CN101132694B - Medical devices with coatings capable of trapping genetically altered cells and methods of use thereof - Google Patents
Medical devices with coatings capable of trapping genetically altered cells and methods of use thereofDownload PDFInfo
- Publication number
- CN101132694B CN101132694BCN200580012581XACN200580012581ACN101132694BCN 101132694 BCN101132694 BCN 101132694BCN 200580012581X ACN200580012581X ACN 200580012581XACN 200580012581 ACN200580012581 ACN 200580012581ACN 101132694 BCN101132694 BCN 101132694B
- Authority
- CN
- China
- Prior art keywords
- cell
- cells
- antibody
- endothelial
- gene product
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Landscapes
- Materials For Medical Uses (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Description
Translated fromChinese本申请要求提交于2004年4月30日的美国临时专利申请No.60/566,829和提交于2004年4月30日的美国临时专利申请No.10/835,767的优先权。This application claims priority to US Provisional Patent Application No. 60/566,829, filed April 30, 2004, and US Provisional Patent Application No. 10/835,767, filed April 30, 2004.
技术领域technical field
本发明涉及用于植入患者血管或中空器官的医疗装置,例如用于治疗各种疾病的经涂覆的支架、支架移植物、合成的血管移植物、心脏瓣膜,导管和血管修复滤筛(vascular prosthetic filter)。具体而言,本发明涉及在与血液接触的表面上具有涂层的医疗装置,所述涂层经工程改造可用于将细胞捕获于所述装置的表面。捕获的细胞可在所述装置的表面上形成单层并可用于许多治疗用途,例如药物递送系统和/或治疗血管疾病中。例如,结合于植入的医疗装置的细胞可为来自循环血的天然内皮祖细胞和/或在体外经遗传修饰的细胞,所述细胞在患者体内表达或分泌具有局部或全身治疗作用的分子或物质。The present invention relates to medical devices for implantation into blood vessels or hollow organs of patients, such as coated stents, stent grafts, synthetic vascular grafts, heart valves, catheters and vascular repair screens ( vascular prosthetic filter). In particular, the invention relates to medical devices having coatings on blood-contacting surfaces engineered to trap cells on the surface of the device. The captured cells can form a monolayer on the surface of the device and can be used in many therapeutic applications, such as in drug delivery systems and/or in the treatment of vascular diseases. For example, the cells incorporated into the implanted medical device may be native endothelial progenitor cells from circulating blood and/or genetically modified cells in vitro that express or secrete molecules with local or systemic therapeutic effects in the patient or substance.
发明背景Background of the invention
疾病中的动脉粥样硬化和癌症,是世界上导致死亡和丧失劳动能力的两种主要原因。动脉粥样硬化与动脉腔表面上的脂肪斑产生有关。这些脂肪斑造成动脉腔横截面狭窄。最终造成在病变部位远端的血流减少,导致受该动脉供血的组织发生缺血性损伤。Atherosclerosis and cancer, among diseases, are two of the leading causes of death and disability in the world. Atherosclerosis is associated with the development of fatty plaques on the luminal surface of arteries. These fatty plaques narrow the arterial lumen cross section. This ultimately results in reduced blood flow distal to the lesion, leading to ischemic damage to tissues supplied by the artery.
冠状动脉向心脏供血。在美国,冠状动脉粥样硬化或冠心病(CAD)是最常见的威胁生命的严重慢性疾病,患此病的人数超过1100万人。冠状动脉粥样硬化造成的社会和经济损失远远超过大多数其他疾病。冠状动脉内腔狭窄对心肌的影响,首先导致心绞痛,然后是心肌梗塞,最后导致死亡,这些患者中有30万人以上在抵达医院之前就已死亡。(《Harrison氏内科医学原理》,Harrison’sPrinciples of Internal Medicine,第14版,1998)。The coronary arteries supply blood to the heart. Coronary atherosclerosis, or coronary artery disease (CAD), is the most common life-threatening serious chronic disease affecting more than 11 million people in the United States. The social and economic costs of coronary atherosclerosis far exceed those of most other diseases. The impact of coronary lumen stenosis on the myocardium first causes angina pectoris, then myocardial infarction, and finally leads to death. More than 300,000 of these patients died before reaching the hospital. (Harrison's Principles of Internal Medicine , 14th Edition, 1998).
对于冠状动脉粥样硬化,可采用经皮腔内冠状动脉内成形术(PTCA)进行治疗。美国每年施行400,000例以上的PTCA手术。在PTCA中,将气囊导管插入外周动脉内,顺着动脉系统推进到受阻塞的冠状动脉。然后使气囊膨胀,将该段动脉管撑开,压平阻塞该处的脂肪斑块,从而增加流经该受损动脉截面的血流。然而,这种治疗通常不能使受损冠状动脉持久地维持张开状态。多达50%的接受PTCA治疗的患者需要在6个月内接受重复治疗以矫正冠状动脉的再狭窄。这种接受PTCA后动脉再度发生的狭窄医学上称为再狭窄。急性的再狭窄涉及血管的回弹和皱缩。血管回弹和皱缩后,中层平滑肌细胞发生增殖以应对由PTCA所引起的动脉损伤。平滑肌细胞的增殖部分由损伤部位释放的各种炎症因子所介导,这类炎症因子包括:血栓素A2、血小板衍生生长因子(PDGF)以及成纤维细胞生长因子(FGF)。已采用了多种不同技术来克服再狭窄问题,这些技术包括:用各种药剂对患者进行治疗、或用支架机械性地保持动脉张开。(《Harrison氏内科医学原理》,Harrison’s Principles of Internal Medicine,第14版,1998)。Coronary atherosclerosis can be treated with percutaneous transluminal coronary angioplasty (PTCA). More than 400,000 PTCA procedures are performed in the United States each year. In PTCA, a balloon catheter is inserted into a peripheral artery and advanced down the arterial system to the blocked coronary artery. The balloon is then inflated, stretching the section of arterial tube apart, flattening the fatty plaque blocking it, thereby increasing blood flow through the damaged section of the artery. However, this treatment usually does not keep the damaged coronary arteries open for long. Up to 50% of patients treated with PTCA require repeat treatment within 6 months to correct coronary restenosis. This reoccurrence of arterial narrowing after PTCA is medically called restenosis. Acute restenosis involves recoil and shrinkage of blood vessels. After vascular rebound and collapse, medial smooth muscle cells proliferate in response to arterial injury caused by PTCA. The proliferation of smooth muscle cells is partially mediated by various inflammatory factors released from the injury site, such inflammatory factors include: thromboxane A2, platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF). A number of different techniques have been used to overcome restenosis, including treating the patient with various agents, or mechanically keeping the artery open with stents. ("Harrison's Principles of Internal Medicine", Harrison's Principles of Internal Medicine, 14th ed., 1998).
已证明支架是克服再狭窄的各种治疗方法中最有效的方法。支架是安置于患病动脉区段内产生正常血管内腔的金属支撑架。将支架置于受损的动脉区段内可防止该动脉的回弹以及继而发生的动脉封闭。支架还可防止该动脉沿动脉中层的局部剥离。通过用支架使动脉内腔保持大于单独采用PTCA所形成的内腔,减少了多达30%的再狭窄。但是,尽管获得这样的成功,支架仍不能完全消除再狭窄。(Suryapranata等,1998。《对于经选择的急性心肌梗死患者中的冠脉支架术与气囊血管成形术的随机比较》, Randomized comparison ofcoronary stenting with balloon angioplasty in selectedpatients with acuteStents have proven to be the most effective of the various therapeutic approaches to overcome restenosis. A stent is a metal support that is placed within a segment of a diseased artery to create a normal vessel lumen. Placing a stent within a damaged segment of an artery prevents recoil of the artery and subsequent closure of the artery. The stent also prevents partial dissection of the artery along the arterial media. Restenosis was reduced by as much as 30% by using stents to maintain the lumen of the artery larger than that created by PTCA alone. However, despite this success, stents do not completely eliminate restenosis. (Suryapranata et al., 1998. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute
动脉狭窄也可发生在冠状动脉之外的其他血管,包括主动脉骼动脉、腹股沟下动脉、远侧深股动脉、远侧腘动脉、胫动脉、锁骨下动脉以及肠系膜动脉等。外周动脉的动脉粥样硬化症(PAD)患病率取决于受损的具体解剖部位,以及诊断阻塞所用的标准。传统上,医师采用间歇性跛行测试法来判定是否存在PAD。然而,这种方法可能大大低估了人群中此病的实际发病率。PAD的发病率随年龄而不同,老年个体PAD的发病率增高。据美国国立医院出院调查的数据估计,每年有55,000例男性和44,000例女性首次诊断为慢性PAD;而有60,000例男性和50,000例女性首次诊断为急性PAD。91%的急性PAD病例都涉及到下肢部位。在PAD患者中,伴发CAD的比率可能超过50%。此外,在PAD患者中,脑血管疾病发病率增高。Arterial stenosis can also occur in vessels other than the coronary arteries, including the aortoiliac artery, infrainguinal artery, distal deep femoral artery, distal popliteal artery, tibial artery, subclavian artery, and mesenteric artery. The prevalence of atherosclerosis (PAD) in peripheral arteries depends on the specific anatomical site of injury and the criteria used to diagnose the obstruction. Traditionally, physicians have used the intermittent claudication test to determine the presence of PAD. However, this approach may substantially underestimate the true incidence of the disease in the population. The incidence of PAD varies with age, with an increased incidence of PAD in older individuals. Data from the National Hospital Discharge Survey estimate that 55,000 men and 44,000 women are first diagnosed with chronic PAD and 60,000 men and 50,000 women are first diagnosed with acute PAD each year. 91% of acute PAD cases involve the lower extremities. In PAD patients, the rate of concomitant CAD may exceed 50%. In addition, in patients with PAD, the incidence of cerebrovascular disease is increased.
可采用经皮腔内气囊血管成形术(PTA)来治疗PAD。采用PTA并结合支架,可减少再狭窄症发病率。然而,在采用诸如支架的医疗装置所获得的术后效果,不如采用标准的血管形成术(即,采用静脉或修复用旁路材料)所获得的效果。(《外科学原理》,Principles of Surgery,Schwartz等编,第20章,“动脉疾病”,ArterialDisease,第7版,McGraw-Hill Health Professions Division,纽约1999)。PAD can be treated with percutaneous balloon angioplasty (PTA). The use of PTA combined with stents can reduce the incidence of restenosis. However, the postoperative results obtained with medical devices such as stents are not as good as those obtained with standard angioplasty (ie, with vein or prosthetic bypass material). ("Principles ofSurgery ", eds. Schwartz et al.,
优选采用建立旁路的方法治疗PAD,在该方法中用移植物绕过阻塞动脉区段建立旁路。(《外科学原理》,Principles of Surgery,Schwartz等编,第20章,“动脉疾病”,Arterial Disease,第7版,McGraw-Hill Health Professions Division,纽约1999)。所述移植物可包括:自体静脉区段(例如,隐静脉)或合成的移植物(例如,用聚酯、聚四氟乙烯(PTFE)或膨胀性聚四氟乙烯(ePTFE)或其他各种聚合物材料制备的移植物)。其术后血管开放率取决于多种不同因素,这些因素包括:旁路移植物的腔内径、用作移植物的合成材料类型,以及流出部位。然而,即便采用了旁路移植物,内膜过度增生以及血栓形成仍然是严重的问题。例如,采用ePTFE旁路移植的腹股沟下动脉旁路术3年后,股-腘旁路的血管开放率为54%,而股-胫旁路则仅为12%。PAD is preferably treated by a bypass approach in which a graft is bypassed around the blocked arterial segment. ("Principles of Surgery ", eds. Schwartz et al.,
因此,显然需要对支架性能、合成的旁路移植物以及其他长期血液接触表面和/或装置性能进行改进,以进一步减少CAD和PAD的发病率和病死率。例如,可采用能使血管径向扩张(向外或正性再塑)的方法来补偿动脉粥样硬化斑块的进行性生长,从而延迟血流受限狭窄(flow-limiting stenosis)的发展。Therefore, there is a clear need for improvements in stent performance, synthetic bypass grafts, and other long-term blood-contacting surfaces and/or device performance to further reduce CAD and PAD morbidity and mortality. For example, approaches that cause radial vessel dilation (outward or positive remodeling) can be used to compensate for progressive atherosclerotic plaque growth, thereby delaying the development of flow-limiting stenosis.
对于支架,已采用的方法是用各种抗凝血剂或抗再狭窄剂来涂覆支架,以减少血栓形成和再狭窄。例如,用放射性物质浸渍支架似乎可通过抑制成肌纤维母细胞的迁移和增殖而抑制再狭窄。(美国专利号5,059,166、5,199,939和5,302,168)。但照射所治疗的血管可引起患者严重的边缘再狭窄问题。此外,照射对受侵害血管处理不均匀。As for the stent, the method that has been adopted is to coat the stent with various anticoagulants or antirestenosis agents in order to reduce thrombus formation and restenosis. For example, impregnating scaffolds with radioactive material appears to inhibit restenosis by inhibiting the migration and proliferation of myofibroblasts. (US Patent Nos. 5,059,166, 5,199,939, and 5,302,168). However, irradiating the treated vessel can cause severe marginal restenosis in patients. In addition, irradiation does not treat affected vessels uniformly.
或者,也已采用化学药剂涂覆支架,例如肝素、磷酸胆碱、雷帕霉素和紫杉酚,所有这些药品都显示可缓减血栓形成和/或再狭窄。虽然肝素和磷酸胆碱显示可在短时明显减少动物模型的血栓形成,但是这类药剂对预防再狭窄似乎没有长时间效果。此外,肝素可能诱发血小板减少症,导致严重的血栓并发症,例如中风。因此,在这种治疗再狭窄的实践方式中,用足够治疗有效量的肝素或磷酸胆碱装载的支架并不可行。Alternatively, stents have also been coated with chemical agents such as heparin, phosphorylcholine, rapamycin, and paclitaxel, all of which have been shown to slow thrombosis and/or restenosis. Although heparin and phosphorylcholine have been shown to significantly reduce thrombosis in animal models in the short term, these agents do not appear to have long-term effects in preventing restenosis. In addition, heparin may induce thrombocytopenia, leading to serious thrombotic complications such as stroke. Therefore, stents loaded with sufficient therapeutically effective amounts of heparin or phosphorylcholine are not feasible in this practice of treating restenosis.
已采用合成的移植物以多种方式治疗来减少术后的再狭窄和血栓形成。(Boe等,1998。《小直径血管移植物修复术:现状》,Small-Diameter Vascular GraftProstheses:Current Status,Archives Physio.Biochem,106:100-115)。例如,据报道,聚氨酯合成物(例如,多孔性聚碳酸酯氨基甲酸乙酯)减少再狭窄的作用与ePTFE移植物相当。还采用了射频辉光放电对所述移植物的表面进行改性,以使聚对二苯甲酸酯移植物氟化。还采用了生物分子浸渍合成的移植物。然而,这些方法无一能长时期显著降低血栓形成或再狭窄发生率。Synthetic grafts have been used in various ways to reduce postoperative restenosis and thrombosis. (Boe et al., 1998. Small-Diameter Vascular Graft Prostheses: Current Status,Archives Physio. Biochem, 106: 100-115). For example, polyurethane compositions (eg, porous polycarbonate urethane) have been reported to reduce restenosis comparable to ePTFE grafts. Radiofrequency glow discharge was also used to modify the surface of the grafts to fluorinate the polyterephthalate grafts. Synthetic grafts impregnated with biomolecules have also been employed. However, none of these approaches significantly reduced the incidence of thrombosis or restenosis over the long term.
内皮细胞(EC)层是正常血管壁的一种重要组分,它提供了血流与血管壁周围组织之间的界面。内皮细胞还可参与生理活动,包括:血管生成、炎症过程以及防止血栓形成(Rodgers GM.FASSEB J 1988;2:116-123)。近年的研究发现,内皮细胞除了组成脉管系统之外,出生后的EC和内皮祖细胞(EPC)还在外周血中进行循环。(Asahara T等。Science,1997;275:964-967;Yin AH,等。Blood,1997;90:5002-5012;Shi Q,等。Blood,1998;92:362-367;Gehling UM,等,Blood,2000;95:3106-3112;Lin Y等,J Clin Invest,2000;105:71-77)。据认为EPC可迁移到循环系统受损内皮层区域,包括创伤和局部缺血性损伤部位(Takahashi T等,Nat Med,1999;5:434-438)。在正常的成年人中,外周血中的EPC浓度为3-10个/mm3(Tkahashi T等,Nat Med,1999;5:434-438;Kalka C,等,Ann Thorac Surg,2000;70:629-834)。如今已经证明,血管对于损伤应答的每一阶段都受到内皮组织的影响(如果不进行控制的话)。据信,在受损且安置支架的血管区段迅速重建功能性内皮层可通过提供对循环细胞因子的屏障、防止血栓的不良影响以及它们所具有的产生保护下层平滑肌细胞层的各种物质的能力,而有助于防止这些潜在的严重并发症。(Van Belle等,1997;《支架的内皮成形术》,Stent Endothelialization,Circulation,95:438-448;Bos等,1998。《小直径血管移植物修复术:现状》,Small-Diameter Vascular Graft Prostheses:Current Status,Archives Physio.Biochem.106:100-115)。The endothelial cell (EC) layer is an important component of normal vessel walls, providing the interface between the blood flow and the tissues surrounding the vessel wall. Endothelial cells are also involved in physiological activities including: angiogenesis, inflammatory processes, and prevention of thrombosis (Rodgers GM. FASSEB J 1988; 2: 116-123). Recent studies have found that, in addition to endothelial cells making up the vasculature, postnatal EC and endothelial progenitor cells (EPC) also circulate in peripheral blood. (Asahara T et al. Science, 1997; 275:964-967; Yin AH, et al. Blood, 1997; 90:5002-5012; Shi Q, et al. Blood, 1998; 92:362-367; Gehling UM, et al. Blood, 2000; 95:3106-3112; Lin Y et al., J Clin Invest, 2000; 105:71-77). EPCs are thought to migrate to damaged endothelial regions of the circulatory system, including sites of trauma and ischemic injury (Takahashi T et al., Nat Med, 1999; 5:434-438). In normal adults, the EPC concentration in peripheral blood is 3-10/mm3 (Tkahashi T et al., Nat Med, 1999; 5:434-438; Kalka C, et al., Ann Thorac Surg, 2000; 70: 629-834). It has now been demonstrated that every stage of the vascular response to injury is influenced, if not controlled, by the endothelium. It is believed that rapid re-establishment of a functional endothelial layer in damaged and stented vessel segments can be achieved by providing a barrier to circulating cytokines, preventing the adverse effects of thrombus and their ability to produce various substances that protect the underlying smooth muscle cell layer. ability to help prevent these potentially serious complications. (Van Belle et al., 1997; "Endothelialization of Stents", Stent Endothelialization,Circulation , 95:438-448; Bos et al., 1998. "Small-Diameter Vascular Graft Prostheses: Current Status", Small-Diameter Vascular Graft Prostheses: Current Status,Archives Physio.Biochem. 106:100-115).
在植入支架后,通过局部递送血管内皮细胞生长因子(VEGF,一种内皮细胞促有丝分裂剂),可促进内皮细胞在支架表面的生长(Van Belle等,1997;《支架的内皮成形术》,Stent Endothelialization,Circulation,95:438-448)。虽然在受损部位可应用重组蛋白质生长因子VEGF的盐水溶液诱发所需的作用效果,VEGF可在植入支架后用通道气囊导管递送。但此项技术并不理想,这是因为据证实单次递送剂量的效果太低不能产生一致的效果。因而,这种方法不能每次精确地重现效果。The growth of endothelial cells on the surface of the stent is promoted by local delivery of vascular endothelial cell growth factor (VEGF, an endothelial cell mitogen) after implantation of the stent (Van Belle et al., 1997; Endothelioplasty of Scaffolds, vol. Stent Endothelialization,Circulation, 95:438-448). Although saline solution of the recombinant protein growth factor VEGF can be used to induce the desired effect at the site of injury, VEGF can be delivered with a tunnel balloon catheter after implantation of the stent. But this technique is not ideal because the effect of a single delivered dose has proven to be too low to produce a consistent effect. Thus, this method cannot reproduce the effect exactly every time.
也已采用内皮细胞种植合成材料移植物,但是种植内皮细胞的临床效果通常都很差,即术后的管腔张开率低(Lio等,1998,《微血管移植术的新概念和新材料:修复性移植物的内皮细胞种植和基因治疗》,New concepts and Materials inMicrovascular Grafting:Prosthetic Graft Endothelial Cell Seeding and GeneTherapy,Microsurgery,18:263-256),其原因极可能是细胞未能妥善地黏附在移植物上和/或由于离体操作致使细胞丧失了其EC功能。Endothelial cells have also been used to plant synthetic material grafts, but the clinical effect of planting endothelial cells is usually very poor, that is, the postoperative lumen opening rate is low (Lio et al., 1998, "New Concepts and New Materials for Microvascular Grafting: Endothelial Cell Seeding and Gene Therapy for Prosthetic Grafts", New concepts and Materials in Microvascular Grafting: Prosthetic Graft Endothelial Cell Seeding and GeneTherapy, Microsurgery, 18: 263-256), the reason is most likely that the cells did not adhere properly to the graft Cells lose their EC function either physically and/or as a result of ex vivo manipulations.
因而,血管损伤部位内皮细胞的黏附、生长以及分化的调节中,原位内皮细胞生长因子以及环境条件是至关重要的。据此,对于再狭窄和其它血管疾病,有必要开发出用于涂覆包括支架和合成的移植物在内的医疗装置的新的方法和组合物,以促进和加速在植入装置上形成功能性内皮,从而在靶血管区段或植入的内腔上形成连续的EC单层,由此抑制新生内膜过度增生。Thus, in situ endothelial cell growth factors and environmental conditions are critical in the regulation of endothelial cell adhesion, growth, and differentiation at sites of vascular injury. Accordingly, for restenosis and other vascular diseases, there is a need to develop new methods and compositions for coating medical devices, including stents and synthetic grafts, to facilitate and accelerate functional formation on implanted devices. Neointimal hyperplasia is inhibited by forming a continuous EC monolayer over the target vessel segment or implanted lumen.
关于癌症等疾病,目前大部分治疗药物会对患者产生全身系统性反应,由于使用常规口服或静脉注射剂型的药物,不仅会影响癌细胞,还会影响体内的任何分裂性细胞(dividing cell)。在许多情况下,由于需治疗的疾病的性质和药物的性质(例如溶解性、体内稳定性、生物利用度等),全身性给药并不有效。在全身性给药时,药物经血液循环运输并分布入包括正常组织在内的机体区域。在病变部位,药物浓度起初低而无效,频繁给药后会提高到毒性水平,而在非病变区域,药物的存在则导致不良的副作用。在某些情况下,药物在给药后易于受代谢降解。因此,常通过提高药物剂量来获得药效并延长时间,这就造成正常组织系统负担的增加以及与患者相关费用的增加。在其它情况下,由于某些有效药物的毒副作用,使其治疗潜能不能完全发挥。With regard to diseases such as cancer, most of the current therapeutic drugs will cause systemic reactions to the patient. Due to the use of conventional oral or intravenous dosage forms of drugs, they will not only affect cancer cells, but also affect any dividing cells in the body. In many cases, systemic administration is not effective due to the nature of the disease being treated and the properties of the drug (eg, solubility, in vivo stability, bioavailability, etc.). When administered systemically, the drug is transported by the blood circulation and distributed into regions of the body including normal tissues. In lesion sites, drug concentrations are initially low and ineffective, increasing to toxic levels after frequent dosing, while in non-lesioned areas, the presence of drug leads to unwanted side effects. In certain instances, the drug is susceptible to metabolic degradation after administration. Therefore, the drug effect is often obtained by increasing the drug dose and prolonging the time, which causes an increase in the burden on the normal tissue system and an increase in the cost associated with the patient. In other cases, the full therapeutic potential of certain effective drugs is not achieved due to toxic side effects.
因此,已做了许多努力来提高药物递送系统的效力和靶向性。例如,采用脂质体递送药物具有的优点在于通常它们能延长药物在血液中的循环时间、通过限制血流中游离药物的浓度来降低副作用、减少药物降解、延长每次给药后的治疗作用、降低对给药频度的需求以及减少所需药物的量。然而,目前可得到的脂质体系统显示在体内将药物递送到靶位点的效果有限。参见Kaye等,1979,Poznansky等,1984,美国专利5,043,165和美国专利4,920,016。Therefore, many efforts have been made to improve the efficacy and targeting of drug delivery systems. For example, the use of liposomes to deliver drugs has advantages in that they generally prolong the time the drug circulates in the blood, reduce side effects by limiting the concentration of free drug in the bloodstream, reduce drug degradation, and prolong the therapeutic effect after each dose , reducing the need for frequency of dosing and reducing the amount of drug needed. However, currently available liposome systems have shown limited effectiveness in delivering drugs to target sites in vivo. See Kaye et al., 1979, Poznansky et al., 1984, US Patent 5,043,165 and US Patent 4,920,016.
为实现对治疗性化合物的高效递送,开发了能掺入转基因DNA的病毒载体,然而临床应用成功的数量仍有限。除了体外和动物模型中所获得的成功数外,已提出将基因转移技术与细胞治疗相结合。将离体基因转移入各种细胞类型中有可能证明比直接体用载体转移具有更大的治疗可行性。参见Kohn等,1987,Bilbao等,1997,和Giannoukakis等,2003。To achieve efficient delivery of therapeutic compounds, viral vectors that incorporate transgenic DNA have been developed, however, the number of successful clinical applications is still limited. In addition to the number of successes obtained in vitro and in animal models, it has been proposed to combine gene transfer techniques with cell therapy. Ex vivo gene transfer into various cell types has the potential to prove greater therapeutic viability than direct in vivo vector transfer. See Kohn et al., 1987, Bilbao et al., 1997, and Giannoukakis et al., 2003.
最近,已开发了局部药物递送运载体,例如药物洗脱支架(drug elutingstent,DES)。参见美国专利6,273,913、美国专利6,258,121和美国专利6,231,600。然而,现有技术中的药物洗脱支架受到许多因素的限制,例如,药物类型、要释放的药物量和释放药物的时间。关于药物洗脱支架需要考虑的其它因素是药物与其它支架涂覆成分(例如聚合物材料)间的相互作用、以及个体药物的性质(例如亲脂性、分子量、灭菌后的完整性和活性、以及效力和毒性)。至于药物洗脱支架中的聚合物材料,必须考虑的是聚合物的类型、聚合比例、装载药物的能力和该聚合物的生物相容性,以及药物-聚合物的相容性(例如,药物的药代动力学)。More recently, local drug delivery vehicles, such as drug eluting stents (DES), have been developed. See US Patent 6,273,913, US Patent 6,258,121 and US Patent 6,231,600. However, drug-eluting stents in the prior art are limited by many factors, such as the type of drug, the amount of drug to be released, and the time of releasing the drug. Other factors to consider with respect to drug-eluting stents are drug interactions with other stent coating components (e.g., polymeric materials), and properties of individual drugs (e.g., lipophilicity, molecular weight, integrity and activity after sterilization, and potency and toxicity). As for polymeric materials in drug-eluting stents, considerations must be given to the type of polymer, the polymerization ratio, the ability to load drug and the biocompatibility of the polymer, as well as drug-polymer compatibility (e.g., drug pharmacokinetics).
此外,药物洗脱支架中的药物剂量是预装载的,不能实现对个体条件和需求的药物剂量调节。至于药物释放时间,药物洗脱支架一旦植入后立即开始释放药物,而不能实现理想的实时释放。In addition, the drug dose in the drug-eluting stent is preloaded, which cannot realize the adjustment of the drug dose to individual conditions and needs. As for the drug release time, the drug-eluting stent starts to release the drug immediately after implantation, and cannot achieve ideal real-time release.
因此,迫切需要开发出一种有效的全身性和局部药物递送系统,以克服目前可用技术的缺陷。本发明正是提供了一种安和且受控方式进行局部或全身性递送治疗药物的系统。Therefore, there is an urgent need to develop an effective systemic and localized drug delivery system to overcome the shortcomings of currently available technologies. It is precisely this invention that provides a system for the local or systemic delivery of therapeutic agents in a safe and controlled manner.
发明概述Summary of the invention
本发明的一个目的是提供一种治疗性药物递送系统以及治疗患者疾病的方法。该治疗或药物递送系统包括具有涂层的医疗装置,该涂层由含有至少一类能识别并结合靶细胞的配体的基质组成,所述靶细胞为例如:内皮祖细胞或遗传改变的哺乳动物细胞、以及已经受到单一或双重转染的遗传改变的哺乳动物细胞。It is an object of the present invention to provide a therapeutic drug delivery system and method of treating a disease in a patient. The therapeutic or drug delivery system includes a medical device having a coating consisting of a matrix containing at least one type of ligand that recognizes and binds target cells, such as endothelial progenitor cells or genetically altered mammalian Animal cells, and genetically altered mammalian cells that have been subjected to single or double transfection.
本发明的医疗装置可为任何可植入患者的装置。例如,在一个实施方式中,所述装置是可插入血管内腔或中空器官的装置,如支架、支架植入物、心脏瓣膜、导管、血管修复滤筛、人造心脏、外置和内置左心室辅助装置(LVAD)和合成血管移植物,它们用于治疗以下疾病:例如癌症;血管疾病,包括再狭窄、动脉粥样硬化、血栓形成、血管梗阻;或这些装置所覆盖的其它任何用途。The medical device of the present invention can be any device that is implantable in a patient. For example, in one embodiment, the device is a device that can be inserted into the lumen of a blood vessel or a hollow organ, such as a stent, stent graft, heart valve, catheter, vascular repair screen, artificial heart, external and internal left ventricle Assistive devices (LVADs) and synthetic vascular grafts for the treatment of diseases such as cancer; vascular disease, including restenosis, atherosclerosis, thrombosis, vascular obstruction; or any other use covered by these devices.
在一个实施方式中,所述医疗装置上的涂层包括:生物相容性基质和至少一种底物或配体,所述底物或配体特异性识别并结合靶细胞(例如内皮祖细胞),以例如预防或治疗再狭窄,或使遗传改变的哺乳动物细胞粘附到所述装置的表面,以例如治疗血管重构和癌症。In one embodiment, the coating on the medical device comprises: a biocompatible matrix and at least one substrate or ligand that specifically recognizes and binds to target cells (e.g. endothelial progenitor cells) ), for example to prevent or treat restenosis, or to adhere genetically altered mammalian cells to the surface of the device, for example to treat vascular remodeling and cancer.
此外,所述医疗装置的涂层可任选地包含至少一种能调节遗传改变细胞的工程改造基因表达和分泌的活性化合物。能激活化合物的活化剂的例子包括但不限于:化学分子和肽,例如生长因子。在涂层包含至少一种化合物的实施方式中,刺激物、活化分子或化合物可用于刺激细胞表达和/或分泌至少一种可治疗疾病的治疗物。In addition, the coating of the medical device may optionally comprise at least one active compound capable of modulating the expression and secretion of the engineered gene of the genetically altered cell. Examples of activators capable of activating compounds include, but are not limited to, chemical molecules and peptides, such as growth factors. In embodiments where the coating comprises at least one compound, the stimulant, activating molecule or compound may be used to stimulate the cell to express and/or secrete at least one disease-treatable therapeutic.
在一个实施方式中,医疗装置上的涂层包括带有外表面的生物相容性基质,所述外表面用于附着治疗有效量的至少一种配体(例如,抗体、抗体片段、或抗体和抗体片段的组合)或至少一种能结合遗传改性细胞表面的工程改造标记的分子。本发明的抗体或抗体片段识别并结合细胞膜或靶细胞表面的抗原或特异性经遗传工程改造的细胞表面标记,从而使细胞固定在所述装置的表面。在一个实施方式中,所述涂层可任选地包含有效量的用于刺激内皮祖细胞固定的至少一种化合物,如果靶细胞是循环祖细胞的话,则可促进成熟、功能化内皮的形成,或者如果靶标是医疗装置表面上的遗传改变细胞的话,则可刺激结合的细胞表达并分泌所需的基因产物。In one embodiment, the coating on the medical device comprises a biocompatible matrix with an outer surface for attachment of a therapeutically effective amount of at least one ligand (e.g., antibody, antibody fragment, or antibody and antibody fragments) or at least one molecule capable of binding an engineered marker on the surface of a genetically modified cell. The antibodies or antibody fragments of the present invention recognize and bind to antigens or specific genetically engineered cell surface markers on the cell membrane or the surface of target cells, thereby immobilizing the cells on the surface of the device. In one embodiment, the coating may optionally comprise an effective amount of at least one compound for stimulating fixation of endothelial progenitor cells and, if the target cells are circulating progenitor cells, promoting the formation of mature, functional endothelium , or if the target is a genetically altered cell on the surface of the medical device, the bound cells can be stimulated to express and secrete the desired gene product.
本发明的医疗装置可为用于植入包含腔道的器官或机体部分的任何装置,也可为但不限于:支架、支架移植物、合成的血管移植物、心脏瓣膜、导管、血管修复滤筛、起搏器、起搏器前导物、除颤器、卵园孔未闭(PFO)中隔闭合装置、血管夹、血管动脉瘤闭锁器、血液透析移植物、血液透析导管、房室分流器、主动脉血管瘤移植物装置或组件、静脉瓣、缝线、血管吻合夹、留置式静脉或动脉导管、血管鞘和药物输送口。根据装置的不同,所述装置可用各种材料制成。例如,本发明的支架可用不锈钢、镍钛合金(NiTi)或铬合金以及生物可降解材料制成。合成的血管移植物可用交联PVA水凝胶、聚四氟乙烯(PTFE)、膨胀性聚四氟乙烯(ePTFE)、多孔型高密度聚乙烯(HDPE)、聚氨酯以及聚对苯二甲酸乙烯酯或生物可降解材料制成。The medical device of the present invention may be any device for implantation into an organ or body part containing a lumen, and may be, but is not limited to: stents, stent-grafts, synthetic vascular grafts, heart valves, catheters, vascular prosthetic filters Screens, pacemakers, pacemaker leads, defibrillators, patent foramen ovale (PFO) septal closure devices, vascular clips, vascular aneurysm sealers, hemodialysis grafts, hemodialysis catheters, atrioventricular shunts devices, aortic aneurysm graft devices or components, venous valves, sutures, vascular anastomotic clips, indwelling venous or arterial catheters, vascular sheaths, and drug delivery ports. Depending on the device, the device can be made of various materials. For example, the stents of the present invention can be made of stainless steel, nickel-titanium (NiTi) or chromium alloys, as well as biodegradable materials. Synthetic vascular grafts are available in cross-linked PVA hydrogels, polytetrafluoroethylene (PTFE), expanded polytetrafluoroethylene (ePTFE), porous high-density polyethylene (HDPE), polyurethane, and polyethylene terephthalate or biodegradable materials.
形成所述医疗装置涂层的生物相容性基质包含但不限于:合成材料,例如,聚氨酯、嵌段聚氨酯-尿素/肝素、聚-L-乳酸、纤维素酯、聚乙二醇、聚乙酸乙烯酯、葡聚糖或明胶;和/或天然存在的材料,例如胶原、弹性蛋白、弹性蛋白原、层连蛋白、纤连蛋白、玻连蛋白之类的基底膜组分,肝素、血纤蛋白、纤维素以及无定形碳或富勒烯(fullerene)。Biocompatible matrices forming the medical device coating include, but are not limited to: synthetic materials such as polyurethane, segmented polyurethane-urea/heparin, poly-L-lactic acid, cellulose esters, polyethylene glycol, polyacetic acid Vinyl esters, dextran, or gelatin; and/or naturally occurring materials such as basement membrane components such as collagen, elastin, tropoelastin, laminin, fibronectin, vitronectin, heparin, fibronectin Proteins, cellulose, and amorphous carbon or fullerenes.
在本发明的一个实施方式中,所述医疗装置中含有包含富勒烯的生物相容性基质。在这一实施方式中,富勒烯的碳原子数可为约C20-C150,更具体而言,所述富勒烯为C60或C70。本发明的富勒烯也可在医疗装置的表面上排列成纳米管(nanotube)。In one embodiment of the invention, the medical device contains a biocompatible matrix comprising fullerenes. In this embodiment, the fullerene may have a carbon number of about C20 -C150 , more specifically, the fullerene is C60 or C70 . The fullerenes of the present invention can also be arranged as nanotubes on the surface of medical devices.
在本发明的一个实施方式中,将所述配体施加于医疗装置与血液接触的表面,所述配体能特异性识别并结合循环血中所需组分或靶细胞表面上的表位。在一个实施方式中,所述配体专门设计为通过仅识别遗传改变细胞的细胞膜上的经遗传工程改造的标记分子,而只能识别并结合遗传改变的哺乳动物细胞。靶细胞的结合将该细胞固定在装置的表面上。In one embodiment of the invention, the ligand is applied to the blood-contacting surface of the medical device, the ligand being capable of specifically recognizing and binding to a desired component in circulating blood or an epitope on the surface of a target cell. In one embodiment, the ligand is specifically designed to recognize and bind only genetically altered mammalian cells by recognizing only genetically engineered marker molecules on the cell membrane of the genetically altered cells. Binding of target cells immobilizes the cells on the surface of the device.
在一个实施方式中,根据经遗传工程改造的细胞膜标记分子来选择医疗装置表面上用于结合遗传改变的细胞的配体。也就是说,所述配体仅与由提供给该细胞的染色体外遗传物质表达的细胞膜标记分子或抗原结合,从而使得所述医疗装置表面上的配体仅识别经遗传修饰的细胞。通过这种方式,只有经遗传修饰的细胞才能结合到医疗装置的表面。例如,如果所述哺乳动物细胞是内皮细胞,所述配体可为至少一种抗体、抗体片段或它们的组合;特异性地产生所述抗体针对靶细胞表面上的特异性靶表位或标记分子。在本发明的这一方面中,所述抗体可为单克隆抗体、多克隆抗体、嵌合抗体或人源化抗体,其通过与遗传改变的内皮细胞的表面标记分子相互反应而仅识别并结合该遗传改变的内皮细胞,并由此调节该细胞以使其粘附到医疗装置表面。可将本发明的抗体或抗体片段共价或非共价连接于基质的表面,或通过接头分子共价连接到涂覆医疗装置的基质的最外层。在这一实施方式中,例如,单克隆抗体还可包括Fab或F(ab’)2片段。本发明的抗体片段包含任何大小的片段,例如,保留了抗体识别并结合靶抗原特性的大分子和小分子。In one embodiment, ligands on the surface of the medical device for binding to genetically altered cells are selected based on genetically engineered cell membrane marker molecules. That is, the ligand only binds to cell membrane marker molecules or antigens expressed by the extrachromosomal genetic material provided to the cell, such that the ligand on the surface of the medical device recognizes only genetically modified cells. In this way, only genetically modified cells are bound to the surface of the medical device. For example, if the mammalian cell is an endothelial cell, the ligand may be at least one antibody, antibody fragment, or combination thereof; the antibody is specifically produced against a specific target epitope or marker on the surface of the target cell molecular. In this aspect of the invention, the antibody may be a monoclonal, polyclonal, chimeric or humanized antibody that recognizes and binds only The genetically altered endothelial cells, thereby conditioning the cells to adhere to the surface of the medical device. Antibodies or antibody fragments of the invention may be covalently or non-covalently attached to the surface of a substrate, or covalently attached via a linker molecule to the outermost layer of a substrate coating a medical device. In this embodiment, for example, the monoclonal antibody may also comprise a Fab or F(ab')2 fragment. Antibody fragments of the present invention include fragments of any size, eg, macromolecules and small molecules that retain the properties of an antibody to recognize and bind a target antigen.
在另一实施方式中,本发明的抗体或抗体片段能特异性地识别并结合被治疗的哺乳动物的抗原,而它们的特异性并不取决于细胞系。在一个实施方式中,例如,在治疗再狭窄中当细胞不经遗传修饰而含有特异性细胞膜标记分子时,所述抗体或片段能特异性地选择并结合循环内皮祖细胞的表面抗原,例如CD133、CD34、CDw90、CD117、HLA-DR、VEGFR-1、VEGFR-2、Muc-18(CD146)、CD130、干细胞抗原(Sca-1)、干细胞因子1(SCF/c-Kit配体)、Tie-2、MHC(例如,H-2Kk和HAD-DR)。In another embodiment, the antibodies or antibody fragments of the invention are capable of specifically recognizing and binding to an antigen in the treated mammal, and their specificity is not dependent on the cell line. In one embodiment, the antibody or fragment is capable of specifically selecting and binding to a surface antigen of circulating endothelial progenitor cells, such as CD133, for example, in the treatment of restenosis when the cells are not genetically modified to contain specific cell membrane marker molecules , CD34, CDw90, CD117, HLA-DR, VEGFR-1, VEGFR-2, Muc-18 (CD146), CD130, stem cell antigen (Sca-1), stem cell factor 1 (SCF/c-Kit ligand), Tie -2. MHC (eg, H-2Kk and HAD-DR).
在另一实施方式中,所述医疗装置的涂层包含至少一层上述的生物相容性基质,该基质含有用于附着治疗有效量的至少一种天然或合成小分子的外表面。所述小分子能识别例如,再狭窄治疗中的内皮祖细胞,并与其相互作用,而将该细胞固定在装置表面从而形成内皮层。所述小分子可用于与该医疗装置联合治疗各种疾病,并可源自各种来源(例如细胞成分,如脂肪酸、蛋白质、核酸、糖类等),并能与内皮祖细胞表面上的抗原相互作用产生与抗体相同的结果或效果。在本发明的这一方面,医疗装置上的涂层还可包含化合物,例如连接于包含抗体或抗体片段的涂层上的本文上述的生长因子。In another embodiment, the coating of the medical device comprises at least one layer of the biocompatible matrix described above, the matrix having an outer surface for attachment of a therapeutically effective amount of at least one natural or synthetic small molecule. The small molecule recognizes and interacts with endothelial progenitor cells, eg, in the treatment of restenosis, to immobilize the cells on the surface of the device to form the endothelial layer. The small molecules can be used in combination with the medical device to treat various diseases and can be derived from various sources (e.g., cellular components such as fatty acids, proteins, nucleic acids, carbohydrates, etc.) and can bind to antigens on the surface of endothelial progenitor cells. The interaction produces the same result or effect as the antibody. In this aspect of the invention, the coating on the medical device may also comprise a compound, such as a growth factor as described herein, attached to the coating comprising the antibody or antibody fragment.
在一个实施方式中,本发明涂层中的化合物(例如用于再狭窄治疗中),包括可刺激或促进祖细胞生长和分化成为成熟的功能性内皮细胞的任何化合物。在另一实施方式中,所述化合物用于刺激经遗传修饰的细胞表达并分泌所需基因产物。例如,用于本发明中的化合物可为生长因子,例如血管内皮生长因子(VEGF)、碱性成纤维细胞生长因子、血小板诱导的生长因子、转化生长因子β1、酸性成纤维细胞生长因子、骨连接素、血管生成素1(Ang-1)、血管生成素2(Ang-2)、胰岛素样生长因子、粒细胞巨噬细胞集落刺激因子、血小板衍生生长因子AA、血小板衍生生长因子BB、血小板衍生生长因子AB以及内皮PAS蛋白1。In one embodiment, compounds in the coatings of the invention (eg, for use in the treatment of restenosis) include any compound that stimulates or promotes the growth and differentiation of progenitor cells into mature, functional endothelial cells. In another embodiment, the compounds are used to stimulate genetically modified cells to express and secrete a desired gene product. For example, compounds useful in the present invention can be growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, platelet-induced growth factor, transforming growth factor beta 1, acidic fibroblast growth factor, bone Connexin, angiopoietin 1 (Ang-1), angiopoietin 2 (Ang-2), insulin-like growth factor, granulocyte-macrophage colony-stimulating factor, platelet-derived growth factor AA, platelet-derived growth factor BB, platelets Derived growth factor AB and endothelial PAS protein 1.
在另一实施方式中,例如当使用遗传改变的哺乳动物细胞时,可用于刺激细胞表达并分泌经遗传工程改造的基因产物的激活剂或化合物,包括但不限于:雌激素、四环素和其它抗生素、他莫昔芬等,可经各种给药途径提供给患者,例如以贴片和皮下形式通过皮肤给药。In another embodiment, such as when genetically altered mammalian cells are used, activators or compounds that can be used to stimulate the cells to express and secrete genetically engineered gene products include, but are not limited to: estrogens, tetracyclines, and other antibiotics , tamoxifen, etc., can be provided to patients through various routes of administration, such as through the skin in the form of a patch and subcutaneously.
本发明还提供用于治疗各种疾病的方法,所述疾病为例如:血管疾病、癌症、血管重构、严重冠心病、动脉粥样硬化、再狭窄、血栓形成、动脉瘤和血管梗阻。在一个实施方式中,提供了一种用于保持或封闭插入血管壁的医疗装置(例如,支架或合成的血管移植物、心脏瓣膜、腹主动脉动脉瘤装置以及它们的部件)、和建立血管稳态内环境,从而防止如再狭窄中的过度内膜增生的方法。在治疗动脉粥样硬化的本发明方法中,所述动脉可为冠状动脉或外周动脉(例如股动脉)。也可用这些技术和医疗装置治疗静脉。The present invention also provides methods for treating various diseases such as: vascular disease, cancer, vascular remodeling, severe coronary heart disease, atherosclerosis, restenosis, thrombosis, aneurysm, and vascular obstruction. In one embodiment, a medical device (e.g., a stent or synthetic vascular graft, heart valve, abdominal aortic aneurysm device, and components thereof) for holding or sealing an inserted vessel wall, and for creating a vessel wall is provided. A method of homeostasis, thereby preventing excessive intimal hyperplasia as in restenosis. In the present methods of treating atherosclerosis, the artery may be a coronary artery or a peripheral artery (eg, femoral artery). Veins can also be treated with these techniques and medical devices.
对于再狭窄的治疗,本发明还提供了一种用于诱导愈合反应的工程改造方法。在一个实施方式中,提供了一种用于在移植血管靶区域中被植入装置的管腔表面上快速诱导形成融合的内皮层的方法,其中所述内皮细胞可表达一氧化氮合酶和其它抗炎和炎症调节因子。本发明还提供了一种医疗装置,其较现有技术装置具有更高的生物相容性,并能通过降低或抑制平滑肌细胞迁移、平滑肌细胞分化和沿医疗装置植入部位的内腔表面的胶原沉积,来降低或抑制组织的过度内膜增生和再狭窄。For the treatment of restenosis, the present invention also provides an engineered method for inducing a healing response. In one embodiment, there is provided a method for rapidly inducing the formation of a confluent endothelial layer on the luminal surface of an implanted device in a target region of a graft vessel, wherein the endothelial cells express nitric oxide synthase and Other anti-inflammatory and inflammation modulators. The present invention also provides a medical device that has higher biocompatibility than prior art devices and can reduce or inhibit smooth muscle cell migration, smooth muscle cell Collagen deposition, to reduce or inhibit excessive intimal hyperplasia and restenosis of the tissue.
在一个实施方式中,用于涂覆医疗装置的方法包括以下步骤:将至少一层生物相容性基质施加于医疗装置表面,其中所述生物相容性基质包含至少一种选自下组的组分:聚氨酯、嵌段聚氨酯-脲/肝素、聚-L-乳酸、纤维素酯、聚乙二醇、聚醋酸乙酯、葡聚糖、明胶、胶原、弹性蛋白、弹性蛋白原、层连蛋白、纤连蛋白、玻连蛋白、肝素、纤维蛋白、纤维素和碳以及富勒烯;并对所述生物相容性基质同时或相继施加:治疗有效量的至少一种抗体、抗体片段或它们的组合,以及至少一种刺激内皮细胞生长和分化的化合物。In one embodiment, the method for coating a medical device comprises the step of: applying at least one layer of biocompatible matrix to the surface of the medical device, wherein said biocompatible matrix comprises at least one selected from the group consisting of Components: polyurethane, segmented polyurethane-urea/heparin, poly-L-lactic acid, cellulose esters, polyethylene glycol, polyethyl acetate, dextran, gelatin, collagen, elastin, tropoelastin, laminin protein, fibronectin, vitronectin, heparin, fibrin, cellulose and carbon and fullerene; and applying to said biocompatible matrix simultaneously or sequentially: a therapeutically effective amount of at least one antibody, antibody fragment or combinations thereof, and at least one compound that stimulates the growth and differentiation of endothelial cells.
本发明还提供了一种治疗哺乳动物血管疾病的方法,所述方法包括:将医疗装置植入哺乳动物的血管或管状器官内腔中,其中所述医疗装置涂覆有(a)生物相容性基质,(b)治疗有效量的至少一种抗体、抗体片段或它们的组合,以及(c)至少一种化合物;其中所述抗体或抗体片段能识别并结合内皮祖细胞表面上的抗原,从而将内皮祖细胞固定在基质表面上,而所述化合物则用于刺激固定的内皮祖细胞在医疗装置表面上形成内皮。The present invention also provides a method of treating vascular disease in a mammal, the method comprising: implanting a medical device into a blood vessel or tubular organ lumen of a mammal, wherein the medical device is coated with (a) a biocompatible a sexual substrate, (b) a therapeutically effective amount of at least one antibody, antibody fragment, or combination thereof, and (c) at least one compound; wherein the antibody or antibody fragment recognizes and binds to an antigen on the surface of an endothelial progenitor cell, The endothelial progenitor cells are thereby immobilized on the substrate surface, and the compound is used to stimulate the immobilized endothelial progenitor cells to form an endothelium on the surface of the medical device.
在一个实施方式中,还提供了治疗患者疾病的治疗/药物递送系统。所述治疗或药物递送系统含有遗传改变的哺乳动物细胞和用于植入患者的医疗装置,其中所述遗传改变的哺乳动物细胞包含编码经遗传工程改造的细胞表面标记和至少一种治疗性基因产物的外源核酸。在一个实施方式中,用适宜的包含外源遗传物质的转染载体体外转染所述基因工程改造的细胞,为所述细胞提供所需的基因。在这一实施方式中,所述细胞可为任何哺乳动物细胞,不论是自体的、同种异体的或是异种的,例如内皮细胞、成纤维细胞、成肌细胞等。在这一实施方式中,所述医疗装置是用生物相容性基质涂覆的,所述基质包括配体,所述配体通过结合经遗传工程改造的细胞膜标记分子或细胞表面上的抗原,而仅结合于遗传改变的哺乳动物细胞。In one embodiment, a therapy/drug delivery system for treating a disease in a patient is also provided. The therapeutic or drug delivery system comprises a genetically altered mammalian cell comprising a genetically engineered cell surface marker encoding a genetically engineered cell surface marker and at least one therapeutic gene and a medical device for implantation into a patient The exogenous nucleic acid of the product. In one embodiment, the genetically engineered cells are transfected in vitro with a suitable transfection vector containing exogenous genetic material to provide the cells with the desired genes. In this embodiment, the cell may be any mammalian cell, whether autologous, allogeneic or xenogeneic, such as endothelial cells, fibroblasts, myoblasts, and the like. In this embodiment, the medical device is coated with a biocompatible matrix comprising a ligand that, by binding to a genetically engineered cell membrane marker molecule or an antigen on the cell surface, Instead, it only binds to genetically altered mammalian cells.
在本发明的治疗和/或药物递送系统中,所述遗传改变的细胞带有外源遗传物质而导入了至少一种编码细胞表面标记分子或抗原的所需基因以及至少一种编码治疗性基因产物的基因。该系统任选地包含信号系统,例如能刺激遗传改变的哺乳动物细胞表达和/或分泌所需基因产物和/或标记基因的活性化合物或分子。In the therapeutic and/or drug delivery system of the present invention, the genetically altered cells carry exogenous genetic material into at least one desired gene encoding a cell surface marker molecule or antigen and at least one encoding therapeutic gene product gene. The system optionally includes a signaling system, such as an active compound or molecule that stimulates expression and/or secretion of the desired gene product and/or marker gene from the genetically altered mammalian cell.
因此,在一个实施方式中,将导入哺乳动物中的所述外源遗传物质经工程改造为可编码可特异性结合装置上配体的细胞膜标记。例如,所述装置用于置入血管内腔时,该外源遗传物质应编码除了提供给患者的经遗传工程改造的细胞以外血流内任何循环性细胞中均没有的细胞膜标记。Thus, in one embodiment, the exogenous genetic material introduced into the mammal is engineered to encode a cell membrane marker that specifically binds to a ligand on the device. For example, where the device is intended to be placed in the lumen of a blood vessel, the exogenous genetic material should encode cell membrane markers that are absent from any circulating cells in the bloodstream other than the genetically engineered cells provided to the patient.
还提供了一种用于治疗各种疾病的经涂覆的医疗装置和方法,所述疾病为:例如血管疾病,包括但不限于动脉粥样硬化、癌症和类风湿性关节炎。本发明的医疗装置包括用于能在体内特异性捕获和固定遗传改变的哺乳动物细胞的涂层,所述遗传改变的哺乳动物细胞是在将所述经涂覆的医疗装置植入患者时同时或随后导入的。Also provided is a coated medical device and method for treating various diseases such as, for example, vascular diseases including, but not limited to, atherosclerosis, cancer, and rheumatoid arthritis. The medical device of the present invention includes a coating for specifically capturing and immobilizing genetically altered mammalian cells in vivo while implanting the coated medical device into a patient. or subsequently imported.
还提供了用于固定表达和/或分泌至少一种用于治疗特定的疾病的物质或治疗剂的固定的遗传改变细胞。在本发明的这一方面中,例如在治疗癌症中,通过在所述细胞内导入外源遗传物质而使所述细胞(例如,内皮细胞)发生遗传改变。在一个实施方式中,将所述遗传物质导入细胞核内,且其为DNA(例如染色体外DNA)。所述染色体外DNA可为载体,例如腺病毒载体、质粒(如裸质粒)、线性或短DNA等。在一个实施方式中,所述DNA包含控制表达所需标记和/或治疗基因的调控/表达盒。在一个实施方式中,所述调控盒可包含用于组成性表达治疗基因的调控元件,或可包含可按患者需要控制或表达的元件。Also provided is a fixed genetically altered cell for fixed expression and/or secretion of at least one substance or therapeutic agent for the treatment of a specific disease. In this aspect of the invention, the cells (eg, endothelial cells) are genetically altered by introducing exogenous genetic material into the cells, eg, in the treatment of cancer. In one embodiment, the genetic material is introduced into the nucleus and is DNA (eg, extrachromosomal DNA). The extrachromosomal DNA can be a vector, such as an adenovirus vector, a plasmid (such as a naked plasmid), linear or short DNA, and the like. In one embodiment, the DNA comprises a regulatory/expression cassette that controls the expression of the desired marker and/or therapeutic gene. In one embodiment, the regulatory cassette may comprise regulatory elements for constitutive expression of the therapeutic gene, or may comprise elements that may be controlled or expressed as desired by the patient.
在一个实施方式中,所述用于植入患者的医疗装置包括涂层;所述涂层包括由基质负载的至少一种能识别并结合靶细胞的配体。在细胞为遗传改变的细胞的实施方式中,所述配体仅识别并结合经工程改造而加入细胞的特异性细胞膜标记分子或抗原。因此在这一实施方式中,配体仅识别已导入患者的遗传改变的哺乳动物细胞,使该遗传改变的哺乳动物细胞结合于所述医疗装置上,表达并分泌标记分子或抗原以及至少一种治疗性基因产物。In one embodiment, the medical device for implantation in a patient comprises a coating; the coating comprising at least one ligand capable of recognizing and binding to target cells supported by a matrix. In embodiments where the cell is a genetically altered cell, the ligand recognizes and binds only specific cell membrane marker molecules or antigens that have been engineered into the cell. Thus in this embodiment, the ligand recognizes only genetically altered mammalian cells that have been introduced into the patient, causing the genetically altered mammalian cells to bind to said medical device, express and secrete marker molecules or antigens and at least one Therapeutic gene products.
在另一实施方式中,所述治疗或药物递送系统还可包含激活分子(activatingmolecula),该激活分子用于刺激所述遗传改变的哺乳动物细胞表达和/或分泌所需的治疗性基因产物。在本发明的这一方面,可通过多种方法将诸如化学刺激物或肽的化合物供给患者,这些方法包括:口服途径、温热贴片(thermal patch)、静脉内、皮内注射等。在这一实施方式中,所述遗传改变的哺乳动物细胞可为自体的或外源的,例如成熟内皮细胞、成纤维细胞、肌细胞、上皮细胞等,它们包含的外源核酸可为染色体外DNA。在一个实施方式中,所述DNA以载体形式提供,例如腺病毒载体、裸质粒DNA、线性DNA等。在一个实施方式中,所述染色体外DNA包含调控盒,即编码细胞膜抗原的基因和至少一种编码用于治疗疾病的肽的基因。在这一实施方式的一个方面中,所述细胞膜特异性基因编码,例如成骨蛋白或前列腺细胞膜蛋白。In another embodiment, the therapeutic or drug delivery system may further comprise an activating molecule for stimulating expression and/or secretion of a desired therapeutic gene product by the genetically altered mammalian cell. In this aspect of the invention, compounds such as chemical stimuli or peptides can be administered to a patient by a variety of methods including: oral route, thermal patch, intravenous, intradermal injection, and the like. In this embodiment, the genetically altered mammalian cells may be autologous or exogenous, such as mature endothelial cells, fibroblasts, muscle cells, epithelial cells, etc., which may contain exogenous nucleic acids that may be extrachromosomal DNA. In one embodiment, the DNA is provided in the form of a vector, such as an adenoviral vector, naked plasmid DNA, linear DNA, and the like. In one embodiment, said extrachromosomal DNA comprises a regulatory cassette, ie a gene encoding a cell membrane antigen and at least one gene encoding a peptide useful in the treatment of a disease. In one aspect of this embodiment, the cell membrane-specific gene encodes, for example, an osteogenic protein or a prostate cell membrane protein.
在一个实施方式中,所述染色体外遗传物质包含编码治疗/药物产物的基因,这些产物为例如用于血管重构的血管内皮生长因子和血管生成因子,或是用于治疗癌症的抗血管生成因子。In one embodiment, the extrachromosomal genetic material comprises genes encoding therapeutic/drug products such as vascular endothelial growth factor and angiogenic factors for vascular remodeling, or anti-angiogenic factors for the treatment of cancer factor.
在另一实施方式中,提供了一种用于治疗疾病的方法。所述方法包括:In another embodiment, a method for treating a disease is provided. The methods include:
将遗传改变的哺乳动物细胞提供给患者;所述细胞包含编码经遗传工程改造的细胞膜标记分子和至少一种治疗性基因产物外源核酸;providing to a patient a genetically altered mammalian cell; said cell comprising an exogenous nucleic acid encoding a genetically engineered cell membrane marker molecule and at least one therapeutic gene product;
将包含涂层的医疗装置植入患者;所述涂层包含负载了至少一种配体的基质,其中该配体能识别并结合所述遗传改变的哺乳动物细胞上的经遗传工程改造的细胞膜标记分子,其中所述遗传改变的哺乳动物细胞结合于医疗装置,并表达和分泌所述治疗性基因产物。在本发明的一个实施方式中,所述治疗性基因和基因产物包括例如血管内皮生长因子、血管生成因子、抗血管生成因子和成纤维细胞生长因子。implanting into a patient a medical device comprising a coating; said coating comprising a matrix loaded with at least one ligand, wherein the ligand recognizes and binds to a genetically engineered cell membrane on said genetically altered mammalian cell A marker molecule wherein said genetically altered mammalian cell is bound to a medical device and expresses and secretes said therapeutic gene product. In one embodiment of the invention, the therapeutic genes and gene products include, for example, vascular endothelial growth factor, angiogenic factors, anti-angiogenic factors, and fibroblast growth factors.
本发明还提供了治疗患者疾病的方法,所述方法包括:将遗传改变的哺乳动物细胞提供给患者;将医疗装置植入患者;所述医疗装置包含涂层,所述涂层包含负载有至少一种配体的基质,所述配体能特异性识别并结合至少一种标记分子,例如该遗传改变的哺乳动物细胞上的受体,其中所述遗传改变的哺乳动物细胞结合于所述医疗装置上,包含用于表达和分泌治疗性基因产物的外源核酸。The present invention also provides a method of treating a disease in a patient, the method comprising: providing genetically altered mammalian cells to the patient; implanting a medical device into the patient; the medical device comprising a coating comprising a load of at least A matrix of ligands capable of specifically recognizing and binding at least one marker molecule, such as a receptor on the genetically altered mammalian cell, wherein the genetically altered mammalian cell binds to the medical The device contains exogenous nucleic acid for expression and secretion of a therapeutic gene product.
在另一实施方式中,提供了一种用于在体内将细胞募集到接触血液的表面的方法。所述方法包括提供位于对象血流中接触血液的表面,所述接触血液的表面经构置能将对象血流中的循环性靶细胞募集到所述接触血液的表面;以及将所述靶细胞募集到所述接触血液的表面。在这一实施方式中,所述接触血液的表面包括植入所述对象的医疗装置的内腔表面。在本发明的这一实施方式中,被募集到接触血液的表面(例如支架或移植物)上的靶细胞可将所述装置的表面自身内皮化(self-endothelialize)而在受损血管部位恢复正常内皮组织。所述接触血液的表面可为生物可降解构架或可涂覆有生物可降解的生物相容性材料。在本发明的这一方面中,所述生物可降解构架在植入血管时将在原位发生降解,而在所述装置内腔上形成的新生内皮重建了通过受伤部位的连贯血管,从而形成功能性的新生血管。In another embodiment, a method for recruiting cells to a blood-contacting surface in vivo is provided. The method includes providing a blood-contacting surface in the bloodstream of a subject, the blood-contacting surface configured to recruit circulating target cells in the bloodstream of the subject to the blood-contacting surface; Recruited to the blood-contacting surface. In this embodiment, the blood-contacting surface comprises a luminal surface of a medical device implanted in the subject. In this embodiment of the invention, target cells recruited to blood-contacting surfaces such as stents or grafts can self-endothelialize the surface of the device to restore damaged vascular sites normal endothelium. The blood contacting surface may be a biodegradable framework or may be coated with a biodegradable biocompatible material. In this aspect of the invention, the biodegradable framework will degrade in situ upon implantation into a vessel, while the neoendothelium formed on the lumen of the device reestablishes a coherent vessel through the injured site, thereby forming Functional neovascularization.
在另一实施方式中,本发明包括一种修复体,所述修复体包括:(a)具有外表面和接触血液表面的载体膜;(b)涂覆于所述载体膜的接触血液表面上的第一层交联聚合化合物;和(c)涂覆于第一层之上的第二层,所述第二层含有在体内对靶细胞具有亲和性的至少一种配体。In another embodiment, the present invention includes a prosthesis comprising: (a) a carrier membrane having an outer surface and a blood-contacting surface; (b) coated on the blood-contacting surface of the carrier membrane and (c) a second layer coated on the first layer, the second layer comprising at least one ligand having an affinity for a target cell in vivo.
在另一实施方式中,提供了一种用于在体内生成自身内皮化移植物的方法,所述方法包括:(a)提供构置的功能为用作血管移植物的构架,所述构架具有内腔表面和外表面,所述内腔表面包含特异性用于结合内皮祖细胞的配体;(b)将所述构架植入对象的血管中;和(c)将循环性内皮祖细胞募集到所述构架的所述内腔表面上以形成新生的内皮。In another embodiment, there is provided a method for generating an autologous endothelialized graft in vivo, the method comprising: (a) providing a framework configured to function as a vascular graft, the framework having a luminal surface and an outer surface, the luminal surface comprising a ligand specific for binding endothelial progenitor cells; (b) implanting the framework in a blood vessel of a subject; and (c) recruiting circulating endothelial progenitor cells onto the luminal surface of the scaffold to form a nascent endothelium.
在另一实施方式中,提供了一种用于原位产生自身内皮化移植物的方法,所述方法包括:(a)提供具有接触循环血液的表面的修复性构建物;(b)将所述修复性构建物植入对象;和(c)从血液中募集循环性细胞(例如,内皮祖细胞和遗传改变的哺乳动物细胞)使其结合到所述修复性构建物的表面上,从而在其上形成新生的内皮。In another embodiment, there is provided a method for in situ generation of an autologous endothelialized graft comprising: (a) providing a prosthetic construct having a surface in contact with circulating blood; (b) converting the implanting the prosthetic construct into the subject; and (c) recruiting circulating cells (e.g., endothelial progenitor cells and genetically altered mammalian cells) from the blood to bind to the surface of the prosthetic construct, thereby A new endothelium forms on it.
在另一实施方式中,提供了原位产生自身内皮化移植物的方法,所述方法包括:(a)提供构置的功能为用作临时血管移植物的生物可降解构架,所述构架具有内腔表面和外表面;(b)将所述生物可降解构架植入血管中;(c)募集循环细胞(例如内皮祖细胞和遗传改变的哺乳动物细胞)使之结合到所述修复体(例如移植物、支架或生物可降解构架)的内腔表面上,从而形成新生的内皮;(d)使血管组织包裹所述构架的外表面以形成外部止血血管结构;和(e)在体内条件下,在可使得所述新生内皮组织和外部血管结构形成功能性新生血管的时间段内,所述生物可降解构架被降解。In another embodiment, there is provided a method of in situ generating an autologous endothelialized graft comprising: (a) providing a biodegradable framework configured to function as a temporary vascular graft, the framework having luminal and external surfaces; (b) implanting the biodegradable framework into blood vessels; (c) recruiting circulating cells (such as endothelial progenitor cells and genetically altered mammalian cells) to bind to the prosthesis ( such as grafts, stents, or biodegradable scaffolds) on the luminal surface, thereby forming a neoendothelium; (d) wrapping vascular tissue around the outer surface of the scaffold to form an external hemostatic vascular structure; and (e) in vivo conditions , the biodegradable framework is degraded within a time period that allows the neoendothelial tissue and external vascular structures to form functional neovascularization.
在一个实施方式中,提供了一种用于原位形成内皮化血管移植物的生物可降解构架,所述构架包含:(a)具有内腔表面和外表面的多孔性生物可降解载体膜;(b)所述内腔表面包含涂覆于所述载体膜上的由至少一种聚合化合物组成的第一层,其中所述化合物可经交联剂自身交联形成共价键,所述共价键在体内条件下可受到酶促裂解或非酶促水解,和(c)对体内结合遗传改变的哺乳动物细胞具有特异性亲和力的配体。In one embodiment, there is provided a biodegradable framework for in situ formation of an endothelialized vascular graft comprising: (a) a porous biodegradable carrier membrane having a luminal surface and an external surface; (b) the lumen surface comprises a first layer coated on the carrier membrane consisting of at least one polymeric compound, wherein the compound is self-crosslinkable via a crosslinking agent to form a covalent bond, the covalent The bond is subject to enzymatic cleavage or non-enzymatic hydrolysis under in vivo conditions, and (c) a ligand with specific affinity for in vivo binding to genetically altered mammalian cells.
在另一实施方式中,提供了一种用于原位产生自身内皮化移植物的方法,所述方法包括:(a)提供具有接触患者循环血的表面的修复性构建物;(b)将所述修复性构建物植入对象或患者;(c)将遗传改变的哺乳动物细胞给予所述患者;和(d)从血液中募集循环性细胞(例如遗传改变的哺乳动物细胞)使其结合到所述修复性构建物的表面上,从而在所述修复性构建物的表面上形成一层遗传改变的细胞。In another embodiment, there is provided a method for in situ generation of an autologous endothelialized graft comprising: (a) providing a prosthetic construct having a surface in contact with a patient's circulating blood; (b) incorporating Implanting the prosthetic construct into a subject or patient; (c) administering the genetically altered mammalian cells to the patient; and (d) recruiting circulating cells (e.g., genetically altered mammalian cells) from the blood to bind them onto the surface of the prosthetic construct, thereby forming a layer of genetically altered cells on the surface of the prosthetic construct.
在另一实施方式中,提供了一种促进血管重构的方法,例如通过向外或正性重构来增大动脉的周长以部分或全部地补偿因形成动脉粥样硬化斑块造成的或动脉受伤后因内膜异常增生造成的内腔侵占,从而防止或抑制受损血管的向内或负性重构。在这一实施方式中,例如提供如上所述的由基质和配体覆盖并能结合基因工程改造细胞的支架,以用于捕获经遗传修饰的自体细胞(例如内皮祖细胞),这些细胞能分泌至少一种强效抗凝血剂和血管舒张剂,例如:前列环素,如前列腺素12,PG12;降钙素基因相关肽,如α-CGRP等。可经工程改造通过细胞产生的其它产物,包括一氧化氮(一氧化氮合酶基因)、基质金属蛋白酶、乙酰胆碱、腺苷、5-羟色胺、P物质(substance P)、肾上腺髓质素等。可采用其产物作为血管舒张剂和/或抗凝血剂或具有血管舒张剂和/或抗凝血剂特性的基因,例如可引起血管平滑肌松弛的血管舒张剂。可通过基因转移技术将编码血管舒张剂的基因(例如前列环素合酶基因)提供给内皮祖细胞或内皮细胞,所述基因转移技术可为例如采用顺反子(cistronic)基因构建物的病毒基因转移,就前列环素而言,例如顺反子环氧合酶-1/前列环素合酶基因构建物可提供连续的局部前列腺素递送。在这一实施方式中,前列腺素局部递送系统可用于治疗例如,脑梗死和冠状血管疾病。还可将血管的正性重构用作调节动脉生成(即成熟血管,例如成人小动脉和动脉的形成)以形成附属血管的治疗方法。In another embodiment, there is provided a method of promoting vascular remodeling, such as increasing the circumference of an artery by outward or positive remodeling to partially or fully compensate for the damage caused by atherosclerotic plaque formation or Lumen encroachment caused by abnormal intimal hyperplasia after arterial injury prevents or inhibits inward or negative remodeling of damaged vessels. In this embodiment, for example, a scaffold as described above covered with a matrix and a ligand capable of binding genetically engineered cells is provided for capturing genetically modified autologous cells (e.g. endothelial progenitor cells) capable of secreting At least one strong anticoagulant and vasodilator, such as: prostacyclin, such as prostaglandin 12, PG12; calcitonin gene-related peptide, such as α-CGRP, etc. Other products that can be engineered to be produced by cells include nitric oxide (nitric oxide synthase gene), matrix metalloproteinases, acetylcholine, adenosine, serotonin, substance P, adrenomedullin, and others. The products thereof may be employed as vasodilators and/or anticoagulants or genes having vasodilator and/or anticoagulant properties, eg vasodilators which cause relaxation of vascular smooth muscle. Genes encoding vasodilators, such as the prostacyclin synthase gene, can be provided to endothelial progenitor or endothelial cells by gene transfer techniques such as viruses employing cistronic gene constructs Gene transfer, in the case of prostacyclins, eg a cistronic cyclooxygenase-1/prostacyclin synthase gene construct can provide continuous local prostacyclin delivery. In this embodiment, the local prostaglandin delivery system can be used to treat, for example, cerebral infarction and coronary vascular disease. Positive remodeling of blood vessels can also be used as a therapeutic method to modulate arteriogenesis (ie, the formation of mature blood vessels, such as adult arterioles and arteries) to form accessory vessels.
在另一实施方式中,可用编码血管舒张化合物和独特细胞表面标记(例如截短的MHC-I)的双顺反子载体转染适宜的细胞,如成纤维细胞、内皮细胞或内皮祖细胞,所述细胞表面标记可被配体(例如固定于血管内修复体上的抗体)识别。例如,可将配体(如抗体)涂覆的支架植入患者的冠状动脉中,然后将遗传改变的细胞(例如遗传改变的内皮细胞)植入需要治疗血管疾病的患者中。在使用遗传改变的细胞的这一实施方式和其它实施方式中,可采用标准的基因工程技术利用例如质粒载体植入细胞之前将外源基因递送入细胞,所述质粒载体可为例如双顺反子pMACSKK.II质粒载体(Miltenyi Biotec,德国),其包含多克隆位点并可将感兴趣的基因插入其中作为所用哺乳动物细胞系的选择标记,所述感兴趣的基因为例如前列环素合酶以及标记基因(如截短的MHC I型分子,H-2K)。In another embodiment, suitable cells, such as fibroblasts, endothelial cells, or endothelial progenitor cells, can be transfected with a bicistronic vector encoding a vasodilating compound and a unique cell surface marker, such as truncated MHC-I, The cell surface markers are recognized by ligands such as antibodies immobilized on the endovascular prosthesis. For example, a ligand (eg, antibody)-coated stent can be implanted in a patient's coronary artery, and then genetically altered cells (eg, genetically altered endothelial cells) can be implanted in a patient in need of treatment for vascular disease. In this and other embodiments using genetically altered cells, standard genetic engineering techniques can be used to deliver the exogenous gene into the cell prior to implanting the cell using, for example, a plasmid vector, which can be, for example, a bicistrans pMACSKK.II plasmid vector (Miltenyi Biotec, Germany), which contains a multiple cloning site and into which a gene of interest, e.g. prostacyclin, can be inserted as a selection marker for the mammalian cell line used Synthases and marker genes (such as truncated MHC class I molecules, H-2K).
在另一实施方式中,用于治疗中的用于转染哺乳动物细胞的外源基因递送系统可包括,例如包含截短的MHC I型抗原和血管舒张剂转基因(例如用于治疗血管疾病的前列环素合酶和/或α-CGRP基因)的慢病毒载体。在这一实施方式中,所述要被转染的哺乳动物细胞可为自体的内皮细胞或内皮祖细胞,而所述修复性装置可用该截短的MHC I型抗原的特异性配体(例如抗-H-2Kk抗体)涂覆。In another embodiment, an exogenous gene delivery system for transfection of mammalian cells used in therapy may include, for example, a transgene comprising a truncated MHC class I antigen and a vasodilator (eg, for the treatment of vascular disease). prostacyclin synthase and/or α-CGRP gene) lentiviral vector. In this embodiment, the mammalian cells to be transfected may be autologous endothelial cells or endothelial progenitor cells, and the prosthetic device may be a ligand specific for the truncated MHC class I antigen (e.g. Anti-H-2Kkappa antibody) coating.
附图简述Brief description of the drawings
图1A为抗体通过交联分子共价偶联于基质的示意图。图1B为C60O分子锚定在基质上的示意图。图1C为本发明涂覆有基质的支架的示意图。Figure 1A is a schematic diagram of the covalent coupling of antibodies to a matrix via cross-linking molecules. Fig. 1B is a schematic diagram of anchoring C60 O molecules on a substrate. Figure 1C is a schematic diagram of a matrix-coated stent of the present invention.
图2A是粘附在由纤连蛋白涂覆的玻片上的内皮祖细胞的相差显微镜照片,所述玻片上含有从富集培养基分离的细胞。图2B是附着在纤连蛋白涂覆的玻片上的内皮祖细胞的相差显微镜照片,所述玻片上含有用抗CD34抗体包被的磁珠分离的细胞。图2D和2F是已孵育7天并用PI进行核染色的内皮祖细胞的显微照片。由这些图可见,如分别对Tie-2(图2E和2G)和VEGFR-2(图2C)抗体反应性的抗体荧光显示的那样,所示细胞表达了成熟内皮细胞的标记。Figure 2A is a phase contrast micrograph of endothelial progenitor cells adhered to fibronectin-coated slides containing cells isolated from enriched media. Figure 2B is a phase contrast micrograph of endothelial progenitor cells attached to fibronectin-coated slides containing cells isolated with anti-CD34 antibody-coated magnetic beads. Figures 2D and 2F are photomicrographs of endothelial progenitor cells incubated for 7 days and nuclear stained with PI. As can be seen from these figures, the indicated cells express markers of mature endothelial cells, as demonstrated by antibody fluorescence for Tie-2 (FIGS. 2E and 2G) and VEGFR-2 (FIG. 2C) antibody reactivity, respectively.
图3A和3B是内皮一氧化氮合酶(eNOS)和磷酸甘油醛脱氢酶(GAPDH)的溴乙锭染色的2%琼脂糖凝胶半定量RT-PCR照片。培养于纤连蛋白涂覆的玻片上3天(图3B)和7天(图3A)后,内皮祖细胞开始表达eNOS mRNA。3A and 3B are ethidium bromide-stained 2% agarose gel semi-quantitative RT-PCR pictures of endothelial nitric oxide synthase (eNOS) and glyceraldehyde phosphate dehydrogenase (GAPDH). After 3 days (Fig. 3B) and 7 days (Fig. 3A) of culture on fibronectin-coated slides, EPCs began to express eNOS mRNA.
图4A-4E是粘附在与HUVEC细胞一起培养并用碘化丙锭染色的:CMDx和抗CD34抗体(4A);明胶和抗CD34抗体(4B);空白的不锈钢圆盘(4C);CMDx涂覆的(4D)和明胶涂覆的(4E)不锈钢圆盘上的HUVEC的照片。Figures 4A-4E are adherents cultured with HUVEC cells and stained with propidium iodide: CMDx and anti-CD34 antibody (4A); gelatin and anti-CD34 antibody (4B); blank stainless steel disc (4C); CMDx coated Photographs of HUVECs on coated (4D) and gelatin-coated (4E) stainless steel discs.
图5A-5C是用人血白细胞组分孵育的无抗体的CMDx涂覆的对照的显微照片。所述细胞以碘化丙锭和FITC标记的抗-KDR抗体染色。图5D-5F是用人血白细胞组分孵育的由无抗体结合于其表面的明胶涂覆的对照不锈钢圆盘的显微照片。所述细胞以碘化丙锭和FITC标记的抗-KDR抗体染色。Figures 5A-5C are photomicrographs of antibody-free CMDx-coated controls incubated with human blood leukocyte fractions. The cells were stained with propidium iodide and FITC-labeled anti-KDR antibody. Figures 5D-5F are photomicrographs of control stainless steel discs incubated with human blood leukocyte fractions coated with gelatin to which no antibody was bound to the surface. The cells were stained with propidium iodide and FITC-labeled anti-KDR antibody.
图6A-6C是用HUVEC孵育的表面上结合有抗CD34抗体的CMDx基质涂覆的不锈钢圆盘的显微照片。所述细胞以碘化丙锭和FITC标记的抗-KDR抗体染色。图6D-6F是用HUVECS孵育的明胶涂覆表面结合有抗体不锈钢圆盘的显微照片。所述细胞以碘化丙锭和FITC标记的抗-KDR抗体染色。Figures 6A-6C are photomicrographs of CMDx matrix-coated stainless steel discs with anti-CD34 antibodies bound to their surfaces incubated with HUVECs. The cells were stained with propidium iodide and FITC-labeled anti-KDR antibody. Figures 6D-6F are photomicrographs of gelatin-coated stainless steel discs with antibody-bound surfaces incubated with HUVECS. The cells were stained with propidium iodide and FITC-labeled anti-KDR antibody.
图7是与祖细胞共孵育24小时的表面结合有抗体的CMDx涂覆的不锈钢圆盘的显微照片。所述细胞以碘化丙锭和FITC标记的抗-KDR抗体染色。Figure 7 is a photomicrograph of a CMDx-coated stainless steel disc with antibody bound to its surface incubated with progenitor cells for 24 hours. The cells were stained with propidium iodide and FITC-labeled anti-KDR antibody.
图8A和8B是与祖细胞共孵育7天的表面结合有抗CD34抗体的CMDx基质涂覆的不锈钢圆盘的显微照片。所述细胞以碘化丙锭和FITC标记的抗-KDR抗体染色。Figures 8A and 8B are photomicrographs of CMDx matrix-coated stainless steel discs with anti-CD34 antibodies bound to their surface incubated with progenitor cells for 7 days. The cells were stained with propidium iodide and FITC-labeled anti-KDR antibody.
图9A和9B是与祖细胞共孵育7天的表面结合有抗CD34抗体的CMDx基质涂覆的不锈钢圆盘的显微照片。所述细胞以碘化丙锭和FITC标记的抗Tie-2抗体染色。Figures 9A and 9B are photomicrographs of CMDx matrix-coated stainless steel discs with anti-CD34 antibodies bound to their surface incubated with progenitor cells for 7 days. The cells were stained with propidium iodide and FITC-labeled anti-Tie-2 antibody.
图10A-10C是在内皮细胞生长培养基中与祖细胞孵育3周的CMDx涂覆的不锈钢圆盘的相差显微镜照片,显示了成熟的内皮细胞。Figures 10A-10C are phase contrast micrographs of CMDx-coated stainless steel discs incubated with progenitor cells in endothelial cell growth medium for 3 weeks, showing mature endothelial cells.
图11是结合有祖细胞的本发明的用功能性富勒烯涂覆的支架表面的示意图。Figure 11 is a schematic representation of the surface of a functional fullerene-coated scaffold of the invention with bound progenitor cells.
图12A-12D是带有或不带有抗CD34抗体的富勒烯涂覆的样品的照片。所述样品与人白细胞组分共孵育,并用碘化丙锭和FITC标记的抗-VEGFR-2抗体染色。12A-12D are photographs of fullerene-coated samples with and without anti-CD34 antibodies. The samples were incubated with human leukocyte fractions and stained with propidium iodide and FITC-labeled anti-VEGFR-2 antibody.
图13A-13D是冠状动脉取出物组织学横切片的低倍和高倍显微照片,所述取出物已植入裸露的不锈钢支架(图13A和13C)和用富勒烯涂覆的样品(图13B和13D)4周。切片用苏木精-伊红染色。Figures 13A-13D are low and high magnification photomicrographs of histological cross-sections of coronary artery extracts implanted in bare stainless steel stents (Figures 13A and 13C) and samples coated with fullerenes (Figures 13A and 13C ). 13B and 13D) 4 weeks. Sections were stained with hematoxylin-eosin.
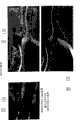
图14A-14G是植入雄性约克猪1和48小时后支架取出物的扫描电镜显微照片。葡聚糖涂覆的(图14A)和葡聚糖/抗CD34抗体涂覆的(14B)支架的取出物为植入后1小时。图14C和14D所示为对照样品取出物,而图14E-G为植入后48小时的葡聚糖/抗CD34抗体涂覆的支架。图14H-14M是取自雄性约克猪的植入4周的冠状动脉取出物的横切面组织学显微照片:未涂覆的(空白不锈钢)(14H和14I)、葡聚糖涂覆对照(14J和14K)以及葡聚糖/抗CD34抗体涂覆的(14L和14M)。Figures 14A-14G are scanning electron micrographs of scaffold removals in male Yorkie pigs 1 and 48 hours after implantation. Removal of dextran-coated (Fig. 14A) and dextran/anti-CD34 antibody-coated (14B) scaffolds was 1 hour post-implantation. Figures 14C and 14D show control sample explants, while Figures 14E-G show dextran/anti-CD34 antibody coated scaffolds 48 hours post-implantation. 14H-14M are cross-sectional histological micrographs of coronary arterial extractions from male Yorkie pigs at 4 weeks implanted: uncoated (blank stainless steel) (14H and 14I), dextran-coated controls ( 14J and 14K) and dextran/anti-CD34 antibody coated (14L and 14M).
图15A、15B和15C分别为植入48小时的18mm长用表面无抗体的葡聚糖-血浆涂覆的支架、葡聚糖-血浆涂覆的/抗CD34抗体涂覆的支架的荧光显微照片。Fig. 15A, 15B and 15C are respectively implanted 48 hours 18mm long with the stent coated with dextran-plasma without antibody on the surface, the stent that dextran-plasma is coated/anti-CD34 antibody is coated with the fluorescence microscope photo.
图16A和16B是碘化丙锭和抗凝集素/FITC偶联的样品的显微照片。Figures 16A and 16B are photomicrographs of propidium iodide and antilectin/FITC conjugated samples.
发明详述Detailed description of the invention
本发明提供了一种涂覆的可植入医疗装置(例如支架或移植物)以及用于涂覆该医疗装置的方法和组合物,以及用所述涂覆的医疗装置治疗血管疾病的方法。本发明还提供了一种用于治疗疾病(例如再狭窄和癌症)的方法,所述方法包括将具有涂层的医疗装置植入需要治疗的患者,并为所述患者提供经遗传工程改造的哺乳动物细胞,所述细胞在体内能结合于所述医疗装置的表面并能产生工程改造的和所需的治疗剂(例如基因产物)。图1A-1C为本发明医疗装置表面涂层的示意图。医疗装置上的涂层包含:生物相容性基质,其可促进在装置表面上形成融合细胞层(例如遗传改变的哺乳动物细胞,如内皮细胞或成纤维细胞)以在患者中调节或产生所需治疗效果,例如产生能防止再狭窄和/或血栓形成的抗血管生成因子或抗凝血剂或产生能抑制内膜过度增生的产物。在一个实施方式中,所述修复装置上的涂层包含的基质含有合成或天然存在的材料,所述材料中包含可促进循环性细胞(例如遗传改变的哺乳动物细胞,如内皮细胞、祖细胞或干细胞)粘附于医疗装置的治疗有效量的至少一种抗体和能刺激内皮细胞生长和分化的至少一种化合物(例如生长因子)。在植入所述装置后,附着于所述装置表面的细胞转化成为成熟和融合的功能性细胞层,例如在所述医疗装置内腔表面形成内皮。在医疗装置上存在的内皮细胞融合层,例如能减少植入部位再狭窄和血栓形成。The present invention provides a coated implantable medical device, such as a stent or graft, and methods and compositions for coating the medical device, as well as methods of treating vascular disease with the coated medical device. The present invention also provides a method for treating diseases such as restenosis and cancer comprising implanting a coated medical device into a patient in need of treatment and providing said patient with a genetically engineered Mammalian cells capable of binding to the surface of the medical device in vivo and producing engineered and desired therapeutic agents (eg, gene products). 1A-1C are schematic illustrations of surface coatings for medical devices of the present invention. Coatings on medical devices include: biocompatible matrices that facilitate the formation of confluent cell layers (e.g., genetically altered mammalian cells such as endothelial cells or fibroblasts) on the device surface to regulate or produce desired A therapeutic effect is desired, such as production of anti-angiogenic factors or anticoagulants that prevent restenosis and/or thrombosis or production of products that inhibit intimal hyperplasia. In one embodiment, the coating on the prosthetic device comprises a matrix comprising a synthetic or naturally occurring material comprising cells that promote cycling (e.g. genetically altered mammalian cells such as endothelial cells, progenitor cells) or stem cells) a therapeutically effective amount of at least one antibody and at least one compound (such as a growth factor) capable of stimulating endothelial cell growth and differentiation to adhere to the medical device. Following implantation of the device, cells attached to the surface of the device transform into a mature and confluent layer of functional cells, eg, forming an endothelium on the luminal surface of the medical device. The presence of a confluent layer of endothelial cells on medical devices, for example, reduces restenosis and thrombosis at implant sites.
如本文所用,“医疗装置”是指暂时或永久引入哺乳动物中以预防或治疗某一医学病状的装置。这些装置包括通过皮下、经皮或手术引入的安置在器官、组织或器官的内腔(例如动脉、静脉、心室或心房)中的任何装置。所述医疗装置可包括:支架、支架移植物、涂覆的支架(例如涂覆有聚四氟乙烯(PTFE)、膨胀型聚四氟乙烯(ePTFE)或是其它天然或合成涂层)、或合成的血管移植物、人造心脏瓣膜、人造心脏以及可连接修复的器官与循环血管的固定件、静脉瓣膜、腹主动脉动脉瘤(AAA)移植物、下腔静脉滤筛片、永久性输药导管、螺卷栓子(emboliccoil)、在血管栓塞形成时使用的栓塞材料(例如,交联PVA水凝胶)、血管缝线、血管吻合固定件、透心肌血管再形成支架和/或其他各种导管。As used herein, "medical device" refers to a device that is introduced into a mammal either temporarily or permanently to prevent or treat a medical condition. These devices include any device introduced subcutaneously, percutaneously or surgically for placement in an organ, tissue or lumen of an organ such as an artery, vein, ventricle or atrium. The medical device may comprise a stent, a stent graft, a coated stent (e.g. coated with polytetrafluoroethylene (PTFE), expanded polytetrafluoroethylene (ePTFE), or other natural or synthetic coating), or Synthetic vascular grafts, artificial heart valves, artificial hearts and fixtures that connect repaired organs to circulatory vessels, venous valves, abdominal aortic aneurysm (AAA) grafts, inferior vena cava filters, permanent drug delivery Catheters, embolic coils, embolic materials used in vascular embolization (e.g., cross-linked PVA hydrogel), vascular sutures, vascular anastomotic anchors, transmyocardial revascularization stents, and/or various other kind of conduit.
用本发明的组合物和方法形成的医疗装置的涂层能在体内刺激所述装置表面上产生融合的哺乳动物细胞层。例如,当所提供的配体结合内皮细胞使其在所述装置的血液接触表面上形成功能性内皮层时,该医疗装置表面上即形成了内皮细胞层,从而防止了再狭窄,并能调节由所述医疗装置的植入而引起的局部慢性炎症反应和血栓形成并发症。Coatings for medical devices formed using the compositions and methods of the present invention can stimulate in vivo the generation of a confluent mammalian cell layer on the surface of the device. For example, when the provided ligand binds endothelial cells to form a functional endothelial layer on the blood-contacting surface of the device, a layer of endothelial cells forms on the surface of the medical device, thereby preventing restenosis and regulating Local chronic inflammatory response and thrombotic complications caused by the implantation of the medical device.
覆盖医疗装置的基质可由合成材料组成,例如,聚合性凝胶泡沫,如由聚乙烯醇(PVA)、聚氨酯、聚-L-乳酸、纤维素酯或聚乙二醇制成的水凝胶。在一个实施方式中,制备基质的合成材料中可包含极为亲水的化合物,例如葡聚糖化合物。在另一实施方式中,所述基质由天然存在的材料组成,例如胶原、纤维蛋白、弹性蛋白、弹性蛋白原和/或无定形碳。所述基质也可包含多层,例如第一层可由合成材料或天然存在的材料组成,而第二层可含有例如配体(如抗体)。各层可相继顺次排列,第一层直接与医疗装置(支架或合成材料移植物)表面相接触,第二层有一表面直接与第一层接触,另一表面则与血管内腔接触。The matrix covering the medical device may consist of a synthetic material, for example, a polymeric gel foam, such as a hydrogel made of polyvinyl alcohol (PVA), polyurethane, poly-L-lactic acid, cellulose esters or polyethylene glycol. In one embodiment, very hydrophilic compounds, such as dextran compounds, may be included in the synthetic material from which the matrix is made. In another embodiment, the matrix is composed of naturally occurring materials such as collagen, fibrin, elastin, tropoelastin and/or amorphous carbon. The matrix may also comprise multiple layers, for example a first layer may consist of a synthetic material or a naturally occurring material and a second layer may contain, for example, a ligand such as an antibody. The layers can be arranged successively, the first layer is in direct contact with the surface of the medical device (stent or synthetic material graft), the second layer has one surface in direct contact with the first layer and the other surface in contact with the lumen of the blood vessel.
所述基质还可包含至少一种生长因子、细胞因子、血管舒张剂、抗凝血剂等。可刺激内皮细胞增殖和分化的生长因子为例如:血管内皮生长因子(VEGF)及其同工型、碱性成纤维细胞生长因子(bFGF)、血小板诱导的生长因子(PIGF)、转化生长因子β1(TGF.β1)、酸性成纤维细胞生长因子(FGF)、骨连接素、血管生成素1、血管生成素2、胰岛素样生长因子(ILGF)、血小板衍生生长因子AA(PDGF-AA)、血小板衍生生长因子BB(PDGF-BB)、血小板衍生生长因子AB(PDGF-AB)、粒细胞巨噬细胞集落刺激因子(GM-CSF)等,或其功能性片段,它们可用于本发明中。血管舒张剂包括:前列环素、α-CGRP等。The matrix may also comprise at least one growth factor, cytokine, vasodilator, anticoagulant, and the like. Growth factors that can stimulate endothelial cell proliferation and differentiation are, for example: vascular endothelial growth factor (VEGF) and its isoforms, basic fibroblast growth factor (bFGF), platelet-inducible growth factor (PIGF), transforming growth factor beta 1 (TGF.β1), acidic fibroblast growth factor (FGF), osteonectin, angiopoietin 1,
在另一实施方式中,所述基质可包含富勒烯,所述富勒烯的碳原子数为约C20-C150。所述富勒烯也可排列成纳米管,其中可掺入分子或蛋白质。所述富勒烯基质也可应用于不锈钢、PTFE或ePTFE医疗装置表面,然后对该层进行功能化并在其表面涂覆抗体和生长因子。或者,可在例如不锈钢医疗装置上先涂覆PTFE或ePTFE层,然后再涂覆第二层富勒烯,再加上抗体和生长因子。In another embodiment, the matrix may comprise fullerenes having a carbon number of about C20 -C150 . The fullerenes can also be arranged into nanotubes into which molecules or proteins can be incorporated. The fullerene matrix can also be applied to the surface of stainless steel, PTFE or ePTFE medical devices, and the layer is then functionalized and coated with antibodies and growth factors. Alternatively, a layer of PTFE or ePTFE can be applied on eg a stainless steel medical device, followed by a second layer of fullerene, plus antibodies and growth factors.
该基质可以非共价或共价的方式附着在医疗装置上。各种抗体和生长因子可通过异基或同基双功能交联试剂,共价附着在该基质上。可采用标准技术将生长因子与抗体一起或在抗体结合之后加入该基质内。The matrix can be attached to the medical device non-covalently or covalently. Various antibodies and growth factors can be covalently attached to the matrix via hetero- or homo-bifunctional cross-linking reagents. Growth factors can be added to the matrix either with the antibody or after binding of the antibody using standard techniques.
如本文所用,术语“抗体”是指一类单克隆、多克隆、人源化或嵌合性抗体或它们的组合,其中,所述单克隆、多克隆、人源化或嵌合抗体与一种抗原或该种抗原的功能性等价物相结合。术语抗体片段包括与抗体具有同样的作用和功效的任何抗体片段(如Fab、F(ab’)2等),且可为任何大小,即大分子或小分子。(包含有多数单独抗体分子的抗体,相当于6.022×1023个分子/摩尔抗体)。As used herein, the term "antibody" refers to a class of monoclonal, polyclonal, humanized or chimeric antibodies or combinations thereof, wherein the monoclonal, polyclonal, humanized or chimeric antibodies are combined with a Antigens or functional equivalents of such antigens. The term antibody fragment includes any antibody fragment (such as Fab, F(ab')2 , etc.) that has the same role and efficacy as an antibody, and can be of any size, ie macromolecule or small molecule. (an antibody containing most individual antibody molecules, equivalent to 6.022×1023 molecules/mole of antibody).
例如,在一个实施方式中,可用生物相容性基质涂覆支架或合成的移植物,该基质包含能调节循环细胞(例如遗传改变的哺乳动物治疗性细胞和内皮祖细胞)粘附于所述医疗装置表面的抗体、抗体片段或它们的组合。例如,本发明的抗体识别并结合特异性的细胞膜标记分子,例如由循环血中遗传改变的哺乳动物细胞产生的内皮祖细胞表面抗原和/或细胞膜分子,从而使得所述细胞固定在装置的表面上形成功能性细胞层(例如功能性内皮组织)。在一个实施方式中,所述抗体包括可与以下物质反应(识别并结合)的单克隆抗体:遗传改变的哺乳动物细胞表面分子、内皮祖细胞表面抗原、或者祖细胞或干细胞表面抗原,例如血管内皮生长因子受体-1、-2和-3(VEGFR-1、VEGFR-2和VEGFR-3以及VEGFR受体家族的同工型)、Tie-1、Tie2、CD34、Thy-1、Thy-2、Muc-18(CD146)、CD30、干细胞抗原-1(Sca-1)、干细胞因子(SCF或c-Kit配体)、CD133抗原、VE-钙粘蛋白、P1H12、TEK、CD31、Ang-1、Ang-2或在所述细胞表面上表达的抗原。在一个实施方式中,可使用与一种抗原反应的单一类型的抗体。或者,可将针对不同内皮祖细胞表面抗原的多种不同的抗体混合在一起并添加到基质中。在另一实施方式中,采用了单克隆抗体的混合物,通过靶向特异性细胞表面抗原来提高内皮形成率。在这一实施方式中,例如可组合使用抗-CD34和抗-CD133,或可将这些与任何或几种上文列出的抗原组合用于附着在医疗装置(例如支架或移植物)上的基质表面。也可用抗体、抗体片段和/或它们的组合来涂覆所述医疗装置。For example, in one embodiment, a stent or synthetic graft can be coated with a biocompatible matrix comprising cells capable of modulating the adhesion of circulating cells, such as genetically altered mammalian therapeutic cells and endothelial progenitor cells, to the Antibodies, antibody fragments, or combinations thereof on the surface of a medical device. For example, antibodies of the invention recognize and bind specific cell membrane marker molecules, such as endothelial progenitor cell surface antigens and/or cell membrane molecules produced by genetically altered mammalian cells in circulating blood, thereby immobilizing the cells on the surface of the device A functional cell layer (such as functional endothelial tissue) forms on the In one embodiment, the antibody comprises a monoclonal antibody reactive with (recognizes and binds to) a genetically altered mammalian cell surface molecule, an endothelial progenitor cell surface antigen, or a progenitor or stem cell surface antigen, such as a blood vessel Endothelial growth factor receptor-1, -2 and -3 (VEGFR-1, VEGFR-2 and VEGFR-3 and isoforms of the VEGFR receptor family), Tie-1, Tie2, CD34, Thy-1, Thy- 2. Muc-18 (CD146), CD30, stem cell antigen-1 (Sca-1), stem cell factor (SCF or c-Kit ligand), CD133 antigen, VE-cadherin, P1H12, TEK, CD31, Ang- 1. Ang-2 or an antigen expressed on the surface of said cell. In one embodiment, a single type of antibody reactive with one antigen can be used. Alternatively, multiple different antibodies against different EPC surface antigens can be mixed together and added to the matrix. In another embodiment, a cocktail of monoclonal antibodies is used to increase the rate of endothelialization by targeting specific cell surface antigens. In this embodiment, for example, anti-CD34 and anti-CD133 may be used in combination, or these may be used in combination with any or several of the above-listed antigens for attachment to medical devices such as stents or grafts. substrate surface. The medical device can also be coated with antibodies, antibody fragments, and/or combinations thereof.
如本文所用,“治疗有效量的抗体”是指可促进细胞附着于医疗装置的抗体用量,其中所述细胞为例如:天然或遗传改变的哺乳动物细胞,包括内皮细胞、祖细胞或干细胞。实施本发明所需的抗体量根据所用抗体的性质而不同。例如,所用抗体的量取决于抗体和与之反应的抗原之间的结合常数和/或亲和力。本领域的普通技术人员熟知如何确定用于具体抗原的抗体的治疗有效量。As used herein, a "therapeutically effective amount of an antibody" refers to an amount of the antibody that promotes attachment of cells, eg, native or genetically altered mammalian cells, including endothelial cells, progenitor cells, or stem cells, to a medical device. The amount of antibody required to practice the invention will vary depending on the nature of the antibody used. For example, the amount of antibody used depends on the binding constant and/or affinity between the antibody and the antigen with which it reacts. Those of ordinary skill in the art are familiar with how to determine a therapeutically effective amount of antibody for a particular antigen.
如本文所用,术语“化合物”是指可刺激遗传改变的哺乳动物细胞表达和/或分泌治疗性基因产物的任何物质。As used herein, the term "compound" refers to any substance that can stimulate the expression and/or secretion of a therapeutic gene product by a genetically altered mammalian cell.
如本文所用,术语“生长因子”是指可刺激细胞(例如遗传改变或未改变的内皮细胞、干细胞或祖细胞)生长和分化为成熟的功能性内皮细胞的肽、蛋白质、糖蛋白、脂蛋白或它们的片段或变体或是合成性分子。成熟的内皮细胞可表达一氧化氮合酶从而将一氧化氮释放入组织。下表1列出了可用于涂覆所述医疗装置的一些生长因子。As used herein, the term "growth factor" refers to a peptide, protein, glycoprotein, lipoprotein that stimulates the growth and differentiation of cells such as genetically altered or unaltered endothelial cells, stem cells, or progenitor cells into mature, functional endothelial cells Or their fragments or variants or synthetic molecules. Mature endothelial cells express nitric oxide synthase to release nitric oxide into tissues. Table 1 below lists some of the growth factors that can be used to coat the medical device.
表1Table 1
生长因子 内皮细胞特异性Growth Factors Endothelial Specificity
酸性成纤维细胞生长因子(aFGF) 无Acidic fibroblast growth factor (aFGF) None
碱性成纤维细胞生长因子(bFGF) 无Basic Fibroblast Growth Factor (bFGF) No
维细胞生长因子3(FGF-3) 无FGF-3 No
成纤维细胞生长因子4(FGF-4) 无Fibroblast Growth Factor 4 (FGF-4) No
成纤维细胞生长因子5(FGF-5) 无Fibroblast Growth Factor 5 (FGF-5) None
成纤维细胞生长因子6(FGF-6) 无Fibroblast Growth Factor 6 (FGF-6) None
成纤维细胞生长因子7(FGF-7) 无Fibroblast Growth Factor 7 (FGF-7) None
成纤维细胞生长因子8(FGF-8) 无Fibroblast Growth Factor 8 (FGF-8) None
成纤维细胞生长因子9(FGF-9) 无Fibroblast Growth Factor 9 (FGF-9) No
血管生成因子1 有Angiogenesis factor 1 Yes
血管生成因子2 有
肝细胞生长因子/分散因子(HGF/SF) 无Hepatocyte Growth Factor/Scatter Factor (HGF/SF) None
血小板衍生生长因子(PDE-CGF) 有Platelet-derived growth factor (PDE-CGF) Yes
转化生长因子-α(TGF-α) 无Transforming Growth Factor-α (TGF-α) None
转化生长因子-β(TGF-β) 无Transforming Growth Factor-β (TGF-β) None
肿瘤坏死因子-α(TNF-α) 无Tumor necrosis factor-α (TNF-α) None
血管内皮生长因子121(VEGF121) 有Vascular endothelial growth factor 121 (VEGF121) Yes
血管内皮生长因子145(VEGF145) 有Vascular endothelial growth factor 145 (VEGF145) Yes
血管内皮生长因子165(VEGF165) 有Vascular endothelial growth factor 165 (VEGF165) Yes
血管内皮生长因子189(VEGF189) 有Vascular endothelial growth factor 189 (VEGF189) Yes
血管内皮生长因子206(VEGF206) 有Vascular endothelial growth factor 206 (VEGF206) Yes
血管内皮生长因子B(VEGF-B) 有Vascular endothelial growth factor B (VEGF-B) Yes
血管内皮生长因子C(VEGF-C) 有Vascular endothelial growth factor C (VEGF-C) Yes
血管内皮生长因子D(VEGF-D) 有Vascular endothelial growth factor D (VEGF-D) Yes
血管内皮生长因子E(VEGF-E) 有Vascular endothelial growth factor E (VEGF-E) Yes
血管内皮生长因子F(VEGF-F) 有Vascular endothelial growth factor F (VEGF-F) Yes
胎盘生长因子 有Placental Growth Factor Yes
血管生成素-1 无Angiopoietin-1 None
血管生成素-2 无Angiopoietin-2 No
血小板反应蛋白(TSP) 无Thrombospondin (TSP) None
增殖蛋白 有Proliferation protein Yes
肝配蛋白-A1(Ephrin-A1,B61) 有Ephrin-A1 (Ephrin-A1, B61) Yes
E-选择蛋白 有E-selectin Yes
鸡趋化和血管生成因子(cCAF) 无Chicken chemotactic and angiogenic factor (cCAF) No
瘦蛋白(Leptin) 有Leptin Yes
肝素亲和力调节肽(HARP) 无Heparin affinity-regulating peptide (HARP) None
肝素 无Heparin None
粒细胞集落刺激因子 无Granulocyte colony stimulating factor None
胰岛素样生长因子 无Insulin-like growth factor No
白介素8 无Interleukin 8 None
甲状腺素 无Thyroxine None
神经鞘氨醇1-磷酸酯 无Sphingosine 1-phosphate No
如本文所用,术语“VEGF“是指上表1中所列血管内皮生长因子的任何同工型,除非可特异性地用该同工的序号或字母缩写对其鉴别。As used herein, the term "VEGF" refers to any isoform of vascular endothelial growth factor listed in Table 1 above, unless it can be specifically identified by the serial number or abbreviation of the isoform.
如本文所用,术语“治疗有效量的生长因子”是指刺激或诱导特异性细胞群(例如天然或经修饰的内皮细胞、祖细胞或干细胞)生长和分化,从而形成融合的成熟和功能性细胞层(例如内皮细胞在医疗装置内腔表面形成功能性内皮组织)的生长因子用量。实施本发明所需的生长因子用量随所用生长因子的性质以及生长因子与其靶细胞受体之间的结合动力学而不同。例如,100μg VEGF已显示可刺激内皮细胞粘附于医疗装置并形成内皮融合层。本领域的普通技术人员熟知如何确定用于刺激细胞(例如,内皮细胞)生长和分化的生长因子的治疗有效量。As used herein, the term "therapeutically effective amount of a growth factor" refers to stimulating or inducing the growth and differentiation of a specific cell population, such as native or modified endothelial cells, progenitor cells, or stem cells, thereby forming confluent mature and functional cells Layer (eg, endothelial cells forming functional endothelial tissue on the luminal surface of a medical device) growth factor dosage. The amount of growth factor required to practice the invention will vary with the nature of the growth factor used and the binding kinetics between the growth factor and its target cell receptor. For example, 100 μg of VEGF has been shown to stimulate endothelial cell adhesion to medical devices and formation of a confluent endothelial layer. One of ordinary skill in the art is familiar with how to determine a therapeutically effective amount of a growth factor for stimulating the growth and differentiation of cells (eg, endothelial cells).
如本文所用,“内膜增生”是指血管壁中平滑肌细胞增殖和/或基质沉积的不良增加。如本文所用,“再狭窄”是指血管内腔的复发性狭窄。血管可因再狭窄而梗阻。在PTCA或PTA后,来自血管中层和外膜且通常不存在于内膜中的平滑肌细胞发生增殖,迁移到内膜并分泌蛋白质,在内膜中形成平滑肌细胞和基质蛋白的积聚。该积聚导致了动脉内腔狭窄、流向狭窄处远端的血流减少。如本文所用,“抑制再狭窄”是指对平滑肌细胞迁移和增殖的抑制并同时防止蛋白质分泌,从而防止再狭窄及其引起的并发症。As used herein, "intimal hyperplasia" refers to an undesirable increase in smooth muscle cell proliferation and/or matrix deposition in the vessel wall. As used herein, "restenosis" refers to recurrent narrowing of the lumen of a blood vessel. Blood vessels can become obstructed by restenosis. After PTCA or PTA, smooth muscle cells from the media and adventitia that are not normally present in the intima proliferate, migrate to the intima and secrete proteins, forming an accumulation of smooth muscle cells and matrix proteins in the intima. This buildup results in a narrowing of the lumen of the artery, reducing blood flow distal to the narrowing. As used herein, "inhibiting restenosis" refers to the inhibition of smooth muscle cell migration and proliferation while preventing protein secretion, thereby preventing restenosis and its resulting complications.
可用本发明的医疗装置、方法和组合物治疗的对象为哺乳动物,包括:人、狗、猫、猪、马、啮齿类和猴。Subjects that can be treated with the medical devices, methods and compositions of the present invention are mammals, including: humans, dogs, cats, pigs, horses, rodents and monkeys.
本发明的治疗方法可应用于体内或体外。The therapeutic methods of the invention may be applied in vivo or in vitro.
术语“内皮祖细胞”是指源自骨髓、血液或局部组织并处于任何发育阶段的内皮细胞(从祖细胞或干细胞到成熟的功能性内皮细胞),它们是非恶性细胞。The term "endothelial progenitor cells" refers to endothelial cells derived from bone marrow, blood or local tissues and at any stage of development (from progenitor cells or stem cells to mature functional endothelial cells), which are non-malignant cells.
可为所述涂覆的医疗装置提供经遗传修饰的哺乳动物细胞,例如可从离体的动脉或静脉(例如人脐静脉)中分离得到的遗传改变的分化的内皮细胞,它们已在体外用所需的核酸构建物进行了遗传改变,而内皮祖细胞则可分离自外周血或骨髓。在一个实施方式中,可通过将内皮细胞与涂覆了基质的医疗装置共同孵育,而使所述内皮细胞能与所述医疗装置结合,其中所述基质掺入了抗体和任选的至少一种生长因子或其它能附着于内皮细胞的配体。在另一实施方式中,所述内皮细胞可为转化的内皮细胞。转染的内皮细胞可包含表达生长因子或其它肽或蛋白质的载体,这些生长因子、肽或蛋白质可直接或间接抑制血栓形成、再狭窄或其它任何治疗结果。The coated medical device can be provided with genetically modified mammalian cells, such as genetically altered differentiated endothelial cells that can be isolated from ex vivo arteries or veins (e.g., human umbilical vein), which have been used in vitro The desired nucleic acid constructs are genetically altered, and endothelial progenitor cells can be isolated from peripheral blood or bone marrow. In one embodiment, endothelial cells are rendered capable of binding to a medical device by incubating the endothelial cells with a matrix that incorporates an antibody and optionally at least one growth factors or other ligands that attach to endothelial cells. In another embodiment, the endothelial cells may be transformed endothelial cells. Transfected endothelial cells may contain vectors expressing growth factors or other peptides or proteins that directly or indirectly inhibit thrombosis, restenosis, or any other therapeutic outcome.
在另一实施方式中,可用哺乳动物表达载体转染内皮细胞或其它类型稳定的哺乳动物细胞(例如成纤维细胞),所述载体包含编码适于特定用途的蛋白质或肽的任何克隆基因。例如,可构建所述载体使其含有表达盒,所述表达盒包含编码血小板衍生生长因子(PDGF)、成纤维细胞生长因子(FGF)或一氧化氮合酶(NOS)的基因,且可用常规方法构建所述表达盒,可由市售货源提供。(参见例如,购自Stratagene,San Diego,CA的哺乳动物表达载体和转染试剂盒)。例如,根据Rosengart等的方法,使用表达VEGF cDNA的腺病毒表达载体,以血管内皮生长因子(VEGF)基因转染纯化的猪内皮祖细胞(“用于治疗冠心病的直接心肌内给予表达VEGF121 cDNA的腺病毒载体的血管生成基因疗法的I期试验的六个月评估”,Six-month assessment ofa phase I trial of angiogenic gene therapy for the treatment of coronaryartery disease using direct intramyocardial administration of an adenovirusvector expressing the VEGF121 cDNA;Ann.Surg.230(4):466-470,1999,纳入本文作为参考)。在这一实施方式中,所述哺乳动物细胞的来源可为自体、同种异体的或异种的。一旦转染了含有所需基因的外源DNA或RNA表达盒而使得所述细胞遗传发生改变,可用标准的组织培养技术来培养所述细胞。可用标准技术在液氮中冻存表达并分泌所需基因的细胞样品。在使用前,可用标准的组织培养技术使冷冻的细胞重新生长。在植入所述装置时将遗传改变的哺乳动物细胞在植入部位局部给予或经静脉内或动脉内给予患者,优选在植入所述涂覆的医疗装置之后给予。转化细胞还可包含给予患者该细胞前用于精确检测和鉴别所述细胞的标记或报告基因。In another embodiment, endothelial cells or other stable mammalian cell types (eg, fibroblasts) can be transfected with a mammalian expression vector comprising any cloned gene encoding a protein or peptide suitable for a particular use. For example, the vector can be constructed to contain an expression cassette comprising a gene encoding platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), or nitric oxide synthase (NOS), and can be expressed using routine Methods The expression cassette can be constructed, which can be provided by commercial sources. (See, e.g., Mammalian Expression Vectors and Transfection Kits available from Stratagene, San Diego, CA). For example, purified porcine endothelial progenitor cells were transfected with the vascular endothelial growth factor (VEGF) gene using an adenoviral expression vector expressing VEGF cDNA according to the method of Rosengart et al. (“Direct intramyocardial administration of VEGF121 cDNA expressing Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA; Ann. Surg. 230(4):466-470, 1999, incorporated herein by reference). In this embodiment, the source of the mammalian cells may be autologous, allogeneic or xenogeneic. Once the cells have been genetically altered by transfection of an exogenous DNA or RNA expression cassette containing the desired gene, the cells can be cultured using standard tissue culture techniques. Samples of cells expressing and secreting the desired gene can be frozen in liquid nitrogen using standard techniques. Frozen cells can be regrowth using standard tissue culture techniques prior to use. The genetically altered mammalian cells are administered locally at the site of implantation or intravenously or intraarterially to the patient upon implantation of the device, preferably after implantation of the coated medical device. Transformed cells may also contain markers or reporter genes for precise detection and identification of the cells prior to administration of the cells to a patient.
可在任何动脉或静脉上实施本发明治疗血管疾病的方法。本发明范围内包括任何动脉的动脉粥样硬化,所述动脉包括冠状动脉、腹股沟下动脉、主动脉髂动脉、锁骨下动脉、肠系膜动脉和肾动脉。本发明还包括其它类型的血管梗阻,例如壁间动脉瘤造成的梗阻。The methods of the present invention for treating vascular disease can be practiced on any artery or vein. Included within the scope of the present invention is atherosclerosis of any artery, including the coronary, inguinal, aortoiliac, subclavian, mesenteric, and renal arteries. Other types of vascular obstruction are also encompassed by the present invention, such as obstruction caused by intramural aneurysms.
治疗患有血管疾病的哺乳动物的方法包括:将涂覆的医疗装置植入所述患者的器官或血管,例如,在血管形成术中植入涂覆的支架的情况下。一旦进入体内,内皮祖细胞通过识别和结合细胞抗原(例如遗传修饰的哺乳动物细胞)而被捕获于所述涂覆的支架的表面上,或通过单独抗体或抗体与其它配体的组合而被捕获于存在于所述装置涂层上的祖细胞表面。一旦祖细胞附着于基质,所述涂层上的生长因子就促进新结合的内皮祖细胞生长和分化并在所述支架的内腔表面形成融合的成熟功能性内皮组织。或者,在植入所述医疗装置前,可在体外用天然或遗传修饰的哺乳动物细胞(例如内皮细胞)涂覆所述医疗装置,这些细胞可为分离自患者血液、骨髓或血管的祖细胞、干细胞或成熟内皮细胞。在两种情况下,所述医疗装置内腔表面上存在的功能性细胞可产生所需的或经工程改造的功能,例如抑制或防止内膜过度增生和血栓形成。A method of treating a mammal suffering from vascular disease comprises implanting a coated medical device into an organ or vessel of said patient, eg, in the case of implanting a coated stent during angioplasty. Once inside the body, endothelial progenitor cells are captured on the surface of the coated scaffold by recognition and binding of cellular antigens, such as genetically modified mammalian cells, or by antibodies alone or in combination with other ligands. Trapped on the surface of progenitor cells present on the coating of the device. Once the progenitor cells are attached to the matrix, the growth factors on the coating promote the growth and differentiation of newly bound endothelial progenitor cells and form confluent mature functional endothelial tissue on the luminal surface of the scaffold. Alternatively, prior to implantation of the medical device, the medical device may be coated in vitro with native or genetically modified mammalian cells such as endothelial cells, which may be progenitor cells isolated from the patient's blood, bone marrow, or blood vessels , stem cells or mature endothelial cells. In both cases, the presence of functional cells on the luminal surface of the medical device can produce desired or engineered functions, such as inhibiting or preventing intimal hyperproliferation and thrombus formation.
内皮细胞Endothelial cells
在某些实施方式中,按照Jaffe等(J.Clin.Invest.,52:2745-2757,1973,在此纳入本文作为参考并用于试验中)的方法,可从人的脐带静脉获得人脐带静脉内皮细胞(HUVEC)。简而言之,用胶原酶处理从血管壁剥离的细胞,放入用明胶涂覆的组织培养瓶中,在含有10%低内毒素的胎牛血清、90微克/毫升无防腐剂的猪肝素、20微克/毫升内皮细胞生长补充剂(ECGS)和谷氨酰胺的M199培养基中培育。In certain embodiments, human umbilical veins can be obtained from human umbilical veins according to the method of Jaffe et al. Endothelial cells (HUVECs). Briefly, cells dissected from vessel walls were treated with collagenase and placed in gelatin-coated tissue culture flasks in 10% endotoxin-low fetal bovine serum, 90 μg/ml preservative-free porcine heparin , 20 μg/ml endothelial cell growth supplement (ECGS) and glutamine in M199 medium.
可按照Asahara等人的方法(用于血管生成的推定的内皮祖细胞的分离,Isolation of putative progenitor endothelial cells for angiogenesis,Science.275:964-967,1997,纳入本文作为参考)从人外周血中分离得到内皮祖细胞(EPC)。将包被有抗CD34的抗体的磁珠与经过组分分离的人外周血液一起孵育。经过孵育后,洗脱结合的细胞,将其培养于EBM-2培养基(Clonetics,San Diego,CA)中。或者,可采用富集培养基分离法来分离这些细胞。简而言之,从志愿者取得外周静脉血,采用密度梯度离心法分离得到单核细胞,将这些细胞接种在包被有纤连蛋白的培养玻片上(Becton Dickinson),在补充5%胎牛血清、人VEGF-A、人成纤维细胞生长因子-2、人表皮细胞生长因子、胰岛素样生长因子-1和抗坏血酸的EC基础培养基-2(EBM-2)(Clonetics)中培养。使内皮祖细胞生长7天,每48小时更换培养基。通过抗CD45、CD34、CD31、VEGFR-2、Tie-2和E-选择素的荧光抗体进行细胞鉴定。(Isolation of putative progenitor endothelial cells for angiogenesis, Science. 275:964-967, 1997, incorporated herein by reference) from human peripheral blood according to the method of Asahara et al. Endothelial progenitor cells (EPCs) were isolated. Magnetic beads coated with anti-CD34 antibody were incubated with fractionated human peripheral blood. After incubation, bound cells were eluted and cultured in EBM-2 medium (Clonetics, San Diego, CA). Alternatively, enriched media isolation can be used to isolate these cells. Briefly, mononuclear cells were isolated from peripheral venous blood obtained from volunteers by density gradient centrifugation and plated on fibronectin-coated culture slides (Becton Dickinson) in the presence of 5% fetal bovine Serum, human VEGF-A, human fibroblast growth factor-2, human epidermal growth factor, insulin-like growth factor-1 and ascorbic acid were cultured in EC basal medium-2 (EBM-2) (Clonetics). EPCs were grown for 7 days with media changes every 48 hours. Cell identification was performed by fluorescent antibodies against CD45, CD34, CD31, VEGFR-2, Tie-2 and E-selectin.
在另一实施方式中,可采用常规方法,用含有任何如下克隆基因的任何表达盒对哺乳动物细胞进行转染,所述克隆基因可含有编码在循环细胞中不常见的特异性标记分子(例如前列腺特异性抗原或骨细胞抗原),且还可表达肽和/或蛋白质,例如血小板衍生生长因子(PDGF)、成纤维细胞生长因子(FGF)或一氧化氮合酶(NOS)等。(参见,例如购自Stratagene,San Diego,CA的哺乳动物表达载体和转染试剂盒)。例如,根据Rosengart等的方法,采用表达VEGF cDNA的腺病毒表达载体,以血管内皮生长因子(VEGF)转染纯化的猪内皮祖细胞(“用于治疗冠心病的直接心肌内给予表达VEGF121 cDNA的腺病毒载体的血管生成基因疗法的I期试验的六个月评估”,Six-month assessment of a phase I trial ofangiogenic gene therapy for the treatment of coronary artery disease usingdirect intramyocardial administration of an adenovirus vector expressingthe VEGF121 cDNA;Ann.Surg.230(4):466-470,1999,纳入本文作为参考)。In another embodiment, mammalian cells can be transfected using conventional methods with any expression cassette containing any cloned gene that may encode a specific marker molecule that is not commonly found in circulating cells (e.g. Prostate-specific antigen or osteocyte antigen), and can also express peptides and/or proteins, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) or nitric oxide synthase (NOS), etc. (See, e.g., mammalian expression vectors and transfection kits available from Stratagene, San Diego, CA). For example, purified porcine endothelial progenitor cells were transfected with vascular endothelial growth factor (VEGF) using an adenoviral expression vector expressing VEGF cDNA according to the method of Rosengart et al. (“Direct intramyocardial administration of VEGF121 cDNA-expressing Six-month assessment of a phase I trial ofangiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA; Ann . Surg. 230(4):466-470, 1999, incorporated herein by reference).
抗体Antibody
可按照Kohler和Milstein的标准技术制备在本发明的方法中采用的单克隆抗体(《分泌预先确定特异性的抗体的融合细胞的连续培育方法》,Continuouscultures of fused cells secreting antibody of predefined specificity,Nature,265:495-497,1975,纳入本文作为参考),或者可直接从市售来源获得用于本发明方法的单克隆抗体。内皮细胞可用作免疫原来产生抗内皮细胞表面抗原的单克隆抗体。Monoclonal antibodies employed in the methods of the present invention can be prepared according to standard techniques of Kohler and Milstein ("Continuous cultures of fused cells secreting antibody of predetermined specificity", Continuous cultures of fused cells secreting antibody of predetermined specificity, Nature, 265:495-497, 1975, incorporated herein by reference), or monoclonal antibodies for use in the methods of the invention may be obtained directly from commercially available sources. Endothelial cells can be used as immunogens to generate monoclonal antibodies against endothelial cell surface antigens.
可将HUVEC或经纯化的内皮祖细胞注射入小鼠或大鼠来制备抗内皮细胞的单克隆抗体。经过足够长的时间后,处死小鼠,取其脾细胞。通过将脾细胞融合于骨髓瘤细胞或淋巴瘤细胞制得永生性细胞,融合通常在含有非离子型表面活性剂(例如聚乙二醇)中进行。使所得包括融合性杂交瘤的细胞在选择性培养基(例如HAT-培养基)中生长,并使用有限稀释条件使存活的细胞在这种培养基中生长。细胞生长于适宜的容器(例如微量滴定孔)中,筛选上清液中具有所需特异性,即能与内皮细胞抗原发生反应的单克隆抗体。Monoclonal antibodies against endothelial cells can be prepared by injecting HUVEC or purified endothelial progenitor cells into mice or rats. After a long enough time, the mice were sacrificed, and the splenocytes were collected. Immortalized cells are produced by fusing spleen cells with myeloma cells or lymphoma cells, usually in a medium containing a non-ionic surfactant such as polyethylene glycol. The resulting cells, including fusion hybridomas, are grown in a selective medium (eg, HAT-medium), and surviving cells are grown in this medium using limiting dilution conditions. Cells are grown in suitable containers (eg, microtiter wells), and the supernatants are screened for monoclonal antibodies of the desired specificity, ie, those capable of reacting with endothelial cell antigens.
已有多种可用于提高单克隆抗体产率的技术,例如:将杂交瘤细胞注射入接受这些细胞的哺乳动物宿主腹腔内,然后收获腹水。当从腹水中不能收集到足量的单克隆抗体时,可从该宿主血液中收获抗体。已有多种可用于分离和纯化单克隆抗体从而使单克隆抗体中不含其它蛋白质或污染物的方法。Various techniques are available to increase the yield of monoclonal antibodies, such as injecting hybridoma cells intraperitoneally into the recipient mammalian host and harvesting the ascites. When a sufficient amount of monoclonal antibody cannot be collected from the ascitic fluid, the antibody can be harvested from the blood of the host. Various methods are available for isolating and purifying monoclonal antibodies so that they are free of other proteins or contaminants.
本发明的范围还包括抗体(例如抗内皮细胞单克隆抗体)的有用结合片段,例如这些抗内皮细胞的单克隆抗体的Fab、F(ab’)2。可通过常规技术获得所述抗体片段。例如,可通过用木瓜蛋白酶或胃蛋白酶对抗体进行肽酶消化来制备有用的结合片段。这些片段可单独使用,或者与其起源抗体或其它类型的抗体及其片段联合使用。Also included within the scope of the invention are useful binding fragments of antibodies (eg, anti-endothelial monoclonal antibodies), eg, the Fab, F(ab')2 of these anti-endothelial monoclonal antibodies. Such antibody fragments can be obtained by conventional techniques. For example, useful binding fragments can be prepared by peptidase digestion of antibodies with papain or pepsin. These fragments can be used alone or in combination with their parent antibody or other types of antibodies and fragments thereof.
本发明的抗体可以是鼠源性IgG类抗体;然而,这并不成为限制。特异性抗体,例如上述的抗体以与其功能相当的那些抗体,无论是鼠源性、哺乳动物源性(包括人源性)、还是其他来源,或它们的组合,都包括在本发明范畴内,还包括含同种型的其它类抗体,例如IgM、IgA、IgE等。这些抗体能特异性识别并以高亲和力结合靶细胞膜上的靶抗原,无论是该靶抗原是在天然分子上或在经遗传工程改造的抗原上。就抗体而言,术语“功能上相当”是指两种不同的抗体各自结合抗原上的相同抗原位点,换言之,所述抗体竞争性结合相同的抗原。所述抗原可位于相同或不同分子上。Antibodies of the invention may be murine IgG class antibodies; however, this is not limiting. Specific antibodies, such as the above-mentioned antibodies and those antibodies that are functionally equivalent to them, whether of murine origin, mammalian origin (including human origin), or other sources, or combinations thereof, are included within the scope of the present invention. Also included are other classes of antibodies comprising isotypes such as IgM, IgA, IgE, and the like. These antibodies can specifically recognize and bind with high affinity to the target antigen on the target cell membrane, whether the target antigen is on a natural molecule or on a genetically engineered antigen. With respect to antibodies, the term "functionally equivalent" means that two different antibodies each bind to the same antigenic site on an antigen, in other words, the antibodies compete for binding to the same antigen. The antigens may be on the same or different molecules.
在一个实施方式中,可采用能与内皮细胞表面抗原(例如,CD34)反应的单克隆抗体和/或其片段。已证明附着在固相载体上的抗CD34单克隆抗体可捕获人外周血中的内皮祖细胞。捕获后,这些祖细胞可分化为内皮细胞。(Asahara等,1997,《用于血管生成的推定的内皮祖细胞的分离》,Isolation of putativeprogenitor endothelial cells for angiogenesis,Science 275:964-967。)杂交瘤产生的抗CD34单克隆抗体可获自美国典型组织收集中心(American TypeTissue Collection,Rockville,MD)。在另一实施方式中,使用了能与内皮细胞表面抗原(例如VEGFR-1和VEGFR-2、CD133或Tie-2)反应的单克隆抗体。在使用遗传改变的细胞的实施方式中,可用与上文所述类似的方式,采用标准技术来产生抗所述经遗传工程改造的基因产物的抗体,在施涂基质后将其施用于所述医疗装置与血液接触的表面上。In one embodiment, monoclonal antibodies and/or fragments thereof reactive with endothelial cell surface antigens (eg, CD34) may be used. Anti-CD34 monoclonal antibody attached to a solid support has been shown to capture endothelial progenitor cells in human peripheral blood. After capture, these progenitor cells can differentiate into endothelial cells. (Asahara et al., 1997, "Isolation of putative progenitor endothelial cells for angiogenesis", Isolation of putative progenitor endothelial cells for angiogenesis, Science 275:964-967.) Anti-CD34 monoclonal antibodies produced by hybridomas can be obtained from US Typical Tissue Collection Center (American TypeTissue Collection, Rockville, MD). In another embodiment, monoclonal antibodies reactive with endothelial cell surface antigens (eg, VEGFR-1 and VEGFR-2, CD133 or Tie-2) are used. In embodiments using genetically altered cells, antibodies against the genetically engineered gene product can be raised using standard techniques in a manner similar to that described above, which can be applied to the substrate following application of the matrix. on blood-contact surfaces of medical devices.
还可使用能与分离自与接受该医疗装置植入的同种动物的抗内皮细胞相反应的多克隆抗体。Polyclonal antibodies reactive with anti-endothelial cells isolated from the same species of animal as which the medical device was implanted may also be used.
支架bracket
术语“支架”在本文中是指当插入或植入血管内腔时,可扩大血管内腔横截面的任何医疗装置。术语“支架”包括:已用本发明的方法涂覆的不锈钢制或其它合金制市售支架;经覆盖的支架,例如用PTFE或ePTFE覆盖。在一个实施方式中,包括经皮递送治疗冠状动脉阻塞或封闭脾脏、颈动脉、骼部和腿腘部血管切口或动脉瘤的支架。在另一实施方式中,将所述支架递送入静脉血管。所述支架可由聚合物或金属构件组成,其上涂覆有含抗体和化合物(例如生长因子)的基质。例如,可采用可变形金属丝支架,如授予Wiktor的美国专利4,886,062中所公开的支架,该专利以其全文纳入本文作为参考。还可使用弹性聚合材料自扩张支架(self-expanding stent),例如名称为“内腔药物洗脱修复术”,IntraluminalDrug Eluting Prosthesis的美国专利5,871,535和公开的国际专利申请WO91/12779中所揭示的支架,将这些专利以其全文纳入本文作为参考。可使用美国专利6,432,132和6,821,292中所揭示的其它支架,将这些专利以其全文纳入本文作为参考。还可使用以下材料制造支架:不锈钢、聚合物、镍-钛、钽、金、铂-铱、钴基合金或Elgiloy或MP35N以及其它含铁材料。将支架置于导管上通过体腔递送到治疗部位,在该部位将支架从导管上释放,使得所述支架扩张为直接接触血管内壁。在另一实施方式中,所述支架包括生物可降解支架(H。Tamai,冠状动脉支架手册,Handbook of Coronary stents,297页,第3版,PW Serruys和MJBKutryk,Martin Dunitz(2000)。本领域的技术人员知道可将本发明的抗体、生长因子和基质与其它自扩张支架设计物(例如弹性金属支架设计物)一起使用。The term "stent" herein refers to any medical device that, when inserted or implanted in the lumen of a blood vessel, expands the cross-section of the lumen of a blood vessel. The term "stent" includes: commercially available stents made of stainless steel or other alloys that have been coated with the method of the present invention; covered stents, for example with PTFE or ePTFE. In one embodiment, percutaneous delivery of a stent to treat blocked coronary arteries or occlude splenic, carotid, iliac, and popliteal vascular incisions or aneurysms of the legs is included. In another embodiment, the stent is delivered into a venous vessel. The scaffold may consist of a polymeric or metallic member coated with a matrix containing antibodies and compounds such as growth factors. For example, a deformable wire stent may be employed, such as that disclosed in US Patent 4,886,062 to Wiktor, which is incorporated herein by reference in its entirety. Self-expanding stents of elastic polymeric material such as those disclosed in U.S. Patent 5,871,535 and published International Patent Application WO 91/12779 entitled "Intraluminal Drug Eluting Prosthesis" may also be used , which are incorporated herein by reference in their entirety. Other scaffolds may be used as disclosed in US Patent Nos. 6,432,132 and 6,821,292, which are incorporated herein by reference in their entirety. Stents can also be fabricated from stainless steel, polymers, nickel-titanium, tantalum, gold, platinum-iridium, cobalt-based alloys or Elgiloy or MP35N and other ferrous materials. The stent is placed on a catheter and delivered through a body lumen to the treatment site where it is released from the catheter such that the stent expands into direct contact with the inner wall of the blood vessel. In another embodiment, the stent comprises a biodegradable stent (H. Tamai, Handbook of Coronary stents, Handbook of Coronary stents, 297 pages, 3rd edition, PW Serruys and MJB Kutryk, Martin Dunitz (2000). Art Those skilled in the art will know that the antibodies, growth factors and matrices of the invention can be used with other self-expanding stent designs, such as elastic metal stent designs.
合成的移植物synthetic graft
术语“合成的移植物”是指具有生物相容性特征的任何人造修复体。在一个实施方式中,所述合成的移植物可用聚对苯二甲酸乙二醇酯(Dacron,PET)或聚四氟乙烯(Teflon,ePTFE)制备。在另一实施方式中,所述合成的移植物由聚氨酯、交联的PVA水凝胶和/或水凝胶的生物相容性泡沫体构成。在另一实施方式中,合成的移植物由网状聚碳酸酯氨基甲酸乙酯(polycarbonate urethane)内层和网状聚对苯二甲酸乙二醇酯外层构成。本领域技术人员知道可将本发明的覆盖组分(例如抗体、生长因子和基质)与任何生物相容性合成的移植物一起使用。(Bos等,1998,小口径的血管修复材料:现状,Small-Diameter Vascular Prostheses:CurrentStatus,Archives Physio Biochem.106:100-115,纳入本文作为参考)。可将合成的移植物用于,例如血管的端-端、端-侧、侧-端、侧-侧或管腔内和吻合血管,或用于患病血管区段的分流,例如作为腹主动脉动脉瘤装置。The term "synthetic graft" refers to any artificial prosthesis having biocompatible characteristics. In one embodiment, the synthetic graft can be made from polyethylene terephthalate (Dacron , PET) or polytetrafluoroethylene (Teflon , ePTFE) preparation. In another embodiment, the synthetic graft is composed of polyurethane, cross-linked PVA hydrogel, and/or biocompatible foam of hydrogel. In another embodiment, a synthetic graft is constructed of a reticulated polycarbonate urethane inner layer and a reticulated polyethylene terephthalate outer layer. Those skilled in the art will recognize that the covering components (eg, antibodies, growth factors, and matrices) of the invention can be used with any biocompatible synthetic graft. (Bos et al., 1998, Small-Diameter Vascular Prostheses: Current Status, Archives Physio Biochem. 106: 100-115, incorporated herein by reference). Synthetic grafts can be used, for example, for end-to-end, end-to-side, side-to-side, side-to-side or intraluminal and anastomotic vessels, or for shunting of diseased vessel segments, for example as abdominal main Artery aneurysm device.
基质matrix
(A)合成材料——所述用于覆盖支架或合成的移植物的基质可选自合成材料,例如:聚氨酯、嵌段聚氨酯-脲/肝素、聚-L-乳酸、纤维素酯、聚乙二醇、交联PVA水凝胶、水凝胶的生物相容性泡沫体或亲水性葡聚糖,例如羧甲基葡聚糖。(A)Synthetic material - the matrix used to cover the stent or synthetic graft can be selected from synthetic materials such as: polyurethane, segmented polyurethane-urea/heparin, poly-L-lactic acid, cellulose ester, polyethylene Diols, cross-linked PVA hydrogels, biocompatible foams of hydrogels or hydrophilic dextran such as carboxymethyl dextran.
(B)天然存在的材料——所述基质可选自天然存在的物质,例如:胶原、纤连蛋白、玻连蛋白、弹性蛋白、层连蛋白、肝素、纤维蛋白、纤维素和碳。对于基质的基本要求是具有足够的弹性和柔韧性以保证其在支架或合成的移植物的接触表面上不断裂。(B)Naturally Occurring Materials - The matrix may be selected from naturally occurring substances such as: collagen, fibronectin, vitronectin, elastin, laminin, heparin, fibrin, cellulose, and carbon. The basic requirement for the matrix is to have sufficient elasticity and flexibility to ensure that it does not break at the contact surface of the stent or synthetic graft.
(C)富勒烯——所述基质还可包含富勒烯(术语“富勒烯”包括多种富勒烯分子)。富勒烯是碳-笼(carbon-cage)分子。富勒烯类中的碳(C)分子数的变化范围为C20-C150。富勒烯是按照本领域技术人员熟知的方法,通过在高温下使碳元素或含碳类物质反应而产生的;例如,通过对碳进行激光汽化、在电弧中加热碳或在碳黑生成火焰中燃烧烃类物质。(授予Patel等的美国专利5,292,813;授予Bhushan等的美国专利5,558,903,其揭示以其全文纳入本文作为参考)。在各种情况下,均产生了含碳的淀积物或烟灰。可从这种烟灰中,通过用适宜的溶剂(例如甲苯)提取而得到不同的富勒烯。用已知的方法分离富勒烯,尤其是通过高效液相色谱法(HPLC)。可合成或从Dynamic Enterpri ses,Ltd.(Berkshire,England or SouthernChemical Group,LLC,Tucker,Georgia)或Bucky USA(Houston Texas)购买富勒烯。(C)Fullerenes - The matrix may also contain fullerenes (the term "fullerene" includes a variety of fullerene molecules). Fullerenes are carbon-cage molecules. The number of carbon (C) molecules in the fullerenes varies from C20 to C150 . Fullerenes are produced by reacting carbon elements or carbon-containing species at high temperatures according to methods well known to those skilled in the art; for example, by laser vaporizing carbon, heating carbon in an electric arc, or forming a flame in carbon black Combustion of hydrocarbons. (US Patent 5,292,813 to Patel et al; US Patent 5,558,903 to Bhushan et al, the disclosures of which are incorporated herein by reference in their entirety). In each case, carbonaceous deposits or soot are produced. Different fullerenes can be obtained from this soot by extraction with a suitable solvent (eg toluene). The fullerenes are separated by known methods, in particular by high performance liquid chromatography (HPLC). Fullerenes can be synthesized or purchased from Dynamic Enterprises, Ltd. (Berkshire, England or Southern Chemical Group, LLC, Tucker, Georgia) or Bucky USA (Houston Texas).
可用不同的方法将富勒烯沉积在表面上,所述方法包括:升华、激光汽化、溅射、离子束、喷涂、浸涂、滚涂或刷涂(如美国专利5,558,903中所揭示,以其全文纳入本文作为参考),或通过支架表面的衍生化。Fullerenes can be deposited on surfaces by various methods including: sublimation, laser vaporization, sputtering, ion beam, spraying, dipping, rolling or brushing (as disclosed in U.S. Patent 5,558,903 for its incorporated herein by reference in its entirety), or by derivatization of the scaffold surface.
富勒烯的一个重要特征是它们能形成“活化碳”。富勒烯的电子结构是重叠的π轨道系统,从而多个成键电子共同存在于围绕分子的表面。(Chemical andEngineering News,Apr.8,1991,第59页,纳入本文作为参考)。作为具有活性的碳的形式,富勒烯显示出对弱相互作用的基本范德华力。富勒烯表面的吸附性能使其具有引导特异性细胞膜相互作用的额外修饰。例如,可将具有选择性结合于特定细胞类型的细胞膜或特定的细胞膜组分(例如凝集素或抗体)的化学性质的特异性分子吸附到富勒烯表面。可对富勒烯表面不同分子的粘附进行操作以产生选择性结合不同细胞类型(例如,内皮祖细胞、内皮细胞、成纤维细胞、原代外植体或T-细胞亚群)的表面,例如授予Richmond等的美国专利5,310,669,其揭示以其全文纳入本文作为参考;Stephen R.Wilson,富勒烯的生物学特性,Biological Aspects ofFullerenes,Fullerenes:Chemistry,Physics and Technology,Kadish等编,John Wiley&Sons,NY 2000,纳入本文作为参考。An important feature of fullerenes is their ability to form "activated carbons". The electronic structure of fullerenes is a system of overlapping π orbitals, whereby multiple bonding electrons co-exist around the surface of the molecule. (Chemical and Engineering News, Apr. 8, 1991, p. 59, incorporated herein by reference). As an active form of carbon, fullerenes exhibit fundamental van der Waals forces for weak interactions. The adsorptive properties of fullerene surfaces allow for additional modifications that direct specific cell membrane interactions. For example, specific molecules with chemistries that selectively bind to cell membranes of specific cell types or to specific cell membrane components such as lectins or antibodies can be adsorbed to the fullerene surface. The adhesion of different molecules to the fullerene surface can be manipulated to create a surface that selectively binds different cell types (e.g., endothelial progenitor cells, endothelial cells, fibroblasts, primary explants, or T-cell subsets), For example, U.S. Patent 5,310,669 to Richmond et al., the disclosure of which is incorporated herein by reference in its entirety; Stephen R. Wilson, Biological Aspects of Fullerenes, Fullerenes: Chemistry, Physics and Technology, eds. Kadish et al., John Wiley & Sons , NY 2000, incorporated herein by reference.
富勒烯还可形成可掺入其它原子或分子的纳米管。(Liu等,Science 280:1253-1256(1998),其揭示纳入本文作为参考)。碳纳米管的合成和制备方法是本领域众所周知的。(授予Olk等的美国专利5,753,088和授予Ebbsen等的美国专利5,641,466,两者的揭示均以其全文纳入本文作为参考)。还可将诸如蛋白质类的分子掺入碳纳米管的内侧。例如,在切开纳米管两端后,可用酶(例如Zn2Cd2-金属硫蛋白、细胞色素C和C3以及β-内酰胺酶)填充纳米管。(Davis等,Inorganica Chim.Acta 272:261(1998);Cook等Full Sci.Tech.5(4):695(1997),两者均纳入本文作为参考)。Fullerenes can also form nanotubes that can incorporate other atoms or molecules. (Liu et al., Science 280:1253-1256 (1998), the disclosure of which is incorporated herein by reference). Methods of synthesis and preparation of carbon nanotubes are well known in the art. (US Patent 5,753,088 to Olk et al. and US Patent 5,641,466 to Ebbsen et al., the disclosures of both are incorporated herein by reference in their entirety). Molecules such as proteins can also be incorporated into the inside of carbon nanotubes. For example, the nanotubes can be filled with enzymes such asZn2Cd2 -metallothionein, cytochromes C and C3,and beta-lactamase after cleavage of the nanotube ends. (Davis et al., Inorganica Chim. Acta 272:261 (1998); Cook et al. Full Sci. Tech. 5(4):695 (1997), both incorporated herein by reference).
还可使用三维富勒烯结构体。以全文纳入本文作为参考的授予Mirkin等的美国专利5,338,571中揭示了通过以下方法在基材表面上形成的三维多层富勒烯结构体:(i)对富勒烯进行化学修饰以产生键形成物质(bond-forming species);(ii)对基材的表面进行化学处理以提供键形成物质,所述物质足以与溶液中具有键形成物质的富勒烯共价结合;以及(iii)将经修饰的富勒烯溶液与经处理的基材表面接触,以形成共价结合于经处理的基材表面的富勒烯层。Three-dimensional fullerene structures can also be used. U.S. Patent No. 5,338,571 to Mirkin et al., which is incorporated herein by reference in its entirety, discloses three-dimensional multilayered fullerene structures formed on substrate surfaces by (i) chemically modifying the fullerenes to produce bond formation material (bond-forming species); (ii) the surface of the substrate is chemically treated to provide a bond-forming species sufficient to covalently bond with the fullerenes in solution having the bond-forming species; and (iii) The modified fullerene solution is contacted with the treated substrate surface to form a fullerene layer covalently bound to the treated substrate surface.
(D)将基质施加于医疗装置(D)Applying the matrix to the medical device
所述基质应能牢固地附着在医疗装置(包括支架或合成的移植物)的表面。在一个实施方式中,这是通过施涂连续的薄层基质而实现的。或者,可仅将抗体和生长因子施涂在与血管内腔直接接触的外表面上。可连续地施涂不同类型的基质连续层。在将基质施涂于支架上之后,可将所述抗体共价或非共价结合涂覆于基质上。The matrix should be capable of being firmly attached to the surface of a medical device, including a stent or a synthetic graft. In one embodiment, this is accomplished by applying a continuous thin layer of the substrate. Alternatively, the antibodies and growth factors can be applied only on the outer surface that is in direct contact with the lumen of the blood vessel. Successive layers of different types of substrates can be applied successively. After the matrix is applied to the scaffold, the antibody can be coated onto the matrix either covalently or non-covalently.
为了覆盖医疗装置,例如支架,可用中等粘度的基质液体溶液浸渍或喷涂所述支架。施涂了各层后,在施涂下一层之前使支架干燥。在一个实施方式中,油漆样基质涂层的总厚度不超过100微米。To cover a medical device, such as a stent, the stent can be dipped or sprayed with a medium viscosity matrix liquid solution. After each layer is applied, the scaffold is allowed to dry before the next layer is applied. In one embodiment, the total thickness of the paint-like base coating does not exceed 100 microns.
在一个实施方式中,所述医疗装置的表面为例如,首先经功能化,然后添加了基质层的支架表面。其后,将抗体以及其它涂覆组分(例如生长因子)偶联到基质表面。在这一方面,将基质施涂于诸如支架表面的技术会产生功能性的化学基团。例如,所述化学基团可为氨基,它能与聚合物的官能团反应以固定作为识别和捕获靶细胞的配体(例如抗体、肽和/或生长因子)的载体的基质中间层。In one embodiment, the surface of the medical device is, for example, a stent surface that is first functionalized and then a matrix layer is added. Thereafter, antibodies and other coating components such as growth factors are coupled to the matrix surface. In this regard, techniques for applying matrices to surfaces such as stents generate functional chemical groups. For example, the chemical group may be an amino group, which is capable of reacting with a functional group of the polymer to immobilize the matrix interlayer as a carrier for ligands (such as antibodies, peptides and/or growth factors) that recognize and capture target cells.
在另一实施方式中,可通过如下方式制备适宜的基质涂覆溶液:在无菌条件下,将480毫克(mg)药物载体,例如聚-D,L-丙交酯(作为R203购自Boehringer Inc.,Ingelheim,Germany)溶于3毫升(ml)三氯甲烷中。然而,原则上可使用血液-和组织-相容性(生物相容性)且可溶解、分散或乳化的生物可降解性(或非生物可降解性)基质,只要施用后能在医疗装置上较快地干燥为自粘附漆样或涂料样涂层。In another embodiment, a suitable matrix coating solution can be prepared by aseptically dissolving 480 milligrams (mg) of a drug carrier, such as poly-D,L-lactide (available as R203 from Boehringer Inc., Ingelheim, Germany) in 3 milliliters (ml) of chloroform. However, biodegradable (or non-biodegradable) matrices that are blood- and tissue-compatible (biocompatible) and can be dissolved, dispersed or emulsified can in principle be used as long as they can be maintained on the medical device after administration. Dries faster to a self-adhesive paint-like or paint-like coating.
例如,本领域的普通技术人员熟知可用纤维蛋白涂覆支架。在授予Muller等的美国专利4,548,736(其揭示全文纳入本文作为参考)中揭示了通过将纤维蛋白原与凝血酶接触可使纤维蛋白凝结。优选地,本发明含纤维蛋白支架中的纤维蛋白含有XIII因子,且在凝结中存在钙,(如授予Gerendas的美国专利3,523,807中所揭示,其揭示以全文纳入本文作为参考,或如公开的欧洲专利申请0366564所揭示,其揭示以全文纳入本文作为参考),以提高植入装置的机械性能和生物稳定性。在这一实施方式中,为了避免任何种间免疫反应(例如人抗牛),用于制备本发明纤维蛋白的纤维蛋白原和凝血酶得自与要植入该支架的动物或人相同种类的动物或人。可通过以下方式生产纤维蛋白精细薄膜形式的纤维蛋白产物:将混合的纤维蛋白原和凝血酶浇注成薄膜,然后通过半透膜渗滤从所述薄膜中去除水分。在欧洲专利申请0366564中(其揭示以全文纳入本文作为参考)揭示了使基材(优选具有高多孔性或者对凝血酶或纤维蛋白原具有高亲和力)与纤维蛋白原溶液和凝血酶溶液接触。结果是通过纤维蛋白原的聚合作用,在医疗装置的表面上形成纤维蛋白层。用该方法施涂的多层纤维蛋白可提供任何所需厚度的纤维蛋白层。或者,可先使纤维蛋白凝结,然后将其研磨成粉末,将该粉末与水混合并在加热的模具中压印成所需形状(美国专利3,523,807)。可通过将纤维蛋白与固定剂(例如戊二醛或甲醛)接触,产生成形的纤维蛋白而获得提高的稳定性。可将本领域技术人员已知的用于制备和形成纤维蛋白的这些方法和其它方法用于本发明中。For example, fibrin-coated scaffolds are well known to those of ordinary skill in the art. Clotting of fibrin by contacting fibrinogen with thrombin is disclosed in US Patent 4,548,736 to Muller et al., the disclosure of which is incorporated herein by reference in its entirety. Preferably, the fibrin in the fibrin-containing scaffolds of the present invention contains Factor XIII, and calcium is present in coagulation, (as disclosed in U.S. Patent 3,523,807 to Gerendas, the disclosure of which is incorporated herein by reference in its entirety, or as disclosed in European Patent application 0366564, the disclosure of which is incorporated herein by reference in its entirety) to improve the mechanical properties and biological stability of implanted devices. In this embodiment, the fibrinogen and thrombin used to prepare the fibrin of the invention are obtained from the same species of animal or human as the scaffold will be implanted in order to avoid any interspecies immune reaction (e.g. human anti-bovine). animals or people. Fibrin products in the form of fibrin fine films can be produced by casting mixed fibrinogen and thrombin into a film and then removing water from the film by diafiltration through a semi-permeable membrane. Contacting a substrate (preferably of high porosity or high affinity for thrombin or fibrinogen) with a solution of fibrinogen and thrombin is disclosed in European Patent Application 0366564, the disclosure of which is incorporated herein by reference in its entirety. The result is the formation of a fibrin layer on the surface of the medical device by polymerization of the fibrinogen. Multiple layers of fibrin applied by this method can provide fibrin layers of any desired thickness. Alternatively, the fibrin can be coagulated first, then ground into a powder that is mixed with water and stamped into the desired shape in a heated mold (US Patent 3,523,807). Increased stability can be obtained by contacting the fibrin with a fixative such as glutaraldehyde or formaldehyde, resulting in shaped fibrin. These and other methods known to those skilled in the art for preparing and forming fibrin can be used in the present invention.
如果用胶原来涂覆所述合成的移植物,制备胶原并使在合成的移植物装置上形成涂层的方法是熟知的,如授予Weadock等的美国专利5,851,230中所揭示,其揭示以全文纳入本文作为参考。该专利描述了用于以胶原涂覆合成的移植物的方法。用于将胶原粘附于多孔性移植物基材的方法通常包括将胶原分散液施涂于基材,使其干燥并重复该过程。胶原分散体的制备通常是通过将不溶性胶原(约1-2重量%)混合成酸性pH(pH为2-4)的分散液。通常将所述分散液以注射器注射入移植物内腔中,并用手揉压使胶原浆液覆盖整个内部表面区域。从所述移植物的两个开口端之一去除过量的胶原浆液。重复涂覆和干燥步骤数次以提供足够的处理。If collagen is used to coat the synthetic graft, methods of preparing collagen and coating synthetic graft devices are well known, as disclosed in U.S. Patent 5,851,230 to Weadock et al., the disclosure of which is incorporated in its entirety. This article is for reference. This patent describes a method for coating synthetic grafts with collagen. Methods for adhering collagen to porous graft substrates generally involve applying a collagen dispersion to the substrate, allowing it to dry, and repeating the process. Collagen dispersions are usually prepared by mixing insoluble collagen (approximately 1-2% by weight) into a dispersion at an acidic pH (pH 2-4). The dispersion is typically syringed into the lumen of the graft and massaged by hand to coat the collagen slurry over the entire interior surface area. Excess collagen slurry was removed from one of the two open ends of the graft. The coating and drying steps were repeated several times to provide adequate treatment.
在另一实施方式中,用无定形碳涂覆支架或合成的移植物。在美国专利5,198,263中(其揭示以全文纳入本文作为参考)描述了一种用于在经氟化气体或含其它卤素的气体的存在下,生产高比率低温沉积无定形碳薄膜的方法。根据该发明的方法进行的沉积可在低于100℃的温度下(包括环境室温),用射频、等离子辅助、化学汽化沉积方法进行。施用本发明的方法产生的无定形碳薄膜可良好地粘附于许多种类的基材,所述基材包括例如:玻璃、金属、半导体和塑料。In another embodiment, the stent or synthetic graft is coated with amorphous carbon. In US Patent No. 5,198,263, the disclosure of which is incorporated herein by reference in its entirety, a method for producing high rate low temperature deposition of amorphous carbon films in the presence of fluorinated gases or other halogen-containing gases is described. Deposition according to the method of the invention can be performed at temperatures below 100° C., including ambient room temperature, by radio frequency, plasma assisted, chemical vapor deposition methods. The amorphous carbon films produced by applying the methods of the present invention adhere well to a wide variety of substrates including, for example: glass, metals, semiconductors, and plastics.
将富勒烯分子与含氨基聚合物的反应性氨基基团位点结合以以形成富勒烯-支架,含氨基的聚合物可按美国专利5,292,813(其揭示以全文纳入本文作为参考)完成。用此方式进行的化学修饰可使得富勒烯直接掺入支架中。在另一实施方式中,可如上所述地将富勒烯沉积在支架或合成的移植物的表面上。(参见,授予Leone等的WO 99/32184,其揭示以全文纳入本文作为参考)。富勒烯(例如C60)也可通过环氧键附着到不锈钢表面上(Yamago等,《有机性富勒烯通过氧化、还原以及C-O与C-C键合形成反应的化学衍生》,Chemical Derivatization of Organofullerenes through Oxidation,Reduction and C-O and C-C Bond FormingReactions,J.Org.Chem.,584796-4798(1998),其揭示以全文纳入本文作为参考)。该附着是通过与氧的共价键完成的。所述化合物和偶联方案可从BuckyUSA.(BuckyUSA,Houston,Texas)购得。Binding of fullerene molecules to reactive amino group sites of amino-containing polymers to form a fullerene-scaffold can be accomplished according to US Pat. No. 5,292,813, the disclosure of which is incorporated herein by reference in its entirety. Chemical modification in this way allows direct incorporation of fullerenes into scaffolds. In another embodiment, fullerenes can be deposited on the surface of a stent or synthetic graft as described above. (See, WO 99/32184 to Leone et al., the disclosure of which is incorporated herein by reference in its entirety). Fullerenes (e.g. C60 ) can also attach to stainless steel surfaces via epoxy bonds (Yamago et al., "Chemical Derivatization of Organic Fullerenes by Oxidation, Reduction, and Bond Formation Reactions of CO and CC", Chemical Derivatization of Organofullerenes through Oxidation, Reduction and CO and CC Bond Forming Reactions, J. Org. Chem., 584796-4798 (1998), the disclosure of which is incorporated herein by reference in its entirety). This attachment is done through a covalent bond with oxygen. The compounds and coupling protocols are commercially available from Bucky USA. (Bucky USA, Houston, Texas).
(E)将配体(例如抗体、肽和/或生长因子)加入基质——将能促进内皮祖细胞粘附的抗体以及能促进细胞生长和分化的生长因子共价或非共价掺入到基质中。可通过如下方式将配体涂层(例如抗体、抗体片段、激素、肽、生长因子和/或诸如此类)掺入基质层:将配体与基质涂层溶液混合,然后将所述溶液施涂在装置的表面上。在某些实施方式中,将抗体、片段或它们的组合和/或生长因子附着在施涂于装置内腔表面的基质的最外层表面,从而使得配体(例如抗体)突出在该表面而能与循环血液接触,并保持它们对靶细胞的亲和力。在这些实施方式中,可采用标准技术将所述配体(例如抗体)施涂在基质表面上。(E)Incorporation of ligands (such as antibodies, peptides, and/or growth factors) into the matrix —antibodies that promote adhesion of EPCs and growth factors that promote cell growth and differentiation are incorporated covalently or non-covalently into the matrix. in the matrix. Ligand coatings (e.g., antibodies, antibody fragments, hormones, peptides, growth factors, and/or the like) can be incorporated into the matrix layer by mixing the ligand with a matrix coating solution and then applying the solution on on the surface of the device. In certain embodiments, the antibody, fragment, or combination thereof, and/or growth factor is attached to the outermost surface of a matrix applied to the lumen surface of the device such that the ligand (e.g., antibody) protrudes from the surface and Can come into contact with circulating blood and maintain their affinity for target cells. In these embodiments, the ligand (eg, antibody) can be applied to the substrate surface using standard techniques.
在一个实施方式中,将所述抗体加入含基质的溶液中。例如,以500-800mg/dl的浓度,将抗CD34单克隆抗体的Fab片段与含有人纤维蛋白原的溶液共同孵育。应理解抗CD34Fab片段的浓度可以不同,本领域的普通技术人员无需经过多的试验就能确定最佳浓度。将支架加入Fab/纤维蛋白混合物中,再加入浓缩的凝血酶(浓度至少为1000U/ml)来活化纤维蛋白。将所得含有Fab片段的聚合纤维蛋白混合液直接加入基质,在支架或合成的移植物的表面上压成薄膜(薄于100pm)。事实上,在涂覆支架或合成的移植物前,用这种方式可将任何类型的抗体或抗体片段掺入基质溶液中。In one embodiment, the antibody is added to the matrix-containing solution. For example, the Fab fragment of anti-CD34 monoclonal antibody is incubated with a solution containing human fibrinogen at a concentration of 500-800 mg/dl. It is understood that the concentration of the anti-CD34 Fab fragment can vary, and those of ordinary skill in the art can determine the optimal concentration without undue experimentation. The scaffold was added to the Fab/fibrin mixture, followed by concentrated thrombin (concentration of at least 1000 U/ml) to activate fibrin. The resulting polymerized fibrin mixture containing Fab fragments is directly added to the matrix and pressed into a thin film (thinner than 100 μm) on the surface of the scaffold or synthetic graft. In this way, virtually any type of antibody or antibody fragment can be incorporated into the matrix solution prior to coating the stent or synthetic graft.
例如,在另一实施方式中,带有或不带有抗体片段的全抗体以及生长因子是共价偶联于基质的。在一个实施方式中,通过异质或同质双功能接头分子将所述抗体和一种或多种生长因子共价连接于基质。如本文所用,术语“连接(tethered)”是指通过接头分子将抗体共价偶联于基质。采用与本发明相关的接头分子的施用通常涉及在将基质附着于支架之前,将接头分子共价偶联于所述基质。在共价偶联于基质后,接头分子为基质提供了许多可用于共价偶联一种或多种抗体的官能活性基团。在这一实施方式的一个实施例中,图1A提供了通过交联分子偶联的示例。内皮细胞1.01通过表面抗原1.02结合于抗体1.03。所述抗体通过交联分子1.04连接于基质1.05-1.06。基质1.05-1.06附着于支架1.07。所述接头分子可直接连接在基质上(即,通过羧基),或通过熟知的偶联化学反应,例如酯化作用、酰胺化作用和酰化作用。所述接头分子可为二或三氨基官能化合物,它们通过直接形成酰胺键而与基质连接,并提供能与抗体反应的氨基官能团。例如,所述接头分子可为多胺官能聚合物,如聚乙烯亚胺(PEI)、聚烯丙基酰胺(PALLA)或聚乙二醇(PEG)。已可由Shearwater Corporation(Birmingham,Alabama)购买到多种聚乙二醇衍生物(例如,mPEG-琥珀酰二酰亚胺基丙酸酯或mPEG-N-羟基琥珀酰亚胺)以及共价偶联的方案。(还可参见,Weiner等,聚乙二醇间隔物对于通过固定化抗体捕获抗原的影响,Influence of a poly-ethyleneglycol spacer on antigen capture byimmobilized antibody,J.Biochem.Biophys.Methods 45:211-219(2000),纳入本文作为参考)。应理解的是具体偶联剂的选择可取决于所用抗体的类型,且此类选择无需经过多试即可作出。还可使用这些聚合物的混合物。这些分子含有多种悬垂性氨基官能团,这些官能团可用于表面固定一种或多种抗体、肽、蛋白质、激素和其它涂层成分。For example, in another embodiment, whole antibodies with or without antibody fragments and growth factors are covalently coupled to the matrix. In one embodiment, the antibody and one or more growth factors are covalently linked to the matrix via a hetero- or homo-bifunctional linker molecule. As used herein, the term "tethered" refers to the covalent coupling of the antibody to the matrix through a linker molecule. Administration using linker molecules in connection with the present invention generally involves covalently coupling the linker molecule to the matrix prior to attaching the matrix to the scaffold. After being covalently coupled to the matrix, the linker molecules provide the matrix with a number of functionally reactive groups that can be used to covalently couple one or more antibodies. In one example of this embodiment, Figure 1A provides an example of coupling by cross-linking molecules. Endothelial cells 1.01 bind to antibody 1.03 via surface antigen 1.02. The antibodies are linked to the matrix 1.05-1.06 via cross-linking molecules 1.04. The matrix 1.05-1.06 is attached to the support 1.07. The linker molecule can be attached directly to the substrate (ie, via a carboxyl group), or via well-known coupling chemistries such as esterification, amidation, and acylation. The linker molecule can be a di- or tri-amino functional compound, which is linked to the substrate by direct formation of an amide bond and provides an amino functional group capable of reacting with the antibody. For example, the linker molecule can be a polyamine functional polymer such as polyethyleneimine (PEI), polyallylamide (PALLA) or polyethylene glycol (PEG). A variety of polyethylene glycol derivatives (e.g., mPEG-succinimidyl propionate or mPEG-N-hydroxysuccinimide) and covalently coupled scheme. (See also, Weiner et al., Influence of a poly-ethyleneglycol spacer on antigen capture by immobilized antibody, Influence of a poly-ethyleneglycol spacer on antigen capture by immobilized antibody, J.Biochem.Biophys.Methods 45:211-219( 2000), incorporated herein by reference). It is understood that the choice of a particular coupling reagent may depend on the type of antibody used, and that such choice can be made without undue trial. Mixtures of these polymers may also be used. These molecules contain a variety of pendant amino functionalities that can be used to surface immobilize one or more antibodies, peptides, proteins, hormones and other coating components.
在一个实施方式中,可将抗体附着于已直接沉积在支架表面上的C60富勒烯层。交联剂可共价附着于富勒烯。然后使抗体附着于交联剂,进而与支架附着。图1B提供了通过富勒烯C60偶联的示例。内皮细胞2.01通过细胞抗原2.02结合于抗体2.03,进而共价或非共价结合于基质2.04。基质2.04通过C60 2.05共价偶联于支架2.06。In one embodiment, the antibody can be attached to aC60 fullerene layer that has been deposited directly on the surface of the scaffold. Crosslinkers can be covalently attached to fullerenes. The antibody is then attached to the cross-linker, which in turn attaches to the scaffold. Figure 1B provides an example of coupling via fullereneC60 . The endothelial cell 2.01 is bound to the antibody 2.03 through the cell antigen 2.02, and then covalently or non-covalently bound to the matrix 2.04. The matrix 2.04 is covalently coupled to the scaffold 2.06 viaC60 2.05.
本发明的小分子可包括用于代替抗体、抗体片段、生长因子等的合成或天然存在的分子或肽。例如,凝集素是一种天然存在的非免疫源性糖结合肽。内皮细胞特异性的凝集素抗原(Ulex Europaeus Uea 1)(Schatz等2000,人子宫内膜内皮细胞:组织因子和1型血血纤蛋白溶酶原活化物抑制剂的分离、特征鉴定以及炎症介导的表达,Human Endometrial Endothelial cells:Isolation,Characterization,and Inflammatory-Mediated Expression of Tissue Factor andType 1 Plasminogen Activator Inhibitor.,Biol Reprod 62:691-697),例如可选择性结合于内皮祖细胞的细胞表面。Small molecules of the invention may include synthetic or naturally occurring molecules or peptides used in place of antibodies, antibody fragments, growth factors, and the like. For example, lectins are naturally occurring non-immunogenic carbohydrate-binding peptides. Endothelial cell-specific lectin antigen (Ulex Europaeus Uea 1) (Schatz et al. 2000, Human endometrial endothelial cells: isolation, characterization, and mediators of inflammation of tissue factor and plasminogen activator type 1 inhibitors. Human Endometrial Endothelial cells: Isolation, Characterization, and Inflammatory-Mediated Expression of Tissue Factor and Type 1 Plasminogen Activator Inhibitor., Biol Reprod 62:691-697), for example, can selectively bind to the cell surface of endothelial progenitor cells.
已创造出能靶向不同细胞表面、蛋白质、糖蛋白、多糖和受体的合成“小分子”。这些分子能选择性结合特定的表面分子,并能靶向特定的细胞类型,例如内皮祖细胞。合成的小分子可识别内皮细胞表面标记,例如VEGF。SU11248(SugenInc.)(Mendel等,2003,《以血管内皮细胞生长因子受体和血小板衍生生长因子受体为靶标的一种新型酪氨酸激酶抑制剂SU11248在体内的抗肿瘤活性:药物动力学/药效学相互关系的测定》,In vivo antitumor activity of SU11248,a novel tyrosine kinase inhibitor targeting vascular endothelial growthfactor and platelet-derived growth factor receptors:determination of apharmacokinetic/pharmacodynamic relationship,Clin Cancer Res.Jan;9(1):327-37)、PTK787/ZK222584(Drevs J.等,2003,《受体酪氨酸激酶:抗癌症治疗的一些新的主要靶标》,Receptor tyrosine kinases:the maintargets for new anticancer therapy,Curr Drug Targets.Feb;4(2):113-21)和SU6668(Laird,AD等,2002,《在体内SU6668对Flk-1/KDR和PDGFR-β的抑制作用在导致小鼠体内的肿瘤血管系统迅速凋亡和肿瘤衰退》,SU6668 inhibitsFlk-1/KDR and PDGFRbeta in vivo,resulting in rapid apoptosis of tumorvasculature and tumor regression in mice.FASEB J.May;16(7):681-90)是结合VEGFR-2的小分子。Synthetic "small molecules" that target different cell surfaces, proteins, glycoproteins, polysaccharides and receptors have been created. These molecules selectively bind specific surface molecules and can target specific cell types, such as endothelial progenitor cells. Synthetic small molecules that recognize endothelial cell surface markers such as VEGF. SU11248 (Sugen Inc.) (Mendel et al., 2003, Antitumor Activity of SU11248, a Novel Tyrosine Kinase Inhibitor Targeting Vascular Endothelial Cell Growth Factor Receptor and Platelet-Derived Growth Factor Receptor, in Vivo: Pharmacokinetics /Determination of Pharmacodynamic Interaction", In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of apharmacokinetic/pharmacodynamic relationship; ): 327-37), PTK787/ZK222584 (Drevs J. et al., 2003, "Receptor tyrosine kinases: some new main targets for anticancer therapy", Receptor tyrosine kinases: the main targets for new anticancer therapy, Curr Drug Targets.Feb;4(2):113-21) and SU6668 (Laird, AD et al., 2002, Inhibition of Flk-1/KDR and PDGFR-β by SU6668 in vivo leads to rapid tumor vasculature in mice Apoptosis and Tumor Regression", SU6668 inhibits Flk-1/KDR and PDGFRbeta in vivo, resulting in rapid apoptosis of tumor and tumor regression in mice. FASEB J.May; 16(7):681-90) is binding to VEGFR-2 Small molecule.
靶向内皮细胞表面的另一亚类合成小分子是α(v)β(3)整合素抑制剂。SM256和SD983(Kerr JS.等,1999,《新型小分子物质αv整合素拮抗物:与已知的各种促血管生成素类抑制物的抗癌症功效相比较》,Novel small molecule alpha vintergrin antagonists:comparative anti-cancer efficacy with knownangiogenesis inhibitors.Anticancer Res Mar-Apr;19(2A):959-68)均为能靶向和结合于内皮细胞表面上的α(v)β(3)的合成小分子。Another subclass of synthetic small molecules that target the surface of endothelial cells are α(v)β(3) integrin inhibitors. SM256 and SD983 (Kerr JS. et al., 1999, "Novel Small Molecule αv Integrin Antagonists: Comparison of Anticancer Efficacy with Various Known Angiopoietin Inhibitors", Novel small molecule alpha vintergrin antagonists: Comparative anti-cancer efficacy with known angiogenesis inhibitors. Anticancer Res Mar-Apr; 19(2A):959-68) are synthetic small molecules that can target and bind to α(v)β(3) on the surface of endothelial cells.
本发明提供了一种药物递送系统,其包括:涂覆的医疗装置,例如支架、支架移植物、心脏瓣膜、导管、血管修复滤筛、人造心脏、外置和内置左心室辅助装置(LVADs)以及合成的血管移植物,它们可用于治疗疾病,这些疾病包括:肿瘤和血管疾病,例如:再狭窄、动脉粥样硬化、血栓形成、血管梗阻等。在一个实施方式中,本发明医疗装置上的涂层包含:生物相容性的基质;至少一种抗体、抗体片段或它们的组合;和/或至少一种化合物,如配体或治疗剂(如雌二醇、血管生成因子、FGF等)。The present invention provides a drug delivery system comprising: coated medical devices such as stents, stent grafts, heart valves, catheters, vascular repair screens, artificial hearts, external and internal left ventricular assist devices (LVADs) And synthetic vascular grafts, which can be used to treat diseases, including: tumors and vascular diseases, such as: restenosis, atherosclerosis, thrombosis, vascular obstruction, etc. In one embodiment, the coating on the medical device of the invention comprises: a biocompatible matrix; at least one antibody, antibody fragment, or combination thereof; and/or at least one compound, such as a ligand or therapeutic agent ( Such as estradiol, angiogenic factors, FGF, etc.).
在一个实施方式中,转基因细胞掺入有至少一种转移基因,所述转移基因可通过以病毒或非病毒为基础的遗传方法导入所述细胞。所述转移基因可编码至少一种治疗药物,可连续表达或在刺激的诱导下表达。在一个实施方式中,所述治疗药物可为激素、肽、蛋白质等。在所述转基因细胞的表面上也存在至少一种抗原,所述抗原能被涂覆于医疗装置表面的抗体所识别并结合。In one embodiment, a transgenic cell incorporates at least one transgene that can be introduced into the cell by viral or non-viral based genetic methods. The transgene can encode at least one therapeutic drug and can be expressed continuously or induced by stimulation. In one embodiment, the therapeutic drug may be a hormone, a peptide, a protein, or the like. Also present on the surface of the transgenic cells is at least one antigen that is recognized and bound by antibodies coated on the surface of the medical device.
如本文所用,“抗体”是指抗体或抗体片段,或抗体和片段的组合,它们可为单克隆抗体、多克隆抗体、嵌合抗体或人源化抗体。本发明的抗体片段包括任何大小的抗体片段(例如大分子和小分子),这些片段保留与该抗体相同的识别和结合靶抗原的特性(图1A、1B和11)。As used herein, "antibody" refers to an antibody or an antibody fragment, or a combination of antibodies and fragments, which may be monoclonal, polyclonal, chimeric, or humanized. Antibody fragments of the invention include antibody fragments of any size (eg, macromolecules and small molecules) that retain the same recognition and binding properties of the antibody as the target antigen (FIGS. 1A, 1B, and 11).
如本文所用,“配体”是指能结合另一种分子(例如哺乳动物细胞上的受体)的分子。例如,配体可为抗体、抗体片段(图1A、1B、11和17)、细胞粘着分子或基底膜组分,它们能识别并结合靶细胞膜上特异性表位或结构。在采用遗传改变的哺乳动物细胞的实施方式中,用于医疗装置涂层上的配体经特别选择而可识别和结合由导入转基因细胞内的外源DNA所产生的基因产物。As used herein, "ligand" refers to a molecule capable of binding another molecule, such as a receptor on a mammalian cell. For example, the ligand can be an antibody, antibody fragment (FIGS. 1A, 1B, 11 and 17), cell adhesion molecule, or basement membrane component that recognizes and binds to a specific epitope or structure on the target cell membrane. In embodiments employing genetically altered mammalian cells, the ligands used in the coating of the medical device are specifically selected to recognize and bind gene products produced by exogenous DNA introduced into the transgenic cells.
如本文所用,“蛋白质”是指任何长度的氨基酸聚合物。所述聚合物可为直链或支链,可包含经修饰的氨基酸,也可间插有非氨基酸。所述聚合物可为天然存在的肽、蛋白质,或其修饰和合成形式,包括其:生物活性片段、衍生物、同类物、模拟物和无功能或显性失活的突变体。As used herein, "protein" refers to a polymer of amino acids of any length. The polymers may be linear or branched, may contain modified amino acids, and may be interspersed with non-amino acids. The polymers may be naturally occurring peptides, proteins, or modified and synthetic forms thereof, including biologically active fragments, derivatives, congeners, mimetics and non-functional or dominant negative mutants thereof.
所述医疗装置可为用于植入带腔的器官或机体部位的任何装置,其可包括但不限于:支架、支架移植物、合成的血管移植物、心脏瓣膜、导管、血管修复滤筛、起搏器、起搏器前导物、除颤器、卵园孔未闭(PFO)中隔闭合装置、血管夹、血管动脉瘤闭锁器、血液透析移植物、血液透析导管、房室分流器、主动脉血管瘤移植物装置或组件、静脉瓣、缝线、血管吻合夹、留置式静脉或动脉导管、血管鞘和药物输送口。根据装置的不同,所述装置可用各种材料制成。例如,本发明的支架可用不锈钢、镍钛合金(NiTi)或铬合金制成。合成的血管移植物可用交联PVA水凝胶、聚四氟乙烯(PTFE)、膨胀性聚四氟乙烯(ePTFE)、多孔性高密度聚乙烯(HDPE)、聚氨酯以及聚对苯二甲酸乙烯酯制成。The medical device may be any device intended for implantation into a lumen organ or body part, which may include, but is not limited to: stents, stent grafts, synthetic vascular grafts, heart valves, catheters, vascular repair screens, Pacemaker, pacemaker lead, defibrillator, patent foramen ovale (PFO) septal closure device, vascular clip, vascular aneurysm sealer, hemodialysis graft, hemodialysis catheter, atrioventricular shunt, Aortic hemangioma graft devices or components, venous valves, sutures, vascular anastomotic clips, indwelling venous or arterial catheters, vascular sheaths, and drug delivery ports. Depending on the device, the device can be made of various materials. For example, stents of the present invention can be made of stainless steel, nickel titanium alloy (NiTi), or chromium alloys. Synthetic vascular grafts are available in cross-linked PVA hydrogels, polytetrafluoroethylene (PTFE), expanded polytetrafluoroethylene (ePTFE), porous high-density polyethylene (HDPE), polyurethane, and polyethylene terephthalate production.
形成本发明装置涂层的生物相容性基质包括:合成材料,例如聚氨酯、嵌段聚氨酯-脲/肝素、聚-L-乳酸、纤维素酯、聚乙二醇、聚醋酸乙酯、葡聚糖和明胶;天然存在的材料,例如基底膜成分,如胶原、弹性蛋白、弹性蛋白原、层连蛋白、纤连蛋白、玻连蛋白、肝素、纤维蛋白、纤维素;以及无定形碳或富勒烯等。Biocompatible matrices that form the coating of the device of the present invention include: synthetic materials such as polyurethane, segmented polyurethane-urea/heparin, poly-L-lactic acid, cellulose esters, polyethylene glycol, polyethyl acetate, dextrose Sugars and gelatin; naturally occurring materials such as basement membrane components such as collagen, elastin, tropoelastin, laminin, fibronectin, vitronectin, heparin, fibrin, cellulose; and amorphous carbon or rich Leene and others.
在一个实施方式中,所述医疗装置包括含有富勒烯的生物相容性基质。在这一实施方式中,所述富勒烯的碳原子数可为约C20-C150,更具体地说,所述富勒烯为C60-C70。本发明的富勒烯还可在医疗装置表面上排列为纳米管。In one embodiment, the medical device comprises a biocompatible matrix comprising fullerenes. In this embodiment, the fullerene may have a carbon number of about C20 -C150 , more specifically, the fullerene is C60 -C70 . The fullerenes of the present invention can also be arranged as nanotubes on the surface of medical devices.
提供给所述医疗装置涂层的抗体包括至少一种能识别并结合转基因细胞表面抗原的抗体,该抗原可由外源基因或转移基因表达并能调节所述细胞对医疗装置表面的粘附。所述抗体可共价或非共价附着于基质表面,或通过接头分子共价连接于覆盖医疗装置的基质的最外层。在本发明的这一方面,例如,所述单克隆抗体还可包括Fab或F(ab’)2片段。Antibodies provided to the coating of the medical device include at least one antibody that recognizes and binds to a surface antigen of a transgenic cell that is expressed by an exogenous gene or a transgene and that regulates the adhesion of the cell to the surface of the medical device. The antibody may be covalently or non-covalently attached to the surface of the substrate, or covalently linked via a linker molecule to the outermost layer of the substrate covering the medical device. In this aspect of the invention, for example, the monoclonal antibody may also comprise a Fab or F(ab')2 fragment.
所述抗体可特异性地识别并结合对接受治疗的哺乳动物的抗原,且它们的特异性并不取决于细胞系。在一个实施方式中,所述抗体对人内皮祖细胞表面抗原具有特异性,所述表面抗原为例如:CD133、CD14、CD34、CDw90、CD117、HLA-DR、VEGFR-1、VEGFR-2、Muc-18(CD146)、CD130、干细胞抗原(Sca-1)、干细胞因子1(SCF/c-Kit配体)、Tie-2、HAD-DR以及其它抗原(例如抗-H-2Kk抗体)。The antibodies specifically recognize and bind to the antigen in the treated mammal and their specificity is not dependent on the cell line. In one embodiment, the antibody is specific to human endothelial progenitor cell surface antigens, such as: CD133, CD14, CD34, CDw90, CD117, HLA-DR, VEGFR-1, VEGFR-2, Muc -18 (CD146), CD130, stem cell antigen (Sca-1), stem cell factor 1 (SCF/c-Kit ligand), Tie-2, HAD-DR and other antigens (eg anti-H-2Kkappa antibody).
在另一实施方式中,所述医疗装置的涂层包含至少一层如上所述的生物相容性基质,该基质包括用于附着治疗有效量的至少一种天然或合成来源的小分子的外表面。所述小分子能识别转基因细胞表面抗原并与其相互作用,从而将转基因细胞固定在装置的表面上,并诱导转移基因的表达。所述小分子可来自各种来源,例如来自细胞组分,如脂肪酸、蛋白质、核酸、糖类等,并可与转基因细胞表面受体相互作用。在本发明的这一实施方式中,医疗装置上的涂层还可包括化合物,例如与含有抗体的涂层结合的配体。In another embodiment, the coating of the medical device comprises at least one layer of a biocompatible matrix as described above comprising an outer coating for the attachment of a therapeutically effective amount of at least one small molecule of natural or synthetic origin. surface. The small molecule is capable of recognizing and interacting with transgenic cell surface antigens, thereby immobilizing the transgenic cells on the surface of the device and inducing the expression of the transgene. The small molecules can be derived from various sources, eg, from cellular components such as fatty acids, proteins, nucleic acids, carbohydrates, etc., and can interact with transgenic cell surface receptors. In this embodiment of the invention, the coating on the medical device may also include a compound, such as a ligand, that binds to the antibody-containing coating.
以病毒和非病毒为基础的遗传方法均可用于导入转移基因以产生转基因细胞。本发明的转基因细胞能表达并分泌由短暂或稳定掺入的转移基因编码的治疗药物。也可结合其它转移基因以赋予其存活、选择性和/或生长优势。可对各种细胞进行修饰以产生非再生或再增殖的转基因细胞,这些细胞为例如:内皮细胞或粒细胞,包括嗜中性粒细胞、嗜曙红粒细胞、嗜碱性粒细胞、单核细胞和淋巴细胞或体细胞,或这些细胞的组合。可在体外培养转基因细胞、对其进行收集和保存。可通过掺入不同的转移基因来生成能产生各种治疗药物的转基因细胞,以用于不同的治疗目的。可通过全身或局部途径,以单一或混合细胞群给予转基因细胞。针对个体病状,可给予不同量的转基因细胞以分泌不同量的治疗药物。在一个实施方式中,所述转基因细胞可再生内皮祖细胞。在另一实施方式中,可使用基于导管的递送方式或用双气囊膨胀法(dual balloon inflation method)局部给予给予转基因内皮祖细胞。Both viral and non-viral based genetic methods can be used to introduce transgenes to generate transgenic cells. The transgenic cells of the invention are capable of expressing and secreting therapeutic agents encoded by transiently or stably incorporated transgenes. Other transgenes may also be incorporated to confer survival, selectivity and/or growth advantages. Various cells can be modified to generate non-regenerative or repopulating transgenic cells such as: endothelial cells or granulocytes including neutrophils, eosinophils, basophils, monocytes cells and lymphocytes or somatic cells, or a combination of these cells. Transgenic cells can be grown, collected and stored in vitro. Transgenic cells capable of producing various therapeutic drugs can be generated by incorporating different transgenes for different therapeutic purposes. Transgenic cells can be administered systemically or locally, in single or mixed cell populations. Different amounts of transgenic cells can be administered to secrete different amounts of therapeutic agents for individual conditions. In one embodiment, the transgenic cells regenerate endothelial progenitor cells. In another embodiment, transgenic endothelial progenitor cells may be administered locally using catheter-based delivery or by dual balloon inflation method.
在一个实施方式中,转基因细胞还包含表达外源性细胞表面抗原的其它转移基因,这些抗原可被涂覆于医疗装置基质中的所述抗体特异性地识别和结合。转移基因的表达和产物的分泌可为连续发生或在外源刺激激活可诱导性启动子而暂时发生。In one embodiment, the transgenic cells further comprise additional transgenes expressing exogenous cell surface antigens that can be specifically recognized and bound by the antibodies coated in the matrix of the medical device. Expression of the transgene and secretion of the product can occur continuously or transiently upon activation of an inducible promoter by an exogenous stimulus.
由本发明的转移基因编码的治疗性化合物可为具有所需生理作用的任何分子,其可为但不限于:所定义的蛋白质类,包括生长因子、趋化因子和细胞因子、配体和受体、以及其它功能性蛋白和非蛋白质性可分泌化合物。在一个实施方式中,治疗性化合物是选自下组的蛋白质:内皮一氧化氮合酶(eNOS)、血管内皮生长因子(VEGF)、抗炎症因子和炎症调节因子。Therapeutic compounds encoded by the transgenes of the invention can be any molecule with the desired physiological effect which can be, but not limited to: defined classes of proteins including growth factors, chemokines and cytokines, ligands and receptors , and other functional proteinaceous and nonproteinaceous secretable compounds. In one embodiment, the therapeutic compound is a protein selected from the group consisting of endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF), anti-inflammatory factors, and inflammation regulators.
药物,例如在采用遗传改变的哺乳动物细胞的实施方式中,是能刺激转移基因表达以及靶产物分泌的化合物,可为所述医疗装置涂层中的配体或其它成分,它们能结合转基因细胞表面抗原并激活染色体外核酸(例如导入靶细胞内的DNA构建物)的下游信号传递途径。在另一实施方式中,遗传改变的哺乳动物细胞的转移基因表达可被例如配体或药物激活,所述配体或药物可被所述转基因细胞摄入并通过可诱导性启动子刺激基因表达。在一个实施方式中,所述配体或药物是全身性给药的。在另一实施方式中,所述配体或药物是涂覆在植入装置的基质中并局部给药的。Drugs, such as in embodiments employing genetically altered mammalian cells, compounds that stimulate expression of the transgene and secretion of the target product, may be ligands or other components of the coating of the medical device that bind to the transgenic cells Surface antigens and activate downstream signaling pathways of extrachromosomal nucleic acids such as DNA constructs introduced into target cells. In another embodiment, transgene expression in genetically altered mammalian cells can be activated by, for example, ligands or drugs that can be taken up by the transgenic cells and stimulate gene expression through an inducible promoter . In one embodiment, the ligand or drug is administered systemically. In another embodiment, the ligand or drug is coated in the matrix of the implant device and administered topically.
本发明提供了用于治疗多种疾病的方法,所述疾病可为但不限于:肿瘤、血管疾病和愈合反应。所述方法相对与现有技术的进步在于根据需要将所需量的对各种药物进行靶位点递送。The present invention provides methods for treating a variety of diseases such as, but not limited to, tumors, vascular diseases, and healing responses. The progress of the method compared with the prior art lies in delivering the required amount of various drugs to the target site according to the needs.
本发明提供了一种治疗肿瘤及其转移的方法。在这一实施方式中,所述转移基因可编码(1)抗血管生成因子,例如干扰素(IFN)、血小板反应素(TSP)、血管抑素、内皮抑素、制瘤素M(OSM)和Rho,它们能抑制作为肿瘤进行性生长前提条件的新生血管生成;或(2)肿瘤抑制蛋白,例如p53、Rb、E1、BRCA1、细胞生长活化剂(例如生长因子)的抗体或显性失活突变体、或细胞周期蛋白依赖的激酶(CDK)或细胞周期蛋白、E2F、NFKB;或这些基因的组合。在一个实施方式中,所述转移基因可包括编码以下物质的基因:例如,前列环素和/或环氧合酶、α-CGRP、基质金属蛋白、和/或内皮一氧化氮合酶。The invention provides a method for treating tumor and its metastasis. In this embodiment, the transgene may encode (1) anti-angiogenic factors such as interferon (IFN), thrombospondin (TSP), angiostatin, endostatin, oncostatin M (OSM) and Rho, which inhibit neovascularization that is a prerequisite for progressive tumor growth; or (2) tumor suppressor proteins, such as antibodies to p53, Rb, E1, BRCA1, cell growth activators (e.g., growth factors), or dominant negative Live mutants, or cyclin-dependent kinases (CDKs) or cyclins, E2F, NFKB; or combinations of these genes. In one embodiment, the transgene may include a gene encoding, for example, prostacyclin and/or cyclooxygenase, alpha-CGRP, matrix metalloprotein, and/or endothelial nitric oxide synthase.
如本文所用,短语“抗血管生成因子”是指能抑制血管生成或血管生长的分子。As used herein, the phrase "anti-angiogenic factor" refers to a molecule that inhibits angiogenesis or blood vessel growth.
本发明还提供了用于治疗血管疾病的方法。在一个实施方式中,提供了一种治疗局部缺血疾病的方法,在该方法中转移基因用于编码血管生成因子,例如多效因子(pleiotrophin)、血管生成因子、血管生成素、整合素刺激因子和/或抗血管生成因子的抗体或显性失活突变体。The invention also provides methods for treating vascular disease. In one embodiment, a method of treating an ischemic disease is provided in which a gene is transferred for encoding an angiogenic factor, such as pleiotrophin, angiogenic factor, angiogenin, integrin stimulating Factors and/or Anti-Angiogenic Factor Antibodies or Dominant Negative Mutants.
如本文所用,短语“血管生成因子”是指能刺激血管生成或血管生长的分子。As used herein, the phrase "angiogenic factor" refers to a molecule that stimulates angiogenesis or growth of blood vessels.
在另一实施方式中,所述发明用于治疗动脉粥样硬化、再狭窄、血栓形成、动脉瘤或血管梗阻。在本发明的这一实施方式中,转移基因用于编码(a)促进内皮重建的eNOS或VEGF;或(b)抗炎症或炎症调节因子,例如IFN-β、IFN-α、TGF-β或白细胞介素-10(IL-10);或(c)用于抑制内膜增生的平滑肌细胞生长、迁移或分化抑制剂;或这些基因的组合。In another embodiment, the invention is used for the treatment of atherosclerosis, restenosis, thrombosis, aneurysm or vascular obstruction. In this embodiment of the invention, the transgenes are used to encode (a) eNOS or VEGF that promote endothelial remodeling; or (b) anti-inflammatory or inflammation modulators such as IFN-β, IFN-α, TGF-β or Interleukin-10 (IL-10); or (c) an inhibitor of smooth muscle cell growth, migration or differentiation for the inhibition of intimal hyperplasia; or a combination of these genes.
本发明还提供了用于诱导愈合反应的工程改造方法。在一个实施方式中,提供了一种在安置于植入血管靶病区中的植入装置内腔表面快速诱导融合内皮层形成的方法,其中的转基因细胞是表达eNOS、VEGF或抗炎症因子或炎症调节因子的内皮祖细胞。在这一实施方式中,与现有技术装置相比,本发明医疗装置的生物相容性有所提高,能通过降低或抑制平滑肌细胞迁移、平滑肌细胞分化和胶原沿内腔表面沉积在医疗装置的植入部位,而降低或抑制基于组织的过度内膜增生和再狭窄。The invention also provides engineered methods for inducing a healing response. In one embodiment, there is provided a method for rapidly inducing the formation of a confluent endothelial layer on the luminal surface of an implant device placed in a target lesion of an implanted vessel, wherein the transgenic cells express eNOS, VEGF or anti-inflammatory factors or Endothelial progenitors of inflammation regulators. In this embodiment, the medical device of the present invention has improved biocompatibility compared to prior art devices by reducing or inhibiting smooth muscle cell migration, smooth muscle cell differentiation, and collagen deposition along the luminal surface of the medical device. to reduce or inhibit tissue-based excessive intimal hyperplasia and restenosis.
在一个实施方式中,用于涂覆医疗装置的方法包括以下步骤:将至少一层生物相容性基质施涂于医疗装置的表面,其中所述生物相容性基质可包含至少-种选自下组的组分:聚氨酯、嵌段聚氨酯-脲/肝素、聚-L-乳酸、纤维素酯、聚乙二醇、聚醋酸乙酯、多糖(例如葡聚糖、明胶)、胶原、弹性蛋白、弹性蛋白原、层连蛋白、纤连蛋白、玻连蛋白、肝素、纤维蛋白、纤维素和碳以及富勒烯,并同时或依次在所述生物相容性基质上施加至少一种抗体和任选的一种能诱导转移基因表达的化合物。In one embodiment, a method for coating a medical device comprises the step of applying at least one layer of a biocompatible matrix to the surface of the medical device, wherein the biocompatible matrix may comprise at least one selected from Components of the following group: Polyurethane, segmented polyurethane-urea/heparin, poly-L-lactic acid, cellulose esters, polyethylene glycol, polyethyl acetate, polysaccharides (eg dextran, gelatin), collagen, elastin , tropoelastin, laminin, fibronectin, vitronectin, heparin, fibrin, cellulose and carbon and fullerene, and simultaneously or sequentially apply at least one antibody and Optionally a compound capable of inducing expression of the transgene.
本发明还提供了一种用于治疗疾病的方法,所述疾病为例如,哺乳动物的肿瘤、血管疾病和伤口愈合。本发明包括将医疗装置植入哺乳动物的血管或管状器官中,其中所述医疗装置涂覆有:(a)生物相容性基质;(b)至少一种抗体;和任选地(c)一种组合物;将转基因细胞导入需要所述治疗的所述哺乳动物,并任选地给予化合物,其中涂覆于医疗装置基质中的抗体识别并结合在转基因细胞表面上表达的抗原,从而将所述转基因细胞固定在基质的表面上,且由转移基因编码的至少一种治疗药物通过由化合物激活的所述被固定的细胞表达,所述化合物为例如药物和在指定位点分泌的治疗性基因产物。The invention also provides a method for treating diseases such as tumors, vascular diseases and wound healing in mammals. The invention includes implanting a medical device into a blood vessel or tubular organ of a mammal, wherein the medical device is coated with: (a) a biocompatible matrix; (b) at least one antibody; and optionally (c) A composition; introducing transgenic cells into said mammal in need of said treatment, and optionally administering a compound wherein antibodies coated in a matrix of a medical device recognize and bind to an antigen expressed on the surface of the transgenic cells, thereby converting The transgenic cells are immobilized on the surface of the matrix, and at least one therapeutic agent encoded by the transgene is expressed by the immobilized cells activated by a compound, such as a drug and a therapeutic agent secreted at a designated site. gene product.
本发明还提供了一种用于治疗哺乳动物中血管疾病的方法,所述方法包括:将医疗装置植入所述哺乳动物的血管或管状器官,其中所述医疗装置涂覆有:(a)生物相容性基质;(b)至少一种抗体;和任选地(c)一种组合物;;将转基因细胞导入需要所述治疗的所述哺乳动物,并任选地给予化合物,其中涂覆于医疗装置基质中的抗体识别并结合仅在转基因细胞膜表面上表达的抗原,从而将所述转基因细胞固定在基质的表面上。所述转基因(遗传改变的)细胞还可包含编码至少一种治疗性基因产物的遗传物质,所述基因产物可为连续表达或在信号(例如包括激素和肽在内的化合物)活化时表达。The present invention also provides a method for treating vascular disease in a mammal, said method comprising: implanting a medical device into a blood vessel or tubular organ of said mammal, wherein said medical device is coated with: (a) (b) at least one antibody; and optionally (c) a composition; introducing transgenic cells into said mammal in need of said treatment, and optionally administering a compound wherein coated Antibodies coated in the matrix of the medical device recognize and bind antigens expressed only on the membrane surface of the transgenic cells, thereby immobilizing the transgenic cells on the surface of the matrix. The transgenic (genetically altered) cells may also comprise genetic material encoding at least one therapeutic gene product, which may be expressed continuously or upon activation of signals such as compounds including hormones and peptides.
本发明的转基因细胞可包含至少一种可表达的转移基因,该基因可用于编码(但不限于):(1)生长因子,包括其家族成员:例如血小板衍生生长因子(PDGF)、转化生长因子(TGF)、内皮生长因子(EGF)、成纤维细胞生长因子(FGF)、胰岛素样生长因子(IGF)、血管内皮生长因子(VEGF)、肝素结合的生长因子、肝癌衍生的生长因子(HDGF)、肝细胞生长因子/分散因子(HGF)、胎盘生长因子(PIGF)、血小板衍生的内皮细胞生长因子(PD-ECGF)、干细胞因子(SCF),以及它们的其它蛋白形式;(2)趋化因子:例如CXC家族、CC家族、C家族,以及它们的其它蛋白形式;(3)细胞因子:例如解聚素和金属蛋白酶(ADAM)、膜联蛋白V、B7&CD28/CTLA-4受体家族、骨形态发生蛋白(BMP)、半胱天冬酶、CD44、CD44H、内皮素-1(ET-1)、eph、促红细胞生成素(Epo)、胞间粘附分子-3/CD50(ICAM-3)、巨噬细胞刺激蛋白(MSP)、基质金属蛋白酶(MMP)、神经营养因子、内皮一氧化氮合酶(eNOS)、NKG2D、血小板内皮细胞粘附分子-1(PECAM-1/CD31)、多效因子/肝素结合细胞因子(PTN/MK)、转铁蛋白受体(sTfR)、防护肽(hedgehog peptide)、STAT、干细胞标记、Th1/Th2、血小板生成素(Tpo)、肿瘤坏死因子家族、VCAM-1/CD16、单克隆非特异性抑制因子β(MNSFβ)、6Ckine(SLC)、B-淋巴细胞趋化诱导物(BCA-1/BLC)、白血病抑制因子、单核细胞衍生的嗜中性粒细胞活化的肽(GRO),以及它们的其它蛋白形式;(4)参与信号传导调节、细胞周期调节、细胞分裂和/或细胞分化的其它功能性蛋白质,它们为例如:配体、受体、磷酸化酶、激酶、转录因子,以及它们的其它蛋白形式。The transgenic cells of the present invention may comprise at least one expressible transgene that encodes (but is not limited to): (1) growth factors, including members of their family: e.g., platelet-derived growth factor (PDGF), transforming growth factor (TGF), endothelial growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), heparin-binding growth factor, liver cancer-derived growth factor (HDGF) , hepatocyte growth factor/dispersion factor (HGF), placental growth factor (PIGF), platelet-derived endothelial growth factor (PD-ECGF), stem cell factor (SCF), and their other protein forms; (2) chemotactic Factors: such as CXC family, CC family, C family, and their other protein forms; (3) cytokines: such as disintegrin and metalloproteinase (ADAM), annexin V, B7&CD28/CTLA-4 receptor family, Bone morphogenetic protein (BMP), caspase, CD44, CD44H, endothelin-1 (ET-1), eph, erythropoietin (Epo), intercellular adhesion molecule-3/CD50 (ICAM- 3), macrophage stimulating protein (MSP), matrix metalloproteinase (MMP), neurotrophic factor, endothelial nitric oxide synthase (eNOS), NKG2D, platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) , pleiotropic factor/heparin-binding cytokine (PTN/MK), transferrin receptor (sTfR), protective peptide (hedgehog peptide), STAT, stem cell markers, Th1/Th2, thrombopoietin (Tpo), tumor necrosis factor Family, VCAM-1/CD16, Monoclonal Nonspecific Suppressor Beta (MNSFβ), 6Ckine (SLC), Inducer of B-lymphocyte Chemotaxis (BCA-1/BLC), Leukemia Inhibitory Factor, Monocyte-derived Phytotropin Neutrophil activating peptides (GRO), and their other protein forms; (4) other functional proteins involved in signal transduction regulation, cell cycle regulation, cell division and/or cell differentiation, such as: ligands, Receptors, phosphorylases, kinases, transcription factors, and their other protein forms.
在一个实施方式中,用于本发明的抗血管生成因子为例如:干扰素(IFNs)、血小板反应素(TSP)、血管抑素和内皮抑素、制瘤素M(OSM)、整合素排列的阻滞剂、金属蛋白酶抑制剂、内皮细胞磷酸化抑制剂、用于血管发生诱导剂的显性失活受体、血管发生诱导剂的抗体、通过其它方式作用的蛋白质,以及它们的其它蛋白形式。其它血管生成因子,包括血管生成因子、血管生成素、整合素刺激因子(例如Del-1),以及它们的其它蛋白形式。In one embodiment, the anti-angiogenic factors used in the present invention are, for example: interferons (IFNs), thrombospondin (TSP), angiostatin and endostatin, oncostatin M (OSM), integrin array Blockers of metalloproteinases, inhibitors of metalloproteinases, inhibitors of endothelial cell phosphorylation, dominant negative receptors for inducers of angiogenesis, antibodies to inducers of angiogenesis, proteins acting by other means, and their other proteins form. Other angiogenic factors, including angiogenic factors, angiopoietins, integrin stimulating factors (eg Del-1), and other protein forms thereof.
用于本发明的其它生长因子为例如:多效因子、肝素结合细胞因子、VEGF家族(包括VEGF-2、VEGF-C和VEGF-D)、FGF家族(包括FGF-1、FGF-2、FGF-5和FGF-18)、肝癌衍生的生长因子(HDGF)、肝细胞生长因子/分散因子(HGF)、内皮生长因子(EGF)家族成员(包括转化生长因子α、EGF和TGF-α-HIII以及血小板衍生生长因子(PDGF)(包括AA、AB和BB亚型)。Other growth factors useful in the present invention are for example: pleiotrophins, heparin binding cytokines, VEGF family (including VEGF-2, VEGF-C and VEGF-D), FGF family (including FGF-1, FGF-2, FGF -5 and FGF-18), liver cancer-derived growth factor (HDGF), hepatocyte growth factor/scatter factor (HGF), endothelial growth factor (EGF) family members (including transforming growth factor α, EGF, and TGF-α-HIII and platelet-derived growth factor (PDGF) (including AA, AB, and BB subtypes).
试验实施例Test Example
在下述的试验性详述部分对本发明进行说明。下述的这些部分是为了理解本发明,而不是且不应解释为以任何方式限制由实施例后的权利要求书中所述的本发明范围。The invention is illustrated in the following experimental detailed description. These sections described below are for the understanding of the present invention and are not and should not be construed as limiting in any way the scope of the present invention which is described in the claims following the examples.
实施例1Example 1
内皮祖细胞的表型Phenotype of endothelial progenitor cells
通过如下方法分离内皮祖细胞(EPC):通过CD34+磁珠分离法(Dynal Biotech)或如最近描述的富集培养基分离法(Asahara T,Murohara T,Sullivan A等,《供血管生成用的推定的内皮祖细胞的分离方法》,I solation of putativeprogenitor endothelial cells for angiogenesis,Science 1997;275:964-7)。简而言之,从健康男性志愿者获取外周静脉血,并通过密度梯度离心获得单核细胞组分,然后将该细胞接种于涂覆有人纤连蛋白的培养玻片上(Becton Dickinson),培养基为EC基础培养基-2(EBM-2)(Clonetics),其中补充有5%胎牛血清、人VEGF-A、人成纤维细胞生长因子-2、人内皮生长因子、胰岛素样生长因子-1和抗坏血酸。使EPC生长7天,每48小时更换培养基。这些试验的结果示于图2A和2B中。图2A和2B显示了抗CD34分离的细胞看似更似纺锤样,这就表明这些细胞正分化为内皮细胞。Endothelial progenitor cells (EPCs) were isolated by CD34+ magnetic bead isolation (Dynal Biotech) or enriched medium isolation as recently described (Asahara T, Murohara T, Sullivan A et al., Putative for angiogenesis Isolation of putative progenitor endothelial cells for angiogenesis, Science 1997; 275: 964-7). Briefly, peripheral venous blood was obtained from healthy male volunteers, and the mononuclear cell fraction was obtained by density gradient centrifugation, and the cells were plated on culture slides (Becton Dickinson) coated with human fibronectin in medium EC basal medium-2 (EBM-2) (Clonetics) supplemented with 5% fetal bovine serum, human VEGF-A, human fibroblast growth factor-2, human endothelial growth factor, insulin-like growth factor-1 and ascorbic acid. EPCs were grown for 7 days with media changes every 48 hours. The results of these experiments are shown in Figures 2A and 2B. Figures 2A and 2B show that anti-CD34 isolated cells appeared to be more spindle-like, suggesting that these cells were differentiating into endothelial cells.
通过免疫组织化学法测定EC的表型。简而言之,将EPC用2%多聚甲醛(PFA)(Sigma)的磷酸盐缓冲溶液(PBS)(Sigma)固定10分钟,用PBS洗涤3次,并用不同的EC特异性标记染色:兔抗人VEGFR-2(Alpha Diagnostics Intl.Inc.)、鼠抗人Tie-2(Clone Ab33,Upstate Biotechnology)、鼠抗人CD34(Becton Dickinson)、EC-凝集素(Ulex Europaeus Ueal)(Sigma)和鼠抗人8因子(Sigma)。使细胞接触异硫氰酸荧光素(FITC)结合的二抗来验证抗体的存在。将碘化丙锭(PI)用作核标记。这些试验的结果示于图2C-2G。图2C显示培养物中24小时后有VEGFR-2表达,这就证实了所述细胞为内皮细胞。图2D和2F所示为7天孵育后结合细胞的核染色,而图2E和2G所示为同一视野中用FITC偶联的抗Tie-2抗体染色的细胞。The phenotype of EC was determined by immunohistochemistry. Briefly, EPCs were fixed with 2% paraformaldehyde (PFA) (Sigma) in phosphate-buffered saline (PBS) (Sigma) for 10 min, washed 3 times with PBS, and stained with different EC-specific markers: rabbit Anti-human VEGFR-2 (Alpha Diagnostics Intl. Inc.), mouse anti-human Tie-2 (Clone Ab33, Upstate Biotechnology), mouse anti-human CD34 (Becton Dickinson), EC-lectin (Ulex Europaeus Ueal) (Sigma) and Mouse anti-human factor 8 (Sigma). The presence of the antibody was verified by exposing the cells to a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Propidium iodide (PI) was used as a nuclear label. The results of these experiments are shown in Figures 2C-2G. Figure 2C shows VEGFR-2 expression after 24 hours in culture, confirming that the cells are endothelial. Figures 2D and 2F show nuclear staining of bound cells after 7 days of incubation, while Figures 2E and 2G show cells in the same field stained with FITC-conjugated anti-Tie-2 antibody.
对eNOS mRNA进行逆转录酶-聚合酶链反应(rt-PCR)来测定EPC表达内皮一氧化氮合酶(eNOS,一种EC功能标志)的能力。使EPC在EBM-2培养基中生长7天,然后用GenElute哺乳动物总RNA试剂盒(Sigma)分离总RNA,并在260nm下通过吸光度定量。用Omni script RT试剂盒(Qiagen)以1μg随机引物对20μL总RNA进行逆转录。对于各个RT产物,在两个平行的PCR反应中对等份的最终反应体积(2-10μL)进行扩增,使用了eNOS特异性引物(299bp产物,有义5’-TTCCGGGGATTCTGGCAGGAG-3’,SEQ IDNO:1,反义5’-GCCATGGTAACATCGCCGCAG-3’,SEQ ID NO:2)或GAPDH特异性引物(343bp产物,有义5’-CTCTAAGGCTGTGGGCAAGGTCAT-3’,SEQ ID NO:3,反义5’-GAGATCCACCACCCTGTTGCTGTA-3’,SEQ ID NO:4)和Taq聚合酶(Pharmacia BiotechAmersham)。PCR循环如下:94℃5分钟,65℃45秒,72℃30秒(对eNOS为35个循环,而对GAPDH则为25个循环)。通过2%琼脂糖凝胶电泳分析rt-PCR产物,用溴化乙锭显色,并用光密度法定量。该试验的结果示于图3A和3B。由图3A和3B可见,细胞在存在或不存在氧的条件下在培养基中孵育3天后,表达了一氧化氮合酶(eNOS)。培养7天后仍继续表达eNOS mRNA。eNOS mRNA的表达表明在3天内所述细胞已分化为成熟的内皮细胞,并开始行使类似于完全分化的内皮细胞的功能。Reverse transcriptase-polymerase chain reaction (rt-PCR) was performed on eNOS mRNA to determine the ability of EPCs to express endothelial nitric oxide synthase (eNOS, a functional marker of EC). EPCs were grown in EBM-2 medium for 7 days, then total RNA was isolated using the GenElute Mammalian Total RNA Kit (Sigma) and quantified by absorbance at 260 nm. 20 μL of total RNA was reverse transcribed with 1 μg of random primers using the Omni script RT kit (Qiagen). For each RT product, aliquots of final reaction volumes (2-10 μL) were amplified in two parallel PCR reactions using eNOS-specific primers (299bp product, sense 5'-TTCCGGGGATTCTGGCAGGAG-3', SEQ IDNO: 1, antisense 5'-GCCATGGTAACATCGCCGCAG-3', SEQ ID NO: 2) or GAPDH specific primer (343bp product, sense 5'-CTCTAAGGCTGTGGGCAAGGTCAT-3', SEQ ID NO: 3, antisense 5'- GAGATCCACCACCCTGTTGCTGTA-3', SEQ ID NO: 4) and Taq polymerase (Pharmacia BiotechAmersham). PCR cycles were as follows: 94°C for 5 minutes, 65°C for 45 seconds, 72°C for 30 seconds (35 cycles for eNOS and 25 cycles for GAPDH). rt-PCR products were analyzed by 2% agarose gel electrophoresis, developed with ethidium bromide, and quantified by densitometry. The results of this experiment are shown in Figures 3A and 3B. As can be seen in Figures 3A and 3B, the cells expressed nitric oxide synthase (eNOS) after incubation in culture medium for 3 days in the presence or absence of oxygen. The expression of eNOS mRNA continued after 7 days of culture. Expression of eNOS mRNA indicated that within 3 days the cells had differentiated into mature endothelial cells and began to function like fully differentiated endothelial cells.
实施例2Example 2
用抗CD34(抗体)涂覆的不锈钢圆盘捕获内皮细胞:在试验过程中,将人脐静脉内皮细胞(HUVEC)(美国典型培养物保藏中心,American Type CultureCollection)培养于内皮细胞生长培养基中。将细胞与表面含或不含结合抗体的用CMDX和明胶涂覆的样品或空白的不锈钢(SST)样品一起孵育。孵育后,去除培养基,并用PBS洗涤样品2次。在2%多聚甲醛(PFA)中固定细胞10分钟,用PBS洗涤3次,每次10分钟以确保去除所有的固定剂。在室温下,将各样品与封闭溶液一起孵育30分钟,以封闭所有非特异性结合。用PBS洗涤样品1次,并接触VEGFR-2抗体的1∶100稀释液培育过夜。然后用PBS洗涤样品3次,以确保已除去所有一抗。在各个样品中加入用封闭溶液作1∶100的稀释的FITC偶联的二抗的,在Belly Dancer装置上室温孵育45分钟。孵育后,用PBS洗涤样品3次,用含有0.1%Tween 20的PBS洗涤一次,再用PBS洗涤。用碘化丙锭(PI)固定样品,并在共聚焦显微镜下观察。Endothelial cell capture using anti-CD34 (antibody)-coated stainless steel discs: During the assay, human umbilical vein endothelial cells (HUVEC) (American Type Culture Collection, American Type Culture Collection) were cultured in endothelial cell growth medium . Cells were incubated with CMDX and gelatin-coated samples or blank stainless steel (SST) samples with or without bound antibody on the surface. After incubation, the medium was removed, and the samples were washed 2 times with PBS. Cells were fixed in 2% paraformaldehyde (PFA) for 10 minutes and washed 3 times with PBS for 10 minutes each to ensure all fixative was removed. Each sample was incubated with blocking solution for 30 minutes at room temperature to block any non-specific binding. Samples were washed once with PBS and incubated overnight with a 1:100 dilution of VEGFR-2 antibody. Samples were then washed 3 times with PBS to ensure all primary antibodies had been removed. Add FITC-conjugated secondary antibody diluted 1:100 with blocking solution to each sample, and incubate on Belly Dancer device for 45 minutes at room temperature. After incubation, the samples were washed 3 times with PBS, once with PBS containing 0.1
图4A-4E是涂覆了如上所述CMDX和抗CD34抗体(图4A)、明胶和抗CD34抗体(图4B)、空白SST(图4C)、CMDX涂层但无抗体(图4D)以及明胶涂层但无抗体(图4E)的SST样品显微照片。附图所示为通过PI染色显示出仅涂覆抗体的样品包含许多粘附于样品表面的细胞。空白SST对照盘显示极少细胞粘附于其表面。Figures 4A-4E are samples coated with CMDX as described above and anti-CD34 antibody (Figure 4A), gelatin and anti-CD34 antibody (Figure 4B), blank SST (Figure 4C), CMDX coated without antibody (Figure 4D), and gelatin Micrograph of SST samples coated but without antibody (Fig. 4E). Shown in the figure is that the sample coated with only antibody, as shown by PI staining, contained many cells adhered to the surface of the sample. Blank SST control discs showed very few cells adhered to their surface.
图5A-5C是涂覆了CMDX而无抗体结合于其表面的对照样品的显微照片。图5A显示通过PI染色可见极少细胞粘附于样品的表面。图5B显示粘附的细胞为VEGFR-2阳性,这表明它们是内皮细胞,而图5C所示为染色的核与VEGFR-2阳性绿色荧光的组合。图5D-F是涂覆了明胶而在其表面上无抗体的对照样品的显微照片。图5D显示无细胞存在,这是由于该样品无PI染色也不发射出的绿色荧光(参见图5E和5F)。Figures 5A-5C are photomicrographs of control samples coated with CMDX without antibody bound to its surface. Figure 5A shows that very few cells adhered to the surface of the sample as seen by PI staining. Figure 5B shows that the adherent cells are positive for VEGFR-2, suggesting that they are endothelial cells, while Figure 5C shows the combination of stained nuclei and VEGFR-2 positive green fluorescence. Figures 5D-F are photomicrographs of control samples coated with gelatin without antibody on their surface. Figure 5D shows the absence of cells, since this sample was neither PI stained nor emitted green fluorescence (see Figures 5E and 5F).
图6A-6C是涂覆了CMDX且具有结合于其表面的抗CD34抗体的SST样品的显微照片。这些附图显示样品包含许多粘附细胞,如绿色荧光显示的那样它们已建立了接近融合的单层(图6A)、为VEGFR-2阳性细胞(图6B和6C)。类似地,图6D-6F是其表面结合有抗CD34抗体的涂覆了明胶的样品的显微照片。这些附图还通过来自VEGFR-2/FITC抗体产生的许多红色染色核和绿色荧光,显示了HUVEC粘附于样品表面(图6E和6F)。6A-6C are photomicrographs of SST samples coated with CMDX and having anti-CD34 antibody bound to its surface. These figures show that the sample contained many adherent cells that had established a near confluent monolayer as indicated by green fluorescence (Figure 6A), VEGFR-2 positive cells (Figures 6B and 6C). Similarly, Figures 6D-6F are photomicrographs of gelatin-coated samples with anti-CD34 antibodies bound to their surface. These figures also show the adherence of HUVECs to the sample surface by numerous red stained nuclei and green fluorescence from the VEGFR-2/FITC antibody (Fig. 6E and 6F).
实施例3Example 3
内皮祖细胞的VEGFR-2和Tie-2染色:如实施例1中所述分离得到人血中的祖细胞,在培养基中体外孵育24小时、7天和3周。孵育后,去除生长培养基,用PBS洗涤样品2次。用2%多聚甲醛(PFA)固定细胞10分钟,然后用PBS洗涤3次,每次10分钟,确保去除所有固定剂。将各样品与440μl的羊(对于VEGFR-2)或马(用于Tie-2)封闭液一起孵育在室温下30分钟,以封闭所有非特异性结合。用PBS洗涤样品1次,然后加入以封闭液作1∶100稀释的VEGFR-2或Tie-2抗体,孵育样品过夜。然后用PBS洗涤3次,以确保所有的一抗都已被洗去。向各样品中加入用马或羊封闭液作1∶100的稀释的FITC偶联的二抗(200μl),并在Belly Dancer装置上在室温下孵育45分钟。孵育后,用PBS洗涤样品3次,用含有0.1%Tween 20的PBS洗涤一次,再用PBS中洗涤。用碘化丙锭(PI)固定样品,并在共聚焦显微镜下观察。VEGFR-2 and Tie-2 staining of endothelial progenitor cells: Progenitor cells from human blood were isolated as described in Example 1 and incubated in culture medium in vitro for 24 hours, 7 days and 3 weeks. After incubation, the growth medium was removed and the samples were washed 2 times with PBS. Cells were fixed with 2% paraformaldehyde (PFA) for 10 minutes, and then washed 3 times with PBS for 10 minutes each, ensuring that all fixative was removed. Each sample was incubated with 440 μl of sheep (for VEGFR-2) or horse (for Tie-2) blocking solution for 30 minutes at room temperature to block any non-specific binding. Wash the sample once with PBS, then add VEGFR-2 or Tie-2 antibody diluted 1:100 with blocking solution, and incubate the sample overnight. Then wash 3 times with PBS to ensure that all the primary antibody has been washed away. FITC-conjugated secondary antibody (200 μl) diluted 1:100 in horse or sheep blocking solution was added to each sample and incubated for 45 minutes at room temperature on a Belly Dancer apparatus. After incubation, the samples were washed 3 times with PBS, once with PBS containing 0.1
图7是在其表面含有CD34抗体的涂覆了CMXD的样品的显微照片,该样品与细胞一起孵育了24小时,显示祖细胞被捕获于样品的表面,这点可由样品表面上存在的红色染色的核证明。该附图还显示了约75%的细胞为VEGFR-2阳性,形态为圆形。Figure 7 is a photomicrograph of a CMXD-coated sample containing CD34 antibody on its surface, which was incubated with cells for 24 hours, showing that progenitor cells are captured on the surface of the sample, which can be seen by the presence of red color on the surface of the sample. Stained nuclear evidence. The figure also shows that about 75% of the cells are positive for VEGFR-2 and are round in shape.
图8A和8B为与细胞一起孵育了7天的样品。如图8A所示,红色染色的核表明细胞存在于样品上,这些细胞为VEGFR-2阳性(图8B,100%),且由细胞的纺锤形所示这些细胞在结构上更为内皮化。图9A和9B是在其表面上包含CD34抗体的涂覆了CMXD的样品显微照片,该样品与细胞一起孵育了7天,在孵育后,使该样品接触Tie-2抗体。如图9A所示,红色染色的核显示了有许多细胞粘附于样品表面。如细胞发射的绿色荧光可见,粘附于样品的细胞也为Tie-2阳性(100%)(图9B)。总而言之,通过许多细胞粘附于样品表面以及粘附细胞表面存在的VEGFR-2和Tie-2受体所示:将细胞与样品一起孵育7天后,涂覆了CD34抗体的样品能将内皮细胞捕获于它们的表面。此外,7天时在样品表面上存在100%的内皮细胞表明了非内皮细胞可能已脱落,或在第7天时所有的粘附细胞都已开始表达内皮细胞标记。Figures 8A and 8B are samples incubated with cells for 7 days. As shown in Figure 8A, red stained nuclei indicated the presence of cells on the sample which were positive for VEGFR-2 (Figure 8B, 100%) and which were more endothelialized in structure as indicated by the spindle shape of the cells. Figures 9A and 9B are photomicrographs of a CMXD-coated sample containing CD34 antibody on its surface, which was incubated with cells for 7 days, after which the sample was exposed to Tie-2 antibody. As shown in Figure 9A, the red stained nuclei showed many cells adhered to the sample surface. Cells adhered to the sample were also Tie-2 positive (100%) as seen by the green fluorescence emitted by the cells (Fig. 9B). In summary, endothelial cells were captured by CD34 antibody-coated samples after 7 days of incubation of the cells with the samples, as shown by the many cells attached to the sample surface and the presence of VEGFR-2 and Tie-2 receptors on the surface of the adherent cells. on their surface. Furthermore, the presence of 100% endothelial cells on the sample surface at 7 days indicates that non-endothelial cells may have detached, or that at day 7 all adherent cells had begun to express endothelial cell markers.
图10A-10C是生长在内皮细胞生长培养基中3周的内皮祖细胞的相差显微镜照片。图10A显示细胞已分化成为成熟的内皮细胞,这点可由箭头处的二维管状结构的血管内腔所证明。图10B显示存在多层的三维细胞叠加,即一个细胞位于另一细胞之上,这就证实了延长时间内生长的内皮细胞开始形成位于另一层之上的层的报道。图10C所示为种板后培养3周的祖细胞,这些细胞具有内皮细胞的外观,且如它们表面存在的CD34/FITC抗体绿色荧光所证明的那样,该附图证实了这些细胞为内皮细胞。Figures 10A-10C are phase contrast micrographs of endothelial progenitor cells grown in endothelial cell growth medium for 3 weeks. Figure 10A shows that the cells have differentiated into mature endothelial cells, as evidenced by the lumen of the blood vessel as a two-dimensional tubular structure at the arrow. Figure 10B shows the presence of three-dimensional cell superposition of multiple layers, one cell on top of another, confirming reports that endothelial cells grown over extended periods of time begin to form layers on top of one another. Figure 10C shows progenitor cells cultured 3 weeks after plating, these cells have the appearance of endothelial cells and as evidenced by the presence of CD34/FITC antibody green fluorescence on their surface, this figure confirms that these cells are endothelial cells .
上述数据显示分离自人血的白细胞含有CD34阳性祖细胞,且这些细胞可发育为成熟内皮细胞并易于表达内皮细胞表面抗原。(VEGFR-2和Tie-2)。该数据还显示可采用针对祖细胞或干细胞表面抗原的抗体来将这些细胞捕获在本发明涂覆的医疗装置的表面上。The above data show that leukocytes isolated from human blood contain CD34-positive progenitor cells and that these cells can develop into mature endothelial cells and readily express endothelial cell surface antigens. (VEGFR-2 and Tie-2). The data also show that antibodies against surface antigens of progenitor or stem cells can be used to capture these cells on the surface of the coated medical devices of the present invention.
实施例4Example 4
涂覆了富勒烯和涂覆了富勒烯并带有抗CD34抗体Fullerene-coated and Fullerene-coated with anti-CD34 antibody
和/或内皮细胞生长因子(Ang-2,VEGF)的不锈钢体and/or endothelial growth factor (Ang-2, VEGF) stainless steel body
采用以下的方法来用产生具有结合抗体和/或生长因子(即,VEGF或Ang-2)的功能性富勒烯层的不锈钢支架和圆盘:The following approach was used to generate stainless steel scaffolds and discs with functional fullerene layers that bind antibodies and/or growth factors (i.e., VEGF or Ang-2):
在第一步中,用0.5M HCl活化SST支架或圆盘的表面,所述HCl还同时清除了表面上的任何钝化污染物。从活化浴中取出金属样品,用蒸馏水冲洗,甲醇干燥并于75℃下烘干。然后将所述支架浸渍于含有氧化富勒烯(C60-O)的甲苯衍生溶液中最多24小时。所述氧化富勒烯可通过支架上的Fe-O、Cr-O和Ni-O结合于支架。将支架从衍生浴中取出,用甲苯冲洗,然后置于Soxhlet Extractor中16小时,用新鲜甲苯去除任何物理吸附的C60。取出支架,并在105℃下烘干过夜。这一反应产生了具有富勒烯单层的完全衍生的支架或圆盘。In a first step, the surface of the SST scaffold or disk is activated with 0.5M HCl, which also simultaneously cleans any passivating contaminants on the surface. The metal samples were removed from the activation bath, rinsed with distilled water, dried with methanol and oven-dried at 75°C. The scaffolds were then immersed in a toluene-derived solution containing fullerene oxide (C60 —O) for up to 24 hours. The fullerene oxide can be bound to the support through Fe-O, Cr-O and Ni-O on the support. The scaffolds were removed from the derivatization bath, rinsed with toluene, and placed in a Soxhlet Extractor for 16 hours to remove any physisorbedC60 with fresh toluene. Remove the stent and dry it overnight at 105 °C. This reaction produces a fully derivatized scaffold or disc with a fullerene monolayer.
在步骤2中,使癸二酸与亚硫酰氯或氯氧化硫(SOCl2)反应形成癸二酰氯,以形成二醛分子溶液。如下所示使所得癸二酰氯与LiAI[t-O丁基]3H和二甘醇二甲醚反应以获得1,10-癸二酰:In
在步骤3中,在获自步骤1的支架或圆盘上形成N-甲基吡咯烷衍生物。如下所示,通过以下反应来对富勒烯分子进行进一步衍生:氮气下,在回流的甲苯溶液中,将等摩尔量的富勒烯和N-甲基甘氨酸与步骤2中的1,10-癸二酰产物反应48小时,以获得N-甲基吡咯烷衍生的富勒烯不锈钢支架或圆盘。In step 3, N-methylpyrrolidine derivatives are formed on the rack or disc obtained from step 1. Fullerene molecules were further derivatized by reacting equimolar amounts of fullerene and N-methylglycine with the 1,10- The sebacyl product was reacted for 48 hours to obtain N-methylpyrrolidine derivatized fullerene stainless steel scaffolds or disks.
洗涤衍生的不锈钢支架或圆盘以去除任何化学残留物,用于以标准方法结合抗体和/或(VEGF或Ang-2)。如实施例1所述分离人血中的祖细胞,使其接触用抗CD34抗体涂覆的富勒烯圆盘。孵育后,去除生长培养基,用PBS洗涤样品2次。用2%多聚甲醛(PFA)固定细胞10分钟,然后用PBS洗涤3次,每次洗涤10分钟,以确保去除所有固定剂。将各样品与封闭溶液一起在室温下孵育30分钟,以封闭所有非特异性结合。用PBS洗涤样品1次,加入1∶100稀释的VEGFR-2抗体孵育过夜。然后用PBS洗涤样品3次,以确保已除去所有一抗。在各个样品中加入用封闭液作1∶100稀释的FITC偶联的二抗,在Belly Dancer装置上室温孵育45分钟。孵育后,用PBS洗涤样品3次,用含有0.1%Tween 20的PBS洗涤一次,再用PBS洗涤。用碘化丙锭(PI)固定样品,并在共聚焦显微镜下观察。图11所示为本发明结合了祖细胞的涂覆了功能性富勒烯的支架表面的示意图。图12A-12B各为用PI染色(12A)和抗-VEGFR-2/FITC-偶联抗体染色的涂覆了富勒烯而无抗体的对照样品的显微照片。图12C和12D是涂覆了富勒烯/抗CD34抗体涂层的样品的显微照片。如图所示,涂覆有抗CD34抗体的样品含有更多粘附于表面的VEGFR-2阳性细胞。Derivatized stainless steel racks or discs are washed to remove any chemical residues for binding antibodies and/or (VEGF or Ang-2) in standard methods. Progenitor cells from human blood were isolated as described in Example 1 and exposed to fullerene discs coated with anti-CD34 antibody. After incubation, the growth medium was removed and the samples were washed 2 times with PBS. Cells were fixed with 2% paraformaldehyde (PFA) for 10 minutes and then washed 3 times with PBS for 10 minutes each to ensure all fixative was removed. Each sample was incubated with blocking solution for 30 minutes at room temperature to block any non-specific binding. The samples were washed once with PBS, and VEGFR-2 antibody diluted 1:100 was added to incubate overnight. Samples were then washed 3 times with PBS to ensure all primary antibodies had been removed. Add FITC-conjugated secondary antibody diluted 1:100 with blocking solution to each sample, and incubate on Belly Dancer device for 45 minutes at room temperature. After incubation, the samples were washed 3 times with PBS, once with PBS containing 0.1
如实施例5所述,将含有或不含有抗体的涂覆了富勒烯的样品植入约克猪。取出支架作组织学分析,用10%福尔马林缓冲液冲洗带支架区段30秒,然后用10%福尔马林缓冲液固定直到处理。从各支架中切取以下5个切片;临近支架1mm处、支架临近末端的1mm处、支架中段处、支架远端1mm处和支架末端1mm处。用苏木精&伊红(HE)和三色弹性蛋白染色切片。图13A-13D是从冠状动脉取出的已植入4周的支架横切面显微照片。数据显示涂覆了富勒烯(图13B和13D)的支架与对照相比抑制了支架所处部位的内膜过度增生(空白支架,图13A和13C)。Yorkie pigs were implanted with fullerene-coated samples with or without antibodies as described in Example 5. Scaffolds were removed for histological analysis, and scaffolded sections were rinsed with 10% buffered formalin for 30 seconds and then fixed with 10% buffered formalin until processing. The following 5 slices were cut from each stent; 1 mm proximal to the stent, 1 mm proximal to the end of the stent, mid-stent, 1 mm distal to the stent, and 1 mm to the end of the stent. Sections were stained with hematoxylin & eosin (HE) and trichrome elastin. 13A-13D are photomicrographs of cross-sections of 4-week-old stents removed from coronary arteries. The data showed that fullerene-coated stents (Figures 13B and 13D) inhibited intimal hyperplasia at the site of the stent compared to controls (blank stent, Figures 13A and 13C).
实施例5Example 5
猪气囊损伤研究:将涂覆了抗体的支架移植入重25-30kg的幼年约克猪。按照《实验动物管理和使用指南》(Guide for the Care and Use of LaboratoryAnimals,NIH出版号80-23,修订于1985)照料动物。禁食过夜后,用盐酸氯胺酮使动物镇静(20mg/kg)。然后用硫喷妥钠(12mg/kg)诱导麻醉,对动物进行插管,并连接给予氧气和氮气混合气(1∶2[体积/体积])的送气管。用0.5-2.5体积%的异氟烷维持麻醉。肌内注射1,000mg普鲁卡因青霉素-G和苄星青霉素-G(链霉素)混合物以提供抗生素性预防。Porcine Air Sac Injury Study: Antibody-coated scaffolds were grafted into juvenile Yorkie pigs weighing 25-30 kg. Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, NIH Publication No. 80-23, revised 1985. After an overnight fast, animals were sedated with ketamine hydrochloride (20 mg/kg). Anesthesia was then induced with sodium thiopental (12 mg/kg), the animals were intubated, and a tracheal tube administered with a mixture of oxygen and nitrogen (1:2 [vol/vol]) was connected. Anesthesia was maintained with 0.5-2.5 vol% isoflurane. Antibiotic prophylaxis was provided by intramuscular injection of 1,000 mg of a mixture of procaine penicillin-G and benzathine penicillin-G (streptomycin).
在无菌条件下,进行左颈动脉切开术,将8F引导鞘置于左颈动脉中。给予所有动物100IU肝素/千克体重。在整个手术过程中定期给予追加的2,500IU的肝素药团(boluse),以将活化凝血时间保持在300秒以上。将6F引导管通过颈动脉鞘导入,推进到冠状动脉口。冠状动脉内给予200μg硝化甘油后,进行血管造影术,用定量冠状动脉血管造影系统分析图像。将3F栓子切除导管插入冠状动脉的近端并向远端推进至选择用于植入支架且内皮裸露区段。将加入抗CD34抗体的经涂覆的R支架通过引导管插入,并在冠状动脉裸露区段中展开。以空白不锈钢支架或涂覆了基质但不含抗体的支架作为对照。以支架对动脉为1.1的比例将支架植入冠状动脉左前降支(LAD)或右冠状动脉(RCA)或冠状动脉回旋支(Cx)。用血管造影术评价支架的尺寸和位置,取出引导鞘,闭合两层皮肤。在试验过程中给予动物300mg的ASA。Under sterile conditions, a left carotid arteriotomy was performed and an 8F introducer sheath was placed in the left carotid artery. All animals were given 100 IU heparin/kg body weight. Additional 2,500 IU heparin boluses were given periodically throughout the procedure to maintain the activated clotting time above 300 seconds. The 6F guide catheter was introduced through the carotid sheath and advanced to the coronary ostium. After intracoronary administration of 200 μg of nitroglycerin, angiography was performed and the images were analyzed with a quantitative coronary angiography system. A 3F embolectomy catheter was inserted into the proximal end of the coronary artery and advanced distally to the endothelial-exposed segment selected for stenting. The coated R stent with added anti-CD34 antibody was inserted through the guide catheter and deployed in the exposed segment of the coronary artery. A blank stainless steel stent or a matrix-coated stent without antibody was used as a control. Stents were implanted in the left anterior descending coronary artery (LAD), right coronary artery (RCA) or circumflex coronary artery (Cx) at a stent-to-artery ratio of 1.1. The size and position of the stent was assessed angiographically, the introducer sheath was removed, and the two layers of skin were closed. Animals were given 300 mg of ASA during the test.
在植入支架后1、3、7、14和28天时处死动物。如上所述先将动物镇静并麻醉。取出植入支架的冠状动脉,在其近端和远端带有1cm无支架的血管。用三种方法处理植入支架的血管:组织学、免疫组化法或扫描电镜法。Animals were sacrificed at 1, 3, 7, 14 and 28 days after stent implantation. Animals were initially sedated and anesthetized as described above. The stented coronary arteries were removed with 1 cm unstented vessels at their proximal and distal ends. Stented vessels were processed using three methods: histology, immunohistochemistry, or scanning electron microscopy.
对于免疫组化检测,用10%福尔马林轻柔冲洗切开的支架30秒,置于10%福尔马林/PBS溶液中直至处理。用含2%多聚甲醛(PFA)的PBS溶液冲洗指定用于免疫组化检测的支架30秒,然后置于2%PFA溶液中15分钟,在用PBS洗涤并储存直至进行使用兔抗人VEGFR-2或鼠抗人Tie-2抗体的免疫组化检测。For immunohistochemistry, the dissected scaffolds were gently rinsed with 10% formalin for 30 seconds and placed in 10% formalin/PBS until processing. Scaffolds designated for immunohistochemical detection were rinsed with 2% paraformaldehyde (PFA) in PBS for 30 seconds, then placed in 2% PFA for 15 minutes, washed with PBS and stored until proceeding using rabbit anti-human VEGFR -2 or mouse anti-human Tie-2 antibody by immunohistochemistry.
通过以下方法来准备用于SEM的支架:用10%福尔马林缓冲液冲洗30秒,然后在含有2.5%戊二醛和2%PFA的0.1M二甲胂酸钠缓冲液中固定过夜。然后用二甲胂酸盐缓冲液洗涤样品三次,并使其清洗过夜。通过以下步骤完成固定后处理:用1%四氧化锇(Sigma)的0.1M二甲胂酸盐缓冲液处理,然后用乙醇脱水(30%乙醇、50%、70%、85%、95%、100%、100%),随后用CO2进行临界点干燥。干燥后,对样品进行喷金,并在SEM下观察。(《对正常猪的冠状动脉采用经肝素涂覆的Palmatz-Schatz支架以减免血栓形成的支持模架》,Reduction in thromboticevents with heparin-coated Palmaz-Schatz stent in normal porcine coronaryarteries,Circulation 93:423-430,纳入本文作为参考)。Scaffolds were prepared for SEM by rinsing with 10% buffered formalin for 30 seconds and then fixing overnight in 0.1 M sodium cacodylate buffer containing 2.5% glutaraldehyde and 2% PFA. Samples were then washed three times with cacodylate buffer and allowed to wash overnight. Post-fixation treatment was accomplished by treatment with 1% osmium tetroxide (Sigma) in 0.1 M cacodylate buffer, followed by ethanol dehydration (30% ethanol, 50%, 70%, 85%, 95%, 100%, 100%) followed by critical point drying withCO2 . After drying, the samples were sprayed with gold and observed under SEM. ("Reduction in thrombotic events with heparin-coated Palmaz-Schatz stent in normal porcine coronary arteries, Circulation 93: 423-430 , incorporated herein by reference).
用10%福尔马林缓冲液冲洗用于组织学分析的支架区段30秒,然后用10%福尔马林缓冲液固定直至处理。从各支架上切取以下5个切片;临近支架1mm处、支架临近末端的1mm处、支架中段处、支架远端1mm处和支架末端1mm处。用苏木精&伊红(HE)以及三色弹性蛋白染色切片。Scaffold segments for histological analysis were rinsed with 10% buffered formalin for 30 s and then fixed with 10% buffered formalin until processing. The following 5 slices were cut from each stent; 1 mm adjacent to the stent, 1 mm near the end of the stent, mid-stent, 1 mm distal to the stent, and 1 mm to the end of the stent. Sections were stained with hematoxylin & eosin (HE) and trichrome elastin.
图14A-14G为植入后1小时(图14A和14B)和48小时(图14C-14G)在扫描电镜下观察到的取出支架。显微照片清晰地显示了用葡聚糖/抗CD34抗体涂覆的支架(14B,14E-G)已捕获了内皮祖细胞,这点可由与用葡聚糖涂覆的对照相比之下,48小时切片在较高倍(400X)下细胞呈纺锤形外观(14A、14C和14D)。Figures 14A-14G are SEM observations of removed stents at 1 hour (Figures 14A and 14B) and 48 hours (Figures 14C-14G) after implantation. Micrographs clearly show that scaffolds coated with dextran/anti-CD34 antibody (14B, 14E-G) have captured EPCs, as compared to controls coated with dextran, Cells at higher magnification (400X) had a spindle-shaped appearance (14A, 14C, and 14D) in the 48-hour section.
猪冠状动脉分离物的横切片也显示与对照相比(空白不锈钢14H和14I;用葡聚糖涂覆的14J和14K),用葡聚糖-抗CD34抗体涂覆(14L、14M)使得内膜过度增生(动脉平滑肌层的厚度)受到显著的抑制。用富勒烯涂覆的支架移植物较空白对照不锈钢支架而言更好地抑制了内膜过度增生(如图13B-13D所示)。Transverse sections of porcine coronary artery isolates also showed that coating with dextran-anti-CD34 antibody (14L, 14M) made endogenous Membrane hyperplasia (thickness of the arterial smooth muscle layer) was significantly inhibited. Fullerene-coated stent-grafts inhibited intimal hyperplasia better than placebo stainless steel stents (as shown in FIGS. 13B-13D ).
图15A和15B分别为经过48小时后取出的18mm长的用葡聚糖-血浆涂覆但表面无抗体的支架分离物和用葡聚糖-血浆涂覆的抗CD34抗体支架的共聚焦显微照片。所述支架曾植入雄性约克幼猪的冠状动脉。对取出的支架进行免疫组化处理和VEGFR-2染色,然后用FITC偶联的二抗处理和在共聚焦显微镜下研究。图15B和15C显示了与不含抗体的支架完全缺乏内皮相比,含有抗体的支架被内皮细胞所覆盖,这点由该切片的绿色荧光所证实(图15A)。Figures 15A and 15B are the confocal microscopy of the 18 mm long dextran-plasma-coated but surface-free scaffold isolate and the anti-CD34 antibody scaffold coated with dextran-plasma that were taken out after 48 hours, respectively photo. The stents had been implanted in the coronary arteries of male Yorkie piglets. The removed scaffolds were processed for immunohistochemistry and stained for VEGFR-2, then treated with FITC-conjugated secondary antibodies and studied under a confocal microscope. Figures 15B and 15C show that the antibody-containing scaffold was covered by endothelial cells, as evidenced by the green fluorescence of the section (Figure 15A), compared to the complete absence of endothelium in the scaffold without antibody.
实施例6Example 6
将内皮生长因子掺入施加于支架的固定了抗体的基质:以下描述了将抗内皮祖细胞表面抗原的抗体固定于施加于血管内支架的生物相容性基质的步骤,内皮生长因子随后吸附于支架以促进循环内皮祖细胞的附着并在与血液接触时成熟为功能性内皮。Incorporation of endothelial growth factor into the antibody-immobilized matrix applied to the stent: The following describes the steps for immobilizing antibodies against endothelial progenitor cell surface antigens on a biocompatible matrix applied to the intravascular stent, and endothelial growth factor is subsequently adsorbed on Scaffold to facilitate attachment of circulating endothelial progenitor cells and maturation into functional endothelium upon contact with blood.
基质沉积:采用了本领域技术人员已知的方法,用等离子沉积技术处理不锈钢支架以在支架表面导入氨基官能性。采用称为水溶性碳二亚胺偶联化学法的标准方法,在水性条件下通过活化的葡聚糖(CMDX)羧基将羧基官能性葡聚糖层结合于沉积在支架上的氨基官能层,在所述水性条件下位于等离子沉积层上的氨基在等离子层和官能性CDMX间形成酰胺键。Matrix Deposition: Using methods known to those skilled in the art, the stainless steel stent was treated with plasma deposition techniques to introduce amino functionalities on the surface of the stent. The carboxyl-functional dextran layer is bound to the amino-functional layer deposited on the scaffold via activated dextran (CMDX) carboxyl groups under aqueous conditions using a standard method known as water-soluble carbodiimide coupling chemistry, Amino groups located on the plasma-deposited layer under the aqueous conditions form amide bonds between the plasma layer and the functional CDMX.
抗体的固定化:通过在含水水溶性碳二亚胺化学试剂的酸性缓冲溶液中孵育,将抗内皮祖细胞表面抗原的抗体(例如鼠单克隆抗人CD34)共价偶联于用CDMX涂覆的支架。Immobilization of antibodies: Covalently couple antibodies against endothelial progenitor cell surface antigens (e.g., mouse monoclonal anti-human CD34) to CDMX-coated bracket.
生长因子的吸附:将单克隆抗人CD34固定在施加于支架的CMDX基质后,将所述装置孵育在含有适宜浓度的内皮生长因子(例如,血管生成素2)的水溶液中,使得生长因子吸附入CMDX基质。用生理盐水溶液冲洗经处理的装置,并储存于叠氮化钠保存剂中。Adsorption of growth factors: After immobilization of monoclonal anti-human CD34 on the CMDX matrix applied to the scaffold, the device was incubated in an aqueous solution containing an appropriate concentration of endothelial growth factor (eg, angiopoietin 2) to allow growth factor adsorption into the CMDX matrix. Treated devices were rinsed with saline solution and stored in sodium azide preservative.
采用标准血管造影技术,当将上述装置植入猪冠状动脉并接触人血液时,产生了增强循环内皮祖细胞捕获和附着于经处理的或涂覆支架表面、并促进细胞成熟为功能性内皮组织的效果。功能性内皮组织的快速建立可降低装置造成的血栓形成和调节内膜过度增生的程度。Using standard angiographic techniques, implantation of the above-described device into porcine coronary arteries and exposure to human blood produced enhanced capture and attachment of circulating endothelial progenitor cells to treated or coated stent surfaces and enhanced cell maturation into functional endothelial tissue Effect. Rapid establishment of functional endothelial tissue reduces device-induced thrombosis and modulates the extent of intimal hyperplasia.
实施例7Example 7
将内皮生长因子和抗体固定于支架上:以下描述了将抗内皮祖细胞细胞表面抗原的抗体和生长因子固定在施加于血管内支架的生物相容性基质上,从而在与血液接触时促进循环内皮祖细胞的附着和成熟为功能性内皮组织步骤。Immobilization of Endothelial Growth Factor and Antibodies to Scaffold: The following describes the immobilization of antibodies against endothelial progenitor cell surface antigens and growth factors on a biocompatible matrix applied to an intravascular stent to facilitate circulation when in contact with blood Attachment and maturation of endothelial progenitor cells into functional endothelial tissue steps.
基质沉积:基质沉积使用了本领域技术人员已知的方法,用等离子沉积技术处理不锈钢支架以在支架表面上导入氨基官能性。采用称为水溶性碳二亚胺偶联化学法的标准方法,在水性条件下通过活化葡聚糖(CMDX)羧基将羧基官能性葡聚糖层结合于沉积在支架上的氨基官能层,在所述水性条件下位于等离子沉积层上的氨基在等离子层和官能性CDMX间形成酰胺键。Matrix Deposition: Matrix deposition employs methods known to those skilled in the art, by treating stainless steel stents with plasma deposition techniques to introduce amino functionality on the surface of the stents. Using a standard method known as water-soluble carbodiimide coupling chemistry, the carboxyl-functional dextran layer was bound to the amino-functional layer deposited on the scaffold by activating the carboxyl groups of dextran (CMDX) under aqueous conditions. Amino groups located on the plasma-deposited layer under the aqueous conditions form amide bonds between the plasma layer and the functional CDMX.
抗体和生长因子的固定化:通过在酸性条件下,将等摩尔浓度的抗内皮祖细胞表面抗原的抗体(例如鼠单克隆抗人CD34)和内皮生长因子(例如,血管生成素-2)孵育于水溶性碳二亚胺试剂溶液中,使其与用CDMX涂覆的支架共价偶联。用生理盐水溶液冲洗经处理的装置,并储存于叠氮化钠保存剂中。Immobilization of antibodies and growth factors: by incubating equimolar concentrations of antibodies against EPC surface antigens (e.g., mouse monoclonal anti-human CD34) and endothelial growth factors (e.g., angiopoietin-2) under acidic conditions It was covalently coupled to the CDMX-coated scaffold in a solution of a water-soluble carbodiimide reagent. Treated devices were rinsed with saline solution and stored in sodium azide preservative.
采用标准血管造影技术,当将上述装置植入猪冠状动脉并接触人血液时,产生了增强循环内皮祖细胞捕获和附着于经处理的或涂覆支架表面、并促进细胞成熟为功能性内皮组织的效果。功能性内皮组织的快速建立可降低装置造成的血栓形成和调节内膜过度增生的程度。Using standard angiographic techniques, implantation of the above-described device into porcine coronary arteries and exposure to human blood produced enhanced capture and attachment of circulating endothelial progenitor cells to treated or coated stent surfaces and enhanced cell maturation into functional endothelial tissue Effect. Rapid establishment of functional endothelial tissue reduces device-induced thrombosis and modulates the extent of intimal hyperplasia.
实施例8Example 8
支架的小分子功能化:如实施例1所述分离得到内皮祖细胞。将细胞接种于纤连蛋白覆盖的玻片并使其在EBM-2培养基中生长7天。固定细胞并用碘化丙锭(PI)和FITC偶联的内皮细胞特异性凝集素染色(Ulex Europaeus Ueal)。这些试验的结果示于图16A和16B中。这些附图显示了内皮祖细胞结合于纤连蛋白涂覆的玻片,且这些细胞在它们的表面上表达有此凝集素的配体。Small molecule functionalization of scaffolds: EPCs were isolated as described in Example 1. Cells were seeded on fibronectin-coated slides and grown in EBM-2 medium for 7 days. Cells were fixed and stained with propidium iodide (PI) and FITC-conjugated endothelial cell-specific lectins (Ulex Europaeus Ueal). The results of these experiments are shown in Figures 16A and 16B. These figures show the binding of endothelial progenitor cells to fibronectin-coated slides and that these cells express ligands for this lectin on their surface.
实施例9Example 9
用编码血管舒张化合物和独特的细胞表面标记(截短的MHC-I)的双顺反子载体转染猪内皮祖细胞(EPC)。MHC-I可被固定于血管内修复体上的特异性抗体识别。将抗体涂覆的支架植入猪冠状动脉中,然后将遗传改变的EPC植入猪。由于抗体-抗原相互作用,EPC被捕获在经涂覆的支架上,并在支架支柱上形成内皮细胞单层。被捕获的细胞可分泌过表达的血管舒张剂、增加远端血流量和触发正性再塑。Porcine endothelial progenitor cells (EPCs) were transfected with a bicistronic vector encoding a vasodilator compound and a unique cell surface marker (truncated MHC-I). MHC-I can be recognized by specific antibodies immobilized on endovascular prostheses. Antibody-coated stents were implanted into porcine coronary arteries, and genetically altered EPCs were then implanted into pigs. Due to antibody-antigen interactions, EPCs were captured on the coated scaffolds and formed endothelial cell monolayers on the scaffold struts. Trapped cells secrete overexpressed vasodilators, increasing distal blood flow and triggering positive remodeling.
质粒的选择:Miltenyi Biotec(德国)开发出了含有pMASCSKk质粒载体的MACSelect K System。所述pMACSK.II质粒是一种含有多克隆位点(MCS)的双顺反子载体(5229bp),在其中克隆有编码前列环素合酶的cDNA以及编码鼠MHC I型分子H-2K的基因。开发该系统是为了选择用于转染的细胞,以截短的MHC分子作为选择标记。天然H-2K的表达限于某些稀有的鼠品系中(例如,AKRiJA或CBNJ),因此针对H-2Kk表面蛋白的单克隆抗体(Miltenyi Biotec)应基本上不会与其它表面抗原发生无关反应。Plasmid selection : Miltenyi Biotec (Germany) developed the MACSelect K System containing the pMASCSKk plasmid vector. The pMACSK.II plasmid is a bicistronic vector (5229bp) containing a multiple cloning site (MCS), in which the cDNA encoding prostacyclin synthase and the cDNA encoding mouse MHC type I molecule H-2K are cloned. Gene. This system was developed to select cells for transfection with truncated MHC molecules as selection markers. Expression of native H-2K is limited to certain rare murine strains (e.g., AKRiJA or CBNJ), so monoclonal antibodies (Miltenyi Biotec) directed against the H-2Kk surface protein should be largely free of unrelated reactions with other surface antigens .
评估与全血的交叉反应性:为了确保抗H-2Kk抗体不与猪全血中的细胞组分发生交叉反应,使全血与FITC偶联的抗H-2K抗体反应,并进行全血FACS分析(BeckmanCoulter Cytomics FC 500)。用表达H-2Kk表面抗原的鼠脾成纤维细胞株AKRIJASp(美国典型培养物保藏中心,American Type Culture Collection,ATCC)“强化”(“spiked”)的全血作为阳性对照。Assessingcross-reactivity with whole blood : To ensure that anti-H-2Kk antibodies do not cross-react with cellular components in porcine whole blood, whole blood was reacted with FITC-conjugated anti-H-2K antibody and whole blood FACS analysis (Beckman Coulter Cytomics FC 500). Whole blood "spiked" from the murine spleen fibroblast cell line AKRIJASp (American Type Culture Collection, ATCC) expressing the H-2Kk surface antigen was used as a positive control.
成纤维细胞的培养:将AKR/JA.Sp成纤维细胞培养于未经涂覆的T-75塑料瓶中(Sarstedt,Montreal),培养条件为:37℃,5%CO2,培养基为含有4mM L-谷胺酰胺、4500mg/L葡萄糖、1mM丙酮酸钠、1500mg/L碳酸氢钠和10%胎牛血清的达尔伯克(氏)改良伊格尔(氏)培养基(DMEM)。用胰蛋白酶/EDTA(Invitrogen)进行细胞分离。通过免疫组织化学分析,使用荧光标记的H-2Kk抗体来证实有H-2Kk的表达。简而言之,以0/5×106个细胞/cm2将细胞接种入2孔未经涂覆的带腔玻片(chamber slide)中。在第1、2、3和4天时,用2%多聚甲醛固定培养物,并用FITC偶联的H-2K抗体(Miltenyi Biotec,德国)和核标记碘化丙锭(PI)(Vectashield Mounting Medium,Vector Laboratories)染色。采用共聚焦显微镜(Nikon Eclipse E800-BioradRadiance 2 100)进行分析和定量。将人成纤维细胞用作阴性对照。Fibroblast culture: AKR/JA.Sp fibroblasts were cultured in uncoated T-75 plastic bottles (Sarstedt, Montreal), the culture conditions were: 37°C, 5% CO2 , and the medium contained Dulbecco's Modified Eagle's Medium (DMEM) with 4 mM L-Glutamine, 4500 mg/L Glucose, 1 mM Sodium Pyruvate, 1500 mg/L Sodium Bicarbonate and 10% Fetal Calf Serum. Cell separation was performed with trypsin/EDTA (Invitrogen). Expression of H-2Kk was confirmed by immunohistochemical analysis using a fluorescently labeled H-2K kantibody . Briefly, cells were seeded into 2-well uncoated chamber slides at 0/5 x106 cells/cm2 . On
不粘附细胞的分析:H-2Kk表面蛋白的保留是不附着形式的AKRIJA.Sp细胞的特征,用于证实血液存在时采用这一系统的可行性。如上所述将细胞培养于未经涂覆的T-75瓶中。在第4天用胰蛋白酶/EDTA使粘附的细胞脱落,并用FITC偶联的H-2Kk抗体和FACS分析(Beckman Coulter Cytomics FC500)测定表达H-2Kk表面蛋白的细胞数量。以FITC标记的鼠IGg2α同种型作为阴性对照。Analysis of non-adherent cells: Retention of the H-2Kk surface protein is characteristic of the non-adherent form of AKRIJA.Sp cells and was used to demonstrate the feasibility of this system in the presence of blood. Cells were grown in uncoated T-75 flasks as described above. Adherent cells were detached on day 4 with trypsin/EDTA, and the number of cells expressing the H-2K kappa surface protein was determined using FITC-conjugated H-2Kkappa antibody and FACS analysis (Beckman Coulter Cytomics FC500). FITC-labeled murine IGg2α isoform served as a negative control.
质粒的构建:利用多克隆位点中的BamHl和HindIII限制性序列将编码前列环素合酶的cDNA克隆入双顺反子载体pMACS Kk.II(Miltenyi Biotec,德国)。使用了位于质粒构建物中的含有前列环素合酶基因和pVAX-1的1153碱基对的cDNA。在选择剂氨苄西林(50ng/ml)存在下进行HG70大肠杆菌的转化。Plasmid construction: The cDNA encoding prostacyclin synthase was cloned into the bicistronic vector pMACS Kk .II (Miltenyi Biotec, Germany) using the BamH1 and HindIII restriction sequences in the multiple cloning site. A 1153 base pair cDNA containing the prostacyclin synthase gene and pVAX-1 in a plasmid construct was used. Transformation of HG70 E. coli was performed in the presence of the selective agent ampicillin (50 ng/ml).
用于人α-CGRP的全长cDNA获自质粒载体pPCR-Script Amp SK(+)中的开放生物系统(Open Biosystems,分类号#MHS 1768-9 1441 17;Huntsville AL)。然后将该片段与BamHl/EcoRl连接入双顺反子质粒载体pMACS K.II。转化JM109大肠杆菌以获得大量所述质粒。Full-length cDNA for human α-CGRP was obtained from Open Biosystems (Catalog #MHS 1768-9 1441 17; Huntsville AL) in the plasmid vector pPCR-Script Amp SK(+). The fragment was then connected to the bicistronic plasmid vector pMACS K.II with BamH1/EcoR1. JM109 E. coli was transformed to obtain a large amount of the plasmid.
EPC的转染:用Ficoll梯度离心法从猪全血中富集猪单核细胞,并用上述的富集培养基分离EPC。培养7天后,用核穿孔的方法(Amaxa Nucleofector,德国),用含有转移基因的双顺反子质粒载体转染EPC,所述转移基因包含α-CGRP或前列环素合酶。利用pVAXt质粒中的报告基因和内皮一氧化氮合酶(eNOS),获得了>70%的EPC电穿孔转染效率(数据未示出)。对成功转染并表达H-2Kk表面蛋白的EPC进行纯化,并用MACS死细胞去除试剂盒(MACS Dead cell removal kit)、MACSelect Kk微珠(MACSelect KkMicroBead)和MS分离柱(MS Separation Column)(Miltenyi Biotec)进行分离。MACSelect Kk微珠是可生物降解的,其在24小时内随细胞培养而消失。Transfection of EPCs: Porcine mononuclear cells were enriched from whole porcine blood by Ficoll gradient centrifugation, and EPCs were isolated with the above-mentioned enrichment medium. After 7 days of culture, the EPCs were transfected with a bicistronic plasmid vector containing a transgene comprising α-CGRP or prostacyclin synthase by nuclear perforation (Amaxa Nucleofector, Germany). Using the reporter gene and endothelial nitric oxide synthase (eNOS) in the pVAXt plasmid, >70% EPC electroporation transfection efficiencies were obtained (data not shown). EPCs successfully transfected and expressing H-2Kk surface protein were purified, and were purifiedwith MACS Dead cell removal kit, MACSelect K kMicroBead and MS Separation Column (MS Separation Column) (Miltenyi Biotec) for separation. MACSelect Kk beads are biodegradable and disappear with cell culture within 24 hours.
血管舒张剂表达的测定:Determination of vasodilator expression:
前列环素合酶活性的测定:在转染2天后,保持培育经转染的EPC。更换培养基,并根据生产商的说明用放射免疫分析法(Amersham Corp.)测定培养基中前列环素合酶代谢物6-酮-前列腺素F1α(6-keto-PGFIcu)的水平来评估前列环素合酶的活性。Determination of prostacyclin synthase activity: 2 days after transfection, the transfected EPCs were kept incubating. The medium was changed and prostacyclin was assessed by measuring the level of the prostacyclin synthase metabolite 6-keto-prostaglandin F1α (6-keto-PGFIcu) in the medium by radioimmunoassay (Amersham Corp.) according to the manufacturer's instructions Cyclone synthase activity.
α-CGRP活性的测定:用免疫组化法色试剂盒(Bachem USA)测定转染细胞中α-CGRP的表达。在-10℃下用甲醇固定培养3天的经转染的EPC5分钟。洗涤所述细胞并风干。将固定的细胞在0.5%过氧化氢的PBS溶液中孵育7分钟以灭活内源性过氧化物活性。将细胞孵育于血清封闭液中20分钟以封闭非特异性结合。然后用抗α-CGRP一抗(兔单克隆,Bachem)以三种稀释度:1∶100、1∶200和1∶500处理细胞2小时。然后洗涤玻片,使其接触生物素化的二抗30分钟。然后冲洗细胞并用HRP-链亲和素复合物处理30分钟。用PBS洗涤后,使细胞接触底物-色原体混合物3分钟。加入去离子水以终止反应。用Mayer氏苏木精复染玻片3分钟。然后用自来水冲洗玻片,置于PBS中直至变蓝色,用蒸馏水漂洗。再用95%和100%乙醇和二甲苯对玻片进行脱水。在玻片上盖上盖玻片并在光镜下检查。Determination of α-CGRP activity: The expression of α-CGRP in the transfected cells was determined by immunohistochemistry kit (Bachem USA). Transfected EPCs cultured for 3 days were fixed with methanol for 5 min at -10°C. The cells were washed and air dried. Fixed cells were incubated in 0.5% hydrogen peroxide in PBS for 7 min to inactivate endogenous peroxide activity. Cells were incubated in serum blocking solution for 20 minutes to block non-specific binding. Cells were then treated with anti-α-CGRP primary antibody (rabbit monoclonal, Bachem) at three dilutions: 1:100, 1:200 and 1:500 for 2 hours. Slides were then washed and exposed to biotinylated secondary antibody for 30 minutes. Cells were then washed and treated with HRP-streptavidin complex for 30 minutes. After washing with PBS, cells were exposed to the substrate-chromogen mixture for 3 minutes. Deionized water was added to terminate the reaction. Slides were counterstained with Mayer's hematoxylin for 3 minutes. Slides were then rinsed with tap water, placed in PBS until blue and rinsed with distilled water. Slides were then dehydrated with 95% and 100% ethanol and xylene. Cover the slides with a coverslip and examine under a light microscope.
用抗体涂覆的支架:如前所述的用葡聚糖和抗H-2Kk抗体涂覆不锈钢支架(9mm长)。Scaffolds coated with antibodies: Stainless steel scaffolds (9 mm long) were coated with dextran and anti-H-2K kappa antibody as described previously.
体内细胞捕获:所有试验均在雄性约克幼猪中(>30kg)中进行。通过在左颈动脉上进行的动脉切开术获得动脉通路。在冠状动脉内给予200pg硝化甘油后,获得冠脉血管造影,并进行在线定量冠状动脉血管造影测定。以1.1∶1的支架对血管比将支架随机在LAD回旋支或右冠状动脉的近端区段展开。一经植入后,就经血管内给予200pg硝化甘油。然后进行血管内超声(IVUS)以测定血管口径,以展开支架的远侧支和远端边缘作为远端和近端参比。利用原型串联气囊导管(CordisCorporation)完成经转染细胞的给予,所述细胞用编码前列环素合酶或α-CGRP的双顺反子载体转染。该导管由位于邻近装置远端的两个高度贴合的气囊构成,通过同一个充气孔膨胀这两个气囊。一旦膨胀后,分离两个气囊间1.0cm长的血管区段,以产生一个局部灌注腔。通过中央腔提供远端血流,并灌注入溶液或通过两个分离的腔将溶液抽入整个腔。灌注腔终止于远端气囊的附近,而排出腔则终止于近端气囊的附近的一个开口。将串联气囊导管向前伸到植入支架的位置,并将气囊膨胀到25psi(1.7个大气压)。通过灌注孔递送盐水,直至分离的区段中不含血液。对植入支架的动脉区段进行随机分组以接受盐水灌注或细胞递送。在10分钟内,以200pL/分钟的灌注速率给予总共为3×10的EPC的2ml细胞悬液,然后孵育10分钟。然后闭合实施动脉切开术的部位,使动物复原。在细胞处理后饲养动物28天。共处理了34只动物(10只为盐水对照,14只为前列环素合酶,14只为α-CGRP)。递送细胞1小时后将每组的两只动物杀死。分离植入支架的区段,并通过以下步骤准备用于SEM的经冲洗的植入支架的动脉:在10%福尔马林缓冲液PBS中固定30秒,然后进一步在含有2.5%戊二醛(BDH Inc.)和2%PFA的0.1M二甲胂酸钠缓冲液(Sigma)中固定过夜。通过以下步骤完成固定后处理:用1%四氧化锇(Sigma)的0.1M砷酸盐缓冲液处理,然后用乙醇梯度脱水,随后用CO2进行临界点干燥。干燥后,对样品进行喷金并在扫描电镜(SEM)下观察是否存在结合于支架支柱的细胞。在植入支架后的第5天处死前列环素合酶组的两只动物和α-CGRP组的两只动物。将取出的含植入支架的动脉区段置于10%福尔马林/PBS溶液中,直至进行标准组织化学分析。从各支架上切取5个切片;临近支架1mm处、支架临近末端的1mm处、支架中段处、支架远端1mm处和支架末端1mm处。用苏木精&伊红(HE)以及三色弹性蛋白对切片进行染色。测定炎症[科诺维奇指数,Kornowski Score(0-3)]指数以评估对输入细胞的排异迹象。在进行指数过程后(约28天),麻醉动物并通过右冠状动脉血管切开术进行冠状动脉血管造影术。进行定量冠状动脉血管造影术,利用IVUS进行血管询问,用标准临床算法记录血管口径的变化。In vivo cell capture: All experiments were performed in male Yorkie piglets (>30 kg). Arterial access was obtained by arteriotomy performed on the left carotid artery. Following intracoronary administration of 200 pg nitroglycerin, coronary angiography was obtained and online quantitative coronary angiography was performed. The stents were randomly deployed in the LAD circumflex branch or the proximal segment of the right coronary artery with a stent-to-vessel ratio of 1.1:1. Once implanted, 200 pg of nitroglycerin was administered intravascularly. Intravascular ultrasound (IVUS) was then performed to measure vessel caliber, with the distal branch and distal edge of the deployed stent as distal and proximal references. Administration of transfected cells transfected with bicistronic vectors encoding prostacyclin synthase or α-CGRP was accomplished using a prototype tandem balloon catheter (Cordis Corporation). The catheter consists of two highly-fitting balloons located adjacent the distal end of the device that are inflated through the same inflation hole. Once inflated, a 1.0 cm long vessel segment between the two balloons was isolated to create a local perfusion lumen. Distal blood flow is provided through a central lumen and solution is infused or pumped across the lumen through two separate lumens. The perfusion lumen terminates in the vicinity of the distal balloon and the drain lumen terminates in an opening in the vicinity of the proximal balloon. The tandem balloon catheter was advanced to the site of the stent and the balloon was inflated to 25 psi (1.7 atmospheres). Saline is delivered through the perfusion hole until the isolated segment is free of blood. Stented arterial segments were randomized to receive saline perfusion or cell delivery. A total of 3 x 10 EPCs in 2 ml of cell suspension were administered at a perfusion rate of 200 pL/min over 10 minutes, followed by incubation for 10 minutes. The site of arteriotomy was then closed and the animal was allowed to recover. Animals were maintained for 28 days after cell treatment. A total of 34 animals were treated (10 saline controls, 14 prostacyclin synthase, 14 α-CGRP). Two animals from each group were sacrificed 1 hour after cell delivery. Isolate the stented segment and prepare the rinsed stented artery for SEM by fixing for 30 s in 10% buffered formalin PBS, followed by further fixation in 2.5% glutaraldehyde containing 2.5% glutaraldehyde. (BDH Inc.) and 2% PFA in 0.1 M sodium cacodylate buffer (Sigma) overnight. Post-fixation treatment was accomplished by treatment with 1% osmium tetroxide (Sigma) in 0.1 M arsenate buffer, followed by dehydration with an ethanol gradient followed by critical point drying with CO2. After drying, the samples were sprayed with gold and observed under a scanning electron microscope (SEM) for the presence of cells bound to the scaffold struts. Two animals in the prostacyclin synthase group and two animals in the α-CGRP group were sacrificed on day 5 after implantation of the stent. The removed arterial segments containing implanted stents were placed in 10% formalin/PBS solution until standard histochemical analysis. Five slices were cut from each stent; 1 mm proximal to the stent, 1 mm proximal to the end of the stent, mid-stent, 1 mm distal to the stent, and 1 mm to the end of the stent. Sections were stained with hematoxylin & eosin (HE) and trichrome elastin. Inflammation [Kornowski Score (0-3)] index was measured to evaluate the signs of rejection of the input cells. Following the exponential procedure (approximately 28 days), animals were anesthetized and coronary angiography was performed by right coronary angiotomy. Quantitative coronary angiography was performed, vascular interrogation was performed using IVUS, and changes in vessel caliber were recorded using standard clinical algorithms.
实施例10Example 10
用于血管重构的体外哺乳动物细胞转染:采用电穿孔的方法将双顺反子质粒转染到内皮祖细胞中,所述质粒包含编码负责腺苷产生的蛋白质和前列腺特异性细胞膜蛋白的基因。两种基因各自受它们自己启动子的控制,从而使得基因得以组成性表达。In vitro transfection of mammalian cells for vascular remodeling: Endothelial progenitor cells were transfected with a bicistronic plasmid containing genes encoding proteins responsible for adenosine production and prostate-specific membrane proteins by electroporation Gene. Both genes are each under the control of their own promoters, allowing constitutive expression of the genes.
用类似于上述的方法构建以下载体,所述载体包含编码前列腺特异性膜蛋白和其天然启动子的基因,以及编码VEGF的基因,这两种基因在同一表达载体中呈串联排列。可将该质粒构建物用于转染实施例9中所述的用于患者的细胞,哺乳动物细胞。The following vector was constructed by a method similar to that described above, the vector comprising the gene encoding the prostate-specific membrane protein and its natural promoter, and the gene encoding VEGF arranged in tandem in the same expression vector. This plasmid construct can be used to transfect the cells described in Example 9 for use in patients, mammalian cells.
序列表sequence listing
<110>奥勃斯医学技术股份有限公司<110> Aobosi Medical Technology Co., Ltd.
(ORBUS MEDICAL TECHNOLOGIES,INC.)(ORBUS MEDICAL TECHNOLOGIES, INC.)
the
<120>具有可捕获遗传改变的细胞的涂层的医疗装置及其使用方法<120> Medical device with coating capable of trapping genetically altered cells and method of use thereof
the
<130>ORB-101(PCT)<130>ORB-101 (PCT)
the
<140>TBA<140>TBA
<141>2005-04-29<141>2005-04-29
the
<150>US60/566,829<150>US60/566,829
<151>2004-04-30<151>2004-04-30
the
<150>US 10/835,767<150>US 10/835,767
<151>2004-04-30<151>2004-04-30
the
<160>4<160>4
the
<170>PatentIn version 3.3<170>PatentIn version 3.3
the
<210>1<210>1
<211>21<211>21
<212>DNA<212>DNA
<213>智人(Homo sapiens)<213> Homo sapiens
the
<220><220>
<221>primer_bind<221>primer_bind
<222>(1)..(21)<222>(1)..(21)
<223>内皮细胞来源的一氧化氮合酶序列的有义PCR引物<223> Sense PCR Primers for Endothelial Cell-Derived Nitric Oxide Synthase Sequence
the
<400>1<400>1
ttccggggat tctggcagga g 21ttccggggat tctggcagga g 21
the
<210>2<210>2
<211>21<211>21
<212>DNA<212>DNA
<213>智人(Homo sapiens)<213> Homo sapiens
the
<220><220>
<221>primer_bind<221>primer_bind
<222>(1)..(21)<222>(1)..(21)
<223>内皮细胞来源的一氧化氮合酶序列的反义PCR引物<223> Antisense PCR Primers for Endothelial Cell-Derived Nitric Oxide Synthase Sequence
the
<400>2<400>2
gccatggtaa catcgccgca g 21gccatggtaa catcgccgca g 21
the
<210>3<210>3
<211>24<211>24
<212>DNA<212> DNA
<213>智人(Homo sapiens)<213> Homo sapiens
the
<220><220>
<221>primer_bind<221>primer_bind
<222>(1)..(24)<222>(1)..(24)
<223>磷酸甘油醛脱氢酶基因的有义PCR引物<223> Sense PCR Primers for Glyceraldehyde Phosphate Dehydrogenase Gene
the
<400>3<400>3
ctctaaggct gtgggcaagg tcat 24ctctaaggct gtgggcaagg tcat 24
the
<210>4<210>4
<211>24<211>24
<212>DNA<212>DNA
<213>智人(Homo sapiens)<213> Homo sapiens
the
<400>4<400>4
gagatccaccaccctgttgc tgta 24gagatccaccaccctgttgc tgta 24
Claims (32)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US56682904P | 2004-04-30 | 2004-04-30 | |
| US10/835,767 | 2004-04-30 | ||
| US60/566,829 | 2004-04-30 | ||
| US10/835,767US9522217B2 (en) | 2000-03-15 | 2004-04-30 | Medical device with coating for capturing genetically-altered cells and methods for using same |
| PCT/US2005/015555WO2005107817A2 (en) | 2004-04-30 | 2005-04-29 | Medical device with coating for capturing genetically-altered cells and methods of using same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201110246552.7ADivisionCN102363051B (en) | 2004-04-30 | 2005-04-29 | Medical device with coating for capturing genetically-altered cells and methods of using same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101132694A CN101132694A (en) | 2008-02-27 |
| CN101132694Btrue CN101132694B (en) | 2011-10-05 |
Family
ID=39129760
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201110246552.7AExpired - Fee RelatedCN102363051B (en) | 2004-04-30 | 2005-04-29 | Medical device with coating for capturing genetically-altered cells and methods of using same |
| CN200580012581XAExpired - Fee RelatedCN101132694B (en) | 2004-04-30 | 2005-04-29 | Medical devices with coatings capable of trapping genetically altered cells and methods of use thereof |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201110246552.7AExpired - Fee RelatedCN102363051B (en) | 2004-04-30 | 2005-04-29 | Medical device with coating for capturing genetically-altered cells and methods of using same |
Country Status (2)
| Country | Link |
|---|---|
| CN (2) | CN102363051B (en) |
| IL (1) | IL176853A0 (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5985040B2 (en) | 2013-02-28 | 2016-09-06 | 株式会社日立ハイテクノロジーズ | Data analysis apparatus and method |
| US11405197B2 (en) | 2020-06-08 | 2022-08-02 | Google Llc | Security token expiration using signing key rotation |
| CN113663125B (en)* | 2021-08-04 | 2023-04-11 | 青岛大学 | Preparation method of urine-derived stem cell capturing scaffold, urine-derived stem cell capturing scaffold and application thereof |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6676931B2 (en)* | 1997-10-01 | 2004-01-13 | Novadel Pharma Inc. | Buccal, polar and non-polar spray or capsule |
- 2005
- 2005-04-29CNCN201110246552.7Apatent/CN102363051B/ennot_activeExpired - Fee Related
- 2005-04-29CNCN200580012581XApatent/CN101132694B/ennot_activeExpired - Fee Related
- 2006
- 2006-07-13ILIL176853Apatent/IL176853A0/enunknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6676931B2 (en)* | 1997-10-01 | 2004-01-13 | Novadel Pharma Inc. | Buccal, polar and non-polar spray or capsule |
Non-Patent Citations (1)
| Title |
|---|
| Takayuki Asahara,et al.Isolation of Putative Progenitor Endothelial Cells forAngiogenesis.Science275.1997,275964-967.* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101132694A (en) | 2008-02-27 |
| CN102363051B (en) | 2014-07-02 |
| CN102363051A (en) | 2012-02-29 |
| IL176853A0 (en) | 2006-10-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2563329C (en) | Medical device with coating for capturing genetically-altered cells and methods for using same | |
| US9522217B2 (en) | Medical device with coating for capturing genetically-altered cells and methods for using same | |
| CN100455275C (en) | Medical device coated with a coating that promotes endothelial cell adhesion and differentiation | |
| US20070191932A1 (en) | Medical device with coating for capturing genetically-altered cells and methods for using same | |
| US20070042017A1 (en) | Medical device with coating that promotes endothelial cell adherence and differentiation | |
| JP5675744B2 (en) | Coating that promotes endothelial cell adhesion | |
| US20070196422A1 (en) | Medical device with coating that promotes endothelial cell adherence and differentiation | |
| CN104013996B (en) | The drug eluting implantable medical device of progenitor endothelial cell capturing | |
| US8088060B2 (en) | Progenitor endothelial cell capturing with a drug eluting implantable medical device | |
| US20070129789A1 (en) | Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device | |
| US20070141107A1 (en) | Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device | |
| US9320829B2 (en) | Progenitor endothelial cell capturing with a drug eluting implantable medical device | |
| US20070128723A1 (en) | Progenitor Endothelial Cell Capturing with a Drug Eluting Implantable Medical Device | |
| CN101132694B (en) | Medical devices with coatings capable of trapping genetically altered cells and methods of use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20111005 Termination date:20170429 | |
| CF01 | Termination of patent right due to non-payment of annual fee |