CN101336094A - Apparatus and method for treating bone - Google Patents
Apparatus and method for treating boneDownload PDFInfo
- Publication number
- CN101336094A CN101336094ACNA2006800521430ACN200680052143ACN101336094ACN 101336094 ACN101336094 ACN 101336094ACN A2006800521430 ACNA2006800521430 ACN A2006800521430ACN 200680052143 ACN200680052143 ACN 200680052143ACN 101336094 ACN101336094 ACN 101336094A
- Authority
- CN
- China
- Prior art keywords
- implant
- vertebral body
- balloon
- expandable
- bone
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Prostheses (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
[0001]本发明涉及外科植入,更具体地是涉及用于骨扩张或扩增的微创装置和方法,优选的是椎骨和/或恢复脊柱前凸。[0001] The present invention relates to surgical implants, and more particularly to minimally invasive devices and methods for expansion or augmentation of bone, preferably vertebrae, and/or restoration of lordosis.
背景技术Background technique
[0002]如图1所示,脊椎压缩性骨折代表了一种常见的脊柱损伤并且可能导致长期残疾(F.Margerl等人:A comprehensive classification ofthoracic and lumbar injuries(胸和腰部损伤的综合分类),Eur Spine J184-201,1994)。这些骨折涉及脊柱10中的一个或多个椎骨体12的骨折。脊柱的压缩性骨折通常发生在胸椎的下椎体或腰椎的上椎体。它们通常涉及受影响的椎体12的前部18(与后侧16相对)的骨折。脊柱压缩性骨折可以导致脊柱受影响区域内椎骨体正常排列或弯曲的变形,例如脊柱前凸。例如,脊柱的转移性癌、外伤能够导致脊柱压缩性骨折和/或相关的脊柱畸形,或与骨质疏松有关。直到最近,医生治疗这种压缩性骨折和相关畸形的手段仍然非常有限。镇痛药、卧床休息、支撑或侵害性脊柱手术是仅有的可行方法。[0002] As shown in Figure 1, vertebral compression fractures represent a common spinal injury and may result in long-term disability (F.Margerl et al.: A comprehensive classification of thoracic and lumbar injuries, Eur Spine J184-201, 1994). These fractures involve fractures of one or more
[0003]最近,发展了用于治疗脊椎压缩性骨折的微创外科手术。这些手术通常涉及用插管或其它介入工具通过椎弓根(pedicle)插入受影响椎骨体的后部。这些手术最基本的是椎体成形术,其字面含义是固定椎骨体,并且不需要首先使骨头复位。[0003] More recently, minimally invasive surgical procedures have been developed for the treatment of vertebral compression fractures. These procedures generally involve insertion of a cannula or other interventional tool through the pedicle into the posterior portion of the affected vertebral body. The most basic of these procedures is vertebroplasty, which literally immobilizes the vertebral bodies without first resetting the bones.
[0004]简而言之,缓慢地使插管或特别的骨针穿过后背的软组织。利用X射线图像引导与小量的X射线染色使得总能看到针的位置。通过针将少量的聚甲基丙烯酸甲酯(PMMA)或其它整形外科接合剂推入椎骨体。PMMA是已经在多种整形外科手术中使用了多年的的医用级物质。通常,接合剂混合有用于减小感染危险的抗生素,以及允许其能在X射线上可见的包含钡或钽的粉末。[0004] Briefly, a cannula or special bone needle is slowly passed through the soft tissue of the back. The use of X-ray image guidance with a small amount of X-ray dye allows the needle position to always be seen. A small amount of polymethylmethacrylate (PMMA) or other orthopedic cement is pushed through the needle into the vertebral body. PMMA is a medical grade substance that has been used for many years in various plastic surgery procedures. Usually, the cement is mixed with antibiotics to reduce the risk of infection, and a powder containing barium or tantalum that allows it to be seen on x-rays.
[0005]椎体成形术对减轻或消除骨折疼痛,防止进一步断裂以及恢复患者的活动是有效的。然而,这个过程不能够对骨折的骨头复位,因此可能引起由于骨折的脊椎畸形问题。通常是不进行椎体成形术,除非是在受影响区域内相邻椎骨体之间的脊柱后凸小于百分之十的情况下。此外,根据最新研究,该手术需要采用低粘度接合剂的高压接合剂灌注,并且可能在30%至80%的手术中导致接合剂渗漏(Truumees,ComparingKyphoplasty and Vertebroplasty,Advances in Osteoporotic FractureManagement,Vol.1,No.4,2002)。在多数情况下,接合剂渗漏是没有危害的。然而,在少数情况下,聚甲基丙烯酸甲酯或其它接合剂渗漏进入脊椎管或椎旁静脉系统时会引发肺栓塞,导致病人死亡(J.S.Jang:Pulmonary Embolism of PMMA after Percutaneous Vertebroplasty,SpineVol.27,NO.19.2002)。[0005] Vertebroplasty is effective in reducing or eliminating fracture pain, preventing further fracture, and restoring the patient's mobility. However, this procedure is not capable of resetting the fractured bone and thus may cause problems with spinal deformity due to the fracture. Vertebroplasty is usually not performed except in cases where the kyphosis between adjacent vertebral bodies in the affected area is less than ten percent. Furthermore, according to recent studies, the procedure requires high-pressure cement infusion with low-viscosity cements and can result in cement leakage in 30% to 80% of procedures (Truumees, Comparing Kyphoplasty and Vertebroplasty, Advances in Osteoporotic Fracture Management, Vol. 1, No.4, 2002). In most cases, cement leaks are harmless. However, in rare cases, leakage of polymethylmethacrylate or other cement into the spinal canal or paraspinal venous system can cause pulmonary embolism, leading to death of the patient (J.S.Jang: Pulmonary Embolism of PMMA after Percutaneous Vertebroplasty, SpineVol. 27, NO.19.2002).
[0006]对脊椎压缩性骨折的更先进的治疗方法通常包括两个阶段:(1)椎骨体原始高度的复位、扩张或恢复以及后续的脊柱曲度的脊柱前凸校正;以及(2)填充或添加材料以支撑或加强骨折的骨头。[0006] More advanced treatments for vertebral compression fractures generally involve two phases: (1) reduction, expansion or restoration of the original height of the vertebral body and subsequent lordotic correction of the spinal curvature; and (2) filling Or adding material to support or strengthen broken bones.
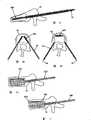
[0007]图2A-D说明了一种这样的治疗方法,即:球囊扩张脊柱后凸成形术(Kyphon,Inc.)。具有可扩张球囊末端的导管通过插管、护套或其它导引器插入骨折的椎骨体的中央部分,骨折的椎骨体包括由骨折的骨皮质包围的相对柔软的骨松质(图2A)。然后脊柱后凸成形术通过使球囊膨胀实现脊柱前凸的重构或实现正常的曲度,球囊在椎骨体内膨胀使椎骨体恢复到其原始高度(图2B)。移去球囊,在椎骨体内留出空腔,然后如上文针对脊柱成形术所述的,通过插管将PMMA或其它填充材料注入空腔(图2C)。移去插管,并且接合剂固化以填充或固定骨头(图2D)。[0007] Figures 2A-D illustrate one such treatment, balloon kyphoplasty (Kyphon, Inc.). A catheter with an expandable balloon tip is inserted through a cannula, sheath, or other guide into the central portion of the fractured vertebral body, which consists of relatively soft spongy bone surrounded by fractured cortical bone (Fig. 2A) . Kyphoplasty then achieves lordotic remodeling or normal curvature by inflating a balloon that is inflated within the vertebral body to restore the vertebral body to its original height (Fig. 2B). The balloon is removed, leaving a cavity within the vertebral body, and PMMA or other filler material is injected into the cavity through the cannula as described above for vertebroplasty (Fig. 2C). The cannula is removed and the cement cures to fill or secure the bone (Fig. 2D).
[0008]该手术的缺点包括成本高,在移去球囊导管之后不能对椎骨体的终板进行复位,以及在手术过程中可能需要对椎体终板进行穿孔。与脊柱成形术一样,虽然不经常发生,但可能最可怕的与脊柱后凸成形术有关的并发症同样涉及接合剂的渗漏。例如,接合剂渗漏进入脊椎管可能引起神经功能缺损。这种接合剂渗漏可能通过椎骨体的低阻静脉或通过先前没有发现的骨头中的裂缝发生。其它并发症包括:附加邻近椎间盘椎体骨折、感染和接合剂栓塞。接合剂栓塞发生的机制与接合剂渗漏相似。接合剂可以被迫进入低阻静脉系统并移动到肺或大脑导致肺栓塞或中风。此外,脊柱后凸球囊是弹性的并且不适于使支架扩大。由于支架阻力,脊柱后凸球囊将在支架前后膨胀并且当球囊达到支架边界时突然爆破。关于球囊脊柱后凸成形术的其它细节例如可以参见Riley等人的美国专利第6,423,083号、第6,248,110号和第6,235,043号;Gantis等人,Ballon kyphoplasty for the treatment of pathological vertebralcompression fractures,Eur Spine J14:250-260,2005;以及Lieberman等人,Initial outcome and efficacy of Kyphoplasty in the treatment of painfulosteoporotic vertebral compression fractures,Spine 26(14):1631-1638,2001,在此将它们的全部内容并入本文作为参考。[0008] Disadvantages of this procedure include the high cost, the inability to reset the endplates of the vertebral bodies after removal of the balloon catheter, and the possible need to perforate the endplates of the vertebral bodies during the procedure. As with kyphoplasty, perhaps the most dreaded, though infrequent, kyphoplasty-related complication involves leakage of cement. For example, leakage of cement into the spinal canal may cause neurological deficits. This cement leakage may occur through low-resistance veins in the vertebral bodies or through previously undetected fissures in the bone. Other complications include: additional adjacent disc vertebral fractures, infection, and cement embolism. Cement embolism occurs by a mechanism similar to cement leakage. Cement can be forced into the low-resistance venous system and travel to the lungs or brain causing pulmonary embolism or stroke. Furthermore, the kyphotic balloon is elastic and not suitable for expanding the stent. Due to stent resistance, the kyphotic balloon will inflate anteriorly and posteriorly to the stent and burst when the balloon reaches the boundary of the stent. Additional details on balloon kyphoplasty can be found, for example, in U.S. Patent Nos. 6,423,083, 6,248,110, and 6,235,043 to Riley et al; Gantis et al, Ballon kyphoplasty for the treatment of pathological vertical compression fractures, Eur Spine J14: 250-260, 2005; and Lieberman et al., Initial outcome and efficacy of Kyphoplasty in the treatment of painful osteoporotic vertebral compression fractures, Spine 26(14):1631-1638, 2001, the entire contents of which are hereby incorporated by reference herein .
[0009]另一种治疗脊椎压缩性骨折的方法是Optimesh(奥泊提美西)系统(Spineology,Inc.,Stillwater,MN),其在所涉及的椎骨体内利用可扩张网孔移植球囊或容器器件提供接合剂或异体移植或自体移植骨头的微创传送。在膨胀后,移植球囊仍留在椎骨体的内侧,这防止了外科手术进行时发生的复位丧失,例如在撤出球囊时在脊柱后凸成形术中可能出现的。然而,这种系统的一个缺点是,网孔植入物(mesh implant)不能很好地结合在椎骨体内。这能够导致植入物和椎骨体之间的相对运动,结果引起术后的复位丧失。例如,可以在公开的公开号为20040073308的美国专利申请中找到关于这个手术的其它细节,在此合并其全部内容以供参考。[0009] Another method of treating vertebral compression fractures is the Optimesh (Spineology, Inc., Stillwater, MN) system, which utilizes an expandable mesh graft balloon or The container device provides minimally invasive delivery of cement or allograft or autograft bone. After inflation, the graft balloon remains inside the vertebral body, which prevents loss of reduction during surgical procedures, such as can occur in kyphoplasty when the balloon is withdrawn. However, a disadvantage of this system is that the mesh implant does not integrate well within the vertebral body. This can lead to relative motion between the implant and the vertebral body, with consequent loss of postoperative reduction. Additional details regarding this procedure can be found, for example, in Published US Patent Application Publication No. 20040073308, which is hereby incorporated by reference in its entirety.
[0010]在脊椎压缩性骨折的治疗中用到的另一种方法是称为SKY骨扩张器的可扩张聚合物扩张物质(polymer augmentation mass)。这种器件能够以受控的方式膨胀到预先设计的尺寸和立方体或梯形结构。与脊柱后凸球囊相似,一旦达到了最佳椎骨高度和空腔,便移去SKY(斯盖)骨扩张器并且将PMMA接合剂或其它填充物注射到空腔内。因此,这种方法具有许多上文针对脊柱后凸成形术描述的相同的缺点和不足。[0010] Another method used in the treatment of vertebral compression fractures is the expandable polymer augmentation mass known as the SKY bone expander. Such devices can be expanded to predesigned dimensions and cubic or trapezoidal configurations in a controlled manner. Similar to the kyphotic balloon, once optimal vertebral height and cavity are achieved, the SKY bone expander is removed and PMMA cement or other filler is injected into the cavity. Thus, this approach suffers from many of the same disadvantages and deficiencies described above for kyphoplasty.
[0011]提出的一种改进的用于对椎骨体压缩性骨折进行复位和扩张的方法是椎骨体支架置放术(vertebral body stenting),例如在以下申请中描述的,等,“Vertebral body stenting”,31:356-361,2002;欧洲专利申请公开号为EP1308134A3;以及公开号为US2003/0088249的美国专利申请,在此合并其全部内容以供参考。例如在图3中描述的椎骨体支架,通常包括将由支架(例如用于血管成形术中的支架)包括的球囊导管(例如脊柱后凸成形球囊)插入椎骨体。在插入球囊之后,例如利用液压使球囊膨胀,从而在椎骨体内使支架扩张。在支架扩张之后,球囊可以缩小并移去,支架仍以扩张状态留在椎骨体内侧以填充椎骨体。[0011] An improved method proposed for reduction and expansion of vertebral body compression fractures is vertebral body stenting, such as described in the following application, etc., "Vertebral body stenting", 31:356-361, 2002; European Patent Application Publication No. EP1308134A3; and US Patent Application Publication No. US2003/0088249, the entire contents of which are hereby incorporated by reference. A vertebral body stent, such as that depicted in Figure 3, typically involves inserting a balloon catheter (eg, a kyphoplasty balloon) contained by a stent (eg, a stent used in angioplasty) into the vertebral body. After insertion of the balloon, the balloon is inflated, eg, hydraulically, thereby expanding the stent within the vertebral body. After the stent is expanded, the balloon can be deflated and removed, leaving the stent in an expanded state inside the vertebral body to fill the vertebral body.
[0012]尽管椎骨体支架的概念对其它已知的治疗压缩性骨折的方法提供了希望,但仍然需要改进支架和其它可扩张植入物以及对骨折的椎骨体和其它骨头进行复位和扩张的相关方法。[0012] Although the concept of a vertebral body scaffold offers hope to other known methods of treating compression fractures, there remains a need for improved scaffolds and other expandable implants and methods for reducing and expanding fractured vertebral bodies and other bones. related methods.
发明内容Contents of the invention
[0013]本发明提供了椎骨体微创扩张的装置和方法。在一个实施例中,本发明提供了用于对脊椎骨折和其它脊柱异常进行校正的植入物和方法。例如,一个或多个支架(stent)或其它可扩张植入物可以被插入到因脊椎压缩性骨折而损坏的椎骨体中。随着一个或多个植入物被插入椎骨体中并膨胀,它们可以填充椎骨体的中央部分并且可以推斥椎骨体终板的内侧,从而提供结构性支撑并且试图将脊椎恢复到其原始高度。任选地,一个或多个可扩张植入物可以包括形状记忆合金或在植入之后膨胀或改变结构(或构形)的其它材料,这可以导致植入物被彻底整合到骨头内和/或帮助恢复受损椎骨体的高度。在植入之后,可以加入骨接合剂(例如,PMMA或磷酸三钙)、骨碎片、脱钙骨或其它填充材料以辅助骨头的稳定并且确保植入物在骨头内就位。[0013] The present invention provides devices and methods for minimally invasive expansion of vertebral bodies. In one embodiment, the present invention provides implants and methods for correcting vertebral fractures and other spinal abnormalities. For example, one or more stents or other expandable implants may be inserted into a vertebral body damaged by a vertebral compression fracture. As one or more implants are inserted into the vertebral body and expand, they can fill the central portion of the vertebral body and can push against the inside of the vertebral body endplates, providing structural support and attempting to return the spine to its original height . Optionally, one or more expandable implants may comprise a shape memory alloy or other material that expands or changes structure (or configuration) after implantation, which may result in the implant being fully integrated into the bone and/or Or help restore the height of the damaged vertebral body. After implantation, bone cement (eg, PMMA or tricalcium phosphate), bone chips, demineralized bone, or other filling materials may be added to aid in the stabilization of the bone and ensure the implant is seated within the bone.
[0014]支架或其它可扩张植入物可以由任意具有期望特性的生物相容性材料组成,例如形状记忆合金(例如,镍钛诺或其它镍钛合金,以铜为基础的合金,以铁为基础的合金等),钛、不锈钢、生物相容性聚合物,其它金属或金属合金,陶瓷,合成物或它们的任意组合。另一个植入物可以具有任意期望的结构以便于扩张,抵抗收缩,和/或在扩张过程中或扩张之后在结构上施加期望的力。一个或多个可扩张植入物可以被独立地插入到骨头中,或者可以被同轴地、并联地或串联地接合或连接在一起以形成具有期望特性的结构。在一些实施例中,支架或其它可扩张植入物可以是可再吸收的。此外,为了在支架应变形的位置处使支架扩张,支架优选应被约束,特别是半约束。[0014] Stents or other expandable implants may be composed of any biocompatible material having desired properties, such as shape memory alloys (e.g., Nitinol or other nickel-titanium alloys, copper-based alloys, iron-based based alloys, etc.), titanium, stainless steel, biocompatible polymers, other metals or metal alloys, ceramics, composites or any combination thereof. The other implant can have any desired structure to facilitate expansion, resist contraction, and/or exert a desired force on the structure during or after expansion. One or more expandable implants may be inserted into the bone independently, or may be joined or connected together coaxially, in parallel, or in series to form a structure with desired properties. In some embodiments, a stent or other expandable implant may be resorbable. Furthermore, the stent should preferably be constrained, in particular semi-constrained, in order to expand the stent at the locations where the stent should deform.
[0015]在一些实施例中,一种治疗骨头的方法可以包括在骨折或骨质疏松的骨头(例如椎骨)内插入相互协作以扩张椎骨体的两个或多个同轴支架。可以与被植入的器件一起添加或单独添加骨接合剂或其它填充物,以辅助骨头的稳定并且确保植入物在骨头内就位。例如,可以将骨移植材料(例如骨碎片或脱钙骨)添加到骨头内,在支架周围一骨接合剂塞可以用于将支架固定在椎骨内。在一些实施例中,一个或多个附加的植入物可以与支架组合使用,例如,可扩张塞、可扩张卷筒、可扩张金属片植入物、链条、锥弓根螺钉等,例如使支架扩张和/或提供附加的扩张。[0015] In some embodiments, a method of treating bone may include inserting into a fractured or osteoporotic bone (eg, a vertebra) two or more coaxial supports that cooperate to expand the vertebral body. Bone cement or other fillers may be added with or separately from the implanted device to assist in the stabilization of the bone and to secure the implant in place within the bone. For example, bone graft material (eg, bone fragments or demineralized bone) can be added to the bone, and a plug of bone cement around the scaffold can be used to secure the scaffold within the vertebrae. In some embodiments, one or more additional implants may be used in combination with the stent, e.g., expandable plugs, expandable rolls, expandable sheet metal implants, chains, pedicle screws, etc., e.g., using The stent expands and/or provides additional expansion.
[0016]在一个实施例中,用于疗骨扩张的装置包括第一可扩张植入物以及至少半约束的扩张器件,所述第一可扩张植入物具有第一结构和第二结构,可扩张植入物能够在其第二结构中经受塑性变形,其中所述植入物包围扩张器件的至少一部分。扩张器件和植入物的结构和尺寸适于通过插管插入骨头的一个区域,其中植入物能够承受施加到其周界上的约5N到300N之间的力。[0016] In one embodiment, an apparatus for osteopathic expansion includes a first expandable implant having a first configuration and a second configuration, and an at least semi-constrained expansion device, The expandable implant is capable of undergoing plastic deformation in its second configuration, wherein the implant surrounds at least a portion of the expansion device. The expansion device and implant are configured and sized for insertion through the cannula into a region of bone where the implant is capable of withstanding a force of between about 5N and 300N applied to its perimeter.
[0017]在另一个实施例中,一种扩张椎骨体的方法包括提供一个球囊插管,其具有带内腔的轴和与内腔可操作地关联的球囊部;提供可扩张植入物,其具有第一可植入尺寸和结构,能够塑性变形到大于可植入尺寸的第二可扩张尺寸和与可植入结构不同的可扩张结构,而且,可扩张植入物安装在球囊插管的球囊部分上,并且将其上安装有植入物的球囊插管插入椎骨体的内部,从而使球囊部分和植入物至少部分位于椎骨体内。所述方法进一步包括使球囊插管的球囊部分膨胀从而使植入物变换到其可扩张尺寸和结构,并且至少从椎骨体移去球囊轴。[0017] In another embodiment, a method of dilating a vertebral body includes providing a balloon catheter having a shaft with a lumen and a balloon portion operably associated with the lumen; providing an expandable implant An object having a first implantable size and structure capable of plastic deformation to a second expandable size greater than the implantable size and an expandable structure different from the implantable structure, and the expandable implant is mounted on a ball the balloon portion of the balloon cannula, and inserting the balloon cannula with the implant mounted thereon into the interior of the vertebral body such that the balloon portion and the implant are at least partially within the vertebral body. The method further includes expanding the balloon portion of the balloon cannula to transform the implant to its expandable size and configuration, and removing at least the balloon shaft from the vertebral body.
[0018]在另一个实施例中,工具包可以包括本发明组件的多种组合。工具包例如可以包括插管和一个或多个可扩张植入物。工具包可以额外包括用于将扩张力施加到一个或多个植入物上的一个或多个球囊或其它可扩张构件。工具包可以额外包括注射器或用于将接合剂或其它填充物注射入椎骨体的其它装置。任选地,一个或多个其它植入物或器件可以被包括在工具包中。[0018] In another embodiment, a kit may include various combinations of components of the present invention. A kit may include, for example, a cannula and one or more expandable implants. The kit may additionally include one or more balloons or other expandable members for applying an expansion force to the one or more implants. The kit may additionally include a syringe or other device for injecting cement or other filler into the vertebral body. Optionally, one or more other implants or devices may be included in the kit.
附图说明Description of drawings
[0019]通过以下示例性附图本发明将得到更详细的描述和更好的理解,其中相同的附图标记表示相同的元件。这些附图仅仅是作为示例来说明某些特征,这些特征可以单独或与其它特征结合使用,并且本发明不应局限于所示的实施例。[0019] The invention will be described in more detail and better understood by the following illustrative drawings, in which like reference numerals denote like elements. The drawings illustrate certain features by way of example only, which may be used alone or in combination with other features, and the invention should not be limited to the embodiments shown.
[0020]图1是对在一个椎骨体内具有脊椎压缩性骨折的脊柱的图解说明;[0020] FIG. 1 is a diagrammatic illustration of a spine with a vertebral compression fracture within one vertebral body;
[0021]图2A-D是对治疗脊椎压缩性骨折的现有技术的图解说明;[0021] Figures 2A-D are illustrations of prior art techniques for treating vertebral compression fractures;
[0022]图3是对描述支撑椎骨体的现有技术的方法的图解说明;[0022] FIG. 3 is an illustration describing a prior art method of supporting a vertebral body;
[0023]图4是根据本发明的实施例的可扩张植入物的透视图;[0023] FIG. 4 is a perspective view of an expandable implant according to an embodiment of the invention;
[0024]图5是带有用于扩张植入物的球囊器件的图4植入物的侧视图;[0024] FIG. 5 is a side view of the implant of FIG. 4 with a balloon device for expanding the implant;
[0025]图6是椎骨的横截面侧视图,示出了通过椎骨的椎弓根插入插管的方法;[0025] FIG. 6 is a cross-sectional side view of a vertebra showing the method of inserting a cannula through the pedicle of the vertebra;
[0026]图7是图6椎骨的横截面侧视图,示出了带有通过插管插入并进入椎骨体的可扩张植入物的球囊插管;[0026] FIG. 7 is a cross-sectional side view of the vertebra of FIG. 6 showing a balloon cannula with an expandable implant inserted through the cannula and into the vertebral body;
[0027]图8是图6椎骨的横截面侧视图,示出了通过引导丝插入的球囊和植入物;[0027] FIG. 8 is a cross-sectional side view of the vertebra of FIG. 6 showing the balloon and implant inserted through the guide wire;
[0028]图9A是具有双侧插入椎骨体的植入物的椎骨的横截面顶视图;Fig. 9 A is the cross-sectional top view of the vertebra with the implant of bilateral insertion vertebral body;
[0029]图9B是具有通过引导丝插入椎骨体内的一个或多个植入物的椎骨的另一个横截面顶视图;Fig. 9 B is another cross-sectional top view of a vertebra having one or more implants inserted into the vertebral body by a guide wire;
[0030]图10是图7椎骨的横截面侧视图,其中球囊膨胀以扩张植入物;[0030] FIG. 10 is a cross-sectional side view of the vertebra of FIG. 7 with the balloon inflated to expand the implant;
[0031]图11是图10椎骨的横截面侧视图,示出了椎骨体内的扩张后的植入物,且球囊已被移去;[0031] FIG. 11 is a cross-sectional side view of the vertebra of FIG. 10 showing the expanded implant in the vertebral body with the balloon removed;
[0032]图12是图11椎骨的横截面侧视图,示出了载有第二植入物的球囊的插入;[0032] FIG. 12 is a cross-sectional side view of the vertebra of FIG. 11 showing insertion of a balloon carrying a second implant;
[0033]图13是图12椎骨的横截面侧视图,示出了载有第二植入物的球囊的膨胀;[0033] FIG. 13 is a cross-sectional side view of the vertebra of FIG. 12 showing inflation of a balloon carrying a second implant;
[0034]图14是图13椎骨的横截面侧视图,示出了球囊的移除;[0034] FIG. 14 is a cross-sectional side view of the vertebra of FIG. 13 showing removal of the balloon;
[0035]图15是图14椎骨的横截面侧视图,示出了在扩张后的第二植入物的内部插入第三植入物;[0035] FIG. 15 is a cross-sectional side view of the vertebra of FIG. 14 showing insertion of a third implant inside the expanded second implant;
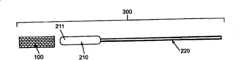
[0036]图16A-C是椎骨的横截面侧视图,示出了在插入可扩张植入物之前注入接合剂;16A-C are cross-sectional side views of a vertebra showing injection of cement prior to insertion of an expandable implant;
[0037]图17是椎骨的横截面侧视图,示出了用于注入接合剂的具有空腔的导管以及球囊植入物;[0037] FIG. 17 is a cross-sectional side view of a vertebra showing a catheter with a cavity and a balloon implant for injecting cement;
[0038]图18是椎骨的横截面侧视图,该椎骨具有扩张的植入物并且在移去球囊之后注入接合剂;[0038] FIG. 18 is a cross-sectional side view of a vertebra with the implant expanded and injected with cement after removing the balloon;
[0039]图19是椎骨的横截面侧视图,示出了处于扩张的植入物内部填充有接合剂的球囊;[0039] FIG. 19 is a cross-sectional side view of a vertebra showing the cement-filled balloon inside the expanded implant;
[0040]图20A和20B分别是在扩张之前和之后的可扩张植入物的侧视图;20A and 20B are side views of the expandable implant before and after expansion, respectively;
[0041]图21是可扩张植入物的另一实施例的侧视图;[0041] FIG. 21 is a side view of another embodiment of an expandable implant;
[0042]图22是可扩张植入物的另一实施例的侧视图;[0042] FIG. 22 is a side view of another embodiment of an expandable implant;
[0043]图23A和B是在扩张之前(左)和之后(右)的可扩张片状植入物的实施例的透视图;23A and B are perspective views of an embodiment of an expandable sheet implant before (left) and after (right) expansion;
[0044]图24A和B分别是在扩张之前和之后的植入物组件的截面端视图;Figure 24 A and B are respectively the sectional end view of the implant assembly before and after expansion;
[0045]图25A-C是植入物组件的另一实施例的横截面端视图;Figure 25 A-C is the cross-sectional end view of another embodiment of implant assembly;
[0046]图26是植入物组件的另一实施例的棘轮机构的端视图;Fig. 26 is the end view of the ratchet mechanism of another embodiment of implant assembly;
[0047]图27是根据本发明的另一实施例的螺旋状植入物的侧视图;[0047] FIG. 27 is a side view of a helical implant according to another embodiment of the present invention;
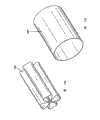
[0048]图28是插入椎骨体的图27螺旋植入物的横截面侧视图;[0048] FIG. 28 is a cross-sectional side view of the helical implant of FIG. 27 inserted into a vertebral body;
[0049]图29A是具有相反卷绕方式的两个螺旋植入物的侧视图;[0049] FIG. 29A is a side view of two helical implants with opposite winding patterns;
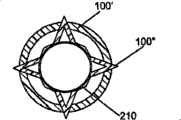
[0050]图29B是结合在一起的图29A螺旋植入物的侧视图;[0050] FIG. 29B is a side view of the helical implant of FIG. 29A joined together;
[0051]图30A-D是装有用于扩张外部植入物的可扩张塞的植入物组件的侧视图;Figure 30A-D is the side view of the implant assembly that is used for expanding the expandable plug of external implant housed;
[0052]图31A-C是示出了使用可扩张植入物组件另一实施例的横截面侧视图;31A-C is a cross-sectional side view illustrating the use of another embodiment of an expandable implant assembly;
[0053]图32是在椎骨体内的可扩张植入物组件的另一实施例的横截面侧视图;及[0053] FIG. 32 is a cross-sectional side view of another embodiment of an expandable implant assembly in a vertebral body; and
[0054]图33A和B是可扩张植入物在内部卷绕构件扩张之前(A)和之后(B)的透视图。[0054] FIGS. 33A and B are perspective views of an expandable implant before (A) and after (B) expansion of the inner coiled member.
具体实施方式Detailed ways
[0055]图4示出了可扩张植入物100在未扩张状态下的三维透视图。这样的植入物100可以插入椎骨体(未示出)或其它骨头内以修复骨头的损伤,例如脊椎压缩性骨折。在一些实施例中,使用球囊导管对脊柱前凸进行重构(例如,如前所述的脊柱后凸成形术),球囊导管载有一个或多个留在椎骨体的内部的可扩张植入物,并且防止在移去用于扩张植入物的球囊导管或其它器件之后的复位丧失。[0055] FIG. 4 shows a three-dimensional perspective view of
[0056]优选地,植入物是可扩张的并且可抵抗破坏力,优选地是例如约5N和约300N之间的力。在一些实施例中,植入物可以具有管的形式并且可以包括一个或多个部件。几个植入物可以彼此插入以获得稳定结构,该结构可以支承作用在椎骨体上的相互作用的压缩力。[0056] Preferably, the implant is expandable and resistant to destructive forces, preferably forces of between about 5N and about 300N, for example. In some embodiments, the implant may have the form of a tube and may comprise one or more components. Several implants can be inserted into each other to obtain a stable structure that can support the interacting compressive forces acting on the vertebral bodies.
[0057]植入物可以由生物相容性形状记忆合金、不锈钢、钴铬合金、钛或其合金、聚合物、磷酸三钙或具有期望特性的任何其它材料制成。在一些实施例中,例如,植入物可以被覆盖或涂覆上生物降解聚合物。[0057] The implant may be made of a biocompatible shape memory alloy, stainless steel, cobalt chromium, titanium or alloys thereof, polymers, tricalcium phosphate, or any other material having the desired properties. In some embodiments, for example, an implant can be covered or coated with a biodegradable polymer.
[0058]在包括形状记忆合金(例如镍钛锘)的实施例中,当植入物被加热到超过作用温度的温度时,植入物可以膨胀,例如由于形状记忆合金经受了马氏体状态(例如,处于较低温度)和奥氏体状态(例如,处于较高温度)之间的相变。例如,植入物内的形状记忆合金纤维的作用温度优选可以为约28℃和约36℃之间。或者,植入体物质在其被能量源(例如紫外线、超声辐射、无线电波、热、电场或磁场)激活时可以膨胀、收缩,或者以其它方式改变形状或结构。[0058] In embodiments comprising a shape memory alloy such as Nitinol, the implant may expand when the implant is heated to a temperature above the action temperature, for example due to the shape memory alloy undergoing a martensitic state (eg, at lower temperatures) and the austenitic state (eg, at higher temperatures). For example, the shape memory alloy fibers within the implant may preferably be exposed to temperatures between about 28°C and about 36°C. Alternatively, the implant substance may expand, contract, or otherwise change shape or configuration when it is activated by an energy source (eg, ultraviolet light, ultrasound radiation, radio waves, heat, electric or magnetic fields).
[0059]未膨胀的植入物可以具有期望的直径,优选适合通过插管的空腔并装入椎骨体内。例如,在一些实施例中,图4植入物100的直径在插入之前可以在约2mm和10mm之间。在膨胀状态下,植入物100的直径可以在约15mm和25mm之间。当然,在不脱离本发明范围的情况下可以使用其它尺寸。[0059] The unexpanded implant may have a desired diameter, preferably to fit through the lumen of the cannula and into the vertebral body. For example, in some embodiments, the diameter of the
[0060]图5至15描述了使用可扩张植入物100对断裂的椎骨体10进行复位和扩张的方法,例如对椎骨体10的终板进行复位并且在对脊柱前凸进行重构后保持该复位。所述方法和植入物可以用于对其它骨头进行复位和扩张。5 to 15 describe a method of reducing and expanding a fractured
[0061]特别地,图5示出了尺寸适于围绕球囊导管组件200或其它器件安装的可扩张植入物,其在此也可被称为用于对植入物施加扩张力的“扩张器件”。球囊导管组件200可以包括球囊210和连接到球囊210一端的导管轴220。设置在球囊210或其它扩张器件上的植入物100在此被称为球囊植入组件300。在一些实施例中,植入物100或其一部分可以与在血管成形术过程中用于保持血管开放的“支架”相似。支架、球囊、球囊支架组件、和/或球囊导管组件可以涂覆有粘合剂、抗生素、骨感应材料或骨传导材料。在扩张之后,使用者可以通过例如热、紫外线、超声辐射、无线电波、电或磁场等能源来激励粘合剂。[0061] In particular, FIG. 5 illustrates an expandable implant sized for installation around a
[0062]优选地,用作将植入物从它的第一插入尺寸扩张到其第二膨胀尺寸的扩张机构的球囊210至少是半约束的,以便球囊210能够在植入物的期待被扩张的区域上施加足够的力。[0062] Preferably, the
[0063]优选地,球囊210或扩张器件不像市场上的一些球囊导管(例如在脊柱后凸成形术中使用的球囊导管)那样是顺应的(弹性的)或半顺应的,在那些球囊中由于支架阻力因而认为球囊将环绕植入物扩张而不提供使支架扩张所需的力。例如,如果球囊的弹性过大,它就可能在支架的前后扩张,并且如果支架的端部插入球囊那么甚至可能爆炸。在这点上,优选球囊相对为非弹性,以便来自球囊的力可以被导入支架的期望区域。或者,与限制弹性扩张的外罩一起使用的相对弹性的球囊能够用作扩张器件。[0063] Preferably, the
[0064]可扩张支架优选为当它扩张到其第二尺寸时经受塑性变形,以便当球囊放气时它能还原到其第一插入尺寸,或在那附近允许使用者移去球囊,如果需要,可将膨胀的植入物留在椎骨体内。the expandable stent preferably undergoes plastic deformation when it expands to its second size, so that it can return to its first insertion size when the balloon is deflated, or thereabout to allow the user to remove the balloon, The expanded implant can be left in the vertebral body if desired.
[0065]图6示出了以锥弓根穿刺术(transpedicular approach)穿过椎骨体10钻出的进入孔11。这种进入孔11可以由钻头、套针或其它器械(未示出)穿过椎骨10的外层骨皮质来形成。在穿过椎骨体10的一个或多个椎弓根形成进入孔之后,为了提供可扩张植入物100的通道可以将插管20插入到每个进入孔11内。[0065] FIG. 6 shows an
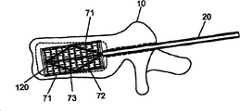
[0066]图7示出了通过单向或双向锥弓根穿刺术引入的球囊导管组件200,可扩张植入物100放置在导管轴220的可扩张球囊端211上。导管220可以与引导器件30(例如图8和9B所示的引导丝)一起插入。引导器件30可以用于将植入物100定位在椎骨体10内的期望位置或方向上。例如,图9A和9B示出了球囊植入组件300(支架器件)的不同的可能位置。插管和引导丝二者可以一同使用或分别使用,以引导球囊植入组件300。或者,插管或引导丝均不能用于对植入物定位,可以通过医师从患者外侧操作导管轴220将球囊植入组件300向下插入到形成在骨头内的通道中。[0066] FIG. 7 shows a
[0067]图10示出了通过(例如利用射线可透的流体)使球囊210膨胀来利用可扩张植入物100对椎骨体10进行复位和扩张的示例性方法,其对围绕的植入物100施加了径向力,从而使植入物100扩张以填充椎骨体10内的空间并且恢复椎骨体10的高度。[0067] FIG. 10 shows an exemplary method of reducing and expanding a
[0068]例如图11所示,在植入物100扩张到期望直径之后,球囊210可以瘪掉并从插管20移去。植入物100优选被构造为保持其膨胀后的尺寸并且即使在移去球囊210之后仍保持椎骨体10的高度。或者,球囊210和植入物可以与导管轴220分开,以便将球囊植入组件300保留在骨头中。[0068]
[0069]如图12所示,第二可扩张球囊植入组件300’(且包括球囊导管组件200’,且所述球囊导管组件200’包括导管轴220’和植入物100B)可以被插入椎骨体10内,例如在第一扩张植入物100A的空腔内以提供进一步的支撑。在插入之后,内部球囊或其它扩张器件可以如图13所示地膨胀或以其他方式扩张以使第二植入物100B扩张,例如,以便使其与第一植入物100A的内表面接合。第二植入物100B可以被同轴地放置在第一植入物100A内以增加抵抗椎骨体10骨折的阻力。如上文图11的情况一样,在插入第二植入物100B之后,内部扩张器件可以如图14和15所示地移去,图14和15描述了插入与第一和第二植入物同轴地设置的第三球囊植入组件300”(其包括球囊导管组件200”,所述球囊导管组件200”包括导管轴220”和植入物100C)。可以在第一植入物内插入任意期望数目的植入物。[0069] As shown in FIG. 12, the second expandable balloon implant assembly 300' (and including the balloon catheter assembly 200', and the balloon catheter assembly 200' includes the catheter shaft 220' and the
[0070]可以加入接合剂、骨碎片或其它填充物60以进一步对椎骨体10进行扩张,并且将一个或多个植入物(支架)锁定就位。填充物60可以进一步包括抗生素、骨形态发生蛋白(BMP)、生长激素等。可以在插入植入物之前、插入过程中或插入以后将这种接合剂或其它填充物60置入椎骨体10内。例如,在一些实施例中,如图16A所示,可以在插入植入物或植入物扩张之前将接合剂或其它填充物60注入椎骨体10。图16B描述在椎骨体10内的扩张球囊植入组件300,以便接合剂或其它填充物60围绕球囊210和植入物分散。图16C描述了在已经移去球囊210后,接合剂或其它填充物60围绕扩张植入物100分散。在一些实施例中,在植入物扩张且移去球囊导管组件之后接合剂或其它填充物60可以注入植入物的内部。在其它实施例中,接合剂或其它填充物60可以在植入物的扩张过程中注入椎骨空间。在另一些实施例中,接合剂或其它填充物60可以被注入球囊导管组件200以扩张球囊从而扩张植入物。其后,可以从球囊上卸下导管轴220,而装有填充物的球囊以及扩张的植入物可以留在骨头内。在导管轴220从球囊卸下之后,导管轴220的通道225可以用于在骨头内注入附加填充物60或不同填充物60’,并且可用于包围扩张后的植入物或者有助于稳定扩张后的植入物。[0070] Cement, bone chips or
[0071]在一些实施例中,导管可以具有多个空腔,例如图17所示的两个空腔221、222,可以用于提供进入椎骨体10的入口和提供将其它材料注入骨头的通道。接合剂或其它填充物60可以通过空腔221注入。通过空腔221注入的材料可以接触可扩张植入物、球囊210的外表面或者与二者均接触。材料可以在球囊扩张之前通过第二空腔222注入,以便材料可以在球囊210或植入物扩张或二者均扩张时散布在骨头空腔内。[0071] In some embodiments, the catheter can have multiple cavities, such as two
[0072]在球囊210、植入物或球囊植入物组合体的外部,或在骨头空腔内分散材料在植入物在骨头内扩张时可以有助于其稳定。附加地或替代性地,诸如生物可容性聚合物的材料可以通过空腔221注入骨头空腔,或置于球囊210的外表面、植入物100或其二者之上,并且在球囊210扩张时在骨头空腔内分散以形成封闭物或袋从而防止任何球囊填充材料从骨头空腔内流失。生物可容性聚合物可以作用于密封另外的多孔骨松质以防止球囊填充物材料的任何泄露。在移去球囊200之后(但植入物仍留在骨头内(图18)),例如还可以使用注射器40注入接合剂或其它填充物60。在另一个实施例中,如图19所示,球囊210可以直接由接合剂或其它填充物60填充。例如,这种球囊210可以在椎骨体10内扩张直至球囊210爆裂。或者,球囊210可以由接合剂或其它填充物60填充和扩张,并且随后使扩张的球囊210与导管轴220分离,以便球囊210留在患者体内。球囊可以由可生物吸收材料形成以便其能够随时间被人体吸收。同样,如等人的公开WO 03/099171A1(PCT/EP03/05407)所描述的,球囊210也可以被打孔。球囊210可以由将在现场硬化的可硬化接合剂来填充。在接合剂在球囊210内硬化之后,可以将球囊210从导管轴220分开或卸下。[0072] Dispersing material on the exterior of the
[0073]图20A至33描述了不同结构和/或图案的植入物或支架,包括具有穿孔的连续圆柱体。重要的是扩张的植入物抵抗由其插入的环境所施加的压力。例如,如果放置在椎骨体内,那么可扩张植入物优选能够承受在插入植入物后患者常规运动过程中施加在椎骨体上的力。植入物优选在扩张之后不会显著地变短(例如,需要使L1-L0之间的差值减小)。图20至22示出了可扩张植入物可以采用的多种网孔状图案。在图20A中,未扩张的植入物的网孔状图案可以形成角α。当植入物扩张时(图20B),网孔状图案形成角β,其中β>α。图23A和B示出了用于插入的由折叠的金属片形成的可扩张植入物。图23A示出了扩张前的植入物400,图23B示出了扩张之后的植入物400’。在优选实施例中,这种折叠的金属片插入器件由形状记忆合金(例如镍钛锘)制成。[0073] FIGS. 20A to 33 depict implants or scaffolds of different configurations and/or patterns, including continuous cylinders with perforations. It is important that the expanded implant resist the pressure exerted by the environment into which it is inserted. For example, if placed within a vertebral body, the expandable implant is preferably capable of withstanding the forces exerted on the vertebral body during routine movement of the patient after insertion of the implant. The implant preferably does not become significantly shorter after expansion (eg, a reduction in the difference between L1-L0 is required). Figures 20 to 22 illustrate various mesh patterns that expandable implants can employ. In FIG. 20A, the mesh-like pattern of the unexpanded implant may form an angle a. When the implant is expanded (FIG. 20B), the mesh-like pattern forms an angle β, where β>α. Figures 23A and B show an expandable implant formed from folded sheet metal for insertion. Figure 23A shows the
[0074]本领域技术人员将意识到能够按以下方式选择植入物的几何形状,即,当它们扩张时几个植入物能够彼此楔入。因此,植入物可以抵抗当它们在其膨胀状态下时的较高压缩力。例如,图24至26示出了植入物/支架(100’和100”)的截面如何被构造为使植入物彼此楔入的不同原理。在图26中,植入物A(内部植入物)和植入物B(外部植入物)的形状可以被设计成与锥体相似,并带有锯齿状(齿形)表面。通过将植入物B从“x1”移动到“x2”,外径可以从“h1”扩张到“h3”。由于存在锯齿表面,因此外部植入物B可保持扩张后的形状。[0074] Those skilled in the art will appreciate that the geometry of the implants can be chosen in such a way that several implants can wedge into each other as they expand. Thus, the implants can resist higher compressive forces when they are in their expanded state. For example, Figures 24 to 26 show different principles of how the cross-sections of implants/stents (100' and 100") can be configured to wedge the implants into each other. In Figure 26, implant A (internal implant) and Implant B (external implant) can be shaped like a cone with a serrated (toothed) surface. By moving Implant B from "x1" to "x2 ”, the outer diameter can expand from “h1” to “h3”. The outer implant B maintains the expanded shape due to the serrated surface.
[0075]如图27至29所示,一个或多个植入物可以具有螺旋结构。几个螺旋植入物可以彼此旋入,以便实现稳定的植入物。图27和28特别描述了具有相同方向螺旋线的螺旋植入物。图29A和B示出了具有不同方向螺旋线的螺旋植入物。不论螺旋线方向如何,螺旋植入物都被构造为彼此旋入,从而加强植入物。[0075] As shown in FIGS. 27-29, one or more implants may have a helical configuration. Several helical implants can be screwed into each other in order to achieve a stable implant. Figures 27 and 28 specifically depict helical implants with helices in the same direction. Figures 29A and B show helical implants with differently oriented helices. Regardless of the direction of the helix, the helical implants are configured to screw into each other, thereby strengthening the implant.
[0076[图30-33示出用于提供扩张植入物100的扩张力的球囊210的替代物。例如,图30A-D示出了植入物100的另一个实施例,其使用可扩张塞50来代替球囊210以扩张植入物100。塞50可以由诸如螺钉(图30A和C)或L手柄(图30B和D)的扩张机构51组成。其它结构的扩张机构51是可预期的。塞50也可以具有远端52、近端53和连接远端52与近端53的多个扩张支架54。扩张机构51拧在远端52和近端53二者上,以致在使用者旋转扩张机构51时,远端52朝向近端53移动,导致多个扩张支架54向外扩张从而扩张植入物110。使用者继续旋转扩张机构51直至植入物110达到其期望直径。如图30A和B所示,植入物110和塞50可以按未扩张状态插入椎骨体(未示出)内。之后,如图30C和D所示,植入物110能够通过旋转塞50的扩张机构51(例如,螺钉)而扩张。在植入物110扩张之后,可以移去塞50。在其它实施例中,塞50可以留在骨头内。[0076] FIGS. 30-33 illustrate an alternative to the
[0077]图31A-C示出了用于扩张植入物的另一种机构。特别地,可以使用起重机构(jack mechanism)70来扩张植入物,例如以与汽车起重机相似的方式工作。如图31A所示,植入物组件500可以通过插管20插入椎骨体10内。植入物组件500可以由植入物120和起重机构70组成。起重机构70可以由多个扩张构件71(优选为至少两个扩张构件71),多个对角拉条72和驱动机构73组成。驱动机构73的操作使得多个对角拉条72从驱动机构73向外延伸,同样地从驱动机构73向外推动多个扩张构件71。扩张构件71的向外运动使得植入物120扩张(图31B)直至达到植入物120的期望直径。在植入物120扩张到期望直径之后,移去驱动机构留下扩张构件71和对角拉条72(图31C)。[0077] FIGS. 31A-C illustrate another mechanism for expanding an implant. In particular, a jack mechanism 70 may be used to expand the implant, for example working in a similar manner to a truck crane. As shown in FIG. 31A ,
[0078]图32示出了使用卷筒联合体80来扩张植入物130因而扩张椎骨体的高度。例如,在美国专利申请第11/471,169号中更详细地说明了这种卷筒联合体80,在此并入其全部内容以供参考。图33示出了使用金属片卷90扩张植入物140的方法,例如同样在美国专利申请第11/471,169号对其进行了说明。[0078] FIG. 32 illustrates the use of the
[0079]尽管在此所述的装置和方法迄今都是针对在脊椎压缩性骨折和脊椎弯曲的变形情况下对脊椎进行复位和扩张进行描述的中,多种其它用途和方法是可预期的。例如,在一些实施例中,包括可扩张支架的一个或多个植入物可以用于对其它受损骨头区域(诸如骨折或软弱的近股骨)进行复位和/或扩张。[0079] Although the devices and methods described herein have heretofore been described for the reduction and expansion of the spine in the context of vertebral compression fractures and deformities of spinal curvature, a variety of other uses and methods are contemplated. For example, in some embodiments, one or more implants comprising expandable scaffolds may be used to reduce and/or expand otherwise damaged bone regions, such as fractures or weakened proximal femurs.
[0080]例如,在这样的实施例中,一个或多个植入物可以通过例如插管或其它引导器插入股骨的顶部,或用于胫骨平台骨折的复位。一旦被插入,植入物可以在股骨的顶部内对材料进行扩张和压缩并且提供牢固的支撑以对该顶部进行扩张。在一些实施例中,植入物可以包括形状记忆合金并且在插入之后(例如,在加热到激活温度以上的温度)扩张或改变其结构。接合剂或其它填充物也可以用于辅助扩张。在其它实施例中,除所述一个或多个植入物以外或代替所述一个或多个植入物,诸如螺钉或其它器件的另一个植入物可以被插入。[0080] For example, in such embodiments, one or more implants may be inserted into the crest of the femur, for example, through a cannula or other guide, or for reduction of a tibial plateau fracture. Once inserted, the implant can expand and compress material within the apex of the femur and provide firm support to expand the apex. In some embodiments, the implant may comprise a shape memory alloy and expand or change its structure after insertion (eg, upon heating to a temperature above the activation temperature). Cement or other fillers may also be used to assist in dilation. In other embodiments, another implant, such as a screw or other device, may be inserted in addition to or instead of the one or more implants.
[0081]在一些实施例中,此处说明的植入物和方法可以与其它装置和方法结合使用以恢复脊柱前凸,对椎骨体进行扩张。例如,一个或多个可扩张植入物可以与已知程序结合使用,例如脊柱后凸球囊,其可以用于开始对椎骨体进行复位和/或在体内建立植入物的空间。在其它实施例中,此处说明的一个或多个植入物可以与其它工具或器件结合使用,例如,辅助将椎骨或其它骨头操作或固定在期望位置上的外部固定装置。[0081] In some embodiments, the implants and methods described herein may be used in conjunction with other devices and methods to restore lordosis and expand the vertebral bodies. For example, one or more expandable implants may be used in conjunction with known procedures, such as a kyphotic balloon, which may be used to initiate reduction of the vertebral body and/or create space for the implant in the body. In other embodiments, one or more of the implants described herein may be used in conjunction with other tools or devices, for example, external fixation devices that assist in manipulating or securing vertebrae or other bones in a desired position.
[0082]在另一个实施例中,工具包可以包括根据本发明的元件的多种组合。例如,工具包可以包括插管和一个或多个可扩张植入物。工具包可以额外包括一个或多个球囊,球囊导管或用于对一个或多个植入物施加扩张力的其它可扩张构件。工具包可以额外包括用于将接合剂或其它填充物注射入椎骨体,或进入球囊或球囊导管的注射器或其它装置。任选地,一个或多个其它植入物或器件可以包括在工具包中。本领域技术人员将理解的是,可以制成器件、元件和组件的多种其它组合并且它们都落在本发明的范围内。[0082] In another embodiment, a kit may include various combinations of elements according to the invention. For example, a kit may include a cannula and one or more expandable implants. The kit may additionally include one or more balloons, balloon catheters, or other expandable members for applying an expanding force to the one or more implants. The kit may additionally include a syringe or other device for injecting cement or other filler into the vertebral body, or into a balloon or balloon catheter. Optionally, one or more other implants or devices may be included in the kit. Those skilled in the art will appreciate that various other combinations of devices, elements and components can be made and are within the scope of the invention.
[0083]在其它实施例中,用于减轻与脊柱相关不适的多种微创植入物和方法可以使用在此说明的具有一个或多个特征的可扩张植入物。例如,包括形状记忆合金的可扩张植入物或其它植入物可以在相邻椎骨的棘状突起之间植入,植入物可以扩张或改变其结构以转移棘状突起并且缓解例如由椎管狭窄、小关节病等引起的疼痛或其它问题。例如,可以将此处说明的扩张系统附加于或代替美国专利公开第2004/018128号和Zucherman等人的美国专利申请第6,419,676号中说明的可扩张内棘状突起装置和方法来使用。[0083] In other embodiments, various minimally invasive implants and methods for alleviating spine-related discomfort may use the expandable implants described herein having one or more features. For example, expandable implants comprising shape memory alloys or other implants can be implanted between the spinous processes of adjacent vertebrae, and the implant can expand or change its configuration to shift the spinous processes and relieve, for example, Pain or other problems caused by tube stenosis, facet joint disease, etc. For example, the expansion systems described herein may be used in addition to or in place of the expandable internal spinous protrusion devices and methods described in US Patent Publication No. 2004/018128 and US Patent Application No. 6,419,676 to Zucherman et al.
[0084]尽管上述说明和附图示出了本发明的优选实施例,但是应该理解,在不脱离所附权利要求限定的本发明精神和范围的情况下,可以作出多种添加、改进和替换。特别地,在不脱离本发明精神和实质特征的情况下,对本领域技术人员而言显而易见的是,可以按其它特定形式、结构、设置、比例并且以其它元件、材料和组分来实施本发明。因此,目前公开的实施例在各方面应被认为是说明性的而非限制性的,本发明的范围由所附权利要求表明,并不局限于上述说明。[0084] Although the foregoing description and accompanying drawings show preferred embodiments of the present invention, it should be understood that various additions, improvements and substitutions may be made without departing from the spirit and scope of the invention as defined by the appended claims . In particular, without departing from the spirit and essential characteristics of the present invention, it will be apparent to those skilled in the art that the present invention may be implemented in other specific forms, structures, arrangements, proportions and with other elements, materials and components . Accordingly, the presently disclosed embodiments are to be considered in all respects as illustrative and not restrictive, with the scope of the invention being indicated by the appended claims and not limited to the foregoing description.
[0085]在此以引用方式将本文引用的所有参考文件的全部内容并入本文,且用于所有目的,以达到如同对每个单独的公开或专利或专利申请进行了特别的、独立的说明,从而通过引用方式将其全部内容并入本文用于所有目的的效果。[0085] All references cited herein are hereby incorporated by reference in their entirety for all purposes as if each individual publication or patent or patent application were specifically and independently indicated , which is hereby incorporated by reference in its entirety for all purposes.
Claims (22)
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US74837705P | 2005-12-08 | 2005-12-08 | |
| US60/748,377 | 2005-12-08 | ||
| US60/753,782 | 2005-12-23 | ||
| US60/789,956 | 2006-04-05 | ||
| US11/471,169 | 2006-06-19 | ||
| US11/523,202 | 2006-09-18 | ||
| US11/527,280 | 2006-09-25 | ||
| US11/546,579 | 2006-10-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN101336094Atrue CN101336094A (en) | 2008-12-31 |
Family
ID=40198290
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA2006800521430APendingCN101336094A (en) | 2005-12-08 | 2006-12-08 | Apparatus and method for treating bone |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN101336094A (en) |
| ZA (1) | ZA200804725B (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102028530A (en)* | 2010-12-20 | 2011-04-27 | 南京市第一医院 | Minimally invasive bone trabecula metal vertebral stent |
| CN103054638A (en)* | 2013-01-05 | 2013-04-24 | 曾融生 | Memory alloy distractor |
| CN104173100A (en)* | 2014-08-14 | 2014-12-03 | 黄哲宇 | Bone cement leakage preventing combined implanting device for spine arthroplasty |
| CN106618714A (en)* | 2015-11-02 | 2017-05-10 | 山东冠龙医疗用品有限公司 | Filling apparatus for injecting bone filling material |
| CN107320173A (en)* | 2017-06-13 | 2017-11-07 | 翎秀生物科技(上海)有限公司 | Vertebral body augmentation formation system and method |
| CN110300557A (en)* | 2016-12-09 | 2019-10-01 | 波士顿科学国际有限公司 | Guide with expansivity energy |
| CN111729180A (en)* | 2020-05-28 | 2020-10-02 | 广州新诚生物科技有限公司 | Vertebral body expansion device and using method |
| CN112294415A (en)* | 2019-07-31 | 2021-02-02 | 中央医疗器材股份有限公司 | Vertebral fixing device |
| CN112334084A (en)* | 2018-06-15 | 2021-02-05 | 尼奥医疗公司 | Vertebral pedicle marker |
| CN113631110A (en)* | 2019-03-19 | 2021-11-09 | Mt奥塞有限责任公司 | Particles made of biocompatible metallic material for vertebroplasty |
| CN114652376A (en)* | 2020-12-23 | 2022-06-24 | 上海中医药大学附属曙光医院 | Bone-implantable full suture bag anchor implantation instrument |
- 2006
- 2006-12-08ZAZA200804725Apatent/ZA200804725B/enunknown
- 2006-12-08CNCNA2006800521430Apatent/CN101336094A/enactivePending
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102028530A (en)* | 2010-12-20 | 2011-04-27 | 南京市第一医院 | Minimally invasive bone trabecula metal vertebral stent |
| CN103054638A (en)* | 2013-01-05 | 2013-04-24 | 曾融生 | Memory alloy distractor |
| CN103054638B (en)* | 2013-01-05 | 2015-11-18 | 兰州西脉记忆合金股份有限公司 | Memory alloy pull-stretch device |
| CN104173100A (en)* | 2014-08-14 | 2014-12-03 | 黄哲宇 | Bone cement leakage preventing combined implanting device for spine arthroplasty |
| CN106618714A (en)* | 2015-11-02 | 2017-05-10 | 山东冠龙医疗用品有限公司 | Filling apparatus for injecting bone filling material |
| CN110300557A (en)* | 2016-12-09 | 2019-10-01 | 波士顿科学国际有限公司 | Guide with expansivity energy |
| CN110300557B (en)* | 2016-12-09 | 2022-06-28 | 波士顿科学国际有限公司 | Introducer with expandable properties |
| CN107320173A (en)* | 2017-06-13 | 2017-11-07 | 翎秀生物科技(上海)有限公司 | Vertebral body augmentation formation system and method |
| CN107320173B (en)* | 2017-06-13 | 2023-12-08 | 依奈德医疗技术(上海)有限公司 | Vertebral body expansion shaping systems and methods |
| CN112334084A (en)* | 2018-06-15 | 2021-02-05 | 尼奥医疗公司 | Vertebral pedicle marker |
| CN113631110A (en)* | 2019-03-19 | 2021-11-09 | Mt奥塞有限责任公司 | Particles made of biocompatible metallic material for vertebroplasty |
| CN112294415A (en)* | 2019-07-31 | 2021-02-02 | 中央医疗器材股份有限公司 | Vertebral fixing device |
| CN112294415B (en)* | 2019-07-31 | 2022-07-29 | 台湾地区“中央医疗器材股份有限公司” | Vertebral fixation device |
| CN111729180A (en)* | 2020-05-28 | 2020-10-02 | 广州新诚生物科技有限公司 | Vertebral body expansion device and using method |
| CN114652376A (en)* | 2020-12-23 | 2022-06-24 | 上海中医药大学附属曙光医院 | Bone-implantable full suture bag anchor implantation instrument |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA200804725B (en) | 2009-03-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20070093899A1 (en) | Apparatus and methods for treating bone | |
| EP1956990A2 (en) | Apparatus and methods for treating bone | |
| CN101336094A (en) | Apparatus and method for treating bone | |
| US8663294B2 (en) | Apparatus and methods for vertebral augmentation using linked expandable bodies | |
| US9956085B2 (en) | Flexible elongated chain implant and method of supporting body tissue with same | |
| JP5366966B2 (en) | Porous containment device and related method for stabilizing vertebral compression fractures | |
| CN102548511B (en) | Method and apparatus for augmenting bone | |
| EP1011464B1 (en) | Systems for percutaneous bone and spinal stabilization, fixation and repair | |
| US8080061B2 (en) | Apparatus and methods for treating bone | |
| US20080009868A1 (en) | Device and method for treating compression fractures | |
| WO2005048856A1 (en) | Expandable implant for treating fractured and/or collapsed bone | |
| WO2010075022A2 (en) | Methods and devices for expanding a spinal canal using balloons | |
| JP2009504332A (en) | Spinal distractor | |
| WO2007076374A2 (en) | Expandable support device and method of using the same | |
| US20110190776A1 (en) | Interosteal and intramedullary implants and method of implanting same | |
| US20160338661A1 (en) | Fracture fragment mobility testing for vertebral body procedures |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | Open date:20081231 |