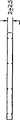CN101472564A - High performance reticulated elastomeric matrix - Google Patents
High performance reticulated elastomeric matrixDownload PDFInfo
- Publication number
- CN101472564A CN101472564ACNA200780023043XACN200780023043ACN101472564ACN 101472564 ACN101472564 ACN 101472564ACN A200780023043X ACNA200780023043X ACN A200780023043XACN 200780023043 ACN200780023043 ACN 200780023043ACN 101472564 ACN101472564 ACN 101472564A
- Authority
- CN
- China
- Prior art keywords
- implantable device
- elastic matrix
- another embodiment
- reticulated
- optionally
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Abstract
Description
Translated fromChinese本申请为2004年5月17日提交的美国申请号US10/848,624的部分继续申请,并且请求该申请,2006年6月22日提交的美国临时申请号US60/816,120和2006年10月3日提交的美国临时申请号US60/849,328的利益,将每一申请披露的内容完整地引入本文作为参考。This application is a continuation-in-part of U.S. Application No. US10/848,624, filed May 17, 2004, and requests that application, U.S. Provisional Application No. US60/816,120, filed June 22, 2006 and filed October 3, 2006 US Provisional Application No. US 60/849,328, the disclosure of each application is incorporated herein by reference in its entirety.
发明领域field of invention
本发明涉及网状弹性基质,其制备,包括通过所谓的“手工”技术和“机器”方法,其后处理,诸如其加强,压缩模制或退火和应用,包括用于将可植入装置植入或局部治疗患者,诸如人和其它动物,用于手术装置,组织加强,组织修复,治疗,营养或其它有用的目的。就这些和其它目的而言,本发明的产品可以单独使用或载有一种或多种可递送的物质。The present invention relates to a reticulated elastic matrix, its preparation, including by so-called "manual" techniques and "machine" methods, its subsequent processing, such as its strengthening, compression molding or annealing and its application, including for implanting implantable devices Injection or local treatment of patients, such as humans and other animals, for surgical devices, tissue reinforcement, tissue repair, therapy, nutrition or other useful purposes. For these and other purposes, the products of the invention may be used alone or loaded with one or more deliverable substances.
发明背景Background of the invention
组织改造(“TE”)手段一般包括生物相容性组织基质的递送,该基质用作细胞附着,生长和/或增殖的支架或支持物,由此通过再生或新组织生长合成新组织以便修复伤口或缺陷。已经公认开放式细胞相容性泡沫具有用于修复和再生组织的重要潜能。然而,由于其被身体分解和吸收,但不会在身体合成新组织以修复伤口过程中和之后产生任何副作用的能力,所以在本领域中在先的工作已经集中于由合成生物吸收性材料构成的组织改造支架。Tissue engineering ("TE") approaches generally involve the delivery of a biocompatible tissue matrix that serves as a scaffold or support for cell attachment, growth and/or proliferation whereby new tissue is synthesized for repair through regeneration or new tissue growth Wounds or Defects. It has been recognized that open cell compatible foams have important potential for tissue repair and regeneration. However, due to its ability to be broken down and absorbed by the body without causing any side effects during and after the body synthesizes new tissue to repair a wound, previous work in this field has focused on tissue transformation scaffolds.
涉及用于组织再生的生物吸收性3-维多孔支架的这些手段的主要弱点在于在该产品寿命周期过程中不需要的组织反应,因为聚合物生物降解并且不能在体内改造TE支架的降解特性,由此严重限制了它们用作有效支架的能力。此外,对例如在通过导管,内窥镜,关节镜(arthoscope)或注射器递送至生物部位过程中可抵抗递送装置中的压缩的植入物仍然存在需求,这种植入物能够通过回弹恢复以便占据和保留在生物部位内并且具有特定的孔径而膨胀,使得该植入物可以在所述部位上与组织一起向内生长,以便适用于有用的治疗目的。此外,从生物耐久性的观点来看,在聚合过程中起泡形成的聚氨基甲酸酯泡沫构成的许多材料不具有吸引力,因为在聚合过程中可能生成可以产生不良生物反应的不需要的材料,例如致癌物,细胞毒素等。相反,本发明的生物耐久性网状弹性基质材料适合于诸如长期TE植入物这类应用,尤其是其中诸如在软组织相关矫形外科应用中经历的动态加载和/或伸展。The main weakness of these approaches involving bioabsorbable 3-dimensional porous scaffolds for tissue regeneration lies in unwanted tissue reactions during the life cycle of this product, since the polymer biodegrades and the degradation properties of the TE scaffold cannot be modified in vivo, Their ability to be used as effective scaffolds is thereby severely limited. In addition, there remains a need for implants that can resist compression in the delivery device during delivery to a biological site, such as by a catheter, endoscope, arthoscope, or syringe, and that can recover by rebounding to Occupying and remaining within a biological site and expanding with a specific pore size such that the implant can ingrow with tissue at the site for useful therapeutic purposes. Furthermore, many materials consisting of polyurethane foams that foam during polymerization are not attractive from a biodurability point of view, since unwanted moieties may be generated during polymerization that can produce adverse biological reactions. Materials such as carcinogens, cytotoxins, etc. In contrast, the biodurable reticulated elastic matrix material of the present invention is suitable for applications such as long term TE implants, especially where dynamic loading and/or stretching is experienced such as in soft tissue related orthopedic applications.
目前大部分的组织支架由生物可降解的聚合物构成,诸如聚乙醇酸(“PGA”),聚乳酸(“PLA”)等的均聚物和共聚物或诸如胶原蛋白,弹性蛋白,基于动物组织的产品,基于人组织的产品等这类生物聚合物。这些材料存在许多缺点,例如,难以改造其特性以接近各种靶向组织中的那些。另外,在体内保持其性能的能力的期限较短,尤其是涉及其弹性和回弹特性。为了花费几周或几个月再生,重塑和/或愈合组织,诸如矫形外科应用软组织或血管组织,不能使用由生物可降解聚合物和生物聚合物构成的支架,因为它们不能维持有效支架所要求的潜在性能,并且特别是就生物聚合物而言,在约2-4周内降解。某些生物可降解聚合物可以在体内存在至多1年或1年以上,但它们通常易脆,易断裂,在体内或体外环境中具有低于约5%的抗张伸展率。由生物聚合物构成的支架的组织改造基质,并且在某些情况中就生物可降解聚合物而言,通常存在不需要的组织反应和装置排斥的高度可能性。后者对基于动物或人组织的产品而言尤其严重。对生物可降解聚合物植入物而言通常在它们长期慢性组织缺陷愈合过程中分解并且降解时观察到不需要的组织反应。Most current tissue scaffolds are composed of biodegradable polymers such as polyglycolic acid ("PGA"), polylactic acid ("PLA") homopolymers and copolymers or such as collagen, elastin, animal-based Tissue products, products based on human tissue, etc. such biopolymers. These materials suffer from a number of disadvantages, such as difficulty in engineering their properties to approximate those in various target tissues. In addition, the ability to maintain its properties in vivo has a shorter duration, especially with regard to its elastic and resilient properties. In order to take weeks or months to regenerate, remodel and/or heal tissue, such as soft tissue or vascular tissue for orthopedic applications, scaffolds composed of biodegradable polymers and biopolymers cannot be used because they cannot maintain the effective scaffold. The required latent properties, and especially in the case of biopolymers, degrade in about 2-4 weeks. Certain biodegradable polymers can persist in the body for up to 1 year or more, but they are generally brittle, prone to fracture, and have a tensile elongation of less than about 5% in an in vivo or in vitro environment. Tissue-engineered matrices for scaffolds composed of biopolymers, and in some cases biodegradable polymers, generally present a high potential for unwanted tissue reactions and device rejection. The latter is especially serious for products based on animal or human tissue. Unwanted tissue reactions are often observed with biodegradable polymer implants as they break down and degrade during the healing process of long-term chronic tissue defects.
可选择地,目前使用冻干技术和可浸出孔生技术(porogens),诸如盐和糖,从而由生物可降解聚合物制成多孔支架;不过,难以控制所得支架的特性,控制多孔性和结构。Alternatively, porous scaffolds are currently made from biodegradable polymers using lyophilization techniques and leachable porogens, such as salts and sugars; however, it is difficult to control the properties of the resulting scaffolds, controlling porosity and structure .
本发明包括网状弹性基质的可植入装置克服了生物吸收性材料,生物可降解聚合物和生物聚合物的上述难题。可以改造这些网状弹性基质材料以便与靶向修复的组织特性基本上匹配或满足导致组织再生,重塑或愈合的具体应用的特定要求。本文披露了成功改造其特性以便接近各种靶向组织中的那些或特性的方式,使得组织再生,改造和/或愈合得以促进。The implantable device of the present invention comprising a reticulated elastic matrix overcomes the aforementioned difficulties with bioabsorbable materials, biodegradable polymers and biopolymers. These reticulated elastic matrix materials can be engineered to substantially match the tissue properties for targeted repair or to meet the specific requirements of specific applications leading to tissue regeneration, remodeling or healing. Disclosed herein are ways to successfully engineer their properties so as to approximate those or properties in various targeted tissues such that tissue regeneration, remodeling and/or healing are promoted.
本文披露了改造本发明网状弹性基质形态和/或特性的方法,通过控制其化学,加工和后处理特征,诸如交联的量,结晶度的量,化学组成,固化条件,网状程度和/或后网状化处理,诸如退火,压缩模制和/或掺入加强。不同于生物可降解聚合物,网状弹性基质在体内长期维持了其物理特性和性能。因此,它无法启动不需要的组织反应,正如对生物可降解植入物在它们分解和降解时观察的。Disclosed herein are methods for modifying the morphology and/or properties of the reticulated elastic matrix of the present invention by controlling its chemical, processing and post-treatment characteristics, such as the amount of crosslinking, the amount of crystallinity, chemical composition, curing conditions, degree of reticulation and / or post reticulation treatments such as annealing, compression molding and / or incorporation of reinforcement. Unlike biodegradable polymers, the reticulated elastic matrix maintains its physical properties and performance in vivo for a long time. Therefore, it is unable to initiate unwanted tissue responses, as observed with biodegradable implants as they disintegrate and degrade.
不同于生物可降解聚合物或生物聚合物,本发明包括网状弹性基质的可植入装置可以在体内长期维持其物理特性和性能。它无法启动不需要的组织反应,正如对生物可降解植入物在它们分解和降解时观察的。本发明网状弹性基质的高空隙量和网状化程度使得组织向内生长和细胞在基质内增殖。不希望受到任何理论约束,认为网状弹性基质的高空隙量和网状化程度不仅使得组织向内生长和基质内细胞增殖,而且能够在最初组织生长入可植入装置后愈合的组织定向和改造。随时间的推移,网状弹性基质和/或可植入装置提供了所修复或替代的原始组织的功能性,诸如具有载荷能力。不希望受到任何理论约束,认为由于网状弹性基质或包括它的可植入装置的高空隙量,所以一旦组织愈合和生物整合发生,则大部分再生或修复的部位由新组织和小体积部分的网状弹性基质或由其构成的可植入装置组成。Unlike biodegradable polymers or biopolymers, implantable devices of the present invention comprising reticulated elastic matrices can maintain their physical properties and performance in vivo for long periods of time. It fails to initiate unwanted tissue responses, as has been observed with biodegradable implants as they break down and degrade. The high void volume and degree of reticulation of the reticulated elastic matrix of the present invention allow tissue ingrowth and cell proliferation within the matrix. Without wishing to be bound by any theory, it is believed that the high void volume and degree of reticulation of the reticulated elastic matrix not only allow tissue ingrowth and cell proliferation within the matrix, but also enable tissue orientation and healing after initial tissue growth into the implantable device. remodel. Over time, the reticulated elastic matrix and/or implantable device provides functionality, such as load-bearing capacity, to the repaired or replaced original tissue. Without wishing to be bound by any theory, it is believed that due to the high void volume of the reticulated elastic matrix or implantable devices incorporating it, once tissue healing and biointegration have occurred, most regenerated or repaired sites consist of new tissue and small volume fractions The reticulated elastic matrix or the implantable device composed of it.
此外,改造可植入装置的压缩变定,回弹性和/或动态压缩恢复的能力以在反复周期性加载后提供网状弹性基质的高恢复力。这类特征在应用中特别有利,例如在矫形外科应用中,其中可植入装置的周期性加载还可以持久地压缩网状弹性基质,由此防止它与促进细胞最佳浸润和组织向内生长所必需的周围软组织发生明显的连续接触。在另一个非限制性实例中,改造本发明可植入装置的密度和孔径以便使网状弹性基质在压缩下达到最大渗透性。如果高载量被置于可植入装置上,则这类特征为有利的。在另一个非限制性实例中,改造网状弹性基质的特性以便使其“软共形拟合”最大化,这在美容外科手术应用中特别有利。In addition, the compression set, resilience and/or dynamic compression recovery capabilities of implantable devices are engineered to provide high resilience of the reticulated elastic matrix after repeated cyclic loading. Such features are particularly advantageous in applications, such as orthopedic applications, where cyclic loading of the implantable device can also durably compress the reticulated elastic matrix, thereby preventing it from interacting with promoting optimal cell infiltration and tissue ingrowth Significant continuous contact with surrounding soft tissues is necessary. In another non-limiting example, the density and pore size of implantable devices of the present invention are engineered so as to achieve maximum permeability of the reticulated elastic matrix under compression. Such features are advantageous if high loads are to be placed on the implantable device. In another non-limiting example, engineering the properties of the reticulated elastic matrix to maximize its "soft conformal fit" is particularly advantageous in cosmetic surgical applications.
Bell等的美国专利US5,891,558,Vyakarnam等的US6,306,424,Plouhar等的US6,638,312和Melican等的US6,599,323和Brown等的美国专利申请号US 2002/0131989,US2003/0147935和Binette等的US 2004/0078077和Chun等的US2004/0175408各自描述了复合植入物或支架。U.S. Patent No. 5,891,558 of Bell et al., U.S. Pat. 2004/0078077 and US2004/0175408 to Chun et al. each describe composite implants or scaffolds.
A.E.S.Clarke等在参考文献proceedings of Blowing Agentsand Foaming Processes 2006,2006年5月16-17日(Munich,Germany)17页的论文“Innovative Manufacture of Olefin Foams”,中描述了通过常规加热以使材料表面膨胀并且通过微波加热使内部膨胀来制备烯烃泡沫。A.E.S.Clarke et al. in the paper "Innovative Manufacture of Olefin Foams" on page 17 of the reference proceedings of Blowing Agents and Foaming Processes 2006, May 16-17, 2006 (Munich, Germany), describe the expansion of the surface of the material by conventional heating. And the olefin foam was prepared by expanding the interior by microwave heating.
上述背景技术的描述可以包括披露内容的洞察力,发现,理解或公开内容或彼此的相关性,我们并不了解本发明之前的,但由本发明提供的相关领域。在本文中特别指出了某些这类本发明的贡献,而本发明的其它这类贡献从上下文中显而易见。仅因为本文引述了对比文件,所以并非允许构成对比文件的领域,它与本发明的领域非常不同,与本发明的领域类似。本申请背景技术部分中引述的参考文献并非允许该参考文献为针对本申请的现有技术。The above description of the background art may include disclosures of insights, discoveries, understandings or disclosures or their relevance to each other that we did not know prior to the present invention, but which are provided by the present invention. Certain such inventive contributions are specifically pointed out herein, while other such inventive contributions will be apparent from the context. Just because a reference is cited herein does not allow for reference to a field that is very different from, and similar to, the field of the present invention. Citation of a reference in the Background section of this application is not an admission that the reference is prior art to the present application.
发明概述Summary of the invention
本发明的可植入装置作为长期TE植入物用于许多应用,尤其是其中在诸如软组织相关矫形外科应用的修复和再生中经历动态加载和/或伸展。The implantable devices of the present invention are useful as long-term TE implants in many applications, especially where dynamic loading and/or stretching is experienced such as in repair and regeneration in soft tissue-related orthopedic applications.
本发明涉及包括含多个孔的网状回弹-可压缩弹性基质的可植入装置,其中该可植入装置进一步包括以至少1-维方式的加强。该可植入装置可以在被加强前或之后退火。该可植入装置可以在被加强前或之后被压缩模制。The present invention relates to an implantable device comprising a reticulated resilient-compressible elastic matrix comprising a plurality of pores, wherein the implantable device further comprises reinforcement in at least a 1-dimensional manner. The implantable device can be annealed before or after being strengthened. The implantable device can be compression molded before or after being reinforced.
本发明还涉及包括含多个孔的网状回弹-可压缩弹性基质的可植入装置,其中该可植入装置在其被网状化后被压缩模制。该可植入装置可以在被压缩模制前或之后退火。该可植入装置可以在被压缩模制前或之后被加强。The present invention also relates to an implantable device comprising a reticulated resilient-compressible elastic matrix comprising a plurality of pores, wherein the implantable device is compression molded after it has been reticulated. The implantable device can be annealed before or after being compression molded. The implantable device can be reinforced before or after being compression molded.
本发明还涉及包括含多个孔的网状回弹-可压缩弹性基质的可植入装置,其中该可植入装置在其网状化后退火。该可植入装置可以在退火前或之后被加强。该可植入装置可以在退火前或之后被压缩模制。The present invention also relates to an implantable device comprising a reticulated resilient-compressible elastic matrix comprising a plurality of pores, wherein the implantable device is annealed after its reticulation. The implantable device can be strengthened before or after annealing. The implantable device can be compression molded before or after annealing.
本发明还涉及制备弹性基质的聚合方法,该方法具有混合如下成分的步骤:The invention also relates to a polymerization process for the preparation of an elastic matrix, the process having the step of mixing the following ingredients:
a)100重量份的多元醇成分,a) 100 parts by weight of the polyol component,
b)约10-约90重量份的异氰酸酯成分,b) from about 10 to about 90 parts by weight of an isocyanate component,
c)约0.5-约6.0重量份的发泡剂,c) about 0.5 to about 6.0 parts by weight of blowing agent,
d)任选约0.05-约8.0重量份的交联剂,d) optionally from about 0.05 to about 8.0 parts by weight of a crosslinking agent,
e)任选约0.05-约8.0重量份的增链剂,e) optionally from about 0.05 to about 8.0 parts by weight of a chain extender,
f)任选约0.05-约3.0重量份的至少一种催化剂,f) optionally from about 0.05 to about 3.0 parts by weight of at least one catalyst,
g)任选约0.1-约8.0重量份的至少一种隔室开放剂,g) optionally from about 0.1 to about 8.0 parts by weight of at least one compartment opener,
h)约0.1-约8.0重量份的表面活性剂,和h) from about 0.1 to about 8.0 parts by weight of a surfactant, and
i)任选约达15重量份的粘度改进剂;从而得到弹性基质。i) optionally up to about 15 parts by weight of a viscosity modifier; thereby obtaining an elastic matrix.
本发明还涉及制备至少部分网状弹性基质的方法,该方法具有如下步骤:The invention also relates to a method for preparing an at least partially reticulated elastic matrix, the method having the steps of:
1)将如下成分混合成混合物:1) Combine the following ingredients into a mixture:
a)100重量份的弹性材料,a) 100 parts by weight of elastic material,
b)任选约2-约70重量份的更具亲水性聚合物材料,b) optionally from about 2 to about 70 parts by weight of a more hydrophilic polymeric material,
c)任选约0.1-约20重量份的交联基,和c) optionally from about 0.1 to about 20 parts by weight of a crosslinking group, and
d)任选约1-约20重量份的发泡剂;d) optionally about 1 to about 20 parts by weight of a blowing agent;
2)使该混合物接触约2.2GHz-约6.0GHz频率下的微波照射,还同时将该混合物加热至约70℃-约225℃的温度;2) exposing the mixture to microwave irradiation at a frequency of from about 2.2 GHz to about 6.0 GHz while also simultaneously heating the mixture to a temperature of from about 70°C to about 225°C;
从而得到至少部分网状的弹性基质。An at least partially reticulated elastic matrix is thus obtained.
本发明还涉及含网状弹性基质的可植入装置,其中配制该网状弹性基质以使细胞向内生长并且增殖入退火的网状弹性基质。The present invention also relates to implantable devices comprising a reticulated elastic matrix, wherein the reticulated elastic matrix is formulated for cell ingrowth and proliferation into the annealed reticulated elastic matrix.
本发明还涉及治疗组织缺陷的方法,该方法具有如下步骤:The invention also relates to a method of treating a tissue defect, the method comprising the steps of:
a)任选使本发明的可植入装置从松弛结构压缩成第一压紧结构;a) optionally compressing the implantable device of the present invention from a relaxed configuration to a first compressed configuration;
b)通过递送装置将压缩的可植入装置递送至体内缺陷部位;并且b) delivering the compressed implantable device to the defect site in the body via the delivery device; and
c)任选使所述的可植入装置在所述体内部位上膨胀成第二工作结构。c) optionally expanding said implantable device at said in vivo site into a second operative configuration.
本发明还涉及治疗组织缺陷的方法,该方法具有通过开放式手术操作插入本发明的可植入装置的步骤。The invention also relates to a method of treating a tissue defect having the step of inserting the implantable device of the invention by an open surgical procedure.
组织缺陷可以涉及矫形外科应用,普通外科手术应用,美容外科手术应用,组织改造应用或其任意的混合物。所述的矫形外科应用可以涉及修复,重建,再生,加强,缺口插入或其任意的混合物的腱,韧带,软骨,关节盘,脊柱盘或其任意的混合物。所述的普通外科手术应用可以涉及腹股沟疝,腹侧疝,股部疝,脐疝或其任意的混合物。Tissue defects may relate to orthopedic applications, general surgical applications, cosmetic surgical applications, tissue modification applications, or any mixture thereof. The orthopedic application may involve repair, reconstruction, regeneration, reinforcement, gap insertion or any mixture thereof of tendon, ligament, cartilage, articular disc, spinal disc or any mixture thereof. Said general surgical application may involve inguinal hernias, ventral hernias, femoral hernias, umbilical hernias or any mixture thereof.
本发明还涉及本文所述任意方法制备的至少部分网状弹性基质产品。The present invention also relates to at least partially reticulated elastic matrix products produced by any of the methods described herein.
附图简述Brief description of the drawings
在下文中详细描述了本发明的某些实施方案和本发明的制备和使用实施方案,与上述描述一起并且依据它们阅读该描述,作为实例,参照附图,其中在贯穿于的视图中类似涉及的字符表示相同或相似的要素,并且其中:Certain embodiments of the invention and embodiments of making and using the invention are described in detail hereinafter, and the above description read together with and in light of them, by way of example, refers to the accompanying drawings, in which similarly referred to throughout the drawings characters denote the same or similar elements, and where:
图1为表示本发明多孔生物耐久性弹性产品的一种实施方案的部分微观结构的一种可能形态的示意图;1 is a schematic diagram showing a possible form of a partial microstructure of an embodiment of the porous biodurable elastic product of the present invention;
图2为制备本发明多孔生物耐久性弹性可植入装置的示意程序框图;Fig. 2 is a schematic procedure block diagram for preparing the porous biodurable elastic implantable device of the present invention;
图3例证了圆柱形预制形式的典型压缩模制过程;Figure 3 illustrates a typical compression molding process for a cylindrical prefabricated form;
图4例证了立方体预制形式的典型压缩模制过程;Figure 4 illustrates a typical compression molding process for a cube prefabricated form;
图5例证了几种不同的典型网状弹性基质加强网格;Figure 5 illustrates several different typical reticulated elastic matrix reinforcement grids;
图6例证了几种不同的典型网状弹性基质加强网格;Figure 6 illustrates several different typical reticulated elastic matrix reinforcement grids;
图7例证了缝线拉出强度试验的几何图;Figure 7 illustrates the geometry for a suture pullout strength test;
图8例证了易于使用本发明可植入装置进行最低侵害和其它再建应用的颜面美容手术的区域;Figure 8 illustrates areas amenable to cosmetic facial procedures for minimally invasive and other reconstructive applications using the implantable device of the present invention;
图9例证了使加强的可植入装置与结节锚固的两种方法;Figure 9 illustrates two methods of anchoring a reinforced implantable device to a nodule;
图10为实施例5网状弹性基质1的扫描电子显微照片影像;Fig. 10 is the scanning electron micrograph image of embodiment 5 reticular
图11为几种网状弹性基质的达西渗透性与可利用的流通区域之间关系的示意图;Fig. 11 is a schematic diagram of the relationship between the Darcy permeability and the available flow area of several reticular elastic matrices;
图12为实施例7网状弹性基质3的扫描电子显微照片影像;Fig. 12 is the scanning electron micrograph image of embodiment 7 reticular elastic matrix 3;
图13表示实施例14的矩形可植入装置的模式;Figure 13 shows a model of the rectangular implantable device of Example 14;
图14表示实施例14的矩形可植入装置模式特征的维量;且Figure 14 represents the dimensions of the pattern features of the rectangular implantable device of Example 14; and
图15表示实施例15的装置的组织学分析照片。FIG. 15 shows photographs of histological analysis of the device of Example 15. FIG.
发明详述Detailed description of the invention
本发明的某些实施方案包括网状生物耐久性弹性体产品,它们也是可压缩的并且在其恢复中表现出回弹性,具有多种应用并且作为实例可以用于生物植入,尤其是植入人体的长期TE植入物,尤其是其中诸如在软组织相关矫形外科应用中经历动态加载和/或伸展;用于组织加强,支持和修复;用于治疗应用;用于美容,再建,泌尿外科或胃食管目的;或作为药物活性剂,例如药物递送基质。其它实施方案包括通过导管,内窥镜,关节镜,腹腔镜,膀胱镜,注射器或其它合适的递送装置进行体内递送的网状生物耐久性弹性体产品,并且可以令人满意地植入,否则就是暴露于活组织和流体延长的是时间期限,例如至少29天。Certain embodiments of the present invention include reticulated biodurable elastomeric products, which are also compressible and exhibit resilience in their recovery, having a variety of applications and as examples may be used in biological implants, especially Long-term TE implants in the human body, especially where dynamic loading and/or stretching is experienced such as in soft tissue related orthopedic applications; for tissue reinforcement, support and repair; for therapeutic applications; for cosmetic, reconstructive, urological or Gastroesophageal purposes; or as a pharmaceutically active agent, such as a drug delivery matrix. Other embodiments include reticulated biodurable elastomeric products that are delivered in vivo by catheter, endoscope, arthroscope, laparoscope, cystoscope, syringe or other suitable delivery device and can be satisfactorily implanted otherwise That is, the exposure to living tissue and fluids is for an extended period of time, eg, at least 29 days.
正如本发明公认的,在医学领域需要无害性的可植入装置,可以将其递送至患者体内部位,例如人体患者体内部位,它可以占据该部位延长的时间期限而不会对宿主有害。在一个实施方案中,这类可植入装置最终还可以变成整合的,诸如生物整合的,例如与组织一起向内生长或生物整合。已经提出各种生物可降解或吸收性多孔聚合物材料用于组织加强和修复。As recognized herein, there is a need in the medical field for non-hazardous implantable devices that can be delivered to a site within a patient's body, such as a human patient, and which can occupy the site for an extended period of time without being harmful to the host. In one embodiment, such implantable devices may also eventually become integrated, such as biointegrated, eg, ingrowth or biointegrated with tissue. Various biodegradable or absorbable porous polymer materials have been proposed for tissue reinforcement and repair.
理想的是形成适合于用作组织改造支架或其它相差无几的基质的可植入装置,以便支持体内细胞增殖应用,例如尤其是在假体器官的软组织连接,再生,加强,支持和向内生长中的大量矫形外科应用中。不受任何特定理论约束,认为具有高空隙量高度网状化可使可植入装置变成与细胞,包括组织一起至少部分向内生长和/或增殖,在某些情况中基本上向内生长和增殖,在某些情况中完全向内生长和增殖,所述的细胞,包括组织诸如成纤维细胞,纤维组织,滑膜细胞,骨髓基质细胞,干细胞和/或纤维软骨细胞。向内生长和/或增殖的组织由此提供了被修复或替代原始组织的缺陷修复的功能性,诸如具有载荷能力。然而,在本发明出现前,尚未得到满足这类可植入装置要求的材料和/或产品。It would be desirable to form implantable devices suitable for use as scaffolds for tissue modification or other comparable matrices to support in vivo cell proliferation applications such as soft tissue attachment, regeneration, reinforcement, support and ingrowth especially in prosthetic organs in a large number of orthopedic applications. Without being bound by any particular theory, it is believed that a high degree of reticulation with a high void volume can cause the implantable device to become at least partially ingrowth and/or proliferate, and in some cases substantially ingrowth, with cells, including tissue and proliferate, and in some cases fully ingrowth and proliferate, said cells, including tissues such as fibroblasts, fibrous tissue, synoviocytes, bone marrow stromal cells, stem cells and/or fibrochondrocytes. The ingrowth and/or proliferating tissue thus provides defective repair functionality, such as load bearing capacity, of the repaired or substituted original tissue. However, prior to the present invention, materials and/or products meeting the requirements for such implantable devices were not available.
从广义上说,本发明某些网状生物耐久性弹性产品的实施方案包括由生物耐久性聚合物弹性体构成的高度渗透性网状基质或如果不是完全,那么就是主要由其构成,所述的生物耐久性聚合物弹性体为回弹-可压缩的,以便在递送至生物部位后恢复其形状。在一个实施方案中,弹性基质具有与动态加载相关的良好疲劳强度。在另一个实施方案中,弹性基质在化学上得到充分表征。在另一个实施方案中,弹性基质在物理上得到充分表征。在另一个实施方案中,弹性基质在化学和物理上均得到充分表征。Broadly speaking, embodiments of certain reticulated biodurable elastic products of the present invention include a highly permeable reticulated matrix composed of, or consisting essentially of, if not entirely, a biodurable polymeric elastomer, said The biodurable polymer elastomer is resilient-compressible to regain its shape after delivery to the biological site. In one embodiment, the elastic matrix has good fatigue strength associated with dynamic loading. In another embodiment, the elastic matrix is chemically well characterized. In another embodiment, the elastic matrix is physically well characterized. In another embodiment, the elastic matrix is well characterized both chemically and physically.
本发明的某些实施方案可以支持细胞生长并且允许细胞在体内向内生长和增殖和用作体内生物可植入装置,例如用于可以在体外或体内使用以便提供细胞增殖基质的组织改造支架。Certain embodiments of the invention can support cell growth and allow cell ingrowth and proliferation in vivo and use as bioimplantable devices in vivo, for example for tissue engineered scaffolds that can be used in vitro or in vivo to provide a matrix for cell proliferation.
本发明的可植入装置作为长期组织改造植入物用于许多应用,尤其是其中在诸如用于修复和再生的软组织相关矫形外科应用中经历动态加载和/或伸展。在某些实施方案中,本发明的网状弹性基质如2004年5月17日提交的美国专利申请US10/848,624(作为美国专利申请公开号US 2005-0043816-A1在2005年2月24日公布)中所述,为所有目的而将该文献完整地引入本文作为参考。The implantable devices of the present invention are useful in many applications as long-term tissue-modifying implants, especially where dynamic loading and/or stretching is experienced in soft tissue-related orthopedic applications such as for repair and regeneration. In certain embodiments, the reticulated elastic matrix of the present invention is described in U.S. Patent Application No. 10/848,624 filed on May 17, 2004 (published on February 24, 2005 as U.S. Patent Application Publication No. US 2005-0043816-A1 ), which is hereby incorporated by reference in its entirety for all purposes.
在一个实施方案中,本发明的网状弹性基质通过提供细胞粘着,迁移,增殖和/或涂敷(例如胶原蛋白)沉积的表面有利于组织向内生长。在另一个实施方案中,任何类型的组织均可以生长入包括本发明网状弹性基质的可植入装置,作为实例包括上皮组织(包括,例如鳞状(squamous),立方和柱状上皮组织),结缔组织(包括,例如疏松(areolar)组织,致密规则和不规则组织,网状组织,脂肪组织,软骨和骨)和肌肉组织(包括,例如骨骼肌,平滑肌和心肌)或其任何的组合,例如纤维血管组织。在本发明的另一个实施方案中,包括本发明网状弹性基质的可植入装置可以具有基本上贯穿于其互联孔体积的组织向内生长。In one embodiment, the reticulated elastic matrix of the present invention facilitates tissue ingrowth by providing a surface for cell adhesion, migration, proliferation and/or coating (eg, collagen) deposition. In another embodiment, any type of tissue can be grown into an implantable device comprising the reticulated elastic matrix of the present invention, including, by way of example, epithelial tissue (including, for example, squamous, cuboidal, and columnar epithelial tissue), Connective tissue (including, for example, areolar tissue, dense regular and irregular tissue, reticular tissue, adipose tissue, cartilage and bone) and muscle tissue (including, for example, skeletal muscle, smooth muscle and cardiac muscle) or any combination thereof, such as fibrovascular tissue. In another embodiment of the present invention, an implantable device comprising a reticulated elastic matrix of the present invention may have tissue ingrowth substantially throughout its interconnected pore volume.
在一个实施方案中,本发明包括具有足够回弹-可压缩性以便通过“递送装置”,即具有包含弹性可植入装置的室的装置递送的可植入装置,同时例如使用导管,内窥镜,关节镜,腹腔镜,膀胱镜或注射器将其递送至所需部位,然后在该部位上释放。在另一个实施方案中,由此递送的弹性可植入装置在递送至生物部位后基本上恢复其形状并且具有足够的生物耐久性和生物相容性特征以适合于长期植入。在另一个实施方案中,由此递送的弹性可植入装置可以跨越缺陷并且用作天然组织中缺口的桥梁。In one embodiment, the present invention includes implantable devices that are sufficiently resilient-compressible to be delivered by a "delivery device", that is, a device having a chamber containing an elastic implantable device, while using, for example, a catheter, endoscopic A scope, arthroscope, laparoscope, cystoscope, or syringe delivers it to the desired site, where it is then released. In another embodiment, the elastic implantable device thus delivered substantially recovers its shape after delivery to the biological site and has sufficient biodurability and biocompatibility characteristics to be suitable for long-term implantation. In another embodiment, the elastic implantable device thus delivered can span defects and serve as a bridge for gaps in native tissue.
可以通过改变材料和/或加工条件在广泛性能范围内改造或定制本发明弹性基质的结构,形态和特性以便不同的功能或治疗应用。The structure, morphology and properties of the elastic matrices of the present invention can be engineered or tailored over a wide range of properties by varying materials and/or processing conditions for different functional or therapeutic applications.
不受任何特定理论约束,认为本发明的目的在于提供轻型耐久性结构,它可以填充生物体积或腔并且包含足够的分布在整体体积内的多孔性,可以通过允许如下情况中的一种或多种满足这一目的:封闭,栓塞,细胞向内生长,细胞增殖,组织再生,细胞粘着,药物递送,由固定酶产生的酶作用和如本文所说的其它有用过程,特别包括请求优先权的应用。Without being bound by any particular theory, it is believed that it is an object of the present invention to provide lightweight durable structures that can fill a biological volume or cavity and contain sufficient porosity distributed throughout the volume by allowing one or more of For this purpose: occlusion, embolization, cell ingrowth, cell proliferation, tissue regeneration, cell adhesion, drug delivery, enzymatic action by immobilized enzymes and other useful processes as described herein, particularly including those for which priority is claimed application.
在一个实施方案中,本发明的弹性基质具有足够的回弹性以便能够基本上恢复,例如为植入人体而压缩后以至少1-维方式恢复至松弛结构大小的至少约50%,例如低压缩变定,例如在25℃或37℃下和针对用于药物活性剂,诸如药物和其它医疗应用控释的足够的强度和流通量。在另一个实施方案中,本发明的弹性基质具有足够的回弹性以便能够为植入人体而压缩后以至少1-维方式恢复至松弛结构大小的至少约60%。在另一个实施方案中,本发明的弹性基质具有足够的回弹性以便能够为植入人体而压缩后以至少1-维方式恢复至松弛结构大小的至少约90%。In one embodiment, the elastic matrices of the present invention are sufficiently resilient to substantially recover, e.g., to at least about 50% of the size of the relaxed structure in at least a 1-dimensional manner after compression for implantation in the human body, e.g., low compression Set, for example at 25°C or 37°C and for sufficient strength and throughput for controlled release of pharmaceutically active agents such as drugs and other medical applications. In another embodiment, the elastic matrix of the present invention is sufficiently resilient to recover to at least about 60% of the relaxed structural size in at least a 1-dimensional manner after compression for implantation in a human body. In another embodiment, the elastic matrix of the present invention is sufficiently resilient to recover to at least about 90% of the relaxed structural size in at least a 1-dimensional manner after compression for implantation in a human body.
在本申请中,术语“生物耐久性”描述了适合于在生物环境中延长时间期限稳定的弹性体和其它产品。这类产品在接触与使用该可植入装置相当的期限的生物环境时相对于其工作状态而言不应表现出分解或降解,侵蚀或机械性能的明显恶化。植入期限可以为:几周,几个月或几年;宿主产品的寿命,其中掺入了本发明弹性产品,诸如移植物或假体;或对弹性产品而言为患者的寿命。在一个实施方案中,应理解理想的接触期限至少约为29天。在另一个实施方案中,应理解理想的接触期限至少为29天。在一个实施方案中,所述的可植入装置在至少2个月为生物耐久性的。在另一个实施方案中,所述的可植入装置在至少6个月为生物耐久性的。在另一个实施方案中,所述的可植入装置在至少12个月为生物耐久性的。在另一个实施方案中,所述的可植入装置在至少12个月以上为生物耐久性的。在另一个实施方案中,所述的可植入装置在至少24个月为生物耐久性的。在另一个实施方案中,所述的可植入装置在至少5年为生物耐久性的。在另一个实施方案中,所述的可植入装置在至少5年以上为生物耐久性的。In this application, the term "biodurable" describes elastomers and other products suitable for being stable for extended periods of time in a biological environment. Such products shall not exhibit decomposition or degradation, corrosion or appreciable deterioration of their mechanical properties relative to their working condition when exposed to the biological environment for a period commensurate with the use of the implantable device. The period of implantation can be: weeks, months or years; the life of a host product into which an elastic product of the invention is incorporated, such as a graft or prosthesis; or in the case of elastic products, the life of the patient. In one embodiment, it is understood that the ideal period of contact is at least about 29 days. In another embodiment, it is understood that the ideal period of contact is at least 29 days. In one embodiment, the implantable device is biodurable for at least 2 months. In another embodiment, the implantable device is biodurable for at least 6 months. In another embodiment, the implantable device is biodurable for at least 12 months. In another embodiment, the implantable device is biodurable for at least 12 months. In another embodiment, the implantable device is biodurable for at least 24 months. In another embodiment, said implantable device is biodurable for at least 5 years. In another embodiment, the implantable device is biodurable for at least 5 years.
在一个实施方案中,本发明的生物耐久性产品也是生物相容性的。在本申请中,术语“生物相容性”意指在植入宿主患者时几乎不会诱导(如果有的话也很少)任何不良生物反应的产品。适合于“生物耐久性”的类似考虑还应用于“生物相容性”特性。In one embodiment, the biodurable product of the invention is also biocompatible. In this application, the term "biocompatible" means a product that induces few, if any, adverse biological reactions when implanted in a host patient. Similar considerations that apply to "biodurability" also apply to the property "biocompatibility".
可以将预计的生物环境理解为在体内,例如所述产品植入患者宿主的体内或局部施用的体内,例如哺乳动物宿主,诸如人或其它灵长类,宠物或运动动物,家畜类或食用动物或实验室动物。所有这类应用均被关注为本发明的范围。本文所用的“患者”为动物。在一个实施方案中,所述的动物为鸟,包括,但不限于小鸡,火鸡,鸭子,鹅或鹌鹑或哺乳动物。在另一个实施方案中,所述的动物为哺乳动物,包括,但不限于牛,马,绵羊,山羊,猪,猫,狗,小鼠,大鼠,仓鼠,家兔,豚鼠,猴子和人。在另一个实施方案中,所述的动物为灵长类或人。在另一个实施方案中,所述的动物为人。The intended biological environment may be understood as in vivo, e.g. the product is implanted in a patient host or administered topically, e.g. a mammalian host, such as a human or other primate, pet or sporting animal, livestock or food animal or laboratory animals. All such applications are contemplated as being within the scope of the present invention. A "patient" as used herein is an animal. In one embodiment, the animal is a bird, including, but not limited to, chickens, turkeys, ducks, geese or quails or mammals. In another embodiment, the animal is a mammal, including, but not limited to, cows, horses, sheep, goats, pigs, cats, dogs, mice, rats, hamsters, rabbits, guinea pigs, monkeys and humans . In another embodiment, the animal is a primate or a human. In another embodiment, said animal is a human.
在一个实施方案中,用于本发明多孔弹性体的结构材料为合成聚合物,尤其是,但不限于耐生物降解的弹性聚合物,例如,在一个实施方案中,聚碳酸酯聚氨基甲酸酯类,聚碳酸酯脲-氨基甲酸酯类,聚醚聚氨基甲酸酯类,聚(碳酸酯共-醚)脲-氨基甲酸酯类,聚硅氧烷类等,在另一个实施方案中,聚碳酸酯聚氨基甲酸酯类,聚碳酸酯脲-氨基甲酸酯类,聚(碳酸酯-共-醚)脲-氨基甲酸酯类和聚硅氧烷类,在另一个实施方案中,聚碳酸酯聚氨基甲酸酯类,聚碳酸酯脲-氨基甲酸酯类和聚硅氧烷类。这类弹性体一般为疏水性的,但根据本发明,可以将它们处理成具有低疏水性或一定亲水性的表面。在另一个实施方案中,可以生产具有低疏水性或一定亲水性的表面的这类弹性体。In one embodiment, the structural material used in the cellular elastomer of the present invention is a synthetic polymer, especially, but not limited to, an elastic polymer resistant to biodegradation, such as, in one embodiment, polycarbonate polyurethane polycarbonate urea-urethanes, polyether polyurethanes, poly(carbonate co-ether) urea-urethanes, polysiloxanes, etc., in another embodiment, poly Carbonate polyurethanes, polycarbonate urea-urethanes, poly(carbonate-co-ether) urea-urethanes and polysiloxanes, in another embodiment, polycarbonate Polyurethanes, polycarbonate urea-urethanes and polysiloxanes. Such elastomers are generally hydrophobic, but according to the invention they can be treated to have a surface that is less hydrophobic or somewhat hydrophilic. In another embodiment, such elastomers can be produced with surfaces of low hydrophobicity or somewhat hydrophilic.
可以将本发明的网状生物耐久性弹性产品描述为具有“宏观结构”和“微观结构”,本文所用的该术语的一般含义描述在下面的段落中。The reticulated biodurable elastic products of the present invention may be described as having a "macrostructure" and a "microstructure," the general meanings of which terms are used herein are described in the following paragraphs.
“宏观结构”意指由本发明生物耐久性弹性产品构成的制品或物品的总体物理特性,诸如:由制品或物品的几何边界描述的外周长,忽略孔或空隙;“宏观结构表面积”指的是最外面的表面积,尽管其上任何孔均被填充,但忽略了孔内的表面积;所述制品或物品占据的“宏观结构体积”或简称“体积”为宏观结构或简称“大”表面积限定的体积;且“堆密度”为每单位体积制品或物品自身中的重量,它不同于结构材料的密度。"Macrostructural" means the overall physical characteristics of an article or article comprised of the biodurable elastic product of the present invention, such as: the outer perimeter described by the geometric boundaries of the article or article, ignoring pores or voids; "macrostructural surface area" refers to The outermost surface area, notwithstanding that any pores thereon are filled, ignoring the surface area within the pores; the "macrostructural volume" or simply "volume" occupied by the article or article in question is defined by the macrostructural or simply "large" surface area volume; and "bulk density" is the weight per unit volume of the article or article itself, which is distinct from the density of the material of construction.
“微观结构”意指构成本发明产品的生物耐久性弹性材料的内部结构特征,诸如孔的尺寸;孔表面积,其为孔中材料表面的总面积;和构成本发明弹性产品某些实施方案的实体结构的小连接体和交点构造。"Microstructure" means the internal structural characteristics of the biodurable elastic materials comprising the products of the present invention, such as pore size; pore surface area, which is the total area of material surfaces in the pores; and Small connectors and intersections of solid structures.
就图1而言,便利显示的是网状泡沫的具体形态的示意图。图1为例证本发明某些实施方案微观结构特征和原理中的某些的便利方式。该图并不指定为本发明弹性产品实施方案的理想描述,也不指定为本发明弹性产品具体实施方案的详细描述。微观结构的其它特征和原理从本说明书中显而易见,或从制备本文所说多孔弹性产品的本发明方法中的一种或多种中显而易见。With respect to Figure 1, conveniently shown is a schematic diagram of a specific morphology of reticulated foam. Figure 1 is a convenient way of illustrating some of the microstructural features and principles of certain embodiments of the invention. This figure is not intended to be an idealized depiction of an embodiment of the elastic product of the present invention, nor is it intended to be a detailed description of a specific embodiment of the elastic product of the present invention. Other features and principles of the microstructure will be apparent from this description, or from one or more of the methods of the invention for making the porous elastic products described herein.
形态form
一般描述了例证的多孔生物耐久性弹性基质10的微观结构,它特别可以为具有不同形状或伸展的连续或无定形本体的单个元件,包括由合适的生物耐久性弹性材料构成并且在其中分散或由此确定的网状实体相12,连续互联空隙相14,后者为网状结构的主要特征。The microstructure of an exemplary porous biodurable
在一个实施方案中,构成弹性基质10的弹性材料可以为多种材料的混合物或掺合物。在另一个实施方案中,弹性材料为单一合成聚合物弹性体,诸如在下文中更具体地描述。在其它实施方案中,尽管弹性基质10进行后网状化加工,诸如退火,压缩模制和/或加强,但是应理解弹性基质10保持了其确定的特征,它保持了生物耐久性,网状化和弹性。In one embodiment, the elastic material comprising
空隙相14在使用前通常为充空气或充气的。在应用中,空隙相14在许多情况,但并非所有情况中填充了液体,例如填充了生物流体或体液。The
如图1中所示的弹性基质10的实体相12具有有机结构并且包括多个相对薄的小连接体16,它们在多个交叉截面18之间延伸并且互联。交叉截面18为主要的结构位置,在此处三个或三个以上小连接体16彼此相遇。可以观察到四个或五个以上小连接体16在交叉截面18相遇或在可以观察到两个交叉截面18彼此合并的位置上相遇。在一个实施方案中,小连接体16在纸平面上下的交叉截面18之间以3-维方式伸展,不偏向特定的平面。因此,任何指定的小连接体16可以以相对于连接在交叉截面18上的其它小连接体16的任何方向从该交叉截面18伸展。小连接体16和交叉截面18一般可以具有弯曲形状并且在它们之间限定多个孔20或实体相12中的间隙。小连接体16和交叉截面18构成互联的连续实体相。The
如图1中所示,弹性基质10的实体相12的结构元件,即小连接体16和交叉截面18可以显示出具有一定的层状结构,就如同从单片中切下的某些,可以理解这种外观可以部分归因于在2-维图中表示复杂的3-维结构的困难性。小连接体16和交叉截面18可以具有并且在许多情况中具有非层状形状,包括圆形,椭圆形和非圆形横截面形状和在面积上沿特定结构改变的横截面,例如,它们锥形化成较小和/或较大的横截面,同时沿其最长尺寸穿过。As shown in FIG. 1, the structural elements of the
弹性基质10的隔室由孔20的簇或组构成,它们可以形成隔室壁,但除外大部分孔20中的隔室壁22因网状化而不存在或基本上不存在的情况。特别地,少量孔20可以具有也称作“窗”或“窗玻璃”的结构材料隔室壁,诸如隔室壁22。当达到阻塞流体和/或组织繁殖和增殖通过孔20的程度时,这类隔室壁是不理想的。在一个实施方案中,隔室壁22可以在合适的加工步骤中被除去,诸如如下所述的网状化步骤。The compartments of the
构成网状弹性基质的各隔室的特征在于其平均隔室直径,或就非球形隔室而言,在于其最大横断面尺寸。网状弹性基质包括构成3-维空间结构的隔室或通过其中的开放孔20互联的空隙相14的网状构造。在一个实施方案中,隔室构成3-维超结构。在图10和12中,可以从显示白色截面的小连接体16和/或交叉截面18观察到各隔室的边界。孔20一般为2-或3-维结构。孔提供了各隔室之间或构成隔室的孔簇或组之间的连接性。The individual compartments making up the reticulated elastic matrix are characterized by their mean compartment diameter, or in the case of non-spherical compartments, by their largest cross-sectional dimension. The reticulated elastic matrix comprises a network of compartments or
除宏观结构表面上的边界终点外,在图1所示的实施方案中,弹性基质10的实体相12几乎不包括(如果有的话)从小连接体16或交叉截面18伸展,但不与另一小连接体或交叉截面连接的游离端、盲端或突出的“小连接体-样”结构。In the embodiment shown in FIG. 1 , the
然而,在可选择的实施方案中,实体相12可以配有多个这类原纤维(未显示),例如每个小连接体16或交叉截面18中有约1-约5个原纤维。在某些应用中,这类原纤维可以用于例如它们提供的额外表面积。However, in alternative embodiments,
可以将小连接体16和交叉截面18视为确定了构成空隙相14的孔20的形状和构造(或反之亦然)。就分散确定而言,孔20中的许多因至少部分缺失隔室壁22而开放进入并且与至少两个额外的孔20相通。在交叉截面18上,三个或三个以上孔20可以被视为相遇并且互通。在某些实施方案中,空隙相14为连续或基本上连续地通过弹性基质10,这意味着几乎不存在封闭的隔室孔(如果有的话)。这类封闭的隔室孔各自内部的容积与任何其它隔室不连通,例如通过隔室壁22与相邻隔室隔离,表示缺乏有用的体积并且可能阻塞有用流体进入弹性基质10内部小连接体和交叉截面结构16和18的通道。The
在一个实施方案中,如果存在,那么封闭的隔室孔包括低于约90%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约80%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约70%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约50%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约30%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约25%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约20%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约15%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约10%的弹性基质10体积。在另一个实施方案中,如果存在,那么封闭隔室孔包括低于约5%的弹性基质10体积。另一个实施方案中,如果存在,那么封闭隔室孔包括低于约2%的弹性基质10体积。封闭隔室孔的存在可以根据其在降低通过弹性基质10的流体的体积流率和/或作为细胞向内生长和增殖入弹性基质10减少中的影响注意到。In one embodiment, closed compartment pores, if present, comprise less than about 90% of the
在另一个实施方案中,弹性基质10被网状化。在另一个实施方案中,弹性基质10基本上网状化。在另一个实施方案中,弹性基质10完全网状化。在另一个实施方案中,弹性基质10的许多隔室壁22被除去:在另一个实施方案中,弹性基质10的大部分隔室壁22被除去。在另一个实施方案中,弹性基质10基本上所有隔室壁22被除去。In another embodiment,
在另一个实施方案中,可以描述为网状的实体相12包括实体结构的连续网状构造,诸如小连接体16和交叉截面18,无任何明显的终止、分离的区或不连续性,这与弹性基质的边界不同,在弹性基质的网状构造中,假拟线通过实体相12的材料可从网状构造的一点被完全跟踪至该网状构造的任何另一点。In another embodiment, the
在另一个实施方案中,空隙相14也为间隙的连续网状构造或用于气体或液体的互通流体通道,该流体通道遍在伸展并且由弹性基质10的实体相12的结构界定(或确定)并且开放进入所有其外表面。如上所述,在其它实施方案中,仅存在几个,基本上无或完全无与空隙网状构造中的至少一个另外的孔20不连通的闭合或封闭的隔室孔。此外,在这一空隙相网状构造中,假拟线可以通过空隙相14从网状构造的一点被完全跟踪至该网状构造的任何另一点。In another embodiment, the
与本发明的目的一致,在一个实施方案中,构成弹性基质10的微观结构以便允许或促使细胞20与实体相12表面粘着,其上新内膜形成及细胞和组织向内生长并且增殖入空隙相14的孔20,此时弹性基质10居留在合适的体内位置一定时间期限。Consistent with the objectives of the present invention, in one embodiment, the microstructure of the
在另一个实施方案中,就某些目的而言包括纤维化的这类细胞或组织向内生长和增殖可以出现或被促使仅仅进入孔20的外层,而进入整个弹性基质10的最深内部和遍在弹性基质10。因此,在该实施方案中,弹性基质10占据的空间变成完全被纤维化,瘢痕或其它组织形式的细胞和组织向生长和增殖填充,但除外弹性实体相12占据的空间。在另一个实施方案中,本发明可植入装置起作用,使得向内生长的组织例如因支持性微脉管系统延长存在而保持生命力。In another embodiment, such cellular or tissue ingrowth and proliferation, including fibrosis for some purposes, may occur or be induced only into the outer layer of the
为了达到这一目的,特别是就空隙相14的形态而言,在一个实施方案中,弹性基质10因具有开放式互联孔而网状化。To this end, particularly with regard to the morphology of the voided
不受任何具体理论约束,认为允许弹性基质10内部被用体液,例如血液自然灌洗,甚至在细胞群居留在弹性基质10内部后也是如此,以便通过向其提供营养物维持该群体并且从其中除去废物。在另一个实施方案中,弹性基质10因具有特定大小范围的开放式互联孔而网状化。在另一个实施方案中,弹性基质10因具有大小范围的分布的开放式互联孔而网状化。Without being bound by any particular theory, it is believed that the interior of the
预计选择弹性基质10的各种物理和化学参数,特别是包括如下所述的参数,以便按照预计弹性基质10的特定应用促使细胞向内生长和增殖。The various physical and chemical parameters of
可以理解这类提供内部细胞灌洗的弹性基质10的构造可以为流体可渗透的并且还可以提供通过并且到达基质内部的流体通道,但目的并非细胞灌洗,例如用于洗脱药物活性剂,例如药物或其它生物有用材料。这类材料可以任选固定到弹性基质10的内表面。It is understood that such constructions of
在本发明的另一个实施方案中,气相12可以被填充或接触可递送治疗气体,例如杀菌剂,诸如臭氧或创伤修补剂,诸如一氧化氮,只要宏观结构表面被例如生物吸收性膜密封,以便在植入产品内包含所述气体,直到所述膜侵蚀,从而释放所述气体以便提供局部治疗或其它作用。In another embodiment of the invention, the
本发明的有用实施方案包括:如图1中所示在一定程度上随机化的结构,其中小连接体16,交叉截面18和孔20的形状和大小基本上改变;更有序的结构,它们也表现出实体和空隙相的3-维互相贯通、结构复杂性和高流体渗透性的所述特征。可以通过本发明的方法生产这类更有序的结构,正如下文进一步描述的。Useful embodiments of the invention include: somewhat randomized structures as shown in FIG. The features of 3-dimensional interpenetration of solid and void phases, structural complexity and high fluid permeability are also exhibited. Such more ordered structures can be produced by the methods of the present invention, as further described below.
多孔性porosity
网状化后,空隙相14可以包括低至10%体积的弹性基质10,这意指在诸如对网状弹性基质施用任何任选内部孔表面涂层或分层前弹性基质10间隙提供的体积,在网状化后,所述的网状弹性基质如本文详细描述的被压缩模制和/或加强。在另一个实施方案中,空隙相14可以包括低至20%体积的弹性基质10。在另一个实施方案中,空隙相14可以包括低至35%体积的弹性基质10。在另一个实施方案中,空隙相14可以包括低至50%体积的弹性基质10。在一个实施方案中,正如所确定的,空隙相14的体积约为10%-约99%体积的弹性基质10。在另一个实施方案中,正如所确定的,空隙相14的体积约为20%-约99%体积的弹性基质10。在另一个实施方案中,正如所确定的,空隙相14的体积约为30%-约97%体积的弹性基质10。在另一个实施方案中,正如所确定的,空隙相14的体积约为50%-约99%体积的弹性基质10。在另一个实施方案中,正如所确定的,空隙相14的体积约为70%-约99%体积的弹性基质10。在另一个实施方案中,空隙相14的体积约为80%-约98%体积的弹性基质10。在另一个实施方案中,空隙相14的体积约为90%-约98%体积的弹性基质10。After reticulation, the
作为本文使用的,在孔为球形或基本上为球形时,其最大的横向尺寸相当于孔的直径。当孔为非球形,例如椭圆形或四面体时,其最大横向尺寸相当于从一个孔表面到另一个孔表面的孔内的最大距离,例如椭圆体孔的长轴长度或四面体孔最长侧长度。本文所用的“平均直径或其它最大横向尺寸”意指球形或基本上球形孔的数均直径或非球形孔的数均最长横向尺寸。As used herein, when a pore is spherical or substantially spherical, its largest transverse dimension corresponds to the diameter of the pore. When the pore is non-spherical, such as ellipsoidal or tetrahedral, its maximum lateral dimension corresponds to the maximum distance inside the pore from one pore surface to the other, such as the length of the major axis of an ellipsoidal pore or the longest length of a tetrahedral pore side length. As used herein, "average diameter or other largest transverse dimension" means the number average diameter of spherical or substantially spherical pores or the number average longest transverse dimension of non-spherical pores.
在一个涉及矫形外科应用等的实施方案中,为了促使细胞向内生长和增殖和提供足够的流体渗透性,孔20的平均直径或其它最大横向尺寸至少约为10μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为20μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为50μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为100μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为150μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为250μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸大于约250μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸大于250μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为450μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸大于约450μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸大于450μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸至少约为500μm。In one embodiment involving orthopedic applications and the like, pores 20 have an average diameter or other largest lateral dimension of at least about 10 μm in order to promote cellular ingrowth and proliferation and to provide adequate fluid permeability. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 20 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 50 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 100 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 150 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 250 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension greater than about 250 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension greater than 250 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 450 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension greater than about 450 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension greater than 450 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of at least about 500 μm.
在一个涉及矫形外科应用等的实施方案中,孔20的平均直径或其它最大横向尺寸不大于约600μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸不大于约500μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸不大于约450μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸不大于约350μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸不大于约250μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸不大于约150μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸不大于约20μm。In one embodiment involving orthopedic applications and the like, pores 20 have an average diameter or other largest lateral dimension of no greater than about 600 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of no greater than about 500 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of no greater than about 450 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of no greater than about 350 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of no greater than about 250 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of no greater than about 150 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of no greater than about 20 μm.
在另一个涉及矫形外科应用等的实施方案中,孔20的平均直径或其它最大横向尺寸约为10μm-约50μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为20μm-约150μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为150μm-约250μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为250μm-约500μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为450μm-约600μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为10μm-约500μM。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为20μm-约600μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为50μm-约600μm。In another embodiment involving orthopedic applications and the like, pores 20 have an average diameter or other largest lateral dimension of from about 10 μm to about 50 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 20 μm to about 150 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 150 μm to about 250 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 250 μm to about 500 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of about 450 μm to about 600 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 10 μm to about 500 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 20 μm to about 600 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 50 μm to about 600 μm.
在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为100μm-约500μm。在另一个实施方案中,孔20的平均直径或其它最大横向尺寸约为150μm-约350μm。In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 100 μm to about 500 μm. In another embodiment, pores 20 have an average diameter or other largest lateral dimension of from about 150 μm to about 350 μm.
在一个涉及矫形外科应用等的实施方案中,为了促使细胞向内生长和增殖和提供足够的流体渗透性,弹性基质10的隔室平均直径或其它最长横向尺寸至少约为100μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸至少约为150μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸至少200μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸至少250μm。In one embodiment involving orthopedic applications and the like, to promote cellular ingrowth and proliferation and to provide sufficient fluid permeability, the average diameter or other longest transverse dimension of the compartments of
在另一个涉及矫形外科应用等的实施方案中,弹性基质10的隔室平均直径或其它最长横向尺寸不大于约1000μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸不大于约850μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸不大于约450μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸不大于约700μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸不大于约650μm。In another embodiment involving orthopedic applications and the like, the average diameter or other longest transverse dimension of the compartments of the
在另一个涉及矫形外科应用等的实施方案中,弹性基质10的隔室平均直径或其它最长横向尺寸约为100μm-约1000μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸约为150μm-约850μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸约为200μm-约700μm。在另一个实施方案中,其隔室平均直径或其它最长横向尺寸约为250μm-约650μm。In another embodiment involving orthopedic applications and the like, the average diameter or other longest transverse dimension of the compartments of the
在另一个实施方案中,由弹性基质10构成的可植入装置可以在单一装置中包括从例如20μm小到例如500μm大之间改变的孔大小。在另一个实施方案中,由弹性基质10构成的可植入装置可以在单一装置中包括从例如100μm小到例如1000μm大之间改变的隔室大小。在另一个实施方案中,这类变化形式可以发生在整个材料的横截面或横截面的任何亚截面。在另一个实施方案中,这类变化形式以有规则逐步过渡的形式出现。在另一个实施方案中,这类变化形式以逐步方式出现。例如,孔大小分布在可植入装置的一端上可以约为20μm-约70μm,并且在该装置的另一端上约为300μm-约500μm。这种孔大小分布的改变可以在一个或多个连续过渡或一个或多个分散步骤中进行。这类孔大小分布的变化形式产生连续过渡区或分散步骤,即从一种孔大小分布过渡到另一种孔大小分布就连续过渡情况而言为更渐进的,或在分散步骤或多个步骤的情况中为更有区别的。就孔的方向而言,类似的过渡可以在孔的方向上发生,其中更具方向性的孔过渡入方向性较低的孔乃至进入基本上无通过横截面或横截面的亚截面的方向的孔。通过由弹性基质10构成的可植入装置横截面的孔大小分布和/或方向的差异可以使得该装置被改造成针对细胞类型,细胞粘着,细胞向内生长和/或细胞增殖方面具有优选性能。可选择地,通过由弹性基质10构成的可植入装置横截面的不同孔大小分布和/或孔方向使得该装置被改造成针对组织类型,组织粘着,组织向内生长和/或组织增殖方面具有优选性能。In another embodiment, implantable devices comprised of
众所周知细胞会沿着并通过由孔大小分布形成的结构轮廓粘着,增殖和分化。细胞定向和细胞形态会产生改造或新形成的组织,它基本上可以复制或模拟实际组织,例如替代组织的解剖特征。这种优选的归因于连续或逐步孔大小分布变化形式细胞形态和定向,其中具有或不具有孔的定向可以在将可植入装置放入组织修复和再生部位而无在先细胞接种时发生。这种优选的归因于连续或逐步孔大小分布变化形式的细胞形态和定向还可以在进行体外细胞培养后将可植入装置放入患者,例如人或动物组织修复和再生部位时发生。这些连续或逐步孔大小分布变化形式,其中具有或不具有孔的定向可以为许多矫形外科应用中TE支架的重要特征,尤其是在包括脊柱,肩,膝,手或关节和假体器官的生长中的软组织连接,修复,再生,加强和/或支持中。Cells are known to adhere, proliferate and differentiate along and through structural contours formed by the pore size distribution. Cell orientation and cell morphology result in engineered or newly formed tissue that essentially replicates or mimics actual tissue, such as the anatomical features of the surrogate tissue. This preference is attributed to continuous or stepwise pore size distribution changes in cell morphology and orientation, where orientation with or without pores can occur upon placement of the implantable device at the site of tissue repair and regeneration without prior cell seeding . This preferred cell morphology and orientation due to continuous or stepwise changes in pore size distribution can also occur when implantable devices are placed in patients, eg, human or animal tissue repair and regeneration sites, following in vitro cell culture. These continuous or stepwise pore size distribution variations, with or without pore orientation, can be an important feature of TE scaffolds in many orthopedic applications, especially in areas including spine, shoulder, knee, hand or joint and prosthetic organ growth. In soft tissue connection, repair, regeneration, strengthening and/or support.
大小和形状size and shape
易于将弹性基质10制成任何所需的大小和形状。本发明的有益性在于弹性基质10适合于通过分割块原料,例如通过切割,模冲,激光切割或压模由这类块原料进行大量生产。在一个实施方案中,可以使用加热表面进行块原料分割。本发明的额外有益性在于弹性基质10的形状和构造可在很大范围内变化并且易于适合于所需解剖形态。The
弹性基质10的大小,形状,构造和其它相关详细描述可以专门用于特定的应用或患者或为大量生产标准化。然而,经济的考虑倾向于标准化。为了达到这一目的,可以将弹性基质10制成为包括不同大小和形状的弹性可植入装置片的试剂盒。此外,正如本说明书中另外部分讨论和请求优先权的申请中披露的,可以将多个,例如两个,三个或四个单个弹性基质10用作用于单一靶向生物部位的可植入装置系统,其为一定大小或成形的或既具有一定大小也成形的以便为治疗个体靶向部位协同其作用。The size, shape, configuration and other relevant details of the
可以为外科医师或其他医疗或兽医从业人员,研究人员等的实施手术操作的开业医生随后可以从可利用范围中选择一种或多种可植入装置用于具体治疗,例如如请求优先权的申请中所述。The medical practitioner performing the procedure, who may be a surgeon or other medical or veterinary practitioner, researcher, etc., may then select one or more implantable devices from available for a particular treatment, e.g. described in the application.
作为实例,弹性基质10的最小尺寸低至0.5mm且最大尺寸高达100mm乃至100mm以上。然而,在一个实施方案中,关注指定用于植入的这类尺寸的弹性基质10可以具有延长的形状,诸如圆柱体,杆体,管或延长棱晶形或折叠,卷曲,螺旋或其它更紧密的构造。类似的,小至0.5mm的尺寸可以为延长形状或条或片-样可植入装置的横向尺寸。As an example, the smallest dimension of the
在一个可选择的实施方案中,具有球形,立方体,四面体,环形或在与任何其它尺寸相比不具备显著延长尺寸的其它形式和约0.5mm-约500mm的直径或其它最大尺寸的弹性基质10可具有效用,例如用于矫形外科应用部位。在另一个实施方案中,具有这类形式的弹性基质10具有约3mm-约20mm的直径或其它最大尺寸。In an alternative embodiment, the
就大部分可植入装置的应用而言,弹性基质10的宏观结构大小包括如下实施方案:致密形状,诸如球体,立方体,角锥体,四面体,锥体,圆柱体,梯形,平行六面体,椭圆体,纺锤形,管形或套管形和许多具有约1mm-约200mm横向尺寸的较低规则形(在另一个实施方案中,这些横向尺寸约为5mm-约100mm.);和具有约0.5-约20For most implantable device applications, the macroscopic structural size of the
mm厚度(在另一个实施方案中,这些厚度约为1-约5mm)和约5-约200mm侧向尺寸(在另一个实施方案中,这些尺寸约为10-约100mm)的片-或条-样形状。mm thickness (in another embodiment, these thicknesses are about 1 to about 5 mm) and about 5 to about 200 mm lateral dimension (in another embodiment, these dimensions are about 10 to about 100 mm) sheet - or strip - shape.
就矫形外科应用治疗而言,本发明的优点在于可以有效地使用可植入弹性基质元件而无须严格地与可能通常为复杂和难以模型化的矫形外科应用部位构造一致。因此,在一个实施方案中,本发明的可植入弹性基质元件具有明显不同和较为简单的构造,例如如请求优先权的申请中所述。With respect to orthopedic application treatments, an advantage of the present invention is that the implantable elastic matrix element can be used effectively without strictly conforming to the orthopedic application site configuration, which can often be complex and difficult to model. Thus, in one embodiment, the implantable elastic matrix element of the present invention has a significantly different and simpler construction, for example as described in the priority-claiming application.
此外,在一个实施方案中,本发明的可植入装置或如果使用一种以上的可植入装置不应完全充满矫形外科应用部位,甚至在原位完全膨胀时也是如此。在一个实施方案中,本发明完全膨胀的可植入装置在尺寸上小于矫形外科应用部位并且在矫形外科应用部位内提供足够的空间,以确保血管化,细胞向内生长和增殖和血液通过可植入装置的可能通道。在另一个实施方案中,本发明完全膨胀的可植入装置在尺寸上基本上与矫形外科应用部位类似。在另一个实施方案中,本发明完全膨胀的可植入装置在尺寸上基本上大于矫形外科应用部位。在另一个实施方案中,本发明完全膨胀的可植入装置在体积上小于矫形外科应用部位。在另一个实施方案中,本发明完全膨胀的可植入装置在体积上基本上与矫形外科应用部位相同。在另一个实施方案中,本发明完全膨胀的可植入装置在体积上大于矫形外科应用部位。在另一个实施方案中,在放入矫形外科应用部位后,本发明膨胀的可植入装置可以溶胀,例如,通过吸收和/或吸附水或其它体液溶胀,在一个实施方案中,以1-维方式溶胀至多1-20%。在一个实施方案中,以1-维方式溶胀至多1-30%。或在另一个实施方案中以1-维方式溶胀至多1-40%。Furthermore, in one embodiment, the implantable device of the present invention, or if more than one implantable device is used, should not completely fill the orthopedic application site, even when fully expanded in situ. In one embodiment, the fully expanded implantable device of the present invention is smaller in size than the orthopedic application site and provides sufficient space within the orthopedic application site to ensure vascularization, cell ingrowth and proliferation, and blood passage. Possible passages for implanted devices. In another embodiment, the fully expanded implantable device of the present invention is substantially similar in size to the orthopedic application site. In another embodiment, the fully expanded implantable device of the present invention is substantially larger in size than the orthopedic application site. In another embodiment, the fully expanded implantable device of the present invention is smaller in volume than the orthopedic application site. In another embodiment, the fully expanded implantable device of the present invention is substantially the same volume as the orthopedic application site. In another embodiment, the fully expanded implantable device of the present invention is larger in volume than the orthopedic application site. In another embodiment, after placement in an orthopedic application site, the expandable implantable device of the present invention can swell, for example, by absorbing and/or absorbing water or other body fluids, in one embodiment, at 1- Dimensional mode swelling up to 1-20%. In one embodiment, swelling is at most 1-30% in a 1-dimensional fashion. Or in another embodiment swell up to 1-40% in a 1-dimensional fashion.
某些有用的可植入装置形状可以接近靶向矫形外科应用部位的外形轮廓。在一个实施方案中,可植入装置成形为相对简单的凸出形,盘-样或半球形或半-椭圆形和适合于治疗不同患者的多个不同部位的大小。Certain useful implantable device shapes can approximate the contours of a targeted orthopedic application site. In one embodiment, the implantable device is shaped as a relatively simple convex shape, disc-like or hemispherical or semi-elliptical and sized to treat a number of different sites in different patients.
在另一个实施方案中,可以想到在植入时,在其孔被生物流体,体液和/或组织充满前,这类用于矫形外科应用等的可植入装置不完全填充、覆盖或跨越它们居留的生物部位,并且在许多情况中尽管不一定必要,但是各植入的弹性基质10具有至少1-维不超过50%其进入的生物部位或超过50%被修复或替代的受损组织。在另一个实施方案中,如上所述的各植入弹性基质10具有至少1-维不超过75%其进入的生物部位或超过75%被修复或替代的受损组织。在另一个实施方案中,如上所述的单个可植入弹性基质10具有至少1-维不超过95%其进入的生物部位或超过95%被修复或替代的受损组织。In another embodiment, it is contemplated that upon implantation, such implantable devices for orthopedic applications and the like do not completely fill, cover or span their pores before their pores are filled with biological fluids, bodily fluids and/or tissue. Inhabited biological site, and in many cases though not necessarily, each implanted
在另一个实施方案中,在植入时,在其孔被充满生物流体,体液和/或组织前,这类用于矫形外科应用等的可植入装置基本上填充、覆盖或跨越它们居留的生物部位,并且在许多情况中尽管不一定必要,但是各植入的弹性基质10具有至少1-维不超过约100%其进入的生物部位或覆盖100%被修复或替代的受损组织。在另一个实施方案中,如上所述的各植入弹性基质10具有至少1-维不超过98%其进入的生物部位或覆盖98%被修复或替代的受损组织。在另一个实施方案中,各植入弹性基质10具有至少1-维不超过102%其进入的生物部位或覆盖102%被修复或替代的受损组织。In another embodiment, such implantable devices for orthopedic applications and the like substantially fill, cover, or span the pore where they reside before their pores are filled with biological fluids, bodily fluids, and/or tissue upon implantation. The biological site, and in many cases though not necessarily, each implanted
在另一个实施方案中,在植入时,在其孔被充满生物流体,体液和/或组织前,这类用于矫形外科应用等的可植入装置过度填充、覆盖或跨越它们居留的生物部位,并且在许多情况中尽管不一定必要,但是各植入的弹性基质10具有至少1-维不超过约105%其进入的生物部位或覆盖105%被修复或替代的受损组织。在另一个实施方案中,如上所述的各植入弹性基质10具有至少1-维不超过125%其进入的生物部位或覆盖125%被修复或替代的受损组织。在另一个实施方案中,如上所述各植入弹性基质10具有至少1-维不超过150%其进入的生物部位或覆盖150%被修复或替代的受损组织。在另一个实施方案中,如上所述各植入弹性基质10具有至少1-维不超过200%其进入的生物部位或覆盖200%被修复或替代的受损组织。在另一个实施方案中,如上所述各植入弹性基质10具有至少1-维不超过300%其进入的生物部位或覆盖300%被修复或替代的受损组织。In another embodiment, such implantable devices for orthopedic applications and the like overfill, cover or span the biological site, and in many cases, although not necessarily, each implanted
用于实施本发明的一个实施方案为足够柔韧和回弹性,即回弹性可压缩的网状弹性基质10,以便能够在环境条件,例如在25℃下最初从松弛结构被压缩成第一致密结构,从而能够通过递送装置进行递送,所述的递送装置例如为用于体外递送的导管,内窥镜,注射器,膀胱镜,套管针或其它合适的插管器仪器;以便在原位膨胀成第二工作结构。此外,在另一个实施方案中,弹性基质在被压缩原始尺寸的约5-95%(例如压缩约为原始尺寸的19/20-1/20)后具有本文所述的回弹-可压缩性。另一个实施方案中,弹性基质在被压缩原始尺寸的约10-90%(例如压缩约为原始尺寸的9/10-1/10)后具有本文所述的回弹-可压缩性。本文所用的弹性基质10具有“回弹-可压缩性”,即在体外第二工作结构至少约为至少1-维形式的松弛结构大小的50%时为“回弹-可压缩的”。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体外至少约为至少1-维形式的松弛结构大小的80%。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体外至少约为至少1-维形式的松弛结构大小的90%。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体外至少约为至少1-维形式的松弛结构大小的97%。One embodiment for practicing the invention is a sufficiently flexible and resilient, i.e., resiliently compressible, reticulated
在另一个实施方案中,弹性基质在被压缩原始体积的约5-95%(例如压缩约为原始体积的19/20-1/20)后具有本文所述的回弹-可压缩性。在另一个实施方案中,弹性基质在被压缩原始体积的约10-90%(例如压缩约为原始体积的9/10-1/10)后具有本文所述的回弹-可压缩性。本文所用的“体积”为除以弹性基质最外面3-维外形的体积。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体内至少约为松弛结构占据体积的50%。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体内至少约为松弛结构占据体积的80%。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体内至少约为松弛结构占据体积的90%。在另一个实施方案中,弹性基质10的回弹-可压缩性使得第二工作结构在体内至少约为弹性基质以其松弛结构形式占据体积的97%。In another embodiment, the elastic matrix has the resilience-compressibility described herein after being compressed by about 5-95% of the original volume (eg, compressed by about 19/20-1/20 of the original volume). In another embodiment, the elastic matrix has the resilience-compressibility described herein after being compressed by about 10-90% of the original volume (eg, compressed by about 9/10-1/10 of the original volume). As used herein, "volume" is the volume divided by the outermost 3-dimensional shape of the elastic matrix. In another embodiment, the resilient-compressibility of the
充分表征的弹性体和弹性可植入装置Well-Characterized Elastomers and Elastic Implantable Devices
在一个实施方案中,用作单独或与掺合物或溶液组合的弹性基质10结构材料的弹性体为充分表征的合成弹性聚合物,它们具有合适的机械性能,这些机械性能已经在化学,物理或生物特性方面得到充分表征而被视为生物耐久性的并且适合于用作患者,特别是哺乳动物且尤其是人的体内可植入装置。在另一个实施方案中,用作弹性基质10结构材料的弹性体在化学,物理或生物特性方面得到充分表征而被视为生物耐久性的并且适合于用作患者,特别是哺乳动物且尤其是人的体内可植入装置。In one embodiment, the elastomers used as structural materials for the
弹性基质物理特性Elastic Matrix Physical Properties
弹性基质10,网状弹性基质,包括网状弹性基质的可植入装置和/或包括压缩模制的网状弹性基质的可植入装置可以具有任何与其其它特性一致的合适的堆密度,也称作比重。例如,在一个实施方案中,如根据ASTM Standard D3574中所述测试方法测定的堆密度可以约为0.005g/cc-约0.96g/cc(约0.31 lb/ft3-约60 lb/ft3)。在另一个实施方案中,堆密度可以约为0.048g/cc-约0.56g/cc(约3.0 lb/ft3-约35 lb/ft3)。在另一个实施方案中,堆密度可以约为0.005g/cc-约0.15g/cc(约0.31 lb/ft3-约9.4 lb/ft3)。在另一个实施方案中,堆密度可以约为0.008g/cc-约0.127g/cc(约0.5lb/ft3-约8 lb/ft3)。在另一个实施方案中,堆密度可以约为0.015g/cc-约0.115g/cc(约0.93 lb/ft3-约7.2 lb/ft3)。在另一个实施方案中,堆密度可以约为0.024g/cc-约0.104g/cc(约1.5 lb/ft3-约6.5 lb/ft3)。The
弹性基质10可以具有与其其它特性一致的任何合适的微观表面积。例如,本领域技术人员通常可以根据多孔材料暴露的平面由孔频率,例如每毫米线中的孔数量估计出微观表面积,并且通常可以由以μm计的平均隔室侧面直径估计出孔频率。
其它合适的物理特性显而易见或对本领域技术人员而言显而易见。Other suitable physical properties will be apparent or apparent to those skilled in the art.
弹性基质机械性能Elastic Matrix Mechanical Properties
在一个实施方案中,网状弹性基质10具有足够的结构完整性以便在体外自承重和自立。然而,在另一个实施方案中,可以给弹性基质10提供结构支持物,诸如肋状物或小连接体。In one embodiment, reticulated
网状弹性基质10具有足够的拉伸强度,使得它可以在其指定应用过程中和后加工步骤过程中经受住正常的手工或机械操作,所述的后加工步骤因没有撕裂,断裂,剥落,破碎或崩解,脱落片或颗粒,或失去其结构完整性而是需要或理想的。原料的拉伸强度不应如此之高,以至于会干扰弹性基质10的制造或其它加工。The reticulated
因此,例如,在一个实施方案中,网状弹性基质10可以具有约700kg/m2-约350,000kg/m2(约1psi-约500psi)的拉伸强度。在另一个实施方案中,弹性基质10可以具有约700kg/m2-约70,000kg/m2(约1psi-约100psi)的拉伸强度。在另一个实施方案中,网状弹性基质10可以具有约7,000kg/m2-约140,000kg/m2(约10psi-约200psi)的拉伸模量。在另一个实施方案中,弹性基质10可以具有约17,500kg/m2-约70,000kg/m2(约25psi-约100psi)的拉伸模量。Thus, for example, in one embodiment, reticulated
充分的极限抗张伸展率也是理想的。例如,在另一个实施方案中,网状弹性基质10具有至少约为25%的极限抗张伸展率。在另一个实施方案中,弹性基质10具有至少约为200%的极限抗张伸展率。Sufficient ultimate tensile elongation is also desirable. For example, in another embodiment, reticulated
在一个实施方案中,弹性基质10在短时内从第一致密结构膨胀成第二工作结构,例如在一个实施方案中,在90秒或90秒内,在另一个实施方案中,在40秒或40秒内,各自从75%压缩应变保持达10分钟后恢复约95%。在另一个实施方案中,弹性基质10在短时内从第一致密结构膨胀成第二工作结构,例如在一个实施方案中在180秒或180秒内,在另一个实施方案中在90秒或90秒内,在另一个实施方案中在60秒或60秒内,各自从75%压缩应变保持达30分钟后恢复约95%。在另一个实施方案中,在75%压缩应变保持达30分钟后,弹性基质10在约10分钟内恢复从而占据其松弛结构所占据的体积的至少约97%。In one embodiment, the
在一个实施方案中,网状弹性基质10可以具有约7,000kg/m2-约140,000kg/m2(约10psi-约200psi)的压缩模量。在另一个实施方案中,弹性基质10具有约17,500kg/m2-约70,000kg/m2(约25psi-约100psi)的压缩模量。在另一个实施方案中,网状弹性基质10在50%压缩应变下具有约700kg/m2-约350,000kg/m2(约1psi-约500psi)的抗压强度。在另一个实施方案中,网状弹性基质10在50%压缩应变下具有约700kg/m2-约70,000kg/m2(约1psi-约100psi)的抗压强度。在另一个实施方案中,网状弹性基质10在75%压缩应变下具有约7,000kg/m2-420,000kg/m2(约10psi-约600psi)的抗压强度。在另一个实施方案中,网状弹性基质10在75%压缩应变下具有约7,000kg/m2-约140,000kg/m2(约10psi-约200psi)的抗压强度。In one embodiment, the reticulated
在另一个实施方案中,网状弹性基质10在约25℃下,即根据ASTMD3574压缩至其厚度的50%时具有不超过约30%的压缩变定。在另一个实施方案中,弹性基质10具有不超过约20%的压缩变定。在另一个实施方案中,弹性基质10具有不超过约10%的压缩变定。在另一个实施方案中,弹性基质10具有不超过约5%的压缩变定。In another embodiment, the reticulated
在另一个实施方案中,正如根据ASTM Standard D3574中所述测试方法测定的,网状弹性基质10具有约0.18kg/线性cm-约8.90kg/线性cm(约1 lbs/线性英寸-约50 lbs/线性英寸)的抗撕裂强度。在另一个实施方案中,正如根据ASTM Standard D3574中所述测试方法测定的,网状弹性基质10具有约0.18kg/线性cm-约1.78kg/线性cm(约1 lbs/线性英寸-约10 lbs/线性英寸)的抗撕裂强度。In another embodiment, the reticulated
在另一个实施方案中,正如根据实施例5中所述测试方法测定的,网状弹性基质10具有约50秒-约2,500秒的静态回复时间t-90%。另一个实施方案中,网状弹性基质10具有约100秒-约2,000秒的静态回复时间t-90%。在另一个实施方案中,网状弹性基质10具有约125秒-约1,500秒的静态回复时间t-90%。In another embodiment, the reticulated
在另一个实施方案中,正如根据实施例5中所述测试方法在空气中和1Hz频率下5,000个循环后测定的,网状弹性基质10具有约5秒-约200秒的动态回复时间t-90%。在另一个实施方案中,正如在空气中和1Hz频率下100,000个循环后测定的,网状弹性基质10具有4,000秒的动态回复时间t-90%。在一个实施方案中,少于约1,750秒。在另一个实施方案中,少于约200秒。在另一个实施方案中,或约50秒-约4,000秒。在另一个实施方案中,在水中和1Hz频率下100,000个循环后测定的,网状弹性基质10具有约3,000秒的动态回复时间t-90%。在一个实施方案中,少于约1,500秒。在另一个实施方案中,少于约100秒。在另一个实施方案中,或约50秒-约3,000秒。In another embodiment, the reticulated
表1概述了适合于网状弹性基质10,包括在网状化后退火的那些网状弹性基质的实施方案的机械性能和其它特性。额外的合适机械性能对本领域技术人员而言显而易见或变得显而易见。Table 1 summarizes mechanical and other properties suitable for embodiments of reticulated
表1:网状弹性基质10的特性Table 1: Properties of
除非另有陈述,否则本文所述多孔材料的机械性能可以根据ASTMD3574-01的标题“Standard TestMethods for Flexible CellularMaterials-Slab,Bonded and Molded Urethane Foams”或其它这类本领域技术人员适当公知的方法测定。Unless otherwise stated, the mechanical properties of the porous materials described herein may be determined according to ASTM D3574-01 titled "Standard Test Methods for Flexible Cellular Materials-Slab, Bonded and Molded Urethane Foams" or other such methods appropriately known to those skilled in the art.
此外,如果在非聚合反应过程后多孔性被赋予用于弹性基质10的弹性体,那么良好的加工性对于聚合后成形和制造而言也是理想的。例如,在一个实施方案中,弹性基质10具有低粘性。Furthermore, if porosity is imparted to the elastomer used for the
生物耐久性和生物相容性Biodurability and Biocompatibility
在一个实施方案中,弹性体具有足够的生物耐久性,以便适合于长期植入患者,例如动物或人。生物耐久性弹性体和弹性基质具有化学,物理和/或生物特性以便提供合理的生物耐久性预期,即所述的弹性体在植入动物,例如哺乳动物时会表现出稳定性至少29天期限。长期植入的预计期限可根据特定应用的不同而改变。就许多应用而言,基本上需要长期植入,并且就这类应用而言,可能需要至少6,12或24个月或5年或5年以上期限的生物耐久性。具有特殊有益性的是可以被视为在患者寿命中为生物耐久性的弹性体。就治疗例如脊柱缺陷的弹性基质10实施方案的可能应用而言,因为这类疾患自身可能存在于相当年轻的人体患者中,所以在其本体中超过50年的生物耐久性可能是有利的。In one embodiment, the elastomer is sufficiently biodurable to be suitable for long-term implantation in a patient, such as an animal or a human. Biodurable elastomers and elastic matrices having chemical, physical and/or biological properties to provide a reasonable expectation of biodurability, i.e. said elastomers will exhibit stability for a period of at least 29 days when implanted in an animal, such as a mammal . The expected duration of long-term implantation may vary depending on the particular application. For many applications, essentially long-term implantation is required, and for such applications, biodurability for a period of at least 6, 12 or 24 months or 5 years or more may be required. Of particular interest are elastomers that can be considered biodurable over the lifetime of the patient. With regard to the possible application of an embodiment of the
在另一个实施方案中,植入期限至少足以使细胞向内生长和增殖开始发生,例如在至少约4-8周内。在另一个实施方案中,弹性体足以得到充分表征以便因显示具有这类提供合理生物耐久性预期的化学,物理和/或生物特性适合于长期植入,即弹性体在植入延长时间期限时会持续表现出生物耐久性。In another embodiment, the period of implantation is at least sufficient for cell ingrowth and proliferation to begin to occur, eg, within at least about 4-8 weeks. In another embodiment, the elastomer is sufficiently well characterized to be suitable for long-term implantation by exhibiting such chemical, physical and/or biological properties that are expected to provide reasonable biodurability, i.e., the elastomer is implanted for an extended period of time. Will continue to exhibit biological durability.
不受任何特定理论约束,由包括聚合、交联、起泡和网状化的方法形成的弹性基质的生物耐久性包括选择为生物耐久性的原料成分和那些成分的化学计量比,使得弹性基质保持其成分的生物耐久性。例如,可以通过将例如在患者体液温度和pH下易水解的化学键和基团,诸如酯基的存在和形成减少至最低限度来提高弹性基质生物耐久性。作为另一个实例,可以在交联和起泡后进行超过约2小时的固化步骤,以便将弹性基质上存在的游离胺基减少至最低限度。此外,重要的是将降解减少至最低限度,所述的降解可能在弹性基质制备过程中出现,例如因接触剪切或热能而出现,诸如可能在作为本领域公知方式的混合,溶解,交联和/或起泡过程中出现。Without being bound by any particular theory, the biodurability of elastic matrices formed by processes including polymerization, crosslinking, foaming, and reticulation includes selection of raw material components for biodurability and stoichiometric ratios of those components such that the elastic matrix Maintains the biodurability of its constituents. For example, elastic matrix biodurability can be enhanced by minimizing the presence and formation of chemical bonds and groups, such as ester groups, that are susceptible to hydrolysis, eg, at the temperature and pH of the patient's body fluids. As another example, a curing step of more than about 2 hours may be performed after crosslinking and foaming in order to minimize the presence of free amine groups on the elastomeric matrix. Furthermore, it is important to minimize degradation which may occur during the preparation of the elastic matrix, for example due to exposure to shear or thermal energy, such as may occur during mixing, dissolution, cross-linking in a manner known in the art. and/or during foaming.
如上所述,生物耐久性弹性体和弹性基质适合于延长时间期限处于生物环境中。这类产品不会表现出明显的分解,降解,侵蚀症状或在接触与其应用相当的时间期限的生物环境和/或体液应力时明显涉及其应用的机械性能退化。然而,某些崩裂,裂化或韧度和强度的缺失-有时称作ESC或环境应激崩裂可能并非与许多如本文所述的矫形外科应用和其它应用相关。许多体内应用,例如在弹性基质10用于治疗矫形外科应用部位时,将其暴露于较小的(如果有的话)机械应力,且由此不会产生导致严重患者后果的机械破坏。因此,不存在ESC对预计本发明的这类应用中的合适的弹性体的生物耐久性而言并非必不可少,因为在内皮化、包囊和细胞向内生长和增殖进展时弹性的重要性程度降低。As noted above, biodurable elastomers and elastic matrices are suitable for prolonged periods of time in biological environments. Such products will not exhibit appreciable symptoms of decomposition, degradation, erosion or degradation of mechanical properties significantly related to their application when exposed to biological environmental and/or body fluid stresses for a period of time comparable to their application. However, some chipping, cracking or loss of toughness and strength - sometimes referred to as ESC or environmental stress cracking - may not be relevant for many orthopedic and other applications as described herein. Many in vivo applications, such as when the
此外,在某些植入应用中,预计弹性基质10随时间过程,例如在2周-1年内变得壁脱落或被组织,瘢痕组织等包裹或掺入和完全整合或生物整合入例如所修复的组织或所治疗的腔。在这种情况中,弹性基质10减少了接触运动或循环的生物流体。因此,生物化学降解或不需要的可能有害的产物释放入宿主生物体的可能性即使无法消除也可以得到减弱。Furthermore, in certain implant applications, it is expected that the
在一个实施方案中,弹性基质具有良好的生物耐久性,并伴随有良好的生物相容性,使得弹性体几乎不会在体内诱导(如果有的话也很少)不良反应。为了达到这一目的,在用于本发明的另一个实施方案中,为在置于指定植入部位延长植入期限时不含可以在体内诱导不良反应或作用的生物不需要或有害物质或结构的弹性体或其它材料。这类弹性体由此应完全缺失或应仅包含极低生物耐受量的生物毒素,诱变剂,致癌物和/或致畸剂。在另一个实施方案中,用于制造弹性基质10的弹性体的生物耐久性生物特征包括抗生物降解和不存在或极低量如下情况中的至少一种:细胞毒性,血液毒性,致癌性,诱变性或致畸性。In one embodiment, the elastic matrix has good biodurability coupled with good biocompatibility such that the elastomer induces few, if any, adverse reactions in vivo. To achieve this purpose, in another embodiment used in the present invention, to be free of biologically undesirable or harmful substances or structures that can induce adverse reactions or effects in vivo when placed at a designated implantation site for extended period of implantation elastomers or other materials. Such elastomers should therefore be completely absent or should contain only very low biologically tolerable amounts of biotoxins, mutagens, carcinogens and/or teratogens. In another embodiment, the biodurability biocharacteristics of the elastomer used to make
来自弹性体聚合,交联和起泡的弹性基质Elastic matrix from elastomer polymerization, crosslinking and foaming
在其它实施方案中,本发明提供了多孔生物耐久性弹性体和聚合,交联和起泡它们的方法,而它们可以用于生产如本文所述的生物耐久性网状弹性基质10。在另一个实施方案中,网状化如下。In other embodiments, the present invention provides porous biodurable elastomers and methods of polymerizing, crosslinking and foaming them, which can be used to produce biodurable reticulated
更具体地说,在另一个实施方案中,本发明提供了制备生物耐久性弹性聚氨基甲酸酯基质的方法,包括由聚碳酸酯多元醇成分和异氰酸酯成分合成该基质,通过聚合,交联和起泡进行,由此形成孔,随后使泡沫网状化而得到网状化产品。将该产品命名为聚碳酸酯聚氨基甲酸酯,为包括由例如聚碳酸酯多元醇成分的羟基和异氰酸酯成分的异氰酸酯基形成的氨基甲酸酯基的聚合物。在该实施方案中,所述方法使用了受控化学以便提供具有良好生物耐久性特征的网状弹性体。按照本发明,进行聚合以便提供使用避免其中生物不需要或有害成分的化学方法得到的产品。More specifically, in another embodiment, the present invention provides a method for preparing a biodurable elastic polyurethane matrix, comprising synthesizing the matrix from a polycarbonate polyol component and an isocyanate component, polymerizing, crosslinking and foaming proceed, whereby pores are formed, followed by reticulation of the foam to obtain a reticulated product. This product is named polycarbonate polyurethane, and is a polymer including urethane groups formed from, for example, hydroxyl groups of a polycarbonate polyol component and isocyanate groups of an isocyanate component. In this embodiment, the method uses controlled chemistry in order to provide a reticulated elastomer with good biodurability characteristics. According to the present invention, polymerization is carried out so as to provide a product obtained using a chemical process which avoids biologically undesirable or harmful components therein.
在一个实施方案中,作为一种原料,所述方法使用至少一种多元醇成分。就本申请的目的而言,术语“多元醇成分”包括平均每个分子含约2个羟基的分子,即双官能多元醇或二元醇;以及平均每个分子含约2个以上羟基的分子,即多元醇或多官能多元醇。典型多元醇类平均每个分子含约2-约5个羟基。在一个实施方案中,作为一种原料,所述方法使用双官能多元醇成分。在该实施方案中,因为二元醇的羟基官能度约为2,所以它不提供所谓的具有软节段交联的“软节段”。在另一个实施方案中,作为一种多元醇成分原料,所述方法使用足量的多官能多元醇成分以便提供受控程度的软节段交联。在另一个实施方案中,该方法提供了足够的软节段交联以便产生稳定的泡沫。在另一个实施方案中,所述的软节段由一般相对低分子量多元醇成分构成,在一个实施方案中,约350-约6,000道尔顿,且在另一个实施方案中,‘约450-约4,000道尔顿。因此,这些多元醇类一般为液体或低熔点固体。这种软节段多元醇被伯或仲羟基封端。在另一个实施方案中,软节段多元醇成分每个分子具有约2个羟基。在另一个实施方案中,软节段多元醇成分每个分子具有约2个以上羟基;每个多元醇分子具有2个以上羟基是某些多元醇分子所需的以便赋予软节段交联。In one embodiment, the process uses at least one polyol component as a feedstock. For the purposes of this application, the term "polyol component" includes molecules having an average of about 2 hydroxyl groups per molecule, i.e., bifunctional polyols or diols; and molecules having an average of about 2 or more hydroxyl groups per molecule , that is, polyols or polyfunctional polyols. Typical polyols contain on average from about 2 to about 5 hydroxyl groups per molecule. In one embodiment, the method uses a bifunctional polyol component as a feedstock. In this embodiment, because the diol has a hydroxyl functionality of about 2, it does not provide so-called "soft segments" with soft segment crosslinks. In another embodiment, as a polyol component feedstock, the method uses a sufficient amount of multifunctional polyol component to provide a controlled degree of soft segment crosslinking. In another embodiment, the method provides sufficient soft segment crosslinking to produce a stable foam. In another embodiment, the soft segment is composed of generally relatively low molecular weight polyol components, in one embodiment, from about 350 to about 6,000 Daltons, and in another embodiment, from about 450 to about 6,000 Daltons. About 4,000 Daltons. Accordingly, these polyols are generally liquids or low-melting solids. Such soft segment polyols are terminated with primary or secondary hydroxyl groups. In another embodiment, the soft segment polyol component has about 2 hydroxyl groups per molecule. In another embodiment, the soft segment polyol component has about 2 or more hydroxyl groups per molecule; having more than 2 hydroxyl groups per polyol molecule is required for certain polyol molecules in order to impart crosslinking to the soft segment.
在一个实施方案中,在多元醇成分中每个分子中的羟基平均数约为2。在另一个实施方案中,在多元醇成分中每个分子中的羟基平均数约大于2。在另一个实施方案中,在多元醇成分中每个分子中的羟基平均数大于2。在一个实施方案中,多元醇成分包含叔碳键。在一个实施方案中,多元醇成分包含多个叔碳键。In one embodiment, the average number of hydroxyl groups per molecule in the polyol component is about 2. In another embodiment, the average number of hydroxyl groups per molecule in the polyol component is greater than about 2. In another embodiment, the average number of hydroxyl groups per molecule in the polyol component is greater than two. In one embodiment, the polyol component contains tertiary carbon bonds. In one embodiment, the polyol component contains multiple tertiary carbon bonds.
在一个实施方案中,多元醇成分为聚醚多元醇,聚酯多元醇,聚碳酸酯多元醇,烃多元醇,聚硅氧烷多元醇,聚(醚-共-酯)多元醇,聚(醚-共-碳酸酯)多元醇,聚(醚-共-烃)多元醇,聚(醚-共-硅氧烷)多元醇,聚(酯-共-碳酸酯)多元醇,聚(酯-共-烃)多元醇,聚(酯-共-硅氧烷)多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯共-硅氧烷)多元醇,聚(烃-共-硅氧烷)多元醇或其混合物。In one embodiment, the polyol component is a polyether polyol, polyester polyol, polycarbonate polyol, hydrocarbon polyol, polysiloxane polyol, poly(ether-co-ester) polyol, poly( Ether-co-carbonate) polyols, poly(ether-co-hydrocarbon) polyols, poly(ether-co-siloxane) polyols, poly(ester-co-carbonate) polyols, poly(ester- co-hydrocarbon) polyols, poly(ester-co-siloxane) polyols, poly(carbonate-co-hydrocarbon) polyols, poly(carbonate co-siloxane) polyols, poly(hydrocarbon-co- - siloxane) polyols or mixtures thereof.
聚醚-类多元醇为例如与乙二醇类或多元醇类聚合的烯化氧,诸如环氧乙烷或环氧丙烷的低聚物,后者导致羟基官能度大于2以便能够进行软节段交联。Polyether-type polyols are, for example, oligomers of alkylene oxides polymerized with glycols or polyols, such as ethylene oxide or propylene oxide, the latter resulting in a hydroxyl functionality of greater than 2 to enable soft-segmentation Segment crosslinking.
聚酯-类多元醇为羧酸与乙二醇或三元醇的反应产物的低聚物,诸如乙二醇己二酸酯,丙二醇己二酸酯,丁二醇丁二醇,二甘醇己二酸酯,邻苯二甲酸酯类,聚已内酯和蓖麻油。当反应剂包括具有大于2的羟基官能度的那些时,例如多元醇类,可能进行软节段交联。Polyester-like polyols are oligomers of reaction products of carboxylic acids with ethylene glycol or trihydric alcohols, such as ethylene glycol adipate, propylene glycol adipate, butanediol butanediol, diethylene glycol Adipates, phthalates, polycaprolactone and castor oil. Soft segment crosslinking is possible when reactants include those having a hydroxyl functionality greater than 2, such as polyols.
聚碳酸酯-类多元醇一般由一类烃二元醇或就多个多元醇而言为各自在羟基之间具有不同烃链长的烃二元酸醇类与碳酸酯单体反应得到。相邻碳酸酯类之间的烃链长与原始二元醇(类)的烃链长相同。例如,可以通过使1,6-己二醇与碳酸盐,诸如碳酸氢钠反应制备双官能聚碳酸酯多元醇,从而得到聚碳酸酯-类多元醇1,6-己二醇碳酸酯。该反应的商购产品的分子量在约500-约5,000道尔顿之间改变。如果聚碳酸酯多元醇在25℃下为固体,那么它一般在进一步加工前被熔化。可选择地,在一个实施方案中,可以由烃二元醇类的混合物,例如1,6-己二醇,环己基二甲醇和1,4-丁二醇的所有三种或任何二元组合制备液体聚碳酸酯多元醇成分。不受任何通道理论约束,认为这类烃二元醇类的混合物可以破坏产物聚碳酸酯多元醇成分的结晶度,从而使得它在25℃下为液体且由此在包含它的泡沫中产生相对较柔软的泡沫。Polycarbonate-like polyols are generally obtained by reacting a class of hydrocarbon diols or, in the case of multiple polyols, hydrocarbon dibasic acid alcohols each having a different hydrocarbon chain length between the hydroxyl groups, with carbonate monomers. The hydrocarbon chain length between adjacent carbonates is the same as that of the original diol(s). For example, bifunctional polycarbonate polyols can be prepared by reacting 1,6-hexanediol with a carbonate, such as sodium bicarbonate, to obtain polycarbonate-
当用于生产聚碳酸酯多元醇的反应剂中包括具有大于2的羟基官能度的那些,例如多元醇类时,可能进行软节段交联。可以通过在制备聚碳酸酯多元醇成分中使用例如己三醇制备每个分子具有大于2的羟基平均数的聚碳酸酯多元醇类,例如聚碳酸酯三元醇。为了制备液体聚碳酸酯三元醇成分,可以使与其它含羟基的材料,例如环己基三甲醇和/或丁三醇的混合物与碳酸酯和己三醇反应。Soft segment crosslinking is possible when the reactants used to produce the polycarbonate polyol include those having a hydroxyl functionality greater than 2, such as polyols. Polycarbonate polyols having an average number of hydroxyl groups per molecule greater than 2, such as polycarbonate triols, can be prepared by using, for example, hexanetriol in the preparation of the polycarbonate polyol component. To prepare liquid polycarbonate triol compositions, mixtures with other hydroxyl-containing materials, such as cyclohexyltrimethanol and/or butanetriol, can be reacted with carbonate and hexanetriol.
商购烃-类多元醇一般由二烯类与乙烯基单体的自由基聚合产生,因此,它们一般为双官能羟基封端的材料。Commercially available hydrocarbon-based polyols generally result from the free radical polymerization of dienes and vinyl monomers, and as such, they are generally difunctional hydroxyl-terminated materials.
聚硅氧烷多元醇为例如含羟基端基的烷基和/或芳基取代的硅氧烷类,诸如二甲基硅氧烷,二苯基硅氧烷或甲基苯基硅氧烷的低聚物。可以通过在制备聚硅氧烷多元醇成分中使用例如甲基羟甲基硅氧烷制备每个分子具有大于2的羟基平均数的聚硅氧烷多元醇类,例如聚硅氧烷三元醇。Silicone polyols are, for example, alkyl- and/or aryl-substituted siloxanes containing hydroxyl end groups, such as dimethylsiloxane, diphenylsiloxane or methylphenylsiloxane Oligomer. Silicone polyols having an average number of hydroxyl groups per molecule greater than 2, such as silicone triols, can be prepared by using, for example, methylolmethylsiloxane in the preparation of the silicone polyol component .
特定类型的多元醇无需限于由单一单体单元构成的那些。例如,聚醚-类多元醇可以由环氧乙烷和氧化丙烯混合物形成。A particular type of polyol need not be limited to those composed of a single monomer unit. For example, polyether-based polyols can be formed from a mixture of ethylene oxide and propylene oxide.
另外,在另一个实施方案中,共聚物或共聚多元醇类可以由上述多元醇类中的任意种通过本领域公知的方法形成。因此,可以使用下列二元成分的多元醇共聚物:聚(醚-共-酯)多元醇,聚(醚-共-碳酸酯)多元醇,聚(醚-共-烃)多元醇,聚(醚-共-硅氧烷)多元醇,聚(酯-共-碳酸酯)多元醇,聚(酯-共-烃)多元醇,聚(酯-共-硅氧烷)多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇和聚(烃-共-硅氧烷)多元醇。例如,聚(醚-共-酯)多元醇可以由聚醚单元形成,所述的聚醚由环氧乙烷与含乙二醇己二酸酯的聚酯单元共聚合形成。在另一个实施方案中,共聚物为聚(醚-共-碳酸酯)多元醇,聚(醚-共-烃)多元醇,聚(醚-共-硅氧烷)多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇,聚(烃-共-硅氧烷)多元醇或其混合物。在另一个实施方案中,共聚物为聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇,聚(烃-共-硅氧烷)多元醇或其混合物。在另一个实施方案中,共聚物为聚(碳酸酯-共-烃)多元醇。例如,可以通过使1,6-己二醇,1,4-丁二醇和烃-类多元醇与碳酸酯聚合形成聚(碳酸酯-共-烃)多元醇。Additionally, in another embodiment, copolymers or copolyols may be formed from any of the polyols described above by methods known in the art. Thus, polyol copolymers of the following binary components can be used: poly(ether-co-ester) polyols, poly(ether-co-carbonate) polyols, poly(ether-co-hydrocarbon) polyols, poly(ether-co-hydrocarbon) polyols, Ether-co-siloxane) polyols, poly(ester-co-carbonate) polyols, poly(ester-co-hydrocarbon) polyols, poly(ester-co-siloxane) polyols, poly(carbonic acid ester-co-hydrocarbon) polyols, poly(carbonate-co-siloxane) polyols and poly(hydrocarbon-co-siloxane) polyols. For example, poly(ether-co-ester) polyols may be formed from polyether units formed by the copolymerization of ethylene oxide and ethylene glycol adipate-containing polyester units. In another embodiment, the copolymer is poly(ether-co-carbonate) polyol, poly(ether-co-hydrocarbon) polyol, poly(ether-co-siloxane) polyol, poly(carbonate - co-hydrocarbon) polyols, poly(carbonate-co-siloxane) polyols, poly(hydrocarbon-co-siloxane) polyols or mixtures thereof. In another embodiment, the copolymer is a poly(carbonate-co-hydrocarbon) polyol, poly(carbonate-co-siloxane) polyol, poly(hydrocarbon-co-siloxane) polyol or mixture. In another embodiment, the copolymer is a poly(carbonate-co-hydrocarbon) polyol. For example, poly(carbonate-co-hydrocarbon) polyols can be formed by polymerizing 1,6-hexanediol, 1,4-butanediol, and hydrocarbon-based polyols with carbonates.
在另一个实施方案中,多元醇成分为聚醚多元醇,聚碳酸酯多元醇,烃多元醇,聚硅氧烷多元醇,聚(醚-共-碳酸酯)多元醇,聚(醚-共-烃)多元醇,聚(醚-共-硅氧烷)多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇,聚(烃-共-硅氧烷)多元醇或其混合物。在另一个实施方案中,多元醇成分为聚碳酸酯多元醇,烃多元醇,聚硅氧烷多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇,聚(烃-共-硅氧烷)多元醇或其混合物。在另一个实施方案中,多元醇成分为聚碳酸酯多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇,聚(烃-共-硅氧烷)多元醇或其混合物。在另一个实施方案中,多元醇成分为聚碳酸酯多元醇,聚(碳酸酯-共-烃)多元醇,聚(碳酸酯-共-硅氧烷)多元醇或其混合物。在另一个实施方案中,多元醇成分为聚碳酸酯多元醇。In another embodiment, the polyol component is polyether polyol, polycarbonate polyol, hydrocarbon polyol, polysiloxane polyol, poly(ether-co-carbonate) polyol, poly(ether-co- -hydrocarbon)polyols, poly(ether-co-siloxane)polyols, poly(carbonate-co-hydrocarbon)polyols, poly(carbonate-co-siloxane)polyols, poly(hydrocarbon-co- - siloxane) polyols or mixtures thereof. In another embodiment, the polyol component is polycarbonate polyol, hydrocarbon polyol, polysiloxane polyol, poly(carbonate-co-hydrocarbon) polyol, poly(carbonate-co-siloxane ) polyols, poly(hydrocarbon-co-siloxane) polyols or mixtures thereof. In another embodiment, the polyol component is polycarbonate polyol, poly(carbonate-co-hydrocarbon) polyol, poly(carbonate-co-siloxane) polyol, poly(hydrocarbon-co-silicone) oxane) polyols or mixtures thereof. In another embodiment, the polyol component is a polycarbonate polyol, poly(carbonate-co-hydrocarbon) polyol, poly(carbonate-co-siloxane) polyol, or mixtures thereof. In another embodiment, the polyol component is a polycarbonate polyol.
此外,在另一个实施方案中,可以在本发明的弹性基质中使用多元醇类和共聚多元醇类的混合物(mixtures),混合物(admixtures)和/或掺合物。在另一个实施方案中,多元醇的分子量可变。在另一个实施方案中,多元醇的官能度可变。Furthermore, in another embodiment, mixtures, admixtures and/or blends of polyols and copolyols may be used in the elastomeric matrix of the present invention. In another embodiment, the molecular weight of the polyol can vary. In another embodiment, the functionality of the polyol can vary.
在另一个实施方案中,当双官能聚碳酸酯多元醇类或双官能烃多元醇类因其自身不能诱导软节段交联时,通过具有大于约2的羟基官能度的增链剂的应用在制剂中引入较高官能度。在另一个实施方案中,通过具有大于约2的异氰酸酯基官能度的异氰酸酯成分的应用引入较高官能度。具有约500-约5,000道尔顿分子量的商品聚碳酸酯二元醇类,诸如来自Arch Chemicals,Inc.(Norwalk,CT)的POLY-CD CD220和来自Stahl USA,Inc.(Peabody,MA)的PC-1733易于得到。商品烃多元醇类购自Sartomer(Exton,PA)。商品聚醚多元醇类易于购自,诸如PLURACOL,例如具有官能度为3的PLURACOL GP430和来自BASFCorp.(Wyandotte,MI)的LUPRANOL系列,来自Dow ChemicalCorp.(Midland,MI.)的VORANOL,来自Bayer Corp.(Leverkusen,Germany)和Huntsman Corp.(Madison Heights,MI)的BAYCOLL B,DESMOPHEN和MULTRANOL。商品聚酯多元醇类易于得到,诸如来自BASF的LUPRAPHEN,来自Dow,BAYCOLL A的TONE聚己内酯和VORANOL和来自Bayer和Huntsman的DESMOPHEN U系列。商品聚硅氧烷多元醇类易于得到,诸如来自Dow。In another embodiment, when the difunctional polycarbonate polyols or difunctional hydrocarbon polyols cannot induce soft segment crosslinking by themselves, through the use of a chain extender having a hydroxyl functionality greater than about 2 Introduce higher functionality into formulations. In another embodiment, the higher functionality is introduced through the use of an isocyanate component having an isocyanate group functionality of greater than about 2. Commercial polycarbonate diols having a molecular weight of about 500 to about 5,000 Daltons, such as POLY-CD CD220 from Arch Chemicals, Inc. (Norwalk, CT) and POLY-CD CD220 from Stahl USA, Inc. (Peabody, MA) PC-1733 is readily available. Commercial hydrocarbon polyols were purchased from Sartomer (Exton, PA). Commercial polyether polyols are readily available from, such as PLURACOL, e.g. PLURACOL GP430 with a functionality of 3 and the LUPRANOL series from BASFCorp. (Wyandotte, MI), VORANOL from Dow Chemical Corp. (Midland, MI.), from Bayer BAYCOLL B, DESMOPHEN and MULTRANOL of Corp. (Leverkusen, Germany) and Huntsman Corp. (Madison Heights, MI). Commercial polyester polyols are readily available such as LUPRAPHEN from BASF, TONE polycaprolactone and VORANOL from Dow, BAYCOLL A and the DESMOPHEN U series from Bayer and Huntsman. Commercial silicone polyols are readily available, such as from Dow.
所述的方法还使用至少一种异氰酸酯成分和任选至少一种增链剂成分以便提供所谓的“硬节段”。就本申请的目的而言,术语“异氰酸酯成分”包括平均每个分子含约2个异氰酸酯基团的分子和每个分子平均含大于约2个异氰酸酯基团的那些分子。异氰酸酯成分的异氰酸酯基团与其它组分的反应性氢基反应,例如与羟基中的氧发生氢键合并且与和多元醇成分中的胺中的氮,增链剂,交联剂和/或水发生氢键合。The method also uses at least one isocyanate component and optionally at least one chain extender component in order to provide so-called "hard segments". For purposes of this application, the term "isocyanate component" includes molecules containing an average of about 2 isocyanate groups per molecule and those molecules containing an average of greater than about 2 isocyanate groups per molecule. The isocyanate group of the isocyanate component reacts with reactive hydrogen groups of other components, such as hydrogen bonding with the oxygen in the hydroxyl group and with the nitrogen in the amine in the polyol component, chain extender, crosslinker and/or Water undergoes hydrogen bonding.
在一个实施方案中,异氰酸酯成分中每个分子中的异氰酸酯基团平均数约为2。在另一个实施方案中,异氰酸酯成分中每个分子中的异氰酸酯基团平均数大于约2。在另一个实施方案中,异氰酸酯成分上每个分子中的异氰酸酯基团平均数大于2。In one embodiment, the average number of isocyanate groups per molecule in the isocyanate component is about two. In another embodiment, the average number of isocyanate groups per molecule in the isocyanate component is greater than about 2. In another embodiment, the average number of isocyanate groups per molecule on the isocyanate component is greater than two.
异氰酸酯指数,即本领域众所周知的数量为可用于反应的制品中异氰酸酯基团数量与能够与那些异氰酸酯基团反应的制品中的基团数量的摩尔比,所述的与那些异氰酸酯基团反应的制品中的基团例如如果存在的话为二元醇,多元醇成分,增链剂和水的反应基团。在一个实施方案中,异氰酸酯指数约为0.9-约1.1。在另一个实施方案中,异氰酸酯指数约为0.9-约1.02。在另一个实施方案中,异氰酸酯指数约为0.98-约1.02。在另一个实施方案中,异氰酸酯指数约为0.9-约1.0。在另一个实施方案中,异氰酸酯指数约为0.9-约0.98。The isocyanate index, a quantity well known in the art, is the molar ratio of the number of isocyanate groups in an article available for reaction to the number of groups in an article capable of reacting with those isocyanate groups The groups in are, for example, diols, polyol components, chain extenders, and water reactive groups if present. In one embodiment, the Isocyanate Index is from about 0.9 to about 1.1. In another embodiment, the Isocyanate Index is from about 0.9 to about 1.02. In another embodiment, the Isocyanate Index is from about 0.98 to about 1.02. In another embodiment, the Isocyanate Index is from about 0.9 to about 1.0. In another embodiment, the Isocyanate Index is from about 0.9 to about 0.98.
典型的二异氰酸酯类包括脂族二异氰酸酯类,包含芳族基团的异氰酸酯类,即所谓的“芳族二异氰酸酯类”或其混合物。脂族二异氰酸酯类包括二异氰酸四亚甲基酯,环己烷-1,2-二异氰酸酯,环己烷-1,4-二异氰酸酯,二异氰酸六亚甲酯,二异氰酸异佛尔酮酯,亚甲基-双-(对-环己基异氰酸酯)(“H12MDI”)或其混合物。芳族二异氰酸酯类包括对-亚苯基二异氰酸酯,4,4’-二苯甲烷二异氰酸酯(“4,4’-MDI”),2,4’-二苯甲烷二异氰酸酯(“2,4’-MDI”),2,4-甲苯二异氰酸酯(“2,4-TDI”),2,6-甲苯二异氰酸酯(“2,6-TDI”),间-四甲基二甲苯二异氰酸酯或其混合物。Typical diisocyanates include aliphatic diisocyanates, isocyanates containing aromatic groups, so-called "aromatic diisocyanates" or mixtures thereof. Aliphatic diisocyanates include tetramethylene diisocyanate, cyclohexane-1,2-diisocyanate, cyclohexane-1,4-diisocyanate, hexamethylene diisocyanate, diisocyanate Acid isophorone ester, methylene-bis-(p-cyclohexylisocyanate) ("H12 MDI") or mixtures thereof. Aromatic diisocyanates include p-phenylene diisocyanate, 4,4'-diphenylmethane diisocyanate ("4,4'-MDI"), 2,4'-diphenylmethane diisocyanate ("2,4 '-MDI"), 2,4-toluene diisocyanate ("2,4-TDI"), 2,6-toluene diisocyanate ("2,6-TDI"), m-tetramethylxylene diisocyanate or its mixture.
每个分子平均含大于约2个异氰酸酯基团的典型异氰酸酯成分包括:二异氰酸六亚甲酯与水的含约3个异氰酸酯基团的加合物,其作为DESMODUR N100商购自Bayer;和含约3个异氰酸酯基团的二异氰酸六亚甲酯的三聚体(trirner),其作为MONDUR N3390商购自Bayer。Typical isocyanate compositions containing on average greater than about 2 isocyanate groups per molecule include: an adduct of hexamethylene diisocyanate with water containing about 3 isocyanate groups, commercially available from Bayer as DESMODUR N100; and a trimer of hexamethylene diisocyanate containing about 3 isocyanate groups, commercially available from Bayer as MONDUR N3390.
在一个实施方案中,异氰酸酯成分含至少约5%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。在另一个实施方案中,异氰酸酯成分含至少5%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。在另一个实施方案中,异氰酸酯成分含约5%-约50%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。在另一个实施方案中,异氰酸酯成分含5%-约50%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。在另一个实施方案中,异氰酸酯成分含约5%-约40%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。在另一个实施方案中,异氰酸酯成分含5%-约40%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。在另一个实施方案中,异氰酸酯成分含5%-约35%重量的2,4’-MDI与平衡物4,4’-MDI的混合物。不受任何特定理论约束,认为大量2,4’-MDI与4,4’-MDI的掺合物的应用由于破坏了不对称2,4’-MDI结构产生的硬节段结晶度而产生较软的弹性基质。In one embodiment, the isocyanate component comprises at least about 5% by weight of a mixture of 2,4'-MDI and the balance 4,4'-MDI. In another embodiment, the isocyanate component comprises at least 5% by weight of a mixture of 2,4'-MDI and balance 4,4'-MDI. In another embodiment, the isocyanate component comprises from about 5% to about 50% by weight of a mixture of 2,4'-MDI and the balance 4,4'-MDI. In another embodiment, the isocyanate component comprises from 5% to about 50% by weight of a mixture of 2,4'-MDI and the balance 4,4'-MDI. In another embodiment, the isocyanate component comprises from about 5% to about 40% by weight of a mixture of 2,4'-MDI and the balance 4,4'-MDI. In another embodiment, the isocyanate component comprises from 5% to about 40% by weight of a mixture of 2,4'-MDI and the balance 4,4'-MDI. In another embodiment, the isocyanate component comprises from 5% to about 35% by weight of a mixture of 2,4'-MDI and the balance 4,4'-MDI. Without being bound by any particular theory, it is believed that the use of large amounts of blends of 2,4'-MDI and 4,4'-MDI produces less crystallinity due to disruption of the hard segmental crystallinity produced by the
合适的二异氰酸酯类包括:MDI,诸如ISONATE 125M,来自Dow的PAPI系列的某些成员和来自Dow的ISONATE 50 OP;含4,4’-MDI和2,4’-MDI混合物的异氰酸酯类,诸如RUBI NATE 9433和RUBINATE9258,它们各自来自Huntsman和MONDUR MRS 2和来自Bayer的MRS 20;TDI,例如来自Lyondell Corp.(Houston,TX);异佛尔酮二异氰酸酯,诸如来自Degussa(Germany)的VESTAMAT;H12MDI,诸如来自Bayer的DESMODUR W;和来自BASF的各种二异氰酸酯类。Suitable diisocyanates include: MDI, such as ISONATE 125M, certain members of the PAPI series from Dow and
每个分子中平均含大于约2个异氰酸酯基团的合适的异氰酸酯成分包括下列修饰的二苯甲烷二异氰酸酯类,它们各自购自Dow:ISOBIND 1088,异氰酸酯基团官能度约为3;ISONATE 143L,异氰酸酯基团官能度约为2.1;PAPI 27,异氰酸酯基团官能度约为2.7;PAPI94,异氰酸酯基团官能度约为2.3;PAPI 580N,异氰酸酯基团官能度约为3;和PAPI 20,异氰酸酯基团官能度约为3.2。Suitable isocyanate components containing an average of greater than about 2 isocyanate groups per molecule include the following modified diphenylmethane diisocyanates, each available from Dow: ISOBIND 1088, having an isocyanate group functionality of about 3; ISONATE 143L, PAPI 27, about 2.7 isocyanate group functionality; PAPI94, about 2.3 isocyanate group functionality; PAPI 580N, about 3 isocyanate group functionality; and
典型的增链剂包括二元醇类,二胺类,链烷醇胺类或其混合物。在一个实施方案中,增链剂为具有2-10个碳原子的脂族二元醇。在另一个实施方案中,二元醇增链剂选自乙二醇,1,2-丙二醇,1,3-丙二醇,1,4-丁二醇,1,5-戊二醇,二甘醇,三甘醇或其混合物。在另一个实施方案中,增链剂为具有2-10个碳原子的二胺。在另一个实施方案中,二胺增链剂选自乙二胺,1,3-二氨基丁烷,1,4-二氨基丁烷,1,5二氨基戊烷,1,6-二氨基己烷,1,7-二氨基庚烷,1,8-二氨基辛烷,异佛尔酮二胺或其混合物。在另一个实施方案中,增链剂为具有2-10个碳原子的链烷醇胺。在另一个实施方案中,链烷醇胺增链剂选自二乙醇胺,三乙醇胺,异丙醇胺,二甲基乙醇胺,甲基二乙醇胺,二乙基乙醇胺或其混合物。Typical chain extenders include glycols, diamines, alkanolamines or mixtures thereof. In one embodiment, the chain extender is an aliphatic diol having 2-10 carbon atoms. In another embodiment, the glycol chain extender is selected from the group consisting of ethylene glycol, 1,2-propanediol, 1,3-propanediol, 1,4-butanediol, 1,5-pentanediol, diethylene glycol , triethylene glycol or a mixture thereof. In another embodiment, the chain extender is a diamine having 2-10 carbon atoms. In another embodiment, the diamine chain extender is selected from ethylenediamine, 1,3-diaminobutane, 1,4-diaminobutane, 1,5-diaminopentane, 1,6-diaminobutane Hexane, 1,7-diaminoheptane, 1,8-diaminooctane, isophoronediamine or mixtures thereof. In another embodiment, the chain extender is an alkanolamine having 2-10 carbon atoms. In another embodiment, the alkanolamine chain extender is selected from diethanolamine, triethanolamine, isopropanolamine, dimethylethanolamine, methyldiethanolamine, diethylethanolamine, or mixtures thereof.
商购增链剂包括二胺类,三胺类的JEFFAMI NE系列和来自Hunt sman的聚醚胺类,来自Creanova的VERSAMIN异佛尔酮二胺,购自Air Products Corp.(Allentown,PA)的二胺类的VERSALI NK系列,乙醇胺,二乙基乙醇胺和购自Dow的异丙醇胺和来自Bayer,BASF和UOP Corp.(Des Plaines,IL)的各种增链剂。Commercially available chain extenders include diamines, the JEFFAMI NE series of triamines and polyetheramines from Huntsman, VERSAMIN isophoronediamine from Creanova, available from Air Products Corp. (Allentown, PA). The VERSALI NK series of diamines, ethanolamine, diethylethanolamine, and isopropanolamine from Dow and various chain extenders from Bayer, BASF, and UOP Corp. (Des Plaines, IL).
在一个实施方案中,存在少量的任选组分,诸如多官能羟基化合物或其它具有超过2的官能度的交联剂,例如甘油以便交联。在另一个实施方案中,任选的多官能交联剂的存在量恰好足以获得稳定的泡沫,即不会崩解成非泡沫样的泡沫。可选择地或此外,脂族和环脂族异氰酸酯类的多官能加合物可以用于赋予与芳族二异氰酸酯类交联。可选择地或此外,脂族和环脂族异氰酸酯类的多官能加合物可以用于赋予与脂族二异氰酸酯类交联。任选该方法在某些实施方案中使用至少一种催化剂,其选自起泡催化剂,例如叔胺,胶凝催化剂,例如二月桂酸二丁基锡或其混合物。此外,本领域中已知叔胺催化剂也可以具有胶凝作用,即它们可以作为起泡和胶凝催化剂起作用。典型的叔胺催化剂包括来自Toyo Soda Co.(Japan)的TOTYCAT系列,来自Texaco Chemical Co.(Austin,TX)的TEXACAT系列,来自Th.Goldschmidt Co.(Germany)的KOSMOS和TEGO系列,来自Rohm和Haas(Philadelphia,PA)的DMP系列,来自Kao Corp.(Japan)的KAOLIZER系列和来自Enterprise Chemical Co.(Altamonte Springs,FL)的QUINCAT系列。典型的有机锡催化剂包括来自WitcoCorporation(Middlebury,CT)的FOMREZ和FOMREZ UL系列,来自CosanChemical Co.(Carlstadt,NJ)的COCURE和COSCAT系列和来自AirProducts的DABCO和POLYCAT系列。In one embodiment, small amounts of optional components such as polyfunctional hydroxyl compounds or other crosslinking agents having a functionality in excess of 2, eg glycerol, are present for crosslinking. In another embodiment, the optional multifunctional crosslinking agent is present in an amount just sufficient to obtain a stable foam, ie, a foam that does not disintegrate into a non-foam-like state. Alternatively or in addition, polyfunctional adducts of aliphatic and cycloaliphatic isocyanates may be used to impart crosslinking with aromatic diisocyanates. Alternatively or in addition, polyfunctional adducts of aliphatic and cycloaliphatic isocyanates may be used to impart crosslinking with aliphatic diisocyanates. Optionally the process uses in certain embodiments at least one catalyst selected from blowing catalysts such as tertiary amines, gelling catalysts such as dibutyltin dilaurate or mixtures thereof. Furthermore, it is known in the art that tertiary amine catalysts can also have a gelling effect, ie they can function as both foaming and gelling catalysts. Typical tertiary amine catalysts include TOTYCAT series from Toyo Soda Co. (Japan), TEXACAT series from Texaco Chemical Co. (Austin, TX), KOSMOS and TEGO series from Th. Goldschmidt Co. (Germany), from Rohm and The DMP series from Haas (Philadelphia, PA), the KAOLIZER series from Kao Corp. (Japan) and the QUINCAT series from Enterprise Chemical Co. (Altamonte Springs, FL). Typical organotin catalysts include the FOMREZ and FOMREZ UL series from Witco Corporation (Middlebury, CT), the COCURE and COSCAT series from Cosan Chemical Co. (Carlstadt, NJ) and the DABCO and POLYCAT series from Air Products.
在某些实施方案中,所述的方法使用至少一种表面活性剂。典型的表面活性剂包括TEGOSTAB BF2370,B-8300,B-8305和B-5055,它们均来自Goldschmidt,来自Dow Corning(Midland,MI)的DC 5241和其它非离子型有机硅,诸如购自Dow Corning,Air Pr oducts和General Electric(Water ford,NY)的聚二甲基硅氧烷类。In certain embodiments, the methods use at least one surfactant. Typical surfactants include TEGOSTAB BF2370, B-8300, B-8305 and B-5055, all ex Goldschmidt, DC 5241 ex Dow Corning (Midland, MI) and other nonionic silicones such as those available from Dow Corning , Air Products and General Electric (Waterford, NY) polydimethylsiloxanes.
在某些实施方案中,所述的方法使用至少一种隔室开放剂。典型的隔室开放剂包括来自Goldschmidt)的ORTEGOL 501。In certain embodiments, the methods use at least one compartment opener. Typical compartment openers include ORTEGOL 501 from Goldschmidt).
可以通过包括预聚物法和单罐法的手段制备交联的聚氨基甲酸酯类。涉及预聚物的实施方案如下。首先,通过常规方法由至少一种异氰酸酯成分(例如MDI)和至少一种具有大于2的官能度的多官能软节段材料(例如具有官能度为3的基于聚醚的软节段)制备预聚物。然后将预聚物,任选至少一种催化剂(例如二月桂酸二丁基锡)和至少一种双官能增链剂(例如1,4-丁二醇)在混合容器内混合以使该混合物固化或交联。在另一个实施方案中,交联在模具中进行。在另一个实施方案中,交联和起泡,即孔成形共同进行。在另一个实施方案中,交联和起泡在模具中共同进行。Crosslinked polyurethanes can be prepared by means including prepolymer methods and one-pot methods. Embodiments involving prepolymers are as follows. First, a preform is prepared by conventional methods from at least one isocyanate component (such as MDI) and at least one multifunctional soft segment material with a functionality greater than 2 (such as a polyether-based soft segment with a functionality of 3). Polymer. The prepolymer, optionally at least one catalyst (such as dibutyltin dilaurate) and at least one difunctional chain extender (such as 1,4-butanediol) are then mixed in a mixing vessel to cure the mixture or crosslinking. In another embodiment, the crosslinking takes place in the mold. In another embodiment, crosslinking and foaming, ie pore formation, are performed together. In another embodiment, crosslinking and foaming are performed together in the mold.
可选择地,可以使用所谓的“单罐”手段。单罐实施方案无需单独的预聚物制备步骤。在一个实施方案中,将原料,诸如上段中所述的那些在混合容器中混合且然后起泡并且交联。在另一个实施方案中,在将所述组分混合前加热。在另一个实施方案中,在将所述组分混合时加热它们。在另一个实施方案中,交联在模具中进行。在另一个实施方案中,起泡和交联共同进行。在另一个实施方案中,交联和起泡在模具中共同进行。在另一个实施方案中,在混合容器中混合除异氰酸酯成分外的所有组分。然后例如以高速搅拌加入异氰酸酯成分并且交联和起泡随之发生。在另一个实施方案中,将这种起泡混合物倾入模具并且使其升出。在另一个实施方案中,将多元醇成分与异氰酸酯成分和其它任选的添加剂,诸如粘度改进剂,表面活性剂和/或隔室开放剂混合成第一液体。Alternatively, so-called "one-pot" approaches can be used. The one-pot embodiment does not require a separate prepolymer preparation step. In one embodiment, raw materials, such as those described in the preceding paragraph, are mixed in a mixing vessel and then foamed and crosslinked. In another embodiment, the components are heated prior to mixing. In another embodiment, the components are heated as they are mixed. In another embodiment, the crosslinking takes place in the mold. In another embodiment, foaming and crosslinking are performed together. In another embodiment, crosslinking and foaming are performed together in the mold. In another embodiment, all components except the isocyanate component are mixed in a mixing vessel. The isocyanate component is then added, eg with high speed stirring, and crosslinking and foaming ensues. In another embodiment, this foaming mixture is poured into molds and allowed to rise. In another embodiment, the polyol component is mixed with the isocyanate component and other optional additives such as viscosity modifiers, surfactants and/or compartment openers into the first liquid.
在另一个实施方案中,所述的多元醇成分在混合温度下为液体。在另一个实施方案中,所述的多元醇成分为固体,因此,升高混合温度,使得在混合前例如通过加热使多元醇成分液化。接下来通过混合发泡剂和任选的添加剂,诸如胶凝催化剂和/或起泡催化剂形成第二液体。然后在混合容器内混合第一和第二液体且然后起泡和交联。In another embodiment, the polyol component is liquid at the mixing temperature. In another embodiment, the polyol component is a solid, therefore, the mixing temperature is increased such that the polyol component is liquefied prior to mixing, eg, by heating. A second liquid is next formed by mixing a blowing agent and optional additives, such as a gelling catalyst and/or a blowing catalyst. The first and second liquids are then mixed in a mixing vessel and then foamed and crosslinked.
在另一个实施方案中,本发明的任何或所有加工手段均可以用于制备具有大于3.4 lbs/ft3(0.054g/cc)密度的泡沫。在该实施方案中,使用交联剂,诸如甘油;异氰酸酯成分的官能度为2.0-2.4;异氰酸酯成分主要由MDI组成;且4,4’-MDI的量大于约50%重量的异氰酸酯成分。多元醇成分的分子量约为1,000-约2,000道尔顿。调整起泡剂,例如水的量以便获得密度大于3.4 lbs/ft3(0.054g/cc)的非网状泡沫。减少量的起泡剂可以减少材料中脲键的数量。可以通过使用双官能增链剂,诸如丁二醇和/或增加泡沫密度和/或通过增加所用交联剂的量补偿较少脲键产生的任何硬度和/或拉伸强度和/或抗压强度的下降。In another embodiment, any or all of the processing means of the present invention can be used to produce foams having a density greater than 3.4 lbs/ft3 (0.054 g/cc). In this embodiment, a crosslinking agent such as glycerin is used; the functionality of the isocyanate component is 2.0-2.4; the isocyanate component consists primarily of MDI; and the amount of 4,4'-MDI is greater than about 50% by weight of the isocyanate component. The molecular weight of the polyol component is from about 1,000 to about 2,000 Daltons. The amount of blowing agent, such as water, is adjusted to obtain a non-reticulated foam with a density greater than 3.4 lbs/ft3 (0.054 g/cc). A reduced amount of blowing agent can reduce the number of urea linkages in the material. Any stiffness and/or tensile and/or compressive strength resulting from fewer urea linkages can be compensated by using difunctional chain extenders such as butanediol and/or increasing foam density and/or by increasing the amount of crosslinker used Decline.
在一个实施方案中,降低交联的程度且由此增加泡沫的韧度和/或破裂伸展度应能够进行更有效的网状化。在另一个实施方案中,产生的较高密度泡沫材料可以更好地经受一个或多个网状化步骤,例如两个网状化步骤的突然冲击并且可以提供对小连接体16的最小损害(如果有的话)。在一个实施方案中,本发明提供了制备柔性聚氨基甲酸酯生物耐久性基质的方法,该基质能够制备基于聚碳酸酯多元醇成分和异氰酸酯成分原料网状化。在另一个实施方案中,提供了制备回弹性聚氨基甲酸酯基质的多孔生物耐久性弹性体聚合方法,该方法包括混合聚碳酸酯多元醇成分和脂族异氰酸酯成分,例如H12MDI。In one embodiment, reducing the degree of crosslinking and thereby increasing the toughness and/or burst stretch of the foam should enable more efficient reticulation. In another embodiment, the resulting higher density foam can better withstand the sudden impact of one or more reticulation steps, such as two reticulation steps, and can provide minimal damage to small connectors 16 ( if so). In one embodiment, the present invention provides a method for preparing a flexible polyurethane biodurable matrix capable of reticulation based on a polycarbonate polyol component and an isocyanate component raw material. In another embodiment, there is provided a porous biodurable elastomeric polymerization process for preparing a resilient polyurethane matrix comprising mixing a polycarbonate polyol component and an aliphatic isocyanate component, such asH12 MDI.
在另一个实施方案中,所述的泡沫基本上不含异氰酸酯键。在另一个实施方案中,所述的泡沫不具有异氰酸酯键。在另一个实施方案中,所述的泡沫基本上不含缩二脲键。在另一个实施方案中,所述的泡沫不具有缩二脲键。在另一个实施方案中,所述的泡沫基本上不含脲基甲酸酯键。在另一个实施方案中,所述的泡沫不具有脲基甲酸酯键。在另一个实施方案中,所述的泡沫基本上不含异氰尿酸酯和缩二脲键。在另一个实施方案中,所述的泡沫不具有异氰尿酸酯和缩二脲键。在另一个实施方案中,所述的泡沫基本上不含异氰尿酸酯和脲基甲酸酯键。在另一个实施方案中,所述的泡沫不具有异氰尿酸酯和脲基甲酸酯键。在另一个实施方案中,所述的泡沫基本上不含异氰尿酸酯和缩二脲键。在另一个实施方案中,所述的泡沫不具有脲基甲酸酯和缩二脲键。在另一个实施方案中,所述的泡沫基本上不含脲基甲酸酯,缩二脲和异氰尿酸酯键。在另一个实施方案中,所述的泡沫不具有脲基甲酸酯,缩二脲和异氰尿酸酯键。不受任何特定理论约束,认为不存在脲基甲酸酯,缩二脲和/或异氰尿酸酯键因较低程度的硬节段交联而为弹性基质提供了加强的柔韧度。In another embodiment, the foam is substantially free of isocyanate linkages. In another embodiment, the foam has no isocyanate linkages. In another embodiment, the foam is substantially free of biuret linkages. In another embodiment, the foam does not have biuret linkages. In another embodiment, the foam is substantially free of allophanate linkages. In another embodiment, the foam has no allophanate linkages. In another embodiment, the foam is substantially free of isocyanurate and biuret linkages. In another embodiment, the foam is free of isocyanurate and biuret linkages. In another embodiment, the foam is substantially free of isocyanurate and allophanate linkages. In another embodiment, the foam is free of isocyanurate and allophanate linkages. In another embodiment, the foam is substantially free of isocyanurate and biuret linkages. In another embodiment, the foam is free of allophanate and biuret linkages. In another embodiment, the foam is substantially free of allophanate, biuret and isocyanurate linkages. In another embodiment, the foam is free of allophanate, biuret and isocyanurate linkages. Without being bound by any particular theory, it is believed that the absence of allophanate, biuret and/or isocyanurate linkages provides enhanced flexibility to the elastic matrix due to a lower degree of hard segment crosslinking.
在某些实施方案中,可以包括有助于获得稳定泡沫的添加剂,例如表面活性剂和催化剂。通过将这类添加剂的量限制在最低的需求,同时维持各添加剂的官能度,可以控制对产品毒性的影响。In certain embodiments, additives such as surfactants and catalysts may be included to assist in obtaining a stable foam. By limiting the amount of such additives to the minimum necessary while maintaining the functionality of each additive, the impact on product toxicity can be controlled.
在一个实施方案中,生产了各种密度的弹性基质,例如约0.005-约0.15g/cc(约0.31-约9.4 lb/ft3)。通过例如制品中起泡剂或发泡剂的量,异氰酸酯指数,异氰酸酯成分含量、反应放热曲线和/或起泡环境的压力来控制密度。In one embodiment, elastic matrices are produced in various densities, for example from about 0.005 to about 0.15 g/cc (about 0.31 to about 9.4 lb/ft3 ). Density is controlled by, for example, the amount of blowing agent or blowing agent in the article, the isocyanate index, the isocyanate component content, the reaction exotherm, and/or the pressure of the foaming environment.
典型的起泡剂包括水和物理起泡剂,例如挥发性有机化学物质,诸如烃类,乙醇和丙酮和各种氟碳化合物及其更对环境有利的替代物,诸如氢氟碳化合物,氯氟碳化合物和氢氯氟碳化合物。水与异氰酸酯基团反应产生用作起泡剂的二氧化碳。此外,在某些实施方案中,可以使用起泡剂,诸如水与氟碳化合物的组合。在另一个实施方案中,将水用作起泡剂。商购的氟碳起泡剂购自Huntsman,E.I.duPont deNemours和Co.(Wilmington,DE),Allied Chemical(Minneapolis,MN)和Honeywell(Morristown,NJ)。Typical blowing agents include water and physical blowing agents such as volatile organic chemicals such as hydrocarbons, ethanol and acetone and various fluorocarbons and their more environmentally friendly alternatives such as hydrofluorocarbons, chlorine Fluorocarbons and Hydrochlorofluorocarbons. Water reacts with isocyanate groups to produce carbon dioxide which acts as a blowing agent. Additionally, in certain embodiments, a blowing agent, such as a combination of water and a fluorocarbon, may be used. In another embodiment, water is used as a blowing agent. Commercially available fluorocarbon blowing agents are available from Huntsman, E.I. duPont de Nemours and Co. (Wilmington, DE), Allied Chemical (Minneapolis, MN) and Honeywell (Morristown, NJ).
就本发明的目的而言,就用于通过起泡和交联制备弹性基质的每100重量份(或100克)的多元醇成分(例如聚碳酸酯多元醇,聚硅氧烷多元醇)而言,在制品中存在的按重量计的其它成分量如下:约10-约90份(或克)的具有约0.85-约1.10的异氰酸酯指数的异氰酸酯成分(例如MDIs,其混合物,H12MDI),约0.5-约6.0份(或克)起泡剂(例如水),约0.1-约2.0份(或克)起泡催化剂(例如叔胺),约0.1-约8.0份(或克)表面活性剂和约0.1-约8.0份(或克)隔室开放剂。当然,所用实际异氰酸酯成分的量涉及并且取决于特定制品的异氰酸酯指数的数量级。另外,就用于通过起泡和交联制备弹性基质的每100重量份(或100克)的多元醇成分而言,如果存在,那么制品中下列任选成分的量按重量计如下:约达20份(或克)增链剂,约达20份(或克)交联剂,约达0.5份(或克)胶凝催化剂(例如包含锡的化合物),约达10.0份(或克)物理起泡剂(例如烃类,乙醇,丙酮,氟碳化合物)和约达15份(或克)粘度改进剂。For the purposes of the present invention, per 100 parts by weight (or 100 grams) of polyol components (e.g. polycarbonate polyols, polysiloxane polyols) used to prepare the elastic matrix by foaming and crosslinking In other words, the other ingredients are present in the article in the following amounts by weight: from about 10 to about 90 parts (or grams) of an isocyanate component (e.g., MDIs, mixtures thereof, H12 MDI) having an isocyanate index of from about 0.85 to about 1.10 , about 0.5-about 6.0 parts (or gram) foaming agent (such as water), about 0.1-about 2.0 parts (or gram) foaming catalyst (such as tertiary amine), about 0.1-about 8.0 parts (or gram) surface active agent and about 0.1 to about 8.0 parts (or grams) of a compartment opener. Of course, the amount of actual isocyanate component used will relate to and depend on the order of the isocyanate index of the particular article. Additionally, for every 100 parts by weight (or 100 grams) of polyol component used to prepare the elastic matrix by foaming and crosslinking, if present, the following optional ingredients are present in the article in amounts by weight as follows: 20 parts (or gram) of chain extender, up to about 20 parts (or gram) of crosslinking agent, up to about 0.5 part (or gram) of gelling catalyst (such as a compound containing tin), up to about 10.0 parts (or gram) of physical Foaming agents (such as hydrocarbons, ethanol, acetone, fluorocarbons) and up to about 15 parts (or grams) of viscosity modifier.
在其它实施方案中,就用于通过起泡和交联制备弹性基质的每100重量份(或100克)的多元醇成分(例如聚碳酸酯多元醇,聚硅氧烷多元醇)而言,在制品中存在的按重量计的其它成分量如下:约10-约90份(或克)的具有约0.85-约1.2,在一个实施方案中,约0.85-约1.019异氰酸酯指数的异氰酸酯成分(例如MDIs,其混合物,H12MDI)。在另一个实施方案中,约0.5-约6.0份(或克)起泡剂(例如水),任选约0.05-约3.0份(或克)催化剂(例如叔胺),诸如起泡催化剂和/或胶凝催化剂,约0.1-约8.0份(或克)表面活性剂,任选约0.1-约8.0份(或克)隔室开放剂,任选约0.05-约8.0份(或克)交联剂,例如甘油和任选约0.05-约8.0份(或克)增链剂,例如1,4-丁二醇。In other embodiments, for every 100 parts by weight (or 100 grams) of polyol components (such as polycarbonate polyols, polysiloxane polyols) used to prepare the elastic matrix by foaming and crosslinking, The other ingredients are present in the article in amounts by weight of from about 10 to about 90 parts (or grams) of an isocyanate component having an isocyanate index of from about 0.85 to about 1.2, and in one embodiment, from about 0.85 to about 1.019 (e.g. MDIs, mixtures thereof, H12 MDI). In another embodiment, from about 0.5 to about 6.0 parts (or grams) of a blowing agent (such as water), optionally from about 0.05 to about 3.0 parts (or grams) of a catalyst (such as a tertiary amine), such as a blowing catalyst and/or or a gelling catalyst, about 0.1 to about 8.0 parts (or grams) of surfactant, optionally about 0.1 to about 8.0 parts (or grams) of a compartment opener, optionally about 0.05 to about 8.0 parts (or grams) of crosslinking agent, such as glycerin and optionally from about 0.05 to about 8.0 parts (or grams) of a chain extender, such as 1,4-butanediol.
然后可以使具有适合于本发明目的特性的基质网状化,所述特性正如通过测试例如在人体温度下可接受的压缩变定,气流,拉伸强度和压缩特性确定的。A matrix may then be reticulated having properties suitable for the purposes of the present invention as determined by testing, for example, acceptable compression set at body temperature, airflow, tensile strength and compression properties.
在另一个实施方案中,省略了胶凝催化剂,例如锡催化剂并且任选用另一种催化剂,例如叔胺替代。在一个实施方案中,叔胺催化剂包括一种或多种非芳族胺类。在另一个实施方案中,使反应进行以便使叔胺催化剂(如果使用的话)完全反应成聚合物并且避免相同的残基。在另一个实施方案中,省略了胶凝催化剂并且代之以使用较高起泡温度。In another embodiment, a gelling catalyst, such as a tin catalyst, is omitted and optionally replaced with another catalyst, such as a tertiary amine. In one embodiment, the tertiary amine catalyst includes one or more non-aromatic amines. In another embodiment, the reaction is carried out so that the tertiary amine catalyst (if used) reacts completely to the polymer and avoids identical residues. In another embodiment, the gelling catalyst is omitted and a higher bubble temperature is used instead.
在另一个实施方案中,为了加强生物耐久性和生物相容性,选择用于聚合过程的组分以避免在终产物中存在生物不良物质或易受生物攻击影响的物质或将这种情况减少至最低限度。In another embodiment, to enhance biodurability and biocompatibility, the components used in the polymerization process are selected to avoid or minimize the presence of biologically undesirable or susceptible to biological attack in the final product to a minimum.
本发明可选择的制备实施方案涉及用可除去的水溶性球体,填充剂或颗粒部分或完全取代水作为起泡剂,所述除去例如在使基质完全交联后通过洗涤,提取或熔化进行。An alternative manufacturing embodiment of the present invention involves the partial or complete replacement of water as foaming agent by water soluble spheres, fillers or particles which are removed eg by washing, extraction or melting after fully crosslinking the matrix.
本发明其它方法的方面Aspects of other methods of the invention
现在参考图2,所示的程序框示意图给出了本发明方法可选择实施方案的广泛概述,由此可以由粗的弹性体或弹性体试剂通过几种不同加工途径中的一种或另一种制备包括生物耐久性多孔网状弹性基质10的可植入装置。Referring now to FIG. 2, a block schematic diagram is shown that provides a broad overview of alternative embodiments of the method of the present invention whereby a crude elastomer or elastomer reagent can be processed by one or the other of several different processing routes. An implantable device comprising a biodurable porous reticulated
在第一途径中,通过使用例如在其制备过程中使用的一种或多种起泡剂使得通过如本文所述方法制备的弹性体包括多个隔室。特别地,可以包括例如多元醇成分,异氰酸酯,任选的交联剂和任意所需的添加剂,诸如表面活性剂等的原料40用于在合成步骤42中合成所需弹性聚合物,其具有或不具有明显的起泡或其它生成孔的活性。选择原料以便提供所需的机械性能并且促进生物相容性和生物耐久性。然后在步骤48中在有关化学性质和纯度,物理和机械性能方面且任选也在生物特性方面均如上所述表征步骤42的弹性聚合物产物,从而得到充分表征的弹性体50。任选表征的数据可以用于控制或改变步骤42以便如途径51中所示加强加工或产品。In a first approach, an elastomer prepared by a method as described herein is rendered to include multiple compartments through the use, for example, of one or more blowing agents used during its preparation. In particular, a
可选择地,由原料40生成充分表征的弹性体50并且由商品出售者60提供至加工设备。根据公知方法合成这类弹性体且随后赋予其多孔性。这类典型的弹性体为BIONATE 80A芳族聚碳酸酯-氨基甲酸酯弹性体(来自Polymer Technology Group Inc.,Berkeley,CA),CARBOTHANE PC 3575 A脂族聚氨基甲酸酯弹性体(Noveon Inc.,Cleveland,OH),CARBOSIL硅氧烷聚碳酸酯氨基甲酸酯(来自PolymerTechnology Group),BIOSPAN节段化聚氨基甲酸酯(来自PolymerTechnology Group)和CHRONOFLEX AL和CHRONOFLEX C(来自CardioTech International Inc.,Wilmington,MA)。可以通过在聚合反应或聚合后步骤中使用的起泡剂赋予弹性体50多孔性。在聚合后步骤(例如以商购典型弹性体或多种弹性体为原料)中一种或多种起泡剂可以例如通过其中吸收和/或其上吸附,任选在升温和/或升压影响下进入原料,此后使起泡气体从起泡剂中释放以便形成包括孔的弹性基质。在一个实施方案中,所述的孔互联。互联的量取决于例如施加于聚合物的温度,施加于聚合物的压力,聚合物内的气体浓度,聚合物表面上的气体浓度,气体释放速率和/或气体释放模式。Alternatively, well-characterized
如果需要,可以选择原料40中使用的弹性聚合物试剂以避免不良副产物或残留物,并且如果需要,在步骤52中纯化。然后在选择和纯化的原料上进行聚合物合成步骤54,并且进行以避免生成不良副产物或残留物。然后如上述对步骤48中所述在步骤56中表征步骤54中产生的弹性聚合物,以便有利于高质量充分确定的产物,即充分表征的弹性体50产生。在另一个实施方案中,这种表征结果为途径58所述过程控制的反馈,从而有利于产生高质量,充分确定的产物,即充分表征的弹性体50。The elastomeric polymer reagent used in
在一个实施方案中,本发明提供了网状生物耐久性弹性基质,其包括特别为生物医学植入设计的聚合物元件。弹性基质包括生物耐久性聚合物材料并且通过避免使该聚合物发生化学改变的一种或多种方法制备,所述的化学改变形成不需要的副产物和包括不需要的未反应原料的残留物。在某些情况中,包括聚氨基甲酸酯类并且通过公知技术生成的泡沫因存在不需要的未反应原料或不需要的副产物而并不适合于长期血管内,矫形外科应用和相关应用。在一个实施方案中,弹性基质由商购生物耐久性聚合物弹性材料构成并且针对原料弹性材料的化学改变在形成多孔和网状弹性基质的一种或多种方法中得以避免。In one embodiment, the present invention provides a reticulated biodurable elastic matrix comprising polymeric elements specifically designed for biomedical implantation. The elastic matrix comprises a biodurable polymeric material and is prepared by one or more methods that avoid chemical alterations of the polymer that form unwanted by-products and residues including unwanted unreacted raw materials . In some cases, foams comprising polyurethanes and produced by known techniques are not suitable for long-term endovascular, orthopedic and related applications due to the presence of unwanted unreacted raw materials or unwanted by-products. In one embodiment, the elastic matrix is constructed of a commercially available biodurable polymeric elastic material and chemical alterations to the raw elastic material are avoided in one or more methods of forming the porous and reticulated elastic matrix.
在另一个实施方案中,用于制造弹性基质10的弹性体的生物耐久性的化学特征包括如下的一种或多种:良好的氧化稳定性;不含或基本上不含易于发生生物降解的键,例如某些聚醚键或可水解酯键的化学特性,所述的键可以通过加入聚醚或聚酯多元醇成分而引入聚氨基甲酸酯;相对精制或纯化且不含或基本上不含不良杂质,反应剂,副产物的化学上充分确定的产物;低聚物等;充分确定的分子量,除非弹性体为交联的;和生物相容性溶剂中的溶解性,当然,除非该弹性体为交联的。In another embodiment, the biodurable chemical characteristics of the elastomer used to make
在另一个实施方案中,涉及用于制备实体相12的弹性体的方法相关特征,例如用于制造弹性基质10的弹性体的生物耐久性包括如下中的一种或多种:方法的可再现性;对产物稠度的过程控制;和避免或基本上除去不良杂质、反应剂、副产物、低聚物等。In another embodiment, process-related characteristics related to the elastomer used to make
在某些实施方案中,谨慎设计并且控制下述本发明成孔的网状和其它聚合后过程。为了这一目的,在某些实施方案中,本发明的方法避免引入不需要的残留物,否则就会对原料的所需生物耐久性产生不良影响。在另一个实施方案中,可以进一步加工和/或表征原料以便促进,提供或证明与生物耐久性相关的特性。在另一个实施方案中,可以将弹性体的必需特性表征为合适的,并且根据本说明书的教导,工艺特征可以为适合的或受控的。In certain embodiments, the pore-forming reticulation and other post-polymerization processes of the present invention described below are carefully designed and controlled. To this end, in certain embodiments, the methods of the present invention avoid the introduction of unwanted residues that would otherwise adversely affect the desired biodurability of the feedstock. In another embodiment, the feedstock can be further processed and/or characterized in order to promote, provide or demonstrate properties associated with biodurability. In another embodiment, the necessary properties of the elastomer can be characterized as suitable, and the process characteristics can be suitable or controlled according to the teachings of this specification.
通过微波照射形成至少部分网状弹性基质Formation of an at least partially reticulated elastic matrix by microwave irradiation
形成本发明至少部分网状弹性基质的另一种方式通过使用微波照射技术进行。在该方法中,将100重量份的弹性材料,诸如聚碳酸酯氨基甲酸酯或聚碳酸酯氨基甲酸酯脲用作原料,优选以颗粒或片状物的形式提供。任选使用合适的熔化掺合机或混合器,诸如挤压机,双螺杆挤出机或Brabender PLASTOGRAPH将弹性材料与例如约2-约70重量份(在一个实施方案中),约10-约35重量份(在另一个实施方案中)的更具亲水性的聚合物材料,诸如聚(乙酸乙烯酯)(PVA),聚乙烯-共-乙酸乙烯酯)(EVA),聚(乙烯醇)或其任意的混合物混合成混合物。所述的掺合机或混合器可以具有螺杆,桨或磁搅拌器。在混合过程中还加入约0.1-约20重量份(在一个实施方案中),约0.25-约5重量份(在另一个实施方案中)的交联剂。在混合过程中还加入约1-约20重量份(在一个实施方案中),约5-约15重量份(在另一个实施方案中)的一种或多种起泡剂。在另一个实施方案中,在混合过程中还加入交联剂和一种或多种起泡剂。Another way of forming the at least partially reticulated elastic matrix of the present invention is through the use of microwave irradiation techniques. In this method, 100 parts by weight of an elastic material such as polycarbonate urethane or polycarbonate urethane urea is used as a raw material, preferably provided in the form of pellets or flakes. Optionally using a suitable melt blender or mixer, such as an extruder, a twin-screw extruder or a Brabender PLASTOGRAPH, the elastomeric material is mixed with, for example, from about 2 to about 70 parts by weight (in one embodiment), from about 10 to about 35 parts by weight (in another embodiment) of a more hydrophilic polymeric material such as poly(vinyl acetate) (PVA), polyethylene-co-vinyl acetate) (EVA), poly(vinyl alcohol ) or any mixture thereof to form a mixture. The blender or mixer may have a screw, paddle or magnetic stirrer. Also added during mixing is about 0.1 to about 20 parts by weight (in one embodiment), about 0.25 to about 5 parts by weight (in another embodiment) of a crosslinking agent. From about 1 to about 20 parts by weight (in one embodiment), from about 5 to about 15 parts by weight (in another embodiment) of one or more blowing agents are also added during mixing. In another embodiment, a crosslinking agent and one or more blowing agents are also added during mixing.
可以使用在约2.2-约6.0Giga Hertz(GHz)(一个实施方案中),在约2.45GHz(在另一个实施方案中)或在约5.8GHz(在另一个实施方案中)频率下生成的微波照射在密闭室内将所得混合物加热成具有互联和互通孔的起泡的至少部分网状弹性基质结构。任选还在进行微波照射的相同密闭室内例如通过加热或对流加热将该混合物加热至约70℃-约225℃的温度(在一个实施方案中)或约100℃-约180℃(在另一个实施方案中),以便有助于形成具有互联和互通孔的起泡的至少部分网状弹性基质结构。因此,如果存在,那么有益性在于更具亲水性的聚合物材料易于在微波照射过程中加热,由此促进包括它的混合物的加热或起泡。在一个实施方案中,选择更具亲水性的聚合物材料,使得其介电损耗和/或介电损耗正切足够大,使得更具亲水性的聚合物材料易于在所用微波照射频率下加热。Microwaves generated at a frequency of about 2.2 to about 6.0 Giga Hertz (GHz) (in one embodiment), at about 2.45 GHz (in another embodiment) or at about 5.8 GHz (in another embodiment) may be used Irradiation heats the resulting mixture in a closed chamber into an at least partially reticulated elastic matrix structure of cells having interconnected and communicating pores. Optionally also heating the mixture to a temperature of from about 70°C to about 225°C in one embodiment, or from about 100°C to about 180°C in another, such as by heating or convective heating, within the same closed chamber in which the microwave irradiation was performed. embodiment) so as to facilitate the formation of an at least partially reticulated elastic matrix structure of cells having interconnected and intercommunicating pores. Thus, if present, it is beneficial that the more hydrophilic polymeric material tends to heat during microwave irradiation, thereby facilitating heating or foaming of mixtures including it. In one embodiment, the more hydrophilic polymeric material is selected such that its dielectric loss and/or dielectric loss tangent are sufficiently large such that the more hydrophilic polymeric material is readily heated at the microwave irradiation frequency used .
该方法可以为批量生产法或连续生产法。任选形成的弹性基质可以如下所述进一步网状化以便获得所需的渗透性。The method can be a batch production method or a continuous production method. The optionally formed elastic matrix can be further reticulated as described below to achieve the desired permeability.
按照本发明的其它实施方案,生物耐久性弹性材料选自聚碳酸酯聚氨基甲酸酯脲,聚碳酸酯聚脲氨基甲酸酯,聚碳酸酯聚氨基甲酸酯,聚碳酸酯聚硅氧烷聚氨基甲酸酯,聚碳酸酯聚硅氧烷聚氨基甲酸酯脲,聚硅氧烷聚氨基甲酸酯,聚硅氧烷聚氨基甲酸酯脲,聚碳酸酯烃聚氨基甲酸酯,聚碳酸酯烃聚氨基甲酸酯脲或其任意的混合物。其中特别关注热塑弹性体,诸如聚氨基甲酸酯类,其化学特性与例如良好的生物耐久性相关。在一个实施方案中,这类热塑性聚氨基甲酸酯弹性体包括聚碳酸酯聚氨基甲酸酯类,聚酯聚氨基甲酸酯类,聚醚聚氨基甲酸酯类,聚硅氧烷聚氨基甲酸酯类,烃聚氨基甲酸酯类(即由每个分子平均包括约2个异氰酸酯基团的至少一种异氰酸酯成分和至少一种羟基封端的烃低聚物和/或烃聚合物形成的那些热塑弹性体聚氨基甲酸酯类),具有所谓的“混合”软节段的聚氨基甲酸酯类及其混合物。混合的软节段聚氨基甲酸酯类为本领域技术人员公知的并且包括,例如聚碳酸酯-聚酯聚氨基甲酸酯类,聚碳酸酯-聚醚聚氨基甲酸酯类,聚碳酸酯聚硅氧烷聚氨基甲酸酯类,聚碳酸酯-烃聚氨基甲酸酯类,聚碳酸酯-聚硅氧烷-烃聚氨基甲酸酯类,聚酯-聚醚聚氨基甲酸酯类,聚酯-聚硅氧烷聚氨基甲酸酯类,聚酯-烃聚氨基甲酸酯类,聚醚-聚硅氧烷聚氨基甲酸酯类,聚醚-烃聚氨基甲酸酯类,聚醚-聚硅氧烷-烃聚氨基甲酸酯类和聚硅氧烷-烃聚氨基甲酸酯类。在另一个实施方案中,热塑性聚氨基甲酸酯弹性体包括聚碳酸酯聚氨基甲酸酯类,聚醚聚氨基甲酸酯类,聚硅氧烷聚氨基甲酸酯类,烃聚氨基甲酸酯类,具有这些混合软节段的聚氨基甲酸酯类或其混合物。在另一个实施方案中,热塑性聚氨基甲酸酯弹性体包括聚碳酸酯聚氨基甲酸酯类,聚硅氧烷聚氨基甲酸酯类,烃聚氨基甲酸酯类,具有这些混合软节段的聚氨基甲酸酯类或其混合物。在另一个实施方案中,热塑性聚氨基甲酸酯弹性体为聚碳酸酯聚氨基甲酸酯或其混合物。在另一个实施方案中,热塑性聚氨基甲酸酯弹性体为聚硅氧烷聚氨基甲酸酯或其混合物。在另一个实施方案中,热塑性聚氨基甲酸酯弹性体为聚硅氧烷聚氨基甲酸酯或其混合物。在另一个实施方案中,热塑性聚氨基甲酸酯弹性体包括异氰酸酯成分中的至少一种二异氰酸酯,至少一种增链剂和至少一种二元醇并且可以由如上文详细描述的二异氰酸酯类,双官能增链剂和二元醇类的任意组合形成。According to other embodiments of the present invention, the biodurable elastic material is selected from polycarbonate polyurethane urea, polycarbonate polyurea urethane, polycarbonate polyurethane, polycarbonate polysiloxane Alkane Polyurethane, Polycarbonate Polysiloxane Polyurethane Urea, Polysiloxane Polyurethane, Polysiloxane Polyurethane Urea, Polycarbonate Hydrocarbon Polyurethane ester, polycarbonate hydrocarbon polyurethane urea or any mixture thereof. Particular attention is paid here to thermoplastic elastomers, such as polyurethanes, whose chemical properties correlate, for example, with good biological durability. In one embodiment, such thermoplastic polyurethane elastomers include polycarbonate polyurethanes, polyester polyurethanes, polyether polyurethanes, polysiloxane polyurethanes Hydrocarbon polyurethanes (i.e., those thermoplastics formed from at least one isocyanate component comprising an average of about 2 isocyanate groups per molecule and at least one hydroxyl-terminated hydrocarbon oligomer and/or hydrocarbon polymer Elastomeric polyurethanes), polyurethanes with so-called "hybrid" soft segments, and mixtures thereof. Hybrid soft segment polyurethanes are well known to those skilled in the art and include, for example, polycarbonate-polyester polyurethanes, polycarbonate-polyether polyurethanes, polycarbonate polysilicon Oxane polyurethanes, polycarbonate-hydrocarbon polyurethanes, polycarbonate-polysiloxane-hydrocarbon polyurethanes, polyester-polyether polyurethanes, polyester-poly Silicone polyurethanes, polyester-hydrocarbon polyurethanes, polyether-polysiloxane polyurethanes, polyether-hydrocarbon polyurethanes, polyether-polysiloxane- Hydrocarbon polyurethanes and polysiloxane-hydrocarbon polyurethanes. In another embodiment, thermoplastic polyurethane elastomers include polycarbonate polyurethanes, polyether polyurethanes, polysiloxane polyurethanes, hydrocarbon polyurethanes, Polyurethanes or mixtures thereof having these mixed soft segments. In another embodiment, thermoplastic polyurethane elastomers include polycarbonate polyurethanes, polysiloxane polyurethanes, hydrocarbon polyurethanes, poly Urethanes or mixtures thereof. In another embodiment, the thermoplastic polyurethane elastomer is polycarbonate polyurethane or a mixture thereof. In another embodiment, the thermoplastic polyurethane elastomer is a silicone polyurethane or a mixture thereof. In another embodiment, the thermoplastic polyurethane elastomer is a silicone polyurethane or a mixture thereof. In another embodiment, the thermoplastic polyurethane elastomer comprises at least one diisocyanate in the isocyanate component, at least one chain extender and at least one diol and can be composed of diisocyanates as described in detail above , any combination of difunctional chain extender and diols.
在一个实施方案中,热塑性弹性体的重均分子量约为30,000-约500,000道尔顿。在另一个实施方案中,热塑性弹性体的重均分子量约为50,000-约250,000道尔顿。In one embodiment, the thermoplastic elastomer has a weight average molecular weight of from about 30,000 to about 500,000 Daltons. In another embodiment, the thermoplastic elastomer has a weight average molecular weight of from about 50,000 to about 250,000 Daltons.
在一个实施方案中,用于实施本发明的适合于如本文所述表征的某些合适的热塑性材料可以包括:如Pinchuk等在美国专利US5,741,331(及其分案的美国专利US6,102,939和6,197,240)所述具有交替仲和季碳的聚烯烃聚合物;具有弹性嵌段,例如聚烯烃和热塑性嵌段的嵌段的共聚物,例如苯乙烯,如Pinchuk等在美国专利申请公开号US 2002/0107330A1中所述;热塑性分节段的聚醚酯,热塑性聚二甲基硅氧烷,二-嵌段聚苯乙烯聚丁二烯,三-嵌段聚苯乙烯聚丁二烯,聚(丙烯基烯醚砜)-聚(碳酸丙烯酯)嵌段共聚物,聚丁二烯和聚异戊二烯的二-嵌段共聚物,乙烯乙酸乙烯酯的共聚物(EVA),分节段的嵌段共-聚苯乙烯聚环氧乙烷,二-嵌段共-聚苯乙烯聚环氧乙烷和三-嵌段共-聚苯乙烯聚环氧乙烷的共聚物,例如如Penhasi在美国专利申请公开号US 2003/0208259 A1(特别参见其中的段落[0035])中所述;和具有混合的软节段的聚氨基甲酸酯类,所述的混合的软节段包括聚硅氧烷与聚醚和/或聚碳酸酯成分,如Meijs等在美国专利US6,313,254中所述;和DiDomenico等在美国专利US 6,149,678,6,111,052和5,986,034中所述的那些聚氨基甲酸酯类。另外适用于实施本发明的是通过如本文所述的本发明方法合成的新或已知的弹性体。在另一个实施方案中,可以将任选的治疗剂载入用于实施本发明的其它弹性体的适当嵌段。In one embodiment, some suitable thermoplastic materials suitable for characterization as described herein for use in the practice of the present invention may include: Pinchuk et al. in U.S. Patent 5,741,331 (and its divisional U.S. 6,197,240) polyolefin polymers with alternating secondary and quaternary carbons; copolymers with elastomeric blocks, such as polyolefins, and thermoplastic blocks, such as styrene, such as Pinchuk et al. in U.S. Patent Application Publication No. US 2002 /0107330A1 described; thermoplastic segmented polyether ester, thermoplastic polydimethylsiloxane, di-block polystyrene polybutadiene, tri-block polystyrene polybutadiene, poly( Propylene ether sulfone)-poly(propylene carbonate) block copolymer, di-block copolymer of polybutadiene and polyisoprene, copolymer of ethylene vinyl acetate (EVA), segmented Co-polymers of block co-polystyrene polyethylene oxide, di-block co-polystyrene polyethylene oxide and tri-block co-polystyrene polyethylene oxide, such as Penhasi described in U.S. Patent Application Publication No. US 2003/0208259 A1 (see especially paragraph [0035] therein); and polyurethanes having a blended soft segment comprising polysilicon Oxane and polyether and/or polycarbonate components, such as those described in Meijs et al., US Pat. No. 6,313,254; and DiDomenico et al., those polyurethanes described in US Pat. Also suitable for use in the practice of the present invention are new or known elastomers synthesized by the methods of the invention as described herein. In another embodiment, optional therapeutic agents may be loaded into appropriate blocks of other elastomers used in the practice of this invention.
适用于实施本发明的某些商购热塑性弹性体包括由PolymerTechnology Group Inc.在商标BIONATE下提供的聚碳酸酯聚氨基甲酸酯类系列。例如,得到非常充分表征等级的聚碳酸酯聚氨基甲酸酯聚合物BIONATE 80A,55和90为可加工的,据报导它们具有良好的机械性能,无细胞毒性,无诱变性,无致癌性和非溶血性。适用于实施本发明的另一种商购弹性体为购自CardioTech International,Inc.的生物耐久性医学等级的聚碳酸酯芳族聚氨基甲酸酯热塑性弹性体CHRONOFLEX C系列。适用于实施本发明的另一种商购弹性体为热塑性聚氨基甲酸酯弹性体的PELLETHANE系列,特别是2363系列产品,且更具体地说为那些命名为81A和85A的由Dow ChemicalCompany(Midland,MI)提供的产品。这些商品聚氨基甲酸酯聚合物为线性的,非交联的聚合物,因此,它们易于分析且易于表征。Certain commercially available thermoplastic elastomers suitable for use in the practice of this invention include the family of polycarbonate polyurethanes offered by Polymer Technology Group Inc. under the trademark BIONATE. For example, very well characterized grades of polycarbonate polyurethane polymers BIONATE 80A, 55 and 90 are processable, they are reported to have good mechanical properties, are non-cytotoxic, non-mutagenic, and non-carcinogenic and non-hemolytic. Another commercially available elastomer suitable for use in the practice of the present invention is the CHRONOFLEX C series of biodurable medical grade polycarbonate aromatic polyurethane thermoplastic elastomers available from CardioTech International, Inc. Another commercially available elastomer suitable for use in the practice of this invention is the PELLETHANE series of thermoplastic polyurethane elastomers, particularly the 2363 series of products, and more specifically those designated 81A and 85A sold by Dow Chemical Company (Midland , MI) products provided. These commercial polyurethane polymers are linear, non-cross-linked polymers, therefore, they are easy to analyze and easy to characterize.
弹性基质的网状化Reticulation of the elastic matrix
弹性基质10可以进行各种加工后处理中的任意种以便促进其应用,其中的某些如本文所述且其中的其它一些对本领域技术人员而言显而易见。在一个实施方案中,如果并非成为所述生产方法的组成部分,那么本发明弹性基质10的网状化可以用于除去至少部分任何存在的内部“窗”,即图1中例证的残留隔室壁22。网状化倾向于增加多孔性和流体渗透性。
具有某些破裂隔室壁的多孔或泡沫材料一般称作“开室”材料或泡沫。相反,称作“网状化”或“至少部分网状化”的多孔材料具有许多,即至少约40%的应存在于相同多孔材料中的隔室壁至少部分被除去,除了仅由封闭的隔室组成的以外。在隔室壁至少部分因网状化被除去的地方,相邻网状隔室彼此开放,互联和连通。更多,即至少约65%的隔室壁被除去的多孔材料称作“进一步网状化”。如果大部分,即至少约80%或基本上所有,即至少约90%的隔室壁被除去,那么保留的多孔材料分别称作“基本上‘网状化”或“完全网状化”。根据本领域的应用,可以理解网状材料或泡沫包括至少部分开放互联隔室的网状构造。Porous or foamed materials with some ruptured cell walls are generally referred to as "open cell" materials or foams. In contrast, a porous material referred to as "reticulated" or "at least partially reticulated" has many, i.e. at least about 40%, of the compartment walls that should be present in the same porous material at least partially removed, except for the compartment walls that are only formed by closed cells. Compartment composed of other. Where compartment walls are at least partially removed by reticulation, adjacent reticulated compartments are open, interconnected and communicate with each other. Porous material in which more, ie at least about 65%, of the compartment walls are removed is referred to as "further reticulated". If a majority, ie at least about 80%, or substantially all, ie at least about 90%, of the compartment walls are removed, then the remaining porous material is said to be "substantially 'reticulated" or "fully reticulated", respectively. Depending on the application in the art, it is understood that the reticulated material or foam comprises a network of at least partially open interconnected compartments.
“网状化”一般意指至少部分除去隔室壁,而不仅是通过破碎方法使其破裂或撕裂。此外,破碎方法很不理想地产生必须通过进一步加工除去的碎屑。在另一个实施方案中,网状化方法基本上完全除去了至少部分隔室壁。例如,可以通过至少部分溶解掉隔室壁,分别称作“溶剂网状化”或“化学网状化”或通过至少部分熔化,燃烧和/或崩开隔室壁,分别称作“燃烧网状化”,“热网状化”或“冲击网状化”进行网状化。可以使来源于熔化隔室壁的熔化材料沉积在小连接体上。在一个实施方案中,这类操作可以用于本发明的方法以便使弹性基质10网状化。在另一个实施方案中,通过在网状化前施加真空抽空在弹性基质10孔中所有截留的空气。在另一个实施方案中,通过多个网状化步骤进行网状化。在另一个实施方案中,使用两次网状化步骤。在另一个实施方案中,在第一次燃烧网状化后进行第二次燃烧网状化。在另一个实施方案中,在燃烧网状化后进行化学网状化。在另一个实施方案中,在化学网状化后进行燃烧网状化。在另一个实施方案中,在第一次化学网状化后进行第二次化学网状化。"Reticulation" generally means the at least partial removal of the compartment walls, not merely their rupture or tearing by fragmentation methods. Furthermore, the crushing method undesirably produces debris that must be removed by further processing. In another embodiment, the reticulation process substantially completely removes at least some of the compartment walls. For example, the compartment walls can be burned and/or ruptured by at least partially dissolving away, respectively termed "solvent reticulation" or "chemical reticulation", or by at least partial melting, respectively termed "burning net". reticulation”, “thermal reticulation” or “impact reticulation” for reticulation. Molten material originating from the melted compartment walls can be deposited on the small connectors. In one embodiment, such manipulations may be used in the method of the present invention to reticulate the
在一个涉及矫形外科应用等的实施方案中,弹性基质10可以网状化成互联孔结构,具有平均直径或其它最大横向尺寸的孔至少约为10μm。In one embodiment involving orthopedic applications and the like,
在另一个实施方案中,弹性基质可以网状化以提出具有至少约为20μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少约为50μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少约为150μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少约为250μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少大于约250μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少大于约250μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少约450μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少大于约450μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少大于450μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有至少约500μm平均直径或其它最大横向尺寸的孔。In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least about 20 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least about 50 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least about 150 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least about 250 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least greater than about 250 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least greater than about 250 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least about 450 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least greater than about 450 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least greater than 450 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of at least about 500 μm.
在另一个涉及矫形外科应用等的实施方案中,弹性基质可以网状化以提出具有不大于约600μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有不大于约450μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有不大于约250μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有不大于约150μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有不大于约20μm平均直径或其它最大横向尺寸的孔。In another embodiment involving orthopedic applications and the like, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of no greater than about 600 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of no greater than about 450 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of no greater than about 250 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of no greater than about 150 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of no greater than about 20 μm.
在另一个涉及矫形外科应用等的实施方案中,弹性基质可以网状化以提出具有约10μm-约50μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有约20μm-约150μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有约150μm-约250μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有约250μm-约500μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有450μm-约600μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有约10μm-约500μm平均直径或其它最大横向尺寸的孔。在另一个实施方案中,弹性基质可以网状化以提出具有约10μm-约600μm平均直径或其它最大横向尺寸的孔。In another embodiment involving orthopedic applications and the like, the elastic matrix can be reticulated to present pores having an average diameter or other largest transverse dimension of from about 10 μm to about 50 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest lateral dimension of from about 20 μm to about 150 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest lateral dimension of from about 150 μm to about 250 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest lateral dimension of from about 250 μm to about 500 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest lateral dimension of 450 μm to about 600 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest lateral dimension of from about 10 μm to about 500 μm. In another embodiment, the elastic matrix can be reticulated to present pores having an average diameter or other largest lateral dimension of from about 10 μm to about 600 μm.
例如,可以任选在网状化前或之后通过溶剂提取纯化网状弹性基质。在一个实施方案中,任何这类诸如使用异丙醇的溶剂提取或其它纯化方法为相对适度的方法,进行它是为了避免对满足本发明目的必不可少的弹性基质的机械或物理性能的可能不良影响或将其降低到最低限度。For example, the reticulated elastic matrix can be purified by solvent extraction, optionally before or after reticulation. In one embodiment, any such solvent extraction with isopropanol or other purification method is a relatively modest method performed in order to avoid the possibility of mechanical or physical properties of the elastic matrix being essential for the purposes of the present invention. adverse effects or minimize them.
一个实施方案使用化学网状化,其中弹性基质在包含无机酸的酸浴中网状化。另一个实施方案使用化学网状化,其中弹性基质在包无机碱的腐蚀性浴中网状化。另一个实施方案使用溶剂网状化,其中不遗留残留物的挥发性溶剂用于该方法。另一个实施方案使用在高于25℃的温度下使用溶剂网状化。在另一个实施方案中,使用溶剂对包括聚碳酸酯聚氨基甲酸酯的弹性基质进行溶剂网状化,所述的溶剂选自四氢呋喃(“THF”),二甲基乙酰胺(“DMAC”),二甲亚砜(“DMSO”),二甲基甲酰胺(“DMF”),N-甲基-2-吡咯烷酮,也称作m-pyrol或其混合物。在另一个实施方案中,使用THF对包括聚碳酸酯聚氨基甲酸酯的弹性基质进行溶剂网状化。在另一个实施方案中,使用N-甲基-2-吡咯烷酮对包括聚碳酸酯聚氨基甲酸酯的弹性基质进行溶剂网状化。在另一个实施方案中,使用强碱对包括聚碳酸酯聚氨基甲酸酯的弹性基质进行化学网状化。在另一个实施方案中,强碱的pH至少约9。One embodiment uses chemical reticulation, wherein the elastic matrix is reticulated in an acid bath comprising a mineral acid. Another embodiment uses chemical reticulation, wherein the elastic matrix is reticulated in a caustic bath containing an inorganic base. Another embodiment uses solvent reticulation, where volatile solvents that leave no residue are used in the process. Another embodiment uses solvent reticulation at temperatures above 25°C. In another embodiment, the elastic matrix comprising polycarbonate polyurethane is solvent reticulated using a solvent selected from the group consisting of tetrahydrofuran ("THF"), dimethylacetamide ("DMAC") ), dimethylsulfoxide ("DMSO"), dimethylformamide ("DMF"), N-methyl-2-pyrrolidone, also known as m-pyrol, or mixtures thereof. In another embodiment, THF is used to solvent reticulate an elastic matrix comprising polycarbonate polyurethane. In another embodiment, N-methyl-2-pyrrolidone is used to solvent reticulate an elastic matrix comprising polycarbonate polyurethane. In another embodiment, a strong base is used to chemically reticulate an elastic matrix comprising polycarbonate polyurethane. In another embodiment, the pH of the strong base is at least about 9.
在这些化学或溶剂网状化实施方案中,可以任选洗涤网状泡沫。在这些化学或溶剂网状化实施方案中,可以任选干燥网状泡沫。In these chemical or solvent reticulation embodiments, the reticulated foam may optionally be washed. In these chemical or solvent reticulation embodiments, the reticulated foam may optionally be dried.
在一个实施方案中,可以使用燃烧网状化,其中用电火花点燃易燃气体,例如氢和氧或甲烷和氧的混合物。在另一个实施方案中,在压力室内进行燃烧网状化。在另一个实施方案中,通过在导入氢气,氧气或其混合物之前排气至少约2分钟将压力室内的压力基本上降至例如低于约50-150毫托。在另一个实施方案中,在一个以上循环中基本上降低了压力室内的压力,例如基本上降低了压力,导入不反应气体,诸如氩气或氮气,然后在导入氢气,氧气或其混合物前再次基本上降低压力。网状化进行的温度可以受例如此时维持室的温度和/或室内氢气/氧气之比的影响。在另一个实施方案中,燃烧网状化后为退火期。在这些燃烧网状化实施方案中的任意一种中,可以任选洗涤网状泡沫。在这些燃烧网状化实施方案中的任意一种中,可以任选干燥网状泡沫。In one embodiment, combustion reticulation may be used, where an electrical spark is used to ignite a flammable gas, such as a mixture of hydrogen and oxygen or methane and oxygen. In another embodiment, combustion reticulation is performed within the pressure chamber. In another embodiment, the pressure in the pressure chamber is substantially reduced, eg, below about 50-150 mTorr, by venting for at least about 2 minutes prior to introducing hydrogen, oxygen, or a mixture thereof. In another embodiment, the pressure in the pressure chamber is substantially reduced in one or more cycles, e.g. substantially reducing the pressure, introducing a non-reactive gas, such as argon or nitrogen, and then again before introducing hydrogen, oxygen, or a mixture thereof. Basically reduce stress. The temperature at which reticulation occurs can be influenced, for example, by the temperature of the maintenance chamber at this time and/or the hydrogen/oxygen ratio in the chamber. In another embodiment, combustion reticulation is followed by an annealing period. In any of these burn reticulation embodiments, the reticulated foam may optionally be washed. In any of these combustion reticulation embodiments, the reticulated foam may optionally be dried.
在一个实施方案中,网状弹性基质对流体,例如液体的渗透性大于对构成网状弹性基质的未网状化基质对流体的渗透性。在另一个实施方案中,进行网状化过程以便提供有利于细胞向内生长并且增殖入基质内部的弹性基质构造。在另一个实施方案中,进行网状化过程以便提供弹性基质构造,其有利于细胞向内生长并且增殖遍布为植入配置的弹性基质。In one embodiment, the reticulated elastic matrix is more permeable to fluids, such as liquids, than the non-reticulated matrix comprising the reticulated elastic matrix. In another embodiment, the reticulation process is performed to provide an elastic matrix configuration that facilitates cell ingrowth and proliferation into the interior of the matrix. In another embodiment, the reticulation process is performed to provide an elastic matrix configuration that facilitates cell ingrowth and proliferation throughout the elastic matrix configured for implantation.
术语“配置”等用于表示该术语所应用的相应结构的排列,成形和尺寸度量。因此,涉及“配置”结构的目的在于指定涉及为适用于所述目的而选择或设计的相关结构或结构部分的完整空间几何形状。The terms "configuration" and the like are used to denote the arrangement, shaping and dimensional measurement of the corresponding structure to which the term applies. Accordingly, reference to a "configured" structure is intended to designate the complete spatial geometry referring to the relevant structure or parts of structures selected or designed to be suitable for the stated purpose.
赋予内部孔特征Give Internal Hole Features
在孔20内,弹性基质10可以任选具有除了上述空隙或填充气体的容积以外的特征。在一个实施方案中,弹性基质10可以具有本文中称作“内部孔”的特征作为其显微结构的组成部分,即,弹性基质10“孔内”的特征。在一个实施方案中,孔20的内表面可以“内孔被涂敷”,即涂敷或处理以便赋予那些表面一定程度的所需特征,例如亲水性。涂敷或处理介质可以具有额外赋予或键合活性组分的能力,然后活性组分优选被递送至孔20。在一个实施方案中,可以使用这种涂敷介质或处理促进材料与孔内表面的共价键合,例如如请求优先权的申请中所述。在另一个实施方案中,涂层包括生物可降解或可吸收聚合物和无机成分,诸如羟磷灰石。可以通过在制成的网状弹性基质10上进行化学或照射处理,通过在弹性体装配过程中使弹性体接触亲水性,例如水环境或通过本领域技术人员公知的其它方式进行亲水性处理。Within the
此外,可以通过在液体涂敷溶液或适合于形成生物相容性聚合物薄膜的条件下的熔化状态中接触成膜生物相容性聚合物在内孔上涂布一种或多种涂层。在一个实施方案中,用于这类涂层的聚合物为具有足够高分子量的成膜生物相容性聚合物,以便不会成为蜡状或粘状。聚合物还应粘附到实体相12。在另一个实施方案中,粘合强度使得聚合物薄膜在网状弹性基质10处理或展开过程中不会破裂或脱出。In addition, one or more coatings may be applied to the inner pores by contacting the film-forming biocompatible polymer in a liquid coating solution or in a molten state under conditions suitable for forming a biocompatible polymer film. In one embodiment, the polymers used in such coatings are film-forming biocompatible polymers of sufficiently high molecular weight so as not to become waxy or sticky. The polymer should also adhere to the
合适的生物相容性聚合物包括聚酰胺类,聚烯烃类(例如聚丙烯,聚乙烯),不可吸收性聚酯类(例如聚对苯二甲酸乙酯)和生物吸收性脂族聚酯类(例如乳酸,乙醇酸,丙交酯,乙交酯,对-二噁烷酮,碳酸环丙酯,s-己内酯的均聚物和共聚物或其混合物)。此外,生物相容性聚合物包括成膜生物吸收性聚合物;它们包括脂族聚酯类,聚(氨基酸),共聚(醚-酯),聚亚烷基草酸酯类,聚酰胺类,聚(亚氨基碳酸酯类),聚原酸酯类,聚氧杂酯类,包括含酰胺基团的聚氧杂酯类,聚酰氨基酯类,聚酸酐类,聚磷腈类,生物分子或其混合物。就本发明的目的而言,脂族聚酯类包括聚合物和丙交酯(包括乳酸d-,1-和内消旋丙交酯),ε-己内酯,乙交酯(包括乙醇酸),羟基丁酸酯,羟基戊酸酯,对-二噁烷酮,碳酸环丙酯(及其烷基衍生物),1,4-二氧杂庚环-2-酮,1,5-二氧杂庚环-2-酮,6,6-二甲基-1,4-二噁烷-2-酮的共聚物或其混合物。在一个实施方案中,加强物可以由生物聚合物,诸如胶原蛋白,弹性蛋白等构成。生物聚合物可以为生物可降解或生物可吸收的。Suitable biocompatible polymers include polyamides, polyolefins (eg, polypropylene, polyethylene), nonabsorbable polyesters (eg, polyethylene terephthalate), and bioabsorbable aliphatic polyesters (for example homopolymers and copolymers of lactic acid, glycolic acid, lactide, glycolide, p-dioxanone, cyclopropyl carbonate, s-caprolactone or mixtures thereof). In addition, biocompatible polymers include film-forming bioabsorbable polymers; they include aliphatic polyesters, poly(amino acids), copoly(ether-esters), polyalkylene oxalates, polyamides, poly (imino carbonates), polyorthoesters, polyoxaesters, including polyoxaesters containing amide groups, polyamidoesters, polyanhydrides, polyphosphazenes, biomolecules or its mixture. For the purposes of this invention, aliphatic polyesters include polymers and lactides (including lactic acid d-, 1- and meso-lactide), ε-caprolactone, glycolide (including glycolic acid ), hydroxybutyrate, hydroxyvalerate, p-dioxanone, cyclopropyl carbonate (and its alkyl derivatives), 1,4-dioxepan-2-one, 1,5- A copolymer of dioxepan-2-one, 6,6-dimethyl-1,4-dioxan-2-one or a mixture thereof. In one embodiment, the reinforcement may be composed of biopolymers, such as collagen, elastin, and the like. Biopolymers can be biodegradable or bioabsorbable.
生物相容性聚合物进一步包括具有相对低长期组织反应的成膜生物耐久性聚合物,诸如聚氨基甲酸酯类,硅氧烷,聚(甲基)丙烯酸酯类,聚酯类,聚烷基环氧(例如聚环氧乙烷),聚乙烯醇类,聚乙二醇类和聚乙烯吡咯烷酮和水凝胶,诸如那些由交联聚乙烯吡咯烷酮和聚酯类形成的那些。其它聚合物也可以用作生物相容性聚合物,只要它们可以溶解,固化或聚合。这类聚合物和共聚物包括聚烯烃类,聚异丁烯和乙烯-α-烯烃共聚物;丙烯酸聚合物(包括甲基丙烯酸酯类)和共聚物;乙烯基卤聚合物和共聚物,诸如聚氯乙烯;聚乙烯醚类,诸如聚乙烯基甲基醚;聚偏卤乙烯诸如聚偏氟乙烯和聚偏二氯乙烯;聚丙烯腈;聚乙烯酮类;聚乙烯芳族化合物,诸如聚苯乙烯;聚乙烯酯类,诸如聚乙酸乙烯酯;乙烯基单体彼此和与ε-烯烃类的共聚物,诸如乙烯-甲基丙烯酸甲酯共聚物和乙烯-乙酸乙烯酯共聚物;丙烯腈-苯乙烯共聚物;ABS树脂;聚酰胺类,诸如尼龙66和聚己内酰胺;醇酸树脂;聚碳酸酯类;聚甲醛类;聚酰亚胺类;聚醚类;环氧树脂;聚氨基甲酸酯类;人造丝;人造丝-三乙酸酯;塞璐玢;纤维素及其衍生物,诸如乙酸纤维素,乙酸丁酸纤维素,硝酸纤维素,丙酸纤维素和纤维素醚类(例如羧甲基纤维素和羟基烷基纤维素);或其混合物。就本发明的目的而言,聚酰胺类包括如下通式的聚酰胺类:Biocompatible polymers further include film-forming biodurable polymers with relatively low long-term tissue response, such as polyurethanes, silicones, poly(meth)acrylates, polyesters, polyalkylene Epoxy (eg polyethylene oxide), polyvinyl alcohols, polyethylene glycols and polyvinylpyrrolidone and hydrogels such as those formed from crosslinked polyvinylpyrrolidone and polyesters. Other polymers can also be used as biocompatible polymers as long as they can be dissolved, solidified or polymerized. Such polymers and copolymers include polyolefins, polyisobutylene and ethylene-α-olefin copolymers; acrylic polymers (including methacrylates) and copolymers; vinyl halide polymers and copolymers, such as polychloride Ethylene; polyvinyl ethers such as polyvinyl methyl ether; polyvinylidene halides such as polyvinylidene fluoride and polyvinylidene chloride; polyacrylonitrile; polyvinyl ketones; polyvinyl aromatics such as polystyrene ; polyvinyl esters, such as polyvinyl acetate; copolymers of vinyl monomers with each other and with ε-olefins, such as ethylene-methyl methacrylate copolymers and ethylene-vinyl acetate copolymers; acrylonitrile-benzene Ethylene copolymers; ABS resins; polyamides such as nylon 66 and polycaprolactam; alkyd resins; polycarbonates; polyoxymethylenes; polyimides; polyethers; epoxy resins; polyurethanes ; Rayon; Rayon-triacetate; Cellophane; Cellulose and its derivatives, such as cellulose acetate, cellulose acetate butyrate, cellulose nitrate, cellulose propionate and cellulose ethers (e.g. carboxylate methylcellulose and hydroxyalkylcellulose); or mixtures thereof. For the purposes of the present invention, polyamides include polyamides of the general formula:
-N(H)-(CH2)n-C(O)-和-N(H)-(CH2)x-N(H)-C(O)-(CH12)y-C(O)-,其中n为约4-约13的整数;x为约4-约12的整数且y为约4-约16的整数。应理解上述材料目录为例证性的,而非限定性的。-N(H)-(CH2 )n -C(O)- and -N(H)-(CH2 )xN(H)-C(O)-(CH12 )y -C(O)-, wherein n is an integer from about 4 to about 13; x is an integer from about 4 to about 12; and y is an integer from about 4 to about 16. It should be understood that the foregoing list of materials is illustrative and not limiting.
一般通过使用聚合物,任选包括药物活性剂,诸如治疗剂或药物进行简单浸渍涂敷或喷涂涂敷由网状弹性基质10构成的装置。在一个实施方案中,涂层为溶液并且在涂敷溶液中的聚合物含量约为1%-约40%重量。在另一个实施方案中,涂敷溶液中聚合物含量约为1%-约20%重量。在另一个实施方案中,涂敷溶液中聚合物含量约为1%-约10%重量。Devices comprised of reticulated
为适当涂敷实体相12,特别根据适当的粘度平衡、聚合物的沉积水平、湿润速率和蒸发的溶剂速率的考虑选择用于涂敷溶液的溶剂或溶剂掺合物,正如本领域公知的。在一个实施方案中,选择溶剂,使得这类聚合物可溶于溶剂。在另一个实施方案中,从涂层中基本上完全除去溶剂。在另一个实施方案中,所述溶剂为无毒性的,非致癌性的和对环境有利的。混合溶剂系统对控制粘度和蒸发速率是有利的。在所有情况中,溶剂均不应与涂敷聚合物反应。溶剂包括,但不限于:丙酮,N-甲基吡咯烷酮(“NMP”),DMSO,甲苯,二氯甲烷,氯仿,1,1,2-三氯乙烷(“TCE”),各种氟利昂类,二噁烷,乙酸乙酯,THF,DMF和DMAC。For proper coating of the
在另一个实施方案中,成膜涂敷聚合物为热塑性聚合物,它熔化,进入弹性基质10的孔20并且在冷却或固化时在至少部分弹性基质10的固体材料12上形成涂层。在另一个实施方案中,热塑性涂敷聚合物在其熔化形式下的加工温度约高于60℃。在另一个实施方案中,热塑性涂敷聚合物在其熔化形式下的加工温度约高于90℃。在另一个实施方案中,热塑性涂敷聚合物在其熔化形式下的加工温度约高于120℃。In another embodiment, the film-forming coating polymer is a thermoplastic polymer that melts, enters the
在如下文中更具体描述的本发明的另一个实施方案中,给弹性基质10的孔20中的某些或全部涂敷或填充细胞向内生长促进剂。在另一个实施方中,可以使促进剂起泡。在另一个实施方案中,促进剂可以为膜的形式。促进剂可以为生物可降解的或可吸收的材料以便促进弹性基质10在体内的细胞侵入。促进剂包括天然存在的材料,它们可以在人体内酶促降解,或在人体内是水解不稳定的,如血纤蛋白、纤维蛋白原、胶原蛋白,弹性蛋白,透明质酸和可吸收性生物相容性多糖类,诸如脱乙酰壳多糖,淀粉,脂肪酸(及其酯类),葡萄糖-聚糖类(glucoso-glycans)和透明质酸。在某些实施方案中,如上述部分中所述涂敷或浸渍弹性基质10的孔表面,但用促进剂取代生物相容性聚合物或将促进剂加入到生物相容性聚合物中,以便促使细胞向内生长和增殖。In another embodiment of the invention, as described in more detail below, some or all of the
在一个实施方案中,进行涂敷或浸渍法以确保本文所用的产品“复合弹性可植入装置”,即网状弹性基质和涂层在压缩后保持足够的回弹性,使得它可以被递送装置,例如递送导管,注射器或内窥镜递送。现在参照胶原蛋白描述这类复合弹性可植入装置的某些实施方案,作为非限制性实例,如上所述,应理解其它材料可以用于替代胶原蛋白。In one embodiment, the coating or impregnation process is performed to ensure that the product "composite elastic implantable device" as used herein, i.e., the reticulated elastic matrix and coating retains sufficient resilience after compression so that it can be delivered by the device , such as a delivery catheter, syringe or endoscopic delivery. Certain embodiments of such composite elastic implantable devices are now described with reference to collagen, as a non-limiting example, as described above, it being understood that other materials may be used in place of collagen.
本发明的一个实施方案为制备复合弹性可植入装置的方法,包括:One embodiment of the invention is a method of making a composite elastic implantable device comprising:
a)使含水胶原蛋白淤浆浸润网状多孔弹性体,诸如弹性基质10的孔,其任选为生物耐久性弹性体产品的孔;和a) infiltrating the pores of a reticulated porous elastomer, such as
b)任选通过冻干除去水,以产生胶原蛋白涂层,其中胶原蛋白涂层任选在至少网状多孔弹性体的部分孔表面上包括孔的互联网状结构。b) removing the water, optionally by lyophilization, to produce a collagen coating, wherein the collagen coating optionally comprises a network of pores on at least a portion of the pore surface of the reticulated cellular elastomer.
可以通过例如使用压力促使胶原蛋白含水淤浆,混悬液或溶液进入弹性基质孔来浸润胶原蛋白。胶原蛋白可以为I,II或III型或其混合物。在一个实施方案中,胶原蛋白类型包括至少90%的胶原蛋白I。胶原蛋白的浓度约为0.3%-约2.0%重量且在冻干时将淤浆,混悬液或溶液的pH调整至约2.6-约5.0。可选择地,可以通过将弹性基质浸入胶原蛋白淤浆来浸润胶原蛋白。Collagen can be infiltrated by forcing an aqueous slurry, suspension or solution of collagen into the pores of the elastic matrix, for example, using pressure. Collagen can be type I, II or III or mixtures thereof. In one embodiment, the collagen type comprises at least 90% collagen I. The concentration of collagen is about 0.3% to about 2.0% by weight and the pH of the slurry, suspension or solution is adjusted to about 2.6 to about 5.0 upon lyophilization. Alternatively, the collagen can be infiltrated by dipping the elastic matrix into the collagen slurry.
与未涂敷的网状弹性体相比,复合弹性可植入装置可以具有在体积上适度减小的空隙相14。在一个实施方案中,复合弹性可植入装置保持了良好的流体渗透性和足够的多孔性以便成纤维细胞或其它细胞向内生长和增殖。Composite elastic implantable devices may have a moderately reduced volume of the
任选可以使冻干的胶原蛋白交联以便控制胶原蛋白涂层的体内酶促降解速率和/或控制胶原蛋白涂层结合弹性基质10的能力。可以通过本领域公知的方法,例如通过在抽真空室内加热,通过在基本上无湿度的惰性气体环境中加热,通过使胶原蛋白接触甲醛蒸汽或通过使用戊二醛来交联胶原蛋白。不受任何特定理论约束,认为在植入复合弹性可植入装置时,对胶原蛋白,诸如成纤维细胞具有高度亲合力的组织形成剂比未涂敷的基质更易于侵入浸渍胶原蛋白的弹性基质10。此外,不受任何特定理论约束,进一步认为随着胶原蛋白酶促降解,新组织侵入并且充满降解胶原蛋白遗留的空隙,同时还浸润和充满弹性基质10中其它可利用的空间。不受任何特定理论约束,认为这类胶原蛋白涂敷或浸渍的弹性基质10对弹性基质10的孔20内胶原蛋白的加强作用提供的结构完整性而言是额外有利的,它可以赋予弹性基质10的各种构造更大的刚性和结构稳定性。Optionally, the lyophilized collagen can be cross-linked in order to control the rate of enzymatic degradation of the collagen coating in vivo and/or to control the ability of the collagen coating to bind the
制备胶原蛋白涂敷的复合弹性可植入装置的方法以实施例3和12中为典型。其它方法对本领域技术人员而言显而易见。The method for preparing a collagen-coated composite elastic implantable device is exemplified in Examples 3 and 12. Other methods will be apparent to those skilled in the art.
涂敷的可植入装置coated implantable device
在某些应用中,由弹性基质10构成的装置可以具有至少部分涂敷或融合的最外或宏观表面,以便提供较小的宏观表面积,因为表面下的孔的内表面积不再受影响。不受任何特定理论的约束,认为这种减小的表面积提供了更可预测性和更便利的通过递送装置内部长弯曲通道的递送和转运。表面涂敷或融合改变了“表面的多孔性”,即至少部分降低了对表面开放的孔的百分比,或在限度内完全隔绝了涂敷或融合表面的孔,即表面为非孔的,因为不具有遗留在涂敷或融合表面上的孔。然而,表面涂敷或融合仍然使得弹性基质10的内部互联多孔结构保持向内和在其它未涂敷或未融合的表面上开放;例如未在该表面上的部分涂敷或融合的孔保持与其它孔互联并且那些遗留的开放表面可以培养细胞向内生长和增殖。在一个实施方案中,涂敷和未涂敷表面彼此正交。在另一个实施方案中,涂敷和未涂敷表面彼此成斜角。在一个实施方案中,涂敷和未涂敷表面彼此相邻。在另一个实施方案中,涂敷和未涂敷表面彼此不相邻。在另一个实施方案中,涂敷和未涂敷表面彼此接触。在另一个实施方案中,涂敷和未涂敷表面彼此不接触。In some applications, a device formed from the
在其它应用中,可以涂敷、融合或熔化由网状弹性基质10构成的可植入装置宏观表面的一个或多个平面,以便改善其与连接装置,例如固定凹或缝合线的连接效率,以便连接装置不会从可植入装置中穿过撕裂或拉出。不受任何特定理论的约束,认为生成锚定如上所述可植入装置上宏观表面的额外接触通过提供较少空隙和较大的阻力而抑制穿过撕裂或拉出。In other applications, one or more planes of the macroscopic surface of the implantable device constituted by the reticulated
可以按照几种不同方式引起弹性基质10的宏观表面层的融合和/或选择性熔化。在一个实施方案中,可以将用于将弹性基质10块切成用于制备最终可植入装置大小和形状的刀或刀片加热至升高的温度,例如,以实施例9中为典型。在另一个实施方案中,通过使用激光切割装置由较大块的弹性基质10切成所需形状和大小的装置,并且在该方法中融合接触激光束的表面。在另一个实施方案中,冷激光切割装置用于切割所需大小和形状的装置。在另一个实施方案中,加热的模具可以用于通过加热压缩方法将所需大小和形状赋予装置。可以将从较大块切成的略大的弹性基质10放入加热模具。模具覆盖切片以便将其总体尺寸减小至所需大小和形状并且融合这些接触加热模具的表面,例如,以实施例10中为典型。在上述各实施方案中,用于成形和定尺寸的加工温度在一个实施方案中,大于约15℃。在另一个实施方案中,用于成形和定尺寸的加工温度超过约100℃。在另一个实施方案中,用于成形和定型的加工温度超过约130℃。在另一个实施方案中,不融合的宏观表面层和/或部分通过在融合宏观表面过程中覆盖它们以防止其暴露。Fusion and/or selective melting of the macroscopic surface layer of
可以由生物相容性聚合物制成宏观表面上的涂层,所述的生物相容性聚合物可以包括生物可降解或可吸收和生物不能降解的或不能吸收的聚合物。合适的可吸收聚合物包括在前面章节中所述的生物相容性聚合物。应理解该材料目录为例证性的,但非限定性的。在一个实施方案中,通过将可吸收的聚合物熔化涂层涂布在成形的弹性基质上来封闭表面孔。弹性基质和涂层共同形成装置。在另一个实施方案中,通过将可吸收的聚合物熔化涂层涂布在成形的弹性基质上封闭表面孔而形成装置。在另一个实施方案中,涂层和弹性基质共同占据大于未涂敷的单独弹性基质的体积。Coatings on macroscopic surfaces can be made from biocompatible polymers, which can include biodegradable or absorbable and nonbiodegradable or nonabsorbable polymers. Suitable absorbable polymers include the biocompatible polymers described in the preceding sections. It should be understood that this list of materials is illustrative, but not limiting. In one embodiment, the surface pores are closed by applying a melt coating of absorbable polymer to the formed elastic substrate. The elastic matrix and coating together form the device. In another embodiment, the device is formed by applying a melt coating of an absorbable polymer to a formed elastic substrate to close surface pores. In another embodiment, the coating and the elastic substrate together occupy a larger volume than the uncoated elastic substrate alone.
例如,可以通过浸渍或喷雾含聚合物或混合有药物活性剂的聚合物的涂敷溶液在弹性基质10上来涂布涂层。在一个实施方案中,在涂敷溶液中的聚合物含量约为1%-约40%重量。在另一个实施方案中,在涂敷溶液中的聚合物含量约为1%-约20%重量。在另一个实施方案中,在涂敷溶液中的聚合物含量约为1%-约10%重量。在另一个实施方案中,未进行溶液涂敷的宏观表面层和/或部分通过在宏观表面溶液涂敷过程中覆盖它们而防止其暴露。例如,基于上述部分(即在“赋予内孔特征”部分)的考虑选择用于涂敷溶液的溶剂或溶剂掺合物。For example, the coating can be applied on the
在一个实施方案中,可以通过熔化成膜涂敷聚合物并且通过浸渍涂布将熔化的聚合物涂布在弹性基质10上来涂布弹性基质10上的涂层,以实施例11中为典型。在另一个实施方案中,可以通过熔化成膜涂敷聚合物并且通过在诸如挤压或共挤压这类过程中通过冲模来应用熔化的聚合物在弹性基质10上涂布涂层,作为弹性基质10形成的心轴上的熔化聚合物薄层。在任一这些实施方案中,熔化的聚合物涂敷宏观表面并且桥连或阻塞该表面的孔,但不会透入内部达任何有效深度。不受任何特定理论的约束,认为它是因熔化的聚合物高粘度所致。因此,维持从宏观表面上取出的部分弹性基质和不接触熔化聚合物的部分弹性基质宏观表面的网状性质。在冷却和固化时,熔化的聚合物在弹性基质10上形成固体涂布层。在一个实施方案中,熔化热塑性涂敷聚合物的加工温度至少约为60℃。在另一个实施方案中,熔化热塑性涂敷聚合物的加工温度至少约为90℃。在另一个实施方案中,熔化热塑性涂敷聚合物的加工温度至少约为120℃。在另一个实施方案中,未进行熔化涂敷的宏观表面层和/或部分通过在宏观表面熔化涂敷过程中覆盖它们而防止暴露。In one embodiment, the coating on the
本发明的另一个实施方案使用了胶原蛋白涂敷的复合物弹性可植入装置,该装置如上所述配置成在可植入装置周围伸展的套管。可以将胶原蛋白基质套管移植在组织修复和再生部位上,与该部位相邻和接触。由此定位的胶原蛋白基质套管可以用于辅助保持弹性基质10,有利于组织封闭形成并且有助于防止渗漏。在一个实施方案中,弹性基质10中存在胶原蛋白可以通过促进成纤维细胞附着于胶原蛋白而促进细胞向内生长和增殖并且改善机械稳定性。存在胶原蛋白可以刺激弹性基质10互联孔的较早和/或更完全浸润。Another embodiment of the present invention utilizes a collagen-coated composite elastic implantable device configured as described above as a sleeve that stretches around the implantable device. The collagen matrix sleeve can be implanted over, adjacent to and in contact with the site of tissue repair and regeneration. The collagen matrix sleeve thus positioned can be used to assist in retaining the
组织培养Tissue culture
本发明的生物耐久性网状弹性基质可以支持细胞类型,包括分泌结构蛋白的细胞和产生表征器官功能的蛋白质的细胞。弹性基质有利于多种细胞类型彼此共存的能力及其支持分泌蛋白质的细胞的能力表明弹性基质在器官体外和体内生长和器官重建中的应用性。此外,所述的生物耐久性网状弹性基质还可以用于扩增人体细胞系,以便对人体植入的许多应用,包括植入成纤维细胞,软骨细胞,成骨细胞,破骨细胞,骨细胞,滑膜细胞,骨髓基质细胞,干细胞,纤维软骨细胞,内皮细胞,平滑肌细胞,脂肪细胞,心肌细胞,肌细胞,角质细胞,肝细胞,白细胞,巨噬细胞,内分泌细胞,泌尿生殖细胞,淋巴管细胞,胰岛细胞,肌细胞,间质细胞,肾细胞,血管细胞,甲状腺细胞,甲状旁腺细胞,肾上腺-下丘脑-垂体轴细胞,胆管细胞,卵巢或睾丸细胞,唾液分泌细胞,肾细胞,上皮细胞,神经细胞,干细胞,先祖细胞,成肌细胞和小肠细胞。The biodurable reticulated elastic matrix of the present invention can support cell types, including cells that secrete structural proteins and cells that produce proteins that characterize organ function. The ability of elastic matrices to facilitate the coexistence of multiple cell types with each other and their ability to support protein-secreting cells suggests the applicability of elastic matrices for organ growth in vitro and in vivo and organ reconstruction. In addition, the biodurable reticulated elastic matrix can also be used to expand human cell lines for many applications in human implantation, including implantation of fibroblasts, chondrocytes, osteoblasts, osteoclasts, bone cells, synoviocytes, bone marrow stromal cells, stem cells, fibrochondrocytes, endothelial cells, smooth muscle cells, adipocytes, cardiomyocytes, myocytes, keratinocytes, hepatocytes, leukocytes, macrophages, endocrine cells, urogenital cells, Lymphatic vessel cells, islet cells, muscle cells, interstitial cells, kidney cells, vascular cells, thyroid cells, parathyroid cells, adrenal-hypothalamic-pituitary axis cells, bile duct cells, ovarian or testicular cells, salivary secreting cells, renal cells , epithelial cells, neural cells, stem cells, progenitor cells, myoblasts and intestinal cells.
可以通过植入在弹性基质中接种的细胞获得改造新组织的手段(在植入前或同时或之后)。在这种情况中,可以以封闭方式配置弹性基质以防止植入细胞接触身体免疫系统,或以开放方式使得可以将新细胞掺入体内。因此,在另一个实施方案中,可以在将弹性基质植入患者前,同时或之后将细胞掺入,即培养和增殖在弹性基质上。The means to engineer new tissues (before or simultaneously or after implantation) can be obtained by implanting cells seeded in elastic matrices. In this case, the elastic matrix can be configured in a closed manner to prevent the implanted cells from contacting the body's immune system, or in an open manner to allow the incorporation of new cells into the body. Thus, in another embodiment, cells may be incorporated, ie, cultured and proliferated, on the elastic matrix prior to, simultaneously with, or after implantation of the elastic matrix into the patient.
在一个实施方案中,可以给由生物耐久性网状弹性基质制成的可植入装置接种细胞类型并且在任选使用递送装置插入患者前培养,以利于组织修复或组织再生的目的。有必要在含有或不含刺激物,诸如应激或定向的合适的培养基中进行组织或细胞培养。所述的细胞包括成纤维细胞,软骨细胞,成骨细胞,破骨细胞,骨细胞,滑膜细胞,骨髓间质细胞,干细胞,纤维软骨细胞,内皮细胞和平滑肌细胞。In one embodiment, an implantable device made of a biodurable reticulated elastic matrix may be seeded with cell types and cultured prior to insertion into a patient, optionally using a delivery device, for the purpose of tissue repair or tissue regeneration. It is necessary to perform tissue or cell culture in a suitable medium with or without stimuli, such as stress or orientation. Said cells include fibroblasts, chondrocytes, osteoblasts, osteoclasts, bone cells, synoviocytes, bone marrow mesenchymal cells, stem cells, fibrochondrocytes, endothelial cells and smooth muscle cells.
可以在具有不同孔形态,大小,形状和定向的生物耐久性网状弹性基质上的表面用不同类型的细胞培养以便研发细胞组织基因工程的可植入装置,它们的目标特别在于矫形外科应用,尤其是在包括脊柱,肩,膝,手或关节和假体器官生长中的软组织连接,修复,再生,加强和/或支持中。在另一个实施方案中,可以如此培养具有类似孔形态,大小,形状和定向的生物耐久性网状弹性基质上的所有表面。Different types of cells can be cultured on the surface of biodurable reticulated elastic matrices with different pore morphology, size, shape and orientation for the development of cell-tissue genetically engineered implantable devices, which are especially aimed at orthopedic applications, Especially in the connection, repair, regeneration, reinforcement and/or support of soft tissue growth involving the spine, shoulder, knee, hand or joints and prosthetic organs. In another embodiment, all surfaces on a biodurable reticulated elastic matrix can be so cultured with similar pore morphology, size, shape and orientation.
在其它实施方案中,本发明的生物耐久性网状弹性基质可以在乳腺假体,起搏器罩,LVAD气囊领域中或作为组织桥连基质的应用。In other embodiments, the biodurable mesh elastic matrix of the present invention can be used in the field of breast prosthesis, pacemaker cover, LVAD balloon or as a tissue bridging matrix.
药物活性剂递送Pharmaceutical Agent Delivery
在另一个实施方案中,用于涂敷网状弹性基质10的成膜聚合物可以提供递送和/或控释药物活性剂,例如药物的载体,诸如如请求优先权的申请中所述。在另一个实施方案中,使药物活性剂与弹性基质10的涂层混合,共价键合,吸附在其上和/或吸附在其内而得到药物组合物。在另一个实施方案中,用于形成泡沫的成分,聚合物和/或掺合物包括药物活性剂。为了形成这些泡沫,将上述成分,聚合物和/或掺合物与药物活性剂混合,此后形成泡沫或在它形成后使药物活性剂载入泡沫。In another embodiment, the film-forming polymer used to coat the reticulated
在一个实施方案中,涂敷聚合物和药物活性剂具有常用的溶剂。这可以提供为溶液的涂层。在另一个实施方案中,药物活性剂可以作为固体分散体存在于涂敷聚合物在溶剂中的溶液中。In one embodiment, the coating polymer and pharmaceutically active agent have common solvents. This can be provided as a coating of solution. In another embodiment, the pharmaceutically active agent may be present as a solid dispersion in a solution of the coating polymer in a solvent.
可以通过混合一种或多种药物活性剂与用于形成泡沫的聚合物,溶剂或聚合物-溶剂混合物配制包括药物活性剂的网状弹性基质并且起泡。可选择地,在一个实施方案中,可以使用药学上可接受的载体将药物活性剂涂敷在泡沫上。在另一个实施方案中,如果使用熔化涂敷,那么药物活性剂耐受熔化加工温度,而基本上不会降低其功效。A reticulated elastic matrix comprising a pharmaceutically active agent can be formulated and foamed by mixing one or more pharmaceutically active agents with a polymer, solvent or polymer-solvent mixture used to form the foam. Alternatively, in one embodiment, the pharmaceutically active agent may be coated on the foam using a pharmaceutically acceptable carrier. In another embodiment, if melt coating is used, the pharmaceutically active agent is resistant to melt processing temperatures without substantially reducing its efficacy.
包含药物活性剂的制剂可以通过使一种或多种药物活性剂与网状弹性基质10涂层混合,共价键合,吸附在其上和/或吸附在其内或通过将药物活性剂掺入额外的疏水性或亲水性涂层来制备。药物活性剂可以作为液体,细分固体或另一种合适的物理形式存在。典型地,但任选所述的基质可以包括一种或多种常用添加剂,诸如稀释剂,载体,赋形剂,稳定剂等。Formulations comprising pharmaceutically active agents can be prepared by mixing, covalently bonding, adsorbing on and/or within the coating of the reticulated
在另一个实施方案中,可以应用涂层以便延缓药物活性剂释放。在另一个实施方案中,上涂层可以用作第二药物活性剂的递送基质。包括快速和缓慢水解聚合物相应层的层化涂层可以用于分阶段或受控释放置于不同层中的不同药物活性剂。聚合物掺合物还可以用于控制不同药物活性剂的释放速率或提供所需的涂层特性(例如弹性,韧性)和药物递送特性(例如释放特性)的平衡。具有不同溶剂溶解性的聚合物可以用于构成不同聚合物层,这些层可以用于递送不同的药物活性剂或控制药物活性剂的释放特性。In another embodiment, a coating may be applied to delay the release of the pharmaceutically active agent. In another embodiment, the topcoat may serve as a delivery matrix for a second pharmaceutically active agent. Layered coatings comprising respective layers of fast and slow hydrolyzing polymers can be used for staged or controlled release of different pharmaceutically active agents placed in different layers. Polymer blends can also be used to control the release rate of different pharmaceutically active agents or to provide a desired balance of coating properties (eg elasticity, toughness) and drug delivery properties (eg release properties). Polymers with different solvent solubilities can be used to construct different polymer layers that can be used to deliver different pharmaceutically active agents or to control the release profile of a pharmaceutically active agent.
药物活性剂的存在量取决于所用的特定药物活性剂和待治疗的医学疾患。在一个实施方案中,药物活性剂以有效量存在。在另一个实施方案中,药物活性剂的量占涂层重量的约0.01%-约60%。在另一个实施方案中,药物活性剂的量占涂层重量的约0.01%-约40%。在另一个实施方案中,药物活性剂的量占涂层重量的约0.1%-约20%。The amount of pharmaceutically active agent present will depend upon the particular pharmaceutically active agent employed and the medical condition being treated. In one embodiment, the pharmaceutically active agent is present in an effective amount. In another embodiment, the amount of pharmaceutically active agent is from about 0.01% to about 60% by weight of the coating. In another embodiment, the amount of pharmaceutically active agent is from about 0.01% to about 40% by weight of the coating. In another embodiment, the amount of pharmaceutically active agent is from about 0.1% to about 20% by weight of the coating.
许多不同的药物活性剂可以与网状弹性基质联用。一般而言,可以通过本发明药物组合物给予的药物活性剂包括,但不限于任何具有施用于植入物部位或通过本发明药物组合物给药的所需生理特性的治疗或药物活性剂(包括,但不限于核酸,蛋白质,脂质和碳水化合物)。治疗剂包括,但不限于:抗感染药,诸如抗生素和抗病毒药;化疗剂(例如抗癌药);抗排斥药;止痛药和止痛药联合用药;抗炎药;激素,诸如类固醇;生长因子(包括,但不限于细胞因子,趋化因子和白细胞介素)和其它天然衍生或遗传改造的蛋白质,多糖类,糖蛋白和脂蛋白。这些生长因子描述在The Cellular and Molecular Basis of BoneFormation and Repair,Vicki Rosen和R.Scott Thies,R.G.LandesCompany出版,将该文献引入本文作为参考。额外的治疗剂包括凝血酶抑制剂,抗血栓形成药,溶栓药,纤维蛋白溶解剂,血管痉挛抑制剂,钙通道阻滞剂,血管舒张药,抗高血压药,抗微生物药,抗生素,表面糖蛋白受体抑制剂,抗血小板药,抗有丝分裂药,微管抑制剂,抗分泌药,肌动蛋白抑制剂,重塑抑制剂,反义核苷酸,抗代谢物,抗增殖药,抗癌化疗剂,抗炎类固醇,非类固醇抗炎药,免疫抑制剂,生长激素拮抗剂,生长因子,多巴胺激动剂,放疗剂,肽类,蛋白质,酶,胞外基质成分,血管紧张素转化酶(ACE)抑制剂,自由基清除剂,螯合剂,抗氧化剂,抗聚合酶,抗病毒药,光动力治疗剂和基因治疗剂。Many different pharmaceutically active agents can be used in combination with the reticulated elastic matrix. In general, pharmaceutically active agents that may be administered by the pharmaceutical compositions of the present invention include, but are not limited to, any therapeutic or pharmaceutically active agent having desirable physiological properties for administration to the implant site or administration by the pharmaceutical compositions of the present invention ( including, but not limited to, nucleic acids, proteins, lipids and carbohydrates). Therapeutic agents include, but are not limited to: anti-infectives, such as antibiotics and antivirals; chemotherapeutics (eg, anticancer drugs); anti-rejection drugs; pain relievers and combinations of pain relievers; anti-inflammatory drugs; hormones, such as steroids; Factors (including, but not limited to, cytokines, chemokines, and interleukins) and other naturally derived or genetically engineered proteins, polysaccharides, glycoproteins, and lipoproteins. These growth factors are described in The Cellular and Molecular Basis of BoneFormation and Repair, published by Vicki Rosen and R. Scott Thies, R.G. Landes Company, which is incorporated herein by reference. Additional therapeutic agents include thrombin inhibitors, antithrombotics, thrombolytics, fibrinolytics, vasospasm inhibitors, calcium channel blockers, vasodilators, antihypertensives, antimicrobials, antibiotics, Surface Glycoprotein Receptor Inhibitors, Antiplatelet Agents, Antimitotic Agents, Microtubule Inhibitors, Antisecretory Agents, Actin Inhibitors, Remodeling Inhibitors, Antisense Nucleotides, Antimetabolites, Antiproliferative Agents, Anticancer chemotherapeutic agents, anti-inflammatory steroids, NSAIDs, immunosuppressants, growth hormone antagonists, growth factors, dopamine agonists, radiotherapeutic agents, peptides, proteins, enzymes, extracellular matrix components, angiotensin conversion Enzyme (ACE) inhibitors, free radical scavengers, chelating agents, antioxidants, antipolymerases, antivirals, photodynamic therapy agents and gene therapy agents.
另外,可以在泡沫形成后的加工过程中向泡沫中加入各种蛋白质(包括短链肽类),生长剂,趋化剂,生长因子受体或陶瓷颗粒,使其吸附在表面上或回填充入泡沫。例如,在一个实施方案中,泡沫的孔中可以部分或完全填充了生物相容性再吸收合成聚合物或生物聚合物(诸如胶原蛋白或弹性蛋白),生物相容性陶瓷材料(诸如羟磷灰石)及其组合并且可以任选包含促进组织通过装置生长的材料。这类组织生长材料包括,但不限于自体移植,同种异体移植或异种移植物骨,骨髓和形态发生蛋白。生物聚合物还可以用作传导材料或趋化材料或用作递送载体生长因子。实例包括重组胶原蛋白,动物衍生的胶原蛋白,弹性蛋白和透明质酸。药物活性剂涂敷或表面处理也可以存在于材料表面上。例如,可以使生物活性肽序列(RGD’s)与表面结合以便有利于蛋白质吸附和随后的细胞组织附着。In addition, various proteins (including short-chain peptides), growth agents, chemoattractants, growth factor receptors, or ceramic particles can be added to the foam during processing after foam formation to allow adsorption on the surface or backfill into the lather. For example, in one embodiment, the cells of the foam may be partially or completely filled with a biocompatible resorbable synthetic or biopolymer such as collagen or elastin, a biocompatible ceramic material such as hydroxyphosphorous Limestone) and combinations thereof and may optionally contain materials that promote tissue growth through the device. Such tissue growth materials include, but are not limited to, autograft, allograft or xenograft bone, bone marrow and morphogenetic proteins. Biopolymers can also be used as conductive or chemotactic materials or as delivery vehicles for growth factors. Examples include recombinant collagen, animal-derived collagen, elastin, and hyaluronic acid. Pharmaceutically active agent coatings or surface treatments may also be present on the surface of the material. For example, biologically active peptide sequences (RGD's) can be bound to surfaces to facilitate protein adsorption and subsequent attachment of cellular tissues.
生物活性分子包括,但不限于蛋白质,胶原蛋白(包括IV和XVIII型),原纤维胶原蛋白(包括I,II,III,V,XI型),FACIT胶原蛋白(IX,XII,XIV型),其它胶原蛋白(VI,VII,XIII型),短链胶原蛋白(VIII,X型),弹性蛋白,巢蛋白-1,微纤维蛋白,纤连蛋白,血纤蛋白,血纤蛋白原,纤聚糖,纤调蛋白,纤蛋白,磷脂酰肌醇聚糖,vitronectin,层粘连蛋白,巢蛋白,胞外基质蛋白,基底膜蛋白多糖,肝素,硫酸类肝素蛋白聚糖,核心蛋白多糖,角质纤丝聚集蛋白,角蛋白,多配体聚糖,聚集蛋白,整联蛋白,聚集蛋白聚糖,二聚糖,骨涎蛋白,软骨基质蛋白,Cat-301蛋白聚糖,CD44,胆碱酯酶,HB-GAM,透明质烷,透明质烷结合蛋白,粘蛋白,骨桥蛋白,纤维蛋白溶酶原,纤溶酶原激活物抑制剂,限制蛋白,丝甘蛋白聚糖,腱糖蛋白,凝血酶敏感蛋白,组织型纤溶酶原激活物,尿激酶型纤溶酶原激活物,多功能蛋白聚糖,冯·维勒布兰德因子,葡聚糖,阿拉伯半乳聚糖,脱乙酰壳多糖,聚丙交酯(polyactide)-乙交酯,藻酸盐,出芽短梗孢糖,明胶和清蛋白。Bioactive molecules include, but are not limited to, proteins, collagen (including types IV and XVIII), fibrillar collagen (including types I, II, III, V, XI), FACIT collagen (types IX, XII, XIV), Other collagens (types VI, VII, XIII), short-chain collagens (types VIII, X), elastin, nestin-1, fibrillin, fibronectin, fibrin, fibrinogen, fibril Sugar, fibromodulin, fibrinoglycan, glypican, vitronectin, laminin, nestin, extracellular matrix protein, basement membrane proteoglycan, heparin, heparan sulfate proteoglycan, decorin, cutin fiber Filaggrin, Keratin, Syndecan, Aggrecan, Integrin, Aggrecan, Diglycan, Bone sialoprotein, Cartilage matrix protein, Cat-301 proteoglycan, CD44, Cholinesterase , HB-GAM, Hyaluronan, Hyaluronan Binding Protein, Mucin, Osteopontin, Plasminogen, Plasminogen Activator Inhibitor, Restriction Protein, Serglycan, Tenascin, Thrombospondin, tissue plasminogen activator, urokinase-type plasminogen activator, versican, von Willebrand factor, dextran, arabinogalactan, Chitosan, polyactide-glycolide, alginate, pullulan, gelatin and albumin.
额外的生物活性分子包括,但不限于细胞黏着分子和母细胞蛋白,包括免疫球蛋白(Ig;包括单克隆和多克隆抗体),钙依粘连蛋白,整联蛋白,选择蛋白和H-CAM超家族中的那些。实例包括,但不限于AMOG,CD2,CD4,CD8,C-CAM(CELL-CAM 105),细胞表面半乳糖基转移酶,连接蛋白,桥粒胶蛋白,桥粒核心糖蛋白,成束蛋白,F11,GP1b-IX复合物,胞间黏着分子,磷酸白细胞表面共同抗原蛋白酪氨酸(LCA,CD45),LFA-1,LFA-3,甘露糖结合蛋白(MBP),MTJC 18,髓鞘相关糖蛋白(MAG),神经细胞粘着分子(NCAM),神经束蛋白,神经胶质蛋白,神经趋化因子,神经生长因子,PECAM-1,PH-20,脑信号蛋白,TAG-1,VCAM-1,SPARC/骨结合素,CCN1(CYR61),CCN2(CTGF;结缔组织生长因子),CCN3(NOV),CCN4(WISP-1),CCN5(WISP-2),CCN6(WISP-3),闭锁蛋白和密蛋白。生长因子包括,但不限于BMP’s(1-7),BMP-类蛋白(GFD-5,-7,-8),表皮生长因子(EGF),红细胞生成素(EPO),成纤维细胞生长因子(FGF),生长激素(GH),生长激素释放因子(GHRF),粒细胞集落刺激因子(G-CSF),粒细胞-巨噬细胞集落刺激因子(GM-CSF),胰岛素,胰岛素样生长因子(IGF-I,IGF-II),胰岛素样生长因子结合蛋白(IGFBP),巨噬细胞集落刺激因子(M-CSF),Multi-CSF(II-3),血小板衍生的生长因子(PDGF),肿瘤生长因子(TGF-α,TGF-β),肿瘤坏死因子(TNF-α),血管内皮细胞生长因子(VEGF’s),血管生成素,胎盘生长因子(PIGF),白细胞介素和受体蛋白或已知结合上述因子的其它分子、短链肽类包括,但不限于(命名为单一字母氨基酸代码),RGD,EILDV,RGDS,RGES,RFDS,GRDGS,GRGS,GRGDTP和QPPRARI。Additional bioactive molecules include, but are not limited to, cell adhesion molecules and parent cell proteins, including immunoglobulins (Ig; including monoclonal and polyclonal antibodies), cadherins, integrins, selectins, and H-CAM those in the family. Examples include, but are not limited to, AMOG, CD2, CD4, CD8, C-CAM (CELL-CAM 105), cell surface galactosyltransferase, connexin, desmoglein, desmoglein, fascin, F11, GP1b-IX complex, intercellular adhesion molecule, phospholeukocyte surface common antigen protein tyrosine (LCA, CD45), LFA-1, LFA-3, mannose-binding protein (MBP),
压缩模制compression molding
除为获得一定范围的所需或靶向可植入装置性能而改变弹性基质10的化学和/或加工外,网状化后步骤,诸如赋予内孔特征(如上所述)也可以用于获得‘一定范围的所需或靶向可植入装置性能。在另一个网状化后的实施方案中,在压缩模制法中将网状弹性基质在至少1-维,例如1-维压缩,2-2-维压缩或3-2-维压缩来进行压缩,并且如果如下文详细描述使用加强物加强,那么在进行加强过程中仍然保持可压缩。In addition to altering the chemistry and/or processing of the
在一个实施方案中,可植入装置由网状弹性基质制成,使得该装置密度约为2.0 lbs/ft3-约4.0 lbs/ft3(约0.032g/cc-约0.064g/cc)。在另一个实施方案中,制成可植入装置,使得该装置密度约为4.01bs/ft3-约8.01bs/ft3(约0.064g/cc-约0.128g/cc)。在另一个实施方案中,制成可植入装置,使得该装置密度约为2.51bs/ft3-约261bs/ft3(约0.040g/cc-约0.417g/cc。In one embodiment, the implantable device is made from a reticulated elastic matrix such that the device has a density of about 2.0 lbs/ft3 to about 4.0 lbs/ft3 (about 0.032 g/cc to about 0.064 g/cc). In another embodiment, the implantable device is fabricated such that the device has a density of about 4.01bs/ft3 to about 8.01bs/ft3 (about 0.064g/cc to about 0.128g/cc). In another embodiment, the implantable device is fabricated such that the device has a density of about 2.5 lbs/ft3 to about 26 lbs/ft3 (about 0.040 g/cc to about 0.417 g/cc.
在一个实施方案中,可植入装置由以1-维形式定向的基质制成。在另一个实施方案中,可植入装置由以2-维形式定向的基质制成。在另一个实施方案中,可植入装置由以3-维形式定向的基质制成。在另一个实施方案中,在基质中基本上无优选的定向。在另一个实施方案中,在最初泡沫形成过程中发生基质定向。在另一个实施方案中,在网状化过程中发生基质定向。在另一个实施方案中,诸如通过压缩模制(可能在网状化后发生)的二次加工过程中出现基质定向。定向结果显示为定向方向中的特性增强和/或性能增强。例如,拉伸特性,诸如拉伸强度可以在泡沫升起方向上得到加强,同时在与泡沫升起方向成直角的方向上出现拉伸强度上轻度改变或无明显改变。In one embodiment, an implantable device is made from a matrix oriented in a 1-dimensional fashion. In another embodiment, an implantable device is made of a matrix oriented in a 2-dimensional fashion. In another embodiment, an implantable device is made from a matrix oriented in a 3-dimensional fashion. In another embodiment, there is substantially no preferred orientation in the matrix. In another embodiment, matrix orientation occurs during initial foam formation. In another embodiment, matrix orientation occurs during reticulation. In another embodiment, matrix orientation occurs during secondary processing such as by compression molding (which may occur after reticulation). Orientation results are shown as feature enhancements and/or performance enhancements in the orientation direction. For example, tensile properties, such as tensile strength, may be enhanced in the direction of foam rise with little or no significant change in tensile strength in a direction at right angles to the direction of foam rise.
在一种本文称作压缩模制的二次加工方法中,通过使用不同温度致密化和/或1-维,2-维或3-维定向获得所需的加强性能。在一个实施方案中,可以在不使用模具的情况下进行致密化和/或定向。在另一个实施方案中,通过使用模具促进致密化和/或定向。如下所述,致密化和/或定向通常在高于25℃,例如约105℃-约180℃在时间期限取决于所用温度的时间期限内进行。在另一个实施方案中,压缩模制过程在批量方法中进行。在另一个实施方案中,压缩模制法过程在连续方法中进行。In a secondary processing method referred to herein as compression molding, the desired reinforcement properties are obtained by using different temperature densification and/or 1-, 2- or 3-dimensional orientation. In one embodiment, densification and/or orientation can be performed without the use of a mold. In another embodiment, densification and/or orientation is facilitated through the use of molds. Densification and/or orientation is generally carried out above 25°C, eg, from about 105°C to about 180°C, for a time period that depends on the temperature used, as described below. In another embodiment, the compression molding process is performed in a batch process. In another embodiment, the compression molding process is performed in a continuous process.
“预制型产品”为成形的未压缩网状弹性基质,它从网状弹性基质块上切下或以机器制造,以用于二次加工,诸如压缩模制。预制型产品可以具有预定的大小和形状。在一个实施方案中,预制型产品的大小和形状根据在压缩模制过程中被赋予的最终或所需压缩比确定。A "preformed product" is a shaped, uncompressed reticulated elastic matrix that is cut or machined from a block of reticulated elastic matrix for secondary processing, such as compression molding. Prefabricated products may have a predetermined size and shape. In one embodiment, the size and shape of the preform is determined according to the final or desired compression ratio imparted during the compression molding process.
当使用模具时,模腔可以具有固定的形状,诸如圆柱体,立方体,球体或椭圆体或它可以具有不规则形状。网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质在被压缩模制时与致密化和/或定向步骤结束时的模具的几何形状具有很大程度的一致性。When using a mold, the mold cavity can have a fixed shape such as a cylinder, cube, sphere or ellipsoid or it can have an irregular shape. The reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix when compression molded conforms to a large degree to the geometry of the mold at the end of the densification and/or orientation steps.
压缩模制还可以在其外形可以在压缩模制过程中改变的模具中进行,例如从最初的形状和/或大小改变成最终的形状和/或大小。这种模具尺寸的改变可以通过施加热或施用荷载来启动或激活。在一个这类实例中,将具有直径d3的网状弹性基质圆柱形预制型产品放入具有大于d3的起始直径d1的薄壁PTFE(聚(四氟乙烯))皱缩-包裹管。在施加外部加热和/或载荷时,PTFE皱缩-包裹管从其起始直径d1皱缩至较小的最终直径d2。将具有直径d3的圆柱形预制型产品压缩成基本上等于或等于d2的最终直径。压缩的网状弹性基质与在该实施方案中为加热皱缩的PTFE管的模具几何形状在很大程度上一致。Compression molding can also be performed in a mold whose shape can be changed during compression molding, for example from an initial shape and/or size to a final shape and/or size. This change in mold size can be initiated or activated by applying heat or applying a load. In one such example, a reticulated elastic matrix cylindrical preform product having a diameter d3 is placed into a thin-walled PTFE (poly(tetrafluoroethylene)) shrink-wrap tube having an initial diameter d1 greater than d3. Upon application of external heat and/or load, the PTFE shrink-wrap tube shrinks from its initial diameter d1 to a smaller final diameter d2. A cylindrical preform product having a diameter d3 is compressed to a final diameter substantially equal to or equal to d2. The compressed reticulated elastic matrix largely conforms to the mold geometry, which in this embodiment is a heat-shrunk PTFE tube.
在一个实施方案中,认为通过压缩模制将致密化和/或定向赋予网状弹性基质导致压缩的网状弹性基质的特性增强和/或性能增强,诸如在其机械性能方面,例如拉伸强度,拉伸模量,抗压强度,压缩模量和/或抗撕裂强度。在另一个实施方案中,认为通过压缩模制将致密化和/或定向赋予网状弹性基质导致涉及在组织愈合部位上递送,一致性,操作性和/或填充性的性能增强。In one embodiment, it is believed that imparting densification and/or orientation to the reticulated elastic matrix by compression molding results in enhanced properties and/or enhanced properties of the compressed reticulated elastic matrix, such as in terms of its mechanical properties, e.g. tensile strength , tensile modulus, compressive strength, compression modulus and/or tear strength. In another embodiment, it is believed that imparting densification and/or orientation to the reticulated elastic matrix by compression molding results in enhanced performance related to delivery, consistency, handling and/or filling at the site of tissue healing.
在压缩模制过程中,在一个实施方案中,预制型产品的至少1-维尺寸,例如圆柱形预制型产品的长度和/或直径在尺寸上减小。用于通过模具的应用使圆柱形预制型产品直径减小而长度基本上不变的非限制性压缩模制方法示出在图3中。可以将图3中61mm直径的典型圆柱形预制型产品放入由圆柱形柔性片,例如薄铝,钢或塑料片构成的模具内部。以任意合适的方式将片的一边固定,而在另一端,即尾部突出。然后施加力以便将尾部从片的圆柱形部分中拉出,由此减小片的内径,并且同时减小保持在片内的预制型产品的直径,如图3中例证的。如其中例证的,可以将图3典型的61mm直径圆柱形预制型产品减小至例如42mm。在这种压缩模制过程中,认为相对于接触模具内表面的预制型产品的外表面,模具内表面运动或位移,此后尾部被拉出;因此,还可以将这种压缩模制方法描述为“运动模具壁”压缩模制方法。During compression molding, in one embodiment at least 1 -dimensional dimension of the preform, eg the length and/or diameter of the cylindrical preform is reduced in size. A non-limiting compression molding method for reducing the diameter of a cylindrical preform product through the application of a mold while maintaining a substantially constant length is shown in FIG. 3 . A typical cylindrical preform of 61 mm diameter in Figure 3 can be placed inside a mold made of a cylindrical flexible sheet, such as a thin aluminum, steel or plastic sheet. One side of the sheet is secured in any suitable manner, while the other end, ie the tail, protrudes. Force is then applied to pull the tail out of the cylindrical portion of the sheet, thereby reducing the inner diameter of the sheet and simultaneously reducing the diameter of the preform held within the sheet, as exemplified in FIG. 3 . As exemplified therein, the typical 61 mm diameter cylindrical preform of Figure 3 can be reduced to, for example, 42 mm. In this compression molding process, it is considered that the inner surface of the mold is moved or displaced relative to the outer surface of the preformed product in contact with the inner surface of the mold, after which the tail is pulled; therefore, this method of compression molding can also be described as "Moving mold wall" compression molding method.
在另一个实施方案中,在压缩模制过程中,预制型产品的1-维,诸如立方体厚度尺寸减小,而另两维基本上保持不变。这一结果例证在图4中。可以将典型的立方体预制型产品放入由两个相对的相当刚性的例如厚铝,钢或塑料的模面构成的模具内部。然后施加力以促使两面彼此靠近,由此减小两面之间保持的立方体的厚度尺寸,如图4中例证的。在这种压缩模制过程中,认为在促使它们彼此接近时每个面相对于接触该面的预制型产品的外表面而言几乎不运动或是固定的;因此,还可以将这种压缩模制方法描述为“固定模具壁”压缩模制方法。In another embodiment, during compression molding, one dimension of the preformed product, such as the cube thickness dimension, is reduced while the other two dimensions remain substantially unchanged. This result is illustrated in Figure 4. A typical cubical preform can be placed inside a mold consisting of two opposing relatively rigid mold faces such as thick aluminum, steel or plastic. A force is then applied to urge the two faces closer to each other, thereby reducing the thickness dimension of the cube held between the two faces, as illustrated in FIG. 4 . In this compression molding process, it is considered that each face hardly moves or is fixed relative to the outer surface of the preform contacting the face when they are brought close to each other; therefore, this compression molding can also be The method is described as a "fixed mold wall" compression molding method.
在另一个实施方案中,基本上预制型产品体积在压缩模制时出现的所有改变可以归因于仅在1-维中出现尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的所有改变可以归因于仅在1-维形式中出现的尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的基本上所有改变可以归因于仅在厚度尺寸中出现的尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的所有改变可以归因于仅在厚度尺寸中出现的尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的基本上所有改变可以归因于仅在长度或高度尺寸中出现的尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的所有改变可以归因于仅在长度或高度尺寸中出现的尺寸改变。In another embodiment, substantially all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes in only 1-dimension. In another embodiment, all changes in the volume of the preformed product upon compression molding can be attributed to the dimensional changes that occur only in the 1-dimensional form. In another embodiment, substantially all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in the thickness dimension. In another embodiment, all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in the thickness dimension. In another embodiment, substantially all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in the length or height dimensions. In another embodiment, all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in the length or height dimension.
本文定义为在压缩模制过程中减小的尺寸的原始大小与压缩模制后最终尺寸的大小之比的线性压缩比约为1.1-约9.9。在另一个实施方案中,线性压缩比约为1.5-约8.0。在另一个实施方案中,线性压缩比约为2.5-约7.0。在另一个实施方案中,线性压缩比约为2.0-约6.0。The linear compression ratio, defined herein as the ratio of the original size of the dimension reduced during compression molding to the size of the final dimension after compression molding, is from about 1.1 to about 9.9. In another embodiment, the linear compression ratio is from about 1.5 to about 8.0. In another embodiment, the linear compression ratio is from about 2.5 to about 7.0. In another embodiment, the linear compression ratio is from about 2.0 to about 6.0.
如果压缩模制过程中被减小尺寸的减小以线性压缩应变,即在原始尺寸上的尺寸改变表示,那么线性压缩应变约为3%-约97%。在另一个实施方案中,线性压缩应变约为15%-约95%。在另一个实施方案中,线性压缩应变约为25%-约90%。在另一个实施方案中,线性压缩应变约为30%-约85%。在另一个实施方案中,线性压缩应变约为40%-约75%。If the reduction in the reduced size during compression molding is expressed in terms of linear compressive strain, ie, a dimensional change from the original dimension, then the linear compressive strain is about 3% to about 97%. In another embodiment, the linear compressive strain is from about 15% to about 95%. In another embodiment, the linear compressive strain is from about 25% to about 90%. In another embodiment, the linear compressive strain is from about 30% to about 85%. In another embodiment, the linear compressive strain is from about 40% to about 75%.
在另一个实施方案中,在压缩模制过程中,圆柱形预制型产品的径向尺寸减小,即圆周减小,使得在二个方向上尺寸减小,而在另一个方向上圆柱体的高度基本上保持不变。在另一个实施方案中,在压缩模制过程中,圆柱形预制型产品的径向尺寸减小,而在另一方向上,该圆柱体的高度保持不变。In another embodiment, during the compression molding process, the radial dimension of the cylindrical preform product is reduced, i.e. the circumference is reduced, so that the dimension is reduced in two directions, while the cylindrical shape is reduced in the other direction. The height remains essentially the same. In another embodiment, during compression molding, the radial dimension of the cylindrical preform decreases, while in the other direction the height of the cylinder remains constant.
在另一个实施方案中,预制型产品体积在压缩模制时出现的基本上所有改变可以归因于仅在2-维中出现的尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的所有改变可以归因于仅在2-维中出现的尺寸维改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的基本上所有改变可以归因于仅在径向尺寸中出现的尺寸改变。在另一个实施方案中,预制型产品体积在压缩模制时出现的所有改变可以归因于仅在径向尺寸中出现的尺寸改变。In another embodiment, substantially all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in 2-dimensions. In another embodiment, all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in 2-dimensions. In another embodiment, substantially all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in the radial dimension. In another embodiment, all changes in the volume of the preformed product upon compression molding can be attributed to dimensional changes that occur only in the radial dimension.
本文定义为圆柱形预制型产品半径的原始大小与压缩模制后最终半径的大小之比的径向压缩比约为1.2-约6.7。在另一个实施方案中,径向压缩比约为1.5-约6.0。在另一个实施方案中,径向压缩比约为2.5-约6.0。在另一个实施方案中,径向压缩比约为2.0-约5.0。The radial compression ratio, defined herein as the ratio of the original size of the radius of the cylindrical preform to the size of the final radius after compression molding, is from about 1.2 to about 6.7. In another embodiment, the radial compression ratio is from about 1.5 to about 6.0. In another embodiment, the radial compression ratio is from about 2.5 to about 6.0. In another embodiment, the radial compression ratio is from about 2.0 to about 5.0.
在另一个实施方案中,本文定义为圆柱形预制型产品横截面积的原始大小与压缩模制后最终横截面积的大小之比的横截面压缩比约为1.5-约47。在另一个实施方案中,横截面压缩比约为1.5-约25。在另一个实施方案中,横截面压缩比约为2.0-约9.0。在另一个实施方案中,横截面压缩比约为2.0-约7.0。In another embodiment, the cross-sectional compression ratio, defined herein as the ratio of the original size of the cross-sectional area of the cylindrical preformed product to the size of the final cross-sectional area after compression molding, is from about 1.5 to about 47. In another embodiment, the cross-sectional compression ratio is from about 1.5 to about 25. In another embodiment, the cross-sectional compression ratio is from about 2.0 to about 9.0. In another embodiment, the cross-sectional compression ratio is from about 2.0 to about 7.0.
如果以横截面压缩应变,即在原始横截面积基础上的横截面积改变来表示圆柱形预制型产品压缩模制过程中横截面积的减小,那么横截面压缩应变约为25%-约90%。在另一个实施方案中,横截面压缩应变约为33%-约88%。在另一个实施方案中,横截面压缩应变约为50%-约88%。If the cross-sectional compressive strain, that is, the change in cross-sectional area based on the original cross-sectional area, is used to represent the decrease in cross-sectional area during compression molding of a cylindrical preformed product, then the cross-sectional compressive strain is about 25% to about 90%. In another embodiment, the cross-sectional compressive strain is from about 33% to about 88%. In another embodiment, the cross-sectional compressive strain is from about 50% to about 88%.
本发明生物耐久性网状弹性基质材料的压缩模制在高于25℃的温度下进行并且在一个实施方案中,可以在约100℃-约190℃下进行。或在另一个实施方案中,约110℃-约180℃。在另一个实施方案中,约120℃-约145℃。在另一个实施方案中,当进行压缩模制过程的温度增加时,进行该压缩模制过程的时间减少。压缩模制时间通常约为10秒-约10小时。在另一个实施方案中,压缩模制时间约为30秒-约5小时。在另一个实施方案中,压缩模制时间约为30秒-约3小时。当进行压缩模制过程的温度升高时,用于压缩模制的时间减少。在较高温度下,压缩模制时间必须短,因为长压缩模制时间可能导致网状弹性基质发生热降解。例如,在一个实施方案中,在约160℃或160℃以上的温度下,压缩模制时间约为30分钟或30分钟以内。在一个实施方案中,约为10分钟或10分钟以内。或在另一个实施方案中,约为5分钟或5分钟以内。在另一个实施方案中,在约150℃温度下,例如约145℃-约155℃下,压缩模制时间约为60分钟或60分钟以内。在一个实施方案中,约为20分钟或20分钟以内。或在另一个实施方案中,约为10分钟或10分钟以内。在另一个实施方案中,在约130℃温度下,例如约125℃-约135℃下,压缩模制时间约为240分钟或240分钟以内。在一个实施方案中,约为120分钟或120分钟以内。或在另一个实施方案中,约为30分钟或30分钟以内。Compression molding of the biodurable reticulated elastic matrix material of the present invention is performed at a temperature above 25°C and in one embodiment, may be performed at a temperature of from about 100°C to about 190°C. Or in another embodiment, from about 110°C to about 180°C. In another embodiment, from about 120°C to about 145°C. In another embodiment, as the temperature at which the compression molding process is performed increases, the time during which the compression molding process is performed decreases. Compression molding times are typically from about 10 seconds to about 10 hours. In another embodiment, the compression molding time is from about 30 seconds to about 5 hours. In another embodiment, the compression molding time is from about 30 seconds to about 3 hours. When the temperature at which the compression molding process is performed increases, the time for compression molding decreases. At higher temperatures, the compression molding time must be short because long compression molding times may lead to thermal degradation of the reticulated elastic matrix. For example, in one embodiment, the compression molding time is about 30 minutes or less at a temperature of about 160°C or greater. In one embodiment, about 10 minutes or less. Or in another embodiment, about 5 minutes or less. In another embodiment, the compression molding time is about 60 minutes or less at a temperature of about 150°C, eg, about 145°C to about 155°C. In one embodiment, about 20 minutes or less. Or in another embodiment, about 10 minutes or less. In another embodiment, the compression molding time is about 240 minutes or less at a temperature of about 130°C, eg, about 125°C to about 135°C. In one embodiment, about 120 minutes or less. Or in another embodiment, about 30 minutes or less.
在压缩模制后,压缩的网状弹性基质密度与压缩模制前的网状弹性基质密度之比可以增加约1.05倍-约25倍。在另一个实施方案中,压缩的网状弹性基质密度可以增加约1.20倍-约7.5倍;例如,在一个实施方案中,从3.5 lbs/ft3(0.056g/cc)的起始密度在压缩模制后增加到4.2 lbs/ft3(0.067g/cc)密度。或在一个实施方案中,增加到压缩模制后的26.3 lbs/ft3(0.421g/cc)密度。在另一个实施方案中,压缩的网状弹性基质的密度可以从例如3.4 lbs/ft3(0.054g/cc)的起始密度增加到压缩模制后的7.9 lbs/ft3(0.127g/cc)。After compression molding, the ratio of the density of the compressed reticulated elastic matrix to the density of the reticulated elastic matrix before compression molding may increase from about 1.05 times to about 25 times. In another embodiment, the compressed reticulated elastic matrix density can be increased by a factor of about 1.20 to about 7.5; for example, in one embodiment, from a starting density of3.5 lbs/ft (0.056 g/cc) Increased to a density of 4.2 lbs/ft3 (0.067g/cc) after molding. Or in one embodiment, increased to a density of 26.3 lbs/ft3 (0.421 g/cc) after compression molding. In another embodiment, the density of the compressed reticulated elastic matrix can be increased from an initial density of, for example, 3.4 lbs/ft3 (0.054 g/cc) to 7.9 lbs/ft3 (0.127 g/cc) after compression molding. ).
压缩模制后,压缩的网状弹性基质的拉伸强度可以比压缩模制前网状弹性基质的拉伸强度增加约1.05倍-约5.0。在另一个实施方案中,压缩的网状弹性基质的拉伸强度可以增加约1.20倍-约2.5倍,例如,在一个实施方案中,从起始的52psi(36,400kg/m2)拉伸强度增加至压缩模制后的62.4psi(43,700kg/m2)拉伸强度。或在一个实施方案中,增加至压缩模制后的130psi(91,000kg/m2)拉伸强度。在另一个实施方案中,压缩的网状弹性基质的拉伸强度可以例如从起始的52psi(36,400kg/m2)拉伸强度增加至压缩模制后的120psi(84,000kg/m2)拉伸强度。在其它实施方案中,拉伸强度的增加出现在1-维,2-维或3-维压缩模制中优选定向的方向上。After compression molding, the tensile strength of the compressed reticulated elastic matrix may be increased by about 1.05 times to about 5.0 compared to the tensile strength of the reticulated elastic matrix before compression molding. In another embodiment, the tensile strength of the compressed reticulated elastic matrix can be increased by a factor of about 1.20 to about 2.5, for example, in one embodiment, from an initial tensile strength of 52 psi (36,400 kg/m2 ) Increased to 62.4 psi (43,700 kg/m2 ) tensile strength after compression molding. Or in one embodiment, increased to 130 psi (91,000 kg/m2 ) tensile strength after compression molding. In another embodiment, the tensile strength of the compressed reticulated elastic matrix can be increased, for example, from an initial tensile strength of 52 psi (36,400 kg/m2 ) to a tensile strength of 120 psi (84,000 kg/m2 ) after compression molding. tensile strength. In other embodiments, the increase in tensile strength occurs in the direction of the preferred orientation in 1-dimensional, 2-dimensional or 3-dimensional compression molding.
压缩模制后,压缩的网状弹性基质的抗压强度可以比压缩模制前网状弹性基质的抗压强度增加约1.05倍-约4.5倍。在另一个实施方案中,压缩的网状弹性基质的抗压强度可以增加约1.20倍-约3.5倍;例如,在一个实施方案中,在50%压缩应变下从2.4psi(1,700kg/m2)的起始压缩强度增加至在压缩模制后50%压缩应变下的2.9psi(2,000kg/m2),或在另一个实施方案中,在压缩模制后的50%压缩应变下增加至8.4psi(5,900kg/m2)。在其它实施方案中,抗压强度的增加出现在1-维,2-维或3-维压缩模制中优选定向的方向上。After compression molding, the compressive strength of the compressed reticulated elastic matrix may be increased by about 1.05 times to about 4.5 times than that of the reticulated elastic matrix before compression molding. In another embodiment, the compressive strength of the compressed reticulated elastic matrix can be increased by a factor of about 1.20 to about 3.5; for example, in one embodiment, from 2.4 psi (1,700 kg/m2 ) to 2.9 psi (2,000 kg/m2 ) at 50% compressive strain after compression molding, or in another embodiment, to 8.4 psi (5,900 kg/m2 ). In other embodiments, the increase in compressive strength occurs in a direction that is preferentially oriented in 1-dimensional, 2-dimensional or 3-dimensional compression molding.
压缩模制后,压缩的网状弹性基质的渗透性通常降低,且由此可能降低压缩的网状弹性基质提供组织向内生长和增殖的能力。因此,重要的是维持压缩模制后的良好渗透性。例如,在一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约50%时,起始网状弹性基质对流体至少约450达西的渗透性降至不低于约250达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约60%时,起始网状弹性基质对流体至少约450达西的渗透性降至不低于100达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约80%时,起始网状弹性基质对流体至少约450达西的渗透性降至不低于约20达西。After compression molding, the permeability of the compressed reticulated elastic matrix is generally reduced, and thus the ability of the compressed reticulated elastic matrix to provide tissue ingrowth and proliferation may be reduced. Therefore, it is important to maintain good permeability after compression molding. For example, in one embodiment, when the cross-sectional area of the reticulated elastic matrix is reduced by about 50% after compression molding, the permeability of the initial reticulated elastic matrix to fluids of at least about 450 Darcy decreases to not less than about 250 darcy. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 450 darcies decreases to no less than 100 darcies when the cross-sectional area of the reticulated elastic matrix is reduced by about 60% after compression molding. West. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 450 Darcy decreases to no less than about 20 when the cross-sectional area of the reticulated elastic matrix is reduced by about 80% after compression molding. Darcy.
在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约50%时,起始网状弹性基质约300达西的渗透性降至不低于约100达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约60%时,起始网状弹性基质对流体至少约300达西的渗透性降至不低于约80达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约75%时,起始网状弹性基质对流体至少约300达西的渗透性降至不低于约15达西。In another embodiment, the permeability of the initial reticulated elastic matrix of about 300 Darcy is reduced to no less than about 100 Darcy when the cross-sectional area of the reticulated elastic matrix is reduced by about 50% after compression molding. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 300 Darcy decreases to no less than about 80 when the cross-sectional area of the reticulated elastic matrix is reduced by about 60% after compression molding. Darcy. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 300 Darcy decreases to no less than about 15 when the cross-sectional area of the reticulated elastic matrix is reduced by about 75% after compression molding. Darcy.
在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约50%时,起始网状弹性基质对流体至少约200达西的渗透性降至不低于约40达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约50%时,起始网状弹性基质对流体至少约200达西的渗透性降至不低于约80达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约60%时,起始网状弹性基质对流体至少约200达西的渗透性降至不低于约40达西。在另一个实施方案中,在该网状弹性基质压缩模制后横截面积减小约70%时,起始网状弹性基质对流体至少约200达西的渗透性降至不低于约15达西。In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 200 darcies decreases to no less than about 40 when the cross-sectional area of the reticulated elastic matrix is reduced by about 50% after compression molding. Darcy. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 200 darcies decreases to no less than about 80 when the cross-sectional area of the reticulated elastic matrix is reduced by about 50% after compression molding. Darcy. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 200 darcies decreases to no less than about 40 when the cross-sectional area of the reticulated elastic matrix is reduced by about 60% after compression molding. Darcy. In another embodiment, the permeability of the initial reticulated elastic matrix to fluids of at least about 200 Darcy decreases to no less than about 15 when the cross-sectional area of the reticulated elastic matrix is reduced by about 70% after compression molding. Darcy.
加强掺入Enhanced incorporation
弹性基质10除进行网状化外还可以进行进一步的一个或多个网状化后加工步骤,从而赋予如上所述的内孔特征和压缩模制。例如,在另一个实施方案中,使用加强物加强网状弹性基质。在其它实施方案中,所述的加强物至少为1-维的,例如1-维加强物(诸如纤维),2-维加强物(诸如由交叉的1-维加强元件构成的2-2-维网状结构)或3-维加强物(诸如3-维网格)。In addition to reticulation,
可以通过将加强物,例如纤维包括或掺入网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质,制备用于各种可植入装置的具体应用目的的多功能的加强弹性基质和/或压缩的加强弹性基质。可以通过使用加强物赋予的增强的功能包括,但不限于增强装置在与手术操作过程中与缝合相关的抗拉出荷载的能力,装置通过在手术操作过程中缝合固定凹在修复部位上来定位的能力和在手术后组织愈合发生时保持装置在修复部位上的能力。在另一个实施方案中,增强的功能性为装置在手术过程中提供了具有额外载荷的能力,以便有利于组织的修复或再生。在另一个实施方案中,增强的功能性为装置至少通过手术后的最初几天提供了具有额外载荷的能力,以便有利于组织的修复或再生。在另一个实施方案中,增强的功能性为手术后装置提供了具有额外载荷的能力,以便有利于组织的修复或再生。Multifunctional reinforced elastics for specific application purposes in various implantable devices can be prepared by including or incorporating reinforcements, such as fibers, into a reticulated cross-linked biodurable elastic polycarbonate urea-urethane matrix matrix and/or compressed reinforced elastic matrix. Enhanced functionality that may be imparted through the use of reinforcements includes, but is not limited to, enhancing the ability of the device to resist pull-out loads associated with sutures during surgical procedures where the device is positioned by suture fixation recessed over the repair site ability and ability to maintain the device on the repair site while tissue healing occurs after surgery. In another embodiment, the enhanced functionality provides the device with the ability to carry additional loads during surgery to facilitate tissue repair or regeneration. In another embodiment, the enhanced functionality provides the device with additional load capacity to facilitate tissue repair or regeneration at least through the first few days after surgery. In another embodiment, the enhanced functionality provides the post-surgical device with additional load capacity to facilitate tissue repair or regeneration.
获得增强功能性的一种方式通过将加强物,例如纤维,纤维网状结构,金属丝和/或缝合线掺入弹性基质进行。另一种获得增强功能性的典型方式通过使用至少一种加强物加强基质进行。将加强物掺入基质可以通过各种方式进行,包括,但不限于缝针,缝合,纺织和编织。在一个实施方案中,可以通过缝合针使加强物与基质连接。在另一个实施方案中,可以通过包括互锁特征的缝合针使加强物与基质连接。在另一个实施方案中,可以通过在由加强物构成的预制或预成形的加强元件周围使弹性基质组分起泡并且使由此形成的复合结构网状化成互通和互联孔结构而将加强物掺入基质。在一个实施方案中,所用的加强物不会干扰基质适应组织向内生长和增殖的能力。One way of achieving enhanced functionality is by incorporating reinforcements, such as fibres, fibrous networks, wires and/or sutures, into the elastic matrix. Another typical way of obtaining enhanced functionality is by reinforcing the matrix with at least one reinforcement. Incorporation of the reinforcement into the matrix can be done in a variety of ways including, but not limited to, stitching, sewing, weaving and braiding. In one embodiment, the reinforcement can be attached to the matrix by suturing needles. In another embodiment, the reinforcement can be attached to the matrix by a suture needle that includes interlocking features. In another embodiment, the reinforcement may be incorporated by foaming an elastic matrix component around a prefabricated or preformed reinforcing element comprised of the reinforcement and reticulating the resulting composite structure into an interconnected and interconnected pore structure. Incorporate matrix. In one embodiment, the reinforcement used does not interfere with the ability of the matrix to accommodate tissue ingrowth and proliferation.
将纤维掺入网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质的弹性基质可以在其密度和/或其定向方面改变。弹性基质的密度可以改变,在一个实施方案中,约2 lbs/ft3-约25 lbs/ft3(约0.032g/cc-约0.401 g/cc),或在另一个实施方案中,约2.5 lbs/ft3-约10 lbs/ft3(约0.040g/cc-约0.160g/cc)。在另一个实施方案中,约3 lbs/ft3-约8.5 lbs/ft3(约0.480g/cc-约0.136g/cc)。定向可以在最初泡沫形成过程中,网状化过程中或网状化和泡沫热固化后的二次加工过程中出现。定向结果表示为在定向方向上的特性增强和/或性能增强。在一个实施方案中,使由加强网状弹性基质构成的装置定位于待修复的组织,以这种方式使得定向基质增强的特性和/或增强的性能以耐受具有较高荷载方向的方向排列。加强物的掺入可以导致基质的性能增强,它优于通过使加强的基质以一个或多个方向定向获得的性能。The elastic matrix incorporating fibers into the reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix can vary in its density and/or its orientation. The density of the elastic matrix can vary, in one embodiment, from about 2 lbs/ft3 to about 25 lbs/ft3 (about 0.032 g/cc to about 0.401 g/cc), or in another embodiment, about 2.5 lbs/ft3 - about 10 lbs/ft3 (about 0.040g/cc - about 0.160g/cc). In another embodiment, about 3 lbs/ft3 to about 8.5 lbs/ft3 (about 0.480 g/cc to about 0.136 g/cc). Orientation can occur during initial foam formation, during reticulation, or during secondary processing after reticulation and thermal curing of the foam. Orientation results are expressed as property enhancements and/or performance enhancements in the orientation direction. In one embodiment, a device comprised of a reinforced reticulated elastic matrix is positioned on the tissue to be repaired in such a way that the enhanced properties and/or enhanced performance of the oriented matrix are aligned in a direction that resists the direction of higher loads . The incorporation of reinforcements can result in enhanced properties of the matrix that are superior to those obtained by orienting the reinforced matrix in one or more directions.
加强物可以包括单丝纤维,复丝,编织,混合单丝纤维,混合复丝,束状单丝纤维,束状复丝等。加强物可以包括非晶态聚合物,半晶体聚合物,例如聚酯或尼龙,碳,例如碳纤维,玻璃,例如玻璃纤维,陶瓷,交联聚合物纤维等或其任意的混合物。所述的纤维可以由可吸收或不能吸收的材料制成。在一个实施方案中,本发明的纤维加强物由生物相容性材料制成。Reinforcements may include monofilament fibers, multifilament fibers, braids, mixed monofilament fibers, mixed multifilament fibers, bundled monofilament fibers, bundled multifilament fibers, and the like. Reinforcements may comprise amorphous polymers, semi-crystalline polymers such as polyester or nylon, carbon such as carbon fibers, glass such as glass fibers, ceramics, cross-linked polymer fibers, etc. or any mixture thereof. The fibers may be made of absorbable or non-absorbable materials. In one embodiment, the fibrous reinforcement of the present invention is made of a biocompatible material.
在一个实施方案中,加强物可以由至少一种不能吸收性材料,诸如生物不能降解或不能吸收性聚合物制成。合适的不能吸收性聚合物的实例包括,但不限于聚酯类(诸如聚对苯二甲酸乙二醇酯和聚对苯二甲酸丁酯);聚烯烃类(诸如聚乙烯和聚丙烯,包括无规立构,全同,间同及其掺合物和聚异丁烯和乙烯-α-烯烃共聚物);丙烯酸聚合物和共聚物;乙烯基卤聚合物和共聚物(诸如聚氯乙烯);聚乙烯醚类(诸如聚乙烯基甲基醚);聚偏卤乙烯(诸如聚偏氟乙烯和聚偏氯乙烯);聚丙烯腈;聚乙烯酮类;聚乙烯芳族化合物(诸如聚苯乙烯);聚乙烯酯类(诸如聚乙酸乙烯酯);乙烯基单体彼此和烯烃类的共聚物(诸如乙烯-甲基丙烯酸甲酯共聚物,丙烯腈-苯乙烯共聚物,ABS树脂和乙烯-乙酸乙烯酯共聚物);聚酰胺类(诸如尼龙4,尼龙6,尼龙66,尼龙610,尼龙11,尼龙12和聚己内酰胺);醇酸树脂;聚碳酸酯类;聚甲醛类;聚酰亚胺类;聚醚类;环氧乙烷树脂;聚氨基甲酸酯类;人造丝;人造丝-三乙酸酯;及其任意的混合物。就本申请的目的而言,聚酰胺类还包括-NH-(CH2)n-C(O)-和-NH-(CH2)x-NH-C(O)-(CH2)y-C(O)-的聚酰胺形式,其中n为6-13的整数,包括两端点;x为6-12的整数,包括两端点;且y为4-16的整数,包括两端点。In one embodiment, the reinforcement may be made of at least one non-absorbable material, such as a non-biodegradable or non-absorbable polymer. Examples of suitable nonabsorbable polymers include, but are not limited to, polyesters (such as polyethylene terephthalate and polybutylene terephthalate); polyolefins (such as polyethylene and polypropylene, including Atactic, isotactic, syndiotactic and their blends and polyisobutylene and ethylene-α-olefin copolymers); acrylic acid polymers and copolymers; vinyl halide polymers and copolymers (such as polyvinyl chloride); Polyvinyl ethers (such as polyvinyl methyl ether); polyvinylidene halides (such as polyvinylidene fluoride and polyvinylidene chloride); polyacrylonitrile; polyvinyl ketones; polyvinyl aromatics (such as polystyrene ); polyvinyl esters (such as polyvinyl acetate); copolymers of vinyl monomers and olefins (such as ethylene-methyl methacrylate copolymer, acrylonitrile-styrene copolymer, ABS resin and ethylene- vinyl acetate copolymer); polyamides (such as nylon 4, nylon 6, nylon 66, nylon 610, nylon 11,
在另一个实施方案中,加强物可以由至少一种生物可降解,生物可吸收或可吸收聚合物制成。合适的可吸收聚合物的实例包括,但不限于脂族聚酯类,例如乳酸,乙醇酸,丙交酯,乙交酯,对-二噁烷酮,碳酸环丙酯,ε-己内酯及其掺合物的均聚物和共聚物。其它典型的生物相容性聚合物包括成膜生物吸收性聚合物,诸如脂族聚酯类,聚(氨基酸),共聚(醚-酯类),聚亚烷基草酸酯类,聚酰胺类,聚(亚氨基碳酸酯类),聚原酸酯类,聚氧杂酯类,包括含酰胺基团的聚氧杂酯类,聚酰氨基酯类,聚酸酐类,聚磷腈类,生物分子及其任意的混合物。就本发明的目的而言,脂族聚酯类包括聚合物和丙交酯(包括乳酸d-,1-和内消旋丙交酯),ε-己内酯,乙交酯(包括乙醇酸),羟基丁酸酯,羟基戊酸酯,对-二噁烷酮,碳酸环丙酯(及其烷基衍生物),1,4-二氧杂庚环-2-酮,1,5-二氧杂庚环-2-酮,6,6-二甲基-1,4-二噁烷-2-酮的共聚物及其任意的混合物。In another embodiment, the reinforcement can be made of at least one biodegradable, bioabsorbable or absorbable polymer. Examples of suitable absorbable polymers include, but are not limited to, aliphatic polyesters such as lactic acid, glycolic acid, lactide, glycolide, p-dioxanone, cyclopropyl carbonate, ε-caprolactone Homopolymers and copolymers and blends thereof. Other typical biocompatible polymers include film-forming bioabsorbable polymers such as aliphatic polyesters, poly(amino acids), copoly(ether-esters), polyalkylene oxalates, polyamides, Poly(iminocarbonates), polyorthoesters, polyoxaesters, including polyoxaesters containing amide groups, polyamidoesters, polyanhydrides, polyphosphazenes, biomolecules and any mixture thereof. For the purposes of this invention, aliphatic polyesters include polymers and lactides (including lactic acid d-, 1- and meso-lactide), ε-caprolactone, glycolide (including glycolic acid ), hydroxybutyrate, hydroxyvalerate, p-dioxanone, cyclopropyl carbonate (and its alkyl derivatives), 1,4-dioxepane-2-one, 1,5- Copolymers of dioxepan-2-one, 6,6-dimethyl-1,4-dioxan-2-one and any mixtures thereof.
可以通过熔化挤压,熔化挤压后进行退火和拉伸,溶液纺丝,静电纺丝和其它本领域公知的方法制备这类纤维/丝。每种纤维均可以为双层化的,具有内芯和外鞘;或多层化的,具有内芯,外鞘和一层或多层中间层。在双-和多-层纤维中,芯,鞘或芯外的任意层均可以包括可降解或可溶解的聚合物。可以不涂敷或用包括非晶态聚合物,半晶体聚合物,碳,玻璃,陶瓷等或其任意的混合物的涂层涂敷纤维。Such fibers/filaments can be prepared by melt extrusion, melt extrusion followed by annealing and drawing, solution spinning, electrospinning and other methods known in the art. Each fiber can be bilayered, having an inner core and sheath, or multilayered, having an inner core, an outer sheath, and one or more intermediate layers. In dual- and multi-layer fibers, the core, sheath or any layer outside the core may comprise degradable or soluble polymers. The fibers may be left uncoated or coated with a coating comprising amorphous polymers, semi-crystalline polymers, carbon, glass, ceramics, etc. or any mixture thereof.
加强物可以由碳,玻璃,陶瓷,生物吸收性玻璃,含硅酸盐的磷酸钙玻璃或其任意的混合物制成。可以控制在人体内降解和/或吸收时间的磷酸钙玻璃可以包含金属,诸如铁,镁,钠,钾或其任意的混合物。The reinforcement may be made of carbon, glass, ceramic, bioabsorbable glass, silicate-containing calcium phosphate glass, or any mixture thereof. Calcium phosphate glasses that allow for controlled degradation and/or absorption time in the human body may contain metals such as iron, magnesium, sodium, potassium, or any mixture thereof.
在另一个实施方案中,所述的1-维加强物包括非晶态聚合物纤维,半结晶聚合物纤维,交联聚合物纤维,生物聚合物纤维,胶原纤维,弹性纤维,碳纤维,玻璃纤维,生物吸收性玻璃纤维,含硅酸盐的磷酸钙玻璃纤维,陶瓷纤维,聚酯纤维,尼龙纤维,非晶态聚合物丝,半结晶聚合物丝,交联聚合物丝,生物聚合物丝,胶原蛋白丝,弹性蛋白丝,碳丝,玻璃丝,生物吸收性玻璃丝,含硅酸盐的磷酸钙玻璃丝,陶瓷丝,聚酯丝,尼龙丝或其任意的混合物。在另一个实施方案中,2-维加强物包括交叉1-维加强元件,其包括非晶态聚合物纤维,半结晶聚合物纤维,交联聚合物纤维,生物聚合物纤维,碳纤维,玻璃纤维,生物吸收性玻璃纤维,含硅酸盐的磷酸钙玻璃纤维,陶瓷纤维,聚酯纤维,尼龙纤维,非晶态聚合物丝,半结晶聚合物丝,交联聚合物丝,生物聚合物丝,碳丝,玻璃丝,生物吸收性玻璃丝,含硅酸盐的磷酸钙玻璃丝,陶瓷丝,聚酯丝,尼龙丝或其任意的混合物。In another embodiment, said 1-dimensional reinforcement comprises amorphous polymer fibers, semi-crystalline polymer fibers, cross-linked polymer fibers, biopolymer fibers, collagen fibers, elastic fibers, carbon fibers, glass fibers , bioabsorbable glass fiber, calcium phosphate glass fiber containing silicate, ceramic fiber, polyester fiber, nylon fiber, amorphous polymer filament, semi-crystalline polymer filament, cross-linked polymer filament, biopolymer filament , collagen filaments, elastin filaments, carbon filaments, glass filaments, bioabsorbable glass filaments, silicate-containing calcium phosphate glass filaments, ceramic filaments, polyester filaments, nylon filaments or any mixture thereof. In another embodiment, the 2-dimensional reinforcement comprises intersecting 1-dimensional reinforcing elements comprising amorphous polymer fibers, semi-crystalline polymer fibers, cross-linked polymer fibers, biopolymer fibers, carbon fibers, glass fibers , bioabsorbable glass fiber, calcium phosphate glass fiber containing silicate, ceramic fiber, polyester fiber, nylon fiber, amorphous polymer filament, semi-crystalline polymer filament, cross-linked polymer filament, biopolymer filament , Carbon filaments, glass filaments, bioabsorbable glass filaments, calcium phosphate glass filaments containing silicate, ceramic filaments, polyester filaments, nylon filaments or any mixture thereof.
可以以不同模式将加强物掺入网状弹性基质。在一个实施方案中,将加强物沿装置的边界放置,从而维持了距装置边缘的固定距离。在另一个实施方案中,将加强物沿装置边界放置,从而维持了距装置边缘的可变距离。在另一个实施方案中,将加强物沿周长,例如圆形装置周长放置,从而维持了距装置边缘的固定距离。在另一个实施方案中,将加强物沿装置的周长放置,从而维持了距装置边缘的可变距离。在另一个实施方案中,加强物作为多个平行和/或基本上平行的1-维加强元件,例如作为多个平行线,诸如平行纤维存在。在另一个实施方案中,可以将加强物作为2-或3-维加强格栅放置,其中所述的1-维加强元件通过彼此的通道。所述的格栅可以具有一个或多个加强元件。在2-或3-维加强格栅的实施方案中,可以使加强物的元件以几何形状模式排列,诸如正方形,矩形,梯形,三角形,菱形,平行四边形,圆形,椭圆形,五边形,六角形和/或具有7个或7个以上侧面的多边形。包括加强格栅的加强元件均可以具有相同的形状和大小或可以具有不同的形状和大小。包括加强格栅的加强元件还可以另外包括边框,周长和/或平行线元件。加强格栅的性能或特性将该加强物掺入基质且由此加强的基质取决于加强物的内在特性以及格栅元件的模式,几何形状和数量。Reinforcements can be incorporated into the reticulated elastic matrix in different modes. In one embodiment, reinforcements are placed along the borders of the device such that a fixed distance from the edge of the device is maintained. In another embodiment, reinforcements are placed along the border of the device such that a variable distance from the edge of the device is maintained. In another embodiment, reinforcements are placed along the perimeter, eg, of a circular device, such that a fixed distance from the edge of the device is maintained. In another embodiment, reinforcements are placed along the perimeter of the device, thereby maintaining a variable distance from the edge of the device. In another embodiment, the reinforcement is present as a plurality of parallel and/or substantially parallel 1-dimensional reinforcing elements, eg as a plurality of parallel threads, such as parallel fibers. In another embodiment, the reinforcement can be placed as a 2- or 3-dimensional reinforcement grid, wherein the 1-dimensional reinforcement elements pass through each other's channels. The grid can have one or more reinforcing elements. In a 2- or 3-dimensional reinforcement grid embodiment, the elements of the reinforcement can be arranged in a pattern of geometric shapes, such as squares, rectangles, trapezoids, triangles, rhombuses, parallelograms, circles, ovals, pentagons , hexagons and/or polygons with 7 or more sides. The reinforcing elements, including the reinforcing grid, may all be of the same shape and size or may be of different shapes and sizes. Stiffening elements comprising reinforcing grids may additionally comprise frame, perimeter and/or parallel line elements. The properties or properties of a reinforced grid that incorporates the reinforcement into the matrix and thus the reinforced matrix depend on the intrinsic properties of the reinforcement as well as the pattern, geometry and number of grid elements.
某些典型的,但非限制性的加强格栅例证在图5和6中。图5a-5c和6a-6d各自包括边框或一个或多个周长加强元件。图5a例证了在矩形格栅加强元件上叠放的椭圆形加强元件。图5b例证了在矩形格栅加强元件上重叠的两个椭圆形加强元件。图5c例证了矩形格栅加强元件。图6a例证了在矩形格栅加强元件上重叠的菱形格栅加强元件。图6b例证了在矩形格栅加强元件上重叠的4-面多边形格栅加强元件。图6c和6d例证了在矩形格栅加强元件上重叠的不同间距和斜角(diagional)加强元件的菱形格栅加强元件。Some typical, but non-limiting reinforcement grids are illustrated in FIGS. 5 and 6 . Figures 5a-5c and 6a-6d each include a frame or one or more perimeter stiffening elements. Figure 5a illustrates oval reinforcement elements superimposed on rectangular grid reinforcement elements. Figure 5b illustrates two oval reinforcement elements superimposed on a rectangular grid reinforcement element. Figure 5c illustrates a rectangular grid reinforcement element. Figure 6a illustrates diamond-shaped grid reinforcement elements superimposed on rectangular grid reinforcement elements. Figure 6b illustrates a 4-sided polygonal grid reinforcement element overlaid on a rectangular grid reinforcement element. Figures 6c and 6d illustrate diamond-shaped grill reinforcement elements of different pitches and diagional reinforcement elements superimposed on rectangular grill reinforcement elements.
在一个实施方案中,单一格栅元件的任意一边可以约为0.25mm-约20mm长或在另一个实施方案中约为5mm-约15mm长。In one embodiment, any side of a single grid element may be from about 0.25mm to about 20mm long or in another embodiment from about 5mm to about 15mm long.
在其它实施方案中,加强元件之间的间隙或间距,诸如在一个实施方案中相邻线性加强元件之间的间隙可以约为0.25mm-约20mm。或在另一个实施方案中约为0.5mm-约15mm。在其它实施方案中,加强元件之间的空隙基本上相同。在其它实施方案中,不同加强元件之间的空隙不同。在其它多-维加强物实施方案中,1-维加强元件之间的空隙与任意其它维加强元件之间的空隙彼此独立。In other embodiments, the gap or spacing between reinforcing elements, such as the gap between adjacent linear reinforcing elements in one embodiment, may be from about 0.25 mm to about 20 mm. Or from about 0.5mm to about 15mm in another embodiment. In other embodiments, the spaces between the reinforcing elements are substantially the same. In other embodiments, the gaps between different reinforcing elements vary. In other multi-dimensional reinforcement embodiments, the interstices between 1 -dimensional reinforcement elements are independent of the interstices between any other dimensional reinforcement elements.
在一个实施方案中,具有基本上为圆形横截面的加强元件的直径可以约为0.03mm-约0.50mm,或在另一个实施方案中,约0.07mm-约0.30mm,或在另一个实施方案中,约0.05mm-约1.0mm,或在另一个实施方案中约0.03mm-约1.0mm。在另一个实施方案中,具有基本上为圆形横截面的加强元件的直径可以等于约8-0号-约0号USP缝合线直径。在一个实施方案中,约8-0号-约2号USP缝合线直径。在另一个实施方案中,约8-0号-约2-0号USP缝合线直径。In one embodiment, the diameter of the reinforcing element having a substantially circular cross-section may be from about 0.03 mm to about 0.50 mm, or in another embodiment, from about 0.07 mm to about 0.30 mm, or in another embodiment In one embodiment, from about 0.05 mm to about 1.0 mm, or in another embodiment from about 0.03 mm to about 1.0 mm. In another embodiment, the diameter of the reinforcing element having a substantially circular cross-section may be equal to about 8-0 gauge to about 0 gauge USP suture diameter. In one embodiment, about 8-
加强元件,例如纤维或缝合线在基质中的加强布局或分布和模式取决于待用装置的设计要求和/或应用。在缝合用于将加强物掺入基质的实施方案中,缝针间距,即连续缝线或相同线内连接点之间的距离约为0.25mm-约4mm或在另一个实施方案中约1mm-约3mm。The reinforcement placement or distribution and pattern of reinforcement elements, such as fibers or sutures, in the matrix depends on the design requirements and/or application of the device to be used. In embodiments where sutures are used to incorporate the reinforcement into the matrix, the needle spacing, i.e. the distance between consecutive sutures or connection points within the same line, is from about 0.25 mm to about 4 mm or in another embodiment from about 1 mm to About 3mm.
在一个实施方案中,在某些应用,诸如可植入装置用于加强作用的旋转套修复术中,可能无需精确的配合来匹配或适合修复或再生的组织。在另一个实施方案中,在使用前,诸如手术修复腱和韧带前使包含加强的网状弹性基质的可植入装置成形。一种典型的成形方法为修整术。当需要成形时,可以沿线或加强纤维将加强的网状弹性基质在长度和/或宽度方向上修整。在一个实施方案中,进行这种修整以便保留约2mm外部加强边界,例如有利于在手术过程中的缝合连接。In one embodiment, in certain applications, such as rotational sleeve repair where implantable devices are used for augmentation, an exact fit may not be required to match or fit the repaired or regenerated tissue. In another embodiment, implantable devices comprising a reinforced reticulated elastic matrix are shaped prior to use, such as prior to surgical repair of tendons and ligaments. A typical shaping method is revision surgery. When shaping is desired, the reinforced reticulated elastic matrix can be trimmed lengthwise and/or widthwise along the threads or reinforcing fibers. In one embodiment, this trimming is done so as to leave about a 2 mm outer reinforcement border, eg, to facilitate suture attachment during surgery.
就本发明包括加强的网状弹性基质的装置而言,在一个实施方案中,与装置厚度垂直的任意横截面的最大尺寸约为0.25mm-约100mm。在另一个实施方案中,装置的最大厚度约为0.25mm-约20mm。For devices of the present invention comprising a reinforced reticulated elastic matrix, in one embodiment, the largest dimension of any cross-section perpendicular to the thickness of the device is from about 0.25 mm to about 100 mm. In another embodiment, the maximum thickness of the device is from about 0.25 mm to about 20 mm.
在一个实施方案中,可以给可植入装置和/或其加强物涂敷本文详细描述的一种或多种生物活性分子,诸如蛋白质,胶原蛋白,弹性蛋白,巢蛋白-1,原纤蛋白,纤连蛋白,细胞黏着分子,母细胞蛋白,钙依粘连蛋白,整联蛋白,选择蛋白,H-CAM超家族等。In one embodiment, the implantable device and/or its reinforcement may be coated with one or more of the bioactive molecules described in detail herein, such as proteins, collagen, elastin, nestin-1, fibrillin , fibronectin, cell adhesion molecule, mother cell protein, cadherin, integrin, selectin, H-CAM superfamily, etc.
在一个实施方案中,将加强物掺入网状弹性基质的装置具有下列性能范围内的至少一种特征。缝合线拉出强度在一个实施方案中约为1.1 lbs/ft-约17 lbs/ft(约5牛顿-约75牛顿)或在另一个实施方案中约2.3 lbs/ft-约9.0 lbs/ft(约10牛顿-约40牛顿)。断裂强度在一个实施方案中约为2.0 lbs/ft-约100 lbs/ft(约8.8牛顿-约440牛顿)或在另一个实施方案中约3.4 lbs/ft-约45 lbs/ft(约15牛顿-约200牛顿)或在另一个实施方案中约6.8 lbs/ft-约22.5lbs/ft(约30牛顿-约100牛顿)。球崩裂强度在一个实施方案中约为3 lbsf-约75 lbsf(约1.35Kgf-约34Kgf)或在另一个实施方案中约8 lbsf-约50 lbsf(约3.65Kgf-约22.5Kgf)。In one embodiment, the device incorporating reinforcement into the reticulated elastic matrix has at least one characteristic within the following property ranges. The suture pull strength is in one embodiment about 1.1 lbs/ft to about 17 lbs/ft (about 5 Newtons to about 75 Newtons) or in another embodiment about 2.3 lbs/ft to about 9.0 lbs/ft ( about 10 Newtons - about 40 Newtons). The breaking strength is in one embodiment from about 2.0 lbs/ft to about 100 lbs/ft (about 8.8 Newtons to about 440 Newtons) or in another embodiment from about 3.4 lbs/ft to about 45 lbs/ft (about 15 Newtons - about 200 Newtons) or in another embodiment about 6.8 lbs/ft to about 22.5 lbs/ft (about 30 Newtons to about 100 Newtons). The ball burst strength is in one embodiment about 3 lbsf to about 75 lbsf (about 1.35 Kgf to about 34 Kgf) or in another embodiment about 8 lbsf to about 50 lbsf (about 3.65 Kgf to about 22.5 Kgf).
使用安装了1kN气动柄上下夹紧颌夹的INSTRON Tester(Model3342)进行缝合线拉出强度试验,所述的颌夹各自具有相对的25mm x25mm橡胶涂敷的柄表面。图7例证了缝合线拉出强度试验实施方案中加强样本和缝合线的几何形状。试验缝合线的长度为2-0ETHIBOND编织聚酯缝合线。在将仪器标准规格长度设定至60mm(2.36英寸)后,将待测试的加强网状弹性基质装置的一端(端2)夹固入仪器下部固定的颌夹。通过使用针头将ETHIBOND试验缝合线插入加强的网状弹性基质装置的另一端(端1)。试验缝合线两端形成环。使试验缝合线在低于最接近装置边缘的水平加强线2-3mm并且优选面对装置宽度中心处与加强装置连接,正如对使用纤维矩形格栅加强的装置的图7例证的。Suture pull strength testing was performed using an INSTRON Tester (Model 3342) fitted with 1 kN pneumatic handle upper and lower clamping jaws, each with opposing 25mm x 25mm rubber-coated handle surfaces. Figure 7 illustrates reinforcement sample and suture geometries for a suture pullout strength test embodiment. The length of test suture was 2-0 ETHIBOND braided polyester suture. After setting the standard specification length of the instrument to 60mm (2.36 inches), clamp one end (end 2) of the reinforced mesh elastic matrix device to be tested into the fixed jaw clamp at the lower part of the instrument. Insert the ETHIBOND trial suture into the other end of the reinforced mesh elastic matrix device (end 1 ) by using a needle. A loop is formed at both ends of the test suture. The test suture was attached to the reinforcement device 2-3 mm below the horizontal reinforcement line closest to the edge of the device and preferably facing the center of the device width, as exemplified in Figure 7 for a device reinforced with a rectangular grid of fibers.
试验缝合线的游离端距离试验缝合线与加强的网状弹性基质装置连接点的长度约为50-60mm。将缝合线的游离端夹紧入仪器上部可移动颌夹子。此后,使用向上移动和远离固定颌夹的可移动颌夹以100mm/min(3.94英寸/min)的速率进行缝合线保持强度试验。将施力-伸长关系曲线中达到的最大力注明为缝合线保持强度,只要加强的网状弹性基质装置中的撕裂限于接近邻近缝合线连接位置的端1水平格栅线的区域。根据对多份样品的测试确定平均值和标准偏差。The free end of the test suture is about 50-60 mm from the connection point of the test suture to the reinforced mesh elastic matrix device. Clamp the free end of the suture into the upper movable jaw clamp of the instrument. Thereafter, a suture retention strength test was performed at a rate of 100 mm/min (3.94 inches/min) using the movable jaw moving up and away from the fixed jaw. The maximum force achieved in the force-elongation curve is noted as suture retention strength, as long as tearing in the reinforced reticulated elastic matrix device is limited to the region near the
按照与上述缝合线拉出强度相同的方式进行断裂强度试验,但不使用编织的聚酯缝合线并且在仪器下部固定颌夹与上部可移动颌夹之间夹紧测试的加强的网状弹性基质装置。此后,使用向上移动和远离固定颌夹的可移动颌夹以100mm/min(3.94英寸/min)的速率进行断裂强度试验。将施力-伸长关系曲线中达到的最大力注明为断裂强度。The breaking strength test was performed in the same manner as above for the suture pull-out strength, but without the braided polyester suture and with the reinforced mesh elastic matrix tested clamped between the fixed lower jaw of the instrument and the upper movable jaw device. Thereafter, a breaking strength test was performed at a rate of 100 mm/min (3.94 inches/min) using the movable jaw moving up and away from the fixed jaw. The maximum force achieved in the force-elongation curve is noted as the breaking strength.
根据ASTM Standard 3787中所述的试验方法测定球崩裂强度,但使用具有10mm直径,18mm直径保持孔和102mm/min(4英寸/min)的十字头速度的较小球。The ball burst strength was determined according to the test method described in ASTM Standard 3787, but using a smaller ball with a 10 mm diameter, 18 mm diameter holding hole and a crosshead speed of 102 mm/min (4 inches/min).
网状弹性基质的其它后加工Other post-processing of reticulated elastic matrix
除上述已经描述的那些外,弹性基质10还可以进行进一步的加工步骤或多个步骤。例如,弹性基质10或由弹性基质10制成的产品可以退火以使结构稳定。In addition to those already described above, the
在一个实施方案中,在高温下退火可以促进聚氨基甲酸酯类中增加的结晶性。在另一个实施方案中,在升温下退火可以促进交联的聚氨基甲酸酯类中增加的结晶性。在升温下退火还可以促进交联聚氨基甲酸酯类和长期贮存期限稳定性中的结构稳定和长期的贮存稳定性。结构稳定和/或额外的结晶性可以对由弹性基质10制成的可植入装置提供提高的贮存稳定性。在一个实施方案中,不受任何特定理论的约束,退火导致泡沫形成和/或网状化过程中网状弹性基质结构中形成的应力松弛。In one embodiment, annealing at high temperature can promote increased crystallinity in polyurethanes. In another embodiment, annealing at elevated temperatures can promote increased crystallinity in crosslinked polyurethanes. Annealing at elevated temperatures can also promote structural stability and long-term storage stability in cross-linked polyurethanes and long-term shelf-life stability. Structural stability and/or additional crystallinity may provide improved storage stability to implantable devices made from
在一个实施方案中,在超过约50℃的温度下进行退火。在另一个实施方案中,在超过约100℃的温度下进行退火。在另一个实施方案中,在超过约125℃的温度下进行退火。在另一个实施方案中,在约100℃-约135℃的温度下进行退火。在另一个实施方案中,在约100℃-约130℃的温度下进行退火。在另一个实施方案中,在约100℃-约120℃的温度下进行退火。在另一个实施方案中,在约105℃-约115℃的温度下进行退火。In one embodiment, the annealing is performed at a temperature in excess of about 50°C. In another embodiment, the annealing is performed at a temperature in excess of about 100°C. In another embodiment, the annealing is performed at a temperature in excess of about 125°C. In another embodiment, the annealing is performed at a temperature of from about 100°C to about 135°C. In another embodiment, the annealing is performed at a temperature of from about 100°C to about 130°C. In another embodiment, the annealing is performed at a temperature of from about 100°C to about 120°C. In another embodiment, the annealing is performed at a temperature of from about 105°C to about 115°C.
在另一个实施方案中,将退火进行至少约2小时。在另一个实施方案中,将退火进行约2-约15小时。在另一个实施方案中,将退火进行约3-约10小时。在另一个实施方案中,将退火进行约4-约8小时。In another embodiment, the annealing is performed for at least about 2 hours. In another embodiment, the annealing is performed for about 2 to about 15 hours. In another embodiment, the annealing is performed for about 3 to about 10 hours. In another embodiment, the annealing is performed for about 4 to about 8 hours.
在限制或不限制装置的情况下进行退火。在另一个实施方案中,在退火时弹性基质10在几何形状上不受约束,例如弹性基质周围未包围有模具。在另一个实施方案中,在退火时弹性基质10在几何形状上受到约束,例如弹性基质在一个或多个侧面上受表面,诸如模具表面约束,使其尺寸,诸如其厚度在退火过程中基本上不会改变。在该实施方案中,弹性基质10未因其受约束压缩至任何明显的程度,因此,在该方面这类退火不同于压缩模制。Annealing was performed with or without confining the device. In another embodiment, the
在一个实施方案中,可以任选在压缩模制后在约110℃-约140℃温度和约15分钟-约4小时时间期限下对压缩的网状弹性基质进一步退火(已经)。与压缩模制一样,可以在将压缩的基质限制在模具中或不使用模具的情况下进行退火。在另一个实施方案中,可以在将压缩的基质限制在模具中的情况下进行退火。如果最初的压缩模制在约150℃或150℃以上的温度下进行,那么退火时间应短以避免压缩的网状弹性基质在长退火时间时热降解的可能性。例如,在约150℃或150℃以上的温度下压缩模制后,在约125℃-约135℃温度下将压缩的网状弹性基质的退火进行约30分钟-约3小时时间期限。In one embodiment, the compressed reticulated elastic matrix may optionally be further annealed (already) after compression molding at a temperature of from about 110°C to about 140°C and a time period of from about 15 minutes to about 4 hours. As with compression molding, annealing can be performed with the compressed matrix confined in a mold or without the use of a mold. In another embodiment, annealing can be performed with the compressed matrix confined in a mold. If the initial compression molding is performed at a temperature of about 150°C or above, the annealing time should be short to avoid the possibility of thermal degradation of the compressed reticulated elastic matrix at long annealing times. For example, after compression molding at a temperature of about 150°C or above, annealing of the compressed reticulated elastic matrix is performed at a temperature of about 125°C to about 135°C for a period of about 30 minutes to about 3 hours.
可以将弹性基质10在其成形或生产过程中模塑成各种形状和大小。形状可以为工作构造,诸如请求优先权申请中所述的任意形状和构造,或形状可以针对的是散装储备料。随后可以切割,修整,冲压储备料,或使其成形以便最终应用。例如,可以通过使用刀片,冲头,钻头或激光进行定尺寸和成形。在这些实施方案中的每种中,用于成形和定尺寸的切割工具的一种或多种加工温度可以高于约100℃。在另一个实施方案中,用于成形和分型的切割工具的加工温度可以高于约130℃。在一个实施方案中,最终步骤可以包括修整可能刺激生物组织的宏观结构表面突出部,诸如小连接体等的修整。在另一个实施方案中,最终步骤可以包括加热退火。可以在最终切割和成形前或之后进行退火。The
成形和定型可以包括定制的成形和定型以使可植入装置与具体患者的具体治疗部位匹配,正如通过成像或本领域公知的其它技术测定的。特别地,弹性基质10在一个实施方案中的一个或小数目,例如少于约6,且在另一个实施方案中少于约2可以包括用于治疗需要修复和/或再生的受损组织的可植入装置系统。Shaping and shaping can include custom shaping and shaping to match the implantable device to the particular treatment site of a particular patient, as determined by imaging or other techniques known in the art. In particular, one or a small number, such as less than about 6 in one embodiment, and less than about 2 in another embodiment, of
由弹性基质10制成的成形和定型装置的尺寸可以根据所治疗特定组织修复和再生位点的不同而改变。在一个实施方案中,压缩和递送前装置的主要尺寸约为0.5mm-约500mm。在另一个实施方案中,压缩和递送前装置的主要尺寸约为10mm-约500mm。在另一个实施方案中,压缩和递送前装置的主要尺寸约为50mm-约200mm。在另一个实施方案中,压缩和递送前装置的主要尺寸约为30mm-约100mm。弹性基质10在通过递送装置,例如导管,注射器或内窥镜压缩和转运时展示出压缩变定。在另一个实施方案中,在设计装置的压缩前尺寸时考虑到压缩变定及其标准偏差。The dimensions of the shaping and shaping device made from
在一个实施方案中,使用可植入装置或装置系统治疗患者,参考所述部位入口内到植入位点限定的体积,所述的可植入装置或装置系统没有在或者其自身完全填充该装置系统居留的靶腔或其它部位。在一个实施方案中其中甚至在生物流体或组织占据弹性基质孔后,可植入装置或装置系统也不完全填充植入物系统仍然居留的靶腔或其它部位。在另一个实施方案中,可植入装置或装置系统原位完全膨胀的体积至少低于所述部位体积的1%。在另一个实施方案中,可植入装置或装置系统原位完全膨胀的体积至少低于所述部位体积的15%。在另一个实施方案中,可植入装置或装置系统原位完全膨胀的体积至少低于所述部位体积的30%。In one embodiment, a patient is treated with an implantable device or system of devices that does not completely fill the volume within or by itself, with reference to a volume defined within the site inlet to the implantation site. The target cavity or other site where the device system resides. In one embodiment wherein the implantable device or device system does not completely fill the target cavity or other site where the implant system still resides even after the biological fluid or tissue has occupied the pores of the elastic matrix. In another embodiment, the fully expanded volume of the implantable device or device system in situ is at least less than 1% of the volume of the site. In another embodiment, the fully expanded volume of the implantable device or device system in situ is at least less than 15% of the volume of the site. In another embodiment, the fully expanded volume of the implantable device or device system in situ is at least less than 30% of the volume of the site.
在另一个实施方案中,可植入装置或装置系统原位完全膨胀的体积大于腔体积约1%-约40%。在另一个实施方案中,可植入装置或装置系统原位完全膨胀的体积大于腔体积约5%-约25%。在另一个实施方案中,可植入装置体积与矫形外科应用部位占据的体积之比约为70%-约90%。在另一个实施方案中,可植入装置体积与矫形外科应用部位占据的体积之比约为90%-约100%。在另一个实施方案中,可植入装置体积与矫形外科应用部位占据的体积之比约为90%-低于约100%。在另一个实施方案中,可植入装置体积与矫形外科应用部位占据的体积之比约为100%-约140%。在另一个实施方案中,可植入装置体积与矫形外科应用部位占据的体积之比约为100%-约200%。在另一个实施方案中,可植入装置体积与矫形外科应用部位占据的体积之比约为100%-约300%。In another embodiment, the fully expanded volume of the implantable device or device system in situ is from about 1% to about 40% greater than the lumen volume. In another embodiment, the fully expanded volume of the implantable device or device system in situ is from about 5% to about 25% greater than the lumen volume. In another embodiment, the ratio of the volume of the implantable device to the volume occupied by the orthopedic application site is from about 70% to about 90%. In another embodiment, the ratio of the volume of the implantable device to the volume occupied by the orthopedic application site is from about 90% to about 100%. In another embodiment, the ratio of the volume of the implantable device to the volume occupied by the orthopedic application site is from about 90% to less than about 100%. In another embodiment, the ratio of the volume of the implantable device to the volume occupied by the orthopedic application site is from about 100% to about 140%. In another embodiment, the ratio of the volume of the implantable device to the volume occupied by the orthopedic application site is from about 100% to about 200%. In another embodiment, the ratio of the volume of the implantable device to the volume occupied by the orthopedic application site is from about 100% to about 300%.
可以通过本领域中公知的任何方法对生物耐久性网状弹性基质10或包括这类基质的可植入装置系统进行灭菌,包括γ照射,高压灭菌,环氧乙烷灭菌,红外线照射和电子束辐照。在一个实施方案中,用于制造弹性基质10的生物耐久性弹性体耐受这类灭菌而不会失去有用的物理和机械特性。γ照射的应用能够提供额外的交联以便提高装置的性能。The biodurable reticulated
在一个实施方案中,可以将灭菌的产品包装在纸张,聚合物或其它合适材料的无菌包装中。在另一个实施方案中,在这类包装中,将弹性基质10压缩在保持元件内以便于其以压缩构造形式载入递送装置,诸如导管或内窥镜中。在另一个实施方案中,弹性基质10包括具有压缩变定的弹性体,这种压缩变定能够使它膨胀至较大比例的其压缩前体积,例如在25℃下,膨胀至其压缩前体积的至少50%。在另一个实施方案中,弹性基质10保持压缩在这类包装中典型商品储存和分配时间后发生膨胀,它通常超过3个月并且从制备到应用可以达到1或5年。In one embodiment, the sterilized product can be packaged in aseptic packaging of paper, polymer or other suitable material. In another embodiment, in such packaging, the
不透射线性radiopacity
在一个实施方案中,例如,可植入装置可以通过使不透射线材料的颗粒粘着,共价键合和/或掺入弹性基质自身而被赋予不透射线性以便于体内成像。不透射线材料包括钛,钽,钨,硫酸钡或其它本领域技术人员公知的合适材料。In one embodiment, for example, an implantable device can be rendered radiopaque to facilitate in vivo imaging by adhering, covalently bonding, and/or incorporating particles of radiopaque material into the elastomeric matrix itself. Radiopaque materials include titanium, tantalum, tungsten, barium sulfate, or other suitable materials known to those skilled in the art.
可植入装置应用Implantable Device Applications
可以如请求优先权申请中所述使用掺入网状弹性基质的可植入装置系统。在一个实施方案中,包括网状弹性基质的可植入装置可以用于治疗组织缺陷,例如在矫形外科应用,普通外科手术应用,美容外科手术应用,组织改造应用或其任意的混合物中用于修复,重建,再生,加强,缺口插入或其任意的组合。Implantable device systems incorporating reticulated elastic matrices may be used as described in the claimed priority application. In one embodiment, an implantable device comprising a reticulated elastic matrix can be used to treat tissue defects, for example in orthopedic applications, general surgical applications, cosmetic surgical applications, tissue modification applications, or any mixture thereof Repair, rebuild, regenerate, enhance, gap insertion or any combination thereof.
在另一个实施方案中,包括网状弹性基质的可植入装置可以用于矫形外科应用,以便腱,韧带,软骨,关节盘,脊柱盘或其任意的混合物的修复,重建,再生,加强,缺口插入或其任意的组合。例如,包括网状弹性基质的可植入装置可以用于广泛的矫形外科应用,包括,但不限于包括脊柱,肩,肘,腕,手,膝,踝或其他关节的修复和再生,正如优先权申请中详细描述的。由生物耐久性网状弹性基质制成的可植入装置为组织向内生长提供了平台,所述的组织向内生长特别有效地治疗所谓的软组织矫形外科应用病症,例如连接,再生,加强或支持软组织,包括腱加强,关节软骨修复,半月板修复和重建,韧带重建,椎间盘脱出稳定和作为核替代物和环形物修复的基板。In another embodiment, an implantable device comprising a reticulated elastic matrix may be used in orthopedic applications for the repair, reconstruction, regeneration, reinforcement, gap insertion or any combination thereof. For example, implantable devices comprising reticulated elastic matrices can be used in a wide variety of orthopedic applications including, but not limited to, the repair and regeneration of spine, shoulder, elbow, wrist, hand, knee, ankle or other joints, as preferred described in detail in the patent application. Implantable devices made of biodurable reticulated elastic matrices provide a platform for tissue ingrowth that is particularly effective in the treatment of conditions for so-called soft tissue orthopedic applications, such as joining, regeneration, strengthening or Supports soft tissue, including tendon reinforcement, articular cartilage repair, meniscus repair and reconstruction, ligament reconstruction, herniated disc stabilization and as a substrate for nuclear replacement and annulus repair.
可以通过使用包括网状弹性基质的可植入装置修复或再生的肩膀区域中的韧带实例包括肩峰锁骨韧带,盂肱韧带,喙肱韧带,横向肱骨韧带,喙肩韧带等。可以通过使用包括网状弹性基质的可植入装置修复或再生的肩膀区域中腱的实例包括冈上肌,冈下肌,臂二头肌长头腱等。还可以通过使用包括网状弹性基质的可植入装置修复或再生肩膀区域中的软骨。Examples of ligaments in the shoulder region that may be repaired or regenerated through the use of implantable devices comprising a mesh elastic matrix include the acromioclavicular ligament, glenohumeral ligament, coracohumeral ligament, transverse humeral ligament, coracoid ligament, and the like. Examples of tendons in the shoulder region that can be repaired or regenerated through the use of implantable devices comprising a mesh elastic matrix include supraspinatus, infraspinatus, long head of biceps brachii, and the like. Cartilage in the shoulder region can also be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix.
可以通过使用包括网状弹性基质的可植入装置修复或再生的肘部区域中韧带的实例包括内侧副韧带(“MCL”),外侧副韧带韧带和环状韧带。可以通过使用包括网状弹性基质的可植入装置修复或再生的肘部区域中腱的实例包括二头肌和三头肌腱。还可以通过使用包括网状弹性基质的可植入装置修复或再生肘部区域中的软骨。Examples of ligaments in the elbow region that may be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix include the medial collateral ligament ("MCL"), the lateral collateral ligament, and the annular ligament. Examples of tendons in the elbow region that may be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix include the biceps and triceps tendons. Cartilage in the elbow region can also be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix.
可以通过使用包括网状弹性基质的可植入装置修复或再生的膝区域中韧带的实例包括后交叉韧带,前交叉韧带(“ACL”),髌韧带,腓骨侧副韧带,胫骨侧副韧带,半月板股骨后韧带,后床突胫腓韧带等。可以通过使用包括网状弹性基质的可植入装置修复或再生的膝区域中腱的实例包括四头肌腱。可以通过使用包括网状弹性基质的可植入装置修复或再生膝区域中的关节软骨。Examples of ligaments in the knee region that may be repaired or regenerated through the use of implantable devices comprising a mesh elastic matrix include the posterior cruciate ligament, anterior cruciate ligament ("ACL"), patellar ligament, fibular collateral ligament, tibial collateral ligament, Meniscal posterior femoral ligament, posterior clinoid tibiofibular ligament, etc. Examples of tendons in the knee region that may be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix include the quadriceps tendon. Articular cartilage in the knee region can be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix.
可以通过使用包括网状弹性基质的可植入装置修复或再生的踝关节区域中韧带的实例包括横向腹股沟韧带,腹股沟交叉韧带,屈肌支持带等。可以通过使用包括网状弹性基质的可植入装置修复或再生的踝关节区域中腱的实例包括腓骨长肌,腓骨短肌,阿基利斯腱等。还可以通过使用包括网状弹性基质的可植入装置修复或再生踝关节区域中的软骨。Examples of ligaments in the ankle region that can be repaired or regenerated through the use of implantable devices comprising a mesh elastic matrix include the transverse inguinal ligament, inguinal cruciate ligament, flexor retinaculum, and the like. Examples of tendons in the ankle region that can be repaired or regenerated through the use of an implantable device comprising a reticulated elastic matrix include peroneus longus, peroneus brevis, Achilles tendon, and the like. Cartilage in the ankle region can also be repaired or regenerated through the use of implantable devices comprising a reticulated elastic matrix.
一般而言,可以通过使用包括网状弹性基质的可植入装置修复或再生脊柱,肩,肘,腕,手,膝,踝或其他身体关节的任何韧带,腱和/或软骨。In general, any ligament, tendon and/or cartilage of the spine, shoulder, elbow, wrist, hand, knee, ankle or other body joints can be repaired or regenerated through the use of an implantable device comprising a mesh elastic matrix.
在一个实施方案中,包括网状弹性基质的可植入装置适合于成形为封闭装置以密封因椎间盘切除(discotomy)产生的环形物中的通道开口,从而在椎间盘脱出,也称作椎间盘突出或滑脱或膨胀盘情况中加强和稳定盘状环形物。可以通过在椎间盘切除操作过程中使用的插管将封闭装置压缩并且递送入环形物开口。通过至少下列两种机制将该装置固定入开口。首先,网状实体相12的外向回弹性可以提供防止偏移的机械方式。第二,网状实体相12可以用作支持纤维软骨生长入弹性基质的互联空隙相14的基质。可以通过使用作为本领域已知的固定凹,缝合线或生物胶水和粘合剂获得额外的固定。封闭装置可以支持纤维软骨向内生长入可植入装置的弹性基质。In one embodiment, an implantable device comprising a reticulated elastic matrix is adapted to be shaped as a closure device to seal the opening of a passageway in an annulus resulting from discectomy (discotomy) in the event of a herniated disc, also referred to as a herniated disc or Reinforces and stabilizes the disc annulus in case of slipping or bulging discs. The closure device may be compressed and delivered into the annulus opening through a cannula used during the discectomy procedure. The device is secured into the opening by at least the following two mechanisms. First, the outward resilience of the network
在另一个实施方案中,将包括网状弹性基质的可植入装置制成补片,可以例如通过缝合,固定凹,U形钉等将其锚定就位以便提供对键愈合时的支持,使得原位腱加强和强化。这特别用于回旋套或库技术修复,其中腱组织恶化或发生慢性缺陷且剩余的腱的强度不足以支持成功锚定腱的所需的缝合,其中腱和肌肉收缩并且不能充分拉伸以便再连接(收缩的腱)或因损伤而破坏的腱,肌肉或组织。包括网状弹性基质的可植入装置可以用作组织向内生长的基质以便在愈合过程中加强腱和提供支持。在一个实施方案中,包括网状弹性基质的可植入装置可以用作缺口插入物或桥接物以便通过提供修复部位和组织向内生长的基质来完全或部分修复撕裂韧带或腱。这类可植入装置还能够修复不能手术的否则就不能再连接的腱。包括网状弹性基质的可植入装置可以用于MCL修复。可以使用常规缝合将可植入装置固定(afixed)在修复部位(在韧带下)上(atop)或使用永久性,例如金属或所谓生物可吸收性U形钉或固定凹/缝合线固定在骨(近中股骨髁或近中胫骨plature)上。还可以使用生物-胶水使补片与指定修复部位连接(诸如腱,韧带或硬脑膜)作为加强装置。In another embodiment, an implantable device comprising a reticulated elastic matrix is fabricated into a patch, which can be anchored in place, for example, by sutures, fixation recesses, staples, etc., to provide support for the key as it heals, Allows tendon strengthening and strengthening in situ. This is especially useful in convoluted cuff or bank technique repairs where the tendon tissue has deteriorated or chronic defects have developed and the remaining tendon is not strong enough to support the sutures needed to successfully anchor the tendon, where the tendon and muscle contract and cannot stretch sufficiently to re-stretch. Tendons, muscles, or tissues that connect (contracted tendons) or that have been disrupted by injury. Implantable devices comprising a reticulated elastic matrix can be used as a matrix for tissue ingrowth to strengthen tendons and provide support during the healing process. In one embodiment, an implantable device comprising a reticulated elastic matrix can be used as a gap insert or bridge to fully or partially repair a torn ligament or tendon by providing a repair site and a matrix for tissue ingrowth. Such implantable devices are also capable of repairing inoperable tendons that would otherwise not reconnect. Implantable devices comprising reticulated elastic matrices can be used for MCL repair. The implantable device can be fixed (afixed) on the repair site (under the ligament) using conventional sutures (atop) or permanently, such as metal or so-called bioabsorbable staples or fixation notches/sutures to the bone (Mesial femoral condyle or mesial tibial plature). Bio-glues can also be used to attach the patch to a designated repair site (such as a tendon, ligament or dura mater) as a reinforcement.
在另一个实施方案中,将网状弹性基质或包括网状弹性基质的可植入装置制成在以无细胞模式时支持组织修复和关节软骨再生的植入生物耐久性基质,由此在膝损伤治疗中具有应用,例如用于半月板修复和ACL重建。包括网状弹性基质的可植入装置可以如近中或侧向关节盘样成形。包括网状弹性基质的可植入装置可以用于完全关节盘或部分关节盘置换。可以缝合完全关节盘或节段或用U形钉与骨或相邻半月板组织固定。In another embodiment, a reticulated elastic matrix or an implantable device comprising a reticulated elastic matrix is made into an implanted biodurable matrix that supports tissue repair and regeneration of articular cartilage in a cell-free mode, whereby in the knee There are applications in injury treatment, eg for meniscus repair and ACL reconstruction. Implantable devices comprising a reticulated elastic matrix can be shaped like mesial or lateral articular discs. Implantable devices comprising reticulated elastic matrices may be used for complete or partial disc replacement. The complete disc or segment can be sutured or stapled to the bone or adjacent meniscal tissue.
包括网状弹性基质的可植入装置的另一种应用在于修复生物结缔组织中虚弱部位的修复,这些虚弱部位使具有导致生物损伤的另一种器官或器官系统膨出或疝出。在一个实施方案中,可植入装置的特征及其功能性使得它适合于普通外科手术应用,诸如在疝气修复中的应用。Another application of implantable devices comprising a reticulated elastic matrix is in the repair of weakened sites in biological connective tissue that bulge or herniate another organ or organ system that has caused biological damage. In one embodiment, the characteristics of the implantable device and its functionality make it suitable for general surgical applications, such as in hernia repair.
可以将疝气描述为腹股沟位置或腹侧与其它较不常见,但众所周知的变化位置,即股骨或脐的疝气。在一个实施方案中,待修复的疝气为腹股沟疝,腹侧疝,股部疝,脐疝或其任意的混合。可以直接或通过腹腔镜手段接近位于手术或创伤前部位上前腹壁或侧腹壁中的疝气。修复主要将包括网状弹性基质的可植入装置置于腹壁内,由此加强或强化腹直肌鞘-横肌,腹外斜肌和/或腹内斜肌的肌肉/筋膜中的缺陷。在一个实施方案中,包括网状弹性基质的可植入装置可以处理成具有在面向腹腔侧上微孔或平滑的一侧和组织向内生长入面向外侧的植入物的另一多孔侧。Hernias can be described as those of the inguinal location or ventral to other less common but well-known variations, namely the femur or umbilical cord. In one embodiment, the hernia to be repaired is an inguinal hernia, ventral hernia, femoral hernia, umbilical hernia, or any mixture thereof. Hernias located in the anterior or lateral abdominal wall on the pre-operative or traumatic site can be accessed directly or by laparoscopic means. Repair of defects in the muscles/fascia of the rectus abdominis sheath—transversus, external oblique, and/or internal oblique—primarily placed into the abdominal wall by placing an implantable device comprising a reticulated elastic matrix . In one embodiment, an implantable device comprising a reticulated elastic matrix can be treated to have a microporous or smooth side on the side facing the abdominal cavity and tissue ingrowth into the other porous side of the implant facing outward. .
可以通过腹膜前手段接近腹股沟疝,所述的腹膜前手段即使用内部环作为通过前路手术与“无紧张性”Lichenstein或填塞或可选择地腹腔镜手术直接进入腹膜前间隙。Inguinal hernias can be accessed by preperitoneal approaches using the internal ring as direct access to the preperitoneal space via an anterior approach with "tension free" Lichenstein or tamponade or alternatively laparoscopic surgery.
在Lichtenstein无张力性修复中,在切开皮肤,斯卡帕筋膜和腹外斜肌腱膜后由前路手术接近腹股沟管。检查间接囊的索,减少任何直接疝气并且通过与相连腱和腹股沟韧带支架边缘缝合的包括网状弹性基质的可植入装置加强底部。可以使包括网状弹性基质的可植入装置裂缝或设计成可容纳索结构。在Kugel技术中,将包括网状弹性基质的可植入装置的单层或双层(具有或不具有自我防卫性的外部记忆回弹环)通过4cm肌肉分离切口前置地放入腹膜前间隙。In the Lichtenstein tension-free repair, the inguinal canal is approached anteriorly after incision of the skin, Scarpa's fascia, and external oblique aponeurosis. The cords of the indirect pouch are inspected, any direct herniation is reduced and the base is reinforced by an implantable device comprising a mesh elastic matrix sutured to the edge of the associated tendon and inguinal ligament scaffold. Implantable devices comprising reticulated elastic matrices can be slit or designed to accommodate cord structures. In the Kugel technique, a single or double layer of an implantable device comprising a reticulated elastic matrix (with or without a self-defensive external memory rebound ring) is placed anteriorly into the preperitoneal space through a 4 cm muscle separation incision .
两种常用的腹腔镜检查技术包括经腹腹膜前修复(“TAPP”)和完全腹膜外修复(“TEP”)。TAPP和TEP都可以将包括网状弹性基质的可植入装置放入腹膜前间隙。在腹部内由在腹膜中做的腹膜前间隙切口进入腹腔内进行TAPP修复。在TEP修复中,在腹膜外间隙中开始完全剥离。在两种手术中适当修复的目的包括:(1)完全剥离肌肉-肌肉-孔口(MPO)和周围结构,其中完全近中暴露耻骨和雷丘斯间隙;(2)除去腹膜前脂肪和索脂肪瘤;(3)评价所有可能的疝气部位;(4)完全减小疝囊;和(5)使索骨架化以确保由输精管和性腺管近端减小间接囊。Two commonly used laparoscopic techniques include transabdominal preperitoneal repair ("TAPP") and total extraperitoneal repair ("TEP"). Both TAPP and TEP can place an implantable device comprising a reticulated elastic matrix into the preperitoneal space. TAPP repair was performed in the abdominal cavity by entering the abdominal cavity through an incision in the preperitoneal space made in the peritoneum. In TEP repair, complete dissection is initiated in the extraperitoneal space. The goals of proper repair in both procedures include: (1) complete dissection of the muscle-muscle-ostium (MPO) and surrounding structures, with full mesial exposure of the pubic bone and the Reicus space; (2) removal of preperitoneal fat and cords; (3) evaluate all possible hernia sites; (4) completely reduce the hernia sac; and (5) skeletonize the cord to ensure reduction of the indirect sac from the proximal vas deferens and gonadal ducts.
在另一个实施方案中,包括网状弹性基质的可植入装置用于美容外科手术应用,包括上颌面,颅,乳腺,泌尿外科,胃食管或其它重建目的。在这类应用中,网状弹性基质可以起占据空间的填料的作用并且提供组织向内生长的平台,它特别有效地治疗这类整形重建病症。In another embodiment, an implantable device comprising a reticulated elastic matrix is used for cosmetic surgical applications, including maxillofacial, cranial, breast, urological, gastroesophageal or other reconstructive purposes. In such applications, the reticulated elastic matrix can act as a space-occupying filler and provide a platform for tissue ingrowth, which is particularly effective in the treatment of such orthopedic conditions.
在一个实施方案中,特别为整形和重建手术,诸如乳腺软组织加强和预防囊形成设计包括网状弹性基质的可植入装置。由于得到了本发明网状弹性基质独特的生物耐久性/生物相容性,所以它特别用于乳腺整形手术。其应用可以减少植入物包囊形成。通常将乳腺植入物放入乳腺自身下或乳腺下肌肉下以手术方式生成的袋中。乳腺植入物(甚至具有纹理表面的那些)会达所有病例25%的人群中形成厚的实体纤维囊或组织变形(折叠/皱褶)。这些囊(按照1-4的等级通常分类为3或4,其中4为“最坏的情况”)对患者和整形外科医生提出了严重的临床挑战。从动物模型和临床经验中可充分接受的是上述聚氨基甲酸酯泡沫成功地避免或明显减弱了囊形成;然而,那些聚氨基甲酸酯泡沫覆盖物还有缺陷。相反,包括网状弹性基质的可植入装置用于避免和或明显减弱囊形成。In one embodiment, implantable devices comprising a reticulated elastic matrix are designed specifically for plastic and reconstructive procedures, such as breast soft tissue augmentation and cyst formation prevention. Due to the unique biodurability/biocompatibility of the reticulated elastic matrix of the present invention, it is particularly useful in breast plastic surgery. Its application can reduce implant cyst formation. Breast implants are usually placed in a surgically created pocket under the mammary gland itself or under the submammary muscle. Breast implants (even those with textured surfaces) develop thick solid fibrous capsules or tissue deformations (folds/folds) in up to 25% of all cases. These cysts (often classified as 3 or 4 on a scale of 1-4, with 4 being the "worst case") present serious clinical challenges to patients and plastic surgeons. It is well accepted from animal models and clinical experience that the polyurethane foams described above successfully avoid or significantly attenuate cyst formation; however, those polyurethane foam coverings also have drawbacks. In contrast, implantable devices comprising reticulated elastic matrices are used to avoid and or significantly reduce cyst formation.
可植入装置可以以几种不同的构造使用。例如,实施方案的正方形或矩形实际上可以与标准手术固定联用,以便将纤维加强物仔细包括到组织结合中。其实例可以用于使用胸壁肌群下的标准乳腺植入物的乳腺重建中的侧乳房下折叠。另一个典型的构造为作为亚-腺或亚-肌乳腺植入物覆盖物的可植入装置。可以定制具有加强网状结构的可植入装置或在其周边上具有存在的唇状构造,以便以无缝合线方式使用标准乳腺植入物覆盖。植入可以在面向外的侧面或两侧上进行,以便增加组织向内生长,稳定植入物并且还减弱乃至预防组织化的增厚植入物纤维囊形成。Implantable devices can be used in several different configurations. For example, the square or rectangular shape of the embodiments can actually be used in conjunction with standard surgical fixation to carefully incorporate fibrous reinforcement into tissue bonding. An example of this can be used for lateral submammary folds in breast reconstruction using standard breast implants under the chest wall muscles. Another exemplary configuration is an implantable device that acts as a covering for sub-glandular or sub-muscular breast implants. The implantable device can be customized with a reinforced mesh structure or with a lip configuration present on its perimeter to allow for sutureless coverage with standard breast implants. Implantation can be done on the outward facing side or both sides in order to increase tissue ingrowth, stabilize the implant and also reduce or even prevent organized thickened implant fibrous capsule formation.
在另一个实施方案中,可植入装置用于颜面美容手术以便最低限度侵害性的和其它重建应用。在颜面美容应用中,可以使用套针(troacar)或其它插管器将可植入装置传送入支持性筋膜软组织。包括网状弹性基质的可植入装置在其整个进程中衔接组织并且随时间连接例如可吸收性缝合线,固定凹,倒钩,钉针,螺丝,U形钉,板,平头钉,胶水等,分散并且可植入装置支持组织向内生长,由此进行安全性生物固定。最常见的是通过开放式或最低程度侵害性/经皮技术解决和手术操作前额、面中部和颈的特殊区域,诸如例证在图8中的鼻唇沟,颊新月体,颊凹陷和下颚垂肉。In another embodiment, the implantable device is used in cosmetic facial surgery for minimally invasive and other reconstructive applications. In cosmetic applications, a trocar or other introducer may be used to deliver the implantable device into the supporting fascial soft tissue. An implantable device comprising a mesh elastic matrix engages tissue throughout its course and attaches over time such as absorbable sutures, fixation notches, barbs, staples, screws, staples, plates, tacks, glue, etc. , a dispersed and implantable device supporting tissue ingrowth, thereby performing safe biofixation. Specialized areas of the forehead, midface, and neck are most commonly addressed and surgically manipulated by open or minimally invasive/percutaneous techniques, such as the nasolabial folds, buccal crescent, buccal depression, and mandible exemplified in Figure 8 wattle.
本发明的可植入装置在所有手术领域具有一般性应用,其中通过组织向内生长至网状弹性基质进行的永久性生物固定和/或悬浮也是需要的。The implantable device of the present invention has general application in all surgical fields where permanent biological fixation and/or suspension by tissue ingrowth into the reticulated elastic matrix is also desired.
包括网状弹性基质的可植入装置也用作体外细胞增殖应用的支持物,例如矫形外科应用,诸如组织连接,再生,加强或腱,韧带,关节盘和环形物和假体器官组织生长中的支持物。Implantable devices comprising reticulated elastic matrices are also used as supports for in vitro cell proliferation applications such as orthopedic applications such as tissue joining, regeneration, reinforcement or growth of tendons, ligaments, joint discs and annulus and prosthetic organ tissue support.
在一个实施方案中,可植入装置可以包含细胞,生长因子和营养物。在另一个实施方案中,生物耐久性可植入装置可以用作非自体细胞或采集自患者的自体细胞的模板,可以将所述细胞在离体实验室环境中培养且然后植入患者缺陷。在另一个实施方案中,可植入装置掺入骨诱导剂,诸如生长因子,例如来源于血小板和白细胞的自体生长因子的能力能够使其被功能化以便调节细胞功能并且积极地诱导组织向内生长。可植入装置由此提供了细胞疗法应用的基础,以便支持宽范围软组织,包括,但不限于关节软骨的组织修复和再生,半月板修复和ACL重建。所得可植入装置填充软骨缺陷,支持自体组织修复和再生且随后能够整合修复或再生部位,例如受损的膝。In one embodiment, the implantable device may contain cells, growth factors and nutrients. In another embodiment, a biodurable implantable device can be used as a template for non-autologous cells or autologous cells harvested from a patient that can be cultured in an ex vivo laboratory setting and then implanted into the patient's defect. In another embodiment, the ability of the implantable device to incorporate osteoinductive agents, such as growth factors, eg, autologous growth factors derived from platelets and leukocytes, enables it to be functionalized to regulate cellular function and actively induce tissue inwards grow. The implantable device thus provides the basis for the application of cell therapy to support tissue repair and regeneration of a wide range of soft tissues, including, but not limited to, articular cartilage, meniscus repair, and ACL reconstruction. The resulting implantable device fills cartilage defects, supports autologous tissue repair and regeneration and can subsequently integrate repair or regeneration sites, such as damaged knees.
在另一个实施方案中,可植入装置用于组织改造应用,包括生成假体器官组织,例如用于肝,肾或乳腺组织的再生。In another embodiment, the implantable device is used in tissue engineering applications, including the generation of prosthetic organ tissue, for example for regeneration of liver, kidney or breast tissue.
在一个非限制性实例中,为指定部位,诸如靶组织愈合部位选择一种或多种包括网状弹性基质的可植入装置。将可植入装置(或多个装置)载入递送装置,诸如导管,内窥镜,套管,套管针等。在一个实施方案中,递送装置用于使用最低限度侵害性方式递送包括网状弹性基质的可植入装置。在可植入装置从递送装置中释放后,可以将其锚定就位以便抵抗从靶修复或再生部位迁移。使可植入装置固定就位的方法包括使用缝线,固定凹,倒钩,插针,螺丝,U形钉,板,平头针,胶或其任意的组合使可植入装置与靶修复部位固定。包括网状弹性基质的可植入装置可以翻转并且通过关节镜套管插入关节。在一个实施方案中,可植入装置与靶组织愈合部位相比超尺寸并且居留在该部位上或通过压缩配合保持在该部位上就位,例如通过网状弹性基质的回弹性。在一个实施方案中,超尺寸的可植入装置在形态上与组织缺陷保持一致。不受任何特定理论的约束,产生这类共形拟合的回弹和可恢复性能导致在可植入装置壁与缺陷之间形成紧密的基本上无间隙的边界,由此提供有助于促进细胞向内生长和组织增殖的界面。一旦在该部位上释放,则包括网状弹性基质的可植入装置以可回弹方式膨胀至约为其原始大小且形状当然服从任何压缩变定限和任何所需的屈曲,覆盖或符合可植入装置弹性所适合的植入部位的解剖和/或几何形状。在另一个实施方案中,通过开放性手术操作插入可植入装置。In one non-limiting example, one or more implantable devices comprising a reticulated elastic matrix are selected for a given site, such as a target tissue healing site. The implantable device (or devices) is loaded into a delivery device, such as a catheter, endoscope, cannula, trocar, or the like. In one embodiment, the delivery device is used to deliver an implantable device comprising a reticulated elastic matrix in a minimally invasive manner. After the implantable device is released from the delivery device, it can be anchored in place so as to resist migration from the target repair or regeneration site. The method of securing the implantable device in place includes using sutures, fixation notches, barbs, pins, screws, staples, plates, tacks, glue, or any combination thereof to secure the implantable device to the target repair site fixed. An implantable device comprising a mesh elastic matrix can be inverted and inserted into the joint through an arthroscopic cannula. In one embodiment, the implantable device is oversized compared to the target tissue healing site and resides at the site or is held in place at the site by a compression fit, such as by the resiliency of the reticulated elastic matrix. In one embodiment, the oversized implantable device is morphologically consistent with the tissue defect. Without being bound by any particular theory, the springback and recoverability properties that produce such a conformal fit result in a tight, substantially gap-free boundary between the implantable device wall and the defect, thereby providing an opportunity to facilitate The interface of cell ingrowth and tissue proliferation. Once released at the site, the implantable device comprising the reticulated elastic matrix resiliently expands to about its original size and shape, of course, subject to any compression set limits and any desired buckling, covering or conforming to the desired shape. The anatomy and/or geometry of the implant site to which the implant device is elastically adapted. In another embodiment, the implantable device is inserted by an open surgical procedure.
在另一个实施方案中,以机械方式使网状弹性基质10固定到缺损。该缺损可能因损伤或疾病导致或可能因手术方式产生。网状弹性基质可以位于靶缺损内部,与之相邻和/或覆盖靶缺损。网状弹性基质可以用作缺陷填料,替代组织,组织加强物和/或加强补片。在另一个实施方案中,网状弹性基质可以跨越缺陷并且用作天然组织内缺口的桥连物。In another embodiment, the reticulated
尽管可以通过许多不同标准或可接受手术方法使包括网状弹性基质的可植入装置与组织修复或再生部位连接,但是两种典型方法如下所述。可以将这些操作应用于其它修复,再生和重建性手术。While an implantable device comprising a reticulated elastic matrix can be attached to a site of tissue repair or regeneration by many different standard or accepted surgical methods, two typical methods are described below. These procedures can be applied to other repair, regenerative and reconstructive procedures.
使用Hall矫形外科应用小圆锯剥去软组织修复部位,诸如受损的冈下肌腱的外皮。剥去骨的标准区域的外皮。将4个Biosuture粘性固定凹放入结节中的正方形构造。握紧冈下肌腱并且使用两个缝合固定凹和Mason-Allen式缝线再附着于肱骨近端。将可植入装置置于修复部位上部,使得在结节侧上有约0.5cm-2cm突出端。装置的剩余部分在腱上伸展。用于腱连接的固定缝线也伴随垂直褥式缝合通过该装置并且使该装置固定在修复腱上,从而生成由可植入装置和腱组成的分层的构造。在侧面,另两个固定缝线通过装置并且将其向下与结节扎紧。在一个实施方案中,将装置固定缝线安排在加强物内部,例如沿装置周长和/或加强格栅最外侧元件内部放置的的加强元件内部。4个固定缝线端如图9a中所示交叉。A small circular saw is used with Hall Orthopedics to deskin the site of soft tissue repair, such as the damaged infraspinatus tendon. Skin the standard area of bone. Deboss 4 Biosuture Adhesive Fixtures into the square configuration in the nodules. The infraspinatus tendon is grasped and reattached to the proximal humerus using two suture fixations and a Mason-Allen suture. The implantable device is placed over the repair site so that there is approximately a 0.5cm-2cm overhang on the nodular side. The remainder of the device is stretched over the tendon. Fixation sutures for tendon attachment were also passed through the device along with vertical mattress sutures and secured the device to the prosthetic tendon, creating a layered construct consisting of the implantable device and tendon. Laterally, two other fixation sutures are threaded through the device and tied down to the tubercle. In one embodiment, the device securing sutures are arranged inside the reinforcement, for example inside reinforcement elements placed along the perimeter of the device and/or inside the outermost elements of the reinforcement grid. The 4 anchor suture ends are crossed as shown in Figure 9a.
在另一个实施方案中,修复如上所述进行,但将可植入装置放置在修复部位上部,使得在结节侧上有约1cm突出端。可植入装置的剩余部分在腱上伸展。用于腱连接的固定缝线也如上所述通过该装置。在侧面,另两个固定缝线如上所述通过装置并且将其向下与结节扎紧。将装置固定缝线如图9b中所示安排在装置加强物内部。In another embodiment, the repair is performed as described above, but with the implantable device placed over the repair site so that there is about a 1 cm overhang on the nodular side. The rest of the implantable device stretches over the tendon. Fixation sutures for tendon connections are also passed through the device as described above. Laterally, two other fixation sutures are passed through the device as described above and tied down to the tubercle. The device securing sutures were arranged inside the device reinforcement as shown in Figure 9b.
在一个实施方案中,由生物耐久性网状弹性基质制成的可植入装置为组织向内生长提供了极佳的平台。在另一个实施方案中,细胞本体,诸如成纤维细胞和组织可以侵入并且生长入包括网状弹性基质的可植入装置。在适当的时机,这类向内生长扩展入插入的网状弹性基质10的内孔20和小间隙。最终,包括网状弹性基质的可植入装置可以基本上被再生细胞向内生长填充,这种向内生长形成了可以占据所述部位或其中的空隙空间的团块。组织向内生长的类型包括,但不限于纤维组织,内皮组织和矫形外科应用软组织。In one embodiment, implantable devices made of biodurable reticulated elastic matrices provide an excellent platform for tissue ingrowth. In another embodiment, cellular bodies, such as fibroblasts and tissues, can invade and grow into implantable devices comprising reticulated elastic matrices. When the time is right, such ingrowth expands into the
在另一个实施方案中,可植入装置促进细胞向内生长和组织再生遍布于所述部位,遍布于部位边界或通过某些暴露表面,由此封闭该部位。随时间的推移,因组织向内生长产生的这一诱导的纤维血管本体可以促进可植入装置掺入靶组织愈合部位。在一个实施方案中,因组织向内生长产生的这一诱导的纤维血管本体可以导致可植入装置至少部分(即使不是基本上完全)生物整合入靶组织愈合部位。在另一个实施方案中,组织向内生长可以导致受损组织修复或受损组织再生和/或重建。在另一个实施方案中,组织向内生长可以导致可植入装置有效抵抗随时间产生的迁移。它还可以填充空隙或缺陷。在另一个实施方案中,组织向内生长为可以长期持续,无害和/或机械稳定的瘢痕组织。在另一个实施方案中,随时间的进程,例如2周到3个月至1年,植入的网状弹性基质10变得完全被组织,纤维组织,瘢痕组织等填充和/或包囊。In another embodiment, the implantable device promotes cellular ingrowth and tissue regeneration throughout the site, across site boundaries or through certain exposed surfaces, thereby sealing the site. Over time, this induced fibrovascular body due to tissue ingrowth can facilitate incorporation of the implantable device into the target tissue healing site. In one embodiment, this induced fibrovascular body due to tissue ingrowth can result in at least partial, if not substantially complete, biointegration of the implantable device into the target tissue healing site. In another embodiment, tissue ingrowth can result in repair of damaged tissue or regeneration and/or reconstruction of damaged tissue. In another embodiment, tissue ingrowth can result in an implantable device that effectively resists migration over time. It can also fill voids or imperfections. In another embodiment, the tissue ingrowth is long lasting, harmless and/or mechanically stable scar tissue. In another embodiment, the implanted reticulated
在另一个实施方案中,可植入装置还具有生物相容性这一有用的永久性生物植入特征。生物相容性包括,但不限于显示无致癌性,诱变性,致畸性,细胞毒性或其它不良生物作用。In another embodiment, the implantable device also has biocompatibility, a useful characteristic of permanent bioimplantation. Biocompatibility includes, but is not limited to, exhibiting no carcinogenicity, mutagenicity, teratogenicity, cytotoxicity or other adverse biological effects.
在另一个实施方案中,将包括网状弹性基质的可植入装置的特性改造成与靶向的组织相容,例如模拟它或满足特定应用的要求。可以通过控制,例如交联的量,结晶度的量,化学组成,固化条件,网状化程度和/或网状化后加工,诸如退火,压缩模制和/或掺入加强物改造网状弹性基质的特性。不同于生物可降解聚合物,网状弹性基质在长时间期限内维持其在体内的物理特征和性能。因此,它不会引起不需要的组织反应,如在生物可降解的植入物分解和降解时可能观察到的。网状弹性基质的高空隙量和网状化程度使得组织向内生长并且使细胞在基质内增殖。在一个实施方案中,向内生长的组织和/或再生的细胞占据原始可植入装置互联空隙相14体积的约25%-约99%,在另一个实施方案中,约为51%-约99%,由此提供所修复或替代的原始组织的功能性,诸如具有荷载能力。In another embodiment, the properties of an implantable device comprising a reticulated elastic matrix are engineered to be compatible with the targeted tissue, eg, mimic it or meet the requirements of a particular application. The reticulation can be modified by controlling, for example, the amount of cross-linking, the amount of crystallinity, the chemical composition, curing conditions, degree of reticulation and/or post-reticulation processing such as annealing, compression molding and/or incorporation of reinforcements properties of elastic substrates. Unlike biodegradable polymers, reticulated elastic matrices maintain their physical characteristics and performance in vivo over long periods of time. Therefore, it does not cause unwanted tissue reactions, as might be observed when biodegradable implants break down and degrade. The high void volume and degree of reticulation of the reticulated elastic matrix allow tissue ingrowth and cell proliferation within the matrix. In one embodiment, the ingrowth tissue and/or regenerated cells occupy from about 25% to about 99% of the volume of the interconnected
在一个非限制性实例中,改造可植入装置的压缩变定,回弹性和/或恢复性以便在反复循环加载后提供网状弹性基质的高度恢复力。这类特征对矫形外科应用而言特别有利,其中可植入装置的循环加载还可以持久性压缩网状弹性基质,由此防止它基本上连续接触允许最佳细胞浸润和组织向内生长所必需的周围软组织。在另一个非限制性实例中,改造可植入装置的密度和孔径以便提供网状弹性基质在压缩下的可接受的渗透性。这类特征和优点有利于脊柱和膝矫形外科应用,其中高载荷被置于可植入装置上。在另一个非限制性实例中,改造网状弹性基质的特性以便将其“软的共形拟合”最大化,这特别有利于美容外科手术应用。在另一个非限制性实例中,使可植入装置的抗张性能最大化以补充所用的固定技术,例如提供对缝合线拉出的最大阻力。In one non-limiting example, the compression set, resilience and/or recovery of the implantable device are engineered to provide a high degree of resilience of the reticulated elastic matrix after repeated cyclic loading. Such features are particularly advantageous for orthopedic applications, where cyclic loading of the implantable device can also permanently compress the reticulated elastic matrix, thereby preventing it from substantially continuous contact necessary to allow optimal cell infiltration and tissue ingrowth surrounding soft tissue. In another non-limiting example, the density and pore size of the implantable device are engineered so as to provide acceptable permeability of the reticulated elastic matrix under compression. Such features and advantages are advantageous for spinal and knee orthopedic applications where high loads are placed on the implantable device. In another non-limiting example, engineering the properties of the reticulated elastic matrix to maximize its "soft conformal fit" is particularly beneficial for cosmetic surgical applications. In another non-limiting example, the tensile properties of the implantable device are maximized to complement the fixation technique used, eg, to provide maximum resistance to suture pullout.
在另一个实施方案中,本文披露的可植入装置可以用作递药载体。例如,可以使治疗剂与生物耐久性实体相12混合,共价键合,吸收在其上和/或吸收入其中。可以通过可植入装置递送各种治疗剂中的任意种,例如上文披露的那些治疗剂。In another embodiment, the implantable devices disclosed herein can be used as drug delivery vehicles. For example, a therapeutic agent may be mixed with, covalently bonded to, absorbed on and/or absorbed into
具体实施方式Detailed ways
实施例Example
列出下列实施例是为了有助于理解本发明,但不应被视为特别限定本文所述的本发明。本发明的这类变化形式,包括属于本领域技术人员范围和配方改变或实验设计中的改变的所有目前已知的等效技术方案的替代方案或随后研发的技术方案均被视为落入本文披露的本发明范围。The following examples are set forth to facilitate the understanding of the present invention, but should not be construed as particularly limiting the invention described herein. Such variants of the present invention, including all presently known alternatives to equivalent technical solutions or subsequently developed technical solutions that fall within the scope of those skilled in the art and formulation changes or changes in experimental design, are deemed to fall within this text scope of the disclosed invention.
实施例1:交联聚氨基甲酸酯基质1的制造Example 1: Manufacture of
芳族异氰酸酯RUBINATE 9258(来自Huntsman)用作异氰酸酯成分。RUBINATE 9258在下25℃为液体。RUBINATE 9258含4,4’-MDI和2,4’-MDI并且具有约2.33的异氰酸酯官能度。具有约2,000道尔顿的二元醇聚(1,6-己烷碳酸酯)二元醇(来自Arch Chemicals的POLY-CD CD220)用作多元醇成分并且在25℃下为固体。蒸馏水用作起泡剂。所用的起泡催化剂为叔胺三乙二胺(在双丙二醇中33%;来自AirProducts的DABCO 33LV)。使用基于硅氧烷的表面活性剂(来自Goldschmidt的TEGOSTAB BF 2370)。使用隔室开放剂(来自Goldschmidt的ORTEGOL 501)。存在粘度改进剂碳酸丙烯酯(来自Sigma-Aldrich)以降低粘度。所用成分的比例如表2中所示。Aromatic isocyanate RUBINATE 9258 (ex Huntsman) was used as isocyanate component. RUBINATE 9258 is a liquid at 25°C. RUBINATE 9258 contains 4,4'-MDI and 2,4'-MDI and has an isocyanate functionality of about 2.33. A diol poly(1,6-hexanecarbonate) diol with about 2,000 Daltons (POLY-CD CD220 from Arch Chemicals) was used as the polyol component and is solid at 25°C. Distilled water was used as a foaming agent. The blowing catalyst used was the tertiary amine triethylenediamine (33% in dipropylene glycol; DABCO 33LV from Air Products). A silicone based surfactant (TEGOSTAB BF 2370 from Goldschmidt) was used. A compartment opener (ORTEGOL 501 from Goldschmidt) was used. The viscosity modifier propylene carbonate (from Sigma-Aldrich) was present to reduce the viscosity. The ratios of the ingredients used are shown in Table 2.
表2Table 2
在循环空气烘箱内使多元醇成分在70℃下液化并且称出其100g放入聚乙烯杯。将5.8g粘度改进剂加入到多元醇成分中以便降低粘度并且使用钻孔混合器的混合轴将所述组分以3100rpm混合15秒而形成“混合物-1”。向混合物-1中加入1.10g表面活性剂并且如上所述将组分混合15秒而形成“混合物-2”。此后向混合物-2中加入1.00g隔室开放剂并且将组分如上所述混合15秒而形成“混合物-3”。向混合物-3中加入62.42g异氰酸酯成分并且将组分混合60±10秒而形成“系统A”。The polyol component was liquefied at 70°C in a circulating air oven and 100 g was weighed out into a polyethylene cup. 5.8 g of a viscosity modifier was added to the polyol component to reduce the viscosity and the components were mixed at 3100 rpm for 15 seconds using the mixing shaft of a drill mixer to form "Mix-1". To Mix-1 was added 1.10 g of surfactant and the components were mixed for 15 seconds as described above to form "Mix-2". Thereafter 1.00 g of compartment opener was added to Mix-2 and the components were mixed for 15 seconds as described above to form "Mix-3". To Mix-3 was added 62.42 g of the isocyanate component and the components were mixed for 60±10 seconds to form "System A".
使用玻璃棒将3.39g蒸馏水与0.53g起泡催化剂在小塑料杯中混合60秒而形成“系统B”。"System B" was formed by mixing 3.39 g of distilled water with 0.53 g of foaming catalyst in a small plastic cup for 60 seconds using a glass rod.
将系统B尽可能快地倾入系统A,同时避免溢出。如上所述使用钻孔混合器将组分剧烈混合10秒,然后倾入22.9cm x 20.3cm x 12.7cm(9英寸x8英寸x5英寸)内表面被铝箔覆盖的厚纸板箱。起泡分布如下:11秒混合时间,27秒成乳液时间和100秒升起时间。Pour System B into System A as quickly as possible while avoiding spillage. The components were vigorously mixed for 10 seconds using a drill mixer as above, then poured into a 22.9cm x 20.3cm x 12.7cm (9"x8"x5") cardboard box with aluminum foil covered interior surfaces. The lather profile was as follows: 11 seconds mix time, 27 seconds emulsion time and 100 seconds rise time.
在开始起泡后2分钟,即在合并系统A和B时,使泡沫进入维持在100-105℃下的循环空气烘箱内固化约55-约60分钟。此后从烘箱内取出泡沫并且在约25℃下冷却10分钟。使用带锯从每侧上取出外膜。此后,将手部压力施加于泡沫每侧以开放隔室窗。使泡沫重新进入循环空气烘箱并且在100-105℃下再后固化4.5小时。Two minutes after initiation of foaming, ie when Systems A and B are combined, the foam is cured in a circulating air oven maintained at 100-105°C for about 55 to about 60 minutes. Thereafter the foam was removed from the oven and cooled at about 25°C for 10 minutes. Remove the adventitia from each side using a band saw. Thereafter, hand pressure was applied to each side of the foam to open the compartment windows. The foam was re-entered into the circulating air oven and post-cured for an additional 4.5 hours at 100-105°C.
如根据光学显微镜观察结果测定的泡沫的平均孔径大于约325μm。The foam has an average pore size greater than about 325 [mu]m as determined by light microscopy observations.
按照ASTM D3574进行下列泡沫测试。使用50mm x 50mm x 25mm尺寸的样本测定堆密度。通过用样品重量除以样本体积计算密度。获得2.29 lbs/ft3(0.037g/cc)的密度值。The following foam tests were performed in accordance with ASTM D3574. Bulk density was determined using a sample of size 50mm x 50mm x 25mm. Density was calculated by dividing the sample weight by the sample volume. A density value of 2.29 lbs/ft3 (0.037 g/cc) was obtained.
对与泡沫升起方向平行或垂直切割的样品进行张力试验。从泡沫块上切下狗骨形的张力样本。经测定每一试验样本约12.5mm厚,约25.4mm宽和约140mm长;每一样本的规格长度为35mm且每一样本的规格宽度为6.5mm。使用INSTRON Universal Testing InstrumentModel 1122以500mm/min(19.6英寸/分钟)的十字头速度测定张力(拉伸强度和断裂时的延长)。与泡沫升起方向平行的平均拉伸强度测定约为33.8psi(23,770kg/m2)。与泡沫升起方向平行的断裂伸长度测定约为123%。与泡沫升起方向垂直的平均拉伸强度测定约为27.2psi(19,150kg/m2)。与泡沫升起方向垂直的断裂伸长度测定约为134%。Tensile tests are performed on samples cut either parallel or perpendicular to the direction of foam rise. Cut a dog-bone-shaped tension sample from the foam block. It was determined that each test sample was about 12.5 mm thick, about 25.4 mm wide and about 140 mm long; the gauge length of each sample was 35 mm and the gauge width of each sample was 6.5 mm. Tension (tensile strength and elongation at break) was measured using an INSTRON Universal Testing Instrument Model 1122 at a crosshead speed of 500 mm/min (19.6 in/min). The average tensile strength parallel to the direction of foam rise was determined to be approximately 33.8 psi (23,770 kg/m2 ). The elongation at break parallel to the direction of foam rise was measured to be approximately 123%. The average tensile strength perpendicular to the direction of foam rise was determined to be approximately 27.2 psi (19,150 kg/m2 ). The elongation at break perpendicular to the direction of foam rise was measured to be about 134%.
实施例2:交联聚氨基甲酸酯基质1的网状化和由其制造可植入装置Example 2: Reticulation of
通过实施例6中所述的操作进行实施例1中所述泡沫的网状化。Reticulation of the foam described in Example 1 was performed by the procedure described in Example 6.
如实施例1中所述测定网状泡沫密度。获得2.13 lbs/ft3(0.034g/cc)的网状化后密度值。Reticulated foam density was determined as described in Example 1. A reticulated density value of 2.13 lbs/ft3 (0.034 g/cc) was obtained.
如实施例1中所述对网状泡沫样品进行张力试验。与泡沫升起方向平行的网状化后拉伸强度平均值测定约为31.1psi(21,870kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为92%。与泡沫升起方向垂直的平均网状化后拉伸强度平均值测定约为22.0psi(15,480kg/m2)。与泡沫升起方向垂直的网状化后伸长度测定约为110%。Tensile testing was performed on reticulated foam samples as described in Example 1. The average post-reticulation tensile strength parallel to the direction of foam rise was measured to be approximately 31.1 psi (21,870 kg/m2 ). The elongation at break after reticulation parallel to the direction of foam rise was determined to be about 92%. The average post-reticulated tensile strength, perpendicular to the direction of foam rise, was measured to be approximately 22.0 psi (15,480 kg/m2 ). The elongation after reticulation perpendicular to the direction of foam rise was measured to be about 110%.
使用测定为50mm x 50mm x 25mm的样本进行压缩试验。使用INSTRON Universal Testing Instrument Model 1122以10mm/min(0.4英寸/分钟)的十字头速度进行试验。将在50%和75%压缩下各自与泡沫升起方向平行的网状化后抗压强度分别测定为1.49psi(1,050kg/m2)和3.49psi(2,460kg,/m2)。将在50%和75%压缩下网状样品在25℃下进行所述量压缩22小时,然后释放压缩应力后各自测定的与泡沫升起方向平行的网状化压缩变定分别测定约为4.7%和7.5%。Compression tests were performed using samples measuring 50mm x 50mm x 25mm. Testing was performed using an INSTRON Universal Testing Instrument Model 1122 at a crosshead speed of 10 mm/min (0.4 inches/min). The post-reticulated compressive strengths parallel to the direction of foam rise were determined to be 1.49 psi (1,050 kg/m2 ) and 3.49 psi (2,460 kg,/m2 ), respectively, at 50% and 75% compression. The reticulated compression set parallel to the direction of foam rise was determined to be approximately 4.7 for each of the reticulated samples at 50% and 75% compression subjected to the stated amount of compression at 25°C for 22 hours and then released from the compressive stress, respectively. % and 7.5%.
用机器由网状泡沫制造蘑菇形可植入装置,它具有约16mm直径和约8mm长度的平坦的圆柱状头或帽和约10mm直径和约8mm长度的狭窄圆柱形杆。此后,通过使其接触约2.3Mr ad剂量的γ射线对样品灭菌。A mushroom-shaped implantable device was machined from reticulated foam with a flat cylindrical head or cap of about 16 mm diameter and about 8 mm length and a narrow cylindrical stem about 10 mm diameter and about 8 mm length. Thereafter, the samples were sterilized by exposing them to gamma radiation at a dose of about 2.3 Mrad.
实施例3:胶原蛋白涂敷的可植入装置的制造Example 3: Fabrication of Collagen-Coated Implantable Devices
洗涤通过从牛来源提取获得的I型胶原蛋白并且切碎成纤丝。通过剧烈搅拌胶原蛋白和水并且加入无机酸至pH约为3.5制备1%重量的胶原蛋白含水淤浆。该淤浆的粘度约为500厘泊。Type I collagen obtained by extraction from bovine sources was washed and minced into fibrils. A 1% by weight aqueous slurry of collagen was prepared by vigorously stirring the collagen and water and adding mineral acid to a pH of approximately 3.5. The viscosity of the slurry was about 500 centipoise.
将按照实施例2制备的蘑菇形可植入装置完全浸入所述的胶原蛋白淤浆,由此用该淤浆浸渍每一可植入装置。此后将胶原蛋白-淤浆浸渍的装置放置在金属托盘上,将该托盘放置在预冷却至-45℃的冻干器架上。在装置中的淤浆冷冻后,使冻干室内的压力降至约100毫托,由此使水离开冷冻的胶原蛋白淤浆升华,遗留在网状化可植入装置孔内的沉积的多孔胶原蛋白基质。此后,使温度缓慢升至约25℃,然后使压力恢复到1个大气压。在冻干器中的总处理时间约为21-22小时。Mushroom-shaped implantable devices prepared according to Example 2 were completely immersed in the collagen slurry, thereby impregnating each implantable device with the slurry. The collagen-slurry impregnated devices were thereafter placed on a metal tray placed on a lyophilizer rack pre-cooled to -45°C. After the slurry in the device is frozen, the pressure in the lyophilization chamber is reduced to about 100 mTorr, thereby sublimating the water out of the frozen collagen slurry, leaving behind the deposited porosity in the pores of the reticulated implantable device. collagen matrix. Thereafter, the temperature was slowly raised to about 25°C, and then the pressure was returned to 1 atmosphere. The total processing time in the lyophilizer was approximately 21-22 hours.
在从冻干器中取出可植入装置后,通过将干燥的胶原蛋白浸渍的植入物接触甲醛蒸汽约21小时使胶原蛋白交联。此后使其接触约2.3Mrad剂量的γ射线对样品灭菌。After removing the implantable device from the lyophilizer, crosslink the collagen by exposing the dried collagen-impregnated implant to formaldehyde vapor for approximately 21 h. Thereafter the samples were sterilized by exposing them to gamma radiation at a dose of about 2.3 Mrad.
实施例4:将植入物植入猪L1-L4腰椎间隙Example 4: Implantation of the implant into the pig L1-L4 lumbar intervertebral space
称重约为55-65kg的Yucatan迷你型猪进行LI-L4(腰椎间隙)椎间盘切除。椎间盘切除由与可接受的人体临床手术操作类似的侧后环形切开术和髓核摘出术(nuclectomy)组成。将通过实施例2和3中所述操作制备的蘑菇形可植入装置在3mm前侧环形切开术中植入以便修复环形缺陷。随后进行标准封闭操作。本发明的可植入装置各自充分起作用,例如它适应性膨胀,封闭环形缺陷并且维持其位置。不存在与该操作相关的不良急性反应并且所有受试动物均无事故地恢复。Yucatan minipigs weighing approximately 55-65 kg underwent LI-L4 (lumbar intervertebral space) discectomy. Discectomy consists of a lateral posterior circular incision and nucleectomy similar to accepted clinical procedures in humans. Mushroom-shaped implantable devices prepared by the procedures described in Examples 2 and 3 were implanted in a 3 mm anterior annular incision to repair annular defects. Standard sealing operations follow. The implantable devices of the present invention each function adequately, eg, it expands adaptively, closes the annular defect and maintains its position. There were no adverse acute reactions associated with the procedure and all tested animals recovered without incident.
实施例5:网状弹性基质1的合成和特性Example 5: Synthesis and Properties of
通过下列操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the following procedure.
芳族异氰酸酯MONDUR MRS-20(来自Bayer Corporation)用作异氰酸酯成分。MONDUR MRS-20在下25℃为液体。MONDUR MRS-20含4,4’-二苯甲烷二异氰酸酯(MDI)和2,4’-MDI且具有约2.2-2.3的异氰酸酯官能度。具有约2,000道尔顿的二元醇聚(1,6-己烷碳酸酯)二元醇(来自Arch Chemicals的POLY-CD220)用作多元醇成分并且在25℃下为固体。蒸馏水用作起泡剂。所用的起泡催化剂为胺类三乙二胺(在双丙二醇中33%重量;来自Air Products的DABCO 33LV)和双(2-二甲氨基乙基)醚(在双丙二醇中23%重量;来自GE Silicones的NIAXA-133)。使用基于硅氧烷的表面活性剂TEGOSTAB BF 2370和TEGOSTABB-8305(来自Goldschmidt)用作细胞稳定剂。使用隔室开放剂(来自Goldschmidt的ORTEGOL 501)。存在粘度改进剂碳酸丙烯酯(来自Sigma-Aldrich)以降低粘度。分别作为交联基和增链剂向该混合物中加入甘油(99.7% USP Grade)和1,4-丁二醇(99.75%重量纯度,来自Lyondell)。所用组分的比例如下表3中所示。The aromatic isocyanate MONDUR MRS-20 (from Bayer Corporation) was used as the isocyanate component. MONDUR MRS-20 is a liquid at 25°C. MONDUR MRS-20 contains 4,4'-diphenylmethane diisocyanate (MDI) and 2,4'-MDI and has an isocyanate functionality of about 2.2-2.3. A diol poly(1,6-hexanecarbonate) diol having about 2,000 Daltons (POLY-CD220 from Arch Chemicals) was used as the polyol component and is solid at 25°C. Distilled water was used as a foaming agent. The blowing catalysts used were the amines triethylenediamine (33% by weight in dipropylene glycol; DABCO 33LV from Air Products) and bis(2-dimethylaminoethyl) ether (23% by weight in dipropylene glycol; from NIAXA-133 from GE Silicones). The silicone-based surfactants TEGOSTAB BF 2370 and TEGOSTAB-8305 (from Goldschmidt) were used as cell stabilizers. A compartment opener (ORTEGOL 501 from Goldschmidt) was used. The viscosity modifier propylene carbonate (from Sigma-Aldrich) was present to reduce the viscosity. To this mixture were added glycerol (99.7% USP Grade) and 1,4-butanediol (99.75% pure by weight from Lyondell) as crosslinkers and chain extenders, respectively. The proportions of the components used are shown in Table 3 below.
表3table 3
异氰酸酯指数,即本领域众所周知的量为异氰酸酯基团在制剂中可用于反应的数量与能够与那些异氰酸酯基团反应的制剂中基团,例如二元醇,多元醇成分,增链剂、水等反应基团(如果存在)数量的摩尔比。将制剂中的异氰酸酯成分放入Edge Sweets Bench Top型氨基甲酸酯混合设备的成分A计量系统并且维持在约20-25℃温度下。Isocyanate Index, a quantity well known in the art is the number of isocyanate groups available in a formulation to react with groups in a formulation capable of reacting with those isocyanate groups, such as diols, polyol components, chain extenders, water, etc. The molar ratio of the number of reactive groups (if present). The isocyanate component of the formulation was placed into the component A metering system of an Edge Sweets Bench Top model urethane mixing equipment and maintained at a temperature of approximately 20-25°C.
使多元醇在约70℃的烘箱内液化并且与粘度改进剂和隔室开放剂按照上述比例合并成均匀混合。将该混合物放入Edge Sweets设备的成分B计量系统。将该多元醇成分维持在约65-70℃温度下的成分B系统中。The polyol was liquefied in an oven at about 70°C and combined with the viscosity modifier and compartment opener in the above proportions to a homogeneous mix. This mixture was placed into the Component B metering system of the Edge Sweets equipment. The polyol component is maintained in the component B system at a temperature of about 65-70°C.
将来自表3的剩余组分按照上述比例混合成单一均匀批量并且放入Edge Sweets设备的成分C计量系统。将该成分维持在约20-25℃温度下。在泡沫形成过程中,来自成分A:成分B:成分C供应的以克/分钟计的流速比例约为8:16:1。The remaining ingredients from Table 3 were mixed in the above proportions into a single homogeneous batch and placed into the Ingredient C metering system of the Edge Sweets equipment. The ingredients are maintained at a temperature of about 20-25°C. During foam formation, the flow rate ratio in grams per minute supplied from component A:component B:component C was approximately 8:16:1.
将上述成分以连续方式在Edge Sweets设备的250cc混合室内合并,该设备安装了置于混合室下的10mm直径喷嘴。通过在混合室内运行的高剪切盘式混合器促进混合。混合成分从喷嘴射入矩形横截面脱模纸壁涂敷的模具。此后泡沫升起至基本上填充模具。所得混合物在接触模具后约10秒开始成乳液,并且在120秒内完全升起。修整所得泡沫的上部并且将泡沫放入100℃固化烘箱内5小时。The above ingredients were combined in a continuous manner in a 250cc mixing chamber of an Edge Sweets apparatus equipped with a 10mm diameter nozzle placed below the mixing chamber. Mixing is facilitated by a high shear disc mixer operating within the mixing chamber. The mixed ingredients are injected from a nozzle into a rectangular cross-section release paper-coated mold. Thereafter the foam rises to substantially fill the mold. The resulting mixture started to emulsion about 10 seconds after contacting the mold and fully rose within 120 seconds. The top of the resulting foam was trimmed and the foam was placed in a 100°C curing oven for 5 hours.
固化后,修整泡沫块的侧面和底部,然后将泡沫放入包括压力室的网状装置,其内部与周围空气隔离。使室内压力降低以便基本上除去固化泡沫中所有的空气。使以足以支持燃烧的比例存在的氢气和氧气混合物进入室。维持室内压力高于大气压足够的时间期限以确保气体透入泡沫。然后通过火花塞点燃室内气体并且这种点火使泡沫内的气体混合物爆炸。为了最小限度地接触任何燃烧产品并且冷却泡沫,从室内除去产生的燃烧气体并且在爆炸后立即用约25℃的氮气替代。然后将上述网状化过程重复一次以上。不受任何特定理论的约束,认为爆炸至少部分除去了泡沫内相邻隔室之间的许多隔室壁或“窗”,由此生成开放的孔并且产生网状弹性基质结构。After curing, the sides and bottom of the foam block are trimmed, and the foam is then placed in a mesh device comprising pressure chambers, the interior of which is isolated from the surrounding air. The chamber pressure is reduced to remove substantially all the air from the cured foam. A mixture of hydrogen and oxygen present in a ratio sufficient to support combustion is admitted to the chamber. The chamber pressure is maintained above atmospheric pressure for a sufficient period of time to ensure gas penetration into the foam. The chamber gas is then ignited by the spark plug and this ignition detonates the gas mixture within the foam. To minimize exposure to any combustion products and cool the foam, the resulting combustion gases were removed from the chamber and replaced with nitrogen at approximately 25°C immediately after the explosion. The reticulation process described above was then repeated one more time. Without being bound by any particular theory, it is believed that the explosion at least partially removes many of the cell walls or "windows" between adjacent cells within the foam, thereby creating open cells and creating a reticulated elastic matrix structure.
正如根据光学显微镜观察结果测定的,网状弹性基质1的平均隔室直径或其它最大横向尺寸约为525μm。图10为网状弹性基质1的扫描电子显微照片(SEM)影像,其表示例如通过其中开放孔互联的隔室网状构造及其互通和互联性。图10的底边上的比例尺条相当于约500μm。正如根据SEM观察结果测定的,网状弹性基质1的平均孔径或其它最大横向尺寸约为205μm。The average cell diameter or other largest lateral dimension of the reticulated
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质1进行下列试验。使用5.0cm x 5.0cm x 2.5cm尺寸的网状弹性基质样本测定堆密度。通过用样本重量除以样本体积计算网状化后密度。获得3.29 lbs/ft3(0.053g/cc)的密度值。The thus formed reticulated
对切成与泡沫升起方向平行或垂直的网状弹性基质1样本进行张力试验。从网状弹性基质沫块上切下狗骨形的张力样本。经测定每一试验样本约1.25cm厚,约2.54cm宽和约14cm长;每一样本的规格长度为3.5cm且每一样本的规格宽度为6.5mm。使用INSTRONUniversal Testing Instrument Model 3342以50cm/min(19.6英寸/分钟)的十字头速度测定张力性质(拉伸强度和断裂时的延长)。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为34.3psi(24,115kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为124%。与泡沫升起方向平行的平均网状化后拉伸强度测定约为61.4psi(43,170kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为122%。Tensile test is carried out on the reticular
使用测定为5.0cm x 5.0cm x 2.5cm的网状弹性基质1样本进行压缩试验。使用INSTRON Universal Testing Instrument Model1122以1mm/min(0.4英寸/分钟)的十字头速度进行试验。将在50%压缩下与泡沫升起方向平行的网状化后抗压强度测定为2.1psi(1,475kg/m2)。网状化样本在25℃下进行50%压缩22小时,然后释放压缩应力后测定的与泡沫升起方向平行的网状化后压缩变定测定约为8.5%。Compression tests were performed using reticulated
在Q800Dynamic Mechanical Analyzer(TA Instruments,NewCastle,DE)中使用标准压缩固定物通过使各12mm直径和6mm厚度的圆柱形样本沿泡沫升起方向进行50%单轴压缩120分钟,随后是120分钟恢复时间测定网状弹性基质1的静态回复。测定6mm(“t-90%”)初始厚度样本恢复至最初厚度90%所需的时间并且测定的平均值为1406秒。Cylindrical specimens each 12 mm in diameter and 6 mm in thickness were subjected to 50% uniaxial compression in the direction of foam rise for 120 minutes using standard compression fixtures in a Q800 Dynamic Mechanical Analyzer (TA Instruments, New Castle, DE), followed by 120 minutes of recovery time The static recovery of reticulated
通过使各1英寸(2.54cm)高(沿泡沫升起方向)x1.25英寸x1.25英寸(3.18cm x 3.18cm)的直角平行六面体样本沿泡沫升起方向进行50%单轴压缩,然后在维持该单轴压缩的同时在空气环境中和1Hz频率下也沿泡沫升起方向赋予±5%应力的动态加载5,000个循环或100,000个循环测定网状弹性基质1的回弹恢复。另外,也如上所述测试100,000个循环的直角平行六面体样本,但在整个测试过程中将样品浸入水。测定并且记录恢复至1英寸(2.54cm)样本最初高度的67%(“t-67%”)和90%(“t-90%”)所需的时间。获得的结果如表4中所示。By subjecting rectangular parallelepiped specimens each 1 inch (2.54 cm) high (in the direction of foam rise) x 1.25 inches by 1.25 inches (3.18 cm x 3.18 cm) to 50% uniaxial compression in the direction of foam rise, and then Resilient recovery of the reticulated
表4Table 4
使用自动化液体渗透仪-LP-101-A型(也来自PorousMaterials,Inc.)测定在泡沫升起方向通过网状弹性基质1的流体,例如液体渗透性。测试的圆柱形网状弹性基质样本的直径为7.0-7.7mm且长度为13-14mm。将样本的平端放置在放在液体渗透仪底部的金属板上。为了测定液体渗透性,通过来自流体储器的压力驱动从样本端沿其轴通过样本向上挤压水。与渗透性测量相关的操作完全自动化并且受Capwin自动化液体渗透仪(6.71.92版本)与均在渗透性计算中执行的Microsoft Excel软件控制。网状弹性基质1渗透性在泡沫升起方向上测定为498达西。Fluid, eg, liquid permeability, was measured through the reticulated
还在网状弹性基质1压缩(与泡沫升起方向垂直)后测定渗透性以便减小可利用的流通面积,由此模拟压缩模制的样品。该步骤通过将具有大于不锈钢样品支架直径的直径的圆柱形样品插入支架进行,由此将样品进行径向压缩。测试的未压缩的圆柱形网状弹性基质1样本直径约为7.0mm且长度约为13-14mm,而压缩的样品的直径在其压入约7.0mm直径不锈钢支架前约为9.0mm-约16.0mm。图11为不同制品网状弹性基质的达西渗透性与可利用流通面积关系的示意图;图11中的线2为这类网状弹性基质1的示意图。图11,100%可利用流通面积代表未压缩的网状弹性基质1并且表明在泡沫升起方向上的最高渗透性498达西。使用可利用流通面积的渗透性改变例证在图11中。例如,网状弹性基质1在泡沫升起方向上的渗透性在压缩后可利用流通面积降至原始面积的47.9%时降至329达西,并且在压缩后可利用流通面积降至原始面积的19.4%时降至28达西。The permeability was also measured after compression of the reticulated elastic matrix 1 (perpendicular to the direction of foam rise) in order to reduce the available flow area, thereby simulating compression molded samples. This step is performed by inserting a cylindrical sample having a diameter larger than that of the stainless steel sample holder into the holder, thereby subjecting the sample to radial compression. The uncompressed cylindrical reticulated
实施例6:网状弹性基质2的合成和特性Example 6: Synthesis and Properties of
通过实施例5中所述操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质,但所用的组分及其比例如下表5中所示。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the procedure described in Example 5, except that the components used and their ratios are shown in Table 5 below.
表5table 5
正如根据光学显微镜观察结果测定的,网状弹性基质2的平均隔室直径或其它最大横向尺寸约为576μm。网状弹性基质2的SEM影像显示了例如通过其中开放孔互联的隔室网状构造。正如根据SEM观察结果测定的,网状弹性基质2的平均孔径或其它最大横向尺寸约为281μm。The average cell diameter or other largest lateral dimension of the reticulated
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质2进行下列试验。如实施例5中所述测定网状弹性基质2的密度;获得3.23 lbs/ft3(0.053g/cc)的密度值。The thus formed reticulated
如实施例5中所述对网状弹性基质2进行张力试验。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为40psi(28,120kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为135%。与泡沫升起方向平行的平均网状化后拉伸强度测定约为55psi(38,665kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为126%。Tensile testing was performed on reticulated
如实施例5中所述对网状弹性基质2进行压缩试验。将在50%压缩下与泡沫升起方向平行的网状化后抗压强度测定约为2.0psi(1,406kg/m2)。在25℃下进行50%压缩22小时,然后释放压缩应力后测定的与泡沫升起方向平行的网状化后压缩变定测定约为7.5%。Compression tests were performed on reticulated
如实施例5中所述测定网状弹性基质2的回弹恢复。获得的结果如表6中所示。The rebound recovery of reticulated
表6Table 6
使用自动化液体渗透仪-LP-101-A型如实施例5中所述在泡沫升起方向上测定通过网状弹性基质2的流体渗透性。将网状弹性基质2的渗透性在泡沫升起方向上测定为314达西。The fluid permeability through the reticulated
如实施例5中所述,还在网状弹性基质2压缩(与泡沫升起方向垂直)后测定渗透性以便减小可利用的流通面积。图11中的线3为达西渗透性与网状弹性基质2的可利用流通面积关系的示意图。在图11中,100%可利用流通面积表示未压缩的网状弹性基质2,并且表明在泡沫升起方向上的最高渗透性为314达西。网状弹性基质2在泡沫升起方向上的渗透性在压缩后可利用流通面积降至原始面积的43.9%时降至224达西,并且在压缩后可利用面积降至原始面积的25.5%时降至54达西。As described in Example 5, the permeability was also measured after compression of the reticulated elastic matrix 2 (perpendicular to the direction of foam rise) in order to reduce the available flow area. Line 3 in FIG. 11 is a schematic diagram of the relationship between the Darcy permeability and the available flow area of the reticulated
实施例7:网状弹性基质3的合成和特性Example 7: Synthesis and Properties of Reticulated Elastic Matrix 3
通过实施例5中所述操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质,但所用的组分及其比例如下表7中所示。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the procedure described in Example 5, except that the components used and their ratios are shown in Table 7 below.
表7Table 7
正如根据光学显微镜观察结果测定的,网状弹性基质3的平均隔室直径或其它最大横向尺寸约为300μm。图12为网状弹性基质3的SEM影像,其表示例如通过其中开放孔互联的隔室网状构造及其互通和互联性。正如根据SEM观察结果测定的,网状弹性基质3的平均孔径或其它最大横向尺寸约为175μm。The average cell diameter or other largest lateral dimension of the reticulated elastic matrix 3 is about 300 [mu]m, as determined from optical microscopic observations. Fig. 12 is a SEM image of a reticulated elastic matrix 3 showing, for example, a network of compartments interconnected by open pores therein and their intercommunication and interconnectivity. The average pore diameter or other largest lateral dimension of the reticulated elastic matrix 3 is about 175 μm, as determined from the SEM observation.
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质3进行下列试验。如实施例5中所述测定网状弹性基质3的密度;获得5.92 lbs/ft3(0.095g/cc)的密度值。The thus formed reticulated elastic matrix 3 obtained from the reticulated foam was subjected to the following tests using a test method based on ASTM Standard D3574. The density of reticulated elastic matrix 3 was determined as described in Example 5; a density value of 5.92 lbs/ft3 (0.095 g/cc) was obtained.
如实施例5中所述对网状弹性基质3样本进行张力试验。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为71.7psi(50,405kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为161%。与泡沫升起方向平行的平均网状化后拉伸强度测定约为104psi(73,110kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为169%。Tensile testing was performed on the reticulated elastic matrix 3 samples as described in Example 5. The average post-reticulated tensile strength perpendicular to the direction of foam rise was measured to be approximately 71.7 psi (50,405 kg/m2 ). The elongation at break after reticulation perpendicular to the direction of foam rise was measured to be about 161%. The average post-reticulated tensile strength parallel to the direction of foam rise was measured to be approximately 104 psi (73,110 kg/m2 ). The elongation at break after reticulation parallel to the direction of foam rise was measured to be about 169%.
如实施例5中所述对网状弹性基质3进行压缩试验。在50%压缩下与泡沫升起方向平行的网状化后抗压强度测定为3.65psi(2,565kg/m2)。Compression tests were performed on reticulated elastic matrix 3 as described in Example 5. The post-reticulated compressive strength parallel to the direction of foam rise at 50% compression was determined to be 3.65 psi (2,565 kg/m2 ).
如实施例5中所述测定网状弹性基质3的静态回复。测定T-90%并且测定平均值为166秒。The static recovery of reticulated elastic matrix 3 was determined as described in Example 5. T-90% was determined and the mean value was determined to be 166 seconds.
如实施例5中所述测定网状弹性基质3的回弹恢复。获得的结果如表8中所示。The rebound recovery of reticulated elastic matrix 3 was determined as described in Example 5. The results obtained are shown in Table 8.
表8Table 8
使用自动化液体渗透仪LP-101-A型如实施例5中所述在泡沫升起方向上测定通过网状弹性基质3的流体渗透性。网状弹性基质3的渗透性在泡沫升起方向上测定为103达西。The fluid permeability through the reticulated elastic matrix 3 was measured in the direction of foam rise as described in Example 5 using an automated liquid penetrometer LP-101-A. The permeability of the reticulated elastic matrix 3 was measured to be 103 Darcy in the direction of foam rise.
实施例8:网状弹性基质4的合成和特性Example 8: Synthesis and Properties of Reticulated Elastic Matrix 4
通过实施例5中所述操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质,但所用的组分及其比例如下表9中所示。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the procedure described in Example 5, except that the components used and their ratios are shown in Table 9 below.
表9Table 9
正如根据光学显微镜观察结果测定的,网状弹性基质4的平均隔室直径或其它最大横向尺寸约为353μm。本实施例的网状弹性基质的SEM影像显示例如通过其中开放孔互联的隔室网状构造。正如根据SEM观察结果测定的,网状弹性基质4的平均孔径或其它最大横向尺寸约为231μm。The average cell diameter or other largest lateral dimension of the reticulated elastic matrix 4 was about 353 μm, as determined from optical microscopic observations. The SEM image of the reticulated elastic matrix of this example shows, for example, a network of compartments interconnected by open pores therein. The average pore diameter or other largest lateral dimension of the reticulated elastic matrix 4 is about 231 μm, as determined from the SEM observation.
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质4进行下列试验。如实施例5中所述测定网状弹性基质4的密度;获得3.81 lbs/ft3(0.061g/cc)的密度值。The thus formed reticulated elastic matrix 4 obtained from the reticulated foam was subjected to the following tests using a test method based on ASTM Standard D3574. The density of reticulated elastic matrix 4 was determined as described in Example 5; a density value of 3.81 lbs/ft3 (0.061 g/cc) was obtained.
如实施例5中所述对网状弹性基质4样本进行张力试验。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为40.9psi(28,753kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为216%。与泡沫升起方向平行的平均网状化后拉伸强度测定约为52.5psi(36,910kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为206%。Tensile testing was performed on the reticulated elastic matrix 4 samples as described in Example 5. The average post-reticulated tensile strength perpendicular to the direction of foam rise was measured to be approximately 40.9 psi (28,753 kg/m2 ). The elongation at break after reticulation perpendicular to the direction of foam rise was measured to be about 216%. The average post-reticulated tensile strength parallel to the direction of foam rise was measured to be approximately 52.5 psi (36,910 kg/m2 ). The elongation at break after reticulation parallel to the direction of foam rise was measured to be about 206%.
如实施例5中所述对网状弹性基质4标本进行压缩试验。在50%压缩下与泡沫升起方向平行的网状化后抗压强度测定为1.3psi(914kg/m2)。Compression tests were performed on the reticulated elastic matrix 4 specimens as described in Example 5. The post-reticulated compressive strength parallel to the direction of foam rise at 50% compression was determined to be 1.3 psi (914 kg/m2 ).
如实施例5中所述测定网状弹性基质4的静态回复。测定T-90%并且测定平均值为466秒。The static recovery of reticulated elastic matrix 4 was determined as described in Example 5. T-90% was determined and the mean value was determined to be 466 seconds.
如实施例5中所述测定网状弹性基质4的回弹恢复。获得的结果如表10中所示。The rebound recovery of reticulated elastic matrix 4 was determined as described in Example 5. The results obtained are shown in Table 10.
表10Table 10
使用自动化液体渗透仪LP-101-A型如实施例5中所述在泡沫升起方向上测定通过网状弹性基质4的流体渗透性。网状弹性基质4的渗透性在泡沫升起方向上测定为380达西。The fluid permeability through the reticulated elastic matrix 4 was measured in the direction of foam rise as described in Example 5 using an automated liquid penetrometer LP-101-A. The permeability of the reticulated elastic matrix 4 was measured at 380 Darcy in the direction of foam rise.
实施例9具有选择性非多孔表面的可植入装置Example 9 Implantable Devices with Selectively Non-Porous Surfaces
使用按照实施例5制备的网状材料片。将具有刀口的加热刀片用于从该片中切割10mm直径和15mm长度的圆柱体。刀片温度高于170℃。接触加热刀片的材料片表面因接触加热刀片而表现出融合和非多孔性。指定保留多孔性即不融合的该材料片的那些表面不接触加热刀片。A sheet of mesh material prepared according to Example 5 was used. A heated blade with a knife edge was used to cut cylinders of 10 mm diameter and 15 mm length from the sheet. The blade temperature is higher than 170°C. The surface of the sheet of material contacting the heated blade exhibits fusion and non-porosity due to contact with the heated blade. Those surfaces of the sheet of material designated to remain porous, ie not fused, do not contact the heating blade.
实施例10具有选择性非多孔表面的可植入装置Example 10 Implantable Devices with Selectively Non-Porous Surfaces
使用按照实施例5制备的适度超尺寸的网状材料片。将适度超尺寸的材料片放入加热至高于170℃温度的模具。然后在该材料片上覆盖模具以便将总尺寸减小至所需大小。在从模具中取出该材料片时,接触模具的材料片表面因接触模具而表现出融合和非多孔性。防止指定保留多孔性即不融合的该材料片的那些表面接触加热模具。具有刀口的加热刀片用于从该片上切割10mm直径和15mm长度的圆柱体。A moderately oversized sheet of mesh material prepared according to Example 5 was used. A moderately oversized sheet of material is placed into a mold heated to a temperature above 170°C. A mold is then overlaid on this sheet of material to reduce the overall dimensions to the desired size. Upon removal of the sheet of material from the mold, the surface of the sheet of material contacting the mold exhibited fusion and non-porosity due to contact with the mold. Those surfaces of the sheet of material designated to remain porous, ie not fused, are prevented from contacting the heated mold. A heated blade with a knife edge was used to cut cylinders of 10 mm diameter and 15 mm length from the sheet.
实施例11具有选择性非多孔性表面的浸渍涂敷的可植入装置Example 11 Dip-coated implantable devices with selectively non-porous surfaces
使用按照实施例5制备的网状材料片。如下将含90mole%PGA和10mole%PLA的共聚物涂层涂布于宏观表面。在205℃下和挤压机中熔化PGA/PLA共聚物并且将该材料片浸入熔化物以便涂敷它。覆盖该材料片的欲保留多孔性,即不用熔化物涂敷的那些表面以保护它们并且使其不接触熔化物。在取出时,熔化物固化并且在它所接触的材料片表面上形成薄的非多孔性涂层。A sheet of mesh material prepared according to Example 5 was used. Copolymer coatings containing 90 mole% PGA and 10 mole% PLA were applied to macroscopic surfaces as follows. The PGA/PLA copolymer was melted in an extruder at 205°C and the sheet of material was dipped into the melt to coat it. Those surfaces of the sheet of material which are to remain porous, ie not coated with the melt, are covered to protect them and keep them from coming into contact with the melt. Upon removal, the melt solidifies and forms a thin, non-porous coating on the surface of the sheet of material it contacts.
实施例12胶原蛋白涂敷的弹性基质的制造Example 12 Fabrication of Collagen-Coated Elastic Matrix
洗涤通过从牛皮中提取获得的I型胶原蛋白并且切碎成纤丝。通过剧烈搅拌胶原蛋白和水并且加入无机酸至pH约为3.5制备1%重量的胶原蛋白含水淤浆。Type I collagen obtained by extraction from bovine hide was washed and chopped into fibrils. A 1% by weight aqueous slurry of collagen was prepared by vigorously stirring the collagen and water and adding mineral acid to a pH of approximately 3.5.
将按照实施例5制备的网状化聚氨基甲酸酯基质切成60mm×60mm×2mm的片。将该片放入浅托盘并且将胶原蛋白淤浆倾在其上,使得该片完全浸入淤浆约15分钟并且任选振摇托盘。如果必要,从该片中滗析出过量的淤浆并且将淤浆浸渍的片放置在塑料托盘上,将该托盘放置在保持在10℃的冻干器托盘上。使冻干器托盘温度以约1℃/分钟的冷却速率从10℃降至-35℃并且使冻干器内的压力降至约75毫托。在保持在-35℃下8小时后,以约1℃/小时的速率使托盘温度升至10℃且然后以约2.5℃/小时的速率使温度达到25℃。在冻干过程中,水从冷冻胶原蛋白淤浆中升华,在网状聚氨基甲酸酯基质片孔内遗留多孔胶原蛋白基质。使压力恢复至1个大气压。The reticulated polyurethane matrix prepared according to Example 5 was cut into pieces of 60 mm x 60 mm x 2 mm. The piece was placed in a shallow tray and the collagen slurry was poured over it so that the piece was completely submerged in the slurry for about 15 minutes and the tray was optionally shaken. If necessary, the excess slurry was decanted from the pieces and the slurry-soaked pieces were placed on a plastic tray placed on a lyophilizer tray maintained at 10°C. The lyophilizer tray temperature was reduced from 10°C to -35°C at a cooling rate of about 1°C/min and the pressure inside the lyophilizer was reduced to about 75 mTorr. After holding at -35°C for 8 hours, the tray temperature was raised to 10°C at a rate of about 1°C/hour and then brought to 25°C at a rate of about 2.5°C/hour. During lyophilization, water sublimes from the frozen collagen slurry, leaving a porous collagen matrix within the cells of the reticulated polyurethane matrix. The pressure was brought back to 1 atmosphere.
任选在约110℃下使多孔胶原蛋白涂敷的聚氨基甲酸酯基质片进一步在氮气流中加热处理约24小时以便交联胶原蛋白,由此提供额外的结构完整性。The porous collagen-coated polyurethane matrix sheet is optionally further heat-treated in a nitrogen stream at about 110° C. for about 24 hours to cross-link the collagen, thereby providing additional structural integrity.
实施例13:网状弹性基质5的合成和特性及其在用于修复大鼠腹壁的Example 13: Synthesis and properties of reticulated elastic matrix 5 and its use in repairing the abdominal wall of rats可植入装置中的应用Applications in Implantable Devices
通过下列操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the following procedure.
芳族异氰酸酯MONDUR MRS 20(来自Bayer;包括4,4’-MDI和2,4’-MDI的混合物)用作异氰酸酯成分。MONDUR MRS 20含约65%-70%重量的4,4’-MDI,约30%-35%重量的2,4’-MDI,具有约2.2-2.3的异氰酸酯官能度并且在25℃下为液体。具有约2,000道尔顿分子量的二元醇,聚(1,6-己烷碳酸酯)二元醇(POLY-CD CD220,ArchChemicals)用作多元醇成分并且在25℃下为固体。蒸馏水用作起泡剂。起泡催化剂为叔胺三乙二胺(在双丙二醇中33%重量;来自AirProducts的DABCO 33LV)。甘油(99.7%USP/EP,来自Dow Chemical)用作交联基且1,4-丁二醇(来自BASF Chemical)用作增链剂。使用基于硅氧烷的表面活性剂(TEGOSTAB BF2370,来自Goldschmidt)。使用隔室开放剂(ORTEGOL 501,来自Goldschmidt)。存在粘度改进剂碳酸丙烯酯(来自Sigma-Aldrich)以便降低粘度。所用组分的比例如下表11中所示。The aromatic isocyanate MONDUR MRS 20 (from Bayer; including a mixture of 4,4'-MDI and 2,4'-MDI) was used as the isocyanate component.
表11Table 11
使二元醇在约70℃的空气循环烘箱内液化并且将其100g称入聚乙烯杯。将5.8g粘度改进剂(碳酸丙烯酯)加入到多元醇中并且使用安装了混合棒的钻孔混合器中以3100rpm混合15秒(混合物-1)。将1.5g表面活性剂(TEGOSTAB BF-2370)加入到混合物-1中并且再混合15秒(混合物-2)。将2.0g隔室开放剂(ORTEGOL 501)加入到混合物-2中并且混合15秒(混合物-3)。将2.15g交联剂(甘油)加入到混合物-3中并且混合15秒(混合物-4)。将0.72g增链剂(1,4-丁二醇)加入到混合物-4中并且混合15秒(混合物-5)。将51.32g异氰酸酯(MONDUR MRS 20)加入到混合物-5中并且混合60秒(系统A)。通过使用小玻璃棒将1.89g蒸馏水与0.56g起泡催化剂(DABCO 33LV)在小塑料杯中混合60秒(系统B)。The diol was liquefied in an air circulating oven at about 70°C and 100 g was weighed into polyethylene cups. 5.8 g of a viscosity modifier (propylene carbonate) was added to the polyol and mixed at 3100 rpm for 15 seconds using a drilled mixer fitted with a mixing rod (Mixture-1). 1.5 g of surfactant (TEGOSTAB BF-2370) was added to Mix-1 and mixed for an additional 15 seconds (Mix-2). 2.0 g of a compartment opener (ORTEGOL 501 ) was added to Mix-2 and mixed for 15 seconds (Mix-3). 2.15 g of crosslinker (glycerin) was added to Mix-3 and mixed for 15 seconds (Mix-4). 0.72 g of chain extender (1,4-butanediol) was added to Mix-4 and mixed for 15 seconds (Mix-5). 51.32 g of isocyanate (MONDUR MRS 20) was added to Mixture-5 and mixed for 60 seconds (System A). 1.89 g of distilled water was mixed with 0.56 g of foaming catalyst (DABCO 33LV) in a small plastic cup for 60 seconds by using a small glass rod (System B).
将系统B尽可能快地倾入系统A,无溢出并且使用钻孔混合器剧烈混合10秒且倾入9英寸x8英寸x5英寸(23cm x 20cm x 13cm)尺寸的厚纸板箱,在纸箱内部用铝箔覆盖。起泡过程如下:混合时间10-12秒,成乳液时间28秒且升起时间120秒。Pour System B into System A as quickly as possible without spillage and mix vigorously for 10 seconds using a drill mixer and pour into a cardboard box measuring 9" x 8" x 5" (23cm x 20cm x 13cm) inside the carton with Cover with aluminum foil. The foaming process was as follows: mix time 10-12 seconds, emulsion time 28 seconds and rise time 120 seconds.
在开始起泡混合后2分钟,将泡沫放入维持在100-105℃下的烘箱内固化约60分钟。从烘箱内取出弹性基质并且在约25℃下冷却10分钟。用锯取出外膜并且用手从所有侧面压弹性基质以开放隔室窗。将弹性基质放回到空气循环烘箱内在100℃-105℃下再进行3.5小时后固化。物理和化学交联均存在于最终的弹性基质中。Two minutes after beginning foam mixing, the foam was cured in an oven maintained at 100-105°C for about 60 minutes. The elastic matrix was removed from the oven and cooled at about 25°C for 10 minutes. The adventitia was removed with a saw and the elastic matrix was pressed by hand from all sides to open the compartment windows. The elastomeric matrix was returned to the air circulating oven for an additional 3.5 hours post cure at 100°C-105°C. Both physical and chemical crosslinks are present in the final elastic matrix.
固化后,修整泡沫块的侧面和底部,然后如实施例5中所述使弹性基质网状化。正如通过光学显微镜观察结果测定的,网状弹性基质5的平均孔径或其它最大横向尺寸约为220μm。After curing, the sides and bottom of the foam block were trimmed, and then the elastic matrix was reticulated as described in Example 5. The average pore diameter or other largest lateral dimension of the reticulated elastic matrix 5 was about 220 μm as determined by optical microscopic observation.
使用基于ASTM Standard D3574的测试方法在获自网状化泡沫的由此形成的网状弹性基质5进行下列试验。如实施例5中所述测定网状弹性基质5的密度;获得4.27 lbs/ft3(0.068g/cc)的密度值。The following tests were performed on the thus formed reticulated elastic matrix 5 obtained from the reticulated foam using a test method based on ASTM Standard D3574. The density of reticulated elastic matrix 5 was determined as described in Example 5; a density value of 4.27 lbs/ft3 (0.068 g/cc) was obtained.
如实施例5中所述对网状弹性基质5样本进行张力试验。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为36.8psi(25,870kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为114%。与泡沫升起方向平行的平均网状化后拉伸强度测定约为66.6psi(46,805kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为117%。Tensile testing was performed on samples of reticulated elastic matrix 5 as described in Example 5. The average post-reticulated tensile strength perpendicular to the direction of foam rise was measured to be approximately 36.8 psi (25,870 kg/m2 ). The elongation at break after reticulation perpendicular to the direction of foam rise was measured to be about 114%. The average post-reticulated tensile strength parallel to the direction of foam rise was measured to be approximately 66.6 psi (46,805 kg/m2 ). The elongation at break after reticulation parallel to the direction of foam rise was measured to be about 117%.
使用测定约为152mm长,25mm宽和12.7mm高的样本,根据ASTM St andard D3574中所述的测试方法测定网状弹性基质5的抗撕裂强度。在每个样本一侧上制作40mm切口。使用INSTRON UniversalTesting Instrument Model 1122与50cm/min(19.6英寸/min)的十字头速度测定抗撕裂强度。将抗撕裂强度测定约为3.15 lbs/线性英寸(526g/线性cm)。The tear resistance of the reticulated elastic matrix 5 was determined according to the test method described in ASTM Standard D3574 using a sample measuring approximately 152mm long, 25mm wide and 12.7mm high. A 40 mm incision was made on one side of each sample. Tear resistance was determined using an INSTRON Universal Testing Instrument Model 1122 with a crosshead speed of 50 cm/min (19.6 in/min). The tear strength was determined to be approximately 3.15 lbs/linear inch (526 g/linear cm).
使用网状弹性基质5并且在其中掺入4-0复丝聚酯纤维(TelflexMedical)制备本发明可植入装置的实例,经测定为1cm长和宽x2mm高的正方形补片。使用具有1型缝线和3mm间距的Viking PlatinumModel 730缝合机将编织的聚酯纤维(具有相当于具有0.20mm最大直径和1.65 lbs(748g)最低拉伸强度的4-0号缝合线的直径)掺入正方形可植入装置。An example of an implantable device of the present invention was prepared using a reticulated elastic matrix 5 into which 4-0 multifilament polyester fibers (Telflex Medical) were incorporated, measuring as a
将可植入装置放入Sprague-Dawley大鼠腹壁。腹壁缺陷具有部分厚度并且遗留腹部筋膜且腹膜和皮肤完整。一定不同的是切下内和外腹斜肌并且在大鼠中被测试可植入装置替代。因此,无装置进入腹腔并且在手术部位实施手术闭合术后皮肤完整。装置周围有天然肌肉组织,皮下组织和筋膜。在植入后16周时处死大鼠。The implantable device was placed into the abdominal wall of a Sprague-Dawley rat. Abdominal wall defects are partial thickness and leave abdominal fascia with intact peritoneum and skin. Certainly the difference was that the internal and external oblique muscles were excised and replaced by test implantable devices in rats. Therefore, no devices were entered into the abdominal cavity and the skin was intact after surgical closure at the surgical site. The device is surrounded by natural musculature, subcutaneous tissue and fascia. Rats were sacrificed at 16 weeks post-implantation.
在16周时的组织学分析显示在整个植入装置中组织向内生长和增殖。植入的装置促进大鼠中腹壁缺陷修复。装置显示出有利的反应并且与良好组织向内生长充分生物整合。Histological analysis at 16 weeks showed tissue ingrowth and proliferation throughout the implanted device. The implanted device promotes repair of abdominal wall defects in rats. The device showed a favorable response and was well biointegrated with good tissue ingrowth.
实施例14:由网状弹性基质4和编织纤维加强物制备可植入装置Example 14: Fabrication of Implantable Devices from Reticulated Elastic Matrix 4 and Braided Fiber Reinforcement
按照实施例8中所述方法制备网状弹性基质4。从该网状弹性基质中切下成形为矩形的可植入装置,诸如手术补片,它具有29mm长,34mm宽和2mm厚度的尺寸。使用具有图13中所示图案的刺绣机(BabyLockEsante BLN型)将编织的聚酯纤维(Telflex Medical;相当于5-0号缝合线并且具有0.15mm最大直径和0.88 lbs(399g)最低拉伸强度的直径)掺入矩形可植入装置。将该图案的特征尺寸提供在图14中。Reticulated elastic matrix 4 was prepared according to the method described in Example 8. A rectangular shaped implantable device, such as a surgical patch, having dimensions of 29 mm long, 34 mm wide and 2 mm thick is cut from the reticulated elastic matrix. Braided polyester fibers (Telflex Medical; equivalent to No. 5-0 suture and having a maximum diameter of 0.15 mm and a minimum tensile strength of 0.88 lbs (399 g) diameter) incorporated into rectangular implantable devices. The feature dimensions of this pattern are provided in FIG. 14 .
使用交叉缝线与下列设置将编织的聚酯纤维掺入矩形可植入装置:缝线运动间距=1.5mm;区域缝合密度=3.9线/mm;机器张力设定=1.4。格栅尺寸为10mm x 8mm,沿四侧各自有2mm边界。Braided polyester fibers were incorporated into rectangular implantable devices using crossed sutures with the following settings: suture travel spacing = 1.5 mm; area suture density = 3.9 threads/mm; machine tension setting = 1.4. The grille measures 10mm x 8mm with 2mm borders along each of the four sides.
测试掺入编织纤维的每一可植入装置的缝合保留强度(SRS),将其定义为从装置中拉出标准缝合线而导致其断裂所需的最大力。还测试了掺入编织纤维的每一装置的抗张强度(TBS),将其定义为完整装置拉伸断裂所需的最大力。使用INSTRON Universal Testing InstrumentModel 3342进行两种测试。Each implantable device incorporating braided fibers was tested for suture retention strength (SRS), defined as the maximum force required to pull a standard suture from the device causing it to break. Each device incorporating the braided fibers was also tested for tensile strength (TBS), defined as the maximum force required to tensile break the complete device. Both tests were performed using the INSTRON Universal Testing InstrumentModel 3342.
在SRS测试中,通过使用针头将2-0 ETHIBOND编织的聚酯缝合线插入可植入装置一端并且使该缝合线以低于第一水平格栅线2mm-3mm和在装置中心线附近与装置连接。通过缝合线两端形成约50mm-60mm长度的环。使装置的自由端(不与缝合线连接)固定在底部固定颌夹的橡胶涂敷的平表面内并且夹紧。以100mm/min(3.94英寸/min)的十字头速度在位移模式下,使用隔离或向上移动并且远离固定颌夹的可移动颌夹进行SRS测试。21牛顿的SRS平均值获自测试这些掺入编织聚酯纤维的可植入装置。In the SRS test, a 2-0 ETHIBOND braided polyester suture is inserted into one end of the implantable device by using a needle and aligning the suture with the device 2mm-3mm below the first horizontal gridline and near the centerline of the device. connect. A loop of about 50mm-60mm in length is formed through both ends of the suture. The free end of the device (not attached to the suture) was secured in the rubber-coated flat surface of the bottom stationary jaw and clamped. The SRS test was performed in displacement mode at a crosshead speed of 100 mm/min (3.94 in/min) with a movable jaw isolated or moved up and away from the fixed jaw. An SRS average of 21 Newtons was obtained from testing these implantable devices incorporating woven polyester fibers.
在这些可植入装置的TBS测试中,将装置的一端安装在固定于固定气动柄上的橡胶涂敷的面之间,并且将装置的另一端固定在固定于可移动气动柄上的橡胶涂敷的面之间。本试验以100mm/min(3.94英寸/min)的十字头速度在位移模式下,使用隔离或向上移动并且远离固定颌夹的可移动颌夹进行。获得57牛顿的TBS平均值。In the TBS testing of these implantable devices, one end of the device is mounted between rubber-coated faces affixed to a fixed pneumatic handle, and the other end of the device is affixed to a rubber-coated surface affixed to a movable pneumatic handle. Between the sides of the application. The test is performed in displacement mode at a crosshead speed of 100 mm/min (3.94 inches/min), using a movable jaw that is isolated or moved upward and away from the fixed jaw. A TBS average of 57 Newtons was obtained.
实施例15:具有网状弹性基质4和编织纤维加强物的可植入装置在大Example 15: Implantable device with reticulated elastic matrix 4 and braided fiber reinforcement at large鼠回旋套加强中的应用The Application of Rat Convoluted Cuff Reinforcement
按照与实施例14中所述方法类似的方式制成具有网状弹性基质4和编织的聚酯纤维的呈矩形补片形状的可植入装置,但使用7-0号编织聚酯纤维。从装置中切下2mm长和宽1mm厚补片形式的小正方形并且植入以便使大鼠的冈上肌腱愈合。An implantable device in the shape of a rectangular patch with a reticulated elastic matrix 4 and braided polyester fibers was fabricated in a manner similar to that described in Example 14, but using 7-0 braided polyester fibers. Small squares in the form of 2 mm long and 1 mm thick patches were cut from the device and implanted to heal the supraspinatus tendon in rats.
使用缝合线通过骨进行的传统腱修复手术治疗,但通过使用上述段落中所述的可植入装置加强。在大鼠中以手术方式生成双侧冈上肌腱撕裂。在大鼠右肩中,实施冈上肌腱的全厚度完全横切。将装置缝在腱的上部并且使用两条5-0PROLENE经骨缝合线将腱-补片结构修复至骨。在手术修复厚8周处死大鼠并且进行腱修复的组织学分析。Surgical treatment of traditional tendon repair through the bone using sutures, but augmented by the use of the implantable device described in the preceding paragraph. Bilateral supraspinatus tendon tears were surgically generated in rats. In the right shoulder of the rat, a full-thickness complete transection of the supraspinatus tendon was performed. The device was sutured over the tendon and the tendon-patch structure was repaired to the bone using two 5-0 PROLENE transosseous sutures. Rats were sacrificed 8 weeks after surgical repair and histological analysis of tendon repair was performed.
在图15中照片例证的组织学分析显示无明显量的炎症或不适当的血管化。根据诸如图15这类照片中组织向内生长占据的面积的分析确定的组织向内生长占据的可植入装置空隙空间百分比至少约为80%。就可植入装置内组织向内生长而言,正如常规H&E染色显示的,最接近装置的细胞形态与在胶原蛋白基质产生中具有活性的结缔组织细胞,诸如成纤维细胞一致,同时远端的细胞(或离最接近可植入装置的细胞更远处)更多显示为静止。使可植入装置周围的组织明显组织化。装置内的组织区域在装置的网状弹性基质的任意指定孔内组织化。然而,可植入装置内的组织在处死大鼠时仍然未完全组织化,因为愈合时间可能不足够长。Histological analysis of the photographs exemplified in Figure 15 showed no appreciable amount of inflammation or inappropriate vascularization. The percentage of void space of the implantable device occupied by tissue ingrowth as determined from an analysis of the area occupied by tissue ingrowth in photographs such as FIG. 15 is at least about 80%. In terms of tissue ingrowth within implantable devices, as shown by conventional H&E staining, the morphology of cells proximal to the device is consistent with connective tissue cells such as fibroblasts active in collagen matrix production, while distal Cells (or cells further away from the cells closest to the implantable device) appear more quiescent. Visibly organizes the tissue surrounding the implantable device. Regions of tissue within the device are organized within arbitrary designated pores of the reticulated elastic matrix of the device. However, the tissue within the implantable device was still not fully organized when the rats were sacrificed, as the healing time may not have been long enough.
实施例16:网状弹性基质6的合成和特性及其在用于修复大鼠回旋套的具有编织纤维加强物的可植入装置中的应用Example 16: Synthesis and properties of reticulated elastic matrix 6 and its use in implantable devices with braided fiber reinforcement for repair of rat cuff
通过与实施例13中所述类似的方法制备网状交联生物耐久性弹性聚碳酸酯脲氨基甲酸酯基质,但将芳族异氰酸酯RUBINATE 9258(来自Huntsman,包括4,4’-MDI和2,4’-MDI的混合物)用作异氰酸酯成分并且不使用交联剂和增链剂。RUBINATE 9258含约68%重量的4,4’-MDI,约32%重量的2,4’-MDI,具有约2.33的异氰酸酯官能度并且在25℃下为液体。具有约2,000道尔顿分子量的多元醇,1,6-聚碳酸六亚甲基酯(POLY-CD CD220),即二元醇用作多元醇成分并且在25℃下为固体。所用组分的比例如下表12中所示。A reticulated crosslinked biodurable elastic polycarbonate ureaurethane matrix was prepared by a method similar to that described in Example 13, except that the aromatic isocyanate RUBINATE 9258 (from Huntsman, including 4,4'-MDI and 2 , a mixture of 4'-MDI) is used as the isocyanate component and does not use crosslinkers and chain extenders. RUBINATE 9258 contains about 68% by weight 4,4'-MDI, about 32% by
表12Table 12
起泡过程如下:混合时间10秒,成乳液时间16秒且升起时间80秒。The foaming process was as follows: mix
在开始起泡混合后2分钟,将弹性基质放入维持在100-105℃下的烘箱内固化60分钟。从烘箱内取出弹性基质并且在约25℃下冷却10分钟。使用锯除去外膜并且用手从所有侧面压弹性基质以开放隔室窗。将弹性基质放回到空气循环烘箱内在100℃下再进行4.0小时后固化。Two minutes after foam mixing started, the elastomeric matrix was cured for 60 minutes in an oven maintained at 100-105°C. The elastic matrix was removed from the oven and cooled at about 25°C for 10 minutes. The adventitia was removed using a saw and the elastic matrix was pressed from all sides by hand to open the compartment windows. The elastomeric matrix was returned to the air circulating oven for an additional 4.0 hours post cure at 100°C.
使用基本上与实施例5中所述网状化方法类似的方法使泡沫网状化一次而得到网状弹性基质6。正如根据光学显微镜观察结果测定的,网状弹性基质6的平均孔径或其它最大横向尺寸为275μm-350μm。The reticulated elastic matrix 6 was obtained by reticulating the foam once using a method substantially similar to the reticulating method described in Example 5. The average pore diameter or other largest lateral dimension of the reticulated elastic matrix 6 is in the range of 275 μm to 350 μm, as determined from optical microscope observations.
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质6进行下列试验。如实施例5中所述测定网状弹性基质6的密度;获得2.99 lbs/ft3(0.046g/cc)的密度值。The resulting reticulated elastic matrix 6 obtained from reticulated foam was subjected to the following tests using a test method based on ASTM Standard D3574. The density of reticulated elastic matrix 6 was determined as described in Example 5; a density value of 2.99 lbs/ft3 (0.046 g/cc) was obtained.
如实施例5中所述对网状弹性基质6样本进行张力试验。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为33.6psi(23,625kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为220%。Tensile testing was performed on reticulated elastic matrix 6 samples as described in Example 5. The average post-reticulated tensile strength perpendicular to the direction of foam rise was determined to be approximately 33.6 psi (23,625 kg/m2 ). The elongation at break after reticulation perpendicular to the direction of foam rise was measured to be about 220%.
如实施例5中所述对网状弹性基质6进行压缩试验。在50%压缩下与泡沫升起方向平行的网状化后抗压强度测定约为1.25psi(878kg/m2)。Compression tests were performed on reticulated elastic matrix 6 as described in Example 5. The post-reticulation compressive strength parallel to the direction of foam rise at 50% compression was measured to be approximately 1.25 psi (878 kg/m2 ).
为了手术植入,通过切割预先用γ射线灭菌的网状弹性基质6的块对基质适当定型和成形。将Sprague-Dawley大鼠(体重约为250g-约275g)用于本实验。使用肌内注射氯胺酮(100mg/kg)和赛拉嗪(5mg/kg)麻醉所有大鼠。此后刮剃上肢,以无菌方式准备和铺手术巾。提供总计7天的抗生素预防。For surgical implantation, the matrix is suitably shaped and shaped by cutting pieces of reticulated elastic matrix 6 that have been sterilized with gamma radiation beforehand. Sprague-Dawley rats (approximately 250 g to approximately 275 g body weight) were used in this experiment. All rats were anesthetized using intramuscular injections of ketamine (100 mg/kg) and xylazine (5 mg/kg). Thereafter the upper extremities were shaved, and surgical drapes were prepared and draped in a sterile manner. Provides a total of 7 days of antibiotic prophylaxis.
手术暴露包括在双侧肩和肩胛骨的背面上的2cm切口。在每侧肩中,鉴定肩胛冈并且在1cm距离内沿其纤维一致性分离三角肌。开放肩峰下囊,但不切除。冈上肌腱显示,因为它在喙肩弓下通过至其在肱骨近端大结节上的插入点。Surgical exposure consisted of 2 cm incisions on both shoulders and the back of the scapula. In each shoulder, identify the spine of the scapula and isolate the deltoid consistently along its fibers within a distance of 1 cm. The subacromial bursa is opened, but not excised. The supraspinatus tendon is shown as it passes under the coracoscapuloid arch to its insertion point on the greater tuberosity of the proximal humerus.
在组织延伸组(1组)中,在双侧切下2mm宽冈上肌腱区域,从插入部位近端1mm开始并且进一步近端延伸2mm,产生2mm×2mm缺陷。这代表了约50%的冈上肌腱宽,相当于人的较大全厚度回旋套撕裂。In the tissue extension group (Group 1), a 2 mm wide supraspinatus tendon region was excised bilaterally, starting 1 mm proximal to the insertion site and extending a further 2 mm proximally, creating a 2 mm x 2 mm defect. This represents approximately 50% of the width of the supraspinatus tendon, which is equivalent to a larger full-thickness cuff tear in humans.
使该缺陷与本实施例插入腱边缘与大结节上插入部位之间的2mm×2mm和1mm厚度网状弹性基质6可植入装置桥连。使用两条5-0 PROLENE(Ethicon Inc.)间断缝合线通过经骨通道在大结节远端固定装置。然后使用两条5-0 PROLENE缝合线使装置的近边与腱的侧边缘连接。然后使用间断4-0 VICRYL(Ethicon Inc.)缝合线使三角肌再接近肩并且使用3-0 MONOCRYL(Ethicon Inc.)封闭皮肤。This defect is bridged with the 2 mm x 2 mm and 1 mm thick reticulated elastic matrix 6 implantable device of this embodiment between the edge of the insertion tendon and the insertion site on the greater tuberosity. The device was secured distal to the greater tuberosity through a transosseous canal using two interrupted 5-0 PROLENE (Ethicon Inc.) sutures. The proximal edge of the device is then attached to the lateral edge of the tendon using two 5-0 PROLENE sutures. The deltoid was then reapproximated to the shoulder using interrupted 4-0 VICRYL (Ethicon Inc.) sutures and the skin was closed using 3-0 MONOCRYL (Ethicon Inc.).
在组织加强组(2组)中,使用#15手术刀片在冈上肌腱插入处近端1mm生成双侧全厚度缺陷,但与1组相反,不取出腱节段。然后使用两条5-0 PROLENE缝合线通过经骨通道将缺陷修复至大结节上的插入部位。还通过与本实施例的网状弹性基质可植入装置对缝加强修复,从而生成由网状弹性基质和腱组成的分层构造。然后使用间断4-0VICRYL(Ethicon Inc.)缝合线使三角肌再接近肩并且使用3-0MONOCRYL(Ethicon Inc.)封闭皮肤。In the tissue augmentation group (Group 2), a #15 scalpel blade was used to generate a bilateral full-
在1组和2组实验中,在术后6周通过二氧化碳吸入处死所有动物。通过肉眼评价大鼠肩的总体愈合证据并且取出冈上肌腱和肱骨近端用于组织学分析。在恢复期时的总体检查揭示出良好整合入腱和骨,无明显炎症改变且瘢痕组织最少。在肩峰下空间和三角肌下区域中发现粘连,与术后改变一致。在组织学上,肩未显示炎症细胞或不适当的血管化。胶原纤维排列在植入装置的任意给定孔室内并且组织化在于具有致密胶原纤维的规则结缔组织的组织化。一般而言,注意到从装置中进一步取出的细胞总体上与直接衬托装置的那些类似,表明网状弹性基质材料对细胞形态无明显的有害影响。1组样本的组织形态测定评价证实装置内修补组织浸润的平均填充比为77.6%(标准偏差+/-8.3%)。In
与1组类似,用于组织加强的植入装置(2组)在体内植入6周后未显示炎症改变或不适当的血管化。此外,遇到与术后改变一致的最低程度的瘢痕形成。植入的装置的组织学分析证实与1组基本上相同的结果。特别地,不存在明显的炎症改变。还注意到浸润装置的修补组织与冈上肌腱和连接肱骨的腱充分生物整合。组织形态测定分析显示装置浸润平均值为79.9%(标准偏差+/-7.7%)。Similar to
实施例17:网状弹性基质2在具有编织纤维加强物的可植入装置中的Example 17:
按照实施例6中所述的操作制备网状弹性基质2。从网状弹性基质2中切下成形为具有54mm长,34mm宽和2mm厚度尺寸的矩形补片的可植入装置。使用Viking Platinum 730缝合机将复丝编织的聚酯纤维(Telflex Medical;相当于具有0.20mm直径和1.65 lbs(748克)最低拉伸强度的4-0号缝合线的丝直径)以格栅的形式插入矩形补片形装置。使用交叉缝线与下列设置将编织的聚酯纤维插入矩形补片:具有2.5mm间距和6.5张力的1型缝线。正方形格栅尺寸为10mm x10mm与沿4个侧面各自的2mm边界。Reticulated
使用与实施例14中所述相同的方法测试SRS和TBS。SRS值为36.5牛顿,其中在经历通过2-0 ETHIBOND缝合线拉出的可植入装置断裂时记录的伸长为25mm。TBS值为56牛顿,在整个装置拉伸断裂时的伸长为7.1mm。SRS and TBS were tested using the same method as described in Example 14. The SRS value was 36.5 Newtons with an elongation of 25 mm recorded upon rupture of the implantable device pulled through the 2-0 ETHIBOND suture. The TBS value was 56 Newtons and the elongation at tensile break of the entire device was 7.1 mm.
实施例18:网状弹性基质1的压缩模制Example 18: Compression molding of reticulated
按照实施例5中所述的操作制备网状弹性基质1。使用下列操作以2-维压缩模制该基质。Reticulated
从网状弹性基质1上切下具有60.5mm直径和62.0mm高度的成形为圆柱体的可植入装置(“圆柱形预制型产品”)。用机器制造圆柱形预制型产品,使得圆柱体轴与泡沫升起方向平行。将圆柱形预制型产品通过在70℃下空气对流烘箱(Blue M Inert Gas Oven Model DCA336F)内加热1.5小时干燥并且储存在干燥环境中。Implantable devices shaped as cylinders ("cylindrical prefabricated products") having a diameter of 60.5 mm and a height of 62.0 mm were cut from the reticulated
40.5mm直径和62.0mm高的圆柱形模具(各自由铝模具基底和覆盖物组成)用于压缩模制干燥圆柱形预制型产品。将干燥的圆柱形预制型产品压配合(在约25℃下)入各模具,以便在与原始泡沫升起方向垂直的径向方向上赋予1.49倍的压缩比。在压缩前后的横截面积比为2.2倍。使用可调整的夹钳使内部各自含压缩的网状弹性基质圆柱形预制型产品的模具保持就位,然后放入烘箱。使用氮气净化烘箱。将模具在130℃温度下在烘箱内的氮气环境中加热3.0小时。此后,从烘箱内取出模具并且使用压缩空气冷却15分钟,此后松弛夹钳。压缩的网状弹性基质1圆柱形预制型产品保持模具的大小和形状。将这些压缩模制的圆柱体储存在干燥环境中。Cylindrical molds of 40.5 mm diameter and 62.0 mm high, each consisting of an aluminum mold base and a cover, were used to compression mold dry cylindrical preformed products. Dry cylindrical preforms were press fit (at about 25°C) into each mold to impart a compression ratio of 1.49 times in the radial direction perpendicular to the original foam rise direction. The cross-sectional area ratio before and after compression was 2.2 times. The molds, each containing a compressed reticulated elastic matrix cylindrical preform inside, were held in place using adjustable clamps and placed in an oven. The oven was purged with nitrogen. The mold was heated at a temperature of 130° C. for 3.0 hours in an oven under a nitrogen atmosphere. Thereafter, the mold was removed from the oven and cooled using compressed air for 15 minutes, after which the clamps were released. The compressed reticulated
使用实施例5和6中所述的操作测定压缩模制的网状弹性基质的特性。将压缩模制前后的网状弹性基质特性列在下表13中,其表明了例如网状弹性基质特性的压缩模制的显著强化。The properties of the compression molded reticulated elastic matrix were determined using the procedure described in Examples 5 and 6. The reticulated elastic matrix properties before and after compression molding are listed in Table 13 below, which shows a significant enhancement of eg compression molding of reticulated elastic matrix properties.
表13Table 13
实施例19:压缩模制的网状弹性基质1及其在用于修复大鼠腹壁的可Example 19: Compression molded reticulated
使用如实施例18中所述制备的压缩模制的网状弹性基质1并且在其中掺入5-0号复丝CP纤维丝(C.P.Medical)制成本发明可植入装置的实例,即测定为1cm长和宽和2mm高的正方形补片。使用Viking Platinum Model 730缝合机与1型缝线和3mm间距将编织纤维掺入矩形装置。An example of an implantable device according to the invention was fabricated using a compression molded reticulated
将可植入装置放入20只Sprague-Dawley大鼠各自的腹壁。腹壁缺陷是具有部分厚度并且遗留腹部筋膜且腹膜和皮肤完整。一定不同的是切下内和外腹斜肌并且在大鼠中被待测可植入装置替代。因此,无装置进入腹腔并且在手术封闭手术部位后皮肤是完整的。植入的装置周围为天然肌肉组织,皮下组织和筋膜。在植入后各1,2,4,8或16周时处死4只大鼠。The implantable device was placed into the abdominal wall of each of 20 Sprague-Dawley rats. Abdominal wall defects are partial thickness and leave abdominal fascia with intact peritoneum and skin. Certainly different the internal and external oblique muscles were excised and replaced in the rat by the implantable device to be tested. Therefore, no devices were entered into the abdominal cavity and the skin was intact after surgical closure of the surgical site. The implanted device is surrounded by native muscle tissue, subcutaneous tissue and fascia. Four rats were sacrificed at 1, 2, 4, 8 or 16 weeks post-implantation.
在上述20只不同Sprague-Dawley大鼠各自的腹壁缺陷上植入了经测定为1cm长和宽和2mm高的正方形补片,它如上所述使用压缩 模制的网状弹性基质1制成,但未掺入5-0号复丝CP纤维丝。还在植入后1,2,4,8或16周时各处死这些大鼠中的4只。还在植入后1,2,4,8或16周时处死这些大鼠。A square patch measuring 1 cm in length and width and 2 mm in height was implanted on each of the above-mentioned 20 different Sprague-Dawley rat abdominal wall defects, made as described above using a compression molded reticulated
在指定的处死时间时,对手术部位+周围天然组织外植入并且通过组织学分析对具有和不具有CP纤维丝的可植入装置进行评价。At the indicated sacrifice times, the surgical site + surrounding native tissue were explanted and implantable devices with and without CP filaments were evaluated by histological analysis.
对加强和未加强的压缩模制的网状弹性基质1可植入装置而言存在类似的宿主反应。愈合反应的特征在于在第1周内主要由单核细胞浸润组成的宿主-移植物相互作用部位上的炎症反应。在整个研究过程中多核巨细胞的数量增加。到第2周时,移植物和结缔组织周围的逐渐组织化的结缔组织囊开始填充可植入装置的孔。结缔组织的组织化随时间进行性增加。到第16周时结缔组织在移植物材料内部和周围极为成熟。在移植物内脉管系统的量增加至第8周。在任何动物中均未观察到下面肌肉组织坏死。Similar host responses exist for reinforced and unreinforced compression molded reticulated
实施例20:具有选择性非多孔性表面的网状弹性基质4在具有多丝编Example 20: Reticulated elastic matrix with selectively non-porous surface织纤维的可植入装置中的应用Applications of Woven Fibers in Implantable Devices
按照实施例8中所述的操作制备网状弹性基质4。从该基质上切下具有50mm长和宽和2mm高的正方形切片。在具有最大表面积的切片的两个表面中,使一面在氮气环境中接触加热板(维持在超过160℃的升温)以便熔化接触的表面,由此在该切片的一侧上生成相对不渗透层或具有相对于网状弹性基质而言低渗透性的层。随后从上述具有不渗透层的切片上切下可植入装置,即经测定42mm长和宽和2mm高的正方形补片。将多丝编织的4-0聚酯纤维(Telflex Medical;相当于4-0号缝合线的直径)以格栅的形式掺入该正方形补片以便形成可以用作例如外科手术用网的可植入装置。正方形格栅的尺寸为8mm x 8mm,且沿四面各自有2mm边界。Reticulated elastic matrix 4 was prepared following the procedure described in Example 8. Square slices having a length and width of 50 mm and a height of 2 mm were cut from the matrix. Of the two surfaces of the slice with the largest surface area, one side was brought into contact with a hot plate (maintained at an elevated temperature in excess of 160°C) in a nitrogen atmosphere in order to melt the contacted surfaces, thereby creating a relatively impermeable layer on one side of the slice Or a layer with low permeability relative to the reticulated elastic matrix. Implantable devices, ie square patches measuring 42 mm long and wide and 2 mm high, were then cut from the above sections with the impermeable layer. Multifilament braided 4-0 polyester fibers (Telflex Medical; diameter equivalent to No. 4-0 suture) are incorporated into the square patch in the form of a grid to form an implantable mesh that can be used, for example, as a surgical mesh. into the device. The square grid measures 8mm x 8mm and has 2mm borders along each of the four sides.
实施例21:具有选择性非多孔性表面的网状弹性基质4在具有可降解Example 21: Reticulated Elastic Matrix 4 with Selectively Non-Porous Surfaces with Degradable多丝编织纤维的可植入装置中的应用Application of Multifilament Braided Fibers in Implantable Devices
按照实施例8中所述的操作制备网状弹性基质4。从该基质上切下具有50mm长和宽和2mm高的正方形切片。在具有最大表面积的切片的两个表面中,一面涂敷热塑性聚碳酸酯聚氨基甲酸酯溶于97%四氢呋喃和3%体积的二甲基甲酰胺的溶液。在溶剂蒸发后,薄涂层遗留在接触表面的孔上,由此在切片的一侧上生成相对不渗透层或具有相对于网状弹性基质而言低渗透性的层。随后从上述具有不渗透层的切片上切下可植入装置,即经测定42mm长和宽和2mm高的正方形补片。将可降解的多丝编织的(Ethicon Inc;乙交酯和丙交酯的共聚物且相当于4-0 VICRYL缝合线的直径)的纤维以格栅的形式掺入该正方形补片以便形成可以用作例如外科手术用网的可植入装置。正方形格栅的尺寸为8mm x 8mm,且沿四面各自有2mm边界。Reticulated elastic matrix 4 was prepared following the procedure described in Example 8. Square slices having a length and width of 50 mm and a height of 2 mm were cut from the matrix. Of the two surfaces of the slice with the largest surface area, one side was coated with a solution of thermoplastic polycarbonate polyurethane in 97% tetrahydrofuran and 3% by volume of dimethylformamide. After the solvent evaporates, a thin coating remains on the pores of the contact surface, thereby creating a relatively impermeable layer or a layer with low permeability relative to the reticulated elastic matrix on one side of the section. Implantable devices, ie square patches measuring 42 mm long and wide and 2 mm high, were then cut from the above sections with the impermeable layer. Fibers of degradable multifilament braid (Ethicon Inc; a copolymer of glycolide and lactide and equivalent to the diameter of a 4-0 VICRYL suture) are incorporated into the square patch in the form of a grid to form a Implantable devices useful as, for example, surgical meshes. The square grid measures 8mm x 8mm and has 2mm borders along each of the four sides.
实施例22:具有编织纤维加强物的网状弹性基质4在用于绵羊回旋套Example 22: Reticulated Elastic Matrix 4 with Braided Fiber Reinforcement for Sheep Convoluted Sleeves加强的可植入装置中的应用Applications in Enhanced Implantable Devices
按照实施例14中所述制成由网状弹性基质4和编织的聚酯纤维构成且形状为经测定40mm长,20mm宽和2mm厚的矩形补片的可植入装置,但使用7-0号编织的聚酯纤维。如下所述将这类可植入装置植入2组的每个绵羊以便愈合绵羊慢性模型中的回旋套撕裂或冈下肌腱,从而评价可植入装置在连接冈下肌腱与肱骨中的强化。An implantable device consisting of reticulated elastic matrix 4 and woven polyester fibers and shaped as a rectangular patch measuring 40 mm long, 20 mm wide and 2 mm thick was made as described in Example 14, but using 7-0 No. woven polyester fiber. These implantable devices were implanted in each sheep in 2 groups as described below to heal rotator cuff tears or the infraspinatus tendon in the ovine chronic model to evaluate the strengthening of the implantable device in connecting the infraspinatus tendon to the humerus .
在每只绵羊的右肩上生成慢性缺陷。使用骨骼成熟的3.5岁以上的Rambouillet X Columbia母羊(绵羊),其体重约为60Kg-约100Kg。23只动物进行该操作。在使用无菌条件全身麻醉中,在右肩关节上做6cm皮肤切口。与切口一致性地分开皮下结肠肌(colimuscle)。沿其肩峰与肩胛头之间的腱分离部位分离三角肌。分离浅头和冈下肌腱插入物。从肱骨上分离冈下肌且然后用5cm x 3cm PRECLUDE DuraSubstitute片(W.L.Gore和Associates,Flagstaff,AZ)包裹。使用常规方法封闭伤口。Generate a chronic defect on the right shoulder of each sheep. Skeletally mature Rambouillet X Columbia ewes (sheep) over 3.5 years old with a body weight of about 60Kg to about 100Kg were used. 23 animals were subjected to this procedure. Under general anesthesia using sterile conditions, a 6 cm skin incision was made on the right shoulder joint. Consistent with the incision, the subcutaneous colimuscle was divided. The deltoid is separated along its tendon separation between the acromion and the head of the scapula. Separate the superficial head and infraspinatus tendon insert. The infraspinatus muscle was isolated from the humerus and then wrapped with 5cm x 3cm PRECLUDE DuraSubstitute sheets (W.L. Gore and Associates, Flagstaff, AZ). Close the wound using conventional methods.
4周后,重新麻醉绵羊并且取出PRECLUDE片。使用Hall矫形外科应用小圆锯剥离冈下肌腱的前部插入部位的外皮。剥离骨标准区域(1cm x 1cm)的外皮。在具有11只动物的对照组(1组)中,在冈下肌结节的1cm x 1cm正方形构造内放置4个Biosuture粘性固定凹(来自Arthrex的3.0mm Biosuture粘性固定凹)后,握住冈下肌腱并且使用固定缝线和Mason-Allen型缝线与肱骨近端再连接。在对照组中所述的另一种方式在于不使用可植入装置使腱与骨再连接。After 4 weeks, the sheep were re-anesthetized and the PRECLUDE tablets were removed. The anterior insertion of the infraspinatus tendon was deskinned using a Hall Orthopedic application with a small circular saw. The skin was stripped off a standard area of bone (1cm x 1cm). In a control group (Group 1) with 11 animals, after placing 4 Biosuture adhesive fixation pockets (3.0mm Biosuture adhesive fixation pockets from Arthrex) within the 1cm x 1cm square configuration of the infraspinatus muscle tubercle, the spinous The inferior tendon was reattached to the proximal humerus using fixation sutures and Mason-Allen type sutures. Another approach, described in the control group, consists in reattaching the tendon to the bone without the use of an implantable device.
在具有12只动物的另一组(2组)中,将可植入装置放置在修复部位上部,使得在结节侧面上存在约1cm突出端。装置的剩余部分在腱上伸展。用于腱连接的固定缝线用垂直褥式缝合连接可植入装置,生成由可植入装置和腱组成的分层的结构。在侧面上,另两条固定缝线通过装置并且将可植入装置向下与结节扎紧。所有可植入装置固定缝合线均至少在该装置中加强格栅的纤维元件上通过。In another group (Group 2) with 12 animals, the implantable device was placed above the repair site such that there was an approximately 1 cm overhang lateral to the nodule. The remainder of the device is stretched over the tendon. Fixation Sutures for Tendon Connections The implantable device is joined with vertical mattress sutures, creating a layered structure consisting of the implantable device and tendon. On the side, another two fixation sutures are threaded through the device and tie the implantable device down to the tubercle. All implantable device fixation sutures are passed over at least the fibrous elements of the reinforcement grid in the device.
在第二次再连接手术后12周时对1和2组动物实施安乐死。收集来自接受可植入装置的组(2组)的9个肩和来自对照组(1组)的8个肩并且即刻准备用于如下的生物机械测试。在除去外部软组织,同时保留肱骨-冈下肌腱结构完整性后,用几个螺丝钻入肱骨近端和肱骨远端,以便获得使用聚甲基甲基丙烯酸酯(PMMA)浇铸型材料与金属固定物连接的区域中肱骨的功能(Purchase)可能性进一步增加。然后使用专门设计的柄将每一测试样本固定在伺服-液压测试机(来自MTSCorp.,Eden Prairie,MN的Model 805)中。下部柄支持肱骨的PMMA-浇铸端。使用基于上述研究研发的铜锌合金冰冷柄将上部柄夹紧在冈下肌腱上作为防止滑脱的预防手段。上部柄以0.5%应力/秒移动以便提供拉伸载荷,直到样本失效并且记录在生物机械试验过程中每个样本达到的最终载荷(定义为最大载荷)。Animals in
对照组(1组)的平均(来自8只动物)最终载荷为762牛顿,标准偏差为474牛顿。接受可植入装置(2组)的平均(来自9只动物)最终载荷为1,328牛顿,标准偏差为427牛顿。使用标准的单向ANOVA统计学分析和在p-值0.05下,将接受可植入装置(2组)的组的最终载荷判定为与不接受装置的对照组(1组)具有显著性差异并且高于对照组(1组)。The mean (from 8 animals) final load for the control group (Group 1) was 762 Newtons with a standard deviation of 474 Newtons. The mean (from 9 animals) final load receiving the implantable device (2 groups) was 1,328 Newtons with a standard deviation of 427 Newtons. Using standard one-way ANOVA statistical analysis and at a p-value of 0.05, the final load of the group receiving the implantable device (Group 2) was judged to be significantly different from the control group (Group 1) not receiving the device and higher than the control group (group 1).
对来自不用于生物机械测试的对照组(1组)的3个修复肩和来自接受可植入装置(2组)的不用于生物机械测试的3个修复肩进行组织学分析。在组织学上,发现可植入装置材料是极为惰性的。炎症反应明显极低。在具有胶原纤维形成的所有可植入装置中均发现了组织向内生长。组织还生长入肱骨。Histological analysis was performed on 3 prosthetic shoulders from the control group not used for biomechanical testing (Group 1 ) and 3 prosthetic shoulders not used for biomechanical testing from those receiving the implantable device (Group 2 ). Histologically, implantable device materials were found to be extremely inert. The inflammatory response was significantly lower. Tissue ingrowth was found in all implantable devices with collagen fiber formation. Tissue also grows into the humerus.
实施例23:网状弹性基质7的合成和特性Example 23: Synthesis and Properties of Reticulated Elastic Matrix 7
通过实施例5中所述的操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质,但所用的组分及其比例如下表14中所示。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the procedure described in Example 5, except that the components used and their ratios are shown in Table 14 below.
表14Table 14
正如根据光学显微镜观察结果测定的,网状弹性基质7的平均隔室直径或其它最大横向尺寸约为481μm。网状弹性基质7的SEM影像表示例如通过其中开放孔互联的隔室网状构造。The average cell diameter or other largest lateral dimension of the reticulated elastic matrix 7 was about 481 μm, as determined from optical microscopic observations. The SEM image of the reticulated elastic matrix 7 shows, for example, a network of compartments interconnected by open pores therein.
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质7进行下列试验。如实施例5中所述测定网状弹性基质7的密度;获得4.96 lbs/ft3(0.080g/cc)的密度值。The thus formed reticulated elastic matrix 7 obtained from the reticulated foam was subjected to the following tests using a test method based on ASTM Standard D3574. The density of reticulated elastic matrix 7 was determined as described in Example 5; a density value of 4.96 lbs/ft3 (0.080 g/cc) was obtained.
如实施例5中所述对网状弹性基质7样本进行张力试验。与泡沫升起方向垂直的平均网状化后拉伸强度测定约为50.2psi(35,300kg/m2)。与泡沫升起方向垂直的网状化后断裂伸长度测定约为162%。与泡沫升起方向平行的平均网状化后拉伸强度测定约为68.2psi(48,000kg/m2)。与泡沫升起方向平行的网状化后断裂伸长度测定约为166%。Tensile testing was performed on samples of reticulated elastic matrix 7 as described in Example 5. The average post-reticulated tensile strength perpendicular to the direction of foam rise was measured to be approximately 50.2 psi (35,300 kg/m2 ). The elongation at break after reticulation perpendicular to the direction of foam rise was measured to be about 162%. The average post-reticulated tensile strength parallel to the direction of foam rise was measured to be approximately 68.2 psi (48,000 kg/m2 ). The elongation at break after reticulation parallel to the direction of foam rise was measured to be about 166%.
如实施例5中所述对网状弹性基质7进行压缩试验。将在50%压缩下与泡沫升起方向平行的网状化后抗压强度测定约为3.31psi(2,325kg/m2)。Compression tests were performed on reticulated elastic matrix 7 as described in Example 5. The post-reticulated compressive strength parallel to the direction of foam rise at 50% compression was determined to be approximately 3.31 psi (2,325 kg/m2 ).
如实施例5中所述测定网状弹性基质7的回弹恢复。获得的结果如表15中所示。The rebound recovery of the reticulated elastic matrix 7 was determined as described in Example 5. The results obtained are shown in Table 15.
表15Table 15
使用自动化液体渗透仪LP-101-A型如实施例5中所述在泡沫升起方向上测定通过网状弹性基质7的流体渗透性。网状弹性基质7的渗透性在泡沫升起方向上测定为282达西。The fluid permeability through the reticulated elastic matrix 7 was measured in the direction of foam rise as described in Example 5 using an Automated Liquid Permeameter Model LP-101-A. The permeability of the reticulated elastic matrix 7 was measured at 282 Darcy in the direction of foam rise.
如实施例5中所述,还在网状弹性基质7压缩(与泡沫升起方向垂直)以便减小可利用的流通面积后测定渗透性。图11中的线1为达西渗透性与网状弹性基质7的可利用流通面积关系的示意图。在图11中,100%可利用流通面积代表未压缩的网状弹性基质7并且表明在泡沫升起方向上的最高渗透性282达西。网状弹性基质7在泡沫升起方向上的渗透性在压缩后可利用流通面积降至原始面积的47.2%时降至136达西,并且在压缩后可利用面积降至原始面积的37.0%时降至95达西。Permeability was also measured after compression of the reticulated elastic matrix 7 (perpendicular to the direction of foam rise) so as to reduce the available flow area, as described in Example 5.
实施例24:网状弹性基质8的合成和特性Example 24: Synthesis and Properties of Reticulated Elastic Matrix 8
通过实施例7中所述的操作制备网状交联生物耐久性弹性聚碳酸酯脲-氨基甲酸酯基质,但所用的组分及其比例如下表16中所示。特别地,使用表面活性剂B-8300和B-5055(各自来自Goldschmidt)替代用于隔室稳定的B-8305表面活性剂。A reticulated crosslinked biodurable elastic polycarbonate urea-urethane matrix was prepared by the procedure described in Example 7, except that the components used and their ratios are shown in Table 16 below. In particular, surfactants B-8300 and B-5055 (each from Goldschmidt) were used instead of B-8305 surfactant for compartment stabilization.
表16Table 16
正如根据光学显微镜观察结果测定的,网状弹性基质8的平均隔室直径或其它最大横向尺寸约为512μm。网状弹性基质8的SEM影像显示了例如通过其中开放孔互联的隔室网状构造。The average cell diameter or other largest lateral dimension of the reticulated elastic matrix 8 is about 512 μm, as determined from optical microscopic observations. The SEM image of the reticulated elastic matrix 8 shows, for example, a network of compartments interconnected by open pores therein.
使用基于ASTM Standard D3574的测试方法对获自网状化泡沫的由此形成的网状弹性基质8进行下列试验。如实施例5中所述测定网状弹性基质8的密度;获得5.25 lbs/ft3(0.084g/cc)的密度值。The thus formed reticulated elastic matrix 8 obtained from the reticulated foam was subjected to the following tests using a test method based on ASTM Standard D3574. The density of reticulated elastic matrix 8 was determined as described in Example 5; a density value of 5.25 lbs/ft3 (0.084 g/cc) was obtained.
然后使网状弹性基质8的块在110℃的烘箱内不受约束地退火5小时或10小时。The blocks of reticulated elastic matrix 8 were then annealed unrestrained in an oven at 110° C. for 5 hours or 10 hours.
如实施例5中所述对未退火和退火的网状弹性基质8样本进行与泡沫升起方向垂直和平行的张力和压缩试验。另外,通过测定在低应变下应力与应变之比各自计算拉伸模量和压缩模量,即每一相应应力的最初斜率与应变曲线之间的关系。正如根据如下的表17中显示的,在110℃下网状化后退火5小时和10小时导致网状弹性基质8的机械性能显著增加。应注意网状弹性基质8的密度在退火后基本上保持不变。The unannealed and annealed reticulated elastic matrix 8 samples were subjected to tension and compression tests perpendicular and parallel to the direction of foam rise as described in Example 5. In addition, the tensile and compressive moduli were each calculated by determining the ratio of stress to strain at low strains, ie the relationship between the initial slope of each corresponding stress versus strain curve. As shown in Table 17 according to the following, post-reticulation annealing at 110° C. for 5 hours and 10 hours resulted in a significant increase in the mechanical properties of the reticulated elastic matrix 8 . It should be noted that the density of the reticulated elastic matrix 8 remains substantially unchanged after annealing.
表17Table 17
引入的指导性公开文献Introduced Guidance Public Documents
特别将本说明书中参照的各自和每篇美国专利和专利申请,各外观和国际专利公开文献和各其它公开文献和各未公开的专利申请全部披露的内容完整地引入本文作为对其进行的相应具体参考。The entire disclosure of each and every U.S. patent and patent application, each Appearance and International Patent Publication, and each other publication and each unpublished patent application referenced in this specification is hereby incorporated in its entirety as its corresponding Specific reference.
尽管上文已经描述了本发明的例证性实施方案,但是应理解许多和各种变型对相关领域技术人员而言显而易见,或在本领域发展时显而易见。将这类变型关注为属于本发明或本说明书披露的本发明的精神和范围。While illustrative embodiments of the invention have been described above, it is to be understood that many and various modifications will be apparent to those skilled in the relevant arts, or as the art develops. Such variations are contemplated as falling within the spirit and scope of the invention or the invention disclosed in this specification.
Claims (101)
Translated fromChineseApplications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US81612006P | 2006-06-22 | 2006-06-22 | |
| US60/816,120 | 2006-06-22 | ||
| US60/849,328 | 2006-10-03 | ||
| US11/652,763 | 2007-01-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN101472564Atrue CN101472564A (en) | 2009-07-01 |
Family
ID=40829474
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA200780023043XAPendingCN101472564A (en) | 2006-06-22 | 2007-06-15 | High performance reticulated elastomeric matrix |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101472564A (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103204747A (en)* | 2013-03-18 | 2013-07-17 | 江苏瑞丰科技实业有限公司 | Nutritional composite gel material for soil improvement |
| CN105748177A (en)* | 2016-04-20 | 2016-07-13 | 华南理工大学 | Personalized spine implantation prosthesis with bionic micropores and manufacturing method thereof |
| CN107296977A (en)* | 2012-01-31 | 2017-10-27 | 托莱多大学 | Injectable, biodegradable bone cement and production and preparation method thereof |
| CN108353237A (en)* | 2015-10-30 | 2018-07-31 | 科利耳有限公司 | Implantable Stimulator Component |
| CN109820613A (en)* | 2019-01-17 | 2019-05-31 | 重庆大学 | A method for constructing an animal model of intervertebral disc degeneration based on posterior ligament injury |
| CN112331013A (en)* | 2020-11-17 | 2021-02-05 | 重庆医药高等专科学校 | Placenta model for teaching demonstration and practical operation examination |
| CN113521401A (en)* | 2021-08-02 | 2021-10-22 | 浙江省人民医院 | A kind of soft tissue expander based on shape memory material and preparation method thereof |
| CN114246977A (en)* | 2021-12-20 | 2022-03-29 | 华南理工大学 | An electrical self-repairing artificial muscle fiber and preparation method thereof |
| WO2024159798A1 (en)* | 2023-02-02 | 2024-08-08 | 安徽省公共卫生临床中心(安徽省传染病医院) | Prosthesis for suturing muscle to femur |
- 2007
- 2007-06-15CNCNA200780023043XApatent/CN101472564A/enactivePending
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107296977A (en)* | 2012-01-31 | 2017-10-27 | 托莱多大学 | Injectable, biodegradable bone cement and production and preparation method thereof |
| CN103204747A (en)* | 2013-03-18 | 2013-07-17 | 江苏瑞丰科技实业有限公司 | Nutritional composite gel material for soil improvement |
| CN108353237A (en)* | 2015-10-30 | 2018-07-31 | 科利耳有限公司 | Implantable Stimulator Component |
| CN108353237B (en)* | 2015-10-30 | 2022-01-04 | 科利耳有限公司 | Implantable stimulation assembly |
| CN105748177A (en)* | 2016-04-20 | 2016-07-13 | 华南理工大学 | Personalized spine implantation prosthesis with bionic micropores and manufacturing method thereof |
| CN105748177B (en)* | 2016-04-20 | 2018-01-02 | 华南理工大学 | A kind of personalized implantable spinal prosthesis and its manufacture method with bionic micropore |
| CN109820613A (en)* | 2019-01-17 | 2019-05-31 | 重庆大学 | A method for constructing an animal model of intervertebral disc degeneration based on posterior ligament injury |
| CN109820613B (en)* | 2019-01-17 | 2020-05-19 | 重庆大学 | Method for constructing intervertebral disc degeneration animal model based on posterior ligament injury |
| CN112331013A (en)* | 2020-11-17 | 2021-02-05 | 重庆医药高等专科学校 | Placenta model for teaching demonstration and practical operation examination |
| CN113521401A (en)* | 2021-08-02 | 2021-10-22 | 浙江省人民医院 | A kind of soft tissue expander based on shape memory material and preparation method thereof |
| CN114246977A (en)* | 2021-12-20 | 2022-03-29 | 华南理工大学 | An electrical self-repairing artificial muscle fiber and preparation method thereof |
| WO2024159798A1 (en)* | 2023-02-02 | 2024-08-08 | 安徽省公共卫生临床中心(安徽省传染病医院) | Prosthesis for suturing muscle to femur |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20070190108A1 (en) | High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair | |
| AU2004241111B2 (en) | Manufacture and use of implantable reticulated elastomeric matrices | |
| US9050176B2 (en) | At least partially resorbable reticulated elastomeric matrix elements and methods of making same | |
| US20100318108A1 (en) | Composite mesh devices and methods for soft tissue repair | |
| US8801801B2 (en) | At least partially resorbable reticulated elastomeric matrix elements and methods of making same | |
| US20050043585A1 (en) | Reticulated elastomeric matrices, their manufacture and use in implantable devices | |
| CN101472564A (en) | High performance reticulated elastomeric matrix | |
| JP2007531557A (en) | Spinal ring defects and ring nucleation regeneration | |
| US20070162131A1 (en) | Repair of spinal annular defects | |
| CN218075338U (en) | Breast implant | |
| HK1130674A (en) | High performance reticulated elastomeric matrix | |
| BRPI0710002A2 (en) | high performance crosslinked elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue addition and / or tissue repair | |
| HK1120419A (en) | Reticulated elastomeric matrices, their manufacture and use in implantable devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code | Ref country code:HK Ref legal event code:DE Ref document number:1130674 Country of ref document:HK | |
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | Application publication date:20090701 | |
| REG | Reference to a national code | Ref country code:HK Ref legal event code:WD Ref document number:1130674 Country of ref document:HK |