CN102885783A - Nanometer medicament microspheres - Google Patents
Nanometer medicament microspheresDownload PDFInfo
- Publication number
- CN102885783A CN102885783ACN2012103615776ACN201210361577ACN102885783ACN 102885783 ACN102885783 ACN 102885783ACN 2012103615776 ACN2012103615776 ACN 2012103615776ACN 201210361577 ACN201210361577 ACN 201210361577ACN 102885783 ACN102885783 ACN 102885783A
- Authority
- CN
- China
- Prior art keywords
- drug
- microspheres
- nano
- nanoparticles
- oil
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000004005microsphereSubstances0.000titleclaimsabstractdescription349
- 239000003814drugSubstances0.000titleclaimsabstractdescription321
- 229940079593drugDrugs0.000claimsabstractdescription259
- 239000002105nanoparticleSubstances0.000claimsabstractdescription214
- 239000000725suspensionSubstances0.000claimsabstractdescription103
- 238000002360preparation methodMethods0.000claimsabstractdescription62
- 239000000839emulsionSubstances0.000claimsabstractdescription44
- 239000000546pharmaceutical excipientSubstances0.000claimsabstractdescription40
- 229940124531pharmaceutical excipientDrugs0.000claimsabstractdescription37
- 229920000642polymerPolymers0.000claimsabstractdescription33
- 239000007900aqueous suspensionSubstances0.000claimsabstractdescription24
- 239000004094surface-active agentSubstances0.000claimsabstractdescription22
- 239000003960organic solventSubstances0.000claimsabstractdescription10
- 239000011259mixed solutionSubstances0.000claimsabstractdescription6
- 229920001223polyethylene glycolPolymers0.000claimsdescription90
- 239000002202Polyethylene glycolSubstances0.000claimsdescription89
- YMWUJEATGCHHMB-UHFFFAOYSA-NDichloromethaneChemical compoundClCClYMWUJEATGCHHMB-UHFFFAOYSA-N0.000claimsdescription87
- 239000003921oilSubstances0.000claimsdescription80
- AOJJSUZBOXZQNB-TZSSRYMLSA-NDoxorubicinChemical compoundO([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1AOJJSUZBOXZQNB-TZSSRYMLSA-N0.000claimsdescription78
- QFJCIRLUMZQUOT-HPLJOQBZSA-NsirolimusChemical compoundC1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1QFJCIRLUMZQUOT-HPLJOQBZSA-N0.000claimsdescription62
- 229960002930sirolimusDrugs0.000claimsdescription60
- ZAHRKKWIAAJSAO-UHFFFAOYSA-NrapamycinNatural productsCOCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)CZAHRKKWIAAJSAO-UHFFFAOYSA-N0.000claimsdescription59
- AQHHHDLHHXJYJD-UHFFFAOYSA-NpropranololChemical compoundC1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1AQHHHDLHHXJYJD-UHFFFAOYSA-N0.000claimsdescription54
- 239000007864aqueous solutionSubstances0.000claimsdescription51
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical compoundO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000claimsdescription41
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsdescription38
- 229960004679doxorubicinDrugs0.000claimsdescription34
- 108010051696Growth HormoneProteins0.000claimsdescription32
- 102000018997Growth HormoneHuman genes0.000claimsdescription32
- 238000011282treatmentMethods0.000claimsdescription32
- 102000003951ErythropoietinHuman genes0.000claimsdescription31
- 108090000394ErythropoietinProteins0.000claimsdescription31
- 229940105423erythropoietinDrugs0.000claimsdescription31
- OXCMYAYHXIHQOA-UHFFFAOYSA-Npotassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanolChemical compound[K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1OXCMYAYHXIHQOA-UHFFFAOYSA-N0.000claimsdescription31
- WEVYAHXRMPXWCK-UHFFFAOYSA-NAcetonitrileChemical compoundCC#NWEVYAHXRMPXWCK-UHFFFAOYSA-N0.000claimsdescription28
- 229910052588hydroxylapatiteInorganic materials0.000claimsdescription28
- XYJRXVWERLGGKC-UHFFFAOYSA-Dpentacalcium;hydroxide;triphosphateChemical compound[OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OXYJRXVWERLGGKC-UHFFFAOYSA-D0.000claimsdescription28
- 229960003712propranololDrugs0.000claimsdescription27
- 102000014150InterferonsHuman genes0.000claimsdescription26
- 108010050904InterferonsProteins0.000claimsdescription26
- 239000000122growth hormoneSubstances0.000claimsdescription26
- 229940079322interferonDrugs0.000claimsdescription26
- 239000002245particleSubstances0.000claimsdescription26
- 239000004632polycaprolactoneSubstances0.000claimsdescription26
- 229920001610polycaprolactonePolymers0.000claimsdescription26
- 238000002347injectionMethods0.000claimsdescription25
- 239000007924injectionSubstances0.000claimsdescription25
- 239000000203mixtureSubstances0.000claimsdescription24
- GWEVSGVZZGPLCZ-UHFFFAOYSA-NTitan oxideChemical compoundO=[Ti]=OGWEVSGVZZGPLCZ-UHFFFAOYSA-N0.000claimsdescription22
- 229940126586small molecule drugDrugs0.000claimsdescription21
- XEKOWRVHYACXOJ-UHFFFAOYSA-NEthyl acetateChemical compoundCCOC(C)=OXEKOWRVHYACXOJ-UHFFFAOYSA-N0.000claimsdescription18
- 238000003756stirringMethods0.000claimsdescription18
- 108090000623proteins and genesProteins0.000claimsdescription17
- XOFYZVNMUHMLCC-ZPOLXVRWSA-NprednisoneChemical compoundO=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1XOFYZVNMUHMLCC-ZPOLXVRWSA-N0.000claimsdescription16
- 229960004618prednisoneDrugs0.000claimsdescription16
- 206010028980NeoplasmDiseases0.000claimsdescription15
- -1pibosufanChemical compound0.000claimsdescription15
- FOIXSVOLVBLSDH-UHFFFAOYSA-NSilver ionChemical compound[Ag+]FOIXSVOLVBLSDH-UHFFFAOYSA-N0.000claimsdescription14
- NOESYZHRGYRDHS-UHFFFAOYSA-NinsulinChemical compoundN1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1NOESYZHRGYRDHS-UHFFFAOYSA-N0.000claimsdescription14
- 102000004169proteins and genesHuman genes0.000claimsdescription14
- 239000004372Polyvinyl alcoholSubstances0.000claimsdescription13
- 229920002451polyvinyl alcoholPolymers0.000claimsdescription13
- 229920002307DextranPolymers0.000claimsdescription12
- CSCPPACGZOOCGX-UHFFFAOYSA-NAcetoneChemical compoundCC(C)=OCSCPPACGZOOCGX-UHFFFAOYSA-N0.000claimsdescription11
- 239000006228supernatantSubstances0.000claimsdescription11
- 239000004408titanium dioxideSubstances0.000claimsdescription11
- 238000012546transferMethods0.000claimsdescription11
- IMNFDUFMRHMDMM-UHFFFAOYSA-NN-HeptaneChemical compoundCCCCCCCIMNFDUFMRHMDMM-UHFFFAOYSA-N0.000claimsdescription10
- RVGRUAULSDPKGF-UHFFFAOYSA-NPoloxamerChemical compoundC1CO1.CC1CO1RVGRUAULSDPKGF-UHFFFAOYSA-N0.000claimsdescription10
- 229960000502poloxamerDrugs0.000claimsdescription10
- 229920001983poloxamerPolymers0.000claimsdescription10
- 239000004626polylactic acidSubstances0.000claimsdescription10
- 239000003102growth factorSubstances0.000claimsdescription9
- 201000011066hemangiomaDiseases0.000claimsdescription9
- 229920000136polysorbatePolymers0.000claimsdescription9
- HEDRZPFGACZZDS-UHFFFAOYSA-NChloroformChemical compoundClC(Cl)ClHEDRZPFGACZZDS-UHFFFAOYSA-N0.000claimsdescription8
- 102000004877InsulinHuman genes0.000claimsdescription7
- 108090001061InsulinProteins0.000claimsdescription7
- 229940125396insulinDrugs0.000claimsdescription7
- VTYYLEPIZMXCLO-UHFFFAOYSA-LCalcium carbonateChemical compound[Ca+2].[O-]C([O-])=OVTYYLEPIZMXCLO-UHFFFAOYSA-L0.000claimsdescription6
- CMSMOCZEIVJLDB-UHFFFAOYSA-NCyclophosphamideChemical compoundClCCN(CCCl)P1(=O)NCCCO1CMSMOCZEIVJLDB-UHFFFAOYSA-N0.000claimsdescription6
- 239000001856Ethyl celluloseSubstances0.000claimsdescription6
- NKANXQFJJICGDU-QPLCGJKRSA-NTamoxifenChemical compoundC=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1NKANXQFJJICGDU-QPLCGJKRSA-N0.000claimsdescription6
- RJURFGZVJUQBHK-UHFFFAOYSA-Nactinomycin DNatural productsCC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21RJURFGZVJUQBHK-UHFFFAOYSA-N0.000claimsdescription6
- 210000000988bone and boneAnatomy0.000claimsdescription6
- 229940044683chemotherapy drugDrugs0.000claimsdescription6
- 229960004397cyclophosphamideDrugs0.000claimsdescription6
- 235000019325ethyl celluloseNutrition0.000claimsdescription6
- 229920001249ethyl cellulosePolymers0.000claimsdescription6
- UQSXHKLRYXJYBZ-UHFFFAOYSA-Niron oxideInorganic materials[Fe]=OUQSXHKLRYXJYBZ-UHFFFAOYSA-N0.000claimsdescription6
- 229960005486vaccineDrugs0.000claimsdescription6
- PMATZTZNYRCHOR-CGLBZJNRSA-NCyclosporin AChemical compoundCC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=OPMATZTZNYRCHOR-CGLBZJNRSA-N0.000claimsdescription5
- 108010036949CyclosporineProteins0.000claimsdescription5
- ZZSNKZQZMQGXPY-UHFFFAOYSA-NEthyl celluloseChemical compoundCCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1ZZSNKZQZMQGXPY-UHFFFAOYSA-N0.000claimsdescription5
- 230000003115biocidal effectEffects0.000claimsdescription5
- TWNQGVIAIRXVLR-UHFFFAOYSA-Noxo(oxoalumanyloxy)alumaneChemical compoundO=[Al]O[Al]=OTWNQGVIAIRXVLR-UHFFFAOYSA-N0.000claimsdescription5
- 229950008882polysorbateDrugs0.000claimsdescription5
- 229920000036polyvinylpyrrolidonePolymers0.000claimsdescription5
- 239000001267polyvinylpyrrolidoneSubstances0.000claimsdescription5
- 235000013855polyvinylpyrrolidoneNutrition0.000claimsdescription5
- DLGOEMSEDOSKAD-UHFFFAOYSA-NCarmustineChemical compoundClCCNC(=O)N(N=O)CCClDLGOEMSEDOSKAD-UHFFFAOYSA-N0.000claimsdescription4
- 108010041308Endothelial Growth FactorsProteins0.000claimsdescription4
- 108050007372Fibroblast Growth FactorProteins0.000claimsdescription4
- 102000018233Fibroblast Growth FactorHuman genes0.000claimsdescription4
- 102000004457Granulocyte-Macrophage Colony-Stimulating FactorHuman genes0.000claimsdescription4
- 108010017213Granulocyte-Macrophage Colony-Stimulating FactorProteins0.000claimsdescription4
- 108010025020Nerve Growth FactorProteins0.000claimsdescription4
- 102000015336Nerve Growth FactorHuman genes0.000claimsdescription4
- 108010079337Tissue Polypeptide AntigenProteins0.000claimsdescription4
- 108010073929Vascular Endothelial Growth Factor AProteins0.000claimsdescription4
- 102000005789Vascular Endothelial Growth FactorsHuman genes0.000claimsdescription4
- 108010019530Vascular Endothelial Growth FactorsProteins0.000claimsdescription4
- HVYWMOMLDIMFJA-DPAQBDIFSA-NcholesterolChemical compoundC1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2HVYWMOMLDIMFJA-DPAQBDIFSA-N0.000claimsdescription4
- 229940126864fibroblast growth factorDrugs0.000claimsdescription4
- 229940053128nerve growth factorDrugs0.000claimsdescription4
- 229920000747poly(lactic acid)Polymers0.000claimsdescription4
- STQGQHZAVUOBTE-UHFFFAOYSA-N7-Cyan-hept-2t-en-4,6-diinsaeureNatural productsC1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1STQGQHZAVUOBTE-UHFFFAOYSA-N0.000claimsdescription3
- GSDSWSVVBLHKDQ-UHFFFAOYSA-N9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acidChemical compoundFC1=CC(C(C(C(O)=O)=C2)=O)=C3N2C(C)COC3=C1N1CCN(C)CC1GSDSWSVVBLHKDQ-UHFFFAOYSA-N0.000claimsdescription3
- 108010006654BleomycinProteins0.000claimsdescription3
- KLWPJMFMVPTNCC-UHFFFAOYSA-NCamptothecinNatural productsCCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=OKLWPJMFMVPTNCC-UHFFFAOYSA-N0.000claimsdescription3
- 108010092160DactinomycinProteins0.000claimsdescription3
- 108010076282Factor IXProteins0.000claimsdescription3
- 108010054218Factor VIIIProteins0.000claimsdescription3
- 102000001690Factor VIIIHuman genes0.000claimsdescription3
- GHASVSINZRGABV-UHFFFAOYSA-NFluorouracilChemical compoundFC1=CNC(=O)NC1=OGHASVSINZRGABV-UHFFFAOYSA-N0.000claimsdescription3
- 101000746367Homo sapiens Granulocyte colony-stimulating factorProteins0.000claimsdescription3
- FBOZXECLQNJBKD-ZDUSSCGKSA-NL-methotrexateChemical compoundC=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1FBOZXECLQNJBKD-ZDUSSCGKSA-N0.000claimsdescription3
- GSDSWSVVBLHKDQ-JTQLQIEISA-NLevofloxacinChemical compoundC([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1GSDSWSVVBLHKDQ-JTQLQIEISA-N0.000claimsdescription3
- 229930192392MitomycinNatural products0.000claimsdescription3
- NWIBSHFKIJFRCO-WUDYKRTCSA-NMytomycinChemical compoundC1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2NWIBSHFKIJFRCO-WUDYKRTCSA-N0.000claimsdescription3
- ZDZOTLJHXYCWBA-VCVYQWHSSA-NN-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxolChemical compoundO([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1ZDZOTLJHXYCWBA-VCVYQWHSSA-N0.000claimsdescription3
- 229930012538PaclitaxelNatural products0.000claimsdescription3
- 239000004793PolystyreneSubstances0.000claimsdescription3
- 108010009583Transforming Growth FactorsProteins0.000claimsdescription3
- 102000009618Transforming Growth FactorsHuman genes0.000claimsdescription3
- JXLYSJRDGCGARV-WWYNWVTFSA-NVinblastineNatural productsO=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)CJXLYSJRDGCGARV-WWYNWVTFSA-N0.000claimsdescription3
- RJURFGZVJUQBHK-IIXSONLDSA-Nactinomycin DChemical compoundC[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21RJURFGZVJUQBHK-IIXSONLDSA-N0.000claimsdescription3
- 230000000692anti-sense effectEffects0.000claimsdescription3
- VSRXQHXAPYXROS-UHFFFAOYSA-Nazanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+)Chemical compound[NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1VSRXQHXAPYXROS-UHFFFAOYSA-N0.000claimsdescription3
- 229960001561bleomycinDrugs0.000claimsdescription3
- OYVAGSVQBOHSSS-UAPAGMARSA-Obleomycin A2Chemical compoundN([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1COYVAGSVQBOHSSS-UAPAGMARSA-O0.000claimsdescription3
- 229910000019calcium carbonateInorganic materials0.000claimsdescription3
- 239000001506calcium phosphateSubstances0.000claimsdescription3
- 229910000389calcium phosphateInorganic materials0.000claimsdescription3
- 235000011010calcium phosphatesNutrition0.000claimsdescription3
- VSJKWCGYPAHWDS-FQEVSTJZSA-NcamptothecinChemical compoundC1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1VSJKWCGYPAHWDS-FQEVSTJZSA-N0.000claimsdescription3
- 229940127093camptothecinDrugs0.000claimsdescription3
- 229960004562carboplatinDrugs0.000claimsdescription3
- 229960005243carmustineDrugs0.000claimsdescription3
- 229960001265ciclosporinDrugs0.000claimsdescription3
- DQLATGHUWYMOKM-UHFFFAOYSA-LcisplatinChemical compoundN[Pt](N)(Cl)ClDQLATGHUWYMOKM-UHFFFAOYSA-L0.000claimsdescription3
- 229960004316cisplatinDrugs0.000claimsdescription3
- 229940105774coagulation factor ixDrugs0.000claimsdescription3
- 229940105778coagulation factor viiiDrugs0.000claimsdescription3
- 229930182912cyclosporinNatural products0.000claimsdescription3
- 229960000640dactinomycinDrugs0.000claimsdescription3
- STQGQHZAVUOBTE-VGBVRHCVSA-NdaunorubicinChemical compoundO([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1STQGQHZAVUOBTE-VGBVRHCVSA-N0.000claimsdescription3
- 229960000975daunorubicinDrugs0.000claimsdescription3
- VSJKWCGYPAHWDS-UHFFFAOYSA-Ndl-camptothecinNatural productsC1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1VSJKWCGYPAHWDS-UHFFFAOYSA-N0.000claimsdescription3
- 229960003668docetaxelDrugs0.000claimsdescription3
- WHWZLSFABNNENI-UHFFFAOYSA-NepinastineChemical compoundC1C2=CC=CC=C2C2CN=C(N)N2C2=CC=CC=C21WHWZLSFABNNENI-UHFFFAOYSA-N0.000claimsdescription3
- 229960002548epinastine hydrochlorideDrugs0.000claimsdescription3
- VJJPUSNTGOMMGY-MRVIYFEKSA-NetoposideChemical compoundCOC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1VJJPUSNTGOMMGY-MRVIYFEKSA-N0.000claimsdescription3
- 229960005420etoposideDrugs0.000claimsdescription3
- 229960002949fluorouracilDrugs0.000claimsdescription3
- 229960002074flutamideDrugs0.000claimsdescription3
- MKXKFYHWDHIYRV-UHFFFAOYSA-NflutamideChemical compoundCC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1MKXKFYHWDHIYRV-UHFFFAOYSA-N0.000claimsdescription3
- 230000002068genetic effectEffects0.000claimsdescription3
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000claimsdescription3
- 239000010931goldSubstances0.000claimsdescription3
- 229910052737goldInorganic materials0.000claimsdescription3
- 229910017053inorganic saltInorganic materials0.000claimsdescription3
- 229960003376levofloxacinDrugs0.000claimsdescription3
- 239000002502liposomeSubstances0.000claimsdescription3
- ZLNQQNXFFQJAID-UHFFFAOYSA-Lmagnesium carbonateChemical compound[Mg+2].[O-]C([O-])=OZLNQQNXFFQJAID-UHFFFAOYSA-L0.000claimsdescription3
- 239000001095magnesium carbonateSubstances0.000claimsdescription3
- 229910000021magnesium carbonateInorganic materials0.000claimsdescription3
- VTHJTEIRLNZDEV-UHFFFAOYSA-Lmagnesium dihydroxideChemical compound[OH-].[OH-].[Mg+2]VTHJTEIRLNZDEV-UHFFFAOYSA-L0.000claimsdescription3
- 239000000347magnesium hydroxideSubstances0.000claimsdescription3
- 229910001862magnesium hydroxideInorganic materials0.000claimsdescription3
- 229960000485methotrexateDrugs0.000claimsdescription3
- 229960004857mitomycinDrugs0.000claimsdescription3
- 230000000921morphogenic effectEffects0.000claimsdescription3
- 108020004707nucleic acidsProteins0.000claimsdescription3
- 102000039446nucleic acidsHuman genes0.000claimsdescription3
- 150000007523nucleic acidsChemical class0.000claimsdescription3
- 239000002773nucleotideSubstances0.000claimsdescription3
- 125000003729nucleotide groupChemical group0.000claimsdescription3
- 229960001699ofloxacinDrugs0.000claimsdescription3
- 229960001592paclitaxelDrugs0.000claimsdescription3
- 229920002223polystyrenePolymers0.000claimsdescription3
- 150000003384small moleculesChemical class0.000claimsdescription3
- 239000000126substanceSubstances0.000claimsdescription3
- 229960001603tamoxifenDrugs0.000claimsdescription3
- RCINICONZNJXQF-MZXODVADSA-NtaxolChemical compoundO([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1RCINICONZNJXQF-MZXODVADSA-N0.000claimsdescription3
- QORWJWZARLRLPR-UHFFFAOYSA-Htricalcium bis(phosphate)Chemical compound[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OQORWJWZARLRLPR-UHFFFAOYSA-H0.000claimsdescription3
- VBEQCZHXXJYVRD-GACYYNSASA-NuroantheloneChemical compoundC([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1VBEQCZHXXJYVRD-GACYYNSASA-N0.000claimsdescription3
- 229960003048vinblastineDrugs0.000claimsdescription3
- JXLYSJRDGCGARV-XQKSVPLYSA-NvincaleukoblastineChemical compoundC([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21JXLYSJRDGCGARV-XQKSVPLYSA-N0.000claimsdescription3
- OGWKCGZFUXNPDA-XQKSVPLYSA-NvincristineChemical compoundC([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12OGWKCGZFUXNPDA-XQKSVPLYSA-N0.000claimsdescription3
- 229960004528vincristineDrugs0.000claimsdescription3
- OGWKCGZFUXNPDA-UHFFFAOYSA-NvincristineNatural productsC1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12OGWKCGZFUXNPDA-UHFFFAOYSA-N0.000claimsdescription3
- 238000005406washingMethods0.000claimsdescription3
- FVLVBPDQNARYJU-XAHDHGMMSA-NC[C@H]1CCC(CC1)NC(=O)N(CCCl)N=OChemical compoundC[C@H]1CCC(CC1)NC(=O)N(CCCl)N=OFVLVBPDQNARYJU-XAHDHGMMSA-N0.000claimsdescription2
- 101800003838Epidermal growth factorProteins0.000claimsdescription2
- 102400001368Epidermal growth factorHuman genes0.000claimsdescription2
- 229940009456adriamycinDrugs0.000claimsdescription2
- 235000012000cholesterolNutrition0.000claimsdescription2
- 229940116977epidermal growth factorDrugs0.000claimsdescription2
- 238000004108freeze dryingMethods0.000claimsdescription2
- 229960003440semustineDrugs0.000claimsdescription2
- 239000002246antineoplastic agentSubstances0.000claims2
- NDLPOXTZKUMGOV-UHFFFAOYSA-Noxo(oxoferriooxy)iron hydrateChemical compoundO.O=[Fe]O[Fe]=ONDLPOXTZKUMGOV-UHFFFAOYSA-N0.000claims2
- 102100022641Coagulation factor IXHuman genes0.000claims1
- 229920000954PolyglycolidePolymers0.000claims1
- 229960004667ethyl celluloseDrugs0.000claims1
- 239000004633polyglycolic acidSubstances0.000claims1
- 229910052814silicon oxideInorganic materials0.000claims1
- 238000000034methodMethods0.000abstractdescription75
- 238000005538encapsulationMethods0.000abstractdescription56
- 239000000463materialSubstances0.000abstractdescription52
- 230000002209hydrophobic effectEffects0.000abstractdescription34
- 206010061218InflammationDiseases0.000abstractdescription21
- 230000004054inflammatory processEffects0.000abstractdescription21
- 230000021164cell adhesionEffects0.000abstractdescription19
- 150000004965peroxy acidsChemical class0.000abstractdescription17
- 238000004945emulsificationMethods0.000abstractdescription8
- 238000013270controlled releaseMethods0.000abstractdescription5
- 238000005119centrifugationMethods0.000abstractdescription3
- 239000002861polymer materialSubstances0.000abstractdescription2
- 239000000243solutionSubstances0.000description39
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description30
- 108010017080Granulocyte Colony-Stimulating FactorProteins0.000description27
- 102000004269Granulocyte Colony-Stimulating FactorHuman genes0.000description27
- 239000002253acidSubstances0.000description24
- 238000000338in vitroMethods0.000description24
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical groupCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description23
- 239000000377silicon dioxideSubstances0.000description20
- 230000000694effectsEffects0.000description18
- 238000004364calculation methodMethods0.000description17
- 201000010099diseaseDiseases0.000description17
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description17
- 230000000844anti-bacterial effectEffects0.000description16
- 235000002639sodium chlorideNutrition0.000description16
- 239000011780sodium chlorideSubstances0.000description15
- SPSXSWRZQFPVTJ-ZQQKUFEYSA-Nhepatitis b vaccineChemical compoundC([C@H](NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCSC)C(=O)N[C@@H](CC1N=CN=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)OC(=O)CNC(=O)CNC(=O)[C@H](C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](N)CCCNC(N)=N)C1=CC=CC=C1SPSXSWRZQFPVTJ-ZQQKUFEYSA-N0.000description13
- 229940124736hepatitis-B vaccineDrugs0.000description13
- 238000011866long-term treatmentMethods0.000description13
- 235000018102proteinsNutrition0.000description12
- 230000001093anti-cancerEffects0.000description10
- 238000002474experimental methodMethods0.000description10
- 238000005297material degradation processMethods0.000description10
- 238000012360testing methodMethods0.000description10
- 230000003285pharmacodynamic effectEffects0.000description9
- 230000008569processEffects0.000description9
- WCUXLLCKKVVCTQ-UHFFFAOYSA-MPotassium chlorideChemical compound[Cl-].[K+]WCUXLLCKKVVCTQ-UHFFFAOYSA-M0.000description8
- 208000007502anemiaDiseases0.000description8
- 210000001519tissueAnatomy0.000description6
- 230000000699topical effectEffects0.000description6
- ZAMOUSCENKQFHK-UHFFFAOYSA-NChlorine atomChemical compound[Cl]ZAMOUSCENKQFHK-UHFFFAOYSA-N0.000description5
- PEDCQBHIVMGVHV-UHFFFAOYSA-NGlycerineChemical compoundOCC(O)COPEDCQBHIVMGVHV-UHFFFAOYSA-N0.000description5
- 239000000460chlorineSubstances0.000description5
- 229910052801chlorineInorganic materials0.000description5
- 230000009467reductionEffects0.000description5
- XBBVURRQGJPTHH-UHFFFAOYSA-N2-hydroxyacetic acid;2-hydroxypropanoic acidChemical compoundOCC(O)=O.CC(O)C(O)=OXBBVURRQGJPTHH-UHFFFAOYSA-N0.000description4
- 208000005189EmbolismDiseases0.000description4
- DHMQDGOQFOQNFH-UHFFFAOYSA-NGlycineChemical compoundNCC(O)=ODHMQDGOQFOQNFH-UHFFFAOYSA-N0.000description4
- 238000001727in vivoMethods0.000description4
- 239000012053oil suspensionSubstances0.000description4
- 239000001103potassium chlorideSubstances0.000description4
- 235000011164potassium chlorideNutrition0.000description4
- 230000002792vascularEffects0.000description4
- 206010016654FibrosisDiseases0.000description3
- 229920003171Poly (ethylene oxide)Polymers0.000description3
- 230000002708enhancing effectEffects0.000description3
- 230000004761fibrosisEffects0.000description3
- 230000005661hydrophobic surfaceEffects0.000description3
- 108091032973(ribonucleotides)n+mProteins0.000description2
- AOJJSUZBOXZQNB-VTZDEGQISA-N4'-epidoxorubicinChemical compoundO([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1AOJJSUZBOXZQNB-VTZDEGQISA-N0.000description2
- 108091003079Bovine Serum AlbuminProteins0.000description2
- VEXZGXHMUGYJMC-UHFFFAOYSA-MChloride anionChemical compound[Cl-]VEXZGXHMUGYJMC-UHFFFAOYSA-M0.000description2
- 102000013831Coagulation factor IXHuman genes0.000description2
- 229930105110Cyclosporin ANatural products0.000description2
- FBPFZTCFMRRESA-FSIIMWSLSA-ND-GlucitolNatural productsOC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)COFBPFZTCFMRRESA-FSIIMWSLSA-N0.000description2
- HTIJFSOGRVMCQR-UHFFFAOYSA-NEpirubicinNatural productsCOc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)COHTIJFSOGRVMCQR-UHFFFAOYSA-N0.000description2
- 239000004471GlycineSubstances0.000description2
- DGAQECJNVWCQMB-PUAWFVPOSA-MIlexoside XXIXChemical compoundC[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+]DGAQECJNVWCQMB-PUAWFVPOSA-M0.000description2
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000description2
- 241001465754MetazoaSpecies0.000description2
- 239000004698PolyethyleneSubstances0.000description2
- DNIAPMSPPWPWGF-UHFFFAOYSA-NPropylene glycolChemical compoundCC(O)CODNIAPMSPPWPWGF-UHFFFAOYSA-N0.000description2
- 208000001647Renal InsufficiencyDiseases0.000description2
- BQCADISMDOOEFD-UHFFFAOYSA-NSilverChemical compound[Ag]BQCADISMDOOEFD-UHFFFAOYSA-N0.000description2
- CDBYLPFSWZWCQE-UHFFFAOYSA-LSodium CarbonateChemical compound[Na+].[Na+].[O-]C([O-])=OCDBYLPFSWZWCQE-UHFFFAOYSA-L0.000description2
- 239000003242anti bacterial agentSubstances0.000description2
- 230000000840anti-viral effectEffects0.000description2
- 229940088710antibiotic agentDrugs0.000description2
- 229940098773bovine serum albuminDrugs0.000description2
- 208000001969capillary hemangiomaDiseases0.000description2
- 230000015556catabolic processEffects0.000description2
- 239000001913celluloseSubstances0.000description2
- 229920002678cellulosePolymers0.000description2
- 238000012512characterization methodMethods0.000description2
- 238000006731degradation reactionMethods0.000description2
- WBKFWQBXFREOFH-UHFFFAOYSA-Ndichloromethane;ethyl acetateChemical compoundClCCl.CCOC(C)=OWBKFWQBXFREOFH-UHFFFAOYSA-N0.000description2
- 239000006185dispersionSubstances0.000description2
- 239000002552dosage formSubstances0.000description2
- 229960001904epirubicinDrugs0.000description2
- 235000011187glycerolNutrition0.000description2
- 208000006454hepatitisDiseases0.000description2
- 231100000283hepatitisToxicity0.000description2
- 201000006370kidney failureDiseases0.000description2
- 201000002364leukopeniaDiseases0.000description2
- 231100001022leukopeniaToxicity0.000description2
- 230000007774longtermEffects0.000description2
- 239000003094microcapsuleSubstances0.000description2
- 238000001000micrographMethods0.000description2
- 229920000573polyethylenePolymers0.000description2
- FGIUAXJPYTZDNR-UHFFFAOYSA-Npotassium nitrateChemical compound[K+].[O-][N+]([O-])=OFGIUAXJPYTZDNR-UHFFFAOYSA-N0.000description2
- 239000000843powderSubstances0.000description2
- 235000012239silicon dioxideNutrition0.000description2
- 229910052709silverInorganic materials0.000description2
- 239000004332silverSubstances0.000description2
- 239000011734sodiumSubstances0.000description2
- 229910052708sodiumInorganic materials0.000description2
- 239000000600sorbitolSubstances0.000description2
- 235000010356sorbitolNutrition0.000description2
- 238000005507sprayingMethods0.000description2
- 239000010414supernatant solutionSubstances0.000description2
- 239000013589supplementSubstances0.000description2
- 238000013268sustained releaseMethods0.000description2
- 239000012730sustained-release formSubstances0.000description2
- 238000002604ultrasonographyMethods0.000description2
- HDTRYLNUVZCQOY-UHFFFAOYSA-Nα-D-glucopyranosyl-α-D-glucopyranosideNatural productsOC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1HDTRYLNUVZCQOY-UHFFFAOYSA-N0.000description1
- 102000040650(ribonucleotides)n+mHuman genes0.000description1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamideChemical compoundCN1CC(C(N)=N)C(=O)NC1=OIXPNQXFRVYWDDI-UHFFFAOYSA-N0.000description1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N100676-05-9Natural productsOC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1OWEGMIWEEQEYGQ-UHFFFAOYSA-N0.000description1
- GUBGYTABKSRVRQ-XLOQQCSPSA-NAlpha-LactoseChemical compoundO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1OGUBGYTABKSRVRQ-XLOQQCSPSA-N0.000description1
- 239000004475ArginineSubstances0.000description1
- 229920001661ChitosanPolymers0.000description1
- 229920000858CyclodextrinPolymers0.000description1
- FBPFZTCFMRRESA-KVTDHHQDSA-ND-MannitolChemical compoundOC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COFBPFZTCFMRRESA-KVTDHHQDSA-N0.000description1
- FBPFZTCFMRRESA-JGWLITMVSA-ND-glucitolChemical compoundOC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)COFBPFZTCFMRRESA-JGWLITMVSA-N0.000description1
- 206010013883DwarfismDiseases0.000description1
- 239000004386ErythritolSubstances0.000description1
- UNXHWFMMPAWVPI-UHFFFAOYSA-NErythritolNatural productsOCC(O)C(O)COUNXHWFMMPAWVPI-UHFFFAOYSA-N0.000description1
- IMROMDMJAWUWLK-UHFFFAOYSA-NEthenolChemical compoundOC=CIMROMDMJAWUWLK-UHFFFAOYSA-N0.000description1
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000description1
- WHUUTDBJXJRKMK-UHFFFAOYSA-NGlutamic acidNatural productsOC(=O)C(N)CCC(O)=OWHUUTDBJXJRKMK-UHFFFAOYSA-N0.000description1
- XEUCQOBUZPQUMQ-UHFFFAOYSA-NGlycoloneChemical compoundCOC1=C(CC=C(C)C)C(=O)NC2=C1C=CC=C2OCXEUCQOBUZPQUMQ-UHFFFAOYSA-N0.000description1
- UWIULCYKVGIOPW-UHFFFAOYSA-NGlycoloneNatural productsCCOC1=C(CC=CC)C(=O)N(C)c2c(O)cccc12UWIULCYKVGIOPW-UHFFFAOYSA-N0.000description1
- ODKSFYDXXFIFQN-BYPYZUCNSA-PL-argininium(2+)Chemical compoundNC(=[NH2+])NCCC[C@H]([NH3+])C(O)=OODKSFYDXXFIFQN-BYPYZUCNSA-P0.000description1
- WHUUTDBJXJRKMK-VKHMYHEASA-NL-glutamic acidChemical compoundOC(=O)[C@@H](N)CCC(O)=OWHUUTDBJXJRKMK-VKHMYHEASA-N0.000description1
- HNDVDQJCIGZPNO-YFKPBYRVSA-NL-histidineChemical compoundOC(=O)[C@@H](N)CC1=CN=CN1HNDVDQJCIGZPNO-YFKPBYRVSA-N0.000description1
- KDXKERNSBIXSRK-YFKPBYRVSA-NL-lysineChemical compoundNCCCC[C@H](N)C(O)=OKDXKERNSBIXSRK-YFKPBYRVSA-N0.000description1
- GUBGYTABKSRVRQ-QKKXKWKRSA-NLactoseNatural productsOC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1OGUBGYTABKSRVRQ-QKKXKWKRSA-N0.000description1
- KDXKERNSBIXSRK-UHFFFAOYSA-NLysineNatural productsNCCCCC(N)C(O)=OKDXKERNSBIXSRK-UHFFFAOYSA-N0.000description1
- 239000004472LysineSubstances0.000description1
- GUBGYTABKSRVRQ-PICCSMPSSA-NMaltoseNatural productsO[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1OGUBGYTABKSRVRQ-PICCSMPSSA-N0.000description1
- 229930195725MannitolNatural products0.000description1
- WHNWPMSKXPGLAX-UHFFFAOYSA-NN-Vinyl-2-pyrrolidoneChemical compoundC=CN1CCCC1=OWHNWPMSKXPGLAX-UHFFFAOYSA-N0.000description1
- 229920001007Nylon 4Polymers0.000description1
- DMGNFLJBACZMRM-UHFFFAOYSA-NO[P]Chemical compoundO[P]DMGNFLJBACZMRM-UHFFFAOYSA-N0.000description1
- 235000008331Pinus X rigitaedaNutrition0.000description1
- 235000011613Pinus brutiaNutrition0.000description1
- 241000018646Pinus brutiaSpecies0.000description1
- 229920002472StarchPolymers0.000description1
- 229930006000SucroseNatural products0.000description1
- CZMRCDWAGMRECN-UGDNZRGBSA-NSucroseChemical compoundO[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1CZMRCDWAGMRECN-UGDNZRGBSA-N0.000description1
- 102000004887Transforming Growth Factor betaHuman genes0.000description1
- 108090001012Transforming Growth Factor betaProteins0.000description1
- HDTRYLNUVZCQOY-WSWWMNSNSA-NTrehaloseNatural productsO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1HDTRYLNUVZCQOY-WSWWMNSNSA-N0.000description1
- 241000700605VirusesSpecies0.000description1
- 239000002671adjuvantSubstances0.000description1
- 230000002776aggregationEffects0.000description1
- 238000004220aggregationMethods0.000description1
- 230000032683agingEffects0.000description1
- HDTRYLNUVZCQOY-LIZSDCNHSA-Nalpha,alpha-trehaloseChemical compoundO[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1HDTRYLNUVZCQOY-LIZSDCNHSA-N0.000description1
- 235000001014amino acidNutrition0.000description1
- ODKSFYDXXFIFQN-UHFFFAOYSA-NarginineNatural productsOC(=O)C(N)CCCNC(N)=NODKSFYDXXFIFQN-UHFFFAOYSA-N0.000description1
- 230000009286beneficial effectEffects0.000description1
- WQZGKKKJIJFFOK-VFUOTHLCSA-Nbeta-D-glucoseChemical compoundOC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-VFUOTHLCSA-N0.000description1
- GUBGYTABKSRVRQ-QUYVBRFLSA-Nbeta-maltoseChemical compoundOC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1OGUBGYTABKSRVRQ-QUYVBRFLSA-N0.000description1
- 210000004204blood vesselAnatomy0.000description1
- 159000000007calcium saltsChemical class0.000description1
- 210000004027cellAnatomy0.000description1
- 230000008859changeEffects0.000description1
- 150000001875compoundsChemical class0.000description1
- 150000001879copperChemical class0.000description1
- 230000007547defectEffects0.000description1
- 230000007812deficiencyEffects0.000description1
- 150000002009diolsChemical class0.000description1
- 238000007876drug discoveryMethods0.000description1
- 230000000857drug effectEffects0.000description1
- 230000010102embolizationEffects0.000description1
- 230000001804emulsifying effectEffects0.000description1
- UNXHWFMMPAWVPI-ZXZARUISSA-NerythritolChemical compoundOC[C@H](O)[C@H](O)COUNXHWFMMPAWVPI-ZXZARUISSA-N0.000description1
- 235000019414erythritolNutrition0.000description1
- 229940009714erythritolDrugs0.000description1
- IDGUHHHQCWSQLU-UHFFFAOYSA-Nethanol;hydrateChemical compoundO.CCOIDGUHHHQCWSQLU-UHFFFAOYSA-N0.000description1
- 239000008103glucoseSubstances0.000description1
- 239000004220glutamic acidSubstances0.000description1
- 235000013922glutamic acidNutrition0.000description1
- 150000004676glycansChemical class0.000description1
- 229960005150glycerolDrugs0.000description1
- 239000008187granular materialSubstances0.000description1
- 208000002672hepatitis BDiseases0.000description1
- HNDVDQJCIGZPNO-UHFFFAOYSA-NhistidineNatural productsOC(=O)C(N)CC1=CN=CN1HNDVDQJCIGZPNO-UHFFFAOYSA-N0.000description1
- 238000011835investigationMethods0.000description1
- 229910052742ironInorganic materials0.000description1
- 239000008101lactoseSubstances0.000description1
- 230000003902lesionEffects0.000description1
- 229920002521macromoleculePolymers0.000description1
- 159000000003magnesium saltsChemical class0.000description1
- 239000000594mannitolSubstances0.000description1
- 235000010355mannitolNutrition0.000description1
- 229960001855mannitolDrugs0.000description1
- 230000007721medicinal effectEffects0.000description1
- 235000013336milkNutrition0.000description1
- 239000008267milkSubstances0.000description1
- 210000004080milkAnatomy0.000description1
- 150000002751molybdenumChemical class0.000description1
- 229940031182nanoparticles iron oxideDrugs0.000description1
- NUKCGLDCWQXYOQ-UHFFFAOYSA-NpiposulfanChemical compoundCS(=O)(=O)OCCC(=O)N1CCN(C(=O)CCOS(C)(=O)=O)CC1NUKCGLDCWQXYOQ-UHFFFAOYSA-N0.000description1
- 229950001100piposulfanDrugs0.000description1
- 229920001282polysaccharidePolymers0.000description1
- 239000005017polysaccharideSubstances0.000description1
- 239000004323potassium nitrateSubstances0.000description1
- 235000010333potassium nitrateNutrition0.000description1
- 229960005205prednisoloneDrugs0.000description1
- OIGNJSKKLXVSLS-VWUMJDOOSA-NprednisoloneChemical compoundO=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1OIGNJSKKLXVSLS-VWUMJDOOSA-N0.000description1
- 230000002265preventionEffects0.000description1
- 230000001737promoting effectEffects0.000description1
- 229960004063propylene glycolDrugs0.000description1
- 239000002994raw materialSubstances0.000description1
- 230000001105regulatory effectEffects0.000description1
- 150000003839saltsChemical class0.000description1
- HFHDHCJBZVLPGP-UHFFFAOYSA-Nschardinger α-dextrinChemical compoundO1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2COHFHDHCJBZVLPGP-UHFFFAOYSA-N0.000description1
- 239000000661sodium alginateSubstances0.000description1
- 235000010413sodium alginateNutrition0.000description1
- 229940005550sodium alginateDrugs0.000description1
- 229910000029sodium carbonateInorganic materials0.000description1
- 235000017550sodium carbonateNutrition0.000description1
- 239000002904solventSubstances0.000description1
- 229960002920sorbitolDrugs0.000description1
- 239000008107starchSubstances0.000description1
- 235000019698starchNutrition0.000description1
- 239000005720sucroseSubstances0.000description1
- 235000000346sugarNutrition0.000description1
- 150000008163sugarsChemical class0.000description1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-NtgfbetaChemical compoundC([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1ZRKFYGHZFMAOKI-QMGMOQQFSA-N0.000description1
- 230000001225therapeutic effectEffects0.000description1
- 231100000331toxicToxicity0.000description1
- 230000002588toxic effectEffects0.000description1
- 231100000419toxicityToxicity0.000description1
- 230000001988toxicityEffects0.000description1
- 230000009466transformationEffects0.000description1
- 229910052721tungstenInorganic materials0.000description1
- 238000002525ultrasonicationMethods0.000description1
- 229940124931vaccine adjuvantDrugs0.000description1
- 239000012646vaccine adjuvantSubstances0.000description1
- 230000004584weight gainEffects0.000description1
- 235000019786weight gainNutrition0.000description1
- 150000003751zincChemical class0.000description1
Images
Landscapes
- Medicinal Preparation (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及药物微球制剂,具体地说,是一种纳米颗粒混悬液包油-油包纳米药物(N/O/N)微球,属于制药技术领域。The invention relates to drug microsphere preparations, in particular to nanoparticle suspension oil-in-oil nano drug (N/O/N) microspheres, belonging to the technical field of pharmacy.
背景技术Background technique
制药行业从药物发现,到临床的应用,最后一个环节是药物制剂。其中有一部分药物需要长期给药才能治愈,还有一部分需要靶向等局部给药。要达到这些目的,原料药必须要制备成相应的剂型。例如需要长期给药但在体内的半衰期短的药物,宜制备成缓释或控释剂型;对于一些肿瘤的治疗,需要一些药物靶向于病灶,例如靶向于肿瘤血管的栓塞微球制剂等。In the pharmaceutical industry, from drug discovery to clinical application, the last link is drug preparation. Some of the drugs need long-term administration to be cured, and some of them need targeted and other local administration. To achieve these goals, raw materials must be prepared into corresponding dosage forms. For example, drugs that require long-term administration but have a short half-life in the body should be prepared as sustained-release or controlled-release dosage forms; for the treatment of some tumors, some drugs need to be targeted to the lesion, such as embolization microsphere preparations that target tumor blood vessels, etc. .
关于微球制剂的制备方法,Shi等 [Double walled POE/PLGA microspheres: encapsulation of water-soluble and water-insoluble proteins and their release properties,Journal of Controlled Release,89 (2003):167–177] 利用W/O/W方法把牛血清白蛋白(BSA)和环孢霉素A(CyA)包封在POE/PLGA壳-核微球里,该方法利用最常见的W/O/W复乳法来制备双层微球,而该复乳的油水界面是公认的蛋白杀手,容易导致水溶性的蛋白在该界面聚集,同样致使包封率不高,存在不完全释放和突释等缺陷。Morita等 [Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly(ethylene glycol) as a protein micronization adjuvant,Journal of Controlled Release,69 (2000):435-444] 用聚乙二醇(PEG)作为蛋白微粉化的赋形剂,即表面活性剂,然后用水包油-油包固体的方法把蛋白微囊包在生物可降解的微球里,该文献虽然利用新的S/O/W 乳化法制备载蛋白微球,但只是改变了表面活性剂,将以前报道较多的聚乙烯醇(PVA)改为聚乙二醇(PEG),这种改变仍不能克服包封率低,疏水性表面易引起局部微囊化及炎症的缺点。Regarding the preparation method of microsphere preparations, Shi et al. [Double walled POE/PLGA microspheres: encapsulation of water-soluble and water-insoluble proteins and their release properties, Journal of Controlled Release, 89 (2003): 167–177] using W/ The O/W method encapsulates bovine serum albumin (BSA) and cyclosporine A (CyA) in POE/PLGA shell-core microspheres, which is prepared by the most common W/O/W double emulsion method Double-layer microspheres, and the oil-water interface of the double emulsion is a recognized protein killer, which easily leads to the aggregation of water-soluble proteins at the interface, which also leads to low encapsulation efficiency, and defects such as incomplete release and burst release. [Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly(ethylene glycol) as a protein micronization adjuvant, Journal of Controlled Release, 69 (2000): 435-444] by Morita et al. PEG) is used as the excipient of protein micronization, that is, surfactant, and then protein microcapsules are encapsulated in biodegradable microspheres by the method of oil-in-water-solid-in-oil. Although this document uses the new S/O/ The W emulsification method prepared protein-loaded microspheres, but only changed the surfactant, and changed the previously reported polyvinyl alcohol (PVA) to polyethylene glycol (PEG). This change still cannot overcome the low encapsulation efficiency. The hydrophobic surface is prone to the disadvantages of local microencapsulation and inflammation.
用常规的S/O、S/O/W和S/O/O方法制备的微球,由于表面疏水,容易导致体内组织微囊化及炎症等副作用,且药物突释易造成药物本身的毒副作用。关于纳米颗粒混悬液包油-油包纳米药物(N/O/N)微球,目前还未见报道。Microspheres prepared by conventional S/O, S/O/W and S/O/O methods are likely to cause side effects such as microencapsulation of tissues in the body and inflammation due to the hydrophobic surface, and the sudden release of the drug is likely to cause the toxicity of the drug itself. side effect. There is no report about nanoparticle suspension oil-in-oil nanomedicine (N/O/N) microspheres.
发明内容Contents of the invention
本发明的目的是针对现有技术中的不足,提供一种纳米颗粒混悬液包油-油包纳米药物(N/O/N)微球,以解决现有技术中微球制剂包封率低,不完全释放和突释,疏水性表面会引起局部微囊化及炎症的缺点。The purpose of the present invention is to address the deficiencies in the prior art, to provide a nanoparticle suspension oil-in-oil nano-drug (N/O/N) microspheres to solve the encapsulation efficiency of microsphere preparations in the prior art Low, incomplete release and burst release, hydrophobic surface can cause disadvantages of local microencapsulation and inflammation.
为实现上述目的,本发明采取的技术方案是:For realizing above-mentioned object, the technical scheme that the present invention takes is:
一种纳米药物微球,所述微球的表面自组装有一层纳米颗粒,微球中药物的重量百分比为0.01%-40%,纳米颗粒的重量百分比为0.01%-96%,聚合物的重量百分比为3.65%-99.98%,药用辅料的重量百分比为0%-30%,微球的粒径为1-500 μm。A nanomedicine microsphere, the surface of the microsphere is self-assembled with a layer of nanoparticles, the weight percentage of the drug in the microsphere is 0.01%-40%, the weight percentage of the nanoparticle is 0.01%-96%, and the weight percentage of the polymer is The percentage is 3.65%-99.98%, the weight percentage of pharmaceutical excipients is 0%-30%, and the particle size of the microspheres is 1-500 μm.
所述的药物包括小分子药物和大分子药物,所述的小分子药物为化学药物,可选自肿瘤化疗类药物、治疗血管瘤的药物或抗生素类药物中的一种,所述的大分子药物为生物大分子药物,可选自蛋白大分子药物、疫苗、抗体、核酸或脂质体药物中的一种或几种,所述的药用辅料为注射用药用辅料,所述微球的粒径为10-100 μm。The drugs include small molecule drugs and macromolecular drugs, and the small molecule drugs are chemical drugs, which can be selected from tumor chemotherapy drugs, drugs for treating hemangioma or antibiotic drugs, and the macromolecule drugs The drug is a biological macromolecular drug, which can be selected from one or more of protein macromolecular drugs, vaccines, antibodies, nucleic acid or liposome drugs. The pharmaceutical excipients are injection drug excipients, and the microspheres The particle size is 10-100 μm.
所述的肿瘤化疗类药物选自阿霉素、环磷酰胺、更生霉素、博莱霉素、柔红霉素、表阿霉素、丝裂霉素、甲氨蝶呤、氟尿嘧啶、卡铂、卡莫司汀、司莫司汀、顺铂、依托泊苷、喜树碱及其衍生物、苯芥胆甾醇、紫杉醇及其衍生物、多西紫杉醇及其衍生物、长春碱、长春新碱、它莫西芬、哌泊舒凡、环磷酰胺或氟他胺及其衍生物中的一种,所述的治疗血管瘤的药物选自泼尼松、普萘洛尔或雷帕霉素中的一种,所述的抗生素类药物选自环孢素、左氧氟沙星、氧氟沙星或盐酸依匹斯汀中的一种。The tumor chemotherapy drugs are selected from doxorubicin, cyclophosphamide, dactinomycin, bleomycin, daunorubicin, epirubicin, mitomycin, methotrexate, fluorouracil, carboplatin , Carmustine, Semustine, Cisplatin, Etoposide, Camptothecin and Its Derivatives, Cholesterol, Paclitaxel and Its Derivatives, Docetaxel and Its Derivatives, Vinblastine, Vincristine Alkaline, tamoxifen, piposulfan, cyclophosphamide or flutamide and its derivatives, the drug for treating hemangioma is selected from prednisone, propranolol or rapamycin A kind of in antibiotics, described antibiotic drug is selected from the one in cyclosporine, levofloxacin, ofloxacin or epinastine hydrochloride.
所述的生物大分子药物选自促红细胞生成素、重组人粒细胞集落刺激因子、粒细胞-巨噬细胞集落刺激因子、疫苗、干扰素、生长激素、胰岛素、表皮生长因子、成纤维细胞生长因子、转化生长因子、胰岛素生长因子、血管内皮细胞生长因子、血小板生长因子、内皮生长因子、神经生长因子、骨衍生性生长因子、骨形成蛋白、组织多肽抗原、抗体、凝血因子VIII、凝血因子IX、遗传因子、反义核苷酸、小分子RNA或基因中的一种或几种。The biomacromolecular drug is selected from the group consisting of erythropoietin, recombinant human granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, vaccine, interferon, growth hormone, insulin, epidermal growth factor, fibroblast growth factor factor, transforming growth factor, insulin growth factor, vascular endothelial growth factor, platelet growth factor, endothelial growth factor, nerve growth factor, bone-derived growth factor, bone morphogenic protein, tissue polypeptide antigen, antibody, coagulation factor VIII, coagulation factor IX. One or more of genetic factors, antisense nucleotides, small molecule RNAs or genes.
所述的纳米颗粒为有机纳米颗粒或无机纳米颗粒,可选自聚苯乙烯纳米颗粒、交联葡聚糖纳米颗粒、二氧化硅纳米颗粒、二氧化钛纳米颗粒、羟基磷灰石纳米颗粒、四氧化三铁纳米颗粒、三氧化二铁颗粒、金纳米颗粒、三氧化二铝纳米颗粒、碳酸钙纳米颗粒、磷酸钙纳米颗粒、碳酸镁纳米颗粒、氢氧化镁纳米颗粒或银纳米颗粒中的一种或几种。The nanoparticles are organic nanoparticles or inorganic nanoparticles, which can be selected from polystyrene nanoparticles, cross-linked dextran nanoparticles, silica nanoparticles, titanium dioxide nanoparticles, hydroxyapatite nanoparticles, One of iron nanoparticles, iron oxide particles, gold nanoparticles, aluminum oxide nanoparticles, calcium carbonate nanoparticles, calcium phosphate nanoparticles, magnesium carbonate nanoparticles, magnesium hydroxide nanoparticles or silver nanoparticles or several.
所述的聚合物选自聚己内酯、聚乳酸、聚乳酸-羟基乙酸、聚乳酸-聚乙二醇、聚羟基乙酸-聚乳酸-聚乙二醇或聚己内酯-聚乙二醇中的一种或几种。The polymer is selected from polycaprolactone, polylactic acid, polylactic acid-glycolic acid, polylactic acid-polyethylene glycol, polyglycolic acid-polylactic acid-polyethylene glycol or polycaprolactone-polyethylene glycol one or more of them.
所述的微球采用以下步骤制备而成:The microspheres are prepared by the following steps:
(1)将药物和药用辅料制备成纳米药物,所述药物在纳米药物中的重量百分比为0.1%-90%,药用辅料在纳米药物中的重量百分比为0%-20 %;(1) Prepare the drug and pharmaceutical excipients into nano-medicines, the weight percentage of the drug in the nano-medicine is 0.1%-90%, and the weight percentage of the pharmaceutical excipients in the nano-medicine is 0%-20%;
(2)将步骤(1)制备的纳米药物按照1:1-1:10的重量比分散在重量百分比浓度为0.5%-80%聚合物的有机溶剂混合溶液中,形成均匀的混悬液,即油包纳米药物混悬液;(2) Dispersing the nanomedicine prepared in step (1) in a weight ratio of 1:1-1:10 in an organic solvent mixed solution with a weight percent concentration of 0.5%-80% polymer to form a uniform suspension, That is, nano-drug suspension in oil;
(3)将步骤(2)形成的油包纳米药物混悬液加入到含重量百分比为1%-80%纳米颗粒的水混悬液或含重量百分比为1%-80%纳米颗粒和重量百分比为0.5%-5%表面活性剂的水混悬液中,进行乳化,形成纳米颗粒混悬液包油-油包纳米药物复乳;(3) Adding the nano-drug suspension in oil formed in step (2) to an aqueous suspension containing 1%-80% by weight nanoparticles or 1%-80% by weight nanoparticles and a weight percent It is emulsified in an aqueous suspension of 0.5%-5% surfactant to form nanoparticle suspension oil-in-oil nano-drug re-emulsion;
(4)将所述纳米颗粒混悬液包油-油包纳米药物复乳转移到含重量百分比为1%-10%无机盐的水溶液中固化1-4小时;(4) Transferring the oil-in-oil-in-oil nano-drug double emulsion of the nanoparticle suspension to an aqueous solution containing 1%-10% by weight of inorganic salt and solidifying for 1-4 hours;
(5)将步骤(4)所得样品进行离心,收集微球,并洗涤所得微球,之后冻干,得到表面自组装有纳米颗粒且内部含有纳米药物的微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash the obtained microspheres, and then freeze-dry to obtain microspheres with nanoparticle self-assembled on the surface and nanomedicine inside.
步骤(1)中所述的纳米药物的制备包括以下步骤:The preparation of the nanomedicine described in step (1) includes the following steps:
将药物和药用辅料溶解在水中,然后加入多孔纳米颗粒,搅拌使得药物和药用辅料充分吸附在多孔纳米颗粒里,离心去除上清液,再充分洗涤,然后冻干形成纳米药物;或Dissolving the drug and pharmaceutical excipients in water, then adding porous nanoparticles, stirring to make the drug and pharmaceutical excipients fully adsorbed in the porous nanoparticles, centrifuging to remove the supernatant, then fully washing, and then freeze-drying to form nano-medicines; or
将药物和药用辅料溶解在水中形成药物水溶液,然后将药物水溶液转移到聚乙二醇水溶液中,充分混匀后于冰箱中预冻,之后冻干,再用二氯甲烷溶解聚乙二醇并离心除去聚乙二醇得到纳米药物。Dissolve the drug and pharmaceutical excipients in water to form an aqueous solution of the drug, then transfer the aqueous solution of the drug to an aqueous solution of polyethylene glycol, mix well, pre-freeze in the refrigerator, and then freeze-dry, then dissolve the polyethylene glycol with dichloromethane And centrifuged to remove polyethylene glycol to obtain nano-medicine.
步骤(2)中所述的有机溶剂混合溶液中还添加有重量百分比为0.1%-20%的聚乙二醇或泊洛沙姆,所述的聚合物重量百分比浓度为5%-30%,所述的有机溶剂选自二氯甲烷、乙酸乙酯、乙腈、庚烷、氯仿或丙酮中的一种或几种。0.1%-20% by weight of polyethylene glycol or poloxamer is also added to the organic solvent mixed solution described in step (2), and the weight percentage concentration of the polymer is 5%-30%, The organic solvent is selected from one or more of dichloromethane, ethyl acetate, acetonitrile, heptane, chloroform or acetone.
步骤(3)中所述的纳米颗粒重量百分比浓度为20%-70%,所述的表面活性剂选自聚乙烯醇、聚乙二醇、聚乙烯吡咯烷酮、泊洛沙姆、聚山梨醇、乙基纤维素或吐温中的一种或几种。The weight percentage concentration of nanoparticles described in step (3) is 20%-70%, and the surfactant is selected from polyvinyl alcohol, polyethylene glycol, polyvinylpyrrolidone, poloxamer, polysorbate, One or more of ethyl cellulose or Tween.
本发明的有益效果在于:The beneficial effects of the present invention are:
1、本发明选择了合适的聚合物材料和制备微球的方法,制备的微球包封率高达80%以上,并且这种表面自组装有一层纳米颗粒的微球具有增强细胞黏附的作用,以及减少局部过酸和疏水材料引起的炎症及微囊化的作用。1. The present invention selects a suitable polymer material and a method for preparing microspheres, and the encapsulation rate of the prepared microspheres is as high as 80%, and the microspheres with a layer of nanoparticles self-assembled on the surface have the effect of enhancing cell adhesion, As well as reducing inflammation and microencapsulation caused by topical peracids and hydrophobic materials.
2、采用本发明方法制备的微球,其粒径大小可以根据不同需要从1 μm到500 μm进行调控,且制备过程不污染环境。2. The particle size of the microspheres prepared by the method of the present invention can be regulated from 1 μm to 500 μm according to different needs, and the preparation process does not pollute the environment.
3、本发明方法制备的微球,大大降低了药物突释,药物几乎完全释放,可以达到零级释放,释放的纳米药物可以局部高效被病变细胞摄取,从而减少药物本身的毒副作用,同时可以使药物在整个制备过程和治疗过程中保持高活性即不失活。3. The microspheres prepared by the method of the present invention greatly reduce the drug burst release, the drug is almost completely released, and can achieve zero-order release. The released nano-medicine can be locally and efficiently taken up by diseased cells, thereby reducing the toxic and side effects of the drug itself, and at the same time can Keep the drug highly active, that is, not inactivated, during the entire preparation process and treatment process.
4、采用本发明方法制成的微球制剂,其微粒表面光滑圆整,颗粒规整无粘连,其冻干粉剂为白色细腻、疏松的粉体,不会塌陷、不粘连,再分散性良好,可以运用到各种药物缓释或控释微球的制备及疫苗的佐剂制备中。4. The microsphere preparation made by the method of the present invention has a smooth and round surface, regular particles without adhesion, and its freeze-dried powder is a white fine and loose powder, which does not collapse or adhere, and has good redispersibility. It can be applied to the preparation of slow-release or controlled-release microspheres of various drugs and the preparation of vaccine adjuvants.
附图说明Description of drawings
附图1是本发明实施例1制备所得微球的扫描电镜(SEM)照片。Figure 1 is a scanning electron microscope (SEM) photo of the microspheres prepared in Example 1 of the present invention.
附图2是本发明实施例1制备所得微球的体外释放曲线。Accompanying
附图3是本发明实施例1制备所得微球的抗菌作用效果曲线。Accompanying
附图4是本发明实施例1制备所得微球的抗癌作用效果曲线。Accompanying
附图5是本发明实施例1制备所得微球与用S/O/W方法制备微球的体内组织相容性扫描电镜(SEM)照片。Figure 5 is a scanning electron microscope (SEM) photograph of the in vivo histocompatibility of the microspheres prepared in Example 1 of the present invention and the microspheres prepared by the S/O/W method.
附图6是本发明实施例3制备所得微球的扫描电镜(SEM)照片。Figure 6 is a scanning electron microscope (SEM) photo of the microspheres prepared in Example 3 of the present invention.
附图7是本发明实施例3制备所得微球的体外释放曲线。Accompanying drawing 7 is the in vitro release curve of the microspheres prepared in Example 3 of the present invention.
附图8是本发明实施例3制备所得微球的药效作用曲线。Accompanying
附图9是本发明实施例3制备所得微球与用S/O/W方法制备微球的体内组织相容性情况。Figure 9 shows the in vivo histocompatibility of the microspheres prepared in Example 3 of the present invention and the microspheres prepared by the S/O/W method.
附图10是本发明实施例5制备所得微球的体外释放曲线。Accompanying
附图11是本发明实施例5制备所得微球的药效作用曲线。Accompanying drawing 11 is the pharmacodynamic action curve of the microspheres prepared in Example 5 of the present invention.
附图12是本发明实施例7制备所得微球的药效作用曲线。Accompanying
附图13是本发明实施例9制备所得微球的药效作用曲线。Accompanying drawing 13 is the pharmacodynamic action curve of the microspheres prepared in Example 9 of the present invention.
附图14是本发明实施例13制备所得微球的药效作用曲线。Accompanying drawing 14 is the pharmacodynamic action curve of the microspheres prepared in Example 13 of the present invention.
附图15是本发明实施例15制备所得微球的药效作用曲线。Accompanying
具体实施方式Detailed ways
下面结合具体实施例并参照附图对本发明作详细说明。The present invention will be described in detail below in conjunction with specific embodiments and with reference to the accompanying drawings.
应该理解,这些实施例仅用于说明本发明,而不用于限定本发明的保护范围。在实际应用中本领域技术人员根据本发明做出的改进和调整,仍属于本发明的保护范围。It should be understood that these examples are only used to illustrate the present invention, not to limit the protection scope of the present invention. Improvements and adjustments made by those skilled in the art according to the present invention in practical applications still belong to the protection scope of the present invention.
一种纳米颗粒混悬液包油-油包纳米药物制备的微球,所述微球的粒径为1-500 μm,较佳地为10-100 μm,其表面组装有一层纳米颗粒;其中所述微球中,药物的重量百分比为0.01%-40%,纳米颗粒的重量百分比为0.01%-96%,聚合物的重量百分比为3.65%-99.98%,药用辅料的重量百分比为0%-30%。A microsphere prepared by oil-in-nanoparticle suspension-oil-in-nanomedicine, the particle size of the microsphere is 1-500 μm, preferably 10-100 μm, and a layer of nanoparticles is assembled on its surface; wherein In the microspheres, the weight percentage of the drug is 0.01%-40%, the weight percentage of the nanoparticles is 0.01%-96%, the weight percentage of the polymer is 3.65%-99.98%, and the weight percentage of the pharmaceutical excipient is 0% -30%.
需要说明的是,本发明技术方案中,所述的小分子药物为化学药物,可以是肿瘤化疗类药物、治疗血管瘤的药物或抗生素类药物中的一种,其中肿瘤化疗类药物选自阿霉素、环磷酰胺、更生霉素、博莱霉素、柔红霉素、表阿霉素、丝裂霉素、甲氨蝶呤、氟尿嘧啶、卡铂、卡莫司汀(BCNU)、司莫司汀、顺铂、依托泊苷、喜树碱及其衍生物、苯芥胆甾醇、紫杉醇及其衍生物、多西紫杉醇及其衍生物、长春碱、长春新碱、它莫西芬、哌泊舒凡、环磷酰胺或氟他胺及其衍生物等中的一种;治疗血管瘤的药物选自泼尼松、普萘洛尔或雷帕霉素等中的一种;抗生素类药物选自环孢素、左氧氟沙星、氧氟沙星或盐酸依匹斯汀等中的一种。It should be noted that, in the technical solution of the present invention, the small molecule drug is a chemical drug, which may be one of tumor chemotherapy drugs, hemangioma treatment drugs or antibiotic drugs, wherein the tumor chemotherapy drugs are selected from Cyclophosphamide, dactinomycin, bleomycin, daunorubicin, epirubicin, mitomycin, methotrexate, fluorouracil, carboplatin, carmustine (BCNU), Mustin, cisplatin, etoposide, camptothecin and its derivatives, phenesinate, paclitaxel and its derivatives, docetaxel and its derivatives, vinblastine, vincristine, tamoxifen, One of pibosufan, cyclophosphamide or flutamide and its derivatives; the drug for treating hemangioma is selected from one of prednisone, propranolol or rapamycin; antibiotics The drug is selected from one of cyclosporine, levofloxacin, ofloxacin or epinastine hydrochloride and the like.
所述的大分子药物为生物大分子药物,选自蛋白大分子药物、疫苗、抗体、核酸或脂质体药物中的一种或几种,具体指选自促红细胞生成素(EPO)、重组人粒细胞集落刺激因子(G-CSF)、粒细胞-巨噬细胞集落刺激因子(GM-CSF)、疫苗、干扰素(IFN)、生长激素(GH)、胰岛素(Insulin)、表皮生长因子(EGF)、成纤维细胞生长因子(FGF)、转化生长因子(TGF-β)、胰岛素生长因子(IGF)、血管内皮细胞生长因子(VEGF)、血小板生长因子(PDGF)、内皮生长因子(EGF)、神经生长因子(NGF)、骨衍生性生长因子(BDGF)、骨形成蛋白(BMP)、组织多肽抗原(TPA)、抗体(Antibody)、凝血因子VIII、凝血因子IX、遗传因子、反义核苷酸(anti-RNA)、小分子RNA或基因(DNA)等中的一种或几种。The macromolecular drug is a biomacromolecular drug, selected from one or more of protein macromolecular drugs, vaccines, antibodies, nucleic acid or liposome drugs, specifically selected from erythropoietin (EPO), recombinant Human granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), vaccine, interferon (IFN), growth hormone (GH), insulin (Insulin), epidermal growth factor ( EGF), Fibroblast Growth Factor (FGF), Transforming Growth Factor (TGF-β), Insulin Growth Factor (IGF), Vascular Endothelial Growth Factor (VEGF), Platelet Growth Factor (PDGF), Endothelial Growth Factor (EGF) , nerve growth factor (NGF), bone-derived growth factor (BDGF), bone morphogenic protein (BMP), tissue polypeptide antigen (TPA), antibody (Antibody), coagulation factor VIII, coagulation factor IX, genetic factors, antisense nuclear One or more of nucleotide (anti-RNA), small molecule RNA or gene (DNA).
所述的药用辅料为注射用药用辅料,可以是小糖类(如蔗糖、海藻糖、葡萄糖、麦芽糖或乳糖等)、多羟基类化合物(如甘露醇、山梨醇、甘油、1,2-丙二醇、赤鲜糖醇、聚乙二醇、聚乙烯醇、聚环氧乙烷或聚吡咯烷酮等)、多糖类化合物(如葡聚糖、海藻酸钠、壳聚糖、淀粉、纤维素或环糊精等)、氨基酸化合物(如甘氨酸、赖氨酸、精氨酸、谷氨酸或组氨酸等)或无机盐类物质(如锌盐、钙盐、铜盐、镁盐或钼盐等)中的一种或任意组合。The pharmaceutical excipients are excipients for injection, which can be small sugars (such as sucrose, trehalose, glucose, maltose or lactose, etc.), polyhydroxy compounds (such as mannitol, sorbitol, glycerin, 1,2- Propylene glycol, erythritol, polyethylene glycol, polyvinyl alcohol, polyethylene oxide or polypyrrolidone, etc.), polysaccharides (such as dextran, sodium alginate, chitosan, starch, cellulose or Cyclodextrin, etc.), amino acid compounds (such as glycine, lysine, arginine, glutamic acid or histidine, etc.) or inorganic salts (such as zinc salts, calcium salts, copper salts, magnesium salts or molybdenum salts etc.), or any combination of them.
所述的聚合物选自聚己内酯(PCL)、聚乳酸(PLA)、聚乳酸-羟基乙酸(PLGA)、聚乳酸-聚乙二醇(PLA-PEG)、聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)或聚己内酯-聚乙二醇(PCL-PEG)中的一种或几种。The polymer is selected from polycaprolactone (PCL), polylactic acid (PLA), polylactic acid-glycolic acid (PLGA), polylactic acid-polyethylene glycol (PLA-PEG), polyglycolic acid-polylactic acid- One or more of polyethylene glycol (PLGA-PEG) or polycaprolactone-polyethylene glycol (PCL-PEG).
制备微球的步骤(2)中,所述的聚合物有机溶剂混合溶液中还添加有重量百分比为0.1%-20%的聚乙二醇(PEG)或泊洛沙姆(Poloxamer)。In the step (2) of preparing the microspheres, polyethylene glycol (PEG) or poloxamer (Poloxamer) is added in a weight percentage of 0.1%-20% to the polymer-organic solvent mixed solution.
所述的有机溶剂选自二氯甲烷、乙酸乙酯、乙腈、庚烷、氯仿或丙酮中的一种或几种,其中以二氯甲烷、乙酸乙酯或乙腈中的一种或几种有机溶剂为佳。Described organic solvent is selected from one or more in methylene chloride, ethyl acetate, acetonitrile, heptane, chloroform or acetone, wherein with one or more organic solvents in methylene chloride, ethyl acetate or acetonitrile Solvents are preferred.
所述的纳米颗粒为有机纳米颗粒或无机纳米颗粒,具体指选自聚苯乙烯纳米颗粒、交联葡聚糖纳米颗粒、二氧化硅纳米颗粒、二氧化钛纳米颗粒、羟基磷灰石纳米颗粒、四氧化三铁纳米颗粒、三氧化二铁颗粒、金纳米颗粒、三氧化二铝纳米颗粒、碳酸钙纳米颗粒、磷酸钙纳米颗粒、碳酸镁纳米颗粒、氢氧化镁纳米颗粒或银纳米颗粒等中的一种或多种。The nanoparticles are organic nanoparticles or inorganic nanoparticles, specifically selected from polystyrene nanoparticles, cross-linked dextran nanoparticles, silicon dioxide nanoparticles, titanium dioxide nanoparticles, hydroxyapatite nanoparticles, four Iron oxide nanoparticles, iron oxide particles, gold nanoparticles, aluminum oxide nanoparticles, calcium carbonate nanoparticles, calcium phosphate nanoparticles, magnesium carbonate nanoparticles, magnesium hydroxide nanoparticles or silver nanoparticles, etc. one or more.
制备微球的步骤(3)中,所述的表面活性剂选自聚乙烯醇(PVA)、聚乙二醇(PEG)、聚乙烯吡咯烷酮(PVP)、泊洛沙姆(Poloxamer)、聚山梨醇、乙基纤维素(EC)或吐温中的一种或几种。In the step (3) of preparing microspheres, the surfactant is selected from polyvinyl alcohol (PVA), polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), Poloxamer, polysorbate One or more of alcohol, ethyl cellulose (EC) or Tween.
制备微球的步骤(2)中,所述的分散方式可选择乳化、涡旋或超声等,分散时间优选为1-5分钟。In the step (2) of preparing microspheres, the dispersion method can be selected from emulsification, vortex or ultrasound, etc., and the dispersion time is preferably 1-5 minutes.
制备微球的步骤(3)中,所述的加入方式可选择滴加、一次性加入、喷雾方式加入或倒入等;所述的乳化方式可选择乳化、涡旋或超声等,乳化时间为0.1-5分钟。In the step (3) of preparing microspheres, the adding method can be selected dropwise, one-time adding, spraying or pouring, etc.; the emulsifying method can be selected from emulsification, vortex or ultrasonic, etc., and the emulsification time is 0.1-5 minutes.
制备微球的步骤(4)中,所述的无机盐可选自氯化钠、氯化钾、硝酸钾或碳酸钠等;所述的转移方式可为滴加、一次性加入、喷雾方式加入或倒入等。In the step (4) of preparing microspheres, the inorganic salt can be selected from sodium chloride, potassium chloride, potassium nitrate or sodium carbonate, etc.; the transfer method can be dropwise, one-time addition, or spraying or pour etc.
制备微球的步骤(5)中,洗涤时可采用水、乙醇或乙醇-水混合液洗涤3-5次。In the step (5) of preparing the microspheres, water, ethanol or ethanol-water mixture may be used for washing for 3-5 times.
实施例1 载有小分子药物阿霉素微球的制备(一)Example 1 Preparation of microspheres loaded with small molecule drug doxorubicin (1)
阿霉素具有抗菌作用和抗癌作用,载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:Doxorubicin has antibacterial and anticancer effects. The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nano-medicine includes the following steps:
(1)取20 mg阿霉素溶解到0.5 ml的水中,然后和多孔二氧化硅纳米颗粒20 mg搅拌24小时,使得阿霉素充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成阿霉素纳米药物;(1) Dissolve 20 mg of doxorubicin in 0.5 ml of water, then stir with 20 mg of porous silica nanoparticles for 24 hours, so that doxorubicin is fully adsorbed in the porous silica nanoparticles, and centrifuge to remove the supernatant solution, fully washed 3 times, and then freeze-dried to form adriamycin nano-medicine;
(2)把上述阿霉素纳米药物和浓度为20%(w/w)的PLGA的二氯甲烷溶液按照重量比1:9混合并超声5分钟形成均匀的混悬液,即油包阿霉素纳米药物(N/O)混悬液;(2) Mix the above-mentioned doxorubicin nanomedicine with 20% (w/w) PLGA in dichloromethane solution at a weight ratio of 1:9 and sonicate for 5 minutes to form a uniform suspension, that is, doxorubicin in oil Suspension of element nano drug (N/O);
(3)把步骤(2)所得油包阿霉素纳米药物(N/O)混悬液滴加到50 ml浓度为10%(w/w)的银纳米颗粒水混悬液中,搅拌5分钟形成纳米颗粒混悬液包油-油包阿霉素纳米药物(N/O/N)复乳;(3) Add the doxorubicin in oil nano drug (N/O) suspension obtained in step (2) dropwise to 50 ml of silver nanoparticle aqueous suspension with a concentration of 10% (w/w), and stir for 5 Form nanoparticle suspension oil-in-oil-in-oil doxorubicin nano-drug (N/O/N) double emulsion in minutes;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包阿霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the nanoparticle suspension oil-in-oil-in-oil doxorubicin nanodrug (N/O/N) double emulsion obtained in step (3) dropwise to 1000 ml of chlorine with a concentration of 5% (w/w). Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nano-medicine.
本实施例所制得的微球中,药物的重量百分比为0.35%,纳米颗粒的重量百分比为96%,聚合物的重量百分比为3.65%,药用辅料的重量百分比为0%。In the microspheres prepared in this example, the weight percentage of the drug is 0.35%, the weight percentage of the nanoparticles is 96%, the weight percentage of the polymer is 3.65%, and the weight percentage of the pharmaceutical excipient is 0%.
对本实施例制备的载有阿霉素的聚乳酸-羟基乙酸(PLGA)微球进行形貌表征、释放曲线测试、抗菌测试、抗癌测试及在体内组织相容性测试,并将其抗菌性、抗癌性及组织相容性与用S/O/W方法(详见:李志平,李云富,张振亚,刘燕,曲燕燕,梅兴国,干扰素A-2b缓释微球的制备及影响因素考察,军事医学科学院院刊,2007,31(5):451-455)制备的微球进行对比,其中抗癌作用的测试条件为:一次给药,总剂量与对照组水溶液组每天一次的共15天的总剂量相同;组织相容性测试中,以微球注射部位出现纤维化的时间为标准计算时间。The polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin prepared in this example were subjected to morphology characterization, release curve test, antibacterial test, anticancer test and in vivo histocompatibility test, and their antibacterial properties , anticancer and histocompatibility and S/O/W method (see: Li Zhiping, Li Yunfu, Zhang Zhenya, Liu Yan, Qu Yanyan, Mei Xingguo, Preparation and influence of interferon A-2b sustained-release microspheres Factor investigation, Journal of the Academy of Military Medical Sciences, 2007, 31(5): 451-455) prepared microspheres were compared, wherein the test conditions for anticancer effect were: once-administered, the total dose was the same as that of the control group aqueous solution group once a day The total dose is the same for a total of 15 days; in the histocompatibility test, the time of fibrosis at the microsphere injection site is used as the standard calculation time.
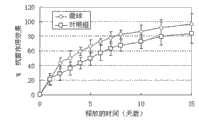
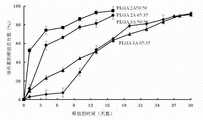
图1是本实施例中载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的扫描电镜(SEM)照片,其中,A为微球的扫描电镜图,B为微球的表面放大图,从图中可以看出,所制备的微球形态好,其表面自组装有一层银纳米颗粒,粒径在1-50 μm。图2是本实施例中载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的体外释放曲线,从图中可以看出,所制备的微球几乎达到100%的药物释放率,突释非常小,几乎完全释放,基本可以达到零级释放,其体外释放性能符合要求。微球中阿霉素相对于其原始投加量的包封率为92.0%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率)。图3是本实施例中载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的抗菌作用曲线,所制备微球的抗菌效果从图中看,其抗菌作用比对照组的好。图4是本实施例中载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的抗癌作用曲线,所制备微球的抗癌作用效果比对照组的好,本实施例制备的微球约为100%,而对照组仅为80%。图5是本实施例中载有阿霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的相容性扫描电镜(SEM)照片,可看出用S/O/W方法制备的微球(图5A)在治疗后的3-6个月出现纤维化组织;而本实施例用N/O/N方法制备的微球(图5B)在治疗一年后也没有纤维组织的出现,即注射部位的微囊化不出现,从而克服了微囊化的产生。Figure 1 is a scanning electron microscope (SEM) photo of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nanomedicine in this example, where A is the scanning electron microscope image of the microsphere, and B is the surface of the microsphere Enlarged picture, it can be seen from the picture that the prepared microspheres have a good shape, and a layer of silver nanoparticles is self-assembled on the surface, with a particle size of 1-50 μm. Figure 2 is the in vitro release curve of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nano-medicine in this example. It can be seen from the figure that the prepared microspheres almost reach 100% drug release rate , the burst release is very small, almost completely released, and can basically reach zero-order release, and its in vitro release performance meets the requirements. The encapsulation rate of doxorubicin in the microspheres relative to its original dosage is 92.0% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation rate of the drug). Figure 3 is the antibacterial effect curve of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nano-medicine in this example, the antibacterial effect of the prepared microspheres can be seen from the figure, and its antibacterial effect is better than that of the control group . Figure 4 is the anti-cancer effect curve of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nano-medicine in this example. The anti-cancer effect of the prepared microspheres is better than that of the control group. The microspheres are about 100%, while the control group is only 80%. Figure 5 is a compatibility scanning electron microscope (SEM) photo of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nanomedicine in this example, it can be seen that the microspheres prepared by the S/O/W method (Figure 5A) Fibrous tissue appeared 3-6 months after treatment; while the microspheres prepared by the N/O/N method in this example (Figure 5B) did not appear fibrous tissue after one year of treatment, that is Microencapsulation at the injection site does not occur, thereby overcoming the occurrence of microencapsulation.
以本实施例方法制备的小分子药物微球可以用于需要长期治疗的疾病,尤其是需要局部治疗的疾病如肿瘤的血管栓塞等。这种方法制备的微球包封率高,可以达到80%以上,且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The small molecule drug microspheres prepared by the method of this example can be used for diseases requiring long-term treatment, especially for diseases requiring local treatment such as tumor vascular embolism. The encapsulation rate of the microspheres prepared by this method is high, which can reach more than 80%, and the microspheres with nanoparticles on the surface, because the histocompatibility of the surface hydrophilic material is better than that of the hydrophobic material, it has the ability to enhance cell Adhesion, reduction of inflammation caused by topical peracids and hydrophobic materials, and microencapsulation.
实施例2 载有小分子药物阿霉素微球的制备(二)Example 2 Preparation of microspheres loaded with small molecule drug doxorubicin (2)
载有阿霉素纳米药物的聚己内酯(PCL)微球的制备,包括如下步骤:The preparation of polycaprolactone (PCL) microspheres loaded with doxorubicin nanomedicine comprises the following steps:
(1)取20 mg阿霉素溶解到0.5 ml的水中,然后和多孔二氧化钛纳米颗粒20 mg搅拌24小时,使得阿霉素充分吸附在多孔的二氧化钛纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成阿霉素纳米药物;(1) Dissolve 20 mg of doxorubicin in 0.5 ml of water, and then stir with 20 mg of porous titanium dioxide nanoparticles for 24 hours, so that the doxorubicin is fully adsorbed in the porous titanium dioxide nanoparticles, centrifuge to remove the supernatant, and then fully Washed 3 times, then freeze-dried to form doxorubicin nanomedicine;
(2)把上述阿霉素纳米药物和浓度为0.5%(w/w)的PCL的乙酸乙酯溶液按照重量比1:10混合并超声5分钟形成均匀的混悬液,即油包阿霉素纳米药物(N/O)混悬液;(2) Mix the above-mentioned doxorubicin nanomedicine with the ethyl acetate solution of PCL with a concentration of 0.5% (w/w) according to the weight ratio of 1:10 and ultrasonicate for 5 minutes to form a uniform suspension, that is, doxorubicin in oil Suspension of element nano drug (N/O);
(3)把步骤(2)所得油包阿霉素纳米药物(N/O)混悬液滴加到50 ml含10%(w/w)银纳米颗粒和1%(w/w)聚乙烯醇(PVA)表面活性剂的水混悬液中,搅拌5分钟形成纳米颗粒混悬液包油-油包阿霉素纳米药物(N/O/N)复乳;(3) Add the doxorubicin in oil nano-drug (N/O) suspension obtained in step (2) dropwise to 50 ml containing 10% (w/w) silver nanoparticles and 1% (w/w) polyethylene In the aqueous suspension of alcohol (PVA) surfactant, stir for 5 minutes to form nanoparticle suspension oil-in-oil-in-oil doxorubicin nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包阿霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为1%(w/w)的氯化钾水溶液中固化3小时;(4) Add the oil-in-oil-in-oil doxorubicin nano-drug (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) to 1000 ml of chlorine with a concentration of 1% (w/w) dropwise. Solidified in potassium chloride aqueous solution for 3 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有阿霉素纳米药物的聚己内酯(PCL)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polycaprolactone (PCL) microspheres loaded with doxorubicin nanomedicine.
本实施例所得的微球中,药物的重量百分比为0.47%,纳米颗粒的重量百分比为83%,聚合物的重量百分比为16.53%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 0.47%, the weight percentage of the nanoparticles is 83%, the weight percentage of the polymer is 16.53%, and the weight percentage of the pharmaceutical excipient is 0%.
本实施例中载有阿霉素纳米药物的聚己内酯(PCL)微球形态好,其表面自组装有一层银纳米颗粒,粒径在10-100 μm。微球的药物体外释放率几乎达到100%,突释非常小,几乎完全释放,基本可以达到零级释放,其体外释放性能符合要求。微球中阿霉素相对于其原始投加量的包封率为93.5%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率)。所制备微球的抗菌、抗癌效果较好,另外,采用N/O/N方法制备的微球在治疗一年后也没有纤维组织的出现,即注射部位的微囊化不出现,从而克服了微囊化的产生。In this example, the polycaprolactone (PCL) microspheres loaded with doxorubicin nano-medicine have a good shape, and a layer of silver nanoparticles is self-assembled on the surface, with a particle size of 10-100 μm. The in vitro drug release rate of the microspheres is almost 100%, the burst release is very small, almost completely released, and can basically achieve zero-order release, and its in vitro release performance meets the requirements. The encapsulation rate of doxorubicin in the microspheres relative to its original dosage is 93.5% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation rate of the drug). The prepared microspheres have good antibacterial and anticancer effects. In addition, the microspheres prepared by the N/O/N method have no fibrous tissue after one year of treatment, that is, microencapsulation at the injection site does not appear, thereby overcoming generation of microencapsulation.
实施例3 载有大分子药物生长激素微球的制备(一)Example 3 Preparation of Microspheres Loaded with Macromolecular Drug Growth Hormone (1)
生长激素具有细胞黏附作用和促进生长或延缓衰老作用,载有生长激素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:Growth hormone has the effect of cell adhesion and promoting growth or delaying aging. The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with growth hormone nano-medicine includes the following steps:
(1)取10 mg生长激素和10 mg葡聚糖溶解到0.4 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到生长激素纳米药物;(1) Dissolve 10 mg growth hormone and 10 mg dextran into 0.4 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of 5% (w/w) polyethylene glycol (PEG8000) aqueous solution Mix well, then pre-freeze in a -80°C refrigerator for 12 hours, then freeze-dry with a lyophilizer, then dissolve PEG with dichloromethane and centrifuge to remove PEG to obtain growth hormone nanomedicine;
(2)把上述生长激素纳米药物和浓度为30%(w/w)的PLGA的乙腈溶液按照重量比为1:8混合,并超声1分钟形成均匀的混悬液,即油包生长激素纳米药物(N/O)混悬液;(2) Mix the above-mentioned somatotropin nanomedicine with 30% (w/w) PLGA acetonitrile solution at a weight ratio of 1:8, and ultrasonicate for 1 minute to form a uniform suspension, that is, somatotropin in oil drug (N/O) suspension;
(3)把步骤(2)所得油包生长激素纳米药物(N/O)混悬液滴加到40 ml浓度为20%(w/w)的羟基磷灰石纳米颗粒水混悬液中并超声0.1分钟形成纳米颗粒混悬液包油-油包生长激素纳米药物(N/O/N)复乳;(3) Add the GH nanodrug (N/O) suspension obtained in step (2) dropwise to 40 ml of 20% (w/w) hydroxyapatite nanoparticle aqueous suspension and Ultrasound for 0.1 minutes to form nanoparticle suspension oil-in-oil growth hormone nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包生长激素纳米药物(N/O/N)复乳滴加到浓度为10%(w/w)的氯化钠水溶液中固化4小时;(4) Add the oil-in-oil-in-oil growth hormone nano-drug (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) dropwise to an aqueous sodium chloride solution with a concentration of 10% (w/w) Medium curing for 4 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用乙醇洗涤5次,冻干后得到载有生长激素纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with ethanol for 5 times, and freeze-dry to obtain polylactic acid-glycolic acid (PLGA) microspheres loaded with growth hormone nano-medicine.
本实施例中所制得微球中,药物的重量百分比为0.17%,纳米颗粒的重量百分比为84.45%,聚合物的重量百分比为15.17%,药用辅料的重量百分比为0.21%。In the microspheres prepared in this example, the weight percentage of the drug is 0.17%, the weight percentage of the nanoparticles is 84.45%, the weight percentage of the polymer is 15.17%, and the weight percentage of the pharmaceutical excipient is 0.21%.
对本实施例制备的载有生长激素的聚乳酸-羟基乙酸(PLGA)微球进行形貌表征、释放曲线测试、药效测试及在体内组织相容性测试,并将其药效及组织相容性与用S/O/W方法制备的微球进行对比,其中药效测试条件为:一次给药,总剂量与对照组水溶液组每天一次的共15天的总剂量相同。The polylactic-glycolic acid (PLGA) microspheres loaded with growth hormone prepared in this example were subjected to morphology characterization, release curve test, drug efficacy test and in vivo histocompatibility test, and their drug efficacy and histocompatibility The performance is compared with the microspheres prepared by the S/O/W method, wherein the drug efficacy test conditions are: once administered, the total dose is the same as the total dose of the control group aqueous solution group once a day for 15 days.
图6是本实施例中载有生长激素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的扫描电镜(SEM)照片,其中,A为微球的扫描电镜图,B为微球的表面放大图,从图中可以看出,所制备的微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在10-120 μm。图7是本实施例中载有生长激素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的体外释放曲线,从图中可以看出,所制备的微球几乎达到100%的药物释放率,突释非常小,几乎完全释放,基本可以达到零级释放(各条曲线代表不同PLGA材料制备的微球,可以调控药物的不同释放时间,其中各个PLGA分别为2A50/50:羟基乙酸-乳酸=1:1和分子量为20000-30000Da、2A65/35:羟基乙酸-乳酸=0.65:0.35和分子量为20000-30000Da、3A50/50:羟基乙酸-乳酸=1:1和分子量为30000-47000Da、3A65/35:羟基乙酸-乳酸=0.65:0.35和分子量为30000-47000Da),其体外释放性能符合要求。微球中生长激素相对于其原始投加量的包封率为91.0%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率)。图8是本实施例中载有生长激素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的药效作用曲线,微球组为本实施例方法制备的微球,对照组为用S/O/W方法制备的微球,空白组为不含药物的微球,可以看出,使用本实施例所制备的微球,体重增加比对照组的体重增加快,药效更好。图9是本实施例中载有生长激素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的组织相容性情况,从图中可以看出,所制备的微球(N/O/N组)的相容性比对照组的好,在治疗期间,在动物组织内没有出现注射部位的微囊化或纤维化。Figure 6 is a scanning electron microscope (SEM) photo of polylactic-glycolic acid (PLGA) microspheres loaded with growth hormone nano-medicines in this example, where A is the scanning electron microscope image of the microspheres, and B is the enlarged surface of the microspheres It can be seen from the figure that the prepared microspheres have a good shape, and a layer of hydroxyapatite nanoparticles is self-assembled on the surface, with a particle size of 10-120 μm. Figure 7 is the in vitro release curve of polylactic-glycolic acid (PLGA) microspheres loaded with somatotropin nano-medicines in this example. It can be seen from the figure that the prepared microspheres almost achieve a drug release rate of 100%. The burst release is very small, almost complete release, and can basically achieve zero-order release (each curve represents microspheres made of different PLGA materials, which can regulate different release times of drugs, and each PLGA is 2A50/50:glycolic acid-lactic acid= 1:1 and molecular weight of 20000-30000Da, 2A65/35:glycolic acid-lactic acid=0.65:0.35 and molecular weight of 20000-30000Da, 3A50/50:glycolic acid-lactic acid=1:1 and molecular weight of 30000-47000Da, 3A65/ 35:glycolic acid-lactic acid=0.65:0.35 and molecular weight 30000-47000Da), its in vitro release performance meets the requirements. The encapsulation rate of growth hormone in the microspheres relative to its original dosage is 91.0% (the calculation method is: drug actually encapsulated in the microspheres/amount of drug input × 100% = drug encapsulation rate). Figure 8 is the pharmacodynamic action curve of polylactic-glycolic acid (PLGA) microspheres loaded with somatotropin nano-medicines in this example. For the microspheres prepared by the /W method, the blank group is microspheres without drugs. It can be seen that the weight gain of the microspheres prepared in this embodiment is faster than that of the control group, and the drug effect is better. Figure 9 shows the histocompatibility of polylactic-glycolic acid (PLGA) microspheres loaded with somatotropin nano-medicines in this example. It can be seen from the figure that the prepared microspheres (N/O/N group ) was better than that of the control group, and no microencapsulation or fibrosis at the injection site occurred in animal tissues during the treatment period.
以本实施例方法制备的微球包封率高,最少可以达到80%,突释非常小,几乎完全释放,基本可以达到零级释放,同时由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,可以使生物大分子药物在整个制备过程和治疗过程中保持高活性,即不失活。而且这种表面具有纳米颗粒的生物大分子药物微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The encapsulation rate of the microspheres prepared by the method of this example is high, at least 80%, the burst release is very small, almost completely released, and can basically achieve zero-order release, and at the same time, the acid produced by the degradation of the nano-particles can interact with hydroxyphosphorus The graystone nanoparticles react to neutralize the acid to ensure the relatively stable internal environment of the microspheres, which can keep the biomacromolecular drugs highly active during the entire preparation process and treatment process, that is, not inactivated. Moreover, the biomacromolecular drug microspheres with nanoparticles on the surface, because the histocompatibility of the hydrophilic material on the surface is better than that of the hydrophobic material, can enhance cell adhesion, reduce local peracidity and inflammation and microbes caused by hydrophobic materials. The role of encapsulation.
本实施例方法制备的生物大分子药物微球可以用于需要频繁注射给药、长期治疗的疾病,尤其是需要局部治疗的疾病如肿瘤、各种病因引起的贫血、侏儒等重大疾病的治疗。The biomacromolecular drug microspheres prepared by the method in this example can be used for diseases that require frequent injections and long-term treatment, especially for diseases that require local treatment such as tumors, anemia caused by various etiologies, dwarfism and other major diseases.
实施例4 载有大分子药物生长激素微球的制备(二)Example 4 Preparation of microspheres loaded with macromolecular drug growth hormone (2)
载有生长激素纳米药物的聚乳酸-聚乙二醇(PLA-PEG)微球的制备,包括如下步骤:The preparation of polylactic acid-polyethylene glycol (PLA-PEG) microspheres loaded with growth hormone nano-medicines comprises the following steps:
(1)取10 mg生长激素和10 mg纤维素溶解到0.4 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到生长激素纳米药物;(1) Dissolve 10 mg growth hormone and 10 mg cellulose into 0.4 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of 5% (w/w) polyethylene glycol (PEG8000) aqueous solution , mixed thoroughly, and then pre-frozen in a -80°C refrigerator for 12 hours, then freeze-dried with a lyophilizer, and then dissolved PEG with dichloromethane and centrifuged to remove PEG to obtain growth hormone nanomedicine;
(2)把上述生长激素纳米药物和浓度为5%(w/w)的PLA-PEG的二氯甲烷溶液按照重量比为1:5混合,并超声1分钟形成均匀的混悬液,即油包生长激素纳米药物(N/O)混悬液;(2) Mix the above-mentioned growth hormone nano-medicine with 5% (w/w) PLA-PEG in dichloromethane solution at a weight ratio of 1:5, and sonicate for 1 minute to form a uniform suspension, that is, oil Including growth hormone nano drug (N/O) suspension;
(3)把步骤(2)所得油包生长激素纳米药物(N/O)混悬液滴加到40 ml含有20%(w/w)羟基磷灰石纳米颗粒和0.5%(w/w)聚乙二醇(PEG)表面活性剂的水混悬液中并超声0.1分钟形成纳米颗粒混悬液包油-油包生长激素纳米药物(N/O/N)复乳;(3) Add the growth hormone nano-drug in oil (N/O) suspension obtained in step (2) dropwise to 40 ml containing 20% (w/w) hydroxyapatite nanoparticles and 0.5% (w/w) In the aqueous suspension of polyethylene glycol (PEG) surfactant and sonicated for 0.1 minutes to form nanoparticle suspension oil-in-oil growth hormone nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包生长激素纳米药物(N/O/N)复乳滴加到浓度为5%(w/w)的氯化钠水溶液中固化1小时;(4) Add the oil-in-oil-in-oil growth hormone nano-medicine (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) dropwise to a sodium chloride aqueous solution with a concentration of 5% (w/w) Medium curing for 1 hour;
(5)把步骤(4)所得样品进行离心,收集微球,并用乙醇水溶液洗涤5次,冻干后得到载有生长激素纳米药物的聚乳酸-聚乙二醇(PLA-PEG)微球。(5) The sample obtained in step (4) was centrifuged to collect the microspheres, washed 5 times with ethanol aqueous solution, and freeze-dried to obtain polylactic acid-polyethylene glycol (PLA-PEG) microspheres loaded with growth hormone nano-medicine.
本实施例中所制得微球中,药物的重量百分比为0.17%,纳米颗粒的重量百分比为74.15%,聚合物的重量百分比为25.47%,药用辅料的重量百分比为0.21%。In the microspheres prepared in this example, the weight percentage of the drug is 0.17%, the weight percentage of the nanoparticles is 74.15%, the weight percentage of the polymer is 25.47%, and the weight percentage of the pharmaceutical excipient is 0.21%.
本实施例中载有生长激素纳米药物的聚乳酸-聚乙二醇(PLA-PEG)微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在12-180 μm。微球的药物体外释放率几乎达到100%,突释非常小,几乎完全释放,基本可以达到零级释放,其体外释放性能符合要求。微球中阿霉素相对于其原始投加量的包封率为94.7%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率)。所制备微球的药效较好,在治疗期间,在动物组织内没有出现注射部位的微囊化或纤维化。这种表面具有纳米颗粒的生物大分子药物微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,可以使大分子药物在整个制备过程和治疗过程中保持高活性,即不失活。In this example, the polylactic acid-polyethylene glycol (PLA-PEG) microspheres loaded with somatotropin nanomedicine have good morphology, and a layer of hydroxyapatite nanoparticles is self-assembled on the surface, with a particle size of 12-180 μm. The in vitro drug release rate of the microspheres is almost 100%, the burst release is very small, almost completely released, and can basically achieve zero-order release, and its in vitro release performance meets the requirements. The encapsulation rate of doxorubicin in the microspheres relative to its original dosage is 94.7% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation rate of the drug). The prepared microspheres have good drug efficacy, and no microencapsulation or fibrosis at the injection site occurs in animal tissues during treatment. This kind of biomacromolecular drug microspheres with nanoparticles on the surface, because the histocompatibility of hydrophilic materials on the surface is better than that of hydrophobic materials, it can enhance cell adhesion, reduce inflammation and microcapsules caused by local peracid and hydrophobic materials. role of transformation. Because the acid produced by the nanoparticle during the material degradation can react with the hydroxyapatite nanoparticle to neutralize the acid to ensure that the internal environment of the microsphere is relatively stable, and the macromolecular drug can be kept at a high level during the entire preparation process and treatment process. Active, that is, not inactivated.
实施例5 载有大分子药物干扰素微球的制备(一)Example 5 Preparation of Macromolecular Drug Interferon Microspheres (1)
干扰素具有抗菌作用和抗病毒效果,载有干扰素纳米药物的聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球的制备,包括如下步骤:Interferon has antibacterial and antiviral effects, and the preparation of polyglycolic acid-polylactic acid-polyethylene glycol (PLGA-PEG) microspheres loaded with interferon nano-medicine includes the following steps:
(1)将5 mg干扰素和5 mg甘氨酸溶解到0.2 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml的浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到干扰素纳米药物;(1) Dissolve 5 mg interferon and 5 mg glycine into 0.2 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of 5% (w/w) polyethylene glycol (PEG8000) aqueous solution , mixed thoroughly, and then pre-frozen in a -80°C refrigerator for 12 hours, then freeze-dried with a lyophilizer, and then dissolved PEG with dichloromethane and centrifuged to remove PEG to obtain interferon nanomedicine;
(2)把上述干扰素纳米药物和浓度为80%(w/w)的PLGA-PEG的乙腈溶液按照重量比为1:1混合并超声1分钟形成均匀的混悬液,即油包干扰素纳米药物(N/O)混悬液;(2) Mix the above-mentioned interferon nanomedicine with PLGA-PEG acetonitrile solution with a concentration of 80% (w/w) according to the weight ratio of 1:1 and sonicate for 1 minute to form a uniform suspension, that is, interferon in oil Nano drug (N/O) suspension;
(3)把步骤(2)所得油包干扰素纳米药物(N/O)混悬液滴加到4 ml浓度为20%(w/w)的二氧化钛纳米颗粒水混悬液中并超声2分钟形成纳米颗粒混悬液包油-油包干扰素纳米药物(N/O/N)复乳;(3) Add the interferon-in-oil nanomedicine (N/O) suspension obtained in step (2) dropwise into 4 ml of titanium dioxide nanoparticle aqueous suspension with a concentration of 20% (w/w) and sonicate for 2 minutes Form nanoparticle suspension oil-in-oil interferon nanomedicine (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包干扰素纳米药物(N/O/N)复乳滴加到1000 ml浓度为10%(w/w)的氯化钠水溶液中固化4小时;(4) Add dropwise the nanoparticle suspension oil-in-oil interferon nanomedicine (N/O/N) double emulsion obtained in step (3) to 1000 ml of 10% (w/w) chloride Cured in sodium aqueous solution for 4 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤4次,冻干后得到载有干扰素纳米药物的聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with
本实施例中所得微球中,药物的重量百分比为0.51%,纳米颗粒的重量百分比为89.89%,聚合物的重量百分比为9.04%,药用辅料的重量百分比为0.56%。In the microspheres obtained in this example, the weight percentage of the drug is 0.51%, the weight percentage of the nanoparticles is 89.89%, the weight percentage of the polymer is 9.04%, and the weight percentage of the pharmaceutical excipient is 0.56%.
对本实施例制备的微球进行形貌表征,结果显示,制备的微球形态好,其表面自组装一层二氧化钛纳米颗粒,粒径在10-150 μm;微球中干扰素相对于其原始投加量的包封率为95.07%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率)。The morphology of the microspheres prepared in this example was characterized, and the results showed that the prepared microspheres had a good shape, and a layer of titanium dioxide nanoparticles was self-assembled on the surface, with a particle size of 10-150 μm; The encapsulation rate of the added dose is 95.07% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug injected × 100% = the encapsulation rate of the drug).
本实施例所制得的微球的体外释放曲线如图10,可看出药物在体外释放几乎达到零级释放。微球的药效曲线如图11,N/O/N组为本实施例方法制备的微球,对照组为用S/O/W方法制备的微球,可看出本实施例微球抗病毒作用优于对照组。The in vitro release curve of the microspheres prepared in this example is shown in Figure 10, and it can be seen that the in vitro release of the drug almost reaches zero-order release. The efficacy curve of the microspheres is shown in Figure 11. The N/O/N group is the microspheres prepared by the method of this embodiment, and the control group is the microspheres prepared by the S/O/W method. It can be seen that the microspheres of this embodiment are resistant to The effect of the virus was better than that of the control group.
这种方法制备的微球包封率高,可以达到80%以上,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与二氧化钛纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,可以使大分子药物在整个制备过程和治疗过程中保持高活性,即不失活。这种方法制备的干扰素微球可以用于需要频繁注射给药、长期治疗的疾病,如肝炎;可以减少注射的频率和减轻病人的痛苦。The encapsulation rate of microspheres prepared by this method is high, which can reach more than 80%, and the burst release is very small and almost complete, and can reach zero-order release. Since the acid produced by nanoparticles during material degradation can react with titanium dioxide nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable, the macromolecular drug can maintain high activity throughout the preparation process and treatment process, that is, Not deactivated. The interferon microspheres prepared by this method can be used for diseases that require frequent injections and long-term treatment, such as hepatitis; the frequency of injections can be reduced and the pain of patients can be relieved.
实施例6 载有大分子药物干扰素微球的制备(二)Example 6 Preparation of Macromolecular Drug Interferon Microspheres (2)
载有干扰素纳米药物的聚己内酯-聚乙二醇(PCL-PEG)微球的制备,包括如下步骤:The preparation of polycaprolactone-polyethylene glycol (PCL-PEG) microspheres loaded with interferon nanomedicine comprises the following steps:
(1)将5 mg干扰素和5 mg甘油溶解到0.2 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml的浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到干扰素纳米药物;(1) Dissolve 5 mg of interferon and 5 mg of glycerol into 0.2 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of 5% (w/w) polyethylene glycol (PEG8000) aqueous solution , mixed thoroughly, and then pre-frozen in a -80°C refrigerator for 12 hours, then freeze-dried with a lyophilizer, and then dissolved PEG with dichloromethane and centrifuged to remove PEG to obtain interferon nanomedicine;
(2)把上述干扰素纳米药物和浓度为10%(w/w)的PCL-PEG的庚烷溶液按照重量比为1:8混合并超声1分钟形成均匀的混悬液,即油包干扰素纳米药物(N/O)混悬液,庚烷溶液中还含有0.1%(w/w)的泊洛沙姆;(2) Mix the above-mentioned interferon nano-medicine with the heptane solution of PCL-PEG with a concentration of 10% (w/w) according to the weight ratio of 1:8 and ultrasonicate for 1 minute to form a uniform suspension, that is, interference in oil Suspension of nano-drugs (N/O) containing 0.1% (w/w) poloxamer in heptane solution;
(3)把步骤(2)所得油包干扰素纳米药物(N/O)混悬液滴加到4 ml含50%(w/w)二氧化钛纳米颗粒和5%(w/w)聚乙烯吡咯烷酮表面活性剂的水混悬液中并超声2分钟形成纳米颗粒混悬液包油-油包干扰素纳米药物(N/O/N)复乳;(3) Add the interferon-in-oil nanomedicine (N/O) suspension obtained in step (2) dropwise to 4 ml containing 50% (w/w) titanium dioxide nanoparticles and 5% (w/w) polyvinylpyrrolidone In the aqueous suspension of surfactant and sonicated for 2 minutes to form nanoparticle suspension oil-in-oil interferon nanomedicine (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包干扰素纳米药物(N/O/N)复乳滴加到1000 ml浓度为8%(w/w)的氯化钠水溶液中固化4小时;(4) Add the nanoparticle suspension oil-in-oil interferon nanomedicine (N/O/N) double emulsion obtained in step (3) dropwise to 1000 ml of 8% (w/w) chloride Cured in sodium aqueous solution for 4 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用乙醇洗涤4次,冻干后得到载有干扰素纳米药物的聚己内酯-聚乙二醇(PCL-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with ethanol four times, and freeze-dry to obtain polycaprolactone-polyethylene glycol (PCL-PEG) microspheres loaded with interferon nano-medicine .
本实施例中所得微球中,药物的重量百分比为0.51%,纳米颗粒的重量百分比为49.89 %,聚合物的重量百分比为49.04 %,药用辅料的重量百分比为0.56%。In the microspheres obtained in this example, the weight percentage of the drug is 0.51%, the weight percentage of the nanoparticles is 49.89%, the weight percentage of the polymer is 49.04%, and the weight percentage of the pharmaceutical excipient is 0.56%.
本实施例制备的微球形态好,其表面自组装一层二氧化钛纳米颗粒,粒径在5-260 μm;微球中干扰素相对于其原始投加量的包封率为93.87%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率)。本实施例所制得的微球在体外释放几乎达到零级释放,微球抗病毒和抗菌作用均优于对照组。这种方法制备的微球包封率高,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与二氧化钛纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,可以使大分子药物在整个制备过程和治疗过程中保持高活性,即不失活。这种方法制备的干扰素微球可以用于需要频繁注射给药、长期治疗的疾病,如肝炎,可以减少注射的频率和减轻病人的痛苦。The microspheres prepared in this example have a good shape, and a layer of titanium dioxide nanoparticles is self-assembled on the surface, with a particle size of 5-260 μm; the encapsulation rate of interferon in the microspheres relative to its original dosage is 93.87% (calculation method It is: the drug actually encapsulated in the microsphere/the amount of the drug input × 100% = the encapsulation rate of the drug). The release of the microspheres prepared in this example almost reached zero-order release in vitro, and the antiviral and antibacterial effects of the microspheres were better than those of the control group. The microspheres prepared by this method have high encapsulation efficiency, very small burst release and almost complete release, and zero-order release can be achieved. Since the acid produced by nanoparticles during material degradation can react with titanium dioxide nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable, the macromolecular drug can maintain high activity throughout the preparation process and treatment process, that is, Not deactivated. The interferon microspheres prepared by this method can be used for diseases requiring frequent injections and long-term treatment, such as hepatitis, which can reduce the frequency of injections and alleviate the pain of patients.
实施例7 载有大分子药物EPO微球的制备(一)Example 7 Preparation of macromolecular drug-loaded EPO microspheres (1)
促红细胞生成素(EPO)具有抗菌作用和治疗贫血作用,载有EPO纳米药物的聚己内酯(PCL)和聚乳酸(PLA)微球的制备,包括如下步骤:Erythropoietin (EPO) has antibacterial and anemia-treating effects. The preparation of polycaprolactone (PCL) and polylactic acid (PLA) microspheres loaded with EPO nanomedicine includes the following steps:
(1)将5 mg EPO和5 mg葡聚糖溶解到0.2 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml的浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到EPO纳米药物;(1) Dissolve 5 mg EPO and 5 mg dextran into 0.2 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of a 5% (w/w) polyethylene glycol (PEG8000) aqueous solution Mix well, then pre-freeze at -80°C for 12 hours, then freeze-dry with a lyophilizer, then dissolve PEG with dichloromethane and centrifuge to remove PEG to obtain EPO nanomedicine;
(2)把上述EPO纳米药物和浓度为10%(w/w)的PCL和10%(w/w)PLA的氯仿溶液按照重量比为1:9混合并超声1分钟形成均匀的混悬液,即油包EPO纳米药物(N/O)混悬液;(2) Mix the above EPO nanomedicine with 10% (w/w) PCL and 10% (w/w) PLA in chloroform according to the weight ratio of 1:9 and sonicate for 1 minute to form a uniform suspension , that is, EPO nano drug (N/O) suspension in oil;
(3)把步骤(2)所得油包EPO纳米药物(N/O)混悬液滴加到5 ml浓度为20%(w/w)的羟基磷灰石纳米颗粒水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包EPO纳米药物(N/O/N)复乳;(3) Add the suspension of EPO nanomedicine in oil (N/O) obtained in step (2) dropwise to 5 ml of 20% (w/w) hydroxyapatite nanoparticle aqueous suspension and
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包EPO纳米药物(N/O/N)复乳滴加到1000 ml浓度为1%(w/w)的氯化钠水溶液中固化1小时;(4) Add the oil-in-oil-in-oil EPO nanomedicine (N/O/N) double emulsion of nanoparticle suspension obtained in step (3) to 1000 ml of sodium chloride with a concentration of 1% (w/w) dropwise Curing in aqueous solution for 1 hour;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有EPO纳米药物的聚己内酯(PCL)和聚乳酸(PLA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polycaprolactone (PCL) and polylactic acid (PLA) microspheres loaded with EPO nanomedicine.
本实施例中所得微球中,药物的重量百分比为0.30%,纳米颗粒的重量百分比为71.79%,聚合物的重量百分比为27.55%,药用辅料的重量百分比为0.36%。In the microspheres obtained in this example, the weight percentage of the drug is 0.30%, the weight percentage of the nanoparticles is 71.79%, the weight percentage of the polymer is 27.55%, and the weight percentage of the pharmaceutical excipient is 0.36%.
对本例制备的微球进行形貌表征,结果显示,所制备微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在50-220 μm;微球中EPO相对于其原始投加量的包封率为93.33%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。本实施例制备的微球的药效曲线见图12,由图中可看出,本实施例制备的微球抗贫血效果和抗菌效果较好,与普通的药物颗粒治疗70天相比,效果都非常好。The morphology of the microspheres prepared in this example was characterized, and the results showed that the prepared microspheres had a good shape, and a layer of hydroxyapatite nanoparticles was self-assembled on the surface, with a particle size of 50-220 μm; The encapsulation rate of the dosage is 93.33% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation rate of the drug), and the experiment shows that its in vitro release curve also meets the requirements. The drug efficacy curve of the microspheres prepared in this example is shown in Figure 12. It can be seen from the figure that the anti-anemia effect and antibacterial effect of the microspheres prepared in this example are better. all very good.
这种方法制备的微球包封率高,可以达到80%以上,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,同时可以使EPO药物在整个制备过程和治疗过程保持高活性,即不失活。且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。本实施例方法制备的EPO微球可以用于需要频繁注射给药、长期治疗各种原因引起的贫血如肿瘤治疗引起的肾衰贫血,可以减少注射的频率和减轻病人的痛苦。The encapsulation rate of microspheres prepared by this method is high, which can reach more than 80%, and the burst release is very small and almost complete, and can reach zero-order release. Because the acid produced by the nano-particles during the material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure the internal environment of the microspheres is relatively stable, and at the same time, it can keep the EPO drug highly active throughout the preparation process and treatment process , that is, not deactivated. And this kind of microspheres with nanoparticles on the surface, because the histocompatibility of hydrophilic materials on the surface is better than that of hydrophobic materials, it can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials . The EPO microspheres prepared by the method in this example can be used for frequent injections and long-term treatment of anemia caused by various causes, such as renal failure anemia caused by tumor treatment, which can reduce the frequency of injections and relieve the pain of patients.
实施例8 载有大分子药物EPO微球的制备(二)Example 8 Preparation of macromolecular drug-loaded EPO microspheres (2)
载有EPO纳米药物的聚己内酯(PCL)和聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球的制备,包括如下步骤:The preparation of polycaprolactone (PCL) and polyglycolic acid-polylactic acid-polyethylene glycol (PLGA-PEG) microspheres loaded with EPO nano-medicine includes the following steps:
(1)将5 mg EPO和5 mg山梨醇溶解到0.2 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml的浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到EPO纳米药物;(1) Dissolve 5 mg EPO and 5 mg sorbitol into 0.2 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of 5% (w/w) polyethylene glycol (PEG8000) aqueous solution , mixed thoroughly, and then pre-frozen in a -80°C refrigerator for 12 hours, then freeze-dried with a lyophilizer, and then dissolved PEG with dichloromethane and centrifuged to remove PEG to obtain EPO nanomedicine;
(2)把上述EPO纳米药物和浓度为20%(w/w)的PCL和60%(w/w)的PLGA-PEG的丙酮溶液按照重量比为1:2混合并超声2分钟形成均匀的混悬液,即油包EPO纳米药物(N/O)混悬液;(2) Mix the above-mentioned EPO nanomedicine with the acetone solution of 20% (w/w) PCL and 60% (w/w) PLGA-PEG according to the weight ratio of 1:2 and sonicate for 2 minutes to form a uniform Suspension, that is, EPO nano drug (N/O) suspension in oil;
(3)把步骤(2)所得油包EPO纳米药物(N/O)混悬液滴加到5 ml含有40%(w/w)羟基磷灰石纳米颗粒和5%(w/w)泊洛沙姆表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包EPO纳米药物(N/O/N)复乳;(3) Add dropwise the EPO nanomedicine in oil (N/O) suspension obtained in step (2) to 5 ml containing 40% (w/w) hydroxyapatite nanoparticles and 5% (w/w) poise In the aqueous suspension of loxamer surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil EPO nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包EPO纳米药物(N/O/N)复乳滴加到1000 ml浓度为1%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil EPO nanomedicine (N/O/N) double emulsion of nanoparticle suspension obtained in step (3) to 1000 ml of sodium chloride with a concentration of 1% (w/w) dropwise Cured in aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有EPO纳米药物的聚己内酯(PCL)和聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polycaprolactone (PCL) and polyglycolic acid-polylactic acid-polyethylene loaded with EPO nanomedicine. Diol (PLGA-PEG) microspheres.
本实施例中所得微球中,药物的重量百分比为0.36%,纳米颗粒的重量百分比为41.79%,聚合物的重量百分比为57.49%,药用辅料的重量百分比为0.36%。In the microspheres obtained in this example, the weight percentage of the drug is 0.36%, the weight percentage of the nanoparticles is 41.79%, the weight percentage of the polymer is 57.49%, and the weight percentage of the pharmaceutical excipient is 0.36%.
对本例制备的微球形貌表征,结果显示所制备微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在40-250 μm;微球中EPO相对于其原始投加量的包封率为89.8%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。本实施例制备的微球抗贫血效果和抗菌效果较好,与普通的药物颗粒治疗70天相比,效果都非常好。The morphology of the microspheres prepared in this example was characterized, and the results showed that the prepared microspheres had a good shape, and a layer of hydroxyapatite nanoparticles was self-assembled on the surface, with a particle size of 40-250 μm; The encapsulation rate of the drug was 89.8% (the calculation method is: the actual drug encapsulated in the microspheres/the input drug amount × 100% = the encapsulation rate of the drug), and the experiment showed that its in vitro release curve also met the requirements. The anti-anemia effect and antibacterial effect of the microspheres prepared in this example are better, and the effect is very good compared with ordinary drug granules for 70 days of treatment.
这种方法制备的微球包封率高,可以达到80%以上,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,同时可以使EPO药物在整个制备过程和治疗过程保持高活性,即不失活。且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。本实施例方法制备的EPO微球可以用于需要频繁注射给药、长期治疗各种原因引起的贫血如肿瘤治疗引起的肾衰贫血,可以减少注射的频率和减轻病人的痛苦。The encapsulation rate of microspheres prepared by this method is high, which can reach more than 80%, and the burst release is very small and almost complete, and can reach zero-order release. Because the acid produced by the nano-particles during the material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure the internal environment of the microspheres is relatively stable, and at the same time, it can keep the EPO drug highly active throughout the preparation process and treatment process , that is, not deactivated. And this kind of microspheres with nanoparticles on the surface, because the histocompatibility of hydrophilic materials on the surface is better than that of hydrophobic materials, it can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials . The EPO microspheres prepared by the method in this example can be used for frequent injections and long-term treatment of anemia caused by various causes, such as renal failure anemia caused by tumor treatment, which can reduce the frequency of injections and relieve the pain of patients.
实施例9 载有大分子药物G-CSF微球的制备(一)Example 9 Preparation of G-CSF Microspheres Loaded with Macromolecular Drugs (1)
粒细胞集落刺激因子(G-CSF)具有抗菌作用和粒细胞增加作用,载有G-CSF纳米药物的聚己内酯(PCL)微球的制备,包括如下步骤:Granulocyte colony-stimulating factor (G-CSF) has antibacterial and granulocyte-increasing effects, and the preparation of polycaprolactone (PCL) microspheres loaded with G-CSF nanomedicine includes the following steps:
(1)将5 mg G-CSF和5 mg葡聚糖溶解到0.2 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml的浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到G-CSF纳米药物;(1) Dissolve 5 mg G-CSF and 5 mg dextran into 0.2 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of polyethylene glycol (PEG8000) with a concentration of 5% (w/w). ) aqueous solution, mixed thoroughly, and then pre-frozen at -80°C for 12 hours, then freeze-dried with a lyophilizer, and then dissolved PEG with dichloromethane and centrifuged to remove PEG to obtain G-CSF nanomedicine;
(2)把上述G-CSF纳米药物和浓度为20%(w/w)的PCL的乙腈溶液按照重量比为1:9混合并超声5分钟形成均匀的混悬液,即油包G-CSF纳米药物(N/O)混悬液,乙腈溶液中还含有20%(w/w)的聚乙二醇;(2) Mix the above-mentioned G-CSF nanomedicine with the acetonitrile solution of PCL with a concentration of 20% (w/w) according to the weight ratio of 1:9 and sonicate for 5 minutes to form a uniform suspension, that is, G-CSF in oil Nano drug (N/O) suspension, the acetonitrile solution also contains 20% (w/w) polyethylene glycol;
(3)把步骤(2)所得油包G-CSF纳米药物(N/O)混悬液滴加到1 ml浓度为70%(w/w)的羟基磷灰石纳米颗粒水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包G-CSF纳米药物(N/O/N)复乳;(3) Add the suspension of G-CSF nano-medicine in oil (N/O) obtained in step (2) dropwise into 1 ml of 70% (w/w) aqueous suspension of hydroxyapatite nanoparticles and stirred for 5 minutes to form nanoparticle suspension oil-in-oil G-CSF nanomedicine (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包G-CSF纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil G-CSF nanomedicine (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) dropwise to 1000 ml of 5% (w/w) chlorine Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用乙醇洗涤3次,冻干后得到载有G-CSF纳米药物的聚己内酯(PCL)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with ethanol three times, and freeze-dry to obtain polycaprolactone (PCL) microspheres loaded with G-CSF nanomedicine.
本实施例中所得微球中,药物的重量百分比为0.70%,纳米颗粒的重量百分比为44.03%,聚合物的重量百分比为54.72%,药用辅料的重量百分比为0.55%。In the microspheres obtained in this example, the weight percentage of the drug is 0.70%, the weight percentage of the nanoparticles is 44.03%, the weight percentage of the polymer is 54.72%, and the weight percentage of the pharmaceutical excipient is 0.55%.
对本实施例制备的微球进行形貌表征,结果显示,制备的微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在1-500 μm;微球中G-CSF相对于其原始投加量的包封率为91.8%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。该实施例微球的药效曲线如图13,其中N/O/N组为本实施例方法制备的微球,对照组为用S/O/W方法制备的微球,空白组为不含药物的微球,从图中可以看出,本实施例微球的药效明显优于对照组。The morphology of the microspheres prepared in this example was characterized, and the results showed that the prepared microspheres had a good shape, and a layer of hydroxyapatite nanoparticles was self-assembled on the surface, with a particle size of 1-500 μm; the G-CSF in the microspheres was relatively The encapsulation efficiency of the original dosage is 91.8% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation efficiency of the drug), and the experiment shows that its release curve in vitro is also meet the requirements. The pharmacodynamic curve of the microspheres of this embodiment is shown in Figure 13, wherein the N/O/N group is the microspheres prepared by the method of this embodiment, the control group is the microspheres prepared by the S/O/W method, and the blank group is the microspheres without As can be seen from the figure, the efficacy of the microspheres of the present embodiment is significantly better than that of the control group.
本实施例方法制备的G-CSF微球可以用于需要频繁注射给药、长期治疗各种原因引起的白细胞减少症,可以减少注射的频率和减轻病人的痛苦。种方法制备的微球包封率高,可以达到80%以上,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,同时可以使EPO药物在整个制备过程和治疗过程中保持高活性,即不失活。且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The G-CSF microspheres prepared by the method in this example can be used for frequent injections and long-term treatment of leukopenia caused by various reasons, which can reduce the frequency of injections and alleviate the pain of patients. The encapsulation rate of the microspheres prepared by the method is high, which can reach more than 80%, and the burst release is very small and almost complete, and can reach zero-order release. Because the acid generated by the degradation of the nano-particles can react with the hydroxyapatite nanoparticles to neutralize the acid, so as to ensure that the internal environment of the microspheres is relatively stable, and at the same time, it can keep the EPO drug at a high level during the entire preparation process and treatment process. Active, that is, not inactivated. And this kind of microspheres with nanoparticles on the surface, because the histocompatibility of hydrophilic materials on the surface is better than that of hydrophobic materials, it can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials .
实施例10 载有大分子药物G-CSF微球的制备(二)Example 10 Preparation of G-CSF Microspheres Loaded with Macromolecular Drugs (2)
载有G-CSF纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with G-CSF nanomedicine includes the following steps:
(1)将5 mg G-CSF和5 mg葡聚糖溶解到0.2 ml的水中形成药物水溶液,然后把上述溶液转移到3.2 ml的浓度为5%(w/w)的聚乙二醇(PEG8000)水溶液中,充分混匀,然后在-80℃冰箱预冻12小时,再用冻干机冻干,然后用二氯甲烷溶解PEG并离心除去PEG得到G-CSF纳米药物;(1) Dissolve 5 mg G-CSF and 5 mg dextran into 0.2 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of polyethylene glycol (PEG8000) with a concentration of 5% (w/w). ) aqueous solution, mixed thoroughly, and then pre-frozen at -80°C for 12 hours, then freeze-dried with a lyophilizer, and then dissolved PEG with dichloromethane and centrifuged to remove PEG to obtain G-CSF nanomedicine;
(2)把上述G-CSF纳米药物和浓度为20%(w/w)的PLGA的二氯甲烷-乙酸乙酯(1:1,v/v)溶液按照重量比为1:9混合并超声5分钟形成均匀的混悬液,即油包G-CSF纳米药物(N/O)混悬液,二氯甲烷-乙酸乙酯溶液中还含有0.1%(w/w)的聚乙二醇;(2) Mix the above G-CSF nanomedicine with 20% (w/w) PLGA in dichloromethane-ethyl acetate (1:1, v/v) solution according to the weight ratio of 1:9 and sonicate A uniform suspension was formed in 5 minutes, that is, G-CSF nano-drug in oil (N/O) suspension, and the dichloromethane-ethyl acetate solution also contained 0.1% (w/w) polyethylene glycol;
(3)把步骤(2)所得油包G-CSF纳米药物(N/O)混悬液滴加到1 ml含有20%(w/w)羟基磷灰石纳米颗粒和2%(w/w)聚山梨醇表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包G-CSF纳米药物(N/O/N)复乳;(3) Add dropwise the G-CSF nanomedicine in oil (N/O) suspension obtained in step (2) to 1 ml containing 20% (w/w) hydroxyapatite nanoparticles and 2% (w/w ) into an aqueous suspension of polysorbate surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil G-CSF nanomedicine (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包G-CSF纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil G-CSF nanomedicine (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) dropwise to 1000 ml of 5% (w/w) chlorine Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有G-CSF纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) microspheres loaded with G-CSF nanomedicine.
本实施例中所得微球中,药物的重量百分比为1.70%,纳米颗粒的重量百分比为43.03%,聚合物的重量百分比为54.12%,药用辅料的重量百分比为1.15%。In the microspheres obtained in this example, the weight percentage of the drug is 1.70%, the weight percentage of the nanoparticles is 43.03%, the weight percentage of the polymer is 54.12%, and the weight percentage of the pharmaceutical excipient is 1.15%.
对本例制备的微球进行形貌表征,结果显示微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在1-450 μm;微球中G-CSF相对于其原始投加量的包封率为89.9%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求,微球的药效较好。本实施例方法制备的G-CSF微球可以用于需要频繁注射给药、长期治疗各种原因引起的白细胞减少症,可以减少注射的频率和减轻病人的痛苦。这种方法制备的微球包封率高,可以达到80%以上,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定,同时可以使G-CSF药物在整个制备过程和治疗过程中保持高活性,即不失活。且这种具有纳米颗粒表面的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The morphology of the microspheres prepared in this example was characterized, and the results showed that the microspheres had a good shape, and a layer of hydroxyapatite nanoparticles self-assembled on the surface, with a particle size of 1-450 μm; The encapsulation rate of the added dose was 89.9% (calculation method: actual drug encapsulated in the microspheres/injected drug amount × 100% = encapsulation rate of the drug), and experiments showed that its in vitro release curve also met the requirements. The effect of the ball is better. The G-CSF microspheres prepared by the method in this example can be used for frequent injections and long-term treatment of leukopenia caused by various reasons, which can reduce the frequency of injections and alleviate the pain of patients. The encapsulation rate of microspheres prepared by this method is high, which can reach more than 80%, and the burst release is very small and almost complete, and can reach zero-order release. Because the acid produced by the nano-particles during the material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable, and at the same time, the G-CSF drug can be used during the entire preparation process and treatment process. Maintain high activity, that is, not inactivate. And this kind of microspheres with nanoparticle surface, because the histocompatibility of surface hydrophilic materials is better than that of hydrophobic materials, it has the effect of enhancing cell adhesion, reducing inflammation and microencapsulation caused by local peracid and hydrophobic materials .
实施例11 载有小分子药物普萘洛尔微球的制备(一)Example 11 Preparation of Microspheres Loaded with Small Molecule Drug Propranolol (1)
普萘洛尔具有抗菌作用和抗癌效果,载有普萘洛尔纳米药物的聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球的制备,包括如下步骤:Propranolol has antibacterial and anticancer effects. The preparation of polyglycolic acid-polylactic acid-polyethylene glycol (PLGA-PEG) microspheres loaded with propranolol nano-medicine includes the following steps:
(1)取20 mg普萘洛尔溶解到0.5 ml的水中,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得普萘洛尔充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成普萘洛尔纳米药物;(1) Dissolve 20 mg of propranolol into 0.5 ml of water, and then stir with 20 mg of porous silica nanoparticles for 24 hours, so that propranolol is fully adsorbed in the porous silica nanoparticles, and removed by centrifugation The supernatant was fully washed 3 times, and then freeze-dried to form propranolol nanomedicine;
(2)把上述普萘洛尔纳米药物和浓度为20%(w/w)的PLGA-PEG的二氯甲烷溶液按照重量比1:9混合并超声5分钟形成均匀的混悬液,即油包普萘洛尔纳米药物(N/O)混悬液;(2) Mix the above-mentioned propranolol nano-medicine with 20% (w/w) PLGA-PEG dichloromethane solution at a weight ratio of 1:9 and ultrasonicate for 5 minutes to form a uniform suspension, that is, oil Including propranolol nano drug (N/O) suspension;
(3)把步骤(2)所得油包普萘洛尔纳米药物(N/O)混悬液滴加到50 ml浓度为10%(w/w)的银纳米颗粒水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包普萘洛尔纳米药物(N/O/N)复乳;(3) Add the propranolol-in-oil nano drug (N/O) suspension obtained in step (2) dropwise to 50 ml of silver nanoparticle aqueous suspension with a concentration of 10% (w/w) and
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包普萘洛尔纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil propranolol nano-drug (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) dropwise to 1000 ml of 5% (w/w) Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有普萘洛尔纳米药物的聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polyglycolic acid-polylactic acid-polyethylene glycol (PLGA-polyethylene glycol) loaded with propranolol nano-medicine. PEG) microspheres.
本实施例中所得微球中,药物的重量百分比为0.35%,纳米颗粒的重量百分比为96%,聚合物的重量百分比为3.65%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 0.35%, the weight percentage of the nanoparticles is 96%, the weight percentage of the polymer is 3.65%, and the weight percentage of the pharmaceutical excipient is 0%.
对本实施例制备的微球进行形貌表征,结果显示,制备的微球形态好,表面自组装一层银纳米颗粒,粒径在20-450 μm;微球中普萘洛尔相对于其原始投加量的的包封率为90%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。本实施例方法制备的普萘洛尔纳米药物微球可以适用于婴幼儿血管瘤治疗,可以减少注射的频率和减轻病人的痛苦。这种方法制备的微球包封率最少可以达到80%,突释非常小和几乎完全释放,可以达到零级释放。由于表面亲水性材料的组织相容性比疏水性材料的好,且这种具有纳米颗粒表面的微球具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The morphology of the microspheres prepared in this example was characterized, and the results showed that the prepared microspheres had a good shape, and a layer of silver nanoparticles self-assembled on the surface, with a particle size of 20-450 μm; The encapsulation rate of the dosage is 90% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug injected × 100% = the encapsulation rate of the drug), and the experiment shows that its in vitro release curve also meets the requirements . The propranolol nano-drug microspheres prepared by the method in this example can be applied to the treatment of infantile hemangioma, which can reduce the frequency of injection and alleviate the suffering of patients. The encapsulation efficiency of the microspheres prepared by this method can reach at least 80%, the burst release is very small and almost complete, and can reach zero-order release. Since the histocompatibility of surface hydrophilic materials is better than that of hydrophobic materials, and the microspheres with nanoparticle surface can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials.
实施例12 载有小分子药物普萘洛尔微球的制备(二)Example 12 Preparation of Microspheres Loaded with Small Molecule Drug Propranolol (2)
载有普萘洛尔纳米药物的聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球的制备,包括如下步骤:The preparation of polyglycolic acid-polylactic acid-polyethylene glycol (PLGA-PEG) microspheres loaded with propranolol nano-medicine comprises the following steps:
(1)取45 mg普萘洛尔溶解到0.5 ml的水中形成药物水溶液,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得普萘洛尔充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成普萘洛尔纳米药物;(1) Dissolve 45 mg of propranolol into 0.5 ml of water to form a drug aqueous solution, and then stir with 20 mg of porous silica nanoparticles for 24 hours, so that propranolol is fully adsorbed in the porous silica nanoparticles , centrifuged to remove the supernatant, and then fully washed 3 times, then freeze-dried to form propranolol nano-medicine;
(2)把上述普萘洛尔纳米药物和浓度为20%的PLGA-PEG的二氯甲烷溶液按照重量比1:10混合并超声5分钟形成均匀的混悬液,即油包普萘洛尔纳米药物(N/O)混悬液,二氯甲烷溶液中还含有20%(w/w)的泊洛沙姆;(2) Mix the above-mentioned propranolol nanomedicine with 20% PLGA-PEG dichloromethane solution at a weight ratio of 1:10 and ultrasonicate for 5 minutes to form a uniform suspension, that is, propranolol in oil Nano drug (N/O) suspension, dichloromethane solution also contains 20% (w/w) poloxamer;
(3)把步骤(2)所得油包普萘洛尔纳米药物(N/O)混悬液滴加到50 ml含浓度为10%(w/w)银纳米颗粒和5%(w/w)乙基纤维素表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包普萘洛尔纳米药物(N/O/N)复乳;(3) Add the propranolol in oil nano-drug (N/O) suspension obtained in step (2) dropwise to 50 ml containing 10% (w/w) silver nanoparticles and 5% (w/w ) in an aqueous suspension of ethyl cellulose surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil propranolol nanomedicine (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包普萘洛尔纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil propranolol nano-drug (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) dropwise to 1000 ml of 5% (w/w) Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有普萘洛尔纳米药物的聚羟基乙酸-聚乳酸-聚乙二醇(PLGA-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polyglycolic acid-polylactic acid-polyethylene glycol (PLGA-polyethylene glycol) loaded with propranolol nano-medicine. PEG) microspheres.
本实施例中所得微球中,药物的重量百分比为14.5%,纳米颗粒的重量百分比为60.18%,聚合物的重量百分比为 25.32%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 14.5%, the weight percentage of the nanoparticles is 60.18%, the weight percentage of the polymer is 25.32%, and the weight percentage of the pharmaceutical excipient is 0%.
对本例制备的微球进行形貌表征,结果显示,本实施例制备的微球形态好,其表面自组装有一层银纳米颗粒,粒径在10-400 μm;微球中普萘洛尔相对于其原始投加量的包封率为93.90%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。以本实施例方法制备的小分子药物微球可以用于需要长期治疗的疾病,尤其是需要局部治疗的疾病如肿瘤的血管栓塞等。这种方法制备的微球包封率高,可以达到80%以上,且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The morphology of the microspheres prepared in this example was characterized, and the results showed that the microspheres prepared in this example had a good shape, and a layer of silver nanoparticles was self-assembled on the surface, with a particle size of 10-400 μm; propranolol in the microspheres was relatively The encapsulation efficiency of the original dosage is 93.90% (the calculation method is: the actual drug encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation efficiency of the drug), and the experiment shows that its in vitro release curve is also meet the requirements. The small molecule drug microspheres prepared by the method of this example can be used for diseases requiring long-term treatment, especially for diseases requiring local treatment such as tumor vascular embolism. The encapsulation rate of the microspheres prepared by this method is high, which can reach more than 80%, and the microspheres with nanoparticles on the surface, because the histocompatibility of the surface hydrophilic material is better than that of the hydrophobic material, it has the ability to enhance cell Adhesion, reduction of inflammation caused by topical peracids and hydrophobic materials, and microencapsulation.
实施例13 载有小分子药物泼尼松微球的制备Example 13 Preparation of Microspheres Loaded with Small Molecule Drug Prednisone
载有泼尼松纳米药物的聚己内酯-聚乙二醇(PCL-PEG)微球的制备,包括如下步骤:The preparation of polycaprolactone-polyethylene glycol (PCL-PEG) microspheres loaded with prednisone nano-medicine comprises the following steps:
(1)取20 mg泼尼松溶解到0.5 ml的水中,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得泼尼松充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成泼尼松纳米药物;(1) Dissolve 20 mg of prednisone into 0.5 ml of water, and then stir with 20 mg of porous silica nanoparticles for 24 hours, so that prednisone is fully adsorbed in the porous silica nanoparticles, and centrifuge to remove the supernatant solution, fully washed 3 times, and then freeze-dried to form prednisone nano-medicine;
(2)把上述泼尼松纳米药物和浓度为30%(w/w)的PCL-PEG的二氯甲烷溶液按照重量比为1:9混合并超声1.5分钟形成均匀的混悬液,即油包泼尼松纳米药物(N/O)混悬液;(2) Mix the above-mentioned prednisone nano-drug and the dichloromethane solution of PCL-PEG with a concentration of 30% (w/w) according to the weight ratio of 1:9 and ultrasonicate for 1.5 minutes to form a uniform suspension, that is, oil Prednisone nano drug (N/O) suspension;
(3)把步骤(2)的油包泼尼松纳米药物(N/O)混悬液滴加到1 ml浓度为60%(w/w)羟基磷灰石纳米颗粒和1%(w/w)吐温表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包泼尼松纳米药物(N/O/N)复乳;(3) Add dropwise the prednisone nano-drug (N/O) suspension in oil in step (2) to 1 ml with a concentration of 60% (w/w) hydroxyapatite nanoparticles and 1% (w/ w) in an aqueous suspension of Tween surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil prednisone nanomedicine (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包泼尼松纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钾水溶液中固化2小时;(4) Add the oil-in-oil-in-oil prednisone nano-drug (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) to 1000 ml of chlorine with a concentration of 5% (w/w). Solidified in potassium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤5次,冻干后得到载有泼尼松纳米药物的聚己内酯-聚乙二醇(PCL-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with
本实施例中所得微球中,药物的重量百分比为1.29%,纳米颗粒的重量百分比为51.72%,聚合物的重量百分比为46.99%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 1.29%, the weight percentage of the nanoparticles is 51.72%, the weight percentage of the polymer is 46.99%, and the weight percentage of the pharmaceutical excipient is 0%.
对本例制备的微球进行形貌表征,结果显示,本实施例制备的微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在1-400 μm;微球中泼尼松相对于其原始投加量的包封率为81.23%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。本实施例制备的泼尼松纳米药物聚己内酯-聚乙二醇(PCL-PEG)微球药效曲线如附图14,其中N/O/N组为本实施例方法制备的微球,对照组为用S/O/W方法制备的微球,从图中可见,本实施例微球的治疗效果明显优于对照组。The morphology of the microspheres prepared in this example was characterized, and the results showed that the microspheres prepared in this example had a good shape, and a layer of hydroxyapatite nanoparticles was self-assembled on the surface, with a particle size of 1-400 μm; prednisolone in the microspheres The encapsulation rate of pine relative to its original dosage is 81.23% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of drug input × 100% = the encapsulation rate of the drug), and the experiment shows that its release in vitro The curves also meet the requirements. The efficacy curve of the prednisone nanomedicine polycaprolactone-polyethylene glycol (PCL-PEG) microspheres prepared in this example is shown in Figure 14, wherein the N/O/N group is the microspheres prepared by the method of this example , the control group is microspheres prepared by the S/O/W method, as can be seen from the figure, the therapeutic effect of the microspheres of this embodiment is obviously better than that of the control group.
这种方法制备的泼尼松纳米药物微球可以适用于婴幼儿血管瘤的治疗,可以减少注射的频率和减轻病人的痛苦。本方法制备的微球包封率高最少可以达到80%,突释非常小和几乎完全释放,可以达到零级释放。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定。且这种表面自组装有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The prednisone nano drug microspheres prepared by this method can be applied to the treatment of infantile hemangioma, can reduce the frequency of injection and alleviate the pain of the patient. The high encapsulation rate of the microspheres prepared by the method can reach at least 80%, the burst release is very small and almost complete, and the zero-order release can be achieved. The acid produced by the nanoparticles during material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable. And this kind of microspheres with self-assembled nanoparticles on the surface, because the histocompatibility of the hydrophilic material on the surface is better than that of the hydrophobic material, it can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials. role.
实施例14 载有大分子药物乙肝疫苗微球的制备Example 14 Preparation of Hepatitis B Vaccine Microspheres Loaded with Macromolecular Drugs
载有乙肝疫苗纳米药物的聚己内酯-聚乙二醇(PCL-PEG)微球的制备,包括如下步骤:The preparation of polycaprolactone-polyethylene glycol (PCL-PEG) microspheres loaded with hepatitis B vaccine nano-medicine comprises the following steps:
(1)将 5 mg乙肝疫苗和10 mg葡聚糖溶解到0.2 ml的水中形成药物水溶液,然后把多孔三氧化铝纳米颗粒20 mg加入上述溶液中搅拌24小时,使得乙肝疫苗及葡聚糖充分吸附在多孔的三氧化铝纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成乙肝疫苗纳米药物;(1) Dissolve 5 mg of hepatitis B vaccine and 10 mg of dextran in 0.2 ml of water to form a drug aqueous solution, then add 20 mg of porous aluminum oxide nanoparticles into the above solution and stir for 24 hours to make the hepatitis B vaccine and dextran fully Adsorbed in porous aluminum oxide nanoparticles, centrifuged to remove the supernatant, then fully washed 3 times, and then freeze-dried to form hepatitis B vaccine nano-medicine;
(2)把上述乙肝疫苗纳米药物和含有浓度为15%(w/w)的PCL-PEG的二氯甲烷溶液按照重量比为1:1混合并超声1.5分钟形成均匀混悬液,即油包乙肝疫苗纳米药物(N/O)混悬液,二氯甲烷溶液中还含有10%(w/w)的泊洛沙姆;(2) Mix the above-mentioned hepatitis B vaccine nano-medicine with the dichloromethane solution containing PCL-PEG at a concentration of 15% (w/w) according to the weight ratio of 1:1 and sonicate for 1.5 minutes to form a uniform suspension, that is, oil-in-oil Hepatitis B vaccine nano drug (N/O) suspension, dichloromethane solution also contains 10% (w/w) poloxamer;
(3)把步骤(2)的油包乙肝疫苗纳米药物(N/O)混悬液滴加到1 ml含有浓度为80%(w/w)交联葡聚糖纳米颗粒和0.5%(w/w)聚乙烯醇(PVA)表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包乙肝疫苗纳米药物(N/O/N)复乳;(3) Add dropwise the hepatitis B vaccine nano drug (N/O) suspension in step (2) to 1 ml containing 80% (w/w) cross-linked dextran nanoparticles and 0.5% (w /w) in an aqueous suspension of polyvinyl alcohol (PVA) surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil hepatitis B vaccine nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得纳米颗粒混悬液包油-油包乙肝疫苗纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil hepatitis B vaccine nano-drug (N/O/N) double emulsion of the nanoparticle suspension obtained in step (3) to 1000 ml of sodium chloride with a concentration of 5% (w/w) dropwise Cured in aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用乙醇洗涤5次,冻干后得到载有乙肝疫苗纳米药物的聚己内酯-聚乙二醇(PCL-PEG)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with ethanol for 5 times, and freeze-dry to obtain polycaprolactone-polyethylene glycol (PCL-PEG) microspheres loaded with hepatitis B vaccine nano-medicine .
本实施例中所得微球中,药物的重量百分比为6.15%,纳米颗粒的重量百分比为61.54%,聚合物的重量百分比为7.69%,药用辅料的重量百分比为24.62%。In the microspheres obtained in this example, the weight percentage of the drug is 6.15%, the weight percentage of the nanoparticles is 61.54%, the weight percentage of the polymer is 7.69%, and the weight percentage of the pharmaceutical excipient is 24.62%.
对本实施例制备的微球进行形貌表征,结果显示,本实施例制备的微球形态好,其表面自组装有一层交联葡聚糖纳米颗粒,粒径在50-300 μm;微球中乙肝疫苗相对于其原始投加量的包封率为82.74%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求,药用效果明显。The morphology of the microspheres prepared in this example was characterized, and the results showed that the microspheres prepared in this example had a good shape, and a layer of cross-linked dextran nanoparticles was self-assembled on the surface, with a particle size of 50-300 μm; The encapsulation efficiency of hepatitis B vaccine relative to its original dosage is 82.74% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of the drug input × 100% = the encapsulation efficiency of the drug), and the experiment shows that its in vitro The release curve also meets the requirements, and the medicinal effect is obvious.
这种方法制备的乙肝疫苗纳米药物微球可以适用于乙肝的防治,可以减少注射的频率和减轻病人的痛苦;本方法制备的微球包封率高,最少可以达到80%,突释非常小和几乎完全释放,可以达到零级释放。这种表面自组装有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The hepatitis B vaccine nano drug microspheres prepared by this method can be applied to the prevention and treatment of hepatitis B, can reduce the frequency of injection and alleviate the pain of patients; the encapsulation rate of the microspheres prepared by this method is high, at least 80%, and the burst release is very small And almost complete release, zero-order release can be achieved. The self-assembled microspheres with nanoparticles on the surface have the advantages of enhancing cell adhesion, reducing inflammation and microencapsulation caused by local peracid and hydrophobic materials because the histocompatibility of hydrophilic materials on the surface is better than that of hydrophobic materials. effect.
实施例15 载有小分子药物雷帕霉素微球的制备(一)Example 15 Preparation of Microspheres Loaded with Small Molecule Drug Rapamycin (1)
雷帕霉素具有抗菌作用和抗癌效果,载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)和聚乳酸(PLA)微球的制备,包括如下步骤:Rapamycin has antibacterial and anticancer effects. The preparation of polylactic-glycolic acid (PLGA) and polylactic acid (PLA) microspheres loaded with rapamycin nano-medicine includes the following steps:
(1)取20 mg雷帕霉素溶解到0.5 ml的水中,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得雷帕霉素充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成雷帕霉素纳米药物;(1) Dissolve 20 mg rapamycin in 0.5 ml of water, then stir with 20 mg porous silica nanoparticles for 24 hours, so that rapamycin is fully adsorbed in the porous silica nanoparticles, and removed by centrifugation The supernatant was fully washed 3 times, and then freeze-dried to form rapamycin nanomedicine;
(2)把上述雷帕霉素纳米药物和浓度为12.5%(w/w)的PLGA的二氯甲烷溶液按照重量比为1:4混合并搅拌2.5分钟,形成均匀的混悬液,再把1.6 ml浓度为12.5%(w/w)的PLA的乙酸乙酯溶液加到上述混悬液中,再搅拌2分钟形成均匀混悬液,即油包雷帕霉素纳米药物(N/O)混悬液;(2) Mix the above-mentioned rapamycin nanomedicine with 12.5% (w/w) PLGA in dichloromethane solution according to the weight ratio of 1:4 and stir for 2.5 minutes to form a uniform suspension, and then put 1.6 ml of PLA ethyl acetate solution with a concentration of 12.5% (w/w) was added to the above suspension, and then stirred for 2 minutes to form a homogeneous suspension, that is, rapamycin nanomedicine in oil (N/O) suspension;
(3)把步骤(2)的油包雷帕霉素纳米药物(N/O)混悬液滴加到2 ml含浓度为10%(w/w)羟基磷灰石纳米颗粒和2%(w/w)聚乙二醇表面活性剂的水混悬液中并超声0.5分钟形成纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳;(3) Add the rapamycin nano-drug (N/O) suspension in step (2) dropwise to 2 ml containing 10% (w/w) hydroxyapatite nanoparticles and 2% ( w/w) Polyethylene glycol surfactant aqueous suspension and ultrasonication for 0.5 minutes to form nanoparticle suspension oil-in-oil rapamycin nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钾水溶液中固化2小时;(4) Add the oil-in-oil-in-oil rapamycin nano-drug (N/O/N) double emulsion obtained in step (3) to 1000 ml of 5% (w/w) Solidified in potassium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)和聚乳酸(PLA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) and polylactic acid (PLA) loaded with rapamycin nano-medicine Microspheres.
本实施例中所得微球中,药物的重量百分比为1.58%,纳米颗粒的重量百分比为60.20%,聚合物的重量百分比为38.22%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 1.58%, the weight percentage of the nanoparticles is 60.20%, the weight percentage of the polymer is 38.22%, and the weight percentage of the pharmaceutical excipient is 0%.
对本实施例制备的微球进行形貌表征,结果显示,制备的微球形态好,其表面自组装有一层羟基磷灰石纳米颗粒,粒径在10-200 μm;微球中雷帕霉素纳米药物相对于其原始投加量的包封率为91.84%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。本实施例制备的雷帕霉素纳米药物聚乳酸-羟基乙酸(PLGA)和聚乳酸(PLA)微球药效曲线如附图15,其中N/O/N组为本实施例方法制备的微球,对照组为用S/O/W方法制备的微球,从图中可见,本实施例微球的药效明显优于对照组。The morphology of the microspheres prepared in this example was characterized, and the results showed that the prepared microspheres had a good shape, and a layer of hydroxyapatite nanoparticles was self-assembled on the surface, with a particle size of 10-200 μm; rapamycin in the microspheres The encapsulation efficiency of nanomedicine relative to its original dosage is 91.84% (the calculation method is: drug actually encapsulated in microspheres/amount of drug input × 100% = encapsulation efficiency of drug), and experiments show that its in vitro The release profile also met the requirements. The pharmacodynamic curves of polylactic-glycolic acid (PLGA) and polylactic acid (PLA) microspheres prepared in this example are shown in Figure 15, and the N/O/N group is the microspheres prepared by the method in this example. Ball, control group is the microsphere prepared by S/O/W method, as can be seen from the figure, the efficacy of the microsphere of the present embodiment is obviously better than that of the control group.
本实施例方法制备的微球可以适用于儿童血管瘤的治疗,可以减少注射的频率和减轻病人的痛苦。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定。同时表面自组装有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The microspheres prepared by the method in this example can be applied to the treatment of hemangioma in children, which can reduce the frequency of injection and alleviate the pain of the patient. The acid produced by the nanoparticles during material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable. At the same time, the microspheres self-assembled with nanoparticles on the surface, because the histocompatibility of the hydrophilic material on the surface is better than that of the hydrophobic material, can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials .
实施例16 载有小分子药物雷帕霉素微球的制备(二)Example 16 Preparation of Microspheres Loaded with Small Molecule Drug Rapamycin (2)
载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine comprises the following steps:
(1)取0.5 mg雷帕霉素溶解到0.5 ml的水中形成药物水溶液,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得雷帕霉素充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成雷帕霉素纳米药物;(1) Take 0.5 mg rapamycin dissolved in 0.5 ml water to form a drug aqueous solution, and then stir with 20 mg porous silica nanoparticles for 24 hours, so that rapamycin is fully adsorbed in the porous silica nanoparticles , centrifuged to remove the supernatant, and then fully washed 3 times, and then freeze-dried to form rapamycin nano-medicine;
(2)把上述雷帕霉素纳米药物和浓度为20%(w/w)的PLGA的二氯甲烷溶液按照重量比1:9混合并超声5分钟形成均匀的混悬液,即油包雷帕霉素纳米药物(N/O)混悬液,二氯甲烷溶液中还含有10%(w/w)的聚乙二醇;(2) Mix the above-mentioned rapamycin nanomedicine with 20% (w/w) PLGA in dichloromethane solution at a weight ratio of 1:9 and sonicate for 5 minutes to form a uniform suspension, that is, rapamycin in oil Pamycin nano drug (N/O) suspension, dichloromethane solution also contains 10% (w/w) polyethylene glycol;
(3)把步骤(2)所得油包雷帕霉素纳米药物(N/O)混悬液滴加到50 ml含有10%(w/w)银纳米颗粒和2%(w/w)聚乙烯吡咯烷酮表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳;(3) The oil-in-oil rapamycin nanodrug (N/O) suspension obtained in step (2) was added dropwise to 50 ml containing 10% (w/w) silver nanoparticles and 2% (w/w) polymer In the aqueous suspension of vinylpyrrolidone surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil rapamycin nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil rapamycin nano-drug (N/O/N) double emulsion obtained in step (3) to 1000 ml of 5% (w/w) Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine.
本实施例中所得微球中,药物的重量百分比为4.17%,纳米颗粒的重量百分比为35.71%,聚合物的重量百分比为 60.12%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 4.17%, the weight percentage of the nanoparticles is 35.71%, the weight percentage of the polymer is 60.12%, and the weight percentage of the pharmaceutical excipient is 0%.
对本实施例制备的微球进行形貌表征,结果显示,本实施例制备的微球形态好,其表面自组装有一层银纳米颗粒,粒径在50-200 μm;微球中雷帕霉素相对于其原始投加量的包封率为98.90%(计算方法为:实际包封在微球的药/投入的药量×100%=药物的包封率),且实验表明其体外释放曲线也符合要求。The morphology of the microspheres prepared in this example was characterized, and the results showed that the microspheres prepared in this example had a good shape, and a layer of silver nanoparticles was self-assembled on the surface, with a particle size of 50-200 μm; rapamycin in the microspheres The encapsulation rate relative to its original dosage is 98.90% (the calculation method is: the drug actually encapsulated in the microspheres / the amount of drug input × 100% = the encapsulation rate of the drug), and the experiment shows that its in vitro release curve also meets the requirements.
本实施例方法制备的小分子药物微球可以用于需要长期治疗的疾病,尤其是需要局部治疗的疾病如肿瘤的血管栓塞等。这种方法制备的微球包封率高,最少可以达到80%。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定。且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The small molecule drug microspheres prepared by the method in this example can be used for diseases requiring long-term treatment, especially diseases requiring local treatment such as tumor vascular embolism. The encapsulation efficiency of microspheres prepared by this method is high, and can reach 80% at least. The acid produced by the nanoparticles during material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable. And this kind of microspheres with nanoparticles on the surface, because the histocompatibility of hydrophilic materials on the surface is better than that of hydrophobic materials, it can enhance cell adhesion, reduce inflammation and microencapsulation caused by local peracid and hydrophobic materials .
实施例17 载有小分子药物雷帕霉素微球的制备(三)Example 17 Preparation of Microspheres Loaded with Small Molecule Drug Rapamycin (3)
载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine comprises the following steps:
(1)取0.01 mg雷帕霉素溶解到0.1 ml的水中形成药物水溶液,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得雷帕霉素充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成雷帕霉素纳米药物;(1) Dissolve 0.01 mg rapamycin into 0.1 ml water to form a drug aqueous solution, and then stir with 20 mg porous silica nanoparticles for 24 hours, so that rapamycin is fully adsorbed in the porous silica nanoparticles , centrifuged to remove the supernatant, and then fully washed 3 times, and then freeze-dried to form rapamycin nano-medicine;
(2)把上述雷帕霉素纳米药物和浓度为10%(w/w)的PLGA的二氯甲烷溶液按照重量比1:10混合并超声5分钟形成均匀的混悬液,即油包雷帕霉素纳米药物(N/O)混悬液,二氯甲烷溶液中还含有1%(w/w)的聚乙二醇;(2) Mix the above-mentioned rapamycin nanomedicine with 10% (w/w) PLGA in dichloromethane solution at a weight ratio of 1:10 and ultrasonicate for 5 minutes to form a uniform suspension, that is, oil-in-rapamycin Pamycin nano drug (N/O) suspension, dichloromethane solution also contains 1% (w/w) polyethylene glycol;
(3)把步骤(2)所得油包雷帕霉素纳米药物(N/O)混悬液滴加到0.1 ml浓度为1%(w/w)银纳米颗粒和2%(w/w)乙基纤维素表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳;(3) Add the oil-in-oil rapamycin nanodrug (N/O) suspension obtained in step (2) dropwise to 0.1 ml with a concentration of 1% (w/w) silver nanoparticles and 2% (w/w) In the aqueous suspension of ethylcellulose surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil rapamycin nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil rapamycin nano-drug (N/O/N) double emulsion obtained in step (3) to 1000 ml of 5% (w/w) Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用乙醇洗涤3次,冻干后得到载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with ethanol three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine.
本实施例中所得微球中,药物的重量百分比为0.01%,纳米颗粒的重量百分比为0.01%,聚合物的重量百分比为 99.98%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 0.01%, the weight percentage of the nanoparticles is 0.01%, the weight percentage of the polymer is 99.98%, and the weight percentage of the pharmaceutical excipient is 0%.
本实施例方法制备的小分子药物微球可以用于需要长期治疗的疾病,尤其是需要局部治疗的疾病如血管瘤等。由于纳米颗粒在材料降解产生的酸可以与羟基磷灰石纳米颗粒发生反应,从而中和酸,以保证微球的内环境相对稳定。这种方法制备的微球包封率高,可以达到80%以上,且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The small molecule drug microspheres prepared by the method in this example can be used for diseases requiring long-term treatment, especially diseases requiring local treatment such as hemangioma. The acid produced by the nanoparticles during material degradation can react with the hydroxyapatite nanoparticles to neutralize the acid to ensure that the internal environment of the microspheres is relatively stable. The encapsulation rate of the microspheres prepared by this method is high, which can reach more than 80%, and the microspheres with nanoparticles on the surface, because the histocompatibility of the surface hydrophilic material is better than that of the hydrophobic material, it has the ability to enhance cell Adhesion, reduction of inflammation caused by topical peracids and hydrophobic materials, and microencapsulation.
实施例18 载有小分子药物雷帕霉素微球的制备(四)Example 18 Preparation of Microspheres Loaded with Small Molecule Drug Rapamycin (4)
载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine comprises the following steps:
(1)取4 mg雷帕霉素溶解到0.2 ml的水中形成药物水溶液,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得雷帕霉素充分吸附在多孔的二氧化硅纳米颗粒里,离心去除上清液,再充分洗涤3次,然后冻干形成雷帕霉素纳米药物;(1) Dissolve 4 mg of rapamycin in 0.2 ml of water to form a drug aqueous solution, and then stir with 20 mg of porous silica nanoparticles for 24 hours, so that rapamycin is fully adsorbed in the porous silica nanoparticles , centrifuged to remove the supernatant, and then fully washed 3 times, and then freeze-dried to form rapamycin nano-medicine;
(2)把上述雷帕霉素纳米药物和浓度为1%(w/w)的PLGA的乙腈溶液按照重量比1:1混合并超声5分钟形成均匀的混悬液,即油包雷帕霉素纳米药物(N/O)混悬液,乙腈溶液中还含有1%(w/w)的泊洛沙姆;(2) Mix the above-mentioned rapamycin nanomedicine with 1% (w/w) PLGA-acetonitrile solution at a weight ratio of 1:1 and sonicate for 5 minutes to form a uniform suspension, that is, rapamycin-in-oil Suspension of Susin Nanomedicine (N/O), the acetonitrile solution also contains 1% (w/w) poloxamer;
(3)把步骤(2)所得油包雷帕霉素纳米药物(N/O)混悬液滴加到0.1ml浓度为4%(w/w)银纳米颗粒和1%(w/w)聚乙烯醇(PVA)表面活性剂和1%(w/w)聚山梨醇表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳;(3) Add the oil-in-oil rapamycin nano-drug (N/O) suspension obtained in step (2) dropwise to 0.1ml with a concentration of 4% (w/w) silver nanoparticles and 1% (w/w) Polyvinyl alcohol (PVA) surfactant and 1% (w/w) polysorbate surfactant in aqueous suspension and stirred for 5 minutes to form nanoparticle suspension oil-in-oil rapamycin nanodrug (N/O/N) double milk;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil rapamycin nano-drug (N/O/N) double emulsion obtained in step (3) to 1000 ml of 5% (w/w) Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine.
本实施例中所得微球中,药物的重量百分比为40%,纳米颗粒的重量百分比为40%,聚合物的重量百分比为 20%,药用辅料的重量百分比为0%。In the microspheres obtained in this example, the weight percentage of the drug is 40%, the weight percentage of the nanoparticles is 40%, the weight percentage of the polymer is 20%, and the weight percentage of the pharmaceutical excipient is 0%.
本实施例方法制备的小分子药物微球可以用于需要长期治疗的疾病,尤其是需要局部治疗的疾病如肿瘤的血管栓塞等。这种方法制备的微球包封率高,可以达到80%以上,且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The small molecule drug microspheres prepared by the method in this example can be used for diseases requiring long-term treatment, especially diseases requiring local treatment such as tumor vascular embolism. The encapsulation rate of the microspheres prepared by this method is high, which can reach more than 80%, and the microspheres with nanoparticles on the surface, because the histocompatibility of the surface hydrophilic material is better than that of the hydrophobic material, it has the ability to enhance cell Adhesion, reduction of inflammation caused by topical peracids and hydrophobic materials, and microencapsulation.
实施例19 载有小分子药物雷帕霉素微球的制备(五)Example 19 Preparation of microspheres loaded with small molecule drug rapamycin (5)
载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球的制备,包括如下步骤:The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine comprises the following steps:
(1)取3 mg雷帕霉素和3 mg聚乙二醇溶解到0.2 ml的水中形成药物水溶液,然后和20 mg多孔二氧化硅纳米颗粒搅拌24小时,使得雷帕霉素充分吸附在多孔的二氧化硅纳米颗粒里,离心去上清液,再充分洗涤3次,然后冻干形成雷帕霉素纳米药物;(1) Dissolve 3 mg rapamycin and 3 mg polyethylene glycol into 0.2 ml of water to form a drug aqueous solution, and then stir with 20 mg porous silica nanoparticles for 24 hours, so that rapamycin is fully adsorbed on the porous In the silicon dioxide nanoparticles, centrifuge to remove the supernatant, then fully wash 3 times, and then freeze-dry to form rapamycin nano-medicine;
(2)把上述雷帕霉素纳米药物和浓度为5%(w/w)的PLGA的二氯甲烷溶液按照重量比1:3混合并超声5分钟形成均匀的混悬液,即油包雷帕霉素纳米药物(N/O)混悬液,二氯甲烷溶液中还含有5%(w/w)的聚乙二醇;(2) Mix the above-mentioned rapamycin nanomedicine with 5% (w/w) PLGA in dichloromethane solution at a weight ratio of 1:3 and sonicate for 5 minutes to form a uniform suspension, that is, rapamycin in oil Pamycin nano drug (N/O) suspension, dichloromethane solution also contains 5% (w/w) polyethylene glycol;
(3)把步骤(2)所得油包雷帕霉素纳米药物(N/O)混悬液滴加到0.1 ml含20%(w/w)银纳米颗粒和2%(w/w)聚乙烯醇(PVA)表面活性剂的水混悬液中并搅拌5分钟形成纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳;(3) Add the oil-in-oil rapamycin nanodrug (N/O) suspension obtained in step (2) dropwise to 0.1 ml containing 20% (w/w) silver nanoparticles and 2% (w/w) polymer In the aqueous suspension of vinyl alcohol (PVA) surfactant and stirred for 5 minutes to form nanoparticle suspension oil-in-oil rapamycin nano drug (N/O/N) double emulsion;
(4)把步骤(3)所得的纳米颗粒混悬液包油-油包雷帕霉素纳米药物(N/O/N)复乳滴加到1000 ml浓度为5%(w/w)的氯化钠水溶液中固化2小时;(4) Add the oil-in-oil-in-oil rapamycin nano-drug (N/O/N) double emulsion obtained in step (3) to 1000 ml of 5% (w/w) Solidified in sodium chloride aqueous solution for 2 hours;
(5)把步骤(4)所得样品进行离心,收集微球,并用水洗涤3次,冻干后得到载有雷帕霉素纳米药物的聚乳酸-羟基乙酸(PLGA)微球。(5) Centrifuge the sample obtained in step (4), collect the microspheres, wash with water three times, and freeze-dry to obtain polylactic-glycolic acid (PLGA) microspheres loaded with rapamycin nano-medicine.
本实施例中所得微球中,药物的重量百分比为30%,纳米颗粒的重量百分比为20%,聚合物的重量百分比为 20%,药用辅料的重量百分比为30%。In the microspheres obtained in this example, the weight percentage of the drug is 30%, the weight percentage of the nanoparticles is 20%, the weight percentage of the polymer is 20%, and the weight percentage of the pharmaceutical excipient is 30%.
本实施例方法制备的小分子药物微球可以用于需要长期治疗的疾病,尤其是需要局部治疗的疾病如血管瘤等。这种方法制备的微球包封率高,最少可以达到80%,且这种表面具有纳米颗粒的微球,由于表面亲水性材料的组织相容性比疏水性材料的好,具有增强细胞黏附、减少局部过酸和疏水材料引起的炎症及微囊化的作用。The small molecule drug microspheres prepared by the method in this example can be used for diseases requiring long-term treatment, especially diseases requiring local treatment such as hemangioma. The encapsulation efficiency of the microspheres prepared by this method is high, at least 80%, and the microspheres with nanoparticles on the surface, because the histocompatibility of the surface hydrophilic material is better than that of the hydrophobic material, it has the ability to enhance cell Adhesion, reduction of inflammation caused by topical peracids and hydrophobic materials, and microencapsulation.
以上所述仅是本发明的优选实施方式,应当指出,对于本技术领域的普通技术人员,在不脱离本发明方法的前提下,还可以做出若干改进和补充,这些改进和补充也应视为本发明的保护范围。The above is only a preferred embodiment of the present invention, it should be pointed out that for those of ordinary skill in the art, without departing from the method of the present invention, some improvements and supplements can also be made, and these improvements and supplements should also be considered Be the protection scope of the present invention.
Claims (10)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210361577.6ACN102885783B (en) | 2012-09-26 | 2012-09-26 | Nanometer medicament microspheres |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210361577.6ACN102885783B (en) | 2012-09-26 | 2012-09-26 | Nanometer medicament microspheres |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102885783Atrue CN102885783A (en) | 2013-01-23 |
| CN102885783B CN102885783B (en) | 2014-04-16 |
Family
ID=47529604
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201210361577.6AExpired - Fee RelatedCN102885783B (en) | 2012-09-26 | 2012-09-26 | Nanometer medicament microspheres |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102885783B (en) |
Cited By (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103143064A (en)* | 2013-02-05 | 2013-06-12 | 山东大学 | Magnetic polycaprolactone tissue engineering scaffold |
| CN103330680A (en)* | 2013-05-31 | 2013-10-02 | 袁伟恩 | Nano drug transdermal preparation and preparation method thereof |
| CN103599070A (en)* | 2013-11-26 | 2014-02-26 | 上海交通大学 | Preparation method of temperature and fluorescence probe of lipidosome loaded with gold nanocluster and anti-cancer drug |
| CN103860392A (en)* | 2014-01-27 | 2014-06-18 | 广东职业技术学院 | Hydrophobic solid microspheres containing epidermal growth factors and preparation method and application of microspheres |
| CN104371021A (en)* | 2014-11-14 | 2015-02-25 | 东华大学 | Preparation method of heptapeptide composite hydrogel microspheres |
| CN104525092A (en)* | 2014-12-17 | 2015-04-22 | 华中科技大学 | Preparation method and product of superparamagnetism nano particles |
| CN105363029A (en)* | 2014-08-12 | 2016-03-02 | 张世艳 | Novel adjuvant vaccine composition associated with HPV |
| CN105456198A (en)* | 2015-12-25 | 2016-04-06 | 正大天晴药业集团股份有限公司 | Entecavir-loaded mesoporous silica nanoparticle and preparation method thereof |
| CN106433685A (en)* | 2016-09-21 | 2017-02-22 | 安徽瀚景观规划设计院有限公司 | Preparation method of soil remediation material on basis of acrylic resin |
| CN104288093B (en)* | 2013-05-31 | 2017-03-08 | 袁伟恩 | Application of the nano drug transdermal preparation in tumour |
| CN106730027A (en)* | 2016-10-26 | 2017-05-31 | 西北大学 | A kind of preparation method of bone cement microballoon |
| CN107224571A (en)* | 2016-03-23 | 2017-10-03 | 张巍 | A kind of PLGA complex microspheres with sciatic nerve defencive function |
| CN107320459A (en)* | 2017-07-21 | 2017-11-07 | 浙江理工大学 | A kind of preparation method based on the double-deck polymer nano particle for cooperateing with controlled release to supply medicine |
| CN107375240A (en)* | 2017-07-21 | 2017-11-24 | 浙江理工大学 | A kind of preparation method with the double-deck collaboration controlled release of targeting for the polymer nano particle of medicine |
| CN107604752A (en)* | 2017-09-18 | 2018-01-19 | 广西工业职业技术学院 | A kind of preparation method for the antibacterial sucrose wrapping paper that develops the color |
| CN107823143A (en)* | 2017-11-10 | 2018-03-23 | 齐鲁工业大学 | A kind of preparation method of bone morphogenetic protein microspheres |
| CN110025406A (en)* | 2019-04-17 | 2019-07-19 | 上海黑焰医疗科技有限公司 | A kind of preparation method of 3D printing bone defect filler |
| CN111349158A (en)* | 2020-03-06 | 2020-06-30 | 西北农林科技大学 | A kind of preparation method of milk goat sex-controlled semen |
| CN111743880A (en)* | 2020-06-05 | 2020-10-09 | 浙江大学医学院附属第一医院 | A kind of oral nano-microsphere preparation of monoclonal antibody drug and preparation method thereof |
| CN111803450A (en)* | 2020-06-15 | 2020-10-23 | 广东省医疗器械研究所 | Nano-reinforced degradable polymer microsphere and preparation method and application thereof |
| CN112386748A (en)* | 2019-08-12 | 2021-02-23 | 湖南早晨纳米机器人有限公司 | Magnesium alloy nano robot and preparation method thereof |
| CN112391599A (en)* | 2019-08-12 | 2021-02-23 | 湖南早晨纳米机器人有限公司 | Magnesium alloy sputtering nano robot and preparation method thereof |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104398480A (en)* | 2014-10-30 | 2015-03-11 | 广西中医药大学附属瑞康医院 | Estrogen-containing nanometer particle and its preparation method |
| WO2018011040A1 (en)* | 2016-07-13 | 2018-01-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Plga microparticles loaded with a fluoroquinolone for the treatment of respiratory diseases |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101461785A (en)* | 2009-01-08 | 2009-06-24 | 上海交通大学 | Oil in water-oil in oil-water in oil method for preparing microballoons |
| CN101474160A (en)* | 2009-01-08 | 2009-07-08 | 上海交通大学 | Oil in oil-oil in oil-water in oil method for preparing microballoons |
| CN101721375A (en)* | 2009-12-30 | 2010-06-09 | 上海交通大学 | Insulin slow release micron sphere composition and preparation method thereof |
| CN101721709A (en)* | 2009-11-13 | 2010-06-09 | 上海市肿瘤研究所 | Calcium phosphate and amphiphilic polymer composite medicament-carrying nano-microsphere, preparation method and application |
| CN101785759A (en)* | 2010-03-09 | 2010-07-28 | 湖南大学 | Nanoparticle for embedding medicinal Adriamycin as well as preparation method and application thereof |
| EP2275094A2 (en)* | 1995-09-29 | 2011-01-19 | Elan Pharma International Limited | Reduction of intravenously administered nanoparticulate-formulation-induced adverse physiological reactions |
- 2012
- 2012-09-26CNCN201210361577.6Apatent/CN102885783B/ennot_activeExpired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2275094A2 (en)* | 1995-09-29 | 2011-01-19 | Elan Pharma International Limited | Reduction of intravenously administered nanoparticulate-formulation-induced adverse physiological reactions |
| CN101461785A (en)* | 2009-01-08 | 2009-06-24 | 上海交通大学 | Oil in water-oil in oil-water in oil method for preparing microballoons |
| CN101474160A (en)* | 2009-01-08 | 2009-07-08 | 上海交通大学 | Oil in oil-oil in oil-water in oil method for preparing microballoons |
| CN101721709A (en)* | 2009-11-13 | 2010-06-09 | 上海市肿瘤研究所 | Calcium phosphate and amphiphilic polymer composite medicament-carrying nano-microsphere, preparation method and application |
| CN101721375A (en)* | 2009-12-30 | 2010-06-09 | 上海交通大学 | Insulin slow release micron sphere composition and preparation method thereof |
| CN101785759A (en)* | 2010-03-09 | 2010-07-28 | 湖南大学 | Nanoparticle for embedding medicinal Adriamycin as well as preparation method and application thereof |
Cited By (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103143064A (en)* | 2013-02-05 | 2013-06-12 | 山东大学 | Magnetic polycaprolactone tissue engineering scaffold |
| CN104288093B (en)* | 2013-05-31 | 2017-03-08 | 袁伟恩 | Application of the nano drug transdermal preparation in tumour |
| CN103330680A (en)* | 2013-05-31 | 2013-10-02 | 袁伟恩 | Nano drug transdermal preparation and preparation method thereof |
| CN103599070A (en)* | 2013-11-26 | 2014-02-26 | 上海交通大学 | Preparation method of temperature and fluorescence probe of lipidosome loaded with gold nanocluster and anti-cancer drug |
| CN103599070B (en)* | 2013-11-26 | 2015-05-20 | 上海交通大学 | Preparation method of temperature and fluorescence probe of lipidosome loaded with gold nanocluster and anti-cancer drug |
| CN103860392A (en)* | 2014-01-27 | 2014-06-18 | 广东职业技术学院 | Hydrophobic solid microspheres containing epidermal growth factors and preparation method and application of microspheres |
| CN103860392B (en)* | 2014-01-27 | 2016-04-06 | 广东职业技术学院 | A kind of hydrophobic solid microsphere containing epidermal growth factor and its preparation method and application |
| CN105363029A (en)* | 2014-08-12 | 2016-03-02 | 张世艳 | Novel adjuvant vaccine composition associated with HPV |
| CN105363029B (en)* | 2014-08-12 | 2021-06-11 | 张世艳 | Novel adjuvant vaccine composition for HPV (human papillomavirus) |
| CN104371021A (en)* | 2014-11-14 | 2015-02-25 | 东华大学 | Preparation method of heptapeptide composite hydrogel microspheres |
| CN104525092A (en)* | 2014-12-17 | 2015-04-22 | 华中科技大学 | Preparation method and product of superparamagnetism nano particles |
| CN105456198A (en)* | 2015-12-25 | 2016-04-06 | 正大天晴药业集团股份有限公司 | Entecavir-loaded mesoporous silica nanoparticle and preparation method thereof |
| CN105456198B (en)* | 2015-12-25 | 2017-04-26 | 正大天晴药业集团股份有限公司 | Entecavir-loaded mesoporous silica nanoparticle and preparation method thereof |
| CN107224571A (en)* | 2016-03-23 | 2017-10-03 | 张巍 | A kind of PLGA complex microspheres with sciatic nerve defencive function |
| CN106433685A (en)* | 2016-09-21 | 2017-02-22 | 安徽瀚景观规划设计院有限公司 | Preparation method of soil remediation material on basis of acrylic resin |
| CN106730027A (en)* | 2016-10-26 | 2017-05-31 | 西北大学 | A kind of preparation method of bone cement microballoon |
| CN106730027B (en)* | 2016-10-26 | 2019-07-30 | 西北大学 | A kind of preparation method of bone cement microballoon |
| CN107375240A (en)* | 2017-07-21 | 2017-11-24 | 浙江理工大学 | A kind of preparation method with the double-deck collaboration controlled release of targeting for the polymer nano particle of medicine |
| CN107320459B (en)* | 2017-07-21 | 2019-07-16 | 浙江理工大学 | A kind of preparation method of polymer nanoparticles based on double layer synergistic controlled release drug delivery |
| CN107375240B (en)* | 2017-07-21 | 2019-10-08 | 浙江理工大学 | It is a kind of to cooperate with controlled release for the preparation method of the polymer nano particle of medicine with targeting bilayer |
| CN107320459A (en)* | 2017-07-21 | 2017-11-07 | 浙江理工大学 | A kind of preparation method based on the double-deck polymer nano particle for cooperateing with controlled release to supply medicine |
| CN107604752A (en)* | 2017-09-18 | 2018-01-19 | 广西工业职业技术学院 | A kind of preparation method for the antibacterial sucrose wrapping paper that develops the color |
| CN107823143A (en)* | 2017-11-10 | 2018-03-23 | 齐鲁工业大学 | A kind of preparation method of bone morphogenetic protein microspheres |
| CN107823143B (en)* | 2017-11-10 | 2023-02-28 | 齐鲁工业大学 | Preparation method of bone morphogenetic protein microspheres |
| CN110025406A (en)* | 2019-04-17 | 2019-07-19 | 上海黑焰医疗科技有限公司 | A kind of preparation method of 3D printing bone defect filler |
| CN112391599A (en)* | 2019-08-12 | 2021-02-23 | 湖南早晨纳米机器人有限公司 | Magnesium alloy sputtering nano robot and preparation method thereof |
| CN112386748A (en)* | 2019-08-12 | 2021-02-23 | 湖南早晨纳米机器人有限公司 | Magnesium alloy nano robot and preparation method thereof |
| CN111349158B (en)* | 2020-03-06 | 2022-11-08 | 西北农林科技大学 | A kind of preparation method of milk goat sex-controlled semen |
| CN111349158A (en)* | 2020-03-06 | 2020-06-30 | 西北农林科技大学 | A kind of preparation method of milk goat sex-controlled semen |
| CN111743880A (en)* | 2020-06-05 | 2020-10-09 | 浙江大学医学院附属第一医院 | A kind of oral nano-microsphere preparation of monoclonal antibody drug and preparation method thereof |
| CN111743880B (en)* | 2020-06-05 | 2022-03-08 | 浙江大学医学院附属第一医院 | Oral nano-microsphere preparation of monoclonal antibody medicines and preparation method thereof |
| CN111803450A (en)* | 2020-06-15 | 2020-10-23 | 广东省医疗器械研究所 | Nano-reinforced degradable polymer microsphere and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102885783B (en) | 2014-04-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102885783B (en) | Nanometer medicament microspheres | |
| CN102885784B (en) | Method for preparing microspheres by using oil-in-nanoparticle mixed suspension-nanometer medicament-in-oil | |
| Ansary et al. | Biodegradable poly (D, L-lactic-co-glycolic acid)-based micro/nanoparticles for sustained release of protein drugs-A review | |
| JP4758607B2 (en) | Biodegradable polymer composition and method of use thereof | |
| CN103330680A (en) | Nano drug transdermal preparation and preparation method thereof | |
| CN101721377B (en) | Method for preparing microspheres with hydrophilic oil in ethanol, oil in hydrophilic oil, solid in oil | |
| US7087246B2 (en) | Controlled release preparation of insulin and its method | |
| Patel et al. | Biodegradable polymers: emerging excipients for the pharmaceutical and medical device industries | |
| Shiehzadeh et al. | Dry powder form of polymeric nanoparticles for pulmonary drug delivery | |
| CN102885785A (en) | Adriamycin-containing nanometer medicament microspheres and preparation method thereof | |
| CN101485627A (en) | Microsphere prepared from glycerol modified solid-in-oil-in-water and preparation method thereof | |
| CN102895192B (en) | Method for producing microsphere through oil-in-nanoparticle suspension-water-in-oil mode | |
| CN102302455B (en) | A kind of preparation method of the microsphere preparation of encapsulating hydrophilic drug | |
| Sonawane et al. | PLGA: a wow smart biodegradable polymer in drug delivery system | |
| CN102895193B (en) | Method for preparing microspheres by oil-in-oil-in-oil-water-in-oil of nanoparticle suspension | |
| CN107126425A (en) | A kind of tanshinone IIA PEG PLGA PEG nanoparticles and preparation method thereof | |
| CN102895191B (en) | Method for preparing microspheres in oil-in-nanosuspension and solid-in-oil mode | |
| CN102885782A (en) | Growth hormone-containing nanometer medicament microspheres and preparation method thereof | |
| CN102895199B (en) | Method for preparing microspheres through oil in nano-particle suspension and sustained-release microspheres | |
| CN102895196B (en) | Method for preparing microspheres by oil-in-nanoparticle suspension-oil-in-oil-solid-in-oil | |
| Wu et al. | The current research status of PLGA as drug and gene carrier | |
| Yang et al. | Advances in drug delivery system based on liposome-composite hydrogel microspheres | |
| CN101773479B (en) | Method for preparing shell-core double-layer microspheres | |
| KR100342746B1 (en) | Controlled release preparation of insulin and its method | |
| Patil et al. | Microstructured Polymer System and Its Application in Pharmacy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20140416 Termination date:20150926 | |
| EXPY | Termination of patent right or utility model |