CN101856509A - Injectable calcium magnesium bone cement and its preparation method and application - Google Patents
Injectable calcium magnesium bone cement and its preparation method and applicationDownload PDFInfo
- Publication number
- CN101856509A CN101856509ACN201010205094ACN201010205094ACN101856509ACN 101856509 ACN101856509 ACN 101856509ACN 201010205094 ACN201010205094 ACN 201010205094ACN 201010205094 ACN201010205094 ACN 201010205094ACN 101856509 ACN101856509 ACN 101856509A
- Authority
- CN
- China
- Prior art keywords
- phosphate
- calcium
- magnesium
- bone cement
- injectable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000002639bone cementSubstances0.000titleclaimsabstractdescription56
- ZFXVRMSLJDYJCH-UHFFFAOYSA-Ncalcium magnesiumChemical compound[Mg].[Ca]ZFXVRMSLJDYJCH-UHFFFAOYSA-N0.000titleclaimsabstractdescription37
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription12
- 239000004375DextrinSubstances0.000claimsabstractdescription58
- 229920001353DextrinPolymers0.000claimsabstractdescription58
- 235000019425dextrinNutrition0.000claimsabstractdescription58
- QORWJWZARLRLPR-UHFFFAOYSA-Htricalcium bis(phosphate)Chemical compound[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OQORWJWZARLRLPR-UHFFFAOYSA-H0.000claimsabstractdescription42
- 239000000843powderSubstances0.000claimsabstractdescription41
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsabstractdescription31
- GVALZJMUIHGIMD-UHFFFAOYSA-Hmagnesium phosphateChemical compound[Mg+2].[Mg+2].[Mg+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OGVALZJMUIHGIMD-UHFFFAOYSA-H0.000claimsabstractdescription24
- 239000004137magnesium phosphateSubstances0.000claimsabstractdescription24
- 229910000157magnesium phosphateInorganic materials0.000claimsabstractdescription24
- 229960002261magnesium phosphateDrugs0.000claimsabstractdescription24
- 235000010994magnesium phosphatesNutrition0.000claimsabstractdescription24
- 239000007790solid phaseSubstances0.000claimsabstractdescription18
- 239000002131composite materialSubstances0.000claimsabstractdescription16
- 239000007788liquidSubstances0.000claimsabstractdescription14
- NBIIXXVUZAFLBC-UHFFFAOYSA-LPhosphate ion(2-)Chemical compoundOP([O-])([O-])=ONBIIXXVUZAFLBC-UHFFFAOYSA-L0.000claimsabstractdescription9
- 238000002156mixingMethods0.000claimsabstractdescription9
- 210000000988bone and boneAnatomy0.000claimsabstractdescription7
- 230000037314wound repairEffects0.000claimsabstractdescription3
- 239000001506calcium phosphateSubstances0.000claimsdescription25
- 229910000389calcium phosphateInorganic materials0.000claimsdescription22
- 235000011010calcium phosphatesNutrition0.000claimsdescription22
- 239000000203mixtureSubstances0.000claimsdescription20
- 229910052588hydroxylapatiteInorganic materials0.000claimsdescription15
- 238000000034methodMethods0.000claimsdescription15
- XYJRXVWERLGGKC-UHFFFAOYSA-Dpentacalcium;hydroxide;triphosphateChemical compound[OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OXYJRXVWERLGGKC-UHFFFAOYSA-D0.000claimsdescription15
- 239000002245particleSubstances0.000claimsdescription14
- BNIILDVGGAEEIG-UHFFFAOYSA-Ldisodium hydrogen phosphateChemical compound[Na+].[Na+].OP([O-])([O-])=OBNIILDVGGAEEIG-UHFFFAOYSA-L0.000claimsdescription13
- 238000007711solidificationMethods0.000claimsdescription11
- 230000008023solidificationEffects0.000claimsdescription11
- 229920002774MaltodextrinPolymers0.000claimsdescription9
- 239000005913MaltodextrinSubstances0.000claimsdescription9
- FUFJGUQYACFECW-UHFFFAOYSA-Lcalcium hydrogenphosphateChemical compound[Ca+2].OP([O-])([O-])=OFUFJGUQYACFECW-UHFFFAOYSA-L0.000claimsdescription9
- 235000019700dicalcium phosphateNutrition0.000claimsdescription9
- 229940035034maltodextrinDrugs0.000claimsdescription9
- 229910000392octacalcium phosphateInorganic materials0.000claimsdescription6
- YIGWVOWKHUSYER-UHFFFAOYSA-Ftetracalcium;hydrogen phosphate;diphosphateChemical compound[Ca+2].[Ca+2].[Ca+2].[Ca+2].OP([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OYIGWVOWKHUSYER-UHFFFAOYSA-F0.000claimsdescription6
- GBNXLQPMFAUCOI-UHFFFAOYSA-Htetracalcium;oxygen(2-);diphosphateChemical compound[O-2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OGBNXLQPMFAUCOI-UHFFFAOYSA-H0.000claimsdescription5
- JUNWLZAGQLJVLR-UHFFFAOYSA-Jcalcium diphosphateChemical compound[Ca+2].[Ca+2].[O-]P([O-])(=O)OP([O-])([O-])=OJUNWLZAGQLJVLR-UHFFFAOYSA-J0.000claimsdescription4
- 229940043256calcium pyrophosphateDrugs0.000claimsdescription4
- 235000019821dicalcium diphosphateNutrition0.000claimsdescription4
- 229910052587fluorapatiteInorganic materials0.000claimsdescription4
- 229940077441fluorapatiteDrugs0.000claimsdescription4
- VSIIXMUUUJUKCM-UHFFFAOYSA-Dpentacalcium;fluoride;triphosphateChemical compound[F-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OVSIIXMUUUJUKCM-UHFFFAOYSA-D0.000claimsdescription4
- ZPWVASYFFYYZEW-UHFFFAOYSA-Ldipotassium hydrogen phosphateChemical compound[K+].[K+].OP([O-])([O-])=OZPWVASYFFYYZEW-UHFFFAOYSA-L0.000claimsdescription3
- 229910000402monopotassium phosphateInorganic materials0.000claimsdescription3
- 235000019796monopotassium phosphateNutrition0.000claimsdescription3
- 229910000403monosodium phosphateInorganic materials0.000claimsdescription3
- 235000019799monosodium phosphateNutrition0.000claimsdescription3
- PJNZPQUBCPKICU-UHFFFAOYSA-Nphosphoric acid;potassiumChemical compound[K].OP(O)(O)=OPJNZPQUBCPKICU-UHFFFAOYSA-N0.000claimsdescription3
- AJPJDKMHJJGVTQ-UHFFFAOYSA-Msodium dihydrogen phosphateChemical compound[Na+].OP(O)([O-])=OAJPJDKMHJJGVTQ-UHFFFAOYSA-M0.000claimsdescription3
- 239000004575stoneSubstances0.000claimsdescription2
- 229940025708injectable productDrugs0.000claims1
- 239000012071phaseSubstances0.000abstractdescription25
- 239000002994raw materialSubstances0.000abstractdescription4
- 239000007791liquid phaseSubstances0.000abstractdescription2
- 239000002002slurrySubstances0.000description24
- 229960001714calcium phosphateDrugs0.000description20
- 239000000243solutionSubstances0.000description12
- 239000008367deionised waterSubstances0.000description10
- 229910021641deionized waterInorganic materials0.000description10
- 239000003795chemical substances by applicationSubstances0.000description9
- 230000008439repair processEffects0.000description9
- 239000008346aqueous phaseSubstances0.000description7
- 230000000694effectsEffects0.000description7
- 239000000463materialSubstances0.000description7
- 206010017076FractureDiseases0.000description5
- 239000004568cementSubstances0.000description5
- 230000007547defectEffects0.000description5
- 238000006703hydration reactionMethods0.000description5
- 239000006072pasteSubstances0.000description5
- 238000004458analytical methodMethods0.000description4
- 239000011575calciumSubstances0.000description4
- 238000011056performance testMethods0.000description4
- 230000008569processEffects0.000description4
- 239000000126substanceSubstances0.000description4
- 229920002472StarchPolymers0.000description3
- 210000004369bloodAnatomy0.000description3
- 239000008280bloodSubstances0.000description3
- 230000008859changeEffects0.000description3
- 238000002474experimental methodMethods0.000description3
- 239000003292glueSubstances0.000description3
- 230000036571hydrationEffects0.000description3
- 238000002347injectionMethods0.000description3
- 239000007924injectionSubstances0.000description3
- 239000011777magnesiumSubstances0.000description3
- CPLXHLVBOLITMK-UHFFFAOYSA-Nmagnesium oxideInorganic materials[Mg]=OCPLXHLVBOLITMK-UHFFFAOYSA-N0.000description3
- 239000000376reactantSubstances0.000description3
- 239000008107starchSubstances0.000description3
- 235000019698starchNutrition0.000description3
- 239000000606toothpasteSubstances0.000description3
- 229940034610toothpasteDrugs0.000description3
- IXPNQXFRVYWDDI-UHFFFAOYSA-N1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamideChemical compoundCN1CC(C(N)=N)C(=O)NC1=OIXPNQXFRVYWDDI-UHFFFAOYSA-N0.000description2
- 229920001661ChitosanPolymers0.000description2
- JLVVSXFLKOJNIY-UHFFFAOYSA-NMagnesium ionChemical compound[Mg+2]JLVVSXFLKOJNIY-UHFFFAOYSA-N0.000description2
- 229910019142PO4Inorganic materials0.000description2
- 230000002378acidificating effectEffects0.000description2
- MXZRMHIULZDAKC-UHFFFAOYSA-Lammonium magnesium phosphateChemical compound[NH4+].[Mg+2].[O-]P([O-])([O-])=OMXZRMHIULZDAKC-UHFFFAOYSA-L0.000description2
- 150000007514basesChemical class0.000description2
- 239000012620biological materialSubstances0.000description2
- 230000015572biosynthetic processEffects0.000description2
- 210000001124body fluidAnatomy0.000description2
- 239000010839body fluidSubstances0.000description2
- 229910052791calciumInorganic materials0.000description2
- YYRMJZQKEFZXMX-UHFFFAOYSA-Lcalcium bis(dihydrogenphosphate)Chemical compound[Ca+2].OP(O)([O-])=O.OP(O)([O-])=OYYRMJZQKEFZXMX-UHFFFAOYSA-L0.000description2
- 239000001913celluloseSubstances0.000description2
- 229920002678cellulosePolymers0.000description2
- 238000011161developmentMethods0.000description2
- 230000018109developmental processEffects0.000description2
- NBIIXXVUZAFLBC-UHFFFAOYSA-MdihydrogenphosphateChemical groupOP(O)([O-])=ONBIIXXVUZAFLBC-UHFFFAOYSA-M0.000description2
- 238000011049fillingMethods0.000description2
- 239000000499gelSubstances0.000description2
- 230000006872improvementEffects0.000description2
- 238000000338in vitroMethods0.000description2
- 230000008595infiltrationEffects0.000description2
- 238000001764infiltrationMethods0.000description2
- 208000014674injuryDiseases0.000description2
- 150000002500ionsChemical class0.000description2
- 230000009916joint effectEffects0.000description2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-Nlactic acidChemical compoundCC(O)C(O)=OJVTAAEKCZFNVCJ-UHFFFAOYSA-N0.000description2
- 229910001425magnesium ionInorganic materials0.000description2
- 239000000395magnesium oxideSubstances0.000description2
- 235000021317phosphateNutrition0.000description2
- 150000003013phosphoric acid derivativesChemical class0.000description2
- 238000007712rapid solidificationMethods0.000description2
- 238000011160researchMethods0.000description2
- 238000007493shaping processMethods0.000description2
- 235000010413sodium alginateNutrition0.000description2
- 239000000661sodium alginateSubstances0.000description2
- 229940005550sodium alginateDrugs0.000description2
- 238000003756stirringMethods0.000description2
- 238000001356surgical procedureMethods0.000description2
- 230000008733traumaEffects0.000description2
- 229910000391tricalcium phosphateInorganic materials0.000description2
- 235000019731tricalcium phosphateNutrition0.000description2
- 229940078499tricalcium phosphateDrugs0.000description2
- QTBSBXVTEAMEQO-UHFFFAOYSA-MAcetateChemical compoundCC([O-])=OQTBSBXVTEAMEQO-UHFFFAOYSA-M0.000description1
- 239000004114Ammonium polyphosphateSubstances0.000description1
- 239000004382AmylaseSubstances0.000description1
- 102000013142AmylasesHuman genes0.000description1
- 108010065511AmylasesProteins0.000description1
- 208000017234Bone cystDiseases0.000description1
- 206010056377Bone tuberculosisDiseases0.000description1
- OYPRJOBELJOOCE-UHFFFAOYSA-NCalciumChemical compound[Ca]OYPRJOBELJOOCE-UHFFFAOYSA-N0.000description1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-NCalcium cationChemical compound[Ca+2]BHPQYMZQTOCNFJ-UHFFFAOYSA-N0.000description1
- 229920002134Carboxymethyl cellulosePolymers0.000description1
- 206010010214Compression fractureDiseases0.000description1
- 235000019739DicalciumphosphateNutrition0.000description1
- 229910019440Mg(OH)Inorganic materials0.000description1
- 229920000881Modified starchPolymers0.000description1
- GXCLVBGFBYZDAG-UHFFFAOYSA-NN-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amineChemical compoundCN(CCC1=CNC2=C1C=CC=C2)CC=CGXCLVBGFBYZDAG-UHFFFAOYSA-N0.000description1
- 208000009360Osteoarticular TuberculosisDiseases0.000description1
- 208000001132OsteoporosisDiseases0.000description1
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical classOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000description1
- 206010037802Radius fractureDiseases0.000description1
- 239000004823Reactive adhesiveSubstances0.000description1
- 208000026137Soft tissue injuryDiseases0.000description1
- 208000007536ThrombosisDiseases0.000description1
- 238000002441X-ray diffractionMethods0.000description1
- YDHWWBZFRZWVHO-UHFFFAOYSA-H[oxido-[oxido(phosphonatooxy)phosphoryl]oxyphosphoryl] phosphateChemical compound[O-]P([O-])(=O)OP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=OYDHWWBZFRZWVHO-UHFFFAOYSA-H0.000description1
- 230000001133accelerationEffects0.000description1
- 239000002253acidSubstances0.000description1
- 230000009471actionEffects0.000description1
- 239000002390adhesive tapeSubstances0.000description1
- LFVGISIMTYGQHF-UHFFFAOYSA-Nammonium dihydrogen phosphateChemical compound[NH4+].OP(O)([O-])=OLFVGISIMTYGQHF-UHFFFAOYSA-N0.000description1
- 229910000387ammonium dihydrogen phosphateInorganic materials0.000description1
- 235000019826ammonium polyphosphateNutrition0.000description1
- 229920001276ammonium polyphosphatePolymers0.000description1
- 235000019418amylaseNutrition0.000description1
- 150000001450anionsChemical class0.000description1
- 238000013459approachMethods0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 239000003462bioceramicSubstances0.000description1
- 230000000740bleeding effectEffects0.000description1
- 230000036770blood supplyEffects0.000description1
- 210000004204blood vesselAnatomy0.000description1
- 239000000316bone substituteSubstances0.000description1
- 210000000459calcaneusAnatomy0.000description1
- 229960005069calciumDrugs0.000description1
- 235000010410calcium alginateNutrition0.000description1
- 239000000648calcium alginateSubstances0.000description1
- 229960002681calcium alginateDrugs0.000description1
- 229940062672calcium dihydrogen phosphateDrugs0.000description1
- 229910001424calcium ionInorganic materials0.000description1
- BRPQOXSCLDDYGP-UHFFFAOYSA-Ncalcium oxideChemical compound[O-2].[Ca+2]BRPQOXSCLDDYGP-UHFFFAOYSA-N0.000description1
- 239000000292calcium oxideSubstances0.000description1
- ODINCKMPIJJUCX-UHFFFAOYSA-Ncalcium oxideInorganic materials[Ca]=OODINCKMPIJJUCX-UHFFFAOYSA-N0.000description1
- OKHHGHGGPDJQHR-YMOPUZKJSA-Lcalcium;(2s,3s,4s,5s,6r)-6-[(2r,3s,4r,5s,6r)-2-carboxy-6-[(2r,3s,4r,5s,6r)-2-carboxylato-4,5,6-trihydroxyoxan-3-yl]oxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylateChemical compound[Ca+2].O[C@@H]1[C@H](O)[C@H](O)O[C@@H](C([O-])=O)[C@H]1O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[C@H]2[C@H]([C@@H](O)[C@H](O)[C@H](O2)C([O-])=O)O)[C@H](C(O)=O)O1OKHHGHGGPDJQHR-YMOPUZKJSA-L0.000description1
- 244000309466calfSpecies0.000description1
- 239000001768carboxy methyl celluloseSubstances0.000description1
- 235000010948carboxy methyl celluloseNutrition0.000description1
- 125000002057carboxymethyl groupChemical group[H]OC(=O)C([H])([H])[*]0.000description1
- 239000008112carboxymethyl-celluloseSubstances0.000description1
- 210000000748cardiovascular systemAnatomy0.000description1
- 238000005266castingMethods0.000description1
- 230000015556catabolic processEffects0.000description1
- 239000003054catalystSubstances0.000description1
- 238000004113cell cultureMethods0.000description1
- 239000006285cell suspensionSubstances0.000description1
- 230000009920chelationEffects0.000description1
- 239000000701coagulantSubstances0.000description1
- 230000015271coagulationEffects0.000description1
- 238000005345coagulationMethods0.000description1
- 150000001875compoundsChemical class0.000description1
- 238000010276constructionMethods0.000description1
- 239000013078crystalSubstances0.000description1
- 238000012258culturingMethods0.000description1
- 230000006378damageEffects0.000description1
- 238000006731degradation reactionMethods0.000description1
- 230000001934delayEffects0.000description1
- 210000004262dental pulp cavityAnatomy0.000description1
- 230000002542deteriorative effectEffects0.000description1
- NEFBYIFKOOEVPA-UHFFFAOYSA-Kdicalcium phosphateChemical compound[Ca+2].[Ca+2].[O-]P([O-])([O-])=ONEFBYIFKOOEVPA-UHFFFAOYSA-K0.000description1
- 229910000390dicalcium phosphateInorganic materials0.000description1
- 229940038472dicalcium phosphateDrugs0.000description1
- 239000003814drugSubstances0.000description1
- 235000013305foodNutrition0.000description1
- 238000007429general methodMethods0.000description1
- 239000011521glassSubstances0.000description1
- 230000012010growthEffects0.000description1
- 238000010438heat treatmentMethods0.000description1
- 239000000017hydrogelSubstances0.000description1
- 230000007062hydrolysisEffects0.000description1
- 238000006460hydrolysis reactionMethods0.000description1
- 229920003063hydroxymethyl cellulosePolymers0.000description1
- 229940031574hydroxymethyl celluloseDrugs0.000description1
- 230000000642iatrogenic effectEffects0.000description1
- 238000011065in-situ storageMethods0.000description1
- 229910010272inorganic materialInorganic materials0.000description1
- 239000011147inorganic materialSubstances0.000description1
- 239000004310lactic acidSubstances0.000description1
- 235000014655lactic acidNutrition0.000description1
- 229910052749magnesiumInorganic materials0.000description1
- AXZKOIWUVFPNLO-UHFFFAOYSA-Nmagnesium;oxygen(2-)Chemical compound[O-2].[Mg+2]AXZKOIWUVFPNLO-UHFFFAOYSA-N0.000description1
- 230000014759maintenance of locationEffects0.000description1
- 238000004519manufacturing processMethods0.000description1
- 230000010534mechanism of actionEffects0.000description1
- 235000019426modified starchNutrition0.000description1
- 235000019837monoammonium phosphateNutrition0.000description1
- 235000019691monocalcium phosphateNutrition0.000description1
- 239000011858nanopowderSubstances0.000description1
- 231100000344non-irritatingToxicity0.000description1
- 230000001590oxidative effectEffects0.000description1
- 239000011148porous materialSubstances0.000description1
- 230000002980postoperative effectEffects0.000description1
- 238000002203pretreatmentMethods0.000description1
- 230000002265preventionEffects0.000description1
- 230000002035prolonged effectEffects0.000description1
- 238000000746purificationMethods0.000description1
- 230000035484reaction timeEffects0.000description1
- 230000009467reductionEffects0.000description1
- 230000001105regulatory effectEffects0.000description1
- 150000003839saltsChemical class0.000description1
- 210000002966serumAnatomy0.000description1
- 239000012890simulated body fluidSubstances0.000description1
- 238000004088simulationMethods0.000description1
- 239000002893slagSubstances0.000description1
- 150000003384small moleculesChemical class0.000description1
- 239000007787solidSubstances0.000description1
- 208000028528solitary bone cystDiseases0.000description1
- 206010041569spinal fractureDiseases0.000description1
- 238000001694spray dryingMethods0.000description1
- 239000003381stabilizerSubstances0.000description1
- 229910052567struviteInorganic materials0.000description1
- 230000002195synergetic effectEffects0.000description1
- 239000002562thickening agentSubstances0.000description1
- 210000001519tissueAnatomy0.000description1
- 230000017423tissue regenerationEffects0.000description1
Images
Landscapes
- Materials For Medical Uses (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于医用修复材料领域,涉及一种用于形成可注射钙镁骨水泥的产品,一种可注射钙镁骨水泥及其制备方法与应用,特别涉及一种用于形成水相高效抗溃散快速固化可注射钙镁骨水泥的产品,一种水相高效抗溃散快速固化可注射钙镁骨水泥及其制备方法与应用。The invention belongs to the field of medical repair materials, and relates to a product for forming an injectable calcium-magnesium bone cement, an injectable calcium-magnesium bone cement and its preparation method and application, in particular to a product for forming an aqueous phase with high efficiency and anti-collapse A fast-curing injectable calcium-magnesium bone cement product, a water-phase high-efficiency anti-collapse rapid-curing injectable calcium-magnesium bone cement and its preparation method and application.
背景技术Background technique
临床上常见的一些骨折,如桡骨远端骨折、胫骨平台骨折、跟骨骨折、椎体压缩性骨折等,只需要闭合复位、经皮注射骨修复材料就可进行缺损修复治疗,避免切开手术部位,简化了手术过程,且可减少医源性的软组织损伤和入路相关并发症,不破坏修复区血供,对患者的创伤最小,费用较低,易于为患者所接受。目前,发展高效微创的可注射性骨替代修复材料已成为必然方向,且为满足临床应用的需要,可注射骨水泥应具有合理的固化时间(一般为8-20分钟)、较高的强度以及良好的抗溃散性能。Some common clinical fractures, such as distal radius fractures, tibial plateau fractures, calcaneal fractures, vertebral compression fractures, etc., only need closed reduction and percutaneous injection of bone repair materials to repair the defect, avoiding open surgery It simplifies the operation process, reduces iatrogenic soft tissue injury and approach-related complications, does not damage the blood supply of the repair area, minimizes trauma to the patient, and is easy to accept by patients. At present, the development of highly efficient and minimally invasive injectable bone substitute repair materials has become an inevitable direction, and in order to meet the needs of clinical applications, injectable bone cement should have a reasonable curing time (generally 8-20 minutes) and high strength. And good anti-collapse performance.
水相可注射磷酸钙骨水泥(Injectable calcium phosphate cement,ICPC)由于具有“定点注射、原位固化、生物相容、逐步降解”的特性,能根据缺损部位任意塑型,近10年来已取得了长足的发展。然而,为了获得更好的可注射性能,相对于常规骨水泥,微创治疗用的ICPC具有较高的液固比,导致了固化时间的延长。与此同时,由于血浆中一些离子和大多数有机物具有阻止或延迟固化产物羟基磷灰石(HA)形成的作用,这又使得ICPC的凝结时间较体外长得多。较长的固化时间既不利于手术进行,又易使ICPC浆体被手术部位的出血冲散或冲走,继而恶化浆体的抗溃散性能。若抗溃散性不好,在注射到体内后固化前,浆体与血浆或体液接触后易被侵蚀而稀散不成型,最终影响浆体的固化甚至限制其临床应用。特别是在经皮椎体成型中,未固化的微纳米粉体一旦随血液进入心血管系统,则会造成血管堵塞,引发血栓及其它并发症。目前,这一缺陷极大地限制了ICPC在临床中的应用(Biomaterials,1996,17:1429-1435;Bioceramics,1996,9:235-238)。因此,有必要在提高浆体抗溃散性能的基础上,对ICPC进行固化调控。Water-phase injectable calcium phosphate cement (ICPC) has the characteristics of "fixed-point injection, in-situ solidification, biocompatibility, and gradual degradation", and can be molded arbitrarily according to the defect site. In the past 10 years, it has achieved Great development. However, in order to obtain better injectability, compared with conventional bone cement, ICPC for minimally invasive treatment has a higher liquid-solid ratio, resulting in a longer curing time. At the same time, because some ions and most organic substances in plasma can prevent or delay the formation of hydroxyapatite (HA), which makes the coagulation time of ICPC much longer than that in vitro. A longer curing time is not conducive to the operation, and it is easy for the ICPC slurry to be dispersed or washed away by bleeding at the surgical site, thereby deteriorating the anti-collapse performance of the slurry. If the collapse resistance is not good, before the slurry is injected into the body and solidified, the slurry will be easily eroded after contact with plasma or body fluid, and will be scattered and not formed, which will eventually affect the solidification of the slurry and even limit its clinical application. Especially in percutaneous vertebral body shaping, once the uncured micro-nano powder enters the cardiovascular system with the blood, it will cause blood vessel blockage, cause thrombosis and other complications. Currently, this defect greatly limits the clinical application of ICPC (Biomaterials, 1996, 17: 1429-1435; Bioceramics, 1996, 9: 235-238). Therefore, it is necessary to control the curing of ICPC on the basis of improving the anti-collapse performance of the slurry.
抗溃散性能的提高可以通过加入抗溃散剂或改变本身的配方来实现。Ishikaw等(J Biomed Mater Res,1997,36(3):393-399)在固化液中添加海藻酸钠,结果发现浆体调和后立即放入水中较少溃散,并能正常固化。其作用机理是海藻酸钠和浆体中的钙离子结合形成不溶于水的藻酸钙水凝胶,从而赋予浆体抗溃散性。Takechi等(J Mater sci:Mater Med,1996,7(6):317-322)通过在ICPC中加入羟甲基纤维素、羧甲基纤维素、壳聚糖醋酸盐、壳聚糖乳酸盐等外加剂,使得ICPC的可注射性提高,但延长了固化时间。王莹等(无机材料学报,2006,21(6):1435-1442)将纤维素作为抗溃散剂加入到ICPC,发现制备的浆体具有优良的抗水性能,抗压强度为20-30MPa,固化产物为HA,生物相容性好,但固化时间为50-55min,无法满足临床的实际需要。上述抗溃散剂在一定程度上限制了外界环境中水相向浆体内部的渗入改善了浆体的抗溃散性,但在固化过程中仍有一定的粉体逸散,且延缓了浆体的固化反应时间。The improvement of anti-collapse performance can be realized by adding anti-collapse agent or changing its own formula. Ishikaw et al. (J Biomed Mater Res, 1997, 36(3): 393-399) added sodium alginate to the solidification solution, and found that the slurry was less collapsible when placed in water immediately after mixing, and it could be cured normally. Its mechanism of action is that sodium alginate combines with calcium ions in the slurry to form a water-insoluble calcium alginate hydrogel, thereby endowing the slurry with anti-collapse properties. Takechi et al. (J Mater sci: Mater Med, 1996, 7 (6): 317-322) by adding hydroxymethyl cellulose, carboxymethyl cellulose, chitosan acetate, chitosan lactic acid in ICPC Admixtures such as salt improve the injectability of ICPC, but prolong the curing time. Wang Ying et al. (Journal of Inorganic Materials, 2006, 21(6): 1435-1442) added cellulose to ICPC as an anti-collapse agent, and found that the prepared slurry had excellent water resistance, and the compressive strength was 20-30MPa. The cured product is HA, which has good biocompatibility, but the curing time is 50-55 minutes, which cannot meet the actual clinical needs. The above-mentioned anti-collapse agent limits the infiltration of the water phase in the external environment to the inside of the slurry to a certain extent and improves the anti-collapse property of the slurry, but there is still a certain amount of powder escaping during the curing process, and delays the curing of the slurry Reaction time.
糊精是淀粉质原料在受到加热、酸或淀粉酶作用下发生分解和水解时而转化生成的小分子中间物质。根据对淀粉的预处理方法及热处理条件的不同,糊精可分为白糊精、黄糊精、英国胶和麦芽糊精。一般采用酸性或氧化性催化剂制备白糊精或黄糊精;直接焙烧而得的糊精称为英国胶。麦芽糊精是由淀粉经低度水解、净化、喷雾干燥制成,不含游离淀粉的淀粉衍生物。糊精易溶于水,具有很强的胶粘性、良好的流动性和优良的保水性能,赋型性强,可形成水溶性膜并粘接材料,在牙膏生产上可代替部分羧甲基纤维素(CMC),作为增稠剂和稳定剂可改善牙膏的结构,并广泛应用于医药、食品、造纸、铸造、壁纸、标签、邮票、胶带纸等领域,具有良好的生物相容性。Dextrin is a small molecule intermediate substance converted from starchy raw materials when they are decomposed and hydrolyzed by heat, acid or amylase. According to the pretreatment method of starch and heat treatment conditions, dextrin can be divided into white dextrin, yellow dextrin, English gum and maltodextrin. Generally, acidic or oxidative catalysts are used to prepare white dextrin or yellow dextrin; the dextrin obtained by direct roasting is called British glue. Maltodextrin is a starch derivative that is made from low-degree hydrolysis, purification, and spray-drying of starch, and does not contain free starch. Dextrin is easily soluble in water, has strong adhesiveness, good fluidity and excellent water retention performance, strong formability, can form water-soluble film and bond materials, and can replace part of carboxymethyl in toothpaste production Cellulose (CMC), as a thickener and stabilizer, can improve the structure of toothpaste, and is widely used in medicine, food, papermaking, casting, wallpaper, labels, stamps, adhesive tape and other fields, and has good biocompatibility.
磷酸镁骨水泥(magnesium phosphate cement,MPC)最早于1945年用作建筑用水泥,之后Brookhaven实验室开发了磷酸镁铵胶凝材料作为快速修补水泥材料。作为一类无机反应型胶粘剂,磷酸镁水泥与磷酸钙骨水泥(CPC)相似,能在人体生理环境下自行固化,水化产物为磷酸镁铵类的生物矿石,生物相容性好(Biomaterials,2002,23:1283-1293)。Lilley等人制备了镁离子替代CaPs的结构(Mg,Ca)Ps体系,认为通过在CaPs体系中加入镁离子能够稳定无定形的CaPs的结构,从而能够调控体系的固化过程及改变体系的固化强度。多年研究表明(Biomed Mater,2008,3(4):1243-1249;J.R.Soc.Interface,2010,doi:10.1098/rsif.2009.0559),MPC凝固快、早期强度高、生物相容性和生物降解性良好,是一种理想的骨缺损修复材料。Magnesium phosphate cement (MPC) was first used as construction cement in 1945, and then Brookhaven Laboratory developed ammonium magnesium phosphate cementitious material as a rapid repair cement material. As a type of inorganic reactive adhesive, magnesium phosphate cement is similar to calcium phosphate bone cement (CPC), which can solidify itself in the physiological environment of the human body. The hydration product is a biomineral of magnesium ammonium phosphate, which has good biocompatibility (Biomaterials, 2002, 23:1283-1293). Lilley et al. prepared a structure (Mg, Ca)Ps system in which magnesium ions replaced CaPs, and believed that adding magnesium ions to the CaPs system could stabilize the structure of amorphous CaPs, thereby regulating the curing process of the system and changing the curing strength of the system. . Years of research have shown (Biomed Mater, 2008, 3(4): 1243-1249; J.R.Soc.Interface, 2010, doi: 10.1098/rsif.2009.0559), MPC has fast solidification, high early strength, biocompatibility and biodegradability Good, it is an ideal material for bone defect repair.
迄今为止未见有糊精提高ICPC浆体抗溃散性、MPC用于调控抗溃散型ICPC固化速度和抗溃散性能的报道。So far, there are no reports that dextrin can improve the collapse resistance of ICPC slurry, and that MPC can be used to regulate the curing speed and collapse resistance of anti-collapse ICPC.
发明内容Contents of the invention
本发明的第一个目的在于克服现有水相可注射磷酸钙骨水泥抗溃散性较差,浆体易被术区出现的血涌冲散或冲走以及降低磷酸钙骨水泥可注射性和延长固化时间等不足,提供一种用于形成可注射钙镁骨水泥的产品。The first object of the present invention is to overcome the poor collapse resistance of the existing water-phase injectable calcium phosphate bone cement, the slurry is easily dispersed or washed away by the blood surge in the operation area and the injectability of the calcium phosphate bone cement is reduced. Insufficient such as prolonging the curing time, a product for forming injectable calcium magnesium bone cement is provided.
本发明的另一个目的在于提供由上述产品制备可注射钙镁骨水泥的方法。Another object of the present invention is to provide a method for preparing injectable calcium-magnesium bone cement from the above product.
本发明的再一个目的在于提供一种由上述方法制备的可注射钙镁骨水泥。Another object of the present invention is to provide an injectable calcium magnesium bone cement prepared by the above method.
本发明的最后一个目的在于提供上述可注射钙镁骨水泥的应用。The last object of the present invention is to provide the application of the above-mentioned injectable calcium-magnesium bone cement.
本发明的用于形成可注射钙镁骨水泥的产品,包括:由复合磷酸钙盐与磷酸镁粉末均匀混合得到的固相粉末;以及由糊精、磷酸氢盐溶于水中得到的固化液。The product for forming injectable calcium-magnesium bone cement of the present invention includes: solid phase powder obtained by uniformly mixing composite calcium phosphate salt and magnesium phosphate powder; and solidified liquid obtained by dissolving dextrin and hydrogen phosphate salt in water.
根据本发明,所述糊精为白糊精、黄糊精、英国胶和麦芽糊精中的至少一种。所述磷酸氢盐为磷酸氢二钠、磷酸二氢钠、磷酸氢二钾和磷酸二氢钾中的至少一种。所述固化液中所述糊精的质量百分比浓度为1%~25%,所述磷酸氢盐的质量百分比浓度1%~18%。According to the present invention, the dextrin is at least one of white dextrin, yellow dextrin, English gum and maltodextrin. The hydrogen phosphate is at least one of disodium hydrogen phosphate, sodium dihydrogen phosphate, dipotassium hydrogen phosphate and potassium dihydrogen phosphate. The mass percentage concentration of the dextrin in the solidified liquid is 1%-25%, and the mass percentage concentration of the hydrogen phosphate is 1%-18%.
根据本发明,所述的复合磷酸钙盐的平均粒径小于10微米,包括:磷酸钙、磷酸四钙、磷酸八钙、磷酸氢钙、羟基磷灰石、氟磷灰石、焦磷酸钙中的一种或它们的混合物。所述复合磷酸钙盐与所述磷酸镁粉末的质量比为(9.5~0.5)∶(0.5~9.5)。According to the present invention, the average particle size of the complex calcium phosphate salt is less than 10 microns, including: calcium phosphate, tetracalcium phosphate, octacalcium phosphate, calcium hydrogen phosphate, hydroxyapatite, fluorapatite, calcium pyrophosphate one or a mixture of them. The mass ratio of the composite calcium phosphate salt to the magnesium phosphate powder is (9.5-0.5): (0.5-9.5).
本发明的可注射钙镁骨水泥的制备方法,将上述产品的所述固化液与所述固相粉末混合均匀,调和形成糊状物;其中,所述固化液与所述固相粉末的质量比为(0.5~3)∶1。优选的,所述固化液与所述固相粉末的质量比为(0.5~0.9)∶1。In the preparation method of the injectable calcium magnesium bone cement of the present invention, the solidified liquid and the solid phase powder of the above product are uniformly mixed to form a paste; wherein, the mass of the solidified liquid and the solid phase powder The ratio is (0.5~3):1. Preferably, the mass ratio of the solidified liquid to the solid phase powder is (0.5-0.9):1.
根据本发明,提供由上述方法制备的可注射钙镁骨水泥,是一种水相高效抗溃散快速固化可注射钙镁骨水泥(fast-setting and anti-washout injectable calcium magnesiium-based bone cement,fa-ICMB)。According to the present invention, the injectable calcium magnesium bone cement prepared by the above method is provided, which is a kind of water-phase high-efficiency anti-collapse fast-setting injectable calcium magnesium bone cement (fast-setting and anti-washout injectable calcium magnesium-based bone cement, fa -ICMB).
本发明的用于形成可注射钙镁骨水泥的产品以及本发明的可注射钙镁骨水泥,均可用于制备骨组织创伤修复用可注射产品。所述糊状物可以通过注射器直接注射填充到手术部位使用,或者在体外人体模拟环境中固化后形成固化体再填充到体内,进行临床应用。本发明制备的水相高效抗溃散快速固化可注射钙镁骨水泥用于治疗骨质疏松和骨组织创伤修复,特别用于桡骨远端、胫骨平台、跟骨等常见骨折及其骨折的辅助固定、髓腔内固定、骨囊肿和骨结核术后修复、根管填充以及椎体成型术,尤其适用于治疗椎体塌陷和防止脊椎骨折。The product for forming injectable calcium-magnesium bone cement of the present invention and the injectable calcium-magnesium bone cement of the present invention can be used to prepare injectable products for bone tissue wound repair. The paste can be directly injected and filled into the surgical site for use through a syringe, or solidified in an in vitro human simulation environment to form a cured body and then filled into the body for clinical application. The water-phase high-efficiency anti-collapse fast-curing injectable calcium-magnesium bone cement prepared by the invention is used for the treatment of osteoporosis and bone tissue trauma repair, especially for common fractures such as distal radius, tibial plateau, calcaneus and auxiliary fixation of fractures , intramedullary fixation, postoperative repair of bone cyst and bone tuberculosis, root canal filling and vertebroplasty, especially for the treatment of vertebral body collapse and prevention of vertebral fractures.
本发明具有原料成本低、制备简单、便于手术操作等优点,制备的fa-ICMB抗溃散性强、可注射性好,且固化速度快。The invention has the advantages of low raw material cost, simple preparation, convenient operation and the like, and the prepared fa-ICMB has strong collapse resistance, good injectability and fast curing speed.
附图说明Description of drawings
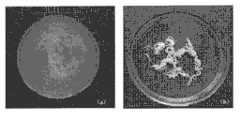
图1显示了白糊精对ICPC抗溃散性能的影响,其中,(a)为不含白糊精的ICPC(b)为含白糊精ICPC。Figure 1 shows the effect of white dextrin on the anti-collapse performance of ICPC, where (a) is ICPC without white dextrin (b) is ICPC with white dextrin.
图2为水相高效抗溃散快速固化可注射钙镁骨水泥固化体的X射线衍射图谱,其中,(a)为麦芽糊精调制的fa-ICMB;(b)为黄糊精调制的fa-ICMB;(c)为白糊精调制的fa-ICMB;(d)为英国胶调制的fa-ICMB;(e)为白糊精调制的ICPC。Figure 2 is the X-ray diffraction pattern of the water-phase high-efficiency anti-collapse fast-curing injectable calcium-magnesium bone cement solidified body, wherein, (a) is fa-ICMB modulated by maltodextrin; (b) is fa-ICMB modulated by yellow dextrin ICMB; (c) fa-ICMB prepared with white dextrin; (d) fa-ICMB prepared with British gum; (e) ICPC prepared with white dextrin.
图3为水相高效抗溃散快速固化可注射钙镁骨水泥固化体的SEM图片。Fig. 3 is a SEM picture of the water-phase high-efficiency anti-collapse fast-curing injectable calcium-magnesium bone cement solidification body.
图4显示了水相高效抗溃散快速固化可注射钙镁骨水泥固化体的生物相容性,其中,(a)为培养1h;(b)为培养2d;(c)为培养3d。Figure 4 shows the biocompatibility of the water-phase high-efficiency anti-collapse fast-curing injectable calcium-magnesium bone cement solidified body, in which (a) is cultured for 1 hour; (b) is cultured for 2 days; (c) is cultured for 3 days.
具体实施方式Detailed ways
本发明人经过广泛而深入的研究,首次意外地发现,将不同配比的复合磷酸钙盐按一定比例与磷酸镁混合形成固相粉末,再与含有良好生物相容性的抗溃散剂所配制的固化液调和,可以制得类牙膏状或稠糊状的水相高效抗溃散快速固化可注射钙镁骨水泥(fa-ICMB)。在此基础上,本发明人完成了本发明。After extensive and in-depth research, the inventor discovered for the first time that the compound calcium phosphate salt with different proportions is mixed with magnesium phosphate in a certain proportion to form a solid phase powder, and then formulated with an anti-collapse agent with good biocompatibility The solidifying liquid is reconciled, and the water phase high-efficiency anti-collapse fast-curing injectable calcium-magnesium bone cement (fa-ICMB) like toothpaste or thick paste can be prepared. On this basis, the present inventors have completed the present invention.
在本发明的上下文中,所述复合磷酸钙盐没有特别限制,为几种磷酸钙按一定比例混合的混合物,按US5525148和US5545254公开的方法配制,所述磷酸钙选自:磷酸钙、磷酸四钙、磷酸八钙、磷酸氢钙、羟基磷灰石、氟磷灰石、焦磷酸钙,且所述复合磷酸钙盐颗粒的平均粒径小于10微米。所述磷酸镁粉末没有特别限制,为碱性化合物和磷酸盐的混合物,按ZL 01 1 05373.9和US 7094286B2公开的方法配制,所述碱性化合物为氧化镁或/和氧化钙;所述磷酸盐为磷酸二氢盐,所述磷酸二氢盐为磷酸二氢铵、磷酸二氢钙、聚磷酸铵中的至少一种。所述人体模拟体液为SBF,按照Kukubo T等(Kukubo T,J Biomed Mater Res,1990,24:721-734)公开的离子浓度进行配制。In the context of the present invention, the complex calcium phosphate salt is not particularly limited, it is a mixture of several calcium phosphates mixed in a certain proportion, prepared according to the method disclosed in US5525148 and US5545254, the calcium phosphate is selected from: calcium phosphate, tetraphosphate Calcium, octacalcium phosphate, calcium hydrogen phosphate, hydroxyapatite, fluorapatite, calcium pyrophosphate, and the average particle size of the composite calcium phosphate salt particles is less than 10 microns. The magnesium phosphate powder is not particularly limited, it is a mixture of basic compounds and phosphates, prepared according to the methods disclosed in ZL 01 1 05373.9 and US 7094286B2, the basic compounds are magnesium oxide or/and calcium oxide; the phosphates It is dihydrogen phosphate, and the dihydrogen phosphate is at least one of ammonium dihydrogen phosphate, calcium dihydrogen phosphate, and ammonium polyphosphate. The human simulated body fluid is SBF, prepared according to the ion concentration disclosed by Kukubo T et al. (Kukubo T, J Biomed Mater Res, 1990, 24:721-734).
以下结合具体实施例,对本发明做进一步说明。应理解,以下实施例仅用于说明本发明而非用于限制本发明的范围。下列实施例中未注明具体条件的实验方法,通常按照常规条件,或按照制造厂商所建议的条件。The present invention will be further described below in conjunction with specific embodiments. It should be understood that the following examples are only used to illustrate the present invention but not to limit the scope of the present invention. For the experimental methods without specific conditions indicated in the following examples, the conventional conditions or the conditions suggested by the manufacturer are usually followed.
通用方法general method
(1)抗溃散率(1) Anti-collapse rate
将所制得的水相可注射钙镁骨水泥灌入注射器,下端接9号针头,用推杆将浆体推出并置于37℃的人体模拟体液(simulated body fluid,SBF)中,在120r/min的振荡培养箱(HZQ-F160,太仓市实验设备厂)中振荡。10分钟后取出,烘干,称量,计算未溃散固化体占初始总固化体的重量百分比,记为抗溃散率。The prepared water-phase injectable calcium-magnesium bone cement was poured into a syringe, and the lower end was connected with a No. /min in a shaking incubator (HZQ-F160, Taicang Experimental Equipment Factory). Take it out after 10 minutes, dry it, weigh it, calculate the weight percentage of the uncollapsed solidified body in the initial total solidified body, and record it as the anti-collapse rate.
(2)固化时间(2) Curing time
将上述制备的骨水泥浆体通过模具制成Φ6mm×5mm的试样,浸入SBF溶液并在37℃、100%湿度环境中固化,测定固化时间。The bone cement paste prepared above was made into a sample of Φ6mm×5mm through a mold, immersed in SBF solution and cured in an environment of 37°C and 100% humidity, and the curing time was measured.
(3)初始粘度(3) Initial viscosity
采用控制应变(CR)的流变仪,通过测量不同剪切速率下粘度η的变化,得到包括初始粘度在内的粘度曲线,以此衡量浆体的可注射性能。Using a controlled strain (CR) rheometer, by measuring different shear rates Viscosity curves including initial viscosity are obtained through the change of viscosity η, which is used to measure the injectability of the slurry.
(4)细胞相容性(4) Cytocompatibility
将上述制备的骨水泥浆体填入到塑胶模具中,置于37℃、100%湿度的模拟人体环境中,待完全固化后取出,脱模获得表面光滑平整的片状(6×6×2mm3)。将上述固化体分装于玻璃瓶、封口,在120℃下高温蒸汽灭菌20分钟后备用。在无菌条件下将样品放于24孔板底部,每孔加入MG63细胞悬液(1×104个/孔)。以10%的小牛血清为培养基,将样品置于37℃,100%湿度,5%CO2的恒温箱中培养,每天换一次培养基。培养1h、2d、3d后利用倒置相差显微镜观察细胞的生长情况。Fill the bone cement slurry prepared above into a plastic mold, place it in a simulated human body environment at 37°C and 100% humidity, take it out after it is completely cured, and demold it to obtain a smooth and flat sheet (6×6×2mm3 ). The above-mentioned cured body was divided into glass bottles, sealed, and sterilized by high-temperature steam at 120°C for 20 minutes before use. The samples were placed on the bottom of a 24-well plate under sterile conditions, and MG63 cell suspension (1×104 cells/well) was added to each well. With 10% calf serum as the medium, the samples were cultured in an incubator at 37°C, 100% humidity, and 5% CO2 , and the medium was changed once a day. After culturing for 1h, 2d, and 3d, the growth of the cells was observed with an inverted phase-contrast microscope.
实施例1Example 1
称取粒径小于10μm的复合磷酸钙盐粉末(成分为磷酸氢钙、磷酸四钙和羟基磷灰石)10g,作为固相粉末;Weigh 10 g of composite calcium phosphate salt powder (composition is calcium hydrogen phosphate, tetracalcium phosphate and hydroxyapatite) with a particle size less than 10 μm as solid phase powder;
称取白糊精0.48g,磷酸氢二钠0.48g,倒入装有6g去离子水的烧杯中,充分搅拌使白糊精、磷酸氢二钠充分溶解到去离子水中,制得固化液;Weigh 0.48g of white dextrin and 0.48g of disodium hydrogen phosphate, pour them into a beaker equipped with 6g of deionized water, stir fully so that white dextrin and disodium hydrogen phosphate are fully dissolved in deionized water, and obtain a solidified solution;
将固化液倒入到固相粉末中,在器皿中分散均匀,用专用调制刀调和均匀,得到水相可注射磷酸钙骨水泥。Pour the solidified liquid into the solid phase powder, disperse evenly in the vessel, and mix evenly with a special mixing knife to obtain the aqueous phase injectable calcium phosphate bone cement.
实施例2~12重复实施例1的实验步骤,不同之处在于实验条件,具体如表1所示。Examples 2-12 Repeat the experimental steps of Example 1, the difference lies in the experimental conditions, as shown in Table 1.
表1实施例2~12的实验条件The experimental condition of table 1
实施例1~12制备的水相可注射磷酸钙骨水泥的性能测试结果如表2所示。由表2可见,在固化液中加入抗溃散剂后,得到的浆体具有良好的可注射性,初始粘度增加,抗溃散率均有所提高,但在不同程度上延长了固化时间。尽管在固化液中加入磷酸氢二钠后,固化速度加快,但仍超过了合理的固化时间。Table 2 shows the performance test results of the aqueous phase injectable calcium phosphate bone cement prepared in Examples 1-12. It can be seen from Table 2 that after the anti-collapse agent is added to the solidification solution, the obtained slurry has good injectability, the initial viscosity increases, and the anti-collapse rate is improved, but the curing time is prolonged to varying degrees. Although the curing speed is accelerated after adding disodium hydrogen phosphate in the curing solution, it still exceeds the reasonable curing time.
在抗溃散剂白糊精和促凝剂磷酸氢二钠的双重作用下,水相可注射磷酸钙骨水泥注射到液相后,形成线,掉落的粉渣较少,水体清澈;未加白糊精时,浆体注射到水中即可溃散,水体浑浊,如图1所示。采用X-射线衍射仪对实施例1的固化产物进行物相分析,结果如图2(e)所示,表明该体系的固化产物主要为羟基磷灰石,白糊精对固化产物组成无明显影响。黄糊精、英国胶、麦芽糊精加入后的效果与白糊精相同。Under the double action of the anti-collapse agent white dextrin and the coagulant disodium hydrogen phosphate, after the water-phase injectable calcium phosphate bone cement is injected into the liquid phase, a line is formed, the falling powder residue is less, and the water is clear; When white dextrin is used, the slurry will collapse when injected into water, and the water will be turbid, as shown in Figure 1. Adopt X-ray diffractometer to carry out phase analysis to the cured product of embodiment 1, the result is as shown in Figure 2 (e), shows that the cured product of this system is mainly hydroxyapatite, and white dextrin has no significant effect on the composition of cured product. Influence. The effect of adding yellow dextrin, British gum and maltodextrin is the same as that of white dextrin.
表2实施例1~12制备的水相可注射磷酸钙骨水泥的性能The performance of the water-phase injectable calcium phosphate bone cement prepared by table 2 embodiment 1~12
实施例13Example 13
称取粒径小于10μm的复合磷酸钙盐粉末(成分为磷酸氢钙、磷酸八钙和羟基磷灰石)0.5g,磷酸镁粉末9.5g,干式混合组成固相粉末;称取白糊精1.2g,磷酸氢二钠1.04g,倒入装有6g去离子水的烧杯中,充分搅拌使白糊精、磷酸氢二钠充分溶解到去离子水中,制得固化液;Weigh 0.5g of composite calcium phosphate salt powder (composition is calcium hydrogen phosphate, octacalcium phosphate and hydroxyapatite) with a particle size of less than 10 μm, and 9.5g of magnesium phosphate powder, and dry mix to form a solid phase powder; weigh white dextrin 1.2g, 1.04g of disodium hydrogen phosphate, poured into a beaker containing 6g of deionized water, fully stirred to fully dissolve white dextrin and disodium hydrogen phosphate into the deionized water to obtain a solidified solution;
将上述制备的固化液倒入固相粉末中,在器皿中分散均匀,用专用调制刀调和均匀,得到具有高效抗溃散性和快速固化的水相可注射钙镁骨水泥(fa-ICMB)。Pour the solidification solution prepared above into the solid phase powder, disperse it evenly in the vessel, and mix it evenly with a special mixing knife to obtain a water-phase injectable calcium-magnesium bone cement (fa-ICMB) with high-efficiency collapse resistance and rapid solidification.
实施例14~24重复实施例13的实验步骤,不同之处在于实验条件,具体如表3所示,性能测试结果如表4所示。Examples 14-24 repeated the experimental steps of Example 13, the difference lies in the experimental conditions, as shown in Table 3, and the performance test results are shown in Table 4.
表3实施例14~24的实验条件The experimental condition of table 3 embodiment 14~24
表4实施例13~24制备的水相可注射磷酸钙骨水泥的性能The performance of the water phase injectable calcium phosphate bone cement prepared by the embodiment 13~24 of table 4
实施例25Example 25
称取粒径小于10μm的复合磷酸钙盐粉末(成分为磷酸氢钙、磷酸八钙和焦磷酸钙)5g,磷酸镁粉末5g,干式混合组成固相粉末;Weigh 5 g of composite calcium phosphate salt powder (composition is calcium hydrogen phosphate, octacalcium phosphate and calcium pyrophosphate) with a particle size less than 10 μm, 5 g of magnesium phosphate powder, and dry mix to form a solid phase powder;
称取白糊精1.2g,磷酸氢二钠1.04g,倒入装有6g去离子水的烧杯中,充分搅拌使白糊精、磷酸氢二钠充分溶解到去离子水中,制得固化液;Weigh 1.2 g of white dextrin and 1.04 g of disodium hydrogen phosphate, pour them into a beaker containing 6 g of deionized water, and stir fully so that white dextrin and disodium hydrogen phosphate are fully dissolved in deionized water to obtain a solidified solution;
将上述制备的固化液倒入固相粉末中,在器皿中分散均匀,用专用调制刀调和均匀,得到具有高效抗溃散性和快速固化的水相可注射钙镁骨水泥fa-ICMB。Pour the solidified liquid prepared above into the solid phase powder, disperse evenly in the container, and mix evenly with a special mixing knife to obtain the water phase injectable calcium-magnesium bone cement fa-ICMB with high-efficiency anti-collapse and fast curing.
实施例26~37重复实施例25的实验步骤,不同之处在于实验条件,具体如表5所示,性能测试结果如表6所示。Examples 26-37 repeated the experimental steps of Example 25, the difference lies in the experimental conditions, as shown in Table 5, and the performance test results are shown in Table 6.
表5实施例26~37的实验条件The experimental conditions of table 5 embodiment 26~37
表6实施例25~37制备的水相可注射磷酸钙骨水泥的性能The performance of the water-phase injectable calcium phosphate bone cement prepared by table 6 embodiment 25~37
如表4和表6所示,在MPC、糊精以及磷酸氢盐的共同作用下,浆体具有良好的可注射性,初始粘度增加,但固化速度明显加快,并且抗溃散率明显提高。As shown in Table 4 and Table 6, under the joint action of MPC, dextrin and hydrogen phosphate, the slurry has good injectability, the initial viscosity increases, but the curing speed is significantly accelerated, and the collapse resistance rate is significantly improved.
采用X-射线衍射仪对实施例34和37的固化产物进行物相分析,如图2(a)和(c)所示,结果表明该体系的固化产物主要为羟基磷灰石、磷酸三钙和未反应的磷酸镁,麦芽糊精和白糊精对固化产物组成无明显影响。Adopt X-ray diffractometer to carry out phase analysis to the curing product of embodiment 34 and 37, as shown in Figure 2 (a) and (c), the result shows that the curing product of this system is mainly hydroxyapatite, tricalcium phosphate And unreacted magnesium phosphate, maltodextrin and white dextrin had no obvious effect on the composition of the cured product.
实施例38~42重复实施例25的实验,不同之处在于:Embodiment 38~42 repeat the experiment of embodiment 25, difference is:
实施例38:复合磷酸钙盐粉末9.5g,磷酸镁粉末0.5g;白糊精0.6g,麦芽糊精0.6g,磷酸氢二钠0.6g,倒入装有6g去离子水的烧杯中。Example 38: 9.5 g of composite calcium phosphate salt powder, 0.5 g of magnesium phosphate powder, 0.6 g of white dextrin, 0.6 g of maltodextrin, and 0.6 g of disodium hydrogen phosphate were poured into a beaker filled with 6 g of deionized water.
实施例39:复合磷酸钙盐粉末4g,磷酸镁粉末6g;白糊精0.96g,黄糊精0.96g,磷酸氢二钠1.08g,倒入装有6g去离子水的烧杯中。Example 39: 4 g of composite calcium phosphate salt powder, 6 g of magnesium phosphate powder; 0.96 g of white dextrin, 0.96 g of yellow dextrin, and 1.08 g of disodium hydrogen phosphate were poured into a beaker filled with 6 g of deionized water.
实施例40:复合磷酸钙盐粉末(成分为磷酸氢钙、磷酸八钙和羟基磷灰石)4g,磷酸镁粉末6g;白糊精0.14g,黄糊精0.35g,英国胶0.12g,麦芽糊精0.35g,磷酸氢二钠0.02g,磷酸二氢钠0.02g,倒入装有4g去离子水的烧杯中。Example 40: Composite calcium phosphate salt powder (composition is calcium hydrogen phosphate, octacalcium phosphate and hydroxyapatite) 4g, magnesium phosphate powder 6g; white dextrin 0.14g, yellow dextrin 0.35g, English gum 0.12g, malt Dextrin 0.35g, disodium hydrogen phosphate 0.02g, sodium dihydrogen phosphate 0.02g were poured into a beaker containing 4g deionized water.
实施例41:复合磷酸钙盐粉末(成分为磷酸氢钙、磷酸四钙和羟基磷灰石)8g,磷酸镁粉末2g;黄糊精0.06g,磷酸二氢钾0.96g,倒入装有6g去离子水的烧杯中。Example 41: Composite calcium phosphate salt powder (composition is calcium hydrogen phosphate, tetracalcium phosphate and hydroxyapatite) 8g, magnesium phosphate powder 2g; yellow dextrin 0.06g, potassium dihydrogen phosphate 0.96g, pour into 6g beaker of deionized water.
实施例42:复合磷酸钙盐粉末(成分为磷酸氢钙、磷酸四钙和氟磷灰石)7g,磷酸镁粉末3g;英国胶0.24g,磷酸氢二钾0.18g,倒入装有6g去离子水的烧杯中。Example 42: Composite calcium phosphate salt powder (composition is calcium hydrogen phosphate, tetracalcium phosphate and fluorapatite) 7g, magnesium phosphate powder 3g; English glue 0.24g, dipotassium hydrogen phosphate 0.18g, pour into a 6g container beaker of ionized water.
实施例38~42制备的水相可注射磷酸钙骨水泥的性能测试结果如表7所示。Table 7 shows the performance test results of the aqueous phase injectable calcium phosphate bone cement prepared in Examples 38-42.
表7实施例38~42制备的水相可注射磷酸钙骨水泥的性能The performance of the water phase injectable calcium phosphate bone cement prepared by the embodiment 38~42 of table 7
如表7所示,在MPC、糊精以及磷酸氢盐的共同作用下,fa-ICMB的固化时间合理,抗溃散性强,且浆体具有良好的可注射性。采用X-射线衍射仪对实施例41的固化产物进行物相分析,如图2(b)所示,结果表明该体系的固化产物主要为羟基磷灰石、磷酸三钙和未反应的磷酸镁,黄糊精对固化产物组成无明显影响。扫描电子显微镜观察实施例42的固化产物,如图3所示,结果表明:该体系的固化产物主要为针状的羟基磷灰石、磷酸二钙和片状的未反应的磷酸镁,如图2(d)所示,英国胶对固化产物组成无明显影响。采用细胞培养实验对实施例40的固化产物进行生物相容性分析,如图4所示,结果表明MG63细胞在fa-ICMB固化产物表面具有良好的粘附作用,不仅能完全铺展,而且增殖明显,具有良好的生物相容性。As shown in Table 7, under the joint action of MPC, dextrin and hydrogen phosphate, the curing time of fa-ICMB is reasonable, the collapse resistance is strong, and the slurry has good injectability. Adopt X-ray diffractometer to carry out phase analysis to the cured product of embodiment 41, as shown in Figure 2 (b), the result shows that the cured product of this system is mainly hydroxyapatite, tricalcium phosphate and unreacted magnesium phosphate , yellow dextrin has no significant effect on the composition of the cured product. The cured product of Example 42 was observed with a scanning electron microscope, as shown in Figure 3, and the results showed that: the cured product of this system is mainly needle-shaped hydroxyapatite, dicalcium phosphate and flaky unreacted magnesium phosphate, as shown in Figure 3 As shown in 2(d), British glue had no significant effect on the composition of the cured product. The biocompatibility analysis of the cured product of Example 40 was carried out by cell culture experiments, as shown in Figure 4, the results show that MG63 cells have good adhesion on the surface of the fa-ICMB cured product, and can not only spread completely, but also proliferate Obviously, it has good biocompatibility.
在水相可注射钙镁骨水泥中加入抗溃散剂糊精,一方面糊精在水溶液中形成了高粘度和强化学结合力的溶液,物理吸附于fa-ICMB颗粒表面,起到固定fa-ICMB颗粒并阻止水分渗入的作用;另一方面糊精链在颗粒表面的桥连作用,糊精和fa-ICMB颗粒间的静电引力作用及Ca2+和糊精中的阴离子的螯合作用,形成致密结构,从而起到抗溃散作用。在复合磷酸钙盐固相粉末中引入MPC,与水混合后,MPC中的酸性组分在水中迅速溶解并发生电离,释放出H+、PO43-,MPC中碱性组分MgO受到水和H+的进攻后,颗粒表面溶解,生成Mg(OH)2和Mg2+。同时Mg2+游离出来,与PO43-作用形成磷酸镁结晶,而Ca2+游离出来同PO43-作用生成磷酸钙-磷酸盐络合物凝胶,加速了fa-ICMB固化进程。随着fa-ICMB水化反应的加速,在浆体表面形成了凝胶状反应物并填塞了fa-ICMB颗粒间的孔隙,可有效改善体系的抗溃散性能。The anti-collapse agent dextrin is added to the water-phase injectable calcium-magnesium bone cement. On the one hand, the dextrin forms a solution with high viscosity and strong chemical binding force in the aqueous solution, and physically adsorbs on the surface of fa-ICMB particles to fix fa- ICMB particles and prevent water infiltration; on the other hand, the bridging effect of dextrin chains on the particle surface, the electrostatic attraction between dextrin and fa-ICMB particles and the chelation of Ca2+ and anions in dextrin, Form a dense structure, thereby playing the role of anti-collapse. MPC is introduced into the composite calcium phosphate solid-phase powder, and after mixing with water, the acidic components in MPC dissolve rapidly in water and ionize, releasing H+ , PO43- , and the alkaline component MgO in MPC is subjected to water After the attack of H+ , the particle surface dissolves to generate Mg(OH)2 and Mg2+ . At the same time, Mg2+ is dissociated and reacts with PO43- to form magnesium phosphate crystals, while Ca2+ is dissociated and reacts with PO43- to form calcium phosphate-phosphate complex gel, which accelerates the curing process of fa-ICMB. With the acceleration of fa-ICMB hydration reaction, a gel-like reactant is formed on the surface of the slurry and fills the pores between fa-ICMB particles, which can effectively improve the anti-collapse performance of the system.
本发明的制备方法,在现有水相可注射磷酸钙骨水泥的基础上,首次在体系中添加白糊精、黄糊精、英国胶和麦芽糊精中的至少一种抗溃散剂,巧妙地利用其较强的保水保形性、溶于水形成高粘度溶液、在表面形成致密水膜以及与反应物和产物高效吸附并形成强化学结合力的特点,有效解决了浆体遇渗液而掉渣、散落甚至不能固化等问题,提高了水相可注射磷酸钙骨水泥的抗溃散能力。且在抗溃散型水相可注射磷酸钙骨水泥的基础上,在体系中添加了快凝、早强并具有良好的可塑性、生物相容性和降解性的磷酸镁。与固化液接触后,fa-ICMB快速发生水化反应,形成凝胶状反应物填塞了颗粒间的空隙和缺陷,协同促进了相当数量接触点的形成,使得浆体具有快速固化和高早强的特点。其快速固化和高早强的特点又进一步促进了fa-ICMB抗溃散性能的提高。水相高效抗溃散快速固化可注射钙镁骨水泥水化后,其最终成分为无定形的镁-磷酸盐络合物凝胶和羟基磷灰石,分别与体内结石和人体硬组织的无机成分相似,生物相容性好,无刺激性。表明由抗溃散剂和磷酸氢盐溶液混合制备的固化液不改变原钙镁骨水泥的固化特性,而且不改变固化后水化产物的组成。In the preparation method of the present invention, on the basis of the existing water-phase injectable calcium phosphate bone cement, at least one anti-collapse agent among white dextrin, yellow dextrin, English gum and maltodextrin is added to the system for the first time. Using its strong water retention and shape retention, dissolving in water to form a high-viscosity solution, forming a dense water film on the surface, and efficiently adsorbing reactants and products to form a strong chemical binding force, it effectively solves the problem of slurry encountering seepage. However, problems such as slag falling, scattering and even failure to solidify have improved the anti-collapse ability of the aqueous phase injectable calcium phosphate bone cement. And on the basis of the anti-collapse type water-phase injectable calcium phosphate bone cement, magnesium phosphate with fast setting, early strength, good plasticity, biocompatibility and degradability is added to the system. After contacting with the curing liquid, fa-ICMB undergoes a rapid hydration reaction, forming a gel-like reactant to fill the gaps and defects between particles, and synergistically promotes the formation of a considerable number of contact points, making the slurry have rapid curing and high early strength specialty. Its rapid curing and high early strength characteristics further promote the improvement of fa-ICMB's anti-collapse performance. The aqueous phase is highly efficient, anti-collapse and fast-curing injectable calcium-magnesium bone cement. After hydration, its final components are amorphous magnesium-phosphate complex gel and hydroxyapatite, which are compatible with stones in the body and inorganic components of human hard tissues. Similar, good biocompatibility, non-irritating. It shows that the solidification solution prepared by mixing the anti-collapse agent and hydrogen phosphate solution does not change the solidification characteristics of the original calcium magnesium bone cement, and does not change the composition of the hydration product after solidification.
本发明的制备方法,糊精和磷酸镁对体系的抗溃散性能起到协同增效作用,与此同时磷酸镁还对体系起到快速固化的作用。制备的fa-ICMB兼具抗溃散性、可注射性和快速固化等性能。In the preparation method of the invention, the dextrin and magnesium phosphate play a synergistic effect on the anti-collapse performance of the system, and at the same time, the magnesium phosphate also plays a role in rapid solidification of the system. The prepared fa-ICMB has the properties of anti-collapse, injectability and fast curing.
本发明将糊精的应用拓展到制备组织修复领域,这既为提高钙镁骨水泥的性能开拓了新途径,也为糊精开辟了一个新的应用领域。制备的水相高效抗溃散快速固化可注射钙镁骨水泥,具有原料成本低、制备简单、便于手术操作等优点,并且抗溃散性强、固化速度快,可在血液/体液环境下安全使用。当应用本发明的可注射高效悬浮稳定的磷酸钙骨水泥时,可以采用直接膏体填充方法,可以通过特制的注射器(换针头)进行直接注射到手术部位的方法,也可以经皮椎体成形术的方法,或者通过预先固化成型的固化体在术中植骨。The invention expands the application of dextrin to the field of preparing tissue repair, which not only opens up a new way for improving the performance of calcium magnesium bone cement, but also opens up a new application field for dextrin. The prepared aqueous-phase high-efficiency anti-collapse fast-curing injectable calcium-magnesium bone cement has the advantages of low raw material cost, simple preparation, convenient operation, etc., and has strong collapse resistance and fast curing speed, and can be used safely in blood/body fluid environment. When the injectable high-efficiency suspended calcium phosphate bone cement of the present invention is used, the direct paste filling method can be used, the method of direct injection into the surgical site can be carried out through a special syringe (needle replacement), and percutaneous vertebral body shaping can also be used. The method of surgery, or the bone grafting in the operation through the pre-cured solidified body.
Claims (10)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201010205094.8ACN101856509B (en) | 2010-06-18 | 2010-06-18 | Injectable calcium magnesium bone cement and its preparation method and application |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201010205094.8ACN101856509B (en) | 2010-06-18 | 2010-06-18 | Injectable calcium magnesium bone cement and its preparation method and application |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101856509Atrue CN101856509A (en) | 2010-10-13 |
| CN101856509B CN101856509B (en) | 2014-02-19 |
Family
ID=42942812
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201010205094.8AActiveCN101856509B (en) | 2010-06-18 | 2010-06-18 | Injectable calcium magnesium bone cement and its preparation method and application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101856509B (en) |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102552985A (en)* | 2012-01-20 | 2012-07-11 | 苏州大学 | Silk fibroin/calcium phosphate bone cement-based porous composite material and preparation method thereof |
| CN104107455A (en)* | 2013-04-19 | 2014-10-22 | 中国人民解放军第二军医大学 | Bone cement and slurry thereof |
| CN104591679A (en)* | 2015-01-31 | 2015-05-06 | 河南理工大学 | Modified magnesium oxychloride bone cement as well as preparation method and application thereof |
| CN104984388A (en)* | 2015-06-24 | 2015-10-21 | 苏州乔纳森新材料科技有限公司 | Skeletal system repairing material and preparation method thereof |
| CN106137371A (en)* | 2016-08-05 | 2016-11-23 | 无锡市第二人民医院 | The interior fixing dry prosthese of bone cement, prefabricated component and preparation method thereof |
| CN106729973A (en)* | 2017-01-19 | 2017-05-31 | 王江林 | A kind of injecting bone cement and preparation method thereof |
| CN107537060A (en)* | 2016-06-24 | 2018-01-05 | 上海瑞邦生物材料有限公司 | One kind development injectable calcium phosphate bone cement and its preparation and application |
| CN111671969A (en)* | 2020-07-30 | 2020-09-18 | 武汉理工大学 | A kind of injectable vertebral body strengthening magnesium phosphate bone cement and preparation method thereof |
| CN111870738A (en)* | 2020-06-11 | 2020-11-03 | 上海蕴邦生物科技有限公司 | Bone repair material and preparation method and application thereof |
| CN111888521A (en)* | 2020-06-11 | 2020-11-06 | 上海蕴邦生物科技有限公司 | Bone repair material and preparation method thereof |
| CN112043862A (en)* | 2020-09-14 | 2020-12-08 | 香港大学深圳医院 | Magnesium slow-release bone cement with self-curing function and preparation method thereof |
| CN112245656A (en)* | 2019-11-20 | 2021-01-22 | 武汉理工大学 | Preparation method of calcium phosphate magnesium bone cement composite scaffold |
| CN113082296A (en)* | 2021-04-26 | 2021-07-09 | 东南大学 | Calcium phosphate bone cement with good injectability and preparation method thereof |
| CN113616852A (en)* | 2021-08-23 | 2021-11-09 | 西北工业大学 | Magnesium powder/calcium phosphate composite bone cement material and preparation method thereof |
| CN115444967A (en)* | 2022-08-08 | 2022-12-09 | 武汉理工大学 | Bioactive hard tissue adhesive and preparation method and application thereof |
| CN115634320A (en)* | 2022-10-09 | 2023-01-24 | 华东理工大学 | Spray-spun absorbable fiber reinforced injectable calcium phosphate bone cement |
| CN116942919A (en)* | 2023-08-02 | 2023-10-27 | 上海市第十人民医院 | Self-curing magnesium phosphate bone cement and preparation method and application thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1446589A (en)* | 2003-01-13 | 2003-10-08 | 华东理工大学 | Medicine controlled functional cement with calcium phosphate being as framework and its preparation method |
| CN101496909A (en)* | 2008-02-01 | 2009-08-05 | 华东理工大学 | Polysaccharide/calcium orthophosphate composite bone cement and preparation method thereof |
| CN101524557A (en)* | 2009-04-21 | 2009-09-09 | 华南理工大学 | Anti-collapsibility calcium phosphate cement, preparation method and application thereof |
- 2010
- 2010-06-18CNCN201010205094.8Apatent/CN101856509B/enactiveActive
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1446589A (en)* | 2003-01-13 | 2003-10-08 | 华东理工大学 | Medicine controlled functional cement with calcium phosphate being as framework and its preparation method |
| CN101496909A (en)* | 2008-02-01 | 2009-08-05 | 华东理工大学 | Polysaccharide/calcium orthophosphate composite bone cement and preparation method thereof |
| CN101524557A (en)* | 2009-04-21 | 2009-09-09 | 华南理工大学 | Anti-collapsibility calcium phosphate cement, preparation method and application thereof |
Non-Patent Citations (2)
| Title |
|---|
| 《Acta Biomaterialia》 20080710 Fan Wu et al. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration 1873-1884 第4卷,* |
| FAN WU ET AL.: "Self-setting bioactive calcium–magnesium phosphate cement with high strength and degradability for bone regeneration", 《ACTA BIOMATERIALIA》* |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102552985A (en)* | 2012-01-20 | 2012-07-11 | 苏州大学 | Silk fibroin/calcium phosphate bone cement-based porous composite material and preparation method thereof |
| CN102552985B (en)* | 2012-01-20 | 2014-03-19 | 苏州大学 | Silk fibroin/calcium phosphate bone cement-based porous composite material and preparation method thereof |
| CN104107455A (en)* | 2013-04-19 | 2014-10-22 | 中国人民解放军第二军医大学 | Bone cement and slurry thereof |
| CN104107455B (en)* | 2013-04-19 | 2018-12-14 | 中国人民解放军第二军医大学 | A kind of bone cement and its slurry |
| CN104591679A (en)* | 2015-01-31 | 2015-05-06 | 河南理工大学 | Modified magnesium oxychloride bone cement as well as preparation method and application thereof |
| CN104984388A (en)* | 2015-06-24 | 2015-10-21 | 苏州乔纳森新材料科技有限公司 | Skeletal system repairing material and preparation method thereof |
| CN107537060A (en)* | 2016-06-24 | 2018-01-05 | 上海瑞邦生物材料有限公司 | One kind development injectable calcium phosphate bone cement and its preparation and application |
| CN106137371A (en)* | 2016-08-05 | 2016-11-23 | 无锡市第二人民医院 | The interior fixing dry prosthese of bone cement, prefabricated component and preparation method thereof |
| CN106729973A (en)* | 2017-01-19 | 2017-05-31 | 王江林 | A kind of injecting bone cement and preparation method thereof |
| CN112245656A (en)* | 2019-11-20 | 2021-01-22 | 武汉理工大学 | Preparation method of calcium phosphate magnesium bone cement composite scaffold |
| CN111870738A (en)* | 2020-06-11 | 2020-11-03 | 上海蕴邦生物科技有限公司 | Bone repair material and preparation method and application thereof |
| CN111888521A (en)* | 2020-06-11 | 2020-11-06 | 上海蕴邦生物科技有限公司 | Bone repair material and preparation method thereof |
| CN111671969A (en)* | 2020-07-30 | 2020-09-18 | 武汉理工大学 | A kind of injectable vertebral body strengthening magnesium phosphate bone cement and preparation method thereof |
| CN112043862A (en)* | 2020-09-14 | 2020-12-08 | 香港大学深圳医院 | Magnesium slow-release bone cement with self-curing function and preparation method thereof |
| CN113082296A (en)* | 2021-04-26 | 2021-07-09 | 东南大学 | Calcium phosphate bone cement with good injectability and preparation method thereof |
| CN113616852A (en)* | 2021-08-23 | 2021-11-09 | 西北工业大学 | Magnesium powder/calcium phosphate composite bone cement material and preparation method thereof |
| CN115444967A (en)* | 2022-08-08 | 2022-12-09 | 武汉理工大学 | Bioactive hard tissue adhesive and preparation method and application thereof |
| CN115634320A (en)* | 2022-10-09 | 2023-01-24 | 华东理工大学 | Spray-spun absorbable fiber reinforced injectable calcium phosphate bone cement |
| CN116942919A (en)* | 2023-08-02 | 2023-10-27 | 上海市第十人民医院 | Self-curing magnesium phosphate bone cement and preparation method and application thereof |
| CN116942919B (en)* | 2023-08-02 | 2025-09-19 | 上海市第十人民医院 | Self-curing magnesium phosphate-based bone binder |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101856509B (en) | 2014-02-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101856509A (en) | Injectable calcium magnesium bone cement and its preparation method and application | |
| Bohner | Design of ceramic-based cements and putties for bone graft substitution | |
| Liu et al. | Novel injectable calcium phosphate/chitosan composites for bone substitute materials | |
| Zhao et al. | An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering | |
| CN107303397B (en) | A kind of biologically active Injectable compound bone cement and its preparation method and application | |
| CN104644455B (en) | A kind of bio-vitric sodium alginate composite biological material and kit and application | |
| CN105396175B (en) | Bone cement and preparation method thereof containing calcium citrate | |
| US20110073006A1 (en) | Rapid setting high strength calcium phosphate cements comprising cyclodextrins | |
| CN102813962B (en) | Injectable and degradable bone cement, and preparation method and application thereof | |
| CN101428153B (en) | Hydrogen phosphate/tricalcium silicate composite self-curing material with biological activity, preparation and uses thereof | |
| CN111870738B (en) | Bone repair material and preparation method and application thereof | |
| CN104591679B (en) | A kind of modified chloromagnesia bone cement and its preparation method and application | |
| Chang et al. | Development of calcium phosphate/sulfate biphasic cement for vital pulp therapy | |
| Qian et al. | Improvement of anti-washout property of calcium phosphate cement by addition of konjac glucomannan and guar gum | |
| Jeong et al. | Preparation, characterization, and in-vitro performance of novel injectable silanized-hydroxypropyl methylcellulose/phase-transformed calcium phosphate composite bone cements | |
| CN107233627B (en) | A kind of calcium phosphate bone cement containing konjac glucomannan and its preparation method and application | |
| CN105731990B (en) | A kind of controlled degradation magnesium phosphate cement and its preparation method and application | |
| CN107569714A (en) | A kind of preparation method of functionalization fracture adhesive | |
| CN113082296B (en) | Calcium phosphate bone cement with good injectability and preparation method thereof | |
| JP2018167016A (en) | Adhesive for jointing hard tissues, adhesive kit for jointing hard tissues and bone cement | |
| TWI388348B (en) | Polymer or oligomer-containing calcium silicate bone cement and methods for the preparation | |
| CHANG et al. | An injectable composite bone cement based on mesoporous borosilicate bioactive glass spheres | |
| CN106693063B (en) | A kind of anti-collapse calcium-silica-based composite bone cement and its preparation method and application | |
| Ahmadi et al. | Evaluation of hMSCs response to sodium alginate/bioactive glass composite paste: effect of CaO/P2O5, sodium alginate concentration and P/L ratios | |
| CN109939261A (en) | A kind of solidifying liquid that can control the injectability of calcium phosphate bone cement and its preparation method and application |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| EE01 | Entry into force of recordation of patent licensing contract | Application publication date:20101013 Assignee:Shanghai Rebone Biomaterials Co., Ltd. Assignor:East China University of Science and Technology Contract record no.:2015310000019 Denomination of invention:Calcium magnesium injectable bone cement and preparation method and application thereof Granted publication date:20140219 License type:Common License Record date:20150130 | |
| LICC | Enforcement, change and cancellation of record of contracts on the licence for exploitation of a patent or utility model |