CN101580244B - Method for preparing mesoscopic material with controllable appearance - Google Patents
Method for preparing mesoscopic material with controllable appearanceDownload PDFInfo
- Publication number
- CN101580244B CN101580244BCN200810106722XACN200810106722ACN101580244BCN 101580244 BCN101580244 BCN 101580244BCN 200810106722X ACN200810106722X ACN 200810106722XACN 200810106722 ACN200810106722 ACN 200810106722ACN 101580244 BCN101580244 BCN 101580244B
- Authority
- CN
- China
- Prior art keywords
- flue
- weight
- parts
- preparation
- och
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034methodMethods0.000titleclaimsabstractdescription37
- 239000000463materialSubstances0.000titleclaimsabstractdescription32
- 239000004094surface-active agentSubstances0.000claimsabstractdescription66
- 238000002360preparation methodMethods0.000claimsabstractdescription47
- 238000005187foamingMethods0.000claimsabstractdescription38
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsabstractdescription33
- 239000002105nanoparticleSubstances0.000claimsabstractdescription32
- 239000002243precursorSubstances0.000claimsabstractdescription6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical compoundO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000claimsdescription94
- VEXZGXHMUGYJMC-UHFFFAOYSA-NHydrochloric acidChemical groupClVEXZGXHMUGYJMC-UHFFFAOYSA-N0.000claimsdescription64
- DBMJMQXJHONAFJ-UHFFFAOYSA-MSodium laurylsulphateChemical compound[Na+].CCCCCCCCCCCCOS([O-])(=O)=ODBMJMQXJHONAFJ-UHFFFAOYSA-M0.000claimsdescription59
- 239000007789gasSubstances0.000claimsdescription43
- 239000000843powderSubstances0.000claimsdescription42
- IJGRMHOSHXDMSA-UHFFFAOYSA-NnitrogenSubstancesN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000claimsdescription41
- 235000019333sodium laurylsulphateNutrition0.000claimsdescription31
- 239000011148porous materialSubstances0.000claimsdescription23
- 229910052757nitrogenInorganic materials0.000claimsdescription21
- 238000006243chemical reactionMethods0.000claimsdescription19
- 239000000178monomerSubstances0.000claimsdescription13
- 239000000203mixtureSubstances0.000claimsdescription10
- GWEVSGVZZGPLCZ-UHFFFAOYSA-NTitan oxideChemical compoundO=[Ti]=OGWEVSGVZZGPLCZ-UHFFFAOYSA-N0.000claimsdescription9
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000claimsdescription9
- 229910052737goldInorganic materials0.000claimsdescription9
- 239000010931goldSubstances0.000claimsdescription9
- 229920000642polymerPolymers0.000claimsdescription9
- QTBSBXVTEAMEQO-UHFFFAOYSA-NAcetic acidChemical compoundCC(O)=OQTBSBXVTEAMEQO-UHFFFAOYSA-N0.000claimsdescription8
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000claimsdescription8
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000claimsdescription8
- 229920001577copolymerPolymers0.000claimsdescription8
- HEMHJVSKTPXQMS-UHFFFAOYSA-MSodium hydroxideChemical compound[OH-].[Na+]HEMHJVSKTPXQMS-UHFFFAOYSA-M0.000claimsdescription6
- ZMANZCXQSJIPKH-UHFFFAOYSA-NTriethylamineChemical compoundCCN(CC)CCZMANZCXQSJIPKH-UHFFFAOYSA-N0.000claimsdescription6
- 239000003999initiatorSubstances0.000claimsdescription6
- QGZKDVFQNNGYKY-UHFFFAOYSA-NAmmoniaChemical compoundNQGZKDVFQNNGYKY-UHFFFAOYSA-N0.000claimsdescription5
- 239000013543active substanceSubstances0.000claimsdescription5
- 230000015572biosynthetic processEffects0.000claimsdescription5
- 239000003795chemical substances by applicationSubstances0.000claimsdescription5
- 239000011734sodiumSubstances0.000claimsdescription5
- XKRFYHLGVUSROY-UHFFFAOYSA-NArgonChemical compound[Ar]XKRFYHLGVUSROY-UHFFFAOYSA-N0.000claimsdescription4
- LZZYPRNAOMGNLH-UHFFFAOYSA-MCetrimonium bromideChemical compound[Br-].CCCCCCCCCCCCCCCC[N+](C)(C)CLZZYPRNAOMGNLH-UHFFFAOYSA-M0.000claimsdescription4
- KDLHZDBZIXYQEI-UHFFFAOYSA-NPalladiumChemical compound[Pd]KDLHZDBZIXYQEI-UHFFFAOYSA-N0.000claimsdescription4
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical compoundOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000claimsdescription4
- ATUOYWHBWRKTHZ-UHFFFAOYSA-NPropaneChemical compoundCCCATUOYWHBWRKTHZ-UHFFFAOYSA-N0.000claimsdescription4
- CDBYLPFSWZWCQE-UHFFFAOYSA-LSodium CarbonateChemical compound[Na+].[Na+].[O-]C([O-])=OCDBYLPFSWZWCQE-UHFFFAOYSA-L0.000claimsdescription4
- -1StNaChemical compound0.000claimsdescription4
- XLOMVQKBTHCTTD-UHFFFAOYSA-NZinc monoxideChemical compound[Zn]=OXLOMVQKBTHCTTD-UHFFFAOYSA-N0.000claimsdescription4
- 239000012159carrier gasSubstances0.000claimsdescription4
- 239000003054catalystSubstances0.000claimsdescription4
- 229910052742ironInorganic materials0.000claimsdescription4
- VNWKTOKETHGBQD-UHFFFAOYSA-NmethaneChemical compoundCVNWKTOKETHGBQD-UHFFFAOYSA-N0.000claimsdescription4
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000claimsdescription4
- 229910052710siliconInorganic materials0.000claimsdescription4
- 229910010271silicon carbideInorganic materials0.000claimsdescription4
- QAOWNCQODCNURD-UHFFFAOYSA-Nsulfuric acid groupChemical groupS(O)(O)(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-N0.000claimsdescription4
- SZEMGTQCPRNXEG-UHFFFAOYSA-Mtrimethyl(octadecyl)azanium;bromideChemical compound[Br-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CSZEMGTQCPRNXEG-UHFFFAOYSA-M0.000claimsdescription4
- VVQNEPGJFQJSBK-UHFFFAOYSA-NMethyl methacrylateChemical compoundCOC(=O)C(C)=CVVQNEPGJFQJSBK-UHFFFAOYSA-N0.000claimsdescription3
- 229910018540Si CInorganic materials0.000claimsdescription3
- PPBRXRYQALVLMV-UHFFFAOYSA-NStyreneChemical compoundC=CC1=CC=CC=C1PPBRXRYQALVLMV-UHFFFAOYSA-N0.000claimsdescription3
- VILCJCGEZXAXTO-UHFFFAOYSA-N2,2,2-tetramineChemical compoundNCCNCCNCCNVILCJCGEZXAXTO-UHFFFAOYSA-N0.000claimsdescription2
- HNUQMTZUNUBOLQ-UHFFFAOYSA-N2-[2-[2-[2-[2-[2-[2-[2-[2-(2-octadecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanolChemical compoundCCCCCCCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOHNUQMTZUNUBOLQ-UHFFFAOYSA-N0.000claimsdescription2
- IVKNZCBNXPYYKL-UHFFFAOYSA-N2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanolChemical compoundCC(C)(C)CC(C)(C)C1=CC=C(OCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO)C=C1IVKNZCBNXPYYKL-UHFFFAOYSA-N0.000claimsdescription2
- WUPHOULIZUERAE-UHFFFAOYSA-N3-(oxolan-2-yl)propanoic acidChemical compoundOC(=O)CCC1CCCO1WUPHOULIZUERAE-UHFFFAOYSA-N0.000claimsdescription2
- XZIIFPSPUDAGJM-UHFFFAOYSA-N6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamineChemical compoundCCN(CC)C1=NC(N)=CC(Cl)=N1XZIIFPSPUDAGJM-UHFFFAOYSA-N0.000claimsdescription2
- OMPJBNCRMGITSC-UHFFFAOYSA-NBenzoylperoxideChemical compoundC=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1OMPJBNCRMGITSC-UHFFFAOYSA-N0.000claimsdescription2
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000claimsdescription2
- OTMSDBZUPAUEDD-UHFFFAOYSA-NEthaneChemical compoundCCOTMSDBZUPAUEDD-UHFFFAOYSA-N0.000claimsdescription2
- 229910000640Fe alloyInorganic materials0.000claimsdescription2
- 229910000990Ni alloyInorganic materials0.000claimsdescription2
- GRYLNZFGIOXLOG-UHFFFAOYSA-NNitric acidChemical compoundO[N+]([O-])=OGRYLNZFGIOXLOG-UHFFFAOYSA-N0.000claimsdescription2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-NSiliconChemical compound[Si]XUIMIQQOPSSXEZ-UHFFFAOYSA-N0.000claimsdescription2
- BQCADISMDOOEFD-UHFFFAOYSA-NSilverChemical compound[Ag]BQCADISMDOOEFD-UHFFFAOYSA-N0.000claimsdescription2
- PMZURENOXWZQFD-UHFFFAOYSA-LSodium SulfateChemical compound[Na+].[Na+].[O-]S([O-])(=O)=OPMZURENOXWZQFD-UHFFFAOYSA-L0.000claimsdescription2
- 229920001807Urea-formaldehydePolymers0.000claimsdescription2
- 239000005083Zinc sulfideSubstances0.000claimsdescription2
- LWZFANDGMFTDAV-BURFUSLBSA-N[(2r)-2-[(2r,3r,4s)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl] dodecanoateChemical compoundCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1OLWZFANDGMFTDAV-BURFUSLBSA-N0.000claimsdescription2
- 229910000147aluminium phosphateInorganic materials0.000claimsdescription2
- 229910021529ammoniaInorganic materials0.000claimsdescription2
- 229910052786argonInorganic materials0.000claimsdescription2
- 239000001273butaneSubstances0.000claimsdescription2
- 229910052980cadmium sulfideInorganic materials0.000claimsdescription2
- 238000006555catalytic reactionMethods0.000claimsdescription2
- 229910000420cerium oxideInorganic materials0.000claimsdescription2
- 229910017052cobaltInorganic materials0.000claimsdescription2
- 239000010941cobaltSubstances0.000claimsdescription2
- GUTLYIVDDKVIGB-UHFFFAOYSA-Ncobalt atomChemical compound[Co]GUTLYIVDDKVIGB-UHFFFAOYSA-N0.000claimsdescription2
- LSXWFXONGKSEMY-UHFFFAOYSA-Ndi-tert-butyl peroxideChemical compoundCC(C)(C)OOC(C)(C)CLSXWFXONGKSEMY-UHFFFAOYSA-N0.000claimsdescription2
- SYELZBGXAIXKHU-UHFFFAOYSA-Ndodecyldimethylamine N-oxideChemical compoundCCCCCCCCCCCC[N+](C)(C)[O-]SYELZBGXAIXKHU-UHFFFAOYSA-N0.000claimsdescription2
- XJWSAJYUBXQQDR-UHFFFAOYSA-Mdodecyltrimethylammonium bromideChemical group[Br-].CCCCCCCCCCCC[N+](C)(C)CXJWSAJYUBXQQDR-UHFFFAOYSA-M0.000claimsdescription2
- 239000003822epoxy resinSubstances0.000claimsdescription2
- 239000001307heliumSubstances0.000claimsdescription2
- 229910052734heliumInorganic materials0.000claimsdescription2
- SWQJXJOGLNCZEY-UHFFFAOYSA-Nhelium atomChemical compound[He]SWQJXJOGLNCZEY-UHFFFAOYSA-N0.000claimsdescription2
- 229910052981lead sulfideInorganic materials0.000claimsdescription2
- 229940056932lead sulfideDrugs0.000claimsdescription2
- 239000010445micaSubstances0.000claimsdescription2
- 229910052618mica groupInorganic materials0.000claimsdescription2
- IJDNQMDRQITEOD-UHFFFAOYSA-Nn-butaneChemical compoundCCCCIJDNQMDRQITEOD-UHFFFAOYSA-N0.000claimsdescription2
- OFBQJSOFQDEBGM-UHFFFAOYSA-Nn-pentaneNatural productsCCCCCOFBQJSOFQDEBGM-UHFFFAOYSA-N0.000claimsdescription2
- 229910052759nickelInorganic materials0.000claimsdescription2
- 239000010955niobiumSubstances0.000claimsdescription2
- 229910017604nitric acidInorganic materials0.000claimsdescription2
- QGLKJKCYBOYXKC-UHFFFAOYSA-NnonaoxidotritungstenChemical compoundO=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1QGLKJKCYBOYXKC-UHFFFAOYSA-N0.000claimsdescription2
- TWNQGVIAIRXVLR-UHFFFAOYSA-Noxo(oxoalumanyloxy)alumaneChemical compoundO=[Al]O[Al]=OTWNQGVIAIRXVLR-UHFFFAOYSA-N0.000claimsdescription2
- BMMGVYCKOGBVEV-UHFFFAOYSA-Noxo(oxoceriooxy)ceriumChemical compound[Ce]=O.O=[Ce]=OBMMGVYCKOGBVEV-UHFFFAOYSA-N0.000claimsdescription2
- 229910052763palladiumInorganic materials0.000claimsdescription2
- 229910052697platinumInorganic materials0.000claimsdescription2
- 229920000647polyepoxidePolymers0.000claimsdescription2
- 238000006116polymerization reactionMethods0.000claimsdescription2
- 239000001294propaneSubstances0.000claimsdescription2
- 230000035484reaction timeEffects0.000claimsdescription2
- 229910052703rhodiumInorganic materials0.000claimsdescription2
- 239000010948rhodiumSubstances0.000claimsdescription2
- MHOVAHRLVXNVSD-UHFFFAOYSA-Nrhodium atomChemical compound[Rh]MHOVAHRLVXNVSD-UHFFFAOYSA-N0.000claimsdescription2
- 239000010703siliconSubstances0.000claimsdescription2
- 229910052814silicon oxideInorganic materials0.000claimsdescription2
- 229910052709silverInorganic materials0.000claimsdescription2
- 239000004332silverSubstances0.000claimsdescription2
- 229910000029sodium carbonateInorganic materials0.000claimsdescription2
- 229940035044sorbitan monolaurateDrugs0.000claimsdescription2
- 235000011067sorbitan monolaureateNutrition0.000claimsdescription2
- XOLBLPGZBRYERU-UHFFFAOYSA-Ntin dioxideChemical compoundO=[Sn]=OXOLBLPGZBRYERU-UHFFFAOYSA-N0.000claimsdescription2
- 239000010936titaniumSubstances0.000claimsdescription2
- 229910052719titaniumInorganic materials0.000claimsdescription2
- 229910001930tungsten oxideInorganic materials0.000claimsdescription2
- 239000011787zinc oxideSubstances0.000claimsdescription2
- DRDVZXDWVBGGMH-UHFFFAOYSA-Nzinc;sulfideChemical compound[S-2].[Zn+2]DRDVZXDWVBGGMH-UHFFFAOYSA-N0.000claimsdescription2
- 238000001354calcinationMethods0.000claims4
- RTZKZFJDLAIYFH-UHFFFAOYSA-NDiethyl etherChemical compoundCCOCCRTZKZFJDLAIYFH-UHFFFAOYSA-N0.000claims2
- CPELXLSAUQHCOX-UHFFFAOYSA-NHydrogen bromideChemical compoundBrCPELXLSAUQHCOX-UHFFFAOYSA-N0.000claims2
- 239000004141Sodium laurylsulphateSubstances0.000claims2
- POULHZVOKOAJMA-UHFFFAOYSA-Ndodecanoic acidChemical compoundCCCCCCCCCCCC(O)=OPOULHZVOKOAJMA-UHFFFAOYSA-N0.000claims2
- 238000009998heat settingMethods0.000claims2
- 229920003987resolePolymers0.000claims2
- DSCFFEYYQKSRSV-UHFFFAOYSA-N1L-O1-methyl-muco-inositolNatural productsCOC1C(O)C(O)C(O)C(O)C1ODSCFFEYYQKSRSV-UHFFFAOYSA-N0.000claims1
- AYAUBWSUZRFVQO-UHFFFAOYSA-N2-[2-(4-phenyl-5-sulfanylidene-1h-1,2,4-triazol-3-yl)ethyl]benzo[de]isoquinoline-1,3-dioneChemical compoundO=C1C(C=23)=CC=CC3=CC=CC=2C(=O)N1CCC1=NNC(=S)N1C1=CC=CC=C1AYAUBWSUZRFVQO-UHFFFAOYSA-N0.000claims1
- SNRUBQQJIBEYMU-UHFFFAOYSA-NDodecaneNatural productsCCCCCCCCCCCCSNRUBQQJIBEYMU-UHFFFAOYSA-N0.000claims1
- VGGSQFUCUMXWEO-UHFFFAOYSA-NEtheneChemical compoundC=CVGGSQFUCUMXWEO-UHFFFAOYSA-N0.000claims1
- 239000005639Lauric acidSubstances0.000claims1
- UEEJHVSXFDXPFK-UHFFFAOYSA-NN-dimethylaminoethanolChemical compoundCN(C)CCOUEEJHVSXFDXPFK-UHFFFAOYSA-N0.000claims1
- 229920003171Poly (ethylene oxide)Polymers0.000claims1
- NSOXQYCFHDMMGV-UHFFFAOYSA-NTetrakis(2-hydroxypropyl)ethylenediamineChemical compoundCC(O)CN(CC(C)O)CCN(CC(C)O)CC(C)ONSOXQYCFHDMMGV-UHFFFAOYSA-N0.000claims1
- GZCGUPFRVQAUEE-SLPGGIOYSA-Naldehydo-D-glucoseChemical compoundOC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=OGZCGUPFRVQAUEE-SLPGGIOYSA-N0.000claims1
- 235000011114ammonium hydroxideNutrition0.000claims1
- XKXHCNPAFAXVRZ-UHFFFAOYSA-Nbenzylazanium;chlorideChemical compound[Cl-].[NH3+]CC1=CC=CC=C1XKXHCNPAFAXVRZ-UHFFFAOYSA-N0.000claims1
- 235000019504cigarettesNutrition0.000claims1
- 229960002887deanolDrugs0.000claims1
- 125000003438dodecyl groupChemical group[H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])*0.000claims1
- 150000002148estersChemical class0.000claims1
- 229910000042hydrogen bromideInorganic materials0.000claims1
- QJGQUHMNIGDVPM-UHFFFAOYSA-Nnitrogen groupChemical group[N]QJGQUHMNIGDVPM-UHFFFAOYSA-N0.000claims1
- VVWRJUBEIPHGQF-UHFFFAOYSA-Npropan-2-yl n-propan-2-yloxycarbonyliminocarbamateChemical groupCC(C)OC(=O)N=NC(=O)OC(C)CVVWRJUBEIPHGQF-UHFFFAOYSA-N0.000claims1
- QQONPFPTGQHPMA-UHFFFAOYSA-NpropyleneNatural productsCC=CQQONPFPTGQHPMA-UHFFFAOYSA-N0.000claims1
- 125000004805propylene groupChemical group[H]C([H])([H])C([H])([*:1])C([H])([H])[*:2]0.000claims1
- 235000017550sodium carbonateNutrition0.000claims1
- APSBXTVYXVQYAB-UHFFFAOYSA-Msodium docusateChemical compound[Na+].CCCCC(CC)COC(=O)CC(S([O-])(=O)=O)C(=O)OCC(CC)CCCCAPSBXTVYXVQYAB-UHFFFAOYSA-M0.000claims1
- 229910052938sodium sulfateInorganic materials0.000claims1
- 235000011152sodium sulphateNutrition0.000claims1
- HFQQZARZPUDIFP-UHFFFAOYSA-Msodium;2-dodecylbenzenesulfonateChemical compound[Na+].CCCCCCCCCCCCC1=CC=CC=C1S([O-])(=O)=OHFQQZARZPUDIFP-UHFFFAOYSA-M0.000claims1
- 238000007711solidificationMethods0.000claims1
- 230000008023solidificationEffects0.000claims1
- 239000004575stoneSubstances0.000claims1
- 125000000383tetramethylene groupChemical group[H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2]0.000claims1
- OGIDPMRJRNCKJF-UHFFFAOYSA-Ntitanium oxideInorganic materials[Ti]=OOGIDPMRJRNCKJF-UHFFFAOYSA-N0.000claims1
- 229960001124trientineDrugs0.000claims1
- 239000002135nanosheetSubstances0.000abstractdescription28
- 239000007788liquidSubstances0.000abstractdescription15
- 239000004005microsphereSubstances0.000abstractdescription11
- 238000003756stirringMethods0.000abstractdescription5
- 238000004519manufacturing processMethods0.000abstractdescription3
- 238000003912environmental pollutionMethods0.000abstractdescription2
- 238000007664blowingMethods0.000abstract1
- 239000000377silicon dioxideSubstances0.000description43
- 230000005587bubblingEffects0.000description32
- 230000005540biological transmissionEffects0.000description29
- 239000011521glassSubstances0.000description28
- BOTDANWDWHJENH-UHFFFAOYSA-NTetraethyl orthosilicateChemical compoundCCO[Si](OCC)(OCC)OCCBOTDANWDWHJENH-UHFFFAOYSA-N0.000description26
- 229920002415Pluronic P-123Polymers0.000description15
- 238000004627transmission electron microscopyMethods0.000description12
- 238000001723curingMethods0.000description11
- 238000002003electron diffractionMethods0.000description11
- 239000002131composite materialSubstances0.000description9
- 239000002245particleSubstances0.000description8
- 235000012239silicon dioxideNutrition0.000description8
- 238000001179sorption measurementMethods0.000description8
- UQSXHKLRYXJYBZ-UHFFFAOYSA-Niron oxideInorganic materials[Fe]=OUQSXHKLRYXJYBZ-UHFFFAOYSA-N0.000description7
- NDLPOXTZKUMGOV-UHFFFAOYSA-Noxo(oxoferriooxy)iron hydrateChemical compoundO.O=[Fe]O[Fe]=ONDLPOXTZKUMGOV-UHFFFAOYSA-N0.000description5
- ISWSIDIOOBJBQZ-UHFFFAOYSA-Nphenol groupChemical groupC1(=CC=CC=C1)OISWSIDIOOBJBQZ-UHFFFAOYSA-N0.000description5
- 239000000443aerosolSubstances0.000description4
- 238000000498ball millingMethods0.000description4
- 239000013335mesoporous materialSubstances0.000description4
- 239000004408titanium dioxideSubstances0.000description4
- KXGFMDJXCMQABM-UHFFFAOYSA-N2-methoxy-6-methylphenolChemical group[CH]OC1=CC=CC([CH])=C1OKXGFMDJXCMQABM-UHFFFAOYSA-N0.000description3
- 238000001000micrographMethods0.000description3
- 238000005191phase separationMethods0.000description3
- 229920001568phenolic resinPolymers0.000description3
- 239000005011phenolic resinSubstances0.000description3
- 238000001338self-assemblyMethods0.000description3
- 229910052708sodiumInorganic materials0.000description3
- OZAIFHULBGXAKX-UHFFFAOYSA-N2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrileChemical compoundN#CC(C)(C)N=NC(C)(C)C#NOZAIFHULBGXAKX-UHFFFAOYSA-N0.000description2
- NLMKTBGFQGKQEV-UHFFFAOYSA-N2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-hexadecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanolChemical compoundCCCCCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCONLMKTBGFQGKQEV-UHFFFAOYSA-N0.000description2
- NLXLAEXVIDQMFP-UHFFFAOYSA-NAmmonia chlorideChemical compound[NH4+].[Cl-]NLXLAEXVIDQMFP-UHFFFAOYSA-N0.000description2
- 239000004793PolystyreneSubstances0.000description2
- MCMNRKCIXSYSNV-UHFFFAOYSA-NZirconium dioxideChemical compoundO=[Zr]=OMCMNRKCIXSYSNV-UHFFFAOYSA-N0.000description2
- 229960000583acetic acidDrugs0.000description2
- 239000003814drugSubstances0.000description2
- 229940079593drugDrugs0.000description2
- 238000013007heat curingMethods0.000description2
- 238000011068loading methodMethods0.000description2
- 229940051841polyoxyethylene etherDrugs0.000description2
- 229920000056polyoxyethylene etherPolymers0.000description2
- 229920002223polystyrenePolymers0.000description2
- 239000010453quartzSubstances0.000description2
- 239000002994raw materialSubstances0.000description2
- 238000000926separation methodMethods0.000description2
- 150000003384small moleculesChemical class0.000description2
- 239000000243solutionSubstances0.000description2
- 239000007921spraySubstances0.000description2
- JMXKSZRRTHPKDL-UHFFFAOYSA-Ntitanium ethoxideChemical compound[Ti+4].CC[O-].CC[O-].CC[O-].CC[O-]JMXKSZRRTHPKDL-UHFFFAOYSA-N0.000description2
- VXNZUUAINFGPBY-UHFFFAOYSA-N1-ButeneChemical compoundCCC=CVXNZUUAINFGPBY-UHFFFAOYSA-N0.000description1
- TZGPACAKMCUCKX-UHFFFAOYSA-N2-hydroxyacetamideChemical compoundNC(=O)COTZGPACAKMCUCKX-UHFFFAOYSA-N0.000description1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N3-azaniumyl-2-hydroxypropanoateChemical compoundNCC(O)C(O)=OBMYNFMYTOJXKLE-UHFFFAOYSA-N0.000description1
- 239000004342Benzoyl peroxideSubstances0.000description1
- 235000013162Cocos nuciferaNutrition0.000description1
- 244000060011Cocos nuciferaSpecies0.000description1
- MWRWFPQBGSZWNV-UHFFFAOYSA-NDinitrosopentamethylenetetramineChemical compoundC1N2CN(N=O)CN1CN(N=O)C2MWRWFPQBGSZWNV-UHFFFAOYSA-N0.000description1
- 239000004593EpoxySubstances0.000description1
- PIICEJLVQHRZGT-UHFFFAOYSA-NEthylenediamineChemical compoundNCCNPIICEJLVQHRZGT-UHFFFAOYSA-N0.000description1
- DGAQECJNVWCQMB-PUAWFVPOSA-MIlexoside XXIXChemical compoundC[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+]DGAQECJNVWCQMB-PUAWFVPOSA-M0.000description1
- 239000004677NylonSubstances0.000description1
- 235000019270ammonium chlorideNutrition0.000description1
- 238000003491arrayMethods0.000description1
- 239000011324beadSubstances0.000description1
- 235000019400benzoyl peroxideNutrition0.000description1
- IAQRGUVFOMOMEM-UHFFFAOYSA-NbuteneNatural productsCC=CCIAQRGUVFOMOMEM-UHFFFAOYSA-N0.000description1
- 239000002775capsuleSubstances0.000description1
- 239000000919ceramicSubstances0.000description1
- 239000000084colloidal systemSubstances0.000description1
- 239000011246composite particleSubstances0.000description1
- 239000013078crystalSubstances0.000description1
- 238000009826distributionMethods0.000description1
- GVGUFUZHNYFZLC-UHFFFAOYSA-Ndodecyl benzenesulfonate;sodiumChemical compound[Na].CCCCCCCCCCCCOS(=O)(=O)C1=CC=CC=C1GVGUFUZHNYFZLC-UHFFFAOYSA-N0.000description1
- 230000000694effectsEffects0.000description1
- 239000000839emulsionSubstances0.000description1
- 150000002191fatty alcoholsChemical class0.000description1
- 239000000835fiberSubstances0.000description1
- 239000010408filmSubstances0.000description1
- 239000012362glacial acetic acidSubstances0.000description1
- 230000005484gravityEffects0.000description1
- 238000000227grindingMethods0.000description1
- 238000010406interfacial reactionMethods0.000description1
- 239000004973liquid crystal related substanceSubstances0.000description1
- 239000007791liquid phaseSubstances0.000description1
- 229910052751metalInorganic materials0.000description1
- 239000002184metalSubstances0.000description1
- 239000011259mixed solutionSubstances0.000description1
- 239000002808molecular sieveSubstances0.000description1
- DVEKCXOJTLDBFE-UHFFFAOYSA-Nn-dodecyl-n,n-dimethylglycinateChemical compoundCCCCCCCCCCCC[N+](C)(C)CC([O-])=ODVEKCXOJTLDBFE-UHFFFAOYSA-N0.000description1
- 239000002077nanosphereSubstances0.000description1
- ZKATWMILCYLAPD-UHFFFAOYSA-Nniobium pentoxideInorganic materialsO=[Nb](=O)O[Nb](=O)=OZKATWMILCYLAPD-UHFFFAOYSA-N0.000description1
- URLJKFSTXLNXLG-UHFFFAOYSA-Nniobium(5+);oxygen(2-)Chemical compound[O-2].[O-2].[O-2].[O-2].[O-2].[Nb+5].[Nb+5]URLJKFSTXLNXLG-UHFFFAOYSA-N0.000description1
- 239000002736nonionic surfactantSubstances0.000description1
- 229920001778nylonPolymers0.000description1
- BPUBBGLMJRNUCC-UHFFFAOYSA-Noxygen(2-);tantalum(5+)Chemical compound[O-2].[O-2].[O-2].[O-2].[O-2].[Ta+5].[Ta+5]BPUBBGLMJRNUCC-UHFFFAOYSA-N0.000description1
- 230000002572peristaltic effectEffects0.000description1
- 239000012071phaseSubstances0.000description1
- 125000001997phenyl groupChemical group[H]C1=C([H])C([H])=C(*)C([H])=C1[H]0.000description1
- 229920003229poly(methyl methacrylate)Polymers0.000description1
- 239000004926polymethyl methacrylateSubstances0.000description1
- 229920002503polyoxyethylene-polyoxypropylenePolymers0.000description1
- 238000004626scanning electron microscopyMethods0.000description1
- HBMJWWWQQXIZIP-UHFFFAOYSA-Nsilicon carbideChemical compound[Si+]#[C-]HBMJWWWQQXIZIP-UHFFFAOYSA-N0.000description1
- LIVNPJMFVYWSIS-UHFFFAOYSA-Nsilicon monoxideChemical compound[Si-]#[O+]LIVNPJMFVYWSIS-UHFFFAOYSA-N0.000description1
- 239000000779smokeSubstances0.000description1
- 229940083542sodiumDrugs0.000description1
- URGAHOPLAPQHLN-UHFFFAOYSA-Nsodium aluminosilicateChemical compound[Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=OURGAHOPLAPQHLN-UHFFFAOYSA-N0.000description1
- 229940080264sodium dodecylbenzenesulfonateDrugs0.000description1
- 239000010935stainless steelSubstances0.000description1
- 229910001220stainless steelInorganic materials0.000description1
- 238000003860storageMethods0.000description1
- 239000000126substanceSubstances0.000description1
- KDYFGRWQOYBRFD-UHFFFAOYSA-Lsuccinate(2-)Chemical compound[O-]C(=O)CCC([O-])=OKDYFGRWQOYBRFD-UHFFFAOYSA-L0.000description1
- BDHFUVZGWQCTTF-UHFFFAOYSA-MsulfonateChemical compound[O-]S(=O)=OBDHFUVZGWQCTTF-UHFFFAOYSA-M0.000description1
- 238000001308synthesis methodMethods0.000description1
- 238000003786synthesis reactionMethods0.000description1
- 238000010189synthetic methodMethods0.000description1
- PBCFLUZVCVVTBY-UHFFFAOYSA-Ntantalum pentoxideInorganic materialsO=[Ta](=O)O[Ta](=O)=OPBCFLUZVCVVTBY-UHFFFAOYSA-N0.000description1
- 238000005287template synthesisMethods0.000description1
- 239000010409thin filmSubstances0.000description1
- 229910001887tin oxideInorganic materials0.000description1
- 229910052984zinc sulfideInorganic materials0.000description1
Images
Landscapes
- Manufacturing Of Micro-Capsules (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明涉及一种制备具有可控形貌的介观材料的方法及其专用装置。 The invention relates to a method for preparing a mesoscopic material with controllable morphology and a special device thereof. the
背景技术Background technique
自从1992年Mobil公司的研究人员成功地合成了MCM-41型介孔分子筛以来,具有单一孔尺寸,高的比表面积和孔隙率的介孔材料得到了科技界广泛的关注(C.T.Kresge,M.E.Leonowicz,W.J.Roth,J.C.Vartuli,J.S.Beck,“Ordered mesoporousmolecular sieves synthesized by a liquid crystal template mechanism”,Nature 1992,359,710)。现在人们不仅能用不同的模板分子控制孔的形状和尺寸,而且能制备不同形貌的介孔材料以适应应用研究的需要。人们通过控制合成条件已经合成出了介孔薄膜、纤维、纳米及微米球、块材及“单晶”等特殊形貌的介孔材料(Y.F.Lu,B.F.Mccaughey,D.H.Wang,J.E.Hampsey,N.Doke,Z.Z.Yang,C.J.Brinker,“Aerosol-assisted formation of mesostructured thin films”,Adv.Mater.2003,15,1733;Z.L.Yang,Z.W.Niu,X.Y.Cao,Z.Z.Yang,Y.F.Lu,Z.B.Hu,C.C.Han,“Templatesynthesis of uniform 1D mesostructured silica materials and their arrays in anodic aluminamembranes”,Angew.Chem.Int.Ed.2003,42,4201;“Composite mesostructures bynano-confinement”,Nat.Mater.2004,3,816;Y.F.Lu,H.Y.Fan,A.Stump,T.L.Ward,T.Rieker,C.J.Brinker,“Aerosol-assisted selfassembly of mesostructured sphericalnanoparticles”,Nature 1999,398,223)。但是对于介孔空心球的关注较少,主要是由于其合成方法的限制。介孔空心球由于兼具介孔材料高的吸附容量和大孔材料优良的物质传输速度在药物装载与释放,低密度材料,吸附分离等领域显示出特殊的应用价值,已经逐渐成为人们感兴趣的研究领域之一。目前,制备介孔中空微球的方法主要集中在硬模板法,软模板,气溶胶辅助的超声喷雾法和界面合成法等。其中,模板法特别是硬模板法与软模板法结合,引起学者广泛的研究兴趣,可广泛制备均一尺寸的介孔空心微球(G.Zhu,S.Qiu,O.Terasaki,Y.Wei,“Polystyrene bead-assistedself-assembly of microstructured silica hollow spheres in highly alkaline media”,J.Am.Chem.Soc.2001,123,7723)。但是模板的除去对球体结构的影响仍然存在,而且球体的壳厚无法控制。Shi发展了利用PVP和CTAB的共模板作用制备了具备装载药物功能的介孔二氧化硅微球(Y.F.Zhu,J.L.Shi,H.R.Chen,W.H.Shen,X.P.Dong,“A facile method to synthesize novel hollow mesoporous silica spheres and advanced storage property”,Micropor.Mesopor.Mater.2005,84,218)。利用受限空间如乳液液滴的界面反应也被用来制备介孔空心微球(J.A.Zasadzinski,E.Kisak,C.Evans,“Complex vesicle-based structures”,Curr.Opin.Colloid Interface Sci.2001,6,85)。上述这两种方法存在的主要问题是在液相中进行,产物分离困难,生产效率极低,而且球壳厚度无法控制。Ward等人利用气溶胶辅助的超声喷雾法结合聚合物的相分离作用,实现了介孔空心二氧化硅微球在气相中的制备(S.B.Rathod,T.L.Ward,“Hierarchical porous and composite particle architectures based on selfassembly and phaseseparation in droplets”,J.Mater.Chem.2007,17,2329)。但是由于所选用的相分离剂和表面活性剂种类有限,无法实现对孔尺寸和结构的控制。因而,如何利用无模板法大批量可控制备介孔空心微球仍然是一个具有挑战性的课题。 Since researchers from Mobil Corporation successfully synthesized MCM-41 mesoporous molecular sieves in 1992, mesoporous materials with a single pore size, high specific surface area and porosity have attracted extensive attention from the scientific community (C.T.Kresge, M.E.Leonowicz , W.J.Roth, J.C.Vartuli, J.S.Beck, "Ordered mesoporousmolecular sieves synthesized by a liquid crystal template mechanism", Nature 1992, 359, 710). Now people can not only use different template molecules to control the shape and size of the pores, but also prepare mesoporous materials with different shapes to meet the needs of applied research. Mesoporous materials with special shapes such as mesoporous films, fibers, nano and microspheres, bulk materials and "single crystals" have been synthesized by controlling the synthesis conditions (Y.F.Lu, B.F.Mccaughey, D.H.Wang, J.E.Hampsey, N. Doke, Z.Z.Yang, C.J.Brinker, "Aerosol-assisted formation of mesostructured thin films", Adv.Mater.2003, 15, 1733; Z.L.Yang, Z.W.Niu, X.Y.Cao, Z.Z.Yang, Y.F.Lu, Z.B.Hu, C.C.Han, "Templatesynthesis of uniform 1D mesostructured silica materials and their arrays in anodic aluminamembranes", Angew.Chem.Int.Ed.2003, 42, 4201; "Composite mesostructures bynano-confinement", Nat.Mater.2004, 3, 816; Y.F.Lu , H.Y.Fan, A.Stump, T.L.Ward, T.Rieker, C.J.Brinker, "Aerosol-assisted selfassembly of mesostructured spherical nanoparticles", Nature 1999, 398, 223). However, less attention has been paid to mesoporous hollow spheres, mainly due to the limitations of their synthetic methods. Due to the high adsorption capacity of mesoporous materials and the excellent mass transfer rate of macroporous materials, mesoporous hollow spheres have shown special application value in the fields of drug loading and release, low-density materials, adsorption and separation, and have gradually become a topic of interest. one of the research fields. At present, the methods for preparing mesoporous hollow microspheres mainly focus on hard template method, soft template method, aerosol-assisted ultrasonic spray method and interfacial synthesis method. Among them, the template method, especially the combination of the hard template method and the soft template method, has aroused extensive research interest of scholars, and can widely prepare mesoporous hollow microspheres of uniform size (G.Zhu, S.Qiu, O.Terasaki, Y.Wei, "Polystyrene bead-assisted self-assembly of microstructured silica hollow spheres in highly alkaline media", J.Am.Chem.Soc.2001, 123, 7723). However, the influence of the removal of the template on the structure of the sphere still exists, and the shell thickness of the sphere cannot be controlled. Shi developed mesoporous silica microspheres with the function of loading drugs by using the co-templating effect of PVP and CTAB (Y.F.Zhu, J.L.Shi, H.R.Chen, W.H.Shen, X.P.Dong, "A facile method to synthesize novel hollow mesoporous silica spheres and advanced storage property”, Micropor. Mesopor. Mater. 2005, 84, 218). Interfacial reactions using confined spaces such as emulsion droplets have also been used to prepare mesoporous hollow microspheres (J.A.Zasadzinski, E.Kisak, C.Evans, "Complex vesicle-based structures", Curr.Opin.Colloid Interface Sci.2001 , 6, 85). The main problems of the above two methods are that they are carried out in the liquid phase, the product separation is difficult, the production efficiency is extremely low, and the thickness of the spherical shell cannot be controlled. Ward et al. used the aerosol-assisted ultrasonic spray method combined with the phase separation of polymers to realize the preparation of mesoporous hollow silica microspheres in the gas phase (S.B. Rathod, T.L. Ward, "Hierarchical porous and composite particle architectures based on selfassembly and phase separation in droplets”, J. Mater. Chem. 2007, 17, 2329). However, due to the limited types of phase separation agents and surfactants selected, the control of pore size and structure cannot be achieved. Therefore, how to prepare large-scale and controllable mesoporous hollow microspheres using a template-free method is still a challenging issue. the
发明内容Contents of the invention
本发明的目的是提供一种制备具有可控形貌的介观材料的方法与专用装置。 The object of the present invention is to provide a method and special device for preparing mesoscopic materials with controllable morphology. the
本发明提供的专用于制备具有可控形貌的介观材料的装置,是一种气泡发生装置,它由包括内气体管、外气体管和进液管在内的三层套管组成,其中,内气体管位于进液管腔内,进液管位于外气体管腔内。 The device specially provided by the present invention for preparing mesoscopic materials with controllable morphology is a bubble generating device, which consists of a three-layer casing including an inner gas tube, an outer gas tube and a liquid inlet tube, wherein , the inner gas pipe is located in the liquid inlet lumen, and the liquid inlet pipe is located in the outer gas lumen. the
上述气泡发生装置中,内气体管和进液管的间距大于0小于2cm。该装置还可包括固化通道,该固化通道的入口与上述内气体管和外气体管的出气口相连。上述气泡发生装置可用石英、玻璃或金属等常用材料制造而得,优选石英。内外气体管可根据需要设计成不同的直径,范围在0.1cm-5cm。可用各种常用的进液装置对该气泡发生装置注入液体,如蠕动泵、压力泵、重力泵、柱塞泵、注射泵、齿轮泵等。 In the above-mentioned bubble generating device, the distance between the inner gas pipe and the liquid inlet pipe is greater than 0 and less than 2 cm. The device may also include a curing channel, the inlet of the curing channel is connected with the gas outlets of the inner gas tube and the outer gas tube. The above-mentioned bubble generating device can be made of common materials such as quartz, glass or metal, preferably quartz. The inner and outer gas pipes can be designed with different diameters according to the needs, ranging from 0.1cm to 5cm. Various common liquid inlet devices can be used to inject liquid into the bubble generating device, such as peristaltic pumps, pressure pumps, gravity pumps, plunger pumps, syringe pumps, gear pumps, etc. the
本发明提供的制备具有可控形貌的介观材料的方法,包括如下步骤: The method for preparing a mesoscopic material with controllable morphology provided by the present invention comprises the following steps:
1)将起泡型表面活性剂加入水中,搅拌至匀,再加入壳层预聚物搅拌至匀,反应得到前体溶液; 1) Add the foaming surfactant into the water, stir until uniform, then add the shell prepolymer and stir until uniform, and react to obtain the precursor solution;
2)将步骤1)得到的前体溶液注入本发明提供的气泡发生装置中的进液管中,在气泡发生装置中内气体管的出气口处产生气泡,经该气泡发生装置中的外气体管吹送成泡,然后将该泡进行固化,得到本发明提供的具有可控形貌的介观材料; 2) Inject the precursor solution obtained in step 1) into the liquid inlet pipe in the bubble generating device provided by the present invention, generate bubbles at the gas outlet of the inner gas pipe in the bubble generating device, and pass through the outer gas in the bubble generating device The tube is blown into bubbles, and then the bubbles are solidified to obtain the mesoscopic material with controllable morphology provided by the present invention;
当内气体管中的气流速度为10-70L/h、外气体管的气流速度为80-400L/h时,形成的具有可控形貌的介观材料为空心微球; When the gas flow rate in the inner gas tube is 10-70L/h and the gas flow rate in the outer gas tube is 80-400L/h, the mesoscopic material with controllable morphology is formed as hollow microspheres;
当内气体管中的气流速度为10-70L/h,外气体管的气流速度为10-100L/h时,形成的具有可控形貌的介观材料为纳米片; When the airflow velocity in the inner gas tube is 10-70L/h, and the airflow velocity in the outer gas tube is 10-100L/h, the formed mesoscopic material with controllable morphology is nanosheet;
当内气体管中的气流速度为60-200L/h,外气体管的气流速度为10-400L/h时,形成的具有可控形貌的介观材料为纳米颗粒。 When the gas flow rate in the inner gas tube is 60-200L/h, and the gas flow rate in the outer gas tube is 10-400L/h, the formed mesoscopic material with controllable morphology is nanoparticles. the
上述制备方法的步骤1)中,所用起泡型表面活性剂为十二烷基硫酸钠、十二烷基苯磺酸钠、十二烷基聚氧乙烯醚硫酸钠、硬脂酸钠、琥珀酸二异辛酯磺酸钠、椰子乙二醇基酰胺、十二烷基二甲基氧化胺或十二烷基二甲基甜菜碱中的任意一种或其任意比例混合的混合物,优选十二烷基硫酸钠; In step 1) of the above-mentioned preparation method, the foaming surfactant used is sodium lauryl sulfate, sodium dodecylbenzenesulfonate, sodium lauryl polyoxyethylene ether sulfate, sodium stearate, succinate Any one of sodium diisooctyl sulfonate, coconut glycol amide, lauryl dimethyl amine oxide or lauryl dimethyl betaine or a mixture thereof in any proportion, preferably ten Sodium Dialkyl Sulfate;
所用壳层预聚物为有机预聚物、单体或由该单体生成的溶胶中的任意一种或其任意比例混合的混合物;其中,有机预聚物选自酚醛树脂、环氧树脂、脲醛树脂、苯乙烯和甲基丙烯酸甲酯; The shell prepolymer used is any one of the organic prepolymer, the monomer or the sol generated by the monomer or its mixture in any proportion; wherein the organic prepolymer is selected from phenolic resin, epoxy resin, Urea-formaldehyde resins, styrene and methyl methacrylate;
单体为R(OCnH2n+1)4、T(CH2)mSi(OCnH2n+1)3、Nb(OCH2CH3)2、Ta(OCH2CH3)2和Na2SiO3; The monomers are R(OCn H2n+1 )4 , T(CH2 )m Si(OCn H2n+1 )3 , Nb(OCH2 CH3 )2 , Ta(OCH2 CH3 )2 and Na2 SiO3 ;
其中,R(OCnH2n+1)4中,R为Si或Ti,n为1~6;T(CH2)mSi(OCnH2n+1)3中,T为CH2=CH-、C6H5-、NH2-或SH-,m为1~6、n为1~2。 Among them, in R(OCn H2n+1 )4 , R is Si or Ti, and n is 1-6; in T(CH2 )m Si(OCn H2n+1 )3 , T is CH2 =CH -, C6 H5 -, NH2 - or SH-, m is 1-6, n is 1-2.
在该步骤1)中,在将起泡型表面活性剂加入水中的步骤中,还可同时加入致孔型表面活性剂, In this step 1), in the step of adding the foaming surfactant to water, the porogenic surfactant can also be added simultaneously,
所用致孔型表面活性剂为小分子、共聚物表面活性剂或者其任意比例的混合物;其中,小分子表面活性剂为十二烷基三甲基溴化铵、十六烷基三甲基溴化铵、十八烷基三甲基溴化铵、月桂酸二甲胺基乙醇酯苄基氯化铵,优选十六烷基三甲基溴化铵或十八烷基三甲基溴化铵;非离子表面活性剂为脂肪醇聚氧乙烯醚、山梨醇单月桂酸酯、OP-10、Brij-56{CH3(CH2)15(OCH2CH2)10OH}、Brij-58{CH3(CH2)15(OCH2CH2)20OH}、Brij-76{CH3(CH2)17(OCH2CH2)10OH}、Span-20等,优选Brij-56{CH3(CH2)15(OCH2CH2)10OH}或Brij-58{CH3(CH2)15(OCH2CH2)20OH};共聚物表面活性剂为聚氧乙烯-聚氧丙烯共聚物Pluronic P123(EO20-PO70-EO20)、F127(EO20-PO106-EO20)、聚氧乙烯-聚氧丁烯共聚物B40-2500(EO17BO14EO17)、B20-5000(EO45BO14EO45)、B20-3800(EO34BO11EO34)、B40-1900(EO13BO11EO13)、苯乙烯-聚氧乙烯共聚物、马来酸-丙烯酸酯共聚物或丙烯酸乙酯-丙烯腈共聚物,优选P123(EO20-PO70-EO20)或F127(EO20-PO106-EO20)。 The porogenic surfactant used is a small molecule, copolymer surfactant or a mixture thereof in any proportion; wherein, the small molecule surfactant is dodecyltrimethylammonium bromide, hexadecyltrimethylbromide Ammonium chloride, stearyltrimethylammonium bromide, dimethylaminoethanol laurate benzyl ammonium chloride, preferably cetyltrimethylammonium bromide or octadecyltrimethylammonium bromide ; Nonionic surfactants are fatty alcohol polyoxyethylene ether, sorbitan monolaurate, OP-10, Brij-56{CH3 (CH2 )15 (OCH2 CH2 )10 OH}, Brij-58{ CH3 (CH2 )15 (OCH2 CH2 )20 OH}, Brij-76{CH3 (CH2 )17 (OCH2 CH2 )10 OH}, Span-20, etc., preferably Brij-56{CH3 (CH2 )15 (OCH2 CH2 )10 OH} or Brij-58{CH3 (CH2 )15 (OCH2 CH2 )20 OH}; the copolymer surfactant is polyoxyethylene-polyoxypropylene copolymer Pluronic P123 (EO20 -PO70 -EO20 ), F127 (EO20 -PO106 -EO20 ), polyoxyethylene-polyoxybutylene copolymer B40-2500 (EO17 BO14 EO17 ), B20- 5000(EO45 BO14 EO45 ), B20-3800(EO34 BO11 EO34 ), B40-1900(EO13 BO11 EO13 ), styrene-polyoxyethylene copolymer, maleic acid-acrylate copolymer or ethyl acrylate-acrylonitrile copolymer, preferably P123 (EO20 -PO70 -EO20 ) or F127 (EO20 -PO106 -EO20 ).
该步骤1)中,可加入催化剂催化该反应进行,所用催化剂为硫酸、盐酸、硝酸、磷酸、醋酸、氢溴酸、氨水、氢氧化钠或碳酸钠。 In the step 1), a catalyst can be added to catalyze the reaction, and the catalyst used is sulfuric acid, hydrochloric acid, nitric acid, phosphoric acid, acetic acid, hydrobromic acid, ammonia, sodium hydroxide or sodium carbonate. the
另外,该步骤1)中所用的壳层预聚物还包括功能纳米颗粒和/或功能单体;其中,所用功能纳米颗粒为二氧化钛、氧化锌、氧化铈、氧化锡、硫化锌、硫化镉、硫化铅、四氧化三铁、氧化铁、镍/铁合金、石墨粉、碳化硅、硼化硅、烟墨粉、氧化铝、云母、氧化硅、氧化钨、铁、镍、钴、铂、钯、铑、银或金中的任意一种或其任意比例混合的混合物: In addition, the shell prepolymer used in step 1) also includes functional nanoparticles and/or functional monomers; wherein, the functional nanoparticles used are titanium dioxide, zinc oxide, cerium oxide, tin oxide, zinc sulfide, cadmium sulfide, Lead sulfide, ferric oxide, iron oxide, nickel/iron alloy, graphite powder, silicon carbide, silicon boride, smoke toner powder, aluminum oxide, mica, silicon oxide, tungsten oxide, iron, nickel, cobalt, platinum, palladium, Any one of rhodium, silver or gold or a mixture thereof in any proportion:
所用功能单体为CH2=C(CH3)COOCH3CH2=C(CH3)COOCH2-CH=CH2、CH2=CHCOO(CH2)11CH3、CH3(CH2)11C≡C-C≡C-(CH2)8CO-(OCH2CH2)5OH或(CH3CH2O)3Si-C6H4-Si(OCH2CH3)3; The functional monomers used are CH2 =C(CH3 )COOCH3 CH2 =C(CH3 )COOCH2 -CH=CH2 , CH2 =CHCOO(CH2 )11 CH3 , CH3 (CH2 )11 C≡CC≡C-(CH2 )8 CO-(OCH2 CH2 )5 OH or (CH3 CH2 O)3 Si-C6 H4 -Si(OCH2 CH3 )3 ;
该壳层预聚物中还可包括引发剂,所用引发剂为偶氮二异丁腈、过氧化苯甲酰或二叔丁基过氧化物。 The shell prepolymer may also include an initiator, and the initiator used is azobisisobutyronitrile, benzoyl peroxide or di-tert-butyl peroxide. the
上述各原料的用量如下:起泡型表面活性剂的重量份数为1-10,水的重量份数为1-10000,壳层预聚物的重量份数为1-300,致孔型表面活性剂的重量份数为1-100,催化剂的重量份数为0.005-5;功能纳米颗粒的重量份数为0.01~50,功能单体的重量份数为10~50,引发剂的重量份数为0.02~0.5。 The consumption of above-mentioned each raw material is as follows: the parts by weight of foaming surfactant is 1-10, the parts by weight of water is 1-10000, the parts by weight of shell layer prepolymer is 1-300, the parts by weight of porogenic surface The parts by weight of the active agent are 1-100, the parts by weight of the catalyst are 0.005-5; the parts by weight of the functional nanoparticles are 0.01-50, the parts by weight of the functional monomer are 10-50, the parts by weight of the initiator The number is 0.02-0.5. the
该步骤的搅拌温度为10-40℃,反应时间为1-4小时; The stirring temperature of this step is 10-40°C, and the reaction time is 1-4 hours;
上述制备方法的步骤2)中,所用气泡发生装置中的载气为氮气、氩气、氦气、甲烷、乙烷、丙烷、丁烷、乙烯、丙烯或丁烯,该载气的流速为10~600L/h; In step 2) of the above-mentioned preparation method, the carrier gas in the bubble generating device used is nitrogen, argon, helium, methane, ethane, propane, butane, ethylene, propylene or butene, and the flow rate of the carrier gas is 10 ~600L/h;
固化步骤中,各种常用的固化方式均适用于本方法,如热固化、紫外光固化、微波催化或固化剂固化等;热固化的固化温度为50-300℃,固化剂为盐酸、氨气、三乙胺、乙二胺或三乙烯四胺。 In the curing step, various commonly used curing methods are applicable to this method, such as heat curing, ultraviolet light curing, microwave catalysis or curing agent curing, etc.; the curing temperature of heat curing is 50-300 °C, and the curing agent is hydrochloric acid, ammonia gas , triethylamine, ethylenediamine or triethylenetetramine. the
另外,上述步骤2)得到的空心微球进行破碎处理,也可得到具有纳米片结构的介观材料;该破碎处理的方式可为球磨或研磨,所用球磨介质是玛瑙、陶瓷、不锈钢、氧化锆、尼龙;球磨时的转速为100-600rpm,球磨时间为10-60min。 In addition, the hollow microspheres obtained in the above step 2) can also be crushed to obtain mesoscopic materials with a nanosheet structure; the crushing method can be ball milling or grinding, and the ball milling medium used is agate, ceramics, stainless steel, zirconia , Nylon; the speed of ball milling is 100-600rpm, and the ball milling time is 10-60min. the
在步骤2)之后,可将上述具有可控形貌的介观材料在惰性气氛中进行灼烧,以去除材料中的表面活性剂。该灼烧温度为400-700℃,优选450℃,灼烧时间为2-6小时。 After step 2), the above-mentioned mesoscopic material with controllable morphology can be burned in an inert atmosphere to remove the surfactant in the material. The burning temperature is 400-700°C, preferably 450°C, and the burning time is 2-6 hours. the
利用本发明得到的介观材料,包括空心微球、纳米片和纳米颗粒,其组成均为单一的二氧化硅、二氧化钛、五氧化二铌、五氧化二钽、交联环氧、交联酚醛、聚苯乙烯、聚甲基丙烯酸甲酯; The mesoscopic material obtained by the present invention includes hollow microspheres, nanosheets and nanoparticles, and its composition is a single silicon dioxide, titanium dioxide, niobium pentoxide, tantalum pentoxide, cross-linked epoxy, cross-linked phenolic , polystyrene, polymethyl methacrylate;
其中,空心微球的尺寸在100-600nm,厚度在10-80nm;纳米片的尺寸在100-800nm,厚度在10-150nm;介孔颗粒的尺寸在100-200nm;可通过改变壳层预聚物的浓度或气流速度来调节尺寸。 Among them, the size of hollow microspheres is 100-600nm and the thickness is 10-80nm; the size of nanosheets is 100-800nm and the thickness is 10-150nm; the size of mesoporous particles is 100-200nm; The size can be adjusted according to the concentration of the substance or the velocity of the gas flow. the
本发明提供的制备具有可控形貌的介观材料的方法,通过调节气流的速度,可实现一步大批量连续制备介观空心微球、纳米片或纳米颗粒,这是任何其他方法如溶胶-凝胶法无法实现的;通过调节表面活性剂的种类和浓度,可以控制介孔的尺寸和结构;通过调节单体的种类,辅以外加功能组分,可在很宽范围内实现对介观材料形态和功能的调控。该方法不需模板,工艺简便,无环境污染,且原料易得,生产成本低,无 污染,且延伸到无机、有机、有机/无机杂化介观材料或功能性介观材料的大批量制备,完全满足工业化的要求。 The method for preparing mesoscopic materials with controllable morphology provided by the present invention can realize the continuous preparation of mesoscopic hollow microspheres, nanosheets or nanoparticles in one step and in large batches by adjusting the speed of the airflow, which is any other method such as sol- It cannot be realized by gel method; by adjusting the type and concentration of surfactant, the size and structure of mesopore can be controlled; by adjusting the type of monomer and adding functional components, it can realize the mesoscopic Modulation of material form and function. The method does not need a template, the process is simple, no environmental pollution, and the raw materials are easy to obtain, the production cost is low, no pollution, and it extends to the mass preparation of inorganic, organic, organic/inorganic hybrid mesoscopic materials or functional mesoscopic materials , fully meet the requirements of industrialization. the
附图说明Description of drawings
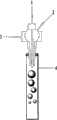
图1为本发明提供的气泡发生装置的结构示意图。 Fig. 1 is a schematic structural view of the bubble generating device provided by the present invention. the
图2为本发明实施例13二氧化硅空心球的透射电镜图。 Fig. 2 is a transmission electron microscope image of a silica hollow sphere in Example 13 of the present invention. the
图3为本发明实施例13介孔二氧化硅空心球的透射电镜图。 Fig. 3 is a transmission electron microscope image of a mesoporous silica hollow sphere in Example 13 of the present invention. the
图4为本发明实施例18介孔二氧化硅纳米片的透射电镜图。 Fig. 4 is a transmission electron microscope image of the mesoporous silica nanosheets of Example 18 of the present invention. the
具体实施方式Detailed ways
下面结合具体实施例对本发明作进一步说明,但本发明并不限于以下实施例。 The present invention will be further described below in conjunction with specific examples, but the present invention is not limited to the following examples. the
实施例1、气泡发生装置
本发明提供的用于制备具有可控形貌的介观材料的方法中的专用装置,为一气泡发生装置,如图1所示,它由包括内气体管1、外气体管3和进液管2在内的三层套管组成,其中,内气体管1位于进液管2腔内,进液管2位于外气体管3腔内。 The special device used in the method for preparing mesoscopic materials with controllable morphology provided by the present invention is a bubble generating device, as shown in Figure 1, it consists of an
上述气泡发生装置中,内气体管1和进液管2的间距大于0小于2cm。该装置还可包括固化通道4,该固化通道4的入口与上述内气体管1和外气体管3的出气口相连。 In the above-mentioned bubble generating device, the distance between the
实施例2、二氧化硅空心球的制备 Embodiment 2, preparation of silica hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,2重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为400L/h,内部气流速度为10L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 2 weight parts are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 400L/h, the internal air velocity is 10L/h, and react at 200°C in a 1m long, 6cm thick glass column, receive it with a flask, and A white powder was collected. the
透射电镜下可看到大约120nm的空心球。 Hollow spheres of about 120nm can be seen under the transmission electron microscope. the
实施例3、二氧化硅空心球的制备
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,2重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为250L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于250℃下反应,用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 2 weight parts are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 250L/h, the internal air velocity is 40L/h, and react at 250°C in a 1m long, 6cm thick glass column, receive it with a flask, and A white powder was collected. the
透射电镜下可看到大约150nm的空心球。 Hollow spheres of about 150nm can be seen under the transmission electron microscope. the
实施例4、二氧化硅空心球的制备
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为60L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 120L/h, the internal air velocity is 60L/h, and react at 200°C in a glass column 1m long and 6cm thick, and receive it with a flask. A white powder was collected. the
透射电镜下可看到大约200nm的空心球。 Hollow spheres of about 200nm can be seen under the transmission electron microscope. the
实施例5、二氧化硅空心球和纳米片的制备 Embodiment 5, preparation of silica hollow spheres and nanosheets
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为90L/h,内部气流速度为60L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 90L/h, the internal air velocity is 60L/h, and react at 200°C in a glass column 1m long and 6cm thick, and receive it with a flask. A white powder was collected. the
扫描和透射电镜下可看到大约200nm的空心球和20nm的纳米片共存,其中空心球质量占30%,纳米片质量占70%。 Under scanning and transmission electron microscopy, it can be seen that hollow spheres of about 200nm and nanosheets of 20nm coexist, wherein the mass of hollow spheres accounts for 30% and the mass of nanosheets accounts for 70%. the
实施例6、二氧化硅纳米片的制备 Embodiment 6, the preparation of silica nanosheet
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为80L/h,内部气流速度为15L/h,并在一根1m长,6cm粗的玻璃柱中于150℃下反应,用烧瓶接收,可收集到白色粉末,透射电镜下可看到大约40纳米厚的纳米片。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 80L/h, the internal air velocity is 15L/h, and react at 150°C in a glass column 1m long and 6cm thick, and receive it with a flask. A white powder was collected, and nanosheets about 40 nm thick could be seen under a transmission electron microscope. the
实施例7、二氧化硅纳米片的制备 Embodiment 7, the preparation of silica nanosheet
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为40L/h,内部气流速度为30L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末,透射电镜下可看到大约55纳米厚的纳米片。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 Hours later, inject bubbles into the bubbling device, the external air velocity is 40L/h, the internal air velocity is 30L/h, and react at 200°C in a glass column 1m long and 6cm thick, and receive it with a flask. A white powder was collected, and nanosheets about 55 nm thick could be seen under a transmission electron microscope. the
实施例8、二氧化硅纳米片的制备 Embodiment 8, the preparation of silica nanosheet
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,5重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为10L/h,内部气流速度为25L/h,并在一根1m长, 6cm粗的玻璃柱中于100℃下反应,用烧瓶接收,可收集到白色粉末,透射电镜下可看到大约80纳米厚的纳米片。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 5 weight parts mix homogeneously, then add 20 weight parts of ethyl orthosilicate, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 10L/h, the internal air velocity is 25L/h, and react at 100°C in a glass column 1m long and 6cm thick, and receive it with a flask. A white powder was collected, and nanosheets about 80 nm thick could be seen under a transmission electron microscope. the
实施例9、二氧化硅纳米片和纳米颗粒的制备 Embodiment 9, preparation of silica nanosheets and nanoparticles
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为40L/h,内部气流速度为65L/h,并在一根1m长,6cm粗的玻璃柱中于100℃下反应,用烧瓶接收,可收集到白色粉末,透射电镜下可看到大约50纳米厚的纳米片和大约100纳米的颗粒,其中纳米片质量占20%,纳米颗粒质量占80%。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After one hour, inject bubbles into the bubbling device, the external air velocity is 40L/h, the internal air velocity is 65L/h, and react at 100°C in a glass column 1m long and 6cm thick, and receive it with a flask. The white powder is collected, and nanosheets with a thickness of about 50 nanometers and particles with a thickness of about 100 nanometers can be seen under a transmission electron microscope, wherein the mass of the nanosheets accounts for 20%, and the mass of the nanoparticles accounts for 80%. the
实施例10、二氧化硅纳米颗粒的制备 Embodiment 10, preparation of silica nanoparticles
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为110L/h,内部气流速度为70L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After 1 hour, inject bubbles into the bubbling device, the external air velocity is 110L/h, the internal air velocity is 70L/h, and react at 200°C in a 1m long, 6cm thick glass column, and receive it with a flask. A white powder was collected. the
透射电镜下可看到大约180纳米左右的纳米颗粒。 Nanoparticles of about 180 nanometers can be seen under the transmission electron microscope. the
实施例11、二氧化硅纳米颗粒的制备 Embodiment 11, preparation of silica nanoparticles
将5重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),400重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为250L/h,内部气流速度为120L/h,并在一根1m长,6cm粗的玻璃柱中于300℃下反应,并用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 5 parts by weight, the water of 400 parts by weight, the 2M hydrochloric acid of 0.1 part by weight are mixed uniformly, then add the tetraethyl orthosilicate of 20 parts by weight, reaction 2 After 1 hour, inject bubbles into the bubbling device, the external air velocity is 250L/h, the internal air velocity is 120L/h, and react at 300°C in a 1m long, 6cm thick glass column, and receive it with a flask. A white powder was collected. the
透射电镜下可看到大约150纳米左右的纳米颗粒。 Nanoparticles of about 150 nanometers can be seen under the transmission electron microscope. the
实施例12、二氧化硅纳米颗粒的制备 Embodiment 12, preparation of silica nanoparticles
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为400L/h,内部气流速度为200L/h,并在一根1m长,6cm粗的玻璃柱中于100℃下反应,并用烧瓶接收,可收集到白色粉末。 The foaming type surfactant SDS (sodium dodecyl sulfate) of 1 weight part, the water of 100 weight parts, the 2M hydrochloric acid of 0.1 weight part are mixed homogeneously, then add the tetraethyl orthosilicate of 20 weight parts, reaction 2 After 1 hour, inject bubbles into the bubbling device, the external air velocity is 400L/h, the internal air velocity is 200L/h, and react at 100°C in a 1m long, 6cm thick glass column, and receive it with a flask. A white powder was collected. the
透射电镜下可看到大约100纳米左右的纳米颗粒。 Nanoparticles of about 100 nanometers can be seen under the transmission electron microscope. the
实施例13、介孔二氧化硅空心球的制备 Example 13, preparation of mesoporous silica hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),30重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应4小时后,注入鼓泡装置鼓泡,外部气流速度为100L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 30 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid is mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate, after reacting for 4 hours, inject the bubbling device to bubble, the external air velocity is 100L/h, the internal air velocity is 40L/h, and in a 1m long, 6cm thick glass column reacted at 200 ℃, received with a flask, and white powder could be collected.
透射电镜下可看到大约200nm的空心球(图2),白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅空心球(图3)。 Hollow spheres with a diameter of about 200 nm can be seen under a transmission electron microscope (Figure 2). The white powder is calcined at 450°C for 6 hours under nitrogen to obtain hollow mesoporous silica spheres (Figure 3). the
小角X射线电子衍射和透射电镜结果表明:介孔结构为无序。氮吸附测试表明,孔径大约在5.1nm,比表面积为380m2.g-1,孔体积0.61cm3.g-1。 Small-angle X-ray electron diffraction and transmission electron microscopy results show that the mesoporous structure is disordered. Nitrogen adsorption test shows that the pore diameter is about 5.1nm, the specific surface area is 380m2 .g-1 , and the pore volume is 0.61cm3 .g-1 .
实施例14、介孔二氧化硅空心球的制备 Example 14, preparation of mesoporous silica hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),5重量份的致孔型表面活性剂P123(EO20-PO70-EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。透射电镜下可看到大约200nm的空心球,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅空心球。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 5 weight parts of porogenous surfactant P123 (EO20 -PO70 -EO20 ), 100 weight parts of water, 0.1 The 2M hydrochloric acid of weight part mixes evenly, then adds the tetraethyl orthosilicate of 20 weight parts, after reacting for 2 hours, injects bubble device bubble, and external air velocity is 120L/h, and internal air velocity is 40L/h, and in React in a 1m long, 6cm thick glass column at 200°C, receive it with a flask, and collect white powder. Hollow spheres with a diameter of about 200 nm can be seen under a transmission electron microscope, and the white powder is calcined at 450°C for 6 hours under nitrogen to obtain hollow mesoporous silica spheres.
X射线电子衍射和透射电镜结果表明:介孔结构为无序。氮吸附测试表明,孔径大约在4.2nm,比表面积为190m2.g-1,孔体积0.22cm3.g-1。 The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered. Nitrogen adsorption test shows that the pore diameter is about 4.2nm, the specific surface area is 190m2 .g-1 , and the pore volume is 0.22cm3 .g-1 .
实施例15、介孔二氧化硅空心球的制备 Example 15, Preparation of Mesoporous Silica Hollow Spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),80重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为150L/h,内部气流速度为60L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。透射电镜下可看到大约200nm的空心球,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅空心球。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 80 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid is mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate, after reacting for 2 hours, inject bubbler bubbles, the external air velocity is 150L/h, the internal air velocity is 60L/h, and in a 1m long, 6cm thick glass column reacted at 200 ℃, received with a flask, and white powder could be collected. Hollow spheres with a diameter of about 200 nm can be seen under a transmission electron microscope, and the white powder is calcined at 450°C for 6 hours under nitrogen to obtain hollow mesoporous silica spheres.
氮吸附测试表明,孔径大约在6.6nm,比表面积为450m2.g-1,孔体积0.89cm3.g-1,X射线电子衍射和透射电镜结果表明:孔结构为有序的层状囊泡结构,因此,通过改变致孔型表面活性剂的量可以控制孔的结构。 Nitrogen adsorption test shows that the pore size is about 6.6nm, the specific surface area is 450m2 .g-1 , and the pore volume is 0.89cm3 .g-1 . The results of X-ray electron diffraction and transmission electron microscopy show that the pore structure is an ordered layered capsule Therefore, the pore structure can be controlled by changing the amount of porogenic surfactant.
实施例16、介孔二氧化硅空心球的制备 Example 16, Preparation of Mesoporous Silica Hollow Spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),15重量份的致孔型表面活性剂F127(EO106PO70EO106),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应3小时后,注入鼓泡装置鼓泡,外部气流速度为250L/h,内部气流速度为50L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。透射电镜下可看到大约200nm的空心球,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅空心球。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 15 weight parts of porogenous surfactant F127 (EO106 PO70 EO106 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid is mixed evenly, then add 20 parts by weight of orthosilicate ethyl ester, after reacting for 3 hours, inject bubble device to bubble, the external air velocity is 250L/h, the internal air velocity is 50L/h, and in a 1m long, 6cm thick glass column reacted at 200 ℃, received with a flask, and white powder could be collected. Hollow spheres with a diameter of about 200 nm can be seen under a transmission electron microscope, and the white powder is calcined at 450°C for 6 hours under nitrogen to obtain hollow mesoporous silica spheres.
氮吸附测试表明,孔径大约在7.4nm,比表面积为360m2.g-1,孔体积0.49cm3.g-1,X射线电子衍射和透射电镜结果表明:孔结构呈有序的六方排列,因此,通过改变致孔型表面活性剂的种类也可以控制孔的结构。 Nitrogen adsorption test shows that the pore diameter is about 7.4nm, the specific surface area is 360m2 .g-1 , and the pore volume is 0.49cm3 .g-1 . The results of X-ray electron diffraction and transmission electron microscopy show that the pore structure is in an orderly hexagonal arrangement. Therefore, the pore structure can also be controlled by changing the type of porogenic surfactant.
实施例17、介孔二氧化钛空心球的制备 Example 17, Preparation of Mesoporous Titanium Dioxide Hollow Spheres
将4重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),15重量份的致孔型表面活性剂F127(EO106PO70EO106),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入150重量份的钛酸四乙酯与冰醋酸的混合溶液(质量比6∶1),反应1小时后,注入鼓泡装置鼓泡,外部气流速度为200L/h,内部气流速度为50L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末。透射电镜下可看到大约150nm的空心球,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化钛空心球。 With 4 parts by weight of foaming surfactant SDS (sodium dodecyl sulfate), 15 parts by weight of porogenous surfactant F127 (EO106 PO70 EO106 ), 100 parts by weight of water, 0.1 parts by weight 2M hydrochloric acid was mixed evenly, and then 150 parts by weight of a mixed solution of tetraethyl titanate and glacial acetic acid (mass ratio 6:1) was added. After reacting for 1 hour, it was injected into a bubbling device for bubbling, and the external air velocity was 200 L/h , the internal air flow rate is 50L/h, and react in a 1m long, 6cm thick glass column at 200°C, receive it with a flask, and white powder can be collected. Hollow spheres with a diameter of about 150 nm can be seen under a transmission electron microscope, and the white powder is calcined at 450°C for 6 hours under nitrogen to obtain hollow mesoporous titanium dioxide spheres.
X射线电子衍射和透射电镜结果表明:介孔结构为无序。 The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered. the
实施例18、介孔二氧化硅纳米片的制备 Example 18, Preparation of Mesoporous Silica Nanosheets
将6重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),20重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为70L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,用烧瓶接收,可收集到白色粉末,透射电镜下可看到大约20纳米厚的纳米片,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅纳米(图4)。 With 6 parts by weight of foaming surfactant SDS (sodium dodecyl sulfate), 20 parts by weight of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 parts by weight of water, 0.1 parts by weight 2M hydrochloric acid was mixed evenly, then 20 parts by weight of tetraethyl orthosilicate was added, and after 2 hours of reaction, it was injected into a bubbling device for bubbling. The external air velocity was 70L/h, and the internal air velocity was 40L/h. React in a 1m long, 6cm thick glass column at 200°C, receive it with a flask, and collect a white powder. Nanosheets about 20 nm thick can be seen under a transmission electron microscope. The white powder is calcined at 450°C for 6 hours under nitrogen, and can Mesoporous silica nanoparticles were obtained (Figure 4).
X射线电子衍射和透射电镜结果表明:介孔结构为无序。氮吸附测试表明,孔径大约在4.1nm,比表面积为450m2.g-1,孔体积0.66cm3.g-1。 The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered. Nitrogen adsorption test shows that the pore diameter is about 4.1nm, the specific surface area is 450m2 .g-1 , and the pore volume is 0.66cm3 .g-1 .
实施例19、介孔二氧化硅纳米片的制备 Example 19, Preparation of Mesoporous Silica Nanosheets
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),40重量份的致孔型表面活性剂F127(EO106PO70EO106),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为60L/h,内部气流速度为50L/h,并在一根1m长、6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到白色粉末。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 40 weight parts of porogenous surfactant F127 (EO106 PO70 EO106 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid was mixed uniformly, then 20 parts by weight of tetraethyl orthosilicate was added, and after 2 hours of reaction, it was injected into a bubbling device for bubbling. The external air velocity was 60L/h, and the internal air velocity was 50L/h, and the React in a 1m long, 6cm thick glass column at 200°C and receive it in a flask, and a white powder can be collected.
透射电镜下可看到大约40纳米厚的纳米片,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅纳米,X射线电子衍射和透射电镜结果表明:介孔结构为无序。 Nanosheets with a thickness of about 40 nm can be seen under the transmission electron microscope. The white powder is calcined under nitrogen at 450 ° C for 6 hours to obtain mesoporous silica nanoparticles. The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered. the
实施例20、介孔二氧化硅纳米颗粒的制备 Example 20, Preparation of Mesoporous Silica Nanoparticles
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为100L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到白色粉末。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid was mixed evenly, then 20 parts by weight of tetraethyl orthosilicate was added, and after 2 hours of reaction, it was injected into the bubbling device for bubbling. The external air velocity was 120L/h, and the internal air velocity was 100L/h, 1m long, 6cm thick glass column reacted at 200 ℃, and received with a flask, white powder can be collected.
透射电镜下可看到大约200纳米左右的纳米颗粒,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅纳米颗粒,X射线电子衍射和透射电镜结果表明:介孔结构为无序。 Nanoparticles of about 200 nanometers can be seen under the transmission electron microscope. The white powder is calcined under nitrogen at 450 ° C for 6 hours to obtain mesoporous silica nanoparticles. The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered . the
实施例21、介孔二氧化硅纳米颗粒的制备 Example 21, Preparation of Mesoporous Silica Nanoparticles
将4重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),20重量份的致孔型表面活性剂Brij-56{CH3(CH2)15(OCH2CH2)10OH},100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入40重量份的正硅酸乙酯,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为300L/h,内部气流速度为150L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到白色粉末。 4 parts by weight of foaming surfactant SDS (sodium dodecyl sulfate), 20 parts by weight of porogenous surfactant Brij-56{CH3 (CH2 )15 (OCH2 CH2 )10 OH }, 100 parts by weight of water and 0.1 parts by weight of 2M hydrochloric acid were mixed evenly, then 40 parts by weight of tetraethyl orthosilicate was added, and after 2 hours of reaction, the bubbler was injected into the bubbling device. The external air velocity was 300L/h, and the internal The gas flow rate is 150L/h, and react at 200°C in a 1m long, 6cm thick glass column, and receive it with a flask, and white powder can be collected.
透射电镜下可看到大约100纳米左右的纳米颗粒,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅纳米颗粒,X射线电子衍射和透射电镜结果表明:介孔结构为无序。 Nanoparticles of about 100 nanometers can be seen under the transmission electron microscope. The white powder is calcined under nitrogen at 450 ° C for 6 hours to obtain mesoporous silica nanoparticles. The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered . the
实施例22、介孔酚醛/二氧化硅复合空心球的制备 Example 22. Preparation of mesoporous phenolic/silicon dioxide composite hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯和20重量份的酚醛树脂反应2小时后,注入鼓泡装 置鼓泡,外部气流速度为120L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于150℃下反应,并用烧瓶接收,可收集到淡红色粉末,透射电镜下可看到大约250nm的空心球,淡红色粉末经450℃氮气下煅烧6hr,可得到介孔酚醛/二氧化硅复合空心球。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate and 20 parts by weight of phenolic resin to react for 2 hours, inject bubbles into the bubbling device, the external air velocity is 120L/h, and the internal air velocity is 40L/h h, and reacted at 150°C in a 1m long and 6cm thick glass column, and received it with a flask, a light red powder can be collected, and a hollow sphere of about 250nm can be seen under a transmission electron microscope. Calcined under nitrogen for 6 hours, the mesoporous phenolic/silicon dioxide composite hollow spheres can be obtained.
X射线电子衍射和透射电镜结果表明:介孔结构为无序。氮吸附测试表明,孔径大约在2.6nm,比表面积为460m2.g-1,孔体积0.29cm3.g-1。 The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disordered. Nitrogen adsorption test shows that the pore diameter is about 2.6nm, the specific surface area is 460m2 .g-1 , and the pore volume is 0.29cm3 .g-1 .
实施例23、介孔酚醛/二氧化硅复合纳米片的制备 Example 23, Preparation of Mesoporous Phenolic/Silicon Dioxide Composite Nanosheets
将6重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),60重量份的致孔型表面活性剂P123(EO20PO70EO20),800重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯和20重量份的酚醛树脂反应2小时后,注入鼓泡装置鼓泡,外部气流速度为30L/h,内部气流速度为60L/h,并在一根1m长,6cm粗的玻璃柱中于150℃下反应,用烧瓶接收,可收集到淡红色粉末。 With 6 parts by weight of foaming surfactant SDS (sodium dodecyl sulfate), 60 parts by weight of porogenous surfactant P123 (EO20 PO70 EO20 ), 800 parts by weight of water, 0.1 parts by weight 2M hydrochloric acid mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate and 20 parts by weight of phenolic resin to react for 2 hours, inject bubbles into the bubbling device, the external air velocity is 30L/h, and the internal air velocity is 60L/h h, and react in a 1m long, 6cm thick glass column at 150°C, receive it with a flask, and collect a light red powder.
透射电镜下可看到大约20nm厚的纳米片,淡红色粉末经450℃氮气下煅烧6hr,可得到介孔酚醛/二氧化硅纳米片,X射线电子衍射和透射电镜结果表明:介孔结构为无序。 Nanosheets with a thickness of about 20nm can be seen under the transmission electron microscope. The light red powder is calcined under nitrogen at 450°C for 6 hours to obtain mesoporous phenolic/silica nanosheets. The results of X-ray electron diffraction and transmission electron microscopy show that the mesoporous structure is disorderly. the
实施例24、介孔二氧化硅/金复合空心球的制备 Example 24. Preparation of mesoporous silica/gold composite hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯和0.1重量份的金纳米粒子,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为150L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到黑色粉末,扫描电镜下可看到大约200纳米的球表面粗糙,分布小颗粒,颗粒尺寸与金纳米粒子尺寸对应,EDS进一步证明空心球外面负载有金纳米粒子。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate and gold nanoparticles of 0.1 parts by weight, after reacting for 2 hours, inject bubbles into the bubbling device, the external air velocity is 150L/h, and the internal air velocity is 40L/h, and react in a 1m long, 6cm thick glass column at 200°C, and receive it with a flask, black powder can be collected, under the scanning electron microscope, it can be seen that the spherical surface of about 200 nanometers is rough, and the distribution of small particles , the particle size corresponds to the size of gold nanoparticles, and EDS further proves that the hollow spheres are loaded with gold nanoparticles.
实施例25、介孔二氧化硅/金复合纳米片的制备 Example 25. Preparation of mesoporous silica/gold composite nanosheets
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯和0.1重量份的金纳米粒子,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为40L/h,内部气流速度为30L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到黑色粉末,透射电镜下可看到大 约20纳米厚的纳米片,一面负载较多的金纳米粒子,另一面较少。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate and gold nanoparticles of 0.1 parts by weight, after reacting for 2 hours, inject the bubbling device for bubbling, the external air velocity is 40L/h, and the internal air velocity is 30L/h, and reacted at 200°C in a 1m long, 6cm thick glass column, and received it with a flask, the black powder can be collected, and nanosheets about 20nm thick can be seen under the transmission electron microscope. More gold nanoparticles, less on the other side.
实施例26、磁性四氧化三铁/二氧化硅复合空心球的制备 Example 26, Preparation of magnetic ferric oxide/silicon dioxide composite hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯和2重量份的磁性四氧化三铁颗粒,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到黑色粉末,透射电镜下可看到大约250nm的空心球,扫描电镜下可以看到表面粗糙,分散着一些小颗粒,并用磁强计表征了其磁性,磁场强度为2000Oe。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate and 2 parts by weight of magnetic iron ferric oxide particles, after reacting for 2 hours, inject bubbles into the bubbling device, the external air velocity is 120L/h, and the internal The air flow rate is 40L/h, and react at 200°C in a 1m long, 6cm thick glass column, and receive it with a flask. Black powder can be collected, and a hollow sphere of about 250nm can be seen under the transmission electron microscope. Scanning electron microscope It can be seen that the surface is rough, and some small particles are dispersed, and its magnetic properties are characterized by a magnetometer, and the magnetic field strength is 2000Oe.
实施例27、磁性四氧化三铁/二氧化硅复合空心球的制备 Example 27. Preparation of magnetic ferric oxide/silicon dioxide composite hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入20重量份的正硅酸乙酯和10重量份的磁性四氧化三铁颗粒,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于200℃下反应,并用烧瓶接收,可收集到黑色粉末,透射电镜下可看到大约250nm的空心球,扫描电镜下可以看到表面粗糙,分散着一些小颗粒,并用磁强计表征了其磁性,磁场强度为4000Oe。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid mixed evenly, then add 20 parts by weight of tetraethyl orthosilicate and 10 parts by weight of magnetic iron ferric oxide particles, after reacting for 2 hours, inject bubbles into the bubbling device, the external air velocity is 120L/h, and the internal The air flow rate is 40L/h, and react at 200°C in a 1m long, 6cm thick glass column, and receive it with a flask. Black powder can be collected, and a hollow sphere of about 250nm can be seen under the transmission electron microscope. Scanning electron microscope It can be seen that the surface is rough and some small particles are dispersed, and its magnetic properties are characterized by a magnetometer, and the magnetic field strength is 4000Oe.
实施例28、功能化介孔空心球的制备 Example 28. Preparation of functionalized mesoporous hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀,然后加入10重量份的正硅酸乙酯和20重量份的(CH3CH2O)3Si-C6H4-Si(OCH2CH3) 3,反应2小时后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于150℃下反应,并用烧瓶接收,可收集到白色粉末。透射电镜下可看到大约200nm的空心球,白色粉末经450℃氮气下煅烧6hr,可得到介孔二氧化硅空心球。红外证明,该空心球的孔壁上含有苯环。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid was mixed evenly, and then 10 parts by weight of ethyl orthosilicate and 20 parts by weight of (CH3 CH2 O)3 Si-C6 H4 -Si(OCH2 CH3 )3 were added, and after 2 hours of reaction , injected into the bubbling device for bubbling, the external air velocity is 120L/h, the internal air velocity is 40L/h, and react at 150°C in a 1m long, 6cm thick glass column, and receive it with a flask, which can be collected White powder. Hollow spheres with a diameter of about 200 nm can be seen under a transmission electron microscope, and the white powder is calcined at 450°C for 6 hours under nitrogen to obtain hollow mesoporous silica spheres. Infrared test proves that the hole wall of the hollow sphere contains benzene rings.
实施例29、功能化介孔空心球的制备 Example 29, Preparation of functionalized mesoporous hollow spheres
将1重量份的起泡型表面活性剂SDS(十二烷基硫酸钠),10重量份的致孔型表面活性剂P123(EO20PO70EO20),100重量份的水,0.1重量份的2M盐酸混合均匀, 然后加入15重量份的正硅酸乙酯、5重量份的(CH3CH2O)3Si-(CH2)6-CH=CH2、1重量份的CH2=CHCOO(CH2)11CH3反应30分钟后,注入鼓泡装置鼓泡,外部气流速度为120L/h,内部气流速度为40L/h,并在一根1m长,6cm粗的玻璃柱中于100℃下反应,并用烧瓶接收,可收集到白色粉末。透射电镜下可看到大约200nm的空心球,白色粉末经350℃氮气下煅烧3hr,除去表面活性剂,同时诱发骨架中的双键聚合,可得到功能化的有机/无机复合介孔空心球,可以提高空心球的强度和韧性。 With 1 weight part of foaming surfactant SDS (sodium dodecyl sulfate), 10 weight parts of porogenous surfactant P123 (EO20 PO70 EO20 ), 100 weight parts of water, 0.1 weight part 2M hydrochloric acid was mixed evenly, and then 15 parts by weight of ethyl orthosilicate, 5 parts by weight of (CH3 CH2 O)3 Si-(CH2 )6 -CH═CH2 , 1 part by weight of CH2 ═ After CHCOO(CH2 )11 CH3 reacted for 30 minutes, it was injected into a bubbling device for bubbling. React at 100°C and receive it in a flask, and white powder can be collected. Hollow spheres with a diameter of about 200 nm can be seen under a transmission electron microscope. The white powder is calcined at 350°C for 3 hours under nitrogen to remove the surfactant and induce the polymerization of double bonds in the skeleton to obtain functional organic/inorganic composite mesoporous hollow spheres. It can improve the strength and toughness of the hollow sphere.
Claims (24)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200810106722XACN101580244B (en) | 2008-05-15 | 2008-05-15 | Method for preparing mesoscopic material with controllable appearance |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200810106722XACN101580244B (en) | 2008-05-15 | 2008-05-15 | Method for preparing mesoscopic material with controllable appearance |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101580244A CN101580244A (en) | 2009-11-18 |
| CN101580244Btrue CN101580244B (en) | 2012-01-11 |
Family
ID=41362585
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN200810106722XAExpired - Fee RelatedCN101580244B (en) | 2008-05-15 | 2008-05-15 | Method for preparing mesoscopic material with controllable appearance |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101580244B (en) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102219179B (en)* | 2010-04-19 | 2012-12-26 | 中国科学院理化技术研究所 | Silver-doped titanium dioxide film and preparation method thereof |
| CN103058206A (en)* | 2012-12-18 | 2013-04-24 | 泰山医学院 | Method for synthesizing highly ordered super-microporous silicon dioxide |
| CN107265437B (en)* | 2017-06-05 | 2019-07-16 | 大连理工大学 | A surface energy-driven self-assembly method for the preparation of multi-cavity carbon spheres and its application |
| CN108176237A (en)* | 2017-12-29 | 2018-06-19 | 广州安赛化工有限公司 | High-efficiency non-phosphate is reverse osmosis to use dirt dispersion agent |
| CN111671660B (en)* | 2020-05-20 | 2022-04-26 | 东南大学 | A kind of moisturizing material and its application in the preparation of moisturizing products |
| CN112397731B (en)* | 2020-11-13 | 2022-02-11 | 安徽大学 | Preparation of a hollow-structured PdCoP/C alloy electrocatalyst and its application in the electrocatalytic oxidation of ethanol |
| CN113070056B (en)* | 2021-03-22 | 2022-11-08 | 南昌大学 | General synthesis method of three-dimensional ordered net-shaped tantalum pentoxide photocatalytic material |
| CN115624928B (en)* | 2022-11-01 | 2024-04-12 | 贵州大学 | Micro-reaction equipment for preparing nano cuprous iodide and use method |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1693377A (en)* | 2004-05-09 | 2005-11-09 | 中国科学院化学研究所 | Use of superhydrophilic and/or superoleophilic nanoporous materials |
| CN1923684A (en)* | 2006-09-15 | 2007-03-07 | 南开大学 | Nano sphericity mesoporous silicon dioxide material and preparation method |
| US7288146B1 (en)* | 2006-03-16 | 2007-10-30 | Kronos International, Inc. | Titanium dioxide pigment coated with hollow bodies and method for its manufacture |
- 2008
- 2008-05-15CNCN200810106722XApatent/CN101580244B/ennot_activeExpired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1693377A (en)* | 2004-05-09 | 2005-11-09 | 中国科学院化学研究所 | Use of superhydrophilic and/or superoleophilic nanoporous materials |
| US7288146B1 (en)* | 2006-03-16 | 2007-10-30 | Kronos International, Inc. | Titanium dioxide pigment coated with hollow bodies and method for its manufacture |
| CN1923684A (en)* | 2006-09-15 | 2007-03-07 | 南开大学 | Nano sphericity mesoporous silicon dioxide material and preparation method |
Non-Patent Citations (1)
| Title |
|---|
| Shailendra B et al.Hierarchical porous and composite particle architectures based on self assembly and phase separation in droplets.《Journal of Materials Chemistry》.2007,第17卷2329-2335.* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101580244A (en) | 2009-11-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101580244B (en) | Method for preparing mesoscopic material with controllable appearance | |
| Guo et al. | Synthesis and characterization of carbon sphere-silica core–shell structure and hollow silica spheres | |
| Zhai et al. | Soft-template synthesis of ordered mesoporous carbon/nanoparticle nickel composites with a high surface area | |
| Sun et al. | Synthesis, functionalization, and applications of morphology-controllable silica-based nanostructures: A review | |
| Xue et al. | Tuning the interfacial activity of mesoporous silicas for biphasic interface catalysis reactions | |
| Chen et al. | One-pot synthesis of thermally stable gold@ mesoporous silica core-shell nanospheres with catalytic activity | |
| Du et al. | Spherical silica micro/nanomaterials with hierarchical structures: Synthesis and applications | |
| Li et al. | Hollow‐structured mesoporous materials: chemical synthesis, functionalization and applications | |
| Blas et al. | Elaboration of monodisperse spherical hollow particles with ordered mesoporous silica shells via dual latex/surfactant templating: radial orientation of mesopore channels | |
| Chen et al. | Synthesis of mesoporous silica hollow nanospheres with multiple gold cores and catalytic activity | |
| Zhang et al. | Surfactant-free synthesis of silica aerogel microspheres with hierarchically porous structure | |
| CN102060300B (en) | Synthesis method of SiO2 hollow spheres with high dispersion, high specific surface area and large pore volume | |
| Zhiani et al. | Synthesis of new class of copper (II) complex-based FeNi3/KCC-1 for the N-formylation of amines using dihydrogen and carbon dioxide | |
| Han et al. | Morphology-controlled synthesis of mesoporous silica with co-template of surfactant P123 and ionic liquid [Dmim] Cl | |
| Deng et al. | Recent advances in the synthesis of Janus nanomaterials of block copolymers | |
| Wu et al. | Dual-templating synthesis of multi-shelled mesoporous silica nanoparticles as catalyst and drug carrier | |
| Soltani et al. | Novel mesoporous crumpled paper-like silica balls | |
| WO2007032436A1 (en) | Thermally expanded microsphere and process for production thereof | |
| CN102050453A (en) | Monox hollow sphere material with multi-stage pore structure and preparation method of monox hollow sphere material | |
| Kosari et al. | Transformation of stöber silica spheres to hollow nanocatalysts | |
| Mizutani et al. | Anomalous pore expansion of highly monodispersed mesoporous silica spheres and its application to the synthesis of porous ferromagnetic composite | |
| Hu et al. | Preparation of hollow alumina nanospheres via surfactant-assisted flame spray pyrolysis | |
| JP2011195813A (en) | Thermally expandable microsphere, hollow microparticle, method of manufacturing the same and application | |
| Yang et al. | A mesoporous hollow silica sphere (MHSS): Synthesis through a facile emulsion approach and application of support for high performance Pd/MHSS catalyst for phenol hydrogenation | |
| Nemec et al. | A versatile interfacial coassembly method for fabrication of tunable silica shells with radially aligned dual mesopores on diverse magnetic core nanoparticles |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20120111 Termination date:20210515 | |
| CF01 | Termination of patent right due to non-payment of annual fee |