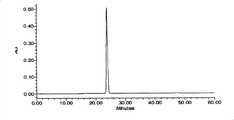
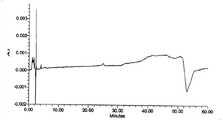
CN101696959B - A kind of method for the content determination of atosiban acetate and its preparation and the determination of related substances - Google Patents
A kind of method for the content determination of atosiban acetate and its preparation and the determination of related substancesDownload PDFInfo
- Publication number
- CN101696959B CN101696959BCN200910008881ACN200910008881ACN101696959BCN 101696959 BCN101696959 BCN 101696959BCN 200910008881 ACN200910008881 ACN 200910008881ACN 200910008881 ACN200910008881 ACN 200910008881ACN 101696959 BCN101696959 BCN 101696959B
- Authority
- CN
- China
- Prior art keywords
- solution
- mobile phase
- atosiban
- sample
- peak
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034methodMethods0.000titleclaimsabstractdescription25
- 239000000126substanceSubstances0.000titleclaimsabstractdescription19
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription12
- SVDWBHHCPXTODI-QIWYXCRTSA-Nacetic acid (2S)-N-[(2S)-5-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxopentan-2-yl]-1-[(4R,7S,10S,13S,16R)-7-(2-amino-2-oxoethyl)-13-[(2S)-butan-2-yl]-16-[(4-ethoxyphenyl)methyl]-10-[(1R)-1-hydroxyethyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamideChemical compoundCC(O)=O.C1=CC(OCC)=CC=C1C[C@@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O)CSSCCC(=O)N1SVDWBHHCPXTODI-QIWYXCRTSA-N0.000titleclaimsdescription8
- 229950010486atosiban acetateDrugs0.000titleclaimsdescription8
- WEVYAHXRMPXWCK-UHFFFAOYSA-NAcetonitrileChemical compoundCC#NWEVYAHXRMPXWCK-UHFFFAOYSA-N0.000claimsabstractdescription36
- 229960002403atosibanDrugs0.000claimsabstractdescription22
- VWXRQYYUEIYXCZ-OBIMUBPZSA-NatosibanChemical compoundC1=CC(OCC)=CC=C1C[C@@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O)CSSCCC(=O)N1VWXRQYYUEIYXCZ-OBIMUBPZSA-N0.000claimsabstractdescription22
- 108700007535atosibanProteins0.000claimsabstractdescription22
- 238000001514detection methodMethods0.000claimsabstractdescription17
- 238000010828elutionMethods0.000claimsabstractdescription10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical compoundO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000claimsabstractdescription9
- 239000000945fillerSubstances0.000claimsabstractdescription5
- YTJSFYQNRXLOIC-UHFFFAOYSA-NoctadecylsilaneChemical compoundCCCCCCCCCCCCCCCCCC[SiH3]YTJSFYQNRXLOIC-UHFFFAOYSA-N0.000claimsabstractdescription5
- 239000008055phosphate buffer solutionSubstances0.000claimsabstract3
- 239000000243solutionSubstances0.000claimsdescription37
- 238000012360testing methodMethods0.000claimsdescription15
- 239000007788liquidSubstances0.000claimsdescription12
- 239000013558reference substanceSubstances0.000claimsdescription10
- ZMANZCXQSJIPKH-UHFFFAOYSA-NTriethylamineChemical compoundCCN(CC)CCZMANZCXQSJIPKH-UHFFFAOYSA-N0.000claimsdescription9
- 239000008363phosphate bufferSubstances0.000claimsdescription7
- 229910000402monopotassium phosphateInorganic materials0.000claimsdescription5
- 235000019796monopotassium phosphateNutrition0.000claimsdescription5
- PJNZPQUBCPKICU-UHFFFAOYSA-Nphosphoric acid;potassiumChemical compound[K].OP(O)(O)=OPJNZPQUBCPKICU-UHFFFAOYSA-N0.000claimsdescription5
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsdescription5
- 239000002994raw materialSubstances0.000claimsdescription4
- 230000035945sensitivityEffects0.000claimsdescription4
- 238000010812external standard methodMethods0.000claimsdescription3
- 238000004128high performance liquid chromatographyMethods0.000claimsdescription3
- 230000014759maintenance of locationEffects0.000claimsdescription3
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical compoundOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000claims4
- 239000012085test solutionSubstances0.000claims3
- 229910000147aluminium phosphateInorganic materials0.000claims2
- 239000000741silica gelSubstances0.000claims2
- 229910002027silica gelInorganic materials0.000claims2
- 238000011003system suitability testMethods0.000claims2
- 239000012088reference solutionSubstances0.000claims1
- QTBSBXVTEAMEQO-UHFFFAOYSA-NAcetic acidChemical compoundCC(O)=OQTBSBXVTEAMEQO-UHFFFAOYSA-N0.000abstractdescription45
- 239000012535impuritySubstances0.000abstractdescription8
- 108090000765processed proteins & peptidesProteins0.000abstractdescription7
- 102000004196processed proteins & peptidesHuman genes0.000abstractdescription5
- 238000004587chromatography analysisMethods0.000abstractdescription4
- 229920001184polypeptidePolymers0.000abstractdescription4
- 239000000377silicon dioxideSubstances0.000abstractdescription3
- 238000000926separation methodMethods0.000abstractdescription2
- 238000004458analytical methodMethods0.000abstract3
- 239000007791liquid phaseSubstances0.000abstract2
- 239000012071phaseSubstances0.000abstract2
- 238000004519manufacturing processMethods0.000abstract1
- 238000003908quality control methodMethods0.000abstract1
- 238000003556assayMethods0.000description8
- DTQVDTLACAAQTR-UHFFFAOYSA-NTrifluoroacetic acidChemical compoundOC(=O)C(F)(F)FDTQVDTLACAAQTR-UHFFFAOYSA-N0.000description4
- 238000010790dilutionMethods0.000description4
- 239000012895dilutionSubstances0.000description4
- QTBSBXVTEAMEQO-UHFFFAOYSA-MAcetateChemical compoundCC([O-])=OQTBSBXVTEAMEQO-UHFFFAOYSA-M0.000description3
- JVTAAEKCZFNVCJ-UHFFFAOYSA-NLactic AcidNatural productsCC(O)C(O)=OJVTAAEKCZFNVCJ-UHFFFAOYSA-N0.000description3
- OAICVXFJPJFONN-UHFFFAOYSA-NPhosphorusChemical compound[P]OAICVXFJPJFONN-UHFFFAOYSA-N0.000description3
- HEMHJVSKTPXQMS-UHFFFAOYSA-MSodium hydroxideChemical compound[OH-].[Na+]HEMHJVSKTPXQMS-UHFFFAOYSA-M0.000description3
- 239000002253acidSubstances0.000description3
- 239000004310lactic acidSubstances0.000description3
- 235000014655lactic acidNutrition0.000description3
- 239000011574phosphorusSubstances0.000description3
- 229910052698phosphorusInorganic materials0.000description3
- 238000011084recoveryMethods0.000description3
- 238000010521absorption reactionMethods0.000description2
- 239000003153chemical reaction reagentSubstances0.000description2
- 239000003814drugSubstances0.000description2
- 238000002474experimental methodMethods0.000description2
- 239000003182parenteral nutrition solutionSubstances0.000description2
- 229910021642ultra pure waterInorganic materials0.000description2
- 239000012498ultrapure waterSubstances0.000description2
- 238000005303weighingMethods0.000description2
- 208000006399Premature Obstetric LaborDiseases0.000description1
- 206010036600Premature labourDiseases0.000description1
- 150000001408amidesChemical class0.000description1
- 150000001413amino acidsChemical group0.000description1
- 239000012491analyteSubstances0.000description1
- -1and intermediumSubstances0.000description1
- 239000005557antagonistSubstances0.000description1
- 238000013459approachMethods0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 230000002860competitive effectEffects0.000description1
- 230000006378damageEffects0.000description1
- 210000003785deciduaAnatomy0.000description1
- 230000008034disappearanceEffects0.000description1
- 229940079593drugDrugs0.000description1
- 238000005516engineering processMethods0.000description1
- 210000002219extraembryonic membraneAnatomy0.000description1
- 238000002347injectionMethods0.000description1
- 239000007924injectionSubstances0.000description1
- 239000002075main ingredientSubstances0.000description1
- 238000005259measurementMethods0.000description1
- 239000000203mixtureSubstances0.000description1
- 208000026440premature laborDiseases0.000description1
- 239000002904solventSubstances0.000description1
- WROMPOXWARCANT-UHFFFAOYSA-Ntfa trifluoroacetic acidChemical compoundOC(=O)C(F)(F)F.OC(=O)C(F)(F)FWROMPOXWARCANT-UHFFFAOYSA-N0.000description1
- 239000003643water by typeSubstances0.000description1
Images
Landscapes
- Investigating Or Analyzing Non-Biological Materials By The Use Of Chemical Means (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
Abstract
Description
Claims (2)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200910008881ACN101696959B (en) | 2009-02-11 | 2009-02-11 | A kind of method for the content determination of atosiban acetate and its preparation and the determination of related substances |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200910008881ACN101696959B (en) | 2009-02-11 | 2009-02-11 | A kind of method for the content determination of atosiban acetate and its preparation and the determination of related substances |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101696959A CN101696959A (en) | 2010-04-21 |
| CN101696959Btrue CN101696959B (en) | 2012-10-17 |
Family
ID=42142074
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN200910008881AExpired - Fee RelatedCN101696959B (en) | 2009-02-11 | 2009-02-11 | A kind of method for the content determination of atosiban acetate and its preparation and the determination of related substances |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101696959B (en) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103421092B (en)* | 2013-09-05 | 2015-05-13 | 杭州阿德莱诺泰制药技术有限公司 | Atosiban purification method |
| CN105503828A (en)* | 2015-12-24 | 2016-04-20 | 北京康立生医药技术开发有限公司 | Preparation method of fumarate of pyrrole derivatives |
| CN106018584A (en)* | 2016-05-12 | 2016-10-12 | 华润双鹤利民药业(济南)有限公司 | Detection method for dezocine related substances |
| CN106932347B (en)* | 2017-05-17 | 2019-04-12 | 浙江遂昌利民科技有限公司 | A kind of mezlocillin and its quality index detection method |
| CN107312072A (en)* | 2017-06-20 | 2017-11-03 | 浙江湃肽生物有限公司 | A kind of method of purifies and separates Atosiban |
| CN109142580A (en)* | 2018-09-12 | 2019-01-04 | 南京康舟医药科技有限公司 | Measure method of the atosiban acetate in relation to substance |
| CN110218242A (en)* | 2019-06-26 | 2019-09-10 | 海南中和药业股份有限公司 | A kind of preparation method of atosiban acetate impurity |
| CN110658297B (en)* | 2019-10-30 | 2021-12-10 | 海南通用三洋药业有限公司 | Method for detecting high-molecular polymer in terlipressin for injection |
| CN110658296A (en)* | 2019-10-30 | 2020-01-07 | 海南通用三洋药业有限公司 | Method for detecting high-molecular polymer in atosiban acetate injection |
| CN118961954A (en)* | 2024-10-18 | 2024-11-15 | 南京恒远科技开发有限公司 | HPLC Content Determination Method of NH-bis(PEG4-t-butyl ester) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101314613A (en)* | 2008-05-08 | 2008-12-03 | 吉尔生化(上海)有限公司 | Solid phase synthesis method for atosiban |
| CN101357937A (en)* | 2007-07-31 | 2009-02-04 | 崔颀 | Method for synthesizing atosiban acetate from solid phase polypeptide |
- 2009
- 2009-02-11CNCN200910008881Apatent/CN101696959B/ennot_activeExpired - Fee Related
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101357937A (en)* | 2007-07-31 | 2009-02-04 | 崔颀 | Method for synthesizing atosiban acetate from solid phase polypeptide |
| CN101314613A (en)* | 2008-05-08 | 2008-12-03 | 吉尔生化(上海)有限公司 | Solid phase synthesis method for atosiban |
Non-Patent Citations (2)
| Title |
|---|
| Alan R. Oyler,et al.Hydrophilic interaction chromatography on amino-silica phases complements reversed-phase high-performance liquid chromatography and capillary electrophoresis for peptide analysis.《Journal of Chromatography A》.1996,第724卷第378-383页.* |
| 李萍等.纳升电喷雾串联质谱在阿托西班一级结构确证中的应用.《分析测试学报》.2008,第27卷第16-18页.* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101696959A (en) | 2010-04-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101696959B (en) | A kind of method for the content determination of atosiban acetate and its preparation and the determination of related substances | |
| Kang et al. | Modern methods for analysis of antiepileptic drugs in the biological fluids for pharmacokinetics, bioequivalence and therapeutic drug monitoring | |
| CN104914185B (en) | A kind of Favipiravir has the HPLC assay method of related substance | |
| CN108828127A (en) | Liquid-phase chromatography method in relation to substance in a kind of detection Parecoxib Sodium and synthetic intermediate | |
| CN104237421A (en) | Related substance detection method for trelagliptin succinate and preparation thereof | |
| CN110146621B (en) | Method for determining content of polymer in cephalosporin antibiotic medicine | |
| CN101502616B (en) | Method for measuring content of Bletilla striata medicinal materials | |
| CN103698424B (en) | Detecting method of detecting organic solvent in slightly-soluble aluminum salt drug | |
| CN107515255A (en) | Utilize high performance liquid chromatograph measure Dapagliflozin and its method about material | |
| CN105842365B (en) | Utilize the method for efficient liquid phase chromatographic analysis Tadalafei | |
| CN103926335B (en) | The high-efficient liquid phase chromatogram process measuring method of related substance in a kind of Dapoxetine hydrochloride | |
| CN1790013B (en) | A method for simultaneous determination of protocatechuic acid and 5-hydroxymethylfurfural in Shengmai injection | |
| CN101661019B (en) | Method for separating and measuring Palonosetron hydrochloride and optical isomers thereof | |
| CN103163228A (en) | Efficient liquid phase analysis method for hydroxyfasudil and preparation thereof | |
| Agrawal | Validated stability indicating method for determination of indapamide in pharmaceutical formulation | |
| CN102109501B (en) | Method for detecting related substances in quinapril hydrochloride and hydrochlorothiazide composition | |
| CN102579961B (en) | Detection method for aristolochic acid A in swelling-reducing and pain-alleviating tincture | |
| CN104965031B (en) | Content measuring method for compound ketoprofen and omeprazole sustained-release capsules | |
| CN114518413B (en) | A method for determining the content of proline in captopril bulk drug | |
| CN104007185A (en) | HPLC determination method for detecting impurities in zanamivir and zanamivir-containing preparation | |
| CN1786706B (en) | Cyclovirobuxine D raw medicine and method for determining its content in preparation by chromatography | |
| CN102109499A (en) | Method for simultaneously detecting acetone and ethyl acetate residues in drug by gas chromatography | |
| CN106153756A (en) | A kind of detect the high performance liquid chromatography of rapamycin in everolimus | |
| Elgawish et al. | Quantitative determination of captopril, perindopril erbumine, moexipril hydrochloride, and ramipril in bulk and pharmaceutical preparations by high performance liquid chromatography | |
| CN104165957B (en) | The assay method of dimer impurity content in a kind of faropenem sodium raw materials |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CP03 | Change of name, title or address | Address after:570216 Haikou Free Trade Zone, Nanhai Road, Hainan, Haikou, China, 168 Co-patentee after:Hainan Zhonghe Polypeptide Research Co.,Ltd. Patentee after:HAINAN ZHONGHE PHARMACEUTICAL Co.,Ltd. Address before:570216 Hainan Zhonghe Pharmaceutical Co., Ltd., Haikou, Nanhai Road, No. 168, Hainan, China Co-patentee before:Hainan Zhonghe Polypeptide Research Co.,Ltd. Patentee before:HAINAN ZHONGHE PHARMACEUTICAL Co.,Ltd. | |
| CP03 | Change of name, title or address | ||
| TR01 | Transfer of patent right | Effective date of registration:20170425 Address after:570216 Haikou Free Trade Zone, Nanhai Road, Hainan, Haikou, China, 168 Patentee after:HAINAN ZHONGHE PHARMACEUTICAL Co.,Ltd. Address before:570216 Haikou Free Trade Zone, Nanhai Road, Hainan, Haikou, China, 168 Co-patentee before:Hainan Zhonghe Polypeptide Research Co.,Ltd. Patentee before:HAINAN ZHONGHE PHARMACEUTICAL Co.,Ltd. | |
| TR01 | Transfer of patent right | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20121017 | |
| CF01 | Termination of patent right due to non-payment of annual fee |