CN102779986A - Preparation method of nanocomposite material with core shell structure - Google Patents
Preparation method of nanocomposite material with core shell structureDownload PDFInfo
- Publication number
- CN102779986A CN102779986ACN2012102362309ACN201210236230ACN102779986ACN 102779986 ACN102779986 ACN 102779986ACN 2012102362309 ACN2012102362309 ACN 2012102362309ACN 201210236230 ACN201210236230 ACN 201210236230ACN 102779986 ACN102779986 ACN 102779986A
- Authority
- CN
- China
- Prior art keywords
- copper
- nano
- preparation
- shell structure
- core
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000011258core-shell materialSubstances0.000titleclaimsabstractdescription16
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription10
- 239000000463materialSubstances0.000titleclaimsabstractdescription7
- 239000002114nanocompositeSubstances0.000titleclaimsabstractdescription5
- 229910052802copperInorganic materials0.000claimsabstractdescription48
- 239000010949copperSubstances0.000claimsabstractdescription48
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000claimsabstractdescription28
- HSFWRNGVRCDJHI-UHFFFAOYSA-Nalpha-acetyleneNatural productsC#CHSFWRNGVRCDJHI-UHFFFAOYSA-N0.000claimsabstractdescription23
- 125000002534ethynyl groupChemical group[H]C#C*0.000claimsabstractdescription23
- 239000002245particleSubstances0.000claimsabstractdescription20
- RYGMFSIKBFXOCR-UHFFFAOYSA-NCopperChemical compound[Cu]RYGMFSIKBFXOCR-UHFFFAOYSA-N0.000claimsabstractdescription18
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000claimsabstractdescription13
- 239000002131composite materialSubstances0.000claimsabstractdescription12
- 239000007789gasSubstances0.000claimsabstractdescription12
- 239000002243precursorSubstances0.000claimsabstractdescription10
- 229910001873dinitrogenInorganic materials0.000claimsabstractdescription7
- 238000006460hydrolysis reactionMethods0.000claimsabstractdescription6
- 238000002485combustion reactionMethods0.000claimsdescription22
- 239000002041carbon nanotubeSubstances0.000claimsdescription21
- 229910021393carbon nanotubeInorganic materials0.000claimsdescription21
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000claimsdescription11
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical groupOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000claimsdescription9
- 238000006243chemical reactionMethods0.000claimsdescription8
- 239000002904solventSubstances0.000claimsdescription7
- ORTQZVOHEJQUHG-UHFFFAOYSA-Lcopper(II) chlorideChemical groupCl[Cu]ClORTQZVOHEJQUHG-UHFFFAOYSA-L0.000claimsdescription6
- 229910000365copper sulfateInorganic materials0.000claimsdescription4
- XTVVROIMIGLXTD-UHFFFAOYSA-Ncopper(II) nitrateChemical compound[Cu+2].[O-][N+]([O-])=O.[O-][N+]([O-])=OXTVVROIMIGLXTD-UHFFFAOYSA-N0.000claimsdescription4
- ARUVKPQLZAKDPS-UHFFFAOYSA-Lcopper(II) sulfateChemical compound[Cu+2].[O-][S+2]([O-])([O-])[O-]ARUVKPQLZAKDPS-UHFFFAOYSA-L0.000claimsdescription4
- 229910052757nitrogenInorganic materials0.000claimsdescription3
- 150000001875compoundsChemical class0.000claimsdescription2
- 229910017053inorganic saltInorganic materials0.000claimsdescription2
- 239000007788liquidSubstances0.000claimsdescription2
- 238000000034methodMethods0.000abstractdescription14
- 229910052799carbonInorganic materials0.000abstractdescription6
- HBBGRARXTFLTSG-UHFFFAOYSA-NLithium ionChemical compound[Li+]HBBGRARXTFLTSG-UHFFFAOYSA-N0.000abstractdescription3
- 239000004020conductorSubstances0.000abstractdescription3
- 229910001416lithium ionInorganic materials0.000abstractdescription3
- 230000001476alcoholic effectEffects0.000abstract1
- 238000010924continuous productionMethods0.000abstract1
- 239000012779reinforcing materialSubstances0.000abstract1
- 239000007921spraySubstances0.000description5
- 239000002105nanoparticleSubstances0.000description4
- 238000007865dilutingMethods0.000description3
- 239000000126substanceSubstances0.000description3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical compoundO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000description2
- 238000003917TEM imageMethods0.000description2
- 239000011248coating agentSubstances0.000description2
- 238000000576coating methodMethods0.000description2
- 229960003280cupric chlorideDrugs0.000description2
- 238000005265energy consumptionMethods0.000description2
- 239000012691Cu precursorSubstances0.000description1
- 238000001241arc-discharge methodMethods0.000description1
- 238000000889atomisationMethods0.000description1
- 229910002092carbon dioxideInorganic materials0.000description1
- 239000001569carbon dioxideSubstances0.000description1
- 238000006555catalytic reactionMethods0.000description1
- 238000005234chemical depositionMethods0.000description1
- 238000009841combustion methodMethods0.000description1
- 238000009776industrial productionMethods0.000description1
- 239000007791liquid phaseSubstances0.000description1
- 239000002086nanomaterialSubstances0.000description1
- 230000003647oxidationEffects0.000description1
- 238000007254oxidation reactionMethods0.000description1
- 238000012805post-processingMethods0.000description1
- 238000012545processingMethods0.000description1
- 235000012239silicon dioxideNutrition0.000description1
- 239000000377silicon dioxideSubstances0.000description1
- 238000005118spray pyrolysisMethods0.000description1
- 239000012808vapor phaseSubstances0.000description1
Images
Classifications
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Landscapes
- Battery Electrode And Active Subsutance (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及一种具有核壳结构的纳米复合材料的制备方法,具体地,涉及铜/纳米碳管复合材料的制备方法,其可应用于锂离子电池、导电材料、场发射增强材料等领域。The invention relates to a method for preparing a nanocomposite material with a core-shell structure, in particular to a method for preparing a copper/carbon nanotube composite material, which can be applied to the fields of lithium ion batteries, conductive materials, field emission enhancement materials and the like.
背景技术Background technique
纳米铜颗粒因为其独特的物理和化学性质,被广泛的应用在催化、光学、电子学等领域。但是,由于为纳米尺寸,在空气不可避免地被氧化,从很大程度上限制了其进一步的发展。为了降低甚至阻止铜纳米颗粒在空气中的氧化,一条可行的途径是对其进行表面修饰,如进行碳或者二氧化硅的包覆,而碳包覆又是最佳之选。目前,制备碳包覆纳米铜颗粒的方法主要有液相化学法、电弧放电法、喷雾热解法、化学气相化学沉积法等。上述方法在一定程度上受到许多条件例如需要对产品的多步处理、高设备要求和高能耗,特别是间歇的操作方式严重制约了其工业化生产。喷雾燃烧法,具有简单快速而且产品纯度高等优点,同时反应本身由溶剂的燃烧为反应提供能量,有效地降低了能耗,是最具工业化前景的纳米材料制备方法之一。Copper nanoparticles are widely used in catalysis, optics, electronics and other fields because of their unique physical and chemical properties. However, due to the nanometer size, it is inevitably oxidized in the air, which greatly limits its further development. In order to reduce or even prevent the oxidation of copper nanoparticles in air, a feasible way is to modify their surface, such as coating with carbon or silicon dioxide, and carbon coating is the best choice. At present, the methods for preparing carbon-coated copper nanoparticles mainly include liquid phase chemical method, arc discharge method, spray pyrolysis method, chemical vapor phase chemical deposition method, etc. The above method is to a certain extent subject to many conditions such as the need for multi-step processing of the product, high equipment requirements and high energy consumption, especially the intermittent operation mode severely restricts its industrial production. The spray combustion method has the advantages of simplicity, speed and high product purity. At the same time, the reaction itself is powered by the combustion of the solvent, which effectively reduces energy consumption. It is one of the most industrialized nanomaterial preparation methods.
发明内容Contents of the invention
本发明的目的在于提供一种工艺简单、易于工业化实施的具有核壳结构的纳米铜/纳米碳管复合材料的制备方法The purpose of the present invention is to provide a method for preparing nano-copper/carbon nanotube composite materials with a core-shell structure that is simple in process and easy to implement industrially
本发明专利的构思如下:The design of the patent of the present invention is as follows:
本发明采用喷雾燃烧反应器,将含铜前驱体的醇溶液雾化后通入燃烧反应器进行水解反应,同时在反应器内通入氮气稀释的乙炔气体。纳米铜颗粒在火焰中首先生成,然后乙炔在纳米铜颗粒的表面分解生成纳米碳管,最终得到一种具有核壳结构的纳米铜/纳米碳管复合材料。该方法设备工艺简单,可连续化生产,易于工业化实施。The invention adopts a spray combustion reactor, atomizes the alcohol solution containing the copper precursor and passes it into the combustion reactor for hydrolysis reaction, and at the same time passes the acetylene gas diluted with nitrogen into the reactor. Nano-copper particles are first generated in the flame, and then acetylene decomposes on the surface of nano-copper particles to form carbon nanotubes, and finally a nano-copper/carbon nanotube composite material with a core-shell structure is obtained. The method has simple equipment and process, can be produced continuously, and is easy to implement industrially.
具体技术方案如下:The specific technical scheme is as follows:
一种具有核壳结构的纳米复合材料的制备方法,包括如下步骤:A method for preparing a nanocomposite with a core-shell structure, comprising the steps of:
将含铜前驱体和溶剂配制成溶液,所述溶液雾化后通入燃烧反应器进行水解反应,同时在所述燃烧反应器内通入氮气稀释的乙炔气体;在所述燃烧反应器的火焰中首先生成纳米铜颗粒,所述乙炔气体在所述纳米铜颗粒的表面分解生成纳米碳管,最终得到一种具有核壳结构的纳米铜/纳米碳管复合材料;The copper-containing precursor and the solvent are prepared into a solution, and the solution is atomized and passed into a combustion reactor for hydrolysis reaction, and at the same time, acetylene gas diluted with nitrogen is passed into the combustion reactor; the flame in the combustion reactor First generate nano-copper particles, the acetylene gas decomposes on the surface of the nano-copper particles to generate carbon nanotubes, and finally obtains a nano-copper/carbon nanotube composite material with a core-shell structure;
其中,in,
所述含铜前驱体溶液的摩尔浓度在0.1~0.5 mol/L,进料量为3~10 ml/min;The molar concentration of the copper-containing precursor solution is 0.1-0.5 mol/L, and the feed rate is 3-10 ml/min;
所述燃烧反应器中空气的进料量为1.0~3.0 m3/h;The feed rate of air in the combustion reactor is 1.0-3.0 m3 /h;
所述乙炔气体的流量为0.02~0.1 m3/h,所述氮气的流量为0.3~1.0 m3/h;The flow rate of the acetylene gas is 0.02-0.1 m3 /h, and the flow rate of the nitrogen gas is 0.3-1.0 m3 /h;
所述水解反应的反应温度为1200~1800℃。The reaction temperature of the hydrolysis reaction is 1200-1800°C.
所述含铜前驱体是铜的无机盐类化合物,所述溶剂为可燃性液态醇类。The copper-containing precursor is an inorganic salt compound of copper, and the solvent is a flammable liquid alcohol.
所述含铜前驱体氯化铜、硝酸铜或硫酸铜,所述溶剂为甲醇或乙醇。The copper-containing precursor is copper chloride, copper nitrate or copper sulfate, and the solvent is methanol or ethanol.
所述核壳结构的纳米铜/纳米碳管复合材料中纳米铜颗粒的粒径为20~50nm,纳米碳管的长度为200~500nm。纳米碳管包覆在纳米铜颗粒的表面。The particle size of the nano-copper particle in the nano-copper/carbon nanotube composite material with core-shell structure is 20-50 nm, and the length of the carbon nano-tube is 200-500 nm. Carbon nanotubes are coated on the surface of copper nanoparticles.
本发明的具有核壳结构的纳米铜/纳米碳管复合材料的制备方法工艺简单,容易操作,无需后处理,且材料结构稳定,可应用在锂离子电池、导电材料、场发射增强材料等领域。The preparation method of the nano-copper/carbon nanotube composite material with a core-shell structure of the present invention is simple in process, easy to operate, does not require post-processing, and has a stable material structure, and can be applied in the fields of lithium-ion batteries, conductive materials, field emission enhancement materials, etc. .
附图说明Description of drawings
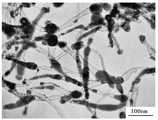
图1是实施例1产物的透射电镜照片;Fig. 1 is the transmission electron micrograph of embodiment 1 product;
图2是实施例2产物的透射电镜照片;Fig. 2 is the transmission electron micrograph of
图3是实施例1产物的XRD谱图。Fig. 3 is the XRD spectrogram of the product of embodiment 1.
具体实施方式Detailed ways
下面通过实施举例对本发明进行具体描述。有必要在此指出的是,以下实施例只用于对本发明作进一步说明,不能理解为对本发明保护范围的限制,该领域的专业技术人员根据本发明的内容作出的一些非本质的改进和调整,仍属于本发明的保护范围。The present invention will be specifically described below by implementing examples. It is necessary to point out here that the following examples are only used to further illustrate the present invention, and cannot be interpreted as limiting the protection scope of the present invention, some non-essential improvements and adjustments made by those skilled in the art according to the contents of the present invention , still belong to the protection scope of the present invention.
各反应参数如下:The reaction parameters are as follows:
含铜前驱体溶液的摩尔浓度在0.1~0.5mol/L,进料量为3~10 ml/min;The molar concentration of the copper-containing precursor solution is 0.1-0.5mol/L, and the feed rate is 3-10 ml/min;
燃烧反应器中空气的进料量为1.0~3.0 m3/h;The feed rate of air in the combustion reactor is 1.0-3.0 m3 /h;
乙炔气体的流量为0.02~0.1 m3/h,氮气的流量为0.3~1.0 m3/h;The flow rate of acetylene gas is 0.02-0.1 m3 /h, and the flow rate of nitrogen gas is 0.3-1.0 m3 /h;
反应温度为1200~1800℃。The reaction temperature is 1200-1800°C.
实施例 1Example 1
利用喷雾燃烧反应器,将氯化铜和乙醇配制成溶液,雾化后通入燃烧反应器进行燃烧反应,其中,氯化铜的摩尔浓度为0.5mol/L,进料速率为3ml/min,燃烧用空气的进料量为3.0 m3/h,反应温度为1200℃。同时在反应器内通入氮气稀释的乙炔气体,用以稀释乙炔的氮气流量为1 m3/h,乙炔0.02 m3/h。纳米铜颗粒在火焰中先生成,乙炔在纳米铜的表面分解生成纳米碳管,最终得到一种具有核壳结构的纳米铜/纳米碳管复合材料,产物的电镜照片如图1所示、XRD照片如图3所示,其中,作为核的纳米铜颗粒粒径为50nm,包覆纳米铜颗粒的碳管长度为200nm。Utilize spray combustion reactor, cupric chloride and ethanol are mixed into solution, pass into combustion reactor after the atomization and carry out combustion reaction, wherein, the molar concentration of cupric chloride is 0.5mol/L, and feed rate is 3ml/min, The feed rate of combustion air is 3.0 m3 /h, and the reaction temperature is 1200°C. At the same time, nitrogen-diluted acetylene gas was fed into the reactor, the flow rate of nitrogen gas for diluting acetylene was 1 m3 /h, and the flow rate of acetylene was 0.02 m3 /h. Nano-copper particles are first formed in the flame, and acetylene decomposes on the surface of nano-copper to form carbon nanotubes, and finally a nano-copper/carbon nanotube composite material with a core-shell structure is obtained. The electron microscope photo of the product is shown in Figure 1, XRD The photo is shown in Figure 3, wherein the diameter of the nano-copper particle as the core is 50nm, and the length of the carbon tube covering the nano-copper particle is 200nm.
实施例 2Example 2
利用喷雾燃烧反应器,将硝酸铜和甲醇配制成溶液,雾化后通入燃烧反应器进行燃烧反应,其中,硝酸铜的摩尔浓度为0.1mol/L,进料速率为10 ml/min,燃烧用空气的进料量为1.0 m3/h,反应温度为1800℃。同时在反应器内通入氮气稀释的乙炔气体,用以稀释乙炔的氮气流量为0.3m3/h,乙炔0.1 m3/h。纳米铜颗粒在火焰中先生成,乙炔在纳米铜的表面分解生成纳米碳管,最终得到一种具有核壳结构的纳米铜/纳米碳管复合材料,产物的电镜照片如图2所示。其中,纳米铜颗粒粒径为20nm,碳管长度为500nm。Using a spray combustion reactor, copper nitrate and methanol are prepared into a solution, which is atomized and passed into the combustion reactor for combustion reaction. The molar concentration of copper nitrate is 0.1mol/L, and the feed rate is 10 ml/min. The feed rate of air is 1.0 m3 /h, and the reaction temperature is 1800°C. At the same time, nitrogen-diluted acetylene gas was introduced into the reactor, the flow rate of nitrogen gas for diluting acetylene was 0.3 m3 /h, and the flow rate of acetylene was 0.1 m3 /h. Nano-copper particles are first formed in the flame, and acetylene decomposes on the surface of nano-copper to form carbon nanotubes, and finally a nano-copper/carbon nanotube composite material with a core-shell structure is obtained. The electron microscope photo of the product is shown in Figure 2. Wherein, the diameter of the nano-copper particle is 20nm, and the length of the carbon tube is 500nm.
实施例 3Example 3
利用喷雾燃烧反应器,将硫酸铜和乙醇配制成溶液,雾化后通入燃烧反应器进行燃烧反应,其中,硫酸铜的摩尔浓度为0.3mol/L,进料速率为7 ml/min,燃烧用空气的进料量为2.0m3/h,反应温度为1500℃。同时在反应器内通入氮气稀释的乙炔气体,用以稀释乙炔的氮气流量为0.5m3/h,乙炔0.05 m3/h。纳米铜颗粒在火焰中先生成,乙炔在纳米铜的表面分解生成纳米碳管,最终得到一种具有核壳结构的纳米铜/纳米碳管复合材料,其中,纳米铜颗粒粒径为30nm,碳管长度为400nm。Using a spray combustion reactor, copper sulfate and ethanol are prepared into a solution, which is atomized and passed into the combustion reactor for combustion reaction, wherein the molar concentration of copper sulfate is 0.3 mol/L, and the feed rate is 7 ml/min. The feed rate of air is 2.0 m3 /h, and the reaction temperature is 1500°C. At the same time, nitrogen-diluted acetylene gas is introduced into the reactor, the flow rate of nitrogen gas for diluting acetylene is 0.5 m3 /h, and the flow rate of acetylene is 0.05 m3 /h. Nano-copper particles are first generated in the flame, and acetylene decomposes on the surface of nano-copper to form carbon nanotubes, and finally a nano-copper/carbon nanotube composite material with a core-shell structure is obtained, wherein the nano-copper particles have a particle size of 30nm and carbon nanotubes. The tube length is 400 nm.
Claims (4)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2012102362309ACN102779986A (en) | 2012-07-10 | 2012-07-10 | Preparation method of nanocomposite material with core shell structure |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2012102362309ACN102779986A (en) | 2012-07-10 | 2012-07-10 | Preparation method of nanocomposite material with core shell structure |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN102779986Atrue CN102779986A (en) | 2012-11-14 |
Family
ID=47124825
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2012102362309APendingCN102779986A (en) | 2012-07-10 | 2012-07-10 | Preparation method of nanocomposite material with core shell structure |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102779986A (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104637569A (en)* | 2013-11-07 | 2015-05-20 | 爱迪克科技股份有限公司 | Lead-free nano conductive paste material |
| CN104841924B (en)* | 2014-02-19 | 2017-11-21 | 中国科学院大连化学物理研究所 | A kind of preparation method of the fully enclosed metal nanoparticle of carbon |
| CN110697686A (en)* | 2019-09-17 | 2020-01-17 | 北京化工大学 | A method for preparing carbon nanotubes by heating powder |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101954480A (en)* | 2010-11-08 | 2011-01-26 | 华东理工大学 | Method for preparing carbon-coated core-shell nanoparticles continuously |
- 2012
- 2012-07-10CNCN2012102362309Apatent/CN102779986A/enactivePending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101954480A (en)* | 2010-11-08 | 2011-01-26 | 华东理工大学 | Method for preparing carbon-coated core-shell nanoparticles continuously |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104637569A (en)* | 2013-11-07 | 2015-05-20 | 爱迪克科技股份有限公司 | Lead-free nano conductive paste material |
| CN104841924B (en)* | 2014-02-19 | 2017-11-21 | 中国科学院大连化学物理研究所 | A kind of preparation method of the fully enclosed metal nanoparticle of carbon |
| CN110697686A (en)* | 2019-09-17 | 2020-01-17 | 北京化工大学 | A method for preparing carbon nanotubes by heating powder |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101954480B (en) | Method for preparing carbon-coated core-shell nanoparticles continuously | |
| Reddy et al. | A new one-step synthesis method for coating multi-walled carbon nanotubes with cuprous oxide nanoparticles | |
| Shen et al. | One step hydrothermal synthesis of TiO 2-reduced graphene oxide sheets | |
| Shen et al. | One-pot hydrothermal synthesis of Ag-reduced graphene oxide composite with ionic liquid | |
| Yusoff et al. | Core-shell Fe3O4-ZnO nanoparticles decorated on reduced graphene oxide for enhanced photoelectrochemical water splitting | |
| MamathaKumari et al. | Nanohybrid of titania/carbon nanotubes–nanohorns: a promising photocatalyst for enhanced hydrogen production under solar irradiation | |
| Fang et al. | CuO/TiO2 nanocrystals grown on graphene as visible-light responsive photocatalytic hybrid materials | |
| Zhang et al. | Co3O4 nanoparticles on the surface of halloysite nanotubes | |
| Feng et al. | Controllable synthesis of hexagon-shaped β-AgI nanoplates in reactable ionic liquid and their photocatalytic activity | |
| CN103143370B (en) | Preparation method of sulfide/graphene composite nano material | |
| Zhang et al. | One–step preparation of CNTs/InVO4 hollow nanofibers by electrospinning and its photocatalytic performance under visible light | |
| CN105161700A (en) | Molybdenum trioxide coated molybdenum doped titanium dioxide nano composite particle and preparation method and application thereof | |
| CN102718209B (en) | Graphene preparation method based on ferrous ion reduction | |
| CN101177245A (en) | Preparation method of nanostructure oxide powder | |
| CN101633498A (en) | Preparation method of boron nitride nano tube with controllable dimension | |
| Wu et al. | One-step synthesis of hierarchical metal oxide nanosheet/carbon nanotube composites by chemical vapor deposition | |
| CN114318406A (en) | A kind of carbon dioxide reduction catalyst for ethylene production, catalytic electrode and preparation method | |
| Chen et al. | A facile hydrothermal strategy for synthesis of SnO2 nanorods-graphene nanocomposites for high performance photocatalysis | |
| CN102779986A (en) | Preparation method of nanocomposite material with core shell structure | |
| CN103043712B (en) | A preparation method for catalytic growth of tin oxide nanowires by noble metal gold particles | |
| Rajarao et al. | GreenApproach to decorate multi-walled carbon nanotubes by metal/metal oxide nanoparticles | |
| CN103203234B (en) | Preparation method of high-dispersion supported nano metal Fe-based catalyst | |
| Tavakol et al. | Gold-decorated sulfur-doped carbon nanotubes as electrocatalyst in hydrogen evolution reaction | |
| CN102010621A (en) | In-situ coating and surface modification method in gas phase powder synthesis process | |
| CN102020264A (en) | Method for preparing phosphorus doped multi-walled carbon nano tube |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C53 | Correction of patent of invention or patent application | ||
| CB03 | Change of inventor or designer information | Inventor after:Li Chunzhong Inventor after:Hu Yanjie Inventor after:Qian Qi Inventor after:Wang Yundong Inventor before:Li Chunzhong Inventor before:Hu Yanjie Inventor before:Qian Qi Inventor before:Wang Yundong | |
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | Application publication date:20121114 |