CN101324579A - A magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and its application method - Google Patents
A magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and its application methodDownload PDFInfo
- Publication number
- CN101324579A CN101324579ACNA2007101188855ACN200710118885ACN101324579ACN 101324579 ACN101324579 ACN 101324579ACN A2007101188855 ACNA2007101188855 ACN A2007101188855ACN 200710118885 ACN200710118885 ACN 200710118885ACN 101324579 ACN101324579 ACN 101324579A
- Authority
- CN
- China
- Prior art keywords
- antibody
- fitc
- solution
- magnetic microparticle
- reagent kit
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于生物技术领域,涉及一种检测糖类抗原的磁性微粒子化学发光酶免疫分析试剂盒及其使用方法。The invention belongs to the field of biotechnology, and relates to a magnetic particle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and a use method thereof.
背景技术Background technique
癌症对人类的生存健康构成了严重威胁。目前肿瘤治疗的关键在于早期发现、早期治疗,这已是人们的共识。但是目前医院诊断早期肿瘤,主要是靠影像学和细胞病理学诊断技术,一般都是在具有明显的占位性病变和临床症状后才得以确诊,但这已不是癌变的最早期。随着肿瘤早期诊断进入蛋白质时代,以血液为代表的体液蛋白质成为肿瘤标志研究领域中的热点。越来越多肿瘤标志物的发现对建立发展快速、高灵敏、高特异肿瘤标志物的新型检测技术提出了更高的要求。Cancer poses a serious threat to human survival and health. At present, the key to tumor treatment lies in early detection and early treatment, which has become a consensus. However, at present, hospitals diagnose early-stage tumors mainly by imaging and cytopathological diagnostic techniques. Generally, they are diagnosed only after they have obvious space-occupying lesions and clinical symptoms, but this is not the earliest stage of canceration. With the early diagnosis of tumors entering the protein era, body fluid proteins represented by blood have become a hot spot in the field of tumor marker research. The discovery of more and more tumor markers has put forward higher requirements for the establishment of new detection technologies for rapid development, high sensitivity, and high specificity of tumor markers.
糖类抗原是发展快应用广的一类肿瘤容量标志物,它包括糖蛋白与糖脂,常表现为粘蛋白形式。其抗原决定簇可定位在糖链上或者在蛋白核上。根据糖基表位的不同和抗原表位大小的差异,一般可分为糖类抗原72-4(carbonhydrate antigen 72-4,CA72-4)、糖类抗原50(carbonhydrate antigen 50,CA50)、糖类抗原19-9(carbonhydrate antigen 19-9,CA19-9)、糖类抗原242(carbonhydrate antigen 242,CA242)、糖类抗原15-3(carbonhydrate antigen 15-3,CA15-3)、糖类抗原27-29(carbonhydrate antigen 27-29,CA27-29)和糖类抗原125(carbonhydrate antigen 125,CA125)。目前对这些标志物进行单检或联检可为疾病的前期筛查、临床诊断、疗效监控、癌细胞转移以及愈后复发情况提供参考依据。Carbohydrate antigens are a class of tumor volume markers that develop rapidly and are widely used. They include glycoproteins and glycolipids, often in the form of mucin. Its antigenic determinant can be located on the sugar chain or on the protein nucleus. According to the different glycosyl epitopes and the differences in the size of antigenic epitopes, they can generally be divided into carbohydrate antigen 72-4 (carbonhydrate antigen 72-4, CA72-4), carbohydrate antigen 50 (carbonhydrate antigen 50, CA50), carbohydrate Carbohydrate antigen 19-9 (carbonhydrate antigen 19-9, CA19-9), carbohydrate antigen 242 (carbonhydrate antigen 242, CA242), carbohydrate antigen 15-3 (carbonhydrate antigen 15-3, CA15-3), carbohydrate antigen 27-29 (carbonhydrate antigen 27-29, CA27-29) and carbohydrate antigen 125 (carbonhydrate antigen 125, CA125). At present, the single or combined detection of these markers can provide a reference for early screening of the disease, clinical diagnosis, efficacy monitoring, cancer cell metastasis, and recurrence after recovery.
目前用于检测糖类抗原的免疫方法除了有酶免疫测定(enzymeimmunoassay,EIA)、放射免疫测定(Radio immunoassay,RIA)、免疫荧光测定(fluoroimmunoassay,FIA)、放射免疫显影(radioimmunoimaging,RII)以及抗体芯片(antibody microarrays)技术之外,化学发光免疫分析(Chemiluminescence immunoassay,CLIA)技术因其高灵敏、高特异、快速、高通量等特点得到越来越广泛的应用。In addition to enzyme immunoassay (enzymeimmunoassay, EIA), radioimmunoassay (Radio immunoassay, RIA), immunofluorescence assay (fluoroimmunoassay, FIA), radioimmunoimaging (radioimmunoimaging, RII) and antibody In addition to the chip (antibody microarrays) technology, Chemiluminescence immunoassay (CLIA) technology has been more and more widely used because of its high sensitivity, high specificity, rapidity, and high throughput.
在实际的免疫检测中,由于待测样品中所含的杂质成分较多,一定程度上影响了检测灵敏度和准确性,所以从复杂的背景中快速分离、纯化出目的待测物,是临床检验工作者面临的难题之一。免疫磁性微粒子技术是利用高分子材料合成一定粒度大小的磁性固相微粒子作载体,以物理吸附、化学偶联等方法包被上具有特异性亲和力的各种免疫活性物质(抗原或抗体),使其致敏为免疫磁性粒子,具有分离速度快、效率高、可重复性好;操作简单、不需要昂贵的仪器设备;不影响被分离细胞或其它生物材料的生物学性状和功能等特点,在外加磁场作用下定向运动,使得某些特殊成分得以分离、浓集或纯化。作为用于CLIA检测技术的一种通用固相分离方法,磁性微粒子可大大提高有效包被量从而节省原料,同时可拓宽检测的线性范围从而有效避免弯钩效应的发生,必将促进CLIA技术在临床检验/检测中的应用与发展。In the actual immunoassay, due to the large amount of impurities contained in the sample to be tested, which affects the detection sensitivity and accuracy to a certain extent, it is a clinical test to quickly separate and purify the target analyte from the complex background. One of the problems workers face. Immunomagnetic microparticle technology uses polymer materials to synthesize magnetic solid-phase microparticles of a certain size as a carrier, and coats various immunoactive substances (antigens or antibodies) with specific affinity by means of physical adsorption and chemical coupling. Its sensitization is immunomagnetic particles, which have the characteristics of fast separation speed, high efficiency and good repeatability; simple operation, no need for expensive equipment; it does not affect the biological properties and functions of separated cells or other biological materials. Directional movement under the action of an external magnetic field enables some special components to be separated, concentrated or purified. As a general solid phase separation method for CLIA detection technology, magnetic microparticles can greatly increase the effective coating amount to save raw materials, and at the same time can broaden the detection linear range to effectively avoid the occurrence of the hook effect, which will definitely promote the development of CLIA technology. Applications and developments in clinical testing/testing.
发明内容Contents of the invention
本发明的目的是提供一种检测糖类抗原的磁性微粒子化学发光酶免疫分析方法及其专用试剂盒。即采用高特异性单克隆抗体及包被抗异硫氰酸荧光素(fluorescein-iso-thiocyanate,FITC)抗体的磁性微粒子固相分离技术,利用FITC和酶分别标记两株单克隆抗体,在液相中形成免疫夹心复合物,然后加入包被抗FITC抗体的磁性微粒子分离剂,进行洗涤分离,并在管式化学发光分析仪中进行测定。The purpose of the present invention is to provide a magnetic particle chemiluminescent enzyme immunoassay method for detecting carbohydrate antigens and a special kit thereof. That is, using highly specific monoclonal antibodies and magnetic microparticle solid-phase separation technology coated with anti-fluorescein-isothiocyanate (FITC) antibodies, using FITC and enzymes to label two monoclonal antibodies respectively, in liquid The immune sandwich complex is formed in the phase, and then the magnetic microparticle separation agent coated with anti-FITC antibody is added for washing and separation, and then measured in a tube chemiluminescence analyzer.
本发明所提供的检测糖类抗原的磁性微粒子化学发光免疫分析试剂盒,包括包被有抗FITC抗体的磁性微粒子;标记FITC的糖类抗原单克隆抗体及标记酶的糖类抗原单克隆抗体混合作为标记物液;糖类抗原标准品溶液;浓缩洗涤液;发光底物液。The magnetic microparticle chemiluminescence immunoassay kit for detecting carbohydrate antigen provided by the present invention includes magnetic microparticles coated with anti-FITC antibody; carbohydrate antigen monoclonal antibody labeled with FITC and carbohydrate antigen monoclonal antibody labeled with enzyme are mixed As marker solution; carbohydrate antigen standard solution; concentrated washing solution; luminescent substrate solution.
所述包被抗FITC抗体的磁性微粒子为粒径为2-3μm、使用浓度为5-10mg/mL、内核为四氧化三铁(Fe3O4)、表面包裹带有活性基团如氨基(NH2-)、羧基(-COOH)的聚合物;所述抗FITC抗体的包被是通过戊二醛两步法将磁性微粒子与抗FITC抗体进行偶联,并以含有0.5%牛血清白蛋白(bovine serum albumin,BSA)、1%酪蛋白的pH为7.2,0.02mol/L的磷酸缓冲液进行封闭完成。The magnetic microparticles coated with anti-FITC antibodies have a particle size of 2-3 μm, a concentration of 5-10 mg/mL, an inner core of ferric oxide (Fe3O4), and surface coatings with active groups such as amino groups (NH2-) , carboxyl (-COOH) polymer; the coating of the anti-FITC antibody is to couple the magnetic microparticles with the anti-FITC antibody by glutaraldehyde two-step method, and to contain 0.5% bovine serum albumin (bovine serum albumin , BSA), 1% casein with a pH of 7.2, and 0.02 mol/L phosphate buffer for blocking.
所述FITC分子与糖类抗原单克隆抗体的偶联物是以常见的碱性液标记法对抗体进行标记。所述标记抗体均为捕获抗体,包括:抗CA72-4单克隆抗体、抗CA50单克隆抗体、抗CA19-9单克隆抗体、抗CA242单克隆抗体、抗CA15-3单克隆抗体、抗CA27-29单克隆抗体和抗CA125单克隆抗体。FITC标记物形式可为冻干粉、浓缩液和工作液;所述FITC标记抗体液以20%小牛血清稀释存放。The conjugate of the FITC molecule and the carbohydrate antigen monoclonal antibody is labeled by a common alkaline solution labeling method. The labeled antibodies are capture antibodies, including: anti-CA72-4 monoclonal antibody, anti-CA50 monoclonal antibody, anti-CA19-9 monoclonal antibody, anti-CA242 monoclonal antibody, anti-CA15-3 monoclonal antibody, anti-CA27- 29 mAb and anti-CA125 mAb. The form of the FITC marker can be freeze-dried powder, concentrated solution and working solution; the FITC-labeled antibody solution is diluted with 20% calf serum for storage.
所述酶标记抗体的标记酶为辣根过氧化物酶或碱性磷酸酶;酶的标记可采用现有技术中的多种方法如戊二醛法或过碘酸钠法,若标记酶为碱性磷酸酶,则优选为戊二醛法,若标记酶为辣根过氧化物酶,则优选为过碘酸钠法。酶标记物形式可为冻干粉、浓缩液和工作液;所述标记抗体均为配对抗体,包括:抗CA72-4单克隆抗体、抗CA50单克隆抗体、抗CA19-9单克隆抗体、抗CA242单克隆抗体、抗CA15-3单克隆抗体、抗CA27-29单克隆抗体和抗CA125单克隆抗体。所述酶标记物工作液所用的稀释液为含有0.05%甘油、0.2g/L的硫柳汞防腐剂溶液(可防止放入-20℃的酶标记物冻结,并保持酶标记物的生物活性)。The labeling enzyme of the enzyme-labeled antibody is horseradish peroxidase or alkaline phosphatase; the labeling of the enzyme can adopt various methods in the prior art such as glutaraldehyde method or sodium periodate method, if the labeling enzyme is Alkaline phosphatase is preferably the glutaraldehyde method, and if the labeled enzyme is horseradish peroxidase, the sodium periodate method is preferable. The form of enzyme markers can be lyophilized powder, concentrated solution and working solution; the labeled antibodies are paired antibodies, including: anti-CA72-4 monoclonal antibody, anti-CA50 monoclonal antibody, anti-CA19-9 monoclonal antibody, anti-CA19-9 monoclonal antibody, CA242 monoclonal antibody, anti-CA15-3 monoclonal antibody, anti-CA27-29 monoclonal antibody and anti-CA125 monoclonal antibody. The diluent used for the working solution of the enzyme marker is a thimerosal preservative solution containing 0.05% glycerin and 0.2 g/L (which can prevent the enzyme marker placed at -20° C. from freezing and maintain the biological activity of the enzyme marker).
所述混合标记物液为体积比为1∶1-1∶3的FITC标记捕获抗体工作液和酶标记配对抗体工作液混合而成。The mixed marker solution is formed by mixing FITC-labeled capture antibody working solution and enzyme-labeled paired antibody working solution with a volume ratio of 1:1-1:3.
所述标准品溶液为六个浓度梯度含有糖类抗原的溶液;所述配制标准品的基质为50%马血清;所述构成标准品的糖类抗原包括:CA72-4、CA50、CA19-9、CA242、CA15-3、CA27-29和CA125。The standard solution is a solution containing carbohydrate antigens in six concentration gradients; the matrix for preparing the standard is 50% horse serum; the carbohydrate antigens that constitute the standard include: CA72-4, CA50, CA19-9 , CA242, CA15-3, CA27-29 and CA125.
所述浓缩洗涤液为pH值为7.4,含有0.1-0.5%吐温-20,0.1%叠氮化钠防腐剂的磷酸盐缓冲液。The concentrated washing solution has a pH value of 7.4 and contains 0.1-0.5% Tween-20 and 0.1% sodium azide preservative phosphate buffer solution.
所述当标记酶为辣根过氧化物酶时发光底物液由A液和B液构成,所述发光底物液A液为过氧化氢溶液,所述发光底物液B液为鲁米诺溶液;当标记酶为碱性磷酸酶时,所述发光底物液为3-(2’-螺旋金刚烷)-4-甲氧基-4-(3”-羟基)苯-1,2-二氧杂环丁烷磷酸钠(4-methoxy-4-(3-phosphatephenyl)-spiro-(1,2-dioxetane-3,2′-adamantane,AMPPD)缓冲液。When the labeled enzyme is horseradish peroxidase, the luminescent substrate liquid is composed of A liquid and B liquid, the luminescent substrate liquid A is a hydrogen peroxide solution, and the luminescent substrate liquid B is a luminescent substrate liquid. Nuo solution; when the labeled enzyme is alkaline phosphatase, the luminescent substrate solution is 3-(2'-helixadamantane)-4-methoxy-4-(3"-hydroxyl) benzene-1,2 - sodium dioxetane phosphate (4-methoxy-4-(3-phosphatephenyl)-spiro-(1,2-dioxetane-3,2'-adamantane, AMPPD) buffer.
所用的反应管材料可为透明聚苯乙烯、聚乙烯、聚丙烯、玻璃等。The material of the reaction tube used may be transparent polystyrene, polyethylene, polypropylene, glass, etc.
本发明提供的检测糖类抗原的方法,是利用所述磁性微粒子化学发光酶免疫试剂盒检测糖类抗原。The method for detecting carbohydrate antigens provided by the invention is to use the magnetic microparticle chemiluminescent enzyme immunoassay kit to detect carbohydrate antigens.
所述检测糖类抗原的方法还可包括样品的前处理。The method for detecting carbohydrate antigens may also include sample pretreatment.
本发明所提供的检测糖类抗原的方法,包括以下步骤:The method for detecting carbohydrate antigen provided by the present invention comprises the following steps:
1)样品前处理1) Sample pretreatment
对临床血清血浆,3000rpm离心5分钟,取上层液即可进行分析测定。For clinical serum and plasma, centrifuge at 3000rpm for 5 minutes, and take the supernatant for analysis and determination.
2)利用上述检测糖类抗原的磁性微粒子化学发光酶免疫分析试剂盒检测样品。2) Using the above-mentioned magnetic particle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens to detect samples.
本发明的试剂盒可定性、定量检测人血清样品中糖类抗原含量。The kit of the invention can qualitatively and quantitatively detect the carbohydrate antigen content in the human serum sample.
本发明所述的检测糖类抗原的磁性微粒子化学发光酶免疫分析试剂盒主要采用磁性微粒子固相分离及双抗夹心的反应模式定性或定量检测人体血清血浆等样品中糖类抗原含量;对样品的前处理要求低,样品前处理过程简单可靠,可快速、高通量检测大批样品。The magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens of the present invention mainly adopts the reaction mode of magnetic microparticle solid-phase separation and double-antibody sandwich to qualitatively or quantitatively detect the content of carbohydrate antigens in samples such as human serum plasma; The pretreatment requirements are low, the sample pretreatment process is simple and reliable, and it can quickly and high-throughput detect a large number of samples.
糖类抗原是一类蛋白大分子,对此类分子一般采用双抗体夹心的方法进行测定。上世纪70年代开始,科学家们通过杂交瘤技术陆续得到了糖类抗原的单克隆抗体,从而为建立双抗体夹心的免疫检测模式提供了保证。Carbohydrate antigens are a kind of protein macromolecules, and the double-antibody sandwich method is generally used for the detection of such molecules. Since the 1970s, scientists have successively obtained monoclonal antibodies to carbohydrate antigens through hybridoma technology, thus providing a guarantee for the establishment of a double-antibody sandwich immune detection model.
本发明的检测原理是当反应液中有FITC标记的捕获抗体和酶标记的配对抗体时,加入系列标准品或样品溶液后,通过“识别”抗原分子上的抗原决定簇,在溶液中形成FITC标记抗体-抗原-酶标记抗体复合物。然后加入包被有抗FITC抗体的磁性微粒子,使其充分分散于溶液中,通过免疫反应即可将免疫复合物偶联富集到磁性微粒子表面,在试管底部施加磁场后即可将偶联有免疫复合物的磁性粒子富集于试管底部,加入发光底物,用管式发光仪测定发光强度,样品的发光强度与样品中抗原含量成正相关,与标准曲线比较即可得出相应的抗原含量。The detection principle of the present invention is that when there are FITC-labeled capture antibodies and enzyme-labeled paired antibodies in the reaction solution, after adding a series of standard products or sample solutions, FITC is formed in the solution by "recognizing" the epitope on the antigen molecule. Labeled antibody-antigen-enzyme labeled antibody complex. Then add magnetic microparticles coated with anti-FITC antibody to fully disperse them in the solution. The immune complex can be coupled and enriched on the surface of the magnetic microparticles through immune reaction. After applying a magnetic field at the bottom of the test tube, the coupled The magnetic particles of the immune complex are enriched at the bottom of the test tube, add a luminescent substrate, and measure the luminous intensity with a tube luminometer. The luminous intensity of the sample is positively correlated with the antigen content in the sample, and the corresponding antigen content can be obtained by comparing with the standard curve .
本发明的检测糖类抗原的磁性微粒子化学发光酶免疫分析试剂盒主要采用直接夹心法定性或定量检测人血清、血浆等样品中糖类抗原的含量;对样品的前处理要求低,前处理过程简单,能快速、高通量检测大批样品;采用了高特异的糖类抗原单克隆抗体和超顺磁、高分散、比表面积大的磁性微粒子,主要试剂以工作液的形式提供,检验方法方便易行,具有特异性高、灵敏度高、精确度高、准确度高等特点。本发明的磁性微粒子化学发光酶免疫分析试剂盒,结构简单、使用方便、价格便宜、携带便利,与市场上的酶联免疫试剂盒、板式化学发光试剂盒相比,线性范围宽,有效避免弯钩效应,不需样品稀释,适用于大批量样品筛选的定性、定量。The magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens of the present invention mainly uses the direct sandwich method to qualitatively or quantitatively detect the content of carbohydrate antigens in samples such as human serum and plasma; the pretreatment requirements for samples are low, and the pretreatment process Simple, fast and high-throughput detection of a large number of samples; using highly specific monoclonal antibodies to carbohydrate antigens and superparamagnetic, highly dispersed, and large specific surface area magnetic particles, the main reagents are provided in the form of working solutions, and the test method is convenient It is easy to operate and has the characteristics of high specificity, high sensitivity, high precision and high accuracy. The magnetic microparticle chemiluminescent enzyme immunoassay kit of the present invention has simple structure, convenient use, cheap price, and convenient portability. Hook effect, without sample dilution, suitable for qualitative and quantitative screening of large batches of samples.
附图说明Description of drawings
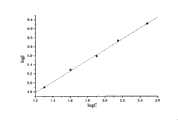
图1是以磁性微粒子化学发光酶免疫分析试剂盒检测CA50的标准曲线(双对数曲线)Figure 1 is the standard curve (double logarithmic curve) for detecting CA50 with the magnetic particle chemiluminescence enzyme immunoassay kit
图2是以磁性微粒子化学发光酶免疫分析试剂盒检测CA125的标准曲线(双对数曲线)Figure 2 is the standard curve (double logarithmic curve) for detecting CA125 with the magnetic particle chemiluminescence enzyme immunoassay kit
具体实施方式 Detailed ways
下述实施例的方法如无特别说明,均为常规方法。The methods in the following examples are conventional methods unless otherwise specified.
下述实施例中的百分含量,如无特别说明,均为质量百分含量。The percentages in the following examples are all mass percentages unless otherwise specified.
实施例1、检测CA50的磁性微粒子化学发光酶免疫分析试剂盒的制备及其检测方法。Example 1. Preparation and detection method of magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting CA50.
检测CA50的磁性微粒子化学发光酶免疫分析试剂盒包括:Magnetic microparticle chemiluminescent enzyme immunoassay kit for detection of CA50 includes:
(1)包被有抗FITC抗体的磁性微粒子:粒径为2-3μm、使用浓度为5-10mg/mL,20mL/瓶,1瓶;(1) Magnetic microparticles coated with anti-FITC antibody: particle size 2-3μm, use concentration 5-10mg/mL, 20mL/bottle, 1 bottle;
(2)标记FITC的CA50单克隆抗体(捕获抗体)及标记酶的CA50单克隆抗体(配对抗体)混合作为标记物液:为体积比为1∶1的FITC标记捕获抗体工作液和酶标记配对抗体工作液混合而成,15mL/瓶,1瓶。其中FITC标记抗体液以20%小牛血清稀释为1.25μg/mL,标记碱性磷酸酶的酶工作液所用的稀释液为含有0.05%甘油、0.2g/L的硫柳汞防腐剂溶液(可防止放入-20℃的酶标记物冻结,并保持酶标记物的生物活性)并稀释为1/2000;(2) FITC-labeled CA50 monoclonal antibody (capture antibody) and enzyme-labeled CA50 monoclonal antibody (paired antibody) are mixed as a labeling solution: FITC-labeled capture antibody working solution and enzyme-labeled pairing at a volume ratio of 1:1 Antibody working solution is mixed, 15mL/bottle, 1 bottle. Among them, the FITC-labeled antibody solution is diluted to 1.25 μg/mL with 20% calf serum, and the diluent used for the enzyme working solution of labeled alkaline phosphatase is a thimerosal preservative solution containing 0.05% glycerin and 0.2g/L (which can prevent release of frozen at -20°C and maintain the biological activity of the enzyme marker) and diluted to 1/2000;
(3)CA50标准品溶液:用50%马血清将CA50浓标准品稀释成标准溶液6瓶,0U/mL,20U/mL,40U/mL,80U/mL,140U/mL,300U/mL,0.5mL/瓶;(3) CA50 standard solution: use 50% horse serum to dilute CA50 concentrated standard into 6 bottles of standard solution, 0U/mL, 20U/mL, 40U/mL, 80U/mL, 140U/mL, 300U/mL, 0.5 mL/bottle;
(4)发光底物液:AMPPD缓冲液,6mL/瓶,1瓶;(4) Luminescence substrate solution: AMPPD buffer solution, 6mL/bottle, 1 bottle;
(5)浓缩洗涤液:pH值为7.4,含有0.1-0.5%吐温-20,0.1%叠氮化钠防腐剂的磷酸盐缓冲液。30mL/瓶,1瓶。使用时用蒸馏水稀释20倍;(5) Concentrated washing solution: a pH value of 7.4, containing 0.1-0.5% Tween-20, 0.1% sodium azide preservative phosphate buffer solution. 30mL/bottle, 1 bottle. Dilute 20 times with distilled water;
(6)反应试管:所用的反应管材料为透明聚苯乙烯、聚乙烯、聚丙烯、玻璃等,铝膜真空封装,100支/包,1包。(6) Reaction test tube: The material of the reaction tube used is transparent polystyrene, polyethylene, polypropylene, glass, etc., sealed in vacuum with aluminum film, 100 tubes/pack, 1 package.
其中,抗FITC抗体与磁性微粒子偶联物、FITC标记的CA50单克隆抗体、碱性磷酸酶标记的CA50单克隆抗体的制备方法如下:Wherein, the preparation method of anti-FITC antibody and magnetic microparticle conjugate, FITC-labeled CA50 monoclonal antibody, and alkaline phosphatase-labeled CA50 monoclonal antibody is as follows:
一、抗FITC抗体与磁性微粒子偶联物的制备1. Preparation of anti-FITC antibody and magnetic microparticle conjugates
1、抗FITC抗体与磁性微粒子偶联物的制备原理:磁性微粒子是内核为Fe3O4、表面包裹了带有活性基团氨基的聚合物,可采用戊二醛两步法将磁性微粒子与抗FITC抗体进行偶联。如此合成的包被有抗FITC抗体的颗粒,不仅有效包被量增加,而且在微观空间具有“长臂”,从而减少空间位阻,使得抗体IgG对抗原的识别域充分展开,有利于抗体-抗原的空间匹配与非共价结合。1. The principle of preparation of anti-FITC antibody and magnetic microparticle conjugates: magnetic microparticles are polymers with Fe3O4 as the core and coated with active amino groups on the surface. The magnetic microparticles and anti-FITC antibody can be combined by glutaraldehyde two-step method. Perform coupling. The particles coated with anti-FITC antibody synthesized in this way not only increase the effective coating amount, but also have "long arms" in the microscopic space, thereby reducing steric hindrance, so that the antibody IgG can fully expand the recognition domain of the antigen, which is beneficial to the antibody- Spatial matching and non-covalent binding of antigens.
2、抗FITC抗体与磁性微粒子偶联物的制备方法:将粒径为2-3μm的磁性微粒子用戊二醛进行活化,室温搅拌,混匀3小时后,加磁场,静置10-15min,倒出上清,用pH值为7.4的0.01mol/L磷酸盐缓冲液清洗三次,并用该溶液进行悬浮,浓度为50-100mg/mL;每毫升悬浮液中加入抗FITC抗体20-80μg,在4℃下搅拌过夜后,加磁场,静置10-15min,倒出上清,用含有0.5%牛血清白蛋白、1%酪蛋白的pH为7.2,0.02mol/L的磷酸缓冲液于室温封闭3-4小时;最后用pH值为7.4、含0.5%BSA的0.02mol/LTris-HCl缓冲液清洗三次,并用该溶液配制成5-10mg/mL的工作液。磁性微粒子分离剂在4℃保存,不应冻存,用时轻轻摇匀。2. The preparation method of anti-FITC antibody and magnetic microparticle conjugates: activate the magnetic microparticles with a particle size of 2-3 μm with glutaraldehyde, stir at room temperature, mix for 3 hours, add a magnetic field, and let stand for 10-15min. Pour off the supernatant, wash three times with 0.01mol/L phosphate buffer with a pH value of 7.4, and use this solution to suspend at a concentration of 50-100 mg/mL; add 20-80 μg of anti-FITC antibody to each ml of suspension, and After stirring overnight at 4°C, add a magnetic field, let it stand for 10-15min, pour off the supernatant, and block with a phosphate buffer solution containing 0.5% bovine serum albumin and 1% casein at a pH of 7.2 and 0.02mol/L at room temperature 3-4 hours; finally wash three times with 0.02mol/LTris-HCl buffer solution with pH value of 7.4 and containing 0.5% BSA, and use this solution to prepare a working solution of 5-10 mg/mL. The magnetic particle separation agent should be stored at 4°C and should not be frozen. Shake gently when using.
二、FITC标记的CA50单克隆抗体的制备2. Preparation of FITC-labeled CA50 monoclonal antibody
1、FITC标记的CA50单克隆抗体的制备原理:FITC分子与糖类抗原单克隆抗体的偶联物是以常见的碱性液标记法对抗体进行标记。1. The principle of preparation of FITC-labeled CA50 monoclonal antibody: the conjugate of FITC molecule and carbohydrate antigen monoclonal antibody is labeled with the common alkaline solution labeling method.
2、FITC标记的CA50单克隆抗体的制备方法:2. Preparation method of FITC-labeled CA50 monoclonal antibody:
(1)A液:取CA50单克隆抗体0.5mg置于50mmol/L pH值为9.3的Na2CO3-NaHCO3缓冲液中;(1) Solution A: Take 0.5 mg of CA50 monoclonal antibody and place it in 50 mmol/L Na2CO3-NaHCO3 buffer solution with a pH value of 9.3;
(2)B液:取FITC2 mg溶于1mL 50mmol/L pH值为9.3的Na2CO3-NaHCO3缓冲液中;(2) Liquid B: Dissolve 2 mg of FITC in 1 mL of 50 mmol/L Na2CO3-NaHCO3 buffer solution with a pH value of 9.3;
(3)将A液置于磁力搅拌器,取B液缓缓滴加至A液中,约在5-8分钟间加完,防止出现气泡,然后置4℃搅拌12~16小时;(3) Put liquid A on a magnetic stirrer, slowly add liquid B to liquid A dropwise, and finish adding in about 5-8 minutes to prevent bubbles, and then stir at 4°C for 12-16 hours;
(4)将反应液装透析袋,置10mmol/L pH值为7.4的磷酸缓冲液中透析2天,每天更换透析液2次;(4) Put the reaction solution into a dialysis bag, dialyze it in phosphate buffer solution with a pH value of 7.4 at 10 mmol/L for 2 days, and change the dialysate twice a day;
(5)将标记物以20%小牛血清稀释为1.25μg/mL,加入适量proclin300,分装,-20℃下保存。(5) Dilute the marker with 20% calf serum to 1.25 μg/mL, add an appropriate amount of proclin300, aliquot and store at -20°C.
三、碱性磷酸酶标记的CA50单克隆抗体的制备3. Preparation of alkaline phosphatase-labeled CA50 monoclonal antibody
1、碱性磷酸酶标记的CA50单克隆抗体的制备原理:可采用现有技术中的多种方法如戊二醛法或过碘酸钠法将酶交联在抗体上,优选戊二醛法;1. Preparation principle of alkaline phosphatase-labeled CA50 monoclonal antibody: various methods in the prior art such as glutaraldehyde method or sodium periodate method can be used to cross-link the enzyme on the antibody, preferably glutaraldehyde method ;
2、碱性磷酸酶标记的CA50单克隆抗体的制备方法:2. Preparation method of alkaline phosphatase-labeled CA50 monoclonal antibody:
(1)取碱性磷酸酶20mg溶于1.5%戊二醛溶液中,室温静置过夜;(1) Dissolve 20 mg of alkaline phosphatase in 1.5% glutaraldehyde solution, and let stand overnight at room temperature;
(2)反应后的酶溶液经Sephadex G-2 5层析柱,用生理盐水洗脱,流速控制1.2mL/min,并收集棕色流出液。放置于25mL烧杯中,电磁搅拌;(2) The reacted enzyme solution was passed through a Sephadex G-25 chromatographic column, eluted with normal saline, the flow rate was controlled at 1.2mL/min, and the brown effluent was collected. Place in a 25mL beaker and stir electromagnetically;
(3)取CA50单克隆抗体2.5mg,以0.1mol/L pH值为7.4的磷酸盐缓冲液稀释至5mL,搅拌下逐滴加入酶溶液中;(3) Take 2.5 mg of CA50 monoclonal antibody, dilute it to 5 mL with 0.1 mol/L phosphate buffer solution with a pH value of 7.4, and add it dropwise to the enzyme solution while stirring;
(4)加入0.2mol/L赖氨酸0.2mL,混匀,置室温2-3小时;(4) Add 0.2mL of 0.2mol/L lysine, mix well, and place at room temperature for 2-3 hours;
(5)反应液装入透析袋,以0.1mol/L pH值为7.4的磷酸盐缓冲液透析24小时后,以含有0.05%甘油、0.2g/L的硫柳汞防腐剂溶液1∶2000稀释,分装,冰冻保存。(5) The reaction solution is packed into a dialysis bag, dialyzed for 24 hours with 0.1mol/L pH value of phosphate buffer saline solution of 7.4, diluted with 1:2000 thimerosal preservative solution containing 0.05% glycerin and 0.2g/L, and divided Assemble and store in the freezer.
利用该试剂盒检测样品中CA50含量的方法如下:The method of using the kit to detect the content of CA50 in the sample is as follows:
一、样品前处理1. Sample pretreatment
对人血清血浆:取空腹晨血样本,3000rpm离心5min,取上层液25μL进行分析。待测样品2-8℃存放不得超过48小时,若48小时未检测,应于-20℃以下保存,但不应超过30天。For human serum and plasma: take a fasting morning blood sample, centrifuge at 3000rpm for 5min, and take 25μL of the supernatant for analysis. The sample to be tested should not be stored at 2-8°C for more than 48 hours. If it has not been tested for 48 hours, it should be stored below -20°C, but not more than 30 days.
二、检测方法2. Detection method
向试管中加入25μL血清样品或同体积的系列标准品溶液,再加入标记物混合液50μL,37℃恒温振荡反应30min。加入磁性微粒子溶液50μL,37℃恒温振荡反应5min后,将试管架置于磁分离器上分离5min,然后倒转分离器倒出上清液,将倒转的试管放在滤纸上,拍击分离器以除去挂壁液体。每管加入洗涤液500μL,用圆周运动使其充分混匀,置于磁分离器上分离5min,然后倒转分离器倒出上清液,将倒转的试管放在滤纸上,拍击分离器以除去挂壁液体,重复3次。加入发光底物300μL,圆周运动使其充分混匀,暗处放置2min后置于磁分离器,待磁颗粒富集于底部后,将试管置于管式发光仪测定。Add 25 μL of serum samples or the same volume of serial standard solutions to the test tube, then add 50 μL of the marker mixture, and react with constant temperature shaking at 37°C for 30 minutes. Add 50 μL of magnetic microparticle solution, shake and react at 37°C for 5 minutes, place the test tube rack on the magnetic separator for 5 minutes, then invert the separator to pour out the supernatant, put the inverted test tube on the filter paper, tap the separator to Remove wall fluid. Add 500 μL of washing solution to each tube, mix well with circular motion, place on a magnetic separator for separation for 5 minutes, then invert the separator to pour out the supernatant, put the inverted test tube on the filter paper, tap the separator to remove Wall hanging liquid, repeat 3 times. Add 300 μL of luminescent substrate, make a circular motion to mix well, place in a dark place for 2 min, and then place in a magnetic separator. After the magnetic particles are enriched at the bottom, place the test tube in a tube-type luminometer for measurement.
三、结果分析3. Results Analysis
发光计数-剂量反应曲线以双对数曲线表示,即所获得的每个浓度标准溶液或样本发光值的平均值(B)减去第一个零标准点的发光值(B0),再取对数,即The luminescence count-dose response curve is expressed as a double-logarithmic curve, that is, the average value (B) of the luminescence value of each concentration standard solution or sample obtained minus the luminescence value (B0) of the first zero standard point, and then take the number, namely
纵轴(Y轴)=log(B-B0)Vertical axis (Y axis) = log (B-B0)
以CA50浓度的自然对数值为横轴(X轴),绘制标准曲线,如图1所示。相应每一个样品中CA50浓度可以从标准曲线上读出。也可以用多参数回归方程法进行拟合,计算出样本溶液中CA50的浓度。由于市场上大多数化学发光板式试剂盒的线性范围在0-150U/mL之间,而本试剂盒提供的线性范围大大增加(0-300U/mL),所以避免了高浓度样品的稀释,弯钩效应也可以得到避免。使用本试剂盒的整个检测过程只需1.5个小时就可以完成,最低检测限为1.0U/mL。Taking the natural logarithm of the CA50 concentration as the horizontal axis (X-axis), draw a standard curve, as shown in Figure 1. The corresponding CA50 concentration in each sample can be read from the standard curve. It can also be fitted with a multi-parameter regression equation method to calculate the concentration of CA50 in the sample solution. Since the linear range of most chemiluminescent plate kits on the market is between 0-150U/mL, and the linear range provided by this kit is greatly increased (0-300U/mL), the dilution of high-concentration samples is avoided, and the The hook effect can also be avoided. The whole detection process using this kit can be completed in only 1.5 hours, and the minimum detection limit is 1.0U/mL.
实施例2、检测CA125的磁性微粒子化学发光酶免疫分析试剂盒的制备及其检测方法。Example 2. Preparation and detection method of magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting CA125.
检测CA125的磁性微粒子化学发光酶免疫分析试剂盒包括:Magnetic microparticle chemiluminescent enzyme immunoassay kit for detection of CA125 includes:
(1)包被有抗FITC抗体的磁性微粒子:粒径为2-3μm、使用浓度为5-10mg/mL,20mL/瓶,1瓶;(1) Magnetic microparticles coated with anti-FITC antibody: particle size 2-3μm, use concentration 5-10mg/mL, 20mL/bottle, 1 bottle;
(2)标记FITC的CA125单克隆抗体(捕获抗体)及标记酶的CA125单克隆抗体(配对抗体)混合作为标记物液:为体积比为1∶2的FITC标记捕获抗体工作液和酶标记配对抗体工作液混合而成,15mL/瓶,1瓶。其中FITC标记抗体液以20%小牛血清稀释为1.25μg/mL,标记辣根过氧化物酶的酶工作液所用的稀释液为含有0.05%甘油、0.2g/L的硫柳汞防腐剂溶液(可防止放入-20℃的酶标记物冻结,并保持酶标记物的生物活性)并稀释为1/1500;(2) FITC-labeled CA125 monoclonal antibody (capture antibody) and enzyme-labeled CA125 monoclonal antibody (paired antibody) are mixed as a labeling solution: FITC-labeled capture antibody working solution and enzyme-labeled pairing at a volume ratio of 1:2 Antibody working solution is mixed, 15mL/bottle, 1 bottle. Wherein the FITC-labeled antibody solution is diluted to 1.25 μg/mL with 20% calf serum, and the diluent used for the enzyme working solution of the labeled horseradish peroxidase is a thimerosal preservative solution containing 0.05% glycerol and 0.2g/L (can be Prevent the enzyme marker placed at -20°C from freezing, and maintain the biological activity of the enzyme marker) and dilute it to 1/1500;
(3)CA125标准品溶液:用50%马血清将CA125浓标准品稀释成标准溶液6瓶,0U/mL,50U/mL,100U/mL,200U/mL,500U/mL,1000U/mL,0.5mL/瓶;(3) CA125 standard solution: use 50% horse serum to dilute CA125 concentrated standard into 6 bottles of standard solution, 0U/mL, 50U/mL, 100U/mL, 200U/mL, 500U/mL, 1000U/mL, 0.5 mL/bottle;
(4)发光底物液:A液:过氧化氢溶液,7mL/瓶,1瓶;B液:鲁米诺溶液,7mL/瓶,1瓶。使用时体积比为1∶1混合。(4) Luminescent substrate solution: A liquid: hydrogen peroxide solution, 7mL/bottle, 1 bottle; B liquid: luminol solution, 7mL/bottle, 1 bottle. When used, the volume ratio is 1:1.
(5)浓缩洗涤液:pH值为7.4,含有0.1-0.5%吐温-20,0.1%叠氮化钠防腐剂的磷酸盐缓冲液。30mL/瓶,1瓶。使用时用蒸馏水稀释20倍;(5) Concentrated washing solution: a pH value of 7.4, containing 0.1-0.5% Tween-20, 0.1% sodium azide preservative phosphate buffer solution. 30mL/bottle, 1 bottle. Dilute 20 times with distilled water;
(6)反应试管:所用的反应管材料为透明聚苯乙烯、聚乙烯、聚丙烯、玻璃等,铝膜真空封装,100支/包,1包。(6) Reaction test tube: The material of the reaction tube used is transparent polystyrene, polyethylene, polypropylene, glass, etc., sealed in vacuum with aluminum film, 100 tubes/pack, 1 package.
其中,抗FITC抗体与磁性微粒子偶联物、FITC标记的CA125单克隆抗体、碱性磷酸酶标记的CA125单克隆抗体的制备方法如下:Wherein, the preparation method of anti-FITC antibody and magnetic particle conjugate, FITC-labeled CA125 monoclonal antibody, alkaline phosphatase-labeled CA125 monoclonal antibody is as follows:
一、抗FITC抗体与磁性微粒子偶联物的制备1. Preparation of anti-FITC antibody and magnetic microparticle conjugates
1、抗FITC抗体与磁性微粒子偶联物的制备原理:磁性微粒子是内核为Fe3O4、表面包裹了带有活性基团氨基的聚合物,可采用戊二醛两步法将磁性微粒子与抗FITC抗体进行偶联。1. The principle of preparation of anti-FITC antibody and magnetic microparticle conjugates: magnetic microparticles are polymers with Fe3O4 as the core and coated with active amino groups on the surface. The magnetic microparticles and anti-FITC antibody can be combined by glutaraldehyde two-step method. Perform coupling.
2、抗FITC抗体与磁性微粒子偶联物的制备方法:将粒径为2-3μm的磁性微粒子用戊二醛进行活化,室温搅拌,混匀3小时后,加磁场,静置10-15min,倒出上清,用pH值为7.4的0.01mol/L磷酸盐缓冲液清洗三次,并用该溶液进行悬浮,浓度为50-100mg/mL;每毫升悬浮液中加入抗FITC抗体20-80μg,在4℃下搅拌过夜后,加磁场,静置10-15min,倒出上清,用含有0.5%牛血清白蛋白、1%酪蛋白的pH为7.2,0.02mol/L的磷酸缓冲液于室温封闭3-4小时;最后用pH值为7.4、含0.5%BSA的0.02mol/LTris-HCl缓冲液清洗三次,并用该溶液配制成5-10mg/mL的工作液。磁性微粒子分离剂在4℃保存,不应冻存,用时轻轻摇匀。2. The preparation method of anti-FITC antibody and magnetic microparticle conjugates: activate the magnetic microparticles with a particle size of 2-3 μm with glutaraldehyde, stir at room temperature, mix for 3 hours, add a magnetic field, and let stand for 10-15min. Pour off the supernatant, wash three times with 0.01mol/L phosphate buffer with a pH value of 7.4, and use this solution to suspend at a concentration of 50-100 mg/mL; add 20-80 μg of anti-FITC antibody to each ml of suspension, and After stirring overnight at 4°C, add a magnetic field, let it stand for 10-15min, pour off the supernatant, and block with a phosphate buffer solution containing 0.5% bovine serum albumin and 1% casein at a pH of 7.2 and 0.02mol/L at room temperature 3-4 hours; finally wash three times with 0.02mol/LTris-HCl buffer solution with pH value of 7.4 and containing 0.5% BSA, and use this solution to prepare a working solution of 5-10 mg/mL. The magnetic particle separation agent should be stored at 4°C and should not be frozen. Shake gently when using.
二、FITC标记的CA125单克隆抗体的制备2. Preparation of FITC-labeled CA125 monoclonal antibody
1、FITC标记的CA125单克隆抗体的制备原理:FITC分子与糖类抗原单克隆抗体的偶联物是以常见的碱性液标记法对抗体进行标记。1. The preparation principle of FITC-labeled CA125 monoclonal antibody: the conjugate of FITC molecule and carbohydrate antigen monoclonal antibody is labeled with the common alkaline solution labeling method.
2、FITC标记的CA125单克隆抗体的制备方法:2. Preparation method of FITC-labeled CA125 monoclonal antibody:
(1)A液:取CA50单克隆抗体0.5mg置于50mmol/L pH值为9.3的Na2CO3-NaHCO3缓冲液中;(1) Solution A: Take 0.5 mg of CA50 monoclonal antibody and place it in 50 mmol/L Na2CO3-NaHCO3 buffer solution with a pH value of 9.3;
(2)B液:取FITC2 mg溶于1mL 50mmol/L pH值为9.3的Na2CO3-NaHCO3缓冲液中;(2) Liquid B: Dissolve 2 mg of FITC in 1 mL of 50 mmol/L Na2CO3-NaHCO3 buffer solution with a pH value of 9.3;
(3)将A液置于磁力搅拌器,取B液缓缓滴加至A液中,约在5-8分钟间加完,防止出现气泡,然后置4℃搅拌12~16小时;(3) Put liquid A on a magnetic stirrer, slowly add liquid B to liquid A dropwise, and finish adding in about 5-8 minutes to prevent bubbles, and then stir at 4°C for 12-16 hours;
(4)将反应液装透析袋,置10mmol/L pH值为7.4的磷酸缓冲液中透析2天,每天更换透析液2次;(4) Put the reaction solution into a dialysis bag, dialyze it in phosphate buffer solution with a pH value of 7.4 at 10 mmol/L for 2 days, and change the dialysate twice a day;
(5)将标记物以20%小牛血清稀释为1.25μg/mL,加入适量proclin300,分装,-20℃下保存。(5) Dilute the marker with 20% calf serum to 1.25 μg/mL, add an appropriate amount of proclin300, aliquot and store at -20°C.
三、辣根过氧化物酶的酶标记的CA125单克隆抗体的制备3. Preparation of horseradish peroxidase enzyme-labeled CA125 monoclonal antibody
1、辣根过氧化物酶标记CA125单克隆抗体的制备原理:使用过碘酸盐氧化法。过碘酸钠将辣根过氧化物酶分子表面的多糖氧化为醛基,醛基与抗体分子上的氨基形成Schiff碱而结合。后者可进一步用NaBH4(或乙醇胺)还原生成稳定的酶标记抗体。1. The preparation principle of horseradish peroxidase-labeled CA125 monoclonal antibody: use the periodate oxidation method. Sodium periodate oxidizes the polysaccharide on the surface of the horseradish peroxidase molecule into an aldehyde group, and the aldehyde group combines with the amino group on the antibody molecule to form a Schiff base. The latter can be further reduced with NaBH4 (or ethanolamine) to generate a stable enzyme-labeled antibody.
2、辣根过氧化物酶的酶标记CA125单克隆抗体的制备方法:2. Preparation method of enzyme-labeled CA125 monoclonal antibody against horseradish peroxidase:
(1)8-10mg辣根过氧化物酶放入1mL玻璃瓶,加入1mlL二次蒸馏水溶解,液体呈棕色;(1) Put 8-10mg of horseradish peroxidase into a 1mL glass bottle, add 1mlL of double distilled water to dissolve, and the liquid turns brown;
(2)电磁搅拌下逐滴缓慢加入新配制的NaIO4 0.2mL,室温下继续搅拌30-40min,液体呈绿色;(2) Slowly add 0.2mL of newly prepared NaIO4 dropwise under electromagnetic stirring, and continue to stir for 30-40min at room temperature, and the liquid turns green;
(3)将反应液对在4℃下对1mmol/L、pH值为4.4的醋酸钠缓冲液透析过夜,中间换液3~5次,每次300mL,溶液呈浅棕色;(3) Dialyze the reaction solution against 1 mmol/L sodium acetate buffer solution with a pH value of 4.4 at 4°C overnight, change the solution 3 to 5 times in the middle, 300 mL each time, and the solution is light brown;
(4)将1mg CA125单克隆抗体溶于2mLDMF,并以0.2mol/L Na2CO3缓冲液稀释至4mL;(4) Dissolve 1 mg of CA125 monoclonal antibody in 2 mL of DMF, and dilute to 4 mL with 0.2 mol/L Na2CO3 buffer;
(5)将透析好的辣根过氧化物酶溶液装入玻璃瓶,加入0.2mol/LNa2CO3溶液将pH值调整至9.0-10.0,混匀后立即加入抗体溶液,边加边电磁搅拌;持续搅拌3-5小时;(5) Put the dialyzed horseradish peroxidase solution into a glass bottle, add 0.2mol/L Na2CO3 solution to adjust the pH value to 9.0-10.0, and immediately add the antibody solution after mixing, and electromagnetically stir while adding; keep stirring 3-5 hours;
(6)向反应液中加入新配制的NaBH4溶液100~200μL,4℃放置2小时。(6) Add 100-200 μL of newly prepared NaBH4 solution to the reaction solution, and place at 4°C for 2 hours.
(7)反应液装入透析袋,以0.1mol/L pH值为7.4的磷酸盐缓冲液透析24小时后,换液4次。以含有0.05%甘油、0.2g/L的硫柳汞防腐剂溶液1∶2000稀释,分装,冰冻保存。(7) The reaction solution was put into a dialysis bag, dialyzed with 0.1 mol/L phosphate buffer solution with a pH value of 7.4 for 24 hours, and the solution was changed 4 times. Dilute it with 1:2000 thimerosal preservative solution containing 0.05% glycerin and 0.2 g/L, subpackage, and store in freezer.
利用该试剂盒检测样品中CA125含量的方法如下:The method of using the kit to detect the content of CA125 in the sample is as follows:
一、样品前处理1. Sample pretreatment
对人血清血浆:取空腹晨血样本,3000rpm离心5min,取上层液25μL进行分析。待测样品2-8℃存放不得超过48小时,若48小时未检测,应于-20℃以下保存,但不应超过30天。For human serum and plasma: take a fasting morning blood sample, centrifuge at 3000rpm for 5min, and take 25μL of the supernatant for analysis. The sample to be tested should not be stored at 2-8°C for more than 48 hours. If it has not been tested for 48 hours, it should be stored below -20°C, but not more than 30 days.
二、检测方法2. Detection method
向试管中加入25μL血清样品或同体积的系列标准品溶液,再加入标记物混合液50μL,37℃恒温振荡反应30min。加入磁性微粒子溶液50μL,37℃恒温振荡反应5min后,将试管架置于磁分离器上分离5min,然后倒转分离器倒出上清液,将倒转的试管放在滤纸上,拍击分离器以除去挂壁液体。每管加入洗涤液500μL,用圆周运动使其充分混匀,置于磁分离器上分离5min,然后倒转分离器倒出上清液,将倒转的试管放在滤纸上,拍击分离器以除去挂壁液体,重复3次。加入发光底物300μL,圆周运动使其充分混匀,暗处放置2min后置于磁分离器,待磁颗粒富集于底部后,将试管置于管式发光仪测定。Add 25 μL of serum samples or the same volume of serial standard solutions to the test tube, then add 50 μL of the marker mixture, and react with constant temperature shaking at 37°C for 30 minutes. Add 50 μL of magnetic microparticle solution, shake and react at 37°C for 5 minutes, place the test tube rack on the magnetic separator for 5 minutes, then invert the separator to pour out the supernatant, put the inverted test tube on the filter paper, tap the separator to Remove wall fluid. Add 500 μL of washing solution to each tube, mix well with circular motion, place on a magnetic separator for separation for 5 minutes, then invert the separator to pour out the supernatant, put the inverted test tube on the filter paper, tap the separator to remove Wall hanging liquid, repeat 3 times. Add 300 μL of luminescent substrate, make a circular motion to mix well, place in a dark place for 2 min, and then place in a magnetic separator. After the magnetic particles are enriched at the bottom, place the test tube in a tube-type luminometer for measurement.
三、结果分析3. Results Analysis
发光计数-剂量反应曲线以双对数曲线表示,即所获得的每个浓度标准溶液或样本发光值的平均值(B)减去第一个零标准点的发光值(B0),再取对数,即The luminescence count-dose response curve is expressed as a double-logarithmic curve, that is, the average value (B) of the luminescence value of each concentration standard solution or sample obtained minus the luminescence value (B0) of the first zero standard point, and then take the number, namely
纵轴(Y轴)=log(B-B0)Vertical axis (Y axis) = log (B-B0)
以CA125浓度的自然对数值为横轴(X轴),绘制标准曲线,如图2所示。相应每一个样品中CA125浓度可以从标准曲线上读出。也可以用多参数回归方程法进行拟合,计算出样本溶液中CA125的浓度。由于市场上大多数化学发光板式试剂盒的线性范围在0-500U/mL之间,而本试剂盒提供的线性范围大大增加(0-1000U/mL),所以避免了高浓度样品的稀释,弯钩效应也可以得到避免。使用本试剂盒的整个检测过程只需1.5个小时就可以完成,最低检测限为1.0U/mL。Taking the natural logarithm value of the CA125 concentration as the horizontal axis (X axis), draw a standard curve, as shown in Figure 2. The corresponding CA125 concentration in each sample can be read from the standard curve. It can also be fitted with a multi-parameter regression equation method to calculate the concentration of CA125 in the sample solution. Since the linear range of most chemiluminescence plate kits on the market is between 0-500U/mL, and the linear range provided by this kit is greatly increased (0-1000U/mL), the dilution of high-concentration samples is avoided, and the The hook effect can also be avoided. The whole detection process using this kit can be completed in only 1.5 hours, and the minimum detection limit is 1.0U/mL.
实施例3、试剂盒精密度、准确度和稳定性实验Embodiment 3, kit precision, accuracy and stability experiment
1、试剂盒精密度测定1. Determination of kit precision
(1)标准品精密度实验(1) Standard precision experiment
将实施例1和实施例2中制备的试剂盒分别取三批进行精密度实验,每批抽取10个试剂盒。以实施例1中所抽取的试剂盒测定40U/mL的CA50标准品5次,以实施例2中所抽取的试剂盒测定100U/mL的CA125标准品5次。计算测定浓度的变异系数。实施例1中的三批试剂盒的测定结果如表1所示,结果表明变异系数在之4.0%-8.2%之间。Three batches of the kits prepared in Example 1 and Example 2 were taken respectively for the precision test, and 10 kits were taken from each batch. Use the kit extracted in Example 1 to measure the 40 U/mL CA50 standard 5 times, and use the kit extracted in Example 2 to measure the 100 U/mL CA125 standard 5 times. Calculate the coefficient of variation for the measured concentrations. The measurement results of the three batches of kits in Example 1 are shown in Table 1, and the results show that the coefficient of variation is between 4.0% and 8.2%.
表1 CA50标准可重复性实验Table 1 CA50 standard repeatability experiment
实施例2中的三批试剂盒的测定结果如表2所示,结果表明变异系数在5.8%-8.8%之间。The measurement results of the three batches of kits in Example 2 are shown in Table 2, and the results show that the coefficient of variation is between 5.8% and 8.8%.
表2 CA125标准可重复性实验Table 2 CA125 standard repeatability experiment
(2)样本可重复性实验(2) Sample repeatability experiment
取两份正常人血清,分别添加CA50和CA125标准品至40U/mL和100U/mL。取实施例1和实施例2中三个不同批次的试剂盒各3个,对样品重复测定5次,分别计算变异系数。测定结果如表3、表4所示,表明本发明所制备的CA50磁性微粒子化学发光酶免疫分析试剂盒测定血清样本的变异系数小于8.6%,CA125磁性微粒子化学发光酶免疫分析试剂盒测定血清样本的变异系数小于5.0%。Take two normal human serum, add CA50 and CA125 standards to 40U/mL and 100U/mL respectively. Take three different batches of test kits in Example 1 and Example 2, respectively, and repeat the measurement of the
表3 CA50血清样品可重复性实验Table 3 Repeatability experiment of CA50 serum samples
表4 CA125血清样品可重复性实验Table 4 CA125 serum sample reproducibility experiment
2、试剂盒准确度测定2. Determination of kit accuracy
取两个CA50临床定值血清,浓度分别为42.56U/mL和83.54U/mL,分别向其中加入CA50的标准品溶液20U/mL、40U/mL和80U/mL,按照实施例1的方法对每个浓度做3个平行,计算回收率。结果如表5所示,表明CA50的添加回收率在82.4%-112%之间。Get two CA50 clinical definite value serums, concentration is respectively 42.56U/mL and 83.54U/mL, adds the standard solution 20U/mL, 40U/mL and 80U/mL of CA50 therein respectively, according to the method for embodiment 1 Three parallel runs were made for each concentration, and the recovery rate was calculated. The results are shown in Table 5, showing that the addition recovery of CA50 is between 82.4%-112%.
取两个CA125临床定值血清,浓度分别为27.78U/mL和93.58U/mL,分别向其中加入CA125的标准品溶液50U/mL、100U/mL和200U/mL,按照实施例2的方法对每个浓度做3个平行,计算回收率。结果如表6所示,表明CA125的添加回收率在98.1%-106%之间。Get two CA125 clinical value serums, concentration is respectively 27.78U/mL and 93.58U/mL, adds the standard solution 50U/mL, 100U/mL and 200U/mL of CA125 therein respectively, according to the method for embodiment 2 Three parallel runs were made for each concentration, and the recovery rate was calculated. The results are shown in Table 6, showing that the addition recovery of CA125 is between 98.1%-106%.
表5实施例1的试剂盒的回收率The recovery rate of the kit of table 5 embodiment 1
表6实施例2的试剂盒的回收率The recovery rate of the kit of table 6 embodiment 2
3、试剂盒稳定性实验3. Kit stability test
对实施例1和实施例2的试剂盒分别进行37℃5天和7天加速实验后,测定试剂盒的最大、最低发光强度、待测物的加标回收率,表明实施例1和实施例2的试剂盒指标均在正常范围之内。对实施例1和实施例2的试剂盒主要组分:标记物工作液、标准品、发光底物液、磁性微粒子工作液进行2-8℃8个月跟踪实验,结果表明各项指标完全符合要求。考虑到试剂盒冷冻情况的发生,将实施例1和实施例2的试剂盒放入-20℃冷冻7天,测定结果也表明试剂盒的各项指标完全正常。从以上结果可以看出试剂盒可以在2-8℃至少可以保存6个月以上。After the kits of Example 1 and Example 2 were subjected to accelerated experiments at 37°C for 5 days and 7 days, respectively, the maximum and minimum luminous intensity of the kits and the recovery rate of the analyte were determined, showing that Example 1 and Example 2 kit indicators are within the normal range. The main components of the kits in Example 1 and Example 2: marker working solution, standard substance, luminescent substrate solution, and magnetic particle working solution were followed up for 8 months at 2-8°C, and the results showed that all indicators were in full compliance with Require. Considering the occurrence of freezing of the kits, the kits of Examples 1 and 2 were frozen at -20°C for 7 days, and the measurement results also showed that the indicators of the kits were completely normal. From the above results, it can be seen that the kit can be stored at 2-8°C for at least 6 months.
Claims (19)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNA2007101188855ACN101324579A (en) | 2007-06-13 | 2007-06-13 | A magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and its application method |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNA2007101188855ACN101324579A (en) | 2007-06-13 | 2007-06-13 | A magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and its application method |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN101324579Atrue CN101324579A (en) | 2008-12-17 |
Family
ID=40188202
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA2007101188855APendingCN101324579A (en) | 2007-06-13 | 2007-06-13 | A magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and its application method |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101324579A (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102288766A (en)* | 2011-08-16 | 2011-12-21 | 内蒙古科慧生物科技有限责任公司 | Carbohydrate antigen 15-3 (CA15-3) quantitative measurement kit and detection method thereof |
| CN102288767A (en)* | 2011-08-16 | 2011-12-21 | 内蒙古科慧生物科技有限责任公司 | Saccharide antigen 72-4(CA72-4) quantitative determination kit and detection method |
| CN102435739A (en)* | 2011-08-31 | 2012-05-02 | 内蒙古科慧生物科技有限责任公司 | Carbohydrate antigen 242(CA242) quantitative determination kit and detection method thereof |
| CN102435736A (en)* | 2011-11-29 | 2012-05-02 | 桂林理工大学 | Method for measuring antigen of ovarian cancer embryo by electrochemical luminescence (ECL) immunosensor |
| CN102565402A (en)* | 2010-12-16 | 2012-07-11 | 北京勤邦生物技术有限公司 | Magnetic particle chemiluminescence kit for detecting furazolidone metabolite and application thereof |
| CN102565032A (en)* | 2010-12-15 | 2012-07-11 | 北京勤邦生物技术有限公司 | Magnetic granule chemiluminescence kit for detecting nitrofurantoin metabolite and application of magnetic granule chemiluminescence kit |
| CN102617816A (en)* | 2012-01-14 | 2012-08-01 | 北京化工大学 | Preparation method for chiral magnetic microspheres containing substituted acetylene polymer |
| CN102901820A (en)* | 2012-09-29 | 2013-01-30 | 江阴泽成生物技术有限公司 | Immunoassay kit and immunodetection method for magnetic particle chemiluminescence of human tumor marker carbohydrate antigen 50 (CA50) |
| CN102901812A (en)* | 2012-11-13 | 2013-01-30 | 江阴泽成生物技术有限公司 | Magnetic particle chemiluminescence immunoassay kit and assay method for human thyroglobulin antibodies (TGAb) |
| CN102914645A (en)* | 2012-11-12 | 2013-02-06 | 武汉伊艾博科技有限公司 | Method for fixing antibody in solid phase carrier in immune adsorption reaction process |
| CN103018445A (en)* | 2012-11-20 | 2013-04-03 | 博奥赛斯(天津)生物科技有限公司 | Chemiluminiscence immunoassay quantitative detection kit for saccharide antigen 50 magnetic particles and preparation method thereof |
| CN103033623A (en)* | 2012-12-10 | 2013-04-10 | 天津市协和医药科技集团有限公司 | Human M2 type pyruvate kinase chemiluminescence immune assay kit and preparation method |
| CN103048461A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminescent assay kit for cancer antigen CA15-3, and preparation method and detection method thereof |
| CN103048453A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminescence detection kit for carbohydrate antigen CA19-9 as well as preparation method thereof and detecting method thereof |
| CN103048452A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminiscence determination kit for antigen CA125 relating to tumor, as well as preparation method and determining method of same |
| CN103194430A (en)* | 2013-04-10 | 2013-07-10 | 深圳市菲鹏生物股份有限公司 | Hybridoma capable of secreting anti-CA125 protein monoclonal antibody and application of hybridoma |
| CN103293313A (en)* | 2012-02-24 | 2013-09-11 | 上海新波生物技术有限公司 | Carbohydrate antigen 15-3 time-resolved immunofluorescence assay and kit |
| CN103592445A (en)* | 2013-10-16 | 2014-02-19 | 北京利德曼生化股份有限公司 | Kit for detecting procalcitonin |
| CN103789271A (en)* | 2014-01-26 | 2014-05-14 | 东北林业大学 | Hybridoma for resisting generation of CA242 monoclonal antibody and preparation of chemiluminescence immune assay kit |
| CN104297492A (en)* | 2014-10-23 | 2015-01-21 | 沈鹤霄 | Marking method based on enzymatic cycling assay and application of marking method based on enzymatic cycling assay |
| CN104330577A (en)* | 2014-11-17 | 2015-02-04 | 南方医科大学南方医院 | C-reactive protein quantitative determination kit and preparation method thereof |
| CN104704373A (en)* | 2012-08-13 | 2015-06-10 | 皇家飞利浦有限公司 | Use of antioxidants in methods and means for detection of target molecules in a blood sample |
| CN104698184A (en)* | 2015-02-10 | 2015-06-10 | 深圳市新产业生物医学工程股份有限公司 | Kit for detecting carbohydrate antigen as well as detection method and application thereof |
| CN104807997A (en)* | 2015-05-05 | 2015-07-29 | 南京格耀生物科技有限公司 | Kit for detecting CA125 and SP17 contents based on chemiluminiscence method as well as method and application thereof |
| CN105353138A (en)* | 2015-12-17 | 2016-02-24 | 艾康生物技术(杭州)有限公司 | Methods for improving accuracy and expanding linear range of immunodetection as well as reagent |
| WO2017008179A1 (en)* | 2015-07-06 | 2017-01-19 | 山东博科生物产业有限公司 | Reagent for high throughput combined detection of hepatitis c virus antigen and antibody |
| CN108152484A (en)* | 2017-12-07 | 2018-06-12 | 珠海霍普金斯医药研究院股份有限公司 | A kind of chemiluminescence magnetic bead enzyme-linked immunization rapid quantitative detection reagent box of PD-L1 |
| CN110297091A (en)* | 2019-07-17 | 2019-10-01 | 中国农业科学院兰州兽医研究所 | A kind of multiple tube-type chemical luminescence detection kit of I type antibody of aftosa O, A, Asia |
| CN110297094A (en)* | 2019-07-01 | 2019-10-01 | 北京大学第一医院 | Detect kit, preparation method and the method for measuring afamin concentration of afamin concentration |
| CN111289759A (en)* | 2020-03-10 | 2020-06-16 | 苏州翊讯生物科技有限公司 | SAA detection kit and SAA quantitative detection method |
| CN111505302A (en)* | 2019-01-31 | 2020-08-07 | 博阳生物科技(上海)有限公司 | Homogeneous immunoassay kit for rapidly detecting N-terminal B-type brain natriuretic peptide, preparation method, detection method and device |
| CN112585445A (en)* | 2018-08-24 | 2021-03-30 | 深圳迈瑞生物医疗电子股份有限公司 | Blood sample analyzer, blood sample analyzing method, and computer storage medium |
| CN115725510A (en)* | 2022-08-10 | 2023-03-03 | 扬州大学 | Monoclonal antibody hybridoma cell strain 2A5 aiming at PCV2b type Cap protein, monoclonal antibody and application |
- 2007
- 2007-06-13CNCNA2007101188855Apatent/CN101324579A/enactivePending
Cited By (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102565032A (en)* | 2010-12-15 | 2012-07-11 | 北京勤邦生物技术有限公司 | Magnetic granule chemiluminescence kit for detecting nitrofurantoin metabolite and application of magnetic granule chemiluminescence kit |
| CN102565402A (en)* | 2010-12-16 | 2012-07-11 | 北京勤邦生物技术有限公司 | Magnetic particle chemiluminescence kit for detecting furazolidone metabolite and application thereof |
| CN102288766B (en)* | 2011-08-16 | 2013-10-09 | 内蒙古科慧生物科技有限责任公司 | Carbohydrate antigen 15-3 (CA15-3) quantitative measurement kit and detection method thereof |
| CN102288767A (en)* | 2011-08-16 | 2011-12-21 | 内蒙古科慧生物科技有限责任公司 | Saccharide antigen 72-4(CA72-4) quantitative determination kit and detection method |
| CN102288766A (en)* | 2011-08-16 | 2011-12-21 | 内蒙古科慧生物科技有限责任公司 | Carbohydrate antigen 15-3 (CA15-3) quantitative measurement kit and detection method thereof |
| CN102288767B (en)* | 2011-08-16 | 2013-12-04 | 内蒙古科慧生物科技有限责任公司 | Saccharide antigen 72-4(CA72-4) quantitative determination kit and detection method |
| CN102435739A (en)* | 2011-08-31 | 2012-05-02 | 内蒙古科慧生物科技有限责任公司 | Carbohydrate antigen 242(CA242) quantitative determination kit and detection method thereof |
| CN102435736A (en)* | 2011-11-29 | 2012-05-02 | 桂林理工大学 | Method for measuring antigen of ovarian cancer embryo by electrochemical luminescence (ECL) immunosensor |
| CN102617816A (en)* | 2012-01-14 | 2012-08-01 | 北京化工大学 | Preparation method for chiral magnetic microspheres containing substituted acetylene polymer |
| CN102617816B (en)* | 2012-01-14 | 2013-07-03 | 北京化工大学 | Preparation method for chiral magnetic microspheres containing substituted acetylene polymer |
| CN103293313A (en)* | 2012-02-24 | 2013-09-11 | 上海新波生物技术有限公司 | Carbohydrate antigen 15-3 time-resolved immunofluorescence assay and kit |
| CN104704373A (en)* | 2012-08-13 | 2015-06-10 | 皇家飞利浦有限公司 | Use of antioxidants in methods and means for detection of target molecules in a blood sample |
| US10591472B2 (en) | 2012-08-13 | 2020-03-17 | Minicare B.V. | Use of antioxidants in methods and means for detection of target molecules in a blood sample |
| CN102901820A (en)* | 2012-09-29 | 2013-01-30 | 江阴泽成生物技术有限公司 | Immunoassay kit and immunodetection method for magnetic particle chemiluminescence of human tumor marker carbohydrate antigen 50 (CA50) |
| CN102914645A (en)* | 2012-11-12 | 2013-02-06 | 武汉伊艾博科技有限公司 | Method for fixing antibody in solid phase carrier in immune adsorption reaction process |
| CN102901812A (en)* | 2012-11-13 | 2013-01-30 | 江阴泽成生物技术有限公司 | Magnetic particle chemiluminescence immunoassay kit and assay method for human thyroglobulin antibodies (TGAb) |
| CN103018445B (en)* | 2012-11-20 | 2015-09-02 | 博奥赛斯(天津)生物科技有限公司 | CA50 magnetic microparticle chemiluminescence immune quantitative detection reagent box and preparation method thereof |
| CN103018445A (en)* | 2012-11-20 | 2013-04-03 | 博奥赛斯(天津)生物科技有限公司 | Chemiluminiscence immunoassay quantitative detection kit for saccharide antigen 50 magnetic particles and preparation method thereof |
| CN103033623A (en)* | 2012-12-10 | 2013-04-10 | 天津市协和医药科技集团有限公司 | Human M2 type pyruvate kinase chemiluminescence immune assay kit and preparation method |
| CN103048452A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminiscence determination kit for antigen CA125 relating to tumor, as well as preparation method and determining method of same |
| CN103048453A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminescence detection kit for carbohydrate antigen CA19-9 as well as preparation method thereof and detecting method thereof |
| CN103048461A (en)* | 2012-12-18 | 2013-04-17 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminescent assay kit for cancer antigen CA15-3, and preparation method and detection method thereof |
| CN103048452B (en)* | 2012-12-18 | 2015-04-15 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminiscence determination kit for antigen CA125 relating to tumor, as well as preparation method and determining method of same |
| CN103048453B (en)* | 2012-12-18 | 2014-11-26 | 苏州浩欧博生物医药有限公司 | Nanometer magnetic particle chemiluminescence detection kit for carbohydrate antigen CA19-9 as well as preparation method thereof and detecting method thereof |
| CN103194430B (en)* | 2013-04-10 | 2014-10-15 | 深圳市菲鹏生物股份有限公司 | Hybridoma capable of secreting anti-CA125 protein monoclonal antibody and application of hybridoma |
| CN103194430A (en)* | 2013-04-10 | 2013-07-10 | 深圳市菲鹏生物股份有限公司 | Hybridoma capable of secreting anti-CA125 protein monoclonal antibody and application of hybridoma |
| CN103592445A (en)* | 2013-10-16 | 2014-02-19 | 北京利德曼生化股份有限公司 | Kit for detecting procalcitonin |
| CN103789271A (en)* | 2014-01-26 | 2014-05-14 | 东北林业大学 | Hybridoma for resisting generation of CA242 monoclonal antibody and preparation of chemiluminescence immune assay kit |
| CN104297492A (en)* | 2014-10-23 | 2015-01-21 | 沈鹤霄 | Marking method based on enzymatic cycling assay and application of marking method based on enzymatic cycling assay |
| CN104330577B (en)* | 2014-11-17 | 2016-05-18 | 南方医科大学南方医院 | A kind of preparation method of c reactive protein quantitative determination reagent kit |
| CN104330577A (en)* | 2014-11-17 | 2015-02-04 | 南方医科大学南方医院 | C-reactive protein quantitative determination kit and preparation method thereof |
| CN104698184A (en)* | 2015-02-10 | 2015-06-10 | 深圳市新产业生物医学工程股份有限公司 | Kit for detecting carbohydrate antigen as well as detection method and application thereof |
| CN104698184B (en)* | 2015-02-10 | 2017-03-01 | 深圳市新产业生物医学工程股份有限公司 | The test kit of detection sugar antigen |
| CN104807997A (en)* | 2015-05-05 | 2015-07-29 | 南京格耀生物科技有限公司 | Kit for detecting CA125 and SP17 contents based on chemiluminiscence method as well as method and application thereof |
| WO2017008179A1 (en)* | 2015-07-06 | 2017-01-19 | 山东博科生物产业有限公司 | Reagent for high throughput combined detection of hepatitis c virus antigen and antibody |
| CN105353138A (en)* | 2015-12-17 | 2016-02-24 | 艾康生物技术(杭州)有限公司 | Methods for improving accuracy and expanding linear range of immunodetection as well as reagent |
| CN108152484A (en)* | 2017-12-07 | 2018-06-12 | 珠海霍普金斯医药研究院股份有限公司 | A kind of chemiluminescence magnetic bead enzyme-linked immunization rapid quantitative detection reagent box of PD-L1 |
| CN112585445A (en)* | 2018-08-24 | 2021-03-30 | 深圳迈瑞生物医疗电子股份有限公司 | Blood sample analyzer, blood sample analyzing method, and computer storage medium |
| CN111505302A (en)* | 2019-01-31 | 2020-08-07 | 博阳生物科技(上海)有限公司 | Homogeneous immunoassay kit for rapidly detecting N-terminal B-type brain natriuretic peptide, preparation method, detection method and device |
| CN110297094A (en)* | 2019-07-01 | 2019-10-01 | 北京大学第一医院 | Detect kit, preparation method and the method for measuring afamin concentration of afamin concentration |
| CN110297091A (en)* | 2019-07-17 | 2019-10-01 | 中国农业科学院兰州兽医研究所 | A kind of multiple tube-type chemical luminescence detection kit of I type antibody of aftosa O, A, Asia |
| CN110297091B (en)* | 2019-07-17 | 2020-12-01 | 中国农业科学院兰州兽医研究所 | A multi-tubular chemiluminescence detection kit for foot-and-mouth disease O, A, Asia I antibodies |
| CN111289759A (en)* | 2020-03-10 | 2020-06-16 | 苏州翊讯生物科技有限公司 | SAA detection kit and SAA quantitative detection method |
| CN111289759B (en)* | 2020-03-10 | 2023-10-27 | 苏州翊讯生物科技有限公司 | SAA detection kit and SAA quantitative detection method |
| CN115725510A (en)* | 2022-08-10 | 2023-03-03 | 扬州大学 | Monoclonal antibody hybridoma cell strain 2A5 aiming at PCV2b type Cap protein, monoclonal antibody and application |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101324579A (en) | A magnetic microparticle chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigens and its application method | |
| CN101324578A (en) | An immunoradiological assay kit for detecting tumor marker CA50 and its application method | |
| CN104698184B (en) | The test kit of detection sugar antigen | |
| CN114594262B (en) | Mycotoxin magnetic chemiluminescence immunoassay kit based on bifunctional fusion protein and its application | |
| CN104530459A (en) | A preparation method of polystyrene fluorescent microspheres coupled with antibodies | |
| CN105693906A (en) | Zwitterionic polymer microspheres and preparing method thereof | |
| CN112269024A (en) | Detection reagent, test paper and kit for interleukin-6 and application thereof | |
| CN101639478A (en) | Kit for detecting estradiol by utilizing magnetic particle chemiluminescence immunoassay | |
| CN108344864A (en) | A kind of preparation and application of the chemiluminescence immunoassay probe based on dendrimer dual amplification label | |
| CN101614742A (en) | Luteinizing Hormone Magnetic Particle Chemiluminescent Immunoassay Assay Kit | |
| CN102323417B (en) | Kit for quantitative determination of pesinogen I (PGI) and detection method thereof | |
| CN104198721A (en) | Preparation and application of Golgi protein 73 (GP73) antigen silicon-based magnetic bead conjugate | |
| CN102707060B (en) | Chemiluminescent test kit for testing activity of MGMT (O6-Methylguanine DNA-Methyltransferase) and test method | |
| CN109239326A (en) | Based on the micro-fluidic immuno-chip analysis method of magnetic particle nano enzyme and application | |
| CN104677889B (en) | Method for detecting alpha fetoprotein by using magnetic immune probe based on luminol functionalization | |
| CN106568949B (en) | A kind of small haptens detection method based on direct competitive fluoroimmunoassay | |
| CN115753716A (en) | Fluorescence biosensor for detecting Golgi protein 73 | |
| Li et al. | Oriented immobilization of antibodies through peptide ligands for ultrasensitive detection of gastrin 17 in human serum via lateral flow immunoassay | |
| CN114563578A (en) | Human Membranous Nephropathy Diagnostic Kit | |
| CN106645686B (en) | One kind being directed to fumonisin B1Sensitive detection method | |
| CN109298178A (en) | Cardiac myosin binding protein C(cMyBP-C based on immunomagnetic beads) time-resolved fluoroimmunoassay kit | |
| CN109633163B (en) | procalcitonin/C reactive protein two-in-one detection kit | |
| CN112710826A (en) | Coating and sealing method for improving stability of reagent | |
| CN101377499B (en) | Nerve specificity olefinic alcohol enzyme chemiluminescence immune analysis determination reagent kit and preparing method thereof | |
| CN116165193A (en) | Carcinoembryonic antigen detection reagent and kit based on double-antibody sandwich chemiluminescence method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication | Open date:20081217 |