CN101724841A - Method for preparing polymer/silver composite membrane by depositing dopamine - Google Patents
Method for preparing polymer/silver composite membrane by depositing dopamineDownload PDFInfo
- Publication number
- CN101724841A CN101724841ACN200810223730ACN200810223730ACN101724841ACN 101724841 ACN101724841 ACN 101724841ACN 200810223730 ACN200810223730 ACN 200810223730ACN 200810223730 ACN200810223730 ACN 200810223730ACN 101724841 ACN101724841 ACN 101724841A
- Authority
- CN
- China
- Prior art keywords
- silver
- polymer
- film
- dopamine
- solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 229910052709silverInorganic materials0.000titleclaimsabstractdescription84
- 239000004332silverSubstances0.000titleclaimsabstractdescription84
- VYFYYTLLBUKUHU-UHFFFAOYSA-NdopamineChemical compoundNCCC1=CC=C(O)C(O)=C1VYFYYTLLBUKUHU-UHFFFAOYSA-N0.000titleclaimsabstractdescription76
- 229960003638dopamineDrugs0.000titleclaimsabstractdescription38
- 239000002131composite materialSubstances0.000titleclaimsabstractdescription32
- 229920000642polymerPolymers0.000titleclaimsabstractdescription29
- 238000000034methodMethods0.000titleclaimsabstractdescription26
- 238000000151depositionMethods0.000titleabstractdescription17
- 239000012528membraneSubstances0.000titledescription9
- BQCADISMDOOEFD-UHFFFAOYSA-NSilverChemical compound[Ag]BQCADISMDOOEFD-UHFFFAOYSA-N0.000claimsabstractdescription83
- 229920001690polydopaminePolymers0.000claimsabstractdescription19
- 238000007747platingMethods0.000claimsabstractdescription18
- 230000008021depositionEffects0.000claimsabstractdescription17
- 229920006254polymer filmPolymers0.000claimsabstractdescription15
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000claimsabstractdescription14
- 239000008103glucoseSubstances0.000claimsabstractdescription14
- SQGYOTSLMSWVJD-UHFFFAOYSA-Nsilver(1+) nitrateChemical compound[Ag+].[O-]N(=O)=OSQGYOTSLMSWVJD-UHFFFAOYSA-N0.000claimsdescription12
- -1polyethylenePolymers0.000claimsdescription11
- 239000004697PolyetherimideSubstances0.000claimsdescription6
- 229920001601polyetherimidePolymers0.000claimsdescription6
- 239000004810polytetrafluoroethyleneSubstances0.000claimsdescription6
- 229920001343polytetrafluoroethylenePolymers0.000claimsdescription6
- 229910001961silver nitrateInorganic materials0.000claimsdescription6
- VHUUQVKOLVNVRT-UHFFFAOYSA-NAmmonium hydroxideChemical compound[NH4+].[OH-]VHUUQVKOLVNVRT-UHFFFAOYSA-N0.000claimsdescription5
- 235000011114ammonium hydroxideNutrition0.000claimsdescription5
- 239000002244precipitateSubstances0.000claimsdescription5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000claimsdescription4
- 239000004696Poly ether ether ketoneSubstances0.000claimsdescription4
- 239000004698PolyethyleneSubstances0.000claimsdescription4
- 239000004743PolypropyleneSubstances0.000claimsdescription4
- 239000004205dimethyl polysiloxaneSubstances0.000claimsdescription4
- 235000013870dimethyl polysiloxaneNutrition0.000claimsdescription4
- 229920000435poly(dimethylsiloxane)Polymers0.000claimsdescription4
- 239000004417polycarbonateSubstances0.000claimsdescription4
- 229920002530polyetherether ketonePolymers0.000claimsdescription4
- 239000004793PolystyreneSubstances0.000claimsdescription3
- 238000006243chemical reactionMethods0.000claimsdescription3
- 229920000515polycarbonatePolymers0.000claimsdescription3
- 229920000573polyethylenePolymers0.000claimsdescription3
- 229920001155polypropylenePolymers0.000claimsdescription3
- 239000004814polyurethaneSubstances0.000claimsdescription3
- CXQXSVUQTKDNFP-UHFFFAOYSA-NoctamethyltrisiloxaneChemical compoundC[Si](C)(C)O[Si](C)(C)O[Si](C)(C)CCXQXSVUQTKDNFP-UHFFFAOYSA-N0.000claimsdescription2
- 238000004987plasma desorption mass spectroscopyMethods0.000claimsdescription2
- 229920000139polyethylene terephthalatePolymers0.000claims2
- 239000005020polyethylene terephthalateSubstances0.000claims2
- 229920002873PolyethyleniminePolymers0.000claims1
- JUPQTSLXMOCDHR-UHFFFAOYSA-Nbenzene-1,4-diol;bis(4-fluorophenyl)methanoneChemical compoundOC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1JUPQTSLXMOCDHR-UHFFFAOYSA-N0.000claims1
- 239000012994photoredox catalystSubstances0.000claims1
- 229920002223polystyrenePolymers0.000claims1
- 229920002635polyurethanePolymers0.000claims1
- 238000002310reflectometryMethods0.000abstractdescription12
- 239000003638chemical reducing agentSubstances0.000abstractdescription9
- 230000004048modificationEffects0.000abstractdescription8
- 238000012986modificationMethods0.000abstractdescription8
- 239000000126substanceSubstances0.000abstractdescription7
- 238000001465metallisationMethods0.000abstractdescription6
- 238000005516engineering processMethods0.000abstractdescription4
- 239000000463materialSubstances0.000abstractdescription2
- 238000005272metallurgyMethods0.000abstract1
- 238000004377microelectronicMethods0.000abstract1
- 229920001721polyimidePolymers0.000description58
- 239000000243solutionSubstances0.000description36
- 239000004642PolyimideSubstances0.000description21
- 238000003756stirringMethods0.000description20
- VEXZGXHMUGYJMC-UHFFFAOYSA-NHydrochloric acidChemical compoundClVEXZGXHMUGYJMC-UHFFFAOYSA-N0.000description12
- 239000011159matrix materialSubstances0.000description11
- 238000006722reduction reactionMethods0.000description8
- 239000007983Tris bufferSubstances0.000description6
- 239000002253acidSubstances0.000description6
- 239000007864aqueous solutionSubstances0.000description6
- 239000012153distilled waterSubstances0.000description6
- LENZDBCJOHFCAS-UHFFFAOYSA-NtrisChemical compoundOCC(N)(CO)COLENZDBCJOHFCAS-UHFFFAOYSA-N0.000description6
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterChemical compoundOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description6
- 229910052751metalInorganic materials0.000description5
- 239000002184metalSubstances0.000description5
- 239000000758substrateSubstances0.000description5
- 230000005611electricityEffects0.000description4
- 239000000203mixtureSubstances0.000description4
- 230000009467reductionEffects0.000description4
- 238000000026X-ray photoelectron spectrumMethods0.000description3
- 229910052757nitrogenInorganic materials0.000description3
- IJGRMHOSHXDMSA-UHFFFAOYSA-NnitrogenSubstancesN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000description3
- 230000008569processEffects0.000description3
- 230000008859changeEffects0.000description2
- 230000000694effectsEffects0.000description2
- 238000011065in-situ storageMethods0.000description2
- 229910052760oxygenInorganic materials0.000description2
- 239000002245particleSubstances0.000description2
- 238000009832plasma treatmentMethods0.000description2
- 102000004169proteins and genesHuman genes0.000description2
- 108090000623proteins and genesProteins0.000description2
- 235000015170shellfishNutrition0.000description2
- RQPKNXVVIBYOBX-KDBLBPRBSA-N(2s)-2-amino-3-(3,4-dihydroxyphenyl)propanoic acid;(2s)-2-(dihydroxyamino)-3-phenylpropanoic acidChemical compoundOC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1.ON(O)[C@H](C(O)=O)CC1=CC=CC=C1RQPKNXVVIBYOBX-KDBLBPRBSA-N0.000description1
- LLLVZDVNHNWSDS-UHFFFAOYSA-N4-methylidene-3,5-dioxabicyclo[5.2.2]undeca-1(9),7,10-triene-2,6-dioneChemical compoundC1(C2=CC=C(C(=O)OC(=C)O1)C=C2)=OLLLVZDVNHNWSDS-UHFFFAOYSA-N0.000description1
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description1
- 239000012190activatorSubstances0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 230000009286beneficial effectEffects0.000description1
- 229910052799carbonInorganic materials0.000description1
- 238000004140cleaningMethods0.000description1
- 230000007812deficiencyEffects0.000description1
- 229940008099dimethiconeDrugs0.000description1
- 238000004870electrical engineeringMethods0.000description1
- 150000002466iminesChemical class0.000description1
- 239000003999initiatorSubstances0.000description1
- 229910010272inorganic materialInorganic materials0.000description1
- 239000011147inorganic materialSubstances0.000description1
- RYZCLUQMCYZBJQ-UHFFFAOYSA-Hlead(2+);dicarbonate;dihydroxideChemical compound[OH-].[OH-].[Pb+2].[Pb+2].[Pb+2].[O-]C([O-])=O.[O-]C([O-])=ORYZCLUQMCYZBJQ-UHFFFAOYSA-H0.000description1
- 239000002932lusterSubstances0.000description1
- 239000002923metal particleSubstances0.000description1
- 238000002715modification methodMethods0.000description1
- 239000000178monomerSubstances0.000description1
- QJGQUHMNIGDVPM-UHFFFAOYSA-Nnitrogen groupChemical group[N]QJGQUHMNIGDVPM-UHFFFAOYSA-N0.000description1
- 239000011368organic materialSubstances0.000description1
- 239000001301oxygenSubstances0.000description1
- 238000004375physisorptionMethods0.000description1
- 229920006289polycarbonate filmPolymers0.000description1
- 229920000307polymer substratePolymers0.000description1
- 238000002360preparation methodMethods0.000description1
- 230000001737promoting effectEffects0.000description1
- 238000011946reduction processMethods0.000description1
Images
Landscapes
- Laminated Bodies (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Electroplating Methods And Accessories (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于聚合物表面改性领域,具体涉及一种在聚合物膜表面还原银的方法,尤其涉及在还原剂存在的条件下通过多巴胺沉积制备具有高导电和高反射性能的聚合物/银复合膜的方法。The invention belongs to the field of polymer surface modification, in particular to a method for reducing silver on the surface of a polymer film, in particular to the preparation of a polymer/silver composite with high conductivity and high reflectivity by dopamine deposition in the presence of a reducing agent membrane method.
背景技术Background technique
聚合物表面的性能与材料在各种工业中的应用密切相关,如何更快速有效地对聚合物表面进行改性多年以来一直是该领域的热点。轻质、可折叠的高导电高反射聚酰亚胺/银复合膜既有聚酰亚胺的高强度、高模量和优异的耐高低温性能,又有银的高导电性和高反射率的特点,使其在电工、电子、信息、军事、航空和航天等方面具有广阔的应用前景。然而,纯聚酰亚胺膜表面存在化学惰性、非亲水性、非极性,且导电性能和反光性能差等问题。由于其表面憎水性,未处理的聚酰亚胺表面与金属层表面的粘结稳定性和结合力较差,需要进行进一步的处理,使其聚合表面能够形成均匀致密的银层。The properties of polymer surfaces are closely related to the application of materials in various industries. How to modify polymer surfaces more quickly and effectively has been a hot spot in this field for many years. Lightweight, foldable, highly conductive and highly reflective polyimide/silver composite film has both the high strength, high modulus and excellent high and low temperature resistance of polyimide, and the high conductivity and high reflectivity of silver The characteristics make it have broad application prospects in electrical engineering, electronics, information, military, aviation and aerospace, etc. However, the surface of pure polyimide film has problems such as chemical inertness, non-hydrophilicity, non-polarity, and poor conductivity and reflective properties. Due to its surface hydrophobicity, the untreated polyimide surface has poor bonding stability and bonding force with the surface of the metal layer, and further treatment is required to allow the polymerized surface to form a uniform and dense silver layer.
在过去的几十年中,科学家们对聚合物表面金属化的各种方法进行了探索和研究,其中在聚合物表面还原银的方法包括等离子体处理、化学还原、紫外接枝、辐照、物理吸附、原位一步自金属化等等。其中,等离子体处理方法对聚合物基体的组成有相关要求,并且处理效果具有时效性;化学还原法中需要有一定的活化剂和还原剂;在紫外接枝法中,反应中需要有相应的光引发剂,光源以及相应可以被引发的基体,接枝能否有效进行与引发剂和基体性能均密切相关;而且上述三种方法制备的复合膜金属与聚合物基体的粘结力较小,对实际应用具有较大影响。而原位一步自金属化方法需要很高的反应温度,而且残留在聚合物基体中的金属粒子对基体的性能也有一定影响。In the past few decades, scientists have explored and studied various methods of metallization on the surface of polymers, among which methods for reducing silver on the surface of polymers include plasma treatment, chemical reduction, ultraviolet grafting, irradiation, Physisorption, in situ one-step self-metallization, etc. Among them, the plasma treatment method has relevant requirements on the composition of the polymer matrix, and the treatment effect is time-sensitive; certain activators and reductants are required in the chemical reduction method; in the ultraviolet grafting method, corresponding The photoinitiator, the light source and the corresponding substrate that can be initiated, whether the grafting can be effectively carried out are closely related to the properties of the initiator and the substrate; and the composite film metal and the polymer substrate prepared by the above three methods have a small bonding force, have great influence on practical applications. However, the in-situ one-step self-metallization method requires a high reaction temperature, and the metal particles remaining in the polymer matrix also have a certain influence on the properties of the matrix.
综上所述,上述改性方法对聚合物基体的性能均有一定的影响,为了保持聚合物基体的物理机械性能,在对其表面进行改性的过程中需要维持聚合物基体的性能不受大的损害。In summary, the above modification methods have a certain impact on the performance of the polymer matrix. In order to maintain the physical and mechanical properties of the polymer matrix, it is necessary to maintain the performance of the polymer matrix in the process of modifying its surface. big damage.
目前,已有研究表明多巴胺可以作为一种有效物质对表面进行功能化改性。多巴胺可以在各种有机、无机材料表面进行黏附,且粘结性能优异,过程简单易行。此种方法源于贝壳类动物分泌的蛋白质物质可以有效地粘结在物体的湿表面,已有科学家对此现象的原理进行研究。研究结果表明,贝壳类动物所分泌出的蛋白质物质中的二羟基苯丙氨酸(DOPA)在其中起到主要作用,并证明多巴胺可以在各种有机或无机基体表面粘结良好,粘结性能与基体无关,且可在基体表面作为有效的平台进行金属还原或其他单体的进一步接枝。在专利WO2008/049108 A1中通过将沉积有多巴胺的聚酰亚胺(PI)膜浸泡于硝酸银溶液中室温反应18小时,得到表面覆盖有金属银的聚酰亚胺膜。但此方法还原时间长,银还原效果不明显,沉积在聚酰亚胺膜表面的银层薄,银颗粒不连续,不具有导电性能。At present, studies have shown that dopamine can be used as an effective substance to functionalize the surface. Dopamine can adhere to the surface of various organic and inorganic materials, and has excellent bonding performance, and the process is simple and easy. This method is derived from the fact that the protein substances secreted by shellfish can effectively bond to the wet surface of objects, and scientists have studied the principle of this phenomenon. The research results show that dihydroxyphenylalanine (DOPA) in the protein substances secreted by shellfish plays a major role in it, and prove that dopamine can bond well on the surface of various organic or inorganic substrates, and the bonding performance It is independent of the substrate and can be used as an effective platform for metal reduction or further grafting of other monomers on the substrate surface. In the patent WO2008/049108 A1, the polyimide (PI) film deposited with dopamine was soaked in a silver nitrate solution and reacted at room temperature for 18 hours to obtain a polyimide film whose surface was covered with metallic silver. However, the reduction time of this method is long, the silver reduction effect is not obvious, the silver layer deposited on the surface of the polyimide film is thin, the silver particles are discontinuous, and have no electrical conductivity.
发明内容Contents of the invention
本发明的目的在于解决现有聚合物表面金属化改性技术中存在的不足,而提供一种通过多巴胺沉积制备聚合物/银复合膜的方法。本发明所提供的方法操作简便、耗时短,所制备的聚合物/银复合膜具有高的导电性能和反射率。The purpose of the present invention is to solve the deficiencies in the existing polymer surface metallization modification technology, and provide a method for preparing a polymer/silver composite film by dopamine deposition. The method provided by the invention is easy to operate and short in time consumption, and the prepared polymer/silver composite film has high conductivity and reflectivity.
本发明通过在碱性条件下将多巴胺沉积在聚合物膜表面之后,将沉积有聚多巴胺的聚合物膜置于银镀液中,加入还原剂葡萄糖溶液,制备具有导电性能的聚合物/银复合膜,具体步骤如下:In the present invention, after dopamine is deposited on the surface of the polymer film under alkaline conditions, the polymer film deposited with polydopamine is placed in a silver plating solution, and a reducing agent glucose solution is added to prepare a polymer/silver composite with conductive properties. film, the specific steps are as follows:
1)将聚合物膜在乙醇中超声波洗净后,将其置于浓度为0.1~0.3mM/mL,pH为6.0~10.0的多巴胺溶液中,以30~100转/min的搅拌速率搅拌0.5~36h,得到表面沉积有聚多巴胺的聚合物膜;1) After ultrasonically cleaning the polymer film in ethanol, place it in a dopamine solution with a concentration of 0.1-0.3 mM/mL and a pH of 6.0-10.0, and stir at a stirring rate of 30-100 rpm for 0.5- 36h, obtain the polymer film that the surface is deposited with polydopamine;
2)将质量浓度为1~6%的硝酸银溶液用氨水滴定至沉淀正好完全消失,得到银镀液;2) Titrating the silver nitrate solution with a mass concentration of 1 to 6% with ammonia water until the precipitate just disappears completely to obtain a silver plating solution;
3)将步骤1)中制备的表面沉积有聚多巴胺的聚合物膜置于步骤2)中制备的银镀液中,加入质量浓度为1~6%的葡萄糖溶液,葡萄糖溶液用量与银镀液的体积比为0.1∶1~1∶1,室温下反应1~10分钟,得到表面具有银白色金属光泽的聚合物/银复合膜。3) Place the polymer film with polydopamine deposited on the surface prepared in step 1) in the silver plating solution prepared in step 2), add a glucose solution with a mass concentration of 1 to 6%, the amount of glucose solution is the same as that of the silver plating solution The volume ratio is 0.1:1 to 1:1, and the reaction is carried out at room temperature for 1 to 10 minutes to obtain a polymer/silver composite film with a silver-white metallic luster on the surface.
由于多巴胺在聚合物基体表面的沉积为物理过程,与聚合物基体的表面形貌和化学组成无关,可适用于各种形态和组成的聚合物基体,如聚醚酰亚胺(PEI)、聚乙烯(PE)、聚丙烯(PP)、聚四氟乙烯(PTFE)、聚苯乙烯(PS)、聚碳酸酯(PC)、对苯二甲酸乙二醇酯(PET)、聚二甲基硅氧烷(PDMS)、聚醚醚酮(PEEK)或聚氨酯(PU)膜等。Since the deposition of dopamine on the surface of the polymer matrix is a physical process and has nothing to do with the surface morphology and chemical composition of the polymer matrix, it can be applied to polymer matrices of various shapes and compositions, such as polyetherimide (PEI), poly Ethylene (PE), Polypropylene (PP), Polytetrafluoroethylene (PTFE), Polystyrene (PS), Polycarbonate (PC), Ethylene Terephthalate (PET), Dimethicone Polyoxane (PDMS), polyetheretherketone (PEEK) or polyurethane (PU) film, etc.
本发明的原理在于:多巴胺中有含氮基团,可以起到还原银的作用,而外加还原剂葡萄糖促进银的还原过程,同时多巴胺加速并且稳固银颗粒在聚酰亚胺膜表面的生长,得到具有高导电性和高反射率的聚酰亚胺/银复合膜。The principle of the present invention is that: there are nitrogen-containing groups in dopamine, which can reduce silver, and the addition of reducing agent glucose promotes the reduction process of silver, and at the same time, dopamine accelerates and stabilizes the growth of silver particles on the surface of the polyimide film. A polyimide/silver composite film with high conductivity and high reflectivity is obtained.
与现有的聚合物表面金属化技术相比较,本发明方法具有以下有益效果:Compared with the existing polymer surface metallization technology, the inventive method has the following beneficial effects:
1)本发明操作简便,耗时短,成本低。1) The present invention has the advantages of simple and convenient operation, short time consumption and low cost.
2)本发明所制备的复合膜表面的银层均匀致密,具有良好的导电性能(电阻为1.5~2.0Ω)以及反射率(大于90%)。2) The silver layer on the surface of the composite film prepared by the present invention is uniform and dense, and has good electrical conductivity (resistance is 1.5-2.0Ω) and reflectivity (greater than 90%).
3)本发明所制备的聚合物/银复合膜,银层与聚合物基体之间具有高的粘结强度。3) The polymer/silver composite film prepared by the present invention has high bonding strength between the silver layer and the polymer matrix.
4)本发明对聚合物膜基体的形貌和组成没有限制,银的沉积不会影响聚合物膜基体的物理机械性能和热性能。4) The present invention has no limitation on the morphology and composition of the polymer film matrix, and the deposition of silver will not affect the physical, mechanical and thermal properties of the polymer film matrix.
附图说明Description of drawings
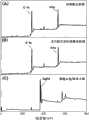
图1、实施例1中的纯聚酰亚胺膜(A),多巴胺沉积的聚酰亚胺膜(B)和聚酰亚胺/银复合膜(C)的X射线光电子能谱(XPS)宽谱图。Pure polyimide film (A) in Fig. 1, embodiment 1, the X-ray photoelectron spectrum (XPS) of the polyimide film (B) of dopamine deposition and polyimide/silver composite film (C) Broad Spectrum.
图2、A、B和C分别为实施例1中的纯聚酰亚胺膜,多巴胺沉积的聚酰亚胺膜和聚酰亚胺/银复合膜的扫描电子显微镜(SEM)图像,标尺为500nm。Fig. 2, A, B and C are the pure polyimide film in embodiment 1 respectively, the scanning electron microscope (SEM) image of the polyimide film of dopamine deposition and polyimide/silver composite film, scale is 500nm.
图3、未经多巴胺沉积改性的聚酰亚胺膜表面,通过化学还原方法沉积的银层(A)和实施例1中经多巴胺沉积改性的聚酰亚胺膜表面沉积的银层(B)的扫描电子显微镜(SEM)图像,样品放大倍数为5万倍。Fig. 3, the polyimide film surface without dopamine deposition modification, the silver layer (A) deposited by chemical reduction method and the silver layer (A) deposited on the polyimide film surface through dopamine deposition modification in embodiment 1 B) Scanning electron microscope (SEM) image of the sample at 50,000X magnification.
图1(XPS宽谱)中Ag 3d的信号峰出现,证明银在聚酰亚胺膜表面的成功沉积。从图2中可以看出纯聚酰亚胺膜,经多巴胺沉积的聚酰亚胺膜和聚酰亚胺/银复合膜的表观形态的变化,同时可以看出本发明制得的聚酰亚胺/银复合膜表面银层致密而且连续,具备良好的导电性能和反射率。从图3看出同样条件下未经多巴胺沉积改性的聚酰亚胺膜表面,通过化学还原方法沉积的银层不够连续致密,而多巴胺沉积后聚酰亚胺膜表面沉积的银层的连续致密,证明了多巴胺在还原银的过程中起到了明显的促进作用。The signal peak of Ag 3d appeared in Figure 1 (XPS broad spectrum), which proved the successful deposition of silver on the surface of polyimide film. As can be seen from Fig. 2, the pure polyimide film, the polyimide film deposited by dopamine and the change of the apparent morphology of the polyimide/silver composite film, can see that the polyimide film that the present invention makes simultaneously The silver layer on the surface of the imine/silver composite film is dense and continuous, and has good electrical conductivity and reflectivity. It can be seen from Fig. 3 that the surface of the polyimide film without dopamine deposition modification under the same conditions, the silver layer deposited by the chemical reduction method is not continuous and dense, and the silver layer deposited on the surface of the polyimide film after dopamine deposition is continuous. dense, proving that dopamine played a significant role in promoting the reduction of silver.
以下结合附图和具体实施方式对本发明作进一步说明。The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments.
具体实施方式Detailed ways
实施例1Example 1
1)配置质量分数为0.15mM/mL的多巴胺水溶液,并用Tris酸和盐酸溶液调节PH至8.5后,将1cm*1cm的聚酰亚胺膜浸泡其中,以60转/min的搅拌速率搅拌24小时,搅拌结束后将沉积有聚多巴胺的聚酰亚胺膜取出,用蒸馏水洗净,真空干燥;1) Prepare a dopamine aqueous solution with a mass fraction of 0.15mM/mL, adjust the pH to 8.5 with Tris acid and hydrochloric acid solution, soak a 1cm*1cm polyimide membrane in it, and stir at a stirring rate of 60 rpm for 24 hours After the stirring is finished, the polyimide film deposited with polydopamine is taken out, washed with distilled water, and dried in vacuum;
2)配置质量分数为3wt%的硝酸银溶液,用氨水滴定至沉淀正好完全消失,得到银镀液;2) Configure a silver nitrate solution with a mass fraction of 3wt%, and titrate with ammonia water until the precipitate just disappears completely to obtain a silver plating solution;
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在10mL银镀液中,加入6wt%的葡萄糖溶液10mL,反应10分钟后,可得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 10mL of silver plating solution, add 10mL of 6wt% glucose solution, and react for 10 minutes to obtain a polyimide/silver film with uniform metallic silver coverage on both sides Composite film.
经测定膜可导电,电阻在1.5~2.0Ω之间,反射率为95~98%。It is determined that the film can conduct electricity, the resistance is between 1.5-2.0Ω, and the reflectivity is 95-98%.
纯聚酰亚胺膜,纯多巴胺以及多巴胺沉积聚酰亚胺膜元素含量见表1。The element contents of pure polyimide film, pure dopamine and dopamine-deposited polyimide film are shown in Table 1.
实施例2Example 2
1)配置质量分数为0.1mM/mL的多巴胺水溶液,并用Tris酸和盐酸溶液调节PH至6后,将1cm*1cm的聚酰亚胺膜浸泡其中,以30转/min的搅拌速率搅拌0.5小时,搅拌结束后将沉积有聚多巴胺的聚酰亚胺膜取出,用蒸馏水洗净,真空干燥;1) Prepare a dopamine aqueous solution with a mass fraction of 0.1mM/mL, and adjust the pH to 6 with Tris acid and hydrochloric acid solution, soak a 1cm*1cm polyimide membrane in it, and stir at a stirring rate of 30 rpm for 0.5 hours After the stirring is finished, the polyimide film deposited with polydopamine is taken out, washed with distilled water, and dried in vacuum;
2)配置质量分数为1wt%的硝酸银溶液,用氨水滴定至沉淀正好完全消失,得到银镀液;2) configure a silver nitrate solution with a mass fraction of 1 wt%, and titrate with ammonia water until the precipitate just disappears completely to obtain a silver plating solution;
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在100mL银镀液中,加入1wt%的葡萄糖溶液10mL,反应1分钟后,得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 100mL silver plating solution, add 10mL of 1wt% glucose solution, and react for 1 minute to obtain a polyimide/silver composite film with uniform metallic silver coverage on both sides. membrane.
实施例3Example 3
1)配置质量分数为0.3mM/mL的多巴胺水溶液,并用Tris酸和盐酸溶液调节PH至10后,将1cm*1cm的聚酰亚胺膜浸泡其中,以100转/min的搅拌速率搅拌36小时,搅拌结束后将沉积有聚多巴胺的聚酰亚胺膜取出,用蒸馏水洗净,真空干燥;1) Prepare a dopamine aqueous solution with a mass fraction of 0.3mM/mL, adjust the pH to 10 with Tris acid and hydrochloric acid solution, soak a 1cm*1cm polyimide membrane in it, and stir at a stirring rate of 100 rpm for 36 hours After the stirring is finished, the polyimide film deposited with polydopamine is taken out, washed with distilled water, and dried in vacuum;
2)配置质量分数为6wt%的硝酸银溶液,用氨水滴定至沉淀正好完全消失,得到银镀液;2) configure a silver nitrate solution with a mass fraction of 6wt%, and titrate it with ammonia water until the precipitate just disappears completely to obtain a silver plating solution;
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在10mL银镀液中,加入4wt%的葡萄糖溶液10mL,反应10分钟后,可得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 10mL of silver plating solution, add 10mL of 4wt% glucose solution, and react for 10 minutes to obtain a polyimide/silver film with uniform metallic silver coverage on both sides. Composite film.
实施例4Example 4
1)同实施例1步骤1);1) with embodiment 1 step 1);
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在50mL银镀液中,加入6wt%的葡萄糖溶液10mL,反应8分钟后,可得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 50mL silver plating solution, add 10mL of 6wt% glucose solution, and react for 8 minutes to obtain a polyimide/silver film with uniform metallic silver coverage on both sides Composite film.
经测定膜可导电,电阻在1.5~2.0Ω之间,反射率为93~97%。It is determined that the film can conduct electricity, the resistance is between 1.5-2.0Ω, and the reflectivity is 93-97%.
实施例5Example 5
1)同实施例1步骤1);1) with embodiment 1 step 1);
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在100mL银镀液中,加入6wt%的葡萄糖溶液10mL,反应5分钟后,可得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 100mL silver plating solution, add 10mL of 6wt% glucose solution, and react for 5 minutes to obtain a polyimide/silver film with uniform metallic silver coverage on both sides Composite film.
经测定膜可导电,电阻在2.0~3.0Ω之间,反射率为85~90%。It is determined that the film is conductive, the resistance is between 2.0-3.0Ω, and the reflectivity is 85-90%.
实施例6Example 6
1)同实施例1步骤1);1) with embodiment 1 step 1);
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在20mL银镀液中,加入6wt%的葡萄糖溶液10mL,反应3分钟后,可得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 20mL of silver plating solution, add 10mL of 6wt% glucose solution, and react for 3 minutes to obtain a polyimide/silver film with uniform metallic silver coverage on both sides Composite film.
经测定膜可导电,电阻在4.0~5.0Ω之间,反射率为80~88%。It is determined that the film can conduct electricity, the resistance is between 4.0-5.0Ω, and the reflectivity is 80-88%.
实施例7Example 7
1)同实施例1步骤1);1) with embodiment 1 step 1);
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)将沉积有聚多巴胺的聚酰亚胺膜浸泡在10mL银镀液中,加入6wt%的葡萄糖溶液10mL,反应1分钟后,可得到两面均有均匀金属银覆盖的聚酰亚胺/银复合膜。3) Soak the polyimide film deposited with polydopamine in 10 mL of silver plating solution, add 10 mL of 6 wt % glucose solution, and react for 1 minute to obtain a polyimide/silver film with uniform metallic silver coverage on both sides Composite film.
经测定膜可导电,电阻在10.0~15.0Ω之间,反射率为75~85%。It is determined that the film can conduct electricity, the resistance is between 10.0-15.0Ω, and the reflectivity is 75-85%.
实施例8Example 8
1)配置质量分数为0.15mM/mL的多巴胺水溶液,并用Tris酸和盐酸溶液调节PH至8.5后,将1cm*1cm的聚醚酰亚胺膜浸泡其中,以60转/min的搅拌速率搅拌24小时,搅拌结束后将沉积有聚多巴胺的聚酰亚胺膜取出,用蒸馏水洗净,真空干燥;1) Prepare a dopamine aqueous solution with a mass fraction of 0.15mM/mL, adjust the pH to 8.5 with Tris acid and hydrochloric acid solution, soak a 1cm*1cm polyetherimide membrane in it, and stir at a stirring rate of 60 rpm for 24 After stirring, the polyimide film deposited with polydopamine was taken out, washed with distilled water, and dried in vacuum;
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)同实施例1步骤3),得到两面均有均匀金属银覆盖的聚醚酰亚胺/银复合膜。3) Same as step 3) of Example 1 to obtain a polyetherimide/silver composite film with uniform metal silver covering on both sides.
实施例9Example 9
1)配置质量分数为0.15mM/mL的多巴胺水溶液,并用Tris酸和盐酸溶液调节PH至8.5后,将1cm*1cm的聚四氟乙烯膜浸泡其中,以60转/min的搅拌速率搅拌24小时,搅拌结束后将沉积有聚多巴胺的聚酰亚胺膜取出,用蒸馏水洗净,真空干燥;1) Prepare a dopamine aqueous solution with a mass fraction of 0.15mM/mL, adjust the pH to 8.5 with Tris acid and hydrochloric acid solution, soak a 1cm*1cm polytetrafluoroethylene membrane in it, and stir at a stirring rate of 60 rpm for 24 hours After the stirring is finished, the polyimide film deposited with polydopamine is taken out, washed with distilled water, and dried in vacuum;
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)同实施例1步骤3),得到两面均有均匀金属银覆盖的聚四氟乙烯/银复合膜。3) Same as step 3) of Example 1 to obtain a polytetrafluoroethylene/silver composite film covered with uniform metallic silver on both sides.
实施例10Example 10
1)配置质量分数为0.15mM/mL的多巴胺水溶液,并用Tris酸和盐酸溶液调节PH至8.5后,将1cm*1cm的聚碳酸酯膜浸泡其中,以60转/min的搅拌速率搅拌24小时,搅拌结束后将沉积有聚多巴胺的聚酰亚胺膜取出,用蒸馏水洗净,真空干燥;1) Prepare a dopamine aqueous solution with a mass fraction of 0.15mM/mL, adjust the pH to 8.5 with Tris acid and hydrochloric acid solution, soak a 1cm*1cm polycarbonate film in it, and stir at a stirring rate of 60 rpm for 24 hours, After stirring, the polyimide film deposited with polydopamine was taken out, washed with distilled water, and dried in vacuum;
2)同实施例1步骤2);2) with embodiment 1 step 2);
3)同实施例1步骤3),得到两面均有均匀金属银覆盖的聚碳酸酯/银复合膜。3) With embodiment 1 step 3), obtain the polycarbonate/silver composite film that both sides all have uniform metal silver to cover.
通过碳氮比的含量变化(表1)说明多巴胺在聚酰亚胺膜表面的存在。表2说明了通过化学方法还原银中,未经多巴胺沉积改性的聚酰亚胺膜表面沉积的银层以及经多巴胺沉积改性的聚酰亚胺膜表面沉积银层制备的聚酰亚胺/银复合膜的导电性能以及反射性能。The presence of dopamine on the surface of the polyimide film was illustrated by the content change of the carbon-to-nitrogen ratio (Table 1). Table 2 illustrates the polyimide prepared by chemically reducing silver, the silver layer deposited on the surface of the polyimide film without dopamine deposition modification and the silver layer deposited on the surface of the polyimide film modified by dopamine deposition Conductive and reflective properties of silver/silver composite films.
表1、实施例1中纯聚酰亚胺膜,纯多巴胺以及多巴胺沉积聚酰亚胺膜元素含量比(注:C At.%、N At.%、O At.%分别表示膜表面碳(C)、氮(N)氧(O)元素的含量)Pure polyimide film in table 1, embodiment 1, pure dopamine and dopamine deposition polyimide film element content ratio (note: C At.%, N At.%, O At.% represent membrane surface carbon respectively ( C), nitrogen (N) oxygen (O) element content)
表2通过化学方法还原银中未经多巴胺改性的聚酰亚胺膜表面沉积的银层以及经多巴胺沉积后制备的聚酰亚胺/银复合膜的导电和反射性能Table 2 The conductive and reflective properties of the silver layer deposited on the surface of the polyimide film without dopamine modification in silver reduced by chemical methods and the polyimide/silver composite film prepared after dopamine deposition
Claims (2)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200810223730ACN101724841A (en) | 2008-10-10 | 2008-10-10 | Method for preparing polymer/silver composite membrane by depositing dopamine |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200810223730ACN101724841A (en) | 2008-10-10 | 2008-10-10 | Method for preparing polymer/silver composite membrane by depositing dopamine |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN101724841Atrue CN101724841A (en) | 2010-06-09 |
Family
ID=42446376
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN200810223730APendingCN101724841A (en) | 2008-10-10 | 2008-10-10 | Method for preparing polymer/silver composite membrane by depositing dopamine |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101724841A (en) |
Cited By (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101913278A (en)* | 2010-06-30 | 2010-12-15 | 中国科学技术大学 | Preparation method of polydopamine-g-polyethylene oxide capillary coating for protein separation by capillary electrophoresis |
| CN102277728A (en)* | 2011-06-27 | 2011-12-14 | 中国科学院宁波材料技术与工程研究所 | Method for preparing conductive ultrahigh molecular weight polyethylene fiber |
| CN102691083A (en)* | 2012-05-29 | 2012-09-26 | 上海大学 | Electrochemical method for improving blood compatibility of surface of metal material |
| CN102698950A (en)* | 2012-05-30 | 2012-10-03 | 上海大学 | Method for coating dopamine on surface of ultrasonic reinforced material |
| CN103232612A (en)* | 2013-04-27 | 2013-08-07 | 上海锦湖日丽塑料有限公司 | Method for coarsening-free electroplating of polycarbonate (PC) alloy resin |
| CN103418250A (en)* | 2013-07-05 | 2013-12-04 | 烟台绿水赋膜材料有限公司 | Method of in-situ generation of nano particle on separating membrane surface |
| CN103834231A (en)* | 2014-01-17 | 2014-06-04 | 哈尔滨工业大学 | A silver-catalyzed colloidal ink for inkjet printing copper patterns on the surface of flexible substrates and its preparation method |
| CN104480455A (en)* | 2014-10-26 | 2015-04-01 | 北京化工大学 | Method for preparing polymer conductive microspheres for anisotropic conductive adhesive film from dopamine |
| CN104888631A (en)* | 2015-06-18 | 2015-09-09 | 天津大学 | Polydopamine/silver modified polymer separating membrane and preparation method |
| CN105016294A (en)* | 2015-06-04 | 2015-11-04 | 天津大学 | Method for preparing high-level micro-structured poly-dopamine film |
| CN105064039A (en)* | 2015-08-07 | 2015-11-18 | 南京理工大学 | Antibacterial PET/PDA-Ag electrospun composite nanofiber, and preparation method thereof |
| CN105148877A (en)* | 2015-09-21 | 2015-12-16 | 武汉大学 | Method for fixing layered double hydroxide on surface of polypropylene and application of method |
| CN105431181A (en)* | 2013-06-20 | 2016-03-23 | 新加坡国立大学 | Surface Modification |
| CN106832885A (en)* | 2017-02-16 | 2017-06-13 | 四川大学 | Polymer composites and its application containing poly-dopamine particle |
| CN107503122A (en)* | 2017-08-23 | 2017-12-22 | 江西诚达工程咨询监理有限公司 | A kind of preparation method of electric wire fire proofing |
| CN107583112A (en)* | 2017-07-24 | 2018-01-16 | 南昌大学 | A kind of preparation method of medical polyurethane antimicrobial nano silver coating |
| CN108385375A (en)* | 2018-03-20 | 2018-08-10 | 华南理工大学 | A kind of preparation method of super-hydrophobic antibiotic fabric |
| CN108440780A (en)* | 2018-02-10 | 2018-08-24 | 朱东洋 | A kind of preparation method of the special PET film of polyester copper plating film |
| CN108584903A (en)* | 2018-03-29 | 2018-09-28 | 聊城大学 | A kind of preparation method of carbonization poly-dopamine/Ag nano compound films |
| CN109402613A (en)* | 2018-10-09 | 2019-03-01 | 全球能源互联网研究院有限公司 | A kind of method in matrix surface coated with silver and silver-plated four acicular type zinc oxide crystal whisker prepared therefrom |
| CN109722900A (en)* | 2019-01-28 | 2019-05-07 | 扬州大学 | Superhydrophobic conductive composite fabric with electromagnetic shielding performance and preparation method thereof |
| CN109881484A (en)* | 2019-02-02 | 2019-06-14 | 东华大学 | A kind of preparation method of electrostatically loaded multi-layer coated yarn or fabric material |
| CN110194847A (en)* | 2019-05-24 | 2019-09-03 | 陕西师范大学 | A kind of preparation method of pet sheet face polymer pattern |
| CN110253995A (en)* | 2019-06-20 | 2019-09-20 | 云南电网有限责任公司电力科学研究院 | A kind of insulating material structure and preparation method thereof |
| CN111501030A (en)* | 2020-05-26 | 2020-08-07 | 电子科技大学 | Surface modification system before chemical plating and method for double modifying polymer substrate surface |
| CN111875841A (en)* | 2020-07-17 | 2020-11-03 | 扬州大学 | Preparation method of conductive latex sponge |
| CN112853409A (en)* | 2020-12-29 | 2021-05-28 | 哈尔滨工业大学(深圳) | Silver-containing plating solution and preparation method of foam metal material |
| CN113501990A (en)* | 2021-06-29 | 2021-10-15 | 浙江中科玖源新材料有限公司 | Composite conductive film and preparation method thereof |
| CN115487686A (en)* | 2022-09-01 | 2022-12-20 | 成都博睿兴材科技有限公司 | Multifunctional electrospun fiber composite membrane and preparation method and application thereof |
| CN119465642A (en)* | 2024-11-15 | 2025-02-18 | 武汉纺织大学 | Conductive filament for use in smart wearable devices and preparation method thereof |
- 2008
- 2008-10-10CNCN200810223730Apatent/CN101724841A/enactivePending
Cited By (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101913278A (en)* | 2010-06-30 | 2010-12-15 | 中国科学技术大学 | Preparation method of polydopamine-g-polyethylene oxide capillary coating for protein separation by capillary electrophoresis |
| CN101913278B (en)* | 2010-06-30 | 2013-07-24 | 中国科学技术大学 | Preparation method of polydopamine-g-polyethylene oxide capillary coating for separating protein by capillary electrophoresis |
| CN102277728B (en)* | 2011-06-27 | 2012-12-26 | 中国科学院宁波材料技术与工程研究所 | Method for preparing conductive ultrahigh molecular weight polyethylene fiber |
| CN102277728A (en)* | 2011-06-27 | 2011-12-14 | 中国科学院宁波材料技术与工程研究所 | Method for preparing conductive ultrahigh molecular weight polyethylene fiber |
| CN102691083B (en)* | 2012-05-29 | 2014-12-03 | 上海大学 | Electrochemical method for improving blood compatibility of surface of metal material |
| CN102691083A (en)* | 2012-05-29 | 2012-09-26 | 上海大学 | Electrochemical method for improving blood compatibility of surface of metal material |
| CN102698950B (en)* | 2012-05-30 | 2014-05-14 | 上海大学 | Method for coating dopamine on surface of ultrasonic reinforced material |
| CN102698950A (en)* | 2012-05-30 | 2012-10-03 | 上海大学 | Method for coating dopamine on surface of ultrasonic reinforced material |
| CN103232612A (en)* | 2013-04-27 | 2013-08-07 | 上海锦湖日丽塑料有限公司 | Method for coarsening-free electroplating of polycarbonate (PC) alloy resin |
| CN105431181A (en)* | 2013-06-20 | 2016-03-23 | 新加坡国立大学 | Surface Modification |
| CN103418250A (en)* | 2013-07-05 | 2013-12-04 | 烟台绿水赋膜材料有限公司 | Method of in-situ generation of nano particle on separating membrane surface |
| CN103834231A (en)* | 2014-01-17 | 2014-06-04 | 哈尔滨工业大学 | A silver-catalyzed colloidal ink for inkjet printing copper patterns on the surface of flexible substrates and its preparation method |
| CN104480455B (en)* | 2014-10-26 | 2017-04-05 | 北京化工大学 | A kind of method that anisotropic conductive film conducting polymer microsphere is prepared by dopamine |
| CN104480455A (en)* | 2014-10-26 | 2015-04-01 | 北京化工大学 | Method for preparing polymer conductive microspheres for anisotropic conductive adhesive film from dopamine |
| CN105016294A (en)* | 2015-06-04 | 2015-11-04 | 天津大学 | Method for preparing high-level micro-structured poly-dopamine film |
| CN104888631A (en)* | 2015-06-18 | 2015-09-09 | 天津大学 | Polydopamine/silver modified polymer separating membrane and preparation method |
| CN105064039A (en)* | 2015-08-07 | 2015-11-18 | 南京理工大学 | Antibacterial PET/PDA-Ag electrospun composite nanofiber, and preparation method thereof |
| CN105148877A (en)* | 2015-09-21 | 2015-12-16 | 武汉大学 | Method for fixing layered double hydroxide on surface of polypropylene and application of method |
| CN106832885A (en)* | 2017-02-16 | 2017-06-13 | 四川大学 | Polymer composites and its application containing poly-dopamine particle |
| CN106832885B (en)* | 2017-02-16 | 2020-03-31 | 四川大学 | Polymer composite material containing polydopamine particles and application thereof |
| CN107583112A (en)* | 2017-07-24 | 2018-01-16 | 南昌大学 | A kind of preparation method of medical polyurethane antimicrobial nano silver coating |
| CN107503122A (en)* | 2017-08-23 | 2017-12-22 | 江西诚达工程咨询监理有限公司 | A kind of preparation method of electric wire fire proofing |
| CN107503122B (en)* | 2017-08-23 | 2020-07-28 | 江西诚达工程咨询监理有限公司 | A kind of preparation method of wire and cable flame retardant material |
| CN108440780A (en)* | 2018-02-10 | 2018-08-24 | 朱东洋 | A kind of preparation method of the special PET film of polyester copper plating film |
| CN108385375A (en)* | 2018-03-20 | 2018-08-10 | 华南理工大学 | A kind of preparation method of super-hydrophobic antibiotic fabric |
| CN108584903A (en)* | 2018-03-29 | 2018-09-28 | 聊城大学 | A kind of preparation method of carbonization poly-dopamine/Ag nano compound films |
| CN109402613A (en)* | 2018-10-09 | 2019-03-01 | 全球能源互联网研究院有限公司 | A kind of method in matrix surface coated with silver and silver-plated four acicular type zinc oxide crystal whisker prepared therefrom |
| CN109722900A (en)* | 2019-01-28 | 2019-05-07 | 扬州大学 | Superhydrophobic conductive composite fabric with electromagnetic shielding performance and preparation method thereof |
| CN109722900B (en)* | 2019-01-28 | 2021-09-17 | 扬州大学 | Super-hydrophobic conductive composite fabric with electromagnetic shielding performance and preparation method thereof |
| CN109881484A (en)* | 2019-02-02 | 2019-06-14 | 东华大学 | A kind of preparation method of electrostatically loaded multi-layer coated yarn or fabric material |
| CN109881484B (en)* | 2019-02-02 | 2021-07-30 | 东华大学 | A kind of preparation method of electrostatically loaded multi-layer coated yarn or fabric material |
| CN110194847A (en)* | 2019-05-24 | 2019-09-03 | 陕西师范大学 | A kind of preparation method of pet sheet face polymer pattern |
| CN110253995B (en)* | 2019-06-20 | 2021-07-27 | 云南电网有限责任公司电力科学研究院 | A kind of insulating material structure and preparation method thereof |
| CN110253995A (en)* | 2019-06-20 | 2019-09-20 | 云南电网有限责任公司电力科学研究院 | A kind of insulating material structure and preparation method thereof |
| CN111501030B (en)* | 2020-05-26 | 2021-03-02 | 电子科技大学 | Surface modification system before chemical plating and method for double modifying polymer substrate surface |
| CN111501030A (en)* | 2020-05-26 | 2020-08-07 | 电子科技大学 | Surface modification system before chemical plating and method for double modifying polymer substrate surface |
| CN111875841A (en)* | 2020-07-17 | 2020-11-03 | 扬州大学 | Preparation method of conductive latex sponge |
| CN111875841B (en)* | 2020-07-17 | 2022-09-23 | 扬州大学 | Preparation method of conductive latex sponge |
| CN112853409A (en)* | 2020-12-29 | 2021-05-28 | 哈尔滨工业大学(深圳) | Silver-containing plating solution and preparation method of foam metal material |
| CN112853409B (en)* | 2020-12-29 | 2022-07-22 | 哈尔滨工业大学(深圳) | A kind of preparation method of silver-containing plating solution and foam metal material |
| CN113501990A (en)* | 2021-06-29 | 2021-10-15 | 浙江中科玖源新材料有限公司 | Composite conductive film and preparation method thereof |
| CN115487686A (en)* | 2022-09-01 | 2022-12-20 | 成都博睿兴材科技有限公司 | Multifunctional electrospun fiber composite membrane and preparation method and application thereof |
| CN115487686B (en)* | 2022-09-01 | 2023-08-29 | 成都博睿兴材科技有限公司 | Multifunctional electrospun fiber composite membrane and preparation method and application thereof |
| CN119465642A (en)* | 2024-11-15 | 2025-02-18 | 武汉纺织大学 | Conductive filament for use in smart wearable devices and preparation method thereof |
| CN119465642B (en)* | 2024-11-15 | 2025-09-19 | 武汉纺织大学 | Conductive filament for smart wearable devices and preparation method thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101724841A (en) | Method for preparing polymer/silver composite membrane by depositing dopamine | |
| Wang et al. | Surface silverized meta-aramid fibers prepared by bio-inspired poly (dopamine) functionalization | |
| Liu et al. | Well-organized organosilane composites for adhesion enhancement of heterojunctions | |
| Chang et al. | Fabrication of copper patterns on flexible substrate by patterning–adsorption–plating process | |
| CN105821396A (en) | Palladium-free chemical copper plating method | |
| CN1481448A (en) | Method for plating metal film on polymer surface | |
| Chen et al. | Formation of reflective and conductive silver film on ABS surface via covalent grafting and solution spray | |
| JP2003213437A (en) | Thin layer metallic film | |
| CN101403183A (en) | Carbon fiber surface modification method | |
| Wang et al. | A facile process to manufacture high performance copper layer on ceramic material via biomimetic modification and electroless plating | |
| Chen et al. | Enhanced interfacial toughness of thermoplastic–epoxy interfaces using ALD surface treatments | |
| Gu et al. | Harnessing superhydrophobic coatings for enhancing the surface corrosion resistance of magnesium alloys | |
| Garcia et al. | Microscopic study of a ligand induced electroless plating process onto polymers | |
| Zhai et al. | Study on the pretreatment of poly (ether ether ketone)/multiwalled carbon nanotubes composites through environmentally friendly chemical etching and electrical properties of the chemically metallized composites | |
| Zhang et al. | Nondestructive grafting of ZnO on the surface of aramid fibers followed by silane grafting to improve its interfacial adhesion property with rubber | |
| Wang et al. | Electroless plating of PVC plastic through new surface modification method applying a semi-IPN hydrogel film | |
| Wu et al. | Organosiloxane monolayers terminated with amine groups as adhesives for Si metallization | |
| Augustine et al. | Dopamine-supported metallization of polyolefins─ a contribution to transfer to an eco-friendly and efficient technological process | |
| Lin et al. | Surface modification of metal substrates using dielectric barrier discharge plasma and the wettability study | |
| Feng et al. | Metallization of polyphenylene sulfide by low-cost mussel-inspired catechol/polyamine surface modification | |
| Qi et al. | Double-surface-silvered polyimide films prepared via a direct ion-exchange self-metallization process | |
| Dang et al. | Ecofriendly preparation of cellulose nanocrystal-coated polyimide fiber: strategy for improved wettability | |
| CN112574578A (en) | Protein/polysaccharide composite nano film and application thereof in preventing conductive coating from generating cracks | |
| CN105887054B (en) | A kind of highly conductive biomass/nano metal flexible compound film and preparation method thereof | |
| Rashid et al. | Nacre inspired tailoring of mechanically strong hydrophobic coatings through Layer-by-Layer assembly |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | Open date:20100609 |