Natural gas liquids
This article does not receive scheduled updates. If you would like to help our coverage grow, considerdonating to Ballotpedia.Contact our team to suggest an update.
 |
|---|
Natural gas liquids (NGLs) arehydrocarbons composed of carbon and hydrogen and include propane, isobutane, butane, ethane, and other liquefied refinery natural gases. Natural gas liquids are separated from their gas state in a natural gas processing plant through condensation, absorption, or other methods. These liquids are used as inputs in petrochemical production. They can also be blended into transportation fuels for use in motor vehicles or used for heating and cooking.[1][2]
Background
Natural gas liquids exist in gaseous form underground and become liquid when exposed to higher pressure and temperatures above the surface. They are separated from natural gas at field facilities or in natural gas processing plants. The content of natural gas liquids varies depending on its form, such as dry gas (which contains little natural gas liquid) and wet gas (which contains higher levels of natural gas liquid). Natural gas liquids have several commercial and industrial uses, including heating, cooking, and gasoline blending. Additionally, the liquids are used as inputs in petrochemical production.[3][4]
Types of NGLs
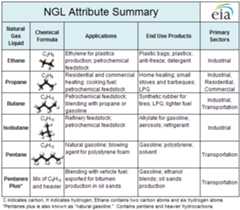
The most commonly used natural gas liquids are propane, ethane, isobutane, and butane. Other natural gas liquids include pentane, a colorless liquid used as a component in various fuels and as a solvent.[3][5]
- Propane is used as an input in petrochemical production or as a fuel for stoves, gas grills, clothes dryers, generators, and water heaters. Propane is generally sold in a compressed cylinder and can be mixed with butane or other natural gas liquids.
- Ethane is used as an input in petrochemical production to produce ethylene, a chemical used to manufacture plastics. Ethane is also used to produce antifreeze, detergent, and other consumer products.
- Isobutane is one of two isomers of butane. Isobutane has three carbon atoms joined to a fourth carbon atom in a clustered branch, while butane has four carbon in a continuous chain. Isobutane is used at oil refineries to produce gasoline and can be used in aerosols and refrigerants.
- Butane is used as an input in petrochemical production to produce synthetic rubber, as a blending component in gasoline, and as lighter fluid.
The graph below shows the types of natural gas liquids, their properties, and their uses by sector.[6]
Production
Oil and natural gas producers extract natural gas liquids during natural gas production at a processing plant. In 1981, the United States produced 580.5 million barrels of natural gas liquids. Natural gas liquid production rose beginning in 2008, when the United States produced 652.8 million barrels of natural gas liquids. In 2016, production of natural gas liquids totaled 1.2 billion barrels. The graph below shows natural gas production by thousand barrels from 1981 to 2016.[7]
Production by liquid type
The table below shows natural gas liquid production by type from 2010 to 2015.[8]
| Natural gas liquid production, 2010-2015 (in thousand barrels of oil) | ||||||
|---|---|---|---|---|---|---|
| Natural gas liquid | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
| Ethane | 317,180 | 337,972 | 356,592 | 354,089 | 398,206 | 412,348 |
| Propane | 213,782 | 230,227 | 260,704 | 300,348 | 359,430 | 417,632 |
| Butane | 56,655 | 57,399 | 65,555 | 80,045 | 100,930 | 121,703 |
| Isobutane | 68,247 | 76,983 | 82,453 | 89,766 | 97,901 | 109,877 |
| Source:U.S. Energy Information Administration, "Natural Gas Plant Field Production" | ||||||
See also
Footnotes
- ↑U.S. Energy Information Administration, “Glossary, N” accessed January 28, 2014
- ↑Investopedia, "Natural gas liquids," accessed March 3, 2017
- ↑3.03.1STI Group, "Guide to Understanding Natural Gas and Natural Gas Liquids," accessed March 3, 2017
- ↑AAPG Wiki, "Natural gas liquids," accessed March 3, 2017
- ↑U.S. Energy Information Administration, "NGL 101 - The Basics," June 6, 2012
- ↑U.S. Energy Information Administration, "What are natural gas liquids and how are they used?" accessed March 3, 2017
- ↑U.S. Energy Information Administration, "U.S. Gas Plant Production of Natural Gas Liquids and Liquid Refinery Gases," accessed March 3, 2017
- ↑U.S. Energy Information Administration, "Natural Gas Plant Field Production," accessed March 3, 2017