CN101781557A - Preparation method of CdSe/CdS core-shell structure quantum dots - Google Patents
Preparation method of CdSe/CdS core-shell structure quantum dotsDownload PDFInfo
- Publication number
- CN101781557A CN101781557ACN 201010140230CN201010140230ACN101781557ACN 101781557 ACN101781557 ACN 101781557ACN 201010140230CN201010140230CN 201010140230CN 201010140230 ACN201010140230 ACN 201010140230ACN 101781557 ACN101781557 ACN 101781557A
- Authority
- CN
- China
- Prior art keywords
- cdse
- quantum dots
- solution
- cadmium
- heptane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Landscapes
- Luminescent Compositions (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及量子点技术领域,更具体涉及CdSe/CdS核-壳结构量子点的制备方法。The invention relates to the technical field of quantum dots, in particular to a preparation method of CdSe/CdS core-shell structure quantum dots.
背景技术Background technique
量子点通常指半径小于或接近于其波尔激子半径的纳米颗粒。量子点材料的研究是一个涉及多学科交叉领域的研究,除半导体量子点外,还有金属和其它物质的量子点,本文中所提及的量子点除特殊说明外均指半导体量子点。半导体量子点是纳米尺度原子和分子的集合体,一般粒径范围在2-40nm。由于量子点具有发射波长可调、量子产率高等特点,在光电器件和生命科学领域中具有广泛的应用前景。量子点分为单一量子点和核-壳结构的量子点,以CdSe量子点为例,由于单一CdSe量子点容易氧化、不稳定,发光效率低,因此,在单一的CdSe量子点表面外延生长一层CdS壳结构,制备CdSe/CdS核-壳结构量子点能够在一定程度上减少量子点表面的大量缺陷,提高量子点的发光性能。Quantum dots generally refer to nanoparticles with radii smaller than or close to their Bohr excitonic radii. The research on quantum dot materials is a research involving interdisciplinary fields. In addition to semiconductor quantum dots, there are also quantum dots of metals and other substances. The quantum dots mentioned in this article refer to semiconductor quantum dots unless otherwise specified. Semiconductor quantum dots are aggregates of nanoscale atoms and molecules, and the general particle size ranges from 2-40nm. Due to the characteristics of adjustable emission wavelength and high quantum yield, quantum dots have broad application prospects in the fields of optoelectronic devices and life sciences. Quantum dots are divided into single quantum dots and quantum dots with core-shell structure. Taking CdSe quantum dots as an example, because single CdSe quantum dots are easy to oxidize, unstable, and have low luminous efficiency, therefore, a single CdSe quantum dot is epitaxially grown on the surface of a single CdSe quantum dot. Layer CdS shell structure, the preparation of CdSe/CdS core-shell structure quantum dots can reduce a large number of defects on the surface of quantum dots to a certain extent, and improve the luminescence performance of quantum dots.
现有技术中,已经公开了多种制备CdSe/CdS核壳结构量子点的方法。例如,中国专利文献CN1317737C公开了一种制备硒化镉/硫化镉核-壳结构量子点的方法,该专利中先在烷基羧酸镉或油酸镉中加入有机包覆剂和甲苯,然后加入硒脲的水溶液,加热到甲苯和水的沸点以上进行反应得到含有硒化镉半导体纳米粒子的透明溶胶;再以合成的硒化镉为原料溶于甲苯中,加入有机酸镉和有机包覆剂,最后加入硫脲的水溶液,加热到甲苯和水的沸点以上进行反应,分离有机相后进行纯化处理得到硒化镉/硫化镉核-壳结构量子点。在上述专利中,使用了毒性较大的甲苯作为有机溶剂,由于该方法对环境的污染大,因此推广应用受到很大限制。In the prior art, various methods for preparing CdSe/CdS core-shell quantum dots have been disclosed. For example, Chinese patent document CN1317737C discloses a method for preparing cadmium selenide/cadmium sulfide core-shell structure quantum dots. In this patent, an organic coating agent and toluene are first added to cadmium alkyl carboxylate or cadmium oleate, and then Add an aqueous solution of selenourea, heat to the boiling point of toluene and water to react to obtain a transparent sol containing cadmium selenide semiconductor nanoparticles; then dissolve the synthesized cadmium selenide in toluene, add organic acid cadmium and organic coating agent, finally add the aqueous solution of thiourea, heat to the boiling point of toluene and water to react, separate the organic phase and carry out purification treatment to obtain cadmium selenide/cadmium sulfide core-shell structure quantum dots. In the above-mentioned patents, toluene, which is more toxic, is used as an organic solvent. Since this method pollutes the environment heavily, its popularization and application is greatly limited.
中国专利文献CN1321466C公开了一种硫化镉量子点的制备方法,该专利选择使用2-18个碳的烷基羧酸镉或氧化镉为镉源,硫脲或硫代乙酰胺为硫源,油酸或三辛基氧膦为包裹剂,水和不溶于水的有机化合物为溶剂;镉源、包裹剂及不溶于水的有机溶剂在80℃-100℃的温度下加热至无色透明,其中,用作溶剂的有机化合物为苯、甲苯、环己烷、正己烷或正庚烷,等冷却到40℃以下后,再与等体积的硫源水溶液共同加入具有聚四氟乙烯衬里的高压釜内,形成一个两相体系;反应在120℃-180℃的温度下在高压釜内完成。在上述专利中,使用了毒性远小于甲苯的正庚烷作为溶剂在高温下制备了硫化镉量子点,但是三辛基氧膦为有机膦,选择它作为包裹剂时,毒性很大。Chinese patent document CN1321466C discloses a preparation method of cadmium sulfide quantum dots. The patent selects cadmium cadmium carboxylate or cadmium oxide as the cadmium source, thiourea or thioacetamide as the sulfur source, and oil Acid or trioctylphosphine oxide is used as encapsulating agent, water and water-insoluble organic compound are used as solvent; cadmium source, encapsulating agent and water-insoluble organic solvent are heated to colorless and transparent at a temperature of 80°C-100°C, wherein , the organic compound used as a solvent is benzene, toluene, cyclohexane, n-hexane or n-heptane, and after cooling to below 40 ° C, it is added to an autoclave with a polytetrafluoroethylene liner together with an equal volume of sulfur source aqueous solution Inside, a two-phase system is formed; the reaction is completed in an autoclave at a temperature of 120°C-180°C. In the above patent, n-heptane, which is much less toxic than toluene, was used as a solvent to prepare cadmium sulfide quantum dots at high temperature, but trioctylphosphine oxide is an organic phosphine, and it is very toxic when it is selected as a coating agent.
本发明人考虑,由于正庚烷的毒性小,因此可以考虑使用正庚烷作为溶剂制备CdSe/CdS量子点,但在现有技术中:无论是采用甲苯制备CdSe/CdS核-壳结构的量子点还是使用正庚烷等溶剂制备硫化镉量子点,均需要将溶液加热至水相和甲苯或正庚烷等有机相的沸点以上的温度进行反应才能制备质量较好、发光效率较高的量子点。现有理论认为:在水相和有机相的沸点以上的温度进行反应时,反应容器内形成气相和液相,在气相和液相的界面处,水相和有机相能够充分的进行接触,因此容易制备质量较好、发光效率较高的单一结构的量子点或核-壳结构的量子点。本发明人经过研究发现,使用正庚烷作为溶剂时,如果在较低温度下通过控制合适的反应条件,在液相中也能够使水相和有机相形成有利于制备质量良好、发光效率较高的CdSe/CdS核-壳结构的量子点的反应界面。The inventor considers that due to the low toxicity of n-heptane, it can be considered to use n-heptane as a solvent to prepare CdSe/CdS quantum dots, but in the prior art: no matter whether toluene is used to prepare CdSe/CdS quantum dots with a core-shell structure Dots or using solvents such as n-heptane to prepare cadmium sulfide quantum dots, the solution needs to be heated to a temperature above the boiling point of the water phase and organic phases such as toluene or n-heptane to react in order to prepare quantum dots with better quality and higher luminous efficiency. point. Existing theory thinks: when reacting at the temperature above the boiling point of water phase and organic phase, gaseous phase and liquid phase are formed in the reaction vessel, at the interface of gaseous phase and liquid phase, water phase and organic phase can fully contact, therefore It is easy to prepare single-structure quantum dots or core-shell quantum dots with better quality and higher luminous efficiency. The inventors have found through research that when n-heptane is used as a solvent, if the appropriate reaction conditions are controlled at a lower temperature, the aqueous phase and the organic phase can also be formed in the liquid phase, which is conducive to the preparation of good quality and high luminous efficiency. High reaction interface of quantum dots with CdSe/CdS core-shell structure.
发明内容Contents of the invention
本发明要解决的技术问题在于提供一种环境污染小、反应条件温和的CdSe/CdS核-壳结构量子点的制备方法,通过该制备方法制备的核-壳结构量子点发光效率高,质量稳定。The technical problem to be solved by the present invention is to provide a method for preparing CdSe/CdS core-shell structure quantum dots with less environmental pollution and mild reaction conditions. The core-shell structure quantum dots prepared by this preparation method have high luminous efficiency and stable quality .
为了解决以上技术问题,本发明提供一种CdSe/CdS核-壳结构量子点的制备方法,包括:In order to solve the above technical problems, the present invention provides a method for preparing CdSe/CdS core-shell structure quantum dots, comprising:
取硒脲水溶液与烷基羧酸镉的正庚烷溶液混合得到第一混合液,将所述第一混合液加热至40℃~70℃,搅拌所述第一混合液进行反应,从反应后的有机相溶液中分离出CdSe量子点;Take the selenourea aqueous solution and the n-heptane solution of cadmium alkyl carboxylate and mix to obtain the first mixed solution, heat the first mixed solution to 40°C-70°C, stir the first mixed solution to react, from the reaction CdSe quantum dots are separated from the organic phase solution;
取所述CdSe量子点加入到烷基羧酸镉的正庚烷溶液中;Get the CdSe quantum dots and join in the n-heptane solution of cadmium alkyl carboxylate;
取硫脲水溶液与所述含有CdSe量子点的烷基羧酸镉的正庚烷溶液混合得到第二混合液,将所述第二混合液加热至40℃~70℃,搅拌所述第二混合液进行反应得到CdSe/CdS核-壳结构的量子点;Mix the thiourea aqueous solution with the n-heptane solution of cadmium alkyl carboxylate containing CdSe quantum dots to obtain a second mixed solution, heat the second mixed solution to 40°C to 70°C, and stir the second mixed solution Liquid reaction to obtain quantum dots with CdSe/CdS core-shell structure;
所述烷基羧酸镉的正庚烷溶液由烷基羧酸镉、有机包覆剂和正庚烷组成。The n-heptane solution of the cadmium alkyl carboxylate is composed of the cadmium alkyl carboxylate, an organic coating agent and n-heptane.
优选的,所述从反应后的有机相溶液中分离出CdSe量子点具体为:Preferably, the separation of CdSe quantum dots from the reacted organic phase solution is specifically:
用丙酮作为沉淀剂从反应后的有机相溶液中沉淀出CdSe量子点,然后分离得到CdSe量子点。The CdSe quantum dots are precipitated from the reacted organic phase solution by using acetone as a precipitant, and then separated to obtain the CdSe quantum dots.
优选的,所述第一混合液的加热温度为60℃~70℃。Preferably, the heating temperature of the first mixed liquid is 60°C to 70°C.
优选的,所述第二混合液的加热温度为60℃~70℃。Preferably, the heating temperature of the second mixed liquid is 60°C-70°C.
优选的,所述烷基羧酸镉的正庚烷溶液中烷基羧酸镉的摩尔浓度为0.015mmol/ml~0.03mmol/ml。Preferably, the molar concentration of the cadmium alkyl carboxylate in the n-heptane solution of the cadmium alkyl carboxylate is 0.015 mmol/ml-0.03 mmol/ml.
优选的,所述烷基羧酸镉的正庚烷溶液中有机包覆剂的体积浓度为0.05ml/ml~0.15ml/ml。Preferably, the volume concentration of the organic coating agent in the n-heptane solution of cadmium alkyl carboxylate is 0.05ml/ml-0.15ml/ml.
优选的,还包括从所述反应后的第二混合液中分离出CdSe/CdS核-壳结构的量子点的步骤。Preferably, the step of separating the CdSe/CdS core-shell quantum dots from the second mixed liquid after the reaction is also included.
优选的,所述从所述反应后的第二混合液中分离出CdSe/CdS核-壳结构的量子点的步骤具体为:Preferably, the step of separating quantum dots with CdSe/CdS core-shell structure from the second mixed solution after the reaction is specifically:
用丙酮作为沉淀剂从第二混合液反应后的有机相溶液中沉淀出CdSe/CdS核-壳结构的量子点,然后离心分离得到CdSe/CdS核-壳结构的量子点。Using acetone as a precipitant to precipitate CdSe/CdS core-shell quantum dots from the organic phase solution after the reaction of the second mixed solution, and then centrifuging to obtain CdSe/CdS core-shell structure quantum dots.
优选的,所述烷基羧酸镉为十四烷基羧酸镉。Preferably, the cadmium alkyl carboxylate is cadmium tetradecyl carboxylate.
优选的,所述有机包覆剂为油酸。Preferably, the organic coating agent is oleic acid.
本发明提供一种CdSe/CdS核-壳结构量子点的制备方法,本发明使用毒性小的正庚烷作为溶剂,先将硒脲水溶液与烷基羧酸镉的正庚烷溶液混合,然后加热至40℃~70℃搅拌进行反应制备CdSe量子点;再将所述CdSe量子点加入到烷基羧酸镉的正庚烷溶液中,然后加入硫脲水溶液,将得到的混合液加热至40℃~70℃搅拌进行反应制备CdSe/CdS量子点。本发明提供的方法中反应温度低,通过搅拌使液相内的水相和有机相均匀的混合得到有利于进行反应的界面,由于反应温度低,量子点的壳层生长均匀。测试结果证明,本发明制备的核-壳结构的量子点质量稳定,发光效率高。The invention provides a preparation method of CdSe/CdS core-shell structure quantum dots. The invention uses n-heptane with low toxicity as a solvent, firstly mixes the selenourea aqueous solution with the n-heptane solution of cadmium alkyl carboxylate, and then heats Stir at 40°C to 70°C for reaction to prepare CdSe quantum dots; then add the CdSe quantum dots to the n-heptane solution of cadmium alkyl carboxylate, then add thiourea aqueous solution, and heat the resulting mixture to 40°C The CdSe/CdS quantum dots were prepared by stirring at ~70°C. In the method provided by the invention, the reaction temperature is low, and the aqueous phase and the organic phase in the liquid phase are uniformly mixed by stirring to obtain an interface favorable for the reaction. Due to the low reaction temperature, the shell layer of the quantum dot grows uniformly. The test result proves that the quantum dot with core-shell structure prepared by the invention has stable quality and high luminous efficiency.
附图说明Description of drawings
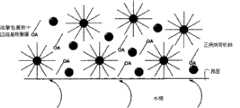
图1为本发明制备CdSe/CdS核-壳结构量子点示意图。Fig. 1 is a schematic diagram of preparing CdSe/CdS core-shell structure quantum dots according to the present invention.
具体实施方式Detailed ways
下面将对本发明实施例中的技术方案进行清楚、完整地描述,显然,所描述的实施例仅仅是本发明一部分实施例,而不是全部的实施例。基于本发明中的实施例,本领域普通技术人员在没有作出创造性劳动前提下所获得的所有其他实施例,都属于本发明保护的范围。The technical solutions in the embodiments of the present invention will be clearly and completely described below. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without creative efforts fall within the protection scope of the present invention.
本发明提供一种CdSe/CdS核-壳结构量子点的制备方法,包括:The invention provides a preparation method of CdSe/CdS core-shell structure quantum dots, comprising:
取硒脲水溶液与烷基羧酸镉的正庚烷溶液混合得到第一混合液,将所述第一混合液加热至40℃~70℃,搅拌所述第一混合液进行反应,从反应后的有机相溶液中分离出CdSe量子点;Take the selenourea aqueous solution and the n-heptane solution of cadmium alkyl carboxylate and mix to obtain the first mixed solution, heat the first mixed solution to 40°C-70°C, stir the first mixed solution to react, from the reaction CdSe quantum dots are separated from the organic phase solution;
取所述CdSe量子点加入到烷基羧酸镉的正庚烷溶液中;Get the CdSe quantum dots and join in the n-heptane solution of cadmium alkyl carboxylate;
取硫脲水溶液与所述含有CdSe量子点的烷基羧酸镉的正庚烷溶液混合得到第二混合液,将所述第二混合液加热至40℃~70℃,搅拌所述第二混合液进行反应得到CdSe/CdS核-壳结构的量子点;Mix the thiourea aqueous solution with the n-heptane solution of cadmium alkyl carboxylate containing CdSe quantum dots to obtain a second mixed solution, heat the second mixed solution to 40°C to 70°C, and stir the second mixed solution Liquid reaction to obtain quantum dots with CdSe/CdS core-shell structure;
所述烷基羧酸镉的正庚烷溶液由烷基羧酸镉、有机包覆剂和正庚烷组成。The n-heptane solution of the cadmium alkyl carboxylate is composed of the cadmium alkyl carboxylate, an organic coating agent and n-heptane.
请参见图1,为本发明制备CdSe/CdS的原理图,在水相和正庚烷有机相的界面处发生量子点的合成反应,在界面生成的量子点被油酸包覆后扩散到有机相中,其它由油酸包裹的十四烷基羧酸镉运动到界面。在搅拌的过程中,水相和有机相的界面面积增加,使前体之间接触机会增多,有利于反应的进行。由于反应温度低,导致量子点的生长速度缓慢,因此所合成的量子点其尺寸比较均匀,有利于制备质量稳定、发光效率高的量子点。Please refer to Figure 1, which is a schematic diagram of the preparation of CdSe/CdS in the present invention. The synthesis reaction of quantum dots occurs at the interface between the water phase and the n-heptane organic phase, and the quantum dots generated at the interface are coated with oleic acid and diffuse into the organic phase. , other cadmium tetradecyl carboxylate wrapped by oleic acid moved to the interface. During the stirring process, the interface area between the aqueous phase and the organic phase increases, which increases the chance of contact between the precursors and facilitates the reaction. Due to the low reaction temperature, the growth rate of quantum dots is slow, so the size of the synthesized quantum dots is relatively uniform, which is conducive to the preparation of quantum dots with stable quality and high luminous efficiency.
按照本发明,所述烷基羧酸镉的正庚烷溶液中的烷基羧酸镉优选为十四烷基羧酸镉,所述有机包覆剂优选为油酸,所述烷基羧酸镉的正庚烷溶液中烷基羧酸镉的摩尔浓度优选为0.015mmol/ml~0.03mmol/ml,更优选为0.018mmol/ml~0.025mmol/ml;所述烷基羧酸镉的正庚烷溶液中的油酸体积浓度优选为0.05ml/ml~0.15ml/ml,更优选为0.08ml/ml~0.12ml/ml。According to the present invention, the cadmium alkyl carboxylate in the n-heptane solution of the cadmium alkyl carboxylate is preferably cadmium tetradecyl carboxylate, the organic coating agent is preferably oleic acid, and the alkyl carboxylic acid The molar concentration of cadmium alkyl carboxylate in the n-heptane solution of cadmium is preferably 0.015mmol/ml~0.03mmol/ml, more preferably 0.018mmol/ml~0.025mmol/ml; The volume concentration of oleic acid in the alkanes solution is preferably 0.05ml/ml-0.15ml/ml, more preferably 0.08ml/ml-0.12ml/ml.
按照本发明,使用油酸作为有机包覆剂,配制十四烷基羧酸镉的正庚烷溶液时,具体可以按照如下步骤:量取十四烷基羧酸镉、油酸和正庚烷加入到容器中进行搅拌加热,加热温度优选为80℃~100℃,更优选为85℃~95℃,更优选为86℃~92℃;对于搅拌速度,本发明并无特别限制,优选为匀速搅拌。对于加热时间,优选加热到直至形成无色透明溶液为止,然后将得到的十四烷基羧酸镉的正庚烷溶液冷却至室温待用。According to the present invention, when using oleic acid as the organic coating agent, when preparing the n-heptane solution of cadmium tetradecyl carboxylate, the specific steps can be as follows: measure cadmium tetradecyl carboxylate, oleic acid and n-heptane and add Stir and heat in a container, the heating temperature is preferably 80°C to 100°C, more preferably 85°C to 95°C, more preferably 86°C to 92°C; the stirring speed is not particularly limited in the present invention, preferably uniform stirring . For the heating time, it is preferred to heat until a colorless transparent solution is formed, and then the n-heptane solution of cadmium tetradecylcarboxylate obtained is cooled to room temperature for use.
取硒脲溶于水中得到硒脲水溶液,其中所述水可以为氮气饱和水,这样可以防止硒脲氧化,对于硒脲水溶液的浓度本发明并无特别限制。将硒脲水溶液与部分制备好的十四烷基羧酸镉的正庚烷溶液混合,其中硒脲与十四烷基羧酸镉的物质量比优选为0.05~0.5∶0.1~1.0,更优选为1∶2。Dissolve selenourea in water to obtain a selenourea aqueous solution, wherein the water can be nitrogen-saturated water, which can prevent selenourea from oxidation, and the concentration of the selenourea aqueous solution is not particularly limited in the present invention. The selenourea aqueous solution is mixed with the partially prepared n-heptane solution of cadmium tetradecyl carboxylate, wherein the mass ratio of selenourea to cadmium tetradecyl carboxylate is preferably 0.05~0.5:0.1~1.0, more preferably It is 1:2.
将硒脲水溶液与十四烷基羧酸镉的正庚烷溶液混合后,优选再将得到的混合液在放置在密闭容器内加热至40℃~70℃进行保温,加热方式优选使用油浴或水浴的加热方式,保温的同时搅拌所述混合液进行反应制备CdSe量子点,对于反应时间,优选为10min以上。对于搅拌速度没有特别限制,优选为匀速搅拌。搅拌所述混合液进行反应时,为防止硒脲氧化,优选在惰性气体保护下搅拌进行反应,惰性气体可以为氮气、氩气等本领域技术人员熟知的惰性气体。搅拌反应制备CdSe量子点后,将反应后的混合液冷却至室温,取出上层有机相,优选使用丙酮作为沉淀剂对有机相中的CdSe量子点进行纯化,然后离心分离得到CdSe量子点。After mixing the selenourea aqueous solution with the n-heptane solution of cadmium tetradecyl carboxylate, it is preferable to place the obtained mixed solution in a closed container and heat it to 40°C to 70°C for heat preservation. The heating method is preferably an oil bath or The heating method of the water bath is to stir the mixed solution while keeping the heat to react to prepare the CdSe quantum dots. The reaction time is preferably more than 10 minutes. There is no particular limitation on the stirring speed, but uniform stirring is preferred. When stirring the mixed solution to react, in order to prevent selenourea from oxidation, it is preferable to stir and react under the protection of an inert gas. The inert gas can be an inert gas well known to those skilled in the art such as nitrogen and argon. After stirring the reaction to prepare CdSe quantum dots, cool the reacted mixed solution to room temperature, take out the upper organic phase, preferably use acetone as a precipitant to purify the CdSe quantum dots in the organic phase, and then centrifuge to obtain CdSe quantum dots.
按照本发明,取纯化后的CdSe量子点与部分制备好的十四烷基羧酸镉的正庚烷溶液混合,其中CdSe与十四烷基羧酸镉的物质的量摩尔比优选为0.05~0.5∶0.1~1.0,更优选为1∶2。将所述CdSe量子点与十四烷基羧酸镉的正庚烷溶液混合时,优选使超声波振荡处理,以使CdSe量子点充分分散在十四烷基羧酸镉的正庚烷溶液中。According to the present invention, the CdSe quantum dot after purification is mixed with partly prepared n-heptane solution of cadmium tetradecylcarboxylate, wherein the molar ratio of CdSe and cadmium tetradecylcarboxylate is preferably 0.05~ 0.5:0.1-1.0, more preferably 1:2. When mixing the CdSe quantum dots with the n-heptane solution of cadmium tetradecyl carboxylate, it is preferable to conduct ultrasonic vibration treatment so that the CdSe quantum dots are fully dispersed in the n-heptane solution of cadmium tetradecyl carboxylate.
取硫脲水溶液加入到上述含有CdSe量子点的烷基羧酸镉的正庚烷溶液中,硫脲与CdSe的物质的量比值优选为2~10∶1,更优选为3∶1。对于所述硫脲水溶液的浓度本发明并无特别限制,配制所述硫脲水溶液时,优选使用二次蒸馏水作为溶剂。The thiourea aqueous solution is added to the above-mentioned n-heptane solution of cadmium alkyl carboxylate containing CdSe quantum dots, the ratio of thiourea to CdSe is preferably 2-10:1, more preferably 3:1. The present invention has no particular limitation on the concentration of the aqueous thiourea solution. When preparing the aqueous thiourea solution, it is preferred to use double distilled water as a solvent.
将硫脲水溶液与所述含有CdSe量子点的烷基羧酸镉的正庚烷溶液混合后,优选将得到的混合液放置在密闭容器中,加热至40℃~70℃搅拌进行反应,对于搅拌速度没有特别限制,优选匀速。对于加热方法没有特别限制,优选使用油浴或水浴等本领域技术人员熟知的加热方式。加热温度更优选为50℃~70℃,更优选为60℃~70℃。反应时间优选为大于10min以上,本发明没有特别限制。反应达到预定时间后,将反应后的混合液冷却至室温,取出有机相,优选使用丙酮作为沉淀剂对CdSe/CdS量子点进行纯化处理,然后离心分离得到CdSe/CdS核-壳结构量子点。After mixing the thiourea aqueous solution with the n-heptane solution of cadmium alkyl carboxylate containing CdSe quantum dots, it is preferable to place the obtained mixed solution in an airtight container, and heat it to 40° C. to 70° C. to stir for reaction. For stirring The speed is not particularly limited, and a uniform speed is preferable. There is no particular limitation on the heating method, and heating methods known to those skilled in the art such as oil bath or water bath are preferably used. The heating temperature is more preferably from 50°C to 70°C, more preferably from 60°C to 70°C. The reaction time is preferably longer than 10 minutes, and the present invention is not particularly limited. After the reaction reaches a predetermined time, cool the reacted mixed solution to room temperature, take out the organic phase, preferably use acetone as a precipitant to purify the CdSe/CdS quantum dots, and then centrifuge to obtain CdSe/CdS core-shell structure quantum dots.
本发明以十四烷基羧酸镉的正庚烷溶液与硒脲水溶液混合在40℃~70℃的条件下搅拌进行反应制备CdSe量子点,然后再将制备的CdSe量子点分散在十四烷基羧酸镉的正庚烷溶液中,取硫脲水溶液与所述含有CdSe量子点分散在十四烷基羧酸镉的正庚烷溶液中,在40℃~70℃的条件下搅拌进行反应制备CdSe/CdS核-壳结构量子点。The present invention prepares CdSe quantum dots by mixing n-heptane solution of cadmium tetradecyl carboxylate and selenourea aqueous solution under the condition of 40°C to 70°C and stirring for reaction, and then dispersing the prepared CdSe quantum dots in tetradecane In the n-heptane solution of cadmium carboxylate, take the aqueous solution of thiourea and disperse the quantum dots containing CdSe in the n-heptane solution of cadmium tetradecyl carboxylate, and stir under the condition of 40 ° C to 70 ° C to react Preparation of CdSe/CdS core-shell quantum dots.
与现有技术相比,本发明先在40℃~70℃的低温下使用十四烷基羧酸镉的正庚烷溶液与硒脲水溶液混合搅拌进行反应制备CdSe量子点,然后再将制备好的CdSe量子点分散在十四烷基羧酸镉的正庚烷溶液中,加入硫脲搅拌进行反应制备CdSe/CdS量子点。由于正庚烷的毒性远远小于甲苯,因此对环境污染小。另外,本发明在低温下通过搅拌的方式使硒脲或硫脲与十四烷基正庚烷的溶液充分混合,在液相中增加水相和有机相的两相界面,搅拌时可以使水相和有机相充分的接触,匀速搅拌时,由于硫脲与十四烷基羧酸镉的反应是逐渐进行的,因此量子点是逐渐均匀的长大的,有利于保证量子点壳层的均匀性,由于量子点壳层均匀性是影响发光效率的一个重要因素,而本发明的对比实验结果表明,制备出的核-壳结构量子点具有很高的发光效率,质量稳定。与高温反应相比,本发明可以在条件更加温和的低温下制备发光效率高的CdSe/CdS核-壳结构的量子点,反应更容易实现。Compared with the prior art, the present invention uses the n-heptane solution of cadmium tetradecyl carboxylate and selenourea aqueous solution to mix and stir at a low temperature of 40°C to 70°C to prepare CdSe quantum dots, and then prepare the quantum dots CdSe quantum dots were dispersed in n-heptane solution of cadmium tetradecyl carboxylate, and thiourea was added to stir for reaction to prepare CdSe/CdS quantum dots. Since the toxicity of n-heptane is far less than that of toluene, it has little environmental pollution. In addition, the present invention fully mixes the solution of selenourea or thiourea and tetradecyl n-heptane by stirring at low temperature, increases the two-phase interface of the water phase and the organic phase in the liquid phase, and can make the water Phase and organic phase are in full contact, and when stirring at a constant speed, since the reaction between thiourea and cadmium tetradecyl carboxylate is carried out gradually, the quantum dots grow uniformly gradually, which is conducive to ensuring the uniformity of the quantum dot shell. Since the uniformity of the quantum dot shell layer is an important factor affecting the luminous efficiency, the comparative experimental results of the present invention show that the prepared core-shell structure quantum dots have high luminous efficiency and stable quality. Compared with the high-temperature reaction, the present invention can prepare CdSe/CdS core-shell quantum dots with high luminous efficiency at a milder and lower temperature, and the reaction is easier to realize.
以下结合具体实施例和比较例说明本发明的技术方案。The technical solution of the present invention is illustrated below in conjunction with specific examples and comparative examples.
实施例1Example 1
制备十四烷基羧酸镉的正庚烷溶液;Preparation of n-heptane solution of cadmium tetradecyl carboxylate;
将0.1134g的十四烷基羧酸镉、1.0ml油酸和10ml正庚烷加入到50ml的圆底烧瓶中,匀速搅拌,加热至80℃~100℃直至形成无色透明的溶液,然后冷却至室温得到十四烷基羧酸镉的正庚烷溶液。Add 0.1134g of cadmium tetradecyl carboxylate, 1.0ml of oleic acid and 10ml of n-heptane into a 50ml round bottom flask, stir at a constant speed, heat to 80℃~100℃ until a colorless and transparent solution is formed, then cool At room temperature, a n-heptane solution of cadmium tetradecyl carboxylate was obtained.
实施例2~实施例5和比较例1~4是制备CdSe量子点的例子。Examples 2 to 5 and Comparative Examples 1 to 4 are examples of preparing CdSe quantum dots.
实施例2Example 2
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入40℃油浴锅中,匀速搅拌圆底烧瓶中的混合液体,反应时间为20min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它的荧光发射峰在467nm处,其发射峰峰形对称,但是有拖尾,是缺陷发光造成的。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round-bottom flask, put it in a 40°C oil bath, stir the mixed liquid in the round-bottom flask at a constant speed, the reaction time is 20min, take out the round-bottom flask and cool to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its fluorescence emission peak is at 467nm, and its emission peak shape is symmetrical, but there is tailing, which is caused by defect emission.
比较例1Comparative example 1
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入40℃油浴锅中,静止反应,反应时间为20min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它的发射峰峰位在436nm处,峰形不对称,在557nm处出现一个很大的宽的缺陷发光峰。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round-bottomed flask, put it in an oil bath at 40°C, and react statically. The reaction time is 20 minutes. Take out the round-bottomed flask and cool it to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its emission peak is at 436nm, the peak shape is asymmetrical, and a large and wide defect luminescence peak appears at 557nm.
实施例3Example 3
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入40℃油浴锅中,匀速搅拌圆底烧瓶中的混合液体,反应时间为40min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它荧光发射峰在470nm处,其发射峰峰形对称,但是有拖尾,是缺陷发光造成的。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round bottom flask, put it in a 40°C oil bath, stir the mixed liquid in the round bottom flask at a constant speed, the reaction time is 40min, take out the round bottom flask and cool to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its fluorescence emission peak is at 470nm, and its emission peak shape is symmetrical, but there is tailing, which is caused by defect emission.
比较例2Comparative example 2
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入40℃油浴锅中,静止反应反应时间为40min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它的发射峰峰位在442nm处,峰形不对称,在560nm处出现一个很大的宽峰,是缺陷发光造成的。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round-bottomed flask, put it in an oil bath at 40°C, and wait for 40 minutes for static reaction. Take out the round-bottomed flask and cool to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its emission peak is at 442nm, the peak shape is asymmetrical, and a large broad peak appears at 560nm, which is caused by defect luminescence.
实施例4Example 4
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入70℃油浴锅中,匀速搅拌圆底烧瓶中的混合液体,反应时间为60min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它的荧光发射峰在572nm处,其发射峰峰形对称,没有拖尾。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round bottom flask, put it in a 70°C oil bath, stir the mixed liquid in the round bottom flask at a constant speed, the reaction time is 60min, take out the round bottom flask and cool to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its fluorescence emission peak is at 572nm, and its emission peak shape is symmetrical without tailing.
比较例3Comparative example 3
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入70℃油浴锅中,静止反应,反应时间为60min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它荧光发射峰在496nm处,其发射峰峰形对称,但是有拖尾,是缺陷发光造成的。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round-bottomed flask, put it in an oil bath at 70°C, and react statically. The reaction time is 60 minutes. Take out the round-bottomed flask and cool it to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its fluorescence emission peak is at 496nm, and its emission peak shape is symmetrical, but there is tailing, which is caused by defect light emission.
实施例5Example 5
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入60℃油浴锅中,匀速搅拌圆底烧瓶中的混合液体,反应时间为45min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它荧光发射峰在515nm处,其发射峰峰形对称,但是有拖尾,是缺陷发光造成的。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round bottom flask, put it in a 60°C oil bath, stir the mixed liquid in the round bottom flask at a constant speed, the reaction time is 45min, take out the round bottom flask and cool to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its fluorescence emission peak is at 515nm, and its emission peak shape is symmetrical, but there is tailing, which is caused by defect emission.
比较例4Comparative example 4
制备CdSe量子点:Preparation of CdSe quantum dots:
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml的圆底烧瓶中氮气保护下混合,密封好圆底烧瓶,放入60℃油浴锅中,静止反应,反应时间为45min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它荧光发射峰在477nm处,其发射峰峰形对称,但是在573nm处有一个很大的缺陷发光峰。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the n-heptane solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared in 10ml embodiment 1 in the round bottom flask of 50ml nitrogen gas Mix under protection, seal the round-bottomed flask, put it in a 60°C oil bath, and react statically. The reaction time is 45min. Take out the round-bottomed flask and cool it to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its fluorescence emission peak is at 477nm, and its emission peak shape is symmetrical, but there is a large defect luminescence peak at 573nm.
实施例6~10、比较例5~9为制备CdSe/CdS核-壳结构量子点的例子。Examples 6-10 and Comparative Examples 5-9 are examples of preparing CdSe/CdS core-shell structure quantum dots.
实施例6(原实施例1)Embodiment 6 (former embodiment 1)
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取实施例2制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中,匀速搅拌下进行反应,反应24小时,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在548nm,峰形对称且无拖尾峰,发光效率为47.2%。Get the CdSe quantum dots prepared in Example 2 and the n-heptane solution of cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dots, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into In an oil bath at 70°C, react under constant stirring, react for 24 hours, take out the round bottom flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, and use acetone as a precipitant for CdSe/CdS nuclei - The quantum dots with shell structure are purified and centrifuged to obtain CdSe/CdS core-shell quantum dots. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 548nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 47.2%.
比较例5Comparative Example 5
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取比较例1制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,静止反应,反应24小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在492nm,峰形对称且无拖尾峰,发光效率为34.7%。Get the n-heptane solution of the CdSe quantum dots prepared by Comparative Example 1 and the cadmium tetradecyl carboxylate prepared by Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to make the CdSe quantum dots fully dispersed, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heating in an oil bath at 70°C, static reaction, after 24 hours of reaction, take out the round bottom flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, use acetone as a precipitant to CdSe/CdS nuclei - The quantum dots with shell structure are purified and centrifuged to obtain CdSe/CdS core-shell quantum dots. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 492nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 34.7%.
实施例7Example 7
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取实施例2制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,同时匀速搅拌圆底烧瓶中的混合液,反应12小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在530nm,峰形对称且无拖尾峰,发光效率为51.0%。Get the CdSe quantum dots prepared in Example 2 and the n-heptane solution of cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dots, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heat it in an oil bath at 70°C, and at the same time stir the mixed solution in the round-bottomed flask at a constant speed. After 12 hours of reaction, take out the round-bottomed flask from the oil bath and cool it to room temperature, take out the organic phase from the reacted solution, and wash it with acetone The CdSe/CdS core-shell structure quantum dots were purified as a precipitant, and the CdSe/CdS core-shell structure quantum dots were obtained by centrifugation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 530nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 51.0%.
比较例6Comparative example 6
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取比较例1制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,静止反应,反应12小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在495nm,峰形对称且无拖尾峰,发光效率为37.7%。Get the n-heptane solution of the CdSe quantum dots prepared by Comparative Example 1 and the cadmium tetradecyl carboxylate prepared by Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to make the CdSe quantum dots fully dispersed, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heating in an oil bath at 70°C, and reacting statically. After 12 hours of reaction, take out the round bottom flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, and use acetone as a precipitant for CdSe/CdS nuclei - The quantum dots with shell structure are purified and centrifuged to obtain CdSe/CdS core-shell quantum dots. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 495nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 37.7%.
实施例8Example 8
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取实施例3制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,同时匀速搅拌圆底烧瓶中的混合液,反应12小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在522nm,峰形对称且无拖尾峰,发光效率为52.1%。Get the CdSe quantum dots prepared in Example 3 and the n-heptane solution of cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dots, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heat it in an oil bath at 70°C, and at the same time stir the mixed solution in the round-bottomed flask at a constant speed. After 12 hours of reaction, take out the round-bottomed flask from the oil bath and cool it to room temperature, take out the organic phase from the reacted solution, and wash it with acetone The CdSe/CdS core-shell structure quantum dots were purified as a precipitant, and the CdSe/CdS core-shell structure quantum dots were obtained by centrifugation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 522nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 52.1%.
比较例7Comparative Example 7
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取比较例2制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,同时静止圆底烧瓶中的混合液,反应12小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在491nm,峰形对称且无拖尾峰,发光效率为36.5%。Get the CdSe quantum dot prepared by Comparative Example 2 and the n-heptane solution of the cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dot, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heating in an oil bath at 70°C while stilling the mixed liquid in the round-bottomed flask. After reacting for 12 hours, take out the round-bottomed flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, and use acetone as The precipitant is used to purify the CdSe/CdS core-shell structure quantum dots, and the CdSe/CdS core-shell structure quantum dots are obtained by centrifugal separation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 491nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 36.5%.
实施例9Example 9
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取实施例3制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,同时匀速搅拌圆底烧瓶中的混合液,反应3小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在510nm,峰形对称且无拖尾峰,发光效率为73.5%。Get the CdSe quantum dots prepared in Example 3 and the n-heptane solution of cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dots, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heat it in an oil bath at 70°C, and at the same time stir the mixed solution in the round-bottomed flask at a constant speed. After reacting for 3 hours, take out the round-bottomed flask from the oil bath and cool it to room temperature, take out the organic phase from the reacted solution, and wash it with acetone The CdSe/CdS core-shell structure quantum dots were purified as a precipitant, and the CdSe/CdS core-shell structure quantum dots were obtained by centrifugation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 510nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 73.5%.
比较例8Comparative Example 8
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取比较例2制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,同时静止圆底烧瓶中的混合液,反应3小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在481nm,峰形对称且无拖尾峰,发光效率为42.7%。Get the CdSe quantum dot prepared by Comparative Example 2 and the n-heptane solution of the cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dot, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heating in an oil bath at 70°C while stilling the mixed solution in the round-bottomed flask. After reacting for 3 hours, take out the round-bottomed flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, and use acetone as The precipitant is used to purify the CdSe/CdS core-shell structure quantum dots, and the CdSe/CdS core-shell structure quantum dots are obtained by centrifugal separation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 481nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 42.7%.
实施例10Example 10
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取实施例2制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,同时匀速搅拌圆底烧瓶中的混合液,反应6小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在505nm,峰形对称且无拖尾峰,发光效率为65.9%。Get the CdSe quantum dots prepared in Example 2 and the n-heptane solution of cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dots, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heat in an oil bath at 70°C while stirring the mixed solution in the round-bottomed flask at a constant speed. After 6 hours of reaction, take out the round-bottomed flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, and wash with acetone The CdSe/CdS core-shell structure quantum dots were purified as a precipitant, and the CdSe/CdS core-shell structure quantum dots were obtained by centrifugation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 505nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 65.9%.
比较例9Comparative Example 9
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取比较例2制备的CdSe量子点与实施例1制备的十四烷基羧酸镉的正庚烷溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的正庚烷溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,静止反应,反应过程中氮气保护,反应6小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用丙酮作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。所得到的CdSe/CdS核-壳结构量子点的发射峰在498nm,峰形对称且无拖尾峰,发光效率为39.8%。Get the CdSe quantum dot prepared by Comparative Example 2 and the n-heptane solution of the cadmium tetradecyl carboxylate prepared in Example 1 and mix in a 50ml round bottom flask, and ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dot, then take 0.0380 1 g of thiourea was dissolved in 10 ml of twice distilled water to obtain an aqueous solution of thiourea, and the aqueous solution of thiourea was added to the above-mentioned n-heptane solution of cadmium tetradecylcarboxylate containing CdSe quantum dots, the round-bottomed flask was sealed, and then put into Heating in an oil bath at 70°C, static reaction, nitrogen protection during the reaction, after 6 hours of reaction, take out the round bottom flask from the oil bath and cool to room temperature, take out the organic phase from the reacted solution, and use acetone as a precipitate The CdSe/CdS core-shell structure quantum dots were purified by a reagent, and the CdSe/CdS core-shell structure quantum dots were obtained by centrifugation. The emission peak of the obtained CdSe/CdS core-shell structure quantum dot is at 498nm, the peak shape is symmetrical and there is no tailing peak, and the luminous efficiency is 39.8%.
在比较例1~4中,静止反应制备了CdSe量子点,然后在比较例5~9中,静止反应制备了CdSe/CdS核-壳结构量子点,静止反应时,水相和有机相的两相界面不能进行充分的接触,量子点的生长不均匀,因此最终得到的核-壳结构量子点质量差,其荧光峰的半峰宽度(FWHM)相对变宽,缺陷发光强烈,发光效率低。In Comparative Examples 1-4, CdSe quantum dots were prepared by static reaction, and then in Comparative Examples 5-9, CdSe/CdS core-shell quantum dots were prepared by static reaction. The phase interface cannot be fully contacted, and the growth of quantum dots is not uniform. Therefore, the quality of the final core-shell structure quantum dots is poor, and the half-maximum width (FWHM) of the fluorescence peak is relatively broadened. The defects emit strong light and low luminous efficiency.
与上述比较例相比,实施例2~5在搅拌状态下制备CdSe量子点,实施例6~10在搅拌状态下制备CdSe/CdS量子点,与现有技术制备量子点需要气液两相不同,本发明在低温的液相下进行反应,在搅拌状态下,水相和有机相能够充分的进行混合,形成有利于反应的界面,而且低温搅拌,能够使量子点生长均匀,因此得到的量子点质量稳定,发光效率高。Compared with the above comparative examples, Examples 2-5 prepared CdSe quantum dots in a stirred state, and Examples 6-10 prepared CdSe/CdS quantum dots in a stirred state, which is different from the gas-liquid two-phase preparation of quantum dots in the prior art. , the present invention reacts in a low-temperature liquid phase, and in a stirring state, the water phase and the organic phase can be fully mixed to form an interface that is beneficial to the reaction, and the low-temperature stirring can make the quantum dots grow uniformly, so the quantum dots obtained The dot quality is stable and the luminous efficiency is high.
比较例10(用甲苯作溶剂,低温静止反应)Comparative example 10 (make solvent with toluene, low temperature static reaction)
将0.1134g的十四烷基羧酸镉、1.0ml油酸和10ml甲苯加入到50ml的圆底烧瓶中,加热至80℃~100℃直至形成无色透明的溶液,然后冷却至室温得到十四烷基羧酸镉的甲苯溶液。Add 0.1134g of cadmium tetradecyl carboxylate, 1.0ml of oleic acid and 10ml of toluene into a 50ml round bottom flask, heat to 80°C to 100°C until a colorless and transparent solution is formed, then cool to room temperature to obtain fourteen A solution of cadmium alkyl carboxylate in toluene.
取0.0125g硒脲溶液溶于10ml氮气饱和水得到硒脲水溶液,将所述硒脲水溶液与10ml制备的十四烷基羧酸镉的甲苯溶液在50ml的圆底烧瓶中混合,密封好圆底烧瓶,放入40℃油浴锅中,静止反应,反应时间为20min,取出圆底烧瓶冷却至室温。取出反应液的上层有机相,用丙酮作为沉淀剂对有机相中CdSe量子点纯化处理,离心分离得到CdSe量子点。它的发射峰峰位在444nm处,峰形不对称,在558nm处出现一个很大的宽峰,是缺陷发光造成的。Get 0.0125g selenourea solution and be dissolved in 10ml nitrogen saturated water to obtain selenourea aqueous solution, the toluene solution of described selenourea aqueous solution and the cadmium tetradecyl carboxylate prepared by 10ml are mixed in the round bottom flask of 50ml, seal the round bottom The flask was placed in an oil bath at 40°C and reacted statically. The reaction time was 20 minutes. The round bottom flask was taken out and cooled to room temperature. Taking out the upper organic phase of the reaction solution, using acetone as a precipitating agent to purify the CdSe quantum dots in the organic phase, and centrifuging to obtain the CdSe quantum dots. Its emission peak is at 444nm, the peak shape is asymmetrical, and a large broad peak appears at 558nm, which is caused by defect luminescence.
制备CdSe/CdS核-壳结构量子点:Preparation of CdSe/CdS core-shell quantum dots:
取上述制备的CdSe量子点与制备的十四烷基羧酸镉的甲苯溶液在50ml圆底烧瓶中混合,超声振荡10分钟,使CdSe量子点充分分散,然后取0.0380g硫脲溶于10ml二次蒸馏水中得到硫脲水溶液,将硫脲水溶液加入到上述含有CdSe量子点的十四烷基羧酸镉的甲苯溶液中,密封好圆底烧瓶,然后放入到70℃的油浴中进行加热,静止反应,反应24小时后,从油浴中取出圆底烧瓶冷却至室温,从反应后的溶液中取出有机相,用甲醇作为沉淀剂对CdSe/CdS核-壳结构量子点进行纯化,离心分离得到CdSe/CdS核-壳结构量子点。它的发射峰峰位在500nm处,峰形不对称,发光效率为29.6%。Get the CdSe quantum dots prepared above and the toluene solution of the cadmium tetradecyl carboxylate prepared and mix in a 50ml round bottom flask, ultrasonically vibrate for 10 minutes to fully disperse the CdSe quantum dots, then get 0.0380g thiourea and dissolve it in 10ml di Obtain thiourea aqueous solution in sub-distilled water, add thiourea aqueous solution to the toluene solution of cadmium tetradecyl carboxylate containing CdSe quantum dots, seal the round bottom flask, and then put it into an oil bath at 70°C for heating , static reaction, after 24 hours of reaction, take out the round bottom flask from the oil bath and cool to room temperature, take out the organic phase from the solution after the reaction, use methanol as a precipitant to purify the CdSe/CdS core-shell structure quantum dots, and centrifuge CdSe/CdS core-shell quantum dots were isolated. Its emission peak is at 500nm, the peak shape is asymmetric, and the luminous efficiency is 29.6%.
在比较例10中,使用甲苯作为溶剂静止反应制备CdSe量子点和CdSe/CdS量子点,甲苯的极性要高于正庚烷的极性,而量子点的合成速度随着极性的增大而变大,当在静止条件下进行反应时,与同样条件的正庚烷相比,量子点生长更加不均匀,因此得到的量子点质量较差,发光效率低。In Comparative Example 10, CdSe quantum dots and CdSe/CdS quantum dots were prepared by using toluene as a solvent for static reaction. The polarity of toluene is higher than that of n-heptane, and the synthesis speed of quantum dots increases with the increase of polarity. However, when the reaction is carried out under static conditions, the growth of quantum dots is more uneven compared with n-heptane under the same conditions, so the quality of the obtained quantum dots is poor and the luminous efficiency is low.
本说明书中各个实施例采用递进的方式描述,每个实施例重点说明的都是与其他实施例的不同之处,各个实施例之间相同相似部分互相参见即可。对于实施例公开的装置而言,由于其与实施例公开的方法相对应,所以描述的比较简单,相关之处参见方法部分说明即可。Each embodiment in this specification is described in a progressive manner, each embodiment focuses on the difference from other embodiments, and the same and similar parts of each embodiment can be referred to each other. As for the device disclosed in the embodiment, since it corresponds to the method disclosed in the embodiment, the description is relatively simple, and for the related information, please refer to the description of the method part.
对所公开的实施例的上述说明,使本领域专业技术人员能够实现或使用本发明。对这些实施例的多种修改对本领域的专业技术人员来说将是显而易见的,本文中所定义的一般原理可以在不脱离本发明的精神或范围的情况下,在其它实施例中实现。因此,本发明将不会被限制于本文所示的这些实施例,而是要符合与本文所公开的原理和新颖特点相一致的最宽的范围。The above description of the disclosed embodiments is provided to enable any person skilled in the art to make or use the invention. Various modifications to these embodiments will be readily apparent to those skilled in the art, and the general principles defined herein may be implemented in other embodiments without departing from the spirit or scope of the invention. Therefore, the present invention will not be limited to the embodiments shown herein, but is to be accorded the widest scope consistent with the principles and novel features disclosed herein.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 201010140230CN101781557B (en) | 2010-04-07 | 2010-04-07 | Preparation method of CdSe/CdS core-shell structure quantum dots |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 201010140230CN101781557B (en) | 2010-04-07 | 2010-04-07 | Preparation method of CdSe/CdS core-shell structure quantum dots |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101781557Atrue CN101781557A (en) | 2010-07-21 |
| CN101781557B CN101781557B (en) | 2013-04-10 |
Family
ID=42521712
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 201010140230ActiveCN101781557B (en) | 2010-04-07 | 2010-04-07 | Preparation method of CdSe/CdS core-shell structure quantum dots |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101781557B (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102634336A (en)* | 2012-04-13 | 2012-08-15 | 南京工业大学 | Light-emitting adjustable ligand-free cadmium sulfide semiconductor quantum dot and preparation method thereof |
| CN105487264A (en)* | 2015-12-29 | 2016-04-13 | 东南大学 | Electro-optical modulating device preparing method based on quantum restriction Stark effect |
| CN105586028A (en)* | 2016-03-10 | 2016-05-18 | 福州大学 | Preparing method for CdSe@CdS core-shell structure quantum dots |
| CN107983272A (en)* | 2016-10-26 | 2018-05-04 | 中国科学院化学研究所 | Sulfide encapsulated particles and preparation method and application |
| CN112011327A (en)* | 2019-05-28 | 2020-12-01 | 苏州星烁纳米科技有限公司 | Preparation method of core-shell structure quantum dot and product prepared by same |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1645559A (en)* | 2004-12-03 | 2005-07-27 | 中国科学院长春应用化学研究所 | Method for synthesizing cadmium selenide and quantum point with cadmium selenide cadmium sulfide nucleocapsid structure |
| US20060157686A1 (en)* | 2005-01-20 | 2006-07-20 | Samsung Electronics Co., Ltd. | Quantum dot phosphor for light emitting diode and method of preparing the same |
- 2010
- 2010-04-07CNCN 201010140230patent/CN101781557B/enactiveActive
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1645559A (en)* | 2004-12-03 | 2005-07-27 | 中国科学院长春应用化学研究所 | Method for synthesizing cadmium selenide and quantum point with cadmium selenide cadmium sulfide nucleocapsid structure |
| US20060157686A1 (en)* | 2005-01-20 | 2006-07-20 | Samsung Electronics Co., Ltd. | Quantum dot phosphor for light emitting diode and method of preparing the same |
Non-Patent Citations (1)
| Title |
|---|
| 《Advanced Materials》 20050131 Daocheng Pan, et al. Synthesis of extremely small CdSe and highly luminescent CdSe/CdS core-shell nanocrystals via a novel two-phase thermal approach 第176-179页 1-10 第17卷, 第2期* |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102634336A (en)* | 2012-04-13 | 2012-08-15 | 南京工业大学 | Light-emitting adjustable ligand-free cadmium sulfide semiconductor quantum dot and preparation method thereof |
| CN105487264A (en)* | 2015-12-29 | 2016-04-13 | 东南大学 | Electro-optical modulating device preparing method based on quantum restriction Stark effect |
| CN105487264B (en)* | 2015-12-29 | 2018-11-09 | 东南大学 | A kind of electro-optical modulation device preparation method based on quantum confined stark effect |
| CN105586028A (en)* | 2016-03-10 | 2016-05-18 | 福州大学 | Preparing method for CdSe@CdS core-shell structure quantum dots |
| CN107983272A (en)* | 2016-10-26 | 2018-05-04 | 中国科学院化学研究所 | Sulfide encapsulated particles and preparation method and application |
| CN112011327A (en)* | 2019-05-28 | 2020-12-01 | 苏州星烁纳米科技有限公司 | Preparation method of core-shell structure quantum dot and product prepared by same |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101781557B (en) | 2013-04-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101781557A (en) | Preparation method of CdSe/CdS core-shell structure quantum dots | |
| Karanikolos et al. | Synthesis and size control of luminescent ZnSe nanocrystals by a Microemulsion− gas contacting technique | |
| CN101913600B (en) | Method for preparing graphene/semiconductor quantum dot composite material | |
| CN101851498B (en) | Method for converting water solubility for reducing biotoxicity of luminous quantum dot synthesized by organic phase | |
| CN1403379A (en) | Prepn of CdSe/CdS or CdSe/ZnS core-shell quantum dot | |
| CN106590644A (en) | Preparation method of cesium-lead-bromine quantum dot | |
| CN106883845A (en) | A kind of perovskite crystallite luminescent material, preparation method and applications | |
| CN111849478B (en) | A kind of preparation method of magnetic fluorescent bifunctional nanomaterial | |
| CN105384189B (en) | A kind of preparation method of cesium lead halide nanorod and the product obtained therefrom | |
| CN101319139A (en) | Preparation method of CdSeS and CdSeS/ZnS core-shell quantum dots | |
| CN109970887A (en) | A kind of Polymeric ligands, quantum dot and preparation method thereof | |
| CN106519098A (en) | A preparing method of core-shell quantum dot/polystyrene fluorescent microspheres | |
| CN102942922A (en) | Surface modification method for hydrophobic Mn doped ZnS quantum dots | |
| CN110615462A (en) | Method for green synthesis of oil-soluble zinc sulfide quantum dots based on liquid paraffin solvent system | |
| CN104876256A (en) | Preparation method of water-soluble zinc sulfate quantum dot | |
| CN104371733B (en) | The method of the CdSe/ZnS nuclear shell structure quantum point that a kind of microfluid synthesis luminescent properties is controlled | |
| CN107138736A (en) | Preparation method and application of aggregation-state phosphorescent copper nanocluster | |
| CN101942299B (en) | A method of synthesizing doped ZnS nano-luminescent material by oil-water interface method | |
| CN111498895B (en) | Cu 2-x Method for regulating and controlling S nanosheet crystal structure | |
| JP2006143526A (en) | Low temperature synthesis of nanoparticles | |
| CN110395760B (en) | A method for preparing near-infrared silver sulfide quantum dots by hypergravity reactor | |
| CN104829763B (en) | There is the preparation method of the organic/inorganic hybridization material POSS-PDMAEMA of temperature and pH-sensitivity | |
| CN104910893A (en) | Preparation method for preparing hydrophilic quantum dots based on novel amphiphilic polymer ultrasonic emulsion process | |
| CN102433124B (en) | Nano-crystal fluorescent powder and preparation method thereof | |
| CN111994948B (en) | A current-free synthesis method of CsPbBr3 nanocrystals |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| ASS | Succession or assignment of patent right | Owner name:CHANGZHOU INSTITUTE OF ENERGY STORAGE MATERIALS + Free format text:FORMER OWNER: CHANGCHUN INST. OF APPLIED CHEMISTRY, CHINESE ACADEMY OF SCIENCES Effective date:20140929 | |
| C41 | Transfer of patent application or patent right or utility model | ||
| COR | Change of bibliographic data | Free format text:CORRECT: ADDRESS; FROM: 130000 CHANGCHUN, JILIN PROVINCE TO: 213017 CHANGZHOU, JIANGSU PROVINCE | |
| TR01 | Transfer of patent right | Effective date of registration:20140929 Address after:Changzhou City, Jiangsu province Hehai road 213017 No. 9 Patentee after:Changzhou Institute of Energy Storage Materials & Devices Address before:130000 Jilin City, Changchun province people's street, No. 5625 Patentee before:Changchun Institue of Applied Chemistry, Chinese Academy of Sciences |