factorSubstances0.000claimsdescription2
- 239000005416organic matterSubstances0.000claimsdescription2
- 230000002138osteoinductive effectEffects0.000claimsdescription2
- 229920000747poly(lactic acid)Polymers0.000claimsdescription2
- 230000000921morphogenic effectEffects0.000claims1
- 102000004169proteins and genesHuman genes0.000claims1
- 108090000623proteins and genesProteins0.000claims1
- 230000007547defectEffects0.000abstractdescription15
- 239000000945fillerSubstances0.000abstractdescription4
- 238000013270controlled releaseMethods0.000abstractdescription3
- 239000011259mixed solutionSubstances0.000abstractdescription2
- 238000010586diagramMethods0.000description8
- HEDRZPFGACZZDS-UHFFFAOYSA-NChloroformChemical compoundClC(Cl)ClHEDRZPFGACZZDS-UHFFFAOYSA-N0.000description6
- 210000002449bone cellAnatomy0.000description6
- 239000002639bone cementSubstances0.000description5
- 102000008143Bone Morphogenetic Protein 2Human genes0.000description4
- 108010049931Bone Morphogenetic Protein 2Proteins0.000description4
- 241001465754MetazoaSpecies0.000description4
- 238000002474experimental methodMethods0.000description4
- 208000015181infectious diseaseDiseases0.000description4
- 239000000243solutionSubstances0.000description4
- 238000003860storageMethods0.000description4
- 238000002054transplantationMethods0.000description4
- 206010028289Muscle atrophyDiseases0.000description3
- 230000010478bone regenerationEffects0.000description3
- 230000015556catabolic processEffects0.000description3
- 238000006731degradation reactionMethods0.000description3
- 230000012010growthEffects0.000description3
- 230000020763muscle atrophyEffects0.000description3
- 201000000585muscular atrophyDiseases0.000description3
- 238000011084recoveryMethods0.000description3
- 230000000735allogeneic effectEffects0.000description2
- 230000002924anti-infective effectEffects0.000description2
- 229910000389calcium phosphateInorganic materials0.000description2
- 239000001506calcium phosphateSubstances0.000description2
- 235000011010calcium phosphatesNutrition0.000description2
- 239000008367deionised waterSubstances0.000description2
- 229910021641deionized waterInorganic materials0.000description2
- 230000028993immune responseEffects0.000description2
- 230000003340mental effectEffects0.000description2
- 238000011160researchMethods0.000description2
- 238000009987spinningMethods0.000description2
- 210000001519tissueAnatomy0.000description2
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterChemical compoundOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description2
- NIXOWILDQLNWCW-UHFFFAOYSA-MAcrylateChemical compound[O-]C(=O)C=CNIXOWILDQLNWCW-UHFFFAOYSA-M0.000description1
- 206010002091AnaesthesiaDiseases0.000description1
- 208000035049Blood-Borne InfectionsDiseases0.000description1
- 206010056377Bone tuberculosisDiseases0.000description1
- 208000027205Congenital diseaseDiseases0.000description1
- 206010028980NeoplasmDiseases0.000description1
- 208000009360Osteoarticular TuberculosisDiseases0.000description1
- 206010031252OsteomyelitisDiseases0.000description1
- 206010031264OsteonecrosisDiseases0.000description1
- 206010061363Skeletal injuryDiseases0.000description1
- 238000010521absorption reactionMethods0.000description1
- 239000003522acrylic cementSubstances0.000description1
- 230000037005anaesthesiaEffects0.000description1
- 239000003242anti bacterial agentSubstances0.000description1
- 239000003462bioceramicSubstances0.000description1
- 230000003115biocidal effectEffects0.000description1
- 230000015572biosynthetic processEffects0.000description1
- 239000000356contaminantSubstances0.000description1
- 230000006378damageEffects0.000description1
- 238000001804debridementMethods0.000description1
- 230000007423decreaseEffects0.000description1
- 201000010099diseaseDiseases0.000description1
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description1
- 238000000635electron micrographMethods0.000description1
- 238000003306harvestingMethods0.000description1
- 230000035876healingEffects0.000description1
- 230000005847immunogenicityEffects0.000description1
- 238000002513implantationMethods0.000description1
- 230000002458infectious effectEffects0.000description1
- 208000014674injuryDiseases0.000description1
- 238000003475laminationMethods0.000description1
- 239000000203mixtureSubstances0.000description1
- 230000036407painEffects0.000description1
- 230000035515penetrationEffects0.000description1
- 238000006116polymerization reactionMethods0.000description1
- 230000001737promoting effectEffects0.000description1
- 230000008929regenerationEffects0.000description1
- 238000011069regeneration methodMethods0.000description1
- 238000011477surgical interventionMethods0.000description1
- 238000001356surgical procedureMethods0.000description1
- 238000012360testing methodMethods0.000description1
- 238000012546transferMethods0.000description1
- 230000008733traumaEffects0.000description1
Images
Landscapes
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于纳米生物医学领域,具体涉及一种具有治疗作用的拼接式人工骨填充缓释复合材料的制备方法。The invention belongs to the field of nano-biomedicine, and in particular relates to a preparation method of a spliced artificial bone filling sustained-release composite material with therapeutic effect.
背景技术Background technique
骨缺损指骨的结构完整性被破坏,是临床常见病。创伤、感染、肿瘤、骨髓炎手术清创、以及各种先天性疾病是导致骨缺损的主要原因。较小的骨缺损(通常指8mm以下),对于一个健康的非吸烟者来说,有可能自行愈合;但骨缺损过大,或者是在较小的骨头上的骨缺损,很难完全愈合,需要手术的干预,目前主要方法有以下几种:Bone defect refers to the destruction of the structural integrity of the bone, which is a common clinical disease. Trauma, infection, tumor, surgical debridement of osteomyelitis, and various congenital diseases are the main causes of bone defects. Smaller bone defects (usually below 8mm) may heal on their own for a healthy non-smoker; but too large a bone defect, or a bone defect on a smaller bone, is difficult to heal completely, Surgical intervention is required, the main methods are as follows:
1、骨移植,又分为自体骨移植,同种异体骨移植,异种骨移植。自体骨移植虽有明显的优点,如无排斥反应、无污染、材料成本低、可以完全吸收、能诱导骨重建。但存在极大缺点,如取骨的量较少、需要额外的取骨手术部位、增加了麻醉及手术的时间、并发症相对较高、取骨部位可能有长达半年或更长时间的疼痛或不适感。同种异体骨移植取自尸体骨,骨组织经过处理后已灭活,具有来源较自体骨丰富,免疫源性低,可提供结构支撑等优点;与自体骨比较,不能诱导骨愈合,同时也存在引起免疫反应、血源性疾病传播的可能性。异种骨移植取自动物的骨骼制成,由于不能100%确定这些材料无传染性污染物,有可能引起免疫反应。1. Bone transplantation is divided into autologous bone transplantation, allogeneic bone transplantation and xenogeneic bone transplantation. Although autologous bone grafting has obvious advantages, such as no rejection, no pollution, low material cost, can be completely absorbed, and can induce bone reconstruction. However, there are great disadvantages, such as a small amount of bone harvested, an additional surgical site for bone harvesting, increased time for anesthesia and surgery, relatively high complications, and pain at the bone harvested site for half a year or longer or discomfort. Allogeneic bone grafts are taken from cadaver bones, and the bone tissue has been inactivated after treatment. It has the advantages of richer source than autologous bone, lower immunogenicity, and can provide structural support. Compared with autologous bone, it cannot induce bone healing and also Potential for immune response, spread of blood-borne disease. Bone xenografts are made from the bones of animals, and since it is not 100% certain that these materials are free from infectious contaminants, it is possible to cause an immune response.
2、人工骨:包括骨水泥(如磷酸钙骨水泥及丙烯酸酯类骨水泥 )和生物陶瓷(如羟基磷灰石)。磷酸钙骨水泥是一种新型的骨修复材料,虽有较好的组织相容性,及可作为抗生素载体,在植入感染性骨缺损处发挥持续抗感染作用,但机械强度较低,不能用于负重骨的修复。丙烯酸酯类骨水泥能够及时塑形,且具有较好的力学性能;但生物相容性较差,缺乏骨传导性,聚合过程会发热引起周围骨坏死,在骨-水泥界面形成纤维组织,及不利于吸收,也不利于骨组织的长入。羟基磷灰石是生物相容性很好的骨修复材料,其修复主要体现在骨传导方面,可以为新骨的形成提供支架。但羟基磷灰石在体内降解的速度慢,不利于新骨的长入。2. Artificial bone: including bone cement (such as calcium phosphate bone cement and acrylate bone cement) and bioceramics (such as hydroxyapatite). Calcium phosphate bone cement is a new type of bone repair material. Although it has good histocompatibility and can be used as an antibiotic carrier to exert a continuous anti-infection effect in implanted infected bone defects, it has low mechanical strength and cannot For the repair of weight-bearing bones. Acrylic bone cement can be shaped in time and has good mechanical properties; however, it has poor biocompatibility and lacks osteoconductivity, and the polymerization process will generate heat and cause surrounding bone necrosis, forming fibrous tissue at the bone-cement interface, and It is not conducive to absorption, nor is it conducive to the ingrowth of bone tissue. Hydroxyapatite is a bone repair material with good biocompatibility, and its repair is mainly reflected in bone conduction, which can provide a scaffold for the formation of new bone. However, hydroxyapatite degrades slowly in the body, which is not conducive to the growth of new bone.
3、骨搬运技术。利用Ilizarov的牵张-成骨原理,在外固定架的辅助下,在骨缺损的近端或远端截骨,并将游离骨段搬运至骨缺损处的方法。在搬运的过程中,截骨处会长出新生的骨组织。虽不存在生物污染或生物相容性等问题,且适合任何类型骨缺损,但是需要的时间较长,期间需要患者及家属的积极配合。3. Bone handling technology. Using Ilizarov's distraction-osteogenesis principle, with the assistance of external fixator, osteotomy at the proximal or distal end of the bone defect, and move the free bone segment to the bone defect. During the transfer process, new bone tissue will grow at the osteotomy site. Although there are no problems such as biocontamination or biocompatibility, and it is suitable for any type of bone defect, it takes a long time and requires the active cooperation of patients and family members.
4、组织工程骨,利用组织工程的手段,构建、培育活的骨组织,以修复或重建骨的结构,该技术目前仍处于研究阶段。4. Tissue-engineered bone, using tissue engineering means to construct and cultivate living bone tissue to repair or rebuild bone structure, this technology is still in the research stage.
综上所述,现有文献的报导中,尚未有关于本发明中所述的生物相容性及生物可降解纳米材料为载体附着物,通过静电纺丝技术制成纳米纤维薄膜,与特制粉末共同压制形成具有长期缓释作用并可进行快速拼接人工骨填充材料,以实现不同治疗作用及增强修复骨部位的耐牵拉性,防止患肢肌肉萎缩功能退化的相关研究报道。In summary, in the reports of the existing literature, there is no information about the biocompatibility and biodegradable nanomaterials described in the present invention as carrier attachments, made of nanofiber films by electrospinning technology, and special powders Co-pressed to form a long-term slow-release effect and can be quickly spliced artificial bone filling materials to achieve different therapeutic effects and enhance the traction resistance of the repaired bone site, and prevent related research reports on muscle atrophy and functional degradation of the affected limb.
发明内容Contents of the invention
本发明的目的在于提供一种具有治疗作用的拼接式人工骨骨填充缓释复合材料的制备方法。The object of the present invention is to provide a preparation method of spliced artificial bone filling slow-release composite material with therapeutic effect.
本发明提出的具有治疗作用的拼接式人工骨骨填充缓释复合材料的制备方法,具体步骤如下:The preparation method of the spliced artificial bone filling slow-release composite material with therapeutic effect proposed by the present invention, the specific steps are as follows:
以生物相容性且生物可降解纳米材料为载体附着物,添加具有不同治疗作用的药物通过静电纺丝技术纺成薄膜,所得薄膜与特制粉末交替放到模具里压制成可相互拼接且具有长期药物缓释作用的人工骨填充缓释复合材料;其中:控制静电纺丝的电压为10-15kv,流量为0.1-1ml/h,时间为1-5h;所述以生物相容性且生物可降解性纳米材料采用壳聚糖、甲壳素、葡聚糖、聚己内酯(PCL)、聚乳酸(PLA)或聚乳酸-羟基乙酸共聚物(PLGA)等的一种或几种。Biocompatible and biodegradable nanomaterials are used as carrier attachments, and drugs with different therapeutic effects are spun into thin films by electrospinning technology. The obtained thin films and special powders are alternately placed in molds and pressed to be spliced with each other and have long-term Artificial bone filling slow-release composite material with drug slow-release effect; wherein: the voltage for controlling electrospinning is 10-15kv, the flow rate is 0.1-1ml/h, and the time is 1-5h; One or more of chitosan, chitin, dextran, polycaprolactone (PCL), polylactic acid (PLA) or polylactic-co-glycolic acid (PLGA) is used as the degradable nanomaterial.
本发明中,所述具有不同治疗作用的药物为硫酸链霉素、青霉素、异烟肼、利福平、β-TCP、HA或促进骨生长的骨诱导生长因子等的一种或几种的组合。In the present invention, the drugs with different therapeutic effects are one or more of streptomycin sulfate, penicillin, isoniazid, rifampicin, β-TCP, HA, or osteoinductive growth factors that promote bone growth. combination.
本发明中,所述特制粉末为β-磷酸三钙(β-TCP)、羟基磷灰石(HA)、氯化钠(NaCl)、骨形态发生蛋白2(BMP2)或聚己内酯(PCL)等的一种或几种。In the present invention, the special powder is β-tricalcium phosphate (β-TCP), hydroxyapatite (HA), sodium chloride (NaCl), bone morphogenetic protein 2 (BMP2) or polycaprolactone (PCL ) etc. one or more.
本发明中,通过对薄膜进行层压或者片压得到有机物和无机物的复合体人工骨。In the present invention, the composite artificial bone of organic matter and inorganic matter is obtained by laminating or sheet pressing the film.
本发明中,对薄膜进行层压或片压时的压力为5KPa-20KPa,时间为0.5-5min。In the present invention, the pressure when laminating or sheet pressing the film is 5KPa-20KPa, and the time is 0.5-5min.
本发明中,压制成的人工骨置于50-85℃下2-30min使表面粉末进一步固化。In the present invention, the pressed artificial bone is placed at 50-85° C. for 2-30 minutes to further solidify the surface powder.
本发明中,压制产生的人工骨在二维或三维空间进行上下或者左右相互拼接成一个平面或者立体,可根据病人的不同需求将人工骨拼接成不同形状。In the present invention, the artificial bone produced by pressing is spliced up and down or left and right in two-dimensional or three-dimensional space to form a plane or three-dimensional, and the artificial bone can be spliced into different shapes according to different needs of patients.
本发明中,根据不同病人的需要可以将纳米纤维薄膜压制成特异的可相互拼接人工骨。In the present invention, according to the needs of different patients, the nanofiber film can be pressed into a specific artificial bone that can be spliced with each other.
本发明中,首次提出了拼接式人工骨,临床上可根据不同的形状的骨缺损拼接成不同的形状对缺损进行填充。In the present invention, spliced artificial bone is proposed for the first time, which can be spliced into different shapes according to different shapes of bone defects clinically to fill the defects.
本发明以生物相容性及生物可降解纳米材料作为载体附着物,利用静电纺丝技术将负有药物的混合溶液纺成薄膜,薄膜与特制粉末交替间隔压制成形,得到具有药物长期缓释,并可进行相互拼接的具有治疗作用的人工骨填充物。通过本发明方法制得的人工骨填充缓释材料:1、包含壳聚糖,甲壳素,葡聚糖,聚己内酯,聚乳酸,聚乳酸-羟基乙酸共聚物等的一种或几种的组合,具有生物相容性及生物可降解性,随着骨细胞的长入人工骨填充物可逐渐被吸收,最后全被新生骨细胞代替;2、通过添加具有不同治疗作用的药物可发挥不同的治疗作用,如加入硫酸链霉素,利福平,异烟肼和HA等的一种或几种组合制得的人工骨可发挥抗骨结核作用,如加入青霉素和BMP2,β-TCP中的任意两种组合或者三种组合制得的人工骨填充物可发挥抗感染及促进骨再生的作用;3、目前临床所用的人工骨绝大多数需要裁剪,这不但延长了手术的时间也增加了患者的感染风险;裁剪出来的人工骨一整个填充进缺损处,骨细胞较难长入中间替代人工骨,导致修复时间加长,病人康复时间慢;已成型的人工骨大多数脆性大,术后病人长时间不能进行正常活动,稍有不慎将发生人工骨碎裂,导致手术失败及感染,这于病人及其家庭都是莫大的精神及经济负担。本发明的方法制得的人工骨比较小,并且可根据病人的缺损不同而拼成不同的形状,临床使用起来也比较方便,缩短手术时间,降低感染率;植入病人体内的人工骨由多个小部分组成,骨细胞可通过每个小部分的间隙深入成骨,有利于修复,缩短病人康复时间;植入的人工骨通过拼图式相互嵌合拼接而成增加了人工骨的韧性,根据拼接的方向不同可从各个方向增强人工骨的耐牵拉性,可能实现病人术后不久即可正常活动,避免患肢长时间不运动长生的肌肉萎缩,减轻病人的精神和经济负担。本发明通过层压法或片压法制得人工骨,随着人工骨的降解可长时间释放具有不同治疗作用的药物。The present invention uses biocompatible and biodegradable nanomaterials as carrier attachments, uses electrospinning technology to spin the mixed solution loaded with drugs into a film, and the film and special powder are alternately pressed and formed to obtain long-term sustained release of drugs. It can also be used as an artificial bone filler with therapeutic effect that can be spliced with each other. The artificial bone filling sustained-release material prepared by the method of the present invention: 1. Contains one or more of chitosan, chitin, dextran, polycaprolactone, polylactic acid, polylactic acid-glycolic acid copolymer, etc. The combination of bone cells is biocompatible and biodegradable. With the growth of bone cells into the artificial bone filler, it can be gradually absorbed, and finally replaced by new bone cells; 2. By adding drugs with different therapeutic effects, it can play a role Different therapeutic effects, such as adding one or more combinations of streptomycin sulfate, rifampicin, isoniazid and HA, etc., can exert anti-bone tuberculosis effect, such as adding penicillin and BMP2, β-TCP The artificial bone filler prepared by any combination of two or three of them can play the role of anti-infection and promoting bone regeneration; 3. Most of the artificial bones currently used in clinical practice need to be cut, which not only prolongs the operation time but also It increases the risk of infection for the patient; the artificial bone that is cut out is filled into the defect, and it is difficult for bone cells to grow into the middle to replace the artificial bone, resulting in longer repair time and slower recovery time for the patient; most of the formed artificial bone is brittle, After the operation, the patient cannot carry out normal activities for a long time, and the artificial bone may be broken if he is not careful, leading to operation failure and infection. This is a great mental and financial burden for the patient and his family. The artificial bone prepared by the method of the present invention is relatively small, and can be assembled into different shapes according to the difference of the defect of the patient. Composed of several small parts, bone cells can go deep into the bone through the gaps of each small part, which is conducive to repair and shortens the patient's recovery time; the implanted artificial bone is made of jigsaw-like interlocking and splicing to increase the toughness of the artificial bone. The different splicing directions can enhance the traction resistance of the artificial bone from all directions, and it is possible to realize the normal activities of the patient shortly after the operation, avoid the long-term muscle atrophy of the affected limb without exercise, and reduce the mental and economic burden of the patient. The artificial bone is prepared by the lamination method or sheet pressing method, and the medicines with different therapeutic effects can be released for a long time along with the degradation of the artificial bone.
本发明的优点:负载在人工骨材料中的药物不同可发挥不同的治疗作用,术后植入体内可长时间缓慢释放,维持局部有效的药物治疗浓度,有助于骨移植物周围骨组织再生同时发挥治疗作用;通过本发明方法所得的人工骨可拼接填充,手术现场可根据病人的不同要求进行不同的拼接;每个拼接的部分都很小,有利于骨细胞渗透长入,可快速修复缺损;植入的人工骨进行拼图式拼接,根据拼接方向不同,可从不同方向增强修复处的耐牵拉性,缩短骨修复时间,防止患肢肌肉萎缩功能退化,促进患者的康复。Advantages of the present invention: different drugs loaded in the artificial bone material can exert different therapeutic effects, can be slowly released after implantation in the body for a long time, maintain local effective drug treatment concentration, and contribute to the regeneration of bone tissue around the bone graft At the same time play a therapeutic role; the artificial bone obtained by the method of the present invention can be spliced and filled, and different splicing can be performed on the surgical site according to the different requirements of patients; each spliced part is very small, which is conducive to the penetration and growth of bone cells and can be repaired quickly Defects: The implanted artificial bone is spliced in a jigsaw style. According to the different splicing directions, the traction resistance of the repaired part can be enhanced from different directions, the bone repair time can be shortened, the muscle atrophy and functional degradation of the affected limb can be prevented, and the patient’s recovery can be promoted.
附图说明Description of drawings
图1 负药溶液静电纺丝薄膜电镜图。Figure 1 Electron micrograph of negative drug solution electrospun film.
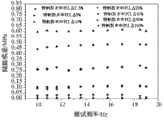
图2 不同药物释药图,骨损伤修复周期一般为2-3个月,药物释放图充分说明了按本发明所得到的产品能保证在骨修复期间具有良好的药物控释功能,促进骨修复的加速进行。Fig. 2 Drug release diagrams of different drugs, the bone injury repair cycle is generally 2-3 months, the drug release diagram fully illustrates that the product obtained according to the present invention can ensure good drug controlled release function during bone repair, and promote bone repair accelerated.
图3 人工骨的内部结构图。Figure 3 Internal structure diagram of artificial bone.
图4 人工骨拼接示意图,其中:为拼接方式示意图,b)为局部拼接图。Figure 4 Schematic diagram of artificial bone splicing, where: is a schematic diagram of the splicing method, and b) is a partial splicing diagram.
图5 为特制粉末中不同含量的PCL制成的人工骨的储能模量。随着PCL含量降低,人工骨的储能模量呈增大趋势,在PCL占10%时,人工骨的储能模量最大,测试表明PCL含量为10%时人工骨的储能模量最高,证明此时人工骨具有良好的力学性能。Figure 5 shows the storage modulus of artificial bone made of different contents of PCL in the special powder. As the PCL content decreases, the storage modulus of the artificial bone tends to increase. When the PCL content is 10%, the storage modulus of the artificial bone is the largest. The test shows that the storage modulus of the artificial bone is the highest when the PCL content is 10%. , proving that the artificial bone has good mechanical properties at this time.
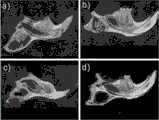
图6 动物实验图,a)为PCL/HA/β-TCP,b)为PCL/HA,C)为PCL/β-TCP,d)为空白对照。通过各组的动物实验图对比可知,存在PCL/HA/β-TCP情况下比空白组具有显著好的修复效果,证明了通过本发明得到的人工骨具有良好骨再生能力。Figure 6 Animal experiment diagram, a) is PCL/HA/β-TCP, b) is PCL/HA, C) is PCL/β-TCP, d) is blank control. Through the comparison of the animal experiment diagrams of each group, it can be seen that in the presence of PCL/HA/β-TCP, the repair effect is significantly better than that of the blank group, which proves that the artificial bone obtained by the present invention has good bone regeneration ability.
图2,图5,图6的实验证明本发明得到的产品具有:1、优良的力学性能;2、良好的药物控释作用;3、动物实验所体现的良好骨再生能力。The experiments in Fig. 2, Fig. 5, and Fig. 6 prove that the product obtained by the present invention has: 1. excellent mechanical properties; 2. good drug controlled release; 3. good bone regeneration ability as reflected in animal experiments.
具体实施方式Detailed ways
下面通过实施例进一步说明本发明。The present invention is further illustrated below by way of examples.
实施例1Example 1
精密称定分子量为8万的PCL 0.24g,利福平粉末0.012g,加入PCL与利福平的总量100/7倍的氯仿,得到的溶液在电压为15kv,流量为0.5ml/h的条件下纺丝4h,得到含利福平的药膜。精密称定分子量为8万的PCL 0.24g,异烟肼粉末0.012g,加入PCL与异烟肼总量的100/7倍的混合液(氯仿:DMF=9:1),得到的溶液在电压为15kv,流量为0.5ml/h的条件下纺丝4h,得到含异烟肼的药膜。将粉末按TCP:HA:PCL:NaCl=(0.4:0.6:1:8)的比例混合研磨均匀,得到特制粉末。精密称定特制粉末0.1g放置于模具中,加入与模具大小相符的异烟肼药膜,加入特制粉末0.1g,加入与模具大小相符的利福平药膜,加入特制粉末0.1g,加入与模具大小相符的异烟肼药膜,加入特制粉末0.1g,加入与模具大小相符的利福平药膜,加入特制粉末0.1g,加入与模具大小相符的异烟肼药膜,加入特制粉末0.1g,加入与模具大小相符的利福平药膜,加入特制粉末0.1g。连同模具置于85℃下20分钟,取出,在10KPa压力下,压制2分钟,取出,放置去离子水中浸泡72h,除去NaCl,取出,晾干,得到产品。Accurately weigh 0.24g of PCL with a molecular weight of 80,000, 0.012g of rifampicin powder, add 100/7 times the total amount of PCL and rifampicin in chloroform, and obtain the solution at a voltage of 15kv and a flow rate of 0.5ml/h. Under the condition of spinning for 4 hours, a drug film containing rifampicin was obtained. Accurately weigh 0.24g of PCL with a molecular weight of 80,000, and 0.012g of isoniazid powder, add a mixture of 100/7 times the total amount of PCL and isoniazid (chloroform:DMF=9:1), and the obtained solution is Spinning for 4 hours under the conditions of 15kv and flow rate of 0.5ml/h, the drug film containing isoniazid was obtained. The powder is mixed and ground according to the ratio of TCP:HA:PCL:NaCl=(0.4:0.6:1:8) to obtain a special powder. Accurately weigh 0.1g of the special powder and place it in the mold, add isoniazid drug film matching the size of the mold, add 0.1g of the special powder, add rifampicin drug film matching the size of the mold, add 0.1g of the special powder, add the Isoniazid drug film matching the size of the mold, add 0.1g of special powder, add rifampicin drug film matching the size of the mold, add 0.1g of special powder, add isoniazid drug film matching the size of the mold, add 0.1 g, add the rifampicin film conforming to the size of the mold, and add 0.1g of special powder. Place it together with the mold at 85°C for 20 minutes, take it out, press it for 2 minutes under a pressure of 10KPa, take it out, soak it in deionized water for 72 hours, remove NaCl, take it out, and dry it to obtain the product.
实施例2Example 2
精密称定壳聚糖 0.24g,青霉素粉末0.012g,加入壳聚糖与青霉素的总量100/7倍的氯仿,得到的溶液在电压为12kv,流量为1ml/h的条件下纺丝3h,得到含青霉素的药膜。将粉末按BMP2:HA:PCL:NaCl=(0.4:0.6:1:8)的比例混合研磨均匀,得到特制粉末。精密称定特制粉末0.1g放置于模具中,加入与模具大小相符的异烟肼药膜,加入特制粉末0.1g,加入与模具大小相符的药膜,加入特制粉末0.1g,加入与模具大小相符的药膜,加入特制粉末0.1g,加入与模具大小相符的药膜,加入特制粉末0.1g,加入与模具大小相符的药膜,加入特制粉末0.1g,加入与模具大小相符的药膜,加入特制粉末0.1g。连同模具置于80℃下20分钟,取出,在8KPa压力下,压制3分钟,取出,放置去离子水中浸泡72h,除去NaCl,取出,晾干,得到产品。Accurately weigh 0.24g of chitosan, 0.012g of penicillin powder, add chitosan and chloroform of 100/7 times the total amount of penicillin, the solution obtained is spun for 3h under the condition of voltage of 12kv and flow rate of 1ml/h, A drug film containing penicillin was obtained. The powder is mixed and ground according to the ratio of BMP2:HA:PCL:NaCl=(0.4:0.6:1:8) to obtain a special powder. Accurately weigh 0.1g of the special powder and place it in the mold, add isoniazid drug film that matches the size of the mold, add 0.1g of the special powder, add the drug film that matches the size of the mold, add 0.1g of the special powder, and add the film that matches the size of the mold Add 0.1g of special powder, add a film that matches the size of the mold, add 0.1g of special powder, add a film that matches the size of the mold, add 0.1g of special powder, add a film that matches the size of the mold, add Special powder 0.1g. Place it together with the mold at 80°C for 20 minutes, take it out, press it for 3 minutes under a pressure of 8KPa, take it out, soak it in deionized water for 72 hours, remove NaCl, take it out, and dry it to obtain the product.
Claims (8)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310170224.2ACN103251983B (en) | 2013-05-10 | 2013-05-10 | Method for preparing spliced artificial bone-filled sustained-release material with treatment effect |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201310170224.2ACN103251983B (en) | 2013-05-10 | 2013-05-10 | Method for preparing spliced artificial bone-filled sustained-release material with treatment effect |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN103251983Atrue CN103251983A (en) | 2013-08-21 |
| CN103251983B CN103251983B (en) | 2014-09-17 |
Family
ID=48956404
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201310170224.2AExpired - Fee RelatedCN103251983B (en) | 2013-05-10 | 2013-05-10 | Method for preparing spliced artificial bone-filled sustained-release material with treatment effect |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN103251983B (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105581998A (en)* | 2016-01-04 | 2016-05-18 | 重庆医科大学附属永川医院 | Sustained-release meshed tablet capable of promoting bone growth and preparation method of sustained-release meshed tablet |
| CN106310357A (en)* | 2016-10-18 | 2017-01-11 | 曹建中 | Bone filling adhesive and preparation method as well as application of bone filling adhesive |
| CN107213529A (en)* | 2017-05-09 | 2017-09-29 | 苏州大学附属第二医院 | A kind of preparation method for being used to improve the degradable medical polymer three-dimensional material of Gegenbaur's cell adhesion and bone formation performance |
| CN114425104A (en)* | 2021-12-21 | 2022-05-03 | 中国人民解放军空军军医大学 | Medicine-carrying bone guiding/inducing composite structure and preparation method and application thereof |
| CN115006599A (en)* | 2022-07-22 | 2022-09-06 | 杭州卫达生物材料科技有限公司 | Bone filling material and preparation method thereof |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL257853A (en)* | 2018-03-04 | 2019-03-31 | Pali Nazir | Process for building artificial multifunctional injectable filler |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101791438A (en)* | 2010-03-16 | 2010-08-04 | 浙江大学 | Method for preparing bioactive poly(lactic-co-glycolic acid)/collagen/hydroxyapatite composite fiber membrane for bone repair |
| CN102580166A (en)* | 2012-02-27 | 2012-07-18 | 浙江大学 | Medical bionic transparent film implanting material, and preparation method and application of material |
- 2013
- 2013-05-10CNCN201310170224.2Apatent/CN103251983B/ennot_activeExpired - Fee Related
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101791438A (en)* | 2010-03-16 | 2010-08-04 | 浙江大学 | Method for preparing bioactive poly(lactic-co-glycolic acid)/collagen/hydroxyapatite composite fiber membrane for bone repair |
| CN102580166A (en)* | 2012-02-27 | 2012-07-18 | 浙江大学 | Medical bionic transparent film implanting material, and preparation method and application of material |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105581998A (en)* | 2016-01-04 | 2016-05-18 | 重庆医科大学附属永川医院 | Sustained-release meshed tablet capable of promoting bone growth and preparation method of sustained-release meshed tablet |
| CN106310357A (en)* | 2016-10-18 | 2017-01-11 | 曹建中 | Bone filling adhesive and preparation method as well as application of bone filling adhesive |
| CN107213529A (en)* | 2017-05-09 | 2017-09-29 | 苏州大学附属第二医院 | A kind of preparation method for being used to improve the degradable medical polymer three-dimensional material of Gegenbaur's cell adhesion and bone formation performance |
| CN114425104A (en)* | 2021-12-21 | 2022-05-03 | 中国人民解放军空军军医大学 | Medicine-carrying bone guiding/inducing composite structure and preparation method and application thereof |
| CN114425104B (en)* | 2021-12-21 | 2023-03-03 | 中国人民解放军空军军医大学 | A drug-loaded bone conduction/induction composite structure and its preparation method and application |
| CN115006599A (en)* | 2022-07-22 | 2022-09-06 | 杭州卫达生物材料科技有限公司 | Bone filling material and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103251983B (en) | 2014-09-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Xue et al. | Bone tissue engineering in the treatment of bone defects | |
| Wang et al. | Collagen-based biomaterials for tissue engineering | |
| Lyons et al. | Nanostructured biomaterials for bone regeneration | |
| Song et al. | Advanced strategies of scaffolds design for bone regeneration | |
| Zizzari et al. | Biologic and clinical aspects of integration of different bone substitutes in oral surgery: a literature review | |
| CN103251983B (en) | Method for preparing spliced artificial bone-filled sustained-release material with treatment effect | |
| Kretlow et al. | Injectable biomaterials for regenerating complex craniofacial tissues | |
| JP5289772B2 (en) | Cartilage-like structure having multi-layer structure and osteochondral substitute and use thereof | |
| TWI316860B (en) | Multi-layered matrix, method of tissue repair using the same and multi-layered implant prepared thereof | |
| Emara et al. | Recent update on craniofacial tissue engineering | |
| Dong et al. | Novel alternative therapy for spinal tuberculosis during surgery: reconstructing with anti-tuberculosis bioactivity implants | |
| Kesharwani et al. | Tissue Engineering: Applications and Advancements | |
| CN1258591C (en) | Tissue engineered peripheral nerve graft | |
| CN114288481A (en) | Multilayer composite medicine-carrying guided bone regeneration membrane and preparation method thereof | |
| Shirsath et al. | Biodegradable Polymer in Medical Implants and Devices | |
| Kharkova et al. | Three-dimensional TCP scaffolds enriched with Erythropoietin for stimulation of vascularization and bone formation. | |
| CN117045857A (en) | Hydrogel-porous zinc bidirectional stent and application thereof | |
| Rodrigues et al. | Biomaterials in preclinical approaches for engineering skeletal tissues | |
| CN103623465A (en) | Tissue-engineering bone scaffold capable of locally regulating osteoclasts activity and preparation method thereof | |
| Salah et al. | 3D Printing and Bioprinting of Biomaterials and Bioceramic Scaffolds: Clinical Outcomes and Implications in Bone Tissue Engineering and Maxillofacial Reconstructive Surgery | |
| Heo et al. | Evaluation of poly (lactide-co-glycolide)/hydroxyapatite nanofibres for reconstruction of critical-sized segmental bone defects in a canine model. | |
| CN1158109C (en) | Biologically cmposite artificial bone and its preparing process | |
| CN1580254A (en) | Tissue engineered peripheral nerve graft | |
| Liu et al. | Comparison of rabbit rib defect regeneration implanted mineralized collagen with and without periosteum | |
| De et al. | 6 Prospects of functional nano-manufactured scaffolds in tissue engineering applications |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20140917 Termination date:20170510 | |
| CF01 | Termination of patent right due to non-payment of annual fee |