DOI:10.1074/JBC.M406576200 - Corpus ID: 26620903
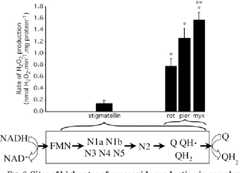
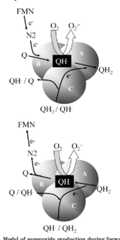
Inhibitors of the Quinone-binding Site Allow Rapid Superoxide Production from Mitochondrial NADH:Ubiquinone Oxidoreductase (Complex I)*
@article{Lambert2004InhibitorsOT, title={Inhibitors of the Quinone-binding Site Allow Rapid Superoxide Production from Mitochondrial NADH:Ubiquinone Oxidoreductase (Complex I)*}, author={Adrian J. Lambert and Martin D. Brand}, journal={Journal of Biological Chemistry}, year={2004}, volume={279}, pages={39414 - 39420}, url={https://api.semanticscholar.org/CorpusID:26620903}}- A. J. LambertM. Brand
- Published inJournal of Biological…17 September 2004
- Chemistry, Biology
Observed rates of superoxide generation by rat skeletal muscle mitochondria under a variety of conditions suggest that quinone-binding site inhibitors can make complex I adopt the highly radical-producing state that occurs during reverse electron transport.
477 Citations
477 Citations
Evidence for Two Sites of Superoxide Production by Mitochondrial NADH-Ubiquinone Oxidoreductase (Complex I)*
- Jason R. TrebergCasey L QuinlanM. Brand
- 2011
Biology, Chemistry
A two-site model of complex I superoxide production is supported; one site in equilibrium with the NAD pool, presumably the flavin of the FMN moiety and the other dependent not only on NAD redox state, but also on protonmotive force and the reduction state of the Q pool.
A Possible Site of Superoxide Generation in the Complex I Segment of Rat Heart Mitochondria
- S. OhnishiT. OhnishiK. Utsumi
- 2005
Biology, Medicine
The results suggest that the major site of superoxide generation is not flavin, but protein-associated ubisemiquinones which are spin-coupled with iron-sulfer cluster N2.
The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria
- L. KussmaulJ. Hirst
- 2006
Biology, Chemistry
The mechanism for the isolated enzyme isolated from bovine heart mitochondria is linked to studies on intact mitochondria, in which superoxide production is enhanced when the NAD+ pool is reduced, and forms a foundation for formulating causative connections between complex I defects and pathological effects.
Superoxide Is Produced by the Reduced Flavin in Mitochondrial Complex I
- Kenneth R PrydeJ. Hirst
- 2011
Biology, Chemistry
The unified mechanism describes how reactive oxygen species production by complex I responds to changes in cellular conditions and establishes a route to understanding causative connections between the enzyme and its pathological effects and to developing rational strategies for addressing them.
Localization of superoxide anion production to mitochondrial electron transport chain in 3-NPA-treated cells.
- A. BácsiMitchell W WoodberryI. Boldogh
- 2006
Biology, Chemistry
The 2-Oxoacid Dehydrogenase Complexes in Mitochondria Can Produce Superoxide/Hydrogen Peroxide at Much Higher Rates Than Complex I*
- Casey L QuinlanRenata L. S. GoncalvesMartin Hey‐MogensenN. YadavaV. BunikM. Brand
- 2014
Biology, Chemistry
The observed rates of H2O2 production over a range of different NAD(P)H reduction levels in isolated skeletal muscle mitochondria under conditions that favored superoxide/H2 O2 production from complex I, the OG DH complex, the BCKDH complex, or the PDH complex were compared.
Superoxide Radical Formation by Pure Complex I (NADH:Ubiquinone Oxidoreductase) from Yarrowia lipolytica*
It is shown that isolated complex I from Yarrowia lipolytica forms superoxide at a rate of 0.15% of the rate measured for catalytic turnover, and that oxygen accepts electrons from FMNH or FMN semiquinone either directly or via more hydrophilic ubiquinone derivatives.
Mitochondrial Complex I superoxide production is attenuated by uncoupling.
- A. DlaskováL. HlavatáJ. JežekP. Ježek
- 2008
Biology, Medicine
High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates.
- F. MullerYuhong LiuH. Van Remmen
- 2008
Biology, Medicine
The present results indicate that reverse-electron transfer-mediated superoxide production can occur under physiologically realistic substrate conditions and suggest that oxaloacetate inhibition of complex II may be an adaptive mechanism to minimize this.
Control of mitochondrial superoxide production by reverse electron transport at complex I
- Ellen L. RobbA. HallM. Murphy
- 2018
Biology, Chemistry
It is concluded that O2̇̄ production by RET is highly responsive to small changes in Δp and the CoQ redox state, indicating that complex I RET represents a major mode of mitochondrial redox signaling.
...
36 References
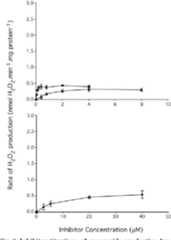
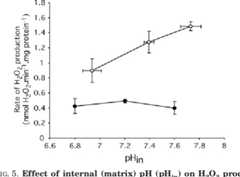
Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane.
- A. J. LambertM. Brand
- 2004
Biology, Chemistry
The conclusions are that the effect of protonmotive force was mostly direct, and not indirect through changes in the redox state of the ubiquinone pool, and that the production of superoxide by complex I during reverse electron transport was at least 3-fold more sensitive to the pH gradient than to the membrane potential.
Topology of Superoxide Production from Different Sites in the Mitochondrial Electron Transport Chain*
- J. St-PierreJulie A. BuckinghamStephen J RoebuckM. Brand
- 2002
Biology, Chemistry
The results do not support the idea that mitochondria produce considerable amounts of reactive oxygen species under physiological conditions and the proportion of electron flow giving rise to hydrogen peroxide with palmitoyl carnitine as substrate is more than an order of magnitude lower than commonly cited values.
The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron–sulfur cluster N2
- M. L. GenovaBarbara VenturaG. Lenaz
- 2001
Chemistry, Biology
Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria.
- J. TurrensA. Boveris
- 1980
Biology, Medicine
An O2- -dependent autocatalytic process that requires NADH, submitochondrial particles and adrenaline is described.
Characterization of Superoxide-producing Sites in Isolated Brain Mitochondria*
- A. KudinN. Bimpong-ButaS. VielhaberC. ElgerW. Kunz
- 2004
Biology, Medicine
It is proposed that reactive oxygen species can activate a self-accelerating vicious cycle causing mitochondrial damage and neuronal cell death in rat and human brain because short-term incubation of rat brain mitochondria with H2O2 induced increased H2 O2 production at this site.
Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria.
- J. TurrensA. AlexandreA. Lehninger
- 1985
Medicine, Chemistry
Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space.
- Derick HanEverett WilliamsE. Cadenas
- 2001
Biology, Medicine
Production and release of superoxide anion towards the cytosolic side of the inner mitochondrial membrane are confirmed and co-treatment of mitoplasts with myxothiazol and antimycin A abolished the EPR signal, suggesting that ubisemiquinone autoxidation at the outer site of the complex-III ubiquinone pool is a pathway for superoxideAnion formation and subsequent release into the intermembrane space.
Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state.
- Y. KushnarevaA. MurphyA. Andreyev
- 2002
Medicine, Biology
A model of thermodynamic control of mitochondrial ROS production is proposed which suggests that the ROS-generating site of complex I is the Fe-S centre N-1a, rather than in complex III, as previously suggested.
Related Papers
Showing 1 through 3 of 0 Related Papers