Chemical compound
Pharmaceutical compound
Tropicamide , sold under the brand nameMydriacyl among others, is a medication used todilate the pupil and help with theexamination of the eye .[ 3] back of the eye .[ 4] eye drops .[ 3] [ 3]
Common side effects includeblurry vision ,increased intraocular pressure , andsensitivity to light .[ 3] psychosis , particularly in children.[ 3] pregnancy is safe for the fetus.[ 5] antimuscarinic part of theanticholinergic family of medications.[ 3] [ 3]
Tropicamide was approved for medical use in the United States in 1960.[ 3] World Health Organization's List of Essential Medicines .[ 6]
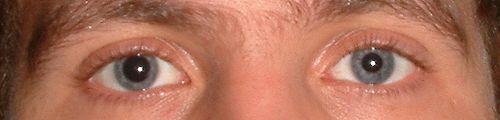
Anisocoria caused by tropicamide instilled into the subject's right eye only.Tropicamide is anantimuscarinic drug that produces short actingmydriasis (dilation of thepupil ) andcycloplegia [ 7] eye drops . It is used to allow better examination of thelens ,vitreous humor , andretina . Due to its relatively short duration of effect (4–8 hours), it is typically used duringeye examinations such as thedilated fundus examination , but it may also be used before or aftereye surgery . Cycloplegic drops are often also used to treat anterioruveitis , decreasing the risk of posteriorsynechiae and decreasing inflammation in the anterior chamber of the eye.[citation needed
Tropicamide is occasionally administered in combination withp -hydroxyamphetamineParemyd ), which is asympathomimetic . The use of the sympathomimetic drug causes the iris dilator muscle to be directly stimulated, causing increased dilation. In the United States, the sympathomimetic drop most commonly used along with tropicamide, is 2.5%phenylephrine hydrochloride (brand name AK-Dilate).[citation needed
Tropicamide induces transient stinging and a slight and transient rise inintraocular pressure in the majority of patients. It may cause redness orconjunctivitis (inflammation) and alsoblurs near vision for a short while after instillation (care must be taken, and the patient must only drive when vision returns to normal). Tropicamide may, in very rare cases,[ 8] acute angle-closure glaucoma . This tends to be in patients with narrowanterior chamber angles , and closure risk must be assessed by the practitioner prior to instillation.[citation needed
Tropicamide is often preferred toatropine because atropine has a longerhalf-life , causing prolonged dilation and blurry vision for up to a week. Atropine has lesssting effect , but can be toxic or fatal if ingested in large quantities by children or adults.[citation needed
Witheye drops ,systemic effects are minimal to nonexistent due to very low absorption into the bloodstream.[ 9]
Tropicamide is ananticholinergic .[ 10] antimuscarinic , acting as aselective muscarinic acetylcholine M1 andM4 receptor antagonist .[ 10] [ 11] deliriant effects.[ 10]
Tropicamide is sometimes abused (injected intravenously e.g. byinsulin syringe ) as an inexpensive recreationaldeliriant drug (along withnaphazoline ). This was initially reported in Russia, but has subsequently spread to various other countries in the former Soviet Union and around Europe, and later in the United States.[ 12] [ 13] [ 14]
Tropicamide severely destroys internal organs when injected.[ 15] [ 16]
Tropicamide has achiral center and twoenantiomers . Medications areracemates .[ 17]
Enantiomers R )-TropicamidCAS: 92934-63-9 S )-TropicamidCAS: 92934-64-0
^ "Summary for ARTG Entry: 25356 Mydriacyl tropicamide 0.5% eye drops bottle" .Therapeutic Goods Administration the original on 5 June 2023. Retrieved5 June 2023 .^ "Mydriacyl 1% eye drops, solution - Summary of Product Characteristics (SmPC)" .Electronic Medicines Compendium the original on 27 June 2022. Retrieved29 July 2020 .^a b c d e f g h "Tropicamide" .Drugs.com American Society of Health-System Pharmacists .Archived from the original on 28 December 2016. Retrieved8 December 2016 .^ Stuart MC, Kouimtzi M, Hill SR, eds. (2009).WHO Model Formulary 2008 .World Health Organization . p. 314.hdl :10665/44053 ISBN 978-92-4-154765-9 ^ "Tropicamide ophthalmic Use During Pregnancy" .Drugs.com Archived from the original on 28 December 2016. Retrieved28 December 2016 .^ World Health Organization model list of essential medicines: 21st list 2019 . Geneva:World Health Organization . 2019.hdl :10665/325771 ^ Manny RE, Hussein M, Scheiman M, Kurtz D, Niemann K, Zinzer K (July 2001)."Tropicamide (1%): an effective cycloplegic agent for myopic children" .Investigative Ophthalmology & Visual Science 42 (8):1728– 35.PMID 11431435 . Archived fromthe original on 2013-01-12. ^ Liew G, Mitchell P, Wang JJ, Wong TY (January 2006)."Fundoscopy: to dilate or not to dilate?" .BMJ 332 (7532): 3.doi :10.1136/bmj.332.7532.3 .PMC 1325111 PMID 16399709 . ^ Vuori ML, Kaila T, Iisalo E, Saari KM (1994-01-01). "Systemic absorption and anticholinergic activity of topically applied tropicamide".Journal of Ocular Pharmacology .10 (2):431– 437.doi :10.1089/jop.1994.10.431 .PMID 8083562 . ^a b c Lakstygal AM, Kolesnikova TO, Khatsko SL, Zabegalov KN, Volgin AD, Demin KA, Shevyrin VA, Wappler-Guzzetta EA, Kalueff AV (May 2019). "DARK Classics in Chemical Neuroscience: Atropine, Scopolamine, and Other Anticholinergic Deliriant Hallucinogens".ACS Chem Neurosci .10 (5):2144– 2159.doi :10.1021/acschemneuro.8b00615 .PMID 30566832 . ^ Lavrador M, Cabral AC, Veríssimo MT, Fernandez-Llimos F, Figueiredo IV, Castel-Branco MM (January 2023)."A Universal Pharmacological-Based List of Drugs with Anticholinergic Activity" .Pharmaceutics .15 (1): 230.doi :10.3390/pharmaceutics15010230 PMC 9863833 PMID 36678858 . ^ Bersani FS, Corazza O, Simonato P, Mylokosta A, Levari E, Lovaste R, Schifano F (2013). "Drops of madness? Recreational misuse of tropicamide collyrium; early warning alerts from Russia and Italy".General Hospital Psychiatry 35 (5):571– 3.doi :10.1016/j.genhosppsych.2013.04.013 .PMID 23706777 . ^ Bersani FS, Imperatori C, Prilutskaya M, Kuliev R, Corazza O (July 2015). "Injecting eye-drops: a mini-review on the non-clinical use of tropicamide".Hum Psychopharmacol .30 (4):262– 4.doi :10.1002/hup.2481 .PMID 26216560 .S2CID 190289 . ^ Bellman V, Ukolova A, Erovichenkova E, Lam S, Srivastava HK, Bruce J, Burgess DM (November 2022)."Abuse of tropicamide eye drops: review of clinical data" .Braz J Psychiatry 44 (5):522– 531.doi :10.47626/1516-4446-2021-2446 .PMC 9561840 PMID 35739063 . ^ "Bellman, V., Ukolova, A., Erovichenkova, E., Lam, S., Srivastava, H. K., Bruce, J., & Burgess, D. M. (2022). Abuse of tropicamide eye drops: review of clinical data. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999)".Braz J Psychiatry .44 . 2 Nov 2022. ^ "Krokodil: Russia's Deadliest Drug (NSFW)" .YouTube ^ Rote Liste (in German). Vol. 57. Frankfurt/Main: Rote Liste Service GmbH. 2017. p. 224.ISBN 978-3-946057-10-9 Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte)
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists 3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g.,brompheniramine ,buclizine ,captodiame ,chlorphenamine (chlorpheniramine) ,cinnarizine ,clemastine ,cyproheptadine ,dimenhydrinate ,dimetindene ,diphenhydramine ,doxylamine ,meclizine ,mequitazine ,perlapine ,phenindamine ,pheniramine ,phenyltoloxamine ,promethazine ,propiomazine ,triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g.,clozapine ,fluperlapine ,olanzapine (+fluoxetine ),rilapine ,quetiapine ,tenilapine ,zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine Bevonium BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol ,+indacaterol ,+neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g.,femoxetine ,paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g.,amoxapine ,maprotiline ,mianserin ,mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g.,amitriptyline (+perphenazine ),amitriptylinoxide ,butriptyline ,cidoxepin ,clomipramine ,desipramine ,desmethyldesipramine ,dibenzepin ,dosulepin (dothiepin) ,doxepin ,imipramine ,lofepramine ,nitroxazepine ,northiaden (desmethyldosulepin) ,nortriptyline ,protriptyline ,quinupramine ,trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Tropine benzilate Trospium chloride Typical antipsychotics (e.g.,chlorpromazine ,chlorprothixene ,cyamemazine (cyamepromazine) ,loxapine ,mesoridazine ,thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (andprodrugs )
Phenethylamines Amphetamines Phentermines Cathinones Phenylisobutylamines (and further-extended) Catecholamines (and close relatives) Cyclized
Phenylalkylpyrrolidines 2-Benzylpiperidines (phenidates ) Phenylmorpholines (phenmetrazines) Phenyloxazolamines (aminorexes) Isoquinolines andtetrahydroisoquinolines 2-Aminoindanes 2-Aminotetralins Others / unsorted 1-Aminomethylindanes (e.g.,2CB-Ind ,AMMI ,bromojimscaline ,jimscaline )2-ADN 2-Benzhydrylpyrrolidine 2C-B-5-hemiFLY-α6 (BNAP) 2C-B-PYR 2CBecca 2CB7 2CJP 2CLisaB 2CLisaH 3-Benzazepines (e.g.,fenoldopam ,lorcaserin ,7-chlorolorcaserin ,SCHEMBL5334361 )3-Benzhydrylmorpholine 3-Phenylpiperidines (e.g.,3-phenylpiperidine ,3-PPP ,OSU-6162 (PNU-96391) ,LPH-5 ,LPH-48 ,2C-B-3PIP ,2C-B-3PIP-NBOMe ,2C-B-3PIP-POMe ,Z3517967757 (Z7757) )6-AB AL-1095 Aminochromes (e.g.,adrenochrome ,adrenolutin )Benzocyclobutenes (e.g.,2CBCB-NBOMe ,bromotomscaline ,S33005 ,TCB-2 ,tomscaline )Benzoxepins (e.g.,BBOX ,IBOX ,TFMBOX )Butyltolylquinuclidine Camfetamine Cypenamine (trans -2-phenylcyclopentylamine) Diphenidine Diphenylprolinol Ergolines (e.g.,LSD )Fencamfamin GYKI-52895 HDMP-29 Ivabradine Methoxphenidine Methylmorphenate Milnacipran MT-45 2-Naphthylamine Org 6582 Partial ergolines (e.g.,NDTDI ,RU-27849 ,DEIMDHPCA ,DEMPDHPCA ,DEMPDHPCA-2C-D ,RU-27251 )PF-592,379 Phenylcyclopropylamines (e.g.,DMCPA ,TMT ,tranylcypromine )Phenylpiracetams (e.g.,phenylpiracetam ,MRZ-9547 ,RGPU-95 )Pyridopyrroloquinoxalines (e.g.,lumateperone ,deulumateperone ,IHCH-7079 ,IHCH-7086 ,IHCH-7113 ,ITI-1549 )Tetrahydrobenzopyranylamines (e.g.,CT-5126 )Tolazoline Tricyclics (e.g.,AMDA ,AMDH ,benzoctamine ,dizocilpine ,SpAMDA )ZC-B
Related compounds 2-Furylethylamine 2-Pyrrolylethylamine 3-Pyrrolylethylamine 3-Pyrrolylpropylamine 2-Tetrahydrofurylethylamine 4-Benzylpiperidine 7-AB Alkylamines (e.g.,1,3-DMBA Tooltip 1,3-dimethylbutylamine ,1,4-DMAA Tooltip 1,4-dimethylamylamine ,heptaminol ,iproheptine ,isometheptene ,methylhexanamine/ 1,3-DMAA ,octodrine ,oenethyl ,tuaminoheptane )Benzylamines (e.g.,benzylamine ,α-methylbenzylamine ,MDM1EA ,ALPHA ,M-ALPHA ,pargyline )Benzylpiperazines (e.g.,benzylpiperazine ,MDBZP ,fipexide )Cyclohexylaminopropanes (e.g.,propylhexedrine ,norpropylhexedrine )Cyclopentylaminopropanes (e.g.,isocyclamine ,cyclopentamine )Phenoxyethylamines (e.g.,3,4,5-trimethoxyphenoxyethylamine ,CT-4719 ,ORG-37684 )Phenylalkenylamines (e.g.,phenylbutenamine )Phenylalkynylamines (e.g.,phenylbutynamine )Phenylpiperazines (e.g.,1-phenylpiperazine ,mCPP Tooltip meta-chlorophenylpiperazine ,TFMPP Tooltip trifluoromethylphenylpiperazine ,oMPP Tooltip ortho-methylphenylpiperazine ,pFPP Tooltip para-fluorophenylpiperazine ,pMeOPP Tooltip para-methoxyphenylpiperazine )Phenylpropylamines (e.g.,phenylpropylamine ,homo-MDA ,homo-MDMA )Thienylaminopropanes (thiopropamines) (e.g.,thiopropamine ,methiopropamine ,thiothinone )Phenylbutylamines (e.g.,4-Phenylbutylamine ,4-Phenylpentan-1-amine )

 N
N Y (what is this?) (verify)
Y (what is this?) (verify)