Drug Summary
What Is Diazepam?
Diazepam is a benzodiazepine indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. In acute alcohol withdrawal, diazepam may be useful in the symptomatic relief of acute agitation,tremor, impending or acutedelirium tremens and hallucinations. Diazepam tablets are a useful adjunct for the relief ofskeletal muscle spasm due to reflex spasm to localpathology (such as inflammation of the muscles or joints, or secondary totrauma),spasticity caused byupper motor neuron disorders (such ascerebral palsy and paraplegia), athetosis, and stiff-man syndrome. Oral diazepam tablets may be used adjunctively in convulsive disorders, although it has not proved useful as the sole therapy. Diazepam is available ingeneric form.
What Are Side Effects of Diazepam?
Common side effects of diazepam include:
- drowsiness
- fatigue
- muscle weakness, and
- problems with coordination (ataxia)
Dosage for Diazepam
Dosage of diazepam is individualized based on the condition being treated and the patient's response to the medication.
What Drugs, Substances, or Supplements Interact with Diazepam?
Diazepam may interact with phenothiazines, antipsychotics, anxiolytics/sedatives, hypnotics, anticonvulsants,narcotic analgesics, anesthetics,sedativeantihistamines, narcotics, barbiturates, MAO inhibitors and otherantidepressants, alcohol, antacids, cimetidine, ketoconazole, fluvoxamine, fluoxetine, omeprazole, phenytoin, Tell your doctor all medications and supplements you use.
Diazepam During Pregnancy and Breastfeeding
Diazepam is not recommended for use during pregnancy; it may harm a fetus. Diazepam passes into breast milk. Breastfeeding while taking diazepam is not recommended.Withdrawal symptoms may occur if you suddenly stop taking diazepam.
Additional Information
Our Diazepam Side Effects Drug Center provides a comprehensive view of available drug information on the potential side effects when taking this medication.
Description for Diazepam
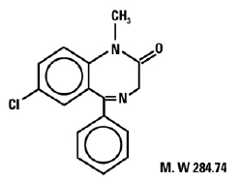
Diazepam is a benzodiazepine derivative. The chemical name of diazepam is 7-chloro-1,3-dihydro-1- methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, insoluble in water. The molecular formula is C16H13ClN2O and the molecular weight is 284.74. The structural formula is as follows:
 |
Diazepam is available for oral administration as tablets containing 2 mg, 5 mg or 10 mg diazepam, USP. In addition to the active ingredient diazepam, each tablet contains the following inactive ingredients: colloidalsilicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch (corn) and sodium lauryl sulfate. The following coloring agents are employed:
2 mg - none
5 mg - FD&C Yellow No.6 Aluminum Lake
10 mg - FD&C Blue No. 1 Aluminum Lake and D&C Yellow No. 10 Aluminum Lake.
Description for Diazepam
Diazepam Injection, USP is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each milliliter (mL) contains 5 mg diazepam; 40% propylene glycol; 10% alcohol; 5% sodium benzoate and benzoic acid added as buffers; and 1.5% benzyl alcohol added as a preservative. pH 6.6 (6.2 to 6.9). Note: Solution may appear colorless to light yellow.
Diazepam is a benzodiazepine derivative chemically designated as 7-chloro-1,3-dihydro-1-methyl-5- phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless crystalline compound, insoluble in water, with the following molecular structure:
 |
Uses for Diazepam
Diazepam tablets, USP are indicated for the management ofanxiety disorders or for the short-term relief of the symptoms of anxiety.Anxiety or tension associated with the stress of everyday life usually does notrequire treatment with an anxiolytic.
In acute alcohol withdrawal, diazepam tablets may beuseful in the symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
Diazepam tablets are a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such asinflammation of the muscles or joints, or secondary to trauma), spasticity causedby upper motor neuron disorders (such as cerebral palsy and paraplegia), athetosis, and stiff-man syndrome.
Oral diazepam tablets may be used adjunctively inconvulsive disorders, although it has not proved useful as the sole therapy.
The effectiveness of diazepam tablets in long-term use,that is, more than 4 months, has not been assessed by systematic clinicalstudies. The physician should periodically reassess the usefulness of the drugfor the individual patient.
Dosage for Diazepam
Dosage should be individualized for maximum beneficialeffect. While the usual daily dosages given below will meet the needs of mostpatients, there will be some who may require higher doses. In such cases dosageshould be increased cautiously to avoid adverse effects.
| ADULTS: | USUAL DAILY DOSE: |
| Management of Anxiety Disorders and Relief of Symptoms of Anxiety | Depending upon severity of symptoms—2 mg to 10 mg, 2 to 4 times daily |
| Symptomatic Relief in Acute Alcohol Withdrawal | 10 mg, 3 or 4 times during the first 24 hours, reducing to 5 mg, 3 or 4 times daily as needed |
| Adjunctively for Relief of Skeletal Muscle Spasm | 2 mg to 10 mg, 3 or 4 times daily |
| Adjunctively in Convulsive Disorders | 2 mg to 10 mg, 2 to 4 times daily |
| Geriatric Patients, or in the presence of debilitating disease | 2 mg to 2.5 mg, 1 or 2 times daily initially; increase gradually as needed and tolerated |
| PEDIATRIC PATIENTS: | |
| Because of varied responses to CNS-acting drugs, initiate therapy with lowest dose and increase as required. Not for use in pediatric patients under 6 months | 1 mg to 2.5 mg, 3 or 4 times daily initially; increase gradually as needed and tolerated |
HOW SUPPLIED
Diazepam Tablets, USP are available containing 2 mg, 5 mgor 10 mg of diazepam, USP.
The2 mg tablets are white, round, scored tabletsdebossed with MYLAN over 271 on one side and the other side being scored. Theyare available as follows:
NDC 0378-0271-01 bottles of 100 tablets
NDC 0378-0271-05 bottles of 500 tablets
The5 mg tablets are orange, round, scored tablets debossed with MYLAN over 345 on one side and the other side being scored. Theyare available as follows:
NDC 0378-0345-01 bottles of 100 tablets
NDC 0378-0345-05 bottles of 500 tablets
The10 mg tablets are green, round, scored tablets debossed with MYLAN over 477 on one side and the other side being scored. Theyare available as follows:
NDC 0378-0477-01 bottles of 100 tablets
NDC 0378-0477-05 bottles of 500 tablets
Store at 20° to 25°C (68° to 77°F). [See USP ControlledRoom Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as definedin the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.: Morgantown, WV 26505 U.S.A. Revised:Sep 2015
Side Effects for Diazepam
Side effects most commonly reported were drowsiness,fatigue, muscle weakness, and ataxia. The following have also been reported:
Central Nervous System: confusion, depression, dysarthria, headache, slurred speech, tremor, vertigo
Gastrointestinal System: constipation, nausea, gastrointestinal disturbances
Special Senses : blurred vision, diplopia,dizziness
Cardiovascular System:hypotension
Psychiatric and Paradoxical Reactions :stimulation,restlessness, acute hyperexcited states, anxiety, agitation, aggressiveness,irritability, rage, hallucinations, psychoses, delusions, increased muscle spasticity,insomnia, sleep disturbances, and nightmares. Inappropriate behavior and otheradverse behavioral effects have been reported when using benzodiazepines.Should these occur, use of the drug should be discontinued. They are morelikely to occur in children and in the elderly.
Urogenital System: incontinence, changes in libido, urinary retention
Skin and Appendages :skin reactions
Laboratories : elevated transaminases and alkalinephosphatase
Other: changes in salivation, including dry mouth,hypersalivation
Antegrade amnesia may occur using therapeutic dosages,the risk increasing at higher dosages. Amnestic effects may be associated withinappropriate behavior.
Minor changes in EEG patterns, usually low-voltage fastactivity, have been observed in patients during and after diazepam therapy andare of no known significance.
Because of isolated reports ofneutropenia andjaundice,periodic blood counts and liver function tests are advisable during long-termtherapy.
Postmarketing Experience
Injury, Poisoning, And Procedural Complications
There have been reports of falls and fractures inbenzodiazepine users. The risk is increased in those taking concomitantsedatives (including alcohol) and in the elderly.
Drug Abuse And Dependence
Diazepam is subject to Schedule IV control under theControlled Substances Act of 1970. Abuse and dependence of benzodiazepines havebeen reported. Addiction-prone individuals (such as drug addicts or alcoholics)should be under careful surveillance when receiving diazepam or otherpsychotropic agents because of the predisposition of such patients tohabituation and dependence. Once physical dependence to benzodiazepines hasdeveloped, termination of treatment will be accompanied by withdrawal symptoms.The risk is more pronounced in patients on long-term therapy.
Withdrawal symptoms, similar in character to those notedwith barbiturates and alcohol have occurred following abrupt discontinuance ofdiazepam. These withdrawal symptoms may consist of tremor, abdominal and musclecramps, vomiting, sweating, headache, muscle pain, extreme anxiety, tension, restlessness,confusion and irritability. In severe cases, the following symptoms may occur: derealization,depersonalization, hyperacusis, numbness and tingling of the extremities,hypersensitivity to light, noise and physical contact, hallucinations orepileptic seizures. The more severe withdrawal symptoms have usually beenlimited to those patients who had received excessive doses over an extendedperiod of time. Generally milder withdrawal symptoms (e.g., dysphoria andinsomnia) have been reported following abrupt discontinuance of benzodiazepinestaken continuously at therapeutic levels for several months. Consequently,after extended therapy, abrupt discontinuation should generally be avoided and agradual dosage tapering schedule followed.
Chronic use (even at therapeutic doses) may lead to thedevelopment of physical dependence: discontinuation of the therapy may resultin withdrawal or rebound phenomena.
Rebound Anxiety
A transient syndrome whereby the symptoms that led totreatment with diazepam recur in an enhanced form. This may occur upondiscontinuation of treatment. It may be accompanied by other reactions includingmood changes, anxiety, and restlessness.
Since the risk of withdrawal phenomena and reboundphenomena is greater after abrupt discontinuation of treatment, it isrecommended that the dosage be decreased gradually.
Drug Interactions for Diazepam
Centrally Acting Agents
If diazepam is to be combined with other centrally actingagents, careful consideration should be given to the pharmacology of the agentsemployed particularly with compounds that may potentiate or be potentiated bythe action of diazepam, such as phenothiazines, antipsychotics,anxiolytics/sedatives, hypnotics, anticonvulsants, narcotic analgesics,anesthetics, sedative antihistamines, narcotics, barbiturates, MAO inhibitorsand otherantidepressants.
Alcohol
Concomitant use with alcohol is not recommended due toenhancement of the sedative effect.
Antacids
Diazepam peak concentrations are 30% lower when antacidsare administered concurrently. However, there is no effect on the extent ofabsorption. The lower peak concentrations appear due to a slower rate ofabsorption, with the time required to achieve peak concentrations on average 20to 25 minutes greater in the presence of antacids. However, this difference wasnot statistically significant.
Compounds Which Inhibit Certain Hepatic Enzymes
There is a potentially relevant interaction betweendiazepam and compounds which inhibit certain hepatic enzymes (particularlycytochrome P450 3A and 2C19). Data indicate that these compounds influence thepharmacokinetics of diazepam and may lead to increased and prolonged sedation.At present, this reaction is known to occur with cimetidine, ketoconazole,fluvoxamine, fluoxetine, and omeprazole.
Phenytoin
There have also been reports that the metabolicelimination of phenytoin is decreased by diazepam.
Warnings for Diazepam
Diazepam is not recommended in the treatment of psychoticpatients and should not be employed instead of appropriate treatment.
Since diazepam has a central nervous system depressanteffect, patients should be advised against the simultaneous ingestion ofalcohol and other CNS-depressant drugs during diazepam therapy.
As with other agents that have anticonvulsant activity,when diazepam is used as an adjunct in treating convulsive disorders, thepossibility of an increase in the frequency and/or severity of grand mal seizuresmay require an increase in the dosage of standard anticonvulsant medication. Abruptwithdrawal of diazepam in such cases may also be associated with a temporaryincrease in the frequency and/or severity of seizures.
Pregnancy
An increased risk of congenital malformations and otherdevelopmental abnormalities associated with the use of benzodiazepine drugsduring pregnancy has been suggested. There may also be nonteratogenic risksassociated with the use of benzodiazepines during pregnancy. There have beenreports of neonatal flaccidity, respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late inpregnancy. In addition, children born to mothers receiving benzodiazepines on aregular basis late in pregnancy may be at some risk of experiencing withdrawalsymptoms during the postnatal period.
Diazepam has been shown to be teratogenic in mice andhamsters when given orally at daily doses of 100 mg/kg or greater(approximately eight times the maximum recommended human dose [MRHD = 1 mg/kg/day]or greater on a mg/m² basis). Cleft palate and encephalopathy are the mostcommon and consistently reported malformations produced in these species byadministration of high, maternally toxic doses of diazepam duringorganogenesis. Rodent studies have indicated that prenatal exposure to diazepamdoses similar to those used clinically can produce long-term changes incellular immune responses, brain neurochemistry, and behavior.
In general, the use of diazepam in women of childbearingpotential, and more specifically during known pregnancy, should be consideredonly when the clinical situation warrants the risk to the fetus. The possibilitythat a woman of childbearing potential may be pregnant at the time ofinstitution of therapy should be considered. If this drug is used during pregnancy,or if the patient becomes pregnant while taking this drug, the patient shouldbe apprised of the potential hazard to the fetus. Patients should also beadvised that if they become pregnant during therapy or intend to becomepregnant they should communicate with their physician about the desirability ofdiscontinuing the drug.
Labor And Delivery
Special care must be taken when diazepam is used duringlabor and delivery, as high single doses may produce irregularities in thefetal heart rate and hypotonia, poor sucking, hypothermia, and moderate respiratorydepression in the neonates. With newborn infants it must be remembered that theenzyme system involved in the breakdown of the drug is not yet fully developed(especially in premature infants).
Nursing Mothers
Diazepam passes into breast milk. Breastfeeding istherefore not recommended in patients receiving diazepam.
Precautions for Diazepam
General
If diazepam is to be combined with other psychotropicagents or anticonvulsant drugs, careful consideration should be given to the pharmacology of the agents to be employed - particularly with known compoundsthat may potentiate the action of diazepam, such as phenothiazines, narcotics, barbiturates,MAO inhibitors and other antidepressants (seeDRUG INTERACTIONS).
The usual precautions are indicated for severelydepressed patients or those in whom there is any evidence of latent depressionor anxiety associated with depression, particularly the recognition that suicidaltendencies may be present and protective measures may be necessary.
Psychiatric and paradoxical reactions are known to occurwhen using benzodiazepines (seeADVERSE REACTIONS). Should this occur,use of the drug should be discontinued. These reactions are more likely tooccur in children and the elderly.
A lower dose is recommended for patients with chronic respiratory insufficiency, due to the risk of respiratory depression.
Benzodiazepines should be used with extreme caution inpatients with a history of alcohol or drug abuse (seeDrug Abuse AndDependence).
In debilitated patients, it is recommended that thedosage be limited to the smallest effective amount to preclude the developmentof ataxia or oversedation (2 mg to 2.5 mg once or twice daily, initially, to beincreased gradually as needed and tolerated).
Some loss of response to the effects of benzodiazepinesmay develop after repeated use of diazepam for a prolonged time.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
In studies in which mice and rats were administereddiazepam in the diet at a dose of 75 mg/kg/day (approximately 6 and 12 times,respectively, the maximum recommended human dose [MRHD = 1 mg/kg/day] on a mg/m²basis) for 80 and 104 weeks, respectively, an increased incidence of liver tumorswas observed in males of both species. The data currently available areinadequate to determine the mutagenic potential of diazepam. Reproductionstudies in rats showed decreases in the number of pregnancies and in the numberof surviving offspring following administration of an oral dose of 100 mg/kg/day(approximately 16 times the MRHD on a mg/m² basis) prior to and during matingand throughout gestation and lactation. No adverse effects on fertility oroffspring viability were noted at a dose of 80 mg/kg/day (approximately 13times the MRHD on a mg/m² basis).
Pregnancy
Teratogenic Effects
Pregnancy Category D.
(see WARNINGS:Pregnancy).
Pediatric Use
Safety and effectiveness in pediatric patients below theage of 6 months have not been established.
Geriatric Use
In elderly patients, it is recommended that the dosage belimited to the smallest effective amount to preclude the development of ataxiaor oversedation (2 mg to 2.5 mg once or twice daily, initially to be increasedgradually as needed and tolerated).
Extensive accumulation of diazepam and its majormetabolite, desmethyldiazepam, has been noted following chronic administrationof diazepam in healthy elderly male subjects. Metabolites of this drug areknown to be substantially excreted by the kidney, and the risk of toxicreactions may be greater in patients with impaired renal function. Becauseelderly patients are more likely to have decreased renal function, care shouldbe taken in dose selection, and it may be useful to monitor renal function.
Hepatic Insufficiency
Decreases in clearance and protein binding, and increasesin volume of distribution and half-life has been reported in patients with cirrhosis. In such patients, a 2-fold to 5-fold increase in mean half-life hasbeen reported. Delayed elimination has also been reported for the activemetabolite desmethyldiazepam. Benzodiazepines are commonly implicated in hepatic encephalopathy. Increases in half-life have also been reported inhepatic fibrosis and in both acute and chronic hepatitis (seeCLINICALPHARMACOLOGY:Pharmacokinetics in Special Populations:HepaticInsufficiency).
Overdose Information for Diazepam
Overdose of benzodiazepines is usually manifested by central nervous system depression ranging from drowsiness to coma. In mildcases, symptoms include drowsiness, confusion, and lethargy. In more seriouscases, symptoms may include ataxia, diminished reflexes, hypotonia, hypotension, respiratory depression, coma (rarely), and death (very rarely).Overdose of benzodiazepines in combination with other CNS depressants(including alcohol) may be fatal and should be closely monitored.
Management Of Overdosage
Following overdose with oral benzodiazepines, generalsupportive measures should be employed including the monitoring of respiration, pulse, and blood pressure. Vomiting should be induced (within one hour) if thepatient is conscious. Gastric lavage should be undertaken with the airwayprotected if the patient is unconscious. Intravenous fluids should be administered.If there is no advantage in emptying the stomach,activated charcoal should begiven to reduce absorption. Special attention should be paid to respiratory andcardiac function in intensive care. General supportive measures should be employed,along with intravenous fluids, and an adequate airway maintained. Shouldhypotension develop, treatment may include intravenous fluid therapy,repositioning, judicious use of vasopressors appropriate to the clinicalsituation, if indicated, and other appropriate countermeasures. Dialysis is of limitedvalue.
As with the management of intentional overdosage with anydrug, it should be considered that multiple agents may have been ingested.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with abenzodiazepine is known or suspected. Prior to the administration offlumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to,not as a substitute for, proper management of benzodiazepine overdose. Patientstreated with flumazenil should be monitored for resedation, respiratorydepression and other residual benzodiazepine effects for an appropriate periodafter treatment.The prescribers hould be aware of a risk of seizure in associationwith flumazenil treatment, particularly in long-term benzodiazepine users andin cyclic antidepressant overdose. Caution should be observed in the use offlumazenil in epileptic patients treated with benzodiazepines. The completeflumazenil package insert, including CONTRAINDICATIONS, WARNINGS, andPRECAUTIONS, should be consulted prior to use.
Withdrawal symptoms of the barbiturate type have occurredafter the discontinuation of benzodiazepines (seeDrug Abuse And Dependence).
Contraindications for Diazepam
Diazepam tablets are contraindicated in patients with aknown hypersensitivity to diazepam and, because of lack of sufficient clinicalexperience, in pediatric patients under 6 months of age. Diazepam is also contraindicatedin patients with myasthenia gravis, severe respiratory insufficiency, severehepatic insufficiency, andsleep apnea syndrome. It may be used in patientswith open-angleglaucoma who are receiving appropriate therapy, but iscontraindicated in acute narrow-angle glaucoma.
Clinical Pharmacology for Diazepam
Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of theseeffects are thought to result from a facilitation of the action of gamma aminobutyricacid (GABA), an inhibitory neurotransmitter in the central nervous system.
Pharmacokinetics
Absorption
After oral administration > 90% of diazepam isabsorbed and the average time to achieve peak plasma concentrations is 1 to 1.5hours with a range of 0.25 to 2.5 hours. Absorption is delayed and decreased whenadministered with a moderate fat meal. In the presence of food mean lag timesare approximately 45 minutes as compared with 15 minutes when fasting. There isalso an increase in the average time to achieve peak concentrations to about2.5 hours in the presence of food as compared with 1.25 hours when fasting.This results in an average decrease in Cmax of 20% in addition to a 27%decrease in AUC (range 15% to 50%) when administered with food.
Distribution
Diazepam and its metabolites are highly bound to plasmaproteins (diazepam 98%). Diazepam and its metabolites cross the blood-brain andplacental barriers and are also found in breast milk in concentrationsapproximately one tenth of those in maternal plasma (days 3 to 9 post-partum).In young healthy males, the volume of distribution at steady-state is 0.8 to 1L/kg. The decline in the plasma concentration-time profile after oraladministration is biphasic. The initial distribution phase has a halflife ofapproximately one hour, although it may range up to > 3 hours.
Metabolism
Diazepam is N-demethylated by CYP3A4 and 2C19 to theactive metabolite N-desmethyldiazepam, and is hydroxylated by CYP3A4 to theactive metabolite temazepam. N-desmethyldiazepam and temazepam are both furthermetabolized to oxazepam. Temazepam and oxazepam are largely eliminated by glucuronidation.
Elimination
The initial distribution phase is followed by a prolongedterminal elimination phase (half-life up to 48 hours). The terminal eliminationhalf-life of the active metabolite N-desmethyldiazepam is up to 100 hours.Diazepam and its metabolites are excreted mainly in the urine, predominantly astheir glucuronide conjugates. The clearance of diazepam is 20 to 30 mL/min inyoung adults. Diazepam accumulates upon multiple dosing and there is someevidence that the terminal elimination half-life is slightly prolonged.
Pharmacokinetics In Special Populations
Children
In children 3 to 8 years old the mean half-life ofdiazepam has been reported to be 18 hours.
Newborns
In full term infants, elimination half-lives around 30hours have been reported, with a longer average half-life of 54 hours reportedin premature infants of 28 to 34 weeks gestational age and 8 to 81 days post-partum.In both premature and full term infants the active metabolite desmethyldiazepamshows evidence of continued accumulation compared to children. Longerhalf-lives in infants may be due to incomplete maturation of metabolicpathways.
Geriatric
Elimination half-life increases by approximately one hourfor each year of age beginning with a halflife of 20 hours at 20 years of age.This appears to be due to an increase in volume of distribution with age and adecrease in clearance. Consequently, the elderly may have lower peakconcentrations, and on multiple dosing higher trough concentrations. It willalso take longer to reach steady-state. Conflicting information has beenpublished on changes of plasma protein binding in the elderly. Reported changesin free drug may be due to significant decreases in plasma proteins due tocauses other than simplyaging.
Hepatic Insufficiency
In mild and moderate cirrhosis, average half-life isincreased. The average increase has been variously reported from 2-fold to5-fold, with individual half-lives over 500 hours reported. There is also an increasein volume of distribution, and average clearance decreases by almost half. Meanhalf-life is also prolonged with hepatic fibrosis to 90 hours (range 66 to 104hours), with chronic active hepatitis to 60 hours (range 26 to 76 hours), andwith acute viral hepatitis to 74 hours (range 49 to 129). In chronic active hepatitis,clearance is decreased by almost half.
Patient Information for Diazepam
To assure the safe and effective use of benzodiazepines,patients should be informed that, since benzodiazepines may producepsychological and physical dependence, it is advisable that they consult withtheir physician before either increasing the dose or abruptly discontinuingthis drug. The risk of dependence increases with duration of treatment; it isalso greater in patients with a history of alcohol or drug abuse.
Patients should be advised against the simultaneousingestion of alcohol and other CNS-depressant drugs during diazepam therapy. Asis true of most CNS-acting drugs, patients receiving diazepam should becautioned against engaging in hazardous occupations requiring complete mentalalertness, such as operating machinery or driving a motor vehicle.
From

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit theFDA MedWatch website or call 1-800-FDA-1088.
Pill Identifier ToolQuick, Easy, Pill Identification
Drug Interaction ToolCheck Potential Drug Interactions
Pharmacy Locator ToolIncluding 24 Hour, Pharmacies