CN100506973C - Lactobacillus fermentum CMS-H002 and its application - Google Patents
Lactobacillus fermentum CMS-H002 and its applicationDownload PDFInfo
- Publication number
- CN100506973C CN100506973CCNB2006101571871ACN200610157187ACN100506973CCN 100506973 CCN100506973 CCN 100506973CCN B2006101571871 ACNB2006101571871 ACN B2006101571871ACN 200610157187 ACN200610157187 ACN 200610157187ACN 100506973 CCN100506973 CCN 100506973C
- Authority
- CN
- China
- Prior art keywords
- cms
- lactobacillus fermentum
- group
- mice
- food
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 241000186840Lactobacillus fermentumSpecies0.000titleclaimsabstractdescription59
- 229940012969lactobacillus fermentumDrugs0.000titleclaimsdescription47
- 206010009900Colitis ulcerativeDiseases0.000claimsabstractdescription28
- 201000006704Ulcerative ColitisDiseases0.000claimsabstractdescription27
- 235000013305foodNutrition0.000claimsabstractdescription12
- 238000004321preservationMethods0.000claimsabstractdescription10
- 230000036541healthEffects0.000claimsabstractdescription9
- 235000013373food additiveNutrition0.000claimsabstractdescription8
- 239000002778food additiveSubstances0.000claimsabstractdescription8
- 239000003814drugSubstances0.000claimsabstractdescription7
- 210000000664rectumAnatomy0.000claimsabstractdescription5
- 210000001072colonAnatomy0.000claimsdescription20
- 230000000112colonic effectEffects0.000claimsdescription19
- 238000002360preparation methodMethods0.000claimsdescription9
- 208000027866inflammatory diseaseDiseases0.000claimsdescription8
- 239000008194pharmaceutical compositionSubstances0.000claimsdescription8
- 229940079593drugDrugs0.000claimsdescription6
- 235000013361beverageNutrition0.000claimsdescription3
- 239000000203mixtureSubstances0.000abstractdescription2
- 208000019399Colonic diseaseDiseases0.000abstract1
- 208000015815Rectal diseaseDiseases0.000abstract1
- 241000699670Mus sp.Species0.000description36
- TVZRAEYQIKYCPH-UHFFFAOYSA-N3-(trimethylsilyl)propane-1-sulfonic acidChemical compoundC[Si](C)(C)CCCS(O)(=O)=OTVZRAEYQIKYCPH-UHFFFAOYSA-N0.000description23
- 102100039019Nuclear receptor subfamily 0 group B member 1Human genes0.000description23
- NCEXYHBECQHGNR-UHFFFAOYSA-NsulfasalazineNatural productsC1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1NCEXYHBECQHGNR-UHFFFAOYSA-N0.000description21
- 241000792859EnemaSpecies0.000description20
- 239000007920enemaSubstances0.000description20
- 229940095399enemaDrugs0.000description18
- 230000001580bacterial effectEffects0.000description16
- 239000007788liquidSubstances0.000description14
- 241000894006BacteriaSpecies0.000description13
- 230000000694effectsEffects0.000description12
- 238000002474experimental methodMethods0.000description12
- 210000004877mucosaAnatomy0.000description12
- 239000000126substanceSubstances0.000description12
- QTBSBXVTEAMEQO-UHFFFAOYSA-NAcetic acidChemical compoundCC(O)=OQTBSBXVTEAMEQO-UHFFFAOYSA-N0.000description11
- 239000008280bloodSubstances0.000description11
- 210000004369bloodAnatomy0.000description11
- 230000006378damageEffects0.000description11
- 230000035622drinkingEffects0.000description11
- 239000002609mediumSubstances0.000description11
- 239000013642negative controlSubstances0.000description11
- 239000013641positive controlSubstances0.000description11
- 239000008186active pharmaceutical agentSubstances0.000description9
- 239000000843powderSubstances0.000description9
- 206010012735DiarrhoeaDiseases0.000description8
- 101710188689Small, acid-soluble spore protein 1Proteins0.000description8
- 101710188693Small, acid-soluble spore protein 2Proteins0.000description8
- 101710166422Small, acid-soluble spore protein AProteins0.000description8
- 101710166404Small, acid-soluble spore protein CProteins0.000description8
- 101710174019Small, acid-soluble spore protein C1Proteins0.000description8
- 101710174017Small, acid-soluble spore protein C2Proteins0.000description8
- 101710174574Small, acid-soluble spore protein gamma-typeProteins0.000description8
- 102100036407ThioredoxinHuman genes0.000description8
- 239000002207metaboliteSubstances0.000description8
- 241000186000BifidobacteriumSpecies0.000description7
- 210000004027cellAnatomy0.000description7
- 239000012634fragmentSubstances0.000description7
- 210000004969inflammatory cellAnatomy0.000description7
- 239000012086standard solutionSubstances0.000description7
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000description6
- CSNNHWWHGAXBCP-UHFFFAOYSA-LMagnesium sulfateChemical compound[Mg+2].[O-][S+2]([O-])([O-])[O-]CSNNHWWHGAXBCP-UHFFFAOYSA-L0.000description6
- QAOWNCQODCNURD-UHFFFAOYSA-NSulfuric acidChemical compoundOS(O)(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-N0.000description6
- 230000037396body weightEffects0.000description6
- 230000002550fecal effectEffects0.000description6
- 239000008103glucoseSubstances0.000description6
- 230000008595infiltrationEffects0.000description6
- 238000001764infiltrationMethods0.000description6
- 238000000034methodMethods0.000description6
- 239000000047productSubstances0.000description6
- 230000028327secretionEffects0.000description6
- 239000007787solidSubstances0.000description6
- 239000000243solutionSubstances0.000description6
- 208000024891symptomDiseases0.000description6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description5
- 208000032843HemorrhageDiseases0.000description5
- 238000004458analytical methodMethods0.000description5
- 208000034158bleedingDiseases0.000description5
- 230000000740bleeding effectEffects0.000description5
- 208000035861hematocheziaDiseases0.000description5
- 239000006041probioticSubstances0.000description5
- 235000018291probioticsNutrition0.000description5
- 238000012360testing methodMethods0.000description5
- 230000004580weight lossEffects0.000description5
- 208000016261weight lossDiseases0.000description5
- 241000304886BacilliSpecies0.000description4
- HEDRZPFGACZZDS-UHFFFAOYSA-NChloroformChemical compoundClC(Cl)ClHEDRZPFGACZZDS-UHFFFAOYSA-N0.000description4
- UETNIIAIRMUTSM-UHFFFAOYSA-NJacareubinNatural productsCC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)OUETNIIAIRMUTSM-UHFFFAOYSA-N0.000description4
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description4
- 229960000583acetic acidDrugs0.000description4
- 210000000436anusAnatomy0.000description4
- 239000003153chemical reaction reagentSubstances0.000description4
- 201000010099diseaseDiseases0.000description4
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description4
- 230000000968intestinal effectEffects0.000description4
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-NisobutanolChemical compoundCC(C)COZXEKIIBDNHEJCQ-UHFFFAOYSA-N0.000description4
- 238000002955isolationMethods0.000description4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-Nlactic acidChemical compoundCC(O)C(O)=OJVTAAEKCZFNVCJ-UHFFFAOYSA-N0.000description4
- 239000013612plasmidSubstances0.000description4
- 238000010186stainingMethods0.000description4
- NCEXYHBECQHGNR-QZQOTICOSA-NsulfasalazineChemical compoundC1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1NCEXYHBECQHGNR-QZQOTICOSA-N0.000description4
- 229960001940sulfasalazineDrugs0.000description4
- 239000000725suspensionSubstances0.000description4
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N100676-05-9Natural productsOC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1OWEGMIWEEQEYGQ-UHFFFAOYSA-N0.000description3
- 229920001817AgarPolymers0.000description3
- SRBFZHDQGSBBOR-IOVATXLUSA-ND-xylopyranoseChemical compoundO[C@@H]1COC(O)[C@H](O)[C@H]1OSRBFZHDQGSBBOR-IOVATXLUSA-N0.000description3
- 208000012671Gastrointestinal haemorrhagesDiseases0.000description3
- 241000186660LactobacillusSpecies0.000description3
- GUBGYTABKSRVRQ-QKKXKWKRSA-NLactoseNatural productsOC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1OGUBGYTABKSRVRQ-QKKXKWKRSA-N0.000description3
- GUBGYTABKSRVRQ-PICCSMPSSA-NMaltoseNatural productsO[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1OGUBGYTABKSRVRQ-PICCSMPSSA-N0.000description3
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000description3
- 241000699666Mus <mouse, genus>Species0.000description3
- MUPFEKGTMRGPLJ-RMMQSMQOSA-NRaffinoseNatural productsO(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1MUPFEKGTMRGPLJ-RMMQSMQOSA-N0.000description3
- VMHLLURERBWHNL-UHFFFAOYSA-MSodium acetateChemical compound[Na+].CC([O-])=OVMHLLURERBWHNL-UHFFFAOYSA-M0.000description3
- 229930006000SucroseNatural products0.000description3
- CZMRCDWAGMRECN-UGDNZRGBSA-NSucroseChemical compoundO[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1CZMRCDWAGMRECN-UGDNZRGBSA-N0.000description3
- MUPFEKGTMRGPLJ-UHFFFAOYSA-NUNPD196149Natural productsOC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1MUPFEKGTMRGPLJ-UHFFFAOYSA-N0.000description3
- 239000008272agarSubstances0.000description3
- 238000010171animal modelMethods0.000description3
- 235000015278beefNutrition0.000description3
- 230000005540biological transmissionEffects0.000description3
- 208000027503bloody stoolDiseases0.000description3
- 229940041514candida albicans extractDrugs0.000description3
- 230000008859changeEffects0.000description3
- 206010009887colitisDiseases0.000description3
- 230000002183duodenal effectEffects0.000description3
- 210000003608feceAnatomy0.000description3
- 239000012530fluidSubstances0.000description3
- 210000004347intestinal mucosaAnatomy0.000description3
- 229940039696lactobacillusDrugs0.000description3
- 239000008101lactoseSubstances0.000description3
- 229910052943magnesium sulfateInorganic materials0.000description3
- 235000019341magnesium sulphateNutrition0.000description3
- 239000000463materialSubstances0.000description3
- 239000006872mrs mediumSubstances0.000description3
- 235000010482polyoxyethylene sorbitan monooleateNutrition0.000description3
- 229920000053polysorbate 80Polymers0.000description3
- 239000001632sodium acetateSubstances0.000description3
- 235000017281sodium acetateNutrition0.000description3
- 239000000758substrateSubstances0.000description3
- KDYFGRWQOYBRFD-UHFFFAOYSA-Nsuccinic acidChemical compoundOC(=O)CCC(O)=OKDYFGRWQOYBRFD-UHFFFAOYSA-N0.000description3
- 239000005720sucroseSubstances0.000description3
- YWYZEGXAUVWDED-UHFFFAOYSA-Ntriammonium citrateChemical compound[NH4+].[NH4+].[NH4+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OYWYZEGXAUVWDED-UHFFFAOYSA-N0.000description3
- 241001148471unidentified anaerobic bacteriumSpecies0.000description3
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description3
- 239000012138yeast extractSubstances0.000description3
- OPIFSICVWOWJMJ-AEOCFKNESA-N5-bromo-4-chloro-3-indolyl beta-D-galactosideChemical compoundO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1OC1=CNC2=CC=C(Br)C(Cl)=C12OPIFSICVWOWJMJ-AEOCFKNESA-N0.000description2
- GUBGYTABKSRVRQ-XLOQQCSPSA-NAlpha-LactoseChemical compoundO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1OGUBGYTABKSRVRQ-XLOQQCSPSA-N0.000description2
- FERIUCNNQQJTOY-UHFFFAOYSA-NButyric acidChemical compoundCCCC(O)=OFERIUCNNQQJTOY-UHFFFAOYSA-N0.000description2
- WQZGKKKJIJFFOK-QTVWNMPRSA-ND-mannopyranoseChemical compoundOC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-QTVWNMPRSA-N0.000description2
- 241000196324EmbryophytaSpecies0.000description2
- 102000004190EnzymesHuman genes0.000description2
- 108090000790EnzymesProteins0.000description2
- WSFSSNUMVMOOMR-UHFFFAOYSA-NFormaldehydeChemical compoundO=CWSFSSNUMVMOOMR-UHFFFAOYSA-N0.000description2
- 206010018001Gastrointestinal perforationDiseases0.000description2
- 206010061218InflammationDiseases0.000description2
- UEZVMMHDMIWARA-UHFFFAOYSA-NMetaphosphoric acidChemical compoundOP(=O)=OUEZVMMHDMIWARA-UHFFFAOYSA-N0.000description2
- 241001465754MetazoaSpecies0.000description2
- LRHPLDYGYMQRHN-UHFFFAOYSA-NN-ButanolChemical compoundCCCCOLRHPLDYGYMQRHN-UHFFFAOYSA-N0.000description2
- 239000001888PeptoneSubstances0.000description2
- 108010080698PeptonesProteins0.000description2
- 208000027418Wounds and injuryDiseases0.000description2
- 230000001919adrenal effectEffects0.000description2
- 238000000246agarose gel electrophoresisMethods0.000description2
- PYMYPHUHKUWMLA-UHFFFAOYSA-NarabinoseNatural productsOCC(O)C(O)C(O)C=OPYMYPHUHKUWMLA-UHFFFAOYSA-N0.000description2
- SRBFZHDQGSBBOR-UHFFFAOYSA-Nbeta-D-Pyranose-LyxoseNatural productsOC1COC(O)C(O)C1OSRBFZHDQGSBBOR-UHFFFAOYSA-N0.000description2
- WQZGKKKJIJFFOK-VFUOTHLCSA-Nbeta-D-glucoseChemical compoundOC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-VFUOTHLCSA-N0.000description2
- GUBGYTABKSRVRQ-QUYVBRFLSA-Nbeta-maltoseChemical compoundOC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1OGUBGYTABKSRVRQ-QUYVBRFLSA-N0.000description2
- 210000004534cecumAnatomy0.000description2
- 230000001684chronic effectEffects0.000description2
- 238000007796conventional methodMethods0.000description2
- 235000015140cultured milkNutrition0.000description2
- 230000003247decreasing effectEffects0.000description2
- 235000014113dietary fatty acidsNutrition0.000description2
- XBDQKXXYIPTUBI-UHFFFAOYSA-NdimethylselenoniopropionateNatural productsCCC(O)=OXBDQKXXYIPTUBI-UHFFFAOYSA-N0.000description2
- ZPWVASYFFYYZEW-UHFFFAOYSA-Ldipotassium hydrogen phosphateChemical compound[K+].[K+].OP([O-])([O-])=OZPWVASYFFYYZEW-UHFFFAOYSA-L0.000description2
- 239000012153distilled waterSubstances0.000description2
- 229940079360enema for constipationDrugs0.000description2
- 238000011156evaluationMethods0.000description2
- 239000000284extractSubstances0.000description2
- 229930195729fatty acidNatural products0.000description2
- 239000000194fatty acidSubstances0.000description2
- 150000004665fatty acidsChemical class0.000description2
- 239000012847fine chemicalSubstances0.000description2
- 208000030304gastrointestinal bleedingDiseases0.000description2
- 210000004907glandAnatomy0.000description2
- 239000003862glucocorticoidSubstances0.000description2
- 239000001963growth mediumSubstances0.000description2
- 230000004054inflammatory processEffects0.000description2
- 208000014674injuryDiseases0.000description2
- PHTQWCKDNZKARW-UHFFFAOYSA-NisoamylolChemical compoundCC(C)CCOPHTQWCKDNZKARW-UHFFFAOYSA-N0.000description2
- KQNPFQTWMSNSAP-UHFFFAOYSA-Nisobutyric acidChemical compoundCC(C)C(O)=OKQNPFQTWMSNSAP-UHFFFAOYSA-N0.000description2
- 239000004310lactic acidSubstances0.000description2
- 235000014655lactic acidNutrition0.000description2
- 231100001231less toxicToxicity0.000description2
- 230000014759maintenance of locationEffects0.000description2
- 229940099596manganese sulfateDrugs0.000description2
- 239000011702manganese sulphateSubstances0.000description2
- 235000007079manganese sulphateNutrition0.000description2
- SQQMAOCOWKFBNP-UHFFFAOYSA-Lmanganese(II) sulfateChemical compound[Mn+2].[O-]S([O-])(=O)=OSQQMAOCOWKFBNP-UHFFFAOYSA-L0.000description2
- BDAGIHXWWSANSR-UHFFFAOYSA-Nmethanoic acidNatural productsOC=OBDAGIHXWWSANSR-UHFFFAOYSA-N0.000description2
- 230000008506pathogenesisEffects0.000description2
- 231100000915pathological changeToxicity0.000description2
- 230000036285pathological changeEffects0.000description2
- 230000001575pathological effectEffects0.000description2
- 235000019319peptoneNutrition0.000description2
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description2
- 230000008569processEffects0.000description2
- 238000000746purificationMethods0.000description2
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-NraffinoseChemical compoundO[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1MUPFEKGTMRGPLJ-ZQSKZDJDSA-N0.000description2
- 239000010802sludgeSubstances0.000description2
- 239000011780sodium chlorideSubstances0.000description2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-Nvaleric acidChemical compoundCCCCC(O)=ONQPDZGIKBAWPEJ-UHFFFAOYSA-N0.000description2
- 238000005303weighingMethods0.000description2
- HDTRYLNUVZCQOY-UHFFFAOYSA-Nα-D-glucopyranosyl-α-D-glucopyranosideNatural productsOC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1HDTRYLNUVZCQOY-UHFFFAOYSA-N0.000description1
- 10802000446516S ribosomal RNAProteins0.000description1
- XYHKNCXZYYTLRG-UHFFFAOYSA-N1h-imidazole-2-carbaldehydeChemical compoundO=CC1=NC=CN1XYHKNCXZYYTLRG-UHFFFAOYSA-N0.000description1
- WLJVXDMOQOGPHL-PPJXEINESA-N2-phenylacetic acidChemical compoundO[14C](=O)CC1=CC=CC=C1WLJVXDMOQOGPHL-PPJXEINESA-N0.000description1
- GWYFCOCPABKNJV-UHFFFAOYSA-M3-Methylbutanoic acidNatural productsCC(C)CC([O-])=OGWYFCOCPABKNJV-UHFFFAOYSA-M0.000description1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N4-(3-methoxyphenyl)anilineChemical compoundCOC1=CC=CC(C=2C=CC(N)=CC=2)=C1OSWFIVFLDKOXQC-UHFFFAOYSA-N0.000description1
- 208000004998Abdominal PainDiseases0.000description1
- PLXMOAALOJOTIY-FPTXNFDTSA-NAesculinNatural productsOC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1Oc2cc3C=CC(=O)Oc3cc2OPLXMOAALOJOTIY-FPTXNFDTSA-N0.000description1
- 208000031648Body Weight ChangesDiseases0.000description1
- WWZKQHOCKIZLMA-UHFFFAOYSA-NCaprylic acidNatural productsCCCCCCCC(O)=OWWZKQHOCKIZLMA-UHFFFAOYSA-N0.000description1
- 102100035882CatalaseHuman genes0.000description1
- 108010053835CatalaseProteins0.000description1
- 229920000742CottonPolymers0.000description1
- FBPFZTCFMRRESA-FSIIMWSLSA-ND-GlucitolNatural productsOC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)COFBPFZTCFMRRESA-FSIIMWSLSA-N0.000description1
- FBPFZTCFMRRESA-KVTDHHQDSA-ND-MannitolChemical compoundOC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COFBPFZTCFMRRESA-KVTDHHQDSA-N0.000description1
- HMFHBZSHGGEWLO-SOOFDHNKSA-ND-ribofuranoseChemical compoundOC[C@H]1OC(O)[C@H](O)[C@@H]1OHMFHBZSHGGEWLO-SOOFDHNKSA-N0.000description1
- 108020004414DNAProteins0.000description1
- 208000027244DysbiosisDiseases0.000description1
- 229930091371FructoseNatural products0.000description1
- 239000005715FructoseSubstances0.000description1
- RFSUNEUAIZKAJO-ARQDHWQXSA-NFructoseChemical compoundOC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1ORFSUNEUAIZKAJO-ARQDHWQXSA-N0.000description1
- 108010010803GelatinProteins0.000description1
- PEDCQBHIVMGVHV-UHFFFAOYSA-NGlycerolNatural productsOCC(O)COPEDCQBHIVMGVHV-UHFFFAOYSA-N0.000description1
- UFHFLCQGNIYNRP-UHFFFAOYSA-NHydrogenChemical compound[H][H]UFHFLCQGNIYNRP-UHFFFAOYSA-N0.000description1
- QIVBCDIJIAJPQS-VIFPVBQESA-NL-tryptophaneChemical compoundC1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1QIVBCDIJIAJPQS-VIFPVBQESA-N0.000description1
- 206010050953Lower gastrointestinal haemorrhageDiseases0.000description1
- 229930195725MannitolNatural products0.000description1
- 241000204031MycoplasmaSpecies0.000description1
- AMQJEAYHLZJPGS-UHFFFAOYSA-NN-PentanolChemical compoundCCCCCOAMQJEAYHLZJPGS-UHFFFAOYSA-N0.000description1
- 238000012408PCR amplificationMethods0.000description1
- 208000005374PoisoningDiseases0.000description1
- 206010057071Rectal tenesmusDiseases0.000description1
- PYMYPHUHKUWMLA-LMVFSUKVSA-NRiboseNatural productsOC[C@@H](O)[C@@H](O)[C@@H](O)C=OPYMYPHUHKUWMLA-LMVFSUKVSA-N0.000description1
- 241000124033SalixSpecies0.000description1
- HDTRYLNUVZCQOY-WSWWMNSNSA-NTrehaloseNatural productsO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1HDTRYLNUVZCQOY-WSWWMNSNSA-N0.000description1
- QIVBCDIJIAJPQS-UHFFFAOYSA-NTryptophanNatural productsC1=CC=C2C(CC(N)C(O)=O)=CNC2=C1QIVBCDIJIAJPQS-UHFFFAOYSA-N0.000description1
- COQLPRJCUIATTQ-UHFFFAOYSA-NUranyl acetateChemical compoundO.O.O=[U]=O.CC(O)=O.CC(O)=OCOQLPRJCUIATTQ-UHFFFAOYSA-N0.000description1
- 241000700605VirusesSpecies0.000description1
- 230000002159abnormal effectEffects0.000description1
- 239000002253acidSubstances0.000description1
- 230000002378acidificating effectEffects0.000description1
- 230000001154acute effectEffects0.000description1
- 238000011166aliquotingMethods0.000description1
- HMFHBZSHGGEWLO-UHFFFAOYSA-Nalpha-D-Furanose-RiboseNatural productsOCC1OC(O)C(O)C1OHMFHBZSHGGEWLO-UHFFFAOYSA-N0.000description1
- WQZGKKKJIJFFOK-PHYPRBDBSA-Nalpha-D-galactoseChemical compoundOC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1OWQZGKKKJIJFFOK-PHYPRBDBSA-N0.000description1
- 238000003556assayMethods0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 230000001363autoimmuneEffects0.000description1
- GONOPSZTUGRENK-UHFFFAOYSA-Nbenzyl(trichloro)silaneChemical compoundCl[Si](Cl)(Cl)CC1=CC=CC=C1GONOPSZTUGRENK-UHFFFAOYSA-N0.000description1
- DLRVVLDZNNYCBX-ZZFZYMBESA-Nbeta-melibioseChemical compoundO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)O1DLRVVLDZNNYCBX-ZZFZYMBESA-N0.000description1
- GWYFCOCPABKNJV-UHFFFAOYSA-Nbeta-methyl-butyric acidNatural productsCC(C)CC(O)=OGWYFCOCPABKNJV-UHFFFAOYSA-N0.000description1
- 230000004579body weight changeEffects0.000description1
- KDYFGRWQOYBRFD-NUQCWPJISA-Nbutanedioic acidChemical compoundO[14C](=O)CC[14C](O)=OKDYFGRWQOYBRFD-NUQCWPJISA-N0.000description1
- 239000002775capsuleSubstances0.000description1
- 239000004202carbamideSubstances0.000description1
- 150000001720carbohydratesChemical class0.000description1
- 235000014633carbohydratesNutrition0.000description1
- 230000034303cell buddingEffects0.000description1
- 210000002421cell wallAnatomy0.000description1
- 238000000546chi-square testMethods0.000description1
- 208000037976chronic inflammationDiseases0.000description1
- 208000037893chronic inflammatory disorderDiseases0.000description1
- 230000008576chronic processEffects0.000description1
- 239000011248coating agentSubstances0.000description1
- 238000000576coating methodMethods0.000description1
- 150000001875compoundsChemical class0.000description1
- 235000009508confectioneryNutrition0.000description1
- 210000000805cytoplasmAnatomy0.000description1
- 235000013365dairy productNutrition0.000description1
- 230000001934delayEffects0.000description1
- 238000001514detection methodMethods0.000description1
- 238000011161developmentMethods0.000description1
- 238000010586diagramMethods0.000description1
- 238000010790dilutionMethods0.000description1
- 239000012895dilutionSubstances0.000description1
- 230000009266disease activityEffects0.000description1
- 238000009826distributionMethods0.000description1
- 239000003937drug carrierSubstances0.000description1
- 230000007140dysbiosisEffects0.000description1
- 238000005516engineering processMethods0.000description1
- 210000002919epithelial cellAnatomy0.000description1
- 238000000855fermentationMethods0.000description1
- 230000004151fermentationEffects0.000description1
- 230000037406food intakeEffects0.000description1
- 235000012631food intakeNutrition0.000description1
- 235000019253formic acidNutrition0.000description1
- 229930182830galactoseNatural products0.000description1
- 239000007789gasSubstances0.000description1
- 238000004817gas chromatographyMethods0.000description1
- 239000008273gelatinSubstances0.000description1
- 229920000159gelatinPolymers0.000description1
- 235000019322gelatineNutrition0.000description1
- 235000011852gelatine dessertsNutrition0.000description1
- 230000002068genetic effectEffects0.000description1
- 239000012362glacial acetic acidSubstances0.000description1
- 239000011521glassSubstances0.000description1
- 235000011187glycerolNutrition0.000description1
- 238000007490hematoxylin and eosin (H&E) stainingMethods0.000description1
- 239000001257hydrogenSubstances0.000description1
- 229910052739hydrogenInorganic materials0.000description1
- 210000003405ileumAnatomy0.000description1
- 238000011534incubationMethods0.000description1
- 230000002757inflammatory effectEffects0.000description1
- 239000007924injectionSubstances0.000description1
- 238000002347injectionMethods0.000description1
- 230000003993interactionEffects0.000description1
- 206010022694intestinal perforationDiseases0.000description1
- 150000002500ionsChemical class0.000description1
- RLJMLMKIBZAXJO-UHFFFAOYSA-Nlead nitrateChemical compound[O-][N+](=O)O[Pb]O[N+]([O-])=ORLJMLMKIBZAXJO-UHFFFAOYSA-N0.000description1
- 238000009630liquid cultureMethods0.000description1
- 230000007774longtermEffects0.000description1
- 239000000594mannitolSubstances0.000description1
- 235000010355mannitolNutrition0.000description1
- 238000004519manufacturing processMethods0.000description1
- WCYAALZQFZMMOM-UHFFFAOYSA-Nmethanol;sulfuric acidChemical compoundOC.OS(O)(=O)=OWCYAALZQFZMMOM-UHFFFAOYSA-N0.000description1
- 229910000402monopotassium phosphateInorganic materials0.000description1
- 235000019796monopotassium phosphateNutrition0.000description1
- 230000000877morphologic effectEffects0.000description1
- 210000003097mucusAnatomy0.000description1
- FUZZWVXGSFPDMH-UHFFFAOYSA-Nn-hexanoic acidNatural productsCCCCCC(O)=OFUZZWVXGSFPDMH-UHFFFAOYSA-N0.000description1
- 235000021590normal dietNutrition0.000description1
- 239000001301oxygenSubstances0.000description1
- 229910052760oxygenInorganic materials0.000description1
- 239000012188paraffin waxSubstances0.000description1
- 239000002245particleSubstances0.000description1
- 230000007170pathologyEffects0.000description1
- PJNZPQUBCPKICU-UHFFFAOYSA-Nphosphoric acid;potassiumChemical compound[K].OP(O)(O)=OPJNZPQUBCPKICU-UHFFFAOYSA-N0.000description1
- 239000002504physiological saline solutionSubstances0.000description1
- 229910052697platinumInorganic materials0.000description1
- 231100000572poisoningToxicity0.000description1
- 230000000607poisoning effectEffects0.000description1
- 230000000529probiotic effectEffects0.000description1
- BDERNNFJNOPAEC-UHFFFAOYSA-Npropan-1-olChemical compoundCCCOBDERNNFJNOPAEC-UHFFFAOYSA-N0.000description1
- 235000019260propionic acidNutrition0.000description1
- IUVKMZGDUIUOCP-BTNSXGMBSA-NquinboloneChemical compoundO([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1IUVKMZGDUIUOCP-BTNSXGMBSA-N0.000description1
- 230000000306recurrent effectEffects0.000description1
- 238000011160researchMethods0.000description1
- 238000013207serial dilutionMethods0.000description1
- 239000000600sorbitolSubstances0.000description1
- 235000013322soy milkNutrition0.000description1
- 238000001228spectrumMethods0.000description1
- 239000001384succinic acidSubstances0.000description1
- 239000006228supernatantSubstances0.000description1
- 230000008961swellingEffects0.000description1
- 229940037128systemic glucocorticoidsDrugs0.000description1
- 208000012271tenesmusDiseases0.000description1
- 231100000331toxicToxicity0.000description1
- 230000002588toxic effectEffects0.000description1
- 230000032258transportEffects0.000description1
- 229940005605valeric acidDrugs0.000description1
Images
Landscapes
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及微生物技术领域,特别是涉及发酵乳杆菌的新的菌株以及该菌株的应用。The invention relates to the technical field of microbes, in particular to a new strain of Lactobacillus fermentum and the application of the strain.
背景技术Background technique
溃疡性结肠炎(ulcerative colitis,UC)是慢性非特异性溃疡性结肠炎的简称,为一种原因未明的直肠和结肠慢性炎性疾病。UC的发病是复杂的、多环境、多因素相互作用的结果,对它的研究很多,但其病因与发病机理仍未完全明确。主要临床表现是腹泻、粘液脓血便、腹痛和里急后重。病情轻重不等,多反复发作或长期迁延呈慢性经过,常常出现并发症,甚至癌变。本病可发生于任何年龄,以20—50岁为多见。男女发病率无明显差别。UC在西方国家相当常见,患病率高达35~100/105。中国发病率较欧、美低,且轻型病例多见,但近二十年来本病的发病率有上升趋势,国内有关UC的报道也明显增加。Ulcerative colitis (UC), short for chronic nonspecific ulcerative colitis, is a chronic inflammatory disease of the rectum and colon of unknown cause. The pathogenesis of UC is the result of complex, multi-environmental, and multi-factorial interactions. There are many studies on it, but its etiology and pathogenesis are still not completely clear. The main clinical manifestations are diarrhea, mucus pus and blood in the stool, abdominal pain and tenesmus. The severity of the disease varies, and many recurrent attacks or long-term delays are chronic processes, often with complications and even canceration. The disease can occur at any age, and it is more common in the age of 20-50. There was no significant difference in incidence between men and women. UC is quite common in western countries, with a prevalence as high as 35-100/105. The incidence rate in China is lower than that in Europe and the United States, and mild cases are more common. However, the incidence rate of this disease has been on the rise in the past two decades, and domestic reports on UC have also increased significantly.
对于该病的治疗,近年来主要采用内科综合治疗,控制急性发作,减少复发,防止并发症。现阶段治疗的药物包括肾上腺糖皮质激素,适用于暴发型或重型患者,可控制炎症,抑制自体免疫过程,减轻中毒症状,有较好疗效。另外柳氮磺吡啶(简称SASP)也常作为首选药物,适用于轻型或重型经肾上腺糖皮质激素治疗已有缓解者,疗效较好。这些药物虽然具有较为理想的疗效,但具有较大的毒副作用,因此期待能开发出更为安全的替代治疗方案。For the treatment of this disease, in recent years, comprehensive medical treatment is mainly used to control acute attacks, reduce recurrence and prevent complications. The current treatment drugs include adrenal glucocorticoids, which are suitable for fulminant or severe patients. They can control inflammation, inhibit the autoimmune process, and relieve symptoms of poisoning, and have a good curative effect. In addition, sulfasalazine (SASP) is often used as the drug of choice, and it is suitable for mild or severe patients who have been relieved by adrenal glucocorticoid treatment, and the curative effect is better. Although these drugs have relatively ideal curative effects, they have relatively large toxic and side effects, so it is expected to develop a safer alternative treatment plan.
最新研究表明,菌群失调是溃疡性结肠炎(ulcerative colitis,UC)的重要病因之一。益生菌在UC治疗中的作用因其安全性优越、毒副作用小的特点而被日益重视,但目前所报道的益生菌治疗UC的疗效尚无法达到理想状态。选择最佳的菌株成为益生菌治疗UC的关键。The latest research shows that dysbiosis is one of the important causes of ulcerative colitis (UC). The role of probiotics in the treatment of UC has been paid more and more attention due to its superior safety and less toxic side effects. However, the curative effect of probiotics in the treatment of UC reported so far has not yet reached the ideal state. Selecting the best strain becomes the key to the treatment of UC with probiotics.
发明内容Contents of the invention
本发明的目的就是为了解决以上问题,提供一种能有效治疗直肠或结肠炎性疾病特别是溃疡性结肠炎的新的发酵乳杆菌菌株及其在制备药物等方面的应用。The purpose of the present invention is to solve the above problems, to provide a new strain of Lactobacillus fermentum that can effectively treat rectal or colonic inflammatory diseases, especially ulcerative colitis, and its application in the preparation of medicines and the like.
本发明的再一目的是提供含有上述新的发酵乳杆菌菌株的药物组合物、食品、保健品及食品添加剂。Another object of the present invention is to provide pharmaceutical compositions, foods, health products and food additives containing the above-mentioned novel Lactobacillus fermentum strains.
为实现上述目的,本发明采用了以下技术方案:To achieve the above object, the present invention adopts the following technical solutions:
本发明公开了一种发酵乳杆菌(Lactobacillus fermenti)CMS-H002,其保藏号为CCTCC No.M206110。The invention discloses a Lactobacillus fermenti CMS-H002, the preservation number of which is CCTCC No. M206110.
本发明还公开了上述发酵乳杆菌在制备用于治疗直肠或结肠炎性疾病的药物中的应用。The invention also discloses the application of the above-mentioned lactobacillus fermentum in the preparation of medicines for treating rectal or colonic inflammatory diseases.
所述直肠或结肠炎性疾病优选为溃疡性结肠炎。The inflammatory disease of the rectum or colon is preferably ulcerative colitis.
本发明还公开了一种药物组合物,该药物组合物包括药学有效剂量的上述发酵乳杆菌(Lactobacillus fermenti)CMS-H002,或其代谢产物、细胞碎片或分泌物。The invention also discloses a pharmaceutical composition, which comprises the above-mentioned Lactobacillus fermenti CMS-H002 in a pharmaceutically effective dose, or its metabolites, cell fragments or secretions.
本发明还公开了一种食品,所述食品含有上述的发酵乳杆菌(Lactobacillus fermenti)CMS-H002,或其代谢产物、细胞碎片或分泌物。The invention also discloses a food, which contains the above-mentioned Lactobacillus fermenti CMS-H002, or its metabolites, cell fragments or secretions.
优选的,所述食品为饮料。Preferably, the food is a beverage.
本发明还公开了一种保健品,该保健品包含上述的发酵乳杆菌(Lactobacillus fermenti)CMS-H002,或其代谢产物、细胞碎片或分泌物。The invention also discloses a health product, which contains the above-mentioned Lactobacillus fermenti CMS-H002, or its metabolites, cell fragments or secretions.
本发明还公开了一种食品添加剂,所述食品添加剂包含上述的发酵乳杆菌(Lactobacillus fermenti)CMS-H002,或其代谢产物、细胞碎片或分泌物。The invention also discloses a food additive, which comprises the above-mentioned Lactobacillus fermenti CMS-H002, or its metabolites, cell fragments or secretions.
本发明的发酵乳杆菌CMS-H002,是一种分离的全新的菌株,该菌株对溃疡性结肠炎能减轻腹泻、出血、体重减轻等症状,也能减轻肠道粘膜的损伤及炎性细胞浸润,效果优于柳氮磺吡啶(SASP);并且作为一种益生菌安全性优越、毒副作用小。该菌株能够安全、有效地治疗溃疡性结肠炎等直肠或结肠炎性疾病。The Lactobacillus fermentum CMS-H002 of the present invention is a brand-new isolated strain, which can alleviate symptoms such as diarrhea, bleeding, and weight loss for ulcerative colitis, and can also reduce intestinal mucosal damage and inflammatory cell infiltration , the effect is better than that of sulfasalazine (SASP); and as a kind of probiotic, it has superior safety and less toxic and side effects. The bacterial strain can safely and effectively treat rectal or colitis inflammatory diseases such as ulcerative colitis.
保藏信息:Preservation information:
菌株名称:发酵乳杆菌(Lactobacillus fermenti)CMS-H002Strain name: Lactobacillus fermenti CMS-H002
保藏日期:2006年10年23日Date of deposit: October 23, 2006
保藏单位:中国典型培养物保藏中心(CCTCC)Deposit unit: China Center for Type Culture Collection (CCTCC)
保藏编号:CCTCC No.M 206110Deposit number: CCTCC No.M 206110
附图说明Description of drawings
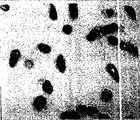
图1为本发明发酵乳杆菌CMS-H002菌液涂片镜下形态(Gram’s染色,×1000)。Fig. 1 is the microscopic morphology of the smear of Lactobacillus fermentum CMS-H002 bacteria liquid of the present invention (Gram's staining, ×1000).
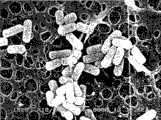
图2为本发明发酵乳杆菌CMS-H002扫描电镜结果(×10000)。Fig. 2 is the scanning electron microscope result (×10000) of Lactobacillus fermentum CMS-H002 of the present invention.
图3为本发明发酵乳杆菌CMS-H002透射电镜结果(×12000)。Fig. 3 is the transmission electron microscope result of Lactobacillus fermentum CMS-H002 of the present invention (×12000).
图4为实施例2中实验前后各实验组小鼠体重变化结果图。4 is a diagram showing the results of body weight changes of mice in each experimental group before and after the experiment in Example 2.
图5为实施例2中各组小鼠死亡率情况结果图。FIG. 5 is a graph showing the results of mortality of mice in each group in Example 2. FIG.
图6为实施例2中各组小鼠结肠长度结果图。FIG. 6 is a graph showing the results of the colon lengths of mice in each group in Example 2. FIG.
图7为实施例2中各组小鼠DAI积分曲线结果图。FIG. 7 is a graph showing the results of DAI integral curves of mice in each group in Example 2. FIG.
图8为实施例2中各组小鼠组织学损伤评分结果图。FIG. 8 is a graph showing the results of histological damage scores of mice in each group in Example 2. FIG.
图9a-1为实施例2中各组小鼠的结肠病理切面图(HE×400),其中:Fig. 9a-1 is the section view of the colon pathology (HE × 400) of each group of mice in Example 2, wherein:
a为正常对照组结肠粘膜;b为模型组结肠粘膜;a is the colonic mucosa of the normal control group; b is the colonic mucosa of the model group;
c为阴性对照组结肠粘膜;d为阳性对照组结肠粘膜;c is the colonic mucosa of the negative control group; d is the colonic mucosa of the positive control group;
e为治疗一组结肠粘膜;f为治疗二组结肠粘膜;e is treating one group of colonic mucosa; f is treating two groups of colonic mucosa;
g为治疗三组结肠粘膜;h为治疗四组结肠粘膜;g is the treatment of three groups of colonic mucosa; h is the treatment of four groups of colonic mucosa;
i为治疗五组结肠粘膜;j为高剂量0501灌肠组结肠粘膜;i is the colonic mucosa of the five treatment groups; j is the colonic mucosa of the high-dose 0501 enema group;
k为高剂量c120灌肠组结肠粘膜;l为治疗六组结肠粘膜。k is the colonic mucosa of the high-dose c120 enema group; l is the colonic mucosa of the six treatment groups.
具体实施方式Detailed ways
本发明的新的菌株:发酵乳杆菌(Lactobacillus fermenti)CMS-H002,已经于2006年10年23日在位于中国武汉市的中国典型培养物保藏中心(CCTCC)进行了保藏,保藏号为CCTCC No.M 206110。The new bacterial strain of the present invention: Lactobacillus fermenti (Lactobacillus fermenti) CMS-H002, has carried out preservation in the Chinese Type Culture Collection Center (CCTCC) located in Wuhan, China on October 23, 2006, and the preservation number is CCTCC No. .M 206110.
本发明的新菌株发酵乳杆菌CMS-H002可从健康婴幼儿粪便或成人十二指肠液中分离得到。该菌株对溃疡性结肠炎能减轻腹泻、出血、体重减轻等症状,也能减轻肠道粘膜的损伤及炎性细胞浸润,效果优于柳氮磺吡啶(SASP)。该菌株能够有效治疗溃疡性结肠炎等直肠或结肠炎性疾病。The new bacterial strain Lactobacillus fermentum CMS-H002 of the present invention can be isolated from healthy infant feces or adult duodenal fluid. The bacterial strain can alleviate symptoms such as diarrhea, bleeding, and weight loss for ulcerative colitis, and can also reduce intestinal mucosal damage and inflammatory cell infiltration, and the effect is better than that of sulfasalazine (SASP). The bacterial strain can effectively treat rectal or colonic inflammatory diseases such as ulcerative colitis.
利用本发明的发酵乳杆菌CMS-H002可以制备成药物组合物。该药物组合物含有药学有效剂量的发酵乳杆菌CMS-H002,或其代谢产物、细胞碎片或分泌物。此外,所述药物组合物还可以含有合适的药物载体。本发明的药物组合物可以为胶囊、溶液或可饮用悬浮液、袋装粉剂等形式,每一单一剂量一般含有发酵乳杆菌CMS-H002菌株约为108~1011细胞。The Lactobacillus fermentum CMS-H002 of the present invention can be used to prepare a pharmaceutical composition. The pharmaceutical composition contains a pharmaceutically effective dose of Lactobacillus fermentum CMS-H002, or its metabolites, cell fragments or secretions. In addition, the pharmaceutical composition may also contain suitable pharmaceutical carriers. The pharmaceutical composition of the present invention can be in the form of capsules, solutions or drinkable suspensions, powder in bags, etc. Each single dose generally contains about 108 to 1011 cells of Lactobacillus fermentum CMS-H002 strain.
利用本发明的发酵乳杆菌CMS-H002还可以制备成食品、保健品或食品添加剂的形式。所述食品、保健品或食品添加剂含有发酵乳杆菌CMS-H002,或其代谢产物、细胞碎片或分泌物。这些食品、保健品或食品添加剂可用于防治溃疡性结肠炎等直肠或结肠炎性疾病,提高使用者的健康水平。本发明的食品可以是含有本发明发酵乳杆菌CMS-H002活菌的饮料的形式,也可以是含有所述活菌的乳制品、发酵乳、酸豆奶等形式。The Lactobacillus fermentum CMS-H002 of the present invention can also be prepared into the form of food, health product or food additive. The food, health product or food additive contains Lactobacillus fermentum CMS-H002, or its metabolites, cell fragments or secretions. These foods, health products or food additives can be used to prevent and treat rectal or colitis inflammatory diseases such as ulcerative colitis, and improve the health level of users. The food of the present invention can be in the form of a beverage containing the live Lactobacillus fermentum CMS-H002 of the present invention, or in the form of dairy products, fermented milk, soy milk, etc. containing the live bacteria.
下面通过具体的实施例对本发明作进一步详细的描述。The present invention will be described in further detail below through specific examples.
实施例1Example 1
发酵乳杆菌CMS-H002的分离鉴定及其生物学特性Isolation, identification and biological characteristics of Lactobacillus fermentum CMS-H002
1、材料1. Materials
试剂:牛肉粉(北京奥博星生物责任有限公司,批号:20040607)、蛋白胨(北京奥博星生物责任有限公司,批号:20040615)、乙酸钠(广东省台山市化工厂,批号:20030401)、硫酸镁(河南省焦作市化工三厂)、硫酸锰(河南焦作市化工厂,批号:981014)、酵母浸粉(北京奥博星生物技术责任有限公司,批号:20030825)、柠檬酸铵(湖南湘中精细化学品厂,批号:20030409)、葡萄糖(湖南师大化学试剂厂,批号:20040208)、吐温-80(西安富力化学厂,批号:021125)、磷酸氢二钾(河南省焦作市化工三厂,批号:20010404)、冰醋酸(广东汕头市西陇化工厂,批号:050414)琼脂粉(北京奥博星生物责任有限公司,批号:040415),X-gal(inalc),API 20A生化鉴定条(法国酶里埃),API 50 CHL生化鉴定条(法国酶里埃)Reagents: beef powder (Beijing Aoboxing Biological Co., Ltd., batch number: 20040607), peptone (Beijing Aoboxing Biological Co., Ltd., batch number: 20040615), sodium acetate (Taishan Chemical Factory, Guangdong Province, batch number: 20030401), magnesium sulfate (No. 3 Chemical Plant of Jiaozuo City, Henan Province), manganese sulfate (Chemical Plant of Jiaozuo City, Henan Province, batch number: 981014), yeast extract powder (Beijing Aoboxing Biotechnology Co., Ltd., batch number: 20030825), ammonium citrate (Hunan Xiangzhong Fine Chemical factory, batch number: 20030409), glucose (Chemical Reagent Factory of Hunan Normal University, batch number: 20040208), Tween-80 (Xi’an R&F Chemical Factory, batch number: 021125), dipotassium hydrogen phosphate (No. , batch number: 20010404), glacial acetic acid (Xilong Chemical Factory, Shantou City, Guangdong, batch number: 050414), agar powder (Beijing Aoboxing Biological Co., Ltd., batch number: 040415), X-gal (inalc), API 20A biochemical identification strip ( French Enzyme),
实验仪器:厌氧培养盒(法国梅里埃)、日本岛津GC2010型气相色谱检测仪(GC)、氢焰离子检测器(FID)、DB-5MS色谱柱、日本岛津UV-2501PC、JSM5600-LV型扫描电镜、日立H-600型透射电镜、Experimental equipment: anaerobic culture box (Mérieux, France), Japan Shimadzu GC2010 gas chromatography detector (GC), hydrogen flame ion detector (FID), DB-5MS chromatographic column, Japan Shimadzu UV-2501PC, JSM5600- LV scanning electron microscope, Hitachi H-600 transmission electron microscope,
MRS-X-gal培养基的制备:Preparation of MRS-X-gal medium:
MRS培养基配方:牛肉粉10克/升、蛋白胨10克/升、乙酸钠5克/升、硫酸镁0.5克/升、硫酸锰0.2克/升、酵母浸粉5克/升、柠檬酸铵2克/升、葡萄糖20克/升、吐温-80(1克/升)、磷酸氢二钾5克/升、琼脂粉15克/升。按配方配置MRS培养基后,以乙酸调节pH值分别至4.5、5.4、6.8。MRS medium formula: beef powder 10 g/L, peptone 10 g/L, sodium acetate 5 g/L, magnesium sulfate 0.5 g/L, manganese sulfate 0.2 g/L, yeast extract powder 5 g/L, ammonium citrate 2 g/L, glucose 20 g/L, Tween-80 (1 g/L), dipotassium hydrogen phosphate 5 g/L, agar powder 15 g/L. After the MRS medium was configured according to the recipe, the pH was adjusted to 4.5, 5.4, and 6.8 with acetic acid.
配制X-gal溶液(20mg/ml)以直径0.22um一次性滤器过滤除菌,取50ul铺于MRS固体培养基上涂布均匀备用。The prepared X-gal solution (20mg/ml) was filtered and sterilized with a disposable filter with a diameter of 0.22um, and 50ul was spread on the MRS solid medium and evenly coated for later use.
2、发酵乳杆菌CMS-H002的分离、纯化:2. Isolation and purification of Lactobacillus fermentum CMS-H002:
无菌棉签挑取健康婴幼儿粪便标本,或胃镜室取十二指肠液,置MRS液体培养基以厌氧盒快速运送至实验室,35℃孵育2—3h,使细菌适应培养基的环境,然后10倍系列稀释成10-1、10-2、10-3等几个稀释度,各取0.1ml铺于MRS固体培养基,以L型玻棒涂布均匀,35℃厌氧培养48~72h。挑取典型菌落于MRS液体培养基厌氧环境培养24h,行Gram’s染色。显微镜下观察形态,选取镜下形态为Gram’s染色阳性杆菌的菌液,划线接种于MRS固体培养基平板。厌氧培养48h,根据平板上菌落形态特征及镜下观察菌体的染色特性、大小、球杆状和分布情况,判断是否纯化。如果细菌不纯,则继续挑取单菌落划线接种于MRS固体培养基平板进一步分离纯化,反复多次分离传代,得到纯化的菌株。Pick up stool samples from healthy infants with sterile cotton swabs, or take duodenal fluid from the gastroscope room, put MRS liquid culture medium in an anaerobic box and quickly transport it to the laboratory, incubate at 35°C for 2-3 hours, so that the bacteria can adapt to the environment of the culture medium. Then 10-fold serial dilution to 10-1 , 10-2 , 10-3 and other dilutions, each take 0.1ml spread on the MRS solid medium, evenly spread with L-shaped glass rod, 35 ℃ anaerobic culture 48~ 72h. Pick typical colonies and culture them in anaerobic environment in MRS liquid medium for 24 hours, and perform Gram's staining. The morphology was observed under a microscope, and the bacterial liquid with Gram's staining positive bacilli was selected and inoculated on the MRS solid medium plate by streaking. Anaerobic culture for 48 hours, according to the morphological characteristics of the colony on the plate and the staining characteristics, size, club shape and distribution of the bacteria observed under the microscope, judge whether it is purified or not. If the bacteria are not pure, continue to pick a single colony and streak inoculate it on the MRS solid medium plate for further isolation and purification, and repeat the isolation and passage several times to obtain purified strains.
3、菌落特征:3. Colony characteristics:
发酵乳杆菌CMS-H002在MRS固体培养基平皿厌氧培养48h后,呈现圆形、白色、凸起、直径约2-4mm、边缘整齐的湿润菌落。After anaerobic culture of Lactobacillus fermentum CMS-H002 on the MRS solid medium plate for 48 hours, it presents a round, white, raised, moist colony with a diameter of about 2-4mm and neat edges.
4、显微镜下形态:4. Morphology under microscope:
发酵乳杆菌CMS-H002菌液涂片:呈革蓝氏染色阳性杆菌,以短杆状杆菌居多,单个或成对,见图1。Lactobacillus fermentum CMS-H002 bacterial liquid smear: Gram-positive bacilli, mostly short bacilli, single or in pairs, see Figure 1.
5、耐氧试验5. Oxygen resistance test
发酵乳杆菌CMS-H002在MRS固体培养基平皿有氧培养48h后,呈现圆形、白色、凸起、直径约2-4mm、边缘整齐的湿润菌落。After aerobic culture of Lactobacillus fermentum CMS-H002 on the MRS solid medium plate for 48 hours, it presents a round, white, raised, moist colony with a diameter of about 2-4mm and neat edges.
6、发酵乳杆菌CMS-H002的生化鉴定6. Biochemical identification of Lactobacillus fermentum CMS-H002
API 20A是一种厌氧菌鉴定系统,有21个测定,能快速、简便地对厌氧菌进行生化鉴定。API 20A试验条是由20个含干燥底物的小管所组成。将菌悬液分装到管内而重新组成这些底物。在35~37℃培养24或48小时之后,所产的代谢物由酸度(pH)指示剂或加入试剂而呈现出来。可根据读表判断反应结果;用分析谱索引或鉴定软件予以鉴定。API 20A is an anaerobic bacteria identification system with 21 assays for quick and easy biochemical identification of anaerobic bacteria. API 20A test strips consist of 20 vials containing dry substrate. These substrates were reconstituted by aliquoting the bacterial suspension into tubes. After incubation at 35-37°C for 24 or 48 hours, the metabolites produced are revealed by acidity (pH) indicators or by addition of reagents. The reaction result can be judged according to the table reading; it can be identified by analysis spectrum index or identification software.
API 50 CHL是用于乳酸杆菌(Lactpbacillus)和相关菌的鉴定,它是由49种可发酵碳水化合物的API 50 CH试验条组成的简易培养基。以测定菌制成悬液接种试验条的每一个小管。当培养时,由于发酵塘水化合物产酸,pH下降,使指示剂变色。结果构成菌株的生化图谱,并用于鉴定或分型。
按API 20A和API 50 CHL厌氧菌鉴定卡说明书操作:将发酵乳杆菌CMS-H002的细菌悬液接种于鉴定卡的反应孔中。35℃微需氧培养48h,根据颜色反应(紫→黄)判断结果,进行编码,查编码检索本或电脑软件。According to the instructions of the API 20A and
API20A生化鉴定结果显示:发酵乳杆菌CMS-H002可发酵葡萄糖、麦芽糖、乳糖、蔗糖、木糖、甘露糖、棉子糖,编码为:46504202,81.5%符合发酵乳杆菌。结果见表1。API20A biochemical identification results show that: Lactobacillus fermentum CMS-H002 can ferment glucose, maltose, lactose, sucrose, xylose, mannose, raffinose, coded as: 46504202, 81.5% conform to Lactobacillus fermentum. The results are shown in Table 1.
表1:发酵乳杆菌CMS-H002 API20A生化鉴定结果Table 1: Biochemical identification results of Lactobacillus fermentum CMS-H002 API20A
API 50 CHL生化鉴定结果显示:发酵乳杆菌CMS-H002可发酵核糖、半乳糖、葡萄糖、果糖、甘露糖、麦芽糖、乳糖、蜜二糖、蔗糖、棉子糖,编码为:44514002,符合发酵乳杆菌。
7、MRS-X-gal培养基显色反应7. MRS-X-gal medium color reaction
将分离纯化鉴定后的发酵乳杆菌CMS-H002接种于MRS-X-gal培养基上,厌氧培养48h,取出平皿放置于空气中,几分钟后观察菌落显色情况。Inoculate the isolated and purified Lactobacillus fermentum CMS-H002 on the MRS-X-gal medium, culture it anaerobically for 48 hours, take out the plate and place it in the air, and observe the color development of the colony after a few minutes.
结果表明,菌落在MRS-X-gal平皿上呈淡蓝色。The results showed that the colony was light blue on the MRS-X-gal plate.
8、发酵乳杆菌CMS-H002的电镜检查8. Electron microscope examination of Lactobacillus fermentum CMS-H002
扫描电镜:SEM:
发酵乳杆菌CMS-H002经固定,并经梯度脱水、梯度置换、喷铂金镀膜,之后于电镜观察,细菌表面光滑、完整,形态均一,呈短棒状,两头钝圆,无芽孢,见图2。Lactobacillus fermentum CMS-H002 was fixed, dehydrated by gradient, replaced by gradient, sprayed with platinum coating, and then observed under the electron microscope. The surface of the bacteria was smooth, complete, uniform in shape, short rod-shaped, blunt at both ends, and without spores.
透射电镜:TEM:
发酵乳杆菌CMS-H002经固定,并经梯度脱水、包埋、切片,醋酸铀、硝酸铅双重染色,之后于透射电镜观察,细菌形态规则,胞壁结构完整,无肿胀及出芽,胞质均匀,未发现异常颗粒,未见病毒、支原体等外源因子,见图3。Lactobacillus fermentum CMS-H002 was fixed, dehydrated by gradient, embedded, sectioned, double-stained with uranyl acetate and lead nitrate, and then observed under a transmission electron microscope. The bacterial shape was regular, the cell wall structure was complete, no swelling and budding, and the cytoplasm was uniform , no abnormal particles were found, and no exogenous factors such as viruses and mycoplasma were found, see Figure 3.
9、质粒检测9. Plasmid detection
收集菌泥按常规方法抽提质粒,1.0%琼脂糖凝胶电泳检测发酵乳杆菌CMS-H002是否存在质粒。检测结果未见质粒。Collect the sludge and extract the plasmid according to the conventional method, and detect whether there is a plasmid in Lactobacillus fermentum CMS-H002 by 1.0% agarose gel electrophoresis. The test results showed no plasmid.
10、遗传特点10. Genetic characteristics
收集发酵乳杆菌CMS-H002的菌泥并按常规方法抽提DNA,用乳酸杆菌的特异性引物进行乳酸菌16s rDNA的PCR扩增,1.0%琼脂糖凝胶电泳检测PCR产物,可见400bp处有一亮带与目标片段大小相同。Collect the sludge of Lactobacillus fermentum CMS-H002 and extract the DNA according to the conventional method, use the specific primers of Lactobacillus to perform PCR amplification of 16s rDNA of Lactobacillus, and detect the PCR product by 1.0% agarose gel electrophoresis. It can be seen that there is a bright spot at 400bp The band is the same size as the target fragment.
11、代谢产物的测定11. Determination of metabolites
(1)标准液的制备:(1) Preparation of standard solution:
醇和挥发性脂肪酸(VFA)标准液的制备:将甲酸37μl、乙酸57μl、丙酸74μl、异丁酸93μl、丁酸92μl、异戊酸109μl、戊酸108μl、己酸125μl、乙醇100μl、丙醇5μl、异丁醇5μl、丁醇10μl、异戊醇0.5μl、戊醇0.5μl加蒸馏水定容至100ml,即为10mmol/L的标准液。Preparation of alcohol and volatile fatty acid (VFA) standard solution: 37 μl of formic acid, 57 μl of acetic acid, 74 μl of propionic acid, 93 μl of isobutyric acid, 92 μl of butyric acid, 109 μl of isovaleric acid, 108 μl of valeric acid, 125 μl of hexanoic acid, 100 μl of ethanol, propanol Add 5μl of isobutanol, 5μl of isobutanol, 10μl of butanol, 0.5μl of isoamyl alcohol, and 0.5μl of amyl alcohol to 100ml with distilled water, which is the standard solution of 10mmol/L.
非挥发性脂肪酸(NVFA)标准液的配制:将乳酸84μl、苯乙酸60mg、琥珀酸60mg加蒸馏水至100ml,即为10mmol/L的标准液。Preparation of non-volatile fatty acid (NVFA) standard solution: Add 84 μl of lactic acid, 60 mg of phenylacetic acid, and 60 mg of succinic acid to 100 ml of distilled water to obtain a 10 mmol/L standard solution.
(2)VFA制备:选用偏磷酸法(2) VFA preparation: use metaphosphoric acid method
取标准液或培养物1ml加偏磷酸0.5ml,调整pH值在2.0以下,37℃匀化2h,10000转/分,4℃离心2min,取上清1μl用于进样分析。Take 1ml of standard solution or culture, add 0.5ml of metaphosphoric acid, adjust the pH value below 2.0, homogenize at 37°C for 2h, centrifuge at 10,000 rpm, 4°C for 2min, and take 1μl of supernatant for sample analysis.
(3)NVFA制备:选用甲醇硫酸法(3) NVFA preparation: choose methanol sulfuric acid method
取标准液或培养物2ml加50%硫酸0.2ml,调整PH值在2.0以下,取硫酸酸性培养物1ml,加入甲醇溶液1ml和50%硫酸0.1ml,将样品煮沸5分钟或室温下过夜,加入氯仿0.5ml,混匀后静置片刻,1000转/分,4℃离心2min,取氯仿层1μl用于进样分析。Take 2ml of standard solution or culture, add 0.2ml of 50% sulfuric acid, adjust the pH value below 2.0, take 1ml of sulfuric acid acidic culture, add 1ml of methanol solution and 0.1ml of 50% sulfuric acid, boil the sample for 5 minutes or overnight at room temperature, add Chloroform 0.5ml, mix well, let stand for a while, centrifuge at 1000 rpm, 4°C for 2min, take 1μl of chloroform layer for sample injection analysis.
(4)标本及标准品中各物质的分析:(4) Analysis of various substances in specimens and standards:
分别取提取后的样品和标准品1μl进样分析,由日本岛津GC2010型气相色谱检测仪(GC)检测各物质的保留时间并以标准品作为样本中该物质的定量标准。1 μl of the extracted samples and standard samples were taken for analysis, and the retention time of each substance was detected by a Shimadzu GC2010 gas chromatograph (GC), and the standard was used as the quantitative standard of the substance in the sample.
结果表明,发酵乳杆菌CMS-H002的VFA主要为乳酸,出峰时间为7.467秒。NVFA主要为乙酸,还有少量琥珀酸,出峰时间为7.947秒。The results showed that the VFA of Lactobacillus fermentum CMS-H002 was mainly lactic acid, and the peak eluting time was 7.467 seconds. NVFA is mainly acetic acid, with a small amount of succinic acid, and the peak time is 7.947 seconds.
实施例2Example 2
发酵乳杆菌CMS-H002灌肠对小鼠溃疡性结肠炎的疗效观察Observation on curative effect of Lactobacillus fermentum CMS-H002 enema on ulcerative colitis in mice
对于经5% DSS诱导Balb/c小鼠溃疡性结肠炎模型,分别给予生理盐水、SASP、发酵乳杆菌CMS-H002、双歧杆菌0501、c120等保留灌肠,观察各组小鼠的体重、死亡情况、大便性状、便血情况、结肠长度、DAI积分以及肠黏膜病理改变等。结果表明:发酵乳杆菌CMS-H002能够减轻小鼠的腹泻、出血、体重减轻等症状,也能减轻肠道粘膜的损伤及炎性细胞浸润等,作用均明显好于模型组、SASP组、双歧杆菌各组。能有效治疗小鼠溃疡性结肠炎。For the ulcerative colitis model of Balb/c mice induced by 5% DSS, the retention enemas of physiological saline, SASP, Lactobacillus fermentum CMS-H002, Bifidobacterium 0501, c120, etc. were given respectively, and the weight and death of mice in each group were observed. condition, stool properties, hematochezia, colon length, DAI score, and intestinal mucosal pathological changes. The results showed that: Lactobacillus fermentum CMS-H002 can relieve symptoms such as diarrhea, bleeding, and weight loss in mice, and can also reduce intestinal mucosal damage and inflammatory cell infiltration, etc., and the effects are significantly better than those of the model group, SASP group, double groups of Mycobacteria. Can effectively treat ulcerative colitis in mice.
一、实验材料和方法:1. Experimental materials and methods:
1、实验材料:1. Experimental materials:
1.1 实验动物:Balb/c小鼠,SPF级,雄性,6-8周龄,体重20±2克,购于湖南农业大学东创实验动物科技服务部,饲养于清洁级动物房。1.1 Experimental animals: Balb/c mice, SPF grade, male, 6-8 weeks old, weighing 20±2 grams, were purchased from Dongchuang Experimental Animal Technology Service Department of Hunan Agricultural University, and were kept in a clean animal room.
1.2 主要试剂:乙酸钠(广东省台山市化工厂、批号20030401)、硫酸镁(河南省焦作市化工三厂)、硫酸锰(河南焦作市化工厂、批号981014)、蛋白胨(北京奥博星生物技术责任有限公司、批号20040615)、酵母浸粉(北京奥博星生物技术责任有限公司、批号20030825)、柠檬酸铵(湖南湘中精细化学品厂、批号20030409)、葡萄糖(湖南师大化学试剂厂、批号20040208)、牛肉粉(北京奥博星生物技术责任有限公司、20040607)、吐温-80(西安富力化学厂、批号021125)、磷酸二氢钾(河南省焦作市化工三厂、批号20010404)、琼脂(北京奥博星生物技术责任有限公司)、柳氮磺胺吡啶片(SASP,上海福达制药有限公司、批号041207)、DSS(分子量5000,Sigma公司)。1.2 Main reagents: sodium acetate (Taishan Chemical Factory, Guangdong Province, batch number 20030401), magnesium sulfate (Jiaozuo Chemical Factory No. Co., Ltd., batch number 20040615), yeast extract powder (Beijing Aoboxing Biotechnology Co., Ltd., batch number 20030825), ammonium citrate (Hunan Xiangzhong Fine Chemical Factory, batch number 20030409), glucose (Hunan Normal University Chemical Reagent Factory, Batch number 20040208), beef powder (Beijing Aoboxing Biotechnology Co., Ltd., 20040607), Tween-80 (Xi'an R&F Chemical Factory, batch number 021125), potassium dihydrogen phosphate (No. 3 Chemical Factory of Jiaozuo City, Henan Province, batch number 20010404), Agar (Beijing Aoboxing Biotechnology Co., Ltd.), Sulfasalazine Tablets (SASP, Shanghai Fuda Pharmaceutical Co., Ltd., batch number 041207), DSS (molecular weight 5000, Sigma Company).
2、实验方法:2. Experimental method:
2.1实验细菌:发酵乳杆菌CMS-H002及双歧杆菌(0501菌株和c120菌株),其中双歧杆菌(0501菌株和c120菌株)是本实验室从健康婴幼儿粪便和成人十二指肠液中分离、鉴定所得。三种菌分别在MRS培养基中厌氧培养24小时后收集菌落,用分光光度计计数后,用无菌生理盐水稀释为1×109CFU/ml、1×107CFU/ml、1×105CFU/ml后备用。2.1 Experimental bacteria: Lactobacillus fermentum CMS-H002 and Bifidobacterium (0501 strain and c120 strain), among which Bifidobacterium (0501 strain and c120 strain) were isolated from healthy infant feces and adult duodenal fluid in this laboratory , The identification proceeds. The three kinds of bacteria were anaerobically cultured in MRS medium for 24 hours, and the colonies were collected, counted by a spectrophotometer, and diluted with sterile saline to 1×109 CFU/ml, 1×107 CFU/ml, 1× 105 CFU/ml for later use.
2.2动物模型:给Balb/c小鼠5% DSS溶液自由饮用7天造成急性溃疡性结肠炎模型。益生菌(发酵乳杆菌CMS-H002及双歧杆菌0501菌株和c120菌株)、生理盐水、SASP等灌肠均从饮用DSS前2天先开始进行,每只小鼠每天灌肠一次,每次0.3ml/20g。2.2 Animal model: give Balb/
2.3实验分组:实验用Balb/c小鼠120只,随机分成12组,各组均为10只分组情况如下:2.3 Experimental grouping: 120 Balb/c mice were used in the experiment, and were randomly divided into 12 groups, with 10 mice in each group. The grouping situation is as follows:
A、正常对照组:正常饮食,无特殊处理A. Normal control group: normal diet, no special treatment
B、模型对照组:饮用DSS造模B. Model control group: drinking DSS to build models
C、阴性对照组:仅用无菌生理盐水灌肠C. Negative control group: enema with sterile normal saline only
D、阳性对照组:用SASP(20mg/ml)灌肠D. Positive control group: Enema with SASP (20mg/ml)
E、治疗一组:DSS造模+1×105CFU/ml 0501菌液灌肠E. Treatment group: DSS modeling + 1×105 CFU/ml 0501 bacterial liquid enema
F、治疗二组:DSS造模+1×107CFU/ml 0501菌液灌肠F. Treatment group two: DSS modeling + 1×107 CFU/ml 0501 bacterial liquid enema
G、治疗三组:DSS造模+1×109CFU/ml 0501菌液灌肠G. Three groups of treatment: DSS modeling + 1×109 CFU/ml 0501 bacterial liquid enema
H、治疗四组:DSS造模+1×109CFU/ml c120菌液灌肠H. Treatment of four groups: DSS modeling + 1×109 CFU/ml c120 bacterial liquid enema
I、治疗五组:DSS造模+1×107CFU/ml 0501菌液灌胃I. Treatment of the fifth group: DSS modeling + 1×107 CFU/ml 0501 bacterial solution orally
J、高剂量0501组:1×109CFU/ml 0501菌液灌肠J. High-dose 0501 group: 1×109 CFU/ml 0501 bacteria liquid enema
K、高剂量c120组:1×109CFU/ml c120菌液灌肠K. High-dose c120 group: 1×109 CFU/ml c120 bacteria liquid enema
L、治疗六组:DSS造模+1×109CFU/ml CMS-H002菌液灌肠L. Six groups of treatment: DSS modeling + 1×109 CFU/ml CMS-H002 bacterial liquid enema
2.4 动物处理:每日观察进食、活动等一般情况,称量体重,观察粪便性状及粪便隐血情况,评估结肠炎严重程度。实验后第9天处死小鼠,游离结肠和远端回肠,取出肛门至盲肠末端的整个结肠和直肠段,观察各组小鼠结肠的大体改变、测量整个结肠长度。用预冷生理盐水将结肠冲洗干净,分别于结肠末端(距肛门1cm)、结肠中段、结肠上段(距盲肠1cm)处各剪取1cm长结肠,福尔马林固定、石蜡包埋、切片,HE染色,观察病理改变。2.4 Animal treatment: daily observation of general conditions such as food intake and activities, weighing of body weight, observation of fecal properties and fecal occult blood, and assessment of the severity of colitis. The mice were sacrificed on the 9th day after the experiment, the colon and distal ileum were freed, and the entire colon and rectum from the anus to the end of the cecum were taken out, and the general changes of the colon of mice in each group were observed and the length of the entire colon was measured. The colon was rinsed with pre-cooled saline, and 1 cm long colons were cut from the end of the colon (1 cm from the anus), the middle of the colon, and the upper part of the colon (1 cm from the cecum), fixed in formalin, embedded in paraffin, and sectioned. HE staining to observe the pathological changes.
2.5 损伤和炎症程度的评估2.5 Assessment of the degree of injury and inflammation
2.5.1 疾病活动情况的评估:每日观察小鼠的体重、大便性状和隐血情况按表3进行评分,得出每只小鼠的DAI积分。2.5.1 Evaluation of disease activity: Daily observation of the body weight, stool properties and occult blood of the mice was scored according to Table 3, and the DAI score of each mouse was obtained.
表3 DAI评分表Table 3 DAI scoring table
*正常大便:成形大便;松散大便:不黏附于肛门的糊状、半成形大便;稀便:可黏附于肛门的稀水样便* Normal stool: formed stool; loose stool: mushy, semi-formed stool that does not adhere to the anus; loose stool: watery stool that can adhere to the anus
2.5.2 组织学损伤的评估:每个切片随机选取至少27个高倍视野(400倍)根据表4评分标准进行计分,取其平均值。2.5.2 Evaluation of histological damage: at least 27 high-power fields (400 times) were randomly selected from each section to be scored according to the scoring standard in Table 4, and the average value was taken.
表4 组织学损伤评分标准Table 4 Scoring criteria for histological damage
二、结果2. Results
1、体重改变1. Weight change
实验前各组小鼠体重差异无显着性,实验后模型组、阴性对照组、阳性对照组、治疗一、二、三、四、五组体重均明显下降,体重差(实验后与实验前的差值)与正常对照组的体重差比较有显着性差异(P<0.05),治疗六组体重稍有下降,与正常对照组的体重差比较无显着性差异(P>0.05),见图4。Before the experiment, there was no significant difference in the body weight of the mice in each group. After the experiment, the body weight of the model group, the negative control group, the positive control group, and the
2、小鼠死亡情况2. Death of mice
实验过程中共有15只小鼠死亡,其中死于下消化道出血10只,慢性肠穿孔1只,不明原因死亡4只。其中死于消化道出血和穿孔的共11只,分别为模型组1只、阴性对照组1只、阳性对照组1只、治疗二组3只、治疗三组2只、治疗四组3只。通过χ2检验死于消化道出血和穿孔的各组小鼠死亡率没有统计学差异,见图5。A total of 15 mice died during the experiment, among which 10 died of lower gastrointestinal bleeding, 1 died of chronic intestinal perforation, and 4 died of unknown reasons. Among them, 11 died of gastrointestinal bleeding and perforation, including 1 in the model group, 1 in the negative control group, 1 in the positive control group, 3 in the second treatment group, 2 in the third treatment group, and 3 in the fourth treatment group. There was no statistical difference in the death rate of mice in each group that died of gastrointestinal bleeding and perforation byχ2 test, as shown in Figure 5.
3、粪便性状及便血情况3. Stool properties and blood in stool
模型组、阴性对照组在饮用DSS 3天后部分小鼠出现粪便隐血阳性,饮用DSS 4天后部分小鼠出现稀糊状肉眼血便;阳性对照组各小鼠饮用DSS 4天后均开始出现肉眼血便;治疗一组、二组、三组、四组均在饮用DSS 2天后部分小鼠出现粪便隐血阳性,饮用DSS 3天后出现稀糊状肉眼血便;治疗五组在饮用DSS 4天后部分小鼠出现粪便隐血阳性,饮用DSS5天后出现稀糊状肉眼血便;治疗六组饮用DSS 3天后有2只小鼠出现粪便隐血阳性,但一直未出现肉眼血便;正常对照组无改变。饮用DSS 7天时各组小鼠便血情况见表5。In the model group and the negative control group, after drinking DSS for 3 days, some mice showed positive fecal occult blood, and after drinking DSS for 4 days, some mice showed loose and mushy gross bloody stools; after drinking DSS for 4 days, all mice in the positive control group began to have gross bloody stools; After drinking DSS for 2 days, some mice in
表5 实验结束时各组小鼠便血情况Table 5 The blood in the stool of each group of mice at the end of the experiment
4、小鼠结肠长度改变4. Changes in the length of the mouse colon
实验后饮用DSS的模型组、阴性对照组、阳性对照组、治疗一、二、三、四、五、六组结肠均缩短,结肠长度与正常对照组比较有显着性差异,但治疗六组结肠长度长于模型组、阴性对照组、阳性对照组、治疗一、二、三、四、五组,与模型组、阴性对照组、阳性对照组比较有显着性差异,见图6。After the experiment, the colons of the model group, negative control group, positive control group, and
5、DAI计分5. DAI scoring
治疗六组的DAI计分较模型组、阳性对照组明显降低,差异具有显着性(P均<0.05),见图7。The DAI score of the six treatment groups was significantly lower than that of the model group and the positive control group, and the difference was significant (all P<0.05), as shown in Figure 7.
6、组织病理学评分:6. Histopathological scoring:
模型组、阴性对照组、阳性对照组及治疗一、二、三、四、五组结肠粘膜上皮细胞广泛缺失,腺体大多数不完整,炎症细胞广泛浸润,呈典型炎症改变。而治疗六组结肠粘膜腺体基本完整,局部有少量炎性细胞浸润或隐窝破坏,组织学评分治疗六组较模型组、阴性对照组、阳性对照组降低,有显着性差异(P<0.05),见图8。各组小鼠结肠病理切片,见图9a~1。In the model group, negative control group, positive control group, and
以上结果表明,发酵乳杆菌CMS-H002菌液灌肠对溃疡性结肠炎小鼠能减轻小鼠的腹泻、出血、体重减轻等症状,也能减轻肠道粘膜的损伤及炎性细胞浸润,较模型组和SASP灌肠组明显减轻。通过使用双歧杆菌菌液灌肠治疗DSS诱导的UC小鼠时,我们发现两株双歧杆菌菌液灌肠能够加重溃疡性结肠炎小鼠的腹泻、出血、和体重减轻,也加重其肠道粘膜的损伤及炎性细胞浸润,较模型组、阴性对照组损伤更严重。但两株双歧杆菌给未用DSS诱导的小鼠灌肠时,小鼠一般情况及肠粘膜病理检查与正常对照组没有差异,因此双歧杆菌或者双歧杆菌的某些菌株可能在UC的发病过程中加重了UC的结肠粘膜损伤。The above results show that the enema of Lactobacillus fermentum CMS-H002 bacteria liquid can reduce the symptoms of diarrhea, bleeding, weight loss and other symptoms of mice with ulcerative colitis, and can also reduce the damage of intestinal mucosa and the infiltration of inflammatory cells, compared with the model group and SASP enema group were significantly relieved. When treating DSS-induced UC mice with bifidobacteria liquid enema, we found that two strains of bifidobacterium liquid enema can aggravate diarrhea, bleeding, and weight loss in mice with ulcerative colitis, and also aggravate their intestinal mucosa The damage and inflammatory cell infiltration were more serious than those in the model group and the negative control group. However, when the two strains of Bifidobacterium were given enema to mice not induced by DSS, the general condition of the mice and the pathological examination of the intestinal mucosa were not different from those of the normal control group. The colonic mucosal injury of UC was aggravated during the process.
Claims (8)
Translated fromChinesePriority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB2006101571871ACN100506973C (en) | 2006-11-29 | 2006-11-29 | Lactobacillus fermentum CMS-H002 and its application |
| HK08104477.6AHK1110097B (en) | 2008-04-23 | Lactobacillus fermenti, cms-h002 and its applying |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB2006101571871ACN100506973C (en) | 2006-11-29 | 2006-11-29 | Lactobacillus fermentum CMS-H002 and its application |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101067119A CN101067119A (en) | 2007-11-07 |
| CN100506973Ctrue CN100506973C (en) | 2009-07-01 |
Family
ID=38879818
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB2006101571871AExpired - Fee RelatedCN100506973C (en) | 2006-11-29 | 2006-11-29 | Lactobacillus fermentum CMS-H002 and its application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN100506973C (en) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101649352B (en)* | 2009-09-21 | 2011-11-09 | 内蒙古农业大学 | Quick qualitative and quantitative measuring method of fermented lactobacillus in probiotic milk products |
| KR101452234B1 (en)* | 2013-07-22 | 2014-10-22 | (주) 피엘바이오 | Novel Lactobacillus fermentum isolated from healthy adults in the Korean longevity villages which promote regular bowel movement |
| CN106434433B (en)* | 2016-09-08 | 2019-05-10 | 济南康多宝生物技术有限公司 | A kind of novel fermentation lactobacillus and its application in sour milk beverage |
| CN106381278B (en)* | 2016-09-08 | 2019-05-10 | 济南康多宝生物技术有限公司 | A kind of lactobacillus preparation and its application in gastric ulcer treatment |
| CN106967630B (en)* | 2016-12-01 | 2019-12-10 | 广东省微生物研究所(广东省微生物分析检测中心) | Lactobacillus fermentum and application thereof |
| WO2018112740A1 (en)* | 2016-12-20 | 2018-06-28 | 深圳华大基因研究院 | Lactobacillus gasseri, culture method therefor and application thereof |
| CN112236154B (en)* | 2018-05-31 | 2024-10-18 | 深圳华大生命科学研究院 | A composition and its application |
| CN109486700A (en)* | 2018-08-31 | 2019-03-19 | 石家庄君乐宝乳业有限公司 | Lactobacillus paracasei N1115 prevents application and the corresponding probiotic powder, application of colitis |
| CN109362882B (en)* | 2018-12-29 | 2022-04-15 | 重庆第二师范学院 | Lactobacillus fermentum CQPC07 and its application in preparing food or medicine for improving ulcerative colitis |
| CN109481476B (en)* | 2018-12-29 | 2021-09-07 | 重庆第二师范学院 | Application of Lactobacillus fermentum CQPC04 in preparing food or medicine for improving ulcerative colitis |
| CN110787334A (en)* | 2019-10-29 | 2020-02-14 | 中国人民解放军陆军军医大学第一附属医院 | Fungus liquid enema device |
| CN113122473B (en)* | 2021-04-10 | 2022-08-02 | 江南大学 | Lactobacillus BB1 enhancing memory ability and its application |
| CN114262678B (en)* | 2021-12-28 | 2023-05-26 | 杭州普元生物技术有限公司 | Lactobacillus fermentum Pm007 and application thereof |
| CN119979364A (en)* | 2023-11-10 | 2025-05-13 | 江南大学 | Application of Limosilactobacillus fermentum WMSN-2 in preventing necrotizing enterocolitis in newborns |
- 2006
- 2006-11-29CNCNB2006101571871Apatent/CN100506973C/ennot_activeExpired - Fee Related
Non-Patent Citations (1)
| Title |
|---|
| 益生菌治疗炎症性肠病的研究进展. 张婵等.中国微生态学杂志,第18卷第2期. 2006* |
Also Published As
| Publication number | Publication date |
|---|---|
| HK1110097A1 (en) | 2008-07-04 |
| CN101067119A (en) | 2007-11-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100506973C (en) | Lactobacillus fermentum CMS-H002 and its application | |
| CN114574390B (en) | Bifidobacterium longum subspecies infantis for relieving colonitis and application thereof | |
| CN114164134B (en) | Long subspecies of Bifidobacterium longum capable of preventing and alleviating symptoms of colitis and its application | |
| CN115786198B (en) | Lactobacillus paracasei and application thereof | |
| CN114231470B (en) | Lactobacillus acidophilus capable of relieving ulcerative colitis and application thereof | |
| CN112535693B (en) | Mixed lactobacillus for preventing and treating ulcerative colitis and application thereof | |
| CN112760250B (en) | Rumen lactobacillus for relieving colitis and application thereof | |
| CN112239739B (en) | Lactobacillus plantarum capable of relieving ETEC (enterotoxigenic enterobacteria) induced diarrhea and application thereof | |
| CN113897302A (en) | A kind of bifidobacteria that can relieve colitis and its application | |
| CN114107121B (en) | Bacillus coagulans and application thereof in treatment of alcoholic liver disease | |
| WO2022011902A1 (en) | Lactobacillus paracasei and application thereof in preparing medicine for treating ulcerative colitis | |
| CN116836879B (en) | Lactobacillus fermentum with colon cancer cell growth inhibition effect and application thereof | |
| CN112592871B (en) | Lactobacillus casei JYLC-374 and application thereof in product for improving male prostate | |
| CN112538441B (en) | Lactobacillus reuteri CCFM1144 for relieving diarrhea caused by ETEC and application thereof | |
| WO2008064521A1 (en) | Lactobacillus fermentum cms-h002 and use thereof | |
| CN119842563B (en) | Dorsal bacteria EMF18-06B1D and its use | |
| CN117099962A (en) | New applications of Lactobacillus fermentans LPFerfectus001 | |
| CN118620789A (en) | A strain of Lactococcus lactis and its application in fermentation of Ganoderma lucidum spores | |
| CN115029270B (en) | Lactobacillus sake capable of reducing intestinal pro-inflammatory cytokines and application thereof | |
| WO2021143621A1 (en) | Anaerostipes sp b2131 strain and use thereof in inflammatory bowel diseases | |
| CN115813950A (en) | Application of lactein in preparing medicine for treating acute ulcerative colitis | |
| WO2018112740A1 (en) | Lactobacillus gasseri, culture method therefor and application thereof | |
| CN118853454A (en) | A strain of Lactobacillus reuteri and its application in fermentation of broken-wall Ganoderma lucidum spore powder | |
| CN117903980A (en) | Enterococcus hirae and its application | |
| CN112592865B (en) | Rumen lactobacillus capable of protecting intestinal barrier and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code | Ref country code:HK Ref legal event code:DE Ref document number:1110097 Country of ref document:HK | |
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| REG | Reference to a national code | Ref country code:HK Ref legal event code:GR Ref document number:1110097 Country of ref document:HK | |
| C17 | Cessation of patent right | ||
| CF01 | Termination of patent right due to non-payment of annual fee | Granted publication date:20090701 Termination date:20111129 |